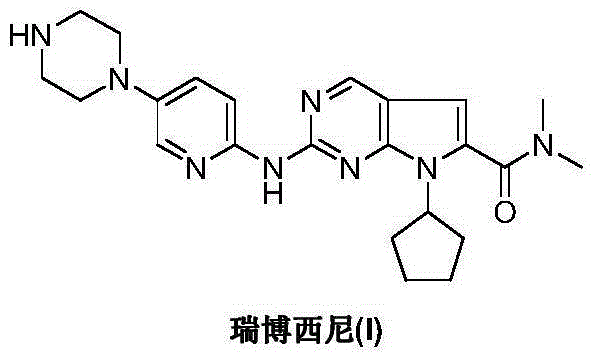
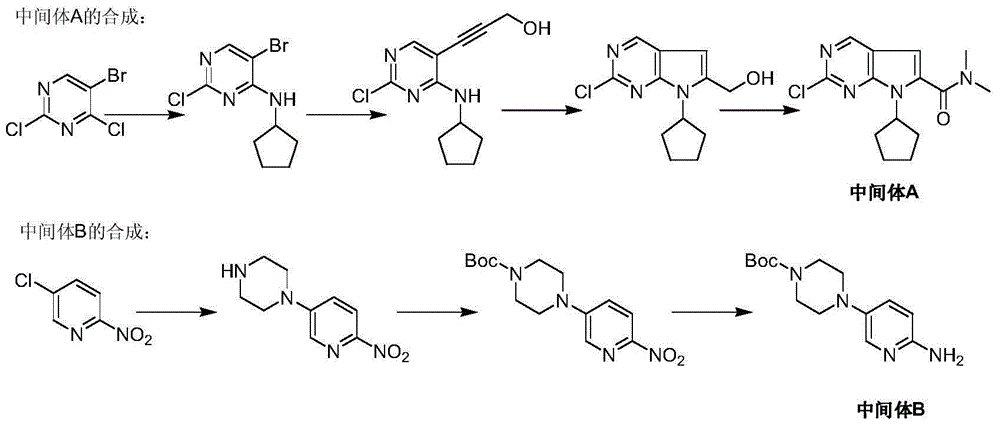
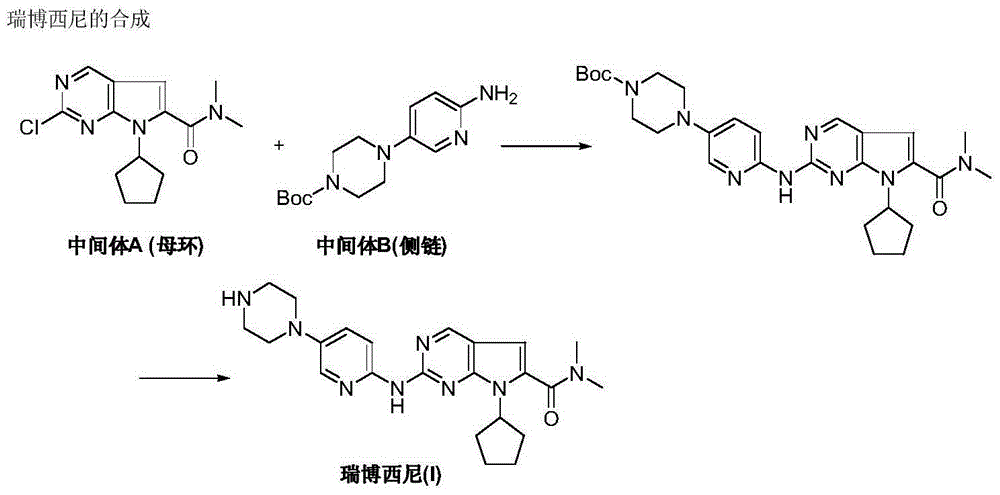
Ribociclib intermediate and preparation method thereof
A dimethyl and methoxy technology, applied in the field of preparation of the drug ribociclib, can solve the problems of cumbersome preparation process, difficult industrialized production, etc., and achieve the effects of easy availability of raw materials, promotion of development, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add N,N-dimethyl-2-carbonyl-propionamide (IV) (5.75g, 50mmol), 0.1g of 98% concentrated sulfuric acid and 30mL of tetrahydrofuran into a dry reaction flask, and heat up to 50-55°C. Bromine (7.9 g, 55 mmol) dried with concentrated sulfuric acid was added dropwise under stirring. After the drop was completed, the reaction was continued with stirring at the temperature for 3-5 hours until the color of bromine basically faded and the reaction was completed. The solvent was recovered under reduced pressure, the residue was recrystallized from ethyl acetate and n-hexane (1:1, V / V), and dried in vacuo at room temperature to obtain a pale yellow solid N,N-dimethyl-1-halogen-2-carbonyl-propane Amide (V) 8.3g, yield 86.0%; EI-MSm / z: 193[M+H] + .
Embodiment 2
[0034] Add N,N-dimethyl-1-halogen-2-carbonyl-propionamide (V) (5.8g, 30mmol), malononitrile (2.2g, 33mmol), diethylamine (6.6g, 90mmol) and silicon dioxide 15g, ground and stirred at room temperature for 30 minutes, and TLC detected that the reaction was complete. Add 100 mL of chloroform into the reaction flask, stir and filter, then wash the filter cake three times with chloroform, combine the organic phases, wash with water and saturated brine in turn, dry over anhydrous sodium sulfate, concentrate, and dry in vacuo to obtain off-white solid N,N- Dimethyl-1,1-dinitrile-3-carbonyl-butanamide (VI) 4.5g, yield 83.8%; EI-MSm / z: 180[M+H] + .
Embodiment 3
[0036] Add N,N-dimethyl-1,1-dinitrile-3-carbonyl-butanamide (VI) (3.6g, 20mmol) and 50mL of methanol into the reaction flask, cool to 4-7°C in an ice bath, and stir Hydrogen chloride gas (5.4 g, 0.15 mol) was slowly introduced under low pressure, and after the completion of the passage, it was raised to room temperature, and the stirring reaction was continued for 24-26 hours. TLC detected that the reaction was complete. Nitrogen was introduced to drive off excess hydrogen chloride gas, concentrated to obtain a viscous solid, and recrystallized with methanol and water (1:1) to obtain light yellow solid 2-methoxy-5-(N,N-dimethyl-formazan Amino)-3-pyrrolecarbonitrile (VII) 3.3g, yield 90.2%; mass spectrum (EI): EI-MSm / z: 194[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com