A crystal form of ribociclib monosuccinate
A technology of monosuccinate and ribociclib, which is applied in the preparation of carboxylate, medical preparations containing active ingredients, drug combinations, etc., can solve the risk of increasing the esterification reaction of succinic acid and isopropanol , reduce the production efficiency of tableting and filling in the preparation, increase the risk of degradation, etc., so as to improve the appearance of the product, ensure the uniformity of mixing and content, and improve the quality of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
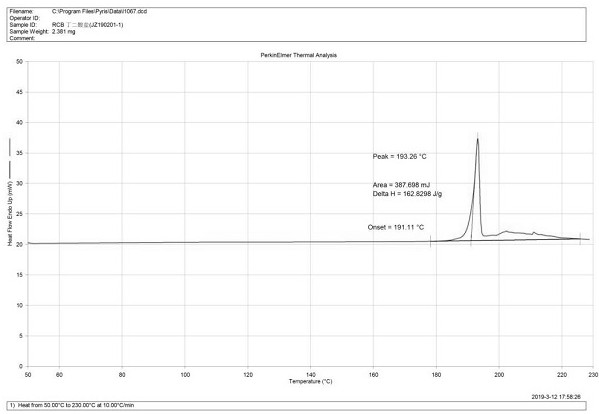
[0053] Weigh 152g ribociclib free base into a 5L reaction flask, add 2.5L isopropanol, stir mechanically, heat to reflux, stir to dissolve. Weigh 43.37g of succinic acid, add it into 450mL of isopropanol, and reflux to dissolve. At 80°C, add the isopropanol solution of succinic acid dropwise into the isopropanol solution of ribociclib free base. After about 15 minutes, the solution becomes clear and the color changes from light yellow to yellow. Incubate at 80°C for 15 minutes. Remove the heat source, lower the temperature slowly, and a solid slowly precipitates out. After the solution was cooled to 15°C, stirred for 1 hour, suction filtered, and the wet product was vacuum-dried at 55°C for 16 hours, 179 g of ribociclib succinate was obtained. Yield 92.7%.
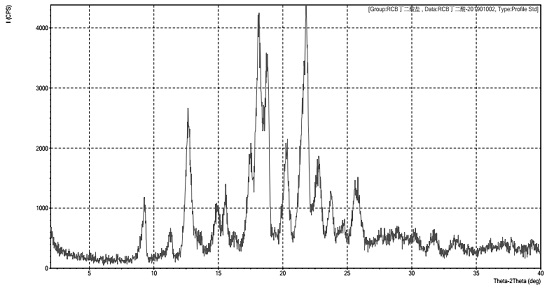
[0054] Table 1 shows the X-ray powder diffraction data of Form I obtained in this example. The XRPD graph is shown in Table 1:
[0055]
[0056] Table 1
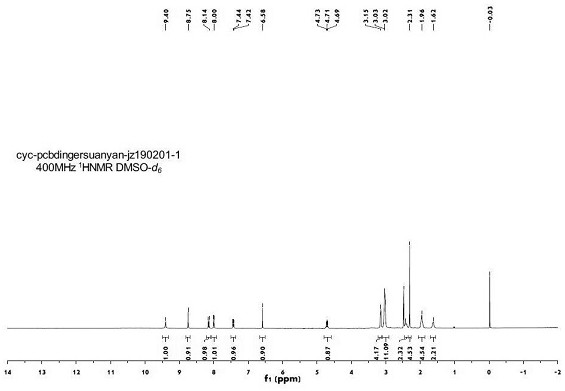
[0057] The NMR spectrum of the crystal form I is as follows...
Embodiment 2
[0061] Stability study of crystal form I of the present invention and monosuccinate anhydrous crystal form of patent CN103201275A under high humidity conditions:
[0062] 1. Take a dry stoppered glass weighing bottle (50mm in outer diameter, 15mm in height) and place it in a suitable constant temperature dryer at 25°C±1°C the day before (place ammonium chloride or ammonium sulfate saturated solution in the lower part) or manually Precise weighing (m1) in the climate chamber (setting temperature is 25°C±1°C, relative humidity is 80%±2%);
[0063] 2. Take an appropriate amount of the test product, put it in the above weighing bottle and lay it flat in the weighing bottle. The thickness of the test product is generally about 1mm, and weigh it precisely (m2);
[0064] 3. Open the weighing bottle and place it together with the bottle cap under the above constant temperature and humidity conditions for 24 hours;
[0065] 4. Close the cap of the weighing bottle and weigh it precisel...
Embodiment 3
[0069] Solubility studies of Form I:
[0070] The monosuccinate crystal form I prepared by the present invention is prepared into a solution with SGF (simulated artificial gastric juice) at pH 1.8, pH 6.5 FaSSIF (artificial intestinal juice in a fasting state) and pH 5.0 FeSSIF (artificial intestinal juice in a fed state) , observed the phenomenon after 1 hour, 4 hours and 24 hours, and found that it was homogeneously dissolved. The experimental results are shown in Table 2:
[0071]
[0072] Table 2
[0073] This experiment shows that the solubility of the crystal form I in the simulated biological medium is greater than 10 mg / mL, which meets the requirements for pharmaceutical use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com