Crystal form of ribociclib succinate as well as preparation method and application thereof
A technology of monosuccinate and ribociclib, applied in the field of medicine, can solve the problems of high equipment requirements, large residual risk of solvents, and unsuitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0120] The present invention provides a method for preparing the above-mentioned crystal form AZT-XXIII. The preparation method comprises: salting the compound of formula (I) and succinic acid in methanol, suction filtration, and drying the filter cake in vacuum. The temperature of the vacuum drying is 25-40°C, preferably 35°C. The compound of formula (I) is a solid compound of formula (I) in any form.
[0121] Crystal form AZT-XXIV and its preparation method
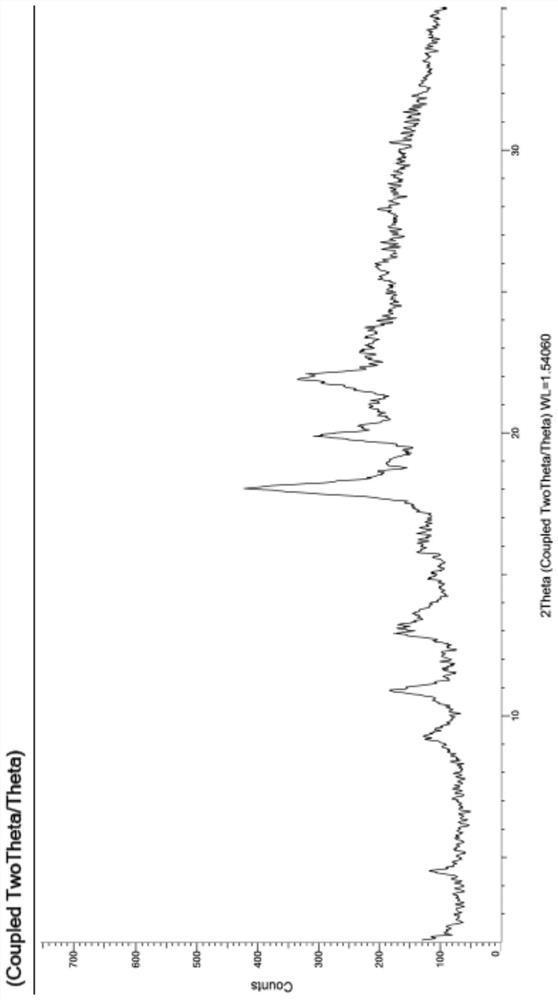
[0122] The crystal form AZT-XXIV provided by the present invention has characteristic peaks in its X-ray diffraction pattern at 2θ values of 8.3°±0.2°, 11.6°±0.2°, 17.7°±0.2° and 20.3°±0.2°.
[0123] Furthermore, the crystal form AZT-XXIV provided by the present invention has an X-ray diffraction pattern with 2θ values of 10.9°±0.2°, 12.7°±0.2°, 14.9°±0.2°, 18.0°±0.2°, 18.9 There are characteristic peaks at °±0.2°, 19.1°±0.2°, 19.4°±0.2°, 19.8°±0.2°, 21.5°±0.2°, 22.7°±0.2°.
[0124] Furthermore, the X-ray diffrac...
Embodiment 1
[0184] Embodiment 1: Preparation of crystal form AZT-XXIII
[0185] Weigh 0.5 g of succinic acid and dissolve it in 30 mL of methanol, weigh 2 g of the compound of formula (I) and suspend it in 30 mL of methanol, add the methanol solution of succinic acid dropwise, filter, and dry the filter cake under vacuum at 40°C to obtain a solid. The obtained solid is the monosuccinate salt form XXIII of the compound of formula (I).
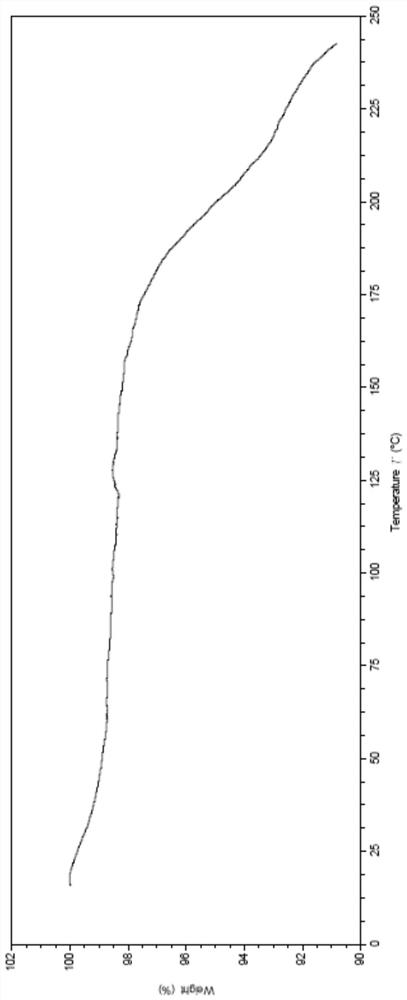
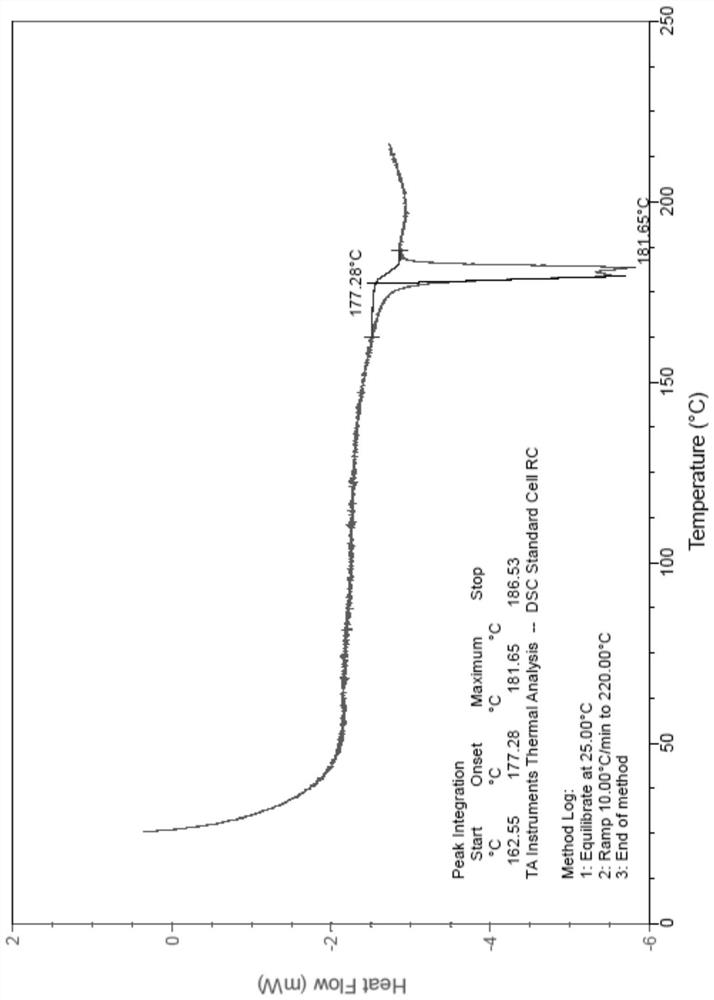
[0186] Carry out XRPD test to the obtained solid, its X-ray powder diffraction pattern is as follows figure 1 As shown, the specific data are shown in Table 1; the obtained solid is tested by TGA, and its spectrogram is as follows figure 2 As shown, there is about 1.5% weight loss when heated to 100°C, which is anhydrous; DSC test is carried out on the obtained solid, and its spectrum is as follows image 3 As shown, the melting point is 181.7°C; 1 H-NMR test, the spectrum is basically as Figure 4 Shown, is monosuccinate.
[0187] Table 1
[0188] ...
Embodiment 2
[0189] Embodiment 2: Preparation of crystal form AZT-XXIV
[0190] Weigh 20 mg of AZT-XXIII in the above Example 1, dissolve in 2 mL of N-methylpyrrolidone / acetone (1:5, v / v) mixed solvent, make a slurry, filter, and vacuum-dry the filter cake at 35°C to obtain a solid, The obtained solid is the monosuccinate salt crystal form AZT-XXIV of the compound of formula (I).
[0191] Carry out XRPD test to the obtained solid, its X-ray powder diffraction pattern is as follows Figure 5 As shown, the specific data are shown in Table 2; the obtained solid is tested by TGA, and its spectrogram is as follows Figure 6 As shown, it has a weight loss of about 7.9% when heated to 145 °C; the resulting solid is subjected to DSC testing, and its spectrogram is as follows Figure 7 As shown, there is an exothermic peak at 131.5°C, an endothermic peak at 132.9°C, and a melting point of 185.8°C.
[0192] Table 2
[0193] 2θ strength 8.3 68.3% 10.9 42.5% 11.6 54.1% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com