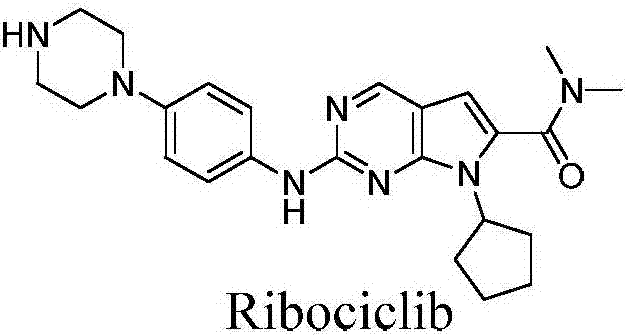
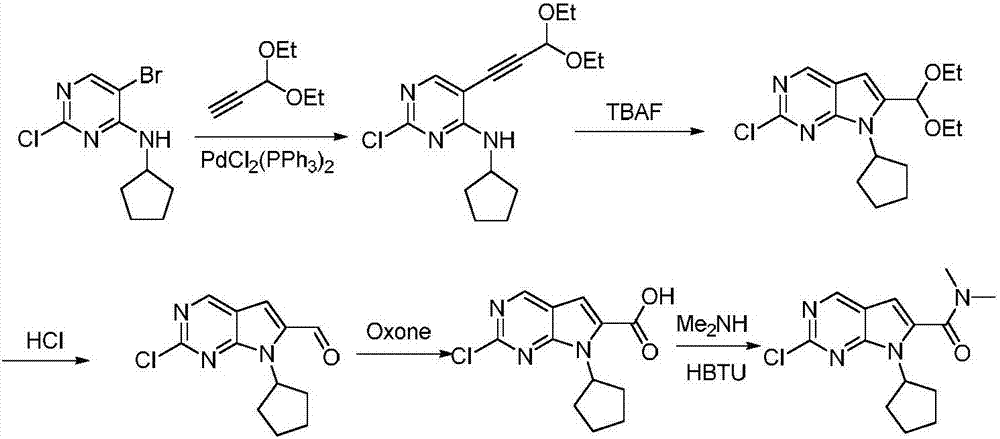
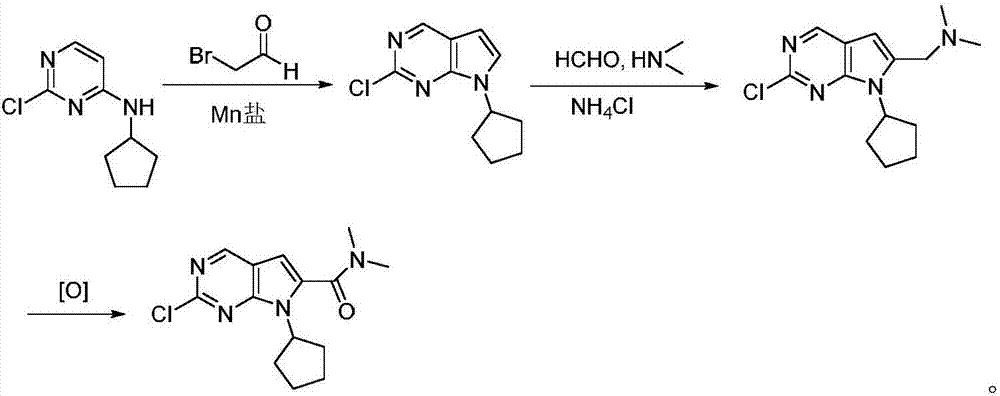
Preparation method for intermediate of Ribociclib for treating breast cancers
A technology of ribociclib and intermediates, applied in the field of drug synthesis, can solve the problems of limiting industrial production and application, unsatisfactory, etc., and achieve the effect of increased yield and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0029] Preparation of 2-chloro-4-cyclopentylaminopyrimidine
[0030] In a 500ml round bottom flask, dissolve 75g of 2,4-dichloropyrimidine in 230ml of DMF, then mix 56g of cyclopentylamine and 70g of triethylamine at room temperature, stir overnight at room temperature, pour the reaction solution into water, dichloromethane Extracted and purified by column chromatography to obtain 91.3 g of 2-chloro-4-cyclopentylaminopyrimidine with a yield of 91.7%, MS (ESI) m / z: 198.07 [M+H] + .
Embodiment 1
[0032] Preparation of 2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidine
[0033] Manganese salt 24.1g (Mn(OAc) 3 2H 2 (2, 90mmol), 19.8g (100mmol) of 2-chloro-4-cyclopentylaminopyrimidine, and 16g (130mmol) of 2-bromoacetaldehyde were added to water, stirred at 35°C for contact reaction for 5 hours, and the reaction solution was cooled to room temperature. Extracted with dichloromethane, concentrated under reduced pressure, and recrystallized from petroleum ether to obtain 20.3 g of 2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidine with a yield of 91.7% and a purity of 99.70% (HPLC area normalization method). MS (ESI) m / z: 222.07, [M+H] + , 1 HNMR(d 6 -DMSO, 300MHz) δ1.55-1.68(m,4H),1.71-1.87(m,4H),4.12(m,1H),6.37(m,1H),8.03(d,1H),8.72(s, 1H).
Embodiment 2
[0035] Preparation of 2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidine
[0036] Manganese salt 32.2g (Mn(OAc) 3 2H 2 (2, 120mmol), 19.8g (100mmol) of 2-chloro-4-cyclopentylaminopyrimidine, and 14.8g (120mmol) of 2-bromoacetaldehyde were added to water, stirred at 50°C for contact reaction for 4 hours, and the reaction solution was cooled to room temperature , extracted with dichloromethane, concentrated under reduced pressure, and recrystallized from petroleum ether to obtain 19.8 g of 2-chloro-7-cyclopentyl-7H-pyrrole[2,3-d]pyrimidine, with a yield of 89.5% and a purity of 99.44% ( HPLC area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com