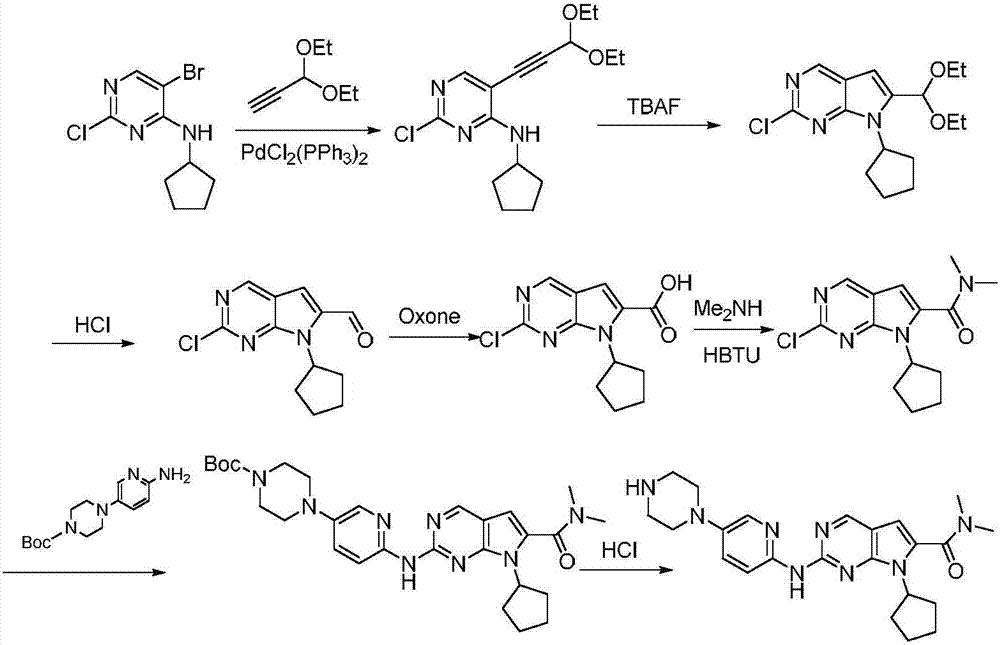
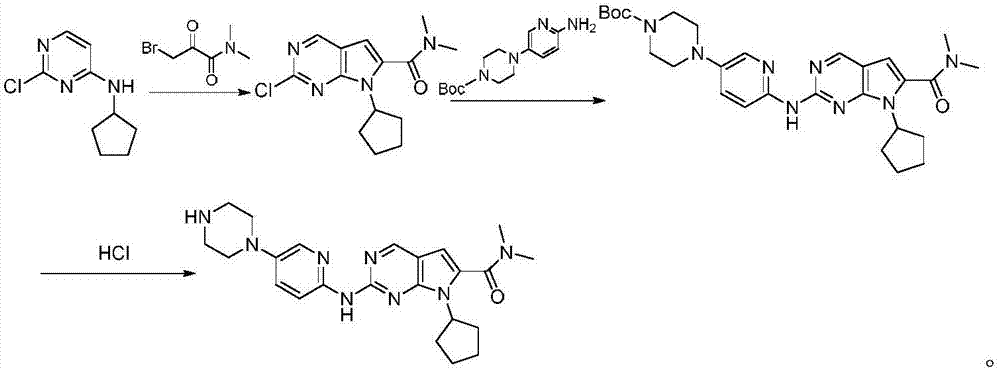
Synthesis technology of ribociclib
A synthesis process and ribociclib technology, which is applied in the field of ribociclib synthesis process, can solve the problems of excessively long reaction steps, low yield and high cost, and achieve mild conditions, high yield and few reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0025] Preparation of 2-chloro-4-cyclopentylaminopyrimidine
[0026] In a 500ml round bottom flask, dissolve 75g of 2,4-dichloropyrimidine in 230ml of DMF, then mix 56g of cyclopentylamine and 70g of triethylamine at room temperature, stir overnight at room temperature, pour the reaction solution into water, dichloromethane Extracted and purified by column chromatography to obtain 91.1 g of 2-chloro-4-cyclopentylaminopyrimidine with a yield of 91.5%, MS (ESI) m / z: 198.07 [M+H] + .
preparation example 2
[0028] Preparation of 3-bromo-2-oxo-N,N-dimethylpropionamide
[0029] In a 500ml round bottom flask, at room temperature, dissolve 26.1g (300mmol) of 2-oxopropionic acid, 136.5g (360mmol) of HBTU, and 7.8g (60mmol) of DIEA in 230ml of DMF, stir for 30min, and then drop into dimethyl Amine 16.2g (360mmol), continued to stir at room temperature overnight, the reaction solution was poured into water, extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, and the organic phase was concentrated under reduced pressure. The concentrate was dissolved in anhydrous dichloromethane, NBS 100g (600mmol) was added dropwise under ice cooling, stirred for 5 hours, quenched with ice water, the organic phase was separated, washed with sodium thiosulfate, the organic phase was concentrated under reduced pressure, and flash column Chromatography yielded 47.7 g of 3-bromo-2-oxo-N,N-dimethylpropionamide, with a yield of 81.9%, MS (ESI) m / z: 193.97 [M+H] +...
Embodiment 1
[0031] Preparation of 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimidine-6-carboxamide
[0032] In a 500ml round bottom flask, add 97.7g (300mmol) of cesium carbonate, 19.8g (100mmol) of 2-chloro-4-cyclopentylaminopyrimidine, 3-bromo-2-oxo-N,N-dimethylpropane Amide 23.3g (120mmol), cuprous iodide 1.5g (8mmol), L-proline 1.2g (10mmol), 220mlTHF, heated up to 40°C and stirred for 4-8 hours, poured into water, extracted with dichloromethane, Recrystallized from n-hexane to obtain 22.6 g of 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimidine-6-carboxamide with a yield of 77.2% and a purity of 99.44% (HPLC area normalization method). MS (ESI) m / z: 293.11 [M+H] + , 1 HNMR(d 6 -DMSO, 300MHz) δ1.57-1.68(m,4H),1.72-1.87(m,4H),3.20(s,6H),4.11(m,1H),6.12(m,1H),8.73(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com