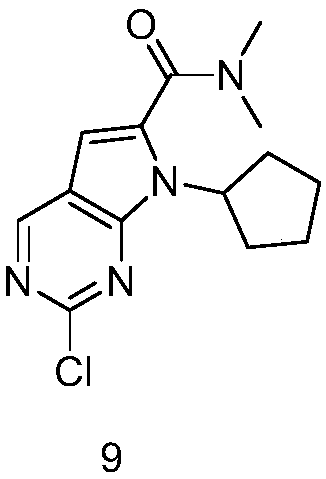
Synthesis method of ribociclib intermediate product and intermediate compound thereof
A synthetic method, ribociclib technology, applied in the synthetic method of ribociclib intermediate products and its intermediate compounds, can solve the problems of long route, difficult reaction amplification, expensive raw materials and reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
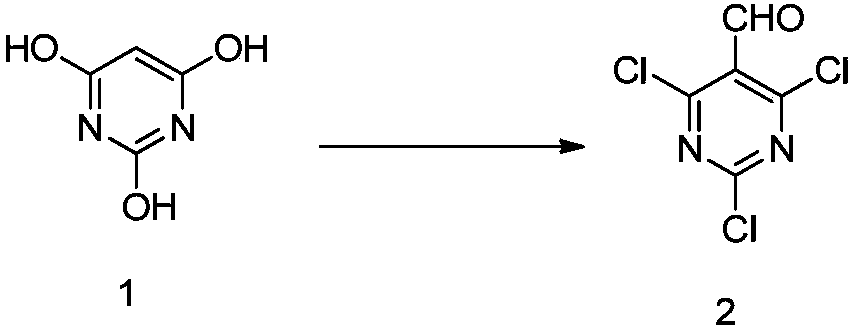
[0070] Add phosphorus oxychloride (153.3g, 1000mmol) into the three-necked flask, stir, cool down to -10~0°C in an ice-salt bath, slowly add N,N-dimethylformamide (36.5g, 500mmol) dropwise, and keep the temperature constant When the temperature is higher than 5°C, after the dropwise addition, the temperature is controlled and stirred for 1 hour, barbituric acid 1 (38.4g, 300mmol) is added, the ice-salt bath is removed, the temperature is naturally returned to room temperature, and then the temperature is raised to 120°C, and stirred overnight. After the completion of the reaction as monitored by TLC, the reaction solution was slowly added to ice water, a large amount of solids precipitated, cold filtered, and the filter cake was washed with water until the effluent was neutral to obtain a light yellow solid, which was dried at 50-60°C by air blast to obtain compound 2 (58.3g , yield 92.5%). ESI m / z = 211.01 (M+1).
Embodiment 2
[0072]
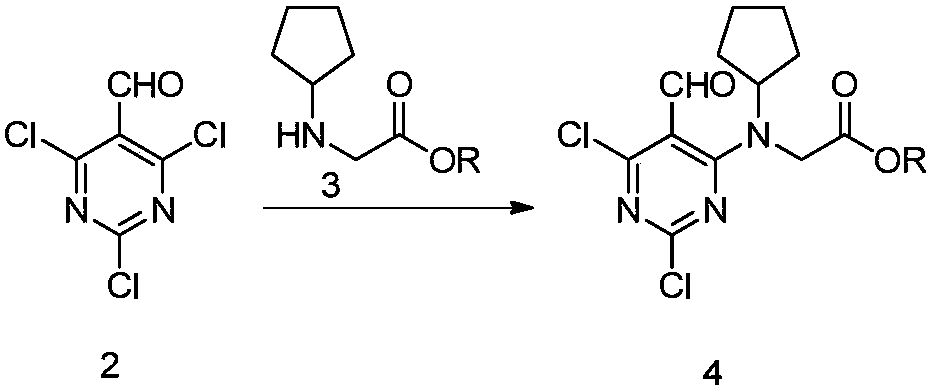
[0073] Methanol (200mL), cyclopentanone (16.8g, 200mmol), glycine methyl ester (17.8g, 200mmol), and acetic acid (0.12g, 2mmol) were sequentially added into the single-port reaction flask. Stir in an ice bath, add sodium triacetate borohydride (63.6 g, 300 mmol) in batches, return to room temperature and stir for 2 hours after the addition, TLC monitors that the reaction is complete, drop water (150 g) to quench the reaction, and concentrate under reduced pressure to remove methanol. Add 1 mol / L hydrochloric acid (200mL), ethyl acetate (200mL), separate liquid, organic layer TLC detects that there is no product, discard it, add solid sodium bicarbonate to the aqueous phase to adjust the pH to about 8, extract three times with ethyl acetate (200mL) , the combined organic phases were concentrated to dryness under reduced pressure to obtain a brown oily compound 3-1 (29.8 g, yield 95.0%). ESI m / z = 158.21 (M+1).
Embodiment 3
[0075]
[0076] Add 2 (31.6g, 150mmol) and dichloromethane (300mL) to the three-necked flask successively, stir in an ice bath, and cool down to 5-15°C; add compound 3-1 (25.9g, 175mmol) and diisopropylethylamine dropwise (38g, 200mmol) in dichloromethane (100mL) solution, control the internal temperature at 10-20°C, remove the ice bath after the dropwise addition, and react at room temperature for 2-5 hours, after TLC monitors the reaction is complete, add 150mL of water, separate the liquid, and extracted with 100 mL of dichloromethane, combined the organic phases, washed once with 200 mL of saturated saline, collected the organic phases, concentrated to dryness to obtain a brown semi-solid, added 50 mL of ethyl acetate and 100 mL of petroleum ether, heated to 50°C and stirred for 1 h, cooled to Filter at 0-5°C, collect the filter cake, and dry under reduced pressure at 40-50°C to obtain a light yellow solid, compound 4-1 (44.3g, yield 89.2%). ESI m / z = 332.12 (M+1). 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com