Compound and application thereof in synthesis of ribociclib
A compound and reaction technology, applied in the field of compound and its application in the synthesis of rebocoxib, can solve the problem of eliminating heavy metal residues and achieve the effect of reducing and removing heavy metal residues and complete substitution reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
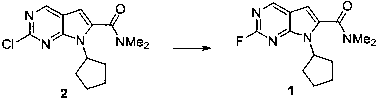
[0019]
[0020] Add 3.0g of compound 2, 3.0g of potassium fluoride and 1.0g of tetrabutylammonium hydrogensulfate into the reaction flask, add 30mL of dimethyl sulfoxide and then raise the temperature to 150°C for 24 hours, monitor the reaction by TLC and use 100mL of water The reaction was quenched, then extracted twice with 50 mL of ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, and the filtrate was collected by filtration. After spinning to dry the solvent, the product was purified by n-heptane / ethyl acetate column chromatography with a yield of 53%.
[0021] M+H molecular ion peak 277.1;
[0022] 1 H NMR (400MHz, CDCl 3 ) δ 8.77 (1H, d), 6.58 (1H, s), 4.75-4.90 (1H, m),3.18 (3H, s), 3.13 (3H, s), 2.30-2.45 (2H, m), 1.95- 2.15 (4H, m), 1.60-1.75 (2H, m).
Embodiment 2
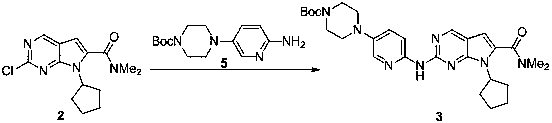
[0024]
[0025] 600 mg of compound 5 was dissolved in 3 mL of toluene solution. At 0~10°C, add 2mL of 1mol / L LiHMDS solution in tetrahydrofuran, and stir for 1h. At 0-10°C, 292 mg of compound 2 in toluene was added, and slowly returned to room temperature for 5 h. After the reaction was monitored by HPLC, 6mL of saturated ammonium chloride was used to quench the reaction, the liquid was separated to obtain the organic phase, dried with anhydrous sodium sulfate, the filtrate was collected by filtration and spin-dried to dry the solvent, and then purified by n-heptane / ethyl acetate column chromatography to obtain Off-white solid, yield 54%.
[0026] M+H molecular ion peak 535.3;
[0027] 1 H NMR (400MHz, CDCl 3 ) δ 8.70 (1H, s), 8.37 (1H, d), 8.02 (1H, d), 7.99(1H, s), 7.33 (1H, dd), 6.44 (1H, s), 4.72-4.85 (1H, ( 2H, m), 1.49 (9H, s).
Embodiment 3
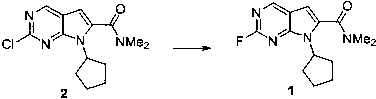
[0029]
[0030] 900 mg of compound 5 was dissolved in 10 mL of toluene solution. At 0~10°C, add 4mL of 1mol / L LiHMDS solution in tetrahydrofuran, and stir for 1h. At 0-10°C, 450 mg of compound 1 in toluene was added, and slowly returned to room temperature for 2.5 h. After the reaction was monitored by HPLC, 9 mL of saturated ammonium chloride was used to quench the reaction, the liquid was separated to obtain an organic phase, and dried using anhydrous sodium sulfate. The filtrate was collected by filtration and spin-dried to dry the solvent, and purified by n-heptane / ethyl acetate column chromatography. A off-white solid was obtained with a yield of 86%.
[0031] M+H molecular ion peak 535.3;
[0032] 1 H NMR (400MHz, CDCl 3 ) δ 8.70 (1H, s), 8.37 (1H, d), 8.02 (1H, d), 7.99(1H, s), 7.33 (1H, dd), 6.44 (1H, s), 4.72-4.85 (1H, ( 2H, m), 1.49 (9H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com