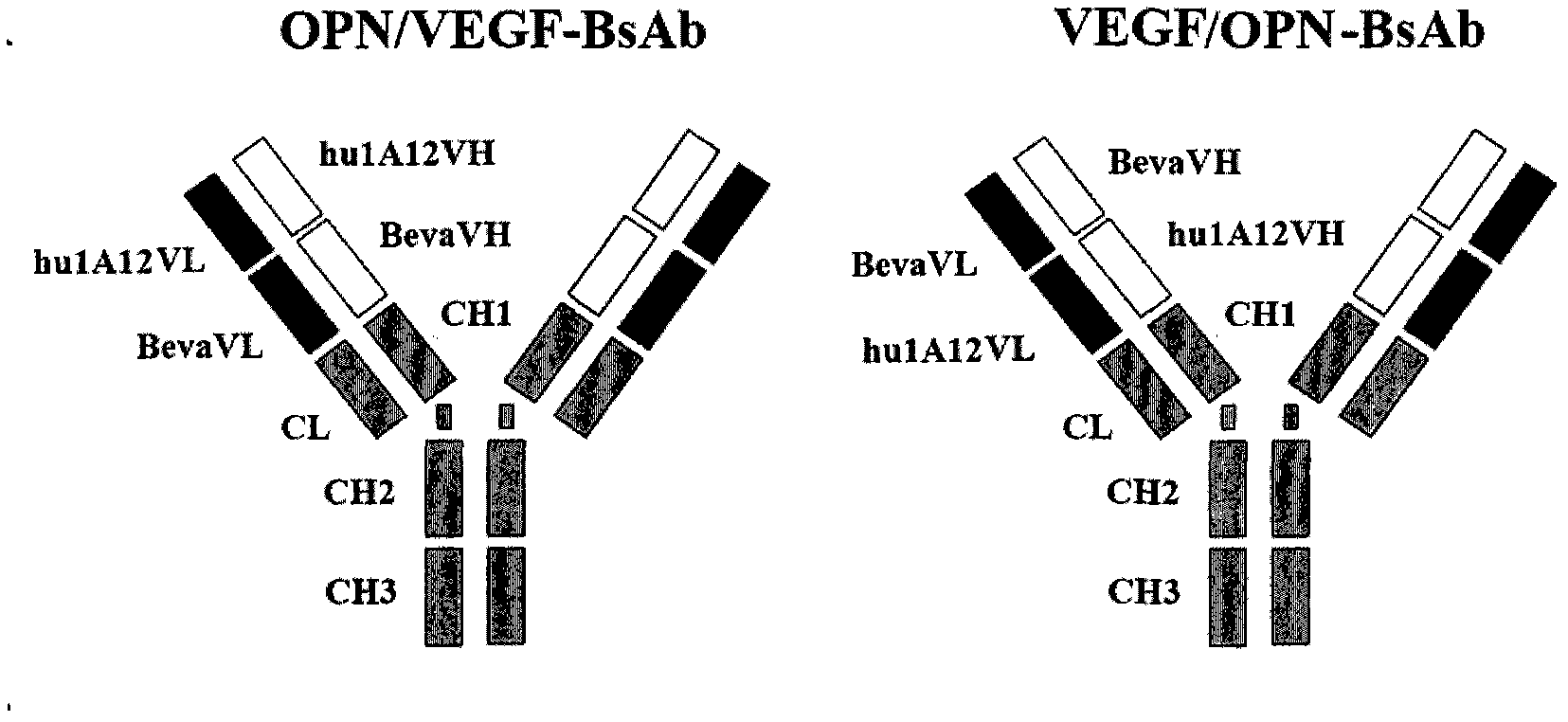
Anti-human VEGF/anti-OPN bispecific antibody, its preparation method and application
A technology for bispecific antibodies and uses, which can be applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-tumor drugs, etc., and can solve problems such as adjustment disorders, safety risks, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Cloning of Human Antibody Light and Heavy Chain Constant Regions and Fc Region Genes
[0050] Isolate healthy human lymphocytes with lymphocyte separation fluid, extract total RNA with Trizol reagent (Invitrogen company product), according to literature (Cell, 1980, 22:197-207) and literature (Nucleic Acids Research, 1982, 10: 4071-4079 ) primers were designed to amplify the heavy chain and light chain constant region genes of the antibody respectively. The PCR reactions were all hot-started. The reaction conditions were: 94°C for minutes; 94°C for 45 seconds; 60°C for 45 seconds; 30 cycles; 10 minutes at 72°C. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector (promega company product). After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO: 1 and SEQ ID NO: 2 show the nucleotide and amino acid sequences of the heavy chain constant region (CH), respectively. SE...
Embodiment 2
[0051] Example 2: Construction of novel bispecific antibodies
[0052]Specific construction method: According to the sequence reported in (Cancer Res.57 (1997) 4593-4599), the heavy and light chain variable region genes of Bevacizumab were synthesized from the whole gene, and the light and heavy chain variable region genes of Hu1A12, please refer to Chinese patent 200710039873.3 , WO2008128455. The heavy chain variable region of Bevacizumab was connected in series with the N-terminus of the heavy chain variable region of hu1A12 cloned by PCR through the Linker (ASTKGP, AST or GGGGS) or directly to construct VEGF / OPN-BsAb, The heavy chain variable regions of VEGF / OPN-BsAb1, VEGF / OPN-BsAb2, and VEGF / OPN-BsAb3 are then linked to the heavy chain constant regions of human IgG1 antibodies to construct VEGF / OPN-BsAb, VEGF / OPN- The heavy chain gene of BsAb1, VEGF / OPN-BsAb2, VEGF / OPN-BsAb3; the light chain variable region of Bevacizumab was passed through the Overlap PCR method via Li...
experiment example 1
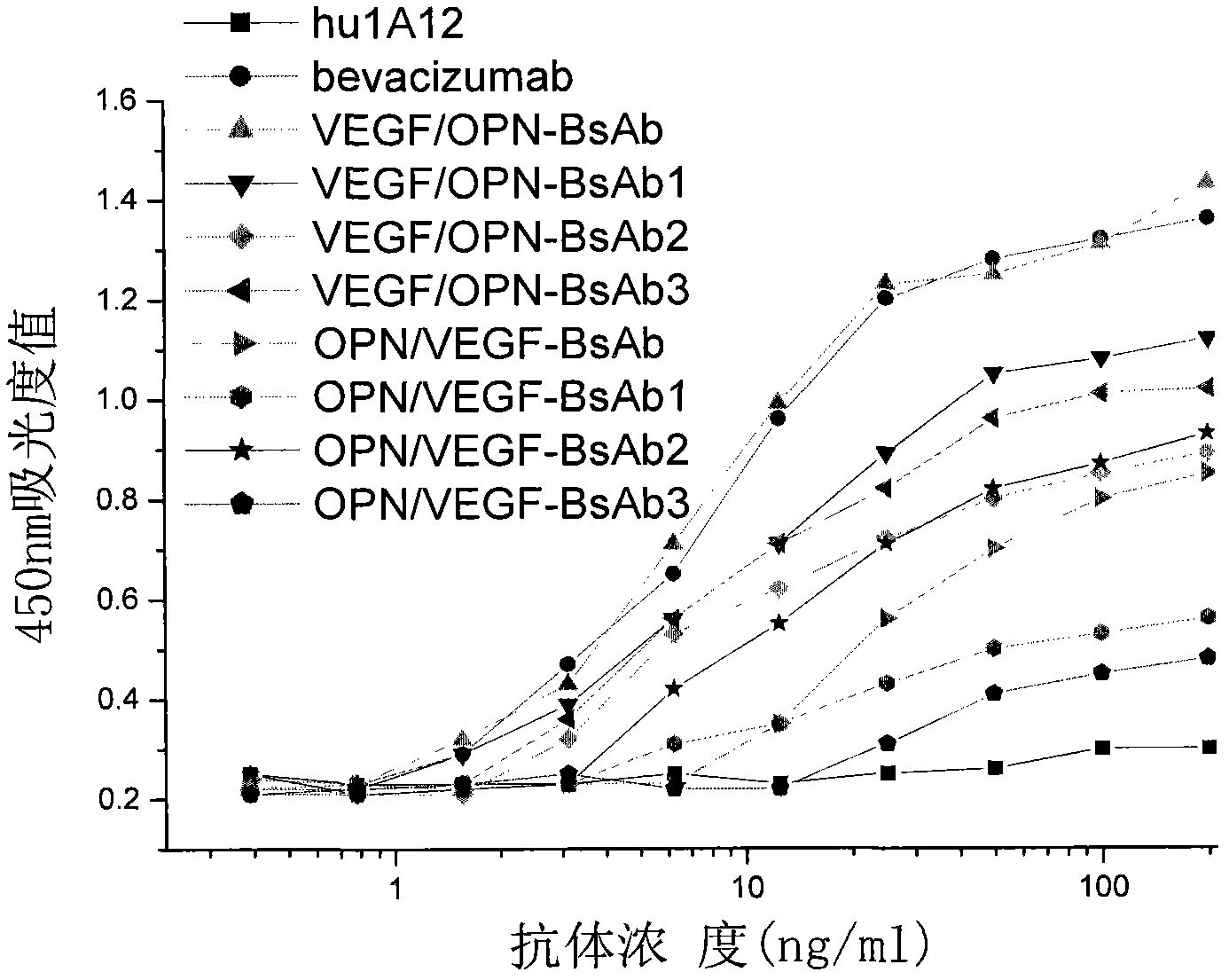
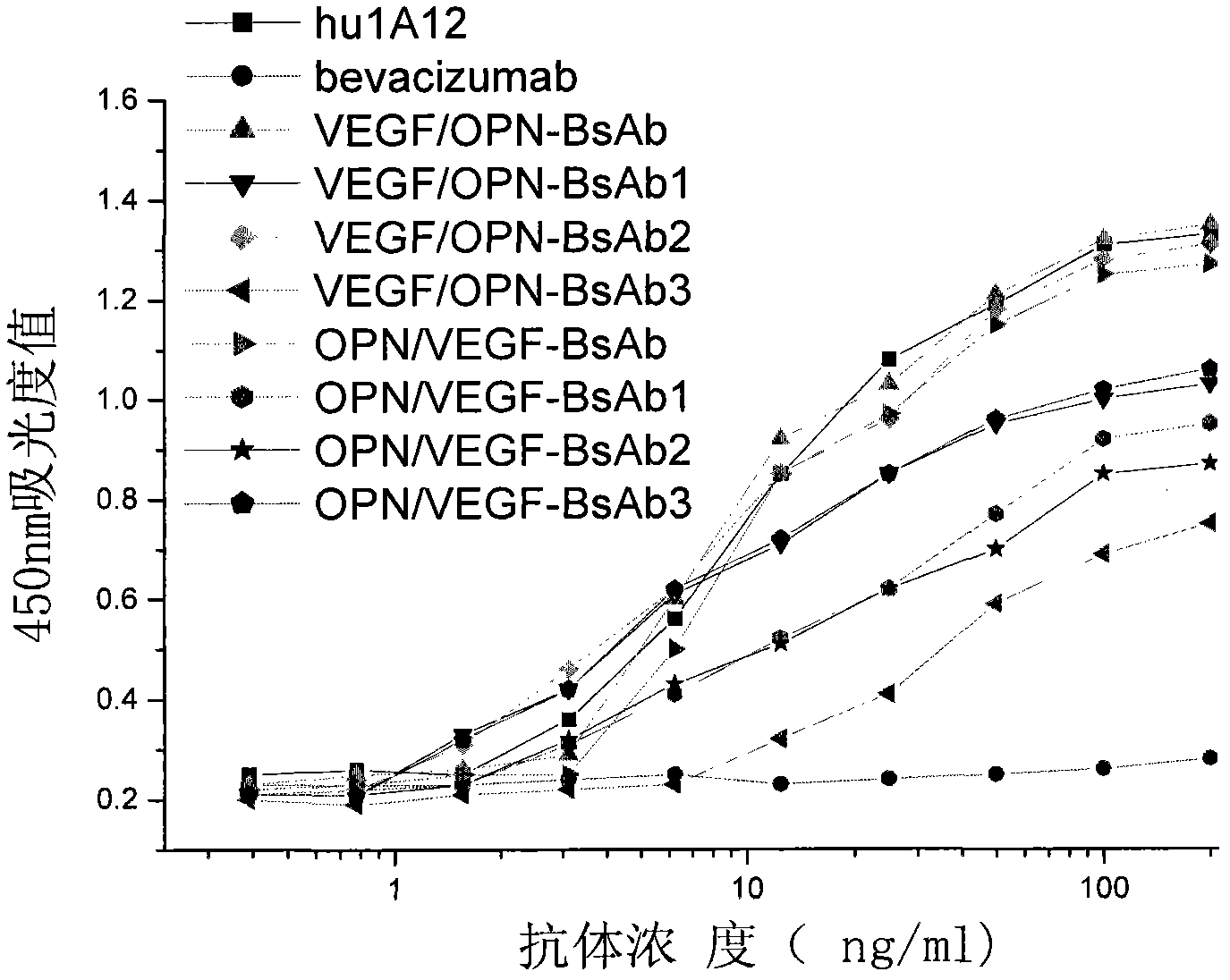
[0057] Experimental example 1: Detection of binding activity of bispecific antibody to VEGF and OPN
[0058] Dilute VEGF (product of R&D Company) with 0.05mmol / L sodium carbonate-sodium bicarbonate buffer solution (pH 9.6) to 2ug / ml, 50ul / well, and coat overnight at 4°C. After blocking with 10% skimmed milk at room temperature for 2 hours, different concentrations of bispecific antibodies and bevacizumab (product of Roche Company) were added, 50ul / well, and 3 parallel wells were taken for each concentration, and incubated at room temperature for 2 hours. Discard the supernatant, wash with PBS three times, add HRP-labeled goat anti-human IgG monoclonal antibody (product of KPL Company) diluted according to the titer, 50ul / well, and incubate at room temperature for 45min. After fully washing with PBS and TMD color development, the absorbance (A450) value at 450 nm was measured in a microplate reader (Elx type automatic enzyme colorimeter, American BIO-TEK company). Experimental...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com