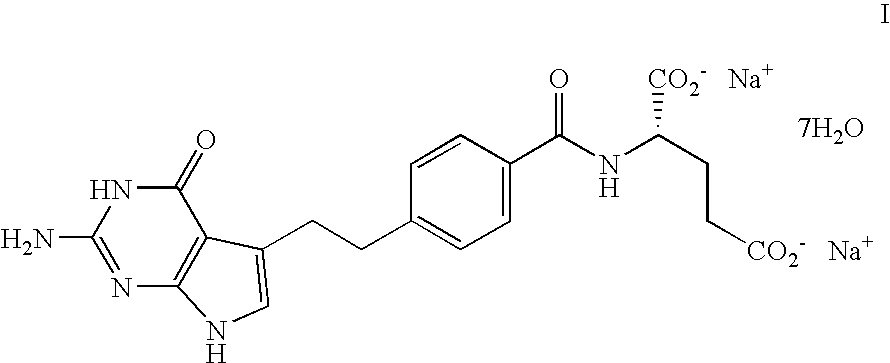
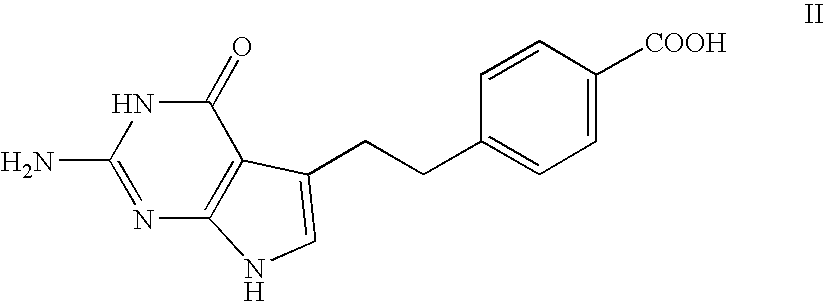
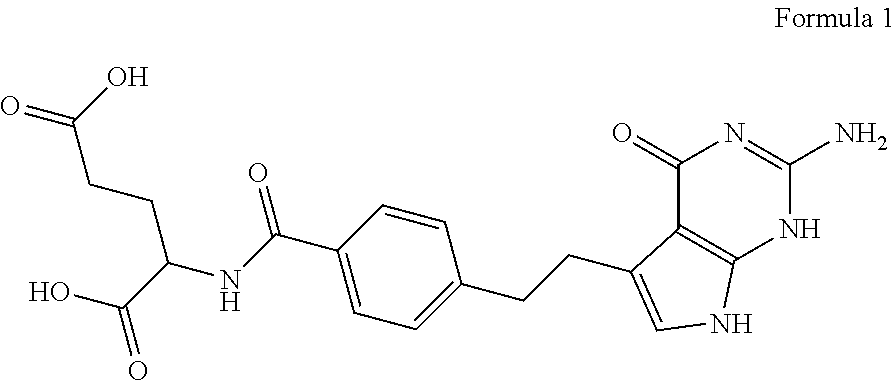
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
97 results about "Pemetrexed" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
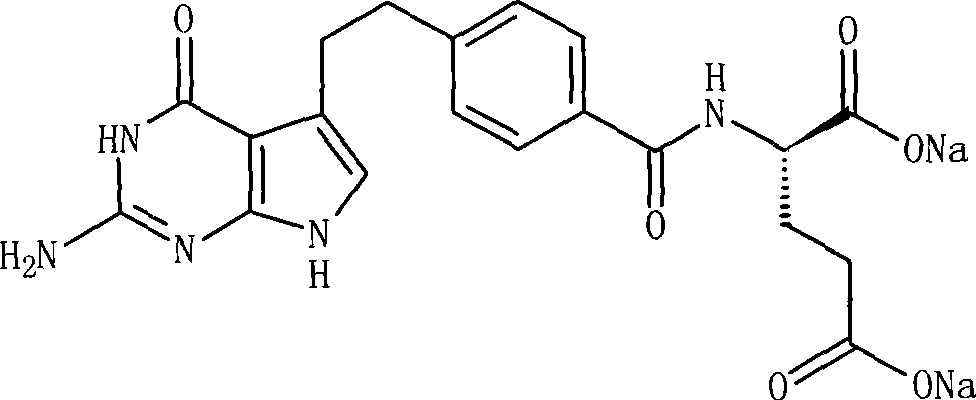
Pemetrexed (brand name Alimta) is a chemotherapy drug manufactured and marketed by Eli Lilly and Company. Its indications are the treatment of pleural mesothelioma and non-small cell lung cancer.
Pemetrexed disodium freeze-dried injection and preparation method thereof
ActiveCN101411710AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLSulfite salt
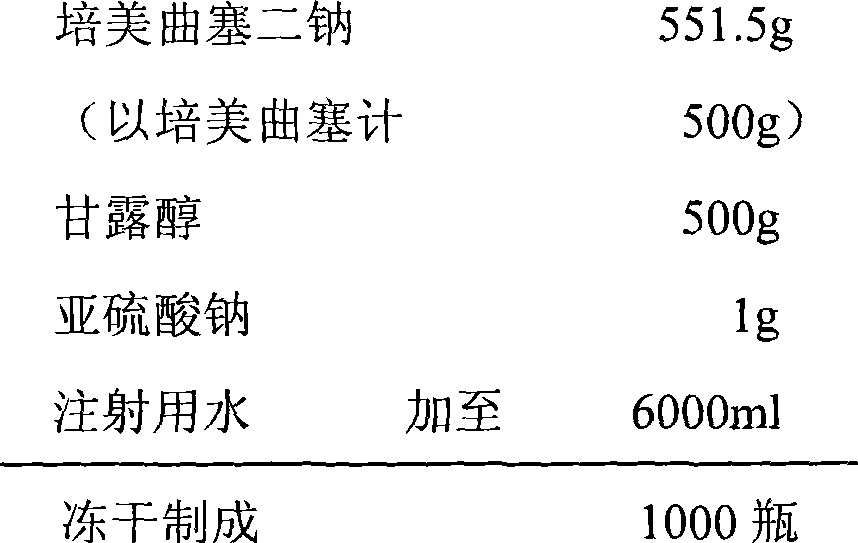
The invention relates to a pemetrexed disodium lyophilized powder injection, which consists of pemetrexed disodium, mannitol and sodium sulfite in the following weight portions: 50 portions of the pemetrexed disodium, 10 to 50 portions of mannitol, and 0.1 to 1 portions of sodium sulfite; and the pH value of the pemetrexed disodium lyophilized powder injection is between 7.0 and 8.0. The process for preparing the pemetrexed disodium lyophilized powder injection comprises the following steps: placing the mannitol in a sterile chamber; adding 80 percent of water for injection into the sterile chamber to dissolve the mannitol; adding the sodium sulfite to the mixture after the water for injection is cooled to a temperature of between 15 and 25 DEG C, and evenly stirring the solution for dissolving the sodium sulfite; then, adding the pemetrexed disodium into the solution, and stirring the solution to completely dissolve the pemetrexed disodium and evenly mixing the pemetrexed disodium, and adjusting the pH value of the solution to between 7.0 and 8.0; decarbidizing; after an intermediate compound passes examination, carrying out volume fixing, filtering, filling, partially stopping, traying, lyophilizing, nitrogen aerating, stopping and unboxing, sealing by a plastic-aluminum combined cap, and packaging after passes quality inspection to obtain the pemetrexed disodium lyophilized powder injection. The invention has the advantages of simple preparation process, convenience and practicality, good repeatability and low production cost, and can realize industrial large-scale production easily.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Medicinal composition containing pemetrexed
InactiveCN101081301AEffective treatmentMaintain stabilityOrganic active ingredientsPharmaceutical delivery mechanismArginineAntioxidant
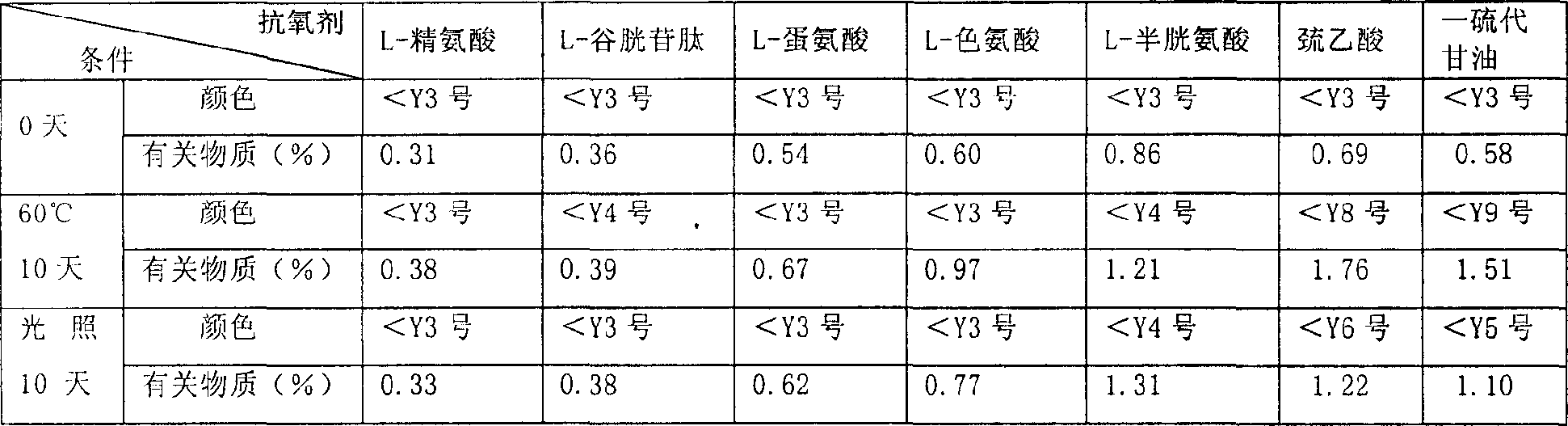
The present invention provides one kind of medicine composition, which contains Pemetrexed, at least one kind of antioxidant selected from L-arginine, L-glutathione, L-methionine and L-tryptophan, and pharmaceutically acceptable excipient. The medicine composition has high stability and acceptable storing period, and is suitable for liquid administration through no gastrointestinal tract.
Owner:海南天源康泽医药科技有限公司
Pharmaceutical compositions containing pemetrexed having extended storage stability
ActiveUS20130231357A1Improve long-term stabilityBiocideOrganic active ingredientsDiseasePharmaceutical medicine
Long term storage stable pemetrexed-containing liquid pharmaceutical compositions are disclosed. The compositions can include pemetrexed or pharmaceutically acceptable salts thereof; an antioxidant selected from lipoic acid, dihydrolipoic acid, methionine and mixtures thereof; a chelating agent selected from lactobionic acid, sodium citrate, tribasic and mixtures thereof; and a pharmaceutically acceptable fluid. The pH of the compositions is in a range of about 8 to about 9.5. The pemetrexed-containing compositions have less than about 5% total impurities, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 227 nm, after at least about 18 months of storage at a temperature of from about 5° C. to about 25° C. Methods of preparing the formulation as well as methods of treatment of pemetrexed-susceptible diseases using the same are also disclosed.
Owner:EAGLE PHARMACEUTICALS INC
Pharmaceutical composition containing pemetrexed
InactiveCN1907284AAvoid degradationColor stableOrganic active ingredientsPharmaceutical delivery mechanismCisplatinPemetrexed
The invention discloses a drug composition with Peimeiqusai and stabilizer at 5: 2-7, wherein the weight rate of Peimeiqusai and shaping agent is 0.5-1: 1. The invention is stable for light and heat, which can be reserved at normal temperature.
Owner:NANJING YINUOWEI PHARM TECH
Nitro compounds and their application in preparation of pemetrexed
ActiveCN1827604AImprove solubilityHigh purityOrganic active ingredientsOrganic chemistryNitro compoundPhotochemistry
The invention relates to a nitro compound and process of its preparation, as well as the use in the preparation of pemetrexed, a kind of anti-tumor medicament, which has the advantages of simplified post-reaction treatment, high product purity, and facilitation of industrialized production.
Owner:QILU PHARMA HAINAN
Combination cancer therapy with an hsp90 inhibitor and an antimetabolite
InactiveUS20140296176A1Increasing side effect profileSurprising biological activityBiocideCarbohydrate active ingredientsCytarabineHsp Inhibitor
The invention provides a method of treating a subject with cancer, particularly leukemia, lymphoma, solid cancer such as colorectal cancer, gastric cancer, bladder cancer, non-small cell lung cancer, and breast cancer, comprising administering to the subject a compound of formulae (I) 40 or (Ia) in combination with an antimetabolite such as methotrexate, pemetrexed, cytarabine or nelarabine, or 5-fluorouracil, or capecitabine or their derivatives.
Owner:SYNTA PHARMA CORP
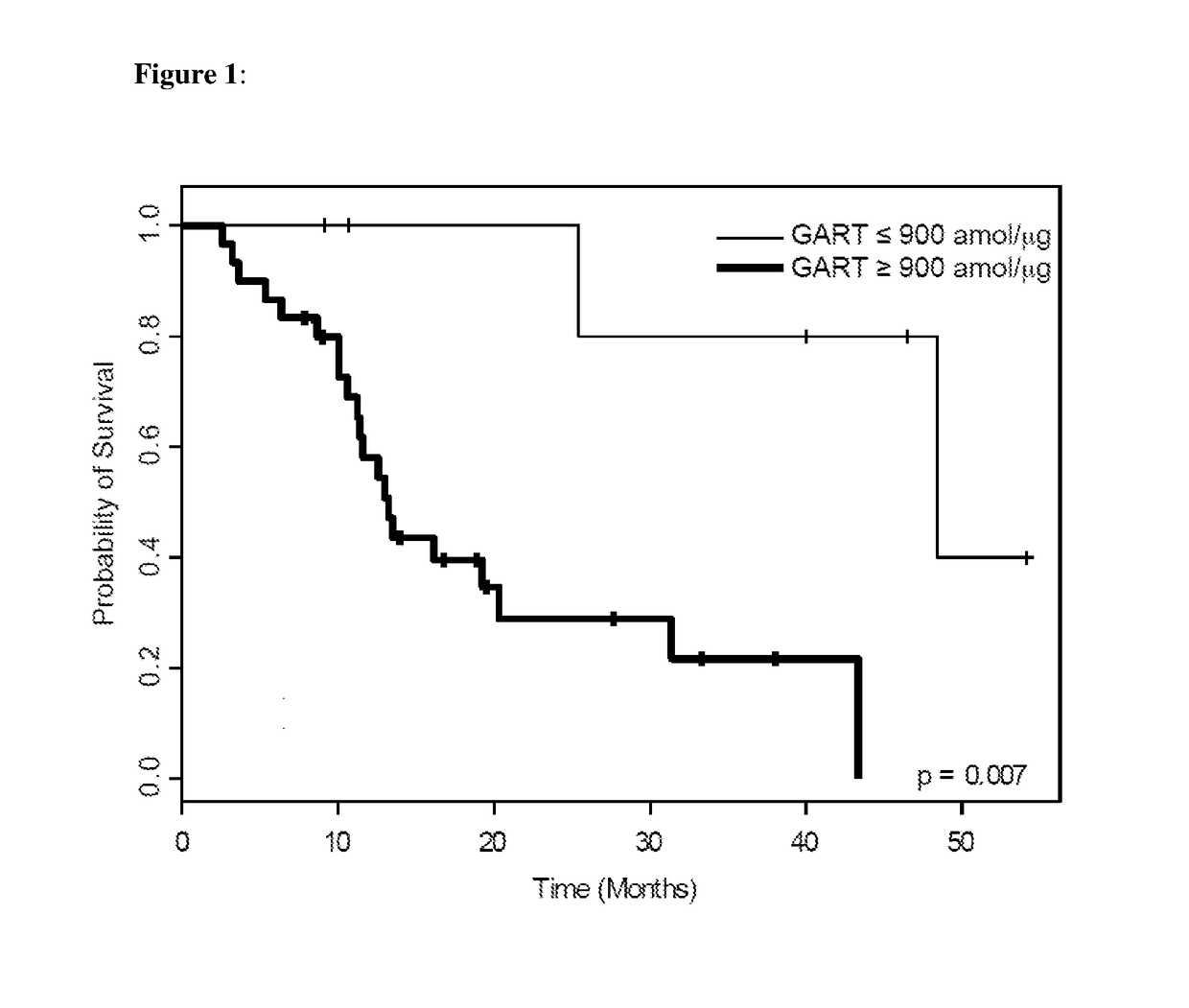
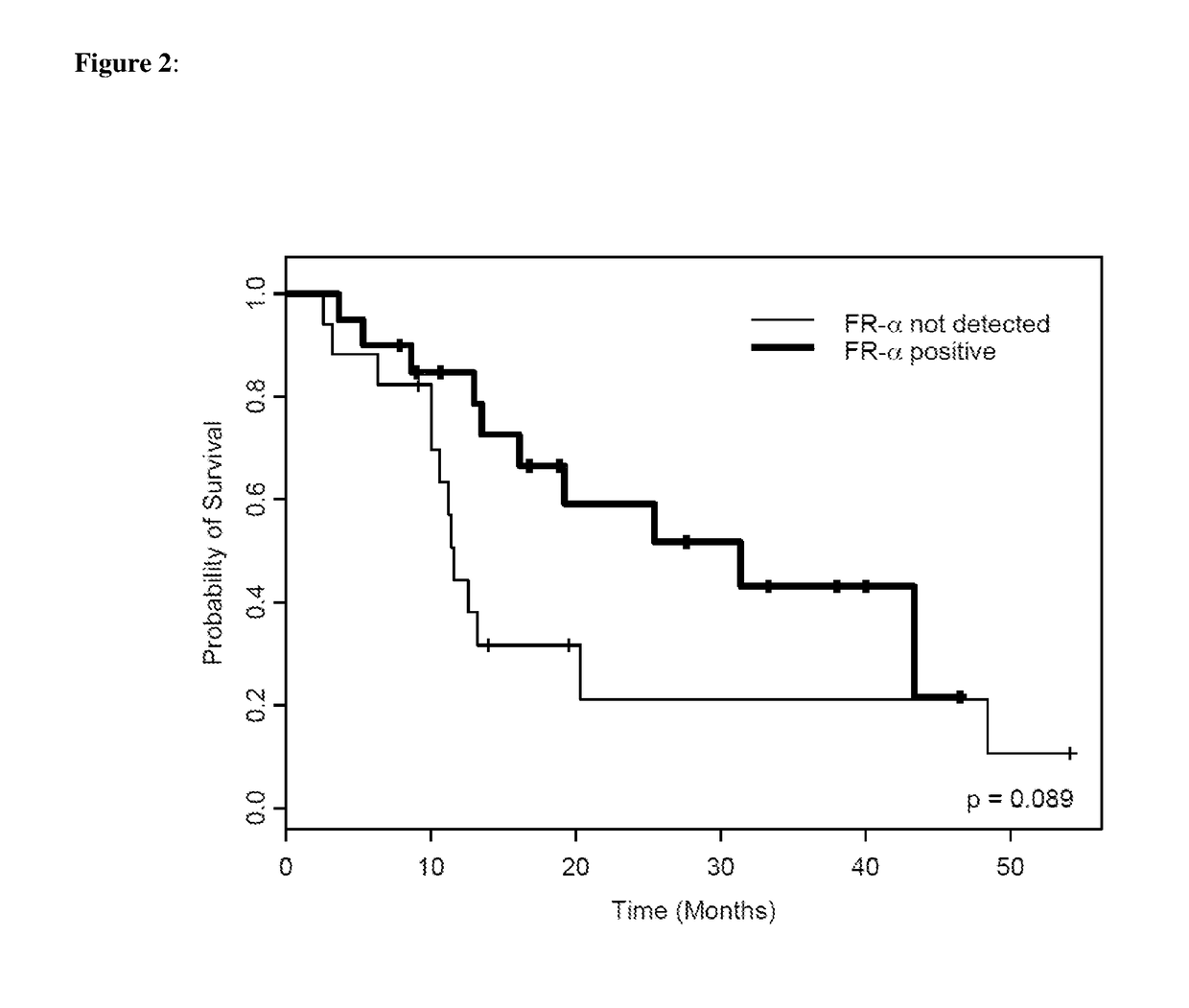
Quantifying FR-α and GART proteins for optimal cancer therapy
Improved methods are provided for treating cancer patients, particularly patients suffering from lung cancer. Methods are provided for identifying whether a lung tumor will be responsive to treatment with a therapeutic regimen that includes pemetrexed and optionally includes cisplatin. A specific FR-α fragment peptide and a specific GART fragment peptide are precisely detected and quantitated by SRM-mass spectrometry directly in lung tumor cells collected from lung tumor tissue that was obtained from a cancer patient and compared to reference levels in order to determine if the lung cancer patient will positively respond to treatment with the c therapeutic regimen.
Owner:EXPRESSION PATHOLOGY
Pemetrexed salt and preparation method thereof
The invention discloses a pemetrexed salt and a preparation method and use thereof, the pemetrexed salt can be dipotassium salt, arginine salt, heme-L-lysinate salt and tromethamine salt of the pemetrexed, and the pemetrexed salt can be used for treatment of various cancers such as mesothelioma, lung cancer and the like.
Owner:CHONGQING PHARMA RES INST
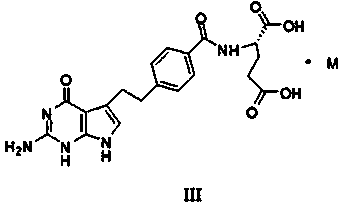
Preparation method of pemetrexed intermediate
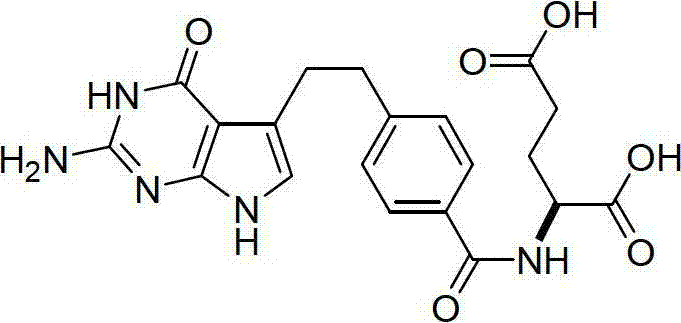
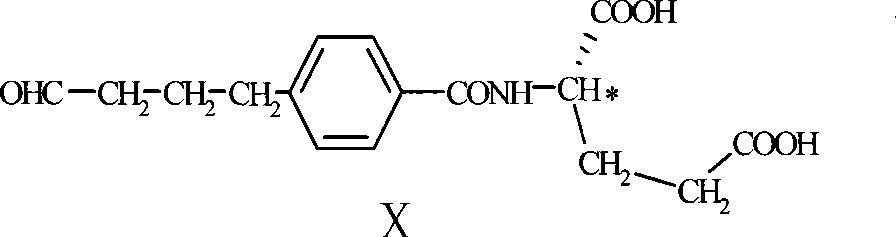
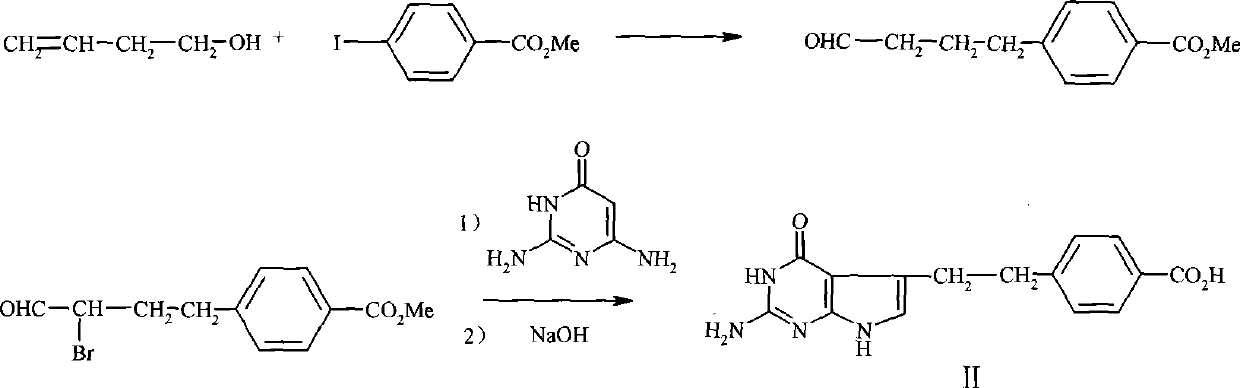
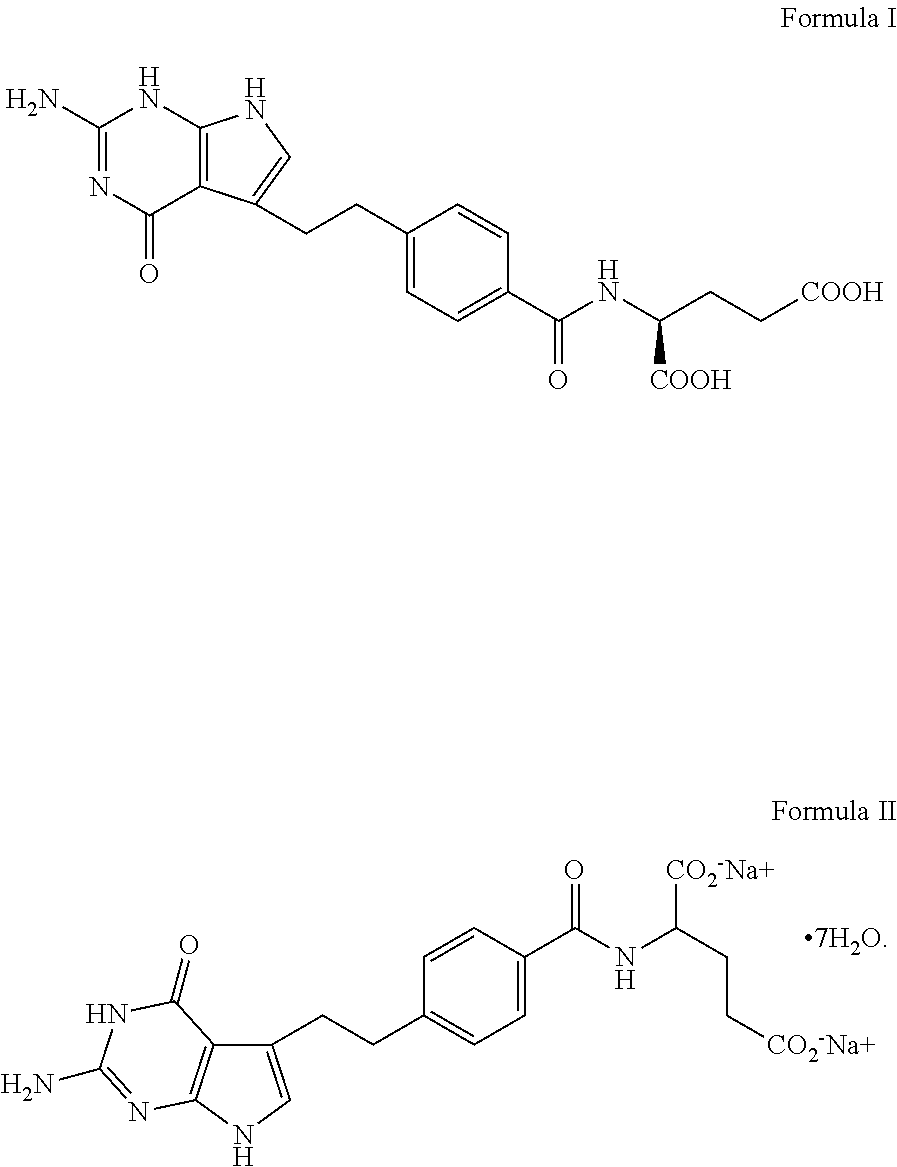
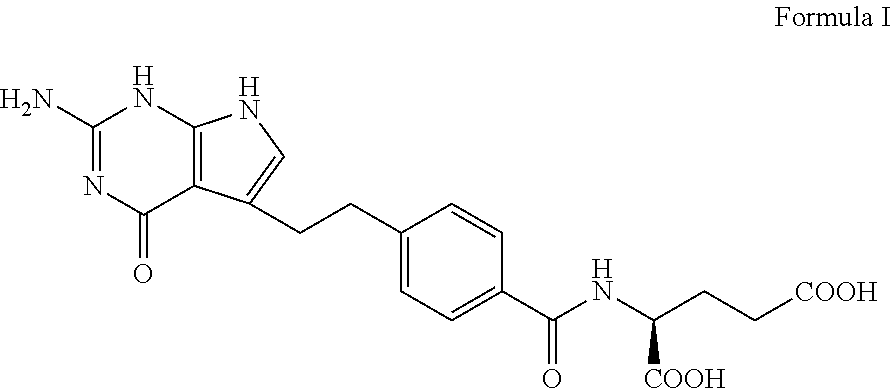
The invention discloses a preparation method of a pemetrexed intermediate. The pemetrexed intermediate is 4-[2-(2-amino-4,7-dihydro-4-oxy-1H-pyrrolo[2,3-d]pyrimidyl-5-yl)ethyl]benzoic acid (I). The preparation method comprises the following steps: carrying out Friedel-Crafts alkylation reaction on benzene and 4-halogen-1-butanol by using aluminum trichloride as a catalyst to generate 4-phenyl-1-butanol (II); carrying out Friedel-Crafts acylation reaction on the compound (II) to obtain 4-(4-carbalkoxyphenyl)-1-butanol (III), and carrying out oxidation reaction on the compound (III) to generate 4-(4-carbalkoxyphenyl)-1-butanal (IV); and carrying out substitution reaction on the compound (IV) and bromine, carrying out condensation and cyclization reaction with 2,4-diamido-6-hydroxypyrimidine (VI) to obtain a compound (VII), and finally, carrying out hydrolysis reaction to obtain the pemetrexed intermediate. The preparation method is simple, convenient, classic and stable, and has the advantage of low cost.
Owner:SUZHOU LIXIN PHARMA
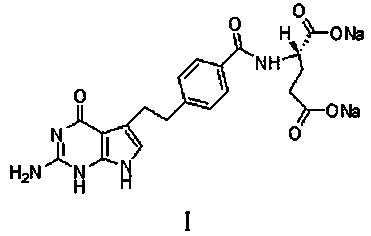
Pharmaceutical compositions of pemetrexed
ActiveUS20150111905A1Stable pharmaceutical compositionOrganic active ingredientsBiocideParenteral nutritionReady to use
A pharmaceutical composition of Pemetrexed represented by formula (I), which is a liquid ready to use solution formulation or a lyophilized pharmaceutical composition for parenteral administration comprising a pharmaceutically acceptable organic amine, an inert gas and optionally containing at least one or more pharmaceutically acceptable excipients. Also provided are processes for preparation of the ready to use solution formulation or lyophilized pharmaceutical composition of the present invention.
Owner:FRESENIUS KABI ONCOLOGY LTD
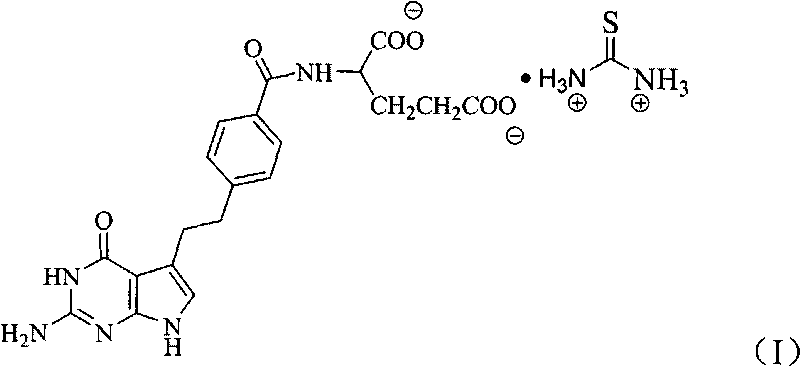
Pemetrexed thiocarbamide salt and preparation method thereof
ActiveCN101691371AEasy to manufactureImprove stabilityOrganic active ingredientsOrganic chemistryOrganic solventThiourea
The invention discloses a pemetrexed thiocarbamide salt and a preparation method thereof, and a medicinal composition containing the pemetrexed thiocarbamide salt. The pemetrexed thiocarbamide salt is generated through the reaction of pemetrexed and thiocarbamide in water and / or an organic solvent. The preparation process is simple, the yield is high and the stability is good.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Pemetrexed liposome and preparation method thereof
InactiveCN104546723AImprove stabilitySimple processOrganic active ingredientsPharmaceutical non-active ingredientsMedicineTrial drug
The invention provides a pemetrexed liposome, which is characterized by being prepared from a pemetrexed and a liposome mixture, wherein the mass ratio of the pemetrexed and the liposome mixture is 1:(0.1-10); through the prepared pemetrexed liposome, the drug resistance can be reduced by more than 60% of non-liposome pemetrexed. Known from stability test, the liposome is favorable in stability. Known from clinical trials, the efficacy of the liposome can be improved by more than 80% compared with the common preparation, the in-vivo retention time can be prolonged by more than 65%, and the targeting effect is obvious.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
New crystalline forms of pemetrexed diacid, and preparations thereof
ActiveUS20110172424A1Good reproducibilitySimple methodOrganic chemistryAntineoplastic agentsPemetrexedMedicinal chemistry
Three new crystalline forms of pemetrexed diacid, preparation methods and uses thereof are disclosed. These preparation processes are simple and have better practicality.
Owner:CHONGQING PHARMA RES INST
Slow released anticancer medicine preparation with both amrubicin and its synergist
The slow released anticancer medicine injection containing both amrubicin and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 25 deg.c. The effective anticancer component is amrubicin, idarubicin, etc and / or antimetabolite composition selected from carmofur, tegafur, zalcitabine, etc. The slow releasing supplementary material is selected from EVAc, sebacic acid copolymer, lactic acid polymer, etc. The slow released microsphere may be also prepared into slow released implanting agent set around or inside the tumor to strengthen the chemotherapy or radiotherapy effect.
Owner:JINAN KANGQUAN PHARMA TECH
Processes for preparing intermediates of pemetrexed
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
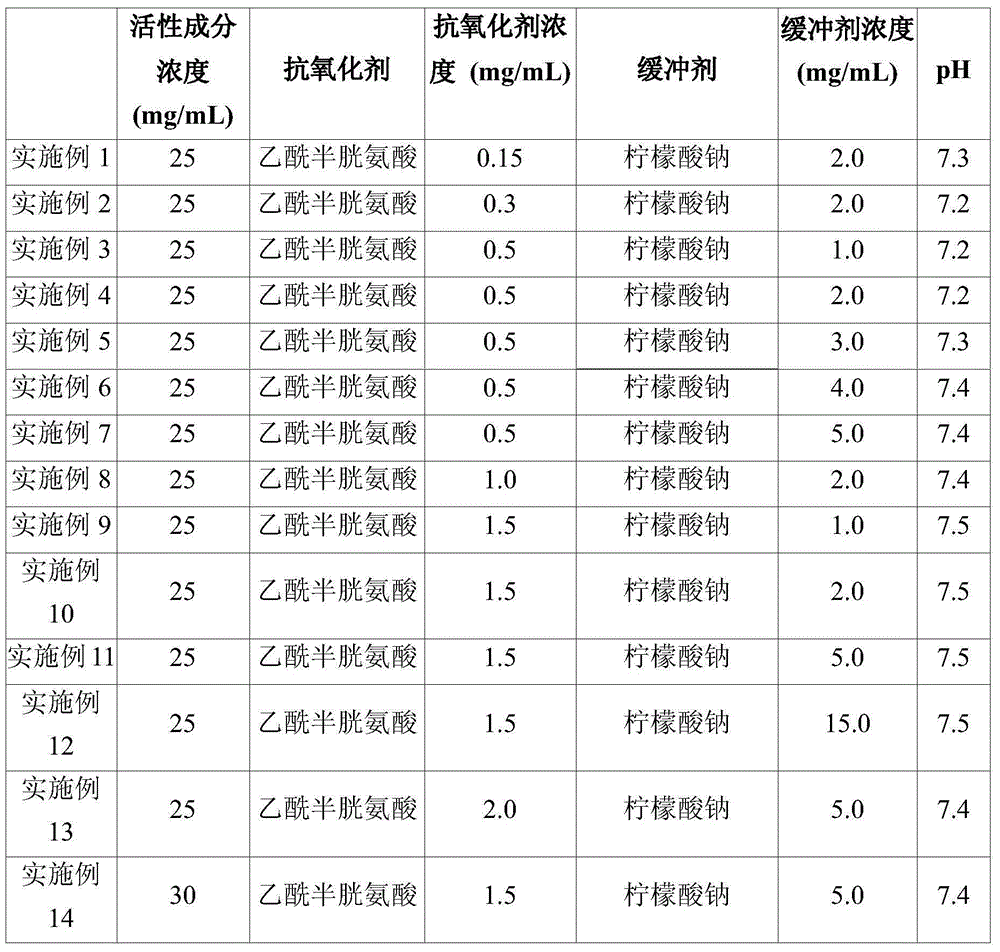
Stabilized pemetrexed formulation
ActiveUS20150297724A1Easy to prepareImprove convenienceOrganic active ingredientsBiocideAntioxidantCysteic acid
The present invention relates to a stabilized pemetrexed formulation, and more particularly to a stabilized pemetrexed formulation comprising acetylcysteine as antioxidant and a citrate salt as buffer.
Owner:CJ HEALTHCARE CORP
Intravenous infusion dosage form
ActiveUS20180036310A1Organic active ingredientsEnergy modified materialsPemetrexedIntravenous Infusions
The present invention relates to an intravenous infusion dosage form of pemetrexed or its pharmaceutically acceptable salt, having long term stability.
Owner:SUN PHARMA INDS
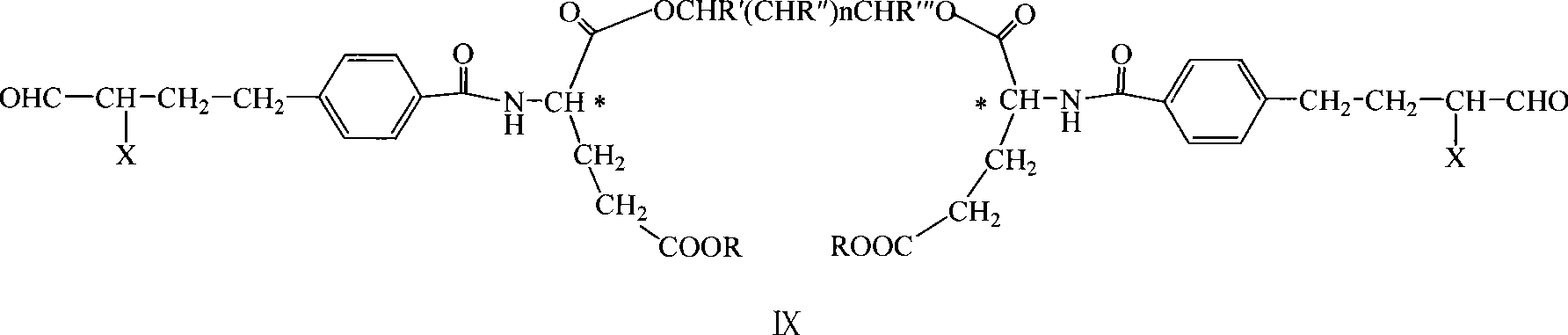
Diglutamate derivates and application thereof in preparation of pemetrexed
The invention relates to a di-glutamate derivative and a preparation method and application in the preparation of pemetrexed thereof. The glutamate derivative is used as a key intermediate for preparing pemetrexed. The method has simple treatment after reaction and high product purity, and facilitates the industrialized production.
Owner:QILU PHARMA CO LTD +1
A stabilized pemetrexed formulation
ActiveCN104812392AEasy to manufactureAvoid pollutionOrganic active ingredientsPharmaceutical containersAntioxidantCitrate salt
The present invention relates to a stabilized pemetrexed formulation, and more particularly to a stabilized pemetrexed formulation comprising acetylcysteine as antioxidant and a citrate salt as buffer.
Owner:CJ HEALTHCARE CORP
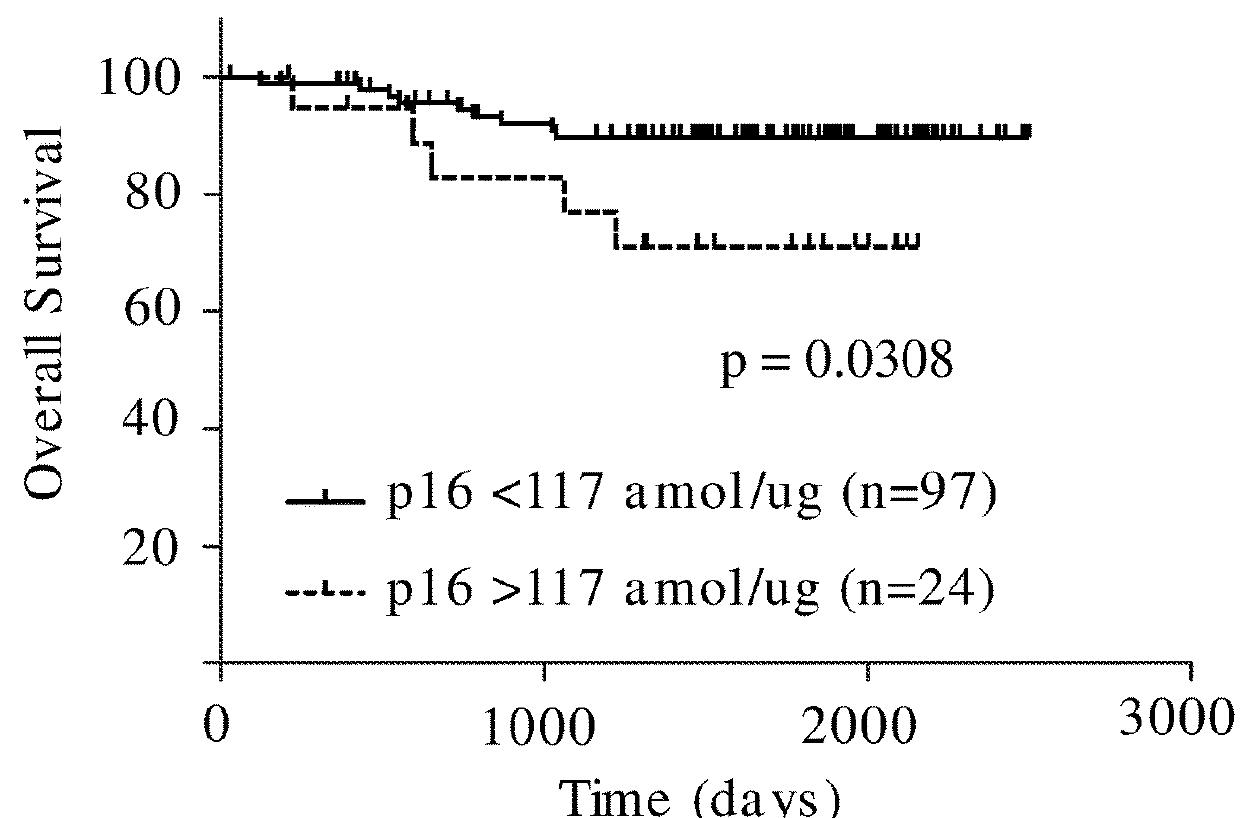
Methods of Treating Lung Cancer by Predicting Responders to Cisplatin-Pemetrexed Combination Therapy
ActiveUS20180177825A1Inhibition formationHeavy metal active ingredientsOrganic active ingredientsPulmonary neoplasmsCisplatin
Methods are provided for identifying whether a lung tumor will be responsive to treatment with the combination of the therapeutic agents cisplatin and pemetrexed. Specified ERCC1, TS, p16, and FRα fragment peptides are precisely detected and quantitated by SRM-mass spectrometry directly in lung tumor cells collected from lung tumor tissue that was obtained from a cancer patient and compared to reference levels in order to determine if the lung cancer patient will positively respond to treatment with the combination of cisplatin and pemetrexed therapeutic agents.
Owner:EXPRESSION PATHOLOGY
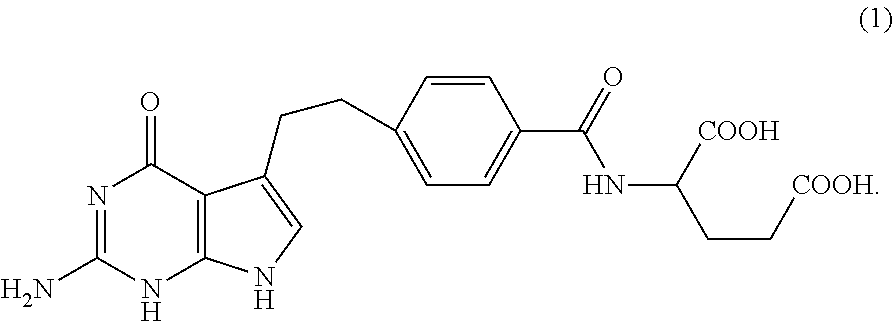
Stable pemetrexed arginine salt and compositions comprising it
The present invention relates to arginine salt of pemetrexed of formula (1), particularly to a stable solid form thereof, and to pharmaceutical compositions comprising such salt.
Owner:SYNTHON BV
Improved Methods Of Treating Lung Cancer Using Multiplex Proteomic Analysis
InactiveUS20190353658A1Inhibition formationDisease diagnosisBiological testingRegimenProteomic Profile
The present invention provides methods for treating cancer patients comprising assaying tumor tissue from patients and identifying those patients most likely to respond to treatment with a platinum-based agent, such as cisplatin, in combination with pemetrexed. Methods are provided for identifying those lung cancer patients most likely to respond to treatment with the combination of cisplatin+pemetrexed chemotherapy agents (“CDDP+PEM”) by determining expression patterns of a set of 38 specific proteins directly in tumor cells derived from patient tumor tissue using SRM mass spectrometry. The method further comprising determining if the patient will respond to treatment with combination therapy, and when proteomic analysis of patient tissue indicates that the patient will respond to treatment with combination therapy, the patient is administered a regimen that includes the pemetrexed / platinum agent combination.
Owner:EXPRESSION PATHOLOGY
Novel midbody of pemetrexed, preparing method and application thereof
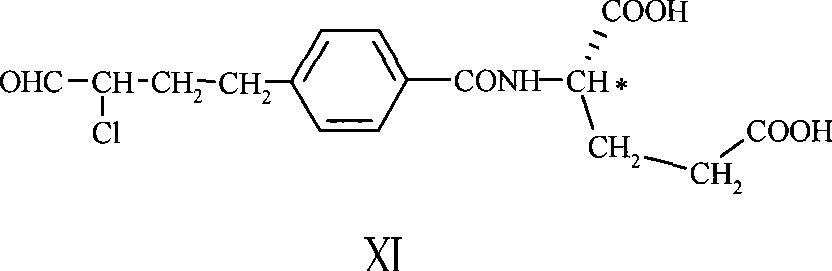
ActiveCN101293854BEasy to purifyHigh yieldOrganic compound preparationCarboxylic acid amides preparationPhenacylBromine
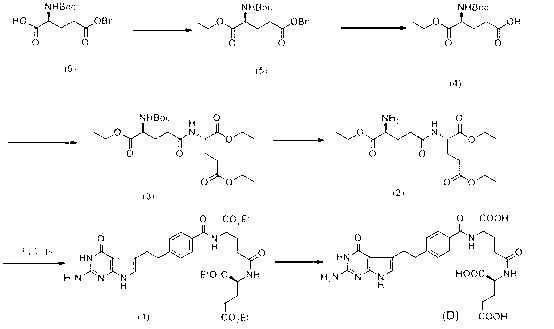
The invention discloses a compound XI, which is a key intermediate for synthesizing pemetrexed with chemical name of N-[4-(2-halo-butyraldehyde-4-base)-benzoyl]-L-glutamic acid and structural formula shown in formula XI, wherein * represents L-configuration carbon, and X represent bromine, chlorine or iodine. The invention also discloses a preparation method of the intermediate and a method for preparing pemetrexed with the intermediate. The method for preparing pemetrexed with the compound XI has the advantages of simple reaction process, easy purification of intermediate, short preparation period, high product purify, and applicability to industrial production.
Owner:QILU PHARMA CO LTD +1
Pharmaceutical compositions comprising pemetrexed and tromethamine
ActiveUS9421207B2Oxygen content can be minimisedOrganic active ingredientsPowder deliveryReady to usePemetrexed
A pharmaceutical composition of Pemetrexed represented by formula (I),which is a liquid ready to use solution formulation or a lyophilized pharmaceutical composition for parenteral administration comprising a pharmaceutically acceptable organic amine, an inert gas and optionally containing at least one or more pharmaceutically acceptable excipients. Also provided are processes for preparation of the ready to use solution formulation or lyophilized pharmaceutical composition of the present invention.
Owner:FRESENIUS KABI ONCOLOGY LTD
Pemetrexed quality control method, and preparation of pemetrexed impurity and salt thereof
ActiveCN102796106AEasy to controlHigh purityOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupQuality control
The invention provides a pemetrexed quality control method, and preparation of a pemetrexed impurity and salt thereof. The pemetrexed impurity is synthesized from the raw material N-tert-butoxycarbonyl-L-glutamic acid-5-benzyl ester. The pemetrexed quality control method mainly uses the pemetrexed impurity prepared by the technique as a standard control substance in detection and analysis. The method provided by the invention solves the problem that the pemetrexed impurity can only be separated and obtained in the preparation process of the pemetrexed and sodium salt thereof in the prior art, and the high-purity pemetrexed impurity can not be obtained. The method is simple to operate, and has the advantages of low facility request, mild reaction conditions, high product purity and high yield.
Owner:JIANGSU SENRAN CHEM +1
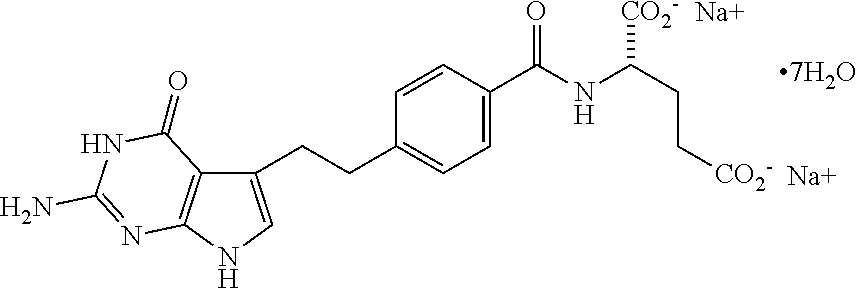
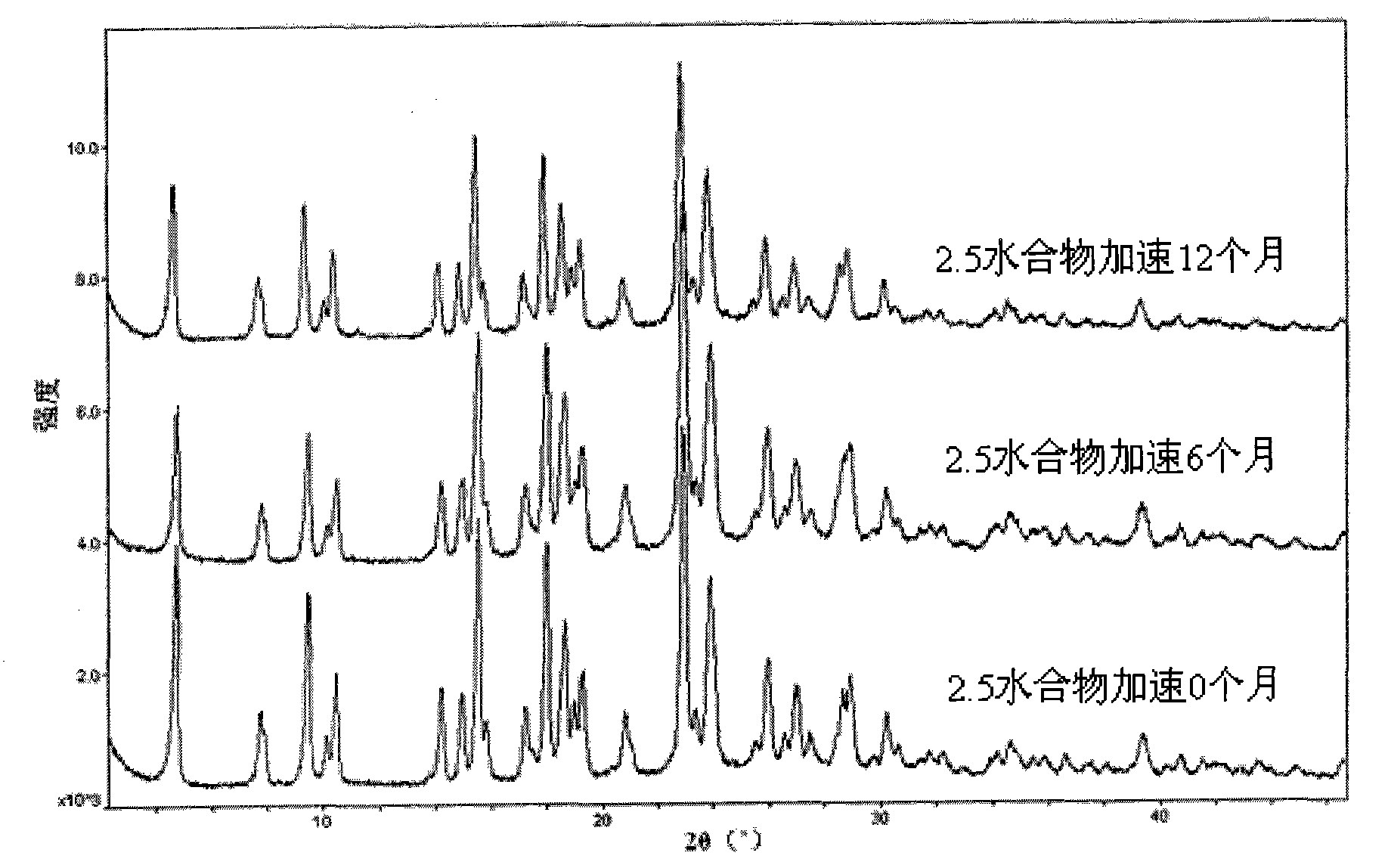
Method for preparing pemetrexed disodium 2.5 water crystal
The invention provides a method for preparing a pemetrexed disodium 2.5 water crystal. The method comprises the following steps of: (1) dissolving a raw material, namely pemetrexed disodium in water; (2) dropwise adding the aqueous solution of the pemetrexed disodium into an organic solvent which can be miscible with the water, and crystallizing; and (3) filtering, collecting a filter cake, and drying to obtain a target product. The preparation method is high in controllability, easy and convenient to operate, high in repeatability, high in production yield, and stable in crystal form and industrial production is easy to implement, and special equipment is not required.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Sustained-released injection including platinum compound and the alkylate agent
The invention relates to an anti-cancer compound as a slow release injection which contains platinum compound and / or alkyl agent, formed by slow release micro ball and solvent. The slow release micro ball comprises the anti-cancer effective components and slow release findings, selected from platinum compound of kpeitabing, peimeiquse, caplatinum, or jxitabing and / or alkyl agent, the solvent is a common solvent or a special solvent with suspending agent, while the viscosity of suspending agent is 100cp-3000cp (at 20-30Deg. C), selected from carboxymethyl cellulose, the anti-cancer effective component is phosphoinositide 3-kinase restrainer and / or the phosphoinositide 3-kinase restrainer booster selected from self-anti-cancer antibiotics and / or tetrazine drug, the slow release finding is selected from phosphate polyester as p (LAEG-EOP) or p (DAPG-EOP), or the polyester or mixture of phosphate, PLA, polyphenyl, PLGA, poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), and the alkyl agent is selected from ranimustine or the like. The anti-cancer compound can be made as slow release plant agent, to inject cancer or around cancer to hold the effective drug density for more than 60 days, while it can significantly reduce the general reaction of drug and selectively strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN SHUAIHUA PHARMA TECH
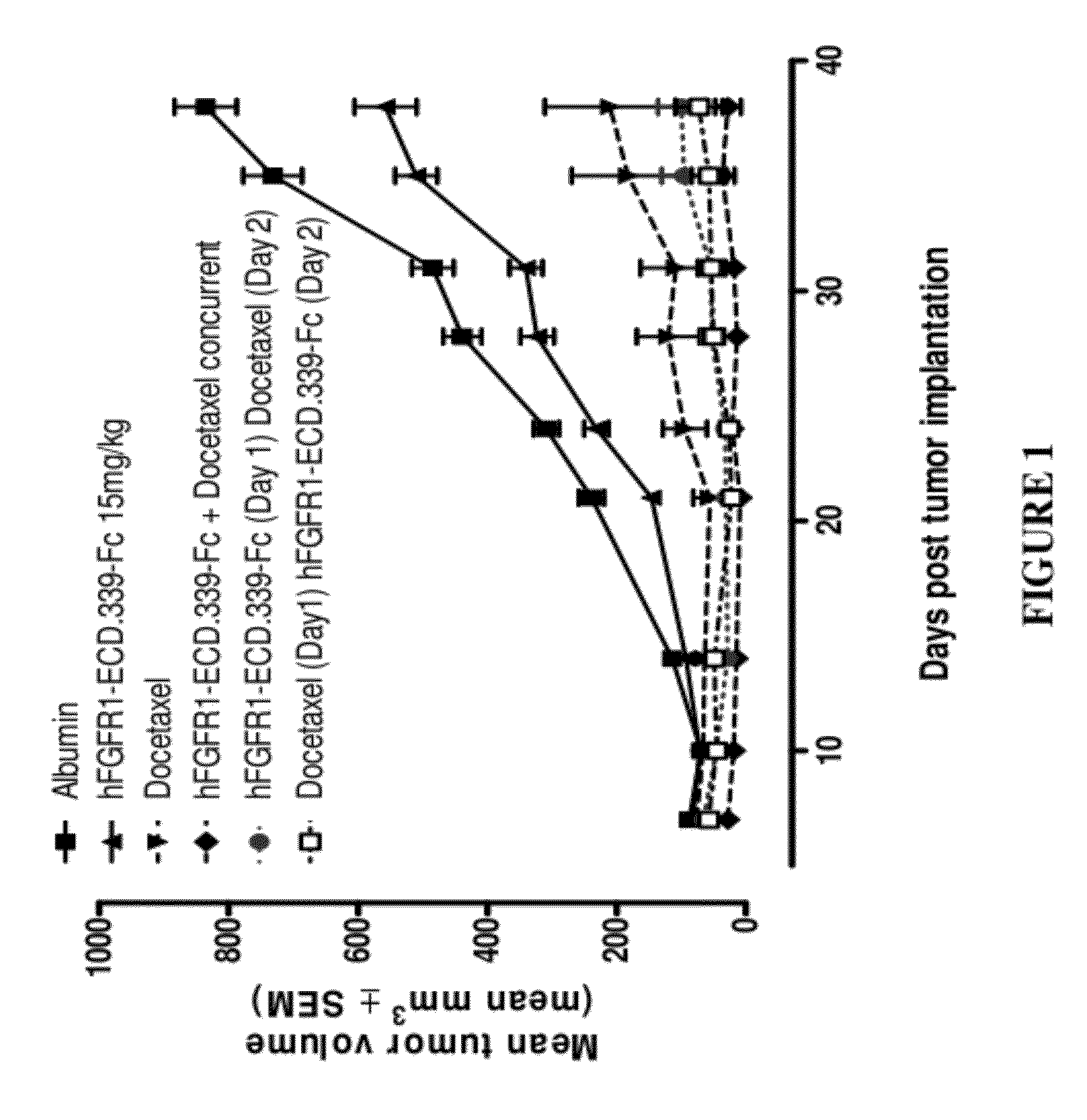
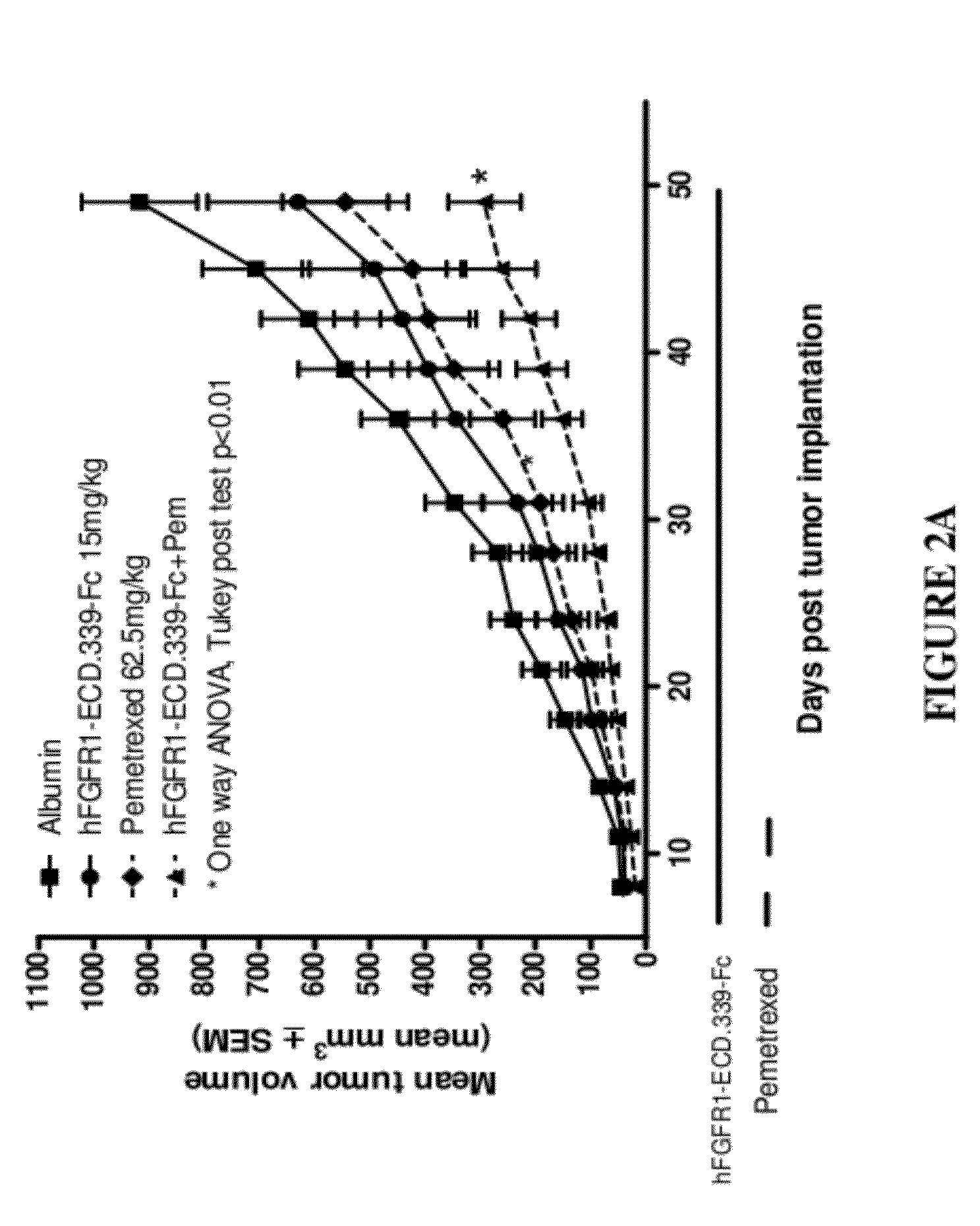
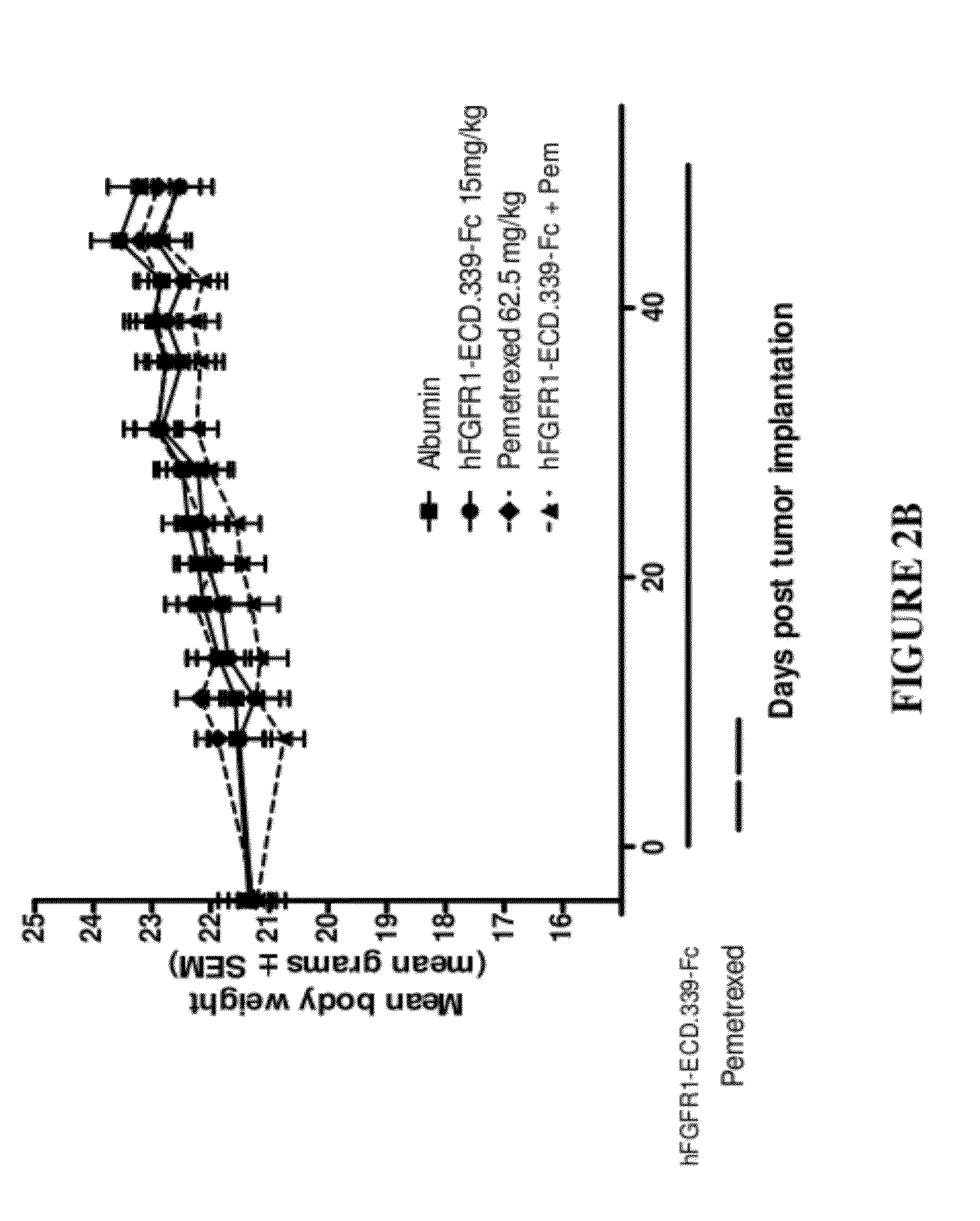
FGFR1 extracellular domain combination therapies for lung cancer
InactiveUS8951972B2Heavy metal active ingredientsOrganic active ingredientsCarboplatinFormyltetrahydrofolic Acids
Methods of treating cancer comprising administering a fibroblast growth factor receptor 1 (FGFR1) extracellular domain (ECD) and / or an FGFR1 ECD fusion molecule in combination with at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are provided. Dosage packs comprising an FGFR1 ECD and / or an FGFR1 ECD fusion molecule and / or at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are also provided. In some embodiments, a dosage pack comprises instructions for administering FGFR1 ECD and / or FGFR1 ECD fusion molecule with at least one additional therapeutic agent.
Owner:FIVE PRIME THERAPEUTICS
Sustained-released injection including antimetabolite medicine and alkylate agent
The invention relates to a slow release injection which contains antimetabolite and / or alkyl agent, formed by slow release micro ball and solvent. The slow release micro ball comprises the anti-cancer effective components selected from platinum compound of kpeitabing, peimeiquse, caplatinum, or jxitabing and / or alkyl agent, the solvent is a common solvent or a special solvent with suspending agent, while the viscosity of suspending agent is 100cp-3000cp (at 20-30Deg. C), selected from carboxymethyl cellulose, the slow release finding is selected from phosphate polyester as p (LAEG-EOP) or p (DAPG-EOP), or the polyester or mixture of phosphate, PLA, polyphenyl, PLGA, poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), and the alkyl agent is selected from ranimustine or the like. The anti-cancer compound can be made as slow release plant agent, to inject cancer or around cancer to hold the effective drug density for more than 50 days, while it can significantly reduce the general reaction of drug and selectively strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN SHUAIHUA PHARMA TECH
Stable Pharmaceutical Formulations of Pemetrexed
ActiveUS20200155556A1Easy to manageReduce exposureOrganic active ingredientsInorganic active ingredientsAntioxidantPharmaceutical medicine
The present invention relates to a long term storage stable multi-dose ready-to use or ready-to dilute pharmaceutical liquid formulation comprising pemetrexed or a pharmaceutically acceptable salt thereof, an antioxidant, a preservative, a buffering agent, and a pharmaceutically acceptable fluid. The invention also relates to a process of preparing the formulation, a kit and a method of treatment of patients having lung cancer by administering the pharmaceutical formulation to a subject in need thereof.
Owner:CIPLA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com