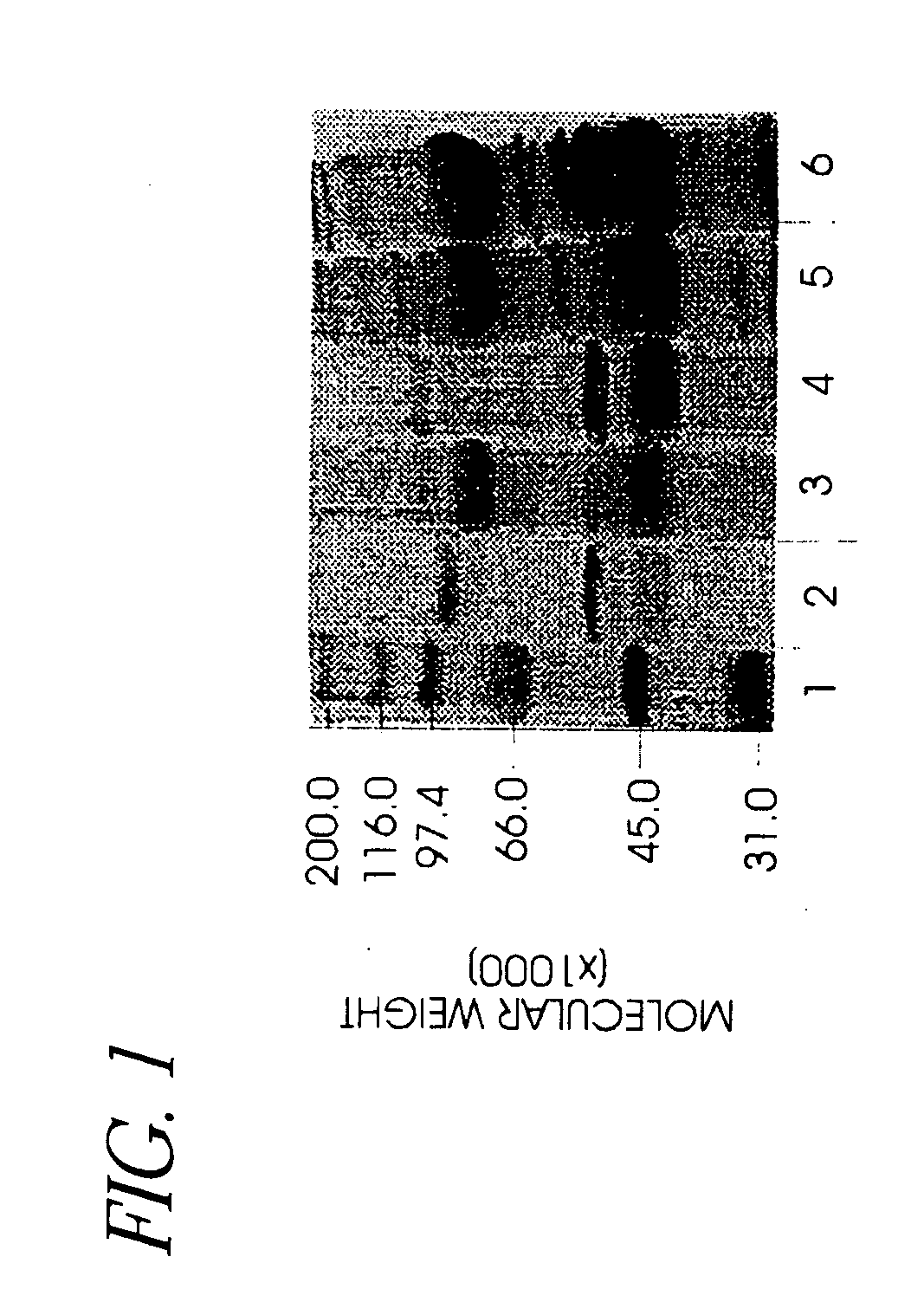
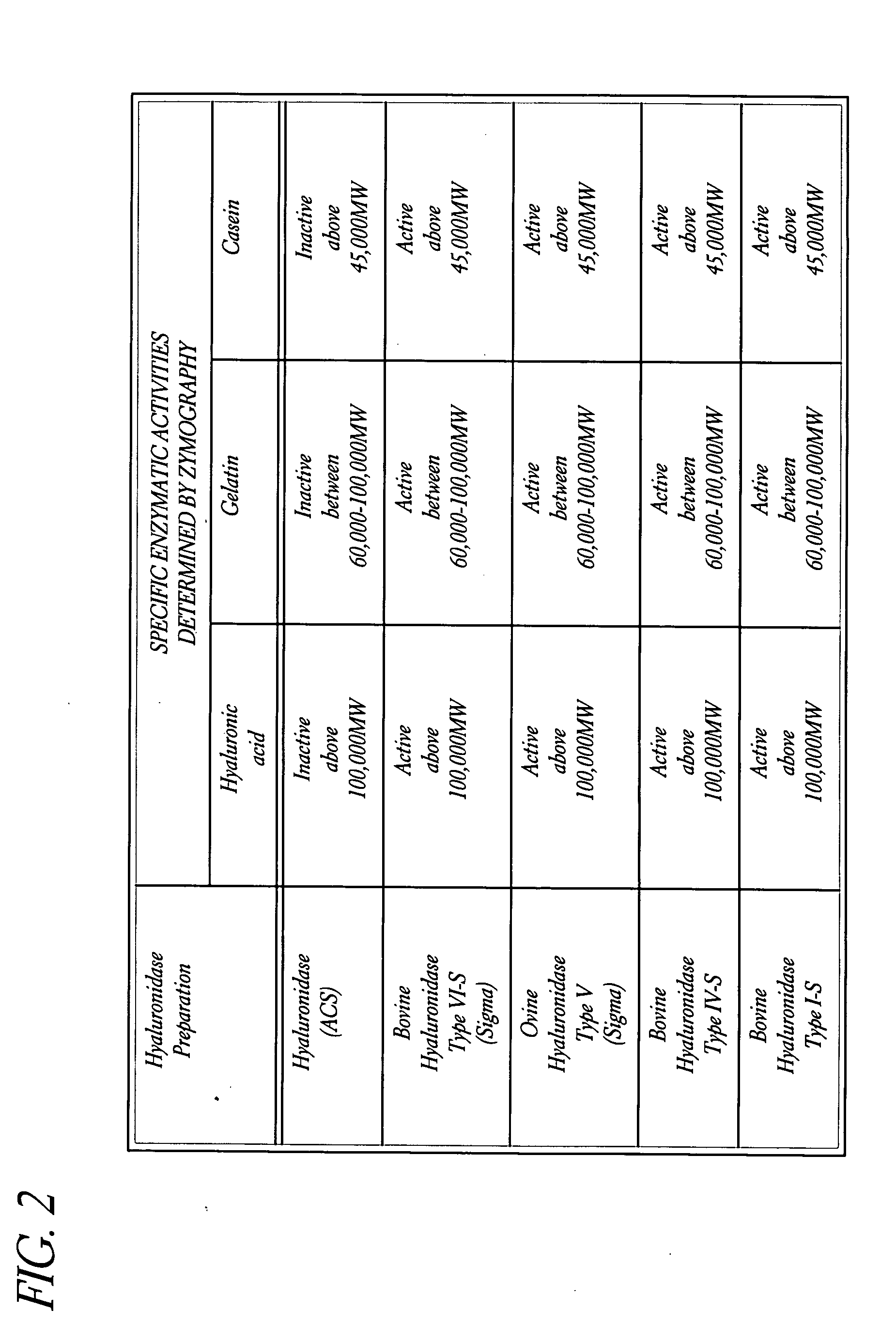
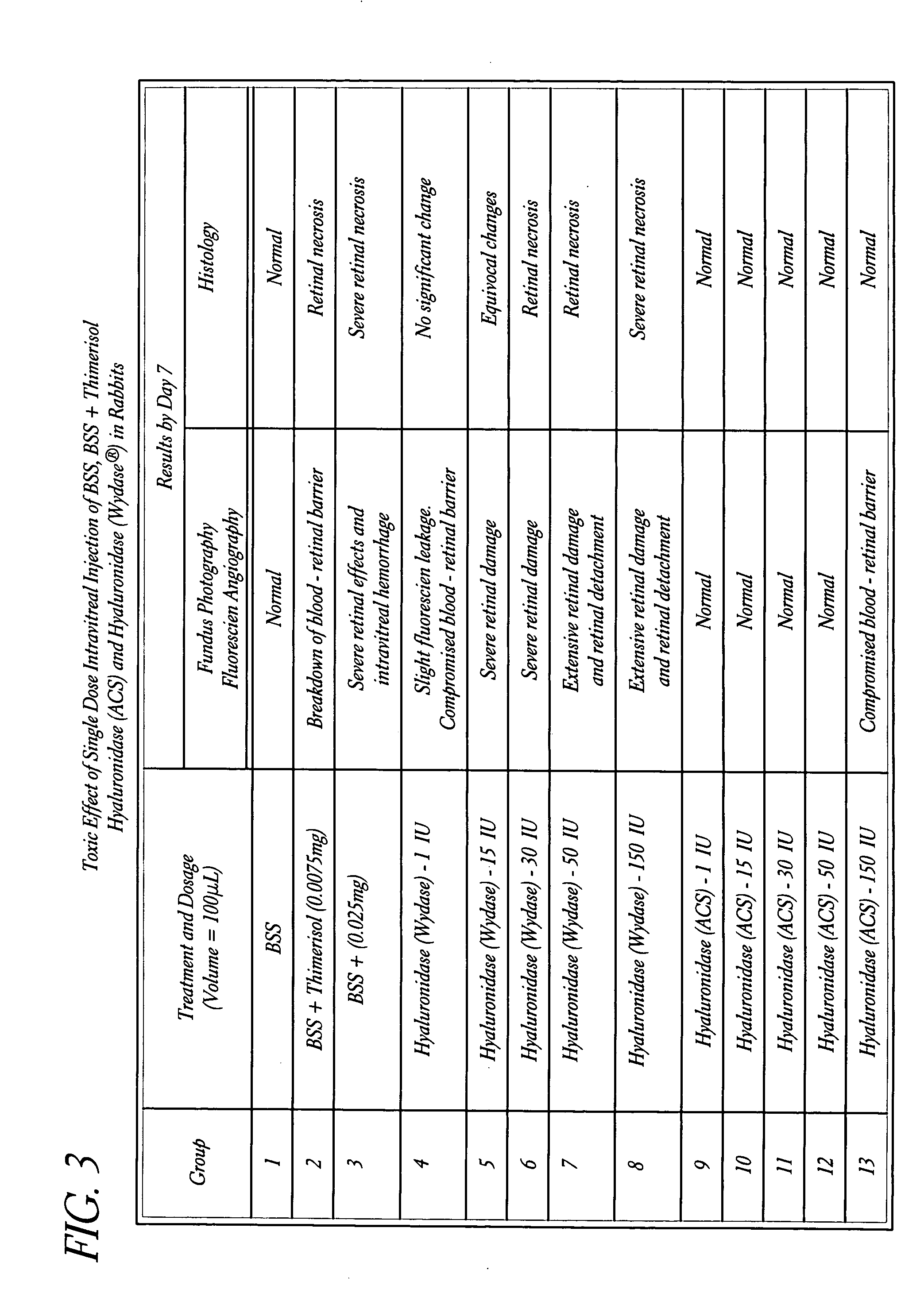
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
a technology of vitreous humor and hyaluronidase, which is applied in the direction of drug compositions, pharmaceutical active ingredients, peptide/protein ingredients, etc., can solve the problems of loss of vision, further vitreous hemorrhage and/or the formation of fibrous tissue at the site of the hemorrhage, and undesirable permanent fibrous attachment, so as to prevent neovascularization and increase the rate of liquid exchange , the effect of increasing the clearan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0063] Fifty-two (52) healthy rabbits of the New Zealand Cross Variety (26 male, 26 female) weighing 1.5 kg to 2.5 kg, were individually marked for identification and were housed individually in suspended cages. The animals received a commercially available pelleted rabbit feed on a daily basis, with tap water available ad libitum.
[0064] The animals were divided into thirteen groups of 4 animals each (2 male, 2 female). Two animals in each group (1 male, 1 female) were selected for pretreatment fundus photography and fluorescein angiography.
[0065] The fundus photography was performed by restraining the animals and visualizing the optic nerve, retinal arcades with fundus with a KOWA® RC-3 Fundus Camera loaded with Kodak Gold 200 ASA film.
[0066] The fluorescein angiography involved a 1.5 ml injection of 2% sterile fluorescein solution via the marginal ear vein. Approximately 30 seconds post-injection the fluorescein was visualized upon localization of the optic nerve, retinal vesse...
example 2
[0083] In this example, 12 healthy rabbits of the New Zealand Cross variety were marked for identification and individually housed in suspended cages. The animals received commercially pelleted rabbit feed on a daily basis and tap water was available ad libitum.
[0084] The animals were randomly divided into four (4) treatment groups of three (3) animals each.
[0085] Initially, the eyes of each animal were examined by dilation with 1-2 drops of 10% tropicamide followed by gross examination, indirect ophthalmoscopy using a 20 diopter lens, and slit lamp examination of the anterior anatomy of the eye.
[0086] Following the initial examination of the animals eyes, 100 μl or 10 μl of blood was injected intravitreally into each eye of each animal.
[0087] On day 2, the animals of each treatment group received a single intravitreal injection of either BSS or hyaluronidase ACS into the right eye, in accordance with the following treatment schedule:
TreatmentGroup #Left EyeRight EyeANoneBSS(3...
example 3
[0094] The primary objective of this study was to determine if a balanced salt solution containing a highly purified hyaluronidase extract from ovine testicular tissue could be injected into the vitreous of visually impaired eyes without eliciting any serious ocular adverse effects.
[0095] Materials and Methods
[0096] Balanced Salt Solution (BSS) was employed as the placebo control, and was obtained from Allergan Pharmaceuticals (Irvine, Calif.). The BSS contained 0.64% sodium chloride, 0.075% potassium chloride, 0.048% calcium chloride dihydrate, 0.03% magnesium chloride hexahydrate, 0.39% sodium acetate trihydrate, 0.17% sodium citrate dihydrate, sufficient sodium hydroxide / hydrochloric acid for adjustment of pH to 7.1-7.2, and water for injection (qs. 100%). Thirty microliter aliquots of BSS or hyaluronidase specific formulation D (Table 6) were loaded into a 300 μl microsyringe fitted with a 29 gauge needle 0.5 inches in length. The loaded microsyringe were then used to inject t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com