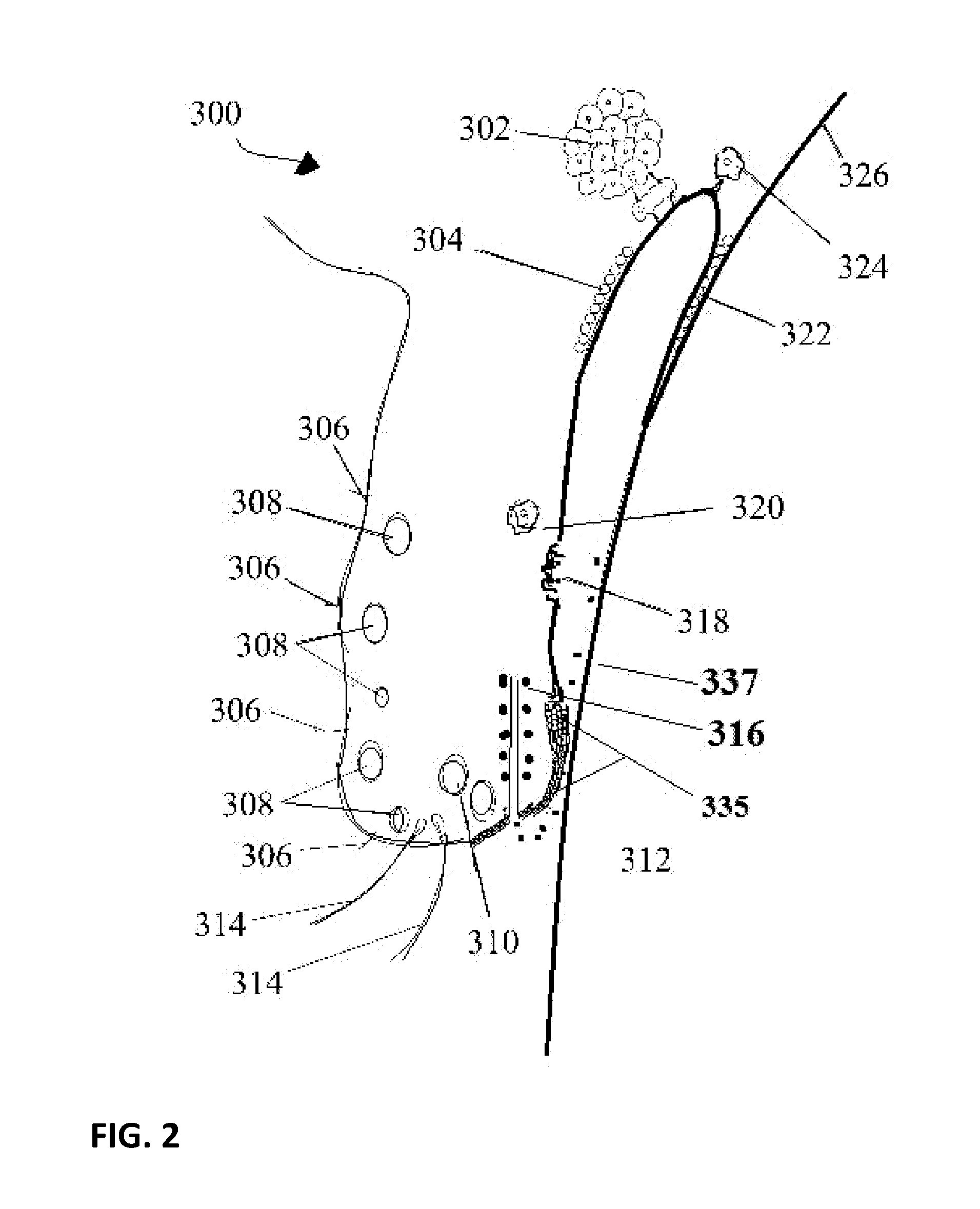
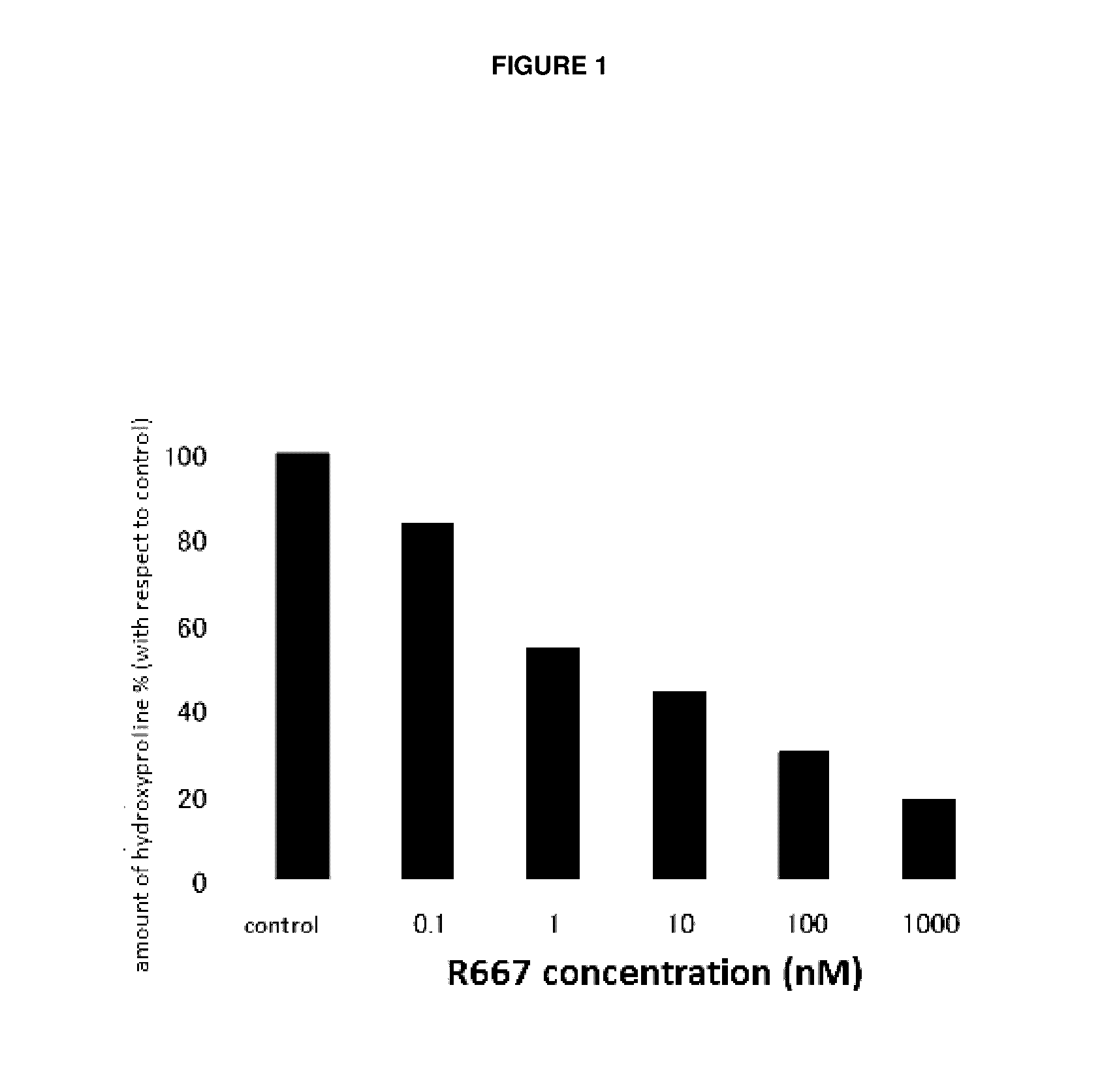
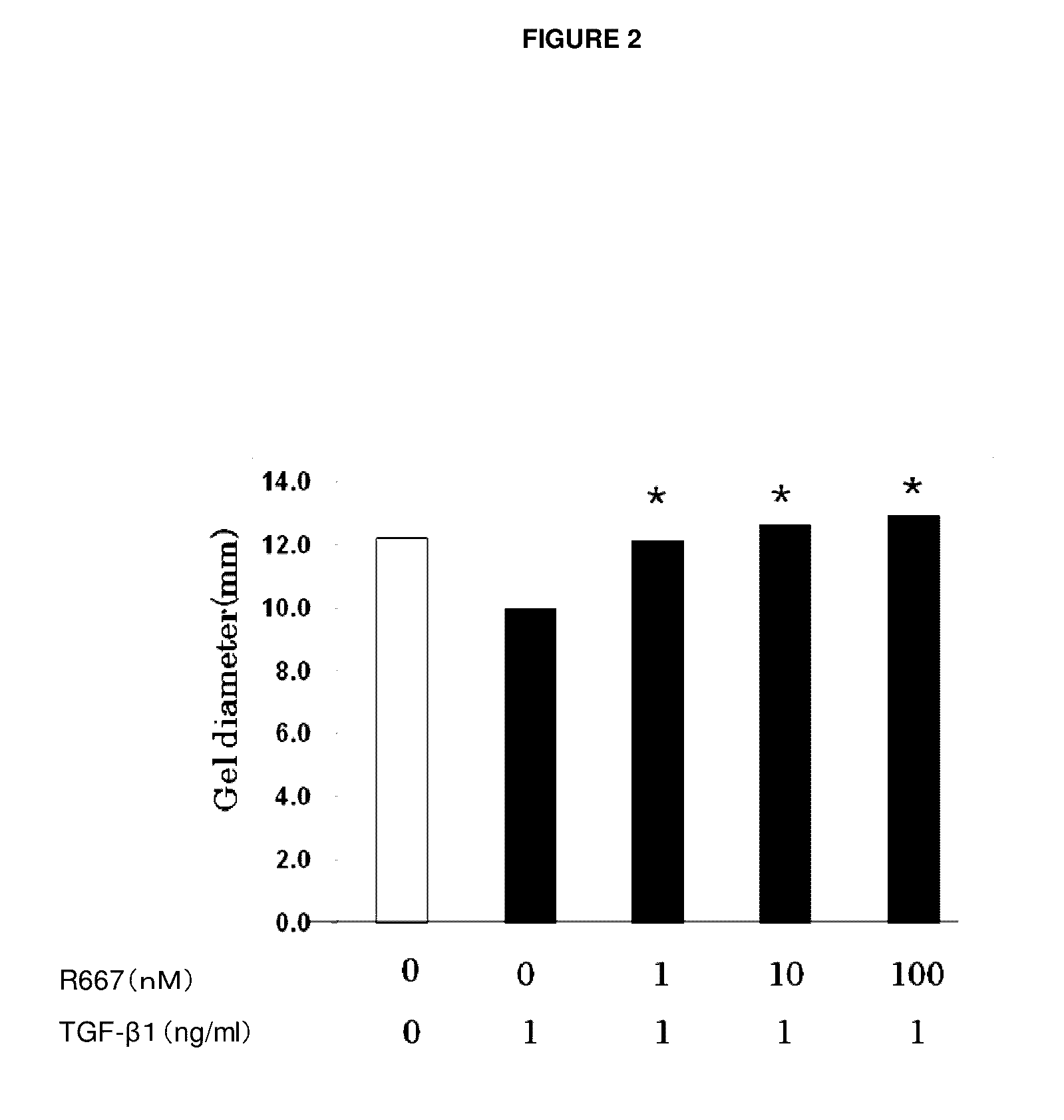
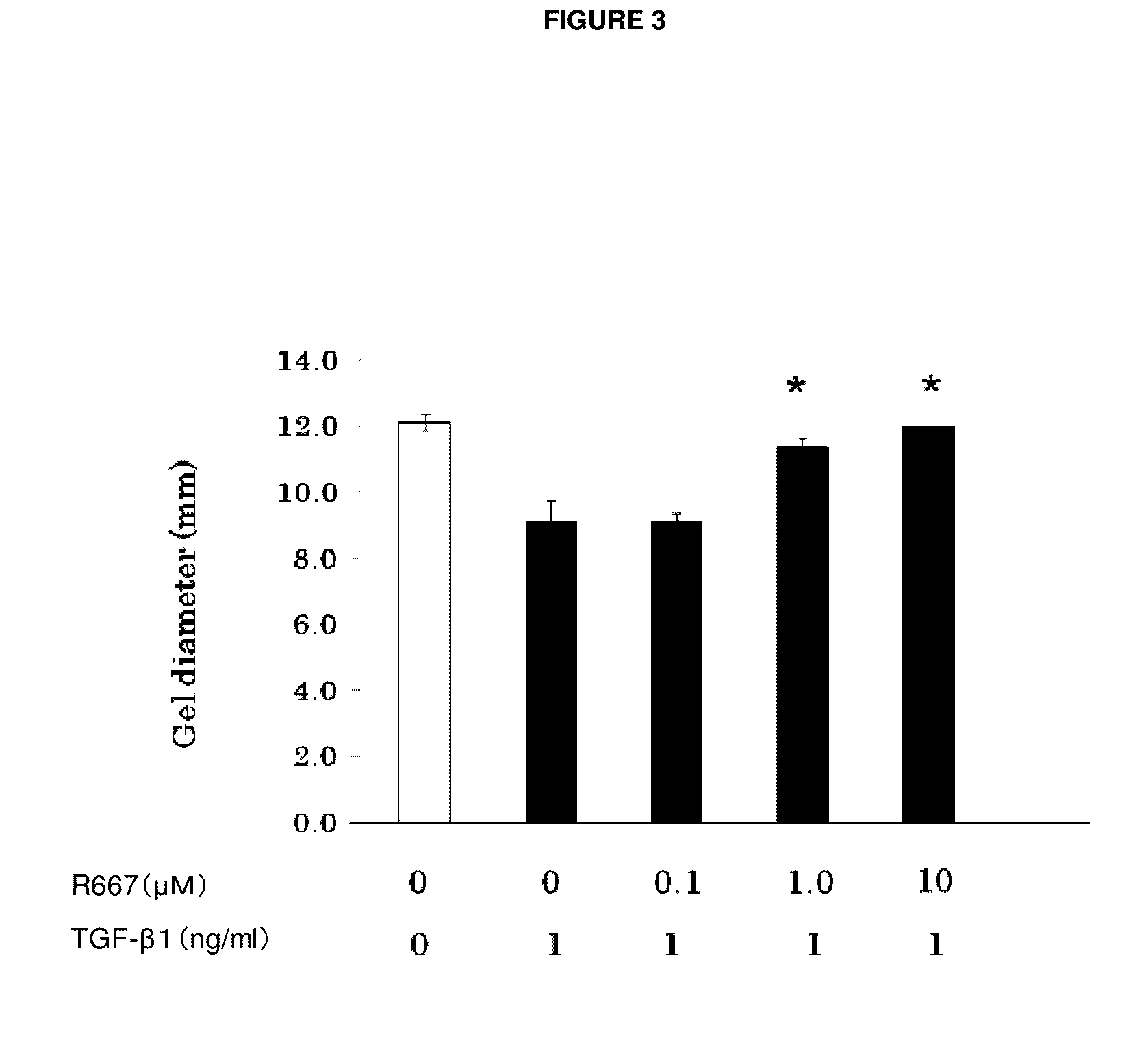
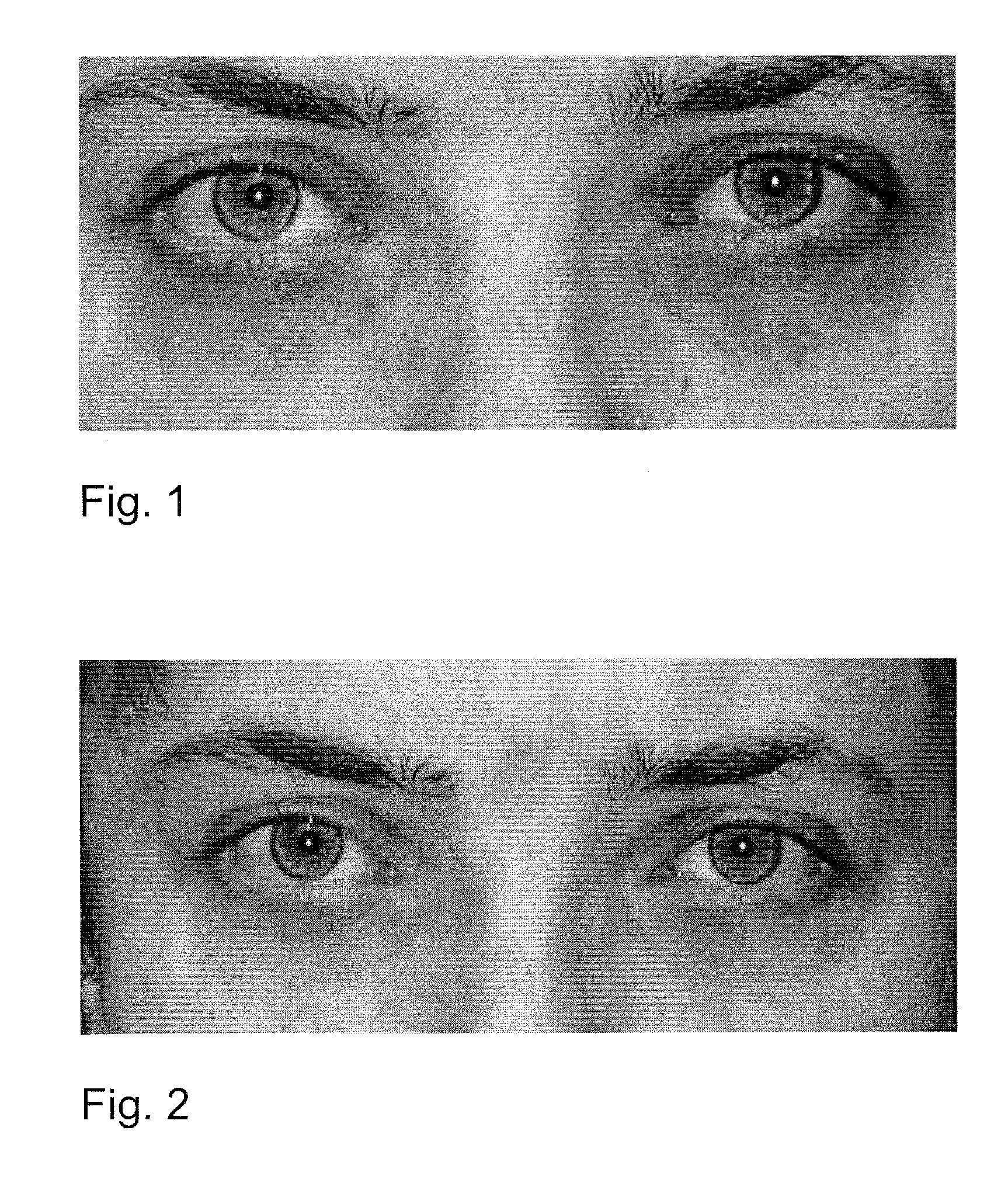
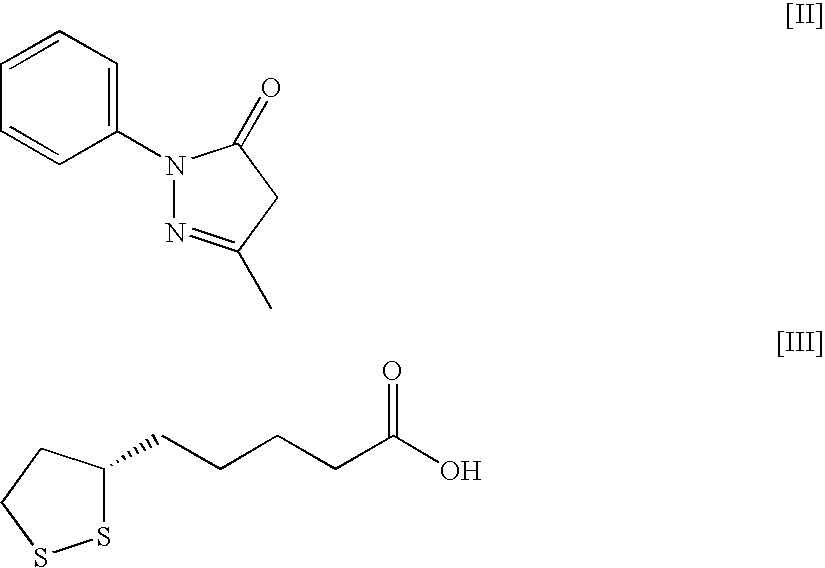
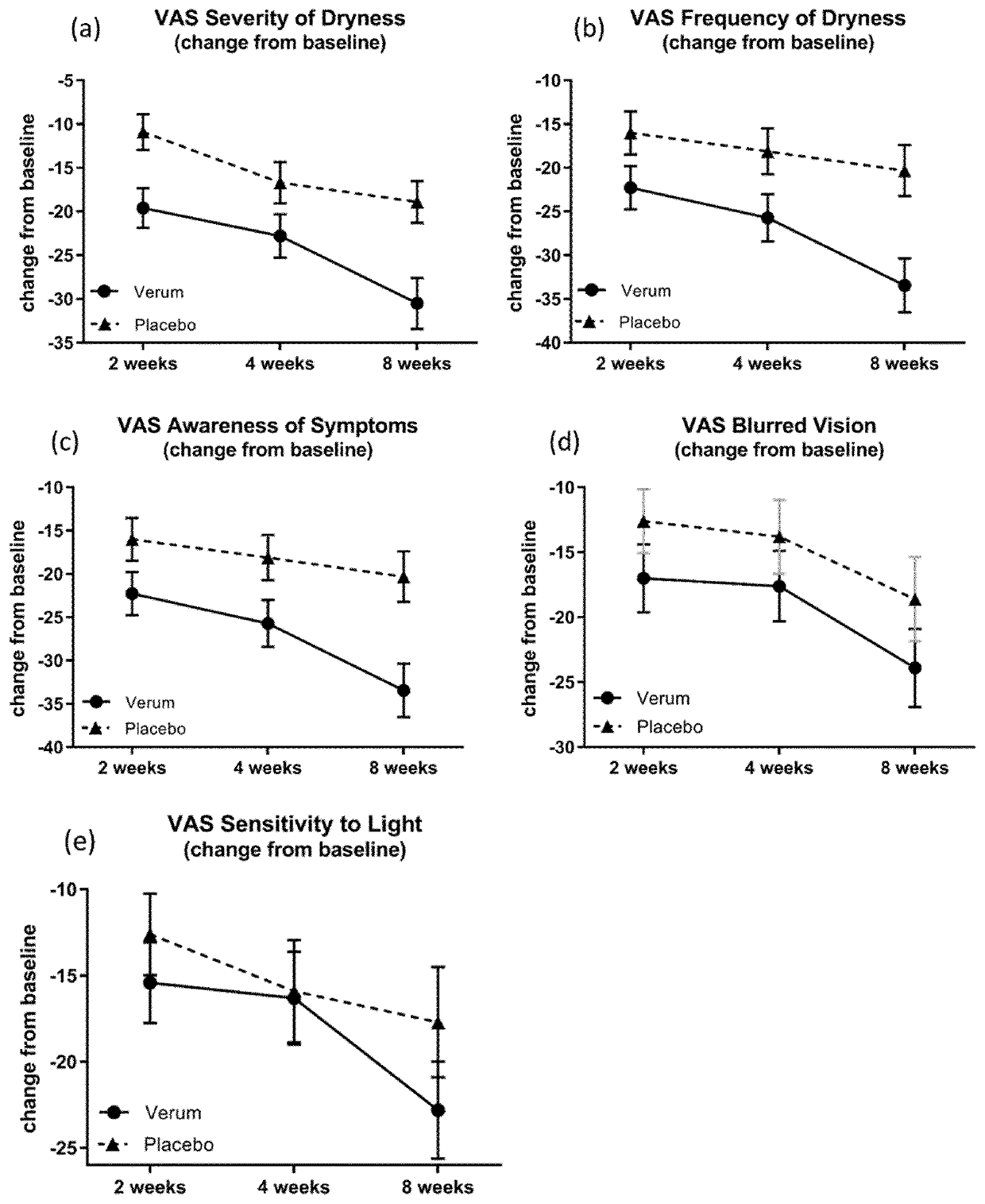
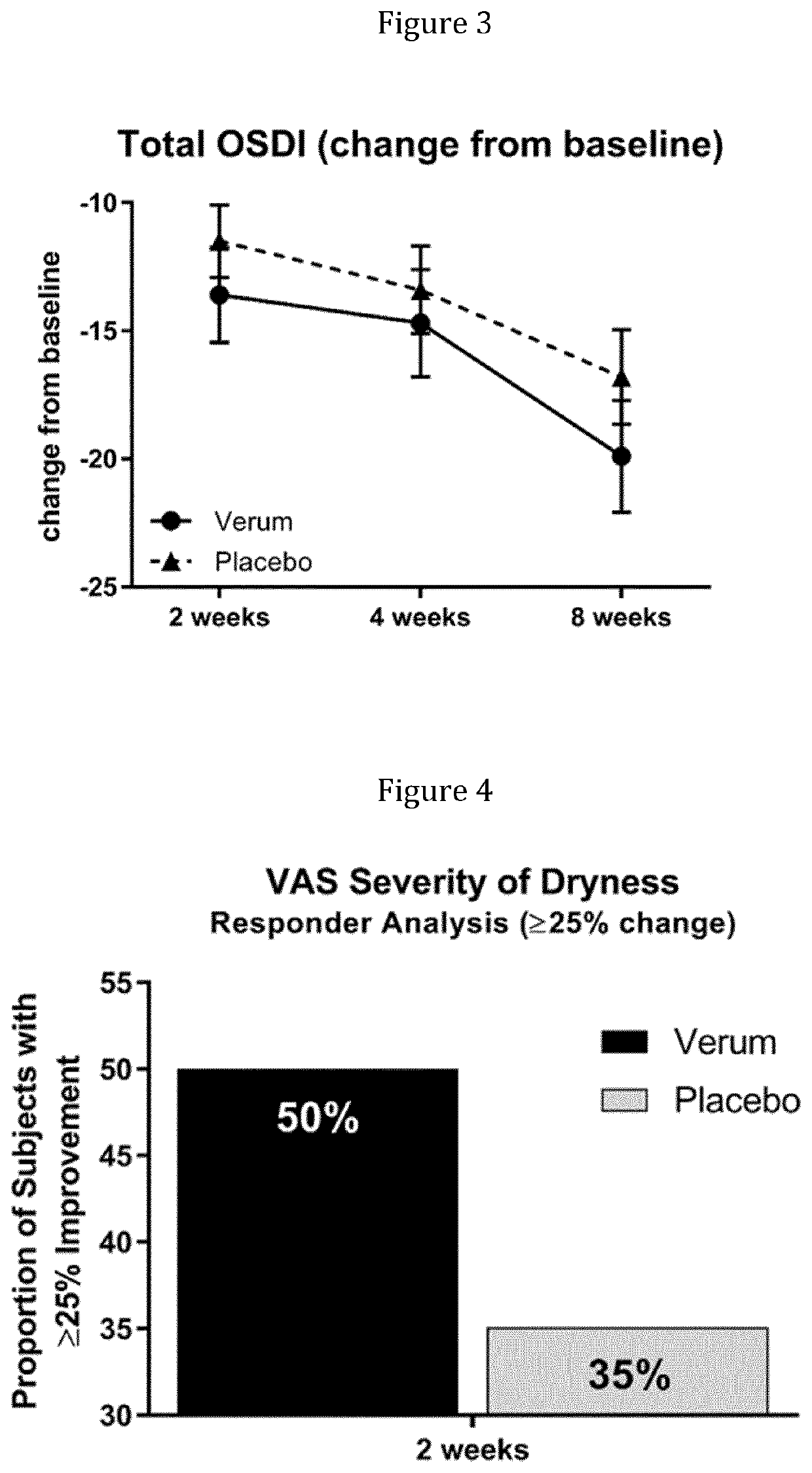
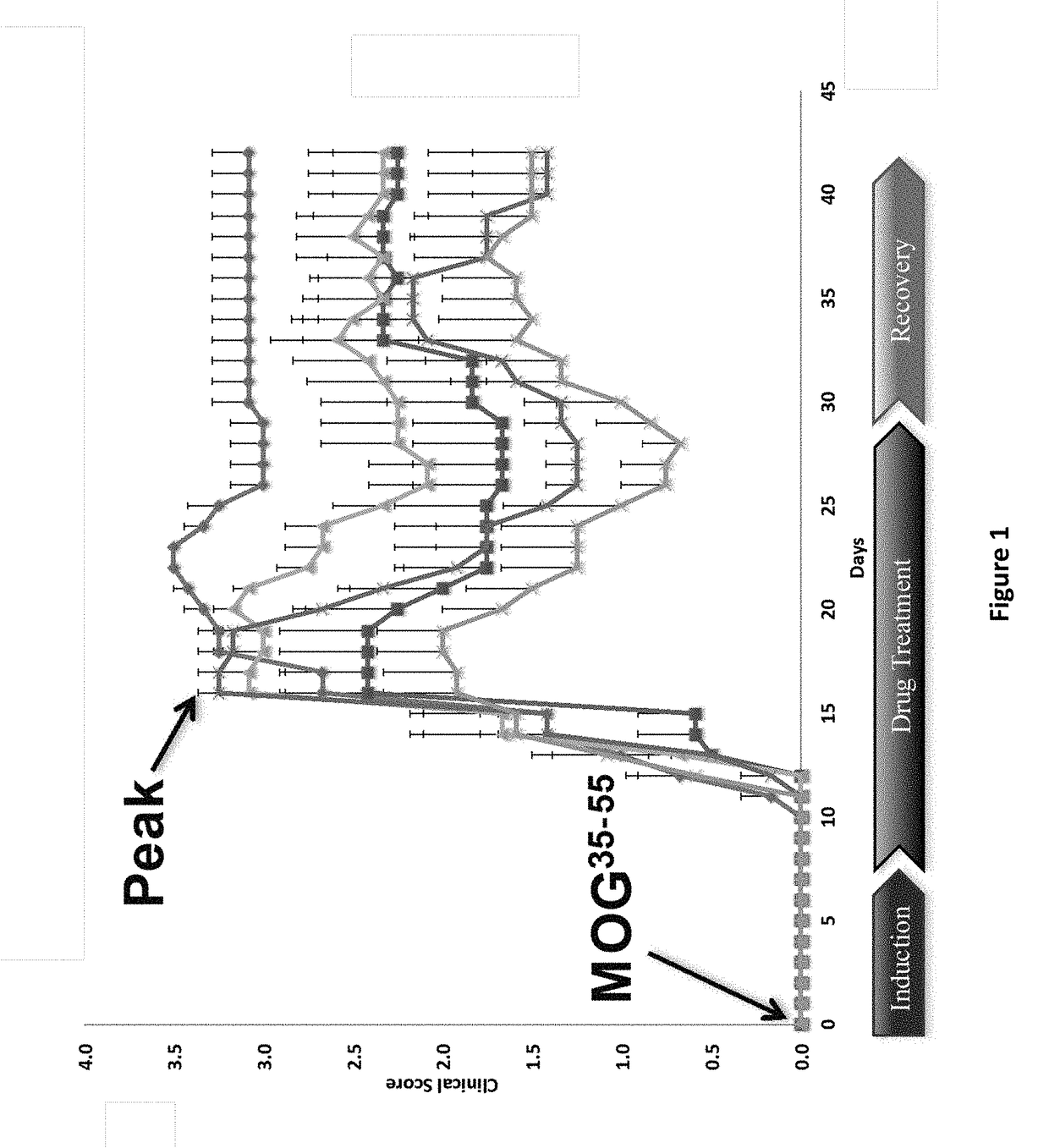
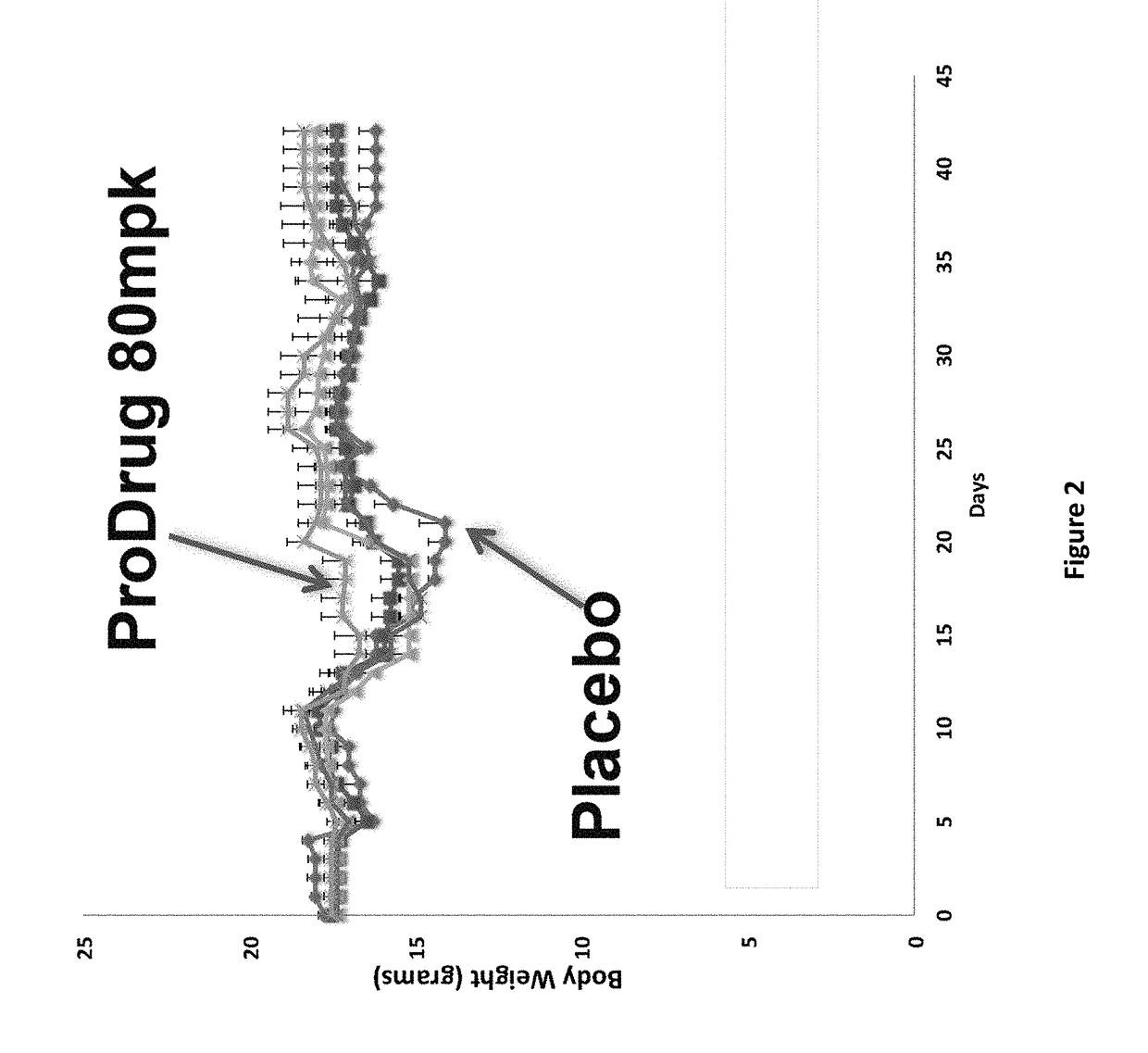
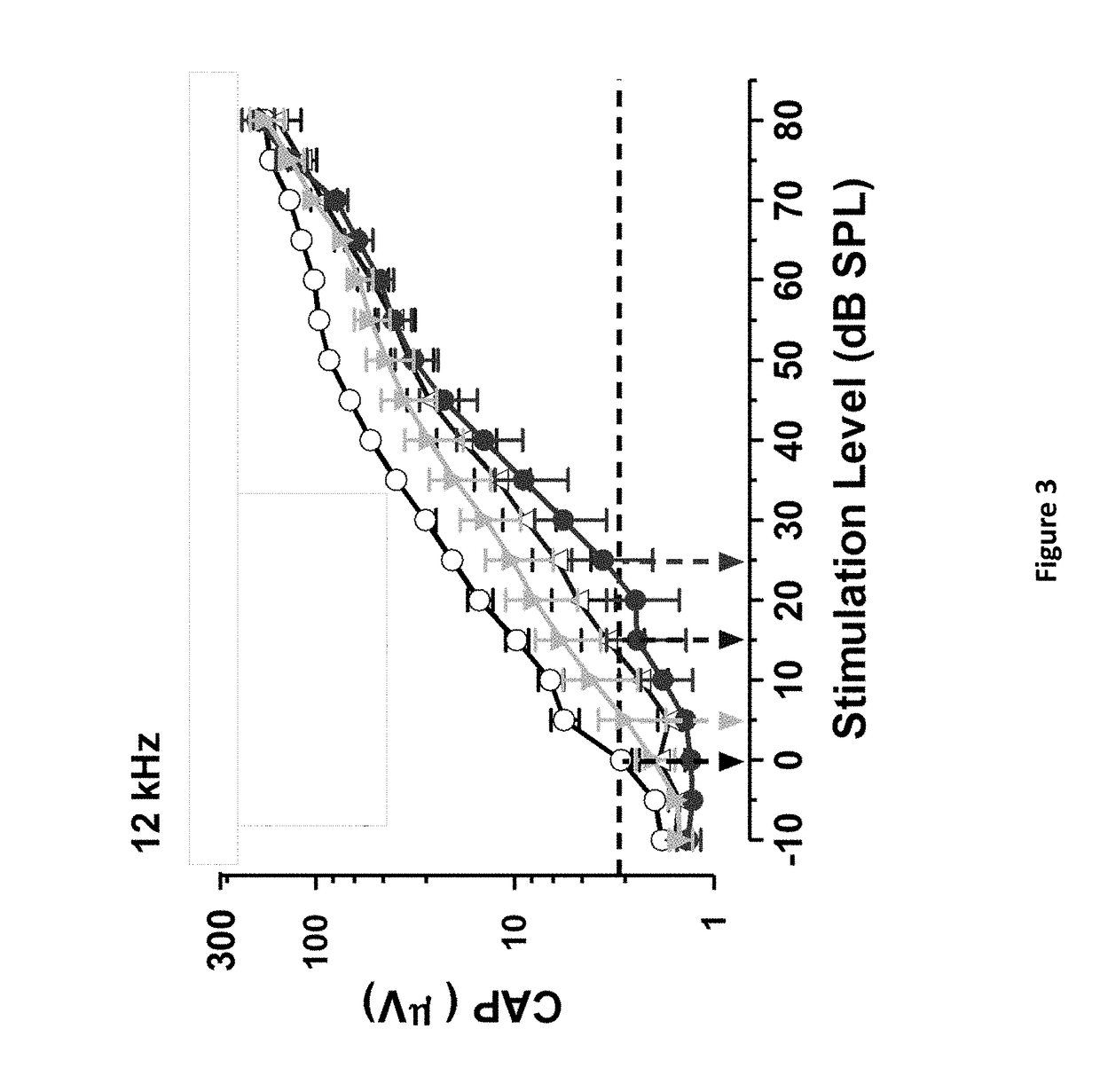
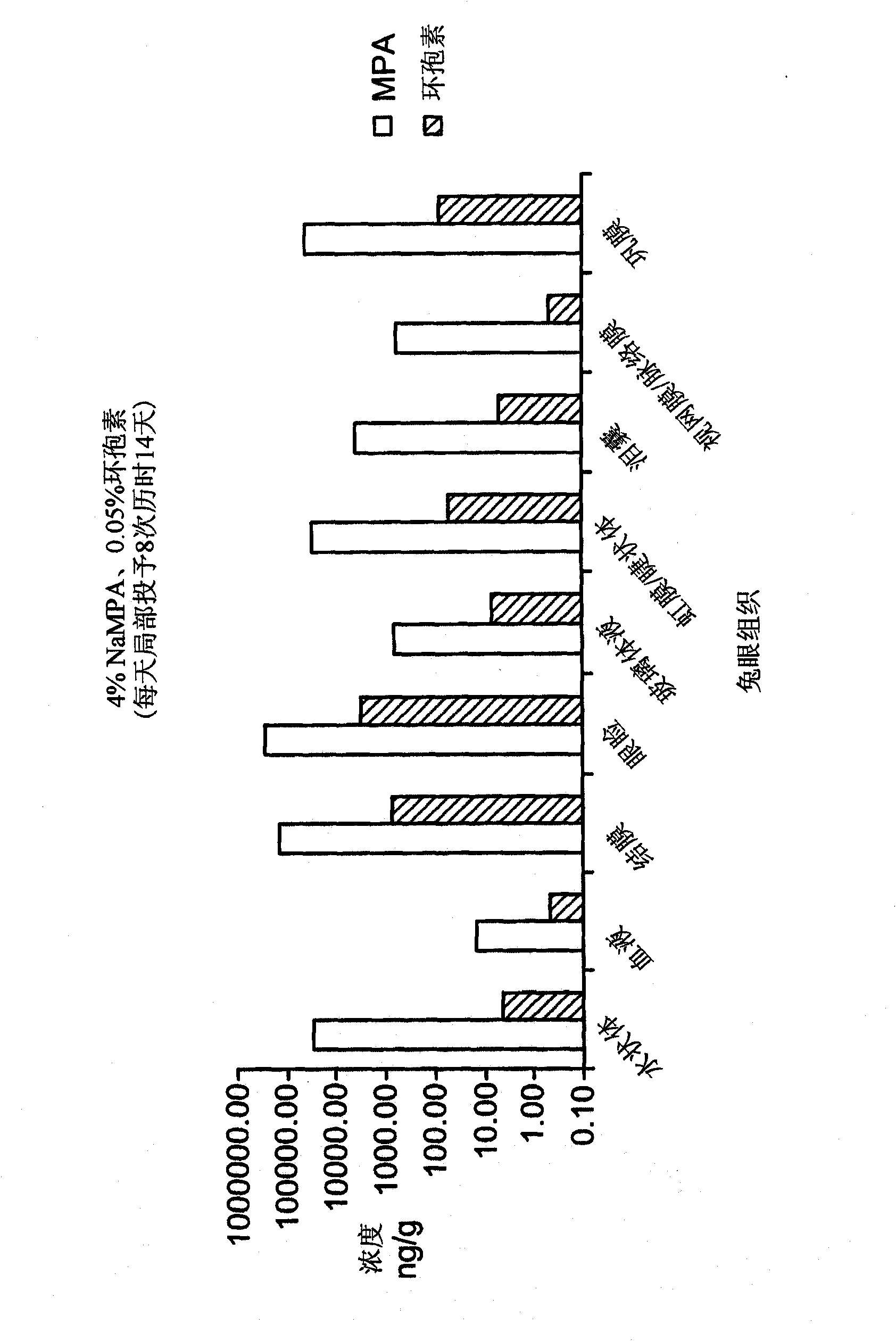
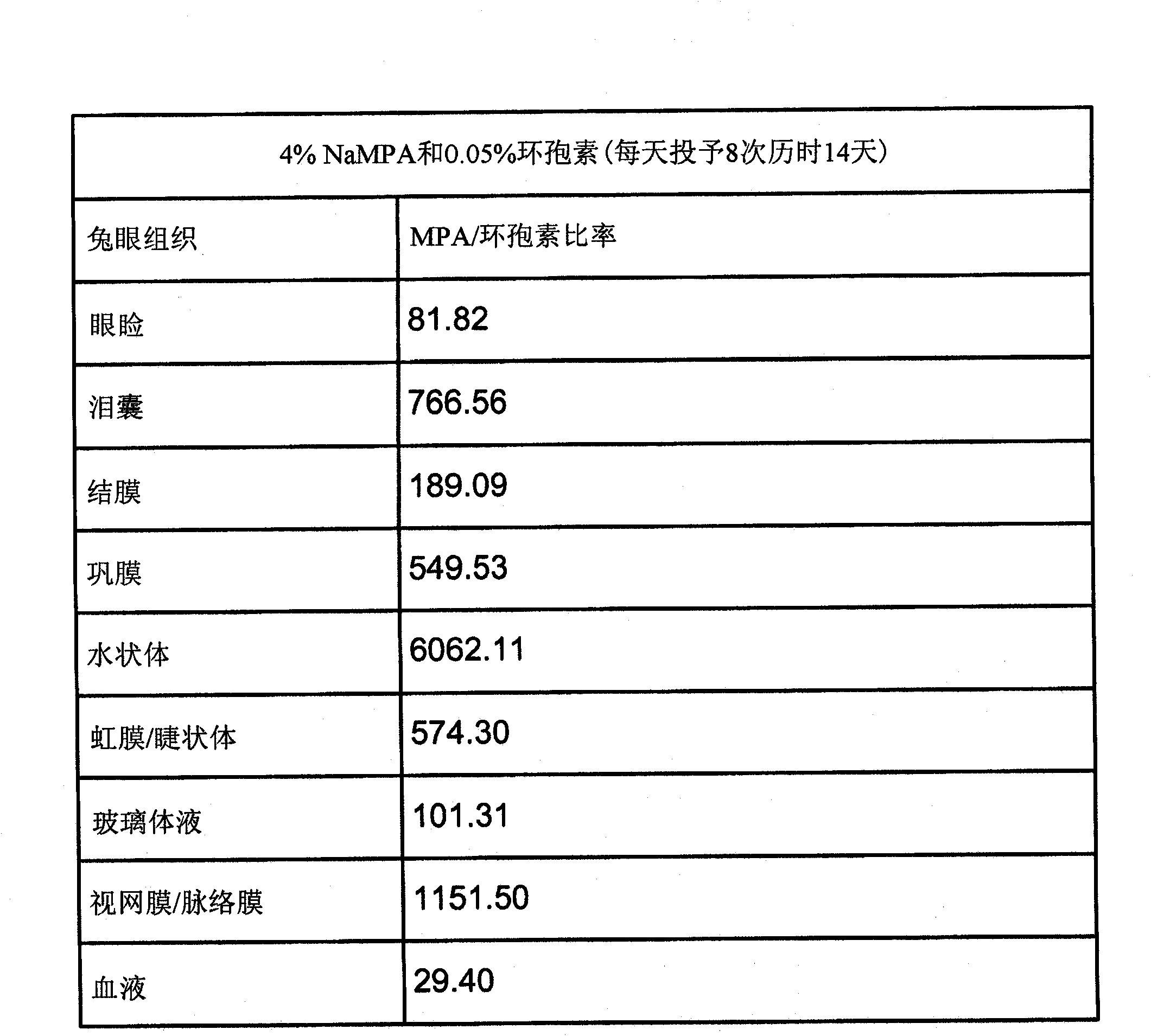
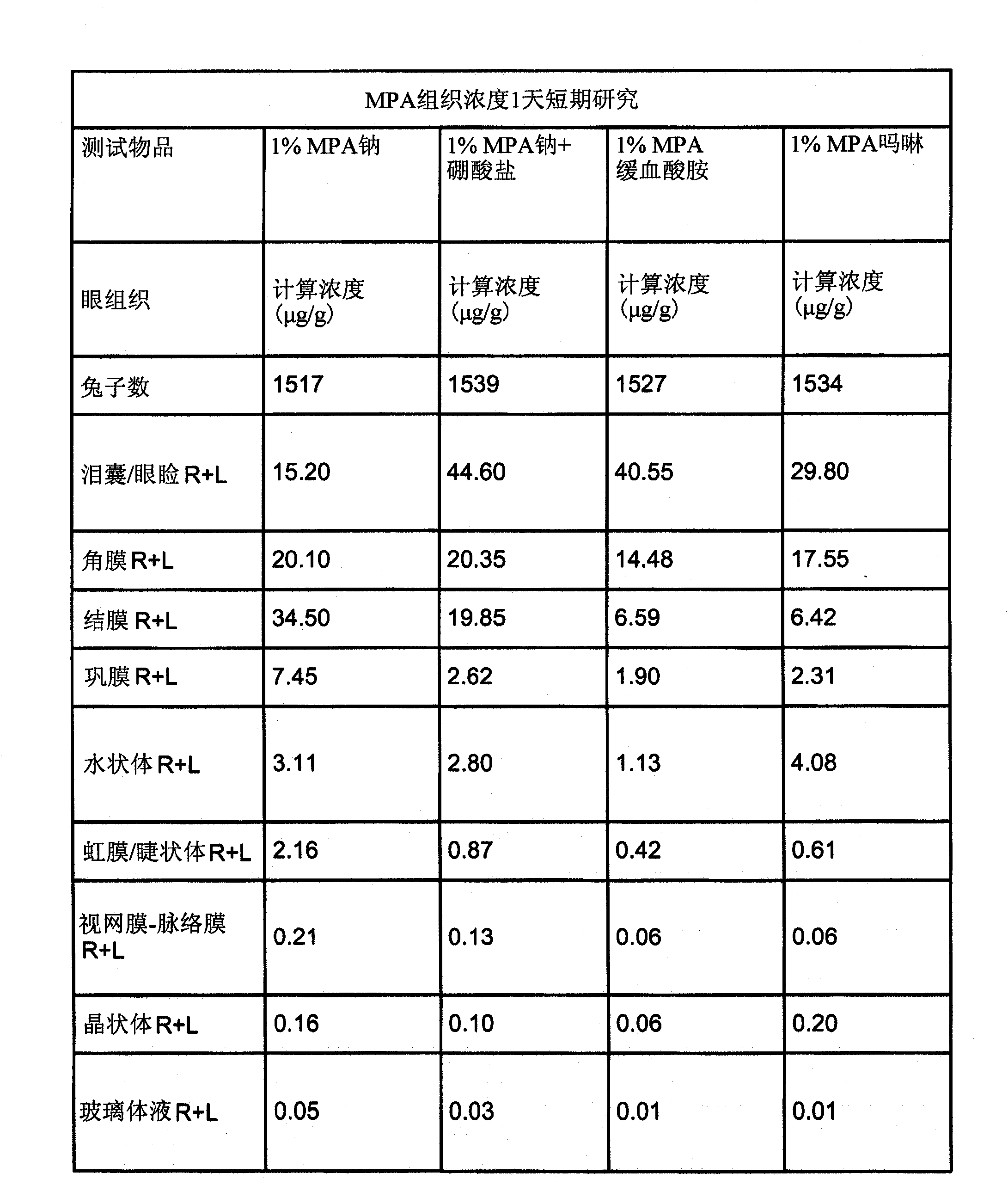
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "KERATOCONJUNCTIVITIS SICCA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic agent for keratoconjunctival disorder
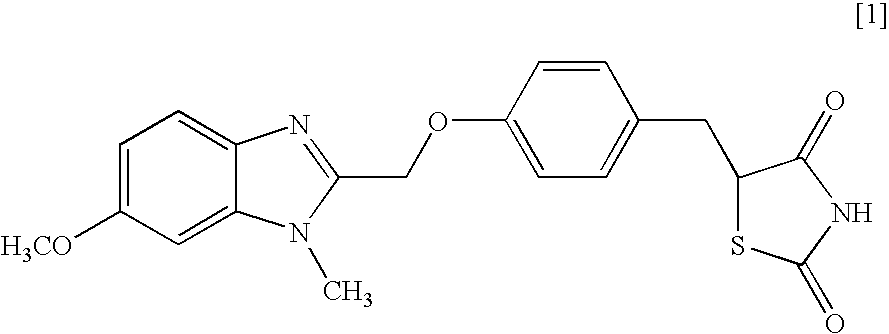
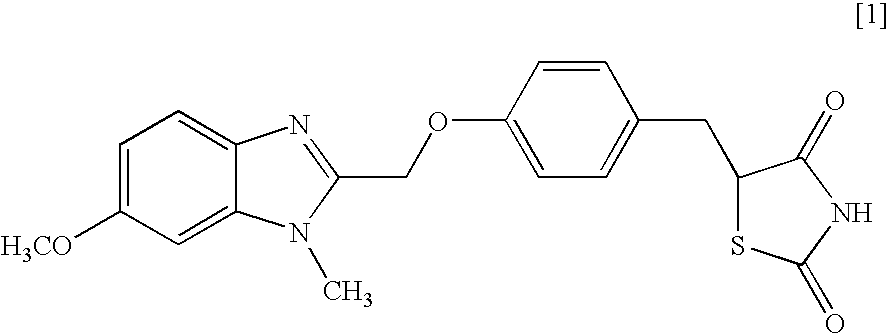
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
New methods of treating dry eye syndrome
The invention relates to a method of insulin eye drops for treating dry eye syndrome due to any and all etiological factors (Keratoconjunctivitis sicca), including Sjogren's syndrome, Meibomian gland dysfunction (MGD) and other glandular malfunction in the eye lids, lacrimal glands, cornea, conjunctiva, and exposed scleral surface of the eye. It is treated with Insulin and / or IGF-I with or without known anti-dry eye syndrome therapeutic, pharmaceutical, biochemical and biological agents or compounds.
Owner:SHANTHA TOTADA R +2
Pharmaceutical composition for treatment of dry eye syndrome
ActiveUS8614178B2Efficient deliveryBetter tolerableSenses disorderPeptide/protein ingredientsAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel pharmaceutical compositions for the treatment of keratoconjunctivitis sicca comprising liquid vehicles which include one or more semifluorinated alkanes. The compositions incorporate an active ingredient selected from the group of macrolide immunosuppressants. They can be administered topically into the eye. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Compositions comprising mixtures of semifluorinated alkanes
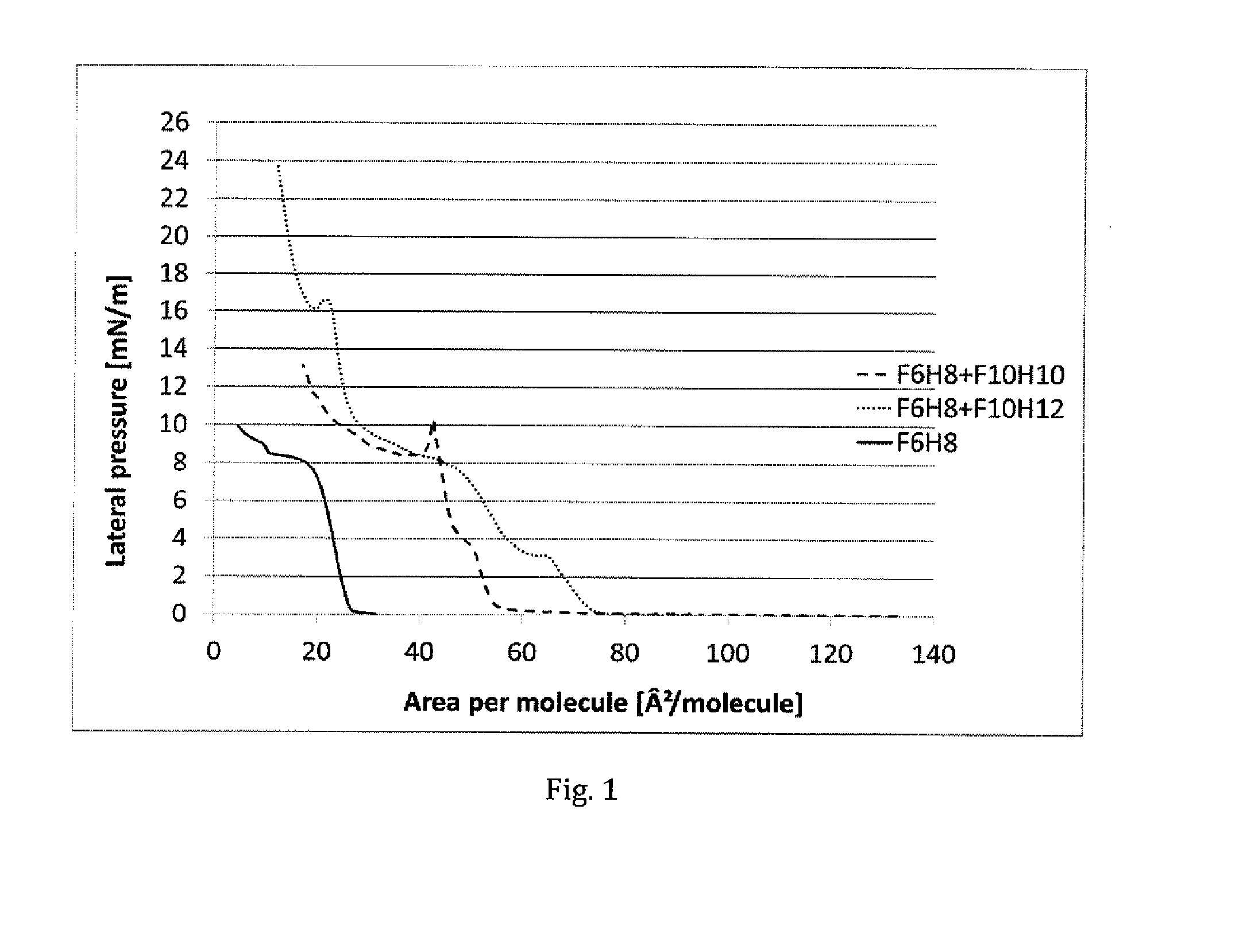
ActiveUS20150224064A1Effective timeTune viscosityBiocideHalogenated hydrocarbon active ingredientsAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel compositions comprising at least two or more semifluorinated alkanes. The compositions can be used as medicines that are topically administered to an eye or ophthalmic tissue, such as for use in the treatment of keratoconjunctivitis sicca (dry eye) and / or meibomian gland dysfunction and symptoms associated therewith. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
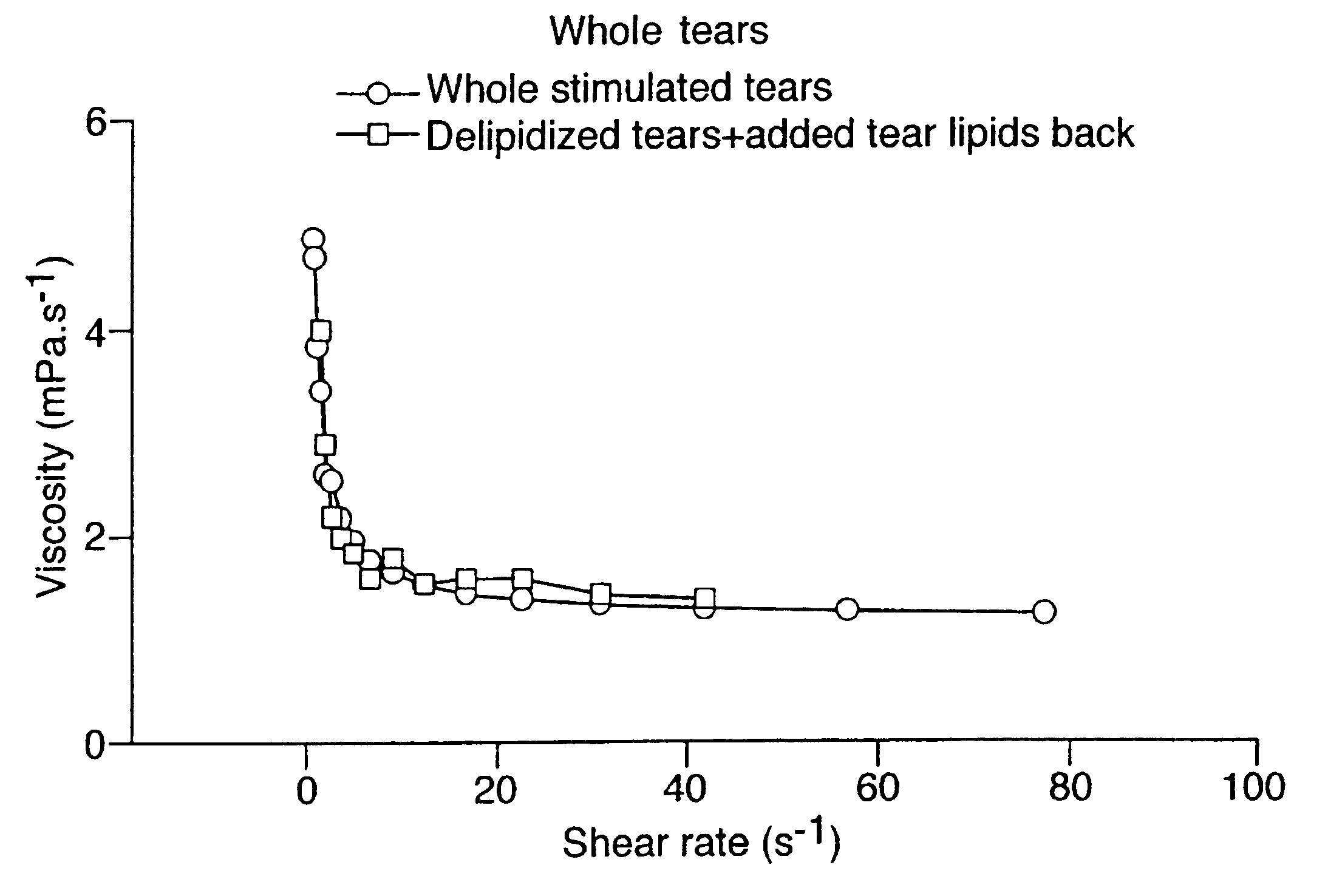
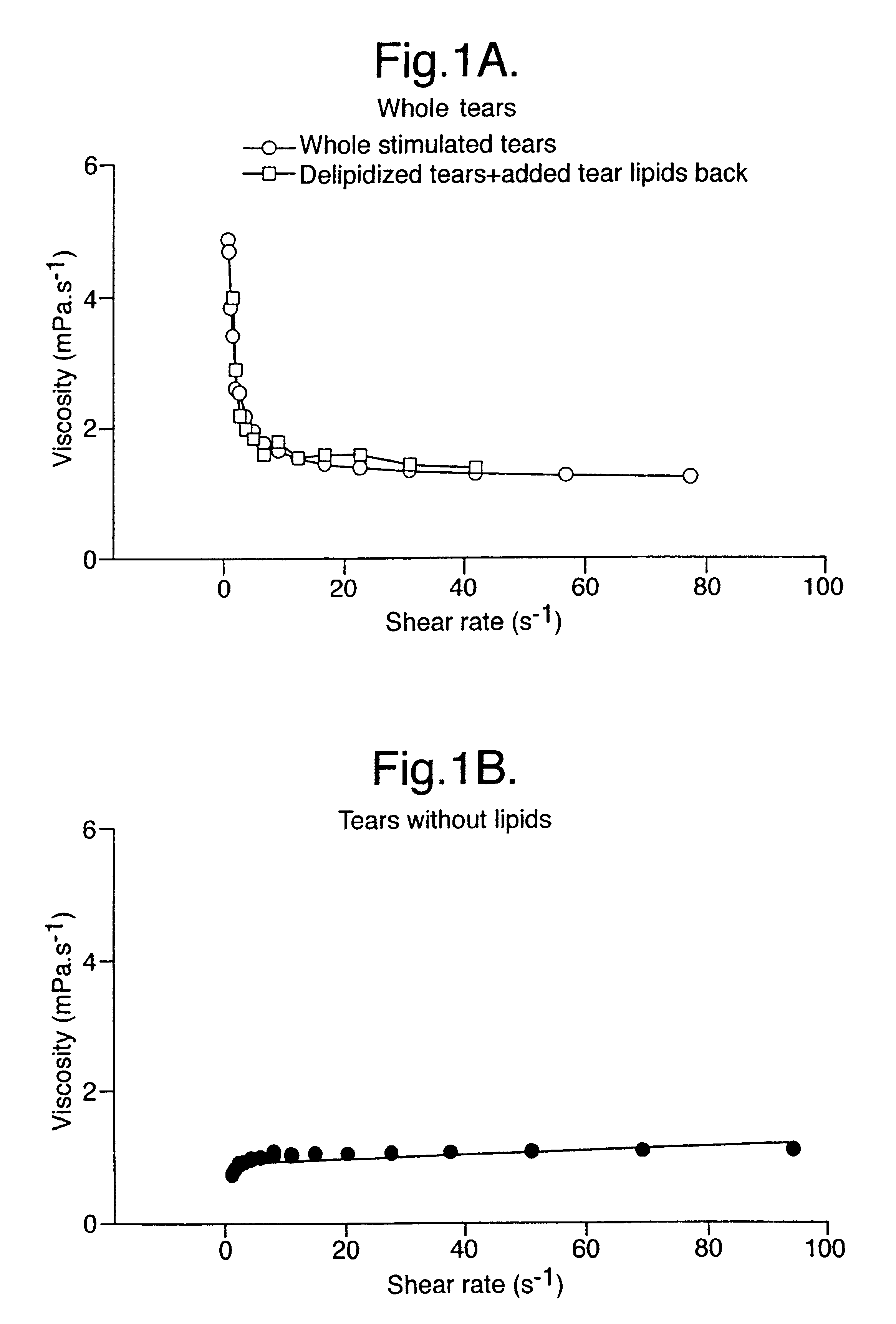
Artificial tear formulation
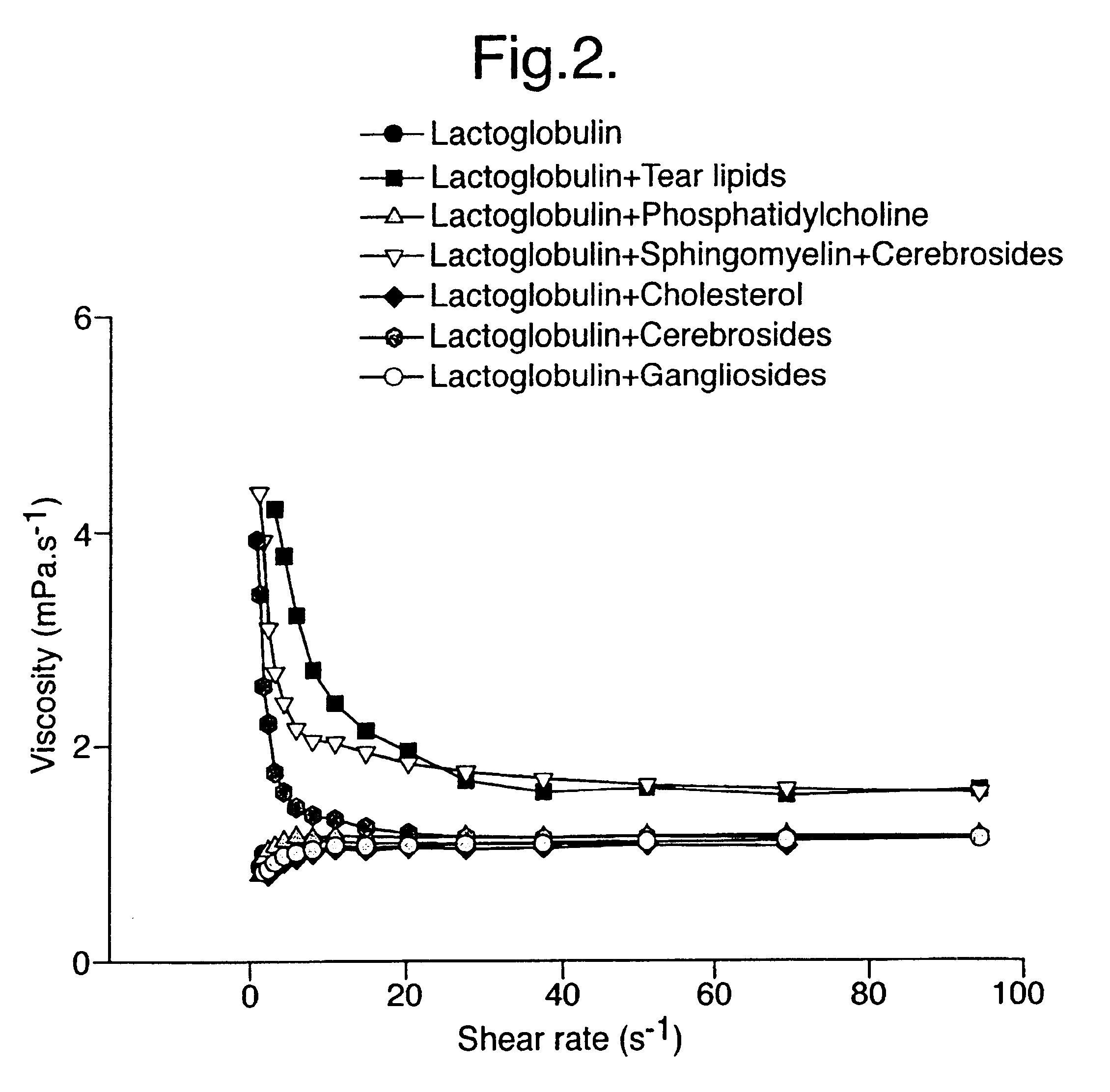
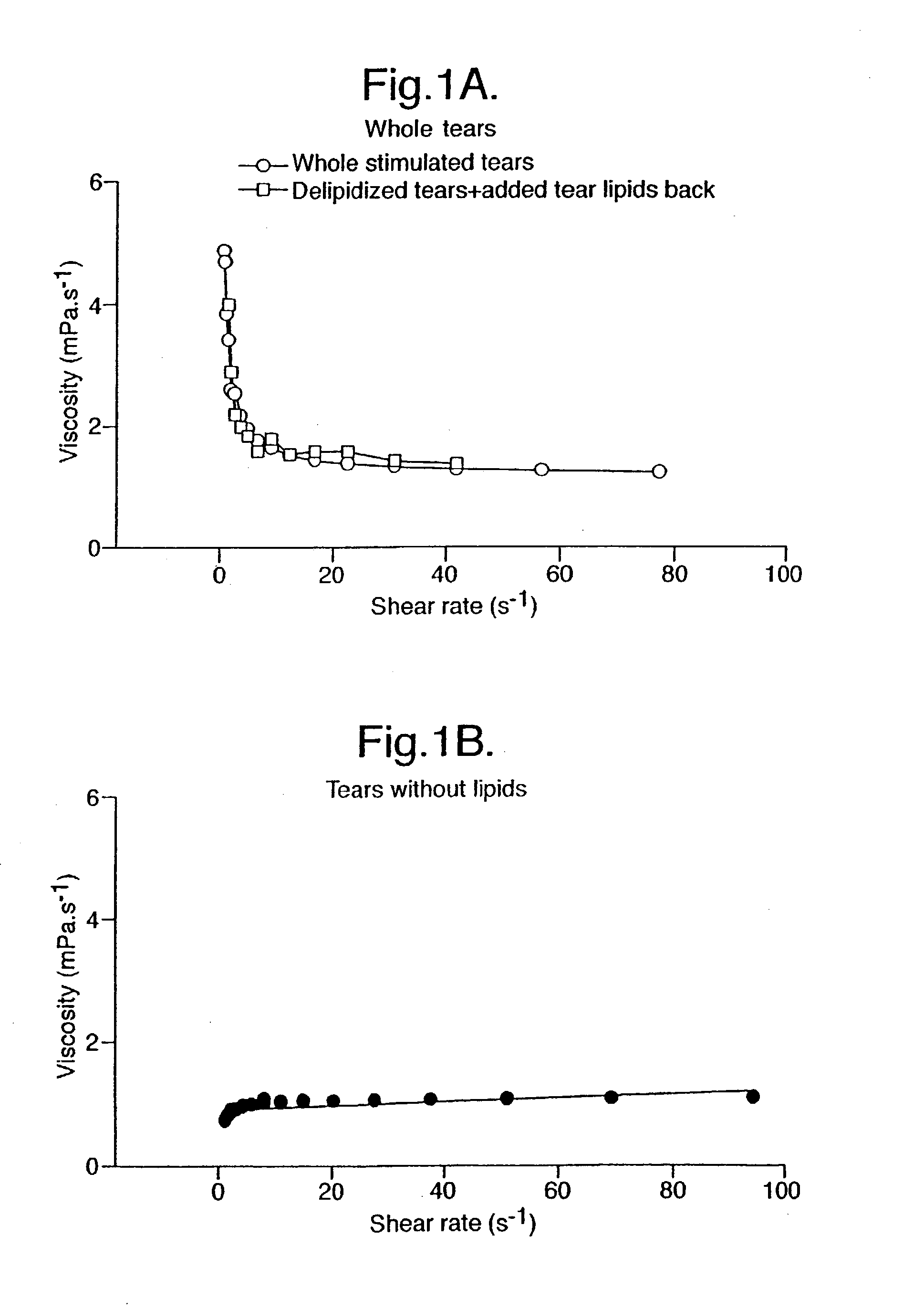
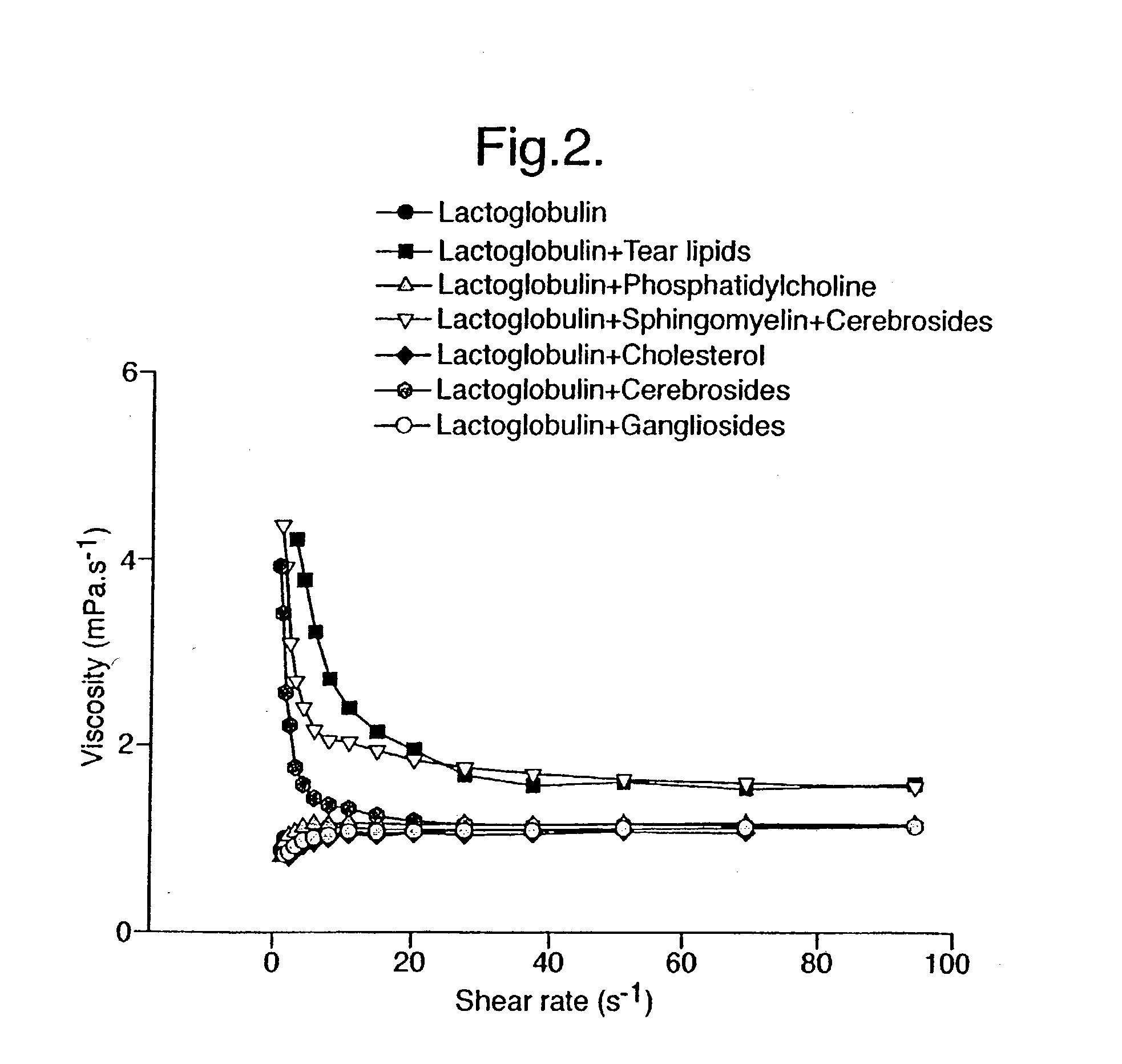
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
Pharmaceutical composition for treatment of dry eye syndrome
ActiveUS20120244177A1Efficient deliveryBetter tolerableSenses disorderAntipyreticAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel pharmaceutical compositions for the treatment of keratoconjunctivitis sicca comprising liquid vehicles which include one or more semifluorinated alkanes. The compositions incorporate an active ingredient selected from the group of macrolide immunosuppressants. They can be administered topically into the eye. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Method And Composition For The Treatment Of Moderate To Severe Keratoconjunctivitis Sicca
InactiveUS20110300097A1Ensuring structureEnsure stabilityBiocideSenses disorderKERATOCONJUNCTIVITIS SICCAKCS - Keratoconjunctivitis sicca
The present invention relates to topical ophthalmic compositions for treating or preventing epithelial lesions or ophthalmic disorders, including dry eye or keratoconjunctivitis sicca.
Owner:AL QAHTANI AHMED H
Therapeutic agent for keratoconjunctive disorders
ActiveUS20150290172A1Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyDisease
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
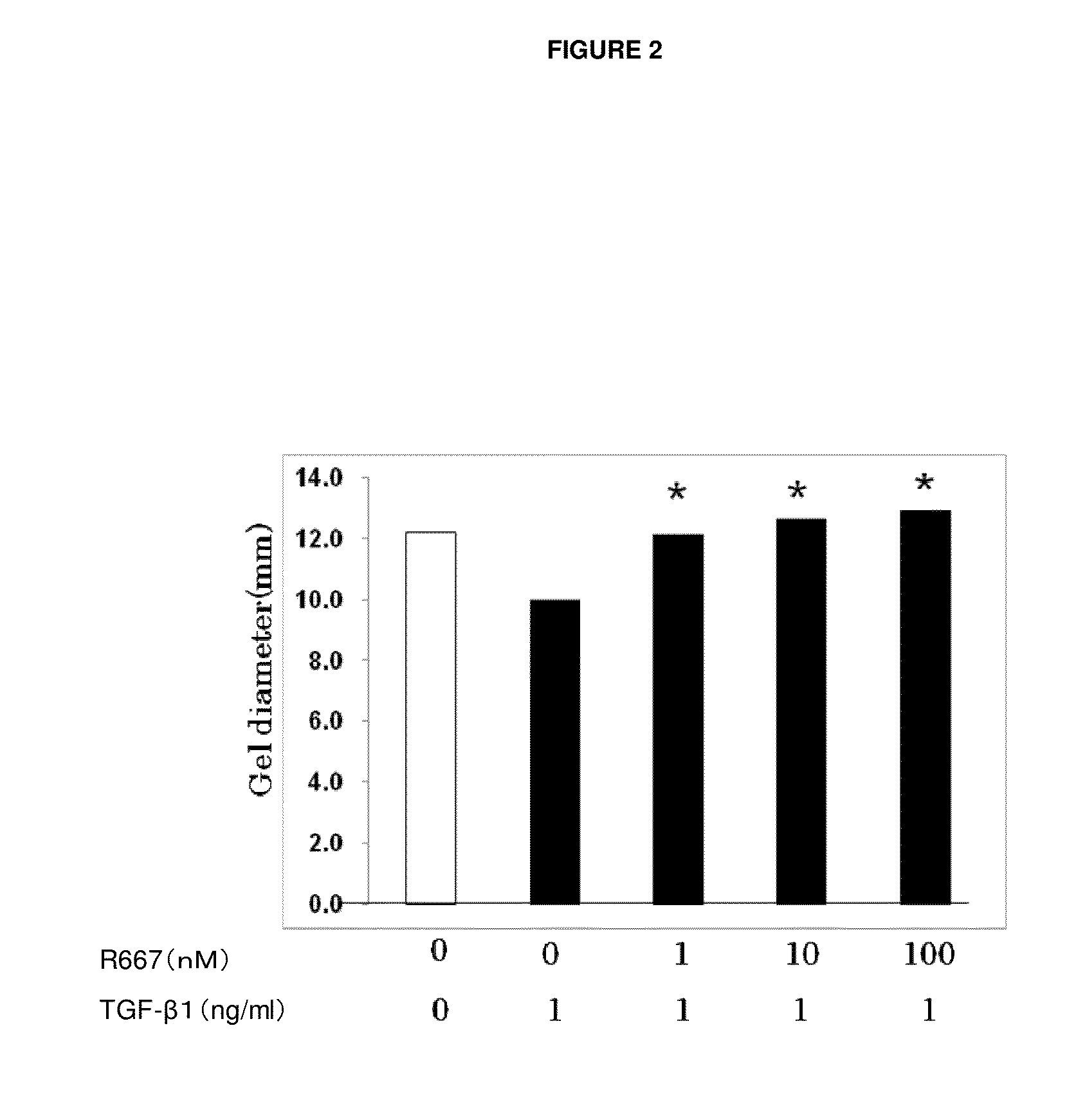
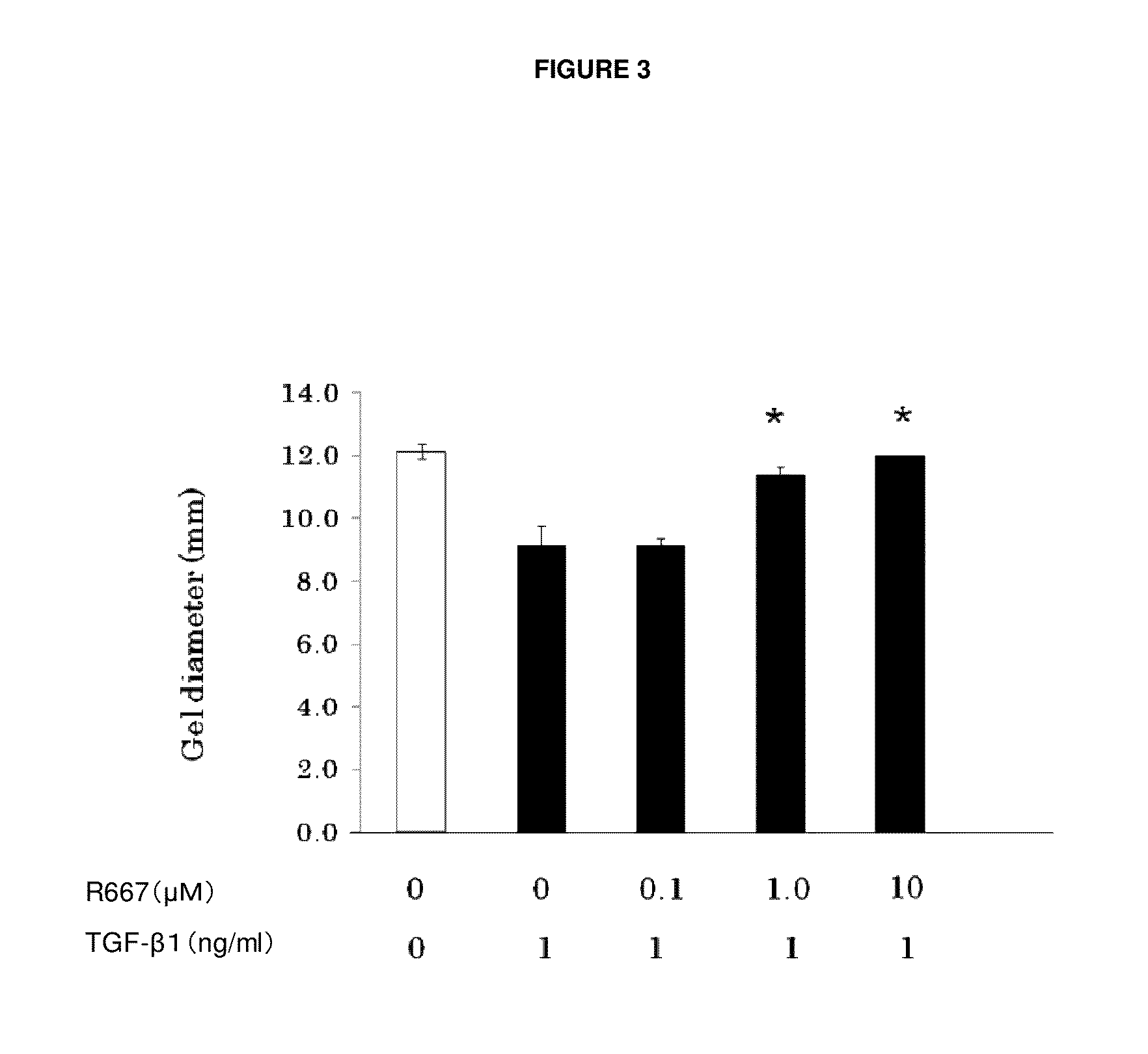
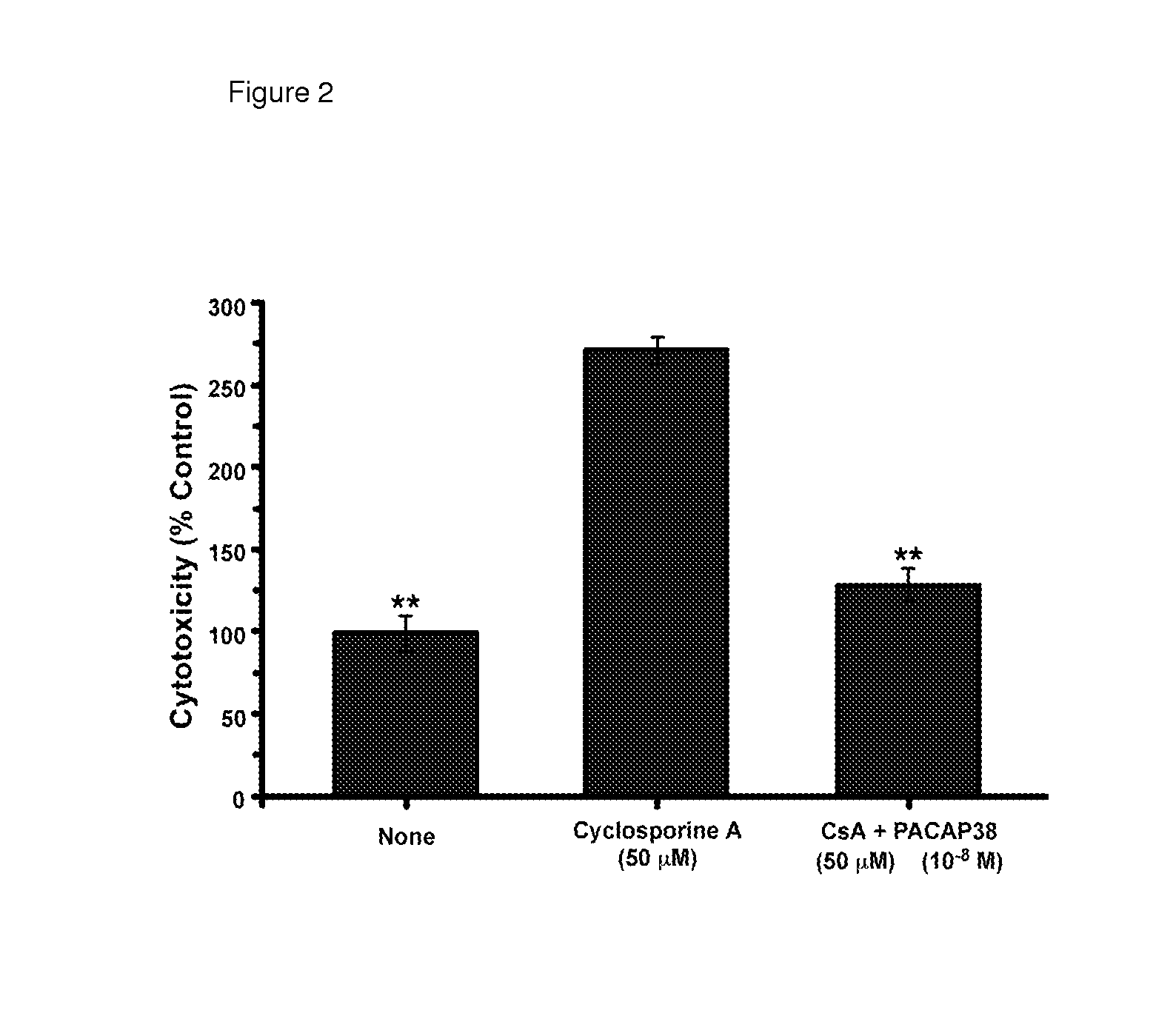
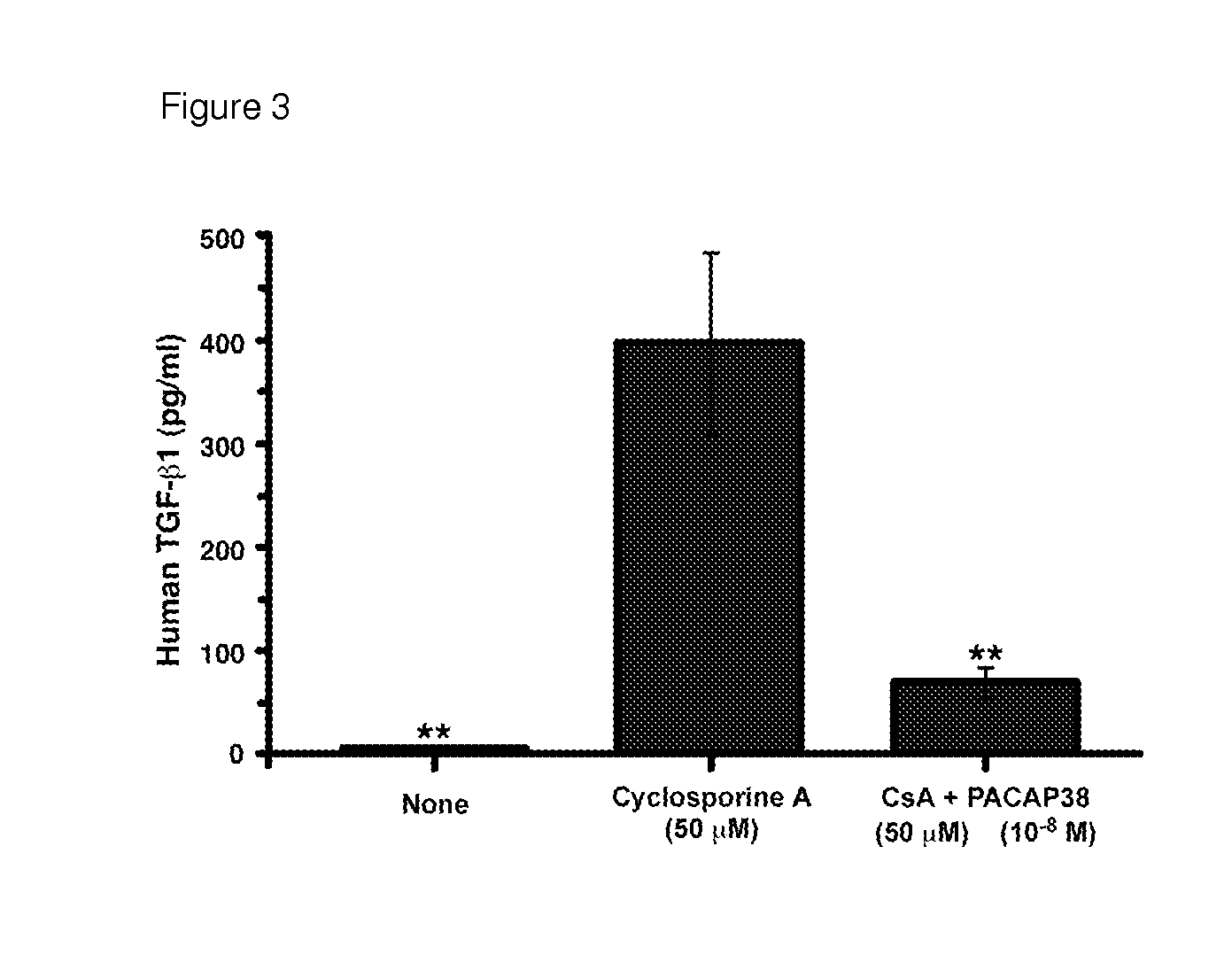
USE OF PITUITARY ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE (PACAP) AND PACAP ANALOGS AS ADJUNCTIVE TREATMENTS WITH INHIBITORS OF CALCINEURIN OR INHIBITORS OF THE MAMMALIAN TARGET OF RAPAMYCIN (mTOR) COMPLEXES
InactiveUS20120309683A1Protecting the major organsEffective protectionNervous disorderMetabolism disorderUveitisAutoimmune responses
This invention relates to methods and compositions for the treatment, management, reduction, or prevention of injuries to one or more major organs of the body, e.g., the brain, heart, lung, kidneys, liver, and gastrointestinal tract, of a mammal (e.g., a human) caused by one or more calcineurin or mammalian target of rapamycin (mTOR) complex inhibitors. The methods include administering an effective amount of one or more pituitary adenylate cyclase-activating polypeptide (PACAP)-like compounds to the mammal. Combination therapy with one or more PACAP-like compounds, either alone or in combination with one or more other prophylactic / therapeutic agents, plus one or more inhibitors of either calcineurin or the mTOR complexes can be used to treat organ transplantation, autoimmune diseases, graft-versus-host disease, Behçet's disease, hematological cancers, noninfectious uveitis, sarcoidosis, tuberous sclerosis complex, acute neurological diseases, age-related neurodegenerative diseases, Huntington's disease and other CAG codon repeat expansion diseases, keratoconjunctivitis sicca, and restenosis.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
Therapeutic agent for keratoconjunctive disorders
ActiveUS9492431B2Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyInfectious Keratitis
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
Compositions and methods of using same for treatment of a disease or disorder of the eye and/or the adnexa of the eye
The present invention relates to compositions comprising at least one flavonoid for the treatment or amelioration of a disease or disorder of the eye and / or the adnexa of the eye in an animal subject, including a human being. More particularly, this invention relates to a composition for the treatment of conjunctivitis, keratoconjunctivitis sicca, and blepharitis. The invention furthermore relates to a pharmaceutical composition comprising at least one flavonoid, such as for example a topical formulation. The source of the flavonoids may be, but are not restricted to, flavonoids extracted from citrus plants. The compositions may furthermore optionally be used in combination with an eyecleaner or eyewash, which may comprise at least one flavonoid.
Owner:OCUMEDIC
Preventive or therapeutic agent for keratoconjunctival disorder
InactiveUS20090105313A1Improve the improvement effectGood prevention effectBiocideSenses disorderConjunctivaDisease
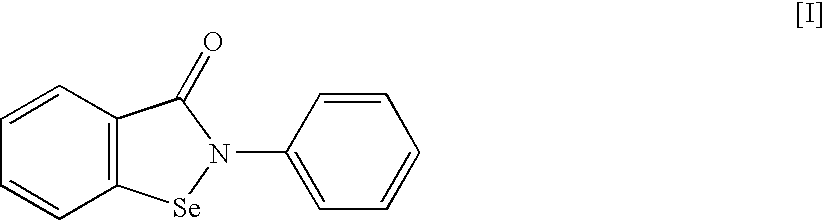
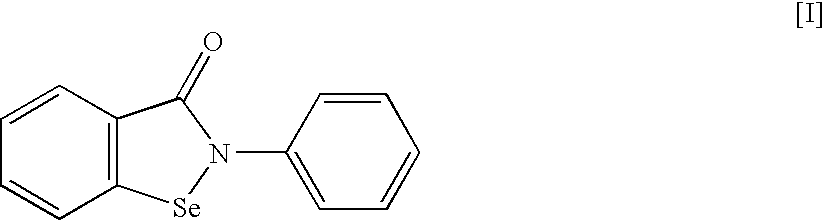
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
Therapeutic agent for keratoconjunctival disorder
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy) benzyl] thiazolidine-2, 4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy) benzyl] thiazolidine-2, 4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Chinese medicament for treating keratoconjunctivitis sicca and preparation method thereof
InactiveCN102920911AConvenient treatmentEffective in treating keratoconjunctival xerosisSenses disorderPlant ingredientsConjunctival xerosisDisease
The invention provides a Chinese medicinal composition for treating keratoconjunctivitis sicca, which comprises the following raw material drugs: heartleaf houttuynia herb, dendrobe, mulberry leaf, medlar, yerbadetajo herb, cassia seed, multiflower knotweed vine, red bean, mulberry, kamuning, liriope spicata, America ginseng, plantain herb, fragrant solomonseal rhizome, figwort root, raw rehmannia, coral ardisia root, buerger pipewort flower, papermulberry fruit, motherwort and liquorice. Compared with a western medicament, the Chinese medicinal composition has good curative effect, relapse prevention, small toxic and side effects and low residue rate in treating keratoconjunctivitis sicca, can regulate a whole human body to achieve the effect of treating both principal and secondary aspect of disease, and is a safe and reliable Chinese medicament for treating keratoconjunctivitis sicca with remarkable curative effect.
Owner:QINGDAO CENT HOSPITAL +1
Method and composition for the treatment of moderate to severe keratoconjunctivitis sicca
ActiveUS20140099355A1Ensuring structureEnsure stabilityPeptide/protein ingredientsHydroxy compound active ingredientsKERATOCONJUNCTIVITIS SICCAKCS - Keratoconjunctivitis sicca
The present invention relates to topical ophthalmic compositions for treating or preventing epithelial lesions or ophthalmic disorders, including dry eye or keratoconjunctivitis sicca.
Owner:AL QAHTANI AHMED H
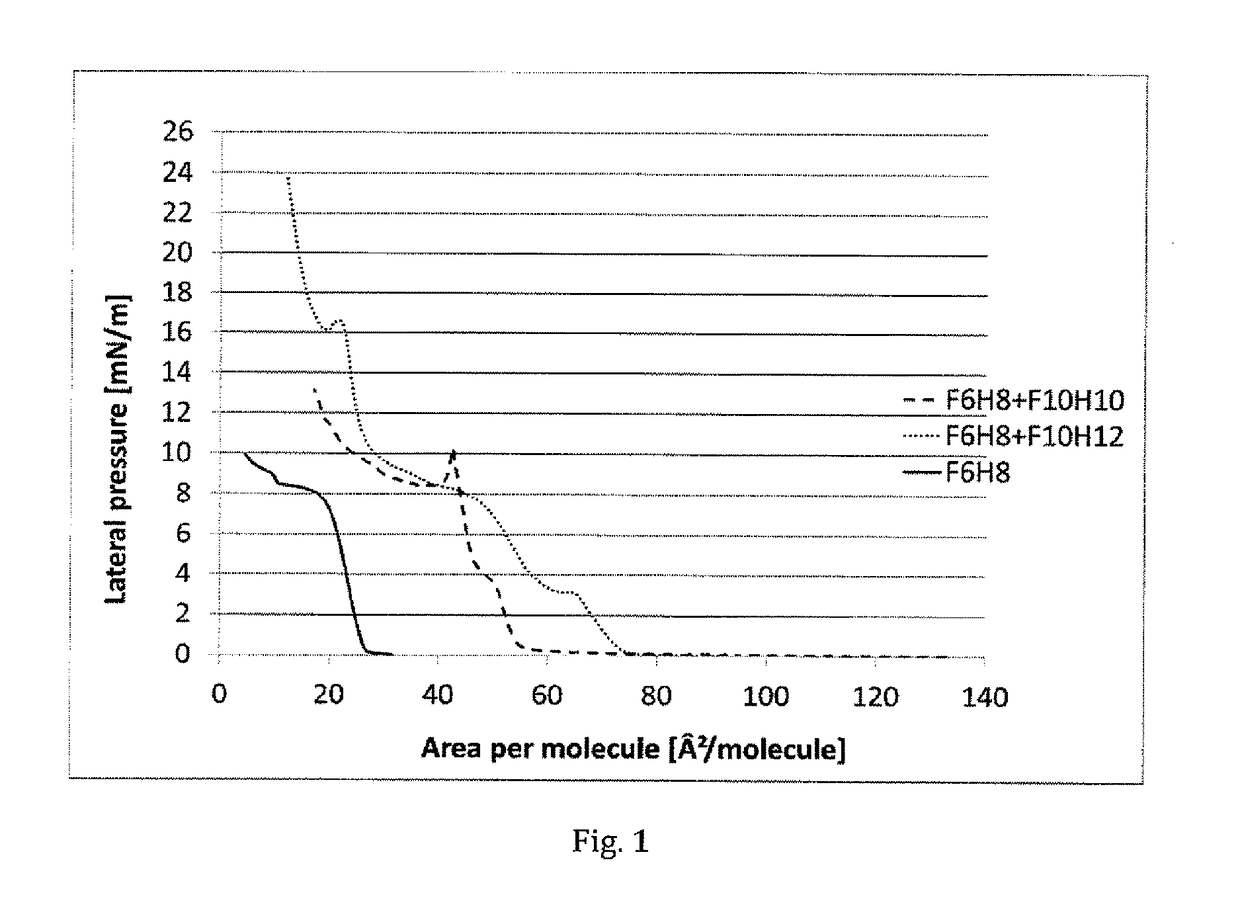
Opthalmic compositions comprising f6h8
PendingUS20200268682A1Delayed ophthalmic releaseHalogenated hydrocarbon active ingredientsSenses disorderConjunctivaAlkane
The present disclosure provides methods of treatment using ophthalmic compositions comprising semifluorinated alkanes for keratoconjunctivitis sicca and / or Meibomian gland dysfunction, which methods provide for the enrichment of an ophthalmic tissue in the semifluorinated alkane, and optionally methods of delayed release of the semifluorinated alkane from the enriched ophthalmic tissue to the surface of the cornea and / or conjunctiva, and / or to the Meibomian gland.
Owner:NOVALIQ GMBH
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
ActiveUS20170252347A1Good blood pressureImprove blood sugar levelsSenses disorderNervous disorderHL - Hearing lossDepressant
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Formulations for treating eye disorders
Ocular delivery of drugs to the eyes is an ongoing challenge due to the unique anatomical and physiological properties of the eye. A solution of the immunosuppressant and anti-inflammatory compound mycophenolic acid with a pH from 6.0 to 8.5 has been demonstrated to exhibit improved bioavailability when topically applied to the eye. Specifically, topical application to the eye of such a solution is effective in penetrating anterior and posterior eye structures. Said solution is effective in treating a variety of inflammatory disorders, including uveitis, allergic conjunctivits, and keratoconjunctivitis sicca.
Owner:ASPREVA INT
Artificial tear formulation
InactiveUS20040126419A1Reproduce viscosityReproduce surface tension propertyAntibacterial agentsBiocideLipid formationKERATOCONJUNCTIVITIS SICCA
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
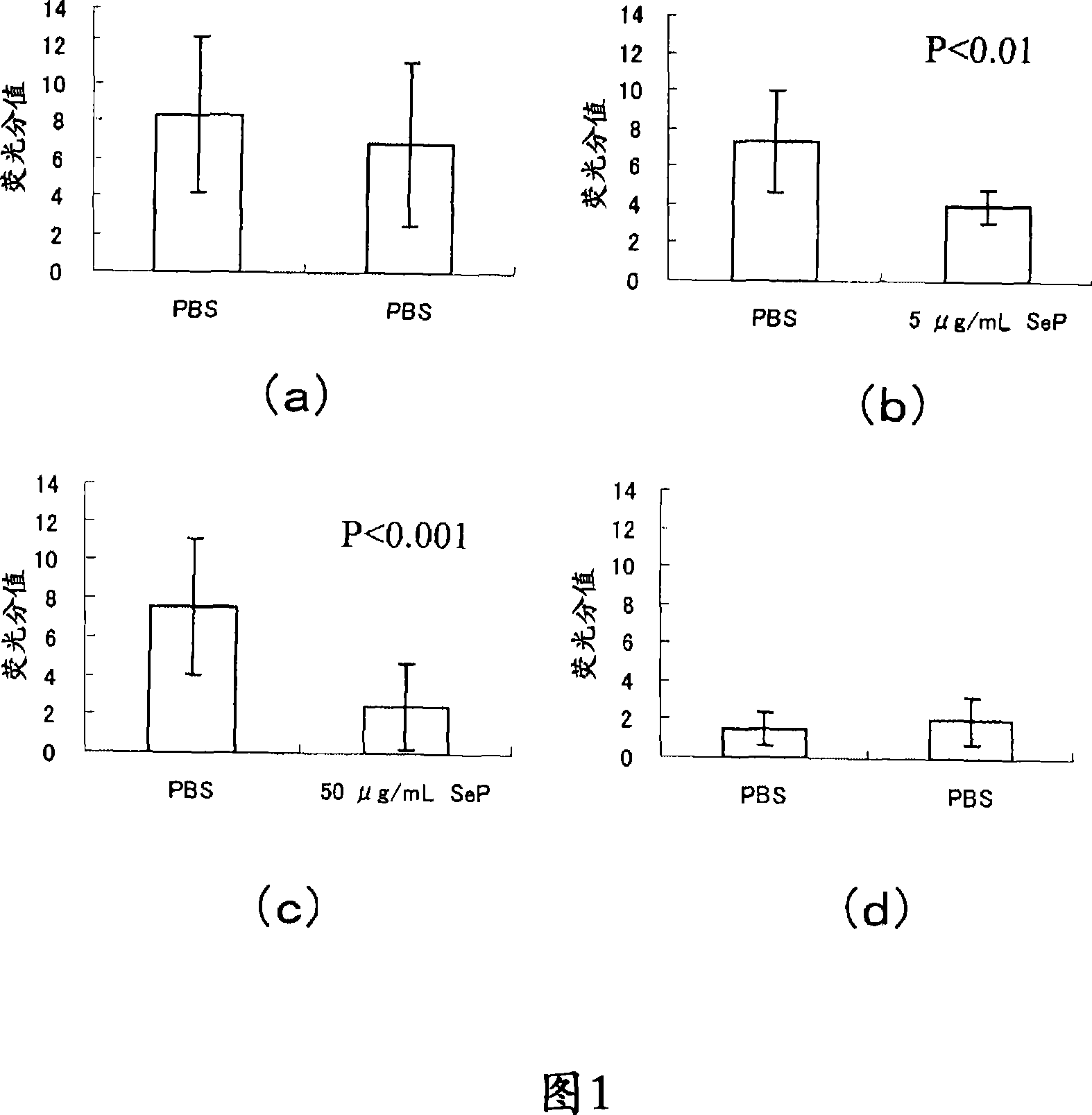
Prophylactic or therapeutic agent for corneal/conjunctival disease
InactiveUS20100113338A1Therapy is simpleGood treatment effectSenses disorderPeptide/protein ingredientsDiseaseSuperficial punctate keratopathy
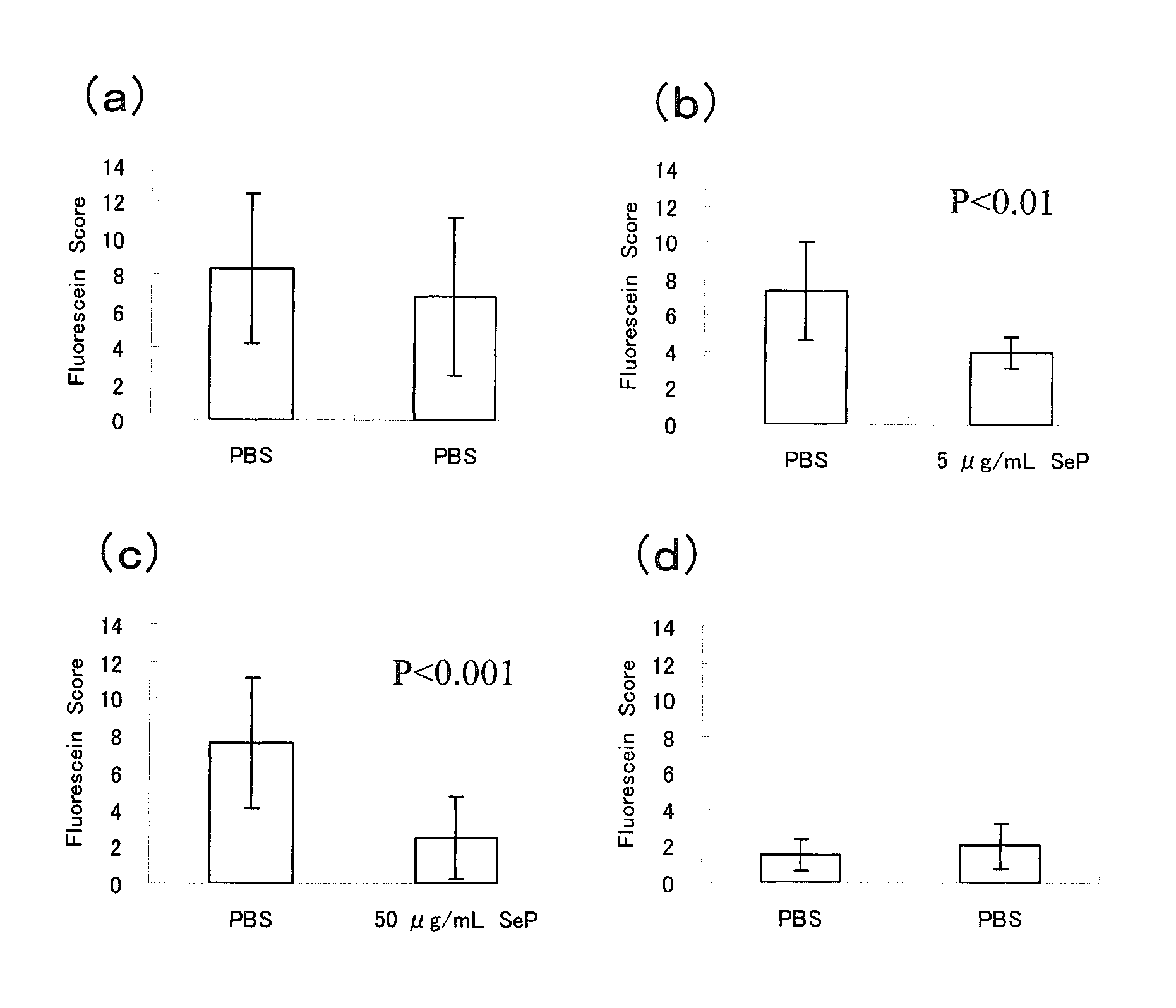
Disclosed is a novel composition for the treatment of a corneal / conjunctival disease. A prophylactic or therapeutic agent for a corneal / conjunctival disease comprising selenoprotein P as an active ingredient, more specifically a prophylactic or therapeutic agent for a corneal / conjunctival disease such as dry eye, keratoconjunctivitis sicca, superficial punctate keratopathy, corneal erosion or corneal ulcer comprising selenoprotein P as an active ingredient, particularly a prophylactic or therapeutic agent for a corneal / conjuncrtival disease such as dry eye, keratoconjunctivitis sicca, superficial punctate keratopathy, corneal erosion or corneal ulcer accompanied by a corneal / conjunctival epithelial discorder.
Owner:TSUBOTA KAZUO +2
Keratoconjunctivitis sicca preventing and treating eye drop and preparation and application method thereof
InactiveCN106963770ARelieve dryness and other symptomsReduce evaporationSenses disorderPharmaceutical delivery mechanismInflammatory factorsKERATOCONJUNCTIVITIS SICCA
The invention relates to a keratoconjunctivitis sicca preventing and treating eye drop and a preparation and application method thereof. The keratoconjunctivitis sicca preventing and treating eye drop is composed of cyoloalliin, anthocyanin, dextranum, hydroxypropyl methyl cellulose, sodium chloride, potassium chloride, sodium borate, pH regulator and purified water. During application, the keratoconjunctivitis sicca preventing and treating eye drop can supplement water for eyes, form protective films on the surfaces of eyes and reduce water evaporation to effective relieve the symptoms caused by keratoconjunctivitis sicca such as dry eyes, and meanwhile, can stimulate secretion of lacrimal glands and increase the number of times of blinking; the cyoloalliin can inhibit secretion of inflammatory factors to release the clinical symptom of dry eyes and meanwhile fundamentally cure the keratoconjunctivitis sicca; besides, the keratoconjunctivitis sicca preventing and treating eye drop is free from preservatives, safe, non-irritative to eyes, high in long-term stability and applicable to wide application of prevention and treatment of various kinds of keratoconjunctivitis sicca.
Owner:MUDANJIANG MEDICAL UNIV
Therapeutic agent for keratoconjunctival disorder
An object of the present invention is to discover a new medicinal use of 5-[4-[[3-methyl-4-oxo-3,4-dihydro-2-quinazolinyl]methoxy]p henylmethyl]thiazolidine-2,4-dione and N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine. Both of the compounds exert an excellent improving effect on corneal disorder models and is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctive epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Ophthalmic medicament composition for forming low-irritation transparent emulsion formulation for surface immune adjustment and inflammation reduction of relevant tissue of eyes or eye periphery
ActiveCN101897949AImprove solubilityLess irritatingSenses disorderCyclic peptide ingredientsPolyoxyethylene castor oilKERATOCONJUNCTIVITIS SICCA
The invention provides an ophthalmic medicament composition for forming a low-irritation transparent emulsion formulation for the surface immune adjustment and inflammation reduction of the relevant tissue of eyes or eye periphery. The ophthalmic medicament composition is used for treating the inflammatory reaction of a serious keratoconjunctivitis sicca and cornea epithelium pathological change patient, forms an emulsion formulation, contains at least one ciclosporin, propylene glycol and polyoxyethylene castor oil derivative, such as polyoxyethylene 35 castor oil derivative, and the like, is transparent, has the characteristics of low irritation, stability and no crystallization phenomenon and is suitable for more sensitive areas, such as eye tissue, and the like.
Owner:RXVISION PHARMA CO LTD
Method for treating a keratoconjunctival disorder
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:BIOVENTURES LLC +1
Compositions comprising mixtures of semifluorinated alkanes
ActiveUS20180071229A1Effective timeTune viscosityHalogenated hydrocarbon active ingredientsSenses disorderAlkaneKERATOCONJUNCTIVITIS SICCA
Owner:NOVALIQ GMBH
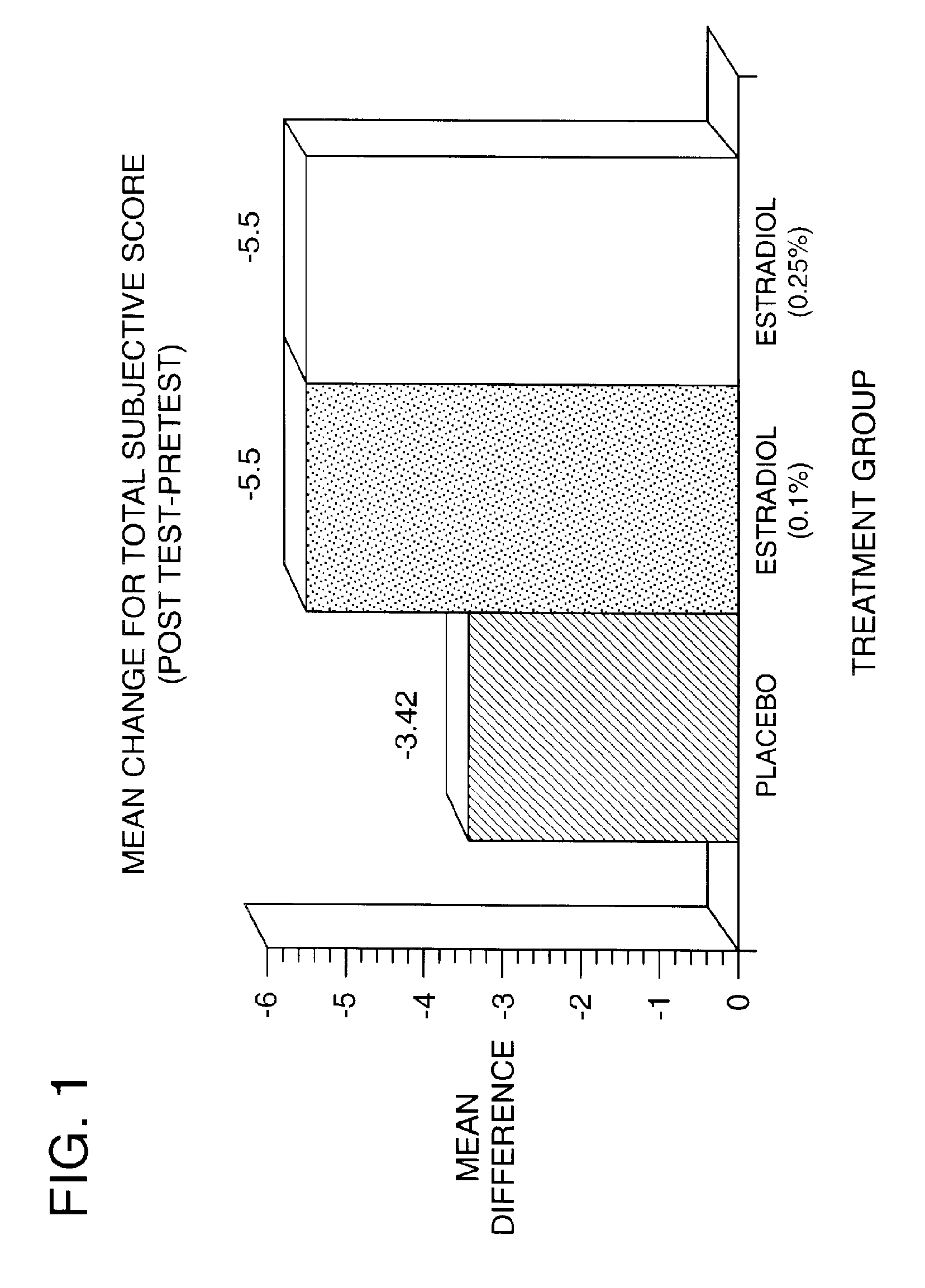
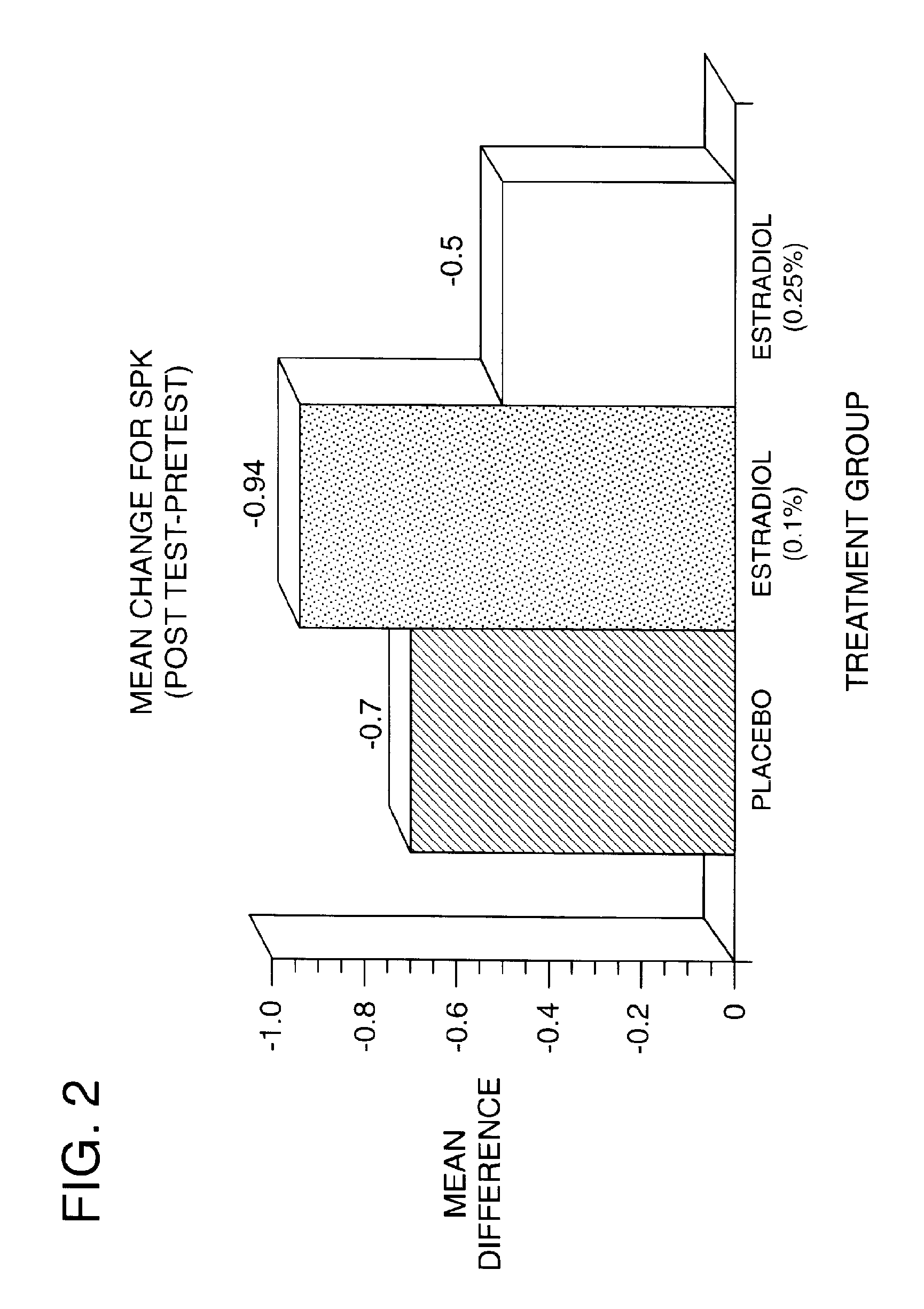
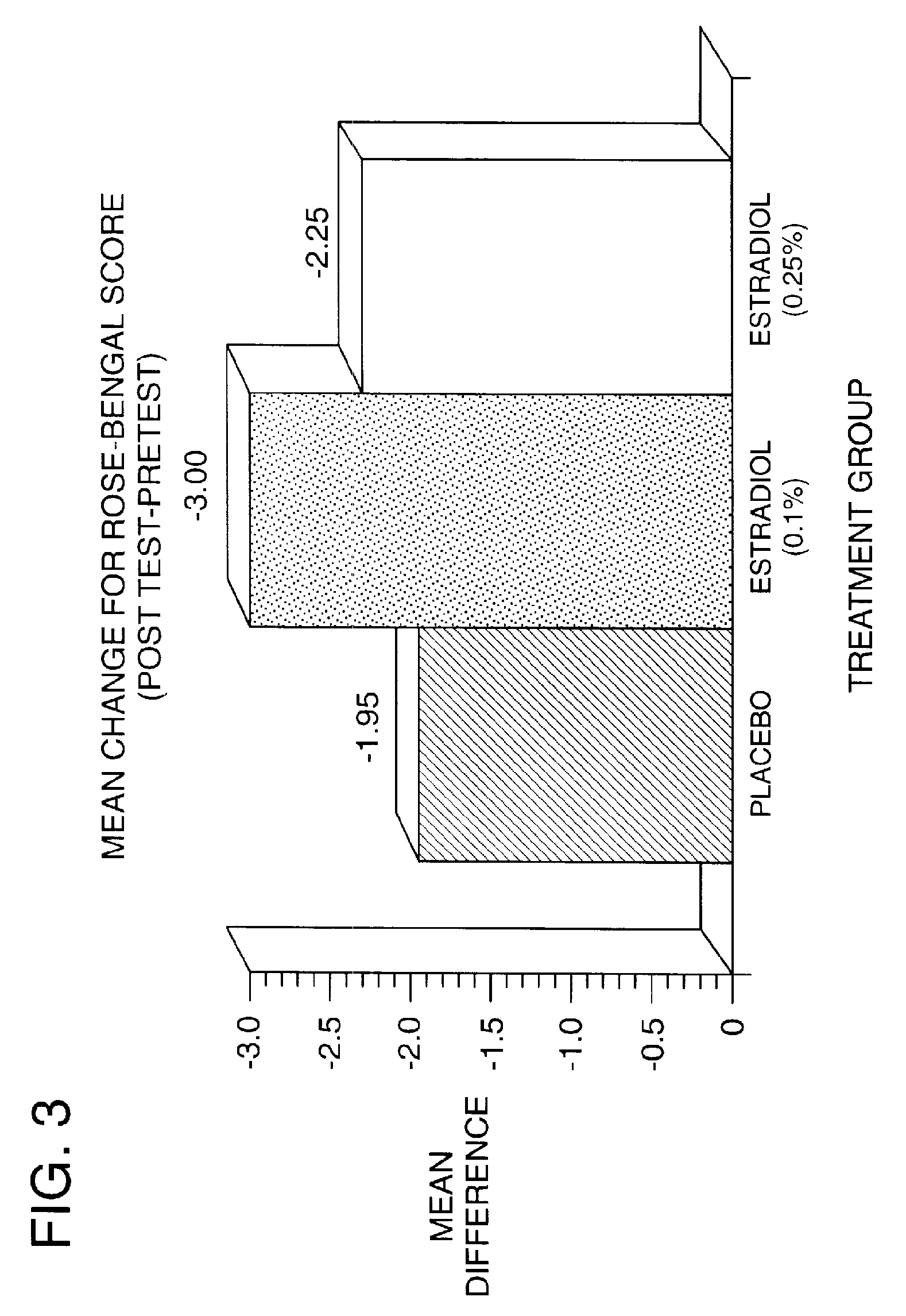
Time-release and micro-dose formulations for topical application of estrogen and estrogen analogs or other estrogen receptor modulators in the treatment of dry eye syndrome, and methods of preparation and application
ActiveUS8987241B2Alleviate dry-eye syndromeFull efficacyOrganic active ingredientsBiocide17 β estradiolGynecology
A topical application formulation of estrogen and estrogen analogs or other estrogen receptor modulators is disclosed for the treatment of primary or secondary dry eye syndrome (also known as keratoconjunctivitis sicca (KCS)). Preferred formulations include 17-β-estradiol and its derivatives in lipid, liposomes, polymers, or aqueous or non-aqueous vehicles for the topical treatment of the ocular surface tissues particularly as time-release or micro-dose formulations. These formulations may also be useful in treating other conditions where KCS may occur, such as post-operative refractive surgery and corneal transplant patients.
Owner:REDWOOD PHARMA AB
Prophylactic or therapeutic agent for conrneal/conjunctival disease
InactiveCN101203236AEasy to solveGood treatment effectSenses disorderPeptide/protein ingredientsConjunctival EpitheliumDisease
Disclosed is a novel composition for the treatment of a corneal / conjunctival disease. A prophylactic or therapeutic agent for a corneal / conjunctival disease comprising selenoprotein P as an active ingredient, more specifically a prophylactic or therapeutic agent for a corneal / conjunctival disease such as dry eye, keratoconjunctivitis sicca, punctate superficial keratitis, corneal erosion or corneal ulcer comprising selenoprotein P as an active ingredient, particularly a prophylactic or therapeutic agent for a corneal / conjunctival disease such as dry eye, keratoconjunctivitis sicca, punctate superficial keratitis, corneal erosion or corneal ulcer accompanied by a corneal and conjunctival epithelial disorder.
Owner:坪田 一男 +2
Traditional Chinese medicine eye drop for treating keratoconjunctivitis sicca and preparation method thereof
InactiveCN104491225AImprove internal and external environmentRelieve symptomsSenses disorderPharmaceutical delivery mechanismMedicinal herbsLiver and kidney
The invention discloses a traditional Chinese medicine eye drop for treating keratoconjunctivitis sicca and a preparation method thereof, and belongs to the field of traditional Chinese medicine. The effective components of the traditional Chinese medicine eye drop disclosed by the invention are composed of the following raw materials: horseweed herb, spreading violet, smallfruit fig aerial root, symplocos lancifolia Sieb. et zucc, hypericum monogynum, semen cuscutae, bullacta flesh, turnip seed, butterflybush flower, pondweed, climbing groundsel herb, three-colored amaranth seed, quercus fabri hance, Japanese buttercup herb, globe amaranth and pennycress seed; according to the traditional Chinese medicine eye drop disclosed by the invention, the selected medicinal materials have good compatibility, meet the theories of traditional Chinese medicine and modern medicine and have the functions of clearing heat and nourishing the liver, engendering liquid and moistening dryness, clearing liver and improving vision, relieving pain and eliminating eye screens, the traditional Chinese medicine eye drop is convenient to use, good in absorption effect and is free of adverse and toxic side effects, and clinical verifications prove that the traditional Chinese medicine eye drop can diminish swelling and relive pain, clear away heat and toxic materials, nourish liver and kidney, and perfect the external and internal environments of organisms, so as to treat or alleviate various symptoms of keratoconjunctivitis sicca, so that the traditional Chinese medicine eye drop is suitable for promotion and application in clinical treatment and nurse of keratoconjunctivitis sicca.
Owner:公慧敏
DNP and DNP prodrug treatment of neuromuscular, neurodegenerative, autoimmune, developmental, traumatic brain injury, concussion, dry eye disease, hearing loss and/or metabolic diseases
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com