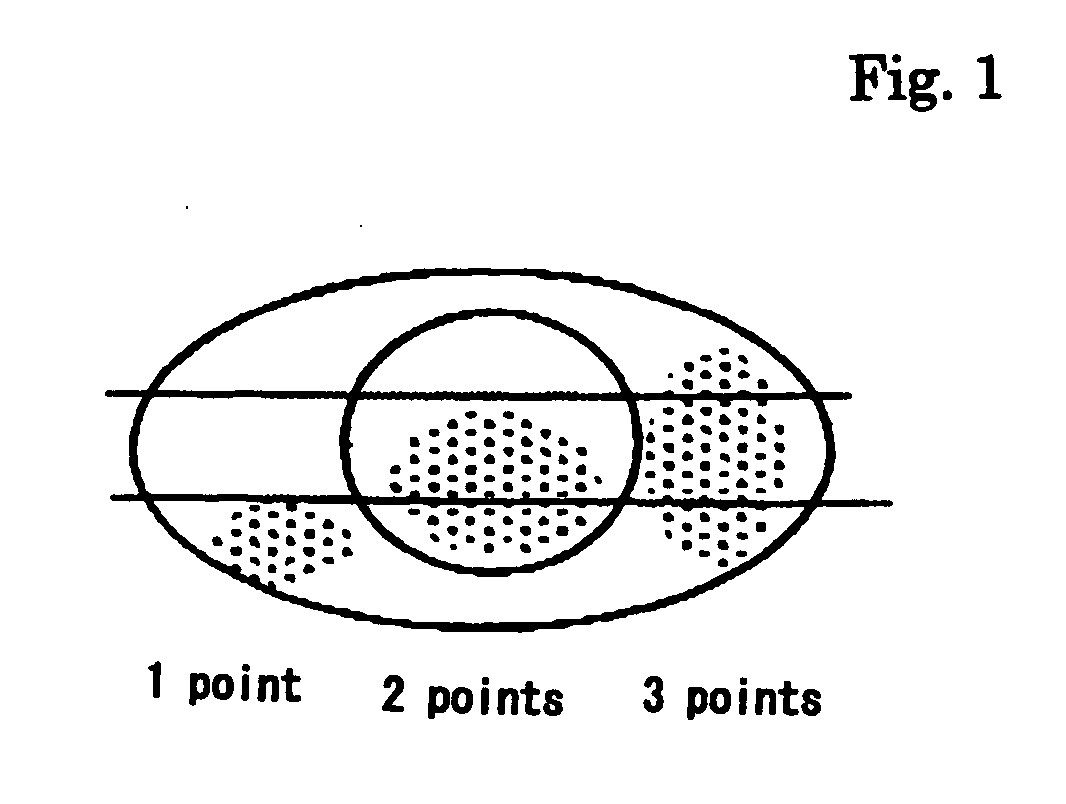
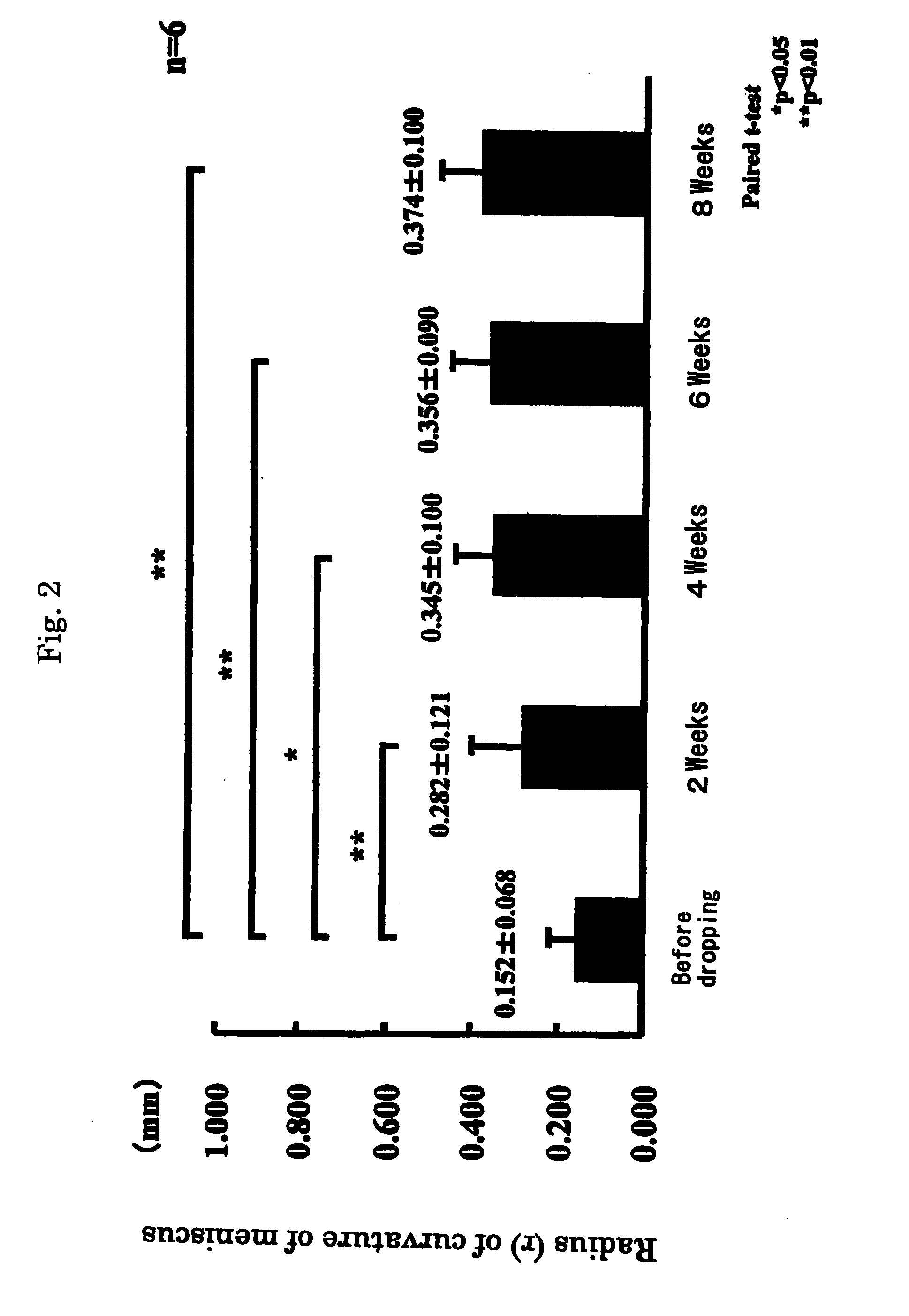
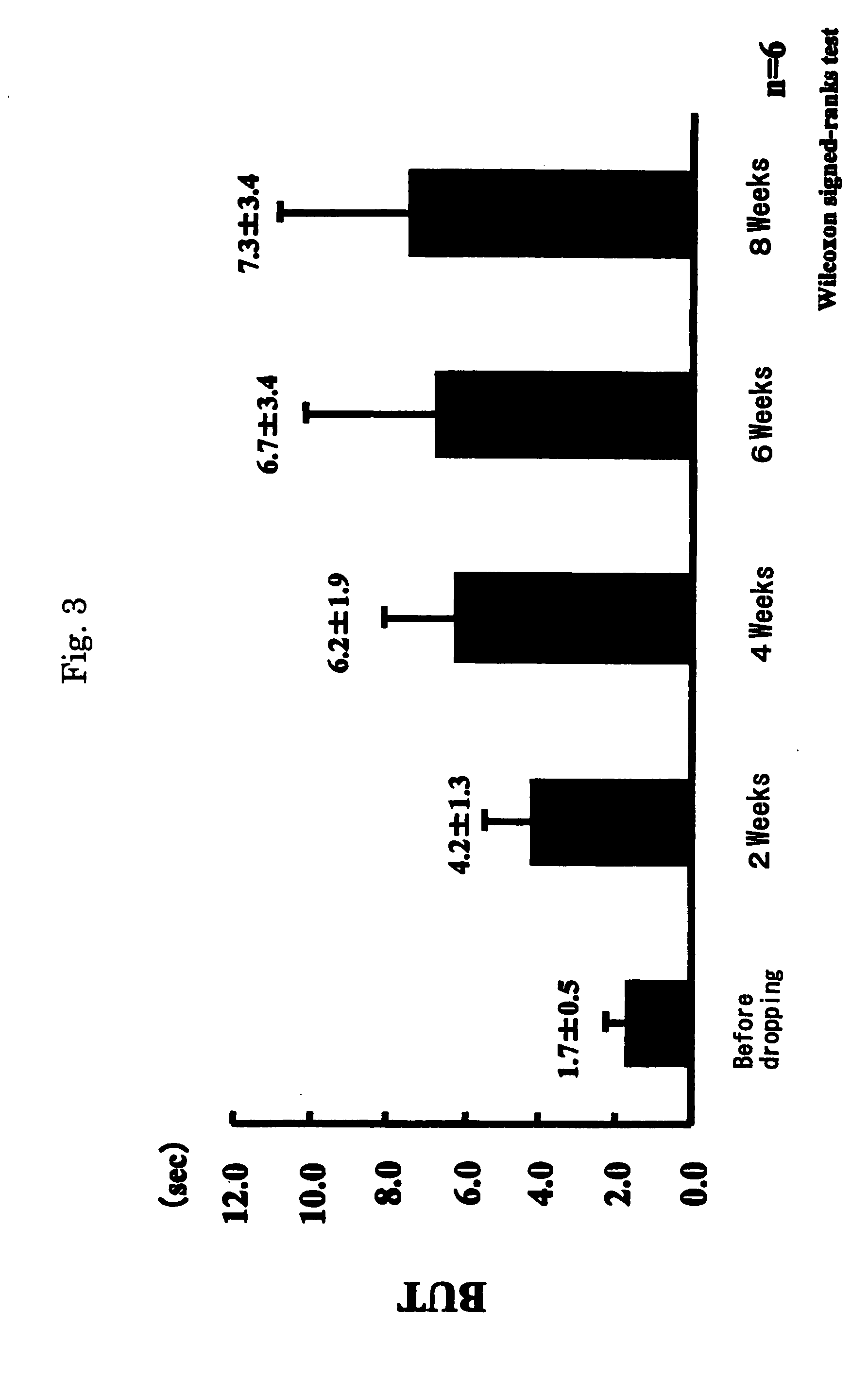
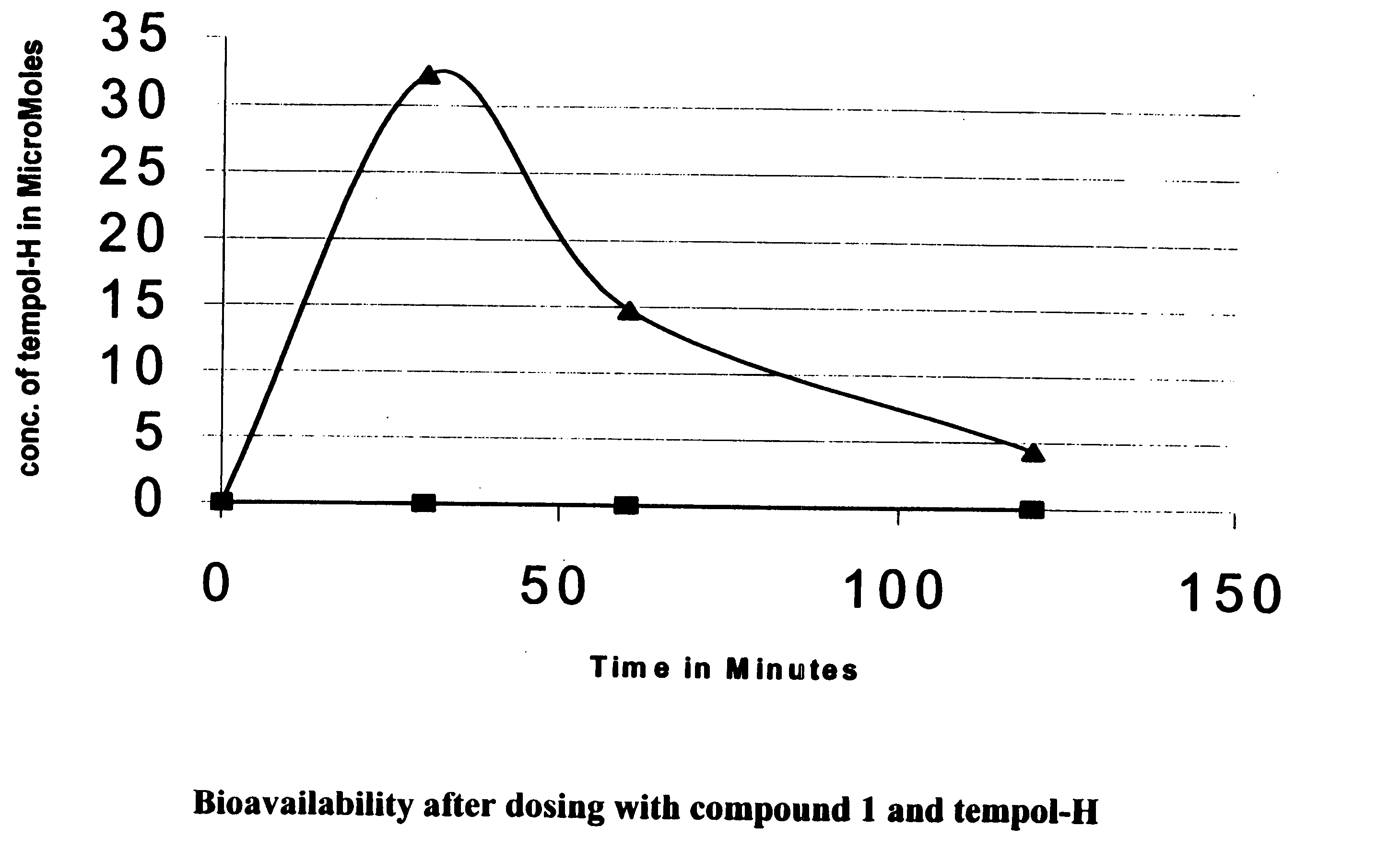
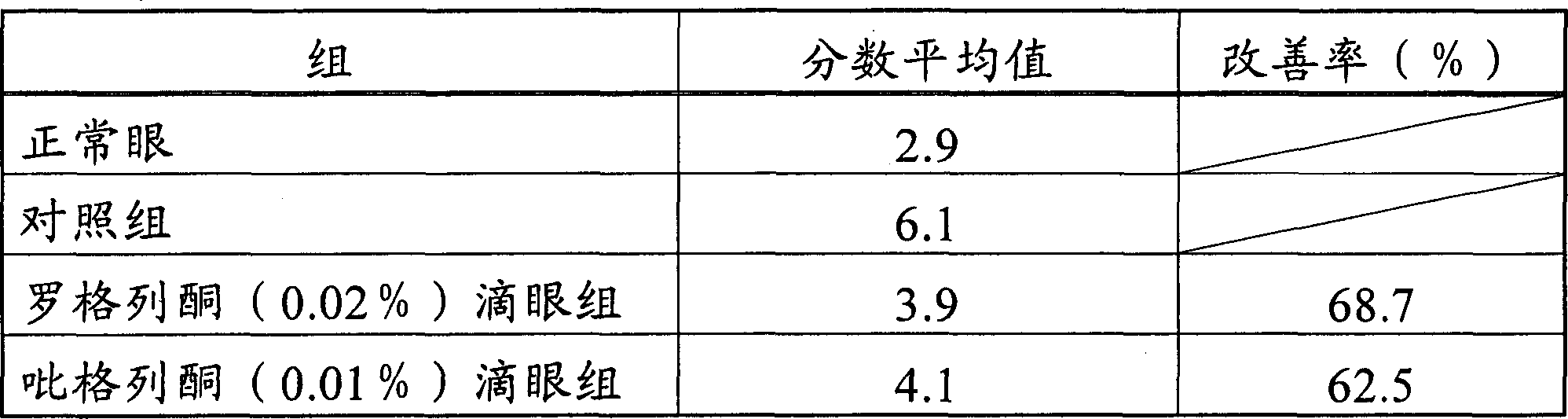
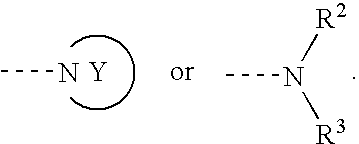Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Corneal Disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A non-neoplastic or neoplastic disorder that affects the cornea. Representative examples include keratitis, bullous keratopathy, and squamous cell carcinoma.
Therapeutic Agent For Ophthalmic Diseases
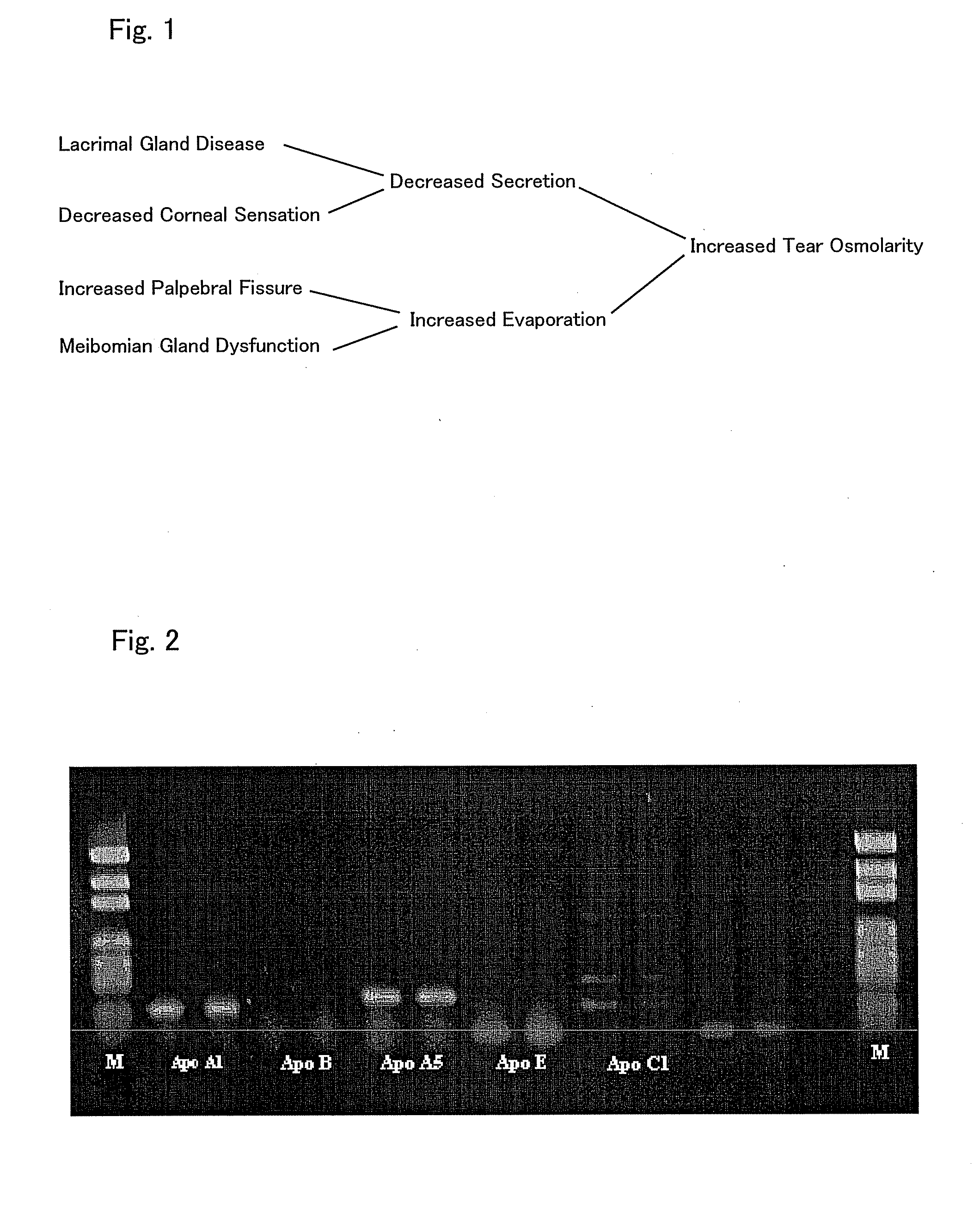
A therapeutic agent for ophthalmic diseases containing Laennec (trade name) as an active ingredient. Laennec, the active ingredient, exhibits a therapeutic effect on a wide variety of ophthalmic diseases by increasing tears and the like and is highly safe even though it is an animal-derived component. Therefore, the therapeutic agent is applicable to the prevention and / or treatment of various types of ophthalmic diseases, particularly corneal disorders, dry eye, asthenopia, inflammatorily ophthalmic diseases (e.g., meibomian gland dysfunction, Stevens-Johnson syndrome, Sjogren syndrome, uveitis) and ophthalmic diseases caused by active oxygen (e.g., cataract, glaucoma, age-related macular degeneration, optic disc atrophy).
Owner:HIBINO SAWAKO
Amelioration of cataracts, macular degeneration and other ophthalmic diseases
InactiveUS20050131025A1Halts development of cataractsInhibit progressBiocidePharmaceutical delivery mechanismDiseaseUveitis
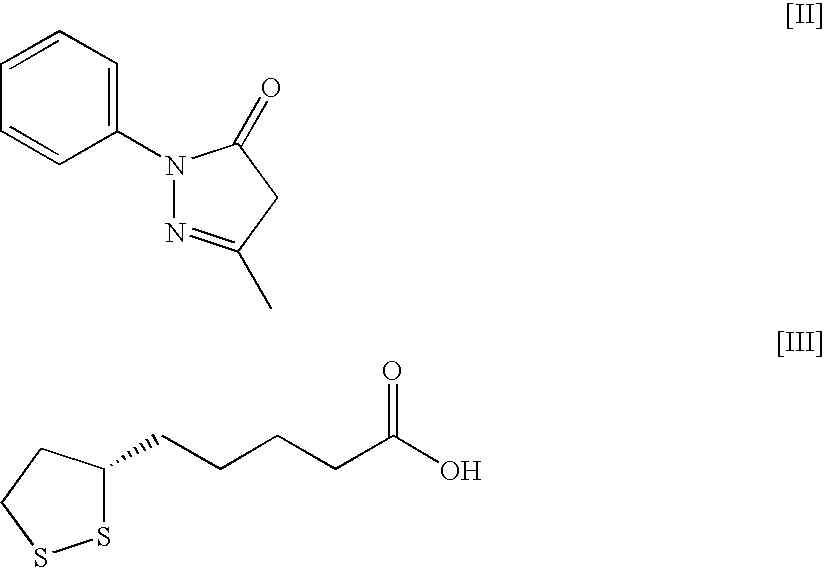
Ophthalmically acceptable compositions used in arresting the development of cataract, presbyopia, macular degeneration and other retinopathies, glaucoma, uveitis and various corneal disorders are disclosed. The compositions are also useful as a prophylactic treatment to prevent or delay development of age-related ocular disorders, which include cataracts, presbyopia, glaucoma and macular degeneration. The compositions comprise a pharmaceutically acceptable carrier or diluent and at least one compound having the formula: [0001]where R1 and R2 are, independently, H or C1 to C3 alkyl; [0002]R3 and R4 are, independently C1 to C3 alkyl; and [0003]where R1 and R2, taken together, or R3 and R4, taken together, or both may be cycloalkyl; [0004]R5 is H, OH, or C1 to C6 alkyl; [0005]R6 is or C1 to C6 alkyl, alkenyl, alkynyl, or substituted alkyl or alkenyl; [0006]R7 is C1 to C6 alkyl, alkenyl, alkynyl, or substituted alkyl or alkenyl or where R6 and R7, or R5, R6 and R7, taken together, form a carbocycle or heterocycle having from 3 to 7 atoms in the ring.
Owner:COLBY PHARMA CO
Therapeutic Compositions for Treatment of Corneal Disorders
ActiveUS20100183587A1Reduce developmentPromote regenerationBiocideOrganic active ingredientsDiseaseCorneal nerve
Owner:THE SCHEPENS EYE RES INST
Ophthalmic composition
InactiveUS20090238810A1Enhanced barrier functionPreventing and easing a corneal disorderSenses disorderApolipeptidesLipid formationDisease
An ophthalmic composition is provided which is capable of enhancing a barrier function of a lipid layer that constitutes a tear film, for thereby restricting and curing corneal disorders such as dry eye. It is also an object of the invention to provide the ophthalmic composition with anti-inflammatory and antimicrobial effects for thereby advantageously preventing and easing corneal / conjunctival disorders such as keratoconjunctivitis. In the present invention, apolipoprotein A-1 is included, as an active ingredient, in the ophthalmic composition.
Owner:MENICON CO LTD
Use of Urokinase Type Plasminogen Activator Inhibitors for the Treatment of Corneal Disorders
InactiveUS20110028397A1Effective treatmentImprove purification effectSenses disorderTripeptide ingredientsDiseaseCorneal ulcer
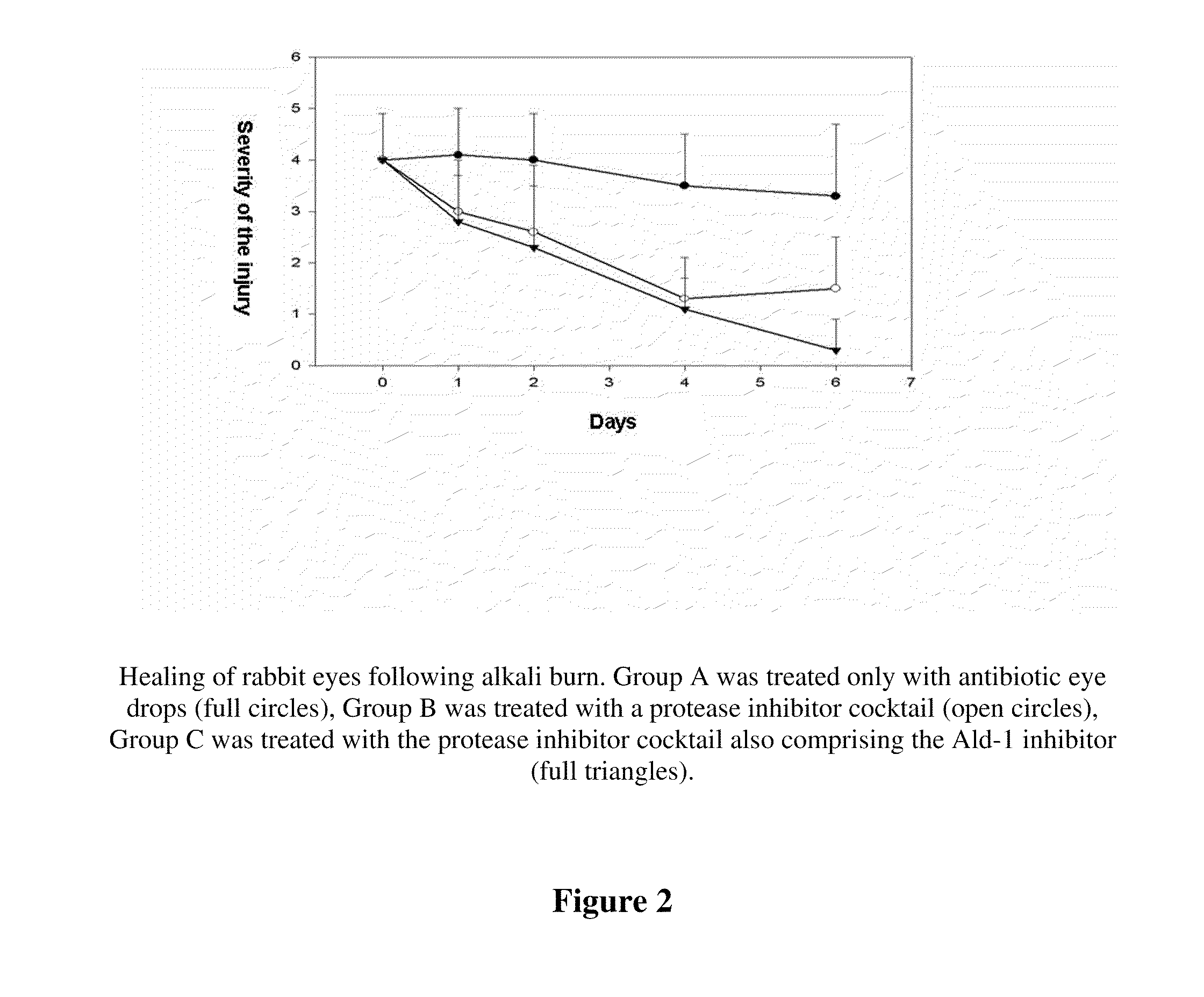
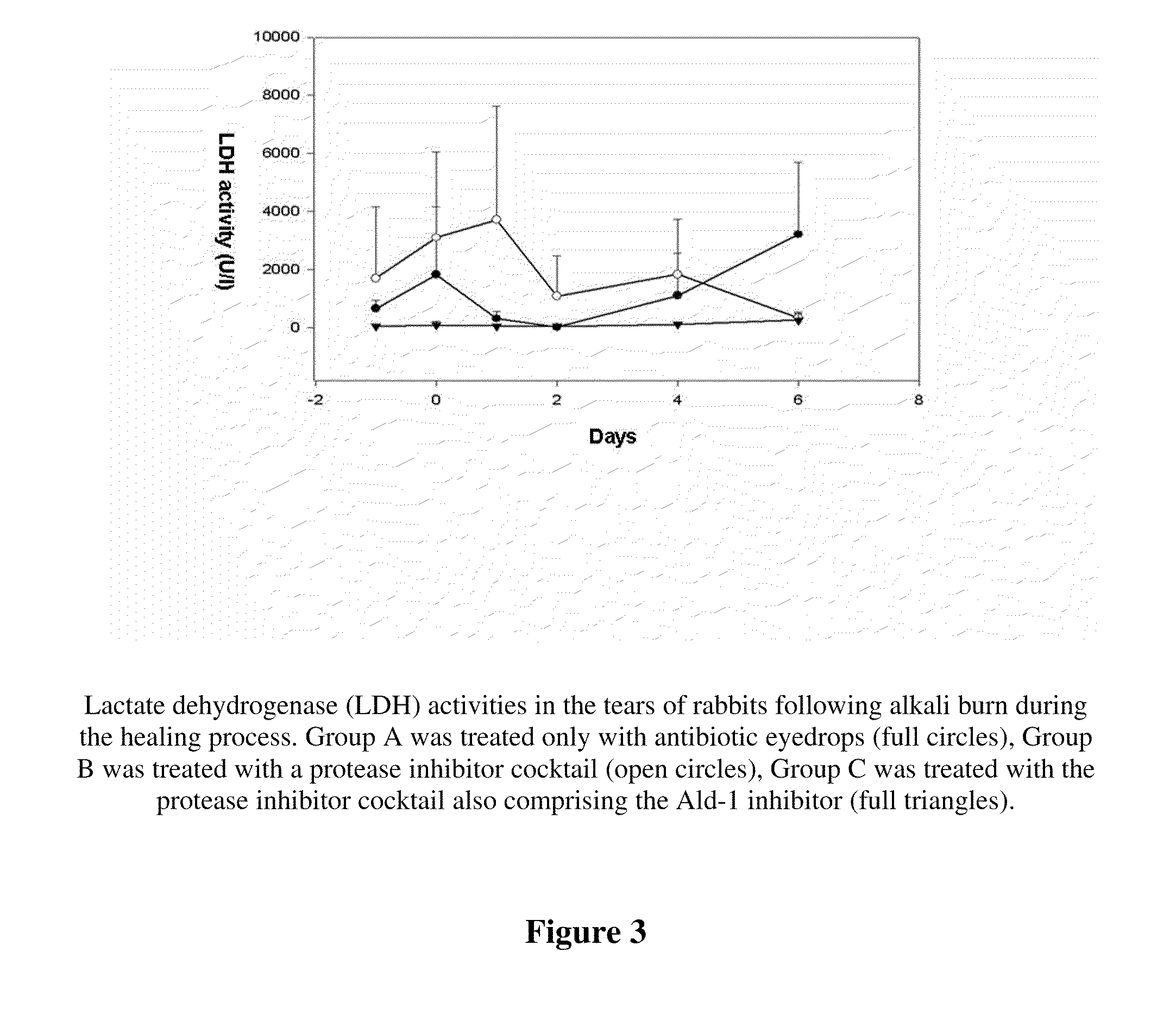
The invention concerns the use of inhibitors of the urokinase type of plasminogen activator (uPA) appearing in the anterior segment of the eye, for the treatment and prevention of corneal ulcers and other disorders. The invention further concerns pharmaceutical compositions, comprising inhibitors of uPA, preferably eye drops and eye ointments. The pharmaceutical compositions according to the invention preferably comprise PAI-2 protein or a derivative thereof retaining uPA-inhibiting capacity, or a tripeptide aldehyde inhibitor, preferably the D-Phe-Pro-Arg-aldehyde (Ald-1). The PAI-2 protein, used according to the invention, is preferably produced through bacterial expression, as a fusion protein.
Owner:UNIVERSITY OF DEBRECEN
Therapeutic Compositions for Treatment of Corneal Disorders
InactiveUS20120014970A1Reducing corneal nerve damageEnhancing corneal nerve regenerationSenses disorderNervous disorderCorneal nerveAnatomy
Owner:THE SCHEPENS EYE RES INST
Therapeutic agent for keratoconjunctive disorders
ActiveUS20150290172A1Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyDisease
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
Therapeutic agent for keratoconjunctive disorders
ActiveUS9492431B2Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyInfectious Keratitis
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
Preventive or therapeutic agent for keratoconjunctival disorder
InactiveUS20090105313A1Improve the improvement effectGood prevention effectBiocideSenses disorderConjunctivaDisease
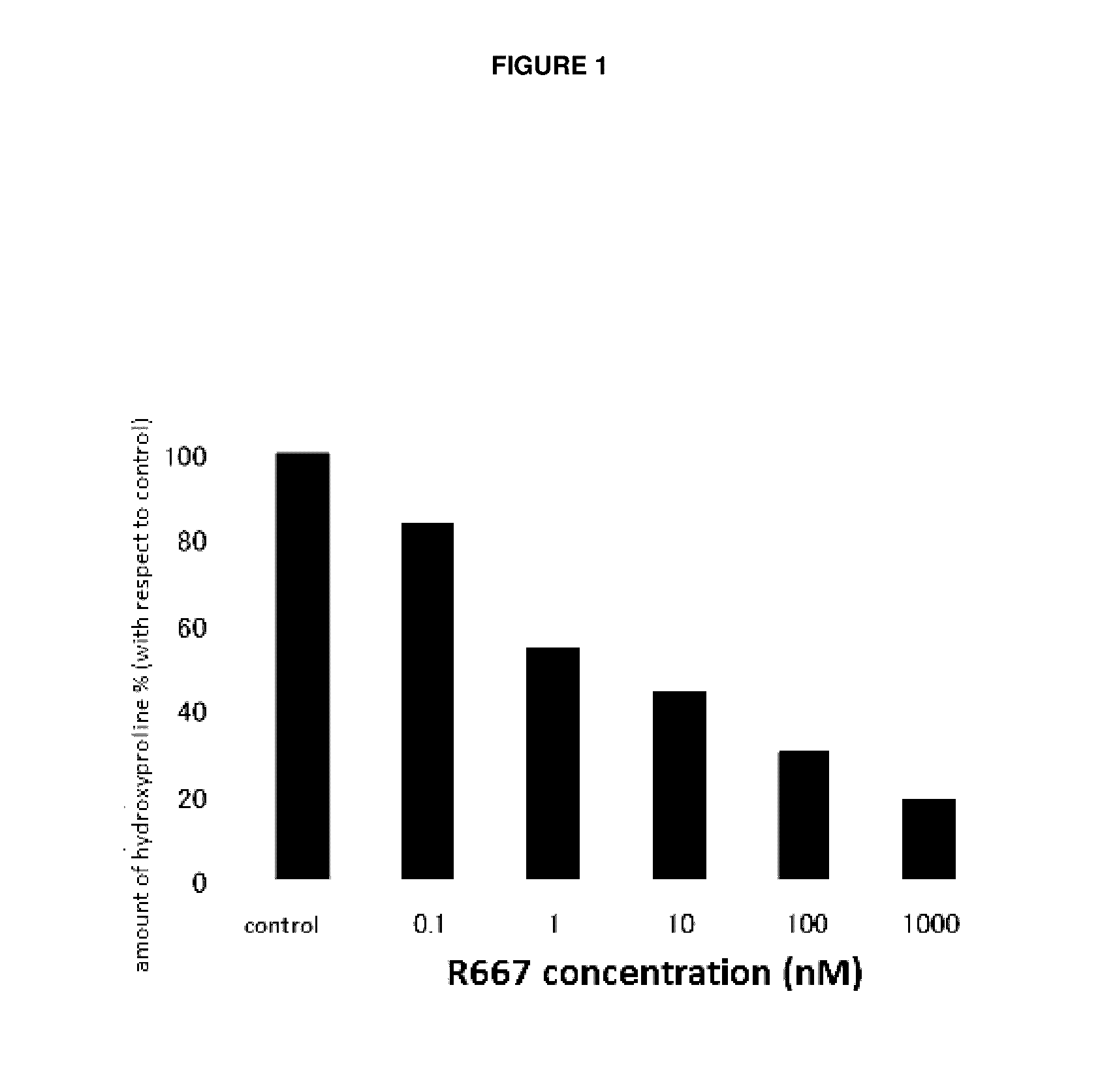
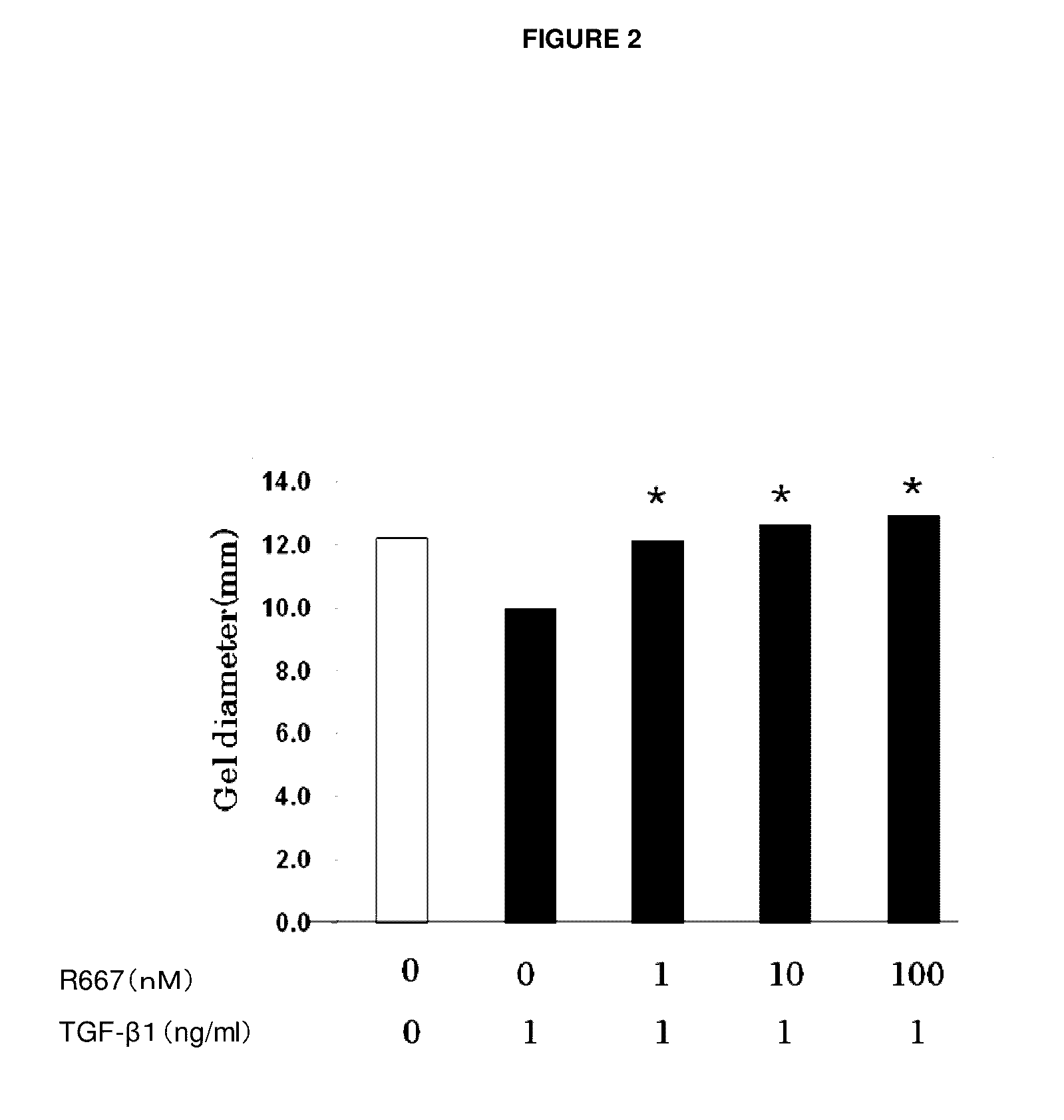
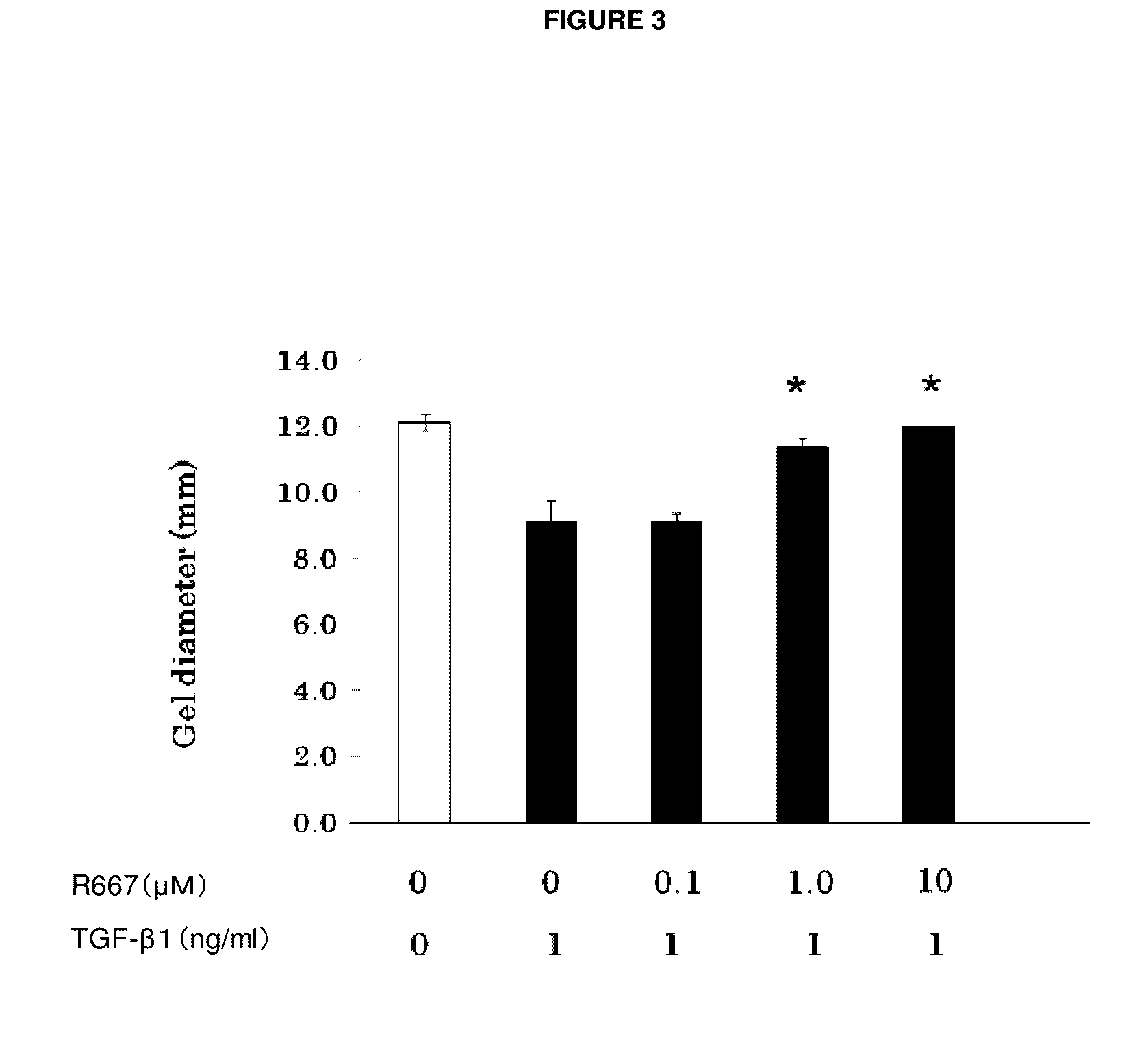
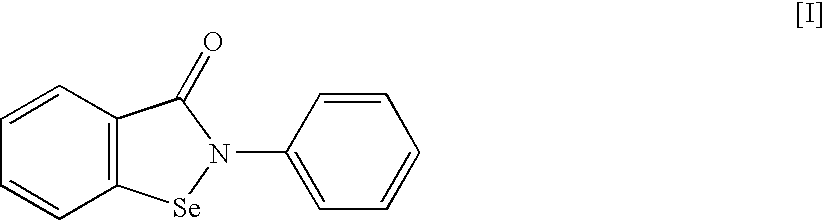
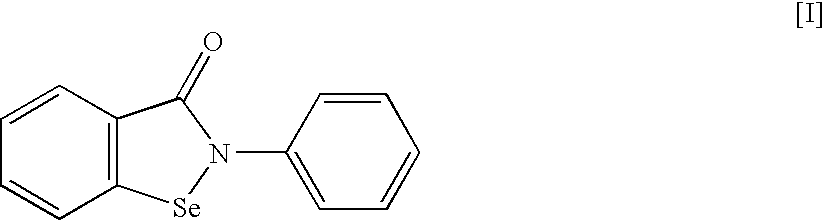
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
Novel peptides and medicinal uses thereof
InactiveUS20050009752A1Easy curingUseful in therapyBiocideSenses disorderDiseaseInsulin-like growth factor
It is an object of the invention to examine the minimum unit of the exhibition of the activity of insulin-like growth factor-I and find a pharmaceutical use thereof in the fields of ophthalmology and dermatology. A joint administration of a peptide containing the amino acid sequence represented by Ser-Ser-Ser-Arg as the minimum unit of the exhibition of the activity of insulin-like growth factor-I and a peptide containing the amino acid sequence represented by Phe-Gly-Leu-Met-NH2 is effective for curing corneal disorders and can significantly promote the healing of skin wounds.
Owner:NISHIDA TERUO +1
Therapeutic agent for ophthalmic diseases
A therapeutic agent for ophthalmic diseases containing Laennec (trade name) as an active ingredient. Laennec, the active ingredient, exhibits a therapeutic effect on a wide variety of ophthalmic diseases by increasing tears and the like and is highly safe even though it is an animal-derived component. Therefore, the therapeutic agent is applicable to the prevention and / or treatment of various types of ophthalmic diseases, particularly corneal disorders, dry eye, asthenopia, inflammatorily ophthalmic diseases (e.g., meibomian gland dysfunction, Stevens-Johnson syndrome, Sjogren syndrome, uveitis) and ophthalmnic diseases caused by active oxygen (e.g., cataract, glaucoma, age-related macular degeneration, optic disc atrophy).
Owner:HIBINO SAWAKO
Therapeutic agent for keratoconjunctival disorder
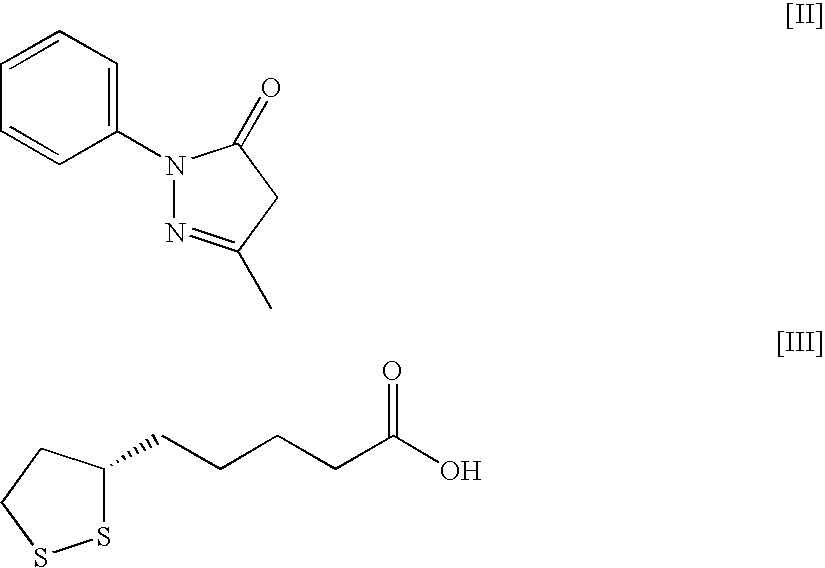
An object of the present invention is to discover a new medicinal use of 5-[4-[[3-methyl-4-oxo-3,4-dihydro-2-quinazolinyl]methoxy]p henylmethyl]thiazolidine-2,4-dione and N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine. Both of the compounds exert an excellent improving effect on corneal disorder models and is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctive epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Method for treating a keratoconjunctival disorder
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
Ocular compositions containing dioleoylphosphatidylglycerol and uses thereof
The present invention provides a method of treating a corneal disorder comprising administering to a patient in need thereof a composition containing pharmaceutically effective amount of dioleoylphosphatidylglycerol and / or palmitoyloleoylphosphatidylglycerol and a pharmaceutically acceptable carrier.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Human functional corneal endothelial cell and application thereof
The present invention complete a technique of treating a corneal disorder or disease by infusion into an anterior chamber of human eyes. Specifically, the present invention based on the findings discovered that cultured human corneal endothelial cells are comprised of a plurality of subpopulations, most of them are not suitable for infusion into patients. The above-described subject was overcome by providing, as a medicament, functionally high grade quality of cells having the function of mature differentiated human corneal endothelial cells which is a specific subpopulation and characterized by their biochemical and functional phenotypes. The present invention provides such a functional mature differentiated corneal endothelial cells, medicament comprising the same, and manufacturing method, quality control and techniques related thereto.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP
Method for the amelioration of ectatic and irregular corneal disorders
Methods for the amelioration of ectatic corneal disorders using corneal augmentations are disclosed. The shape of the augmentation is determined using data obtained from mapping of a patient's cornea based on computerized corneal topography and tomography. Factors considered include the maximum keratometry and specific iso-deviation contours. In one embodiment, an augmentation is inlayed into a femtosecond created, intrastromal pocket. In a further embodiment, an onlay augmentation is positioned over a region of the cornea from which the epithelial layer has been removed. The onlay is held in place by glue, sutures, tucking under a perimeter chamfer, or some combination thereof, until the epithelial layer regrows over the augmentation. In a further embodiment, the inlay or only augmentation is followed by a post-augmentation, further reshaping of the corneal augmentation. In one embodiment, this further reshaping is photorefractive keratectomy (PRK) surgery. In another and a phototherapeutic keratectomy (PTK) surgery.
Owner:CTAK LLC
Ophthalmological composition
InactiveUS20100099629A1Promote healingGood effectSenses disorderConnective tissue peptidesOphthalmologyPharmaceutical drug
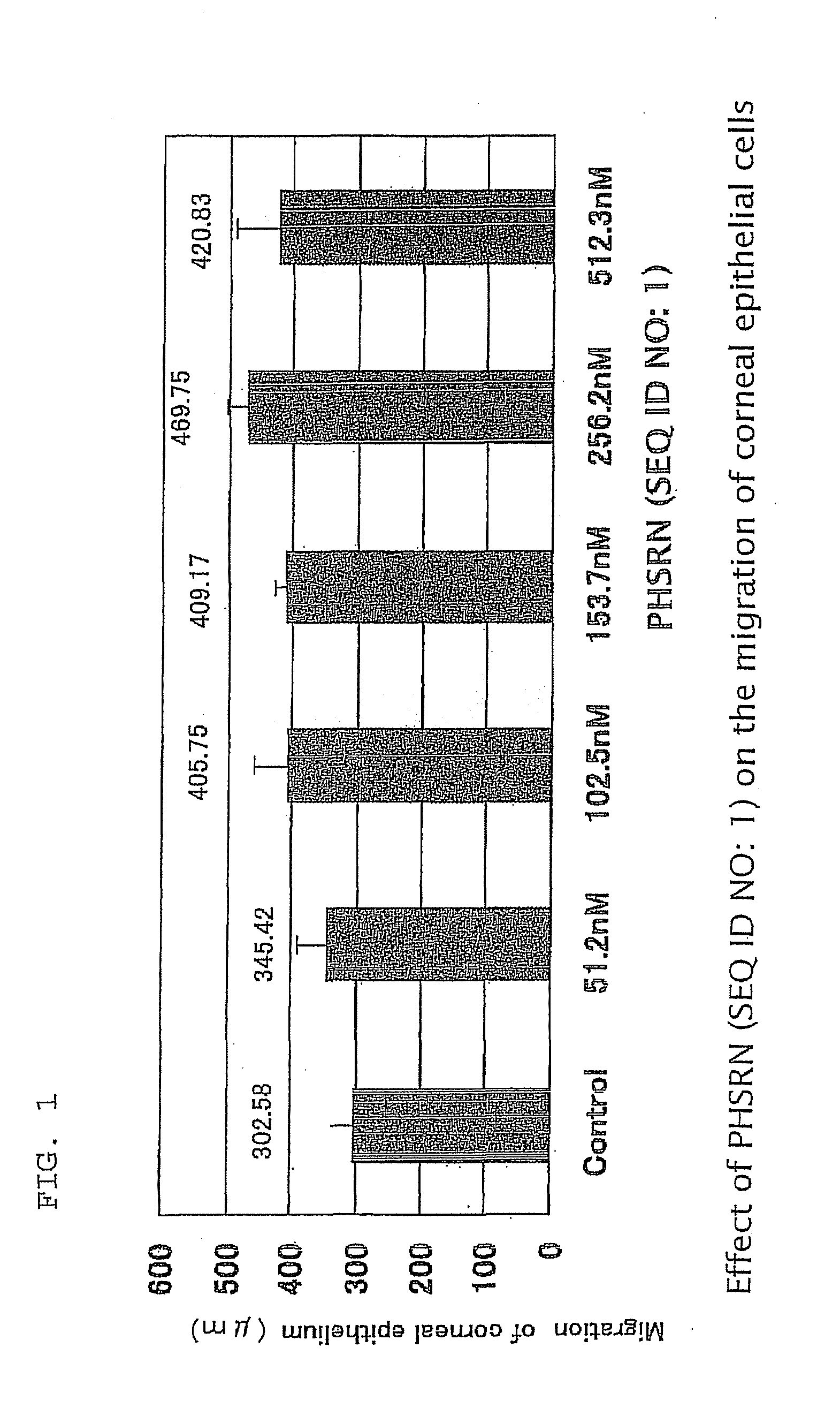
An object is to find the minimum activity expression site of fibronectin, clarify the actions of this minimum unit in relation to opthalmological fields, and provide an opthalmological composition having this minimum unit as an effective component. This invention provides an opthalmological composition, in particular, a corneal disorder treatment agent containing the peptide, PHSRN (SEQ ID NO: 1) (Pro-His-Ser-Arg-Asn (SEQ ID NO: 1)), or Ac-Pro-His-Ser-Arg-Asn-NH2, which is a derivative thereof, or a salt thereof that is allowable as a medical drug as an effective component. The preferred dosage form is an ophthalmic formulation.
Owner:NISHIDA
Therapeutic agent for keratoconjunctival disorder
InactiveCN101229166ASignificant improvementOrganic active ingredientsSenses disorderFilamentary keratitisConjunctiva
An object of the present invention is to discover a new medicinal use of 5-[4-[[3-methyl-4-oxo-3,4-dihydro-2-quinazolinyl]methoxy]p henylmethyl]thiazolidine-2,4-dione and N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine. Both of the compounds exert an excellent improving effect on corneal disorder models and is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctive epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Therapeutic agent for keratoconjunctival disorder
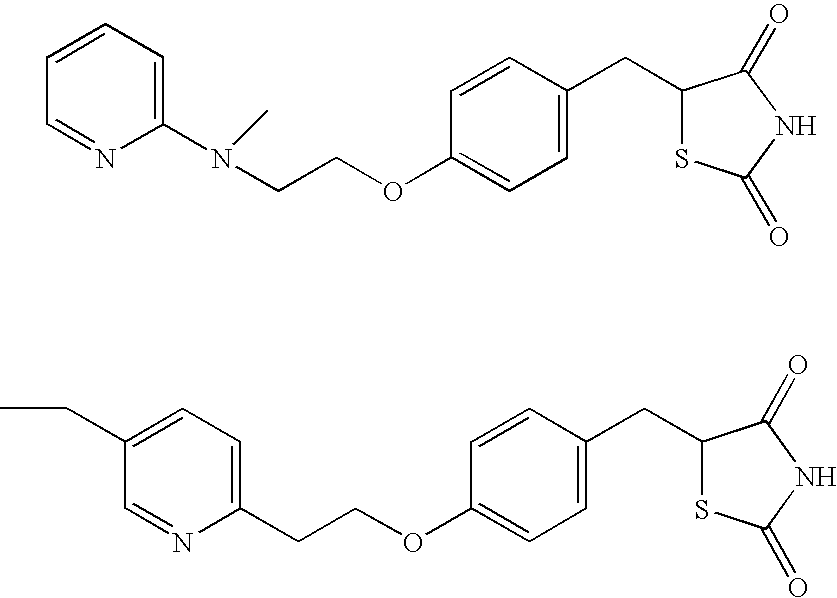
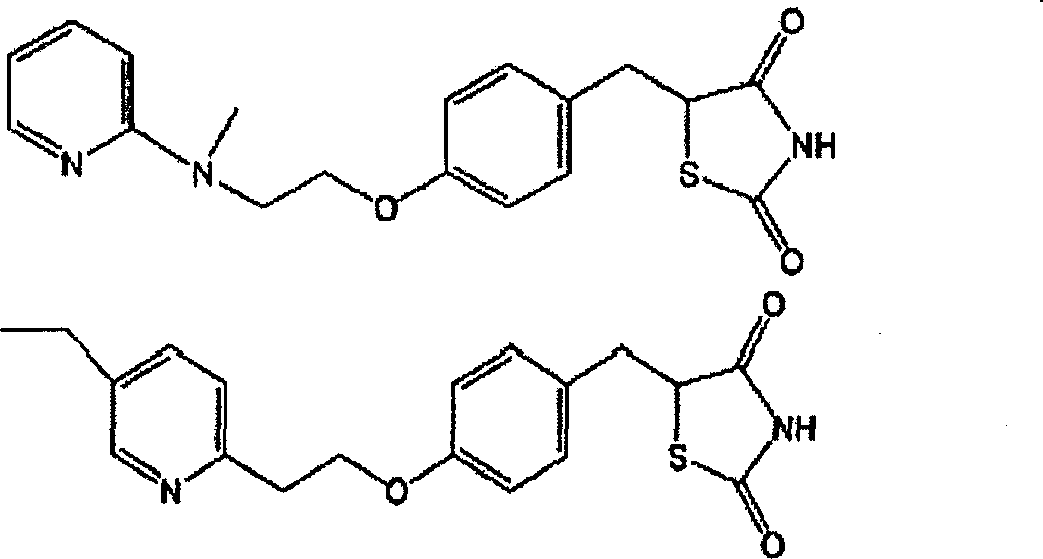
An object of the present invention is to search a new medicinal use of 5-[p-[2-(methyl-2-pyridylamino) ethoxy]benzyl]-2,4-thiazolidinedione and 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione. 5-[p-[2-(methyl-2-pyridylamino)ethoxy]benzyl]-2,4-thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione or a salt thereof exerts an excellent improving effect on corneal disorder models, therefore, it is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
Drug for curing corneal and conjunctival disease
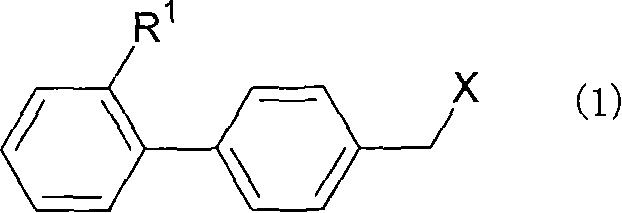
The purpose is to provide a therapeutic agent for a corneal / conjunctival disorder. A compound represented by the general formula (1) or a salt thereof shows an excellent ameliorating effect in a corneal disorder model, and therefore is useful as a therapeutic agent for a corneal / conjunctival disorder such as dry eye, corneal ulcer, keratitis and conjunctivitis. In the general formula, the ring Y represents a substituted or unsubstituted nitrogenated heterocyclic ring; R1 represents a carboxyl group or a substituted or unsubstituted nitrogenated 5-membered heterocyclic ring; and R2 and R3 independently represent a hydrogen atom, a substituted or unsubstituted alkyl group or a substituted or unsubstituted alkylcarbonyl group.
Owner:SANTEN PHARMA CO LTD
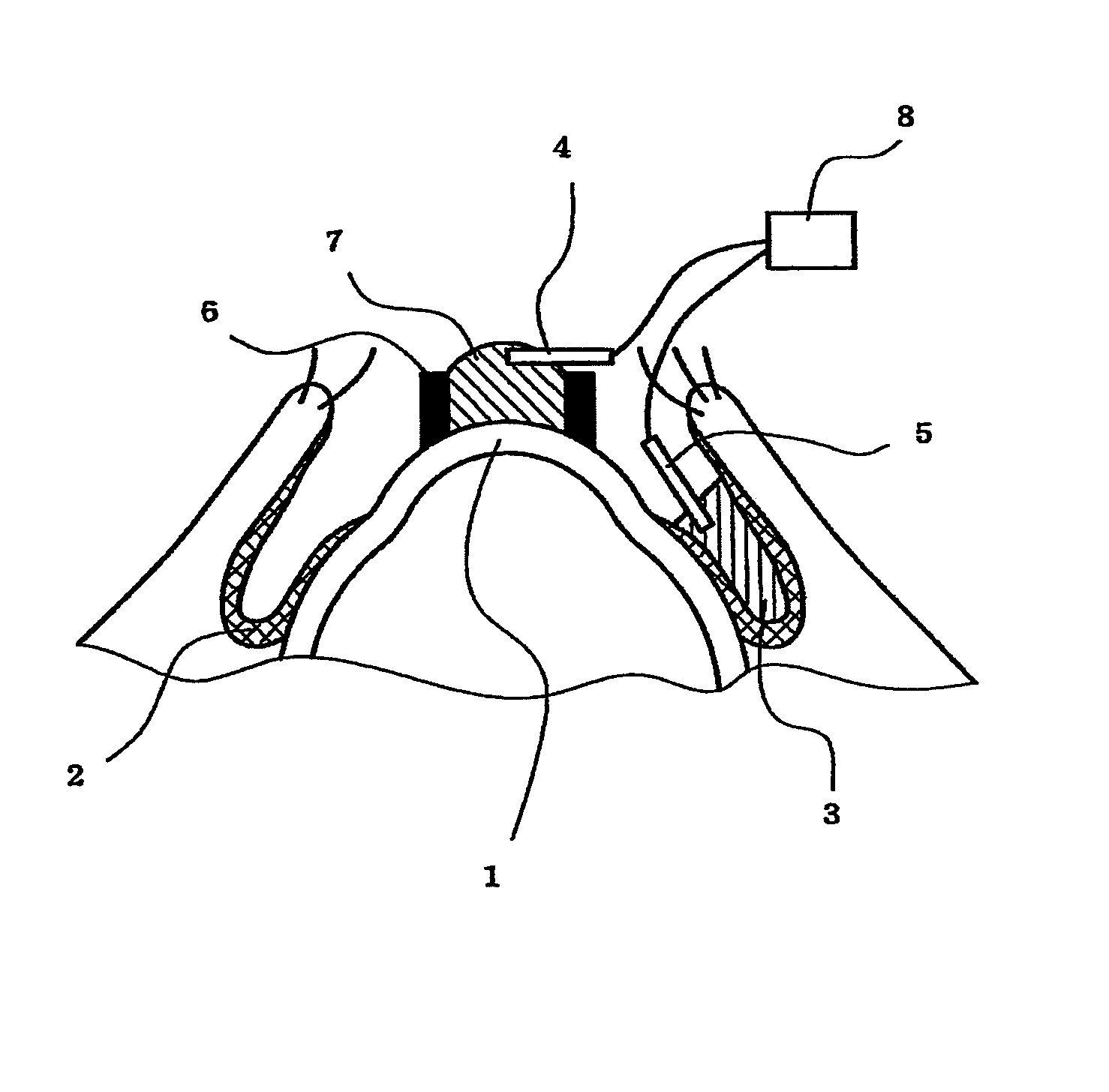
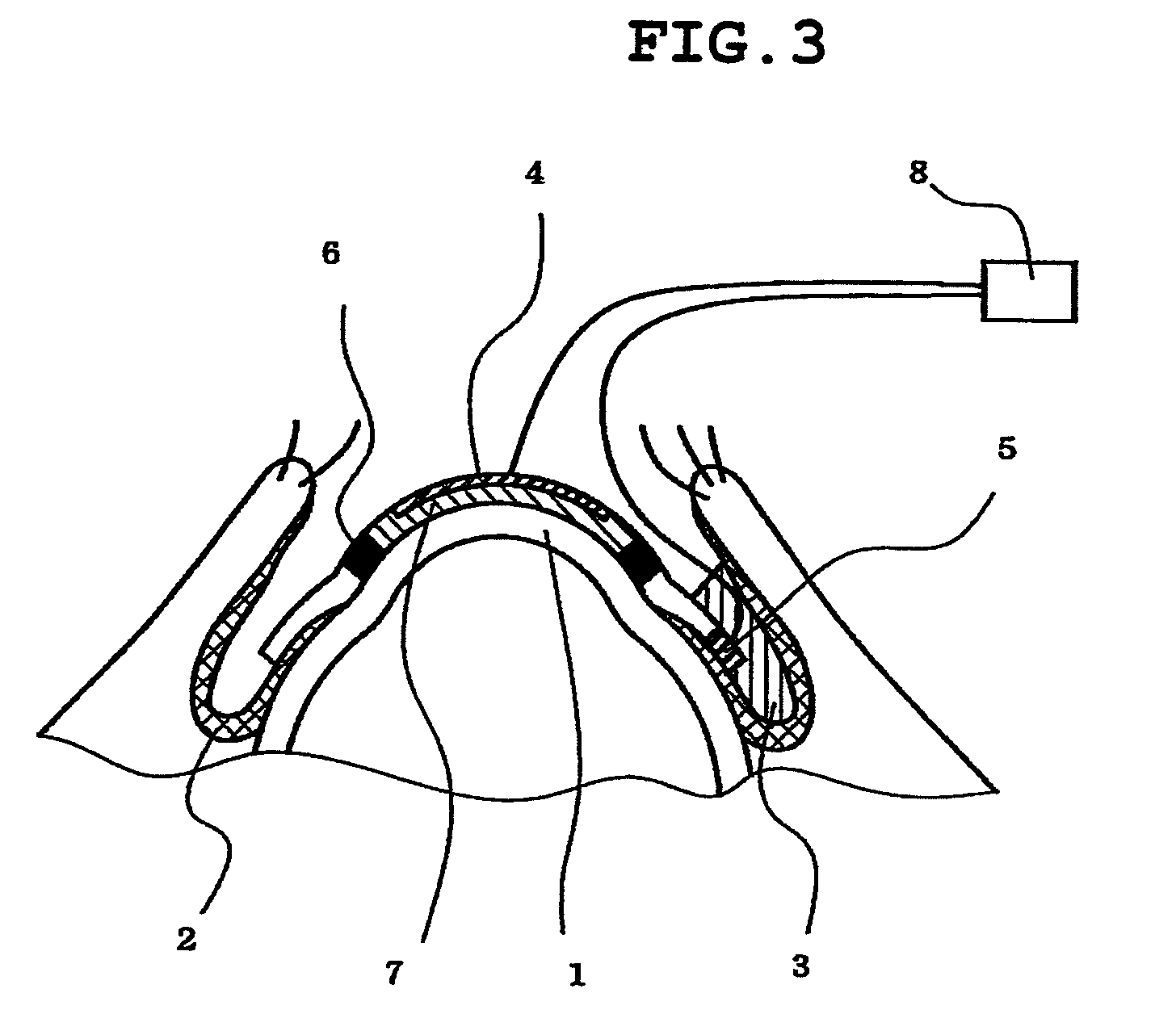
Method of measuring electrical resistance value of corneal trans-epithelium
InactiveUS8478395B2Great contributionAccurate measurementDiagnostic recording/measuringSensorsEpitheliumConjunctiva
The invention provides a method for evaluating a corneal disorder quantitatively and is applicable to living eyes. In particular, the invention provides a method for measuring a corneal transepithelial electric resistance, which method comprises: (1) a step of placing a first electrode on the cornea and a second electrode on the conjunctiva; and (2) a step of flowing an electric current between the first electrode and the second electrode to measure the electric resistance. The invention also provides a device for measuring a corneal transepithelial electric resistance value.
Owner:NAGASAKI UNIVERSITY +1
Proteasome modulation for treatment of corneal disorders
ActiveUS20180369316A1Reduces corneal transparencyShorten the progressSenses disorderDipeptide ingredientsDiseaseProteasome degradation
Disclosed herein are methods and compositions useful for preventing or reducing corneal haze of opacification resulting from Limbal Stem Cell Deficiency (LSCD). The invention comprises a method of preventing or treating corneal opacification, comprising administering to a subject a sufficient amount of a proteasome modulator. The invention also comprises a method of preventing or treating corneal opacification, comprising administering to a subject a sufficient amount of a proteasome modulator. In addition, the invention comprises a method of administering to a subject suffering from corneal opacification with a sufficient amount of proteasome modulator, resulting in reduction of Keratin proteins in the cornea of the subject.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Agent for treating keratoconjunctival trouble
An object of the present invention is to search a new medicinal use of 5-[p-[2-(methyl-2-pyridylamino)ethoxy]benzyl]-2,4-thiazolidinedione and 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione. 5-[p-[2-(methyl-2-pyridylamino)ethoxy]benzyl]-2,4-thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione or a salt thereof exerts an excellent improving effect on corneal disorder models, therefore, it is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
Therapeutic Agent for Keratoconjunctival Disorder
An object of the present invention is to research a new medicinal use of E-4-[4-(5-methyl-2-phenyl-4-oxazolylmethoxy) benzyloxyimino]-4-phenylbutyric acid, Z-2-[4-(5-methyl-2-phenyl-4-oxazolylmethoxy) benzyloxyimino]-2-(4-phenoxyphenyl)acetic acid, 2-[2-propyl-3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]propoxy]phenoxy] benzoic acid, 2(S)-methoxy-3-[4-[3-(4-phenoxyphenoxy)propoxy]phenyl] propionic acid, or a salt thereof. Any of the above-mentioned carboxylic acid compounds and a salt thereof exhibit an excellent improving effect on corneal disorder models and are useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis or filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Proteasome modulation for treatment of corneal disorders
ActiveUS10736934B2Improve eyesightImprove the situationSenses disorderDipeptide ingredientsDiseaseOphthalmology
Disclosed herein are methods and compositions useful for preventing or reducing corneal haze of opacification resulting from Limbal Stem Cell Deficiency (LSCD). The invention comprises a method of preventing or treating corneal opacification, comprising administering to a subject a sufficient amount of a proteasome modulator. The invention also comprises a method of preventing or treating corneal opacification, comprising administering to a subject a sufficient amount of a proteasome modulator. In addition, the invention comprises a method of administering to a subject suffering from corneal opacification with a sufficient amount of proteasome modulator, resulting in reduction of Keratin proteins in the cornea of the subject.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Therapeutic Agent for Keratoconjunctival Disorder
An object of the present invention is to research a therapeutic agent for a keratoconjunctival disorder. A compound represented by the following general formula (1) or a salt thereof exhibits an excellent improving effect on corneal disorder models, and therefore is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis or conjunctivitis. In the formula, the ring Y represents a substituted or unsubstituted nitrogen-containing heterocyclic ring; R1 represents a carboxy group or a substituted or unsubstituted nitrogen-containing 5-membered heterocyclic ring; and R2 and R3 may be the same or different and represent a hydrogen atom, a substituted or unsubstituted alkyl group or a substituted or unsubstituted alkylcarbonyl group.In the formula, X represents
Owner:SANTEN PHARMA CO LTD
Therapeutic Agent for Keratoconjunctival Disorder
InactiveUS20090270474A1Good effectEasy to optimizeOrganic active ingredientsBiocideFilamentary keratitisSuperficial punctate keratopathy
An object of the present invention is to discover a new use of eprosartan or a salt thereof. Eprosartan or a salt thereof exhibits an excellent improving effect in a corneal disorder model, and therefore is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
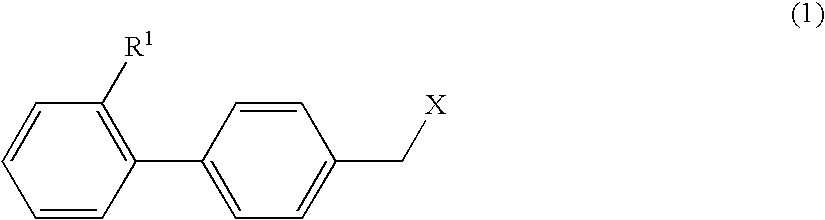
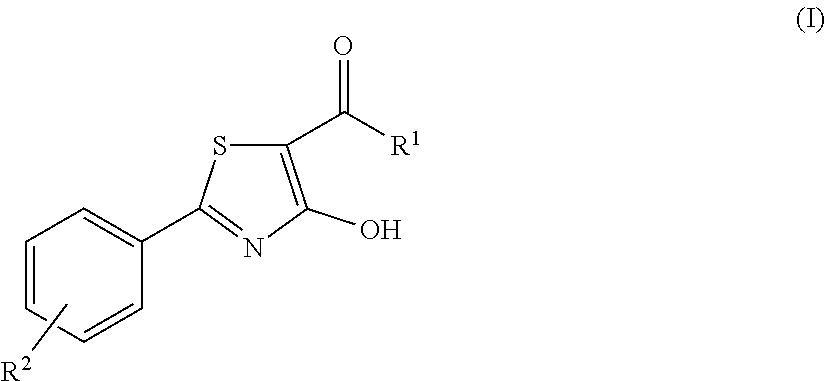
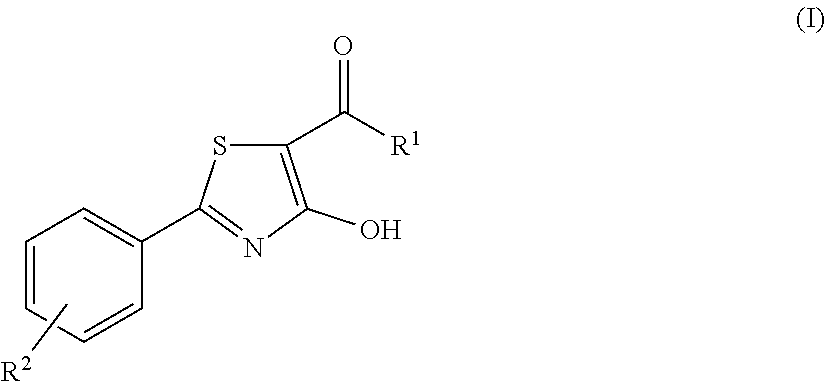
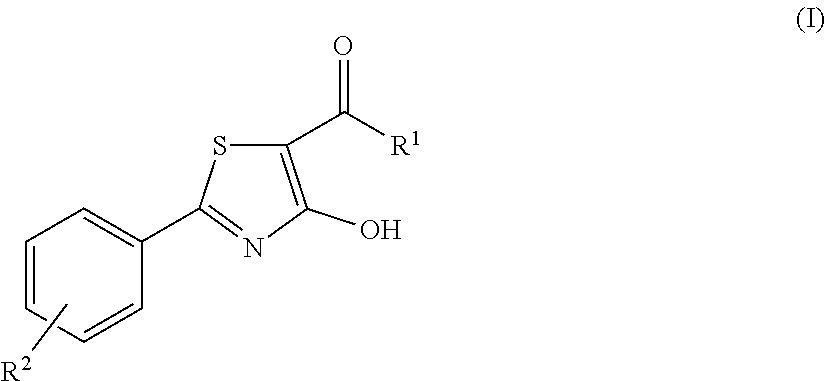
4-hydroxy-2-phenyl-1,3-thiazol-5-yl methanone derivatives as trpm8 antagonists
ActiveUS20180319785A1Improve oral bioavailabilityImprove performanceOrganic active ingredientsNervous disorderIrritable bowelUrological Disorders
The invention relates to compounds acting as antagonists of Transient Receptor Potential cation channel subfamily M member 8 (TRPM8), and having formula (I):Said compounds are useful in the treatment of diseases associated with activity of TRPM8 such as pain, ischaemia, neurodegeneration, stroke, psychiatric disorders, itch, irritable bowel diseases, cold-induced and / or exacerbated-respiratory disorders, urological disorders, corneal disorders associated to disturbances in the production of the tears and / or altered blinking such as epiphora and dry eye disease.
Owner:DOMPE FARM SPA
Donor overlay for treatment or alleviation of anterior corneal disorders
The invention relates to methods for the treatment or alleviation of an anterior corneal disorder in a subject in need thereof comprising removing corneal epithelial cells from an eye of said subject without removing any corneal tissue or other ocular tissue located posterior to the corneal epithelial cell layer; and positioning an overlay comprising a Bowman layer (BL), Descemet membrane (DM) and / or crystalline lens capsule on the anterior surface of said corneal tissue located posterior to the corneal epithelial cell layer. The invention further relates to freeze-dried and / or gamma irradiated Bowman layer, Descemet membrane and / or crystalline lens capsule and compositions comprising the same that are useful in such methods.
Owner:NIIOS USA INC
Ocular compositions containing dioleoylphosphatidylglycerol and uses thereof
The present invention provides a method of treating a corneal disorder comprising administering to a patient in need thereof a composition containing pharmaceutically effective amount of dioleoylphosphatidylglycerol and / or palmitoyloleoylphosphatidylglycerol and a pharmaceutically acceptable carrier.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com