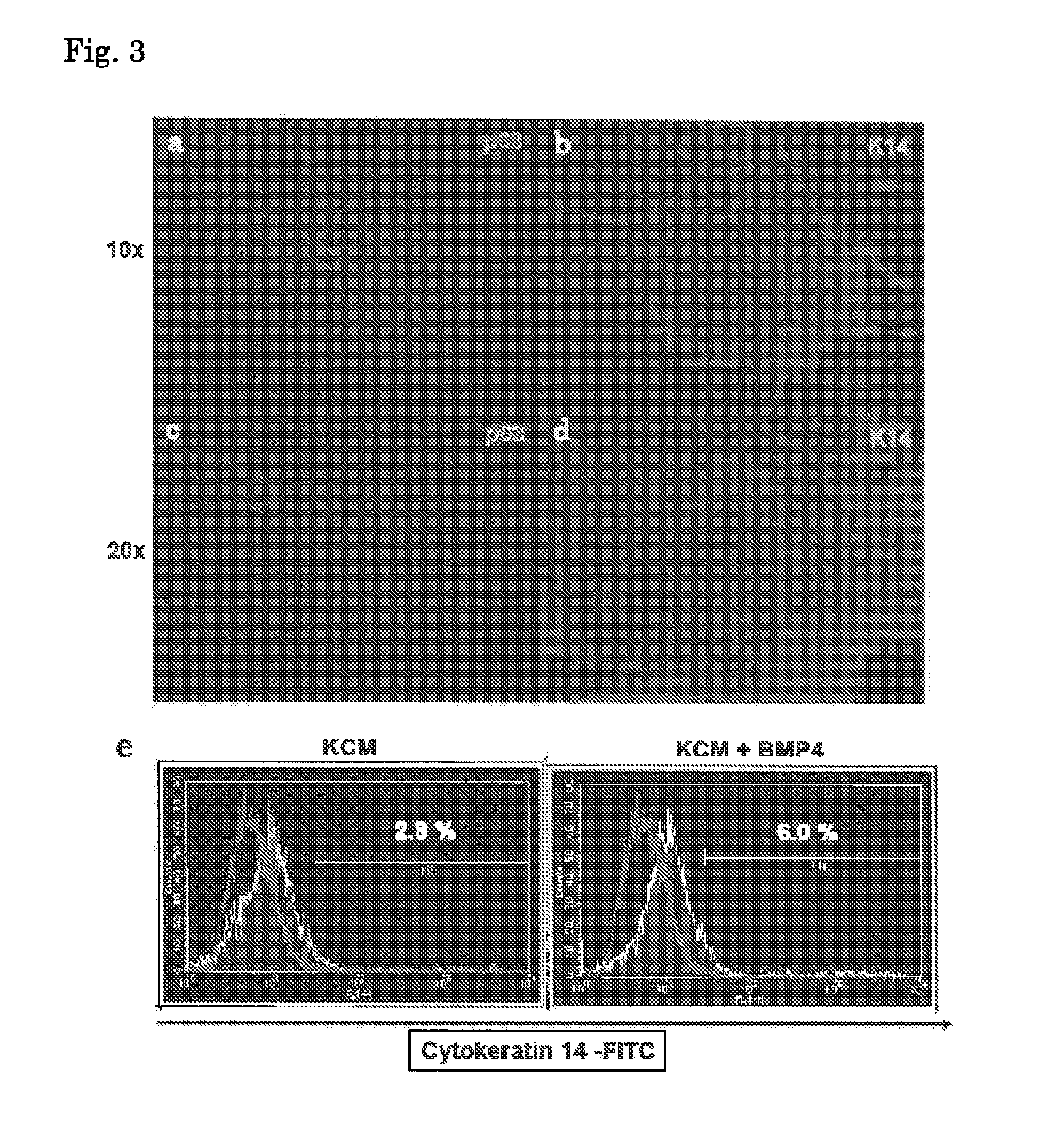
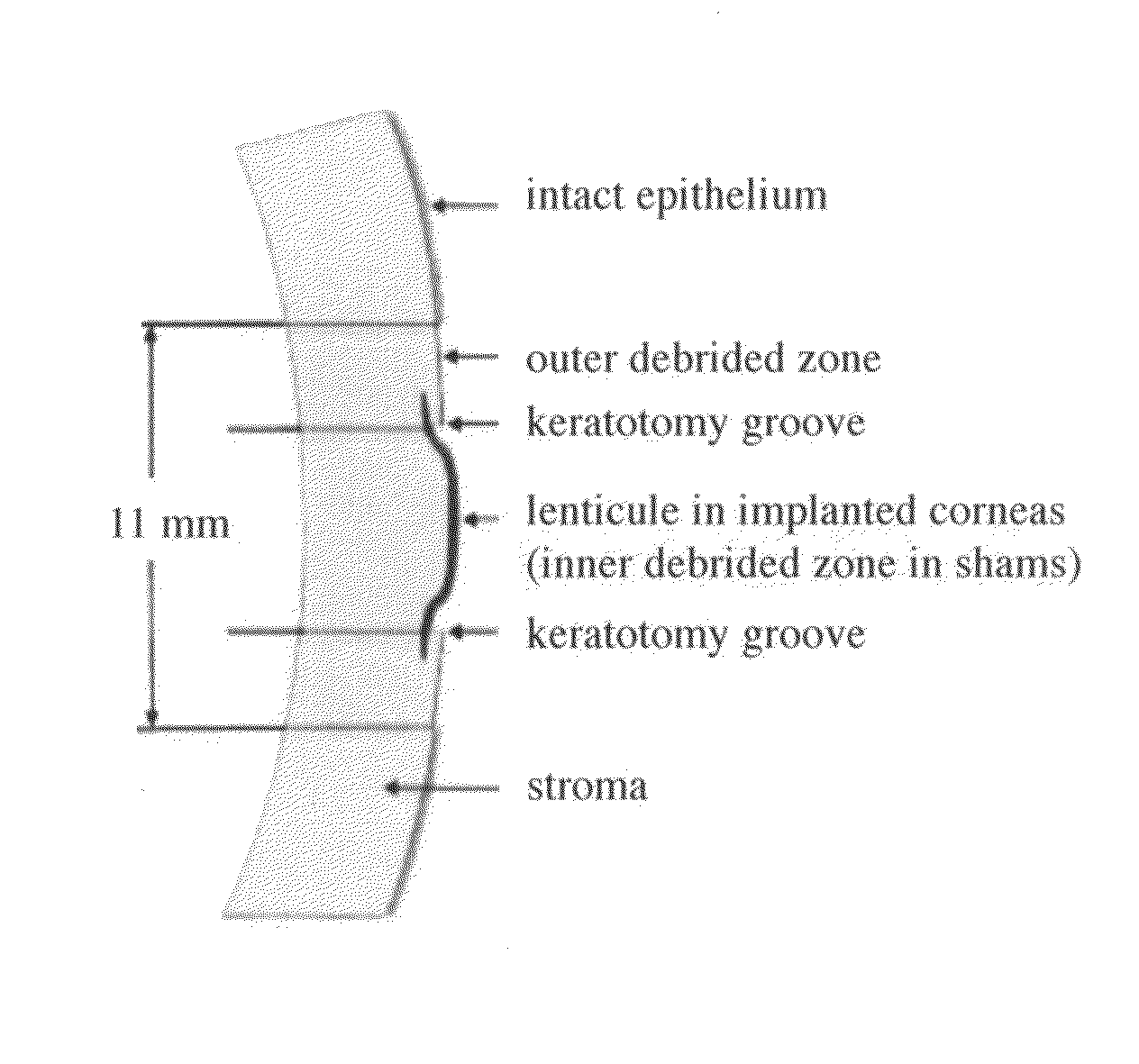
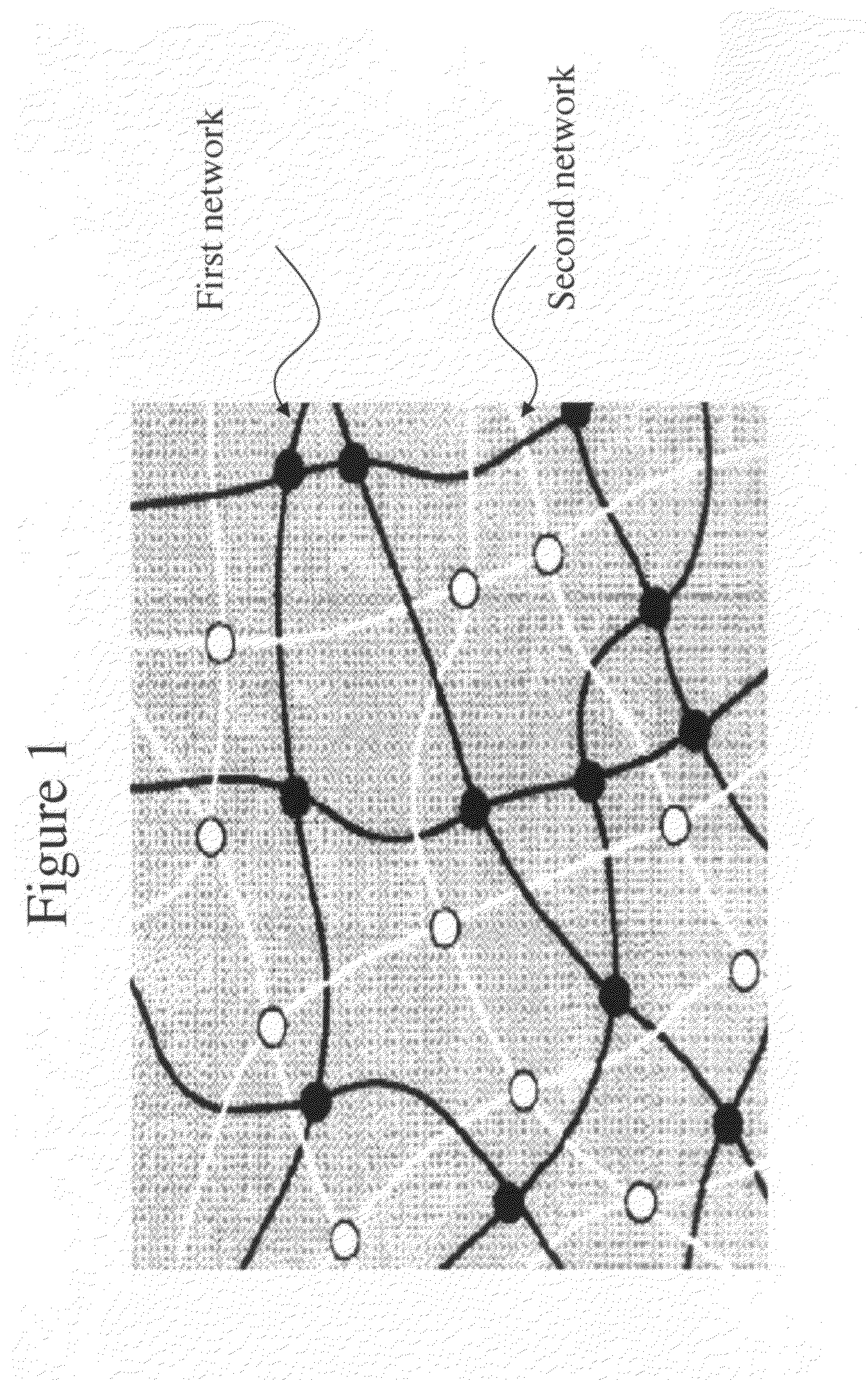
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
145 results about "Corneal epithelial cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Artificial corneal implant
A material that can be applied as implants designed to artificially replace or augment the cornea, such as an artificial cornea, corneal onlay, or corneal inlay (intrastromal lens) is provided. The artificial corneal implant has a double network hydrogel with a first network interpenetrated with a second network. The first network and the second network are based on biocompatible polymers. At least one of the network polymers is based on a hydrophilic polymer. The artificial cornea or implant has epithelialization promoting biomolecules that are covalently linked to the surface of the double network hydrogel using an azide-active-ester chemical linker. Corneal epithelial cells or cornea-derived cells are adhered to the biomolecules. The double network has a physiologic diffusion coefficient to allow passage of nutrients to the adhered cells.
Owner:SANTA CLARA UNIVERSITY
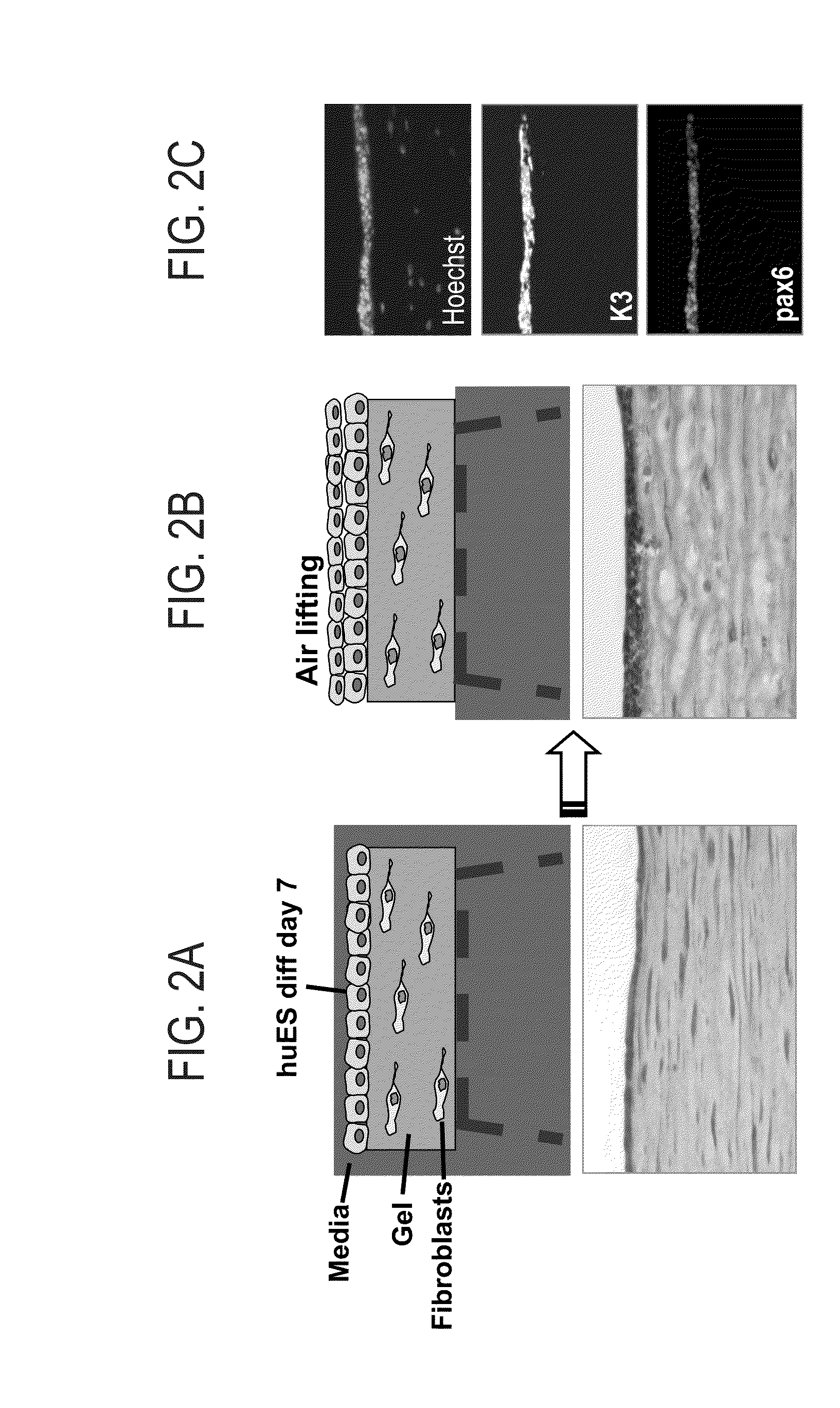
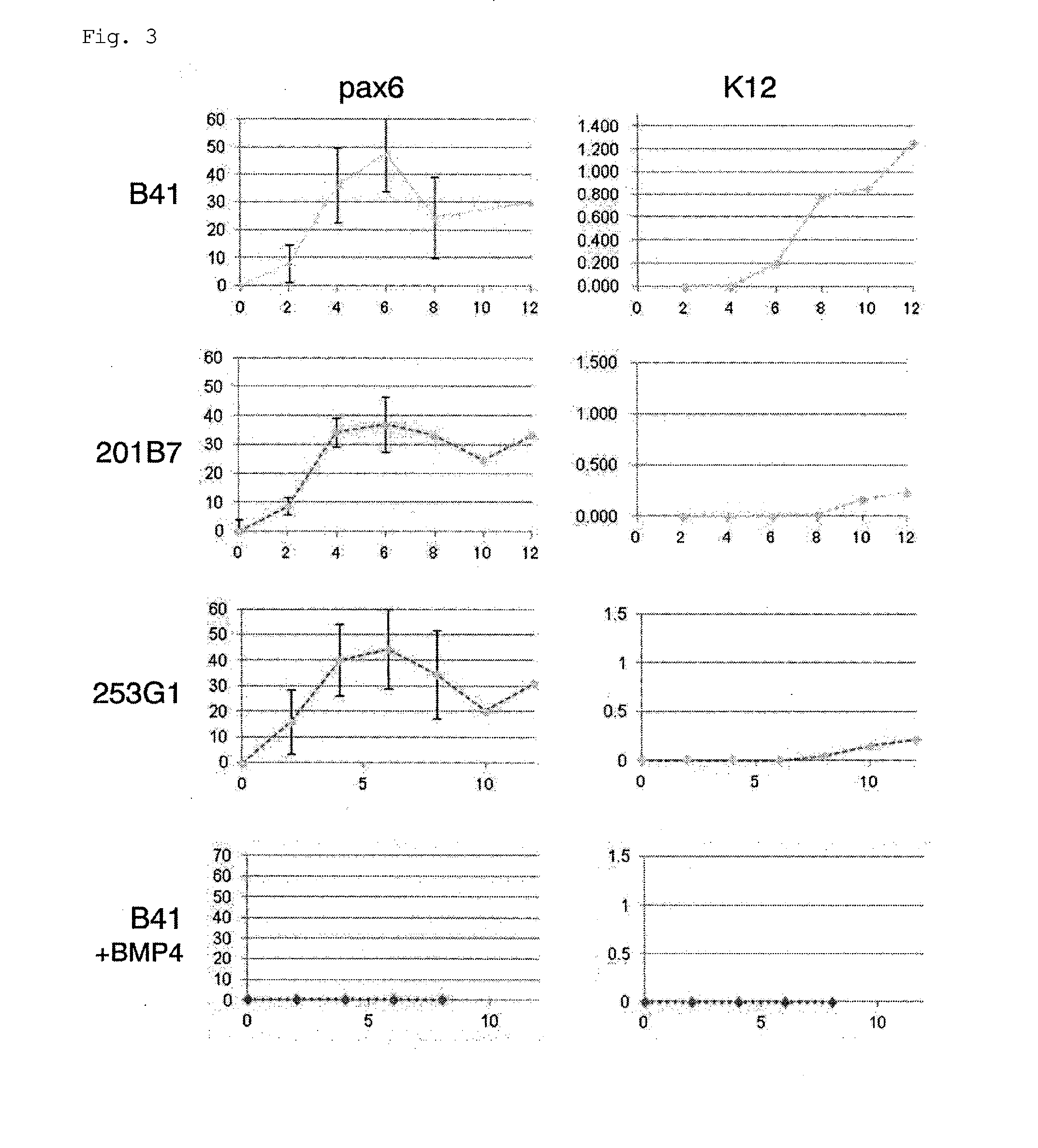
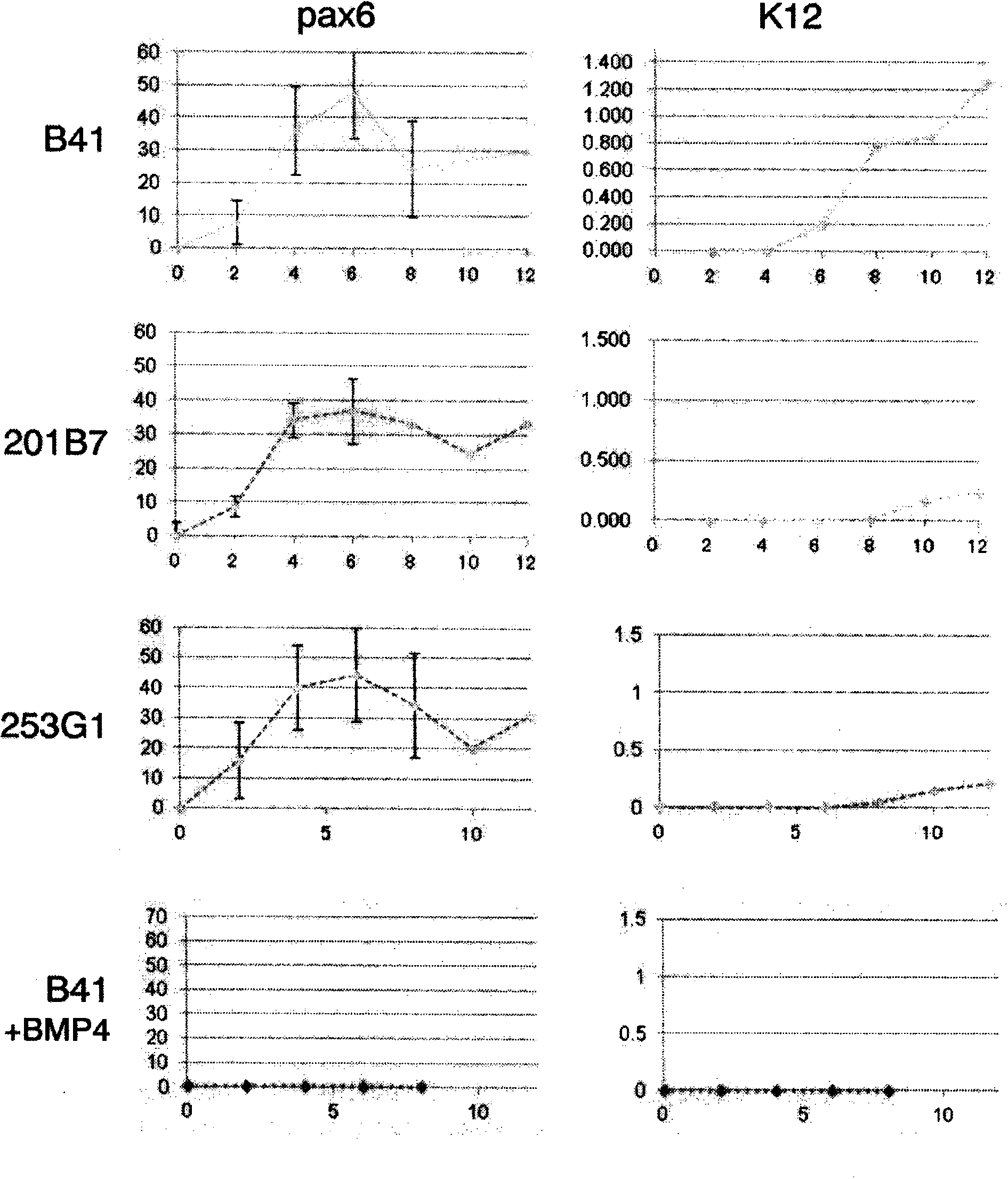
Method for inducing differentiation into epithelial progenitor cell/stem cell population and corneal epithelial cell population from induced pluripotent stem cells
InactiveUS20120142103A1Reduce rejectionEpidermal cells/skin cellsNervous system cellsDiseasePluripotential stem cell
The present invention relates to: a method for inducing differentiation into an epithelial progenitor cell / stem cell population or a corneal epithelial cell population by culturing, under particular conditions, induced pluripotent stem cells induced from mammalian somatic cells or undifferentiated stem cells; an epithelial progenitor cell / stem cell population or a corneal epithelial cell population obtained by the method; and a cell preparation for the treatment of epithelial disease and a cell sheet, which are prepared using these cell populations.
Owner:TOHOKU UNIV +1
Tissue engineered cornea epithelial transplantation membrane and preparation method and use thereof
InactiveCN101306207AMaintain biological characteristicsElasticEye implantsTransplanted corneaOphthalmology
The invention discloses a tissue engineering cornea epithelial transplantation membrane, a preparing method and an application thereof. The invention aims to provide a tissue engineering cornea epithelial transplantation membrane, the tissue engineering cornea epithelial transplantation membrane comprises a porcine cornea epithelial cell and nonantigenic tissue engineering cornea bracket material, wherein, the porcine cornea epithelial cell and the nonantigenic tissue engineering cornea bracket material are combined into a whole by adopting a tissue engineering method. And meanwhile, The invention further provides a method for preparing the tissue engineering cornea epithelial transplantation membrane, and a use of the tissue engineering cornea epithelial transplantation membrane during the process of preparing medical treatment material used for remedying blind eye disease caused by the pathological state and the damage of corneas.
Owner:PEKING UNIV THIRD HOSPITAL
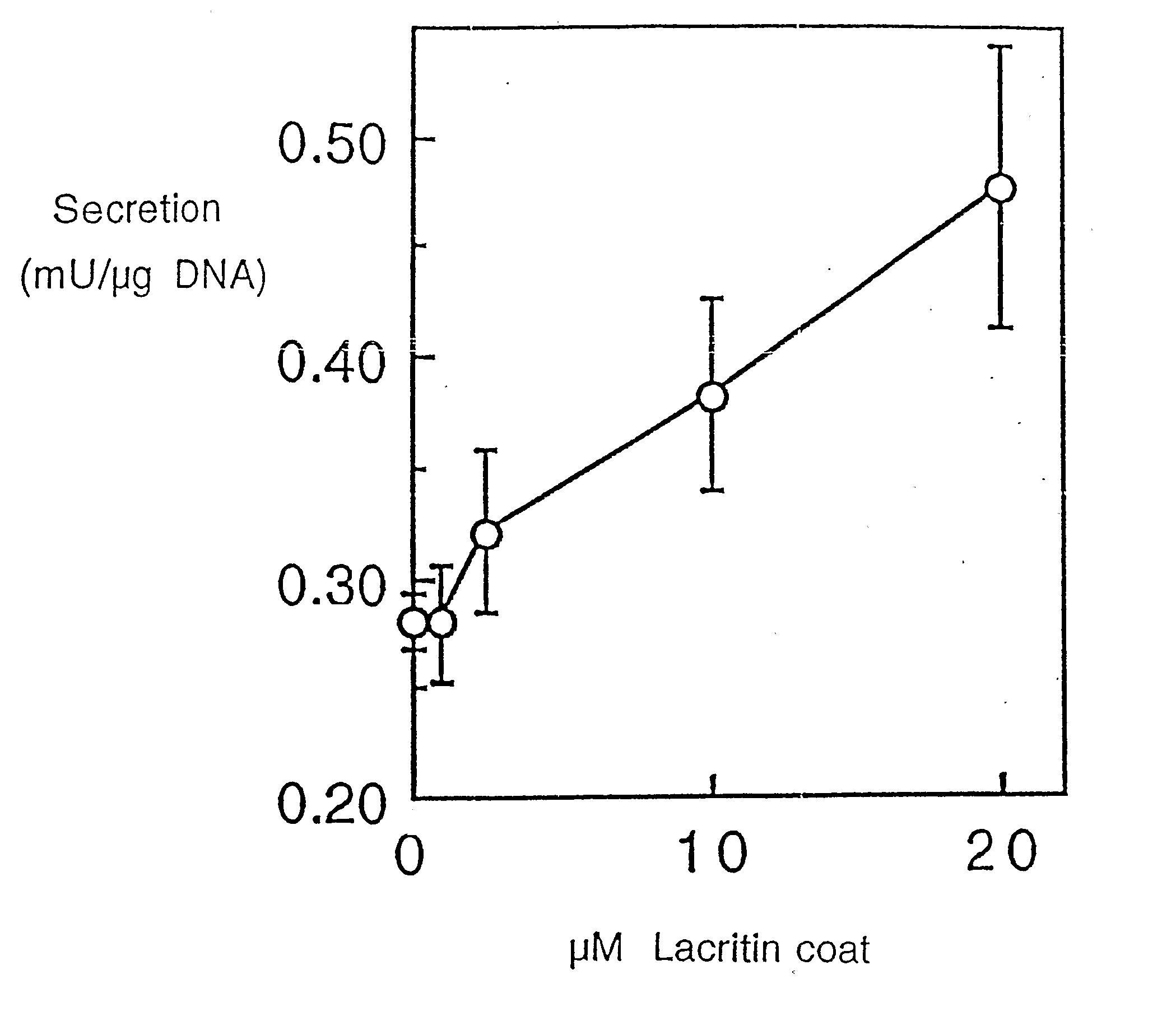
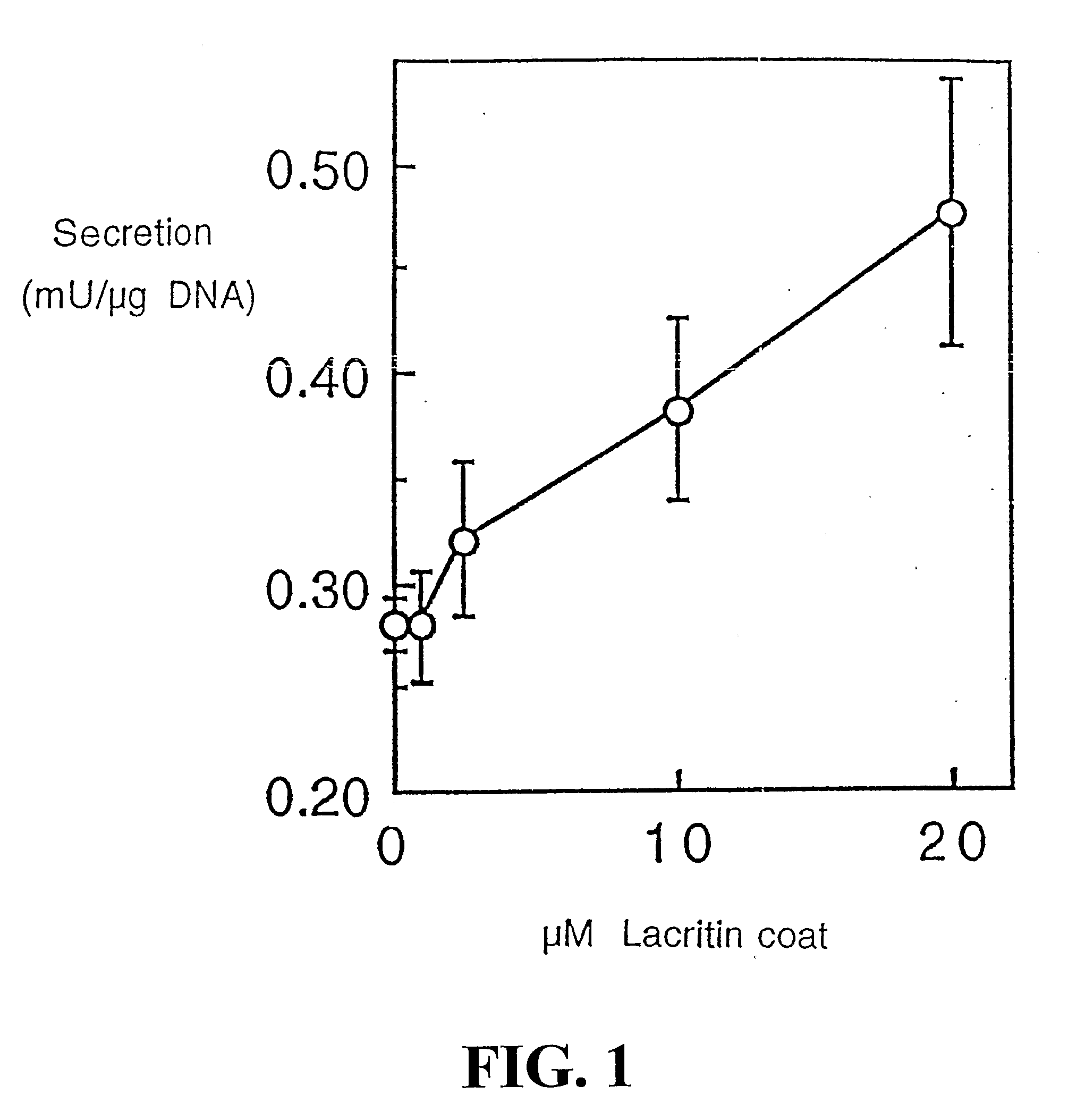
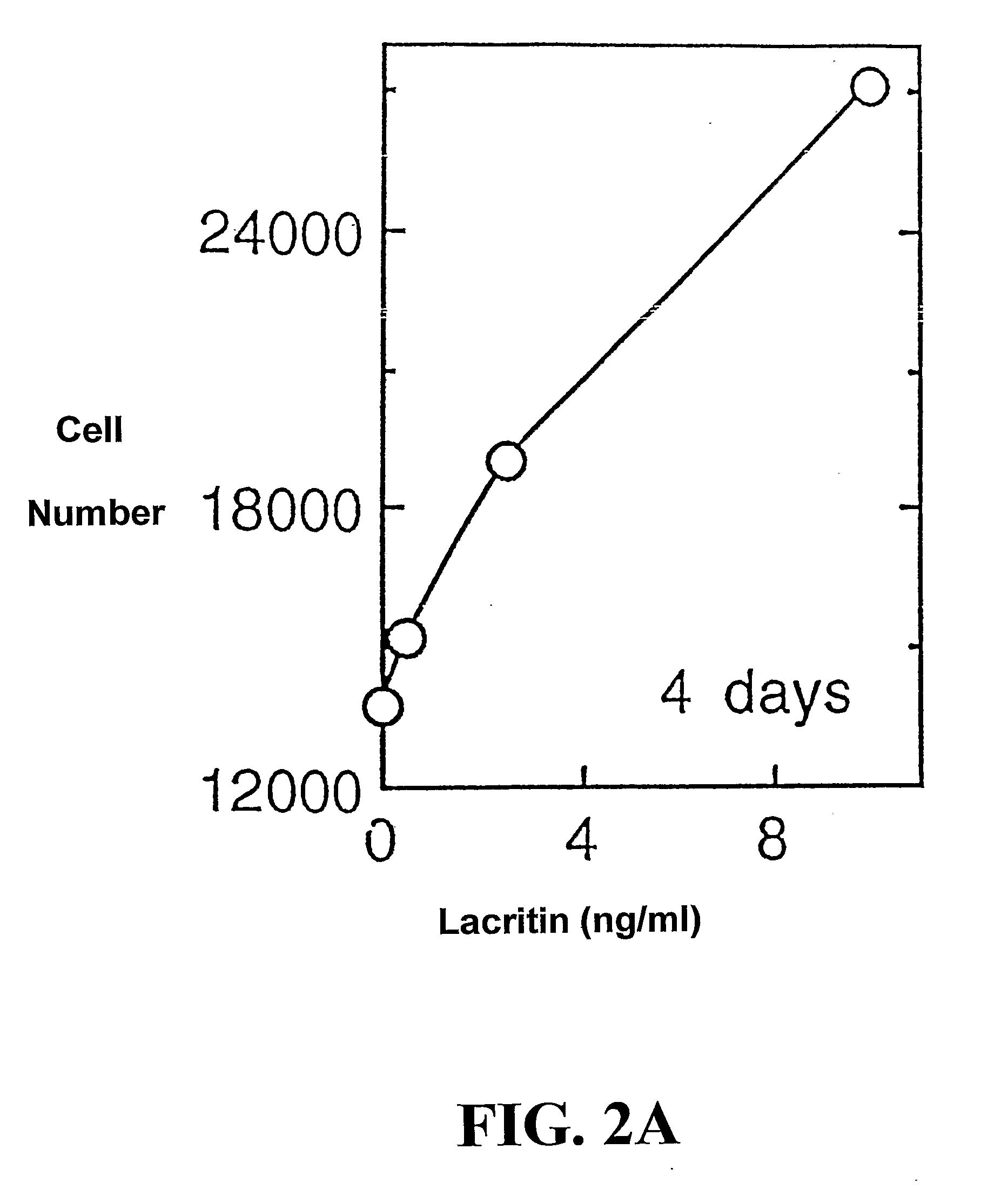
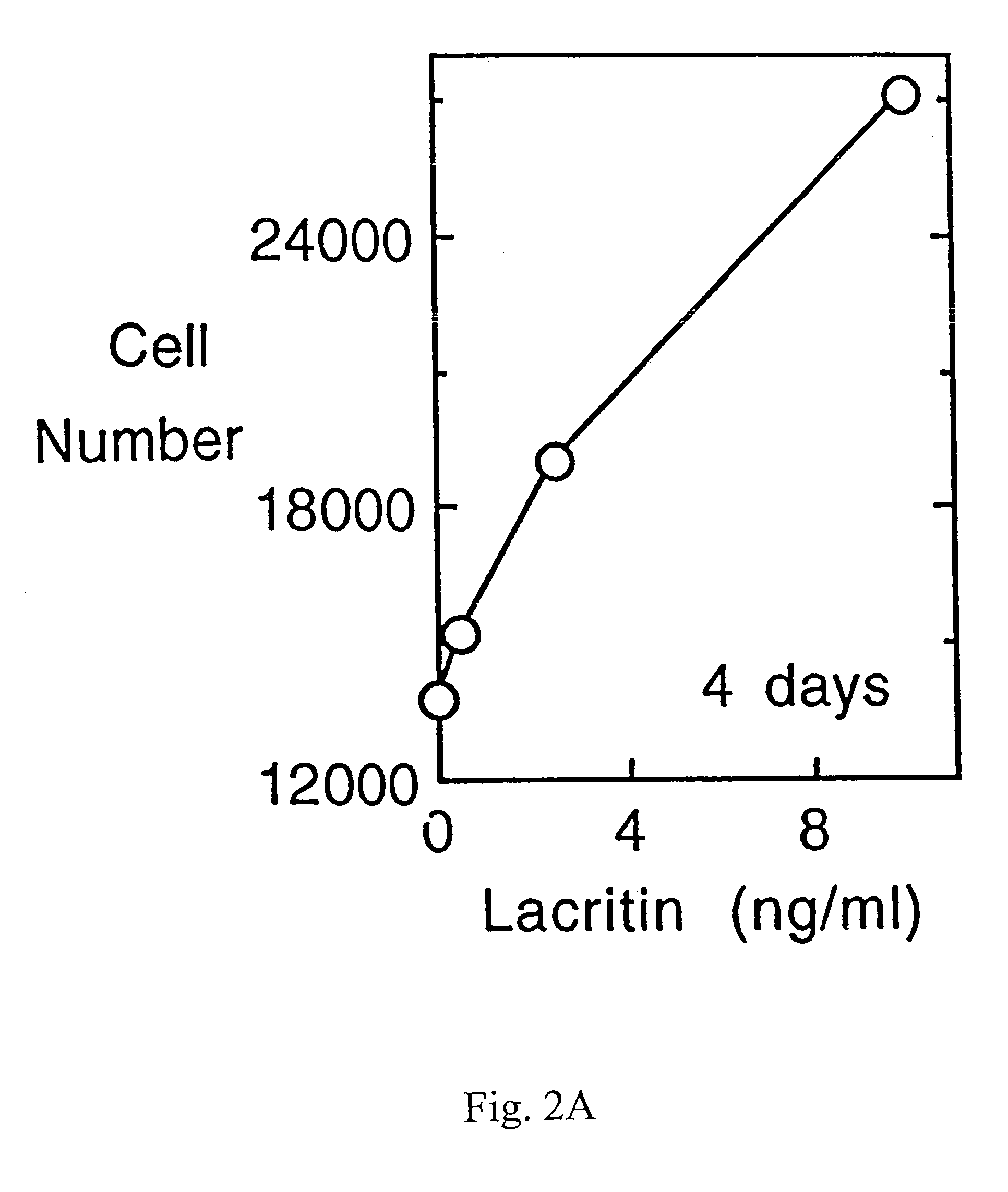
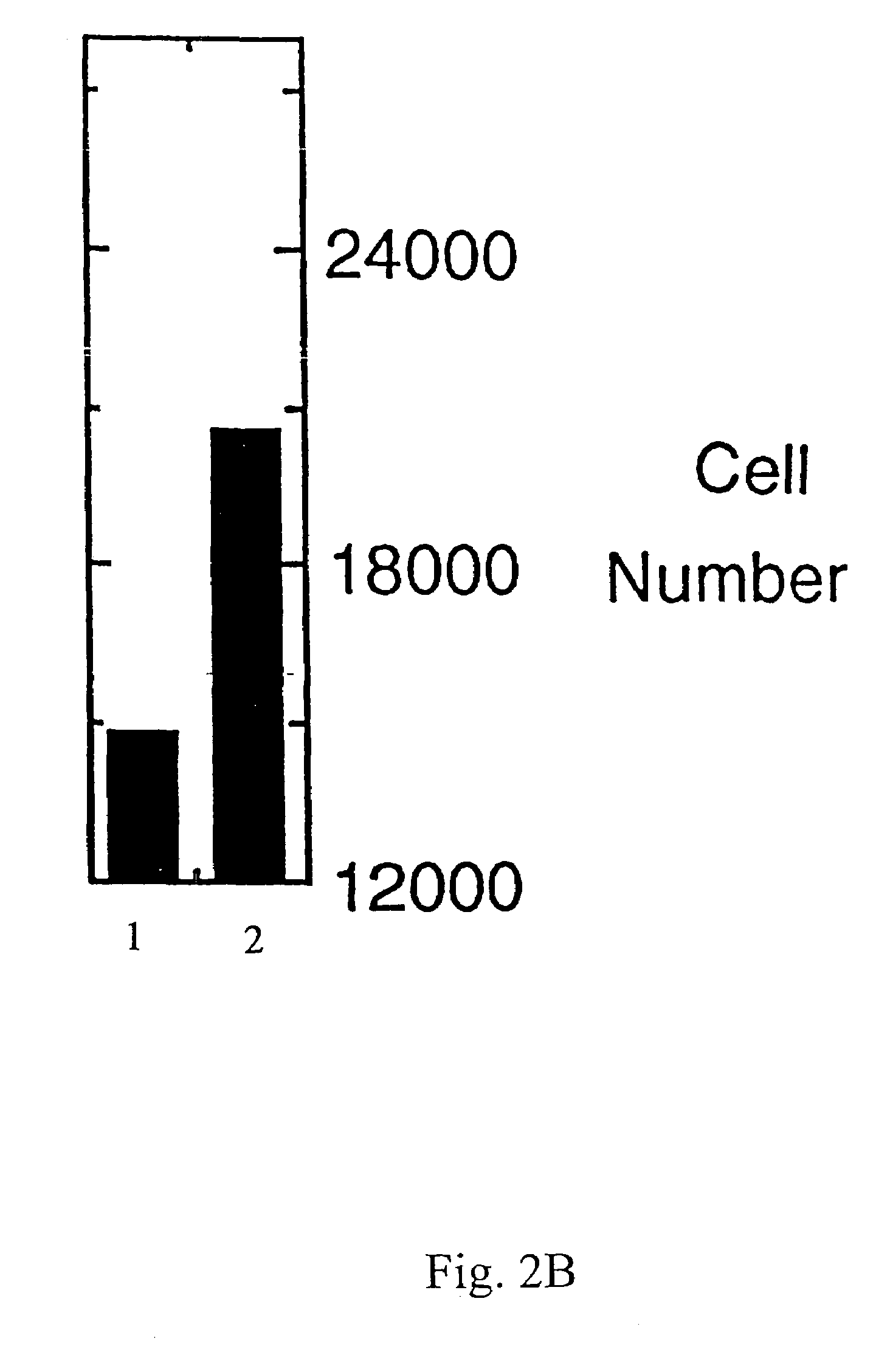
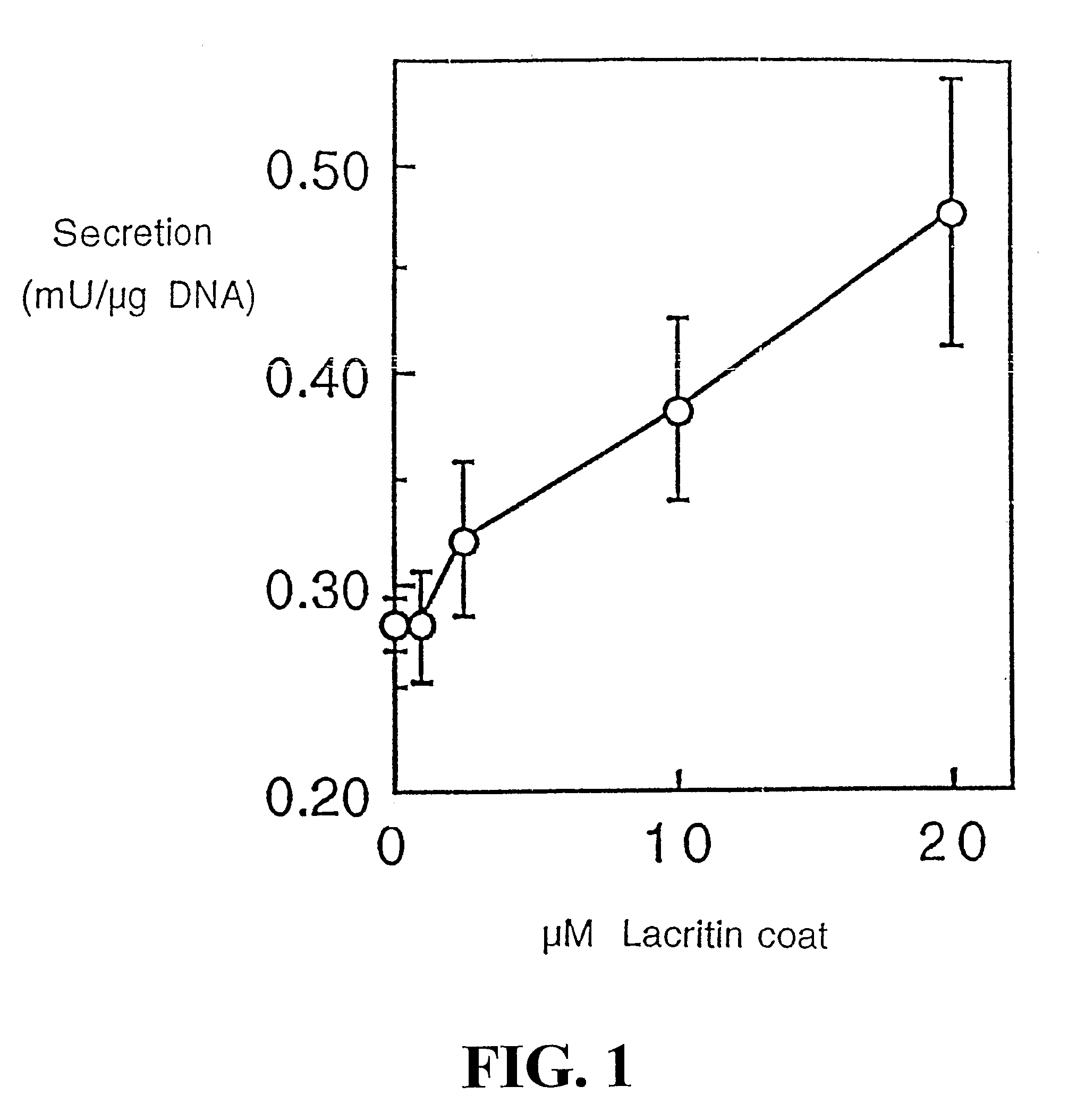
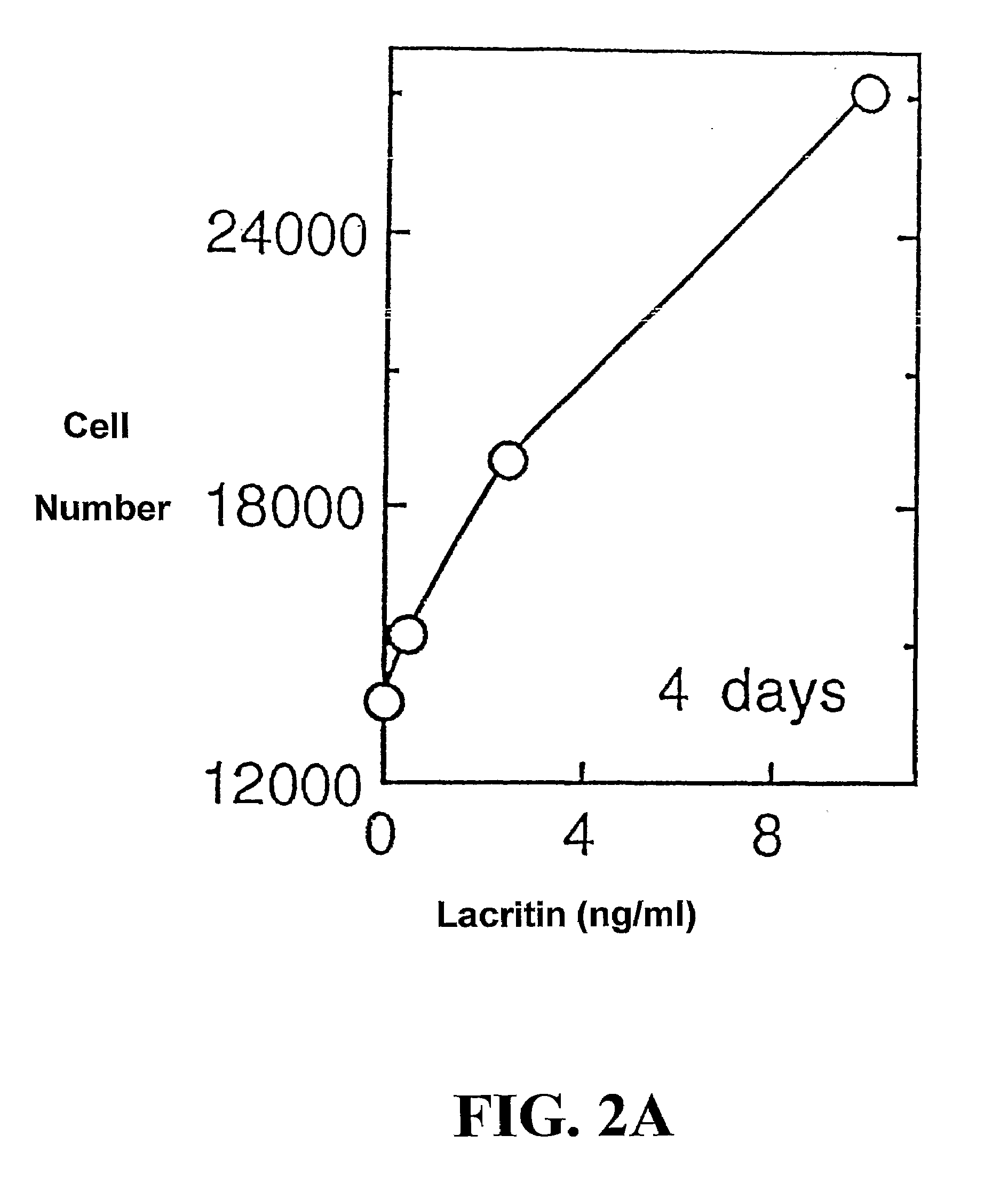
Use of Lacritin in Promoting Ocular Cell Survival
ActiveUS20070207522A1Promote ocular cell survivalPrevent and treat corneal infectionSenses disorderPeptide/protein ingredientsLacritinCell survival
The present invention relates to the use of lacritin, and fragments, derivatives, and homologs thereof, and nucleic acid sequences encoding that protein. The invention relates to the use of lacritin as a growth factor human corneal epithelial cells and other select epithelial cells. The invention also relates to the use of lacritin in promoting ocular cell survival in response to an insult or injury, in protecting against ocular inflammation, and in promoting ocular wound repair.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Preparation method for artificial corneas
The invention discloses a preparation method for artificial corneas. The preparation method comprises the following steps of (1) collecting age-appropriate pig eyeballs; (2) scraping corneal epithelial cells and corneal endothelial cells of the pig eyeballs, reserving bowman layers, preparing lamellar corneas by using a lamellar blade, and drilling size-specific corneal slices; (3) putting the corneal slices obtained in step (2) in liquid nitrogen for 8-35 minutes, slowly rewarming the corneal slices to room temperature under normal temperature and normal pressure after the corneal slices are taken out, repeating the operation for 4-12 times; (4) putting the corneal slices which are subjected to freezing-thawing treatment in step (3) in normal saline for carrying out ultrasonic cleaning, wherein the ultrasonic working frequency is 20-250 kHz, and the ultrasonic time is 15-65 minutes; (5) putting the corneal slices which are subjected to ultrasonic treatment in a buffer system, carrying out enzymolysis on DNA (Deoxyribose Nucleic Acid) of corneal stromal cells by using endonuclease, and obtaining the artificial corneas. According to the artificial corneas prepared by the preparation method disclosed by the invention, gaps between natural collagen fibers of the artificial corneas are beneficial for inducing the growth of the corneal cells and the growth of nerve fibers of an acceptor and promoting the repairing and the reconstructing of corneas of a donor.
Owner:XIAMEN DAKAI BIOTECH CO LTD
Corneal contact lens nursing composition containing mycose and preparation method thereof
InactiveCN101691478AReduce drynessRelieve sorenessOther chemical processesSurface-active agentsTrehalose
The invention provides a corneal contact lens nursing composition containing mycose, and also relates to the compositions of the type. The composition comprises the following components in weight percent: 0.001-8% of mycose, 0.01-3% of isotonic regulator, 0.001-4% of buffering agent, 0.01-3% of lubricating moisturizing agent, 0.001-1% of complexing agent, 0.01-1% of surface active agent and 0.00001-0.15% of antimicrobial agent. The invention is used for the storage, sterilization, cleaning, lubrication, moisture preservation and dry resistance of the lens of the corneal contact lens and for the rehumidification of the lens, can relieve the dryness and acid bilges of the eyes, reduce the death rate of the corneal epithelial cell in a dry environment, and improve the restoration of the corneal epithelial and intradermal cells.
Owner:HAICHANG CONTACT LENSES
3D model constructed in vitro by means of serum-free culture medium as well as construction method of 3D model
ActiveCN107326003AEnsure nutritional needsPromote growthEpidermal cells/skin cellsCulture processBiological materialsLiquid surfaces
The invention discloses a 3D model constructed in vitro by means of a serum-free culture medium as well as a construction method of the 3D model and relates to the technical field of biological materials in tissue engineering. Due to selection variety of seed cells and good in-vitro construction repeatability, industrial preparation of the 3D model can be realized. The model is an in-vitro model formed by primary corneal epithelial cells subjected to tissue isolation, limbal stem cells, immortalized corneal epithelial cells, primary oral epithelial cells or oral mucosa squamous cancer cell lines TR146 subjected to in-vitro amplification and stratified through the serum-free culture medium with an undersurface-gas-liquid surface staged culture method.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Method for constructing human corneal epithelial cell system
ActiveCN102168064ANot tumorigenicLow costMicroorganism based processesArtificial cell constructsCuticleCell system
The invention relates to a method for constructing a human corneal epithelial cell system. The construction method comprises the following steps of: disinfecting cornea, digesting the cornea, taking off corneal epithelium with a front elastic layer, cutting the corneal epithelium into small tissue blocks, flatly attaching the tissue blocks to the bottom of culture holes downwards, adding culture solution into a carbon dioxide culture box, and performing plate-attaching culture at the temperature of 37 DEG C, changing the culture solution during culturing, performing subculture when the cells grow into a single layer, and presently, completing the construction of the human corneal epithelial cell system above 60 generations, wherein the culture solution contains 20 percent of fetal calf serum, 0.002 to 0.004 percent of human epidermic cell growth factor, 0.001 to 0.002 percent of human alkali fibroblast growth factor, 0.04 to 0.1 percent of carboxymethyl chito-oligosaccharide, 0.025 to0.1 percent of DMEM / F12 culture solution of chondroitin sulfate, and 0.008 to 0.01 percent of IV type collagen. In the construction method, the non-transfection human corneal epithelial cell system is successfully constructed by utilizing the human corneal epithelial tissues, and the constructed cell system can realize continuous transfer of culture, and a great number of human corneal epithelialcells are provided.
Owner:青岛彩晖生物科技有限公司
Three-dimensional bioprinted artificial cornea
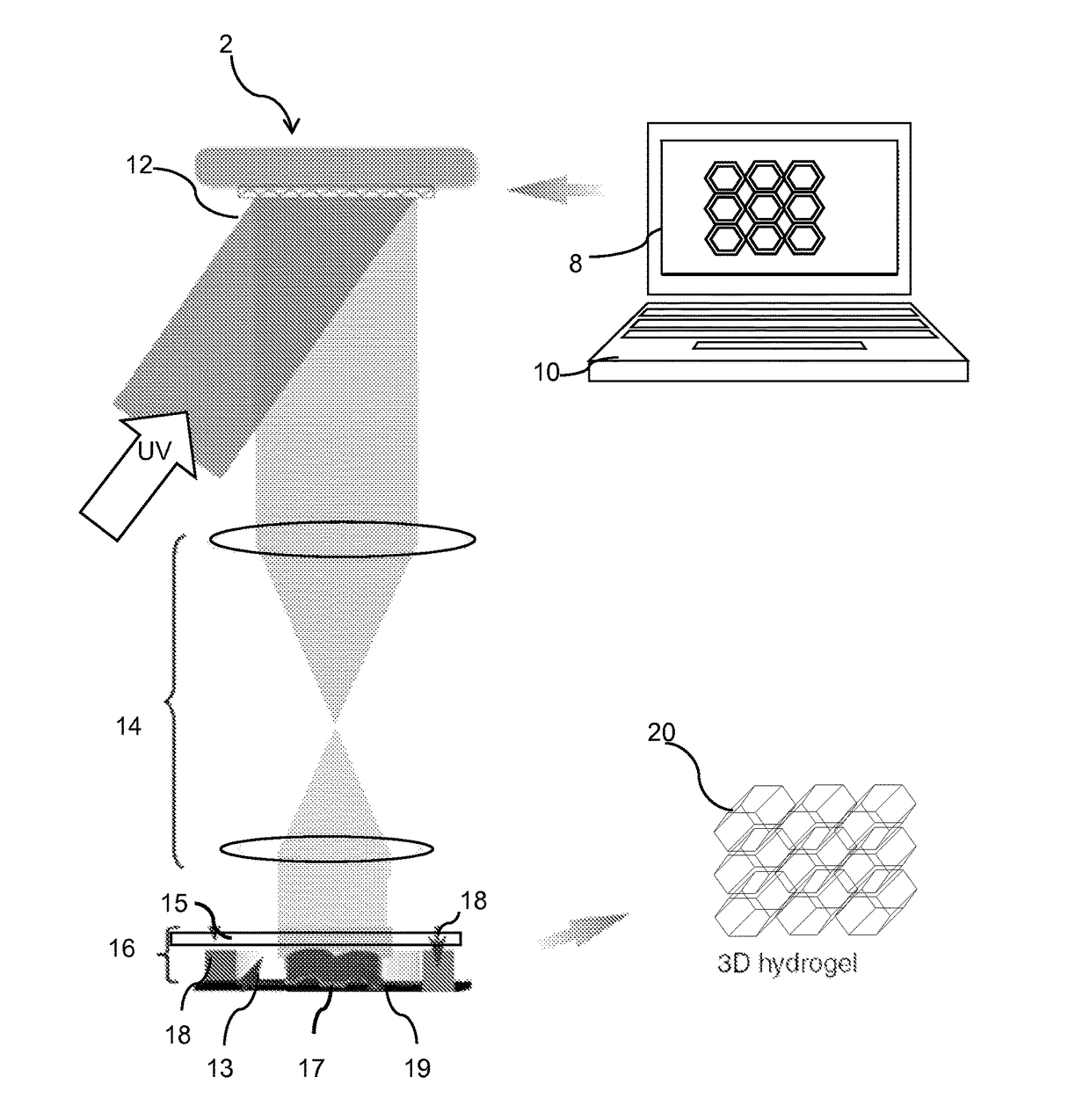
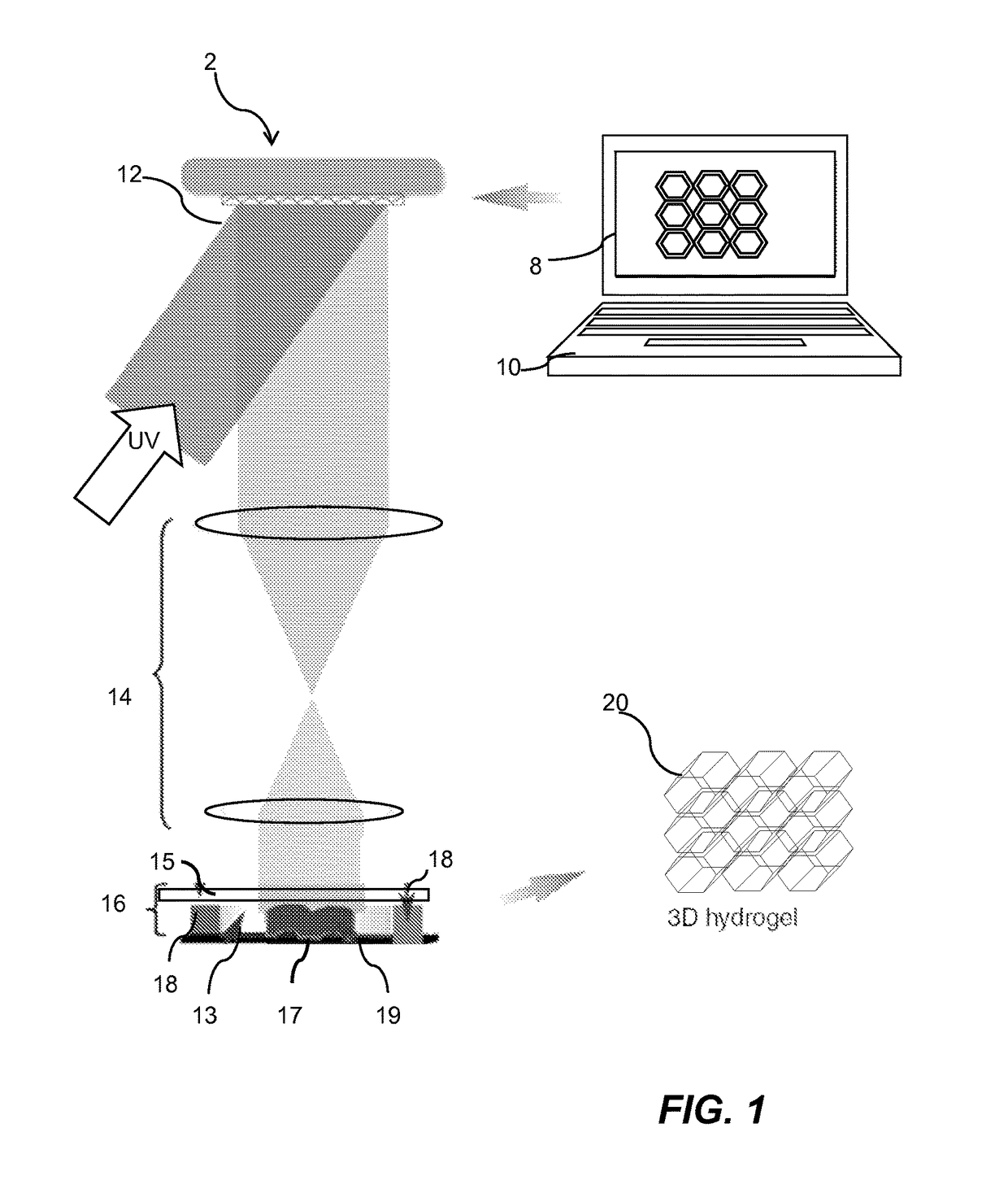
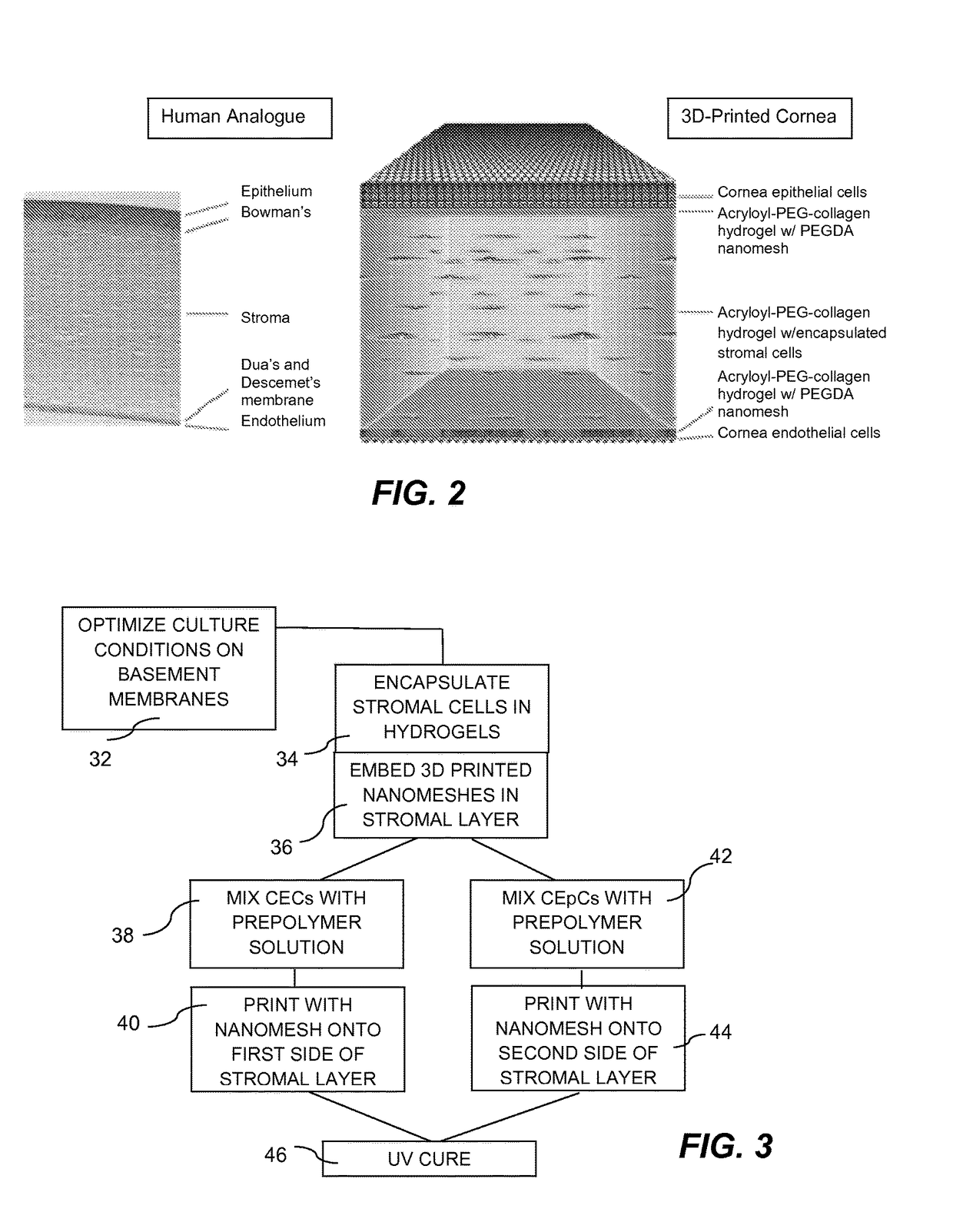
InactiveUS20170281828A1Restore visionEliminate dependenciesAdditive manufacturing apparatusPharmaceutical delivery mechanismCorneal endothelial cellStromal cell
An artificial cornea is fabricated by separately culturing live stromal cells, live corneal endothelial cells (CECs) and live corneal epithelial cells (CEpCs), and 3D bioprinting separate stromal, CEC and CEpC layers to encapsulate the cells into separate hydrogel nanomeshes. The CEC layer is attached to a first side of the stromal layer and the CEpC layer to a second side of the stromal layer to define the artificial cornea.
Owner:RGT UNIV OF CALIFORNIA
Mesenchymal stem cell-derived exosome
ActiveCN107849535APromote proliferationIncreased undifferentiated maintenance activitySenses disorderMicrobiological testing/measurementMesenchymal stem cellGrowth promoting
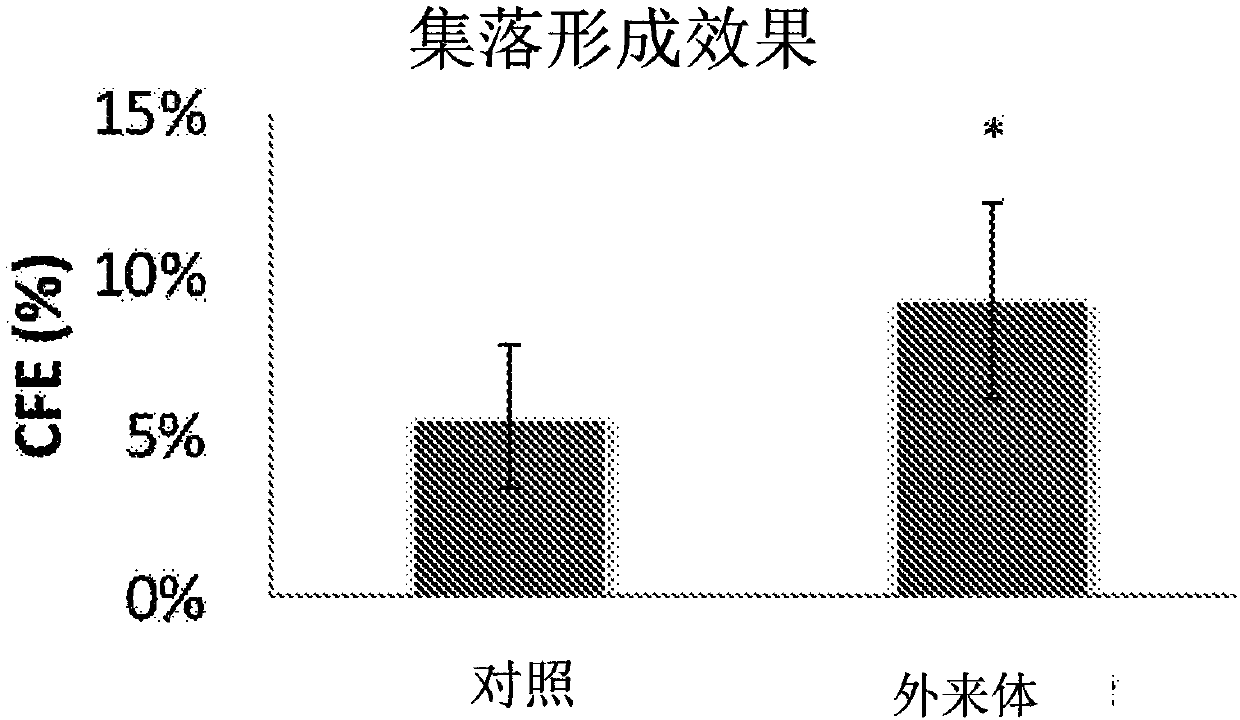
The present invention relates to a mesenchymal stem cell-derived microparticle that has corneal epithelial stem cell and / or corneal epithelial cell growth-promoting activity, undifferentiated cornealepithelial stem cell maintenance activity, corneal epithelial stem cell colony formation-promoting activity, or a corneal epithelium protection effect.
Owner:OSAKA UNIV +1
Tissue-engineered cornea and preparation method thereof
ActiveCN110613862AIncreasing the thicknessIncreased mechanical toughnessEye implantsTissue regenerationFiberCell-Extracellular Matrix
The invention discloses a tissue-engineered cornea and a preparation method thereof. The tissue-engineered cornea comprises three or more layers of laminated structures, each layer of the laminated structure is a composite layer formed in the mode that a fiber scaffold prepared by electrospinning or electrostatic direct writing or light curing 3D printing is cured and composited with one or more of collagen, an acellular matrix and silk fibroin, the space between every two adjacent layers is inoculated with corneal stromal cells and neurons, the corneal stromal cells and the neurons can grow in the direction of fibers of the scaffold, the corneal stromal cells between every two adjacent layers secrete extracellular matrixes to form a tissue structure similar to the human corneal stroma, and the upper surfaces and the lower surface of the laminated structures are inoculated with corneal epithelial cells and corneal endothelial cells. The tissue-engineered cornea can simulate the corneastructure well, is easy to prepare and good in biocompatibility and is easily fused with tissue.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Ocular tear growth factor-like protein
The present invention relates to a novel lacrimal gland protein (designated lacritin) and the nucleic acid sequences encoding that protein. Lacritin has activity as a growth factor on both human corneal epithelial cells and on the lacrimal acinar cells that produce it. Accordingly, one embodiment of the present invention is directed to the use of lacritin to treat Dry Eye and other disorders requiring the wetting of the eye.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
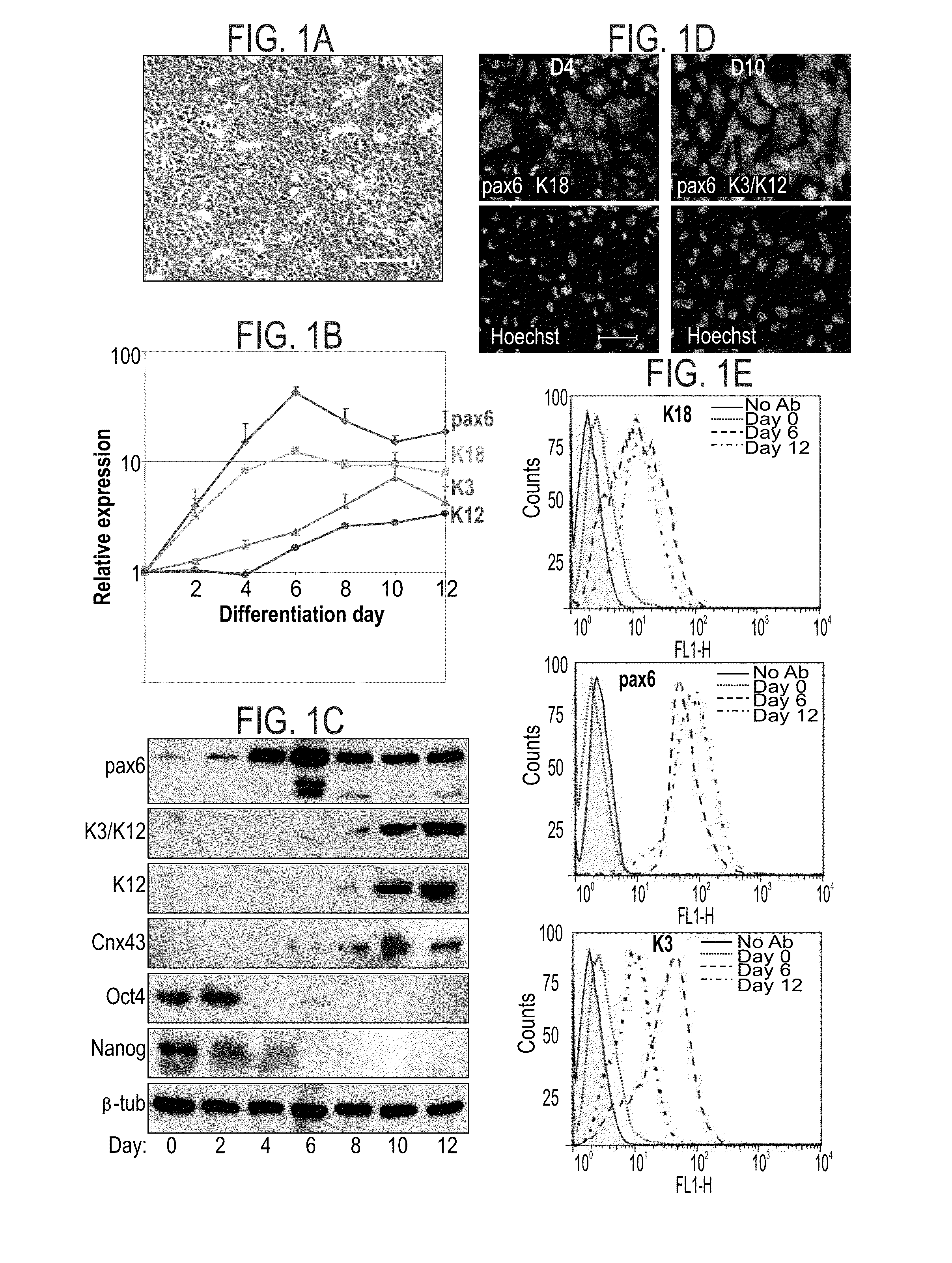
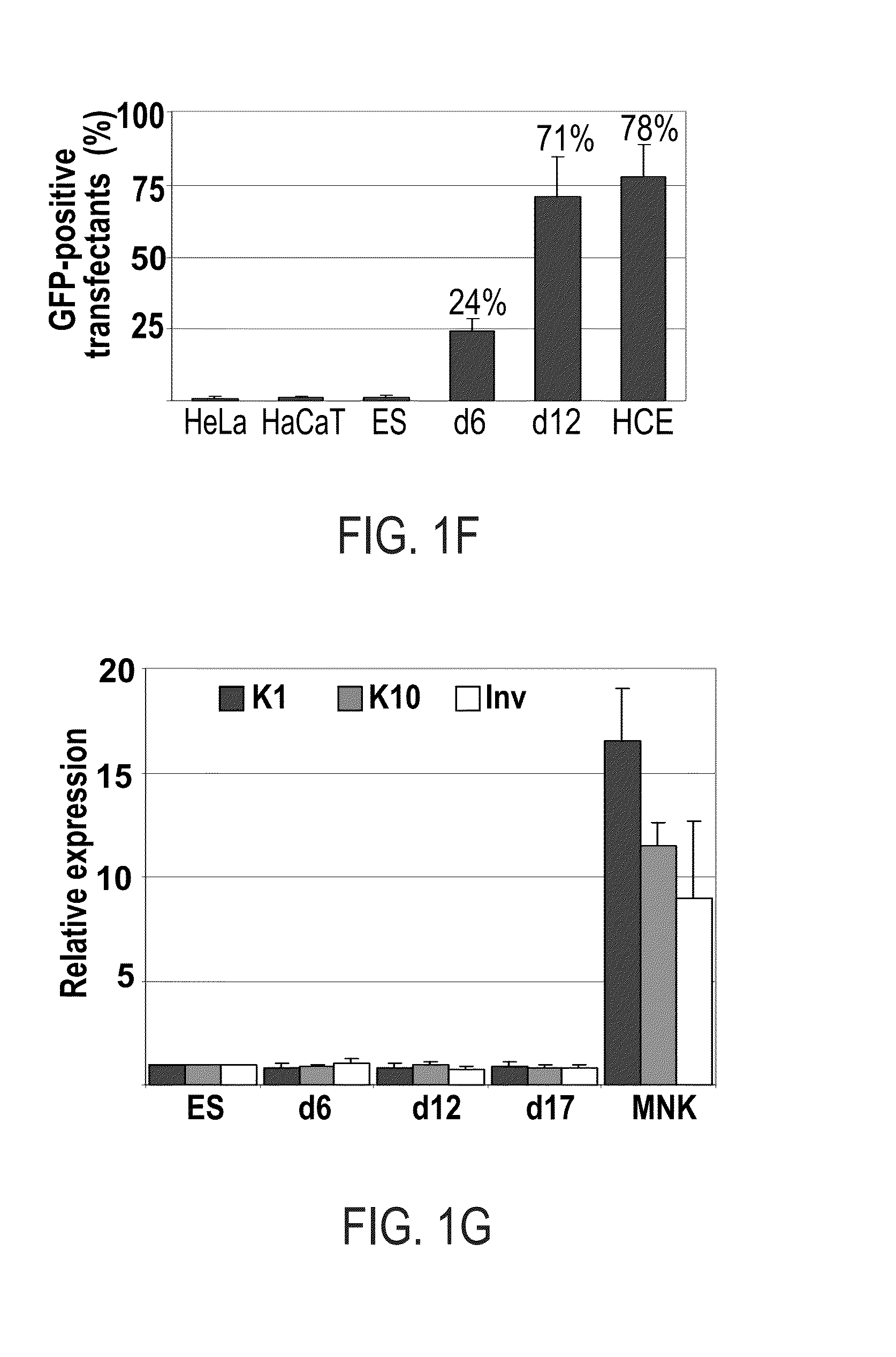
Methods of generating corneal cells and cell populations comprising same
A method of generating a population of corneal epithelial cells is disclosed. The method comprises culturing human pluripotent stem cells in corneal fibroblast-conditioned medium on a solid surface comprising an extracellular matrix component thereby generating the population of corneal epithelial cells. Isolated cell populations and corneal tissues are also disclosed, as well as uses thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Bletilla striata polysaccharide hydrogel, culture medium and application thereof as well as method of inducing differentiation of umbilical cord mesenchymal stem cells to corneal epithelial cells
ActiveCN105085938AGood hygroscopicityImprove hydrophilicityVertebrate cellsArtificial cell constructsPorosityHuc mscs
The invention relates to the field of cell culture, in particular relates to letilla striata polysaccharide hydrogel, a culture medium and application thereof as well as a method of inducing differentiation of umbilical cord mesenchymal stem cells (hUC-MSCs) to corneal epithelial cells. The method comprises the steps of sulfating letilla striata polysaccharide and then crosslinking with Epsilon-polylysine carrying polyfunctional groups to prepare the polysaccharide hydrogel. The letilla striata polysaccharide hydrogel is of a semi-transparent membrane, has good porosities, and is suitable for the growth and the differentiation of cells. The experiment shows that by taking the letilla striata polysaccharide hydrogel provided by the invention as a scaffold, after the hUC-MSCs and amniotic epithelial cells are subjected to co-culture for 7 days, the differentiation rate of the hUC-MSCs can be up to 31.96%. The differentiation rate (p is less than 0.01) is obviously superior to that of a contrasting example which does not adopt the hydrogel scaffold. In addition, the hydrogel provided by the invention can promote the cells to grow vigorously, and shorten the induction time.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Artificial corneal implant
InactiveUS20110184513A1High molecular weightEye implantsNervous system cellsCell adhesionDouble network
A material that can be applied as implants designed to artificially replace or augment the cornea, such as an artificial cornea, corneal onlay, or corneal inlay (intrastromal lens) is provided. The artificial corneal implant has a double network hydrogel with a first network interpenetrated with a second network. The first network and the second network are based on biocompatible polymers. At least one of the network polymers is based on a hydrophilic polymer. The artificial cornea or implant has epithelialization promoting biomolecules that are covalently linked to the surface of the double network hydrogel using an azide-active-ester chemical linker. Corneal epithelial cells or cornea-derived cells are adhered to the biomolecules. The double network has a physiologic diffusion coefficient to allow passage of nutrients to the adhered cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
iPS CELL HAVING DIFFERENTIATION PROPENSITY FOR CORNEAL EPITHELIUM
InactiveUS20140127803A1Improve securitySimple processGenetically modified cellsEpidermal cells/skin cellsInduced pluripotent stem cellStromal cell
A major object of the present invention is to provide a method for inducing cell differentiation into corneal epithelial stem cells and / or corneal epithelial cells, for the easy production of a corneal epithelial cell sheet having superior safety in view of the possibility of vascularization and the like occurring. A method for inducing differentiation of a pluripotent stem cell into a corneal epithelial stem cell and / or a corneal epithelial cell, the method comprising the step of culturing a pluripotent stem cell in the presence of a stromal cell or an amnion-derived factor.
Owner:OSAKA UNIV
Culture medium and method for inducing differentiation of umbilical cord mesenchymal stem cells to corneal epithelial cells
ActiveCN105087466AIncrease percentageIncrease the percentage of differentiationArtificial cell constructsVertebrate cellsHuc mscsStem cell culture
The invention relates to the field of stem cell culture, in particular to a culture medium and method for inducing differentiation of umbilical cord mesenchymal stem cells to corneal epithelial cells. An epidermal growth factor and insulin are added into a basic culture solution, so that the percentage of differentiation of hUC-MSCs to the corneal epithelial cells is increased. Moreover, the culture solution does not contain heterogenous serum, so that the risk is lowered. According to the method provided by the invention, the umbilical cord mesenchymal stem cells and amniotic epithelial cells are co-cultured, so that the differentiation percentage can be increased remarkably, and the required culture period is short. The adopted umbilical cord mesenchymal stem cells are derived from umbilical cords and placentas, so that the source is wide, and legal and ethical limitations are avoided.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
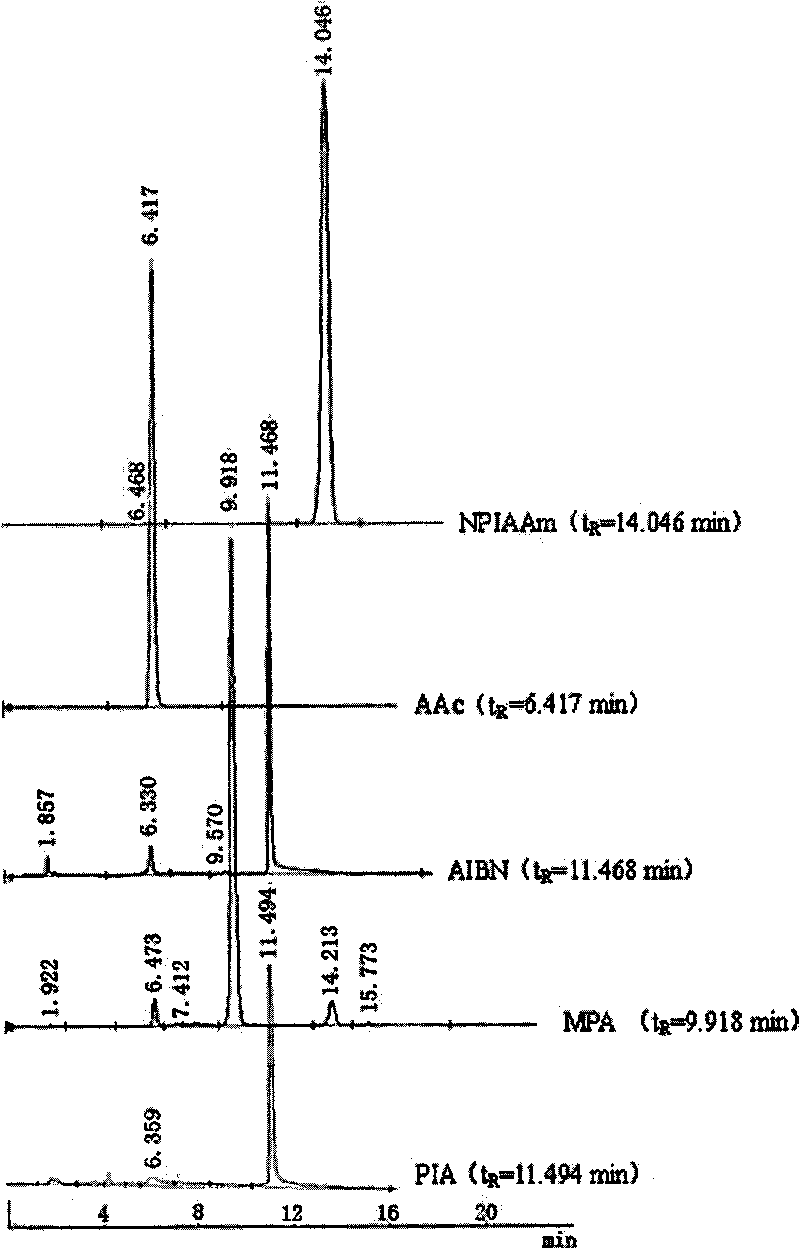
Preparation method for temperature sensing cornea epithelial cell culture material and application
InactiveCN101701207APrevent functional impairmentGood biocompatibilityArtificial cell constructsVertebrate cellsBiocompatibility TestingPolystyrene
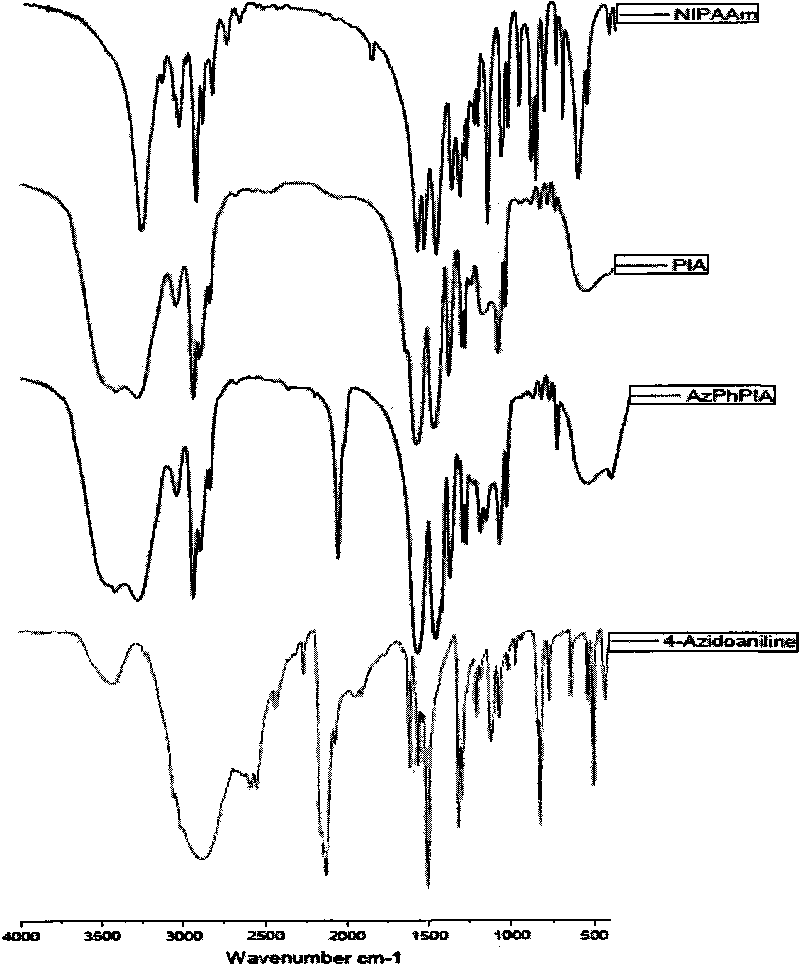
The invention discloses a preparation method for temperature sensing cornea epithelial cell culture material and application. The method is as follows: combining nitrine-aniline derivative of N- isopropyl acrylamide / acrylic acid, and then fixing the nitrine aniline derivative of N-isopropyl acrylamide / acrylic acid on a polystyrene board used for tissue culture by a UV-light chemical graft method, thus obtaining the culture material. The invention also discloses the application of the temperature sensing cornea epithelial cell culture material in culturing human corneal epithelial cells. The temperature sensing cornea epithelial cell culture material prepared by the preparation method has good plasticity and biocompatibility, can support the good growth of the cornea epithelial cells, does not cause inflammatory reaction or cell functional damage, and has good clinical application prospect.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Tissue engineered cornea
InactiveCN107929811AMaintain propertiesAnti-fibrosis propertiesTissue regenerationProsthesisCell-Extracellular MatrixECM Protein
The invention discloses a tissue engineered cornea. The tissue engineered cornea comprises at least three amnion tissue layers, wherein corneal stromal cells are grown between two adjacent amnion tissue layers, extracellular matrixes which are secreted by the corneal stromal cells and contain keratocan, lumican, I type collagen and V type collagen are also contained, and the extracellular matrixesare regularly arranged, so that a tissue structure similar to corneal stroma is formed. Corneal epithelial cells and corneal endothelial cells are respectively inoculated on the upper surface and thelower surface of the structure, so that a total cornea is formed. Surface collagenous fibers of the amnion tissue layers are regularly arranged, are parallel to each other and are transversally intersected and closely arranged to form a fiber sheet, so that the corneal stromal cells and the extracellular matrixes secreted by the corneal stromal cells are guided to be regularly arranged; and at least three amnion tissue layers have the characteristic of inhibiting fibrosis, so that the characteristics of the corneal stromal cells are maintained, and differentiation of the corneal stromal cellsto fibroblasts is inhibited.
Owner:XIAMEN UNIV
Ocular tear growth factor-like protein
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Applications of human placenta peptide in preparing drugs for treating dry eye syndrome and other ocular surface diseases
InactiveCN101947307AReduce the risk of infectionEnsure safetySenses disorderPeptide/protein ingredientsNew zealand whiteSecretion
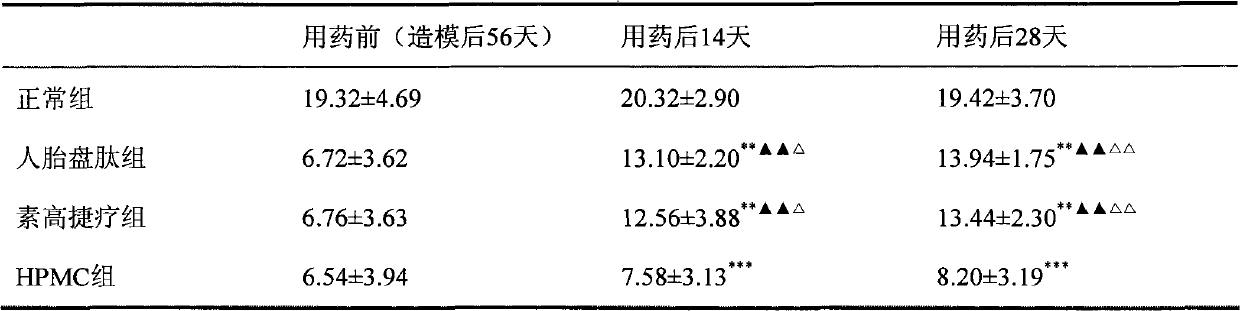
The invention discloses applications of human placenta peptide in preparing drugs for treating simple keratitis, dry eye syndrome, corneal epithelium erosion, corneal alkali burn, various keratititides, LASIK corneal laser surgery and other ocular surface diseases. The experiment proves that the human placenta peptide can increase the tear liquid secretion amount of a dry eye model of New Zealand white rabbits, promote the repairation of corneal epithelium cell damage, increase the quantity of conjunctiva goblet cells, has no stimulation to eyes, and has therapeutic effect on the dry eye model of the New Zealand white rabbit with obvious curative effect, thus the human placenta peptide can be used for treating the dry eye syndrome, the corneal epithelium erosion, corneal epithelium defect, the corneal alkali burn, keratitis and the LASIK corneal laser surgery and the other ocular surface diseases.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency
InactiveCN105688282ACurvature maintenanceQuick fitEye implantsTissue regenerationDepyrogenationBiocompatibility
The invention relates to a novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency. The novel biological artificial cornea is prepared with a method in the steps as follows: 1, obtaining of a cornea raw material; 2, preparation of a cornea scaffold; 3, deep modification of the cornea scaffold; 4, deep inactivation of the cornea for removal of pyrogen; 5, irradiation sterilization of the cornea; 6, cultivation and implantation of corneal endothelial cells. Deep and light lamellar biological artificial corneas with different thicknesses and a full-thickness biological artificial cornea can be established. After being implanted, the novel biological artificial cornea can be quickly healed, becomes transparent quickly and keeps good refraction, the eyesight can be recovered and the eyeball is beautified. The obtained biological artificial cornea has good mechanical performance and biocompatibility, a full-thickness cornea graft is formed after endothelial cells are implanted on a full-thickness cornea stroma scaffold and can be rebuilt in vivo, perform in-vivo induction to promote growth of corneal limbal stem cells and growth of corneal epithelial cells and become transparent quickly on the basis, the treatment efficiency and effects are improved, and an application of lamellar keratoplasty and an application of penetrating keratoplasty are both considered.
Owner:广州宏畅生物科技有限公司
Corneal contact lens nursing composition containing trehalose quaternary ammonium salt
InactiveCN103083710AReduce drynessRelieve sorenessSpectales/gogglesBiocideEscherichia coliMortality rate
A corneal contact lens nursing composition containing trehalose quaternary ammonium salt is provided; the corneal contact lens nursing composition is characterized by consisting of the following components in percentage by weight: 0.001-2% of trehalose quaternary ammonium salt, 0.01-2% of isotonic regulator, 0.05-2% of surfactant, 0.01-1% of complexing agent, 0.01-3% of buffer, 0.00001-1% of anti-microorganism compound and 89-99.91899% of water. The composition provided by the invention has corneal contact lens storing, sterilizing, cleaning, lubricating, hydrating and anti-drying functions and is very effective for relieving dryness and stiffness of eyes; by adopting the composition, the death rate of corneal epithelial cells under a dry condition is reduced and the repairing of corneal epithelial cells and endothelial cells is prompted. The kill rate of each composition to escherichia coli, pseudomonas aeruginosa and staphylococcus aureus is greater than 99.9% and the kill rate to candida albica and solanaceae fusarium is greater than 90.0%.
Owner:JIANGSU HORIEN CONTACT LENS
Corneal epithelial sheet, method of constructing the same and transplantation method using the sheet
InactiveCN1929877AImprove securityImprove survivabilityNervous system cellsArtificial cell constructsFibroblastTherapeutic effect
A corneal epithelial sheet having potential for the achievement of a favorable therapeutic effect and being highly safe in transplantation. The corneal epithelial sheet is constructed by: (a) preparing corneal epithelial cells; (b) separately culturing human fibroblasts in a collagen gel; (c) sowing or placing the corneal epithelial cells on the collagen gel; and (d) culturing and proliferating the corneal epithelial cells in the absence of xenogeneic animal cells.
Owner:阿如布勒斯特有限公司 +1
Use of lacritin in promoting ocular cell survival
The present invention relates to the use of lacritin, and fragments, derivatives, and homologs thereof, and nucleic acid sequences encoding that protein. The invention relates to the use of lacritin as a growth factor human corneal epithelial cells and other select epithelial cells. The invention also relates to the use of lacritin in promoting ocular cell survival in response to an insult or injury, in protecting against ocular inflammation, and in promoting ocular wound repair.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
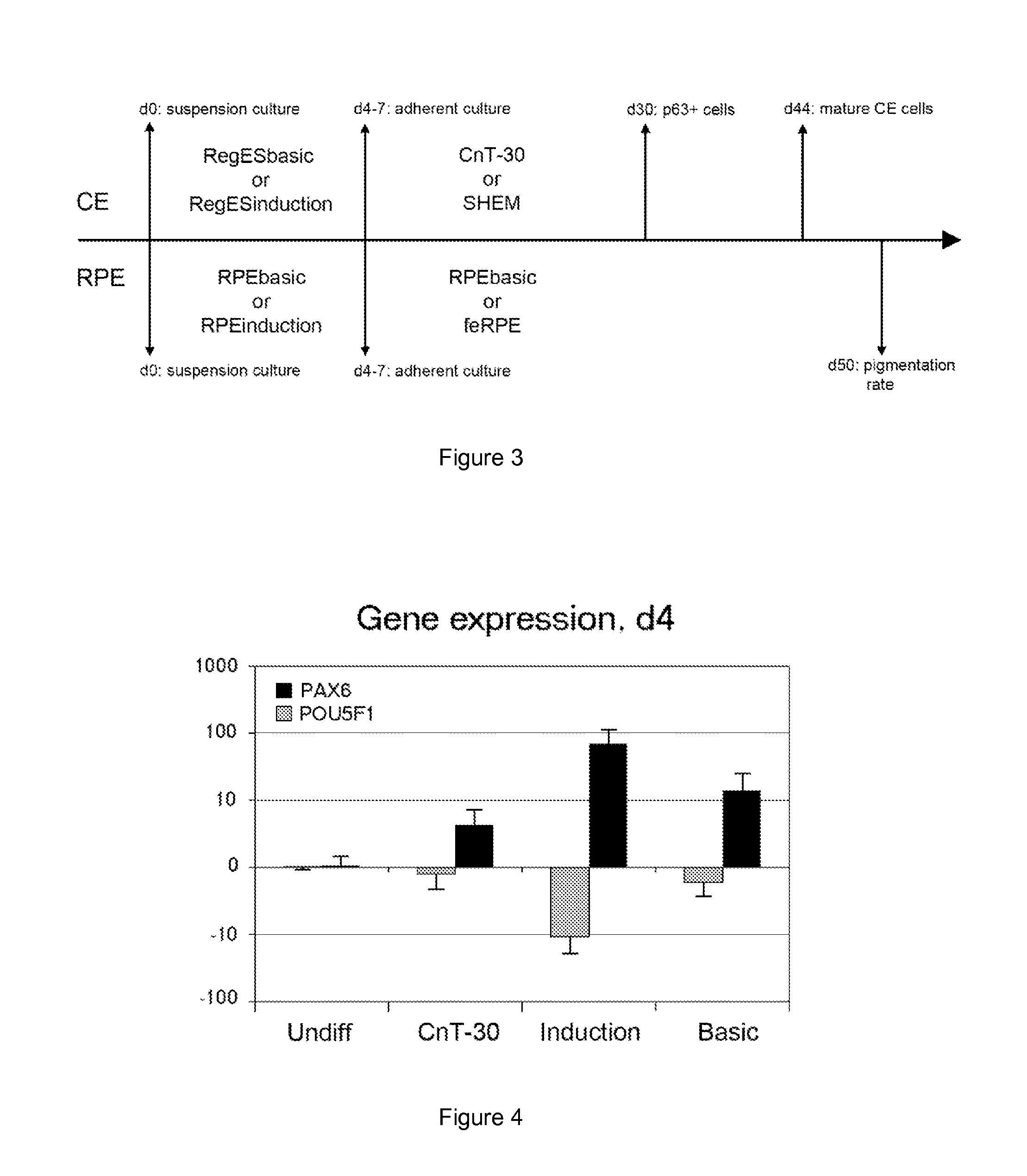
Methods and media for differentiating eye cells
Here is provided a novel differentiation protocol, which was experimentally shown to give rise to corneal epithelial precursor cells or early pigmented RPE precursor cells in defined and xeno-free conditions. The early precursor cells may be further maturated towards corneal epithelium cells, stratified corneal epithelium or mature RPE cells. Such cells may contribute to treatment and research of corneal and retinal conditions, diseases, pathologies as well as toxicology and drug development.
Owner:UNIVERSITY OF TAMPERE
Ips cell having differentiation propensity for corneal epithelium
InactiveCN103492555AImprove securityEasy to getGenetically modified cellsEpidermal cells/skin cellsInduced pluripotent stem cellStromal cell
The main purpose of the present invention is to provide a method for inducing the differentiation into a corneal epithelial stem cell and / or a corneal epithelial cell, which can be utilized for the production of a corneal epithelial cell sheet that has higher safety from the viewpoint of the possibility of occurrence of vascularization and the like and that can be produced readily. A method for inducing the differentiation of a pluripotent stem cell into a corneal epithelial stem cell and / or a corneal epithelial cell comprises a step of culturing the pluripotent stem cell in the presence of a stromal cell or an amnion-derived factor.
Owner:OSAKA UNIV
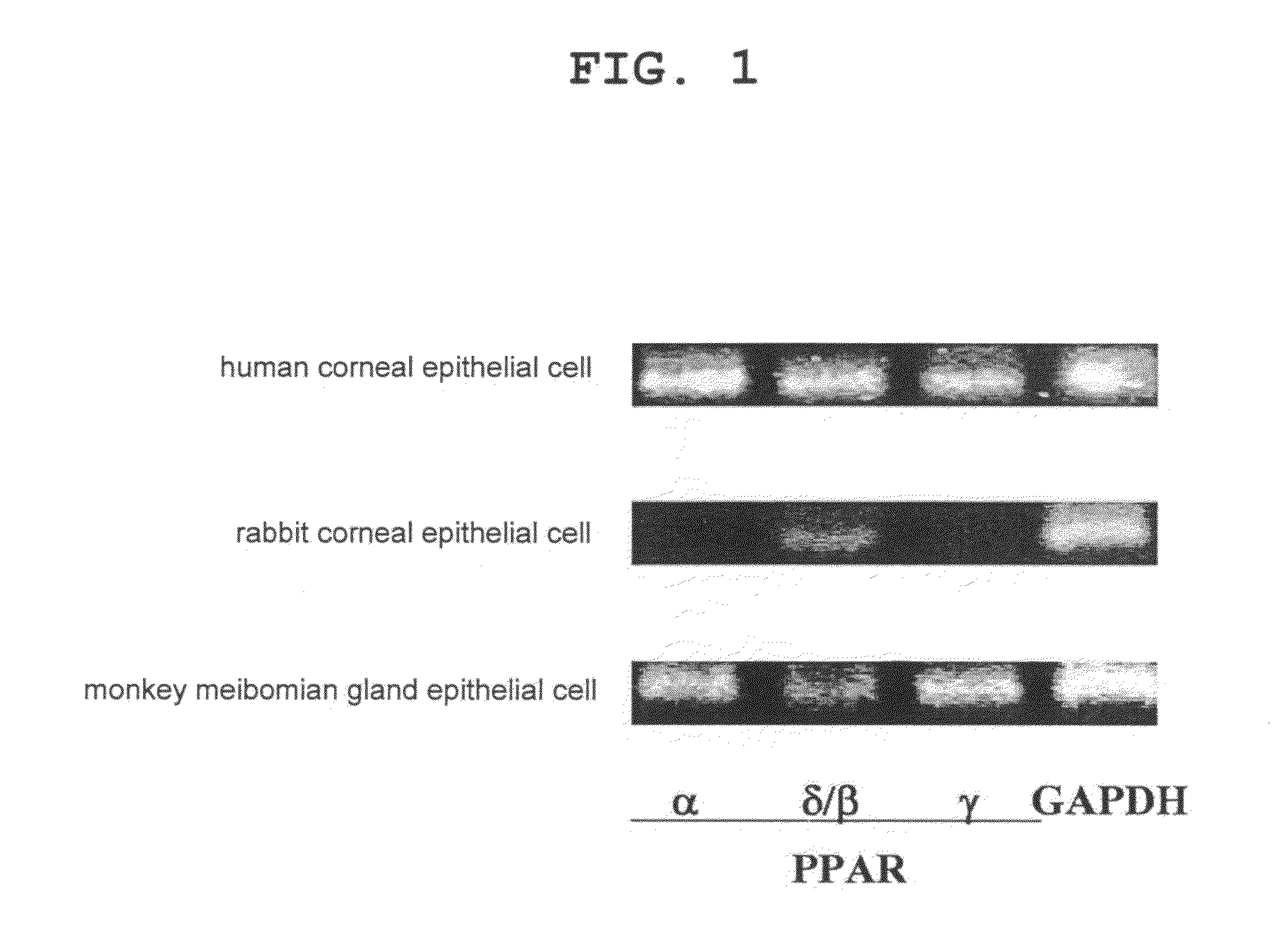
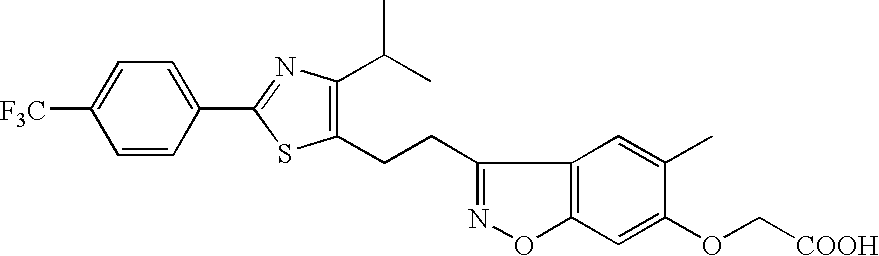
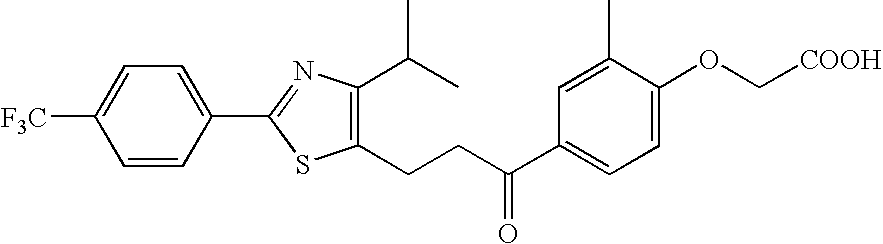
Pharmaceutical containing ppara agonist
Provision of an agent for promoting proliferation of meibomian gland epithelial cells or corneal epithelial cells, and a therapeutic agent for ocular diseases such as meibomian gland dysfunction, dry eye and the like.A preparation containing [3-[2-[4-isopropyl-2-(4-trifluoromethyl)phenyl-5-thiazolyl]ethyl]-5-methyl-1,2-benzisoxazol-6-yl]oxyacetic acid, [4-[3-[2-(4-trifluoromethyl)phenyl-4-isopropyl-5-thiazolyl]propionyl]-2-methylphenoxy]acetic acid or [4-[3-[2-(2-hydroxy-4-chlorophenyl)-5-isopropyl-4-oxazolyl]propionyl]-2-methylphenoxy]acetic acid, or a pharmacologically acceptable salt thereof as an active ingredient is used as an agent for promoting proliferation of meibomian gland epithelial cells or corneal epithelial cells, as well as a therapeutic agent for ocular diseases such as meibomian gland dysfunction, dry eye and the like.
Owner:NIPPON CHEMIPHAR CO LTD +1
Suture-resistant collagen-based corneal regeneration and repair material and preparation method thereof
ActiveCN110404110ALow costSimple molding processTissue regenerationProsthesisCross-linkComposite film
The invention belongs to the field of biomedical materials, and discloses a suture-resistant collagen-based corneal regeneration and repair material and a preparation method thereof. The preparation method comprises the following steps: adding collagen to hydrochloric acid to dissolve collagen, then adding an EDC / NHS cross-linking agent for a cross-linking reaction, heating and concentrating to obtain a cross-linked collagen solution; defoaming, adding the defoamed product to a petri dish to reach a certain thickness, then tiling a PCL circular ring to the bottom of the cross-linked collagen solution, air-drying, and washing to obtain a composite film; and hot-pressing the composite film in a die at 100-120 DEG C for secondary molding to obtain the suture-resistant collagen-based corneal regeneration and repair material. The suture-resistant collagen-based corneal regeneration and repair material obtained by the invention has good biocompatibility and is resistant to conventional suture during surgery. In addition, corneal epithelial cells can adhere to and proliferate on the surface of the material. Thereby, rapid epithelization of the corneal epithelial cells on the graft duringsurgery is realized, and finally the purpose of corneal defect repair and corneal replacement is achieved.
Owner:SOUTH CHINA UNIV OF TECH
Method for large-scale preparation of high-purity unsaturated hyaluronic acid disaccharide
ActiveCN110982862AHigh purityLess impuritiesSugar derivativesDisaccharidesOrganic solventHyaluronidase
The invention discloses a method for large-scale preparation of high-purity unsaturated hyaluronic acid disaccharide. The method adopts two-step enzymolysis and two-step purification, and specificallyincludes the steps of: firstly, carrying out enzymolysis on macromolecular hyaluronic acid or salt thereof by hyaluronidase, purifying the obtained product with a low organic solvent multiple to obtain low-molecular-weight hyaluronic acid or salt thereof with high purity, further performing thorough enzymolysis on the low-molecular-weight hyaluronic acid or salt thereof, and purifying the obtained product with a high organic solvent multiple to obtain the high-purity unsaturated hyaluronic acid disaccharide. The method has simple technological process operation, mild conditions, high production efficiency, low energy consumption, and is suitable for large-scale industrial production. The obtained product has high purity and low cytotoxicity, has the characteristic of promoting the proliferation of human umbilical vein endothelial cells and human corneal epithelial cells, can be used as a standard substance for content and purity detection of hyaluronic acid and related products thereof, and also has wide application prospect in the field of medicines.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com