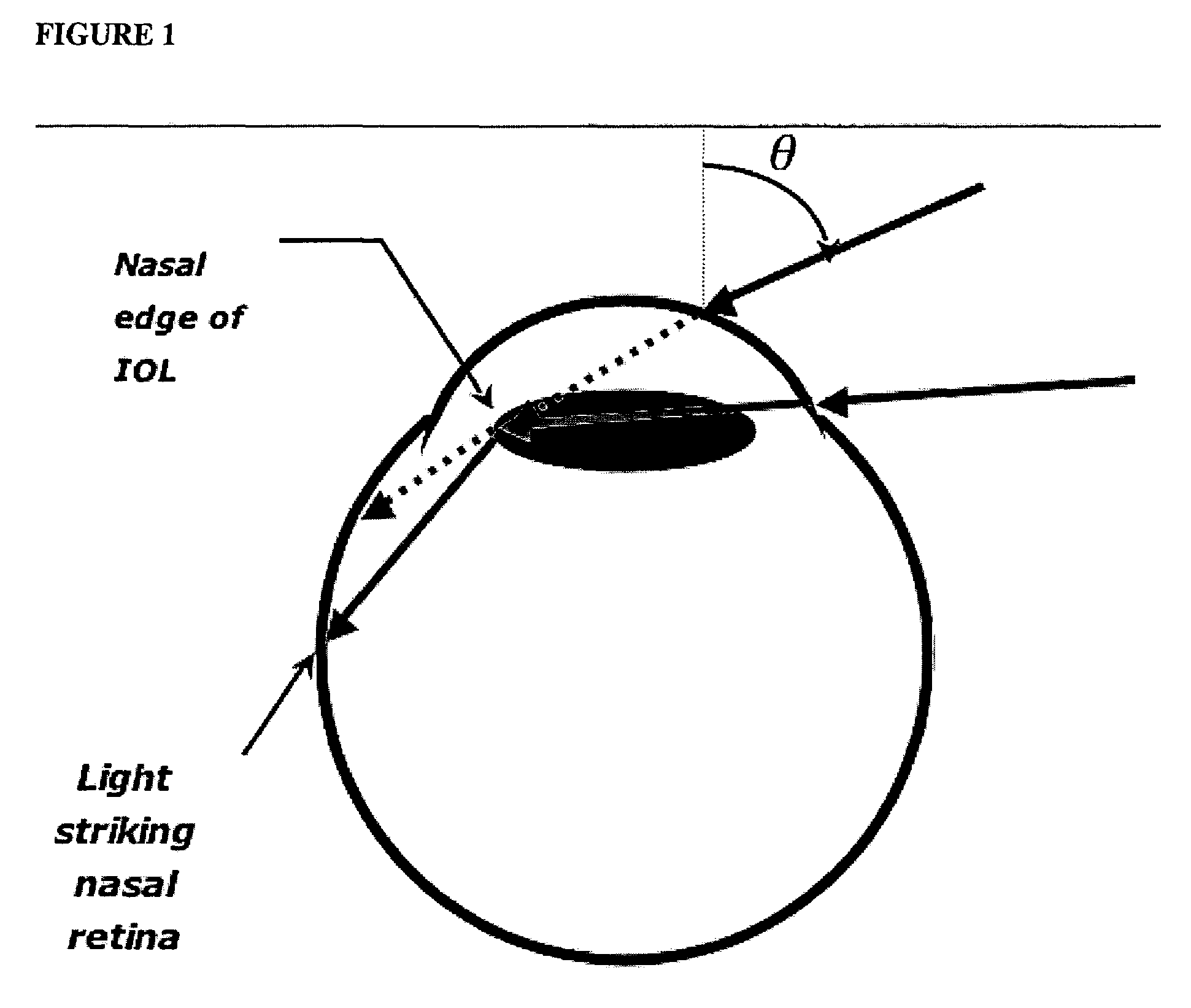
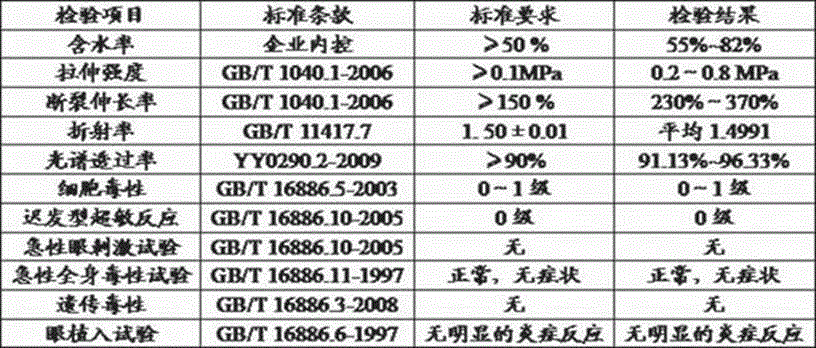
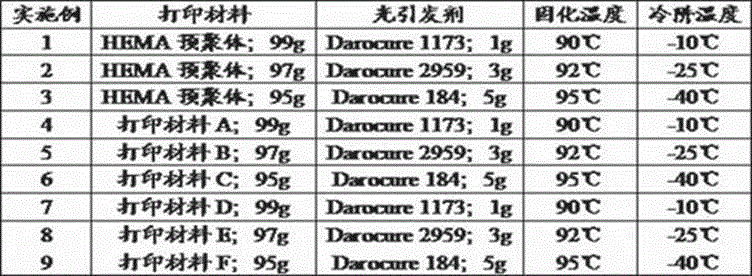
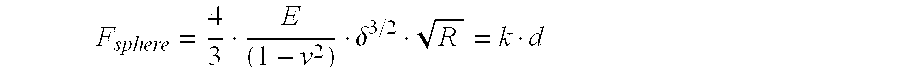Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
167 results about "Artificial cornea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
More recently, a less invasive, non-penetrating artificial cornea has been developed which can be used in more routine cases of corneal blindness. While conventional cornea transplant uses donor tissue for transplant, an artificial cornea is used in the Keratoprosthesis procedure.
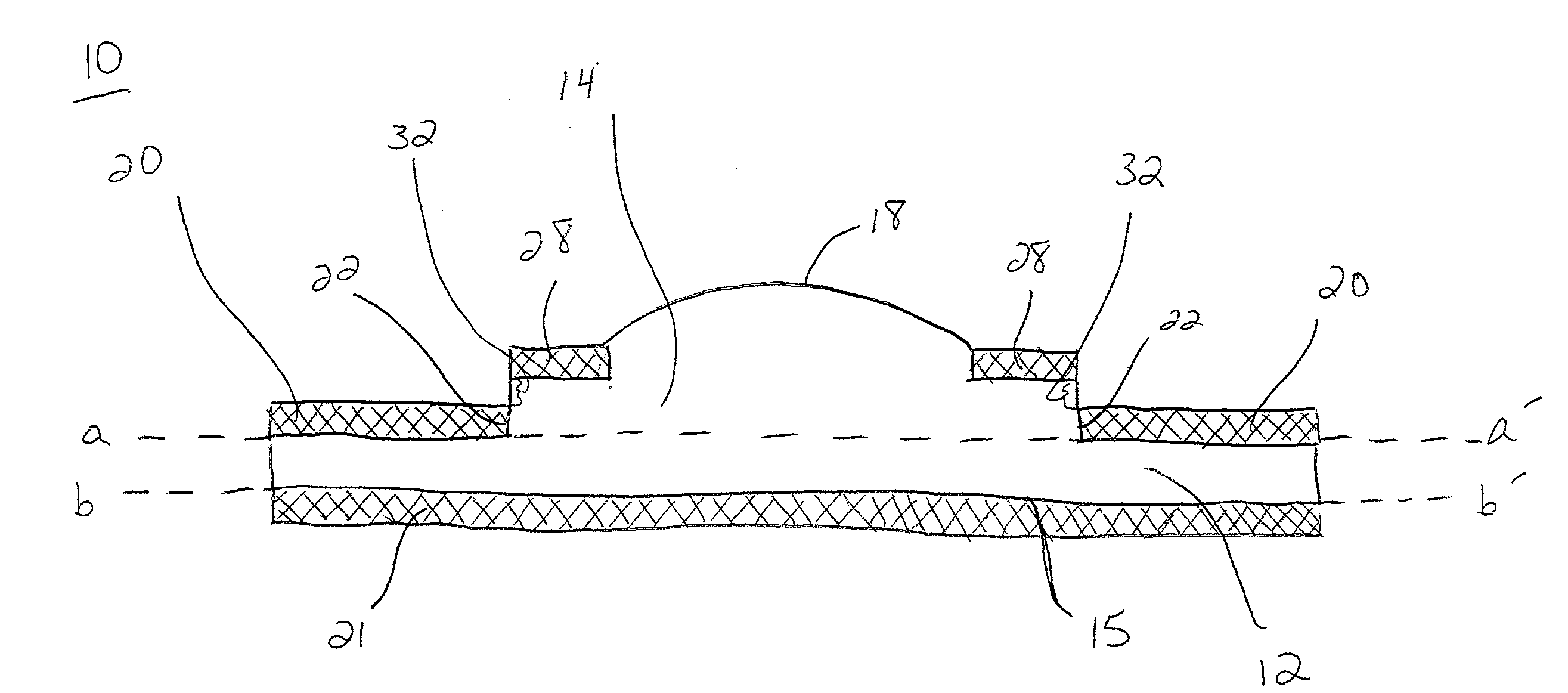
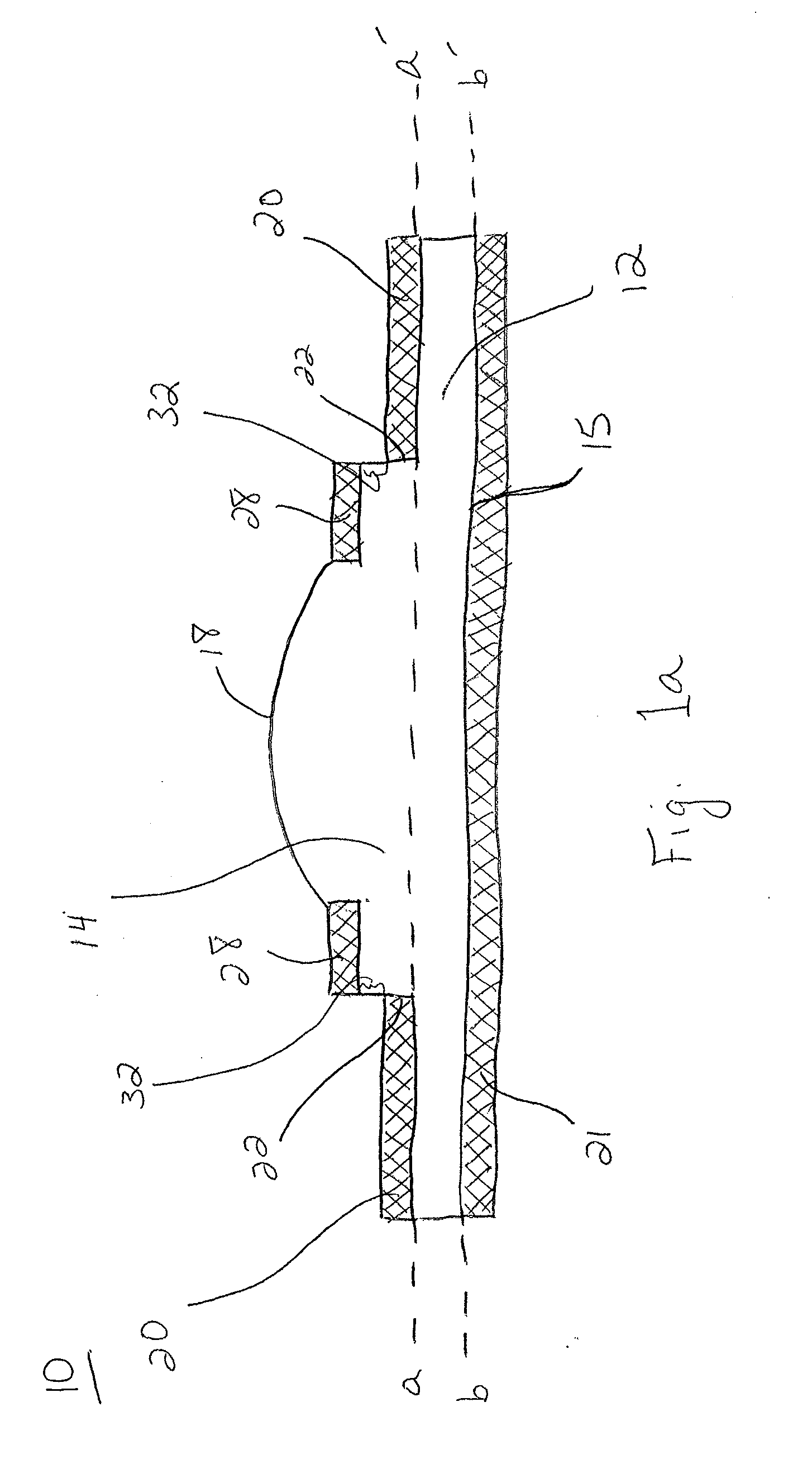
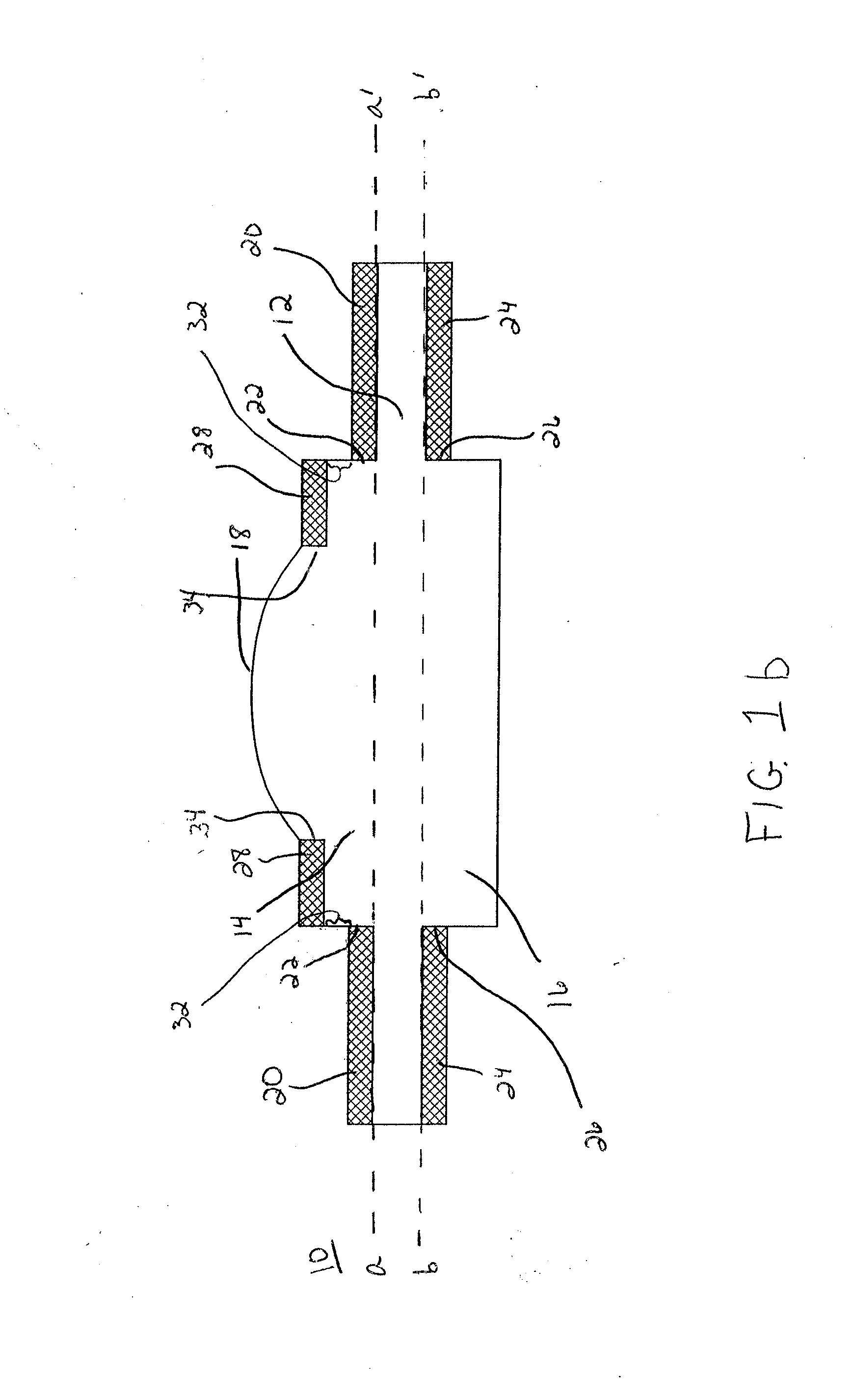
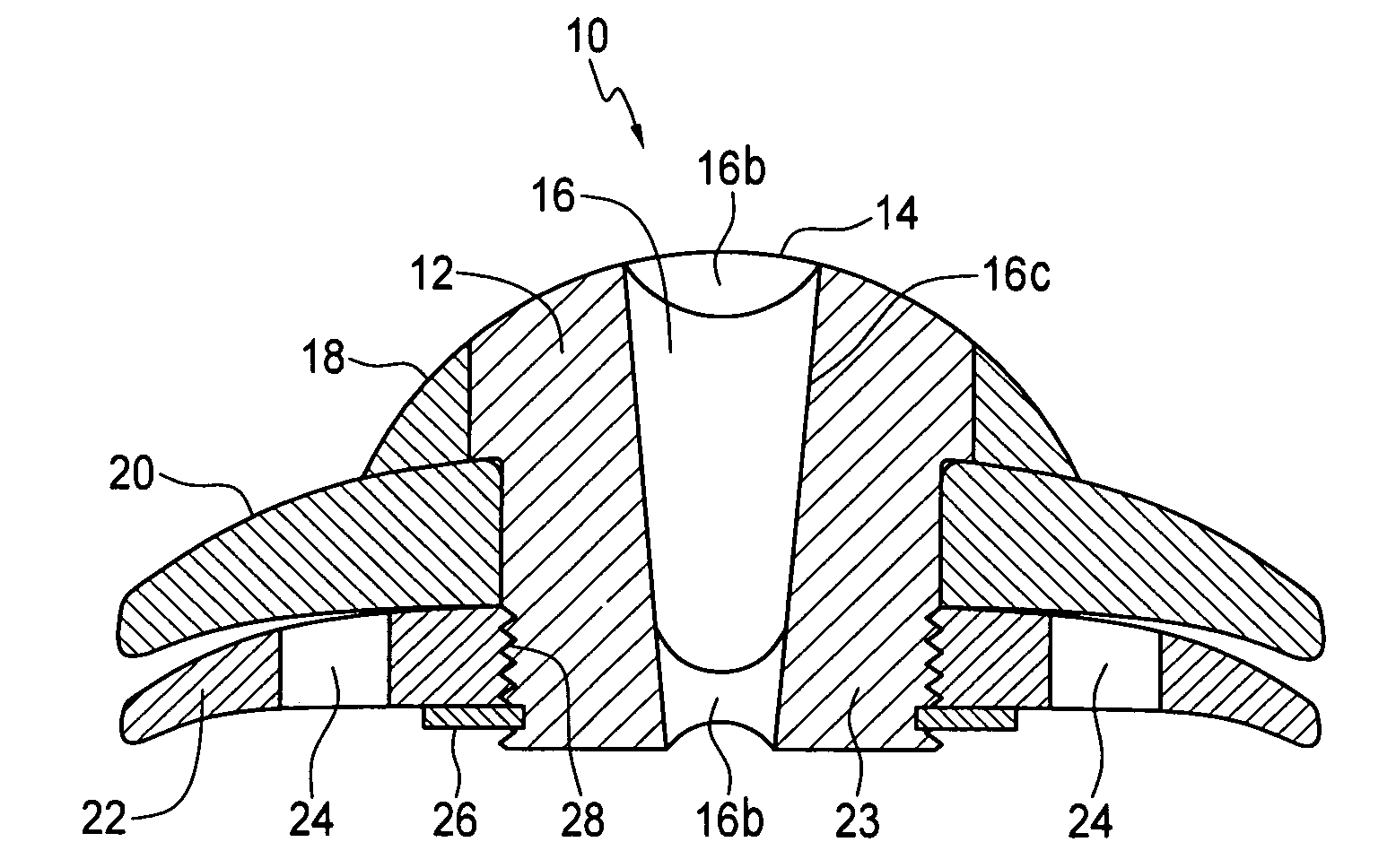
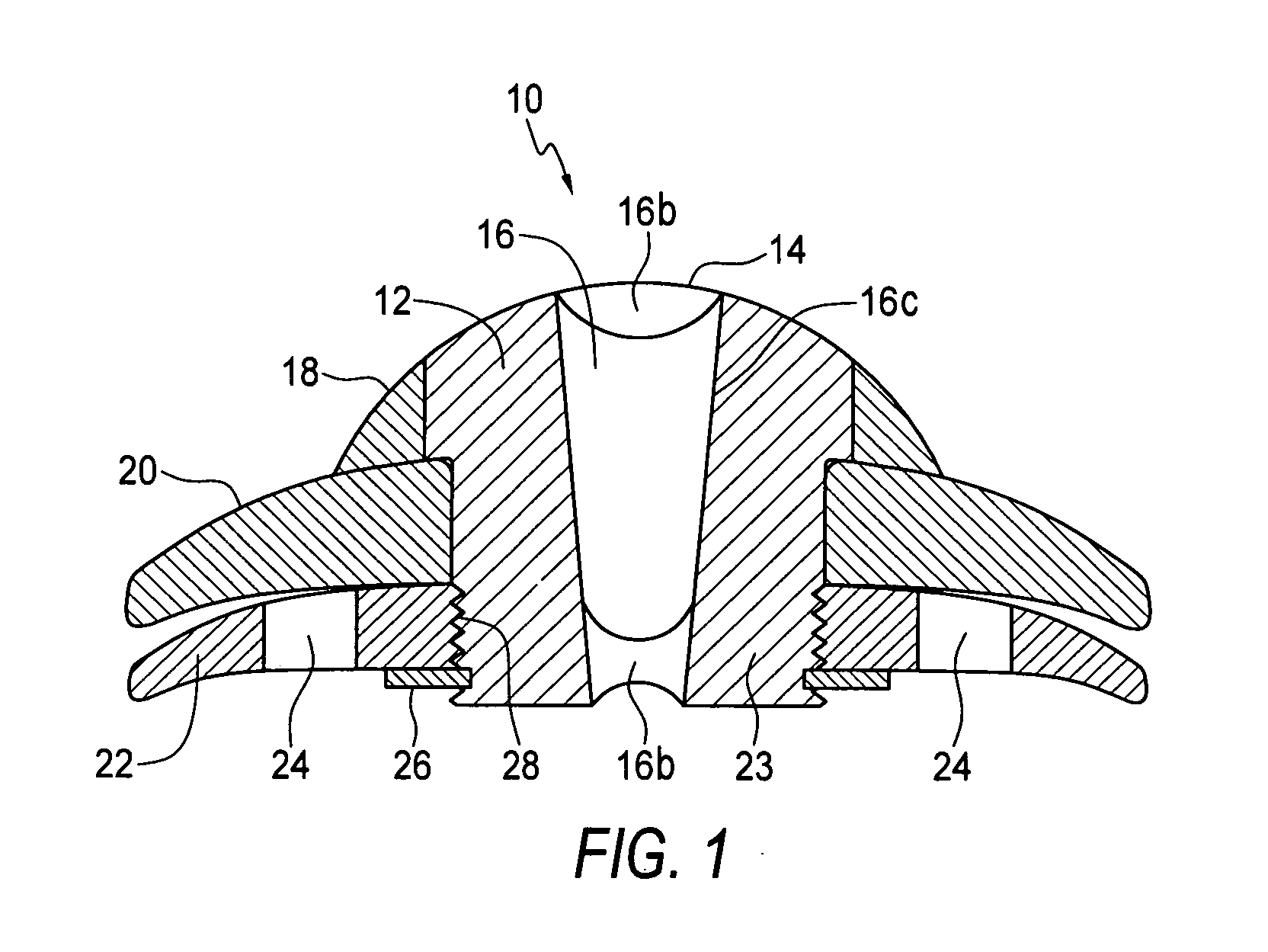
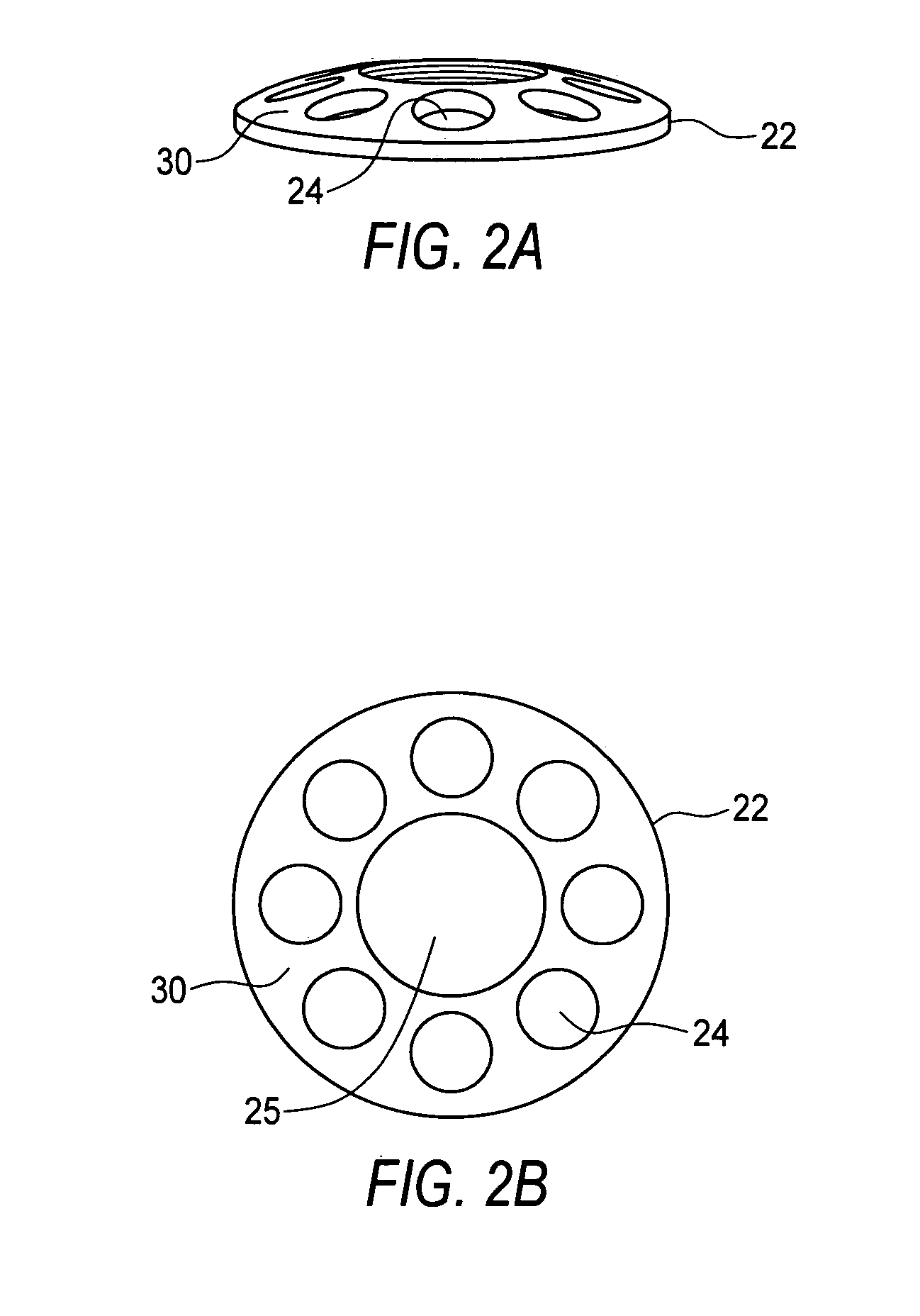
Artificial Cornea and Method of Making Same
Owner:WL GORE & ASSOC INC
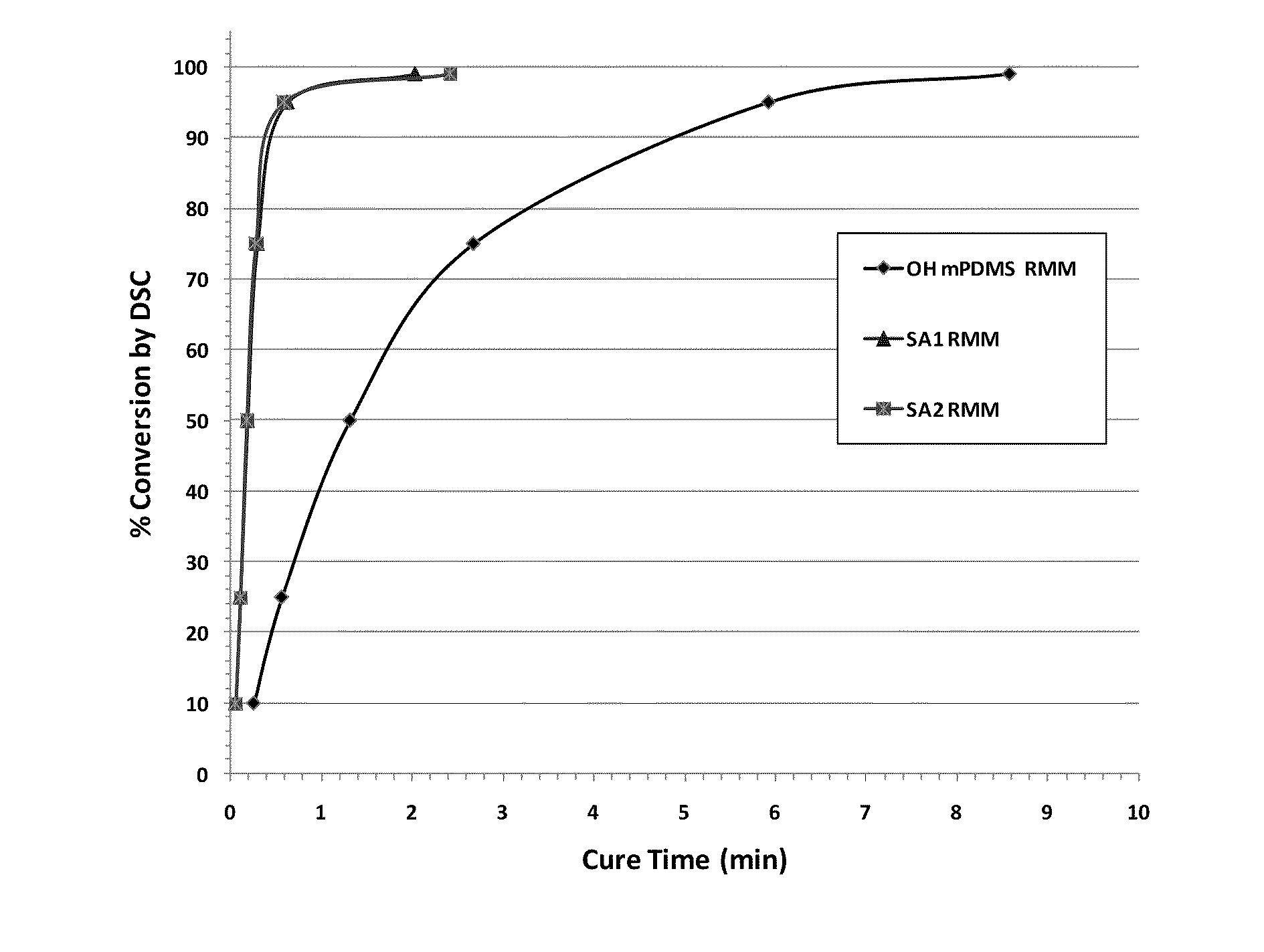
Silicone (METH)acrylamide monomer, polymer, ophthalmic lens, and contact lens
ActiveUS20110237766A1Fast aggregationPromote recoverySilicon organic compoundsCoatingsArtificial corneaContact lens
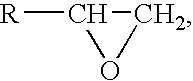
The present invention relates to a silicone (meth)acrylamide monomer, and this silicone (meth)acrylamide monomer is particularly suitable for use in contact lenses, intraocular lenses, artificial cornea, and the like.
Owner:JOHNSON & JOHNSON VISION CARE INC
Artificial cornea
InactiveUS6976997B2Improve mechanical propertiesEasy to suture onto recipient bedMaterial nanotechnologyCoatingsDiseasePostoperative inflammation
The invention provides implants suitable for use as an artificial cornea, and methods for making and using such implants. Artificial corneas having features of the invention may be two-phase artificial corneas, or may be three phase artificial corneas. These artificial corneas have a flexible, optically clear central core and a hydrophilic, porous skirt, both of which are biocompatible and allow for tissue integration. A three-phase artificial cornea will further have an interface region between the core and skirt. The artificial corneas have a high degree of ocular tolerance, and allow for tissue integration into the skirt and for epithelial cell growth over the surface of the prosthesis. The use of biocompatible material avoids the risk of disease transmission inherent with corneal transplants, and acts to minimize post-operative inflammation and so to reduce the chance or severity of tissue necrosis following implantation of the synthetic cornea onto a host eye.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Artificial corneal implant
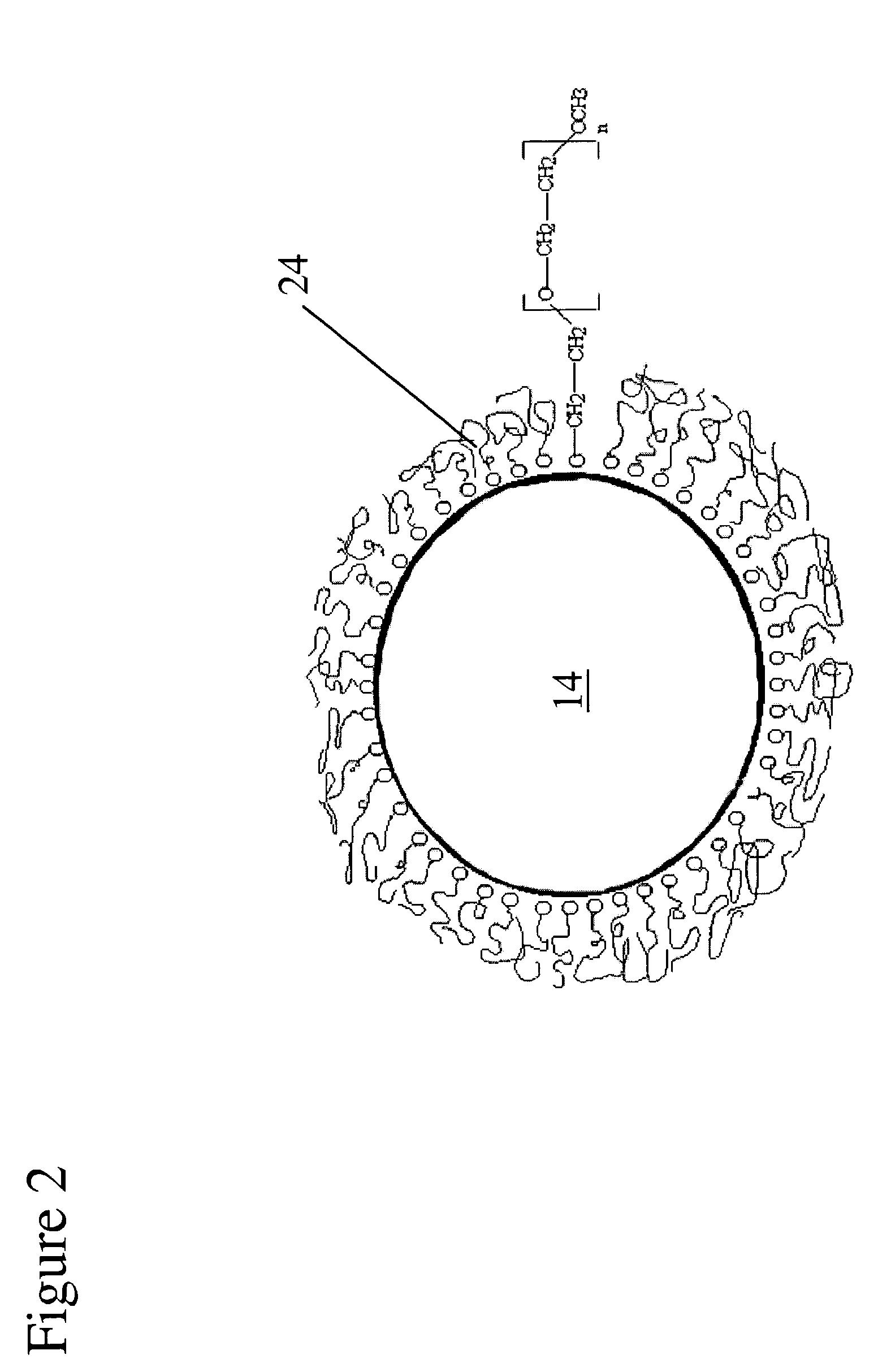
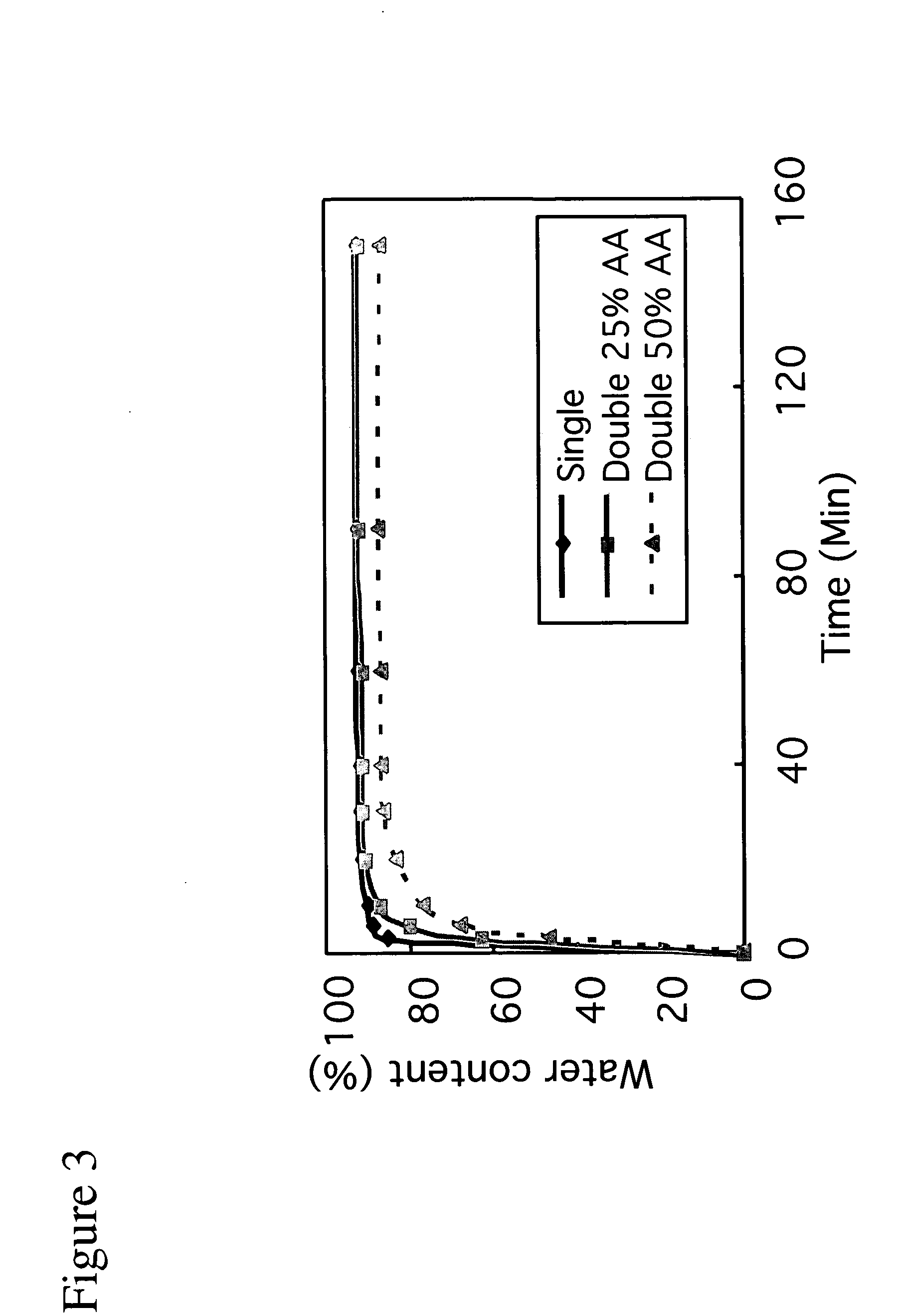
A material that can be applied as implants designed to artificially replace or augment the cornea, such as an artificial cornea, corneal onlay, or corneal inlay (intrastromal lens) is provided. The artificial corneal implant has a double network hydrogel with a first network interpenetrated with a second network. The first network and the second network are based on biocompatible polymers. At least one of the network polymers is based on a hydrophilic polymer. The artificial cornea or implant has epithelialization promoting biomolecules that are covalently linked to the surface of the double network hydrogel using an azide-active-ester chemical linker. Corneal epithelial cells or cornea-derived cells are adhered to the biomolecules. The double network has a physiologic diffusion coefficient to allow passage of nutrients to the adhered cells.
Owner:SANTA CLARA UNIVERSITY
Collagen material and its production process
InactiveUS7084082B1Avoid stickingPromote regenerationSuture equipmentsSynthetic resin layered productsEndocraniumBiocompatibility Testing
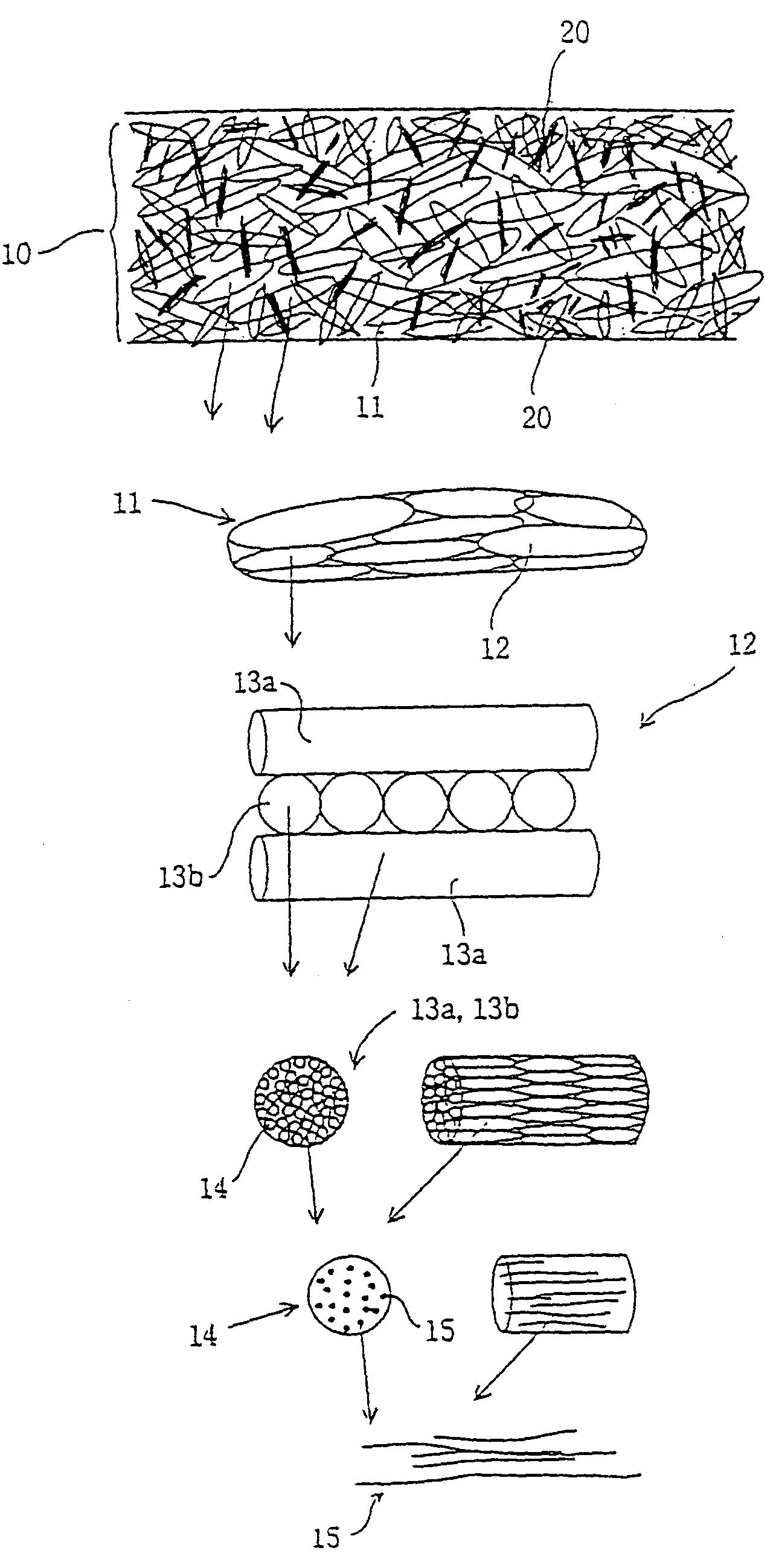
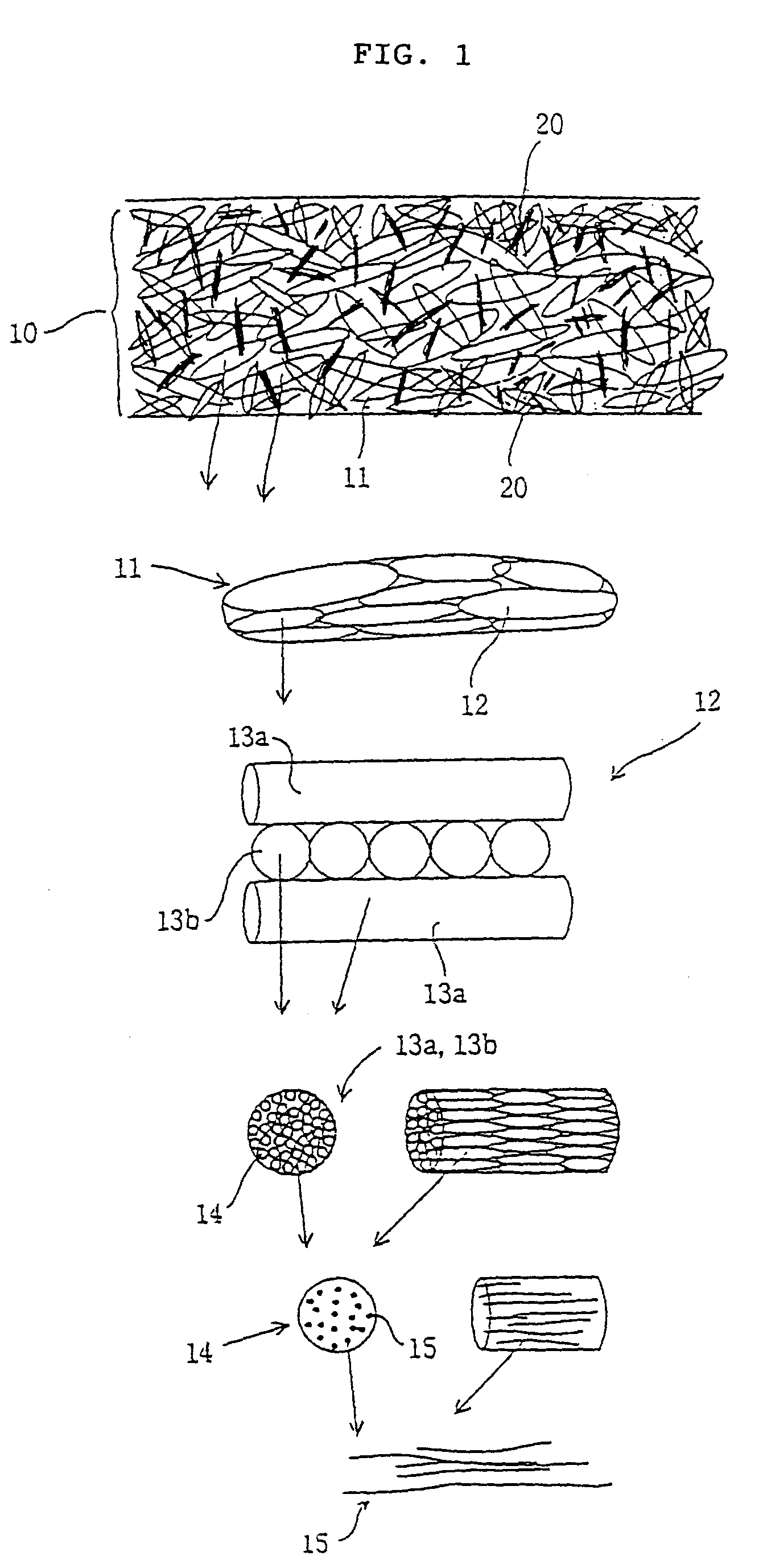
The objective of the present invention is to provide a collagen material that possesses physical properties to an extent that allows suturing while still maintaining the biochemical properties inherently possessed by collagen, and retains its shape for a certain amount of time even after application to the body; its production process; and, a medical material on which it is based, examples of which include a artificial tube for nerve, artificial tube for spinal cord, artificial esophagus, artificial trachea, artificial blood vessel, artificial valve or alternative medical membranes such as artificial endocranium, artificial ligaments, artificial tendons, surgical sutures, surgical prostheses, surgical reinforcement, wound protecting materials, artificial skin and artificial cornea, characterized by filling or having inside a substance having biocompatibility that can be degraded and absorbed in the body into a matrix of a non-woven fabric-like multi-element structure of collagen fibers having ultra-fine fibers of collagen as its basic unit.
Owner:TAPIC INT
Artificial cornea
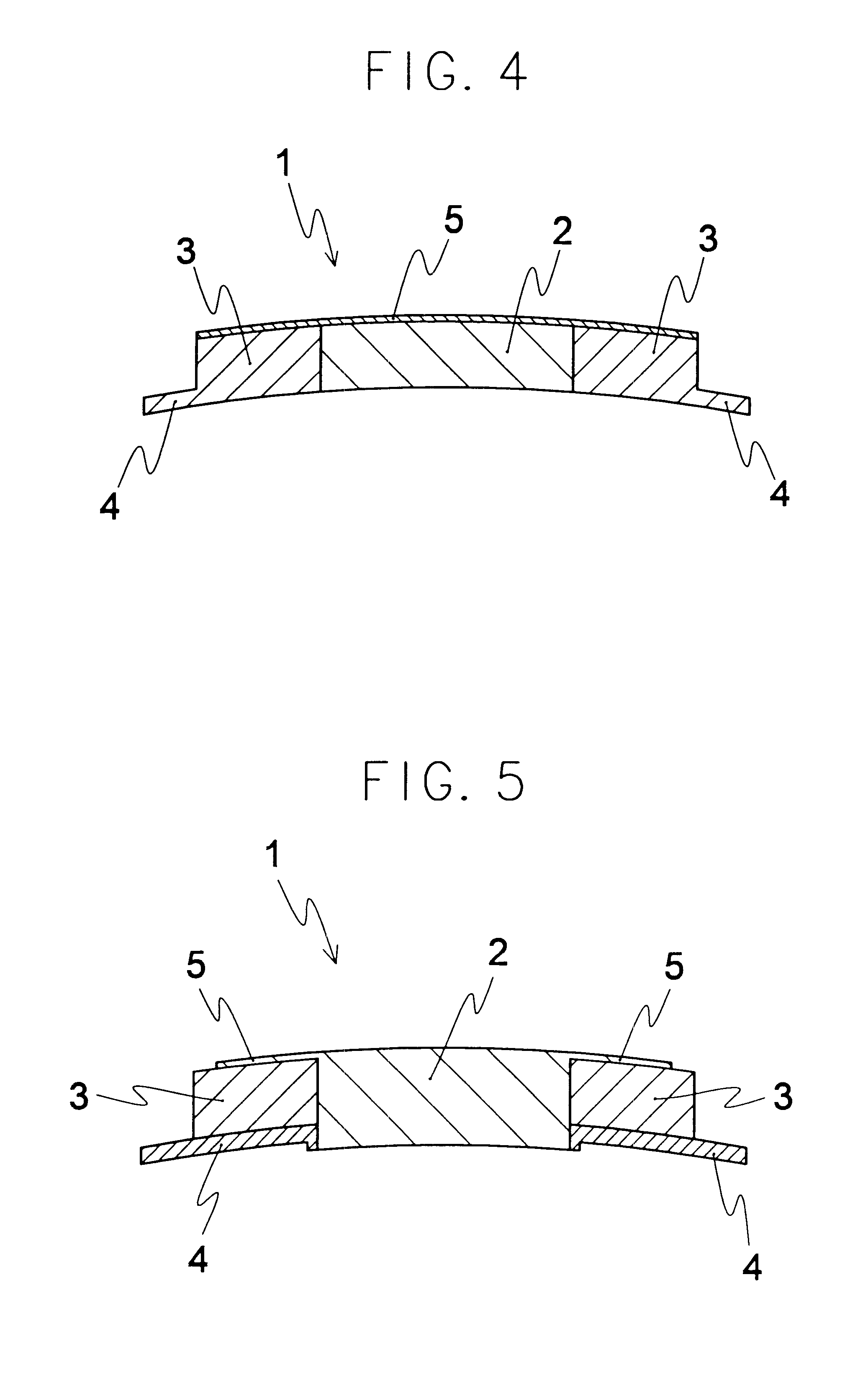
An artificial cornea comprising an optical element made of an optically transparent material, having a front surface and a posterior surface, and a skirt provided so as to support with surrounding at least a part of the optical element, characterized in that the skirt is provided with a flange on its side facing the interior of eyes during implantation of the artificial cornea and the flange radially protrudes outward from the skirt. The artificial cornea can be well compatible with ocular tissue, prevent leakage of intraocular aqueous humor and intraocular invasion of bacteria, reduce stimulation on palpebral conjunctiva and further, inhibit progression of the down growth, and which has no possibility of reduction in transparency of the optical element due to the down growth as well as detachment and extrusion from the implanted state.
Owner:MENICON CO LTD
Exogenous cornea substrate without cells and its preparation method and use
The invention provides a cell-less heterogenetic cornea material and manufacturing method and use, the manufacturing method for the product is: acquires the fresh animals' cornea at first; eliminates the cell membrane of the cornea material and nucleic acid with chemical method and enzyme method; carries on dehydration process to the cell-less heterogenetic cornea material; stores for backup. The method eliminates the antigenicity of the heterogenetic cornea material through eliminating the cell elements in the cornea material. At the same time, it reserves the integrity of glue fiber and transparency of the cornea. The material can be applied to construction of manmade cornea or used as the medical material for cornea transplantation, and cornea refraction operation directly.
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
Biological artificial cornea and method of making
InactiveUS20070142908A1Minimizing antigenHigh stability and biocompatibilityEye implantsPharmaceutical containersHuman bodyAntigen
An artificial cornea for implantation into a human body is made by a method that includes the steps of providing a natural animal cornea that has a substrate, crosslinking and fixing the substrate, minimizing the antigens from the substrate, and coupling an active layer to the substrate.
Owner:SUMMIT GD BIOTECH
Acellular cornea or acellular corneal stroma, preparation method and application thereof
InactiveCN101985051ARetain toughnessLow immunogenicityProsthesisFreeze thawingVaccine Immunogenicity
The invention discloses acellular cornea or acellular corneal stroma, a preparation method and application thereof. The method comprises the following steps of: (1) obtaining fresh animal full-thickness cornea or corneal stroma; (2) removing corneal epithelium, corneal endothelium and stroma cells, namely 1, soaking the full-thickness cornea or the corneal stroma in pure water at room temperature; 2, placing the soaked full-thickness cornea or corneal stroma into enzyme solution, digesting with oscillating, and washing with balanced salt solution with oscillating; and 3, repeating freeze-thaw processes of the full-thickness cornea or the corneal stroma for 4 to 8 times and washing with balanced salt solution with oscillating to obtain the acellular cornea or the acellular corneal stroma; (3) dehydrating; and (4) sterilizing and storing. In the method, the decellularization processing time of the cornea is short; the influence on the structure and the physiological property of the cornea is small; and the processed cornea has very low immunogenicity which is similar to the property of natural cornea. The acellular cornea or the acellular corneal stroma can be applied to artificial cornea construction of tissue engineering and also can serve as a medical material applied to corneal transplantation and refraction surgery.
Owner:JINAN UNIVERSITY
Artificial cornea
The present invention provides an artificial corneal implant having an optically clear central core and a porous, hydrophilic, biocompatible skirt peripheral to the central core. In one embodiment, the central core is made of an interpenetrating double network hydrogel and the skirt is made of poly(2-hydroxyethyl acrylate) (PHEA). In another embodiment, both the central core and the skirt are made of interpenetrating double network hydrogels. The artificial corneal implant may also have an interdiffusion zone in which the skirt component is interpenetrated with the core component, or vice versa. In a preferred embodiment, biomolecules are linked to the skirt, central core or both. These biomolecules may be any type of biomolecule, but are preferably biomolecules that support epithelial and / or fibroblast cell survival and growth. Preferably, the biomolecules are linked in a spatially selective manner. The present invention also provides a method of making an artificial corneal implant using photolithography.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Keratoprosthesis
InactiveUS20140142200A1Easy to controlImprove consistent qualityBiocideAbsorbent padsWound dressingArtificial cornea
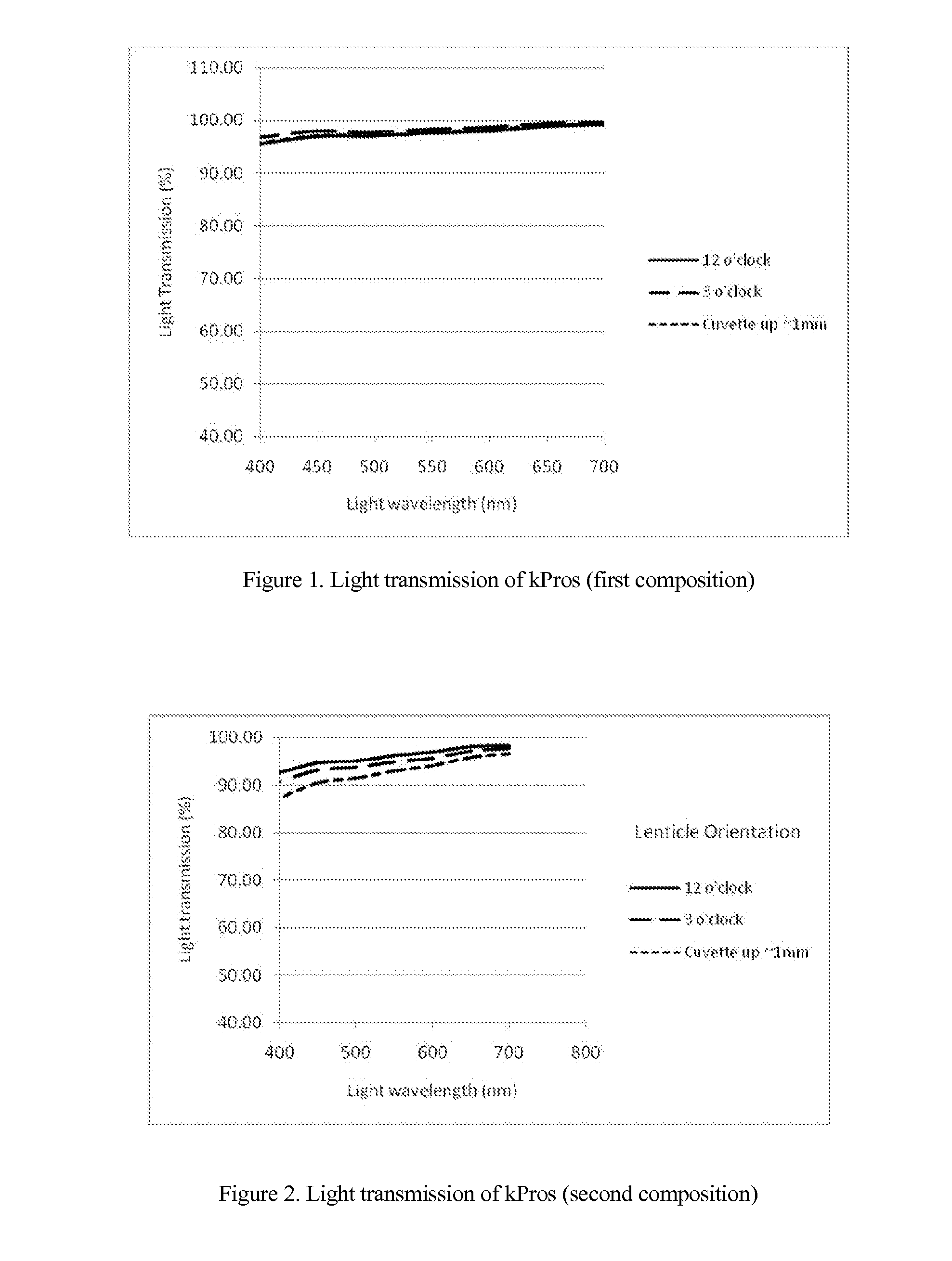
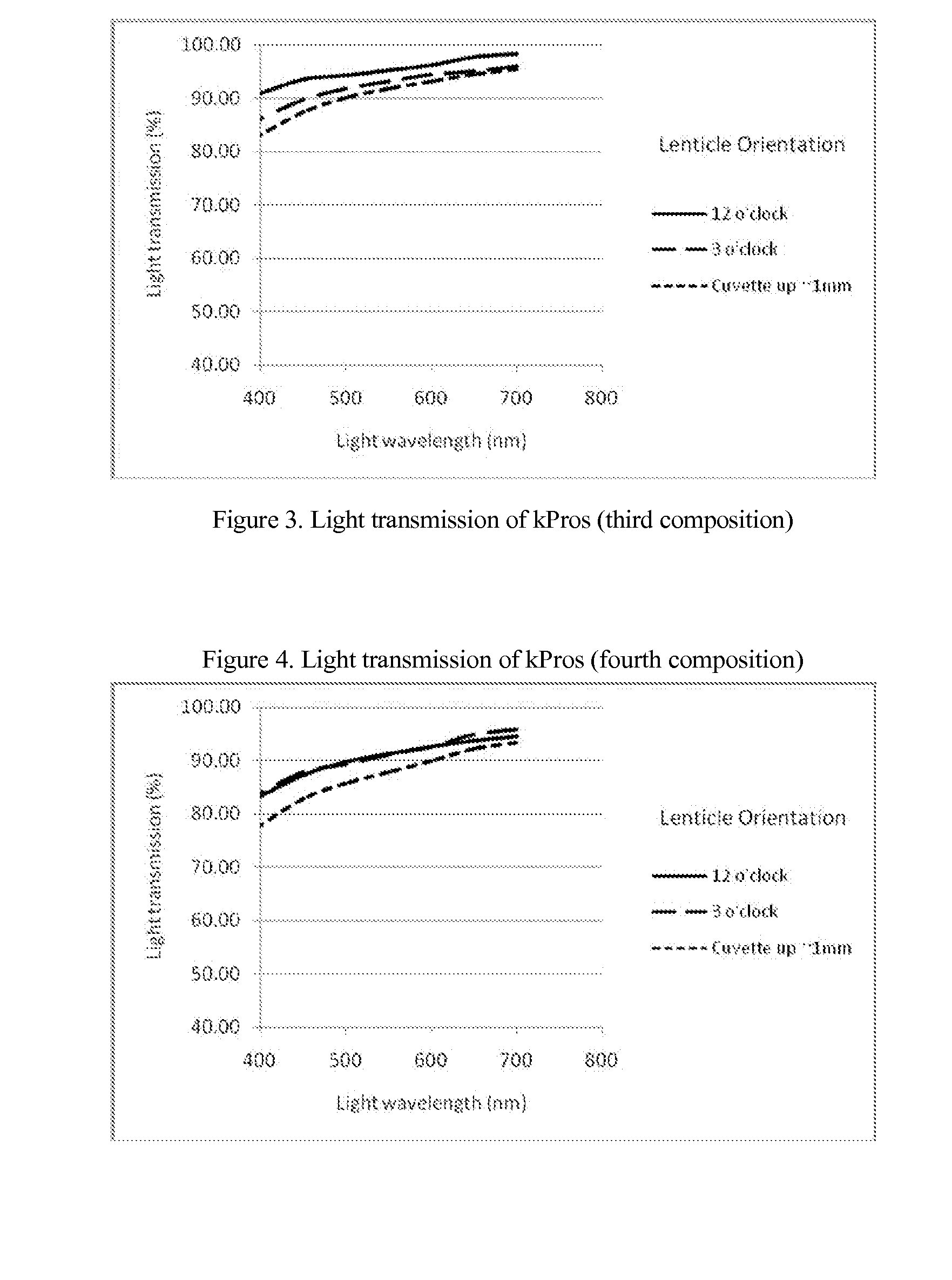
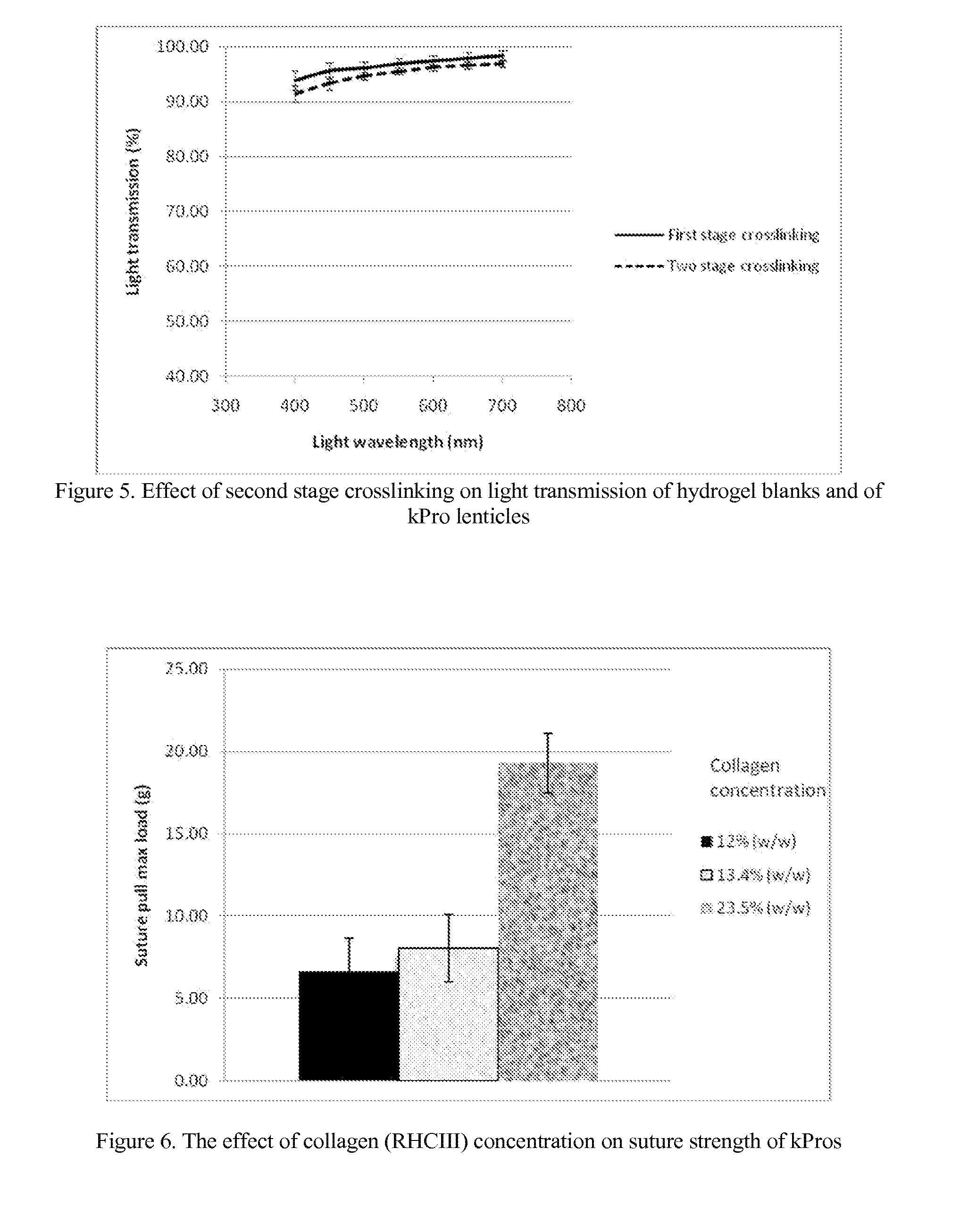
The invention comprises a method of making molded, double-crosslinked (i.e., two stages of crosslinking), transparent, collagen materials using a novel combination of diafiltration, lyophilization, and homogenization. The collagen material can be used not only as an ophthalmic device, but also as a tissue scaffold, drug delivery device, wound dressing, or other collagen hydrogel based device.
Owner:EYEGENIX
Treatment of photic disturbances in the eye
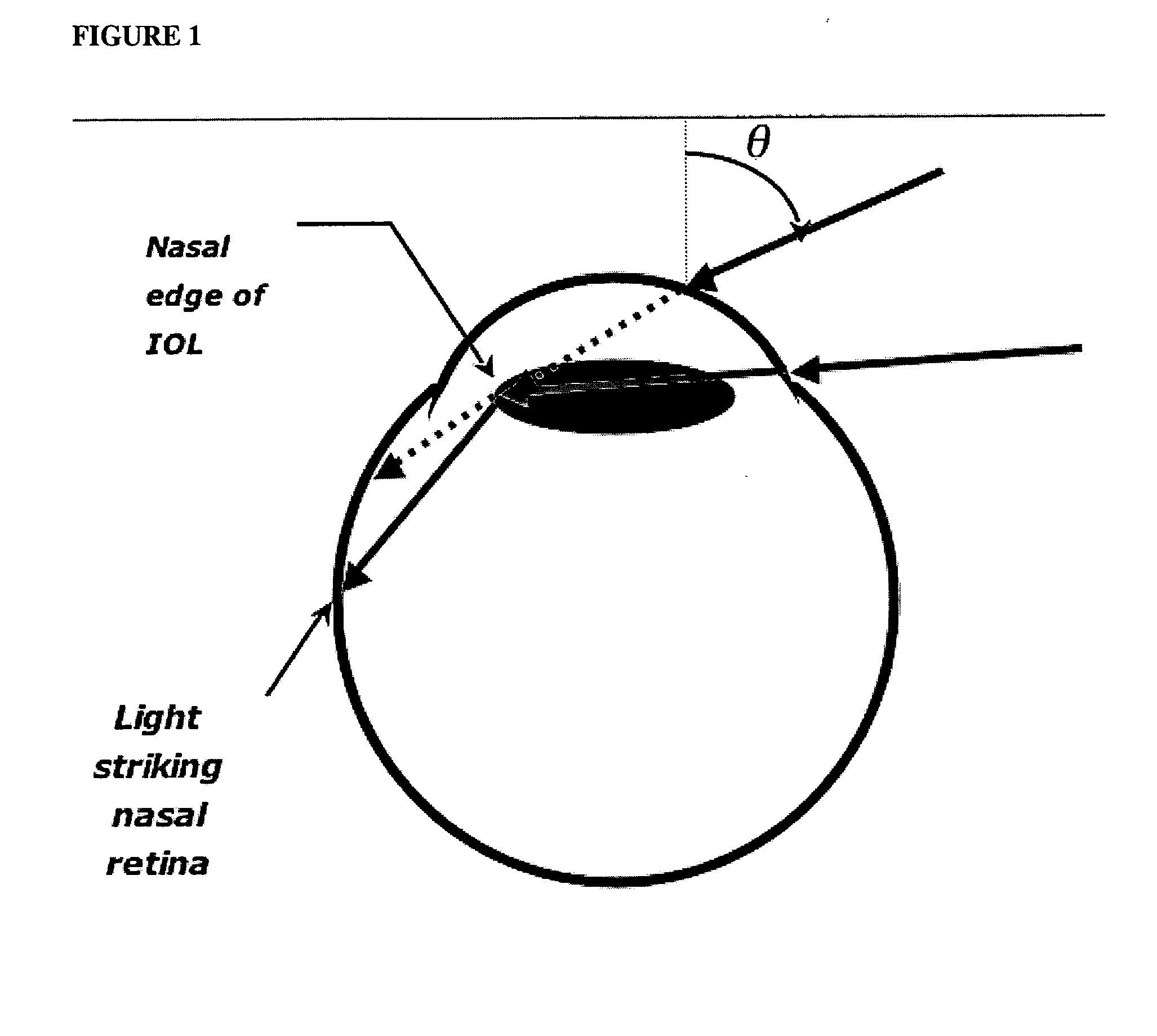
InactiveUS20050060031A1Good effectReduce and eliminate incident light photic disturbanceIntraocular lensOptical partsArtificial corneaOptometry
An ocular lens treated so that at least a portion of the lens perimeter diminishes peripheral light focus on the interior of the eye in use, so as to ameliorate photic eye disturbances. The lens perimeter of intraocular lenses, artificial corneas and contact lenses are treated to diminish peripheral light focus.
Owner:CORONCO MINAS THEODORE
Polymer having interconnected pores for drug delivery and method
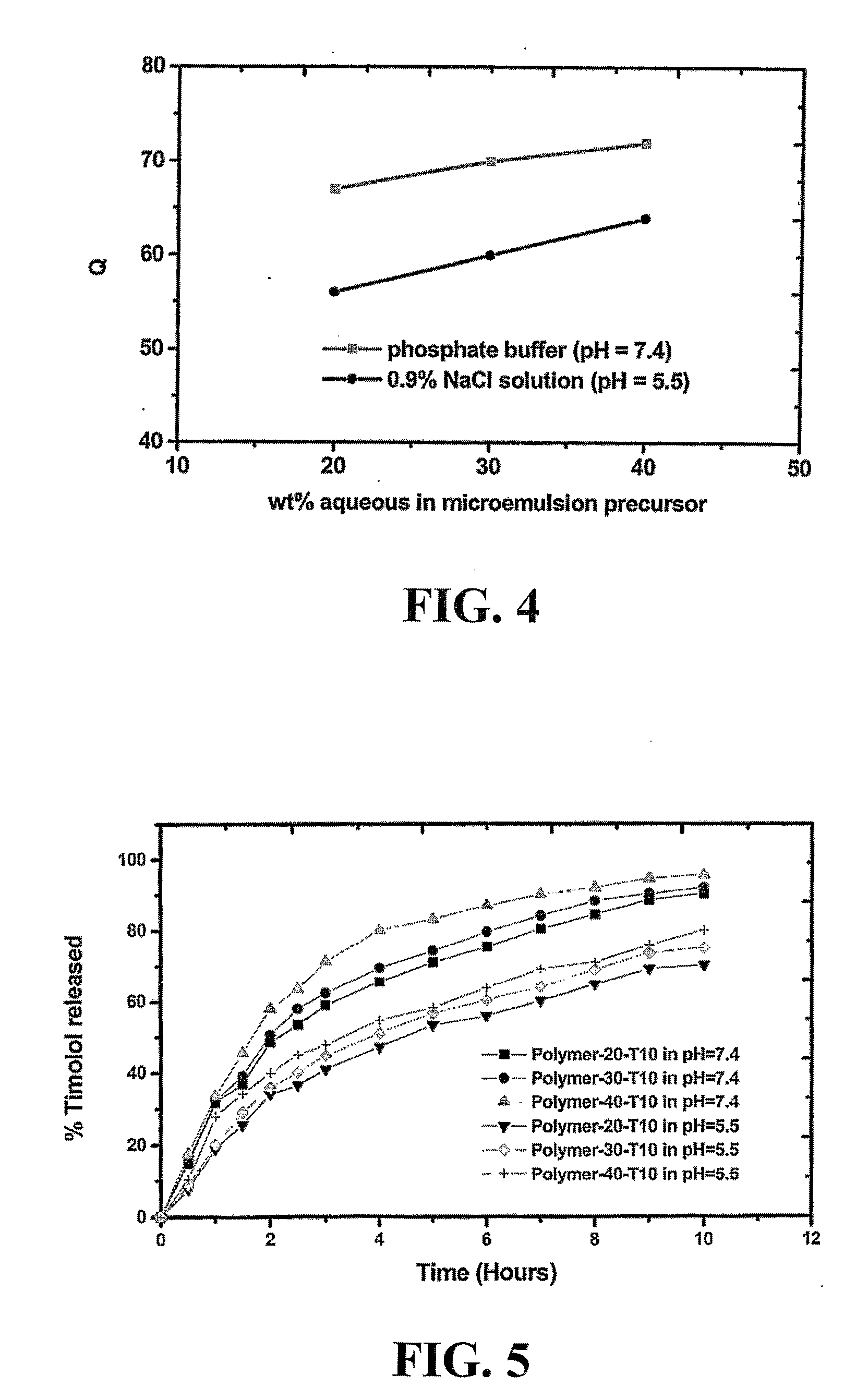
A bicontinuous microemulsion of water, a monomer, and a surfactant copolymerizable with the monomer is polymerized to form a transparent and porous polymer defining interconnected pores. The pores may have a pore diameter in the range of 10 to 100 mm. The microemulsion may further include a drug such that, when the polymer is formed, the drug is dispersed in one or both of the polymer and the pores and is releasable therefrom when the polymer is in contact with a liquid. The drug may be an ophthalmic drug and the polyer can be used to form drug delivery devices, such as contact lenses and artificial corneas.
Owner:AGENCY FOR SCI TECH & RES
Methods and compositions for growing corneal endothelial and related cells on biopolymers and creation of artifical corneal transplants
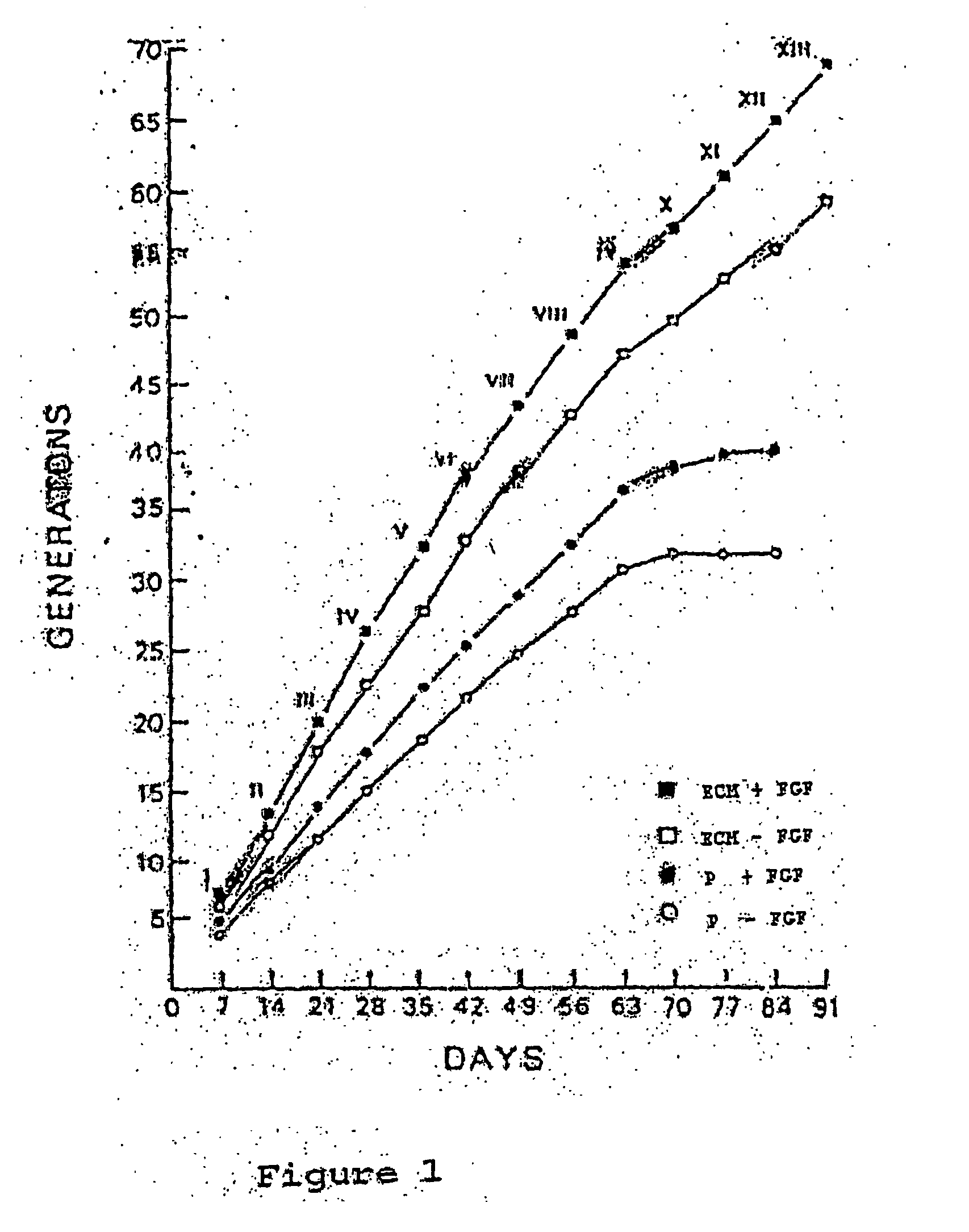
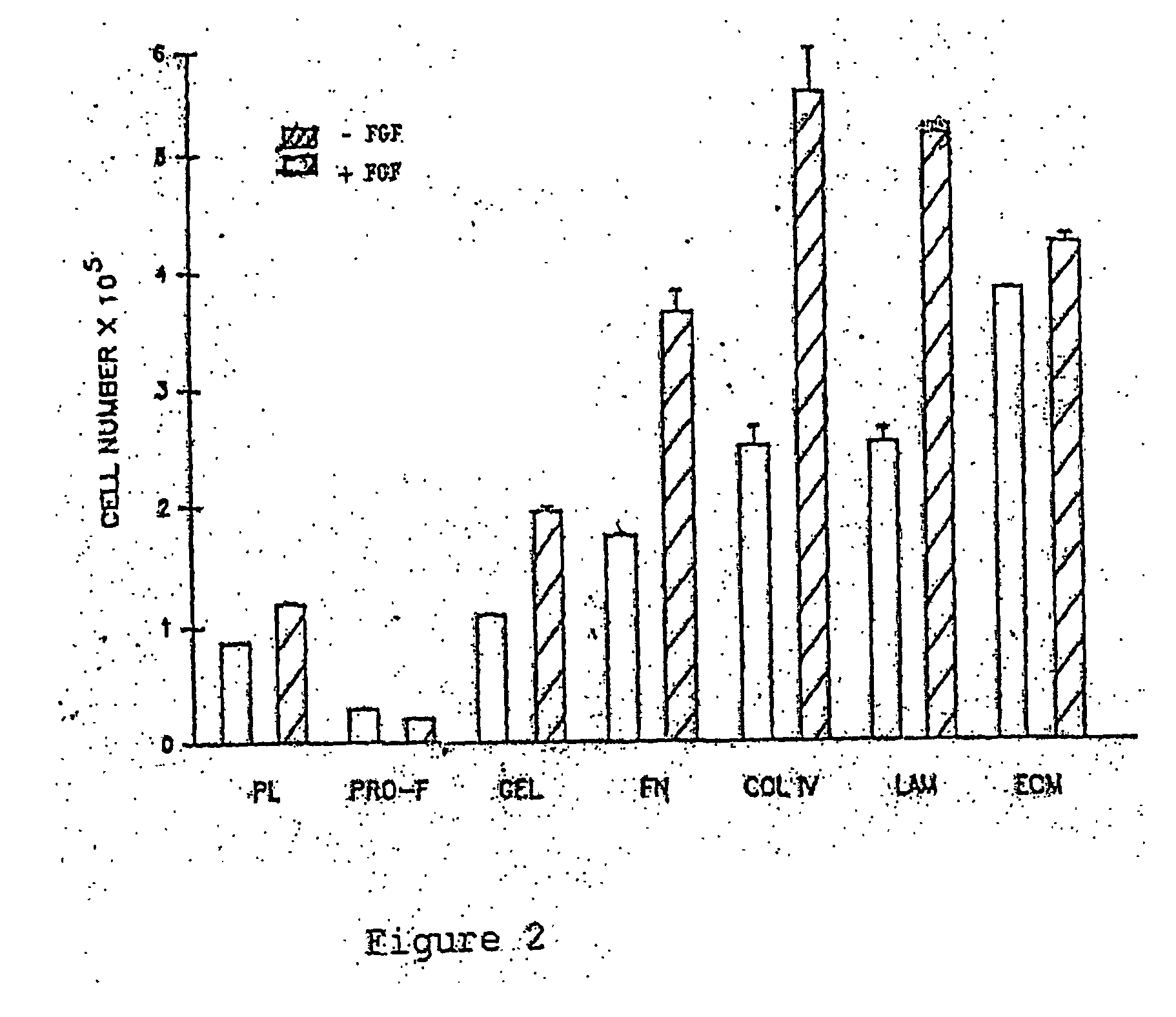
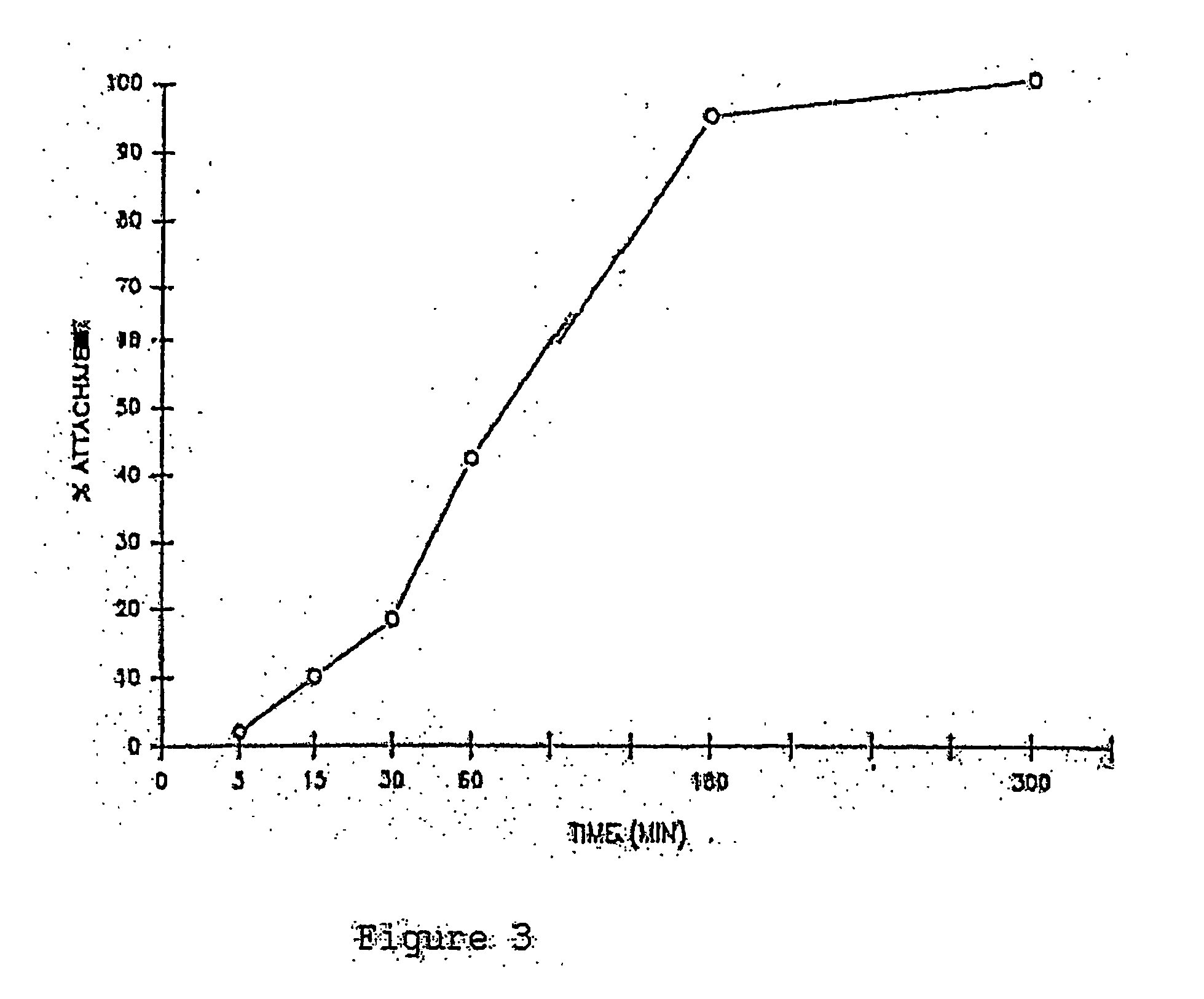
This invention discloses methods to attach and grow a monolayer of cultured human corneal endothelial cells onto the endothelial side of the stroma synthesized from biopolymer to generate a more bio-equivalent artificial cornea. The approaches will include the use of attachment and growth promoting agents such as fibronectin, laminin, RGDS, collagen type IV, bFGF conjugated with polycarbophil, and EGF conjugated with polycarbophil. The patent also describes a method to create a self-sustaining polymer containing adhesive molecules and growth factors to support the attachment and proliferation of cultured human corneal endothelial cells for corneal transplantation either as a half-thickness device or full-thickness button replacement. An approach for the implantation of cultured retinal pigment epithelial (RPE) cells into the sub-retinal space for treatment of age-related macular degeneration (ARMD) is disclosed in this invention. This method will enable the delivery of the transplanted RPE in a sheet of monolayer cells and will be better suited to perform their physiological function.
Owner:CELLULAR BIOENG
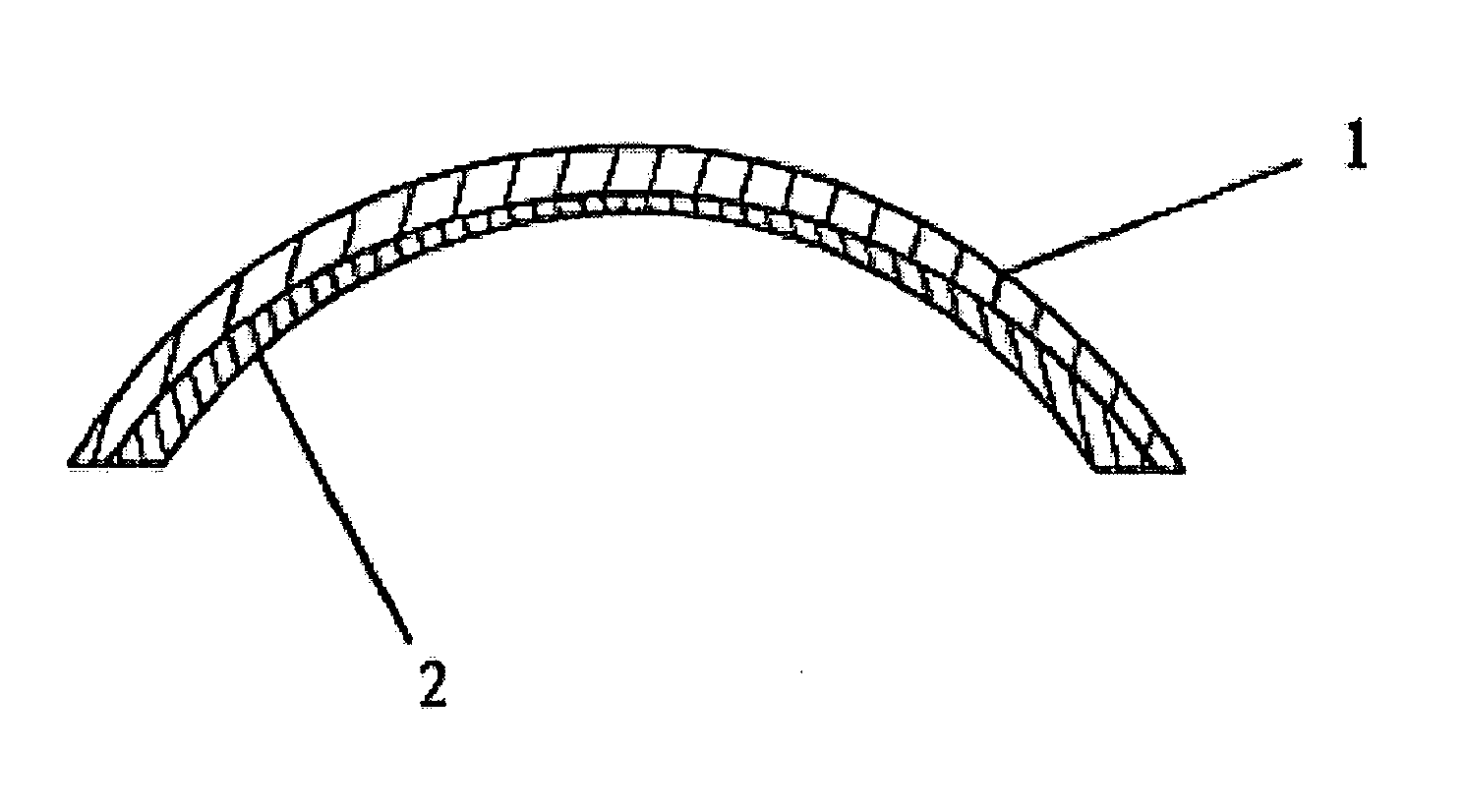
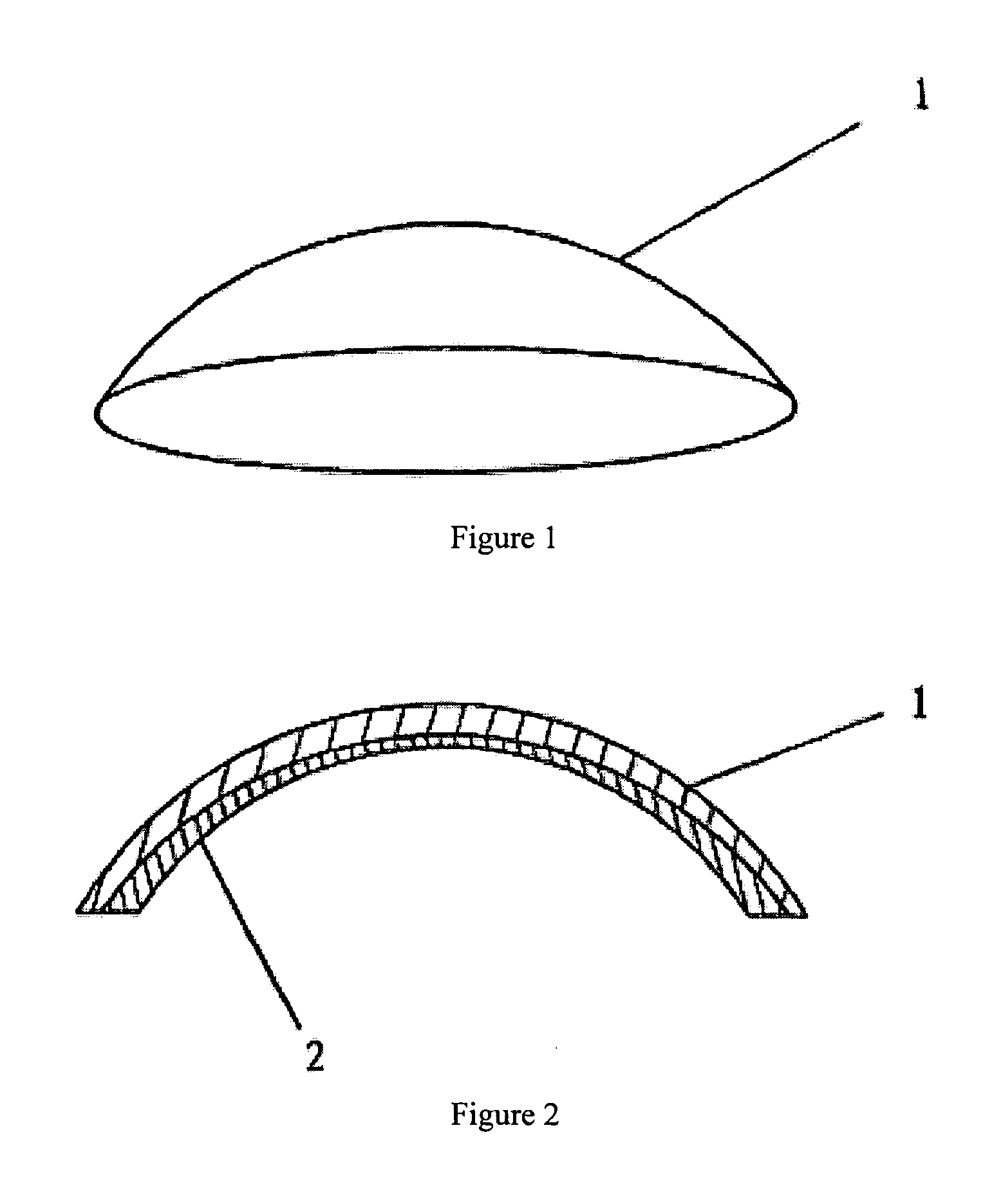
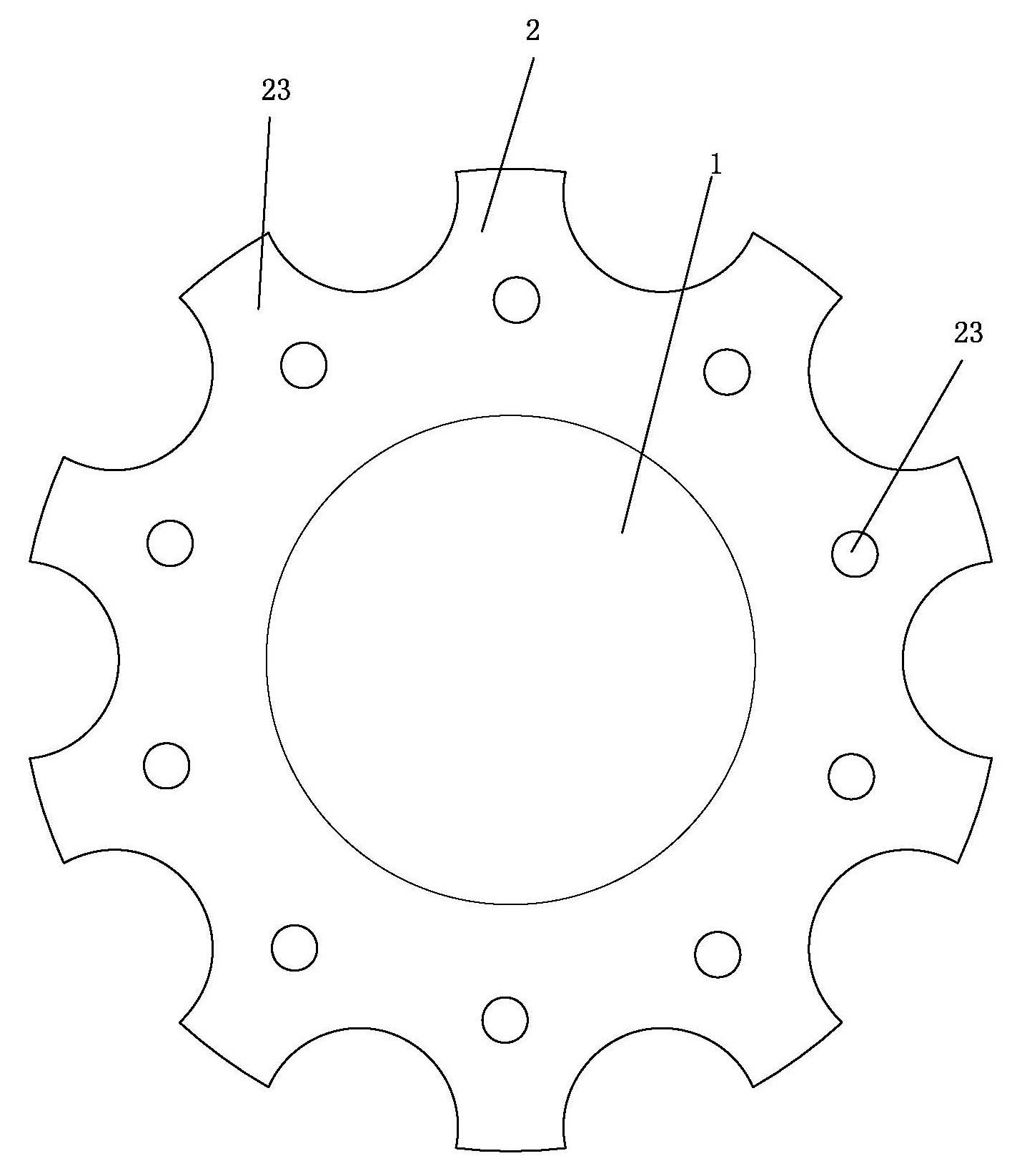
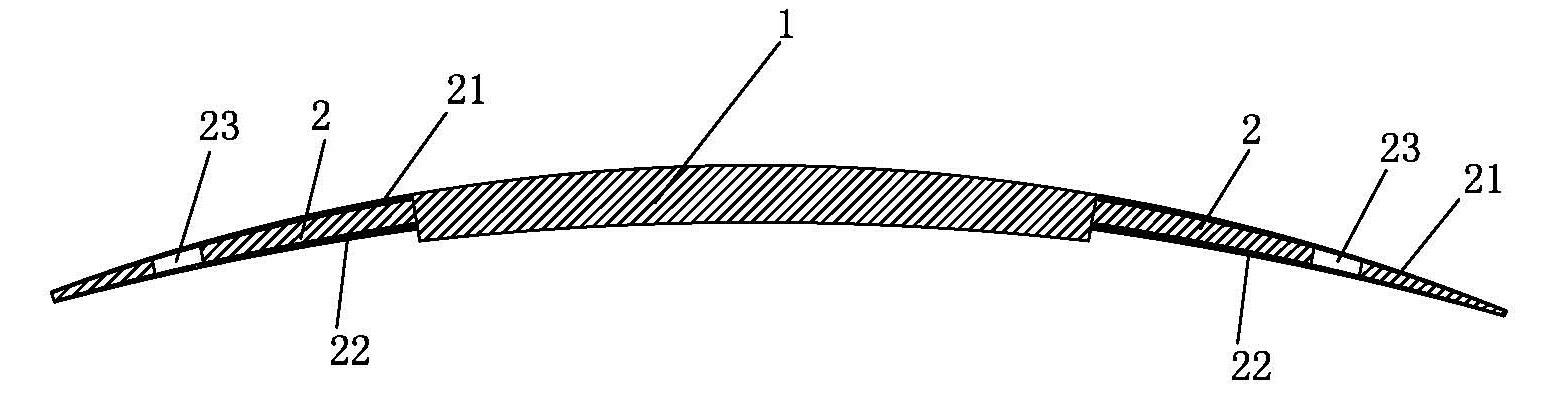
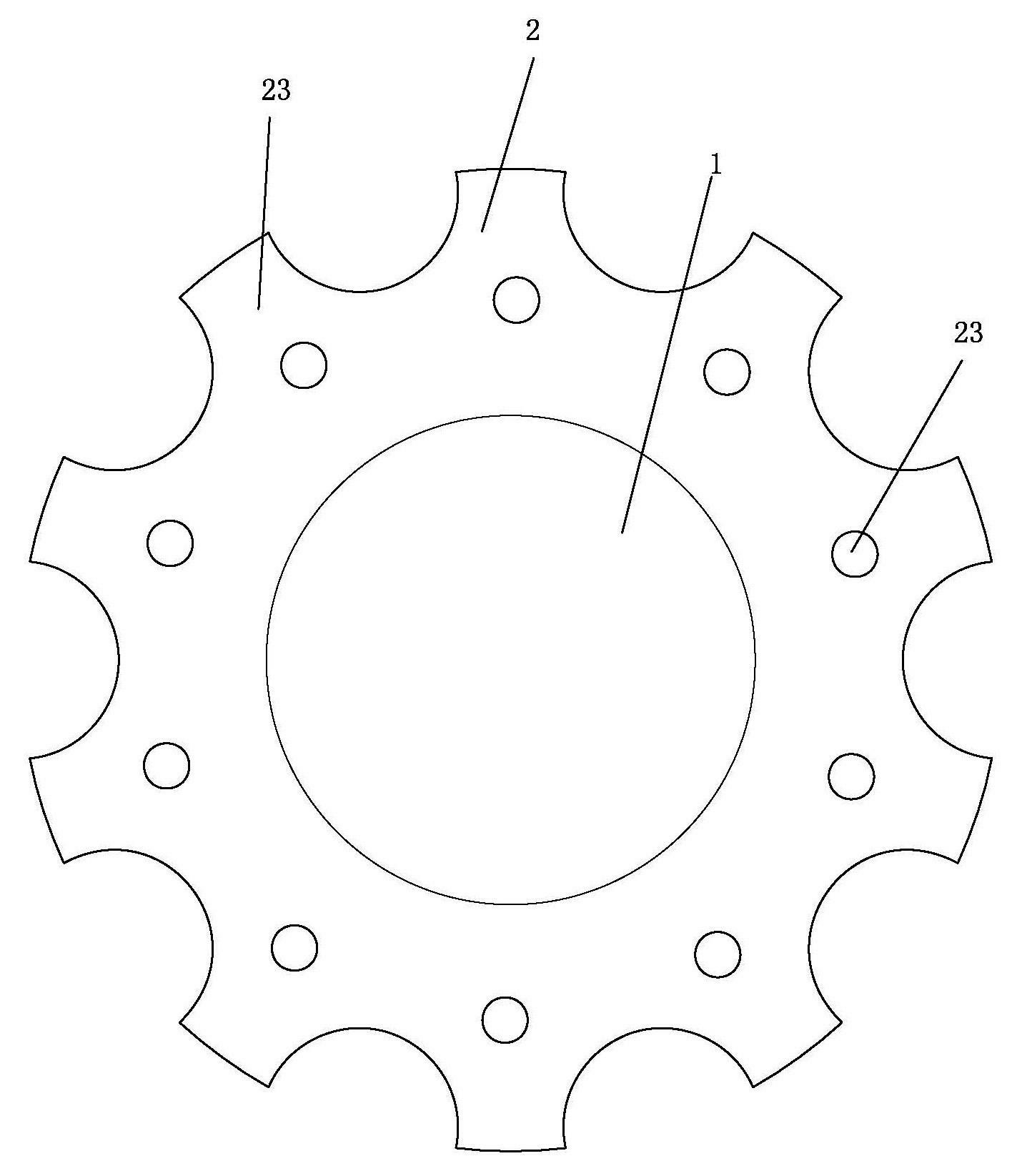
an artificial cornea
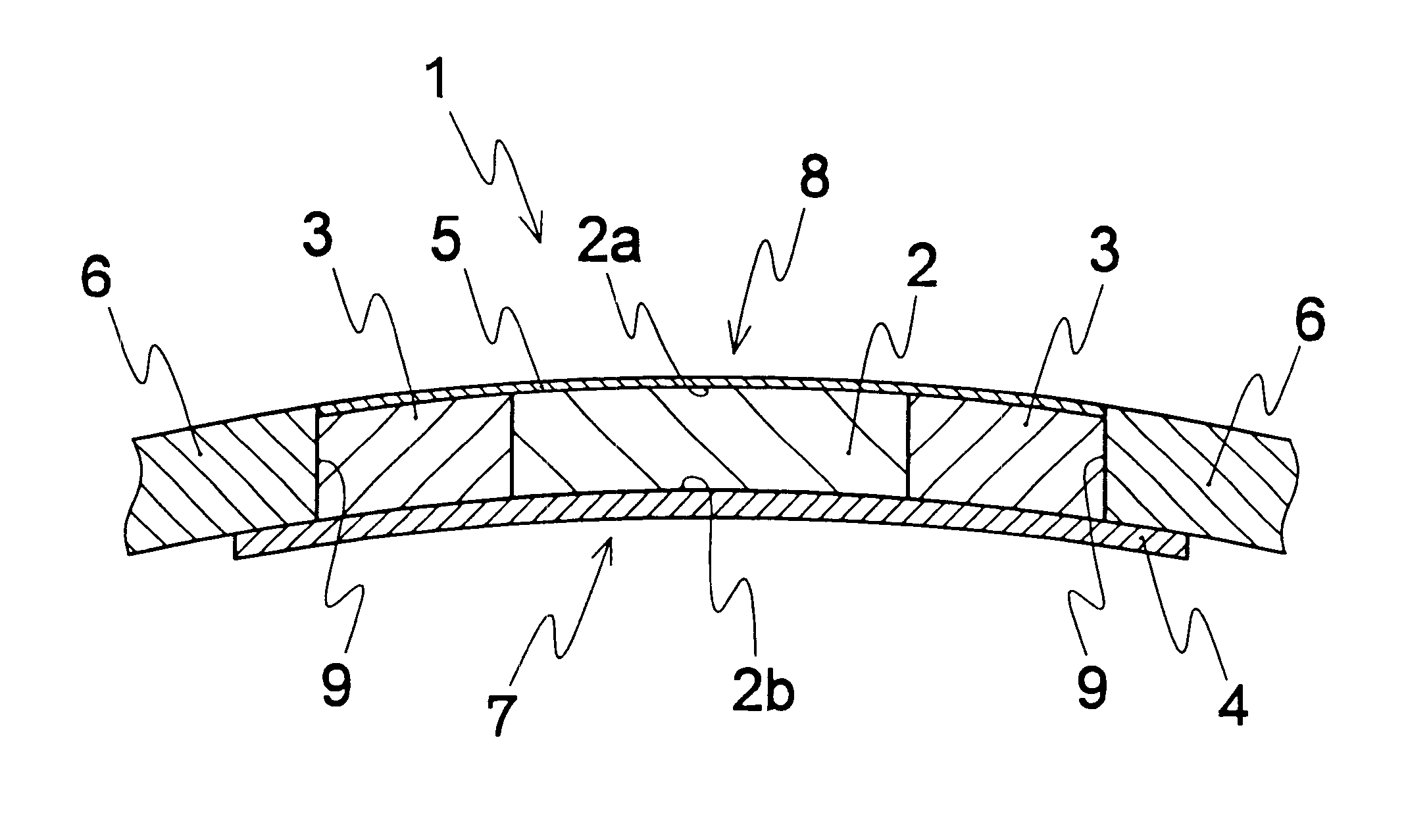
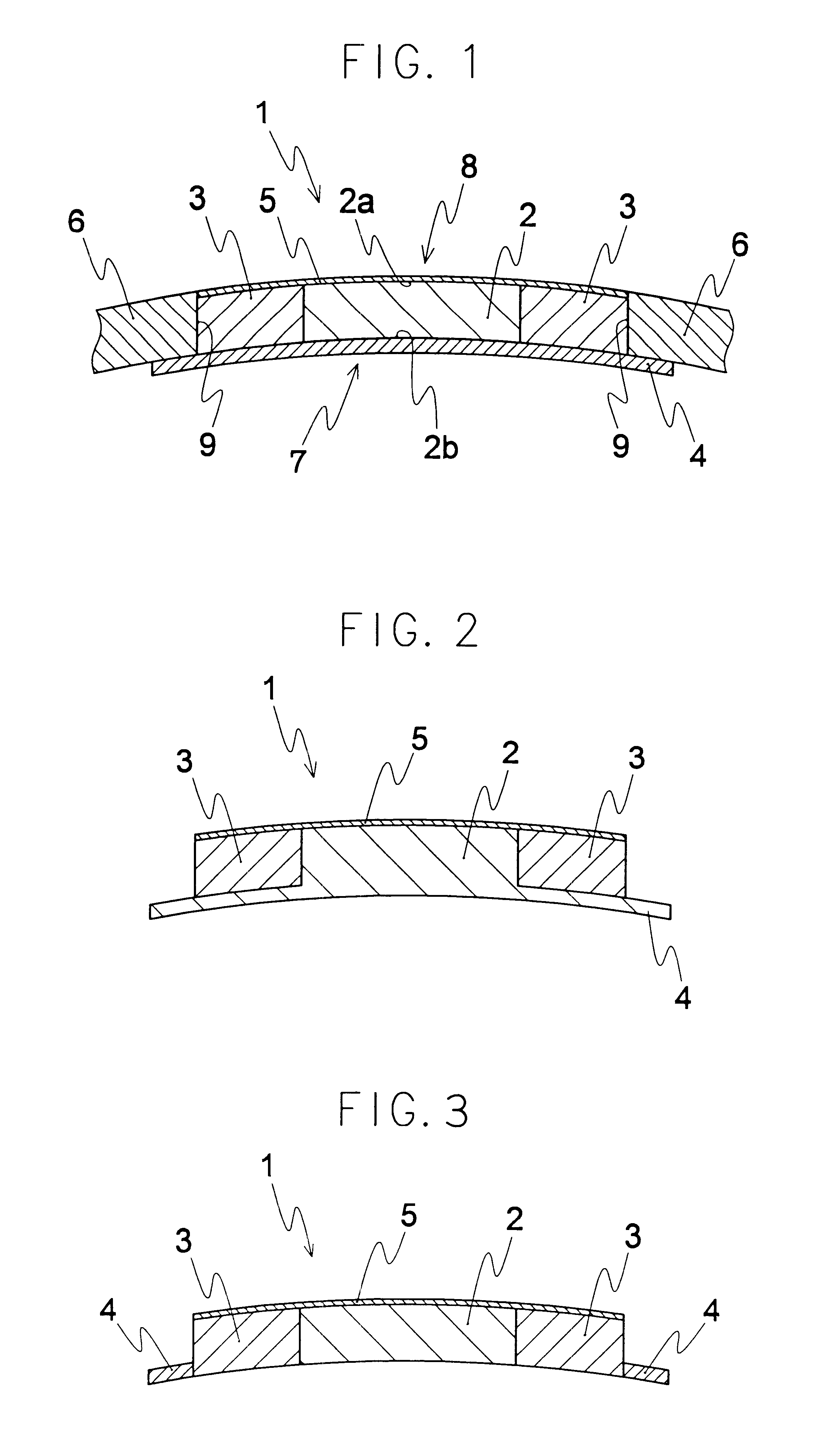
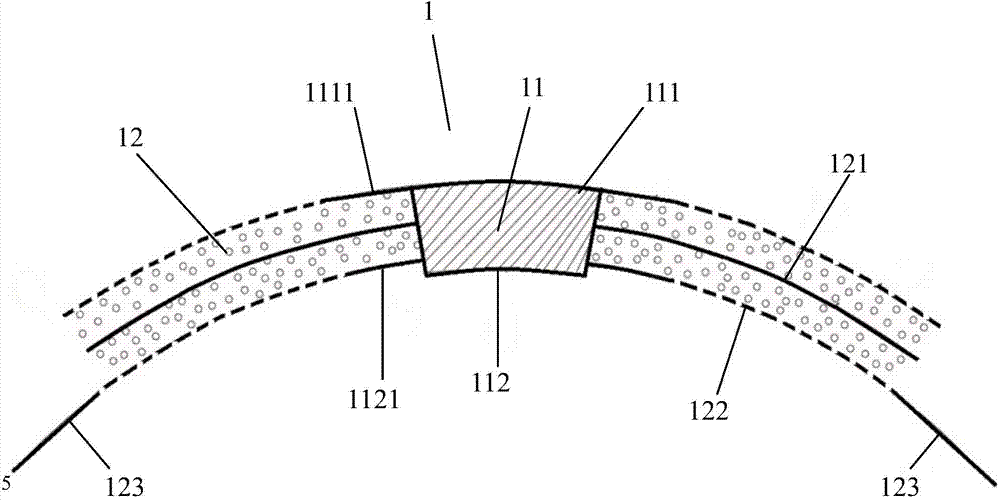
An artificial cornea has an optical zone (1) and a skirt (2) equipped with the periphery of the optical zone (1). The inner surface (22) and outer surface (21) of the skirt (2) are equipped with biomaterial coatings compatible to human eye tissues. An edge of the skirt (2) is zigzag, and more than four through holes (23) are disposed on the skirt (2). The edge of the skirt (2) is zigzag, so that the skirt (2) can adapt to varied cornea radian easily, and the irregular jointing caused by the heaving of the skirt (2) is avoided. More than four through holes (23) are disposed on the skirt (2), such that the fixation of sutures is facilitated, fusion, fiber growth, nutrition communication and the like of anterior and posterior lamellar corneas and the artificial cornea are strengthened, and the biocompatibility of the cornea is enhanced.
Owner:姚晓明 +2
Integrated artificial cornea and preparing method thereof
InactiveCN101658445AAvoid spontaneous dislocationStop the leakEye implantsBiocompatibility TestingFreeze and thaw
The invention relates to an integrated artificial cornea and a preparing method thereof. The optical center of the artificial cornea is polyvinyl alcohol hydrogel containing 80 to 90 weight percent ofwater; a skirt stent is porous polyvinyl alcohol hydrogel which contains 70 to 90 weight percent of water and 0 to 15 weigh percent of graphite powder and is distributed with micropores; and an neighboring periphery combining part of the optical center part and the skirt stent part is an integrated transitional structure in mutual crossing integration. The integrated artificial cornea is preparedby the following steps: firstly, fully dissolving polyvinyl alcohol by a solvent, adding the graphite powder and a pore forming agent of water soluble particles to perform compression molding, and fully soaking and washing the mixture in deionized water after freezing and thawing to obtain a skirt stent blank; and then, drilling a central hole in the optical center, dripping polyvinyl alcohol solution into the hole and performing compression molding, performing freezing and thawing repeatedly, and fully soaking and washing the skirt stent blank to obtain the artificial cornea. The artificialcornea has better biocompatibility and flexibility, is resistant to tear and drag, is favorable for the biological healing between regenerated tissues and the skirt stent, can prevent the leakage andthe infection of aqueous humor, and can effectively avoid spontaneous dislocation after transplant.
Owner:SICHUAN UNIV
Preparation method for artificial corneas
The invention discloses a preparation method for artificial corneas. The preparation method comprises the following steps of (1) collecting age-appropriate pig eyeballs; (2) scraping corneal epithelial cells and corneal endothelial cells of the pig eyeballs, reserving bowman layers, preparing lamellar corneas by using a lamellar blade, and drilling size-specific corneal slices; (3) putting the corneal slices obtained in step (2) in liquid nitrogen for 8-35 minutes, slowly rewarming the corneal slices to room temperature under normal temperature and normal pressure after the corneal slices are taken out, repeating the operation for 4-12 times; (4) putting the corneal slices which are subjected to freezing-thawing treatment in step (3) in normal saline for carrying out ultrasonic cleaning, wherein the ultrasonic working frequency is 20-250 kHz, and the ultrasonic time is 15-65 minutes; (5) putting the corneal slices which are subjected to ultrasonic treatment in a buffer system, carrying out enzymolysis on DNA (Deoxyribose Nucleic Acid) of corneal stromal cells by using endonuclease, and obtaining the artificial corneas. According to the artificial corneas prepared by the preparation method disclosed by the invention, gaps between natural collagen fibers of the artificial corneas are beneficial for inducing the growth of the corneal cells and the growth of nerve fibers of an acceptor and promoting the repairing and the reconstructing of corneas of a donor.
Owner:XIAMEN DAKAI BIOTECH CO LTD
Treatment of photic disturbances in the eye
InactiveUS7217289B2Reduce and eliminate oblique incident light photic disturbanceIntraocular lensOptical partsUses eyeglassesEyewear
An ocular lens treated so that at least a portion of the lens perimeter diminishes peripheral light focus on the interior of the eye in use, so as to ameliorate phobic eye disturbances. The lens perimeter of intraocular lenses, artificial corneas and contact lenses are treated to diminish peripheral light focus.
Owner:CORONCO MINAS THEODORE
Artificial cornea and manufacturing method thereof
InactiveCN104546222AMeet individual diopter needsGood restorativeEye implantsLiquid stateEngineering
The invention discloses artificial cornea and a manufacturing method thereof and relates to the technical field of artificial organs. The artificial cornea is manufactured based on 3D (three dimensional) printing technology. The manufacturing method includes setting up a human vision model; setting up a personal eyeball model by 3D scanning; importing data into a 3D printer; preparing printing materials; scanning the printing materials point by point through ultraviolet laser beams with wave length in 355 nm and beam quality M2 in 1.0-1.3, curing the liquid-state printing materials from point to line and line to surface, controlling a lifting platform to move by a computer, curing the liquid-state printing materials layer by layer, and finally stacking layer by layer to obtain a 3D-printed cured product; freezing and forming and successively processing. The manufacturing method is quick and accurate, simple and feasible to operate, low in cost, and capable of accurately controlling the microstructure of the materials. The artificial cornea is perfectly matched with an original optometric system of a patient, and the requirements of different patients for individual diopter can be met.
Owner:深圳华明生物科技有限公司
Acrylic polymer material with high refractive index
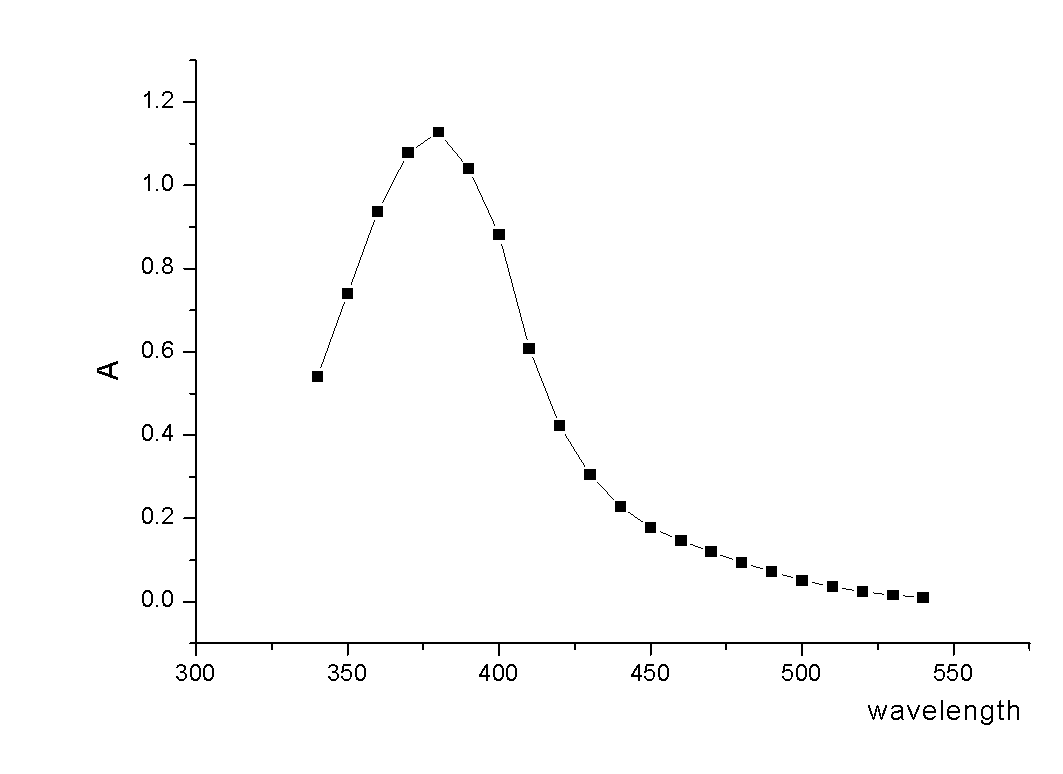
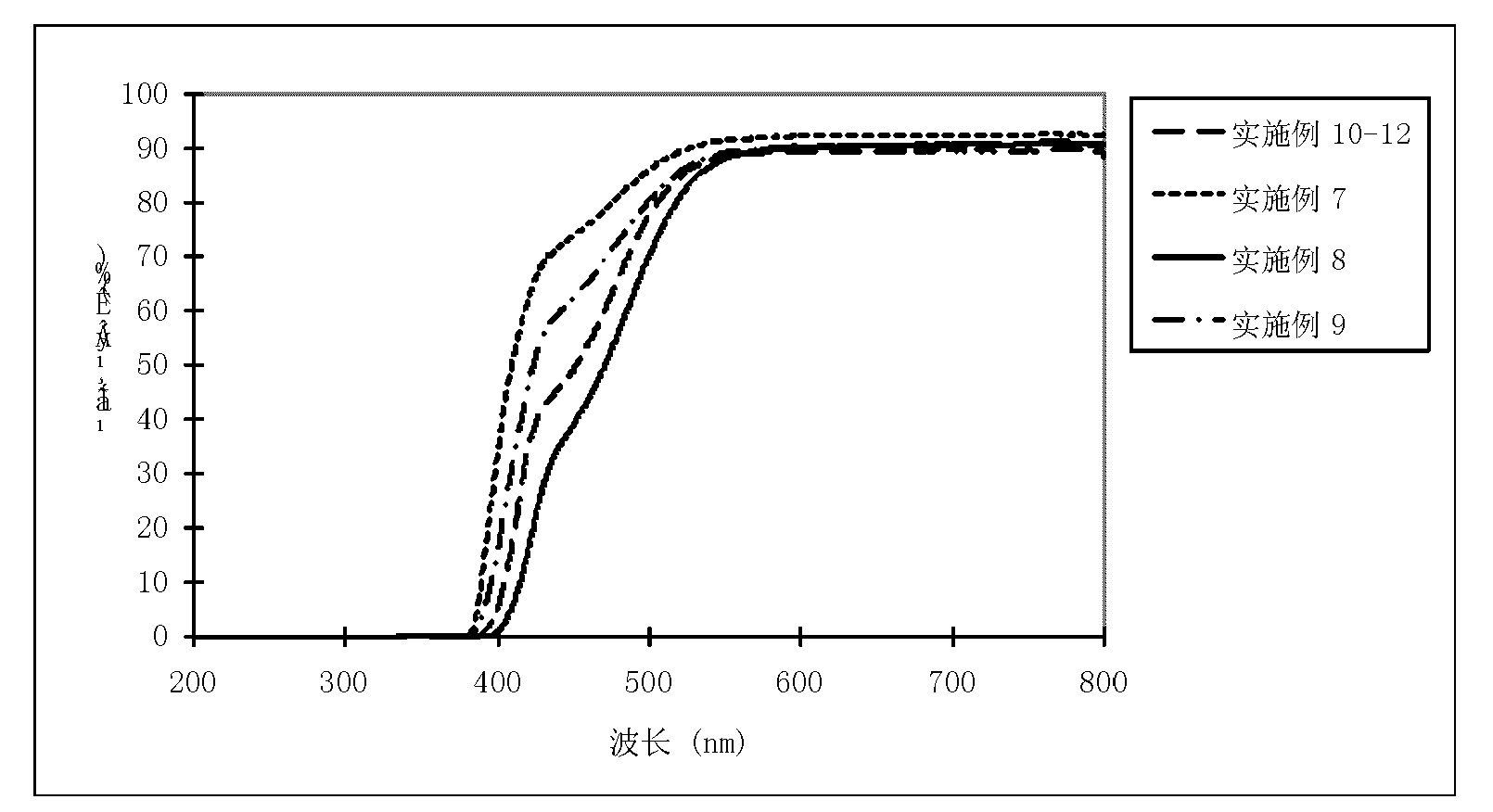
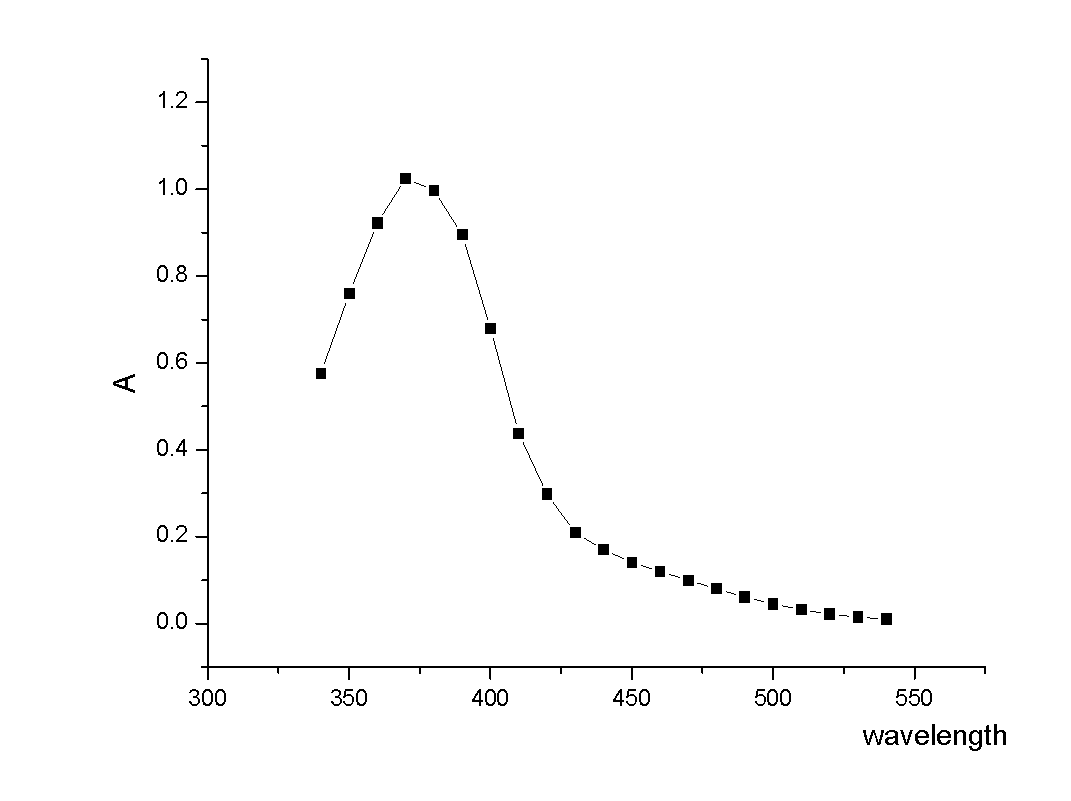
The invention relates to an acrylic polymer material with high refractive index, particularly an acrylic polymer material which has high refractive index and low dispersion deviation and optionally contains blue-light radiation resistance. The material can be used for preparing medical devices, especially preparing eye medical devices, such as artificial lens, contact lens, artificial cornea, cornea inner ring, cornea inner lens, glaucoma valve, drug controlled release carrier, glasses, goggles, medical device lens, telescope, observation mirror. The invention further relates to a polymerisable yellow azo dye which has a maximum absorption wavelength of about 360-370 nm and has methacrylamide nonsaturated group in the structure, thus the effects of effectively absorbing blue lights and / or ultraviolet rays can be achieved.
Owner:EYEBRIGHT MEDICAL TECH BEIJING
Artificial cornea
The invention discloses an artificial cornea which is composed of an optical portion and a supporting portion. The optical portion and the supporting portion are integrally formed through three-dimensional printing, the optical portion is arranged in the center of the artificial cornea, the supporting portion is arranged at the periphery of the optical portion and of a net-shaped multi-hole structure, and accordingly the biological synthesis between the artificial cornea and a receptor tissue is improved. An artificial cornea in the prior art uses a lens column-supporting two-piece mode, an optical portion and a supporting portion of the artificial cornea in the prior art are separated, and aqueous fluid leakage and serous complication like endoophthalmitis are prone to happening after operation to cause transplantation failure. The integrated artificial cornea is manufactured through a three-dimensional printing technique. By means of an integrated structure, the whole artificial cornea is firm and stable in structure, not prone to deforming and capable of solving the problems caused by the two-piece artificial cornea. Individual data models can be manufactured according to parameters of an eyeball in different age groups or CT and UBM three-dimensional scanning parameters of a patient, so that individual artificial cornea can be manufactured.
Owner:XIAMEN UNIV
Transcorneal vision assistance device
The invention provides a transcorneal vision assistance device implantable in the eye of a patient. A preferred embodiment transcorneal microtelescope vision assistance device is implantable in the eye of a patient and includes a keratoprosthesis configured to replace a portion of the cornea of a patient and to secure the keratoprosthesis to a remaining front portion of the cornea. A microtelescope is carried by the keratoprosthesis for transcorneal mounting of the microtelescope.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
A method for preparing bioactivity possessed artificial cornea
The invention relates to a method for preparing biologically active artificial cornea. It takes animal cornea basic material as raw material, promoting cornea growth or / and preventing partial lesion by enzyme slaking, repeated unfreezing, washing, and irradiating with 60Co. It employs physical and chemical method to remove cell component and soluble protein which will cause immune response, and retains external base material construction, adds multifunctional component which is favor for cornea cell growth or / and preventing partial lesion, freeze dries and stores it. The tensile strength and diopter of prepared artificial cornea are similar to that of normal cornea, it can prevent lesion and dissolution after being implanted in, and promote cornea epithelium regeneration and collagen synthesis; the biocompatibility is good, no obvious immune rejection reaction and no toxic to cell, and can be used to treat various eye injury.
Owner:西安组织工程工程技术研究中心
Durably-transparent silk fibroin film and preparation method thereof
ActiveCN103861149AGood biocompatibilityGood light transmissionAbsorbent padsProsthesisBiocompatibility TestingDrug control
The invention relates to a durably-transparent silk fibroin film and a preparation method thereof. The preparation method comprises the steps of: by taking silk fibroin as a raw material, adding amide with the molecular weight of less than 150 into fibroin solution to serve as a transparent crosslinking agent, and pouring into a film. The transmittance of the fibroin film in the visible light range of 400-800nm is more than 90%, and has durability. The prepared silk fibroin film is easily degradable and good in biocompatibility, and can be used as a cell carrier material on which cells normally grow. The material can maintain stable transparency for a long time, and thus has certain advantages as a cornea carrier or cornea substitution material, and can be used for contact lenses, artificial cornea, cell culture, wound repair materials and temporary coverage, tissue isolation and drug controlled release materials, and the like.
Owner:PHARSUN MEDICAL BIOTECHNICS (SHANGHAI) CO LTD
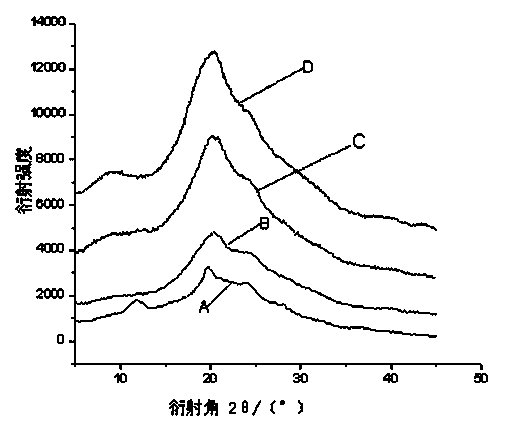
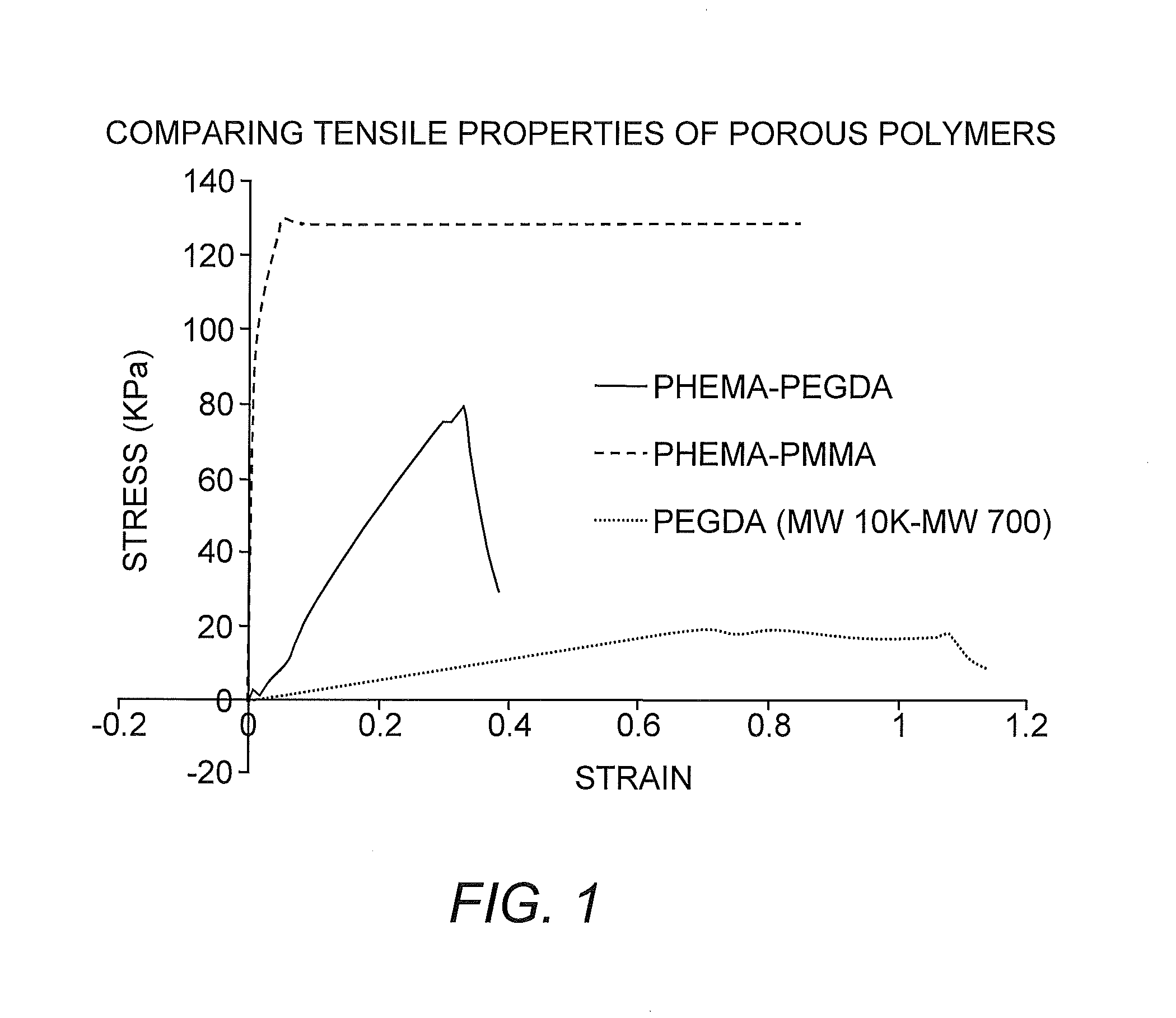
Suturable Hybrid Superporous Hydrogel Keratoprosthesis for Cornea
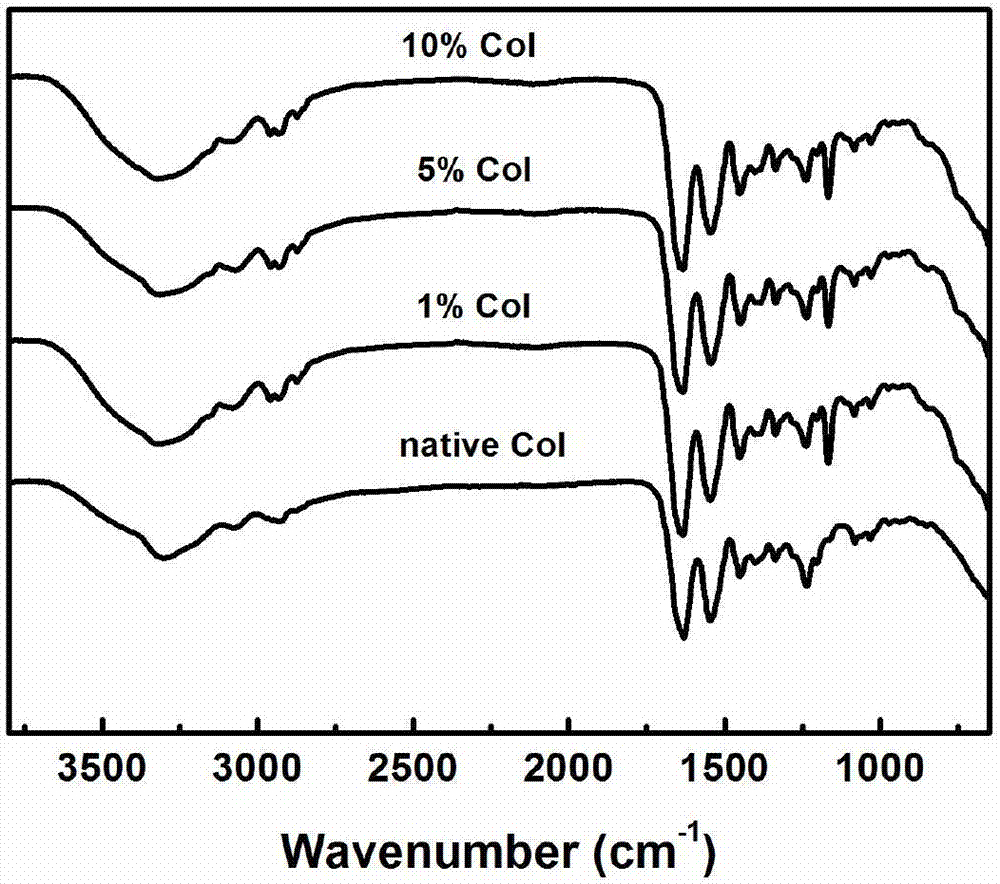
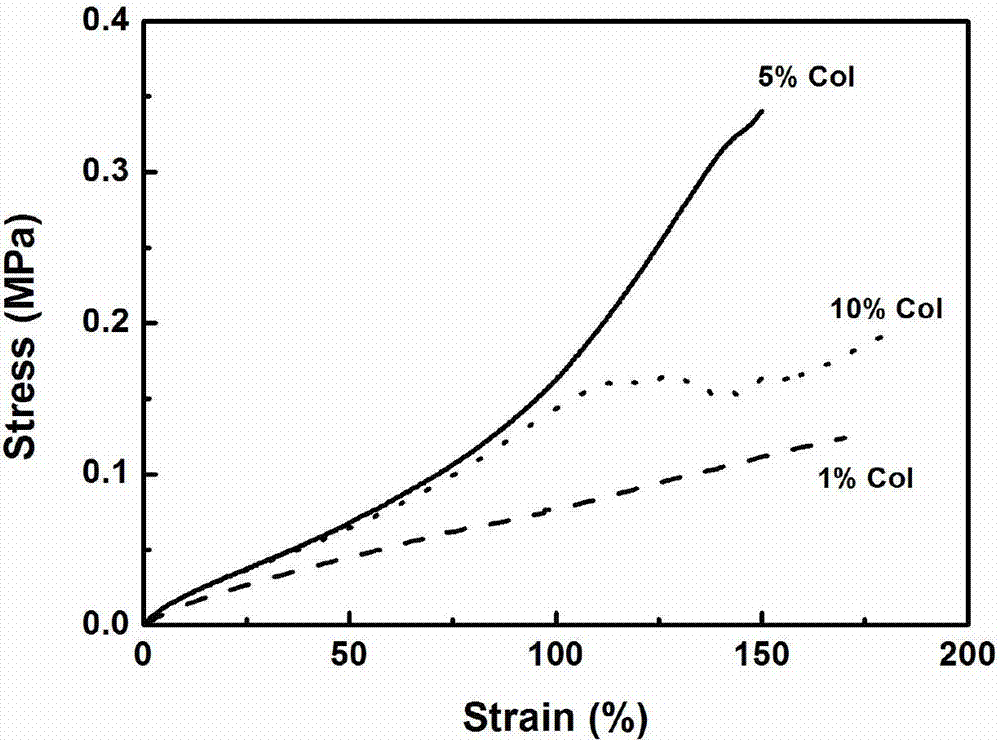
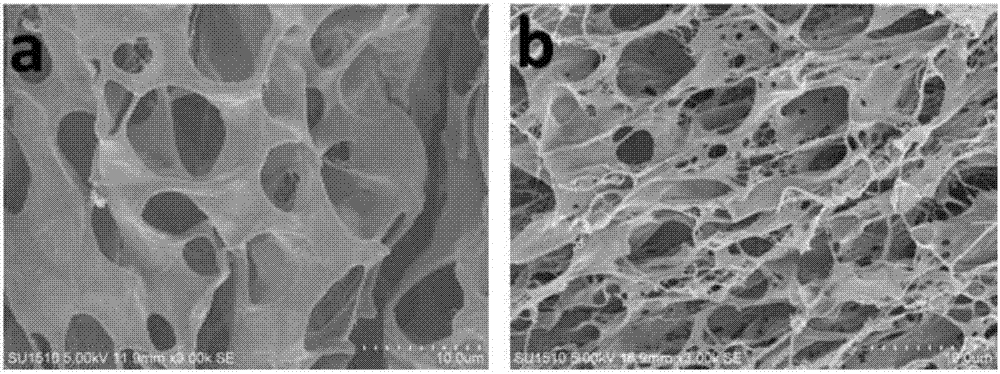
The present invention features a hybrid superporous hydrogel scaffold for cornea regeneration and a method for producing the same. The hybrid hydrogel is composed of a superporous poly(2-hydroxyethyl methacrylate) (PHEMA) and poly(methyl methacrylate) (PMMA) copolymer mixed with collagen. The hybrid scaffold can be used as a suturable hybrid corneal implant or keratoprosthesis.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Constructing method for human corneal endothelium cell system
The construction of human corneal endothelium cell line with corneal endothelium as material includes the steps of: the first adherent culture of human corneal endothelium cell inside DMEM / F12 culture liquid with ox embryo serum in 20 wt%, chondroitin sulfate oxidizing and degrading matter, epidermal cell growth factor, basic fibroblast growth factor and ox eye auxin to obtain pure corneal endothelium cell; and the subsequent secondary culture in trypsinization process. The technological process is scientific and reasonable, and up to now, passage cell of 106-th generation has been obtained. The human corneal endothelium cell line of the present invention has no any virus or cancer genetic transfection, has no any tumorigenicity, and is expected to be used in direct artificial cornea production and clinical application.
Owner:青岛彩晖生物科技有限公司
Three-dimensional bioprinted artificial cornea
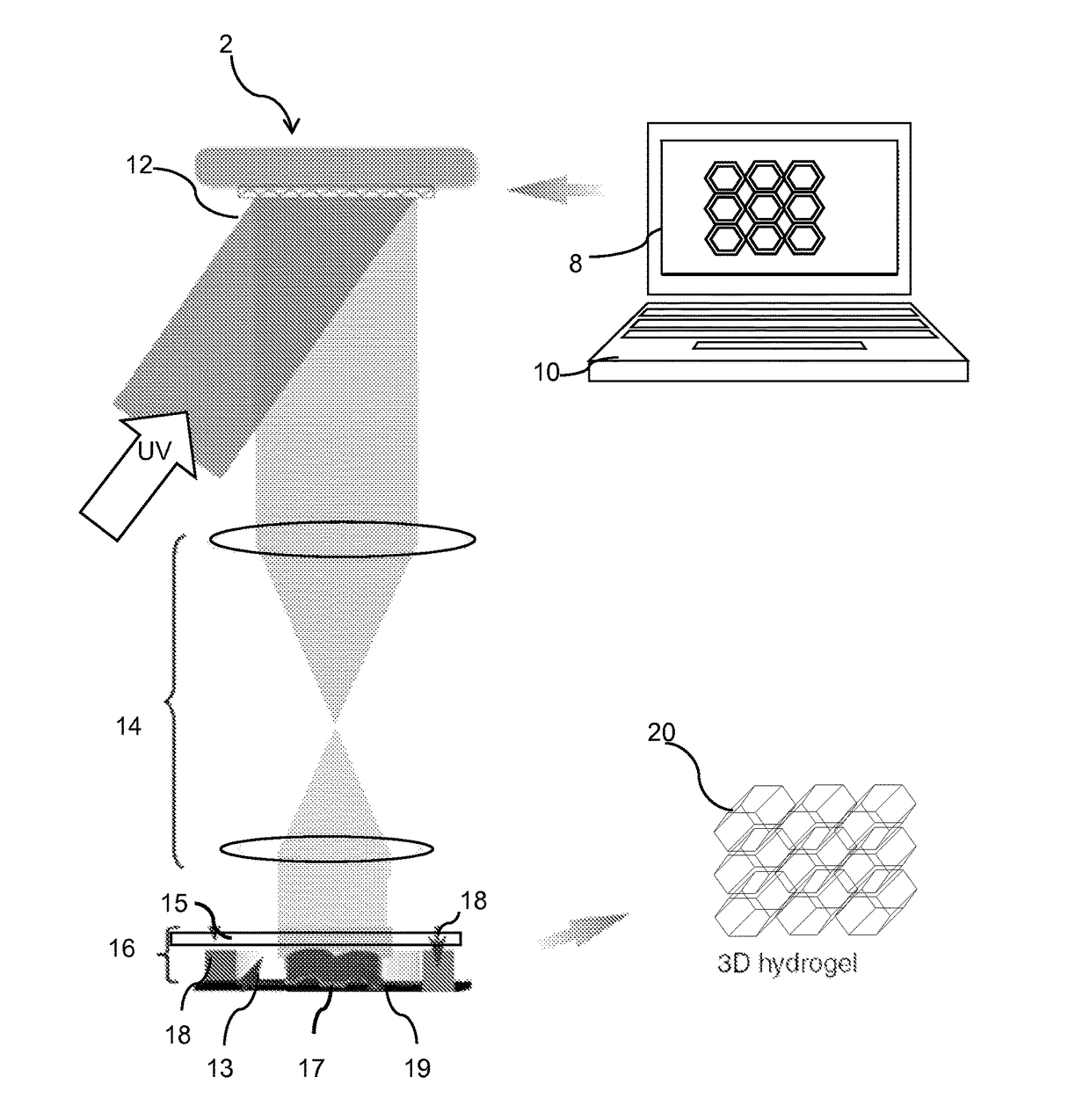
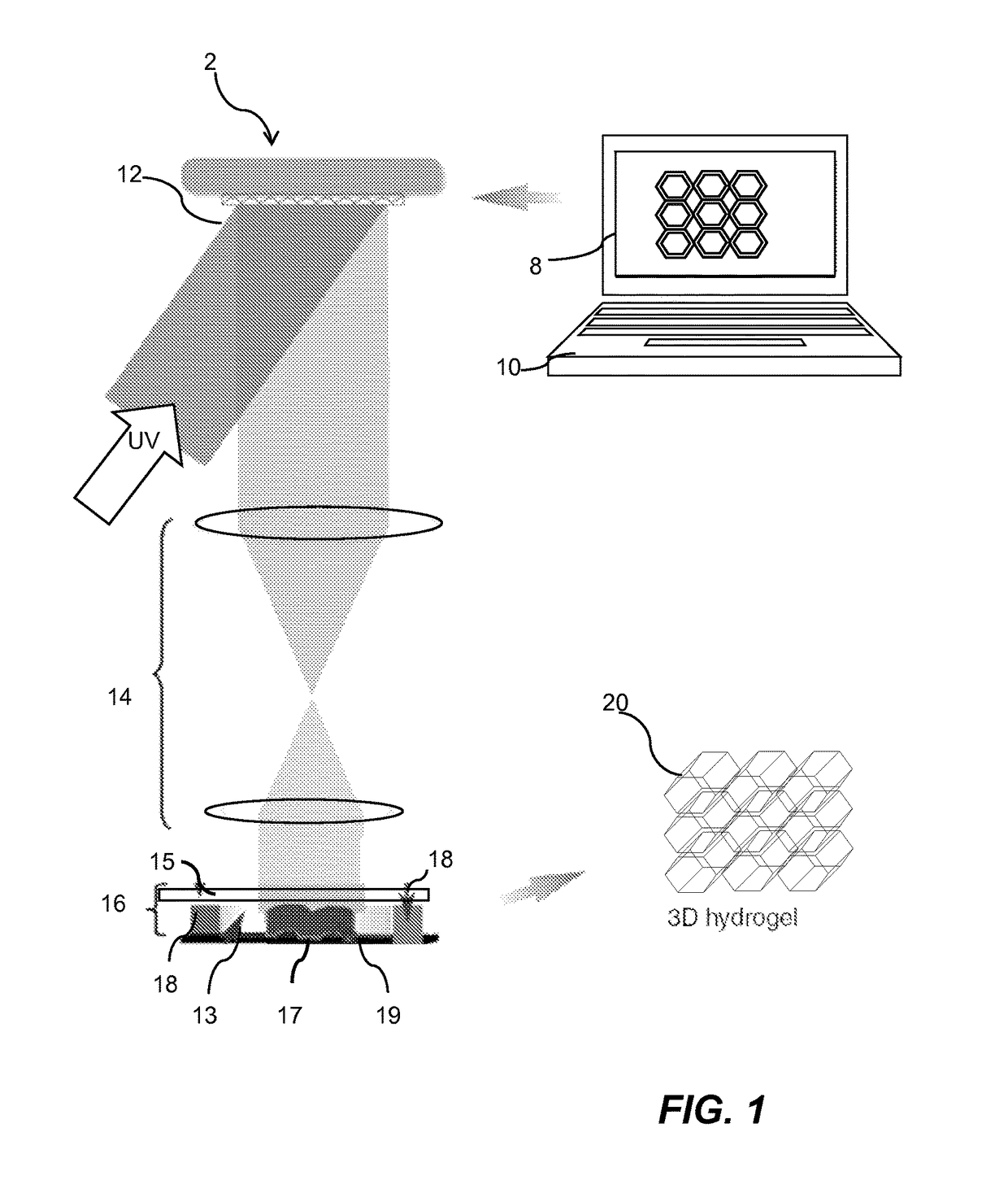
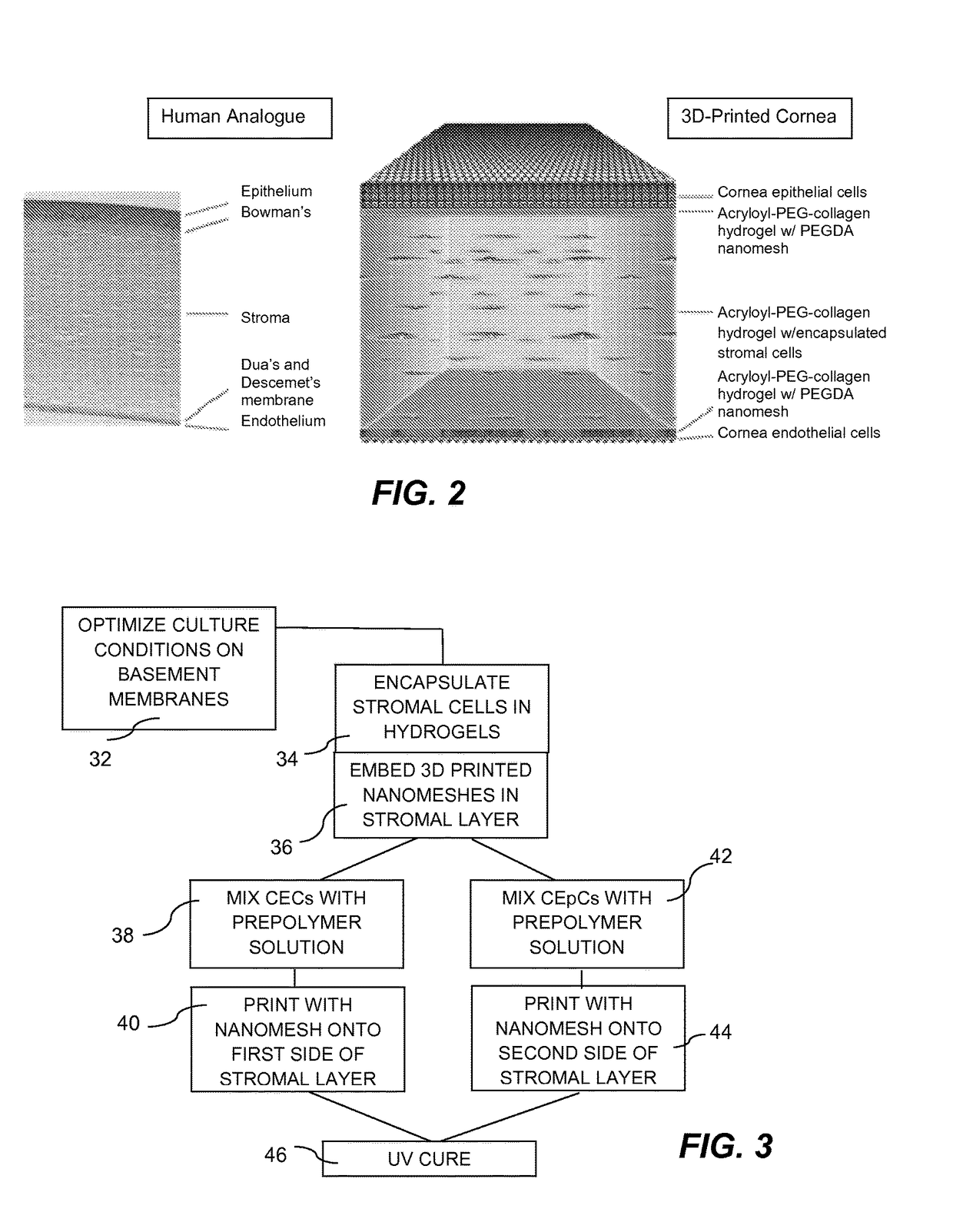
InactiveUS20170281828A1Restore visionEliminate dependenciesAdditive manufacturing apparatusPharmaceutical delivery mechanismCorneal endothelial cellStromal cell
An artificial cornea is fabricated by separately culturing live stromal cells, live corneal endothelial cells (CECs) and live corneal epithelial cells (CEpCs), and 3D bioprinting separate stromal, CEC and CEpC layers to encapsulate the cells into separate hydrogel nanomeshes. The CEC layer is attached to a first side of the stromal layer and the CEpC layer to a second side of the stromal layer to define the artificial cornea.
Owner:RGT UNIV OF CALIFORNIA
Tussah silk fibroin film and preparation method thereof
The invention relates to technology of medical biological material with natural polymer, and discloses a tussah silk fibroin film and a preparation method thereof. Nontoxic dihydric alcohol is added into tussah silk solution so as to be taken as a cross-linking agent which is injected into a mold, and the tussah silk fibroin film is formed after the mixture drying. According to weight, the tussah silk fibroin film contains 40-90% of tussah silk fibroin; the protein leaching loss of the film in water with the temperature 37 DEG C for 24 hours is less than 3%, the tensile strength at break is greater than 20Mpa, and the tensile elongation at the break is greater than 20%. As an extra chemical cross-linking agent or chemical denaturant does not need to be added in the preparation process, the biocompatibility of the tussah silk fibroin can not be reduced, and can be kept good. The tussah silk fibroin difficult to dissolve can be used for covering materials for artificial cornea, endocranium, gullet, heart valve, blood vessels and wound surfaces, and can be used for temporal covering, and isolating tissues, and can be taken as medicine releasing materials and the like.
Owner:苏州盛泽科技创业园发展有限公司
Collagen/chondroitin sulfate composite artificial cornea and preparation method thereof
InactiveCN106943632APromote growthPromote growth and proliferationProsthesisBiocompatibility TestingPhosphoric acid
The invention discloses a preparation method of a collagen / chondroitin sulfate composite artificial cornea. The preparation method comprises the following steps: dissolving collagen into a water solution of imidazole ionic liquid to obtain a collagen solution (1-30%); then dissolving chondroitin sulfate into a water solution to obtain a chondroitin sulfate water solution (1-20 mg / mL); evenly mixing two solutions according to a certain ratio, adding a natural crosslinking agent (EDC / NHS) with a good biocompatibility to prepare collagen / chondroitin sulfate in-situ crosslinked composite hydrogel; soaking the composite hydrogel in a coagulating bath to carry out moulding and regeneration, washing the hydrogel by water to remove the solvent, naturally drying to obtain a collagen / chondroitin sulfate composite membrane; dissolving a corneal growth factor FGF-10 into a phosphoric acid buffer solution to obtain a FGF-10 water solution (1-100 [mu]g / mL) ; and soaking the composite membrane into the FGF-10 water solution to prepare a collagen based membrane Col / Cs / FGF with a sustained release effect. The provided novel artificial cornea has the advantages of high strength, sustained release, and biocompatibility, and has an important application prospect.
Owner:JIANGNAN UNIV
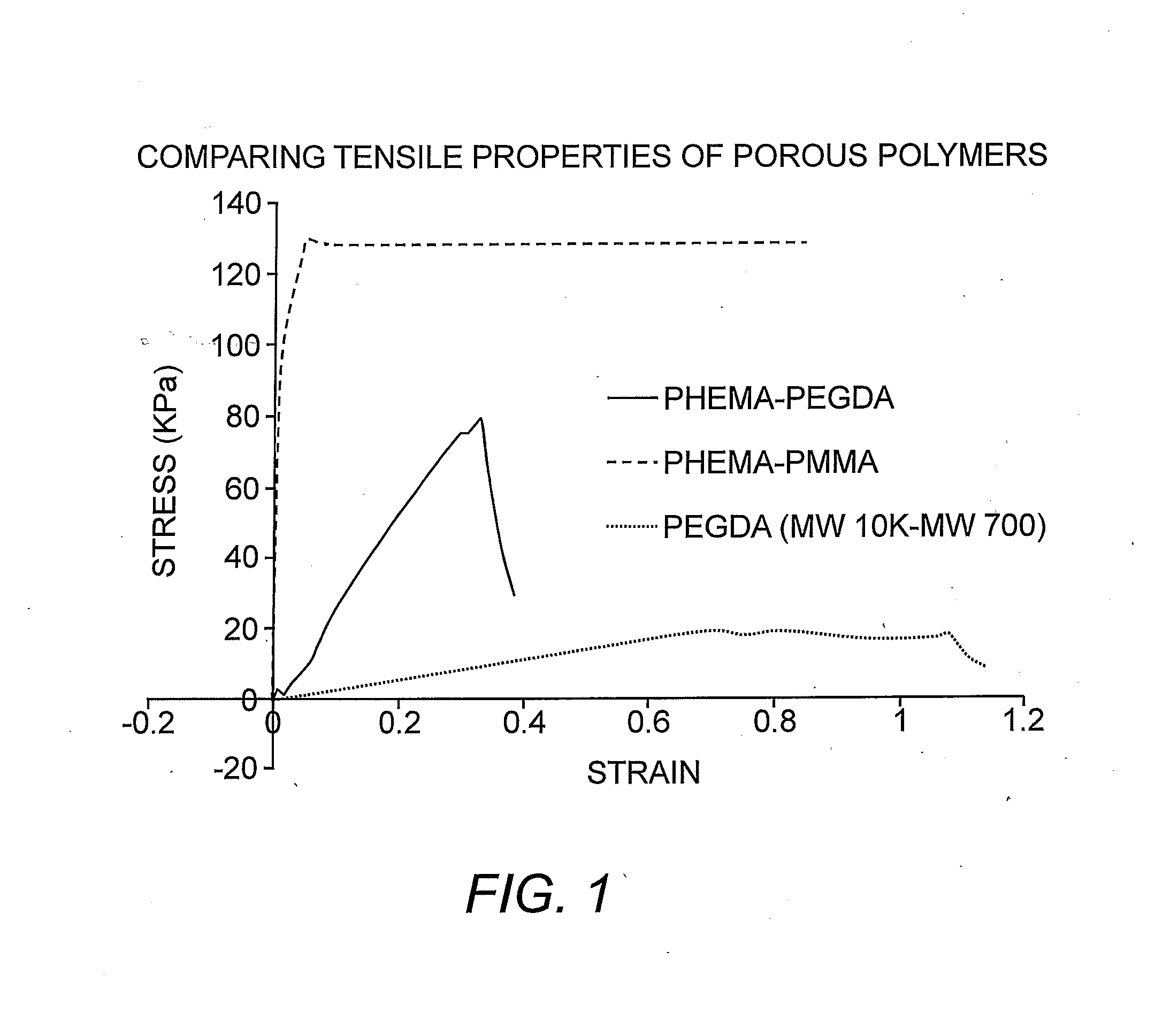
Suturable hybrid superporous hydrogel keratoprosthesis for cornea
InactiveUS20160144069A1Eye implantsPharmaceutical delivery mechanismProsthesisPoly(methyl methacrylate)
The present invention features a superporous hydrogel scaffold for corneal regeneration or replacement and a method for producing the same. The superporous hydrogel is composed of a poly(2-hydroxyethyl methacrylate) (PHEMA) and poly(methyl methacrylate) (PMMA) copolymer mixed with collagen. The scaffold can be used as a suturable hybrid corneal implant or keratoprosthesis.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com