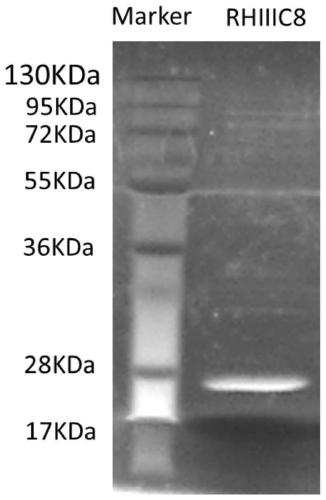
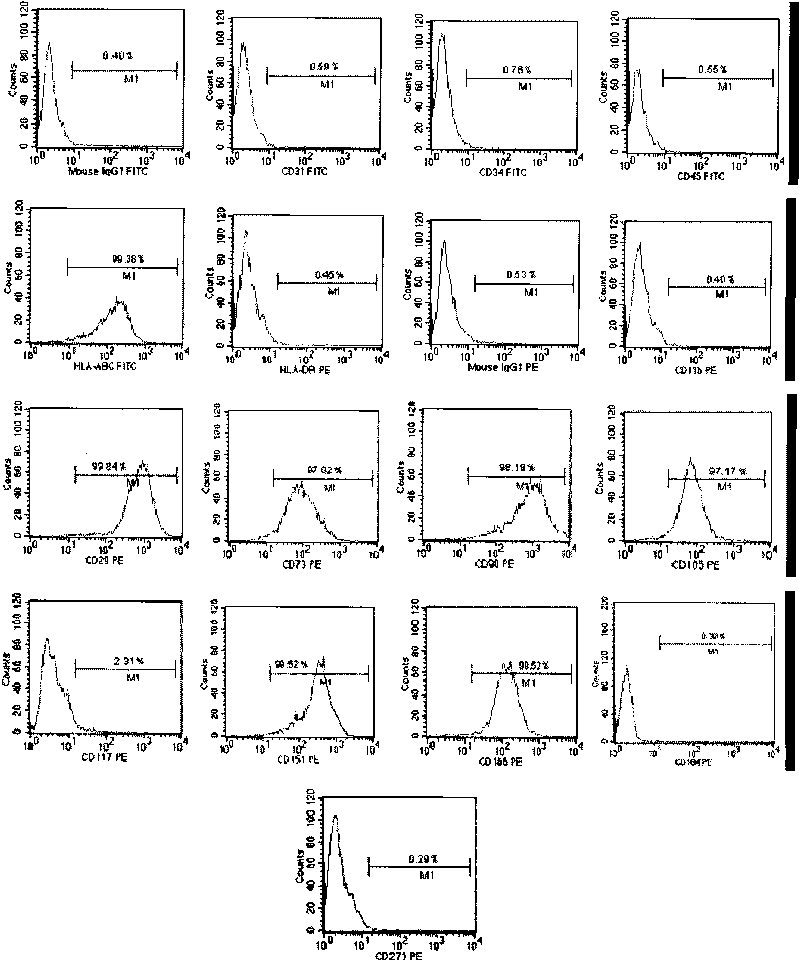
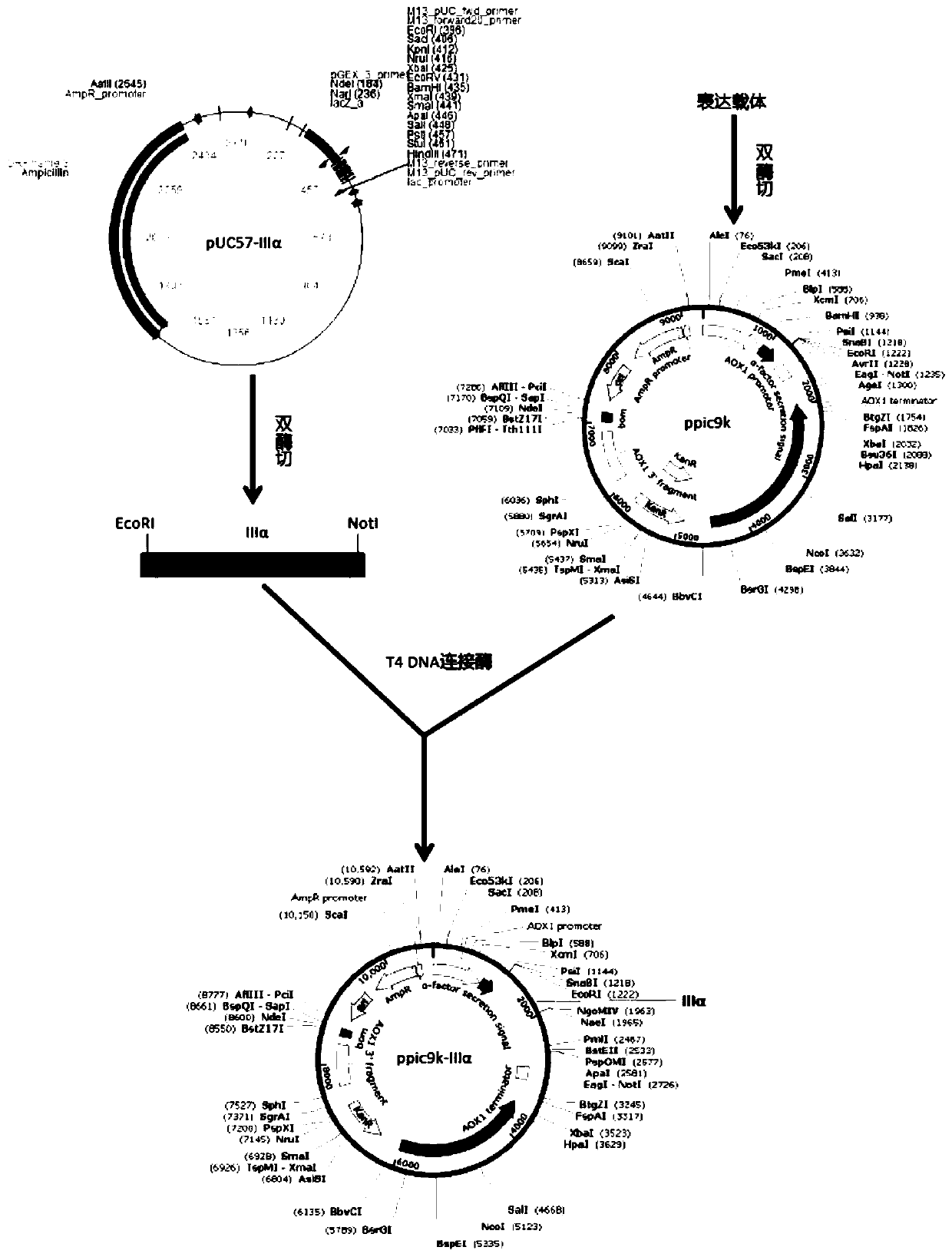
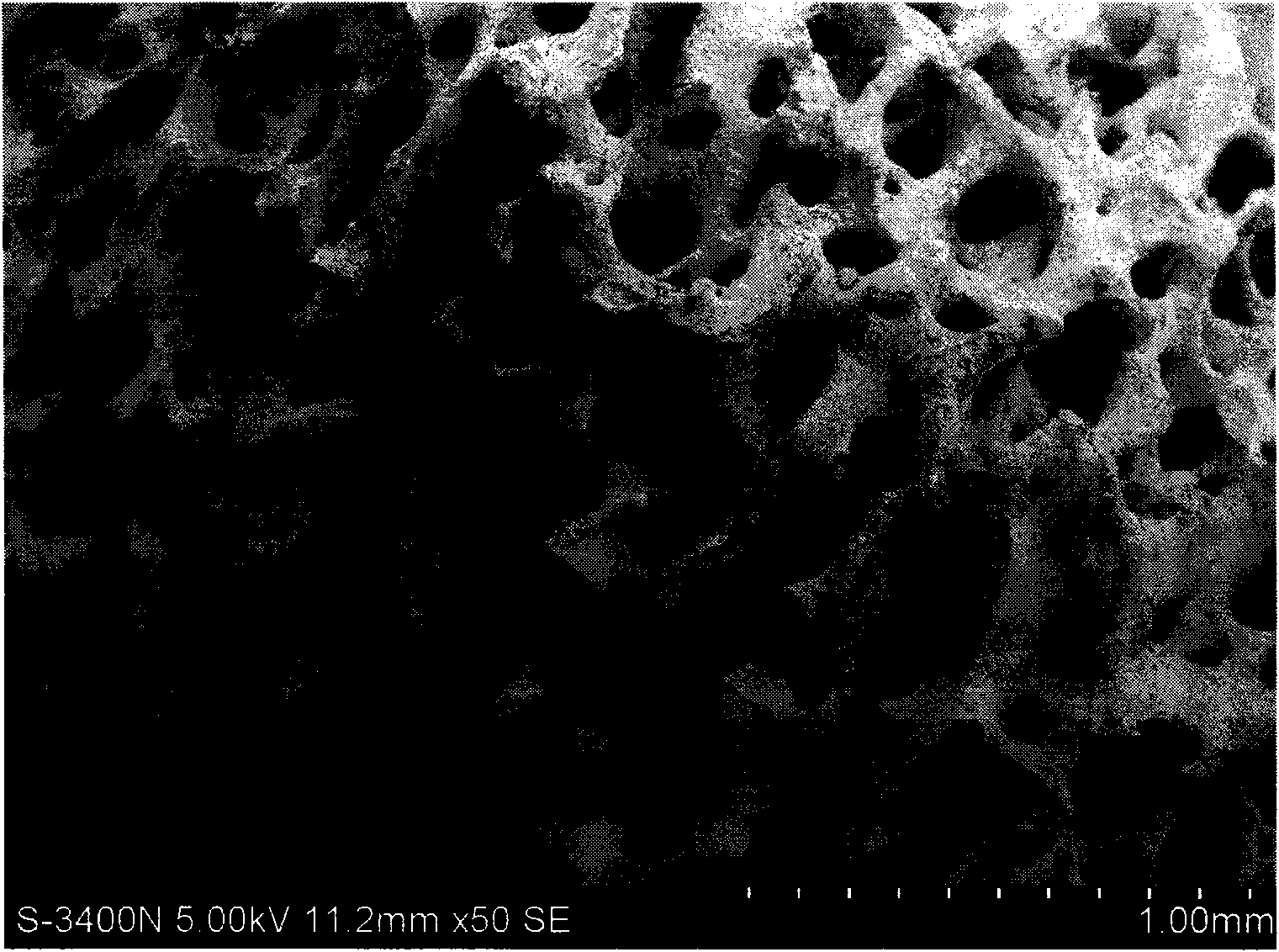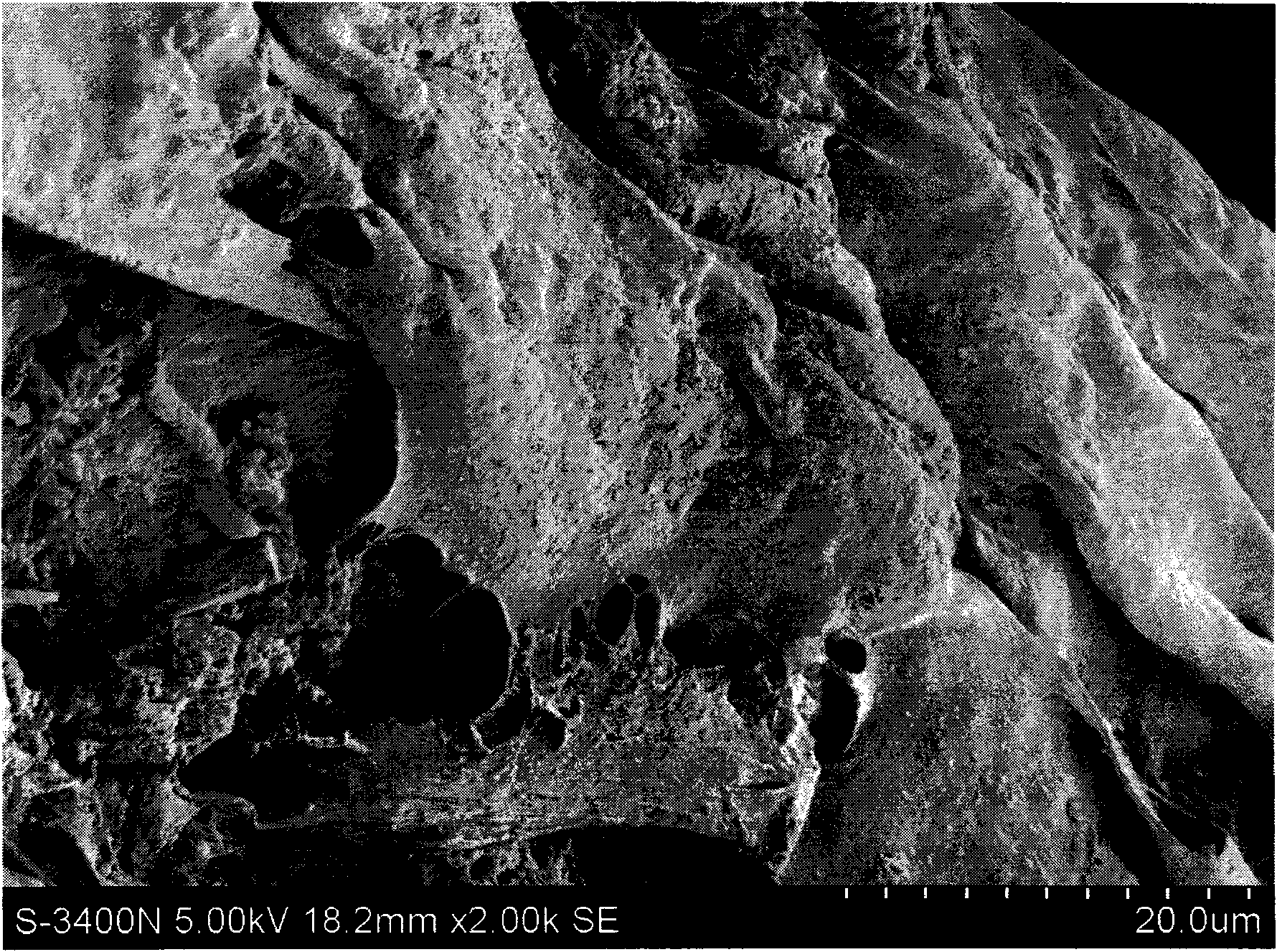Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
383 results about "Immune rejection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
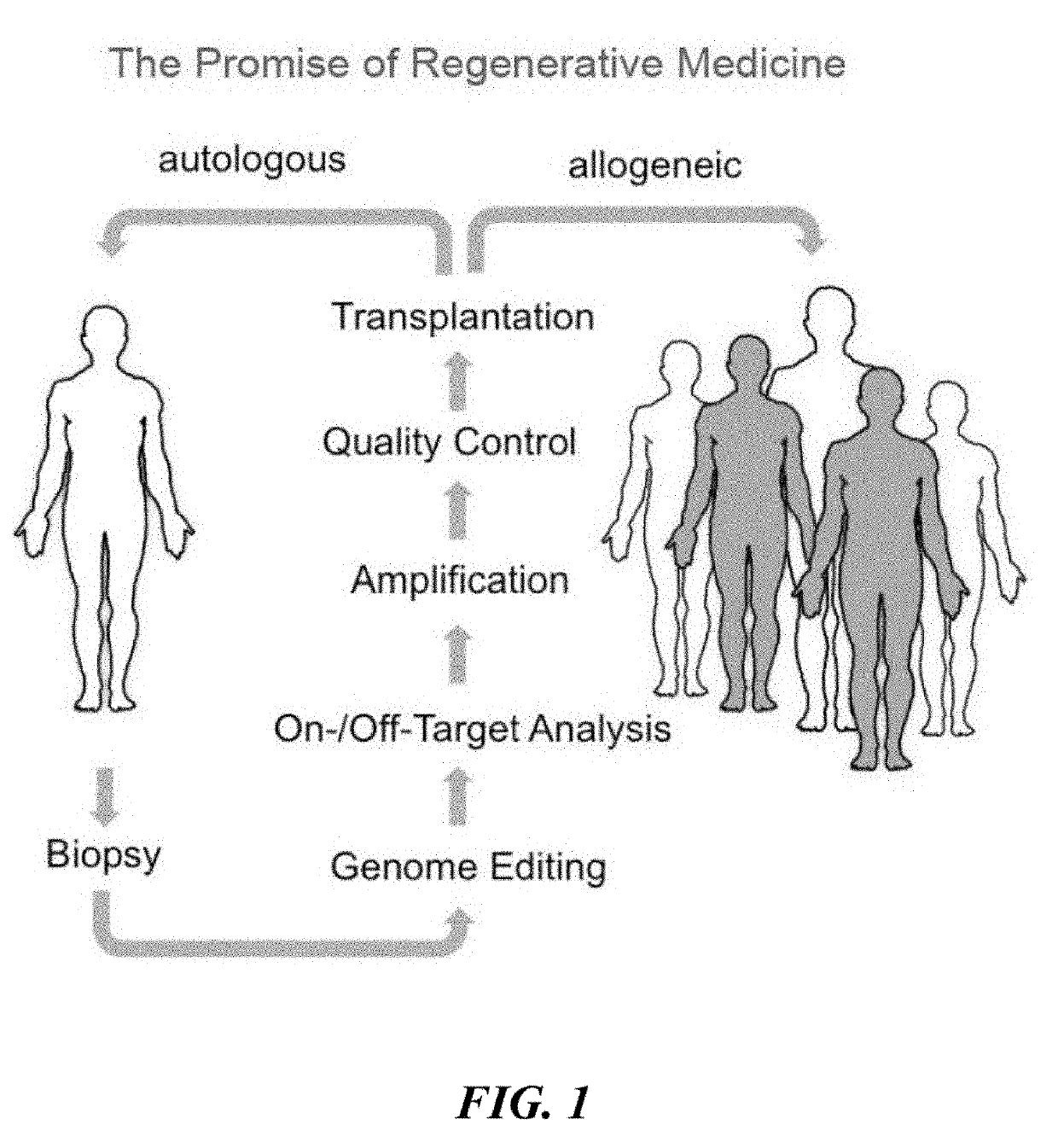
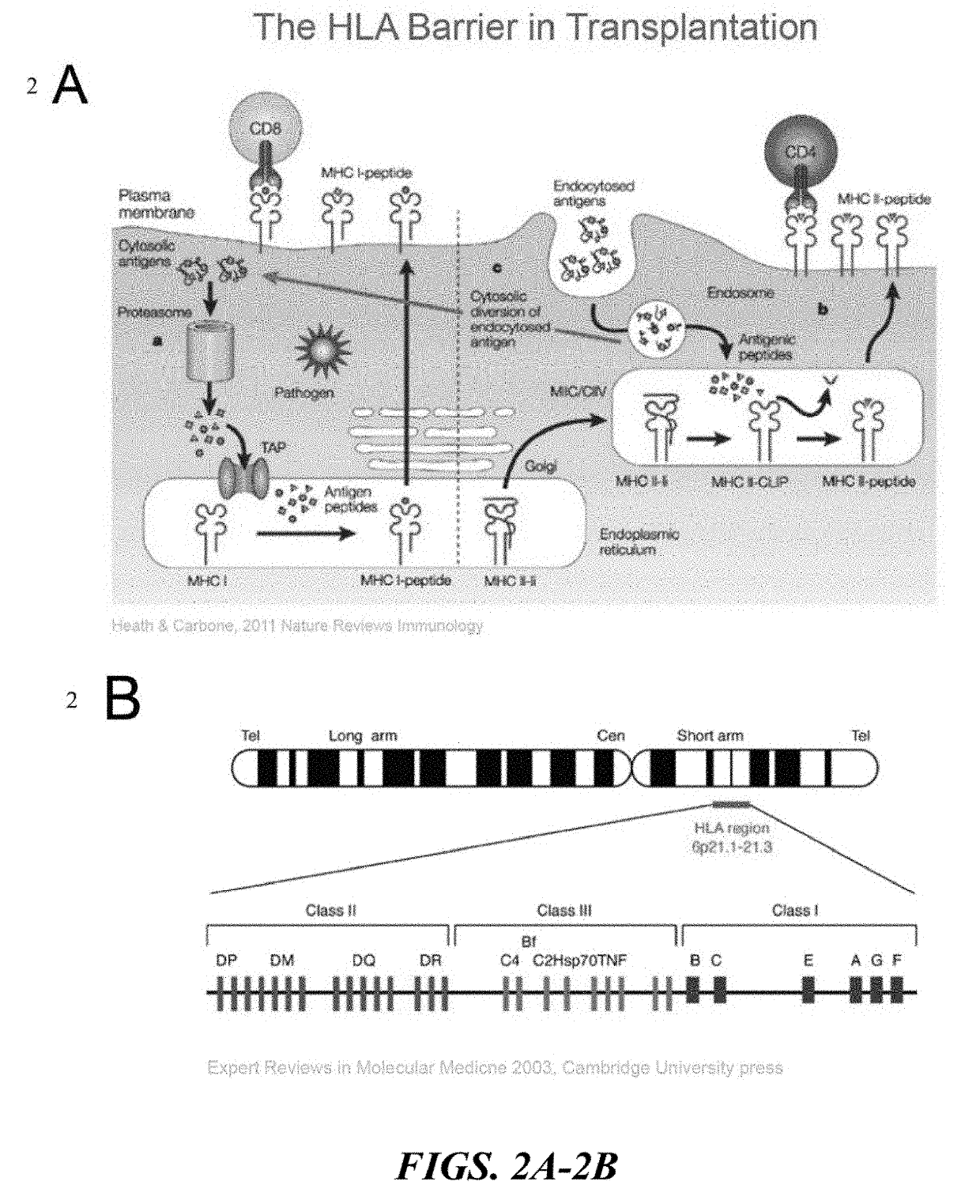
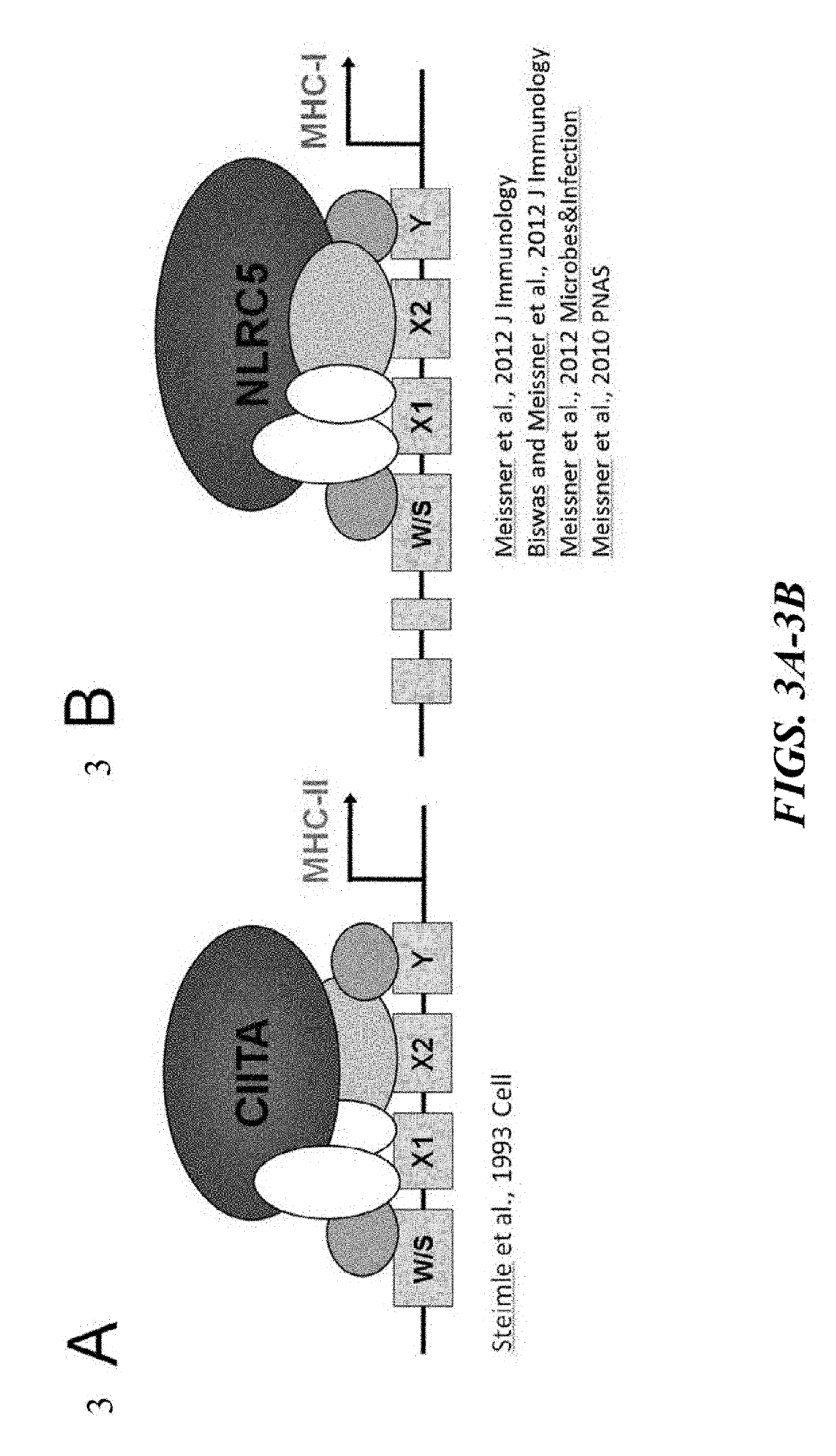
Immune rejection is a complication that may occur with stem cell transplantation. When it occurs the immune system of a person sees the transplanted cells as 'foreign' and thus begins a fast and possibly aggressive response to attack those cells that are not recognized as 'self.' During a chronic rejection response, the attack is:
Preparation method for cellfree intestinum tenue submucosa biological material
ActiveCN101366975ARepair defectRepair membranous defectsProsthesisBiocompatibility TestingIntestinal submucosa
The invention relates to a method for preparing a biomaterial of acellular small intestinal submucosa, which comprises the steps of preposition treatment, acellular treatment, enzyme treatment, preparations of membranous products and particle products. Compared with the prior art, the biomaterial has higher bioactivity and biocompatibility, no obvious immune rejection and no toxic effect on cells; besides, the biomaterial has a certain mechanical strength and toughness, and variable shape, size and thickness, thereby being convenient for clinical suturing and fixing. At the same time, the preparation method has the advantages of unlimited raw material source, cell-free residues, no ethical issues and being capable of effectively inactivating virus. The prepared products are applicable to the biomedical engineering fields, such as repairing defections of body tissue, serving as tissue filling materials, repairing facial depression deformity, serving as tissue reinforcements to replace fascia, repairing membranous defections and malnourished and infected surfaces of wound, serving as materials for biodegradable stents, and serving as injectable filling materials.
Owner:SHAANXI RUISHENG BIOTECH
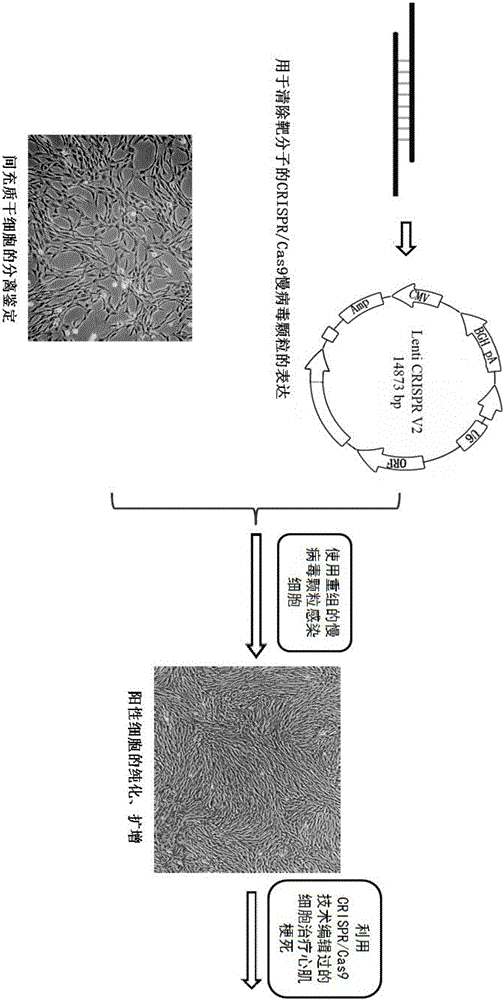
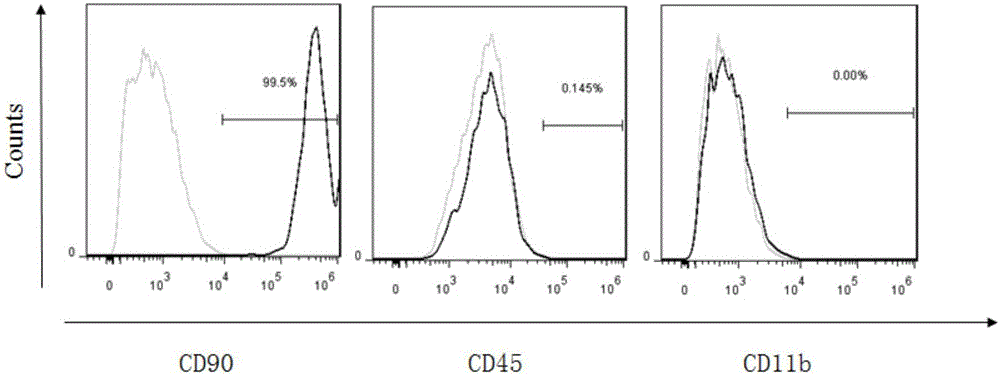
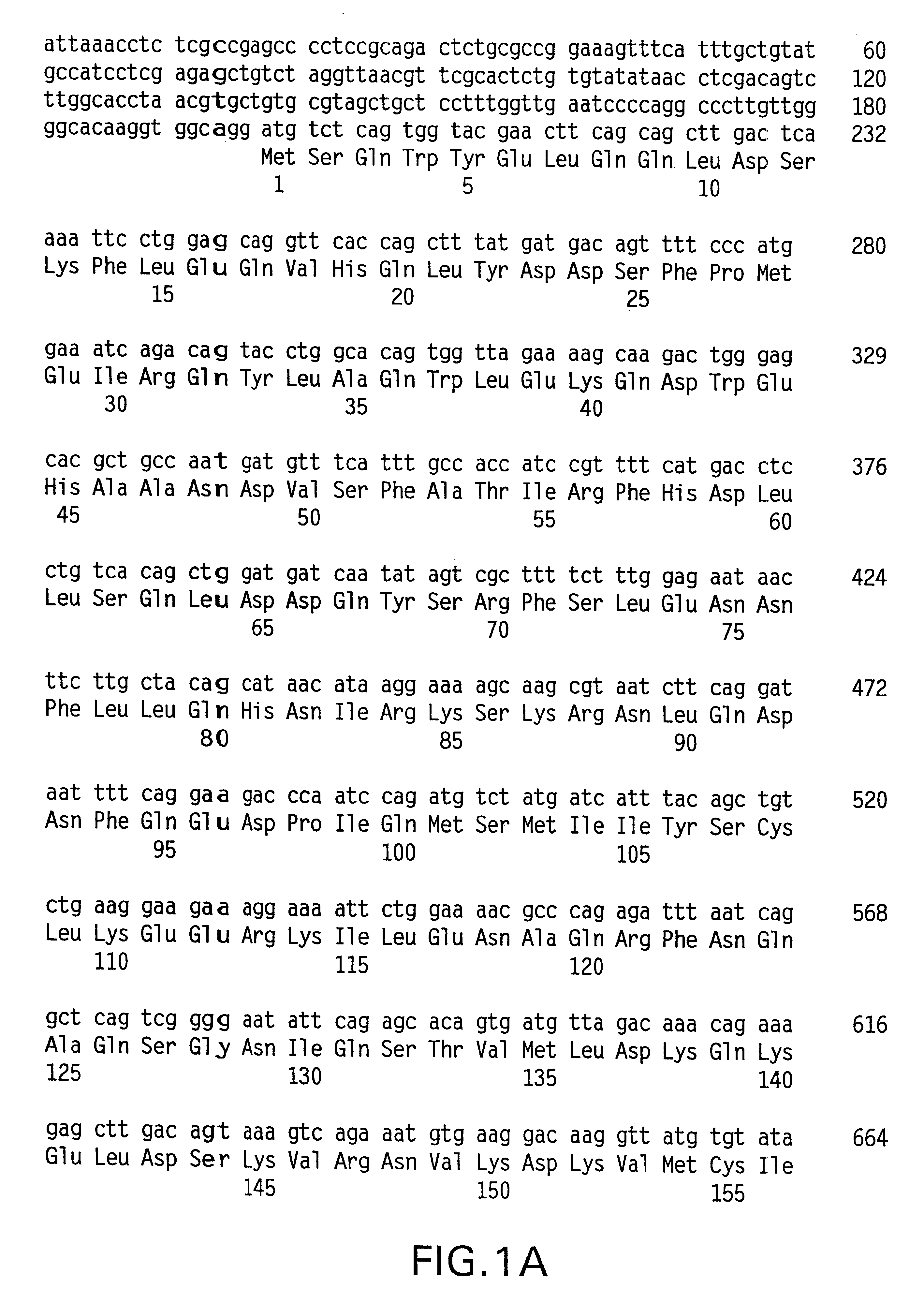
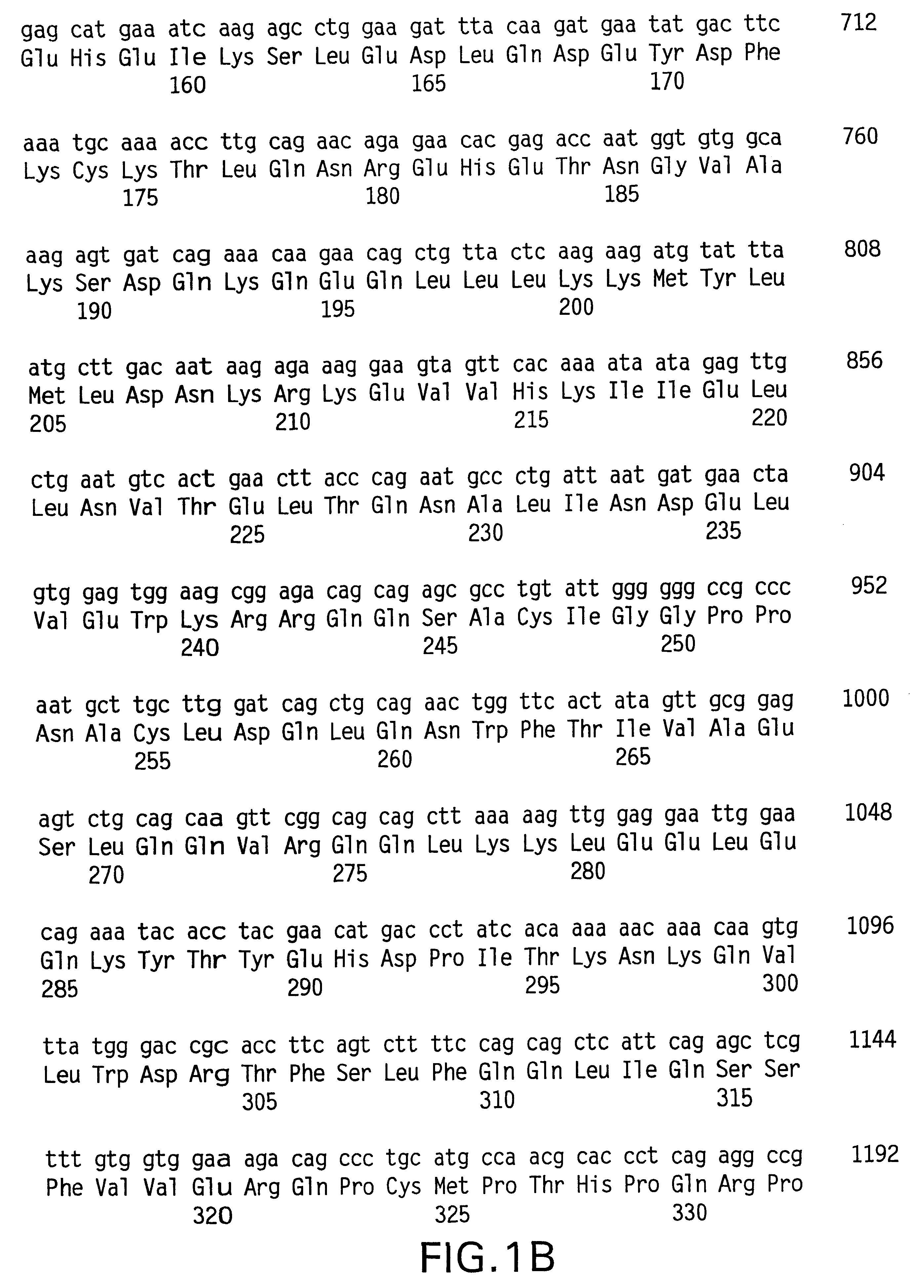
Preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of allogenic mesenchymal stem cells in treating myocardial infarction
ActiveCN105985985AImprove anti-apoptotic abilityPromote homingUnknown materialsFermentationAntigenInflammatory factors
The invention belongs to the field of allogenic mesenchymal stem cells, and particularly relates to a preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of the allogenic mesenchymal stem cells in treating myocardial infarction. The preparation method comprises the following steps: carrying out separation by density gradient centrifugation to obtain allogenic single karyocytes, and carrying out adherent culture to obtain mesenchymal stem cells; designing a mesenchymal stem cell surface antigen B2M-gRNA and an inflammatory factor TNF-alpha-gRNA; establishing recombinant slow virus particles, and transfecting the mesenchymal stem cells; optimizing the mesenchymal stem cells by using IGF-1; and preparing drugs for treating myocardial infarctions by using the modified and optimized mesenchymal stem cells. The CRISPR / Cas9 technique is utilized to remove the antigens capable of causing immunological rejection and the inflammatory factors capable of causing inflammatory reaction on the mesenchymal stem cell surface, and the IGF-1 is utilized to enhance the apoptosis resistance of the mesenchymal stem cells and promote the homing of the mesenchymal stem cells, thereby providing a new technical scheme for preparing drugs for treating cardiovascular diseases in clinic. The prepared allogenic mesenchymal stem cells can not cause immunological rejection after cell transplantation.
Owner:SUZHOU UNIV
Method for identifying a compound to be tested for an ability to reduce immune rejection by determining Stat4 and Stat6 proteins
InactiveUS6534277B1Easy to useReduce immune rejectionMicrobiological testing/measurementLibrary screeningDiseaseStat signaling
The present invention relates to methods for identifying compounds that can reduce immune rejection, for example, transplant- or autoimmune disorder-related immune rejection. The present invention is based, in part, on the discovery, demonstrated herein, that immune rejection can be monitored by determining the amount of particular members of the Jak / Stat signal transduction pathway present within an affected tissue. The present invention is further based, in part, on the discovery, demonstrated herein, that immune rejection can be reduced and tolerance can be induced by modulating the amount of these particular members of the Jak / Stat signal transduction pathway present, expressed or active within an affected tissue. In particular, the results demonstrate that immune rejection can be monitored by determining the amount of mRNA or protein of Stat1, Stat3, Stat4, Stat6, SOCS1, or SOCS3 present, e.g., in an affected tissue.
Owner:MILLENNIUM PHARMA INC
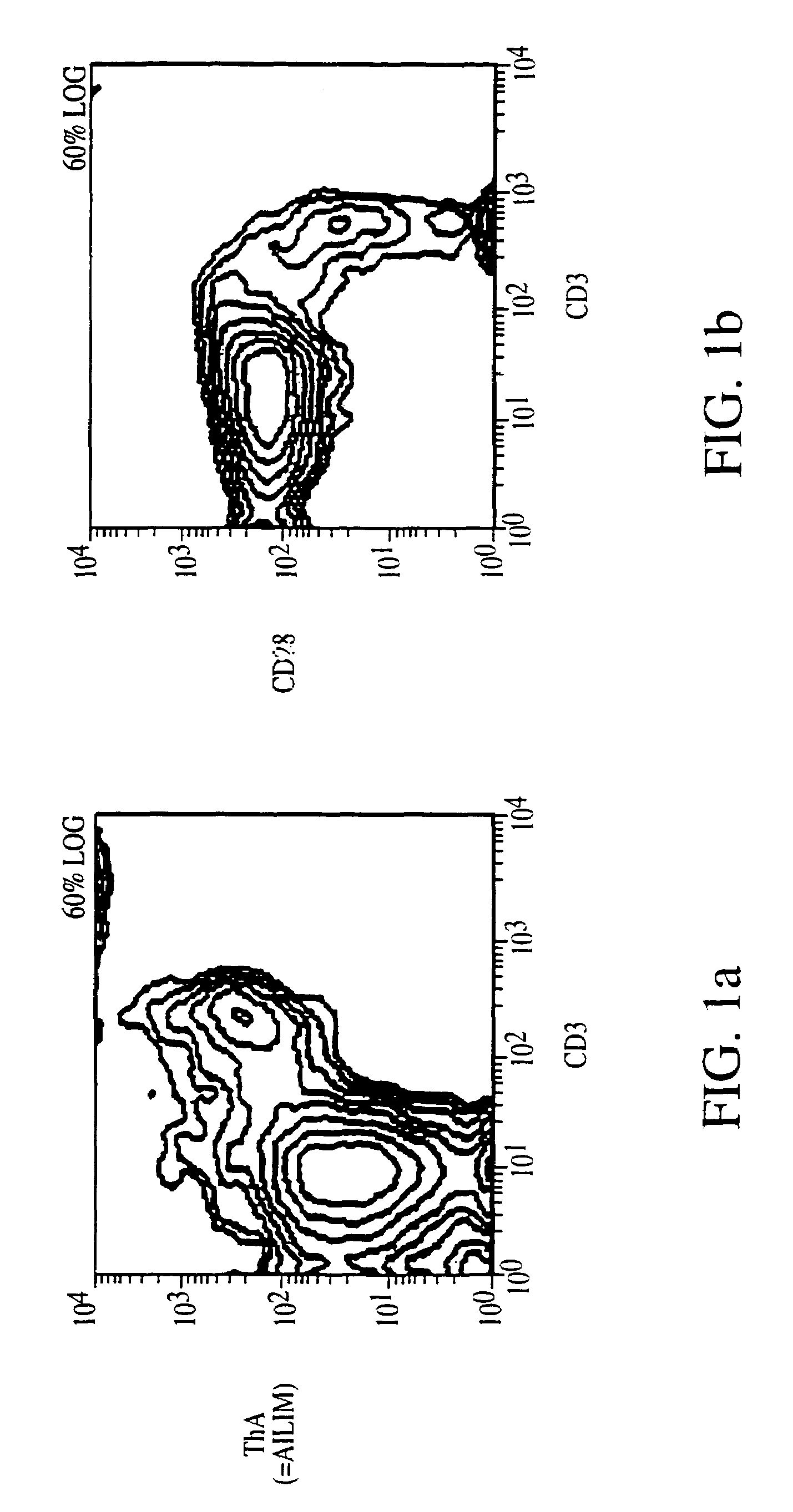
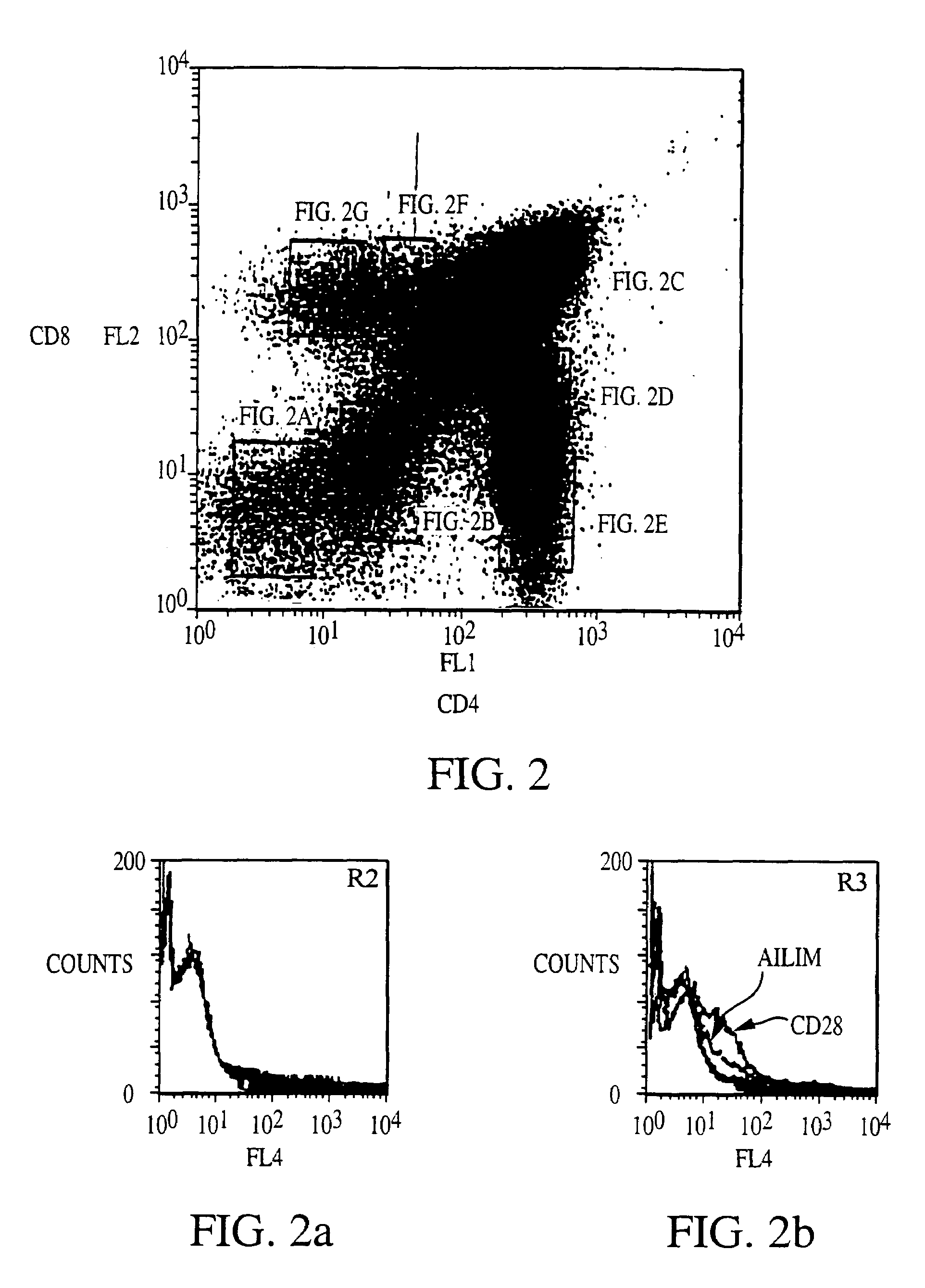
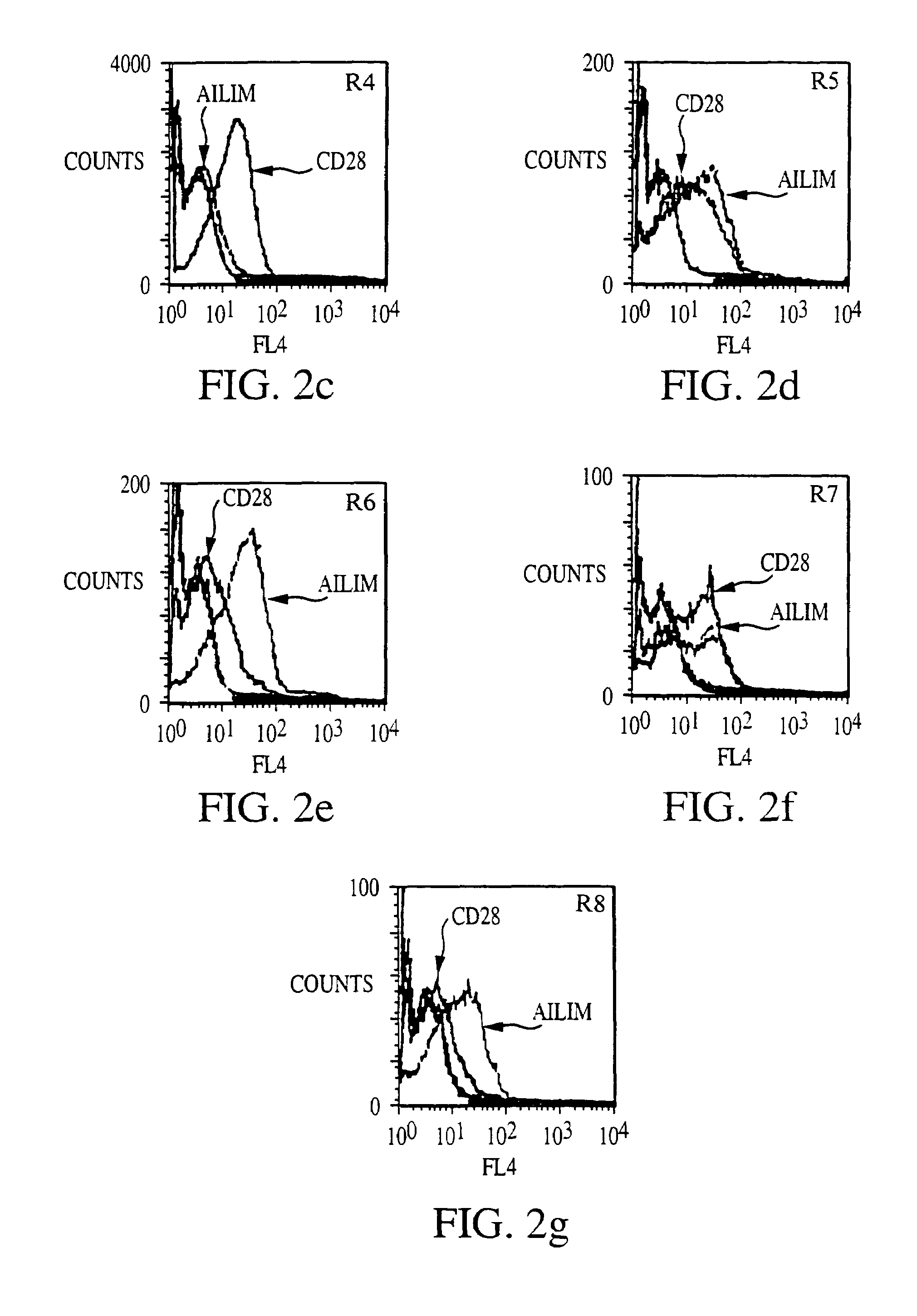
Methods of preventing or treating graft versus host reaction by administering an antibody or portion thereof that binds to AILIM
InactiveUS7465445B2Modulate productionPrevent diseaseOrganic active ingredientsBiocideAntigenTherapeutic effect
An antibody against AILIM (alternatively called JTT-1 antigen, JTT-2 antigen, ICOS and 8F4) was found to have a significant therapeutic effect on arthrosis, for example, rheumatoid arthritis and osteoarthritis, graft versus host disease, graft immune rejection, inflammation (hepatitis and inflammatory bowel diseases), diseased condition accompanied by the excessive production of an antibody against a foreign antigen triggered by immunological sensitization by the antigen.
Owner:JAPAN TOBACCO INC
Tailor-made pluripotent stem cell and use of the same
InactiveUS20080003560A1Lower Level RequirementsTransplantation rejection reaction could be significantly reducedGenetically modified cellsHybrid cell preparationEgg cellSomatic cell
An object of the present invention is to efficiently establish cells, tissues, and organs capable of serving as donors for treating diseases, without eliciting immune rejection reactions, without starting with an egg cell. This object was achieved by providing a pluripotent stem cell having a desired genome. The cell was produced by treating with a reprogramming agent, producing a fusion cell of an MHC deficient stem cell with a somatic cell, or after producing a fusion cell of a stem cell with a somatic cell, removing a gene derived from the stem cell by performing genetic manipulation with a retrovirus.
Owner:REPROCELL
Double-layer composite regeneration film and making method thereof
ActiveCN103191085AHigh mechanical strengthDegradation time controllableKetone active ingredientsAmide active ingredientsCompound aFiber
The invention relates to a double-layer composite regeneration film and a making method thereof. The regeneration film comprises a compact layer and a loose layer, the compact layer is formed through compounding a degradable high-molecular fiber web with a collagen film, or with collagen and nano-silver particles, or with collagen and a medicine having a treatment effect, and the loose layer is formed by treating one or more selected from chitosan, silk fibroins, keratoprotein and collagens as a matrix material. The preparation method mainly comprises a step of preparing the fiber strengthened compact layer, a step of preparing the loose layer, and a step of preparing a guide film through compounding. The regeneration film has the advantages of good biocompatibility and mechanical performances, biodegradability, low immune rejection response, simple operation and the like, and can be widely used for restoring tissues, especially bone tissues and periodontal tissues.
Owner:SHENZHEN LANDO BIOMATERIALS
Tissue engineering artificial skin and preparation method thereof
ActiveCN104984407AWith mechanical strengthOrientational structureProsthesisCollagenanViral infection
The invention provides a tissue engineering artificial skin and a preparation method thereof. The artificial skin comprises an epidermal layer and a dermal layer, wherein the epidermal layer material is made of collagen or a mixed material of collagen and chitosan; the dermal layer material is made of collagen or a mixed material of collagen and chitosan, hyaluronic acid, chondroitin sulfate, alginate or polyving akohol. The epidermal layer comprises a semipermeable film; the dermal layer comprises two layers of three-dimensional porous supporting structures with different porosities. The used collagen is end-free collagen, so that the problems of immune rejection, foreign body reaction, viral infection and the like of the artificial skin during application are solved. The invention further provides the preparation method for the tissue engineering artificial skin.
Owner:BEIJING PAISHENG BIOTECH CO LTD
Recombinant human collagen and application thereof
InactiveCN110194795AHigh purityIncrease productionConnective tissue peptidesBacteriaBiotechnologyProtein target
The invention discloses a recombinant human collagen and an application thereof. An amino acid sequence of the protein is shown as SEQ ID NO. 3, the nucleotide sequence of the protein-coding gene is shown as SEQ ID NO. 1. The recombinant human collagen of the invention has very good hydrophilicity and stability, and the amino acid composition thereof is 100% identical to the corresponding amino acid sequence of the natural collagen, and the amount of expressed protein can account for about 25% of the total protein of the thalline, per milliliter of the bacteria liquid precipitate contains 0.25mg of the target protein, and the production cost is very low and the cycle is short. The prepared collagen is applied to the human body without immunological rejection and allergic reaction, and canbe widely applied to the biomedicine and cosmetics industries.
Owner:郭伟
Humanization active forging bone and preparation method thereof
ActiveCN101564553APromotes Adhesive GrowthPromote ingrowthProsthesisBone structureCell-Extracellular Matrix
The invention discloses a humanization active forging bone and a preparation method thereof. The humanization active forging bone has main components of hydroxyapatite crystal, natural animal cancellous bones, particles of which the particle size is between 0.1 and 5 millimeters and having three-dimensional netlike porous structures, and a coating of which the pore-size distribution is between 50 and 600 microns and the surface is compounded with extracellular matrix synthesized and excreted by human cells. The forging bone not only has a similar structure with a human bone and is advantageous for cell ingrowth, formation of new bones, and bone structure reconstruction at a transplantation position, but also can be firmly combined with the coating of which the surface is coated with the extracellular matrix synthesized and excreted by the human cells and cell growth factors, promotes the attached growth of in vivo osteoblasts, and accelerates the formation of the new bones; and simultaneously, the forging bone has the advantages of molding randomly and not causing immunological rejection reactions, and can satisfy treatment needs of clinical bone defect and nonunion.
Owner:SHAANXI RUISHENG BIOTECH
Tissue engineering corium and its preparation method
A tissue-engineered epidermis is prepared from skin fibroblasts through obtaining the skin fibroblasts, preparing culture medium, amplifying culture, preparing extocytic matrix compound, preparing biologic scaffold, compounding the skin fibroblasts on the surface of biologic scaffold, and 3D culture. Its advantages are a certain elasticity and toughness, short culture time, and no obvious immunorejection reaction.
Owner:陕西艾尔肤组织工程有限公司
Method for reshaping and beautifying by using tissue engineering fat regeneration technology
The invention provides a method for reshaping and beautifying by using tissue engineering fat regeneration technology, which is applicable to plastic surgery and reshaping and beautifying and belongs to the field of tissue engineering. The method comprises the following technical routes: directly extracting mesenchyme matrix cells with multiple differentiation capabilities from fatty tissues of a patient, culturing and propagating the mesenchyme matrix cells in vitro, wrapping a specific amount of cells and other necessary components in a hydrogel stent material, and implanting the hydrogel stent material into the corresponding part in the body of the patient. The cells are grown into fatty tissues along with degradation and absorption of the stent material so as to perform regenerative repair on the soft tissue defect of the patient. The method has the advantages that the cells of the patient do not bring immunological rejection to the patient, and the regenerative repair effect is safe, natural and durable because the used hydrogel stent material is finally degraded and absorbed in the body of the patient.
Owner:王影
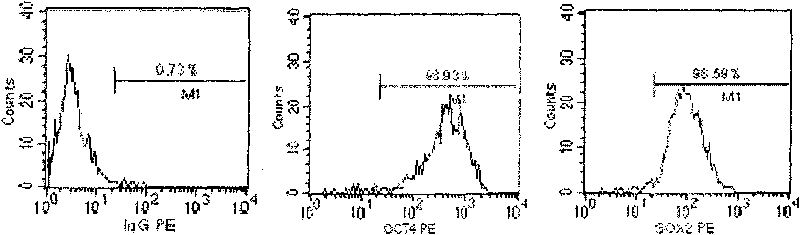
Sub totipotential stem cell and preparation method and application thereof
The invention discloses a method for preparing a population of?human pluripotent stem cells and the application thereof. The preparation of stem cells is characterized by comprising the following steps: CD151<+>, CD31<->, Sox<2+> pluripotent stem cells are separated and collected from human umbilical cord and or placenta tissues; the cells adhere to grow in a culture vessel under a predetermined condition and expand through passage 20 or above to be still stable in gene expression. The population of cells of this invention do not form teratoma after injection into animals. The human pluripotent stem cells highly express CD151, OCT4 and Sox-2 as specific markers of embryonic stem cells, as well as specific markers of epidermic cells, endothelial cells, thrombocytes, dendritic cells, while lack expression of CD31, CD34, CD45 and HLA-II. The pluripotent stem cells are also characterized as being able to adhere to tissue culture plastic and having the potential to differentiate into three germ layers: endoderm, mesoderm and ectoderm. These pluripotent stem cells are able to be used as carrier cells of gene therapy and for the treatment of diseases caused by cell damage or cell aging. The present invention provides a method of isolating, purifying and culturally expanding of a population of human pluripotent stem cell for preparing the high purity injection preparation. The preparation of stem cells has a good therapeutic effect on the treatment of diseases caused by cell damage or cell aging in animal and human clinical trials. The preparation also has no toxic side effect and no immune rejection.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Preparation method and device of duramater/spinal dural transplanting substitute
ActiveCN102727935ASimple Surface Functional StructureWidely sourced and cheapProsthesisAntigenDefect repair
The invention provides a preparation method of a duramater / spinal dural transplanting substitute which is obtained by repeated freezing and thawing of dural tissue, rolling and cracking of cells, crosslinking fixed protection, accellular antigen extraction, dense surface fibrosis modification, packaging and sterilization, and has the advantages of simple method, wide raw material sources, cheap raw materials, and low cost. The prepared dural substitute completely removes components of cells and other antigen components simultaneously when protecting dural tissue natural structure and properties, is good in biocompatibility, free of immune rejection, safe and reliable, good in mechanical performance, and easy in clinical operation, can meet the needs of defect repair, has the function of promoting tissue regeneration as a loose surface is beneficial to the tissue fluid adsorption, active factor enrichment, and growth of blood vessels and cells, and has the advantages of rapidness in fusion with a host, biodegradable absorption, and good repair effect. The animal test shows that the defect can be completely repaired without brain or spinal fluid leakage, or adhesion with brain tissue, and significant rejection is not found.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Tissue patch and preparation method thereof
ActiveCN101366979AImprove biological activityGood biocompatibilityProsthesisCell freeBiocompatibility Testing
The invention provides a tissue patch and a preparation method thereof. The tissue patch uses an acellular small intestinal submucosa as an inner layer, the two sides of the tissue patch are covered with acellular amniotic membranes; the prepared tissue patch isolates from immunotoxicity of the acellular small intestinal submucosa, and the acellular small intestinal submucosa compensates for the deficiencies of a mechanical strength of the acellular amniotic membrane. Compared with the prior art, the tissue patch has higher biological activity and biocompatibility, no obvious immune rejection and no toxic effect on cells; and the preparation method has the advantages of unlimited raw materials source, cell-free residues, no ethical issues and capability of effectively inactivating virus. The prepared product is applicable to serving as a tissue reinforcement to repair a shortcoming or a defect of a strength of a body soft tissue; the prepared product is used as a tissue filling material and is applicable to the repair of facial depression deformity.
Owner:SHAANXI RUISHENG BIOTECH
3D bio-printing medical dressing and preparation method thereof
InactiveCN105031713ASimple manufacturing processEasy to operateAdditive manufacturing apparatusAbsorbent padsCell freeSide effect
The invention discloses a 3D bio-printing medical dressing and a preparation method thereof. The medical dressing takes cell-free collagen as a main component and is prepared by a rapid prototyping technology. According to the invention, the medical dressing prepared from a bio-material containing cell-free collagen has the advantages of good biocompatibility, no toxic or side effect and no immunological rejection; meanwhile, by using the rapid prototyping technology for preparing the dressing with a 3D structure, the preparation process is convenient and fast, the operation steps are simple, the geometric shape, pore diameter, porosity and pore distribution of the dressing can be accurately controlled according to wound sizes of different patients and the characteristics of the material, and thus personalized medical dressings with good air permeability and hydroscopic property for patients can be prepared.
Owner:SOUTH CHINA UNIV OF TECH
Universal donor stem cells and related methods
ActiveUS20190309259A1Reduce and eliminate and activityReduce and eliminate surface expressionGenetically modified cellsStable introduction of DNAHLA-BImmunogenicity
Disclosed herein are universal donor stem cells and related methods of their use and production. The universal donor stem cells disclosed herein are useful for overcoming the immune rejection in cell-based transplantation therapies. In certain embodiments, the universal donor stem cells disclosed herein do not express one or more MHC-I and MHC-II human leukocyte antigens. Similarly, in certain embodiments, the universal donor stem cells disclosed herein do not express one or more human leukocyte antigens (e.g., HLA-A, HLA-B and / or HLA-C) corresponding to MHC-I and MHC-II human leukocyte antigens, thereby rendering such cells hypoimmunogenic.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for regenerating a derma tissue by utilizing fat mesenchymal cell
One purpose of the invention is to provide a method and a formula, which is characterized in that mesenchymal stem cells from an autologous fat cell tissue are proliferated by in-vitro culture, then is mixed into a support material and finally is transplanted into the body of a patient; the fat mesenchymal cell in a transplant is differentiated to derma fibroblast under the action of the local microenvironment, the newly generated derma fibroblast can further activate secretion of cell collagenstroma at an injection part so as to carry out reproductive repair on coloboma and aging of human skin tissue. The application scope of the invention includes: subcutaneous padding for face lift, subcutaneous padding cosmetology, subcutaneous padding for repairing depressed deletion or impairment and the like. Seed cells utilized in the invention are prepared by separating and purifying the fat tissues of the patients, so that ethical disputes and immunological rejection reaction are avoided. The fat tissues can be obtained by the instrument suction method; and the operation of the method is simple, patients suffer from minor damages and pains.
Owner:王影
Preparation method and application thereof of acellular conjunctiva matrix
InactiveCN101590292AEasy to prepareGood biocompatibilityEye implantsDead animal preservationTreatment effectBiocompatibility Testing
The invention relates to a preparation method and application thereof of an acellular conjunctiva matrix used as a tissue engineering corneal scaffold material. The method comprises the following steps: removing cell components in a bulbar conjunctiva first; preparing the acellular conjunctiva matrix; and taking the acellular conjunctiva matrix as a tissue engineering corneal scaffold. A rabbit corneal epithelium or an endothelial cell can form a good cell single layer on the scaffold, can construct a tissue engineering corneal epithelium or endothelium, and can successfully transplant the tissue engineering corneal epithelium or endothelium onto rabbit animal model eyes. The degradation time of the built tissue engineering cornea is longer, rabbit eyes have no obvious immune reject reaction, and the therapeutic effects on the lack of corneal limbus stem cells or the decompensation of the corneal endothelial cell function are obvious. The method and the application thereof have the advantages of simple preparation method, good biocompatibility, low antigenicity, easy growth and proliferation of seed cells, slow degradation, extensive bulbar conjunctiva sources and wide application and development prospects; and the transparency can be maintained all the time in the training process and after transplantation.
Owner:SHANDONG EYE INST
Bioactive polypeptide QEPVL, and preparation and application thereof
ActiveCN102964427AImproves antioxidant activityBoosts immune activityPeptide/protein ingredientsMicroorganism based processesLymphocyteDrug biological activity
The invention relates to the field of a protein and particularly relates to a milk-derived bioactive polypeptide QEPVL with in-vitro antioxidant activity and body immunity promoting activity, wherein an amino acid sequence of the bioactive polypeptide QEPVL is Gln-Glu-Pro-Val-Leu. Through an in-vitro antioxidant test and an in-vitro immunity promoting test, the polypeptide QEPVL is proved to have relatively good antioxidant biological activity and immunity improving function; on one hand, free radicals in the body can be removed to reduce the harm on the human body caused by the free radicals; on the other hand, according to the bioactive polypeptide QEPVL, the immunity of the body can be further enhanced and the multiplication capacity of lymphocytes in vitro can be promoted, thereby improving the ability of the body to resist infections of external pathogens and reducing the incidence rate of the body without causing immunological rejections. The bioactive polypeptide QEPVL has great significance for the development of milk products, healthcare products and medicines with the antioxidant function and the immunity enhancement.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Preparation method and application thereof for cell-biological bracket compound based on biological print technology
InactiveCN103272288ABioprinting technology is simple and easyLow costSurgerySpinal cord lesionNerves regeneration
The invention provides a preparation method and the application thereof for a cell-biological bracket compound based on biological print technology. The cell-biological compound is formed by fibrous protein which is drawn out from the autoblood of a patient and processed by an ink-jet print technology. The surface and the interior of the cell-biological compound encompass one or more trophic factors and bone mesenchymal stem cells derived from self. According to the invention, the biological print technology is adopted, the appearance shape, cells and encompassing state model of the trophic factors can be designed according to requirements and practical situation, the cell-biological bracket compound is obtained through printing accurately, the fibrous protein and the bone mesenchymal stem cells are derived from self, so that the problem of immunological rejection is avoided, the trophic factors are cultivated in the bracket and are released slowly with the degradation of the bracket material, the cell-biological compound is applied to the spinal cord injured part to promote nerve regeneration and functional reconstruction on the injured part.
Owner:谢杨 +1
Articular cartilage graft and preparation method thereof
ActiveCN103920190AAvoid allergiesLower immune responseJoint implantsCell-Extracellular MatrixCartilage lesion
An articular cartilage graft and a preparation method thereof are provided. The prepared articular cartilage graft is composed of a superficial layer, a middle layer and a deep layer from outside to inside; the thickness, shape and size of each layer are all matched with a cartilage injury part, and thus preoperative shaping is not required; after grafting, the articular cartilage graft has layer distribution corresponding to distribution of each layer of surrounding normal cartilages, is conducive to intercellular signal transmission, transduction and regulation, and can be better integrated with surrounding normal cartilage tissues; the articular cartilage graft has structure characteristics consistent with those of the natural cartilages, the collagen type II content is gradually decreased from the superficial layer to the deep layer, the GAG content is increased gradually from the superficial layer to the deep layer, and compression resistance and wear resistance are good; and chondrocytes in the cartilage graft are wrapped with an extracellular matrix, have low immunogenicity, allow generation of immunologic rejection to be avoided after grafting, can survive for a long term and exert functions, and improve cartilage repair long-term curative effects.
Owner:西安博鸿生物技术有限公司
Implantable bioartificial perfusion system
ActiveUS20130289540A1High densityEnhanced interactionMedical devicesPharmaceutical delivery mechanismAutonomic functionTissue fluid
The disclosure provides an implantable bioartificial active secretion system for providing a physiological regulating secretion such as insulin necessary for functionality of a physiologic activity such as glucose metabolism of a living-being host. The system includes a housing implantable within the host, in fluidic communication with tissue fluid indicative of a physiological regulating secretion need. A chamber within the housing contains a plurality of physiologically active, autonomously functioning, live secretory cells for producing the physiological regulating secretion. A continually operating two pump apparatus moves tissue fluid into contact with the secretory cells for pick up of the physiological regulating secretion for subsequent physiologically-effective dispensing into the host, while avoiding immunorejection of the host body or of the host to the secretory cells.
Owner:ZELTSER GREGORY +1
Method for processing porcine cornea for decellularization
ActiveUS20110183404A1Efficient processingMinimizing immune responseHormonesEye implantsMedicineDecellularization
Disclosed is a method for processing porcine cornea using an aqueous NaCl solution and an aqueous trypsin / EDTA solution to decellularize enucleated porcine cornea. The porcine cornea processed by the method causes neither inflammation nor immune rejection. The porcine corneal stroma decellularized by the method can be recellularized together with host keratocytes after transplantation.
Owner:SEOUL NAT UNIV R&DB FOUND
Recombinant human type III collagen, expression strain and construction method
ActiveCN111363029AHigh purityImprove hydrophilicityFungiConnective tissue peptidesHuman bodyEngineering
The invention discloses a recombinant human type III collagen protein, an expression strain and a construction method. The recombinant human type III collagen contains 498 amino acids and has a theoretical molecular weight of about 54.5kd. The constructed recombinant human type III collagen expression strain can effectively, stably and largely express the recombinant human type III collagen. The recombinant human collagen has good hydrophilicity and stability, the structure is 100% identical to the corresponding part of a natural collagen gene sequence, immune rejection does not caused when the recombinant human collagen is used in a human body, and the recombinant human collagen can be widely used in fields such as biomedical materials and cosmetics.
Owner:JIANGSU JLAND BIOTECH CO LTD
Construction method of tissue engineering blood vessel
InactiveCN101259292AGood cell compatibilityHigh mechanical strengthProsthesisVascular endotheliumSurgery
The invention relates to a construction method of a tissue engineering blood vessel which belongs to the tissue engineering field. The construction method of the tissue engineering blood vessel includes the following steps: (1) tubular autologous living tissue bed frame material is connected to a continuous perfusion bioreactor under the vitro aseptic condition, and the outside of a vessel lumen is filled with culture medium; (2) endothelial cells obtained from the vitro culture medium is suspended at M199 or vascular endothelium-like cells generating from the induction of bone marrow mono nucleus cells are suspended at endothelial progenitor cell culture medium and are inoculated on the inner surface of the vessel lumen according to a high density planting method; (3) after the endothelial cells or the vascular endothelium-like cells are adhered, the inner cycle and the external cycle of the vessel lumen are communicated and the blood circulation is simulated to provide proper pressure stimulation and shear stress stimulation and the endothelial cells or the vascular endothelium-like cells are cultured for 5-7 days in a carbon dioxide incubator. The construction method has the advantages that perfect cell compatibility and enough mechanical strength are provided, the defects of immunological rejection and inflammation reaction, etc. caused by heterogenetic material are overcome, and the preparation time is short.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Tissue mending material with biological activity and preparation method thereof
The invention relates to a tissue repairing material with bioactivity and a preparation method thereof. The material is made by compounding human body living cells, and extracellular matrixes and cell growth factors synthesized and excreted by the human body living cells on the acellular small intestine submucosa. Natural antigen components are removed from the prepared tissue repairing material with bioactivity, and when the tissue repairing material is applied to a wound, the tissue repairing material can survive and directly take part in the repair of the wound to continuously synthesize and excrete growth factors, guide the cells around the wound to grow in and the creation of blood vessel, and induce the differentiation of stem cells to skin cells, thereby obviously promoting the wound healing; besides, the tissue repairing material not only has part of the characteristics of human body tissues and the good mechanical property of the acellular small intestine submucosa, but also has high biocompatibility to human body, obviously reduces the immune rejection; at the same time, the prepared tissue repairing material can cover the surface of wound, fill the defection of soft tissues, promote the growth and proliferation of the cells around the wound, repair the defection of the soft tissue organs, and promote the wound healing.
Owner:SHAANXI RUISHENG BIOTECH
Biological degradable hemostatic sponge material and its preparing method
ActiveCN1820789AImprove adhesionLower immune responseAbsorbent padsBandagesDamages tissueFreeze-drying
The present invention discloses a kind of biodegradable hemostatic sponge material and its preparation process. The preparation process includes the following steps: 1. dissolving collagen like human collagen in distilled water to form 0.5-3 % concentration solution; 2. dissolving chitosan in dilute acid solution to form 0.5-2 % concentration solution and neutralizing with alkali; and 3. mixing the two kinds of obtained solution, vacuum defoaming and freeze drying to form sponge and Co-60 irradiating sterilization. The biodegradable hemostatic sponge material has high adhesion to tissue, less immune rejection, high toughness, excellent pain relieving and sterilizing effect, capability of promoting the recovery of damaged tissue, high hemostatic effect and greatly raised safety.
Owner:NORTHWEST UNIV
Nerve conduit and preparation method thereof
InactiveCN104689376AImmune rejectionFree from virus transmissionTubular organ implantsMedicineElectrospinning
The invention provides a nerve conduit. The nerve conduit is characterized by comprising an inner layer, an outer layer and a cavity; the outer surface of the inner layer is wrapped with the outer layer; the cavity is formed between the inner layer and the outer layer; the inner layer is a hydrophilic cytoskeleton layer manufactured by the electrostatic spinning method; the outer layer is a hydrophobic nerve conduit skeleton layer by the electrostatic spinning method; the cavity is used for storing a bioactive factor solution. The nerve conduit has the characteristics of being proper in strength and hardness, degradable, capable of providing proper neurotrophic active substances, ideal in double-layer or multi-layer structure, semi-permeable, low in cost, short in production cycle, easy to be stored and transported, free of viruses, free of immunological rejection or extremely small in immunological rejection, and wide in applicable scope; the requirement on nerve regeneration process can be met well.
Owner:邹蓉
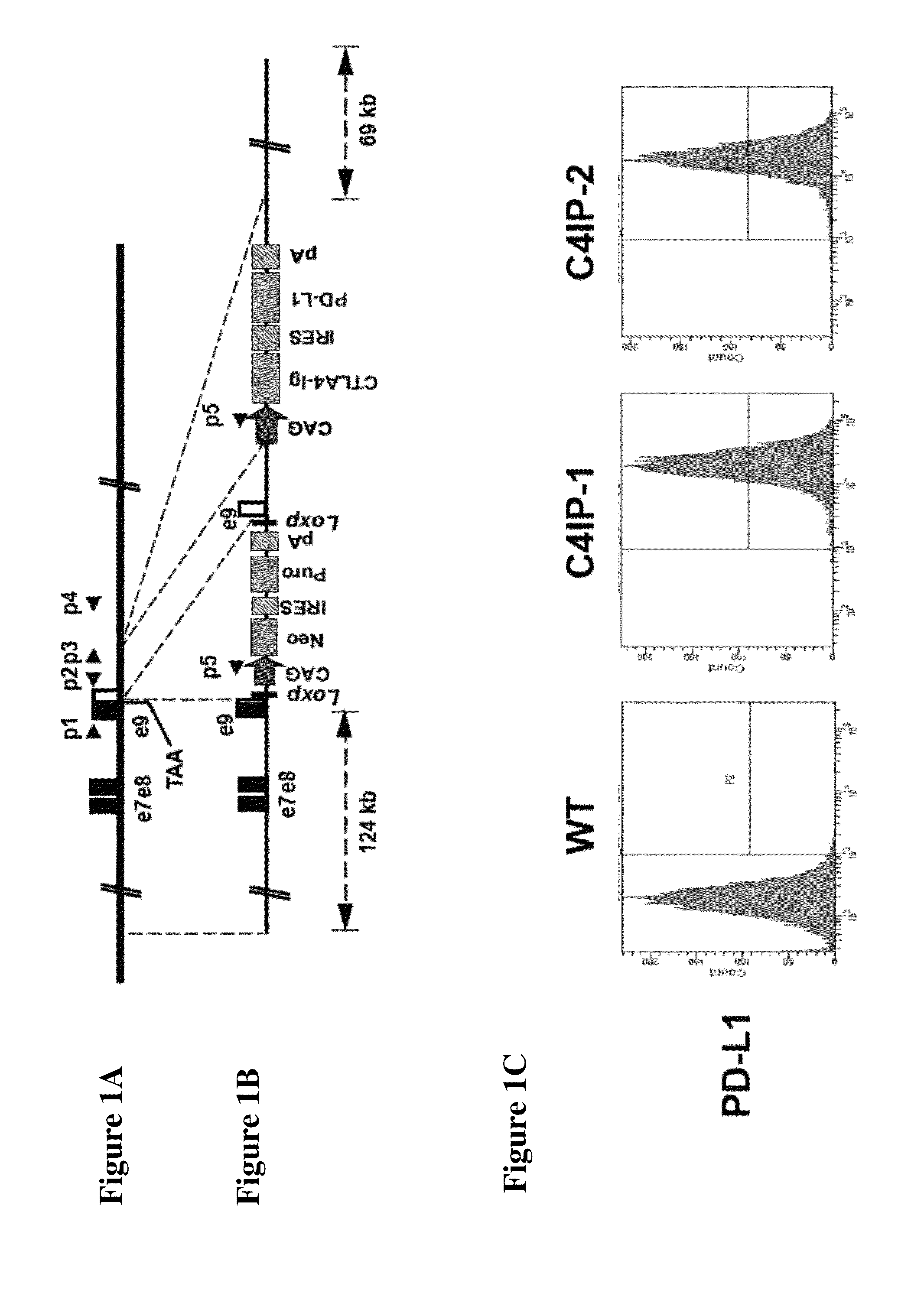
Compositions and methods for preventing allogeneic immune rejection
InactiveUS20150139994A1Prevent rejectionPrevent allogeneic rejectionPeptide/protein ingredientsAntibody mimetics/scaffoldsAllogeneic cellPd l1 expression
The present invention provides methods for preventing the allogeneic immune rejection of allogeneic cells, such as cells derived from human Embryonic Stem Cells (hESCs), without suppressing the entire immune system. Also provided a vector containing a CTLA4-Ig and PD-L1 expression cassette, and compositions containing a CTLA4-Ig and PD-L1 for use in preventing allogeneic immune rejection of allogeneic cells.
Owner:RGT UNIV OF CALIFORNIA
Conformal coating of cells for immunoisolation
ActiveUS20140147483A1Lower the volumeMinimal diffusion barrierBiocideDispersion deliveryConformal coatingBiological materials
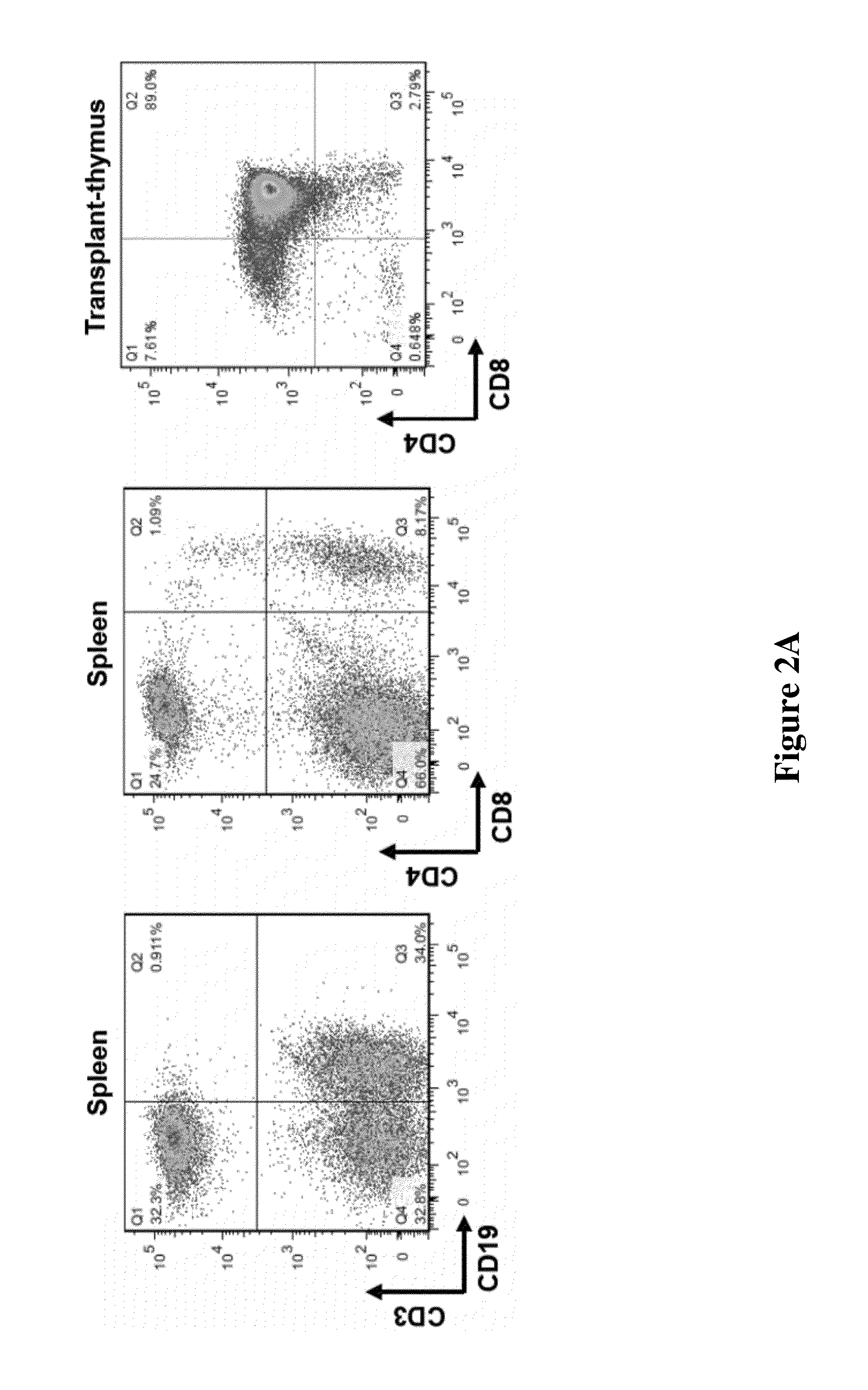
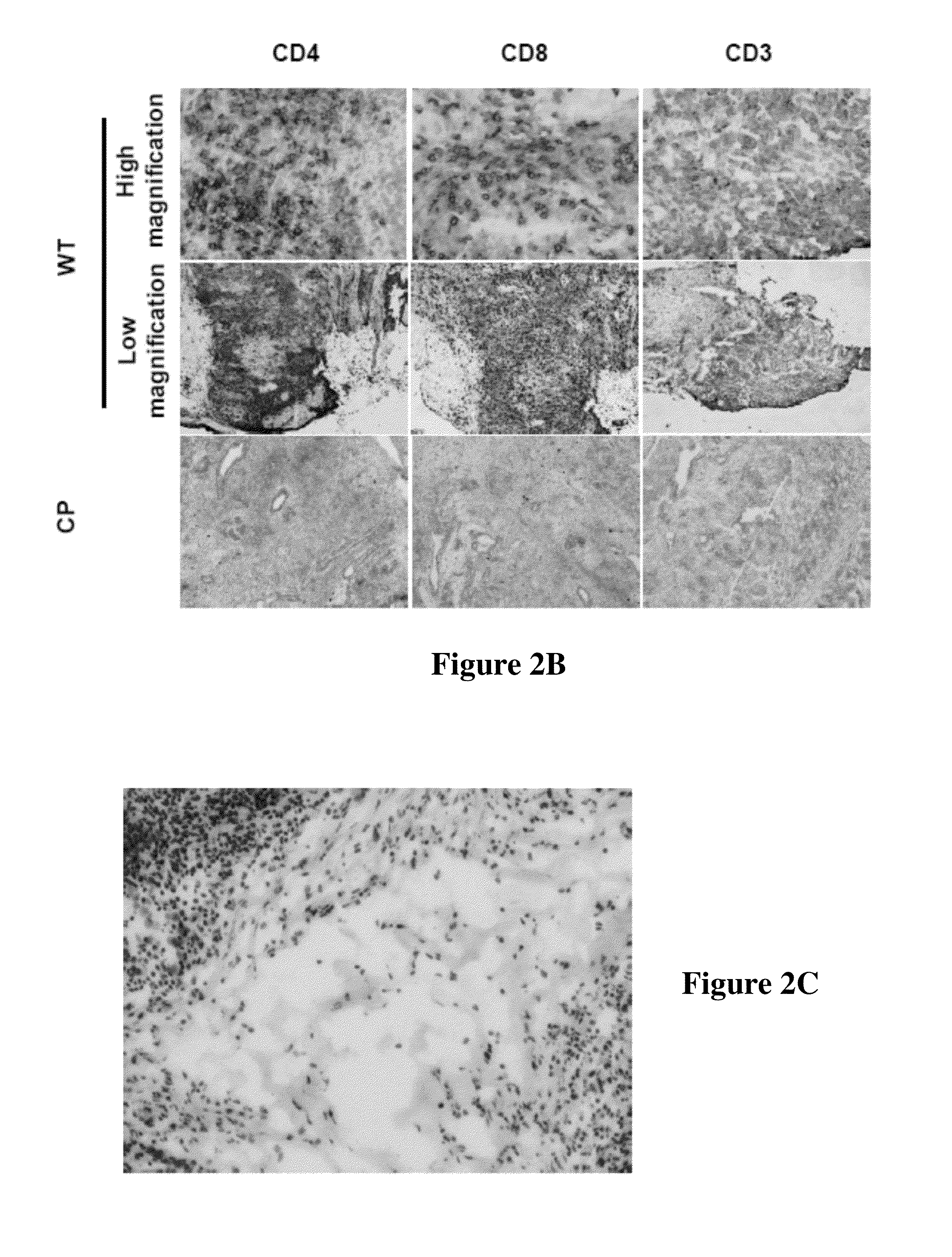
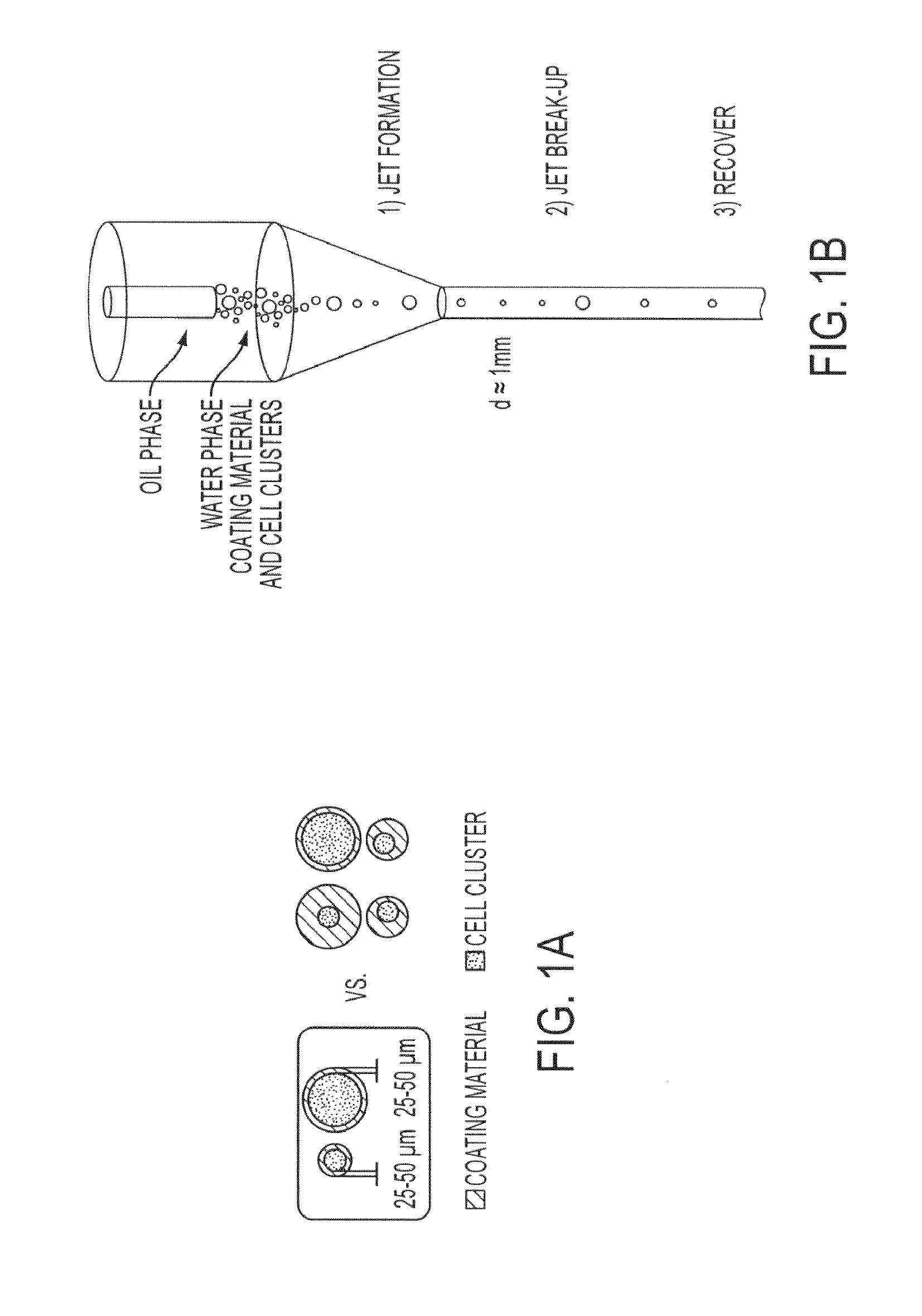
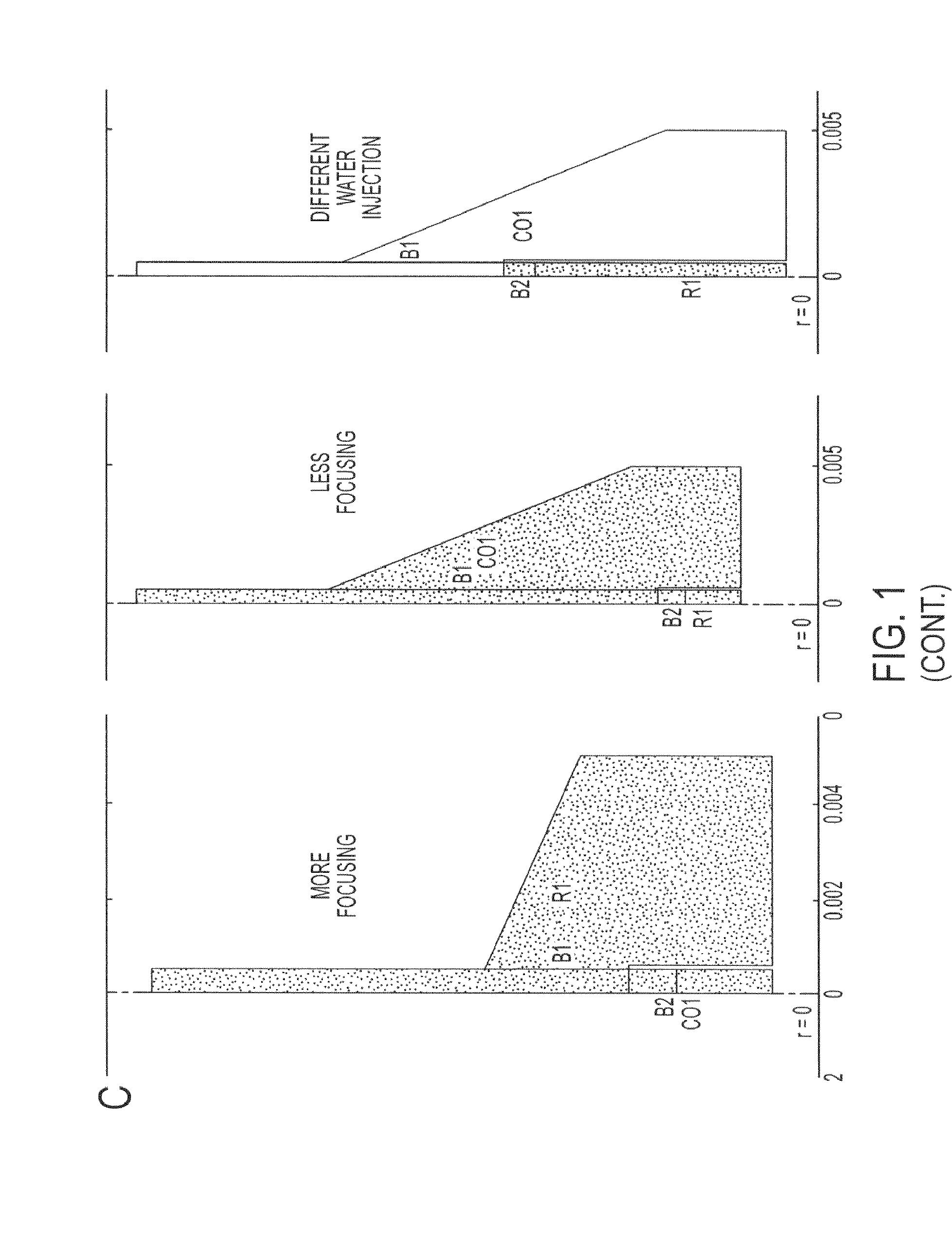
Hydrodynamic methods for conformally coating non-uniform size cells and cell clusters for implantation, thus preventing immune rejection or inflammation or autoimmune destruction while preserving cell functionality. A method for conformally coating cells and c clusters with hydrogels that are biocompatible, mechanically and chemically stable and porous, with an appropriate pore cut-off size. The methods of the invention are advantageously reproducible and result in a relatively high yield of coated versus non-coated cell clusters, without compromising cell functionality. Conformal coating devices configured to perform the methods of the invention, methods of optimally utilizing said devices and purifying the coated islets, and coated biomaterials made by said methods.
Owner:SERNOVA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com