Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
342 results about "Spinal cord lesion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
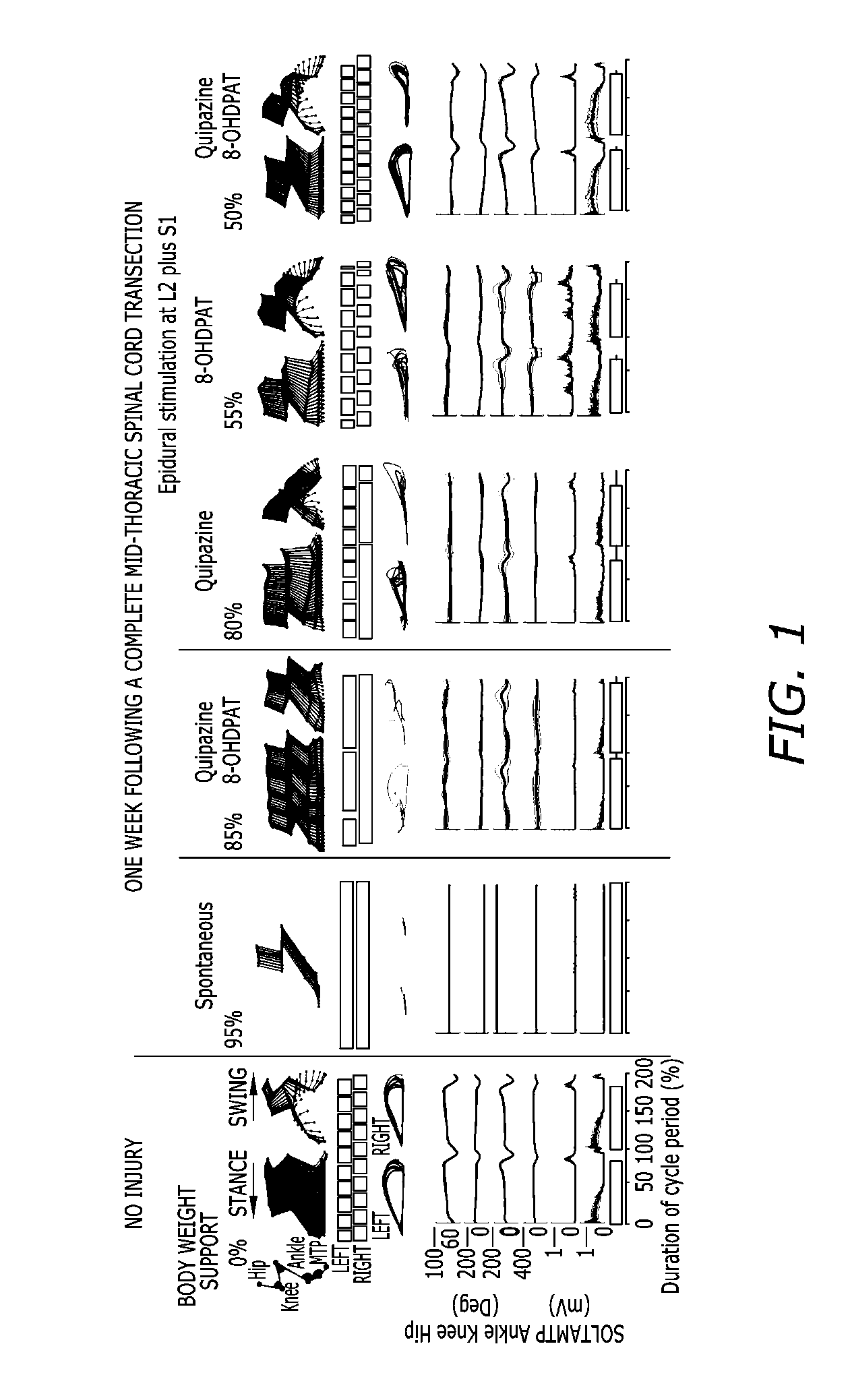
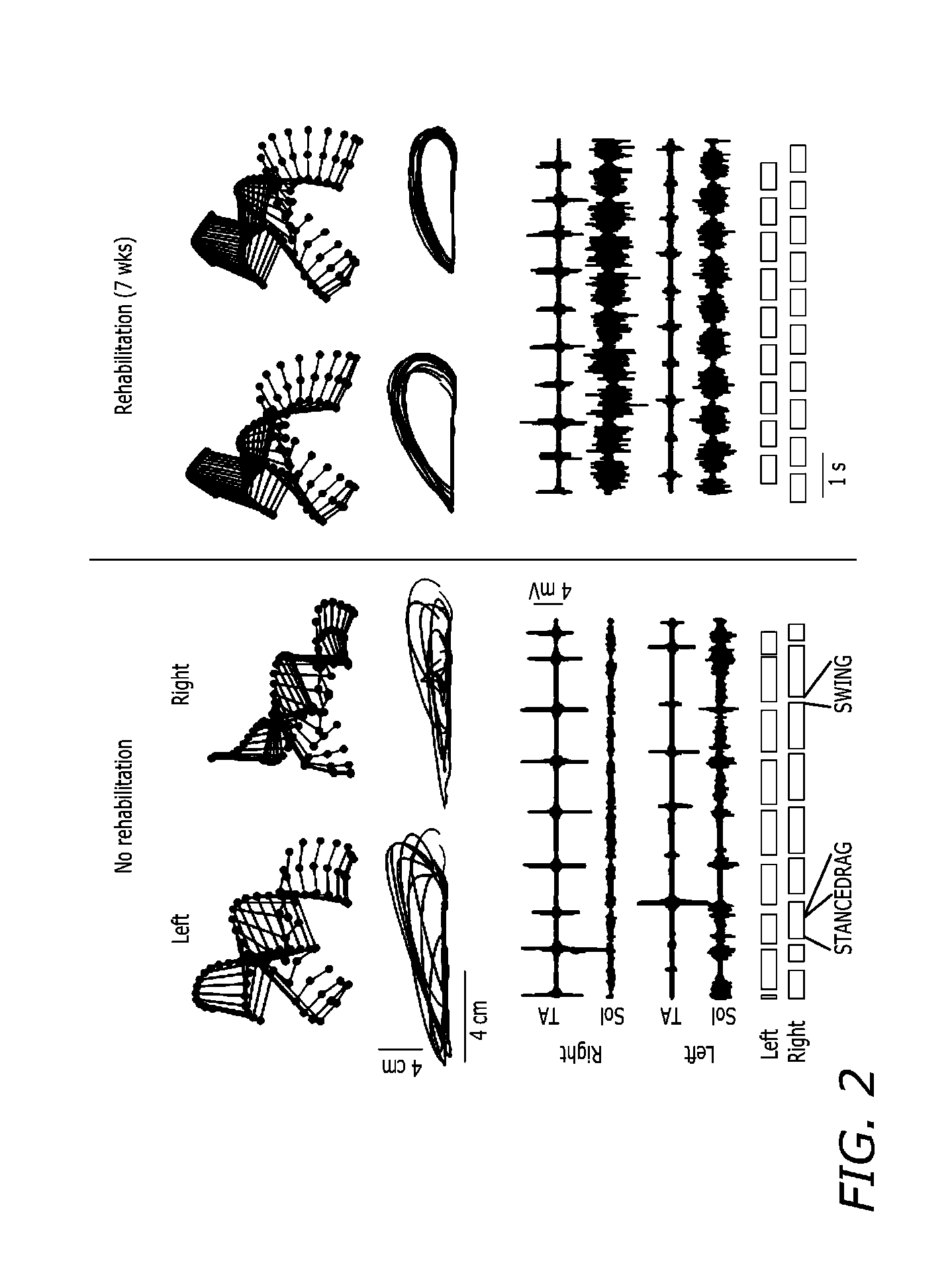
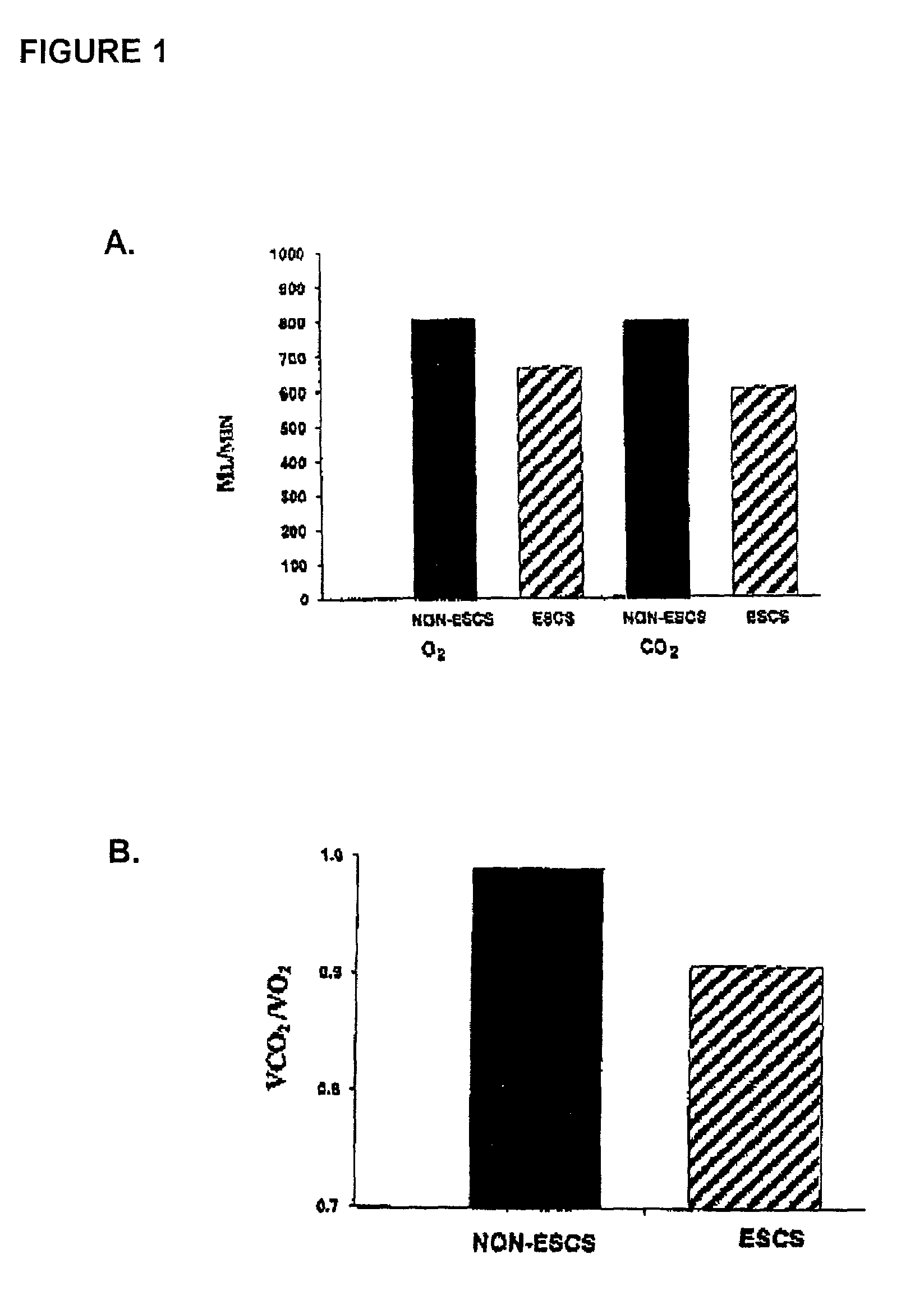
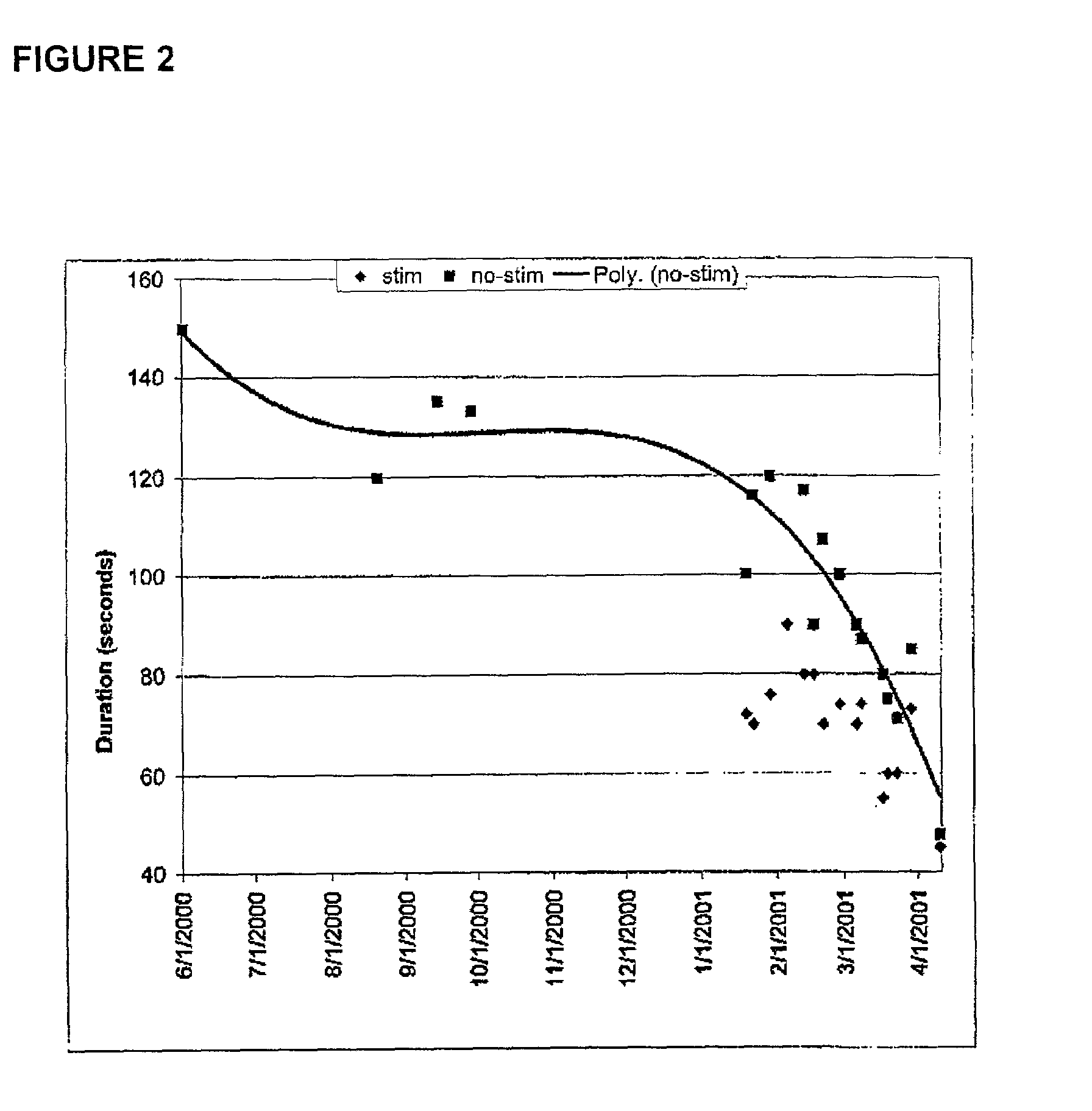
Method for restoring gait in individuals with chronic spinal cord injury
InactiveUS7065408B2Increase chanceElectrotherapyChiropractic devicesGait trainingFull weight bearing
A method for restoring functional ambulation in subjects with incomplete spinal cord injuries which includes partial weight bearing therapy followed by epidural spinal cord stimulation (ESCS) to facilitate partial weight bearing therapy and over-ground walking. Electrical epidural stimulation (EES) is generated by an implanted device during partial weight bearing therapy on a treadmill. The subject is then transitioned to full weight bearing gait training on a treadmill with EES to over-ground gait training with EES and a walker, and finally to full weight bearing independent stepping over-ground with EES with or without an assistive device.
Owner:HERMAN RICHARD M +3
Pain abatement catheter system
InactiveUS7022109B1Increase the areaSafely advancedInfusion syringesSurgical needlesEpidural spaceDevice breakage
A pain abatement catheter system particularly suited for treatment within the epidural space of a patient which includes an epidural introducer assembly, an epidural catheter and a steerable epidural guidewire assembly. The introducer assembly provides a one-piece introduction into the epidural space and includes an elongated introducer needle, an inner stylet, and a flexible outer sheath with a hub. The improved epidural catheter may be safely introduced into the epidural space under fluoroscopic guidance and can be effectively steered through tortuous anatomy, adhesions, or scar tissue for the purpose of delivering medications or contrast dyes to highly selective areas of the epidural space including nerve-root-sleeve injections. The epidural catheter / hub assembly is advanced over a soft atraumatic tip guidewire which significantly decreases the risk of device breakage, nerve root damage, or spinal cord injury. The improved catheter also increases efficacy, maneuverability, and safety in the diagnosis and intervening treatment of acute and chronic back and limb pain. The steerable epidural guidewire assembly allows for safely introducing a guidewire into the epidural space through a flexible sheath cannula and effectively maneuvering the catheter into highly specific areas while reducing the risk of epidural, nerve root, or spinal cord injury.
Owner:DITTO DEBORAH L
Fluorine-containing amino acid derivatives
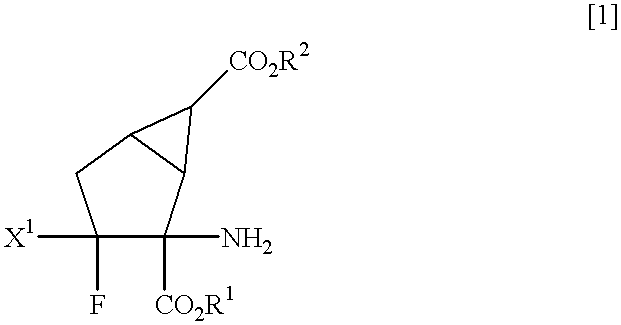
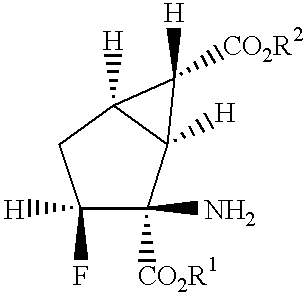
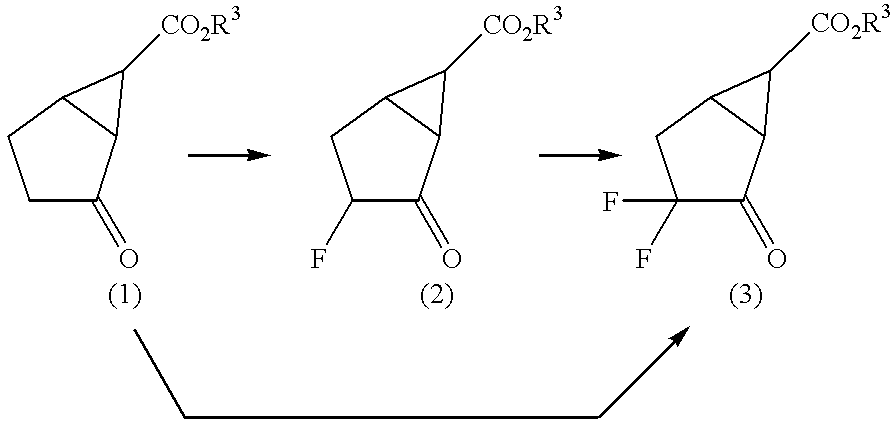
Fluorine-containing amino acid derivatives represented general formula (I), pharmaceutically acceptable salts thereof or hydrates of the same, wherein X1 represents hydrogen or fluorine; and R1 and R2 are the same or different and each represents hydrogen or lower C1-10 alkyl. These compounds are useful as drugs, in particular, group 2 metabotropic glutamate receptor agonists for treating and preventing psychiatric disorders such as schizophrenia, anxiety and associated diseases, depression, bipolar disturbance and epilepsy, and neurological diseases such as drug addiction, cognition disorder, Alzheimer's disease, Huntington's chorea, Parkinson's disease, motility disturbance associating muscular stiffness, cerebral ischemia, cerebral insufficiency, spinal cord lesion and head disturbance.
Owner:TAISHO PHARMACEUTICAL CO LTD
Stem cell therapy for the treatment of central nervous system disorders
The invention provides a method for treating CNS disorders by administering a neural stem cell composition and a mesenchymal stem cell composition on opposing sides of the blood brain barrier. The neural stem cell composition is administered to the central nervous system, while the mesenchymal stem cell composition is administered to the circulatory system, such as by intravenous injection. The method finds use in the treatment of degenerative GNS disorders, as well as traumatic CNS disorders such as stroke and spinal cord injury.
Owner:MIRONOV NIKOLAY
Three-dimensional scaffolds, methods for fabricating the same, and methods of treating a peripheral nerve or spinal cord injury
ActiveUS20130110138A1Promote extensive axonal regenerationRobust and long regenerationFilament/thread formingBiochemical treatment with enzymes/microorganismsFiberMedicine
One aspect of the invention provides a three-dimensional scaffold including at least one layer of highly-aligned fibers. The at least one layer of highly-aligned fibers is curved in a direction substantially perpendicular to a general direction of the fibers. Another aspect of the invention provides a method for fabricating a three-dimensional scaffold. The method includes: electro spinning a plurality of fibers to produce at least one layer of highly-aligned fibers and forming the at least one layer of highly-aligned fibers into a three-dimensional scaffold without disturbing the alignment of the highly-aligned polymer fibers. A further aspect of the invention provides methods for using a three-dimensional scaffold to treat nerve or spinal cord injury.
Owner:RENESSELAER POLYTECHNIC INST +1
Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives
This invention provides compounds of the formula: 1 wherein R.sub.1 and R.sub.2 are each, independently, H, alkyl, cycloalkyl, --CH.sub.2-cycloalkyl, alkoxy, halogen, fluorinated alkyl, --CN, --NH--SO.sub.2-alkyl, --SO.sub.2--NH-alkyl, alkyl amide, amino, alkylamino, dialkylamino, fluorinated alkoxy, acyl, or phenoyl or thiophenoyl; R.sub.3 and R.sub.4 are each, independently, H, alkyl or cycloalkyl; R.sub.5 is H or alkyl; R.sub.6 is H or; and the dashed line indicates an optional double bond; or a pharmaceutically acceptable salt thereof, and pharmaceutical compositions and methods utilizing these compounds for the treatment or prevention of disorders including obsessive-compulsive disorder, depression, anxiety, generalized anxiety disorder, schizophrenia, migraine, sleep disorders, eating disorders, obesity, epilepsy, and spinal cord injury.
Owner:WYETH LLC
Conpositions and method to prevent and treat brain and spinal cord injuries
The cerebrospinal fluid and intracranial pressure are two major reasons why central nervous system is so vulnerable to injuries. A composition and method for treating injured central nervous tissue, or preventing injury to central nervous system tissue is provided. The composition is comprised of a combination of colloidal osmotic agents and crystal osmotic agents. The method comprise of: a). Withdrawing cerebrospinal fluid from the subarachnoid spaces around the tissue to be treated or protected and b). Injecting the composition which is dissolved by patient's own cerebrospinal fluid into subarachnoid spaces. The treatment can be augmented with agents that suppress production of cerebrospinal fluid, or with other known neuroprotective agents.
Owner:WANG YANMING
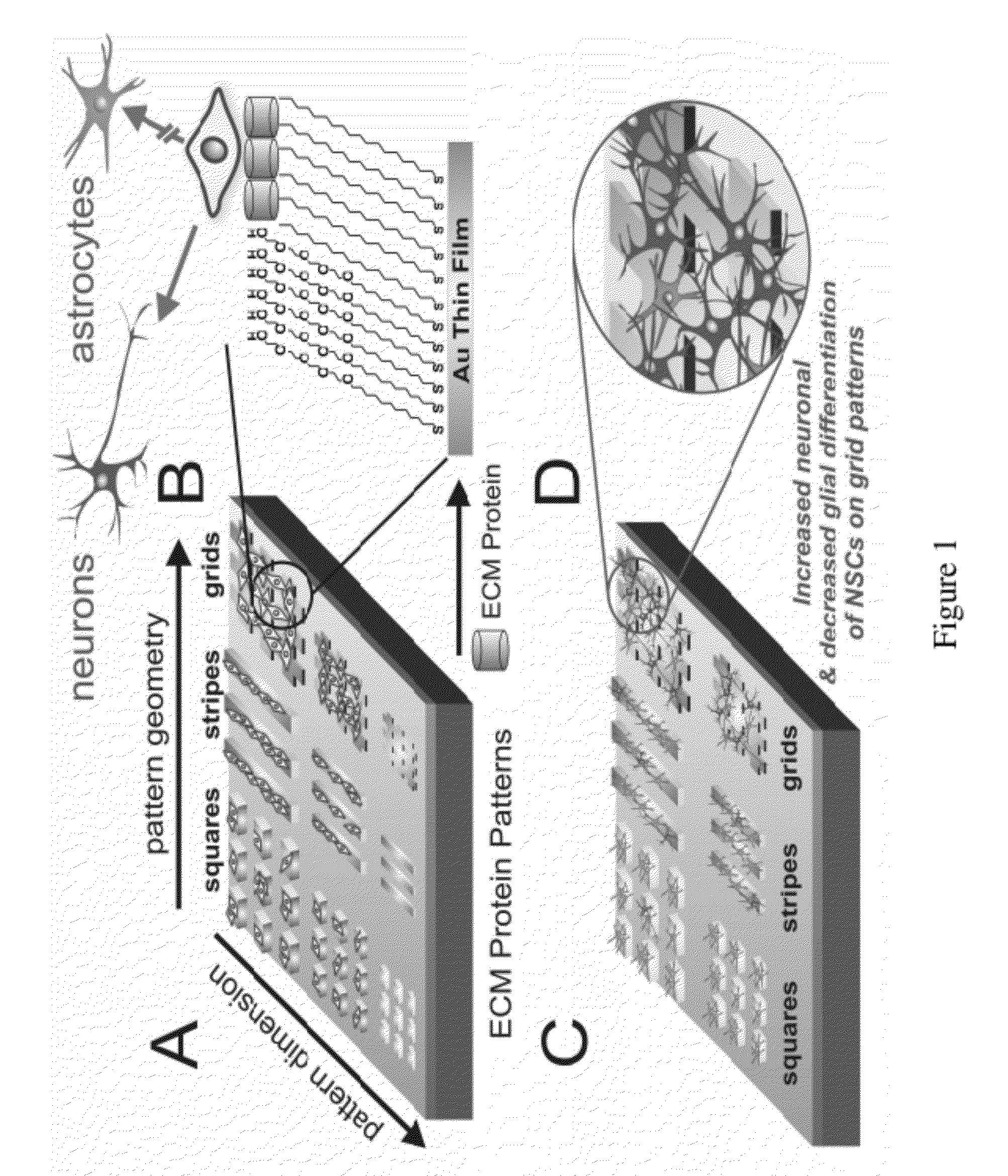
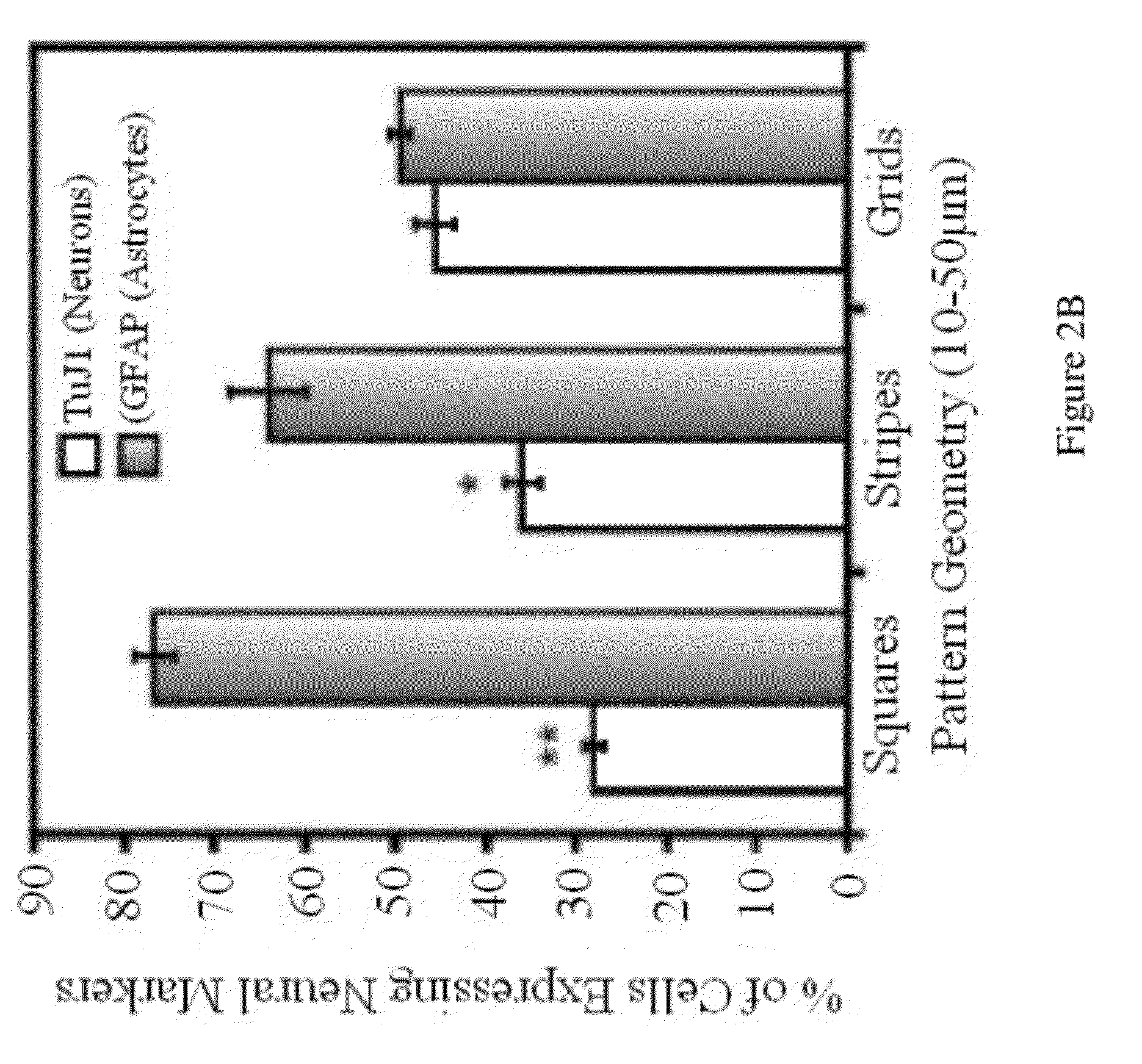
Stem cell differentiation using novel light-responsive hydrogels
This application discloses a light-responsive hydrogel-based platform that can modulate multiple microenvironmental signals to direct the differentiation of human induced pluripotent stem cell-derived neural progenitor cells (hiPSC-NPCs) into neuronal cells. The invention provides novel methods for directing differentiation of neural stem cells into neurons useful for treatment of degenerative diseases or disorders, including but not limited to Alzheimer's, Parkinson's, or spinal cord injury (SCI).
Owner:RUTGERS THE STATE UNIV
High density epidural stimulation for facilitation of locomotion, posture, voluntary movement, and recovery of autonomic, sexual, vasomotor, and cognitive function after neurological injury
ActiveUS9101769B2Easy to controlPromote recoverySpinal electrodesChiropractic devicesDiseaseImpaired proprioception
Methods of enabling locomotor control, postural control, voluntary control of body movements (e.g., in non-weight bearing conditions), and / or autonomic functions in a human subject having spinal cord injury, brain injury, or neurological neuromotor disease. In certain embodiments, the methods involve stimulating the spinal cord of the subject using an epidurally placed electrode array, subjecting the subject to physical training thereby generating proprioceptive and / or supraspinal signals, and optionally administering pharmacological agents to the subject. The combination of stimulation, physical training, and optional pharmacological agents modulate in real time electrophysiological properties of spinal circuits in the subject so they are activated by supraspinal information and / or proprioceptive information derived from the region of the subject where locomotor activity is to be facilitated.
Owner:CALIFORNIA INST OF TECH +2
Composition and method to prevent and treat brain and spinal cord injuries
A composition and method for treating and preventing injury to central nervous system tissue are provided. The composition is comprised of agents that can increase colloidal osmotic pressure and osmolality. The method comprise of: a). Withdrawing cerebrospinal fluid from the subarachnoid spaces around the tissue to be treated or protected and b). Injecting the composition into subarachnoid spaces.
Owner:WANG YANMING
Gene therapy for neurodegenerative disorders
Compositions and methods for treating disorders affecting motor function, such as motor function affected by disease or injury to the brain and / or spinal cord, are disclosed.
Owner:GENZYME CORP
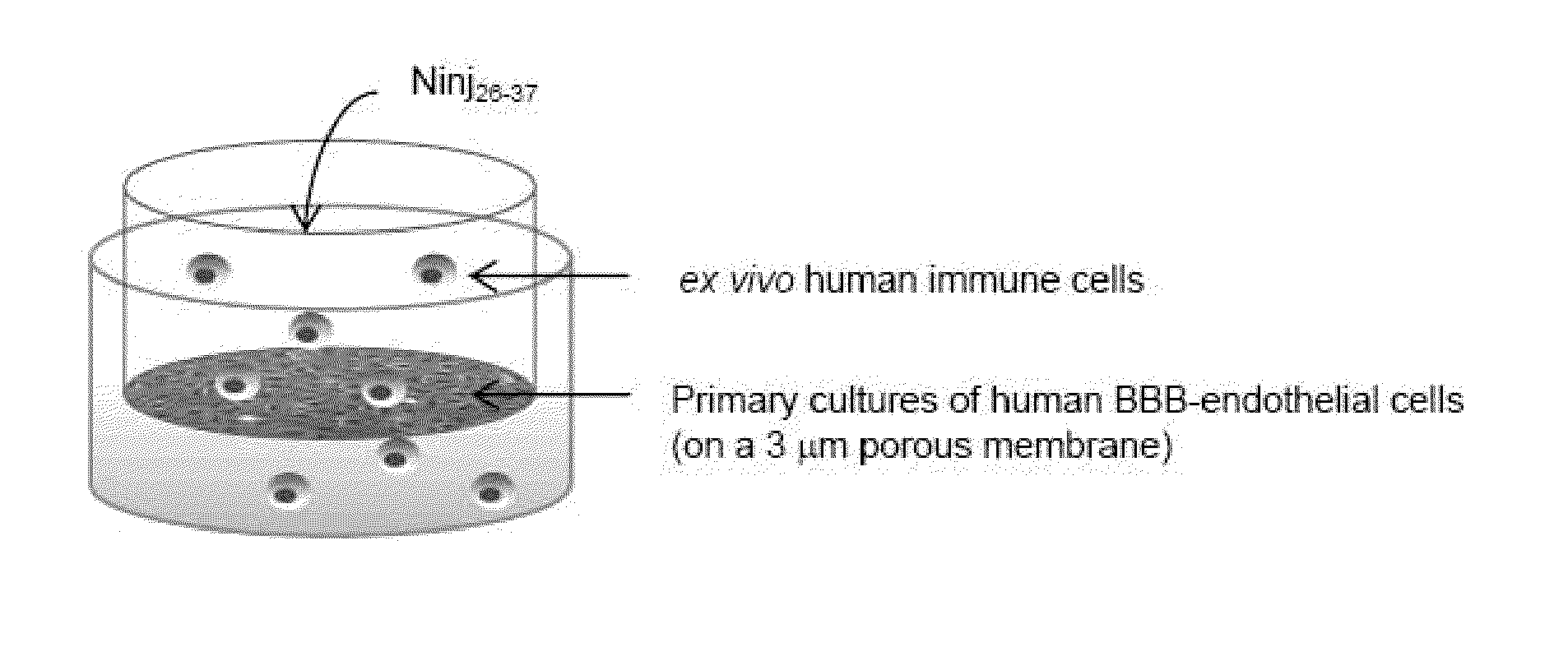
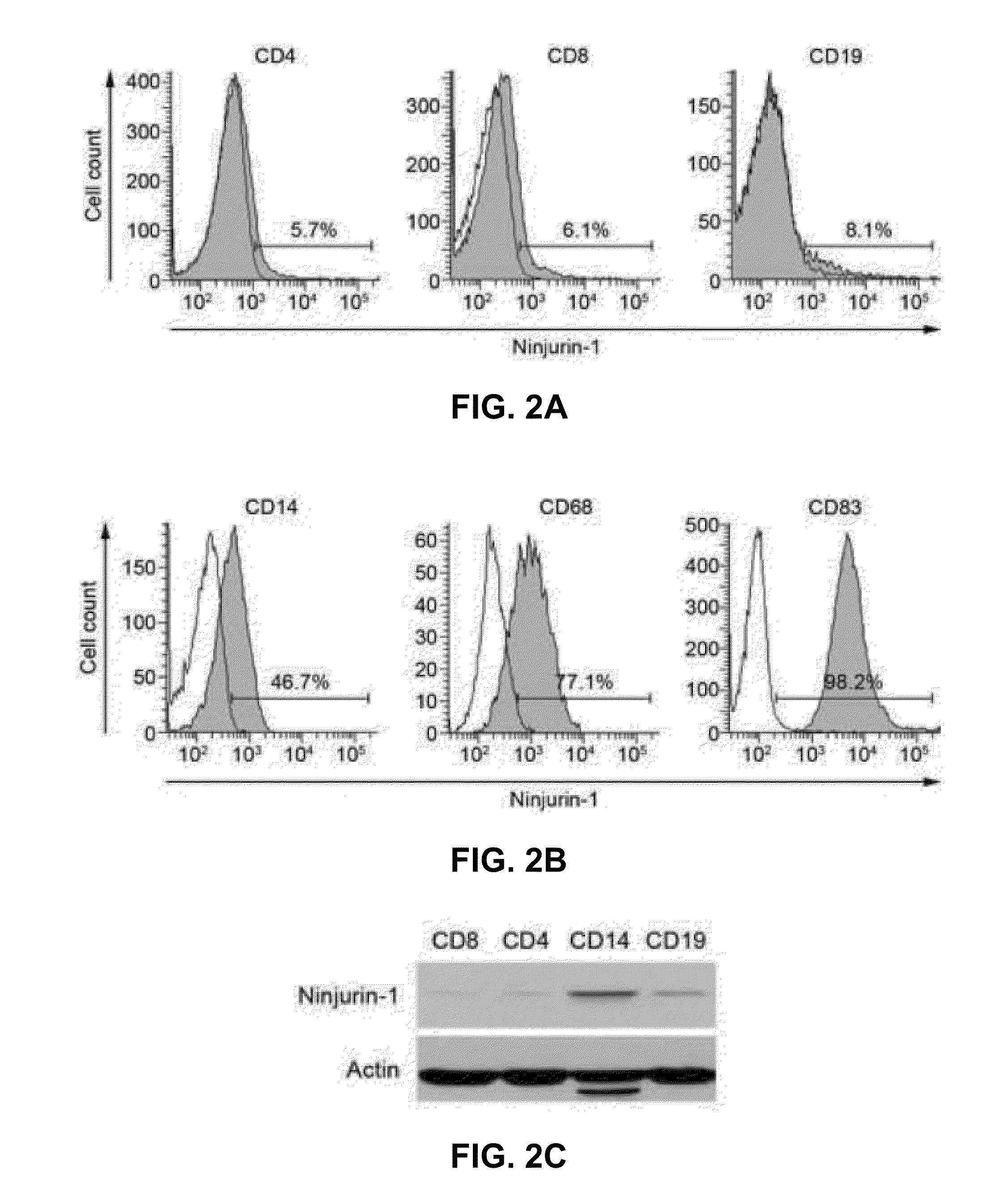
Ninjurin-1 modulation and uses thereof
ActiveUS20100310568A1Relieve symptomsReduce tissue damageNervous disorderPeptide/protein ingredientsMedicineInflammation
Methods, uses, agents and compositions useful for the prevention, treatment and / or diagnosis of neuroinflammatory conditions such as multiple sclerosis and spinal cord injury based on the modulation of nerve injury-induced protein-1 (Ninjurin-1) are disclosed.
Owner:VAL CHUM PARTNERSHIP
Preparation method and application thereof for cell-biological bracket compound based on biological print technology
InactiveCN103272288ABioprinting technology is simple and easyLow costSurgerySpinal cord lesionNerves regeneration
The invention provides a preparation method and the application thereof for a cell-biological bracket compound based on biological print technology. The cell-biological compound is formed by fibrous protein which is drawn out from the autoblood of a patient and processed by an ink-jet print technology. The surface and the interior of the cell-biological compound encompass one or more trophic factors and bone mesenchymal stem cells derived from self. According to the invention, the biological print technology is adopted, the appearance shape, cells and encompassing state model of the trophic factors can be designed according to requirements and practical situation, the cell-biological bracket compound is obtained through printing accurately, the fibrous protein and the bone mesenchymal stem cells are derived from self, so that the problem of immunological rejection is avoided, the trophic factors are cultivated in the bracket and are released slowly with the degradation of the bracket material, the cell-biological compound is applied to the spinal cord injured part to promote nerve regeneration and functional reconstruction on the injured part.
Owner:谢杨 +1
Neuronal differentiation method of adult stem cells using small molecules
The present invention relates to a neuronal differentiation method of adult stem cells using small molecules, more particularly to a method for inducing differentiation of adult stem cells into nerve cells using small molecules, which enables effective differentiation into nerve cells and, thus, is useful in treating intractable CNS disorders such as Parkinson's disease, dementia, Alzheimer's disease and spinal cord injury.
Owner:KOREA RES INST OF CHEM TECH
Composition for treating a disease caused by neuronal insult comprising a human umbilical cord blood-derived mesenchymal stem cell as an active ingredient
InactiveUS20080131405A1Improve abilitiesResilienceBiocideNervous disorderHuntingtons choreaRisk stroke
Provided is a composition for treating nerve damage-related diseases. The composition includes a human umbilical cord blood-derived mesenchymal stem cell as an active ingredient. The mesenchymal stem cell isolated and incubated from the human umbilical cord blood migrates to an injured area to be differentiated into a nerve cell or a neuroglial cell at the time of in vivo transplantation. Thus, the mesenchymal stem cell and a composition including the same can be effectively used in cell replacement therapy and gene therapy for treating diseases caused by nerve damage including a stroke, Parkinson's disease, Alzheimer's disease, Pick's disease, Huntington's disease, amyotrophic lateral sclerosis, traumatic central nervous system disease and a spinal cord injury.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Therapeutic encapsulated embryonic stem cells and mesenchymal stromal cells
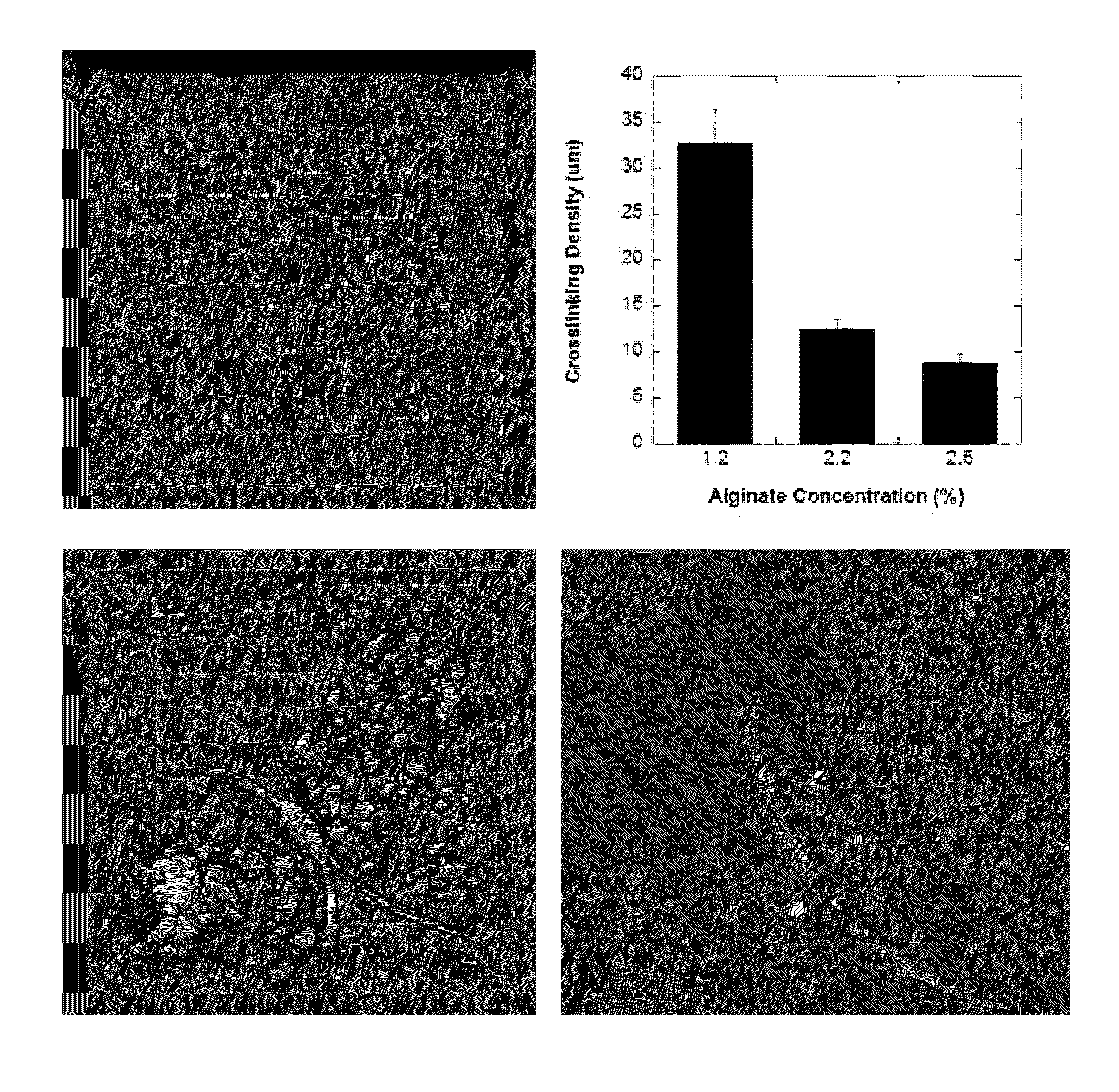
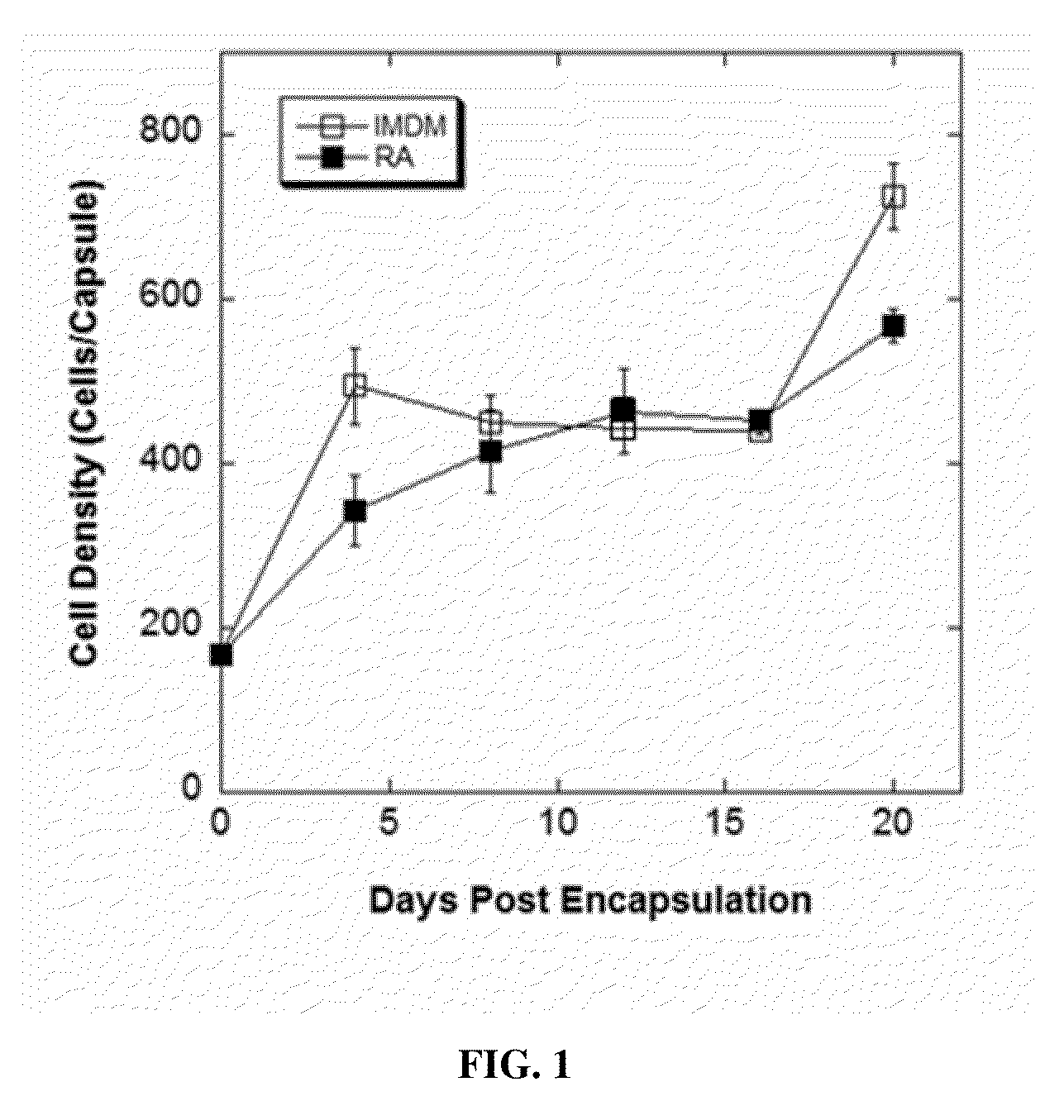
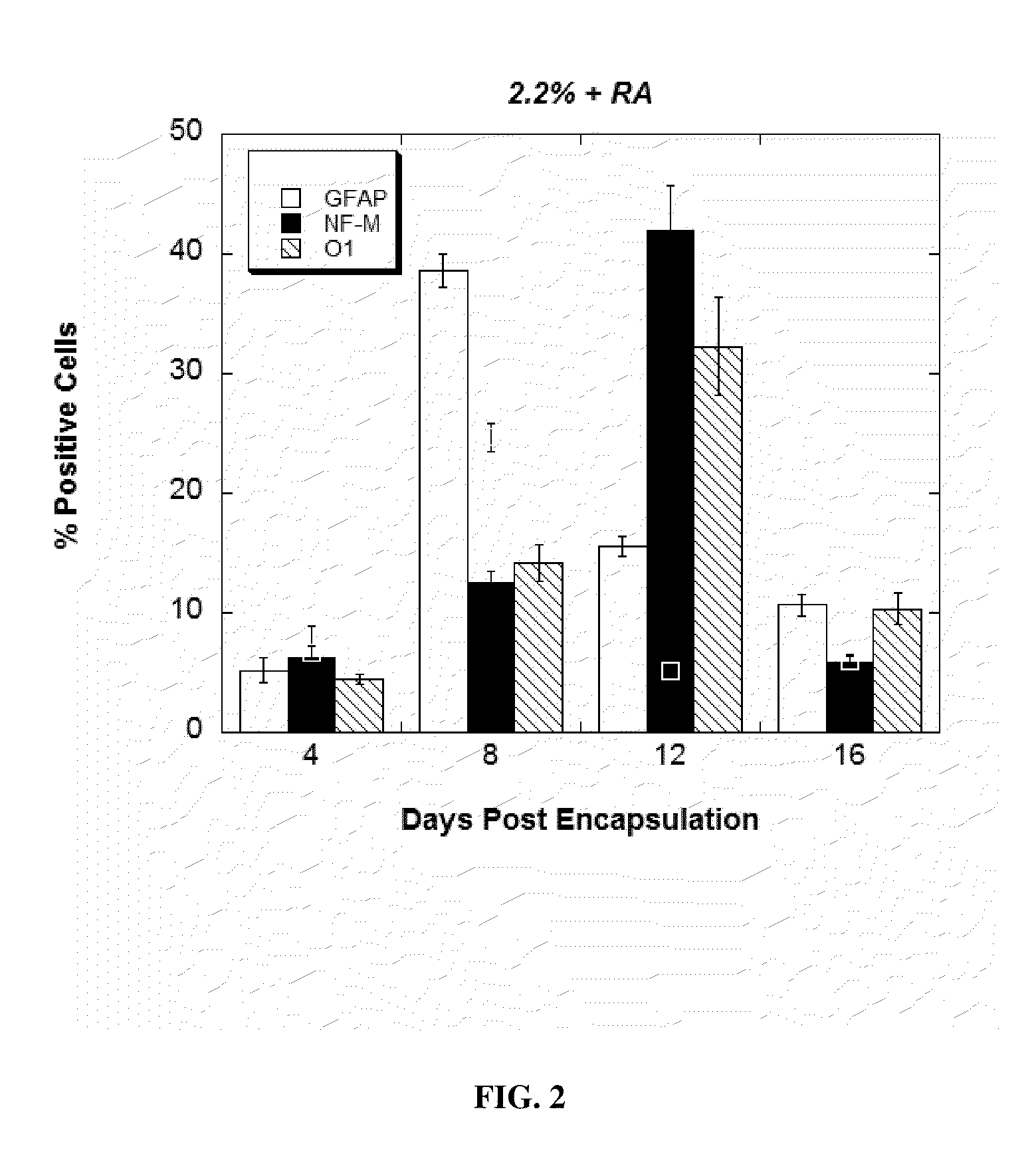
InactiveUS20120020931A1Reduce aggregationIncrease differentiationBiocideNervous disorderDiseaseSpinal cord lesion
This application discloses alginate microencapsulation-mediated differentiation of embryonic stem cells and use of the stem cell differentiation method for the development of effective treatment of various diseases and disorders. The microencapsulation of embryonic stem (ES) cells results in decreased cell aggregation and enhanced neural lineage differentiation through incorporating the soluble inducer retinoic acid (RA) into the permeable microcapsule system. This application also discloses a micro-encapsulation system for immobilizing mesenchymal stromal cells (MSCs) while sustaining the molecular communication. Thus, the invention provides the use of encapsulated mesenchymal stromal cells in the cellular transplantation therapies. Moreover, the invention provides methods for delivery of encapsulated MSCs into the central nervous system and therapies derived therefrom, such as, the treatment of spinal cord injury (SCI) and other inflammatory conditions.
Owner:RUTGERS THE STATE UNIV
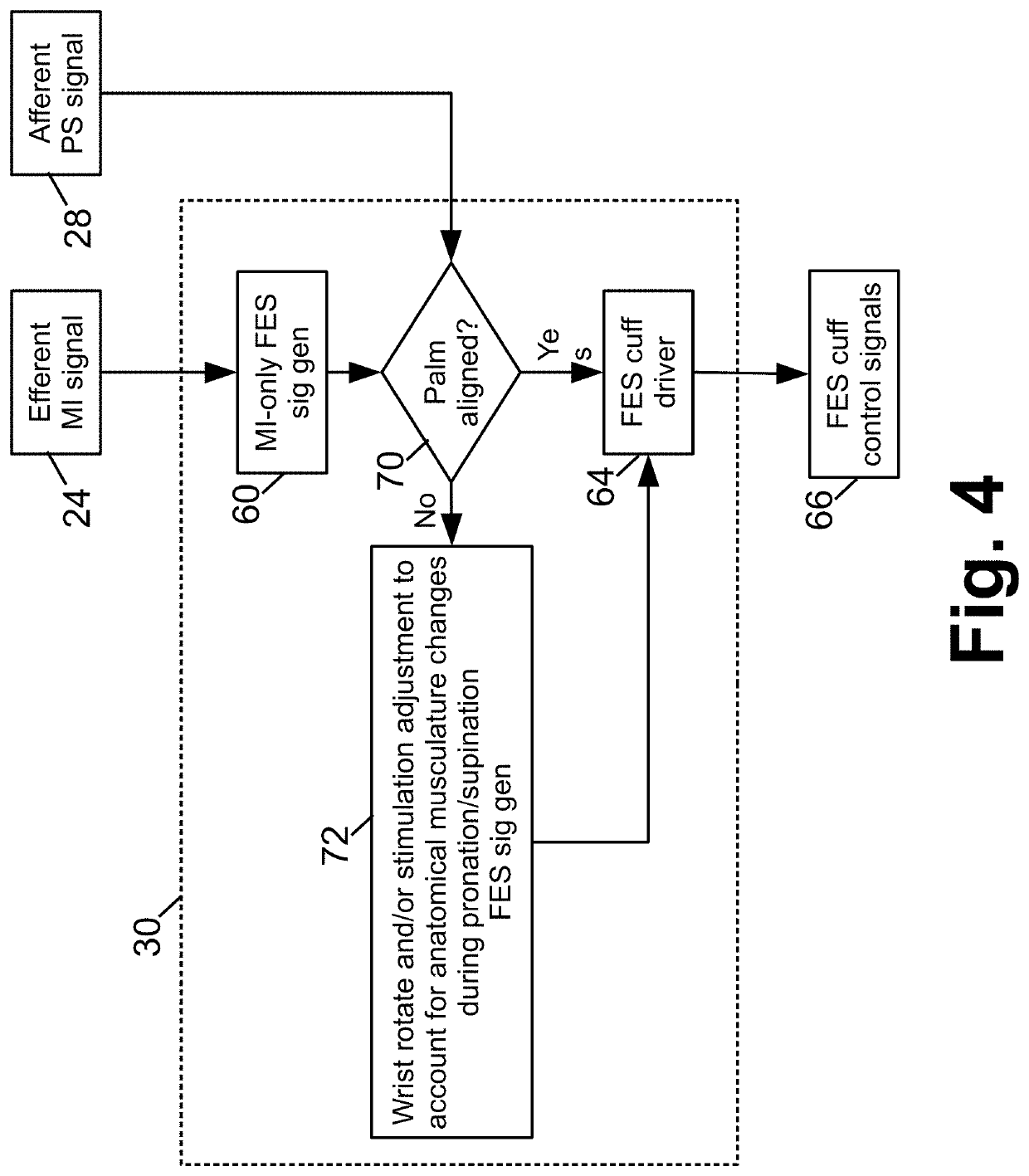
Motor function neural control interface for spinal cord injury patients
At least one electrical brain signal is received from a patient and is demultiplexed into an efferent motor intention signal and at least one afferent sensory signal (such as an afferent touch sense signal and / or an afferent proprioception signal). A functional electrical stimulation (FES) device is controlled to apply FES to control a paralyzed portion of the patient that is paralyzed due to a spinal cord injury of the patient. The controlling of the FES device is based on at least the efferent motor intention signal. A demultiplexed afferent touch sense signal may be used to control a haptic device. The afferent sensory signal(s) may be used to adjust the FES control.
Owner:BATTELLE MEMORIAL INST
Human IgM antibodies, and diagnostic and therapeutic uses thereof particularly in the central nervous system
InactiveUS20090274690A1Promote safer self-therapiesExtended half-lifeNervous disorderPeptide/protein ingredientsNervous systemSpinal cord lesion
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including neural dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. The neuromodulatory agent has one or more of the following characteristics: it is capable of inducing remyelination; binding to neural tissue; promoting Ca−− signaling with oligodendrocytes; and promoting cellular proliferation of glial cells. Amino acid and DNA sequences of exemplary antibodies are disclosed. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in diagnostic and therapeutic applications, including screening assays for the discovery of additional antibodies that bind to cells of the nervous system, particularly oligodendrocytes.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
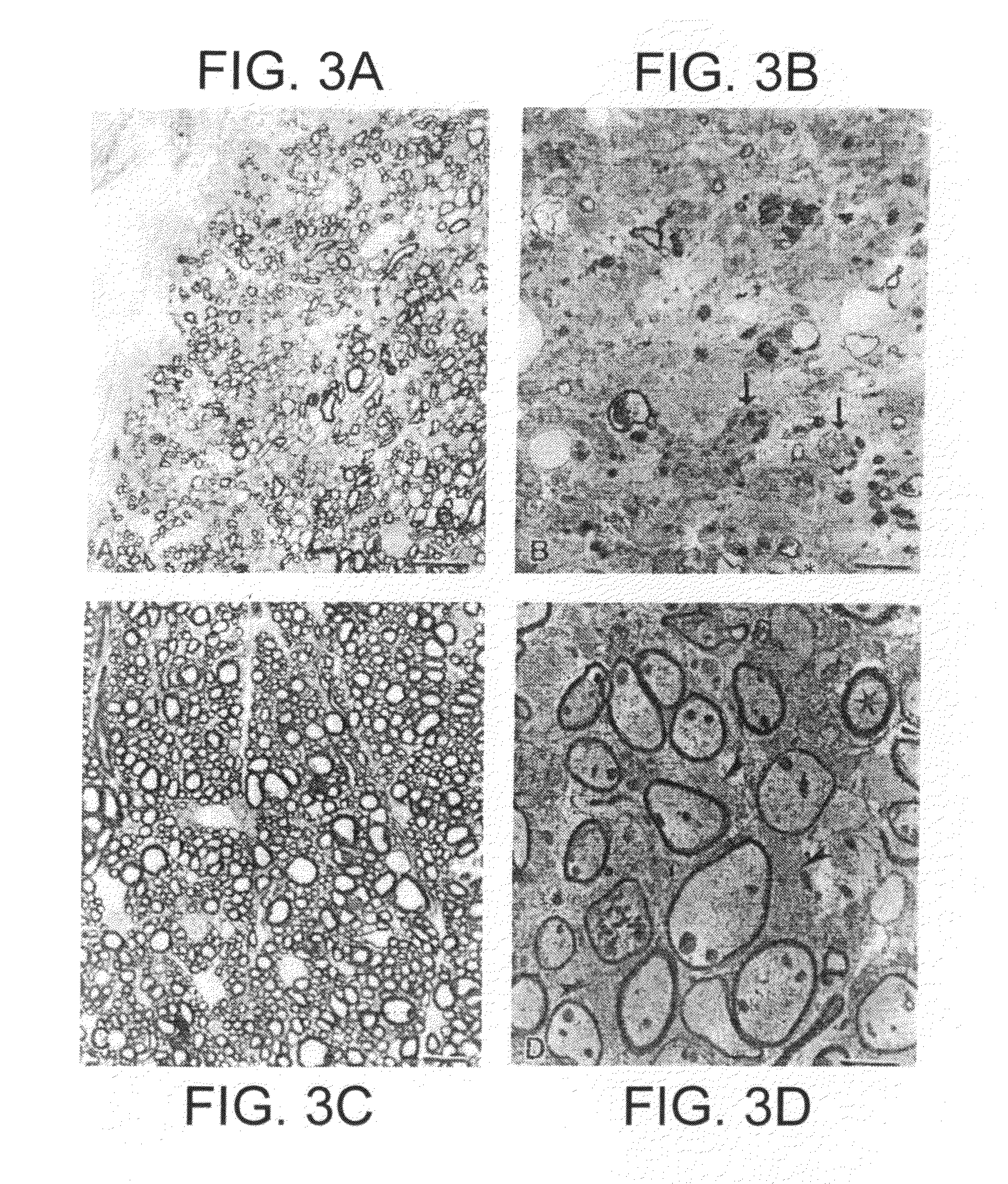
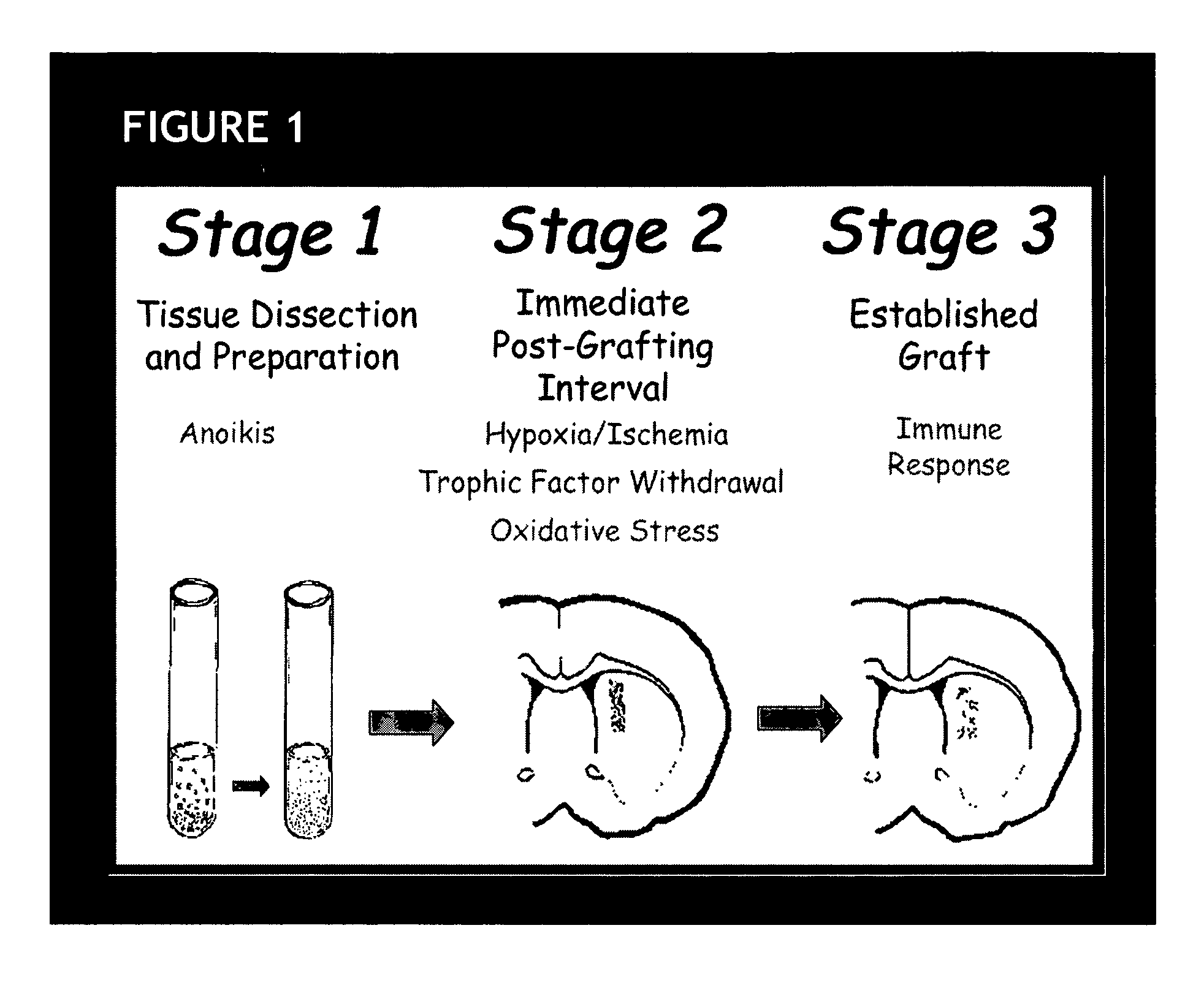
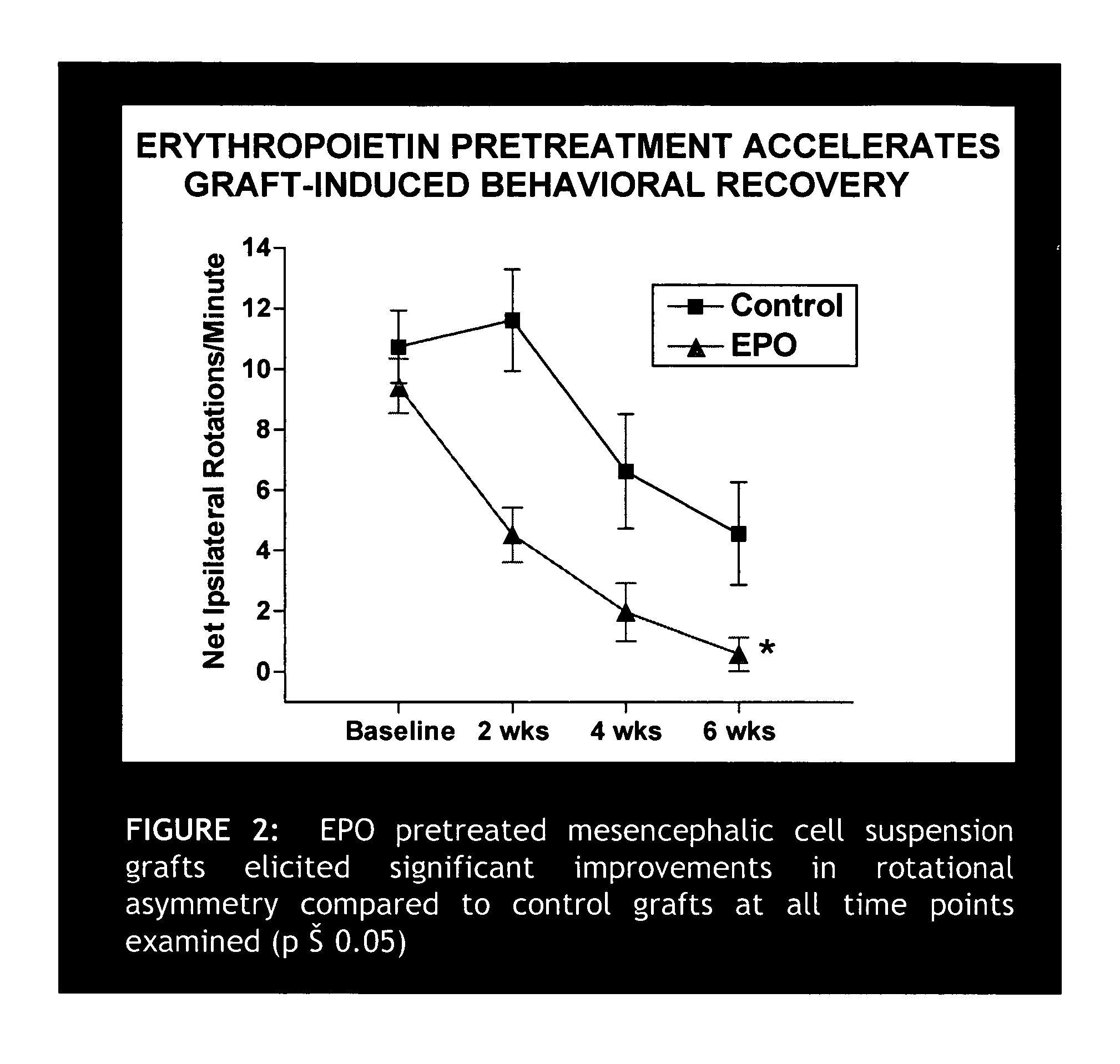
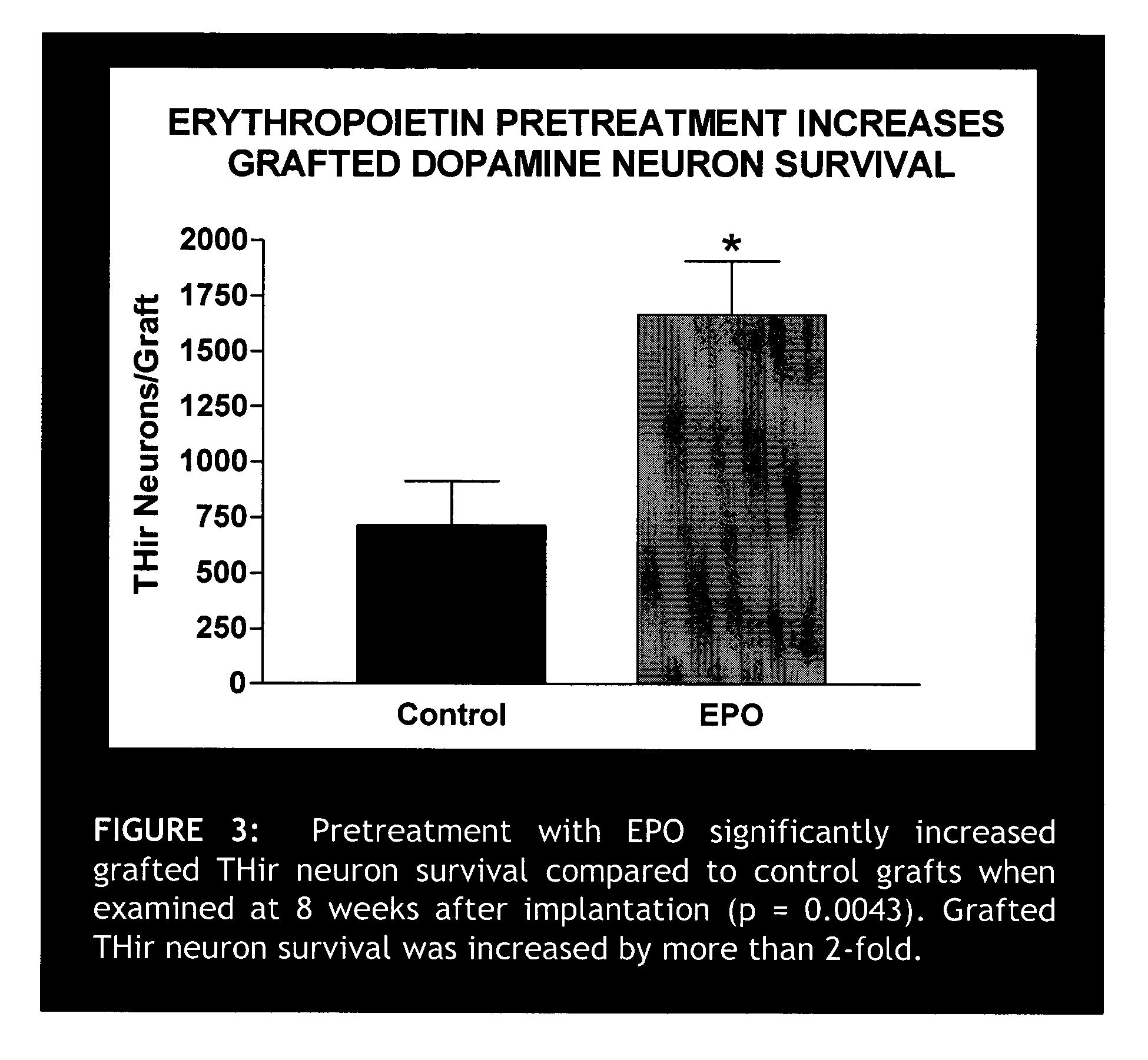
Erythropoietin administration to improve graft survival
ActiveUS7459152B2Enhance cell viabilityImprove survivalBiocideNervous disorderErythropoietinGraft survival
The present invention provides methods, compounds and kits for increasing the viability of cells. The methods involve treating cells that make up a tissue graft with erythropoietin before, during or after delivery or administration. The method can employ cells of different types, including cells of neural or paraneural origin, such as adrenal chromaffin cells. Also useful are cell lines grown in vitro. Cells not of neural or paraneural origin, such as fibroblasts, may also be used following genetic alteration to express a desired neural product such as a neurotransmitter or a neuronal growth factor. The method is used to treat neurological diseases such as Parkinson's disease, Alzheimer's disease, Huntington's disease, epilepsy, and traumatic brain or spinal cord injury.
Owner:RUSH UNIV MEDICAL CENT
Wheelchair power apparatus for electronic driving conversion
ActiveUS10888474B2Reduce the burden onWheelchairs/patient conveyanceRod connectionsPhysical medicine and rehabilitationWheelchair
Disclosed is a wheelchair power apparatus for electronic driving conversion. It is an object of the present invention to provide a wheelchair power apparatus for electronic driving conversion, which can provide severely disabled people, for instance, patients with spinal cord injury, the weak or the old, who use wheelchairs, with convenience in movement, and convert a manual four-wheel wheelchair into an electronic three-wheel wheelchair just by detachably mounting the electronic module having electronic wheels to the existing manual four-wheel wheelchair.
Owner:ROBO3
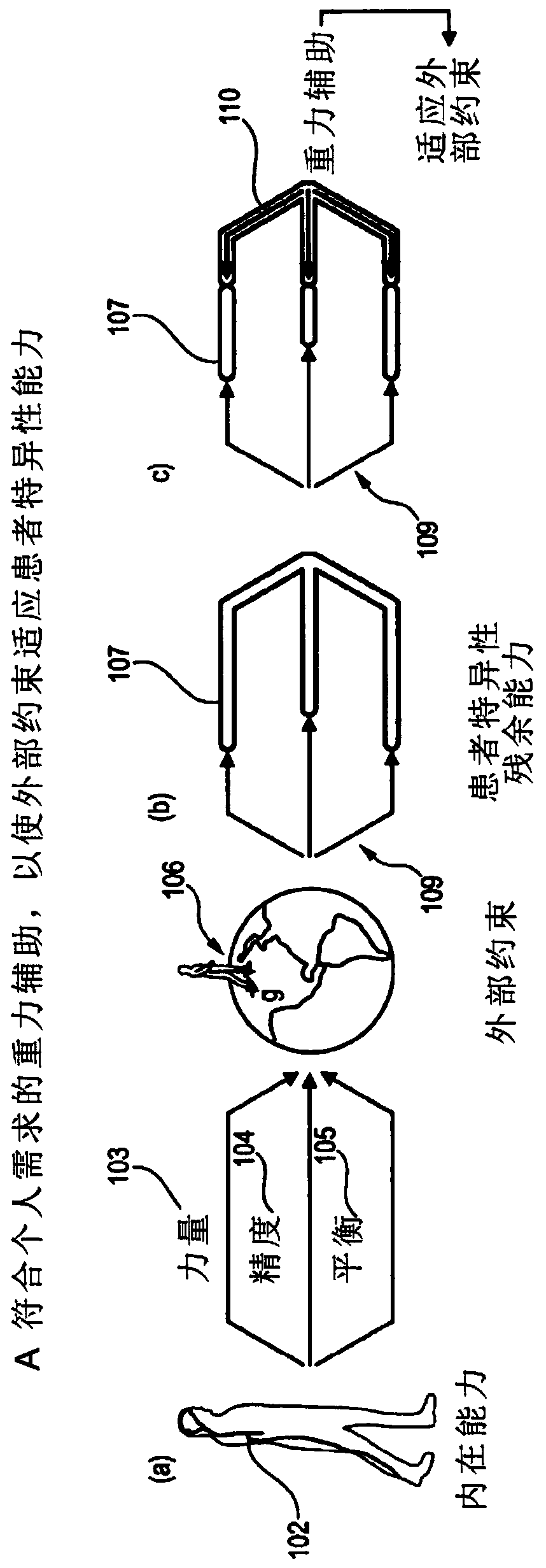
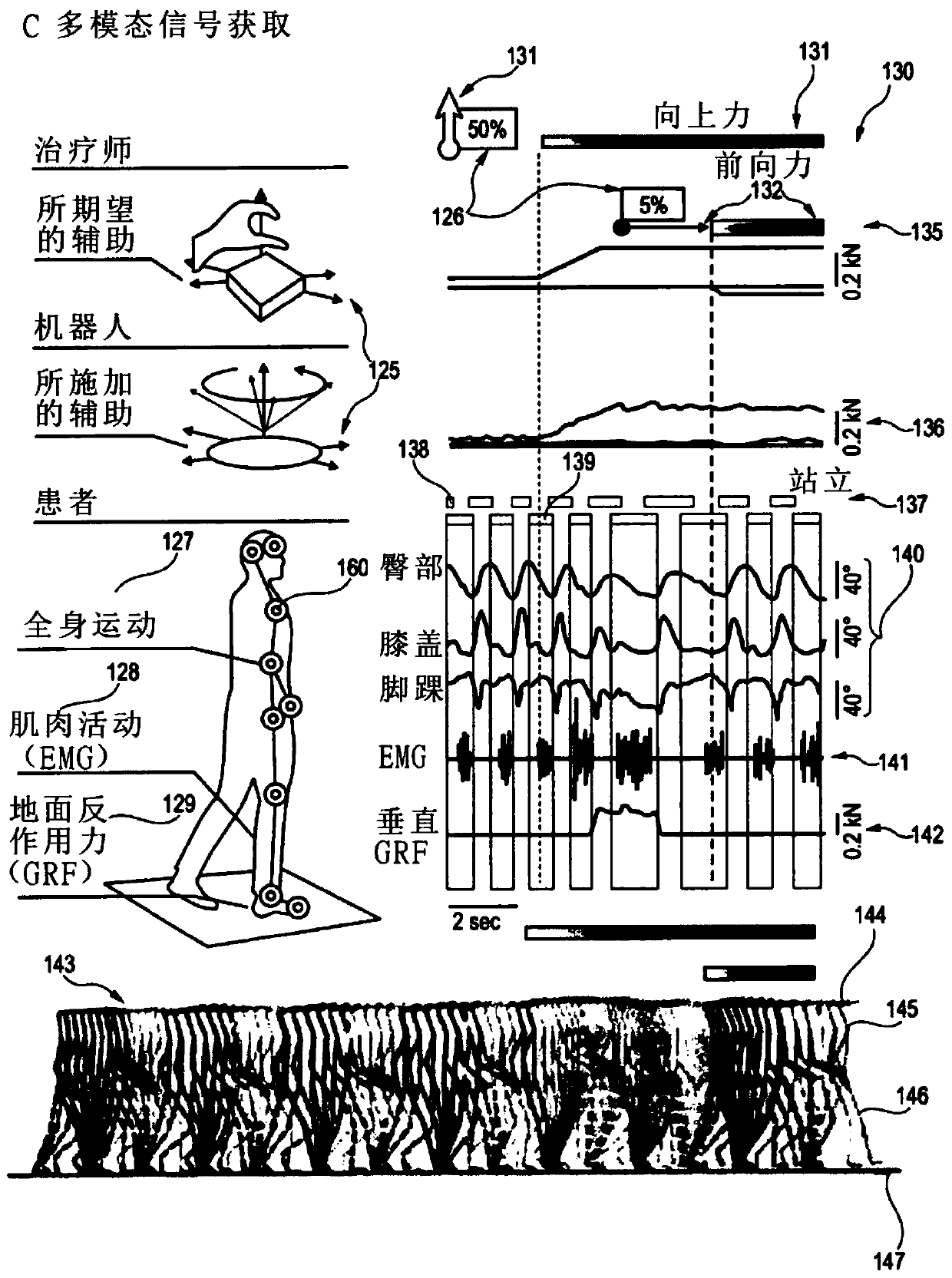
Apparatus comprising a support system for a user and its operation in a gravity-assist mode
InactiveCN109843371AEasy to placeRestore repetitive natureProgramme-controlled manipulatorChiropractic devicesPhysical medicine and rehabilitationSpinal cord lesion
The present application relates to devices and systems for rehabilitation of the locomotor system, for example limbs. In particular, the present application discloses an apparatus, more in particulara robotic platform capable of optimizing gravity-dependent trunk movements, enabling overground locomotion in non-ambulatory individuals with spinal cord injury and stroke, while promoting durable motor improvement when delivered during gait rehabilitation facilitated by electrical spinal cord stimulation.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
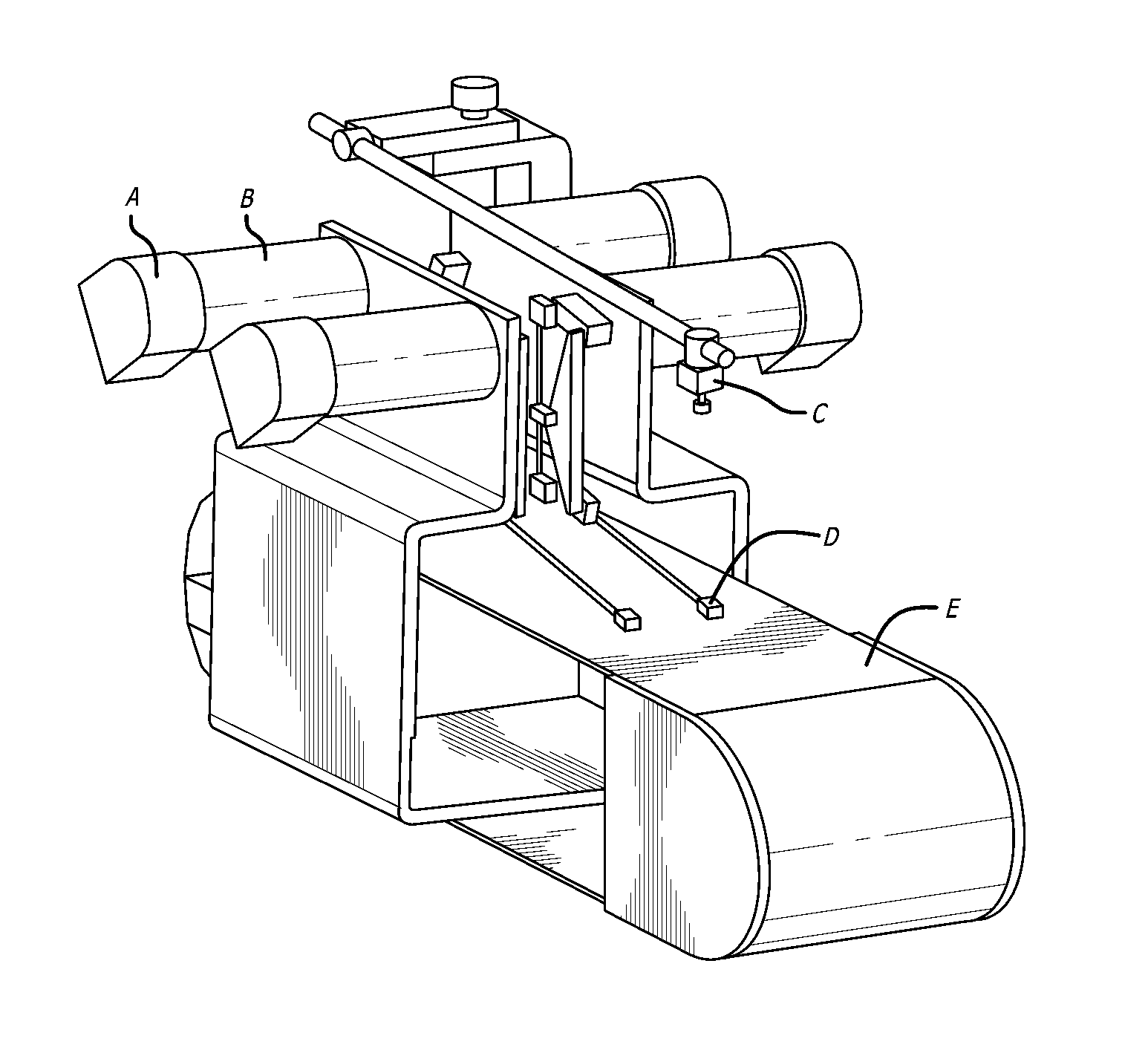
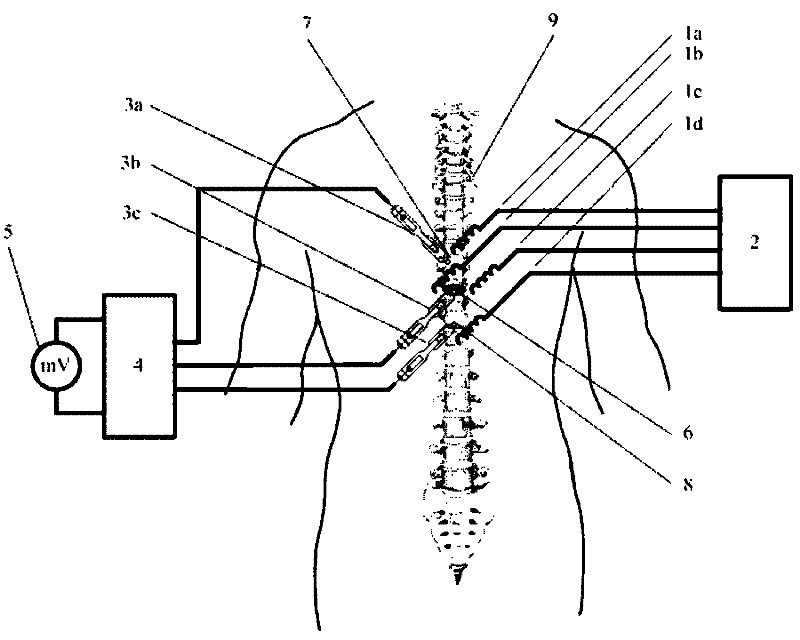
Method and device for injury potential compensation after spinal cord injury
ActiveCN102512757AAchieve compensationSpinal microenvironment stabilizationInternal electrodesExternal electrodesPotential measurementSignal amplification
The invention discloses a method and a device for injury potential compensation after spinal cord injury. The method comprises the following steps of: detecting an injury potential after spinal cord injury, observing the value of injury potential in real time, and controlling a stimulation voltage according to the observed value of injury potentials to allow the injury potential close to zero. The method achieves injury potential compensation to ensure that the spinal cord microenvironment is relatively stable. The device for implementing the compensation method comprises four stimulating electrodes, a stimulation voltage generating circuit (2), an injury potential measuring electrode, an injury potential amplifying circuit (4) and voltage display equipment (5). The stimulation voltage generating circuit (2) generates an adjustable stimulation voltage, the stimulation voltage is applied onto the spinal cord of a patient through the stimulating electrodes to stimulate the spinal cord, the injury potential measuring electrode is used for measuring an injury potential, the injury potential amplifying circuit (4) magnifies the injury potential and filters off high-frequency interference in the injury potential, the voltage display equipment (5) displays the value of magnified injury potential, and the stimulation voltage is regulated according to the value of magnified injury potential.
Owner:INST OF ELECTRICAL ENG CHINESE ACAD OF SCI
Composite mesenchymal stem cell functional collagen scaffold and applications thereof
The invention discloses a composite mesenchymal stem cell functional collagen scaffold and applications thereof. According to the present invention, isolated animal fascia is soaked sequentially withacetone, a sodium deoxycholate solution, a Triton X-100 solution, a pepsin aqueous solution and a papain solution, and free-drying is performed to obtain the composite mesenchymal stem cell functionalcollagen scaffold; the composite mesenchymal stem cell functional collagen scaffold is used for acute spinal cord injury and old spinal cord injury, and the comprehensive evaluation results from various aspects show that the composite mesenchymal stem cell functional collagen scaffold can substantially promote the movement function recovery, nerve regeneration, axon myelination and synapse formation of acute and old spinal cord injury dogs; and the functional biomaterial can provide great significance in the repair of spinal cord injury, and can provide theoretical support for the applicationof functional biomaterials in the future.
Owner:BEIJING ZKZKTECH CO LTD
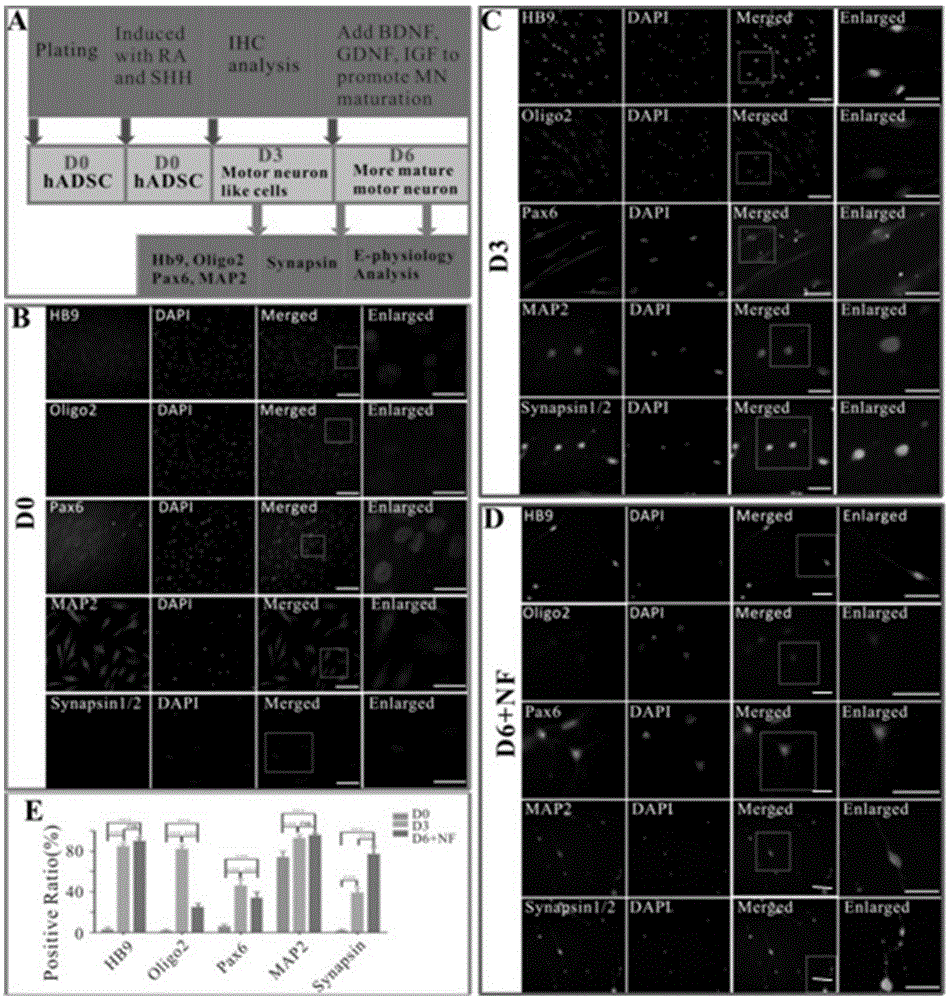
Motoneuron-like cells derived from adipose stem cells as well as preparation method and application of motoneuron-like cells
The invention provides a method for preparing motoneuron-like cells derived from adipose stem cells. The method comprises the following steps: treating 3-6 generations of high-purity adipose stem cells by using a motoneuron-like cell inducer to obtain motoneuron-like cells; and treating the adipose stem cells by using the motoneuron-like cell inducer, and then treating continuously by using neurotrophic factors, thereby obtaining more mature motoneuron-like cells. By adopting the method provided by the invention, the more mature motoneuron-like cells derived from the adipose stem cells can be obtained in an extremely short time, and the obtained motoneuron-like cells can be used for preventing or treating motoneuron injuries and spinal cord injuries; and the pre-induced motoneuron-like cells obtained by the method provided by the invention can be better used for preventing and treating the motoneuron injuries and spinal cord injuries.
Owner:SHANGHAI EAST HOSPITAL +1
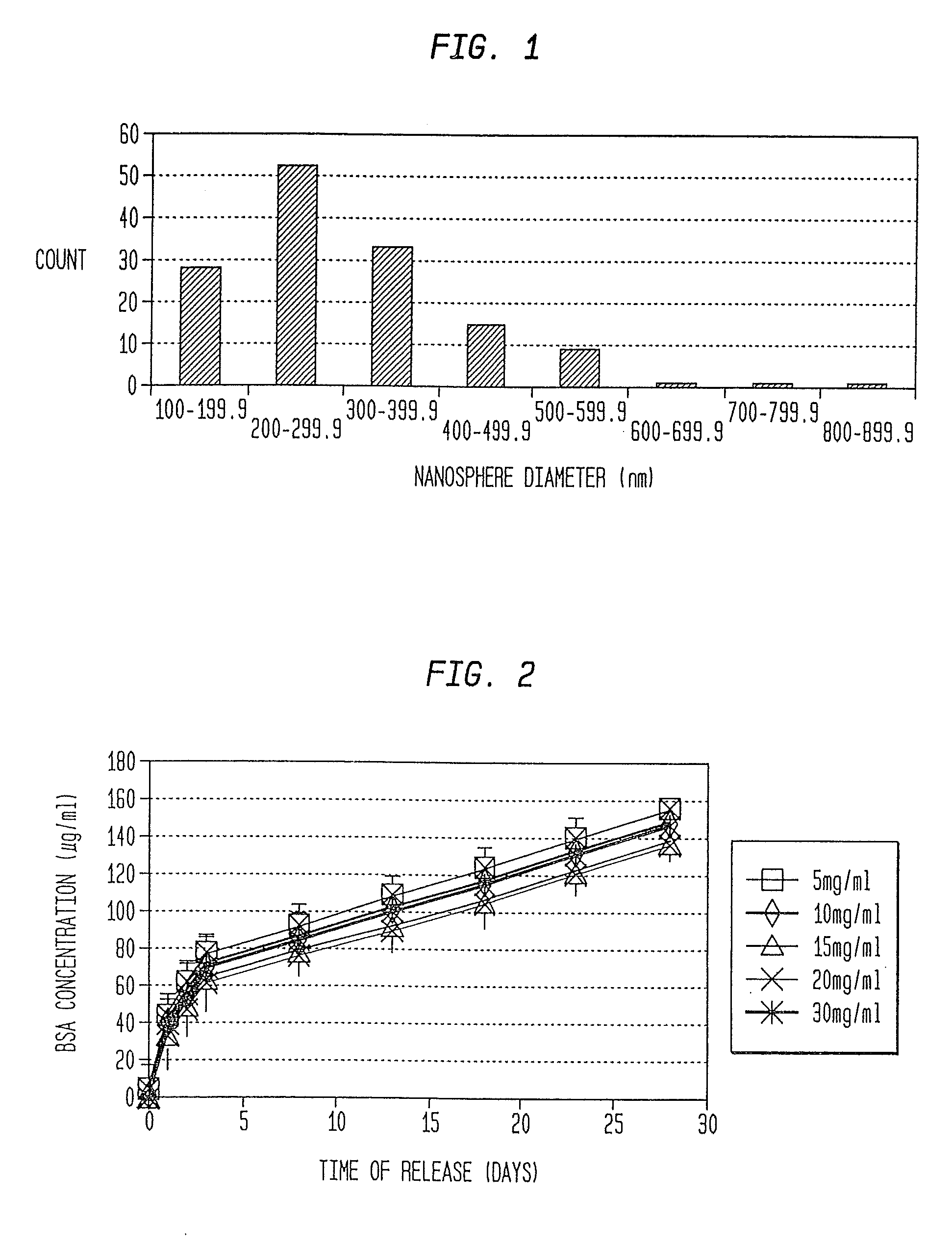
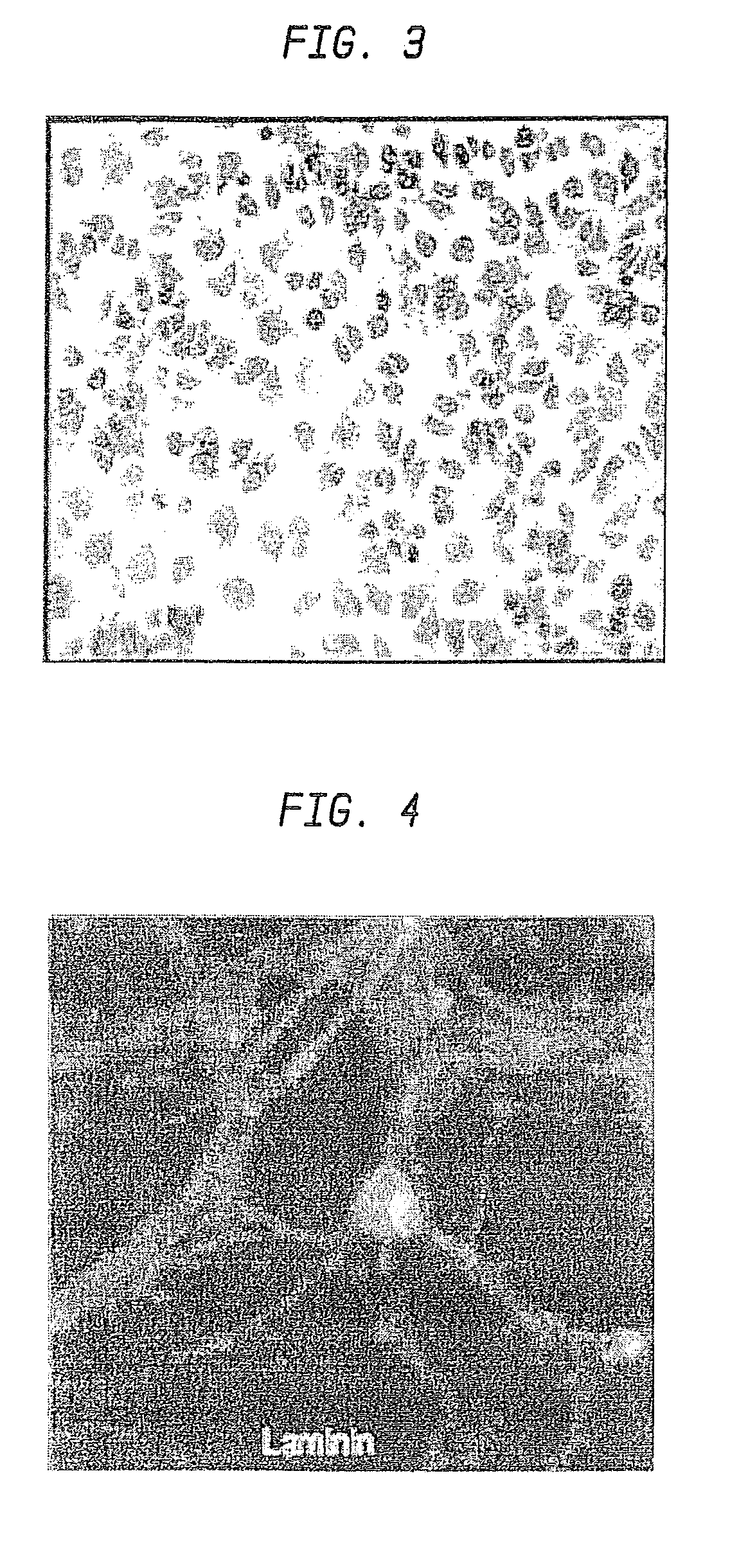
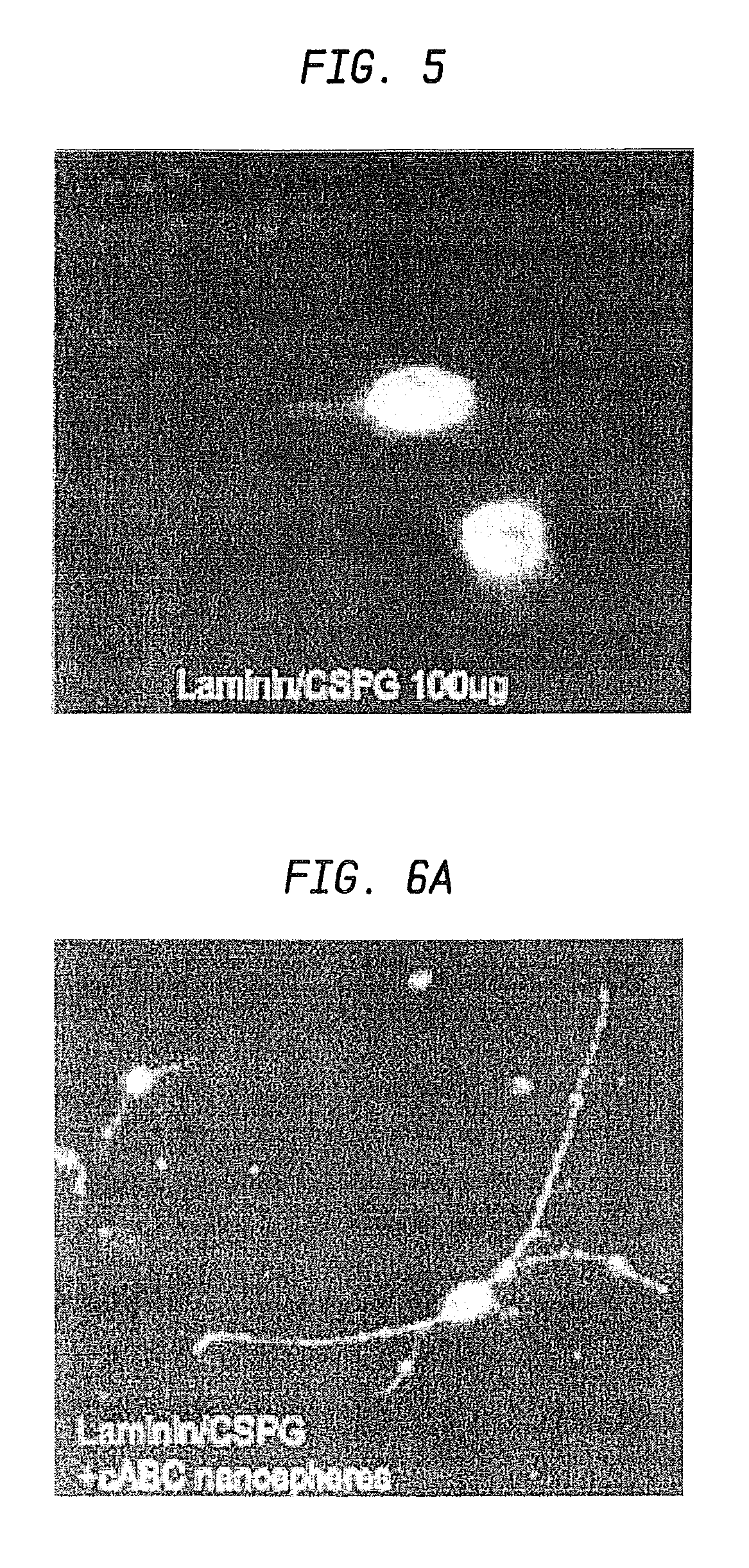
Nanosphere/Microsphere Delivery System for the Treatment of Spinal Cord Injury
InactiveUS20110212136A1Promote recoveryAvoid scaringNervous disorderPeptide/protein ingredientsMicrosphereMedicine
A formulation including injectable biodegradable nanospheres and / or microspheres as a delivery system for chondroitinase ABC (cABC) or a functional derivative of cABC to treat acute and chronic spinal chord injury in a mammal having the same is provided. The biodegradable nanosphere / microsphere formulation releases cABC or a functional derivative of cABC in a time-released manner at the site of the spinal cord injury. cABC infusion can promote axon regrowth and some behavioral recovery. The nanospheres and / or microspheres provided herein include cABC or a functional derivative of cABC loaded within and / or on a biodegradable polymer matrix. In some embodiments of the present invention, the surface of the biodegradable polymer matrix can be modified to target a specific scar site. In addition to providing a nanosphere formulation that include polymeric incorporated cABC, a method of treating a mammal having a spinal cord injury is also provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Novel tetrahydro-isoquinolines
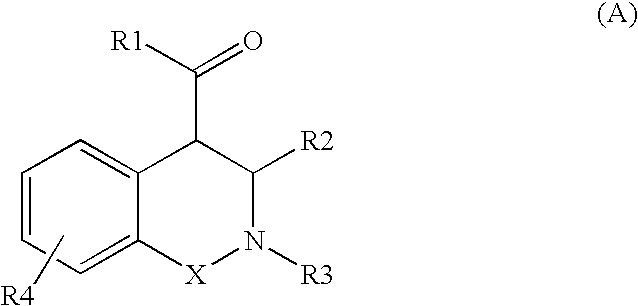
ActiveUS20090306130A1Therapeutic utility in cancer therapyInduce apoptosisBiocideOrganic chemistrySide effectMulti organ
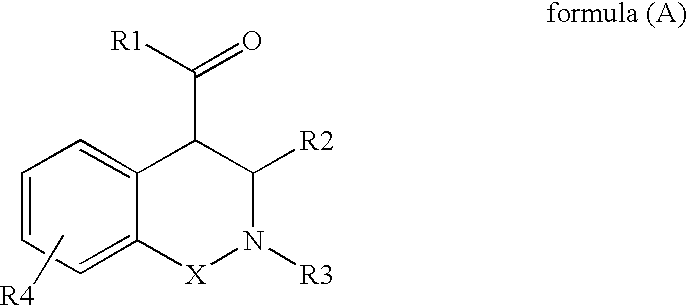
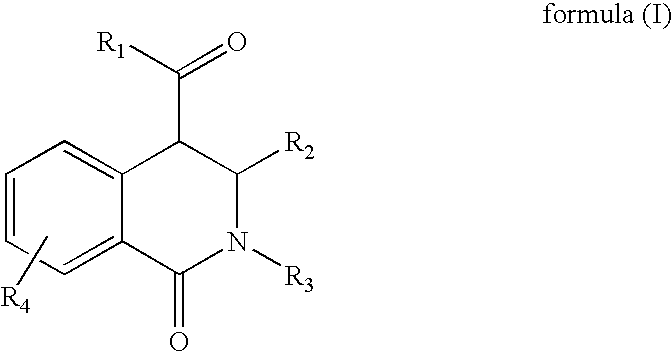
The present invention provides a compound selected from compounds of formula (A) as ligand binding to the HDM2 protein, inducing apoptosis and inhibiting proliferation, and having therapeutic utility in cancer therapy and prevention. Compounds of formula (A) can be used as therapeutics for treating stroke, myocardial infarction, ischemia, multi-organ failure, spinal cord injury, Alzheimer's Disease, injury from ischemic events and heart valvular degenerative disease. Moreover, compounds of formula (A) can be used to decrease the side effects from cytotoxic cancer agents, radiation and to treat viral infections.
Owner:NEXUS PHARM INC
Neurite growth regulatory factors
InactiveUS6103232AAvoid environmentIncrease the areaNervous disorderHydrolasesNerve fiber bundleMyelin
The present invention relates to methods of inducing neurite outgrowth in the central nervous system by antagonizing neural growth inhibitory factors. More particularly, the present invention is directed to use of antibodies to the central nervous system (CNS) myelin associated proteins; such antibodies can be used in the diagnosis and therapies of nerve damage resulting from trauma, infarction, and degenerative disorders of the CNS. In a specific embodiment of the invention, the monoclonal antibody IN-1 may be used to promote neurite outgrowth of nerve fibers over long distances in spinal cord lesions.
Owner:ZURICH UNIV OF
Intravenous omega-3 fatty acid compositions & method of use
The invention encompasses intravenous pharmaceutical compositions containing omega-3 fatty acids and methods of treating traumatic brain injury, traumatic spinal cord injury and / or stroke using these pharmaceutical compositions.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Establishment method of severe spinal cord injury prognosis prediction model
ActiveCN112992346AAccurate predictionDemonstrating Forecast AccuracyMedical data miningMedical automated diagnosisData setSpinal cord lesion
The invention discloses a method for establishing a severe spinal cord injury prognosis prediction model, and the method is characterized by comprising the following steps: extracting clinical data of cases of patients diagnosed as spinal cord injury: 1) incorporating the following clinical characteristics; 2) preprocessing the clinical features: processing missing data through different filling methods according to the types of the clinical features; 3) incorporating an algorithm combination of a feature selection method and a machine learning classification algorithm, wherein the feature selection method is used for screening clinical features with significant prediction values, and the selected clinical features are used for training the machine learning classification algorithm; 4) selecting the algorithm combination with the maximum area AUC under the micro average curve from the prediction performance of the algorithm combination in the step 3) in the training data set, and integrating the algorithm combination by using a stacking method to obtain a prediction model. The invention has accurate and objective performance for predicting the prognosis of the patient with severe spinal cord injury.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
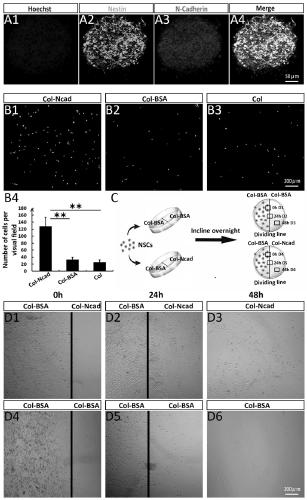
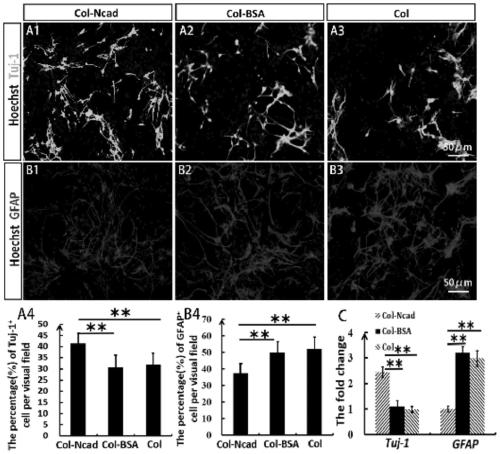
Tissue engineering material for nerve injury repair as well as preparation method and application of tissue engineering material
ActiveCN111110924AEfficient migration inductionPromote differentiationNervous system cellsCell culture supports/coatingSpinal cord lesionCollagen scaffold
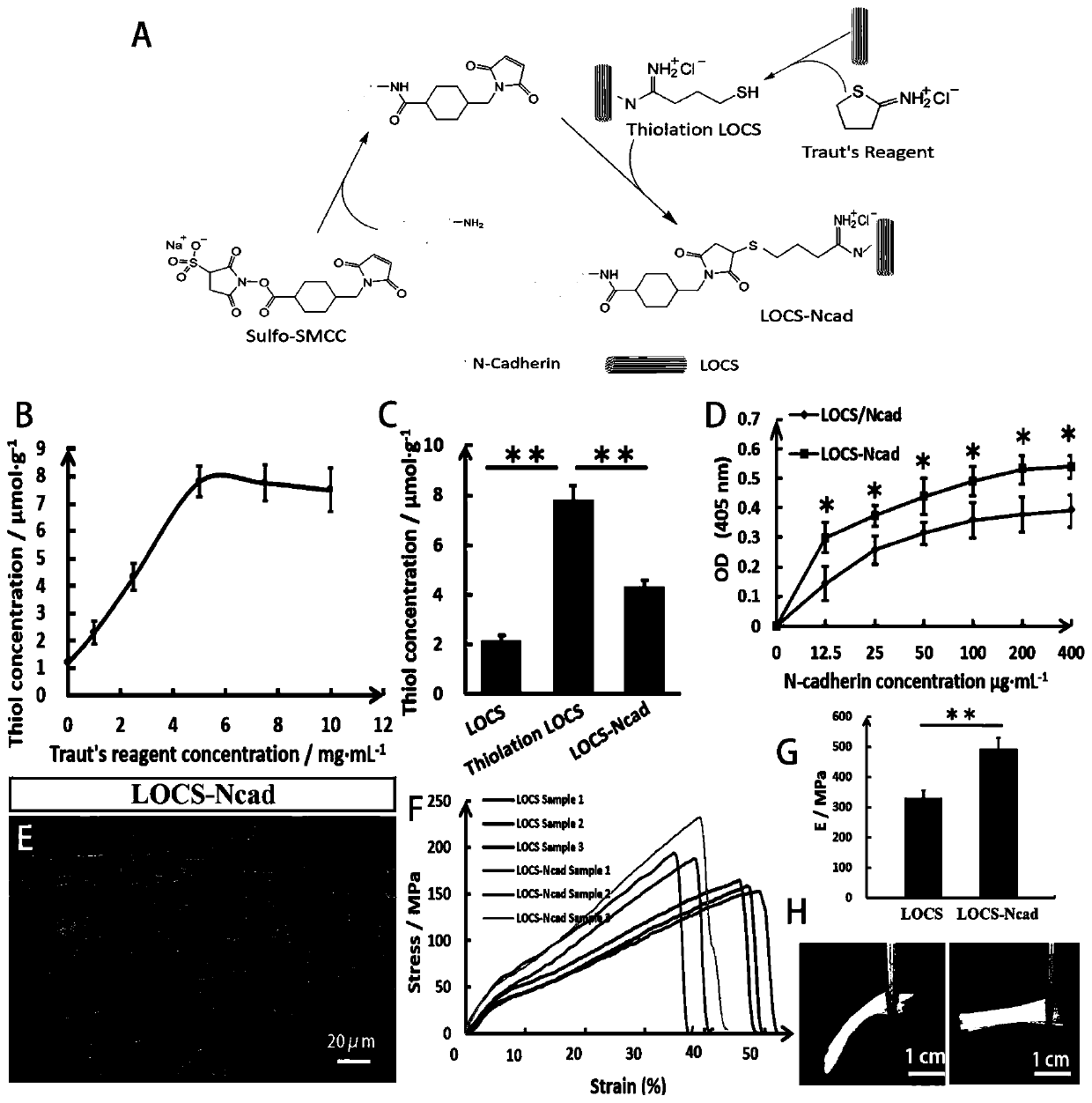
The invention relates to the technical field of tissue engineering, in particular to a tissue engineering material for nerve injury repair as well as a preparation method and application of the tissueengineering material. The tissue engineering material for nerve injury repair is a linear ordered collagen stent of crosslinked N-cadherin. The tissue engineering material prepared by crosslinkingtheN-cadherin in the linear ordered collagen stent can efficiently induce neural stem cells to migrate towards an injury region, enrich neutral stem cells in the injury region, effectively inhibit deposition of inhibitors such as chondroitin sulfate proteolgycan and promote differentiation of the neutral stem cells towards neurons so as to promote recovery of electrophysiological and motion functions; and meanwhile, the N-cadherin crosslinked linear ordered collagen stent also has stable and ordered topological structures and excellent mechanical properties and thus can be used for repairing nerve injuries such as spinal cord injury.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18b7c78f-e97e-45ca-abd5-187dfef2a747/US20020128261A1-20020912-C00001.png)
![Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18b7c78f-e97e-45ca-abd5-187dfef2a747/US20020128261A1-20020912-C00002.png)
![Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18b7c78f-e97e-45ca-abd5-187dfef2a747/US20020128261A1-20020912-C00003.png)