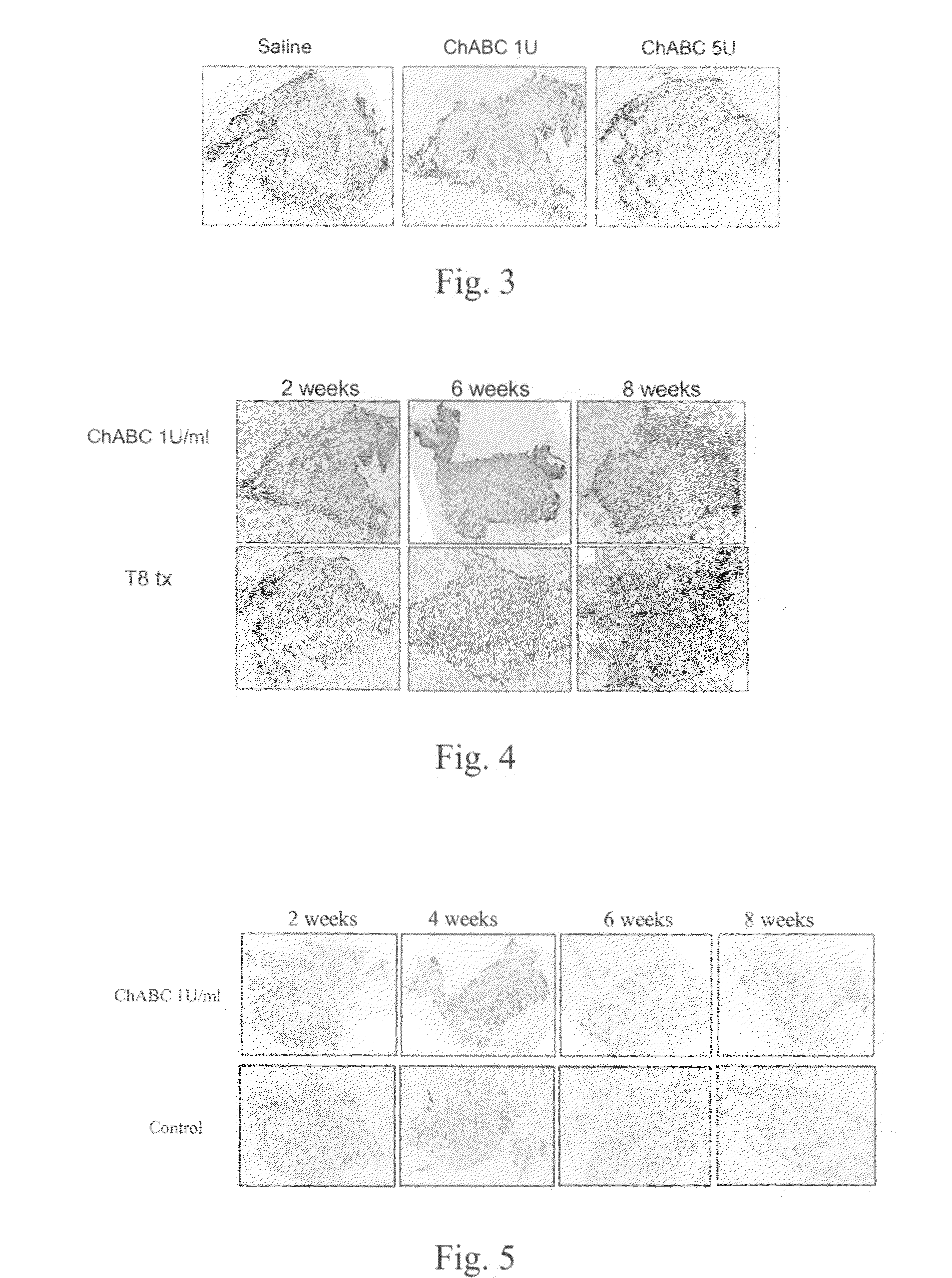
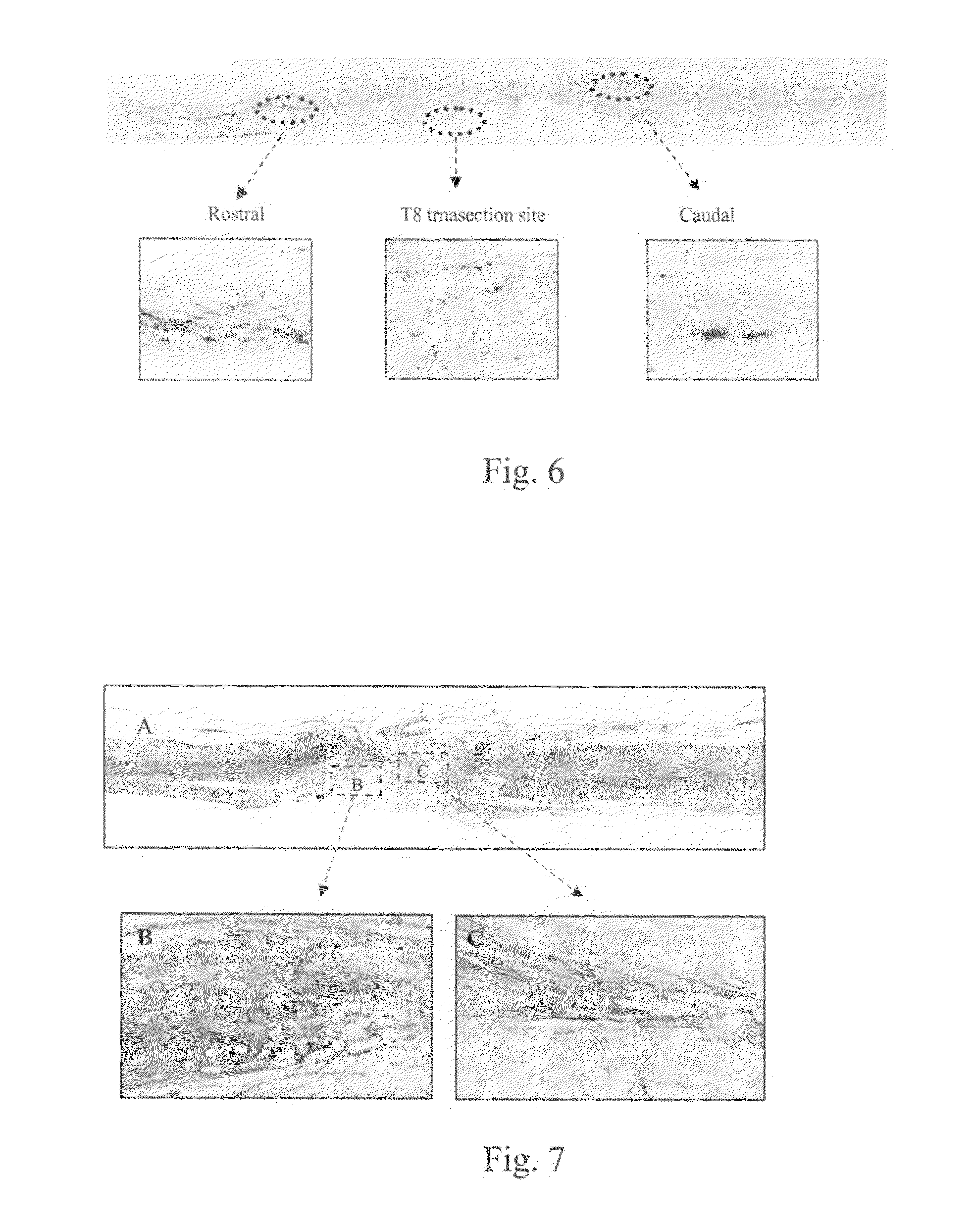
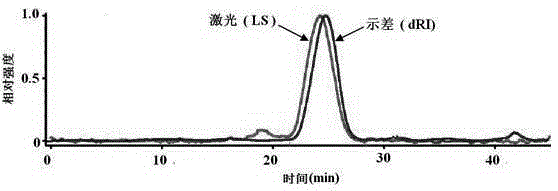
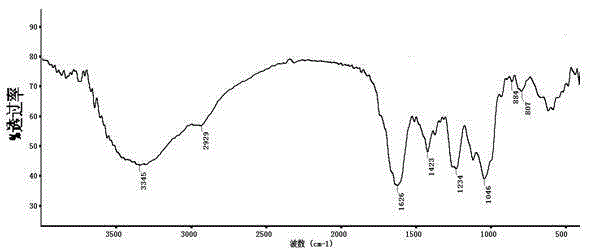
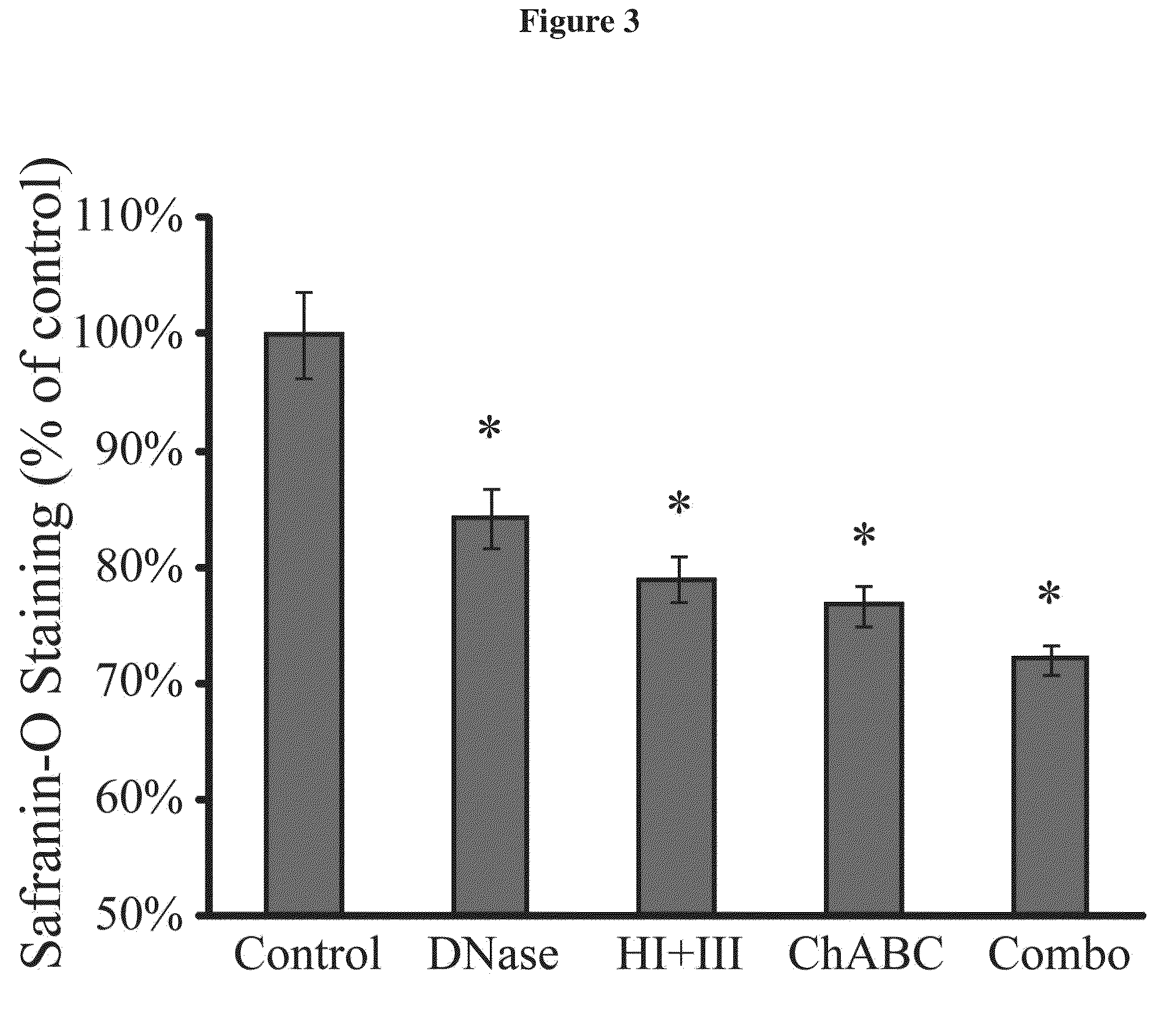
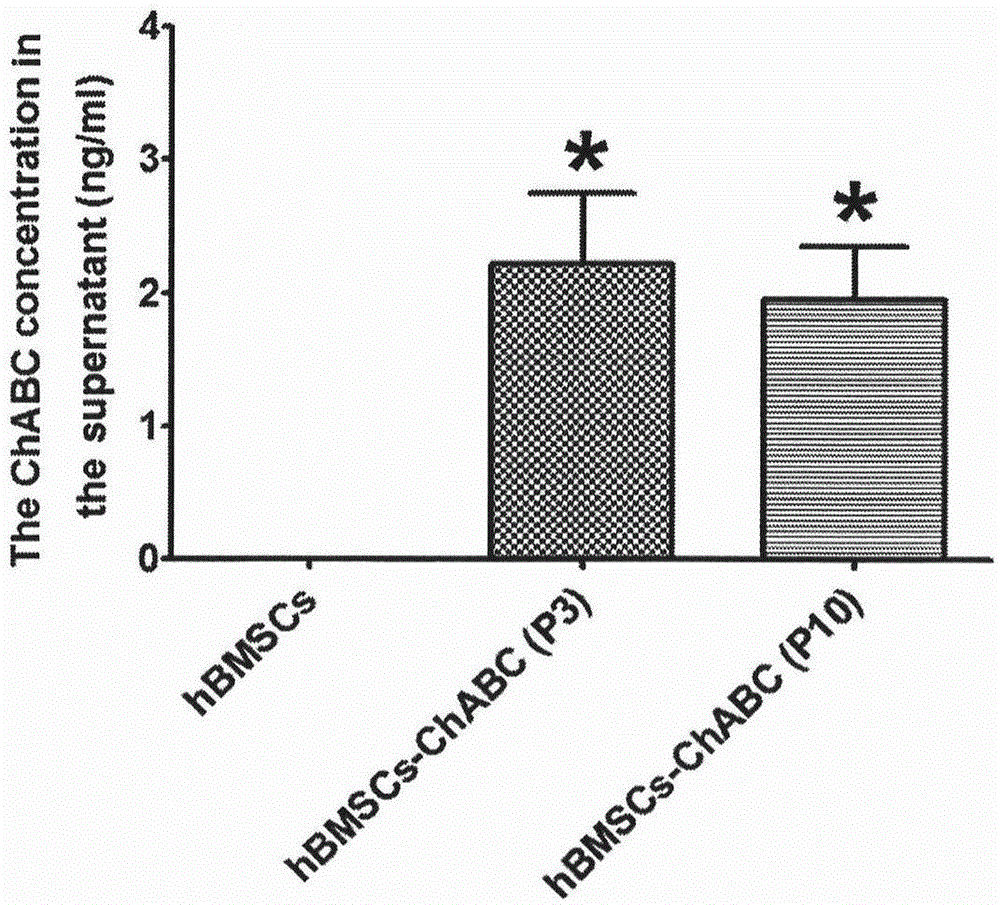
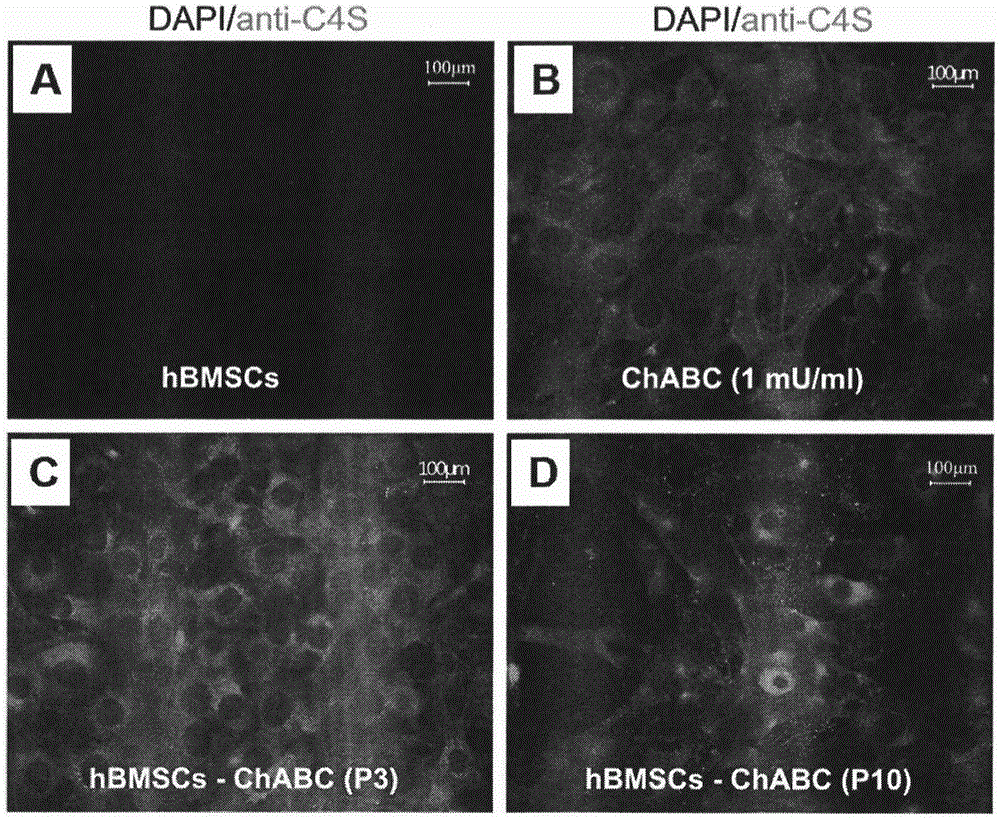
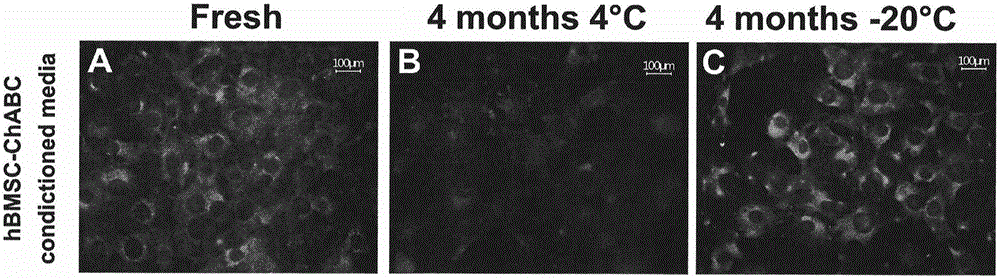
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Chondroitinase ABC" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
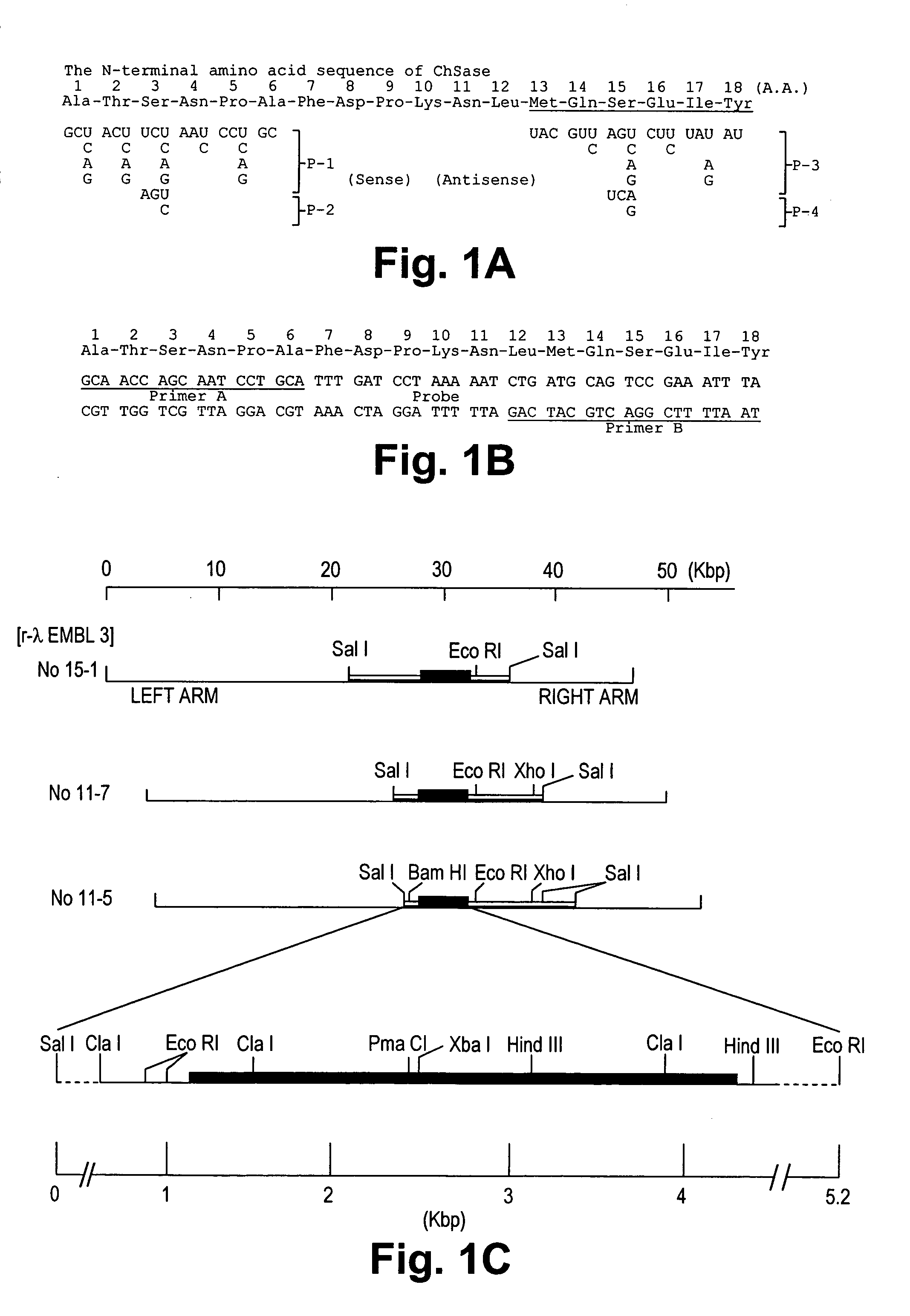
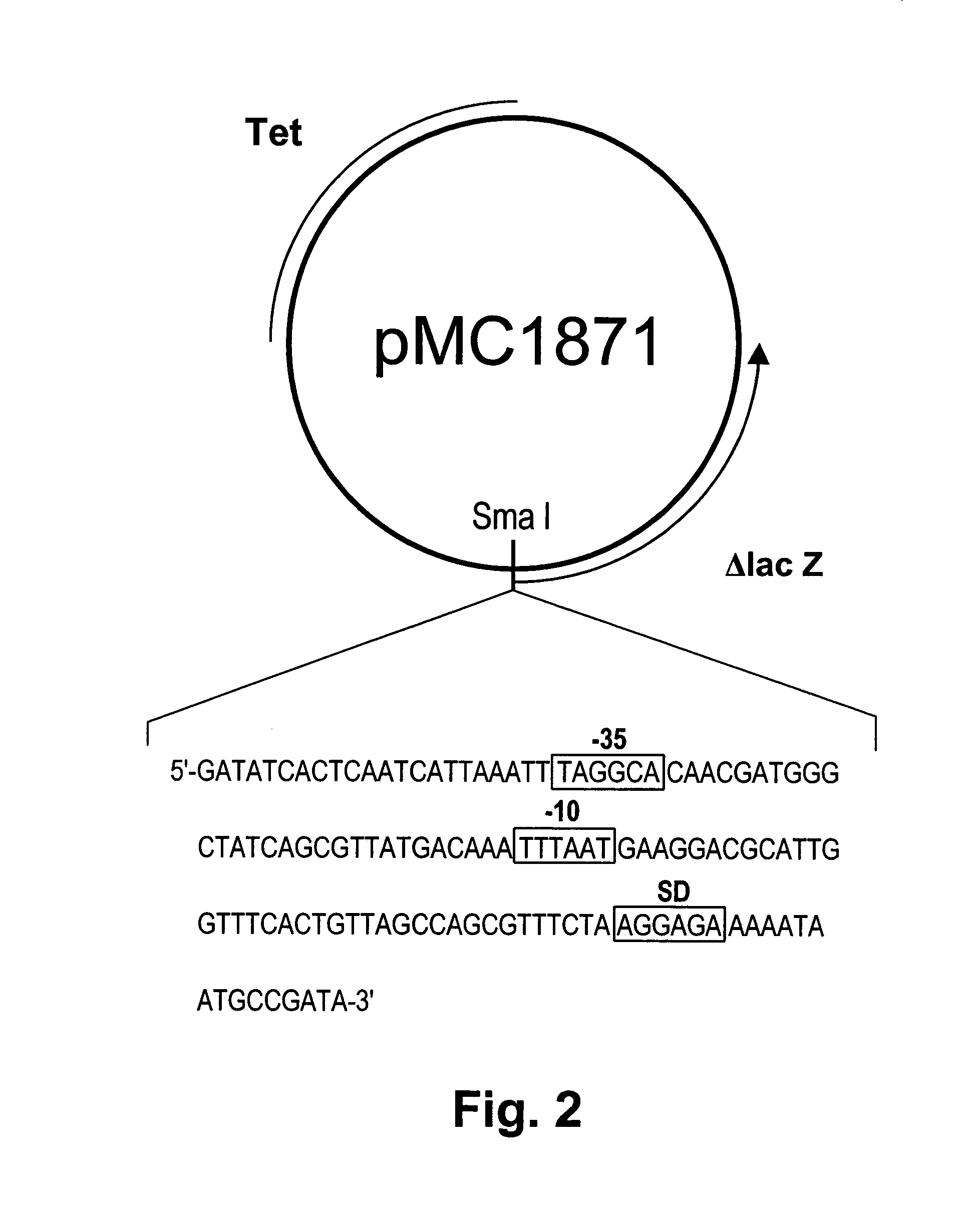
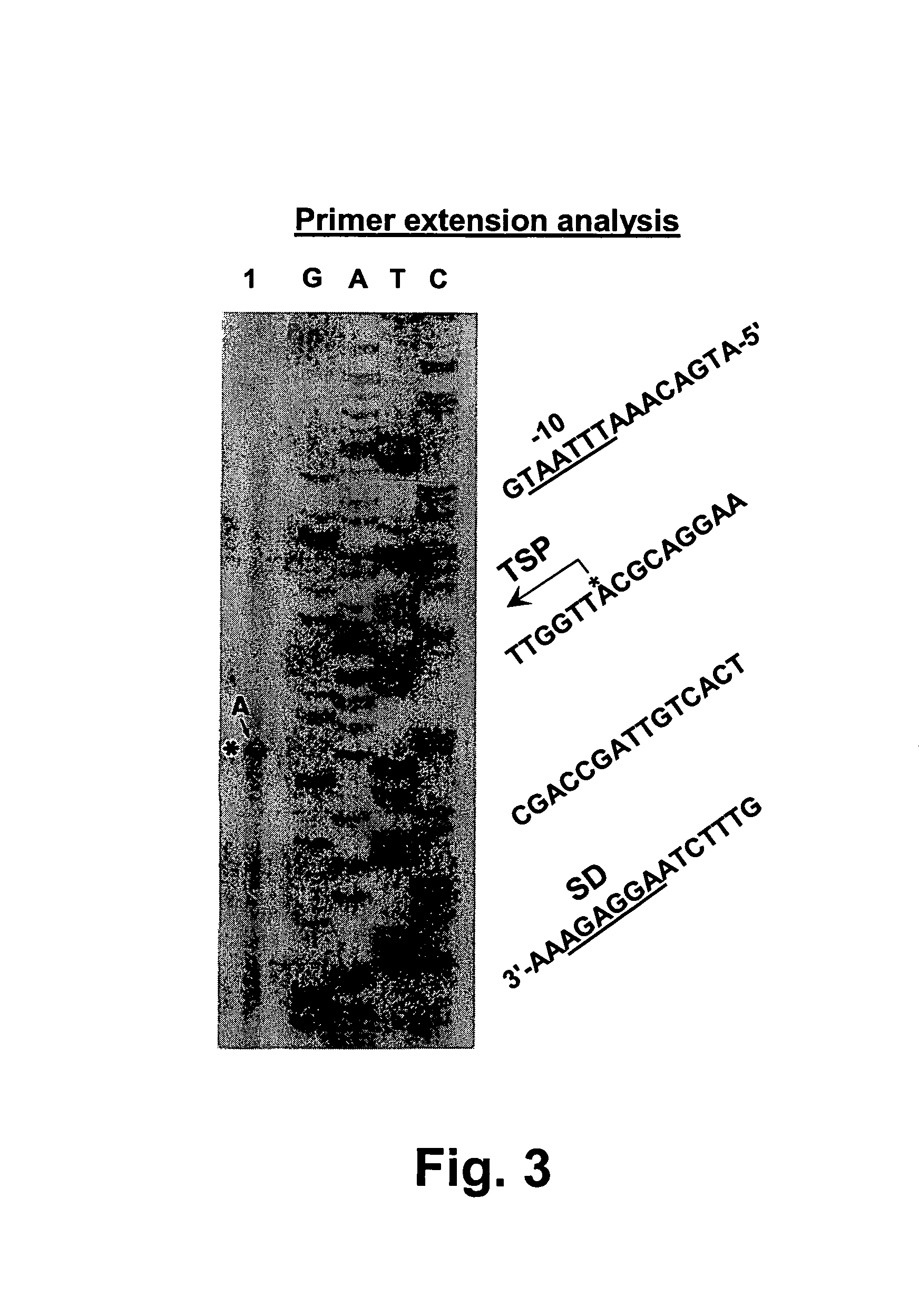
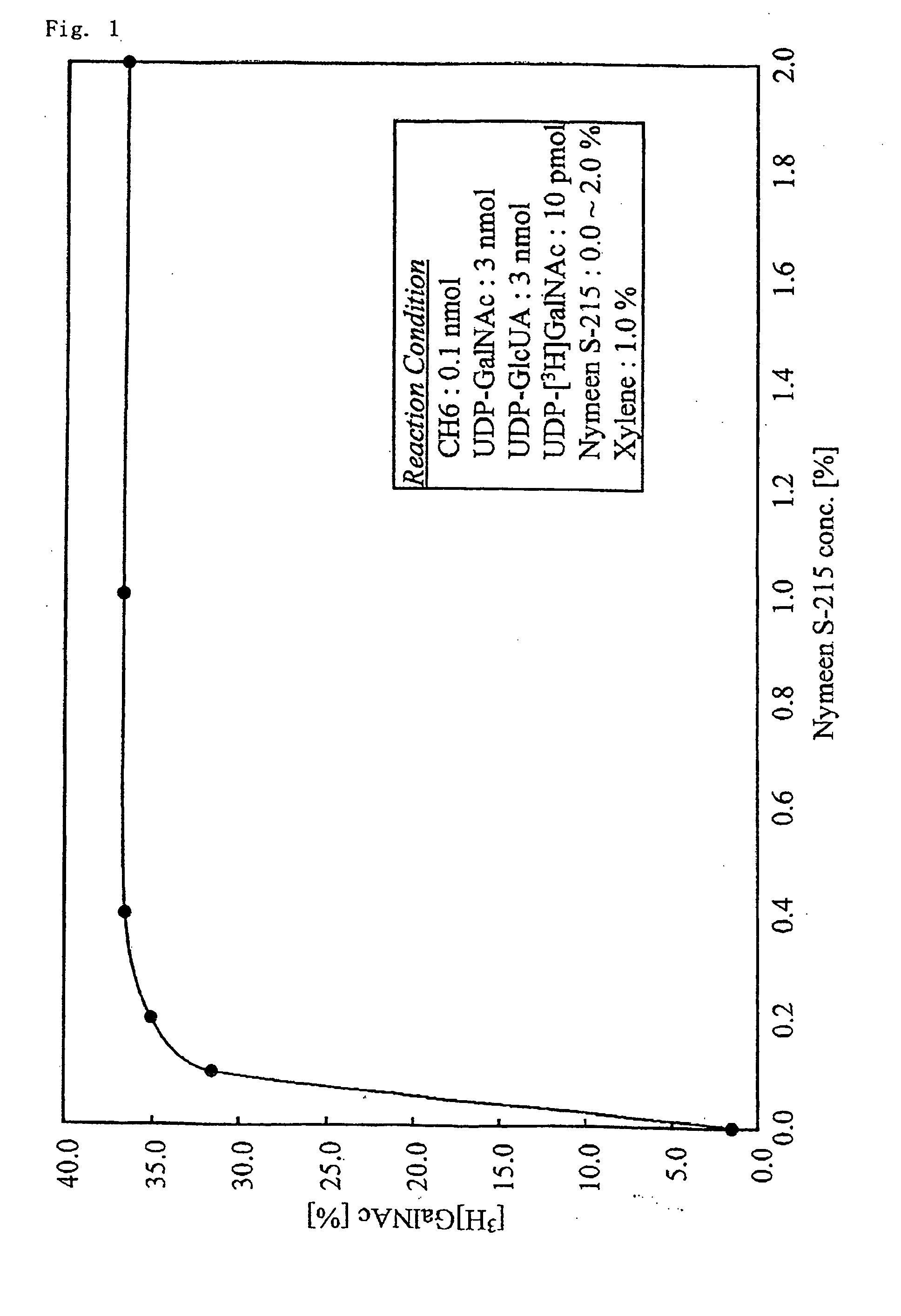
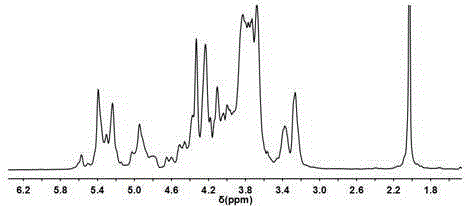
Chondroitinase ABC is purified from Proteus vulgaris. by cation exchange chromatography catalyzes the degradation of chondroitin sulfate A, chondroitin sulfate C, dermatan sulfate, chondroitin and hyaluronan to mainly disaccharideswith D-4hexuronate by the eliminative cleavage of 1,4-B-hexosaminyl linkages.
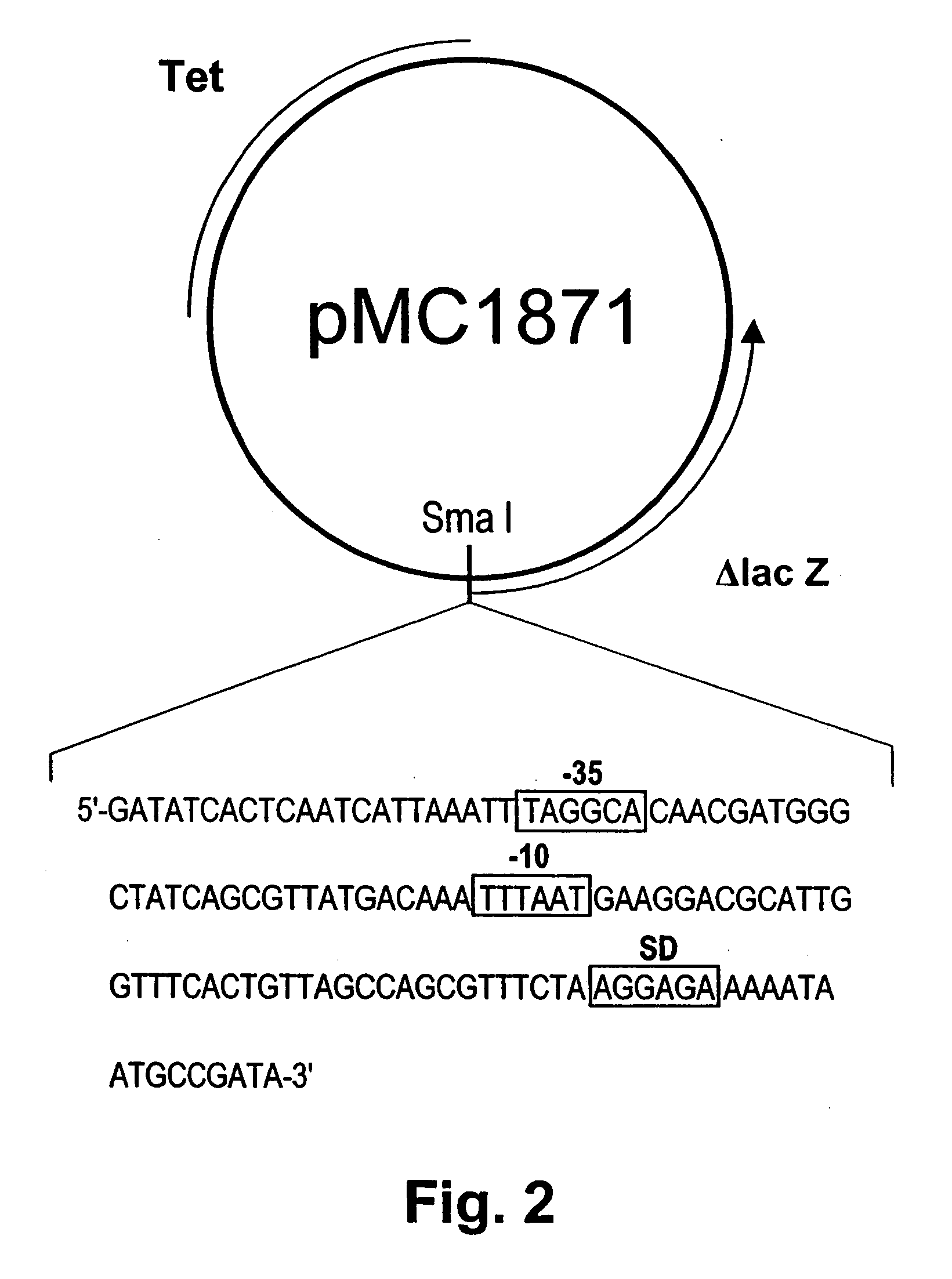
Chondroitinase, process for preparing the same, and pharmaceutical composition comprising the same
InactiveUS6184023B1Avoid stickingInhibit productionBacteriaHydrolasesChondroitinase ABCConcentration gradient
A crystallizable, purified chondroitinase ABC having a molecular weight of about 100,000 dalton by the measurement of the SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the measurement by the gel permeation chromatography method, having alanine as the N-terminal amino acid and proline as the C-terminal amino acid. A process for the purification of the crystallizable purified chondroitinase ABC comprising removing nucleic acid from an surfactant solution extract obtained from cells of chondroitinase ABC-producing microorganisms and chromatographically treating by concentration gradient elution using a weak cation exchange resin or a strong cation exchange resin. A composition comprising a chondroitinase and serum albumin, gelatin, or a nonionic surfactant.
Owner:SEIKAGAKU KOGYO CO LTD
Treatment of central nervous system damage
InactiveUS20050118157A1Decreased inhibitory propertiesImprove plasticityCompound screeningNervous disorderNervous systemChondroitinase ABC
The invention provides a method of promoting neuronal plasticity in the CNS of a mammal, the method comprising administering to the CNS of the mammal an agent that reduces the inhibitory properties of chondroitin sulphate proteoglycans. Preferred agents are chondroitinases and sulfatases, e.g., chondroitinase ABC. Also provided are methods of identifying further agents.
Owner:KING'S COLLEGE LONDON +1
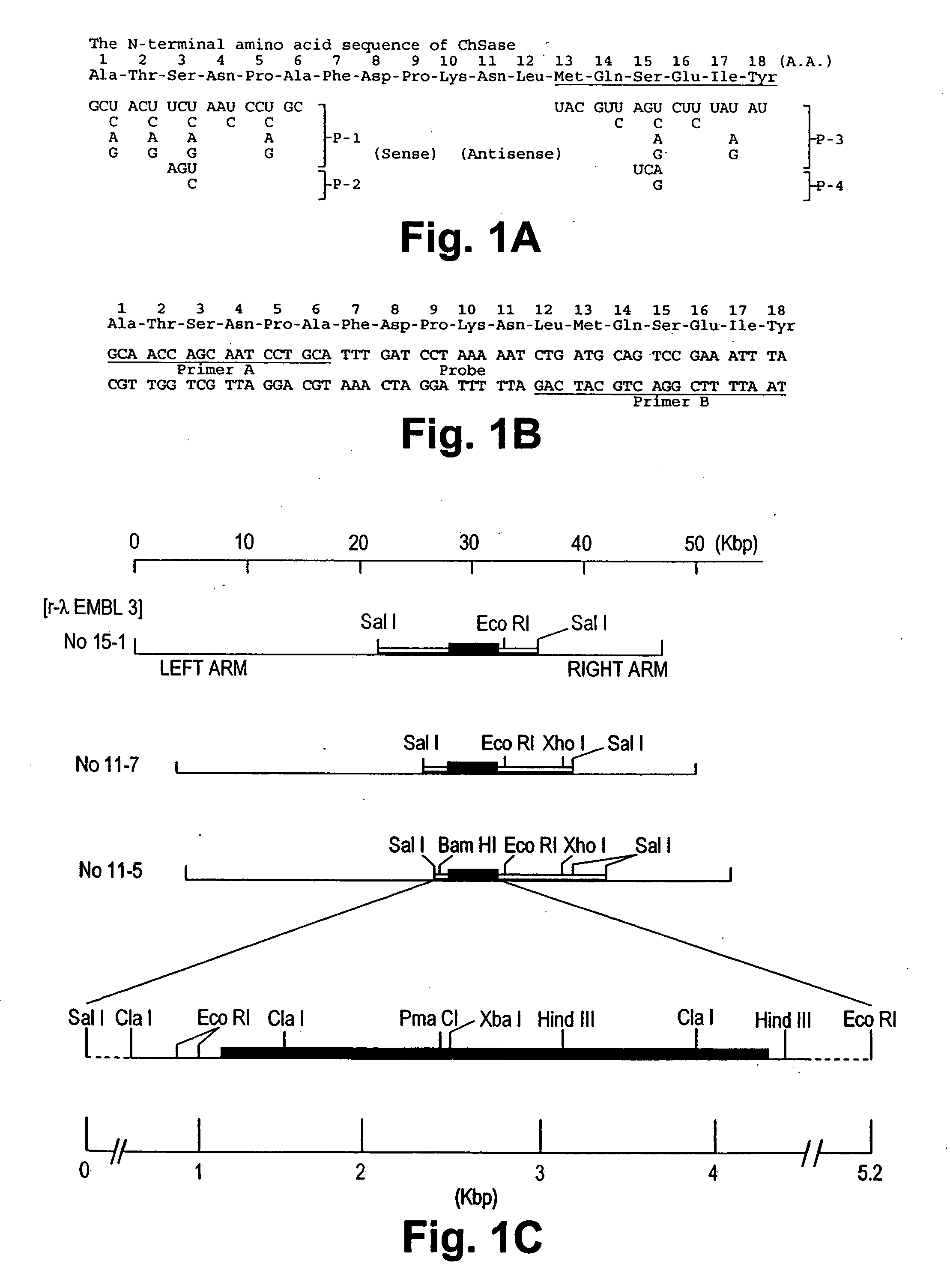
Gene encoding chondroitinase ABC and uses therefor
Nucleic acid sequences coding for the chondroitinase ABC gene and isolated chondroitinase ABE protein produced in a host cell transformed with a nucleic acid vector directing the expression of a nucleotide sequence coding for chondroitinase ABE protein described. Chondroitinase ABC prepared by chemical synthesis also described. Monoclonal and polyclonal antibodies which are specifically reactive with chondroitinase ABC protein are disclosed. The isolated chondroitinase ABC can be used in methods of treating intervertebral disc replacement, promoting neurite regeneration, and detecting galactosaminoglycans.
Owner:MARUHA NICHIRO
Long-chain chondroitin sugar chain and method for producing the same and method for promoting synthesis of chondroitin
ActiveUS20090263867A1Efficient productionEasy to produceBacteriaSugar derivativesFiltrationChondroitinase ABC
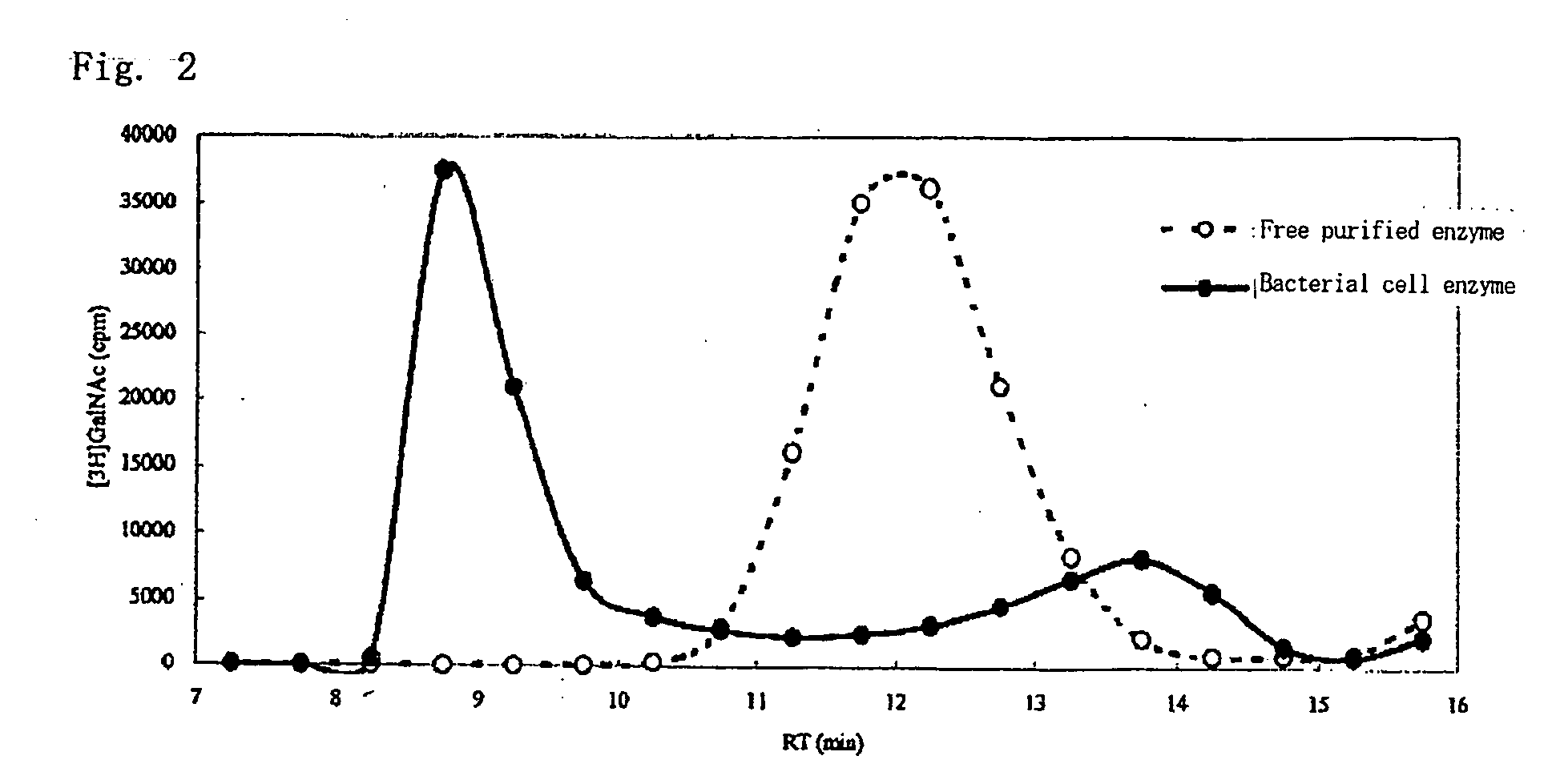
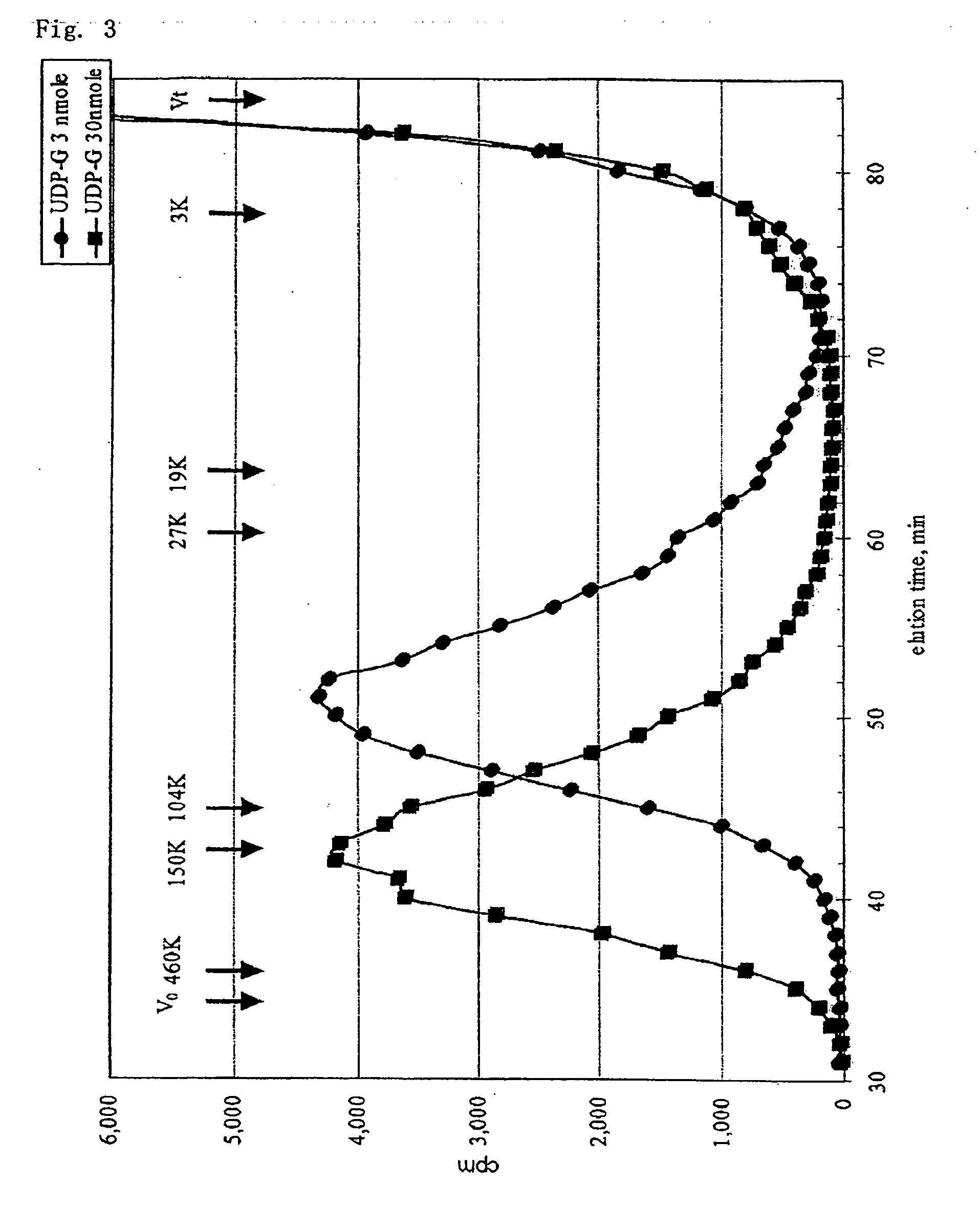
A method for producing a chondroitin sugar chain comprises at least the following step: a step of allowing “a glucuronic acid donor”, “an N-acetyl galactosamine donor”, “a sugar receptor” and “a bacterial cell enzyme which synthesizes chondroitin” to coexist in a reaction system in the presence of a surfactant. Here, the surfactant is preferably selected from n-nonyl-β-D-thiomaltopyranoside, sucrose monocaproate and sucrose monolaurate. The chondroitin sugar chain has all the following properties 1) to 3): 1) a weight average molecular weight: 50,000 or more when it is measured by gel filtration chromatography, 2) it is completely degraded to disaccharides with chondroitinase ABC, 3) when the sugar chain is decomposed with chondroitinase ABC and the decomposed products are subjected to a disaccharide analysis, substantially all of them correspond to an unsaturated disaccharide unit of chondroitin.
Owner:SEIKAGAKU KOGYO CO LTD
Tissue engineering nerval stent and preparation method and application thereof
The invention discloses a tissue engineering nerval stent and a preparation method and an application thereof. The chemical extraction method is adopted for removing main antigens, namely cells, axons and myelin sheaths, which can cause the rejection, and chondroitinase ABC is further adopted for removing chondroitin sulfate proteoglycan in xenogeneic nerves, thereby getting acellular xenogenic nerves with a complete vessel of a basilar membrane of an extracellular matrix and can be used as the tissue engineering nerval stent. The tissue engineering nerval stent is applicable to restoring peripheral nerve defect of rats, rabbits, dogs, human beings and the like, plays a very obvious role in promoting the regeneration of the axons and can further increase the length of restoring the peripheral nerve defect, thereby being a great nerve transplantation medical material.
Owner:卢世璧
Gene encoding chondroitinase ABC and uses therefor
Nucleic acid sequences coding for the chondroitinase ABC gene and isolated chondroitinase ABC protein produced in a host cell transformed with a nucleic acid vector directing the expression of a nucleotide sequence coding for chondroitinase ABC protein are described. Chondroitinase ABC prepared by chemical synthesis is also described. Monoclonal and polyclonal antibodies which are specifically reactive with chondroitinase ABC protein are disclosed. The isolated chondroitinase ABC can be used in methods of treating intervertebral disc displacement, promoting neurite regeneration, and detecting galactosaminoglycans.
Owner:MARUHA NICHIRO
Agents for suppressing neural fibrotic degeneration
InactiveUS20090202515A1Suppress neural fibrotic degenerationGreat medical and industrial significanceOrganic active ingredientsNervous disorderSide chainNeural cell
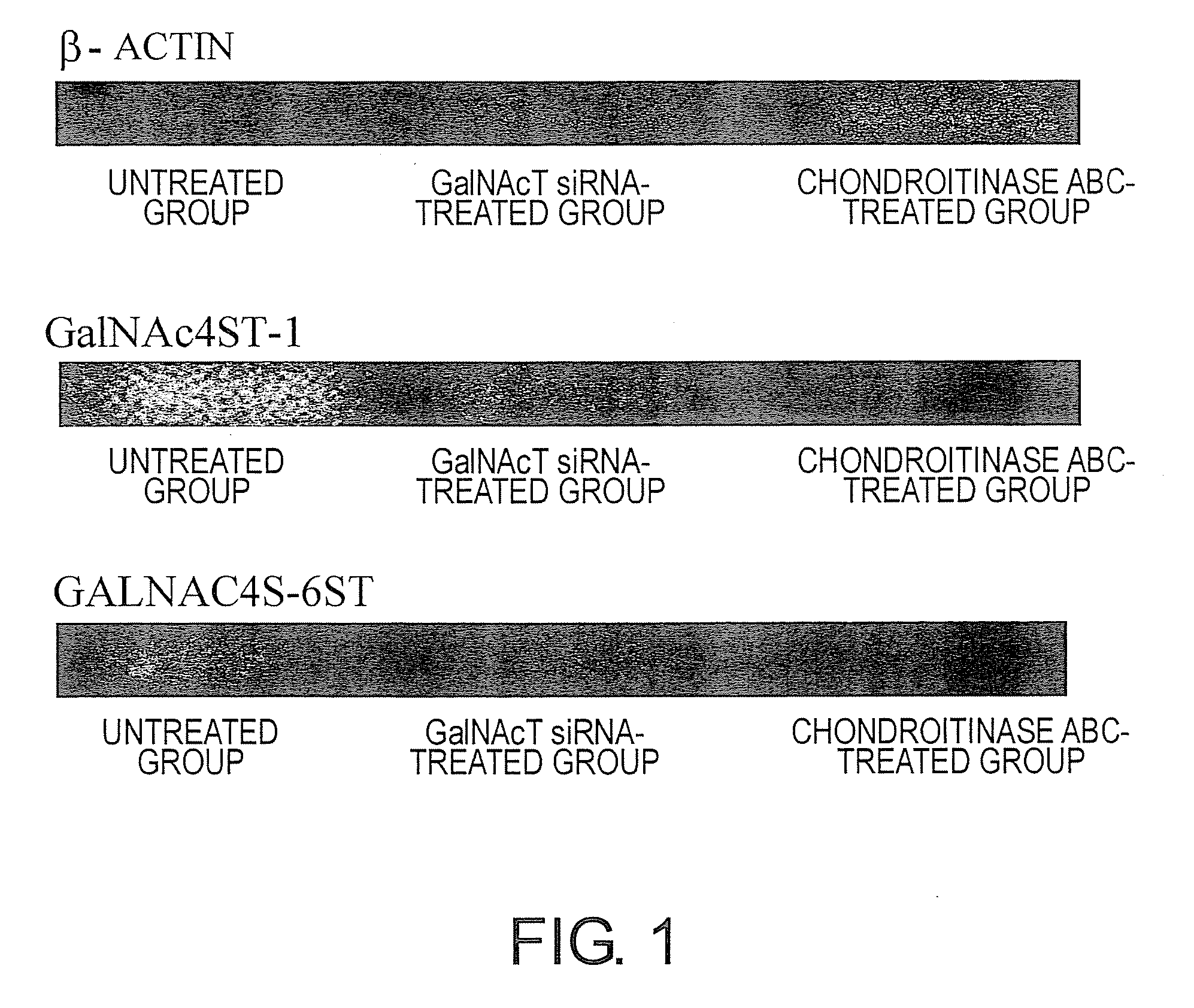
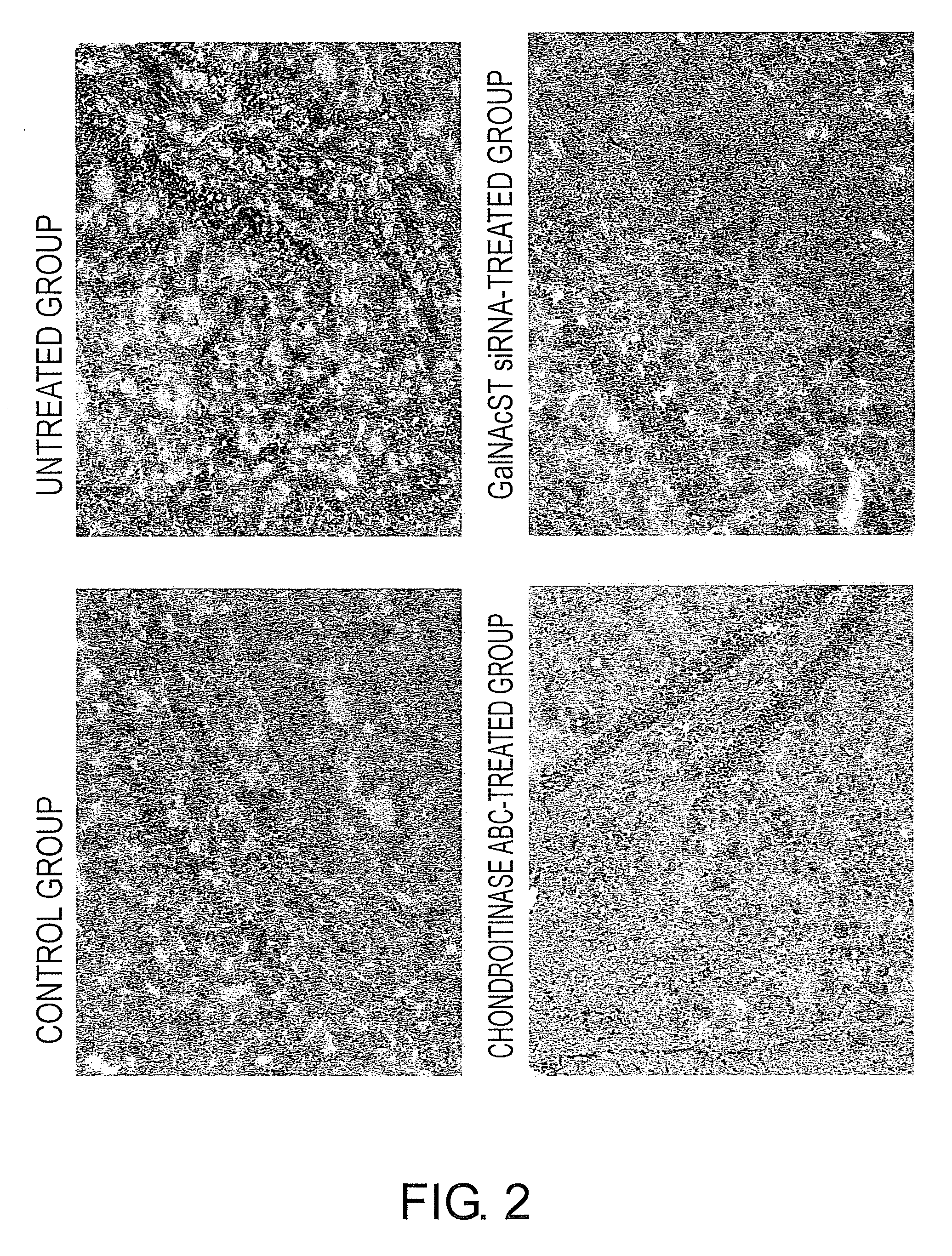
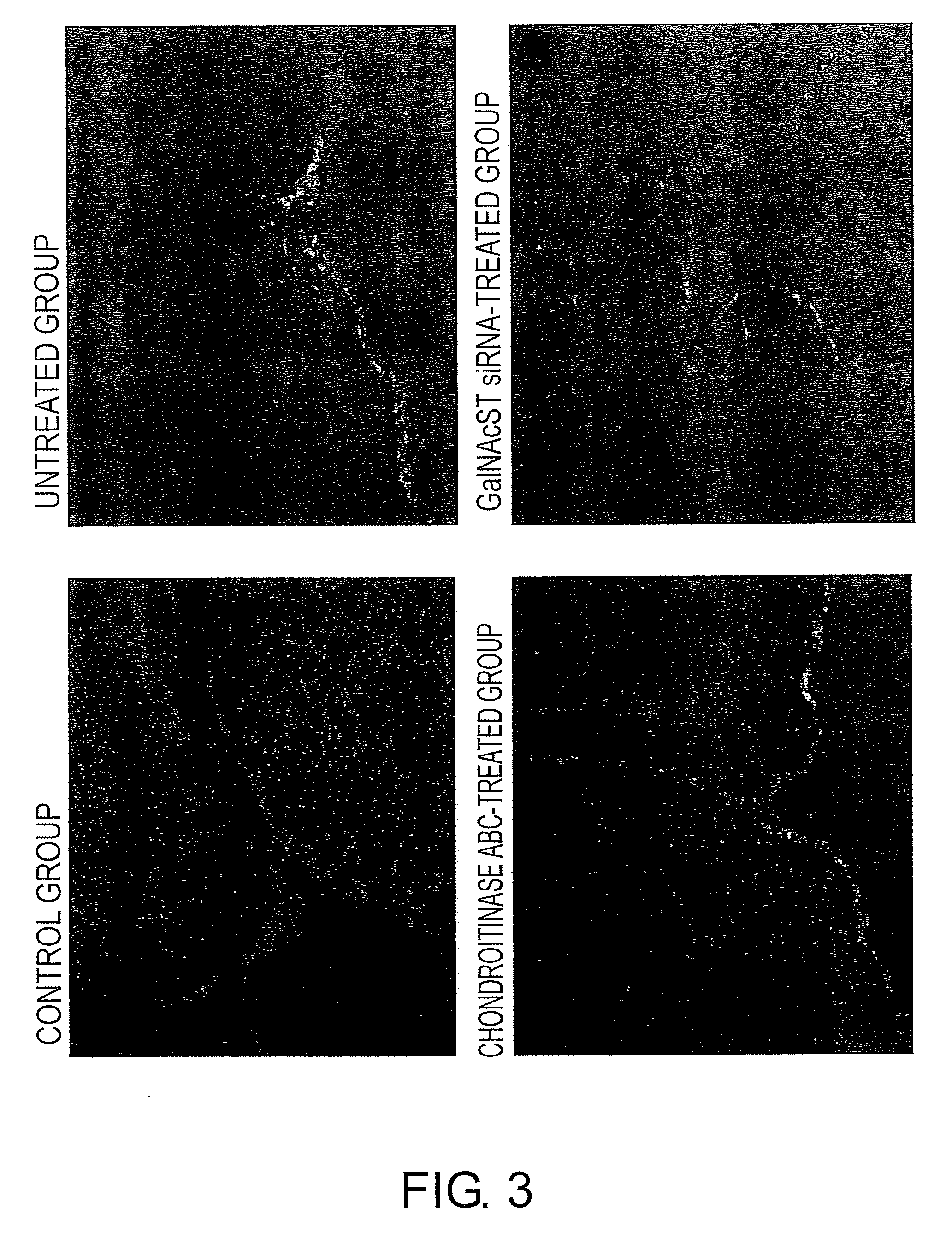
The present invention examined the accumulation of chondroitin sulfate proteoglycans (CSPGs). The present invention relates to neurodegeneration-suppressing agents that are suitable for gene therapy or prevention of neural fibrotic degenerative diseases which induce neural cell death due to an accumulation of abnormal proteins, where the therapies are based on siRNAs against N-acetylgalactosamine-4-O-sulfotransferases (N-acetylgalactosamine-4-O-sulfotransferase-1, N-acetylgalactosamine-4-O-sulfotransferase-2, and N-acetylgalactosamine-4-sulfate 6-O-sulfotransferase (GalNAc4ST-1, GalNAc4ST-2, and GALNAC4S-6ST, respectively)), which are sulfotransferases for acetylgalactosamine, a CSPG side chain, and chondroitinase ABC, an enzyme that degrades chondroitin sulfate, another CSPG side chain.
Owner:STELIC INST OF REGENERATIVE MEDICINE STELIC INST
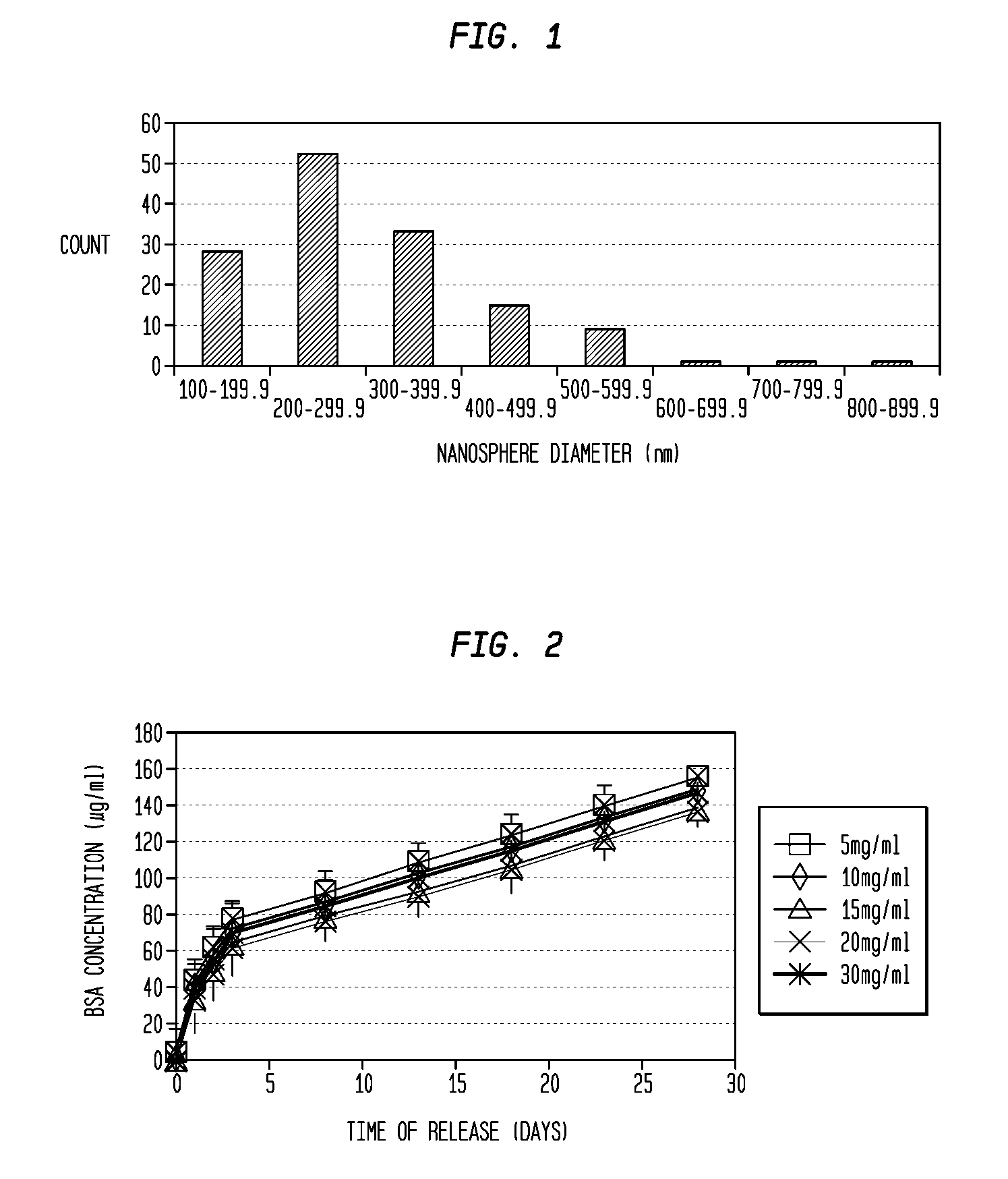
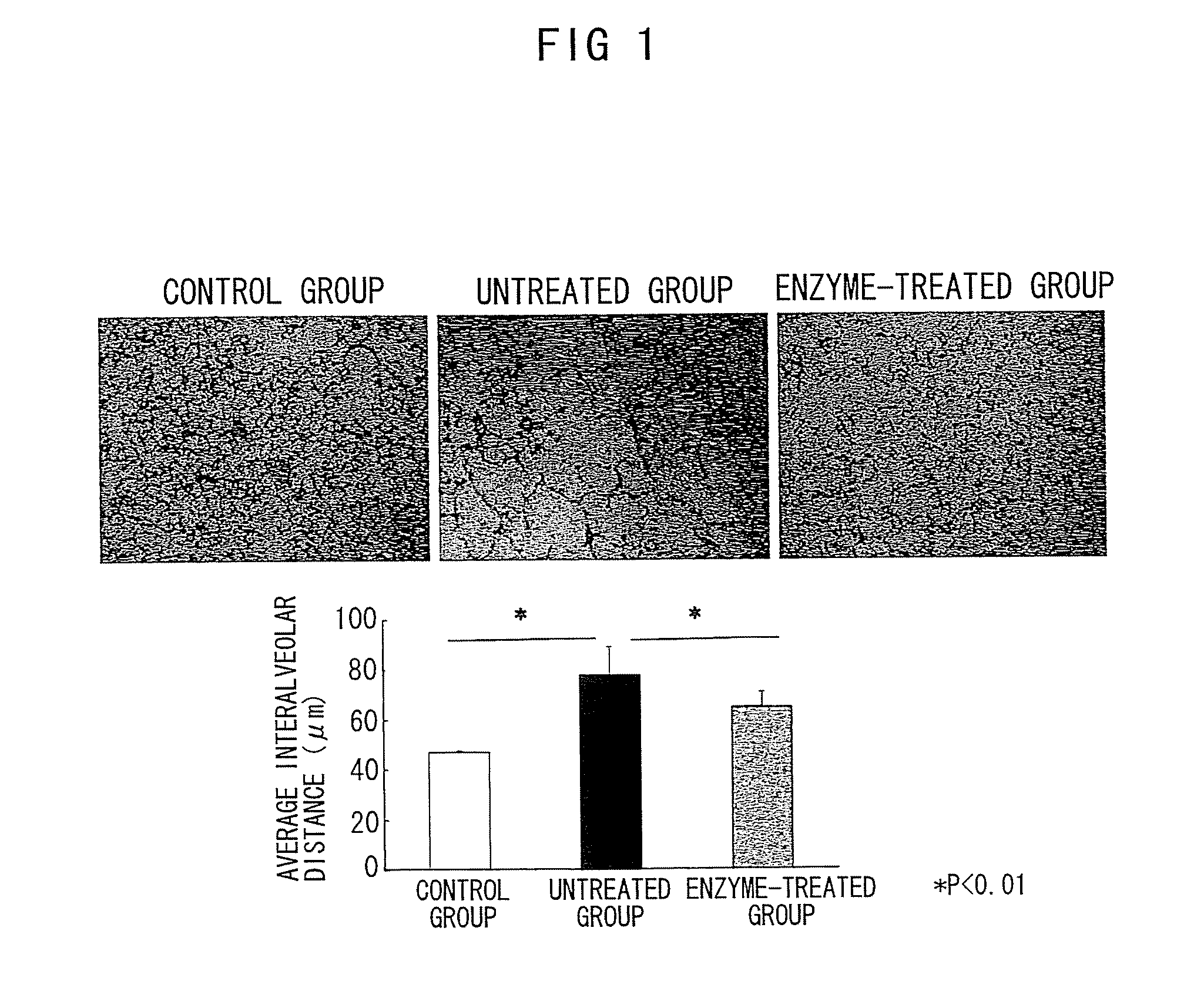
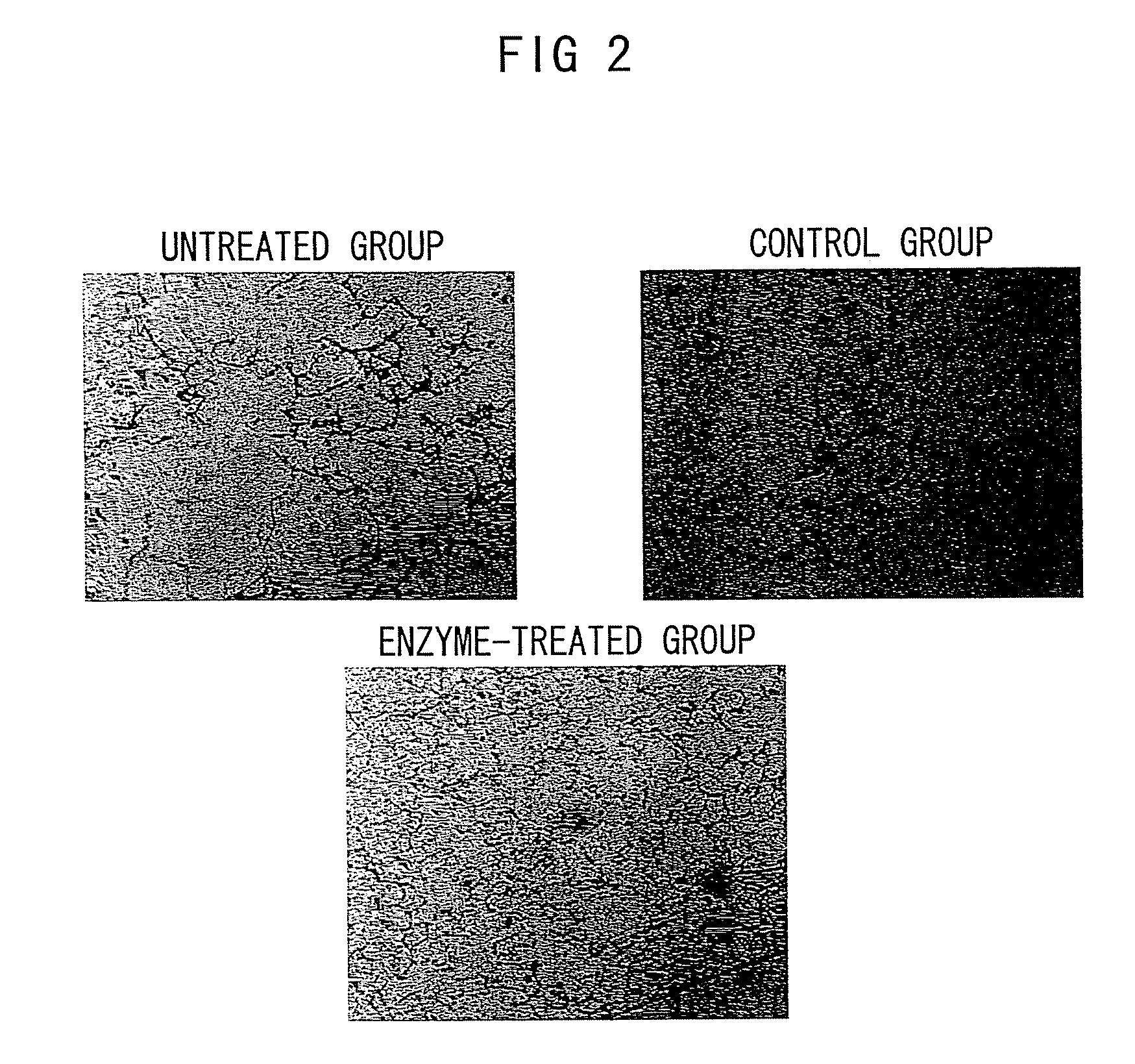
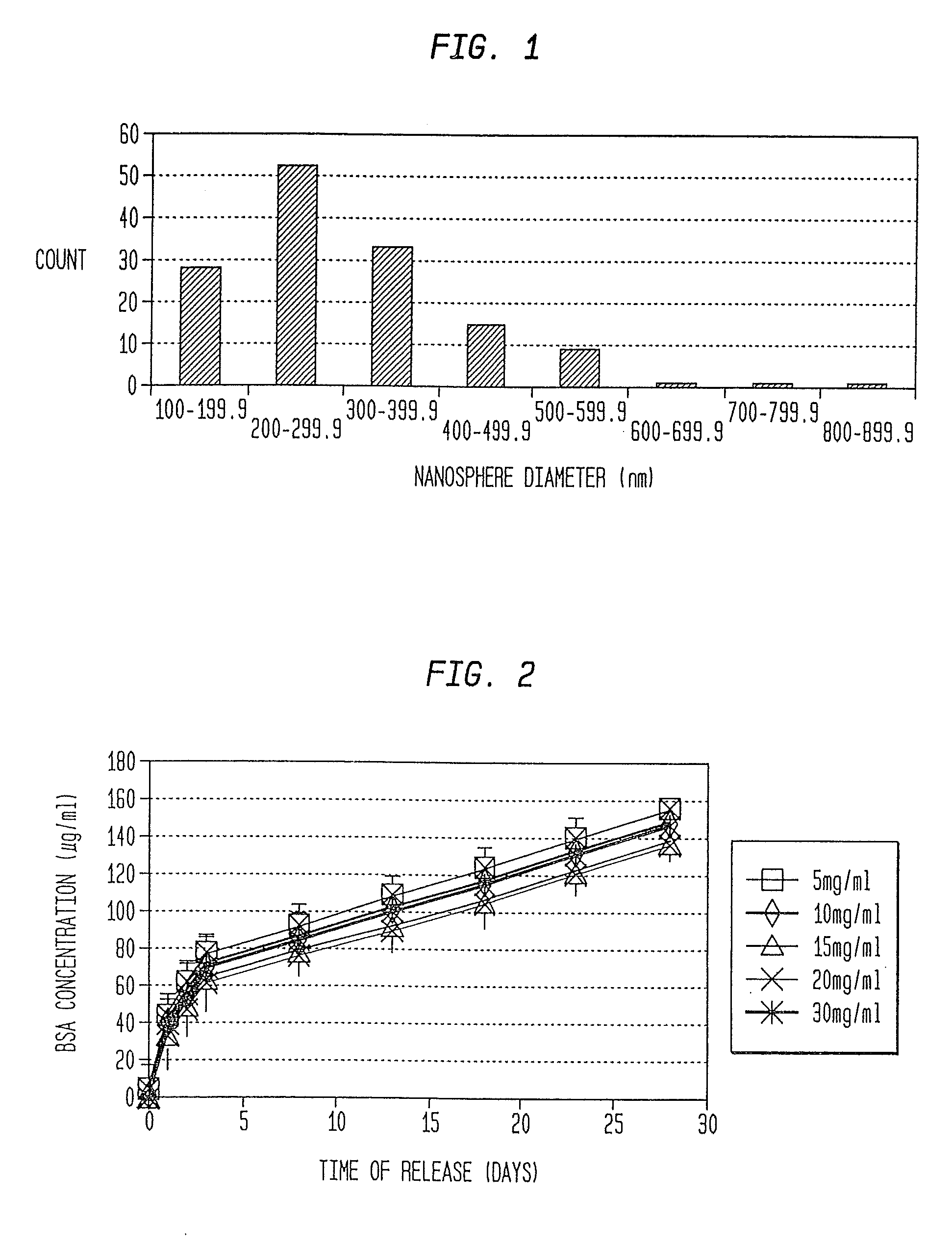
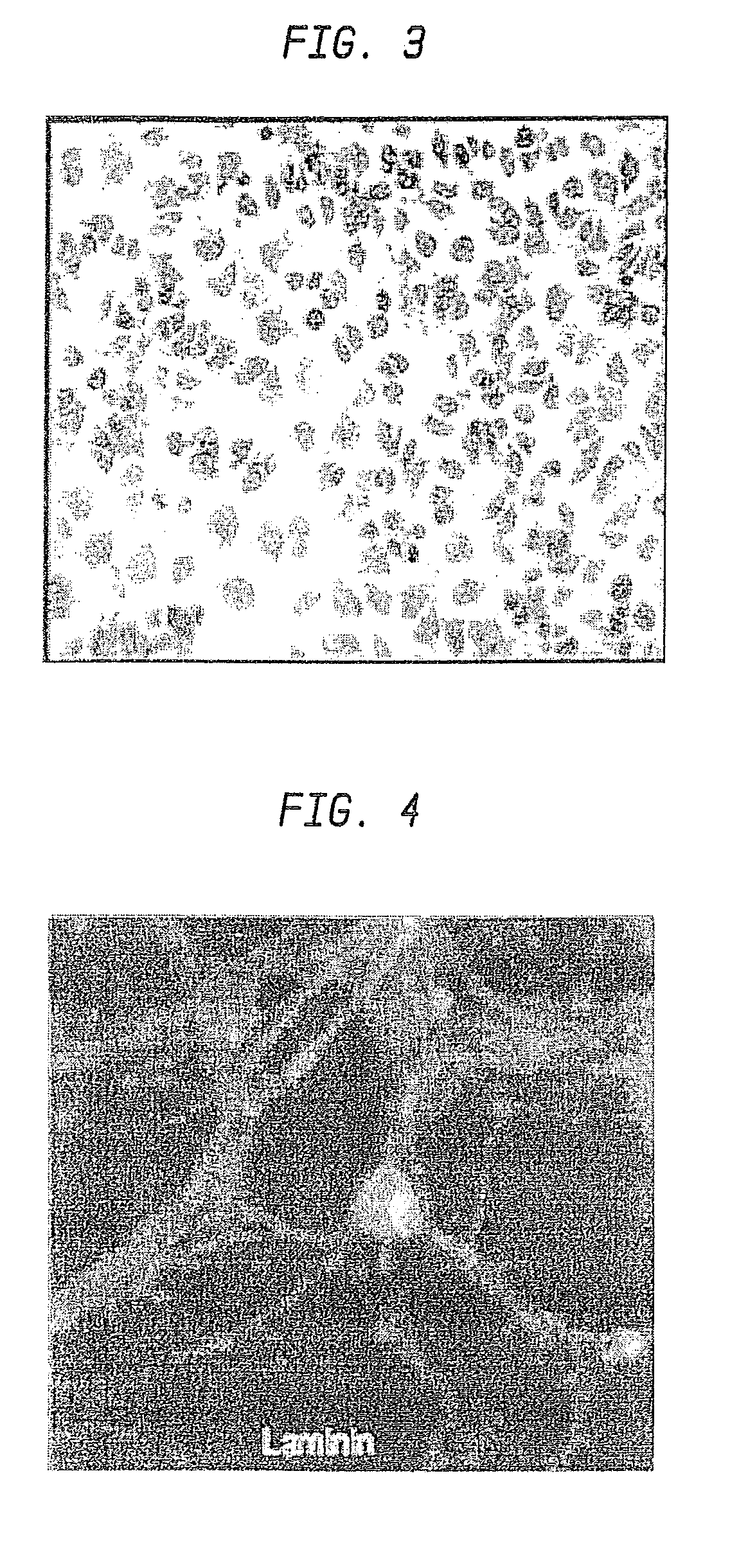
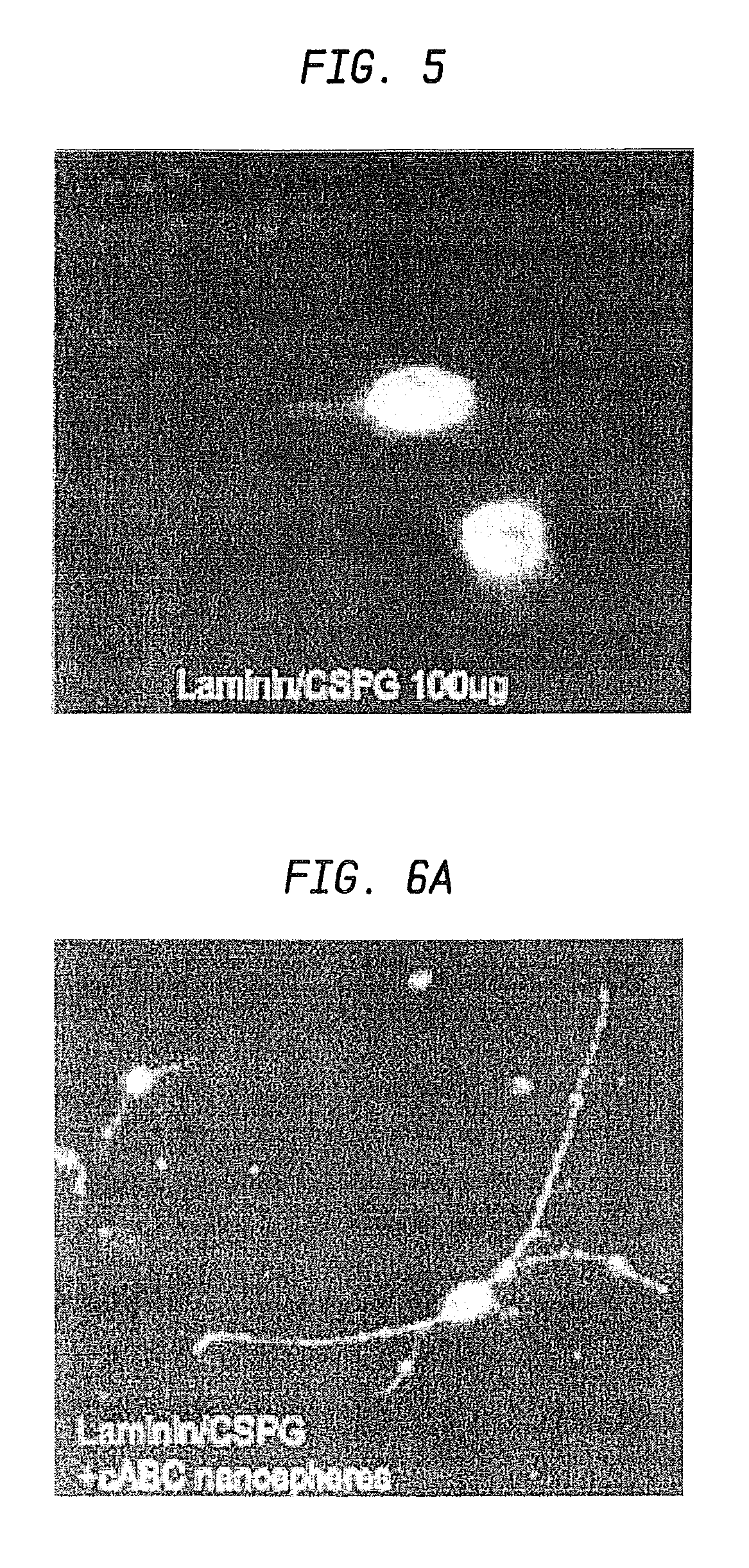
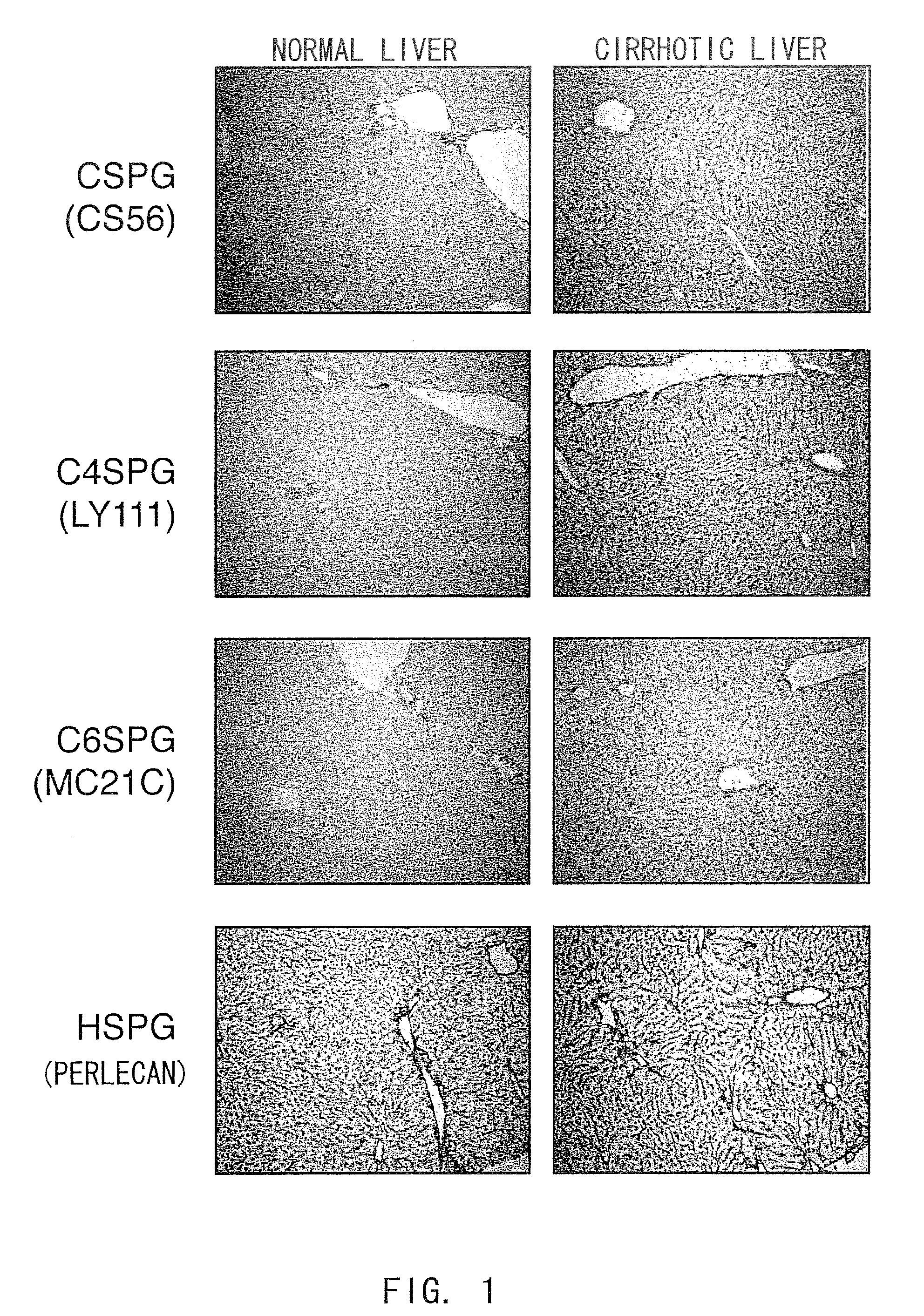
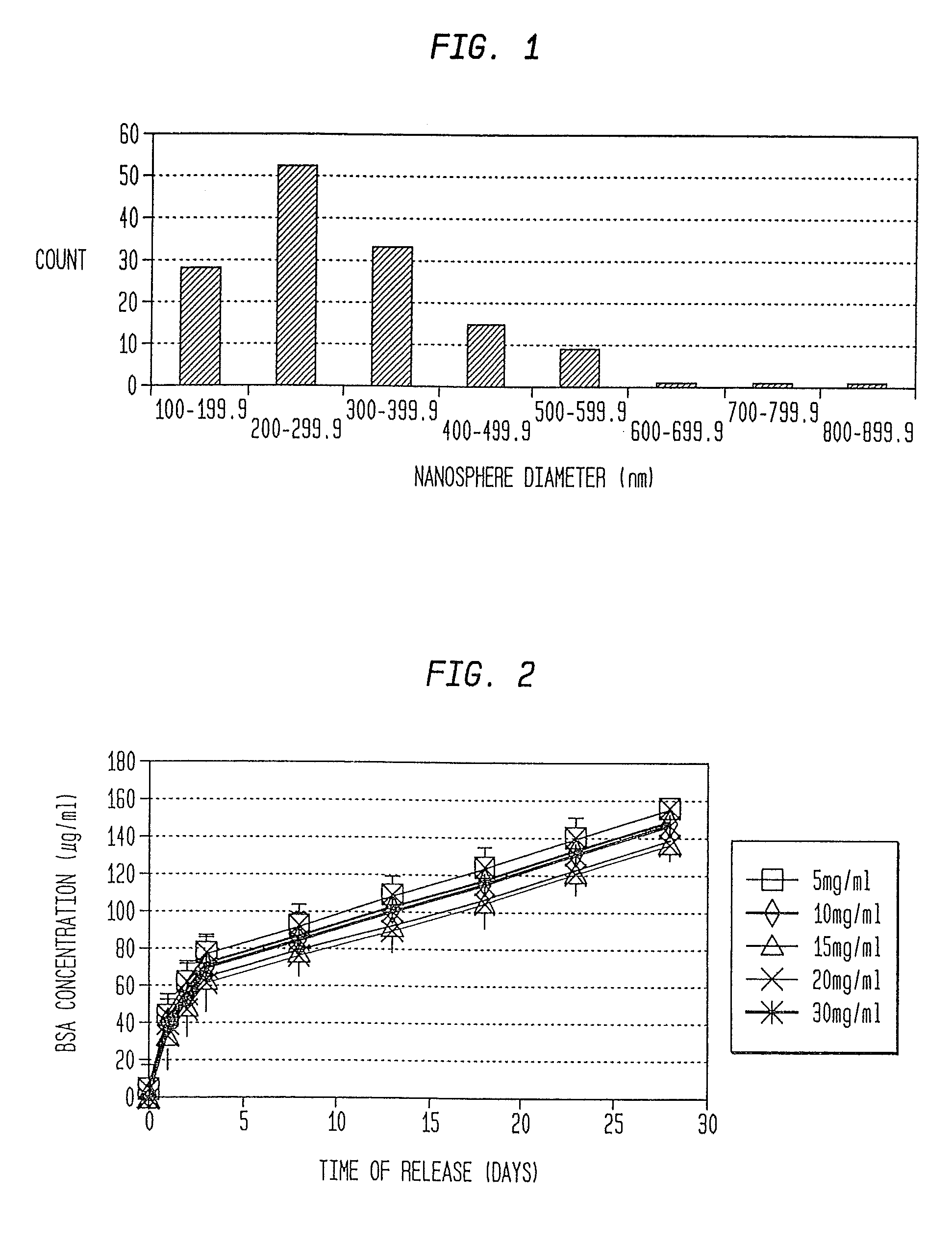
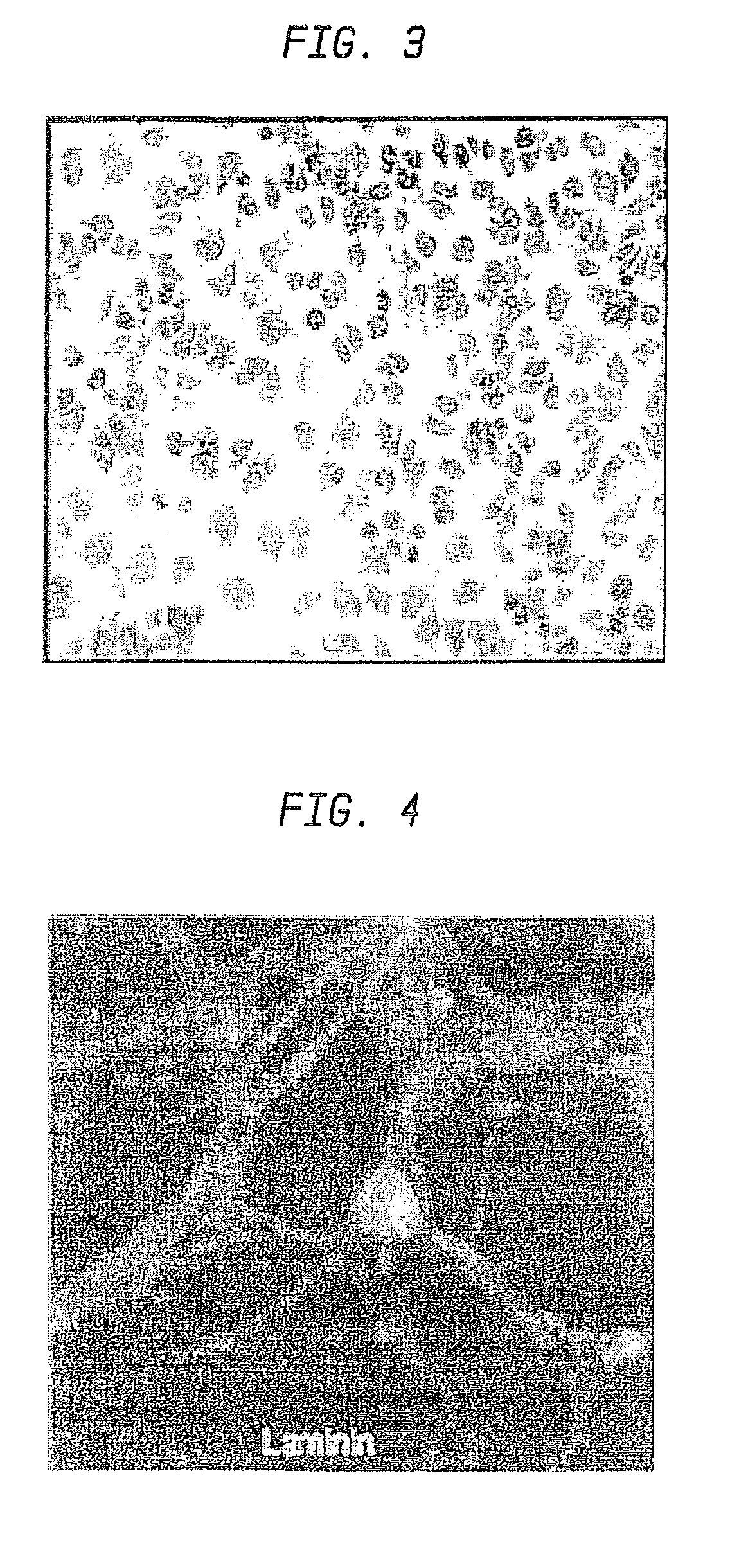
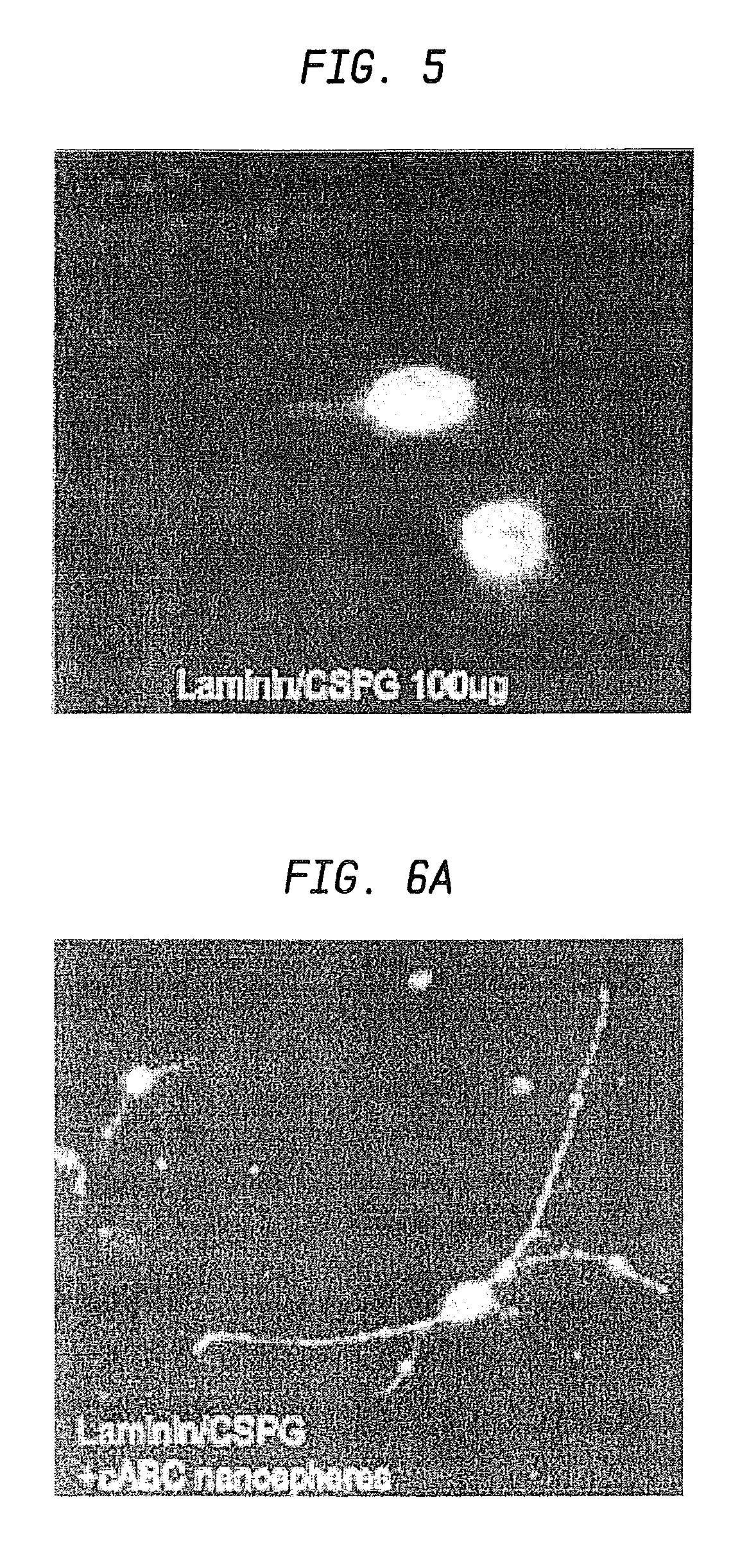
Nanosphere/microsphere delivery system for the treatment of spinal cord injury
InactiveUS20070116697A1Promote recoveryAvoid scaringPowder deliveryPeptide/protein ingredientsMedicineMicrosphere
A formulation including injectable biodegradable nanospheres and / or microspheres as a delivery system for chondroitinase ABC (cABC) or a functional derivative of cABC to treat acute and chronic spinal chord injury in a mammal having the same is provided. The biodegradable nanosphere / microsphere formulation releases cABC or a functional derivative of cABC in a time-released manner at the site of the spinal cord injury. cABC infusion can promote axon regrowth and some behavioral recovery. The nanospheres and / or microspheres provided herein include cABC or a functional derivative of cABC loaded within and / or on a biodegradable polymer matrix. In some embodiments of the present invention, the surface of the biodegradable polymer matrix can be modified to target a specific scar site. In addition to providing a nanosphere formulation that include polymeric incorporated cABC, a method of treating a mammal having a spinal cord injury is also provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Heparitin sulfate from rat tissue and production thereof
Heparan sulfate originating from rat tissue and its production are disclosed. The procedures is carried out by homogenizing rat tissue, degreasing, hydrolyzing by protease, hydrolyzing by alkali, deproteinizing, centrifugalizing, taking out purified liquor, dialyzing, depositing dialyzate by cetylpyridinium chloride, hydrolyzing by RNase and chondroitinase ABC, and separating by ionic exchanging column. It has special raw material, better product anticoagulant and antiradiation performances, and various Heparan sulfate. It can be used for research on medicinal molecular model structure and protein interaction. I
Owner:OCEAN UNIV OF CHINA
Agents for improving chronic obstructive pulmonary diseases
InactiveUS20090202514A1Promoting CSPG degradationAvoid depositionOrganic active ingredientsPeptide/protein ingredientsSulfationChondroitinase ABC
The present invention relates to agents for suppressing pulmonary emphysematous lesions and agents for suppressing emphysematous lesions which are suitable for treating and / or preventing COPD, which comprise as an active ingredient a substance having an activity of promoting CSPG degradation, inhibiting CSPG synthesis, or inhibiting CSPG sulfation (examples of such agents are: chondroitinase ABC, C6ST antisense agents, and GalNAc antisense agents); agents for treating and / or preventing COPD; methods for suppressing emphysematous lesions; and methods for treating and / or preventing COPD.
Owner:STELIC INST OF REGENERATIVE MEDICINE
Nanosphere/Microsphere Delivery System for the Treatment of Spinal Cord Injury
InactiveUS20110212136A1Promote recoveryAvoid scaringNervous disorderPeptide/protein ingredientsMicrosphereMedicine
A formulation including injectable biodegradable nanospheres and / or microspheres as a delivery system for chondroitinase ABC (cABC) or a functional derivative of cABC to treat acute and chronic spinal chord injury in a mammal having the same is provided. The biodegradable nanosphere / microsphere formulation releases cABC or a functional derivative of cABC in a time-released manner at the site of the spinal cord injury. cABC infusion can promote axon regrowth and some behavioral recovery. The nanospheres and / or microspheres provided herein include cABC or a functional derivative of cABC loaded within and / or on a biodegradable polymer matrix. In some embodiments of the present invention, the surface of the biodegradable polymer matrix can be modified to target a specific scar site. In addition to providing a nanosphere formulation that include polymeric incorporated cABC, a method of treating a mammal having a spinal cord injury is also provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
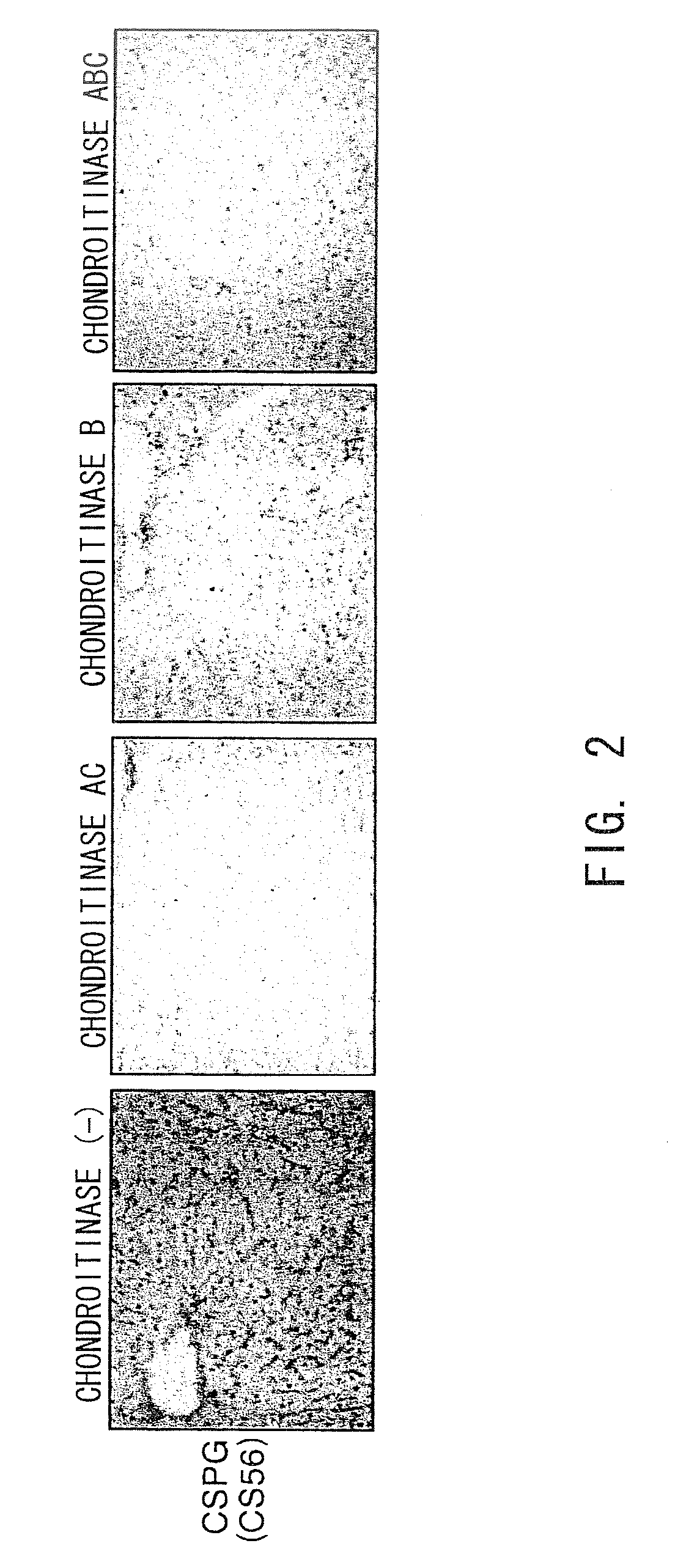
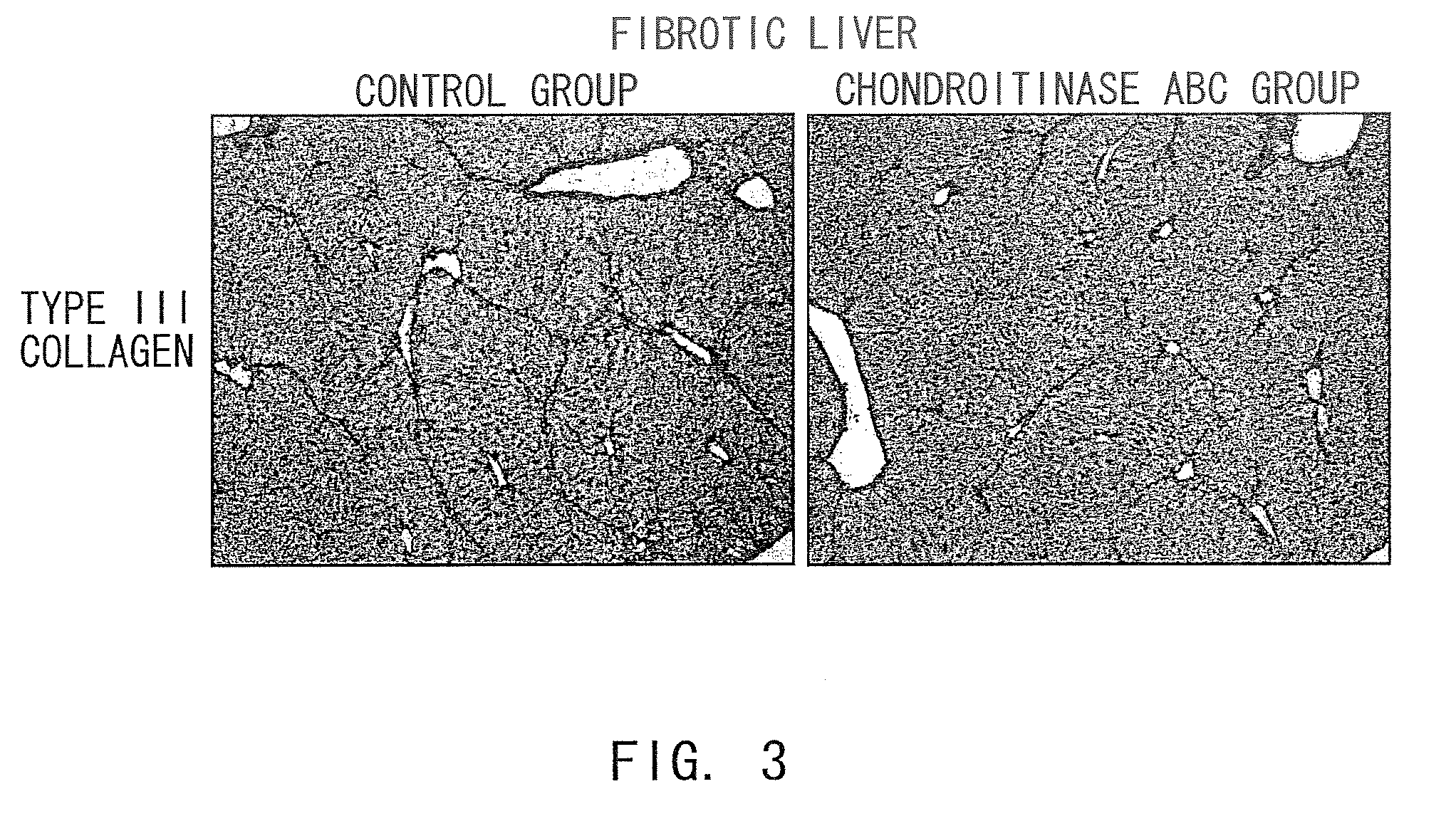
Agents for suppressing hepatic fibrosis
InactiveUS20090060892A1Enhance liver tissue fibrosisEfficient degradationPeptide/protein ingredientsMetabolism disorderLiver tissueMedicine
The present invention relates to hepatic fibrosis-suppressing agents that are suitable for treating or preventing fibrotic liver diseases such as cirrhosis, which comprise as an active ingredient a substance that inhibits the production or accumulation of chondroitin sulfate proteoglycans including chondroitinase ABC and ADAMTS-4; and methods of screening for the agents.The present inventors discovered for the first time that hepatic fibrosis could be efficiently suppressed by suppressing the production or accumulation of chondroitin sulfate proteoglycans. Specifically, fibrosis of liver tissues can be suppressed by administering chondroitinase ABC, a chondroitin sulfate proteoglycan-degrading enzyme, or by using siRNA to suppress the expression of C4ST-1, C6ST-1, or C6ST-2, a sulfotransferase for chondroitin sulfate proteoglycans. Compounds such as nucleic acids that are used as siRNA can be used as effective agents for suppressing hepatic fibrosis. Furthermore, hepatic fibrosis-suppressing agents can be found by screening for compounds that suppress the production or accumulation of chondroitin sulfate proteoglycans.
Owner:STELIC INST OF REGENERATIVE MEDICINE
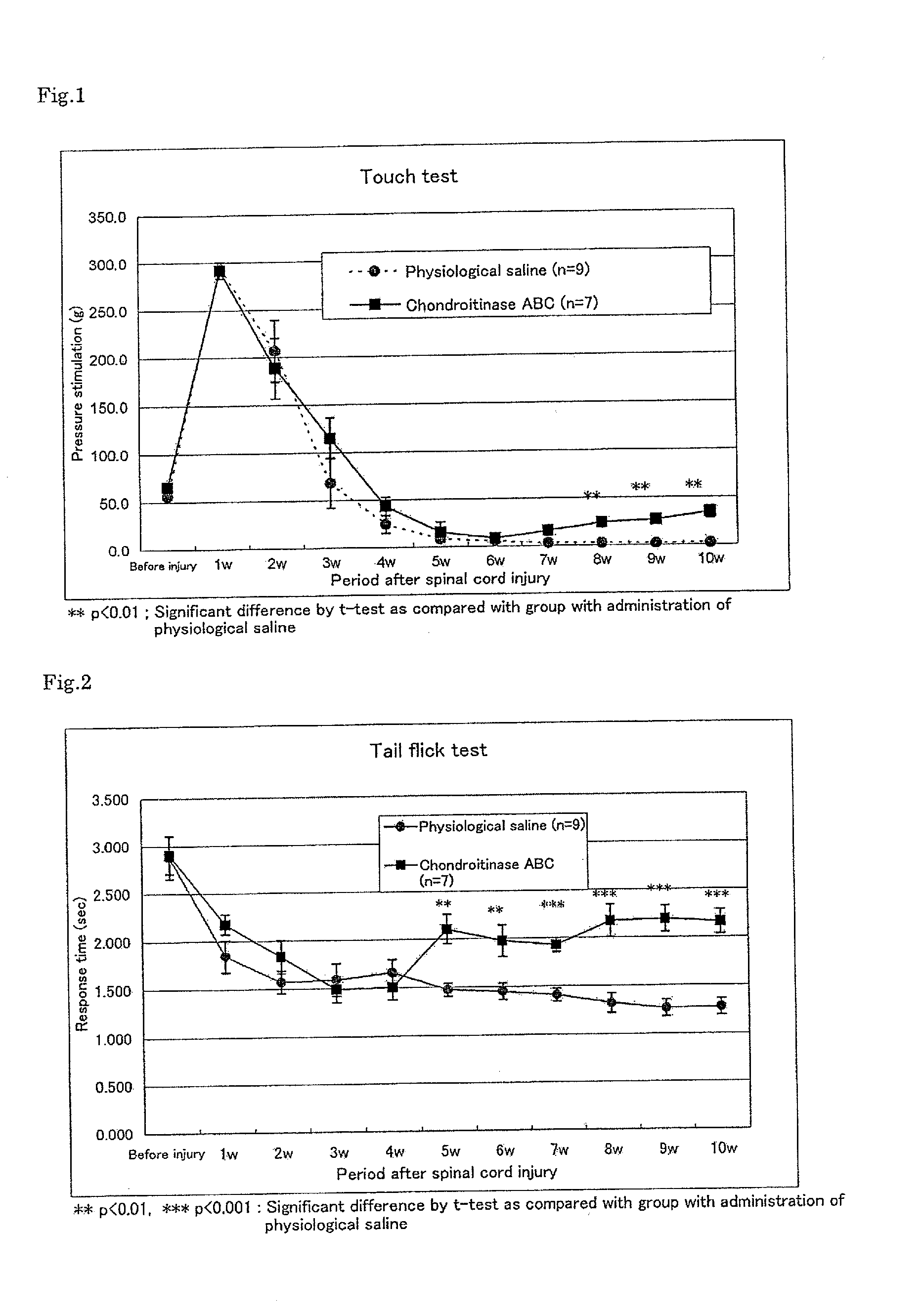
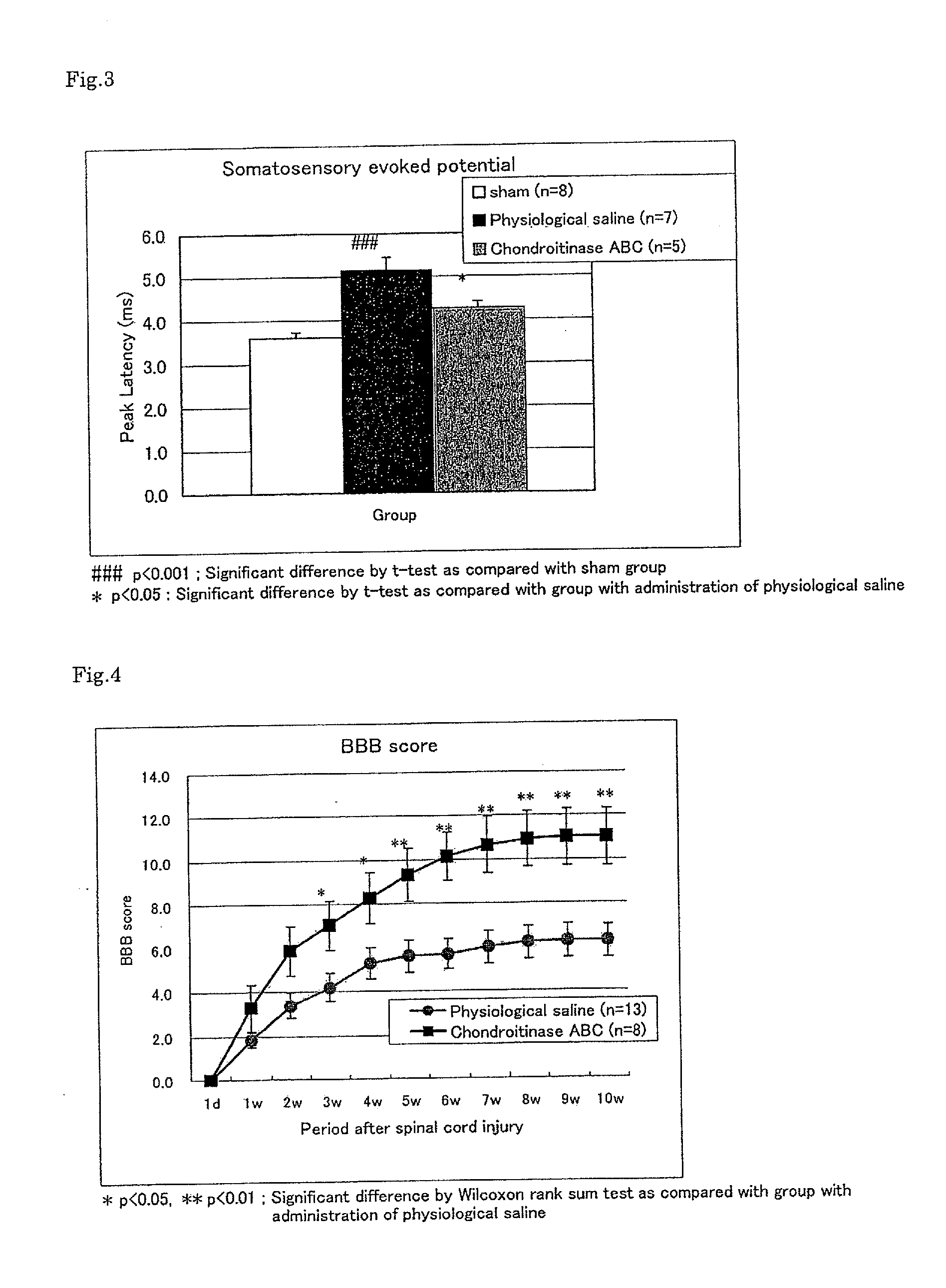
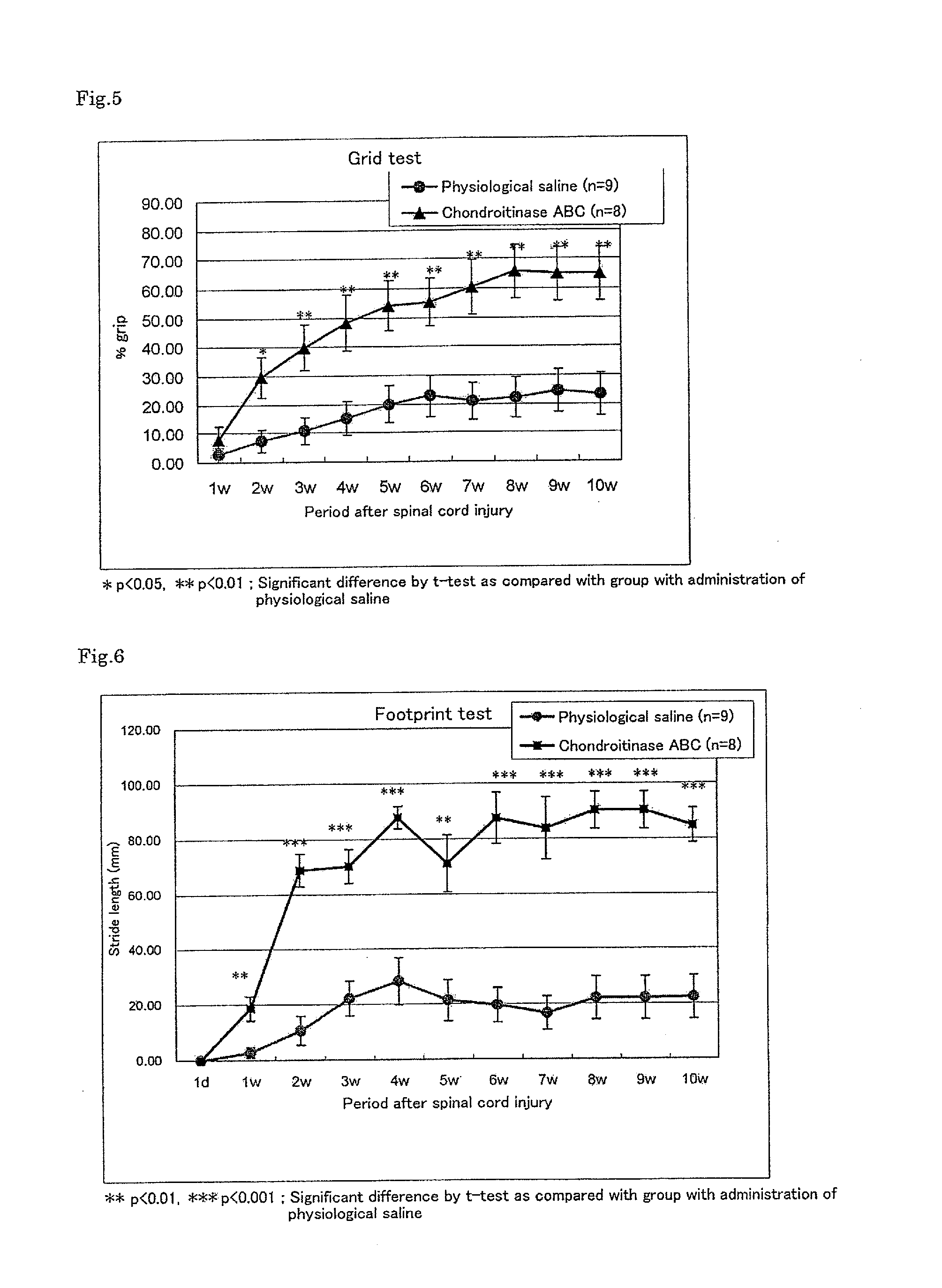
Pharmaceutical composition for promoting axon regeneration after spinal cord damage and behavior function recovery
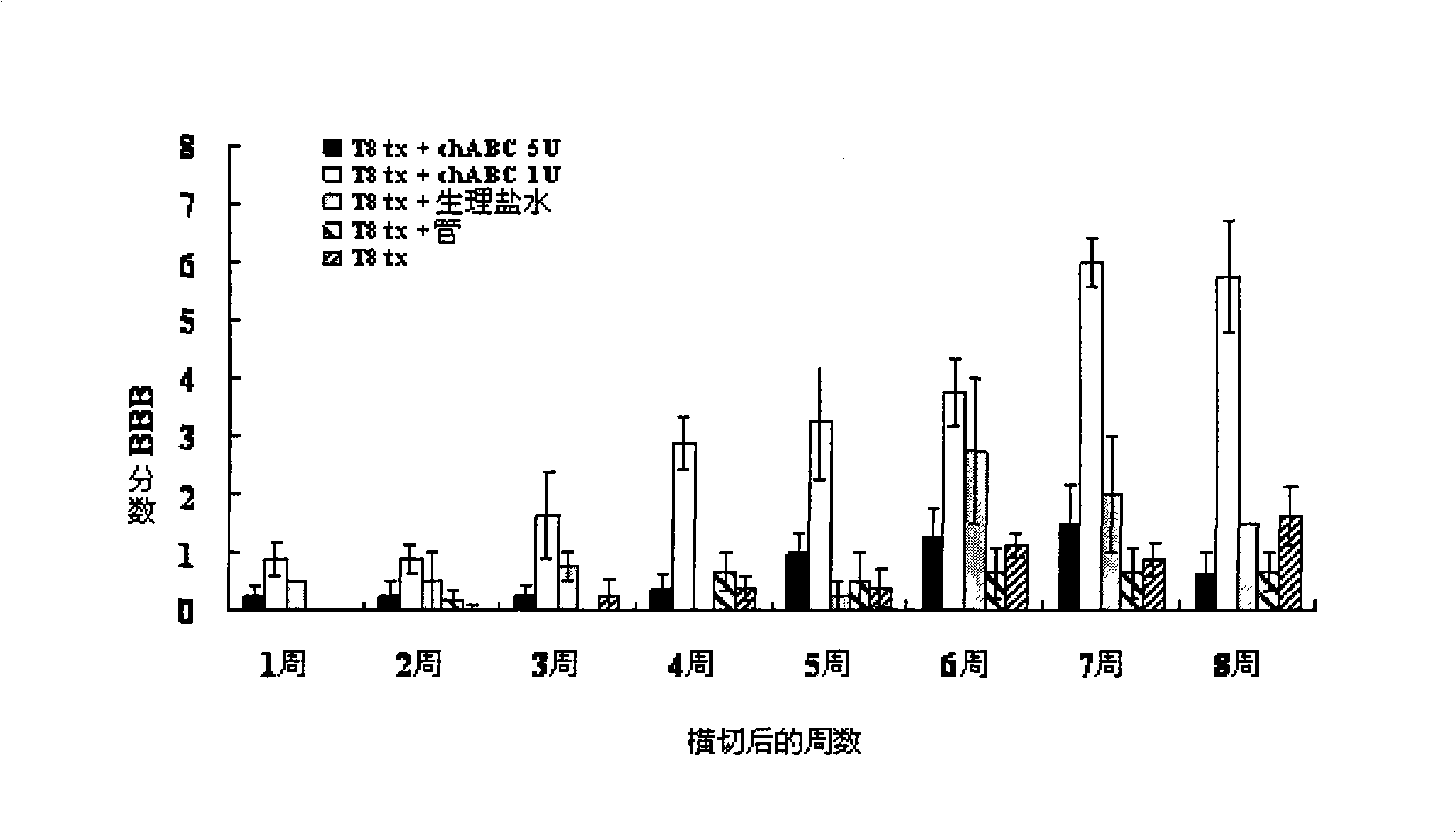
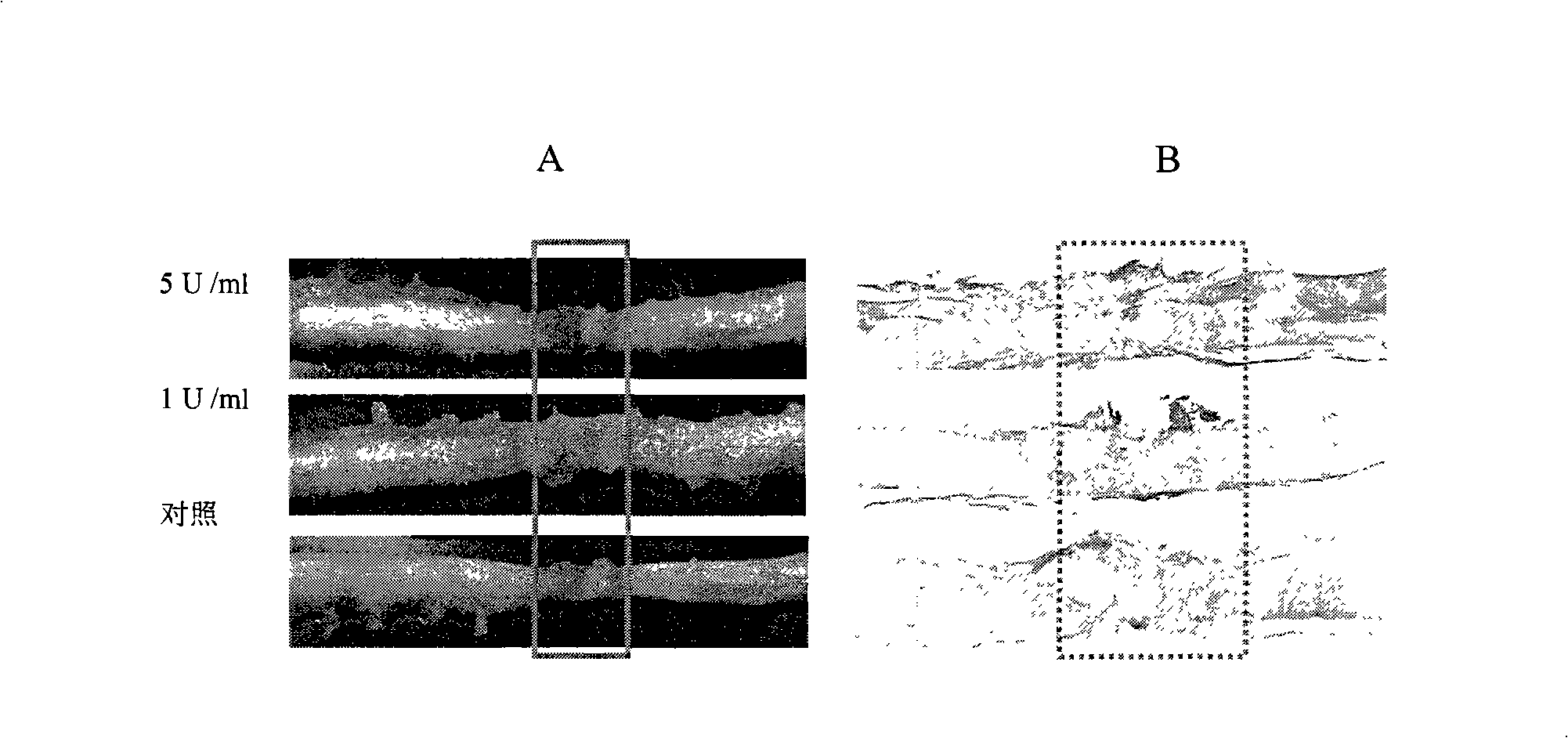
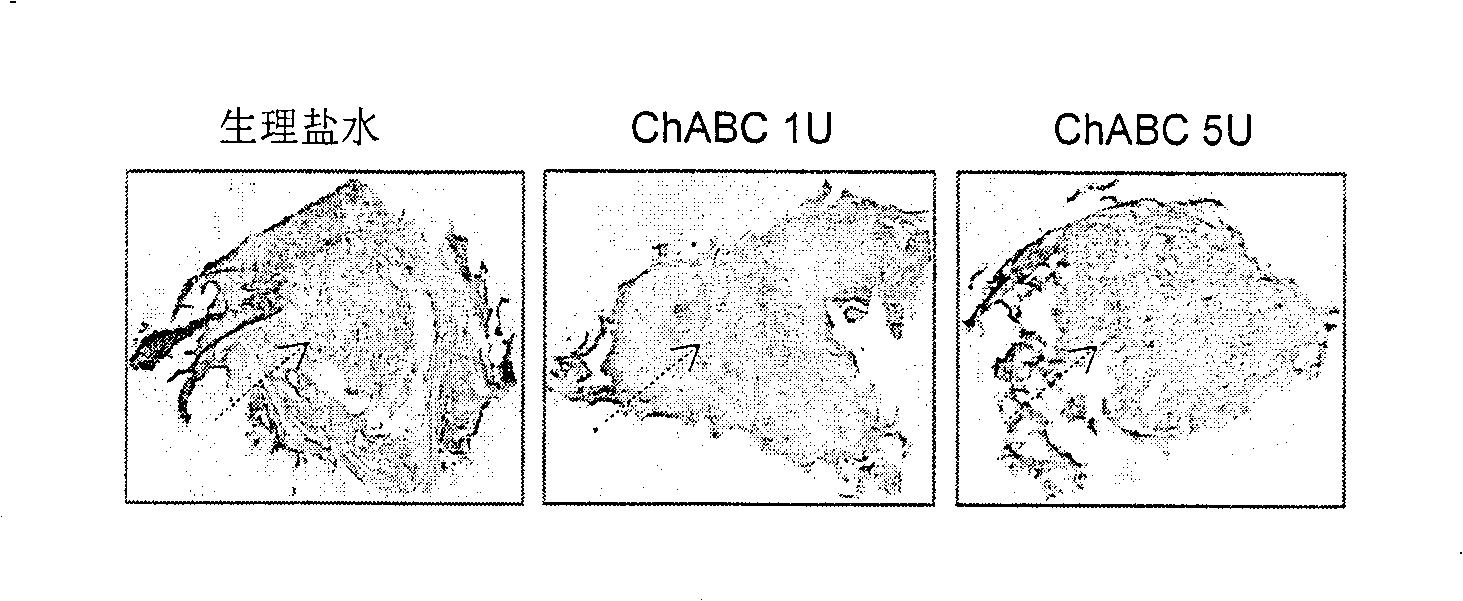
The invention provides a pharmaceutical composition for promoting the regeneration of axons and the recovery of behavioral functions of examinees who suffer neurologic damage. The pharmaceutical composition contains chondroitinase ABC (ChABC) with safe and effective amount and a pharmaceutically accepted carrier. The invention also provides application of the ChABC in preparation of the pharmaceutical composition for promoting the regeneration of the axons and the recovery of the behavioral functions of the examinees, wherein the pharmaceutical composition contains the ChABC with the safe and effective amount which is used for promoting the regeneration of the axons and the recovery of the behavioral functions of the examinees who suffer neurologic damage. The pharmaceutical composition contains about 0.1 to 10U / ml of ChABC, wherein the pharmaceutical composition is infused to injured parts. After being used, the ChABC of the pharmaceutical composition has obvious function of improving the regeneration, and outstanding recovery effect on the behavioral functions.
Owner:郑宏志
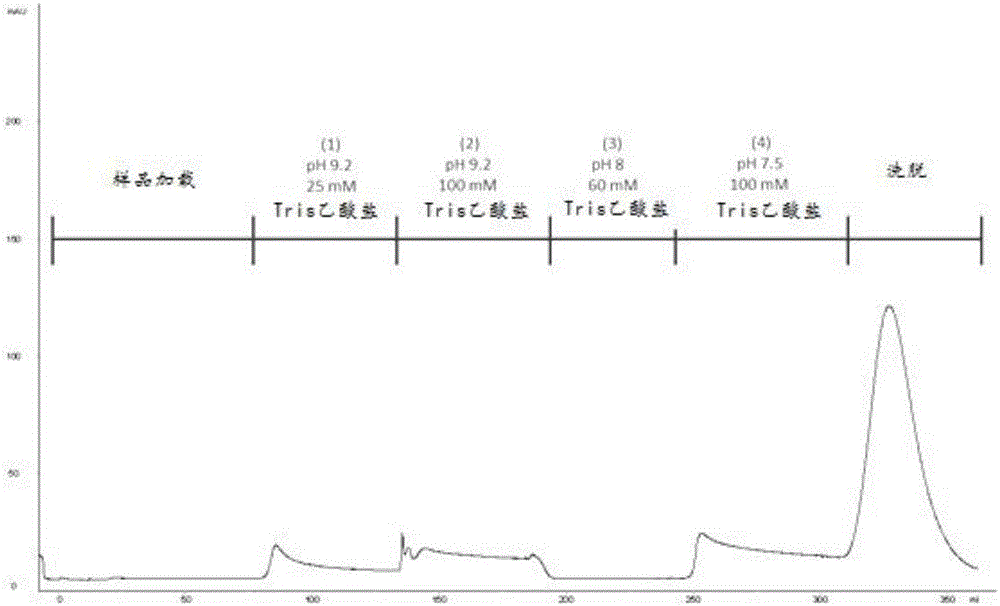
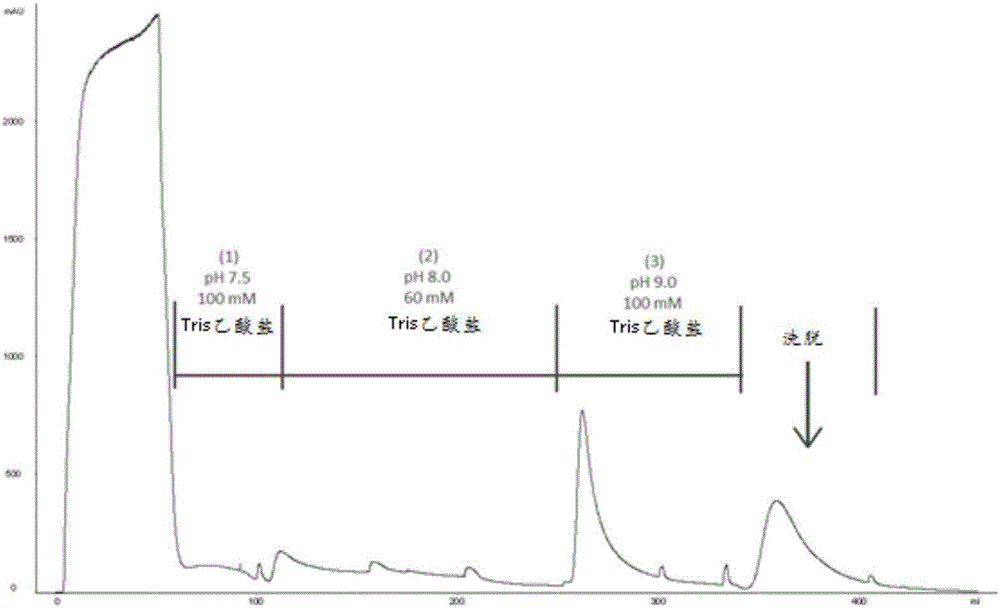
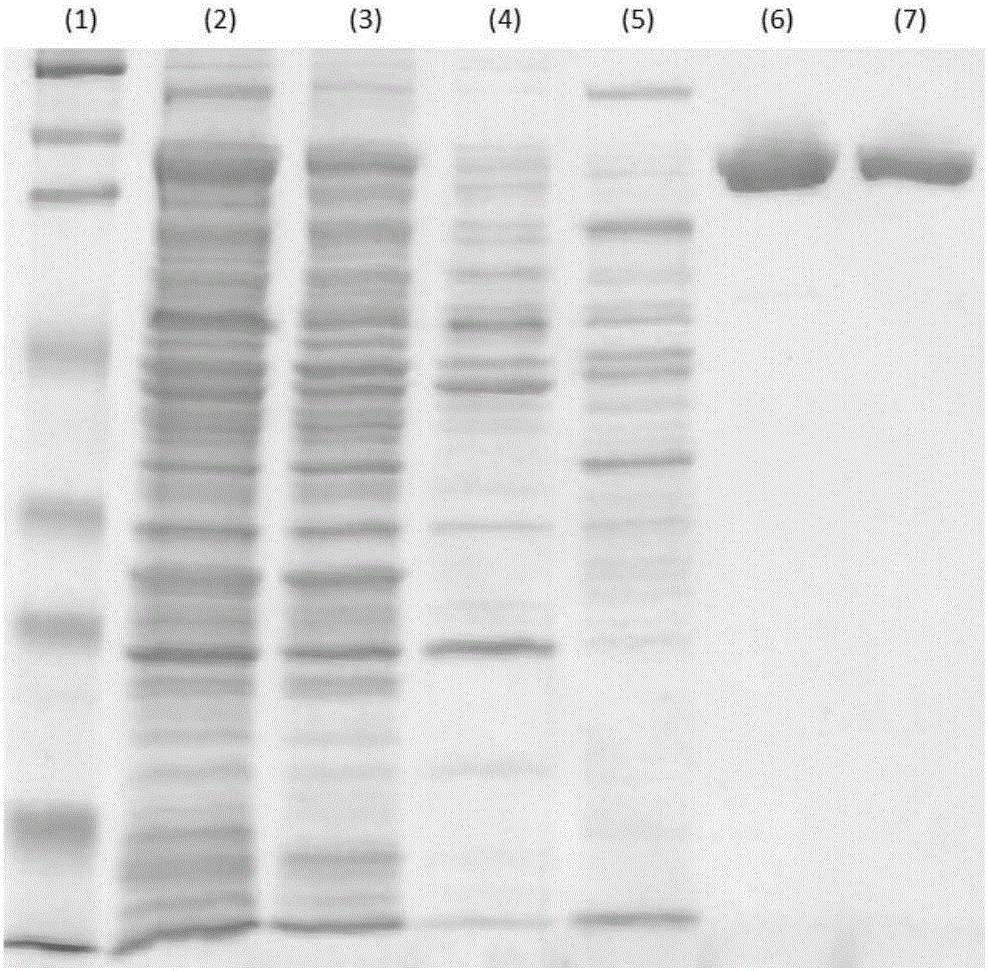
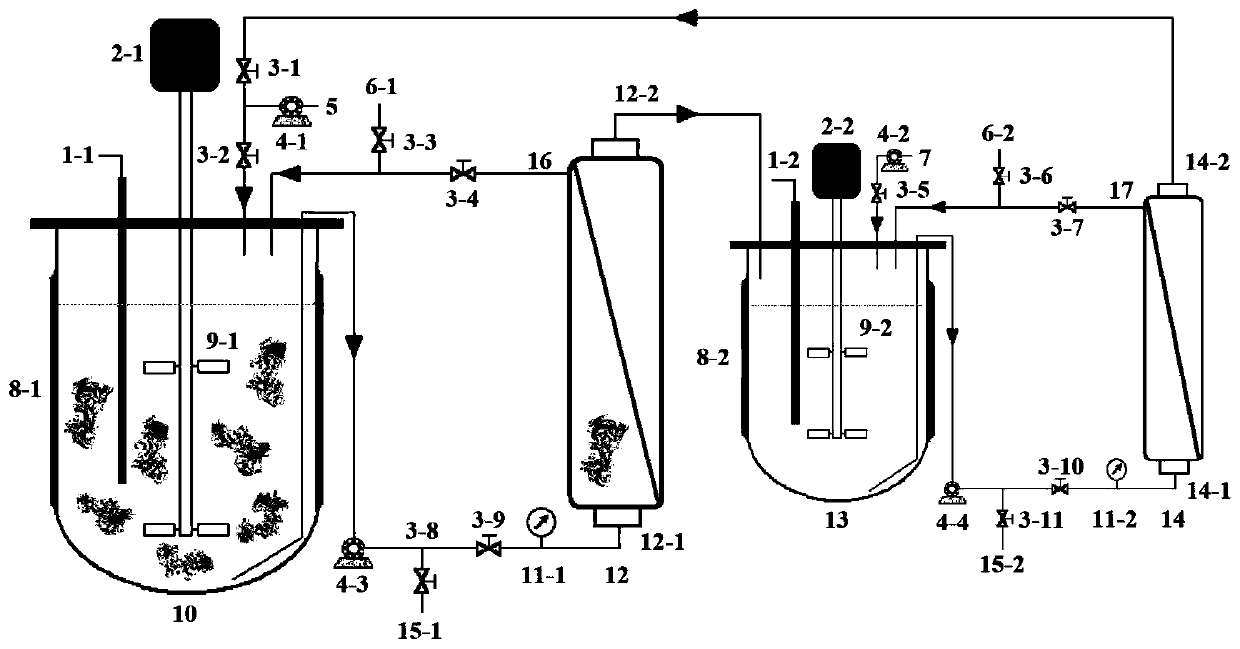
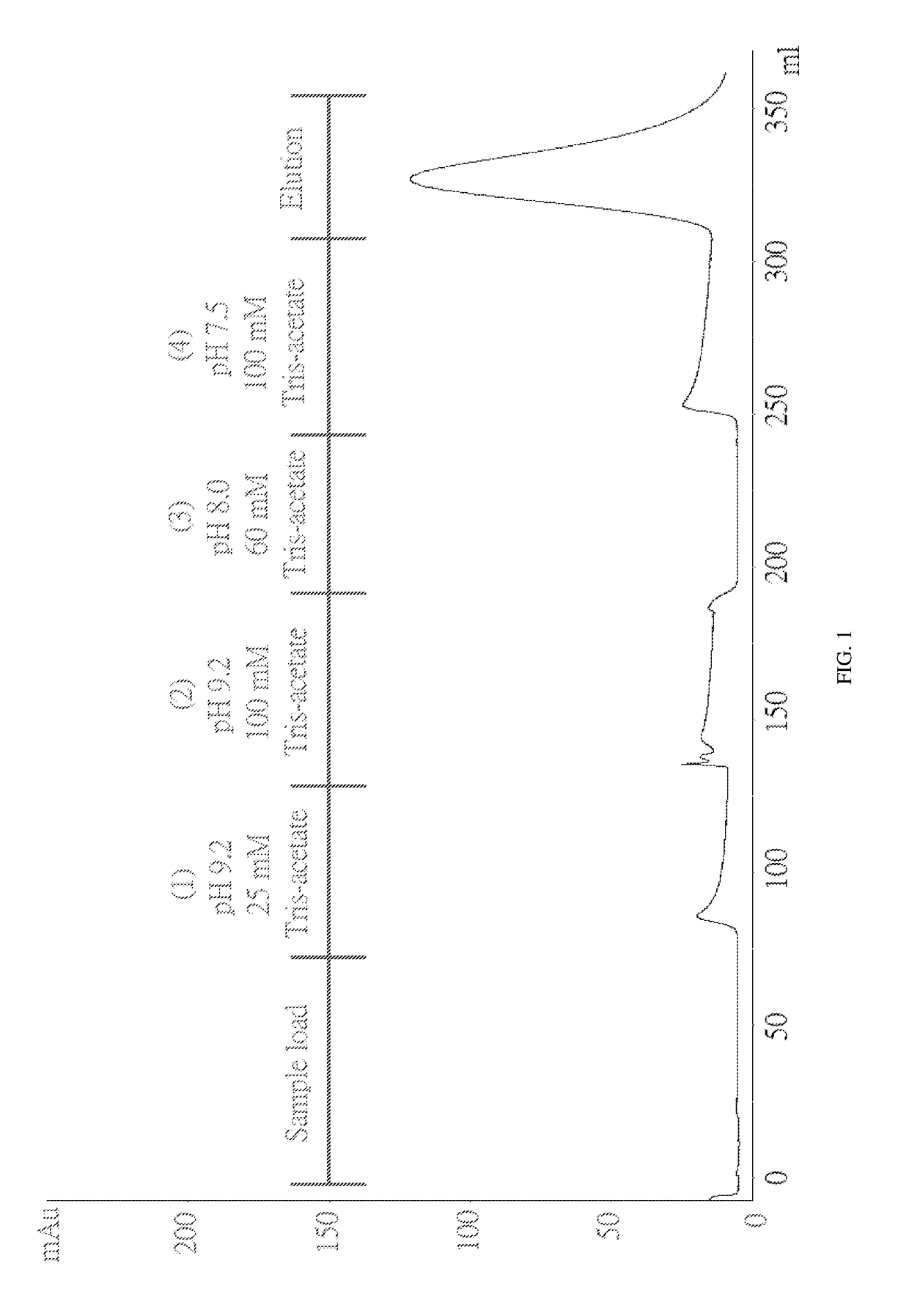
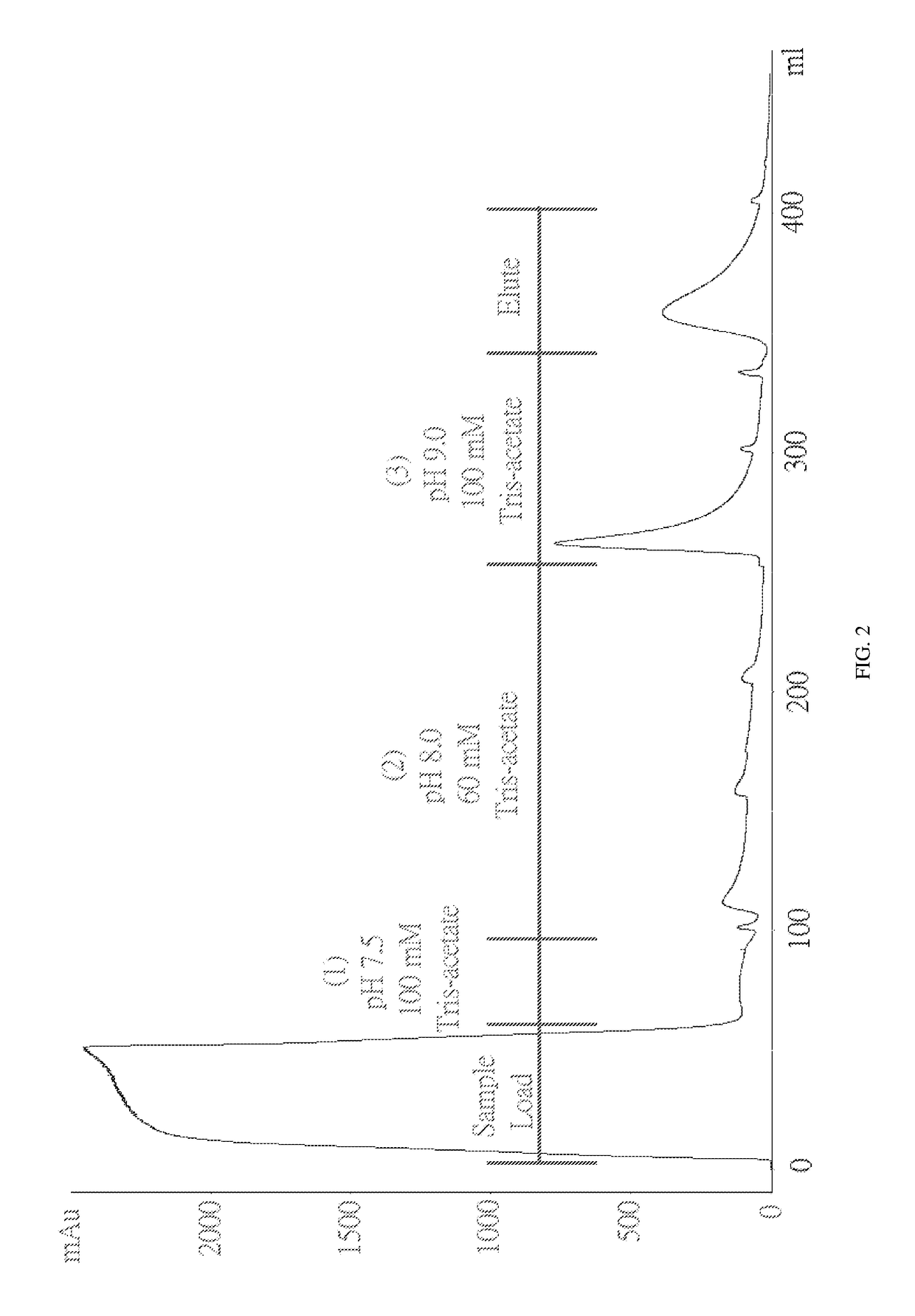
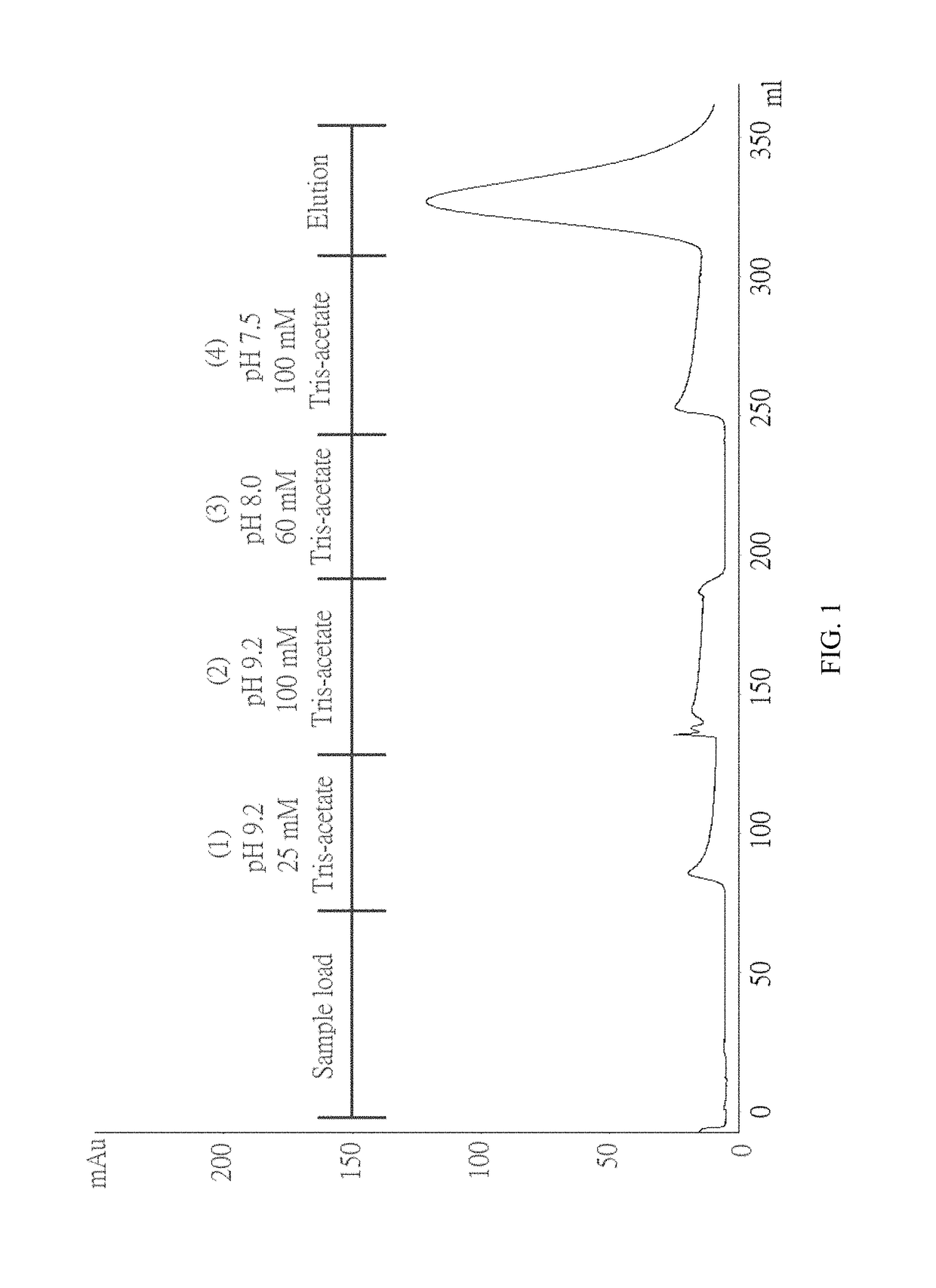
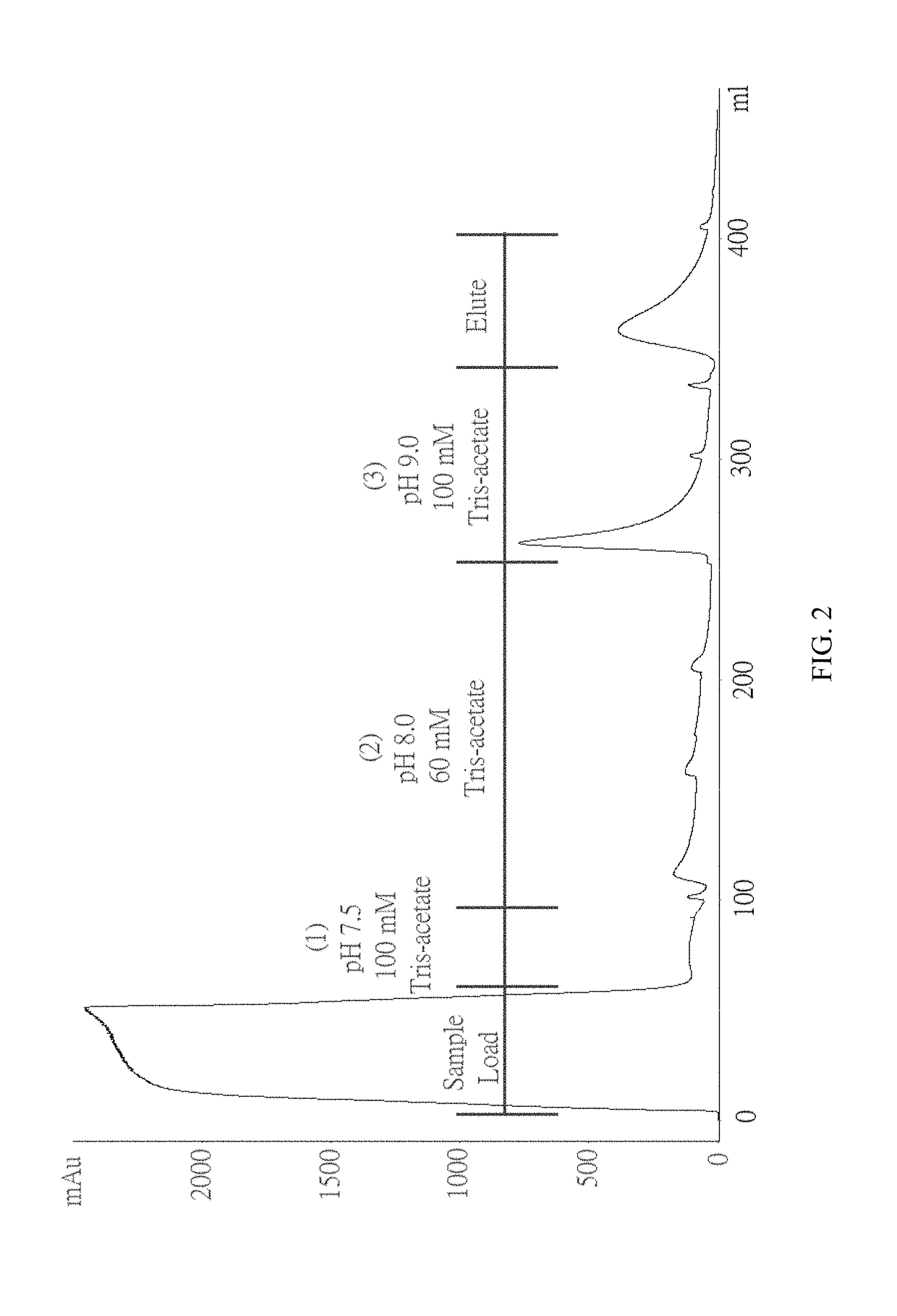
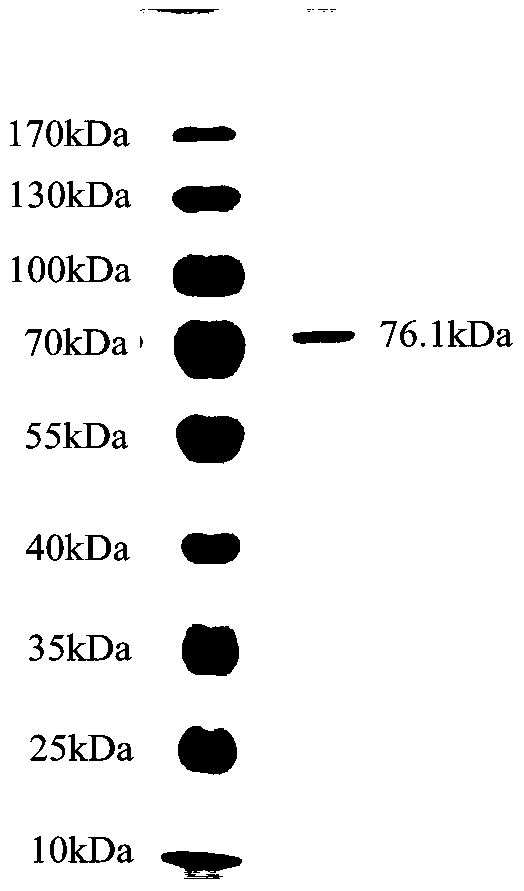
Method for purifying chondroitinase ABC
The invention discloses a method for purifying chondroitinase ABC. According to the method, an affinity column, on which heparin is fixed, is used for obtaining purified chondroitinase ABC from a substrate containing chondroitinase ABC by means of a chromatography. The method can be used for obtaining the high-purity chondroitinase ABC and has the advantages of being simple and high in recovery rate.
Owner:ADVANTEK SERUM LAB
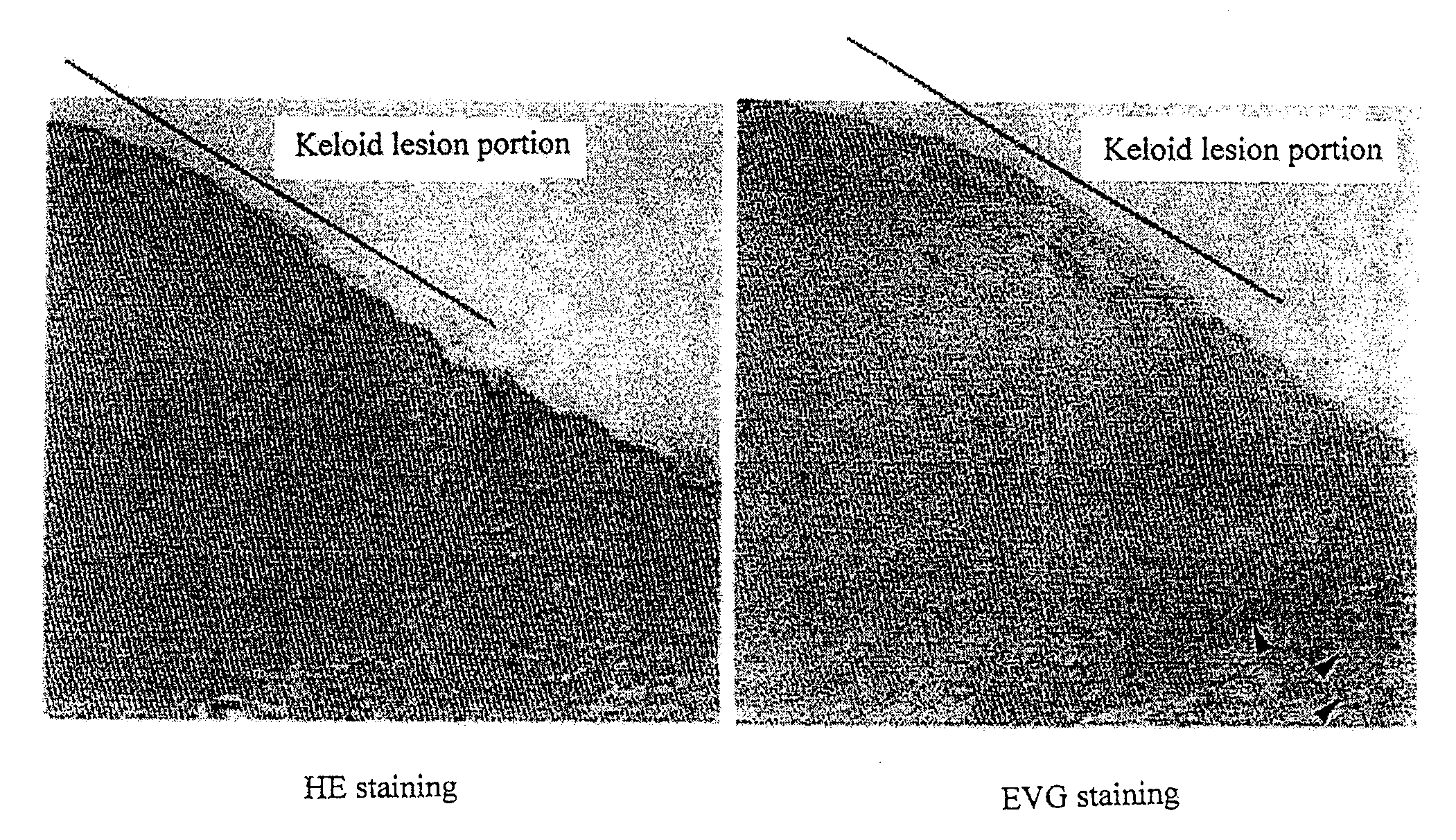
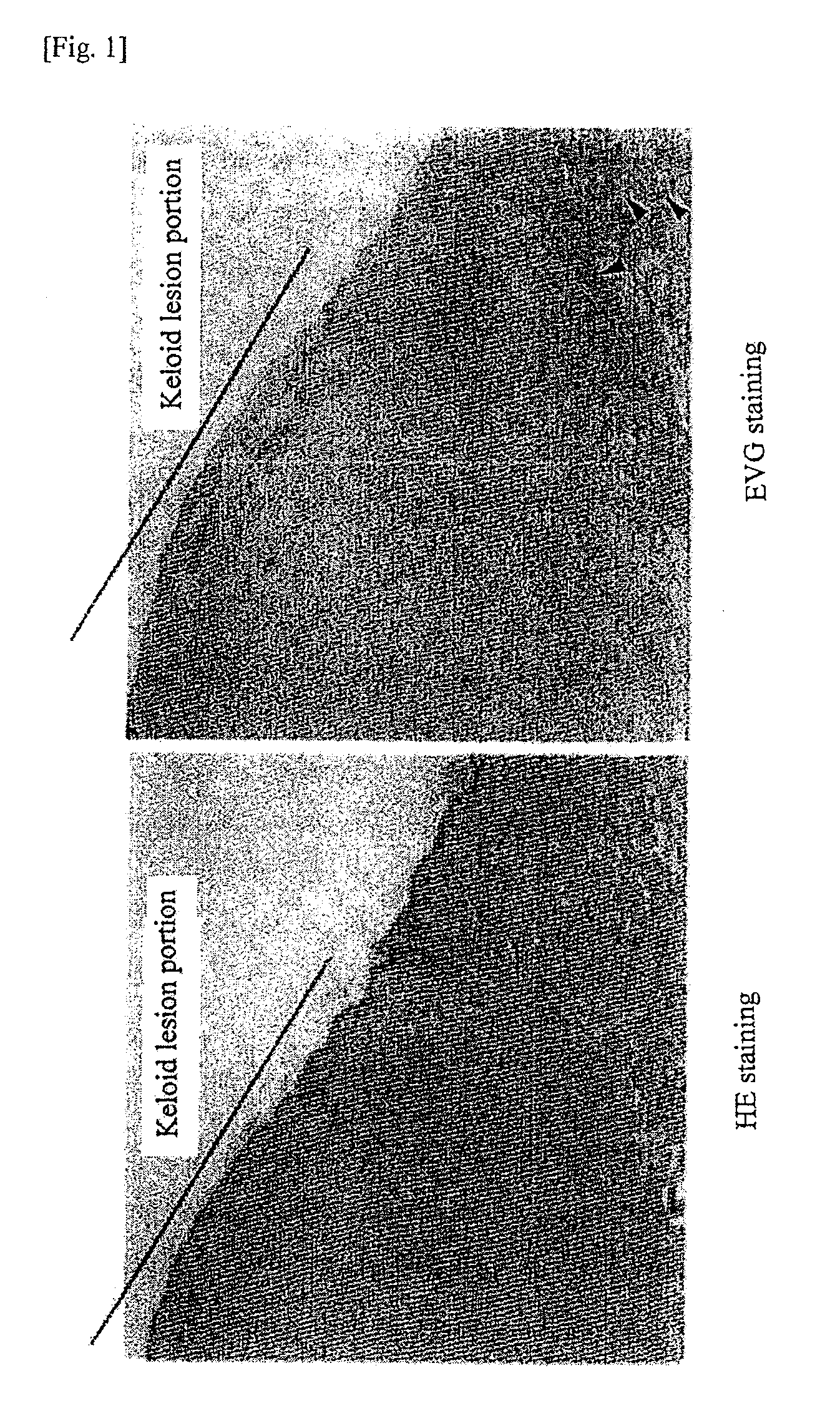
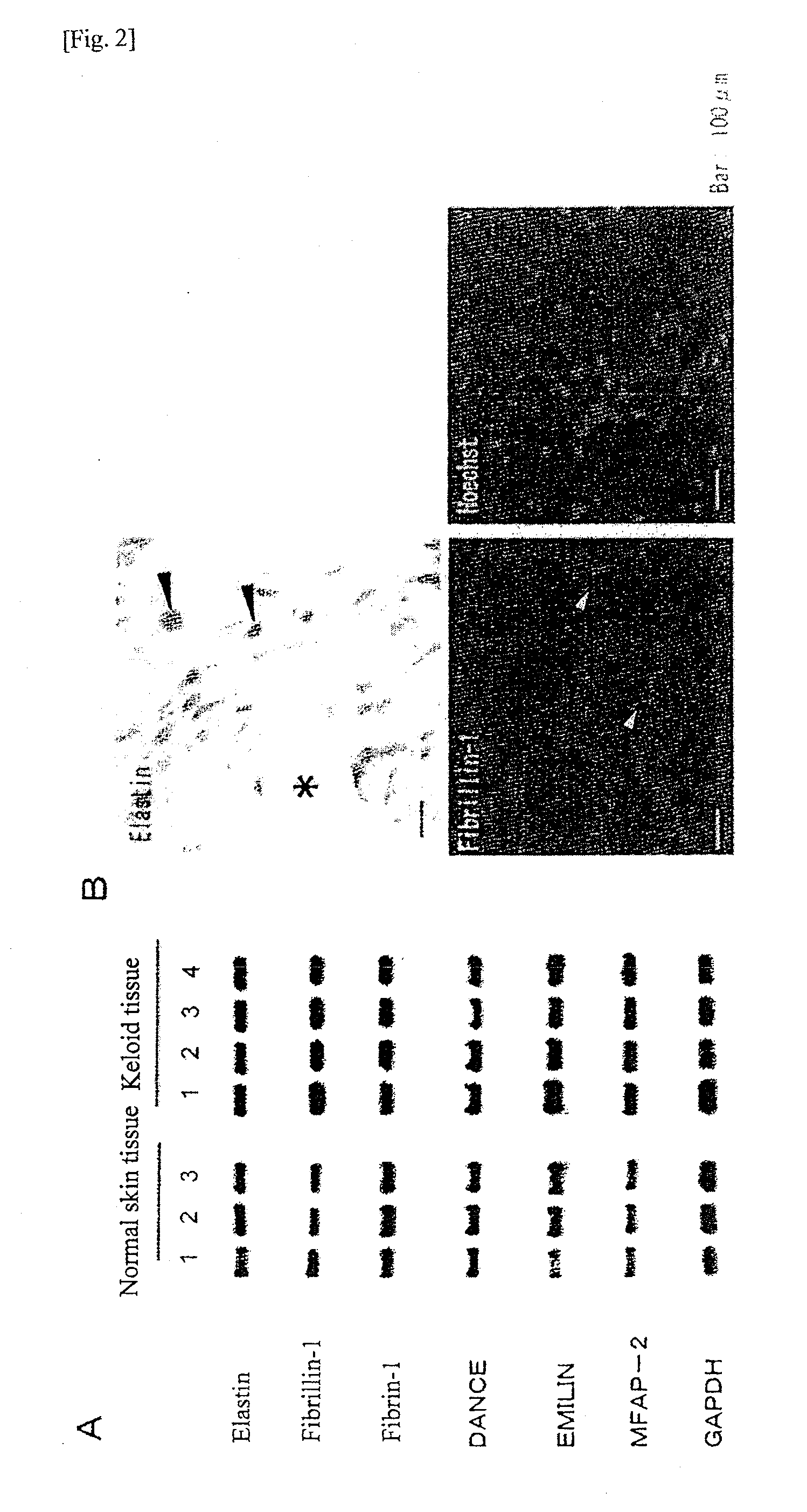
Radical therapeutic agent for keloid and hypertrophic scar
InactiveUS20110008312A1Image obtainedMore inhibitory effectPeptide/protein ingredientsPeptidesKeloidHypertropic
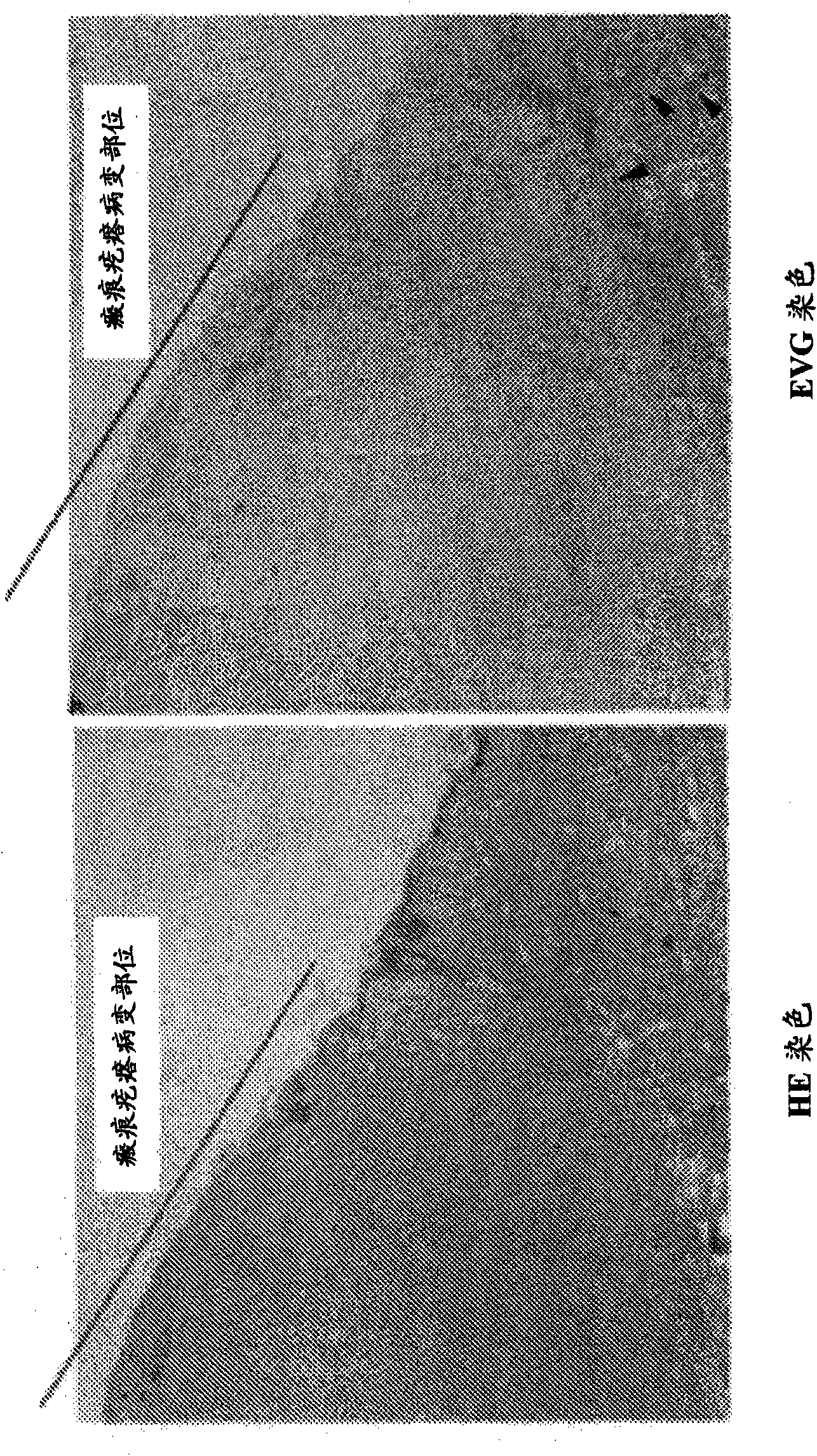
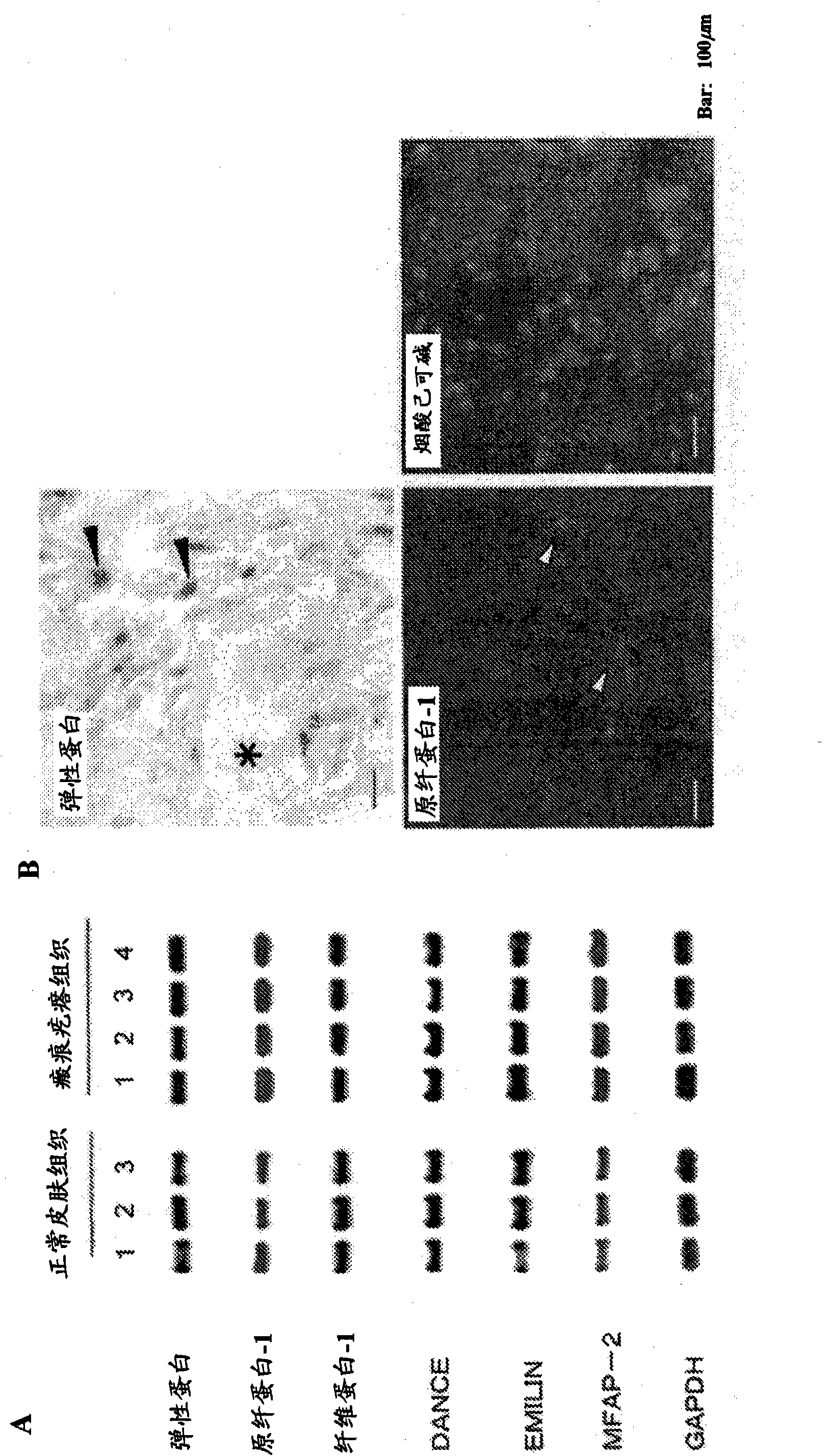
An effective therapeutic agent for keloids and / or hypertrophic scars is provided. Specifically, an elastic fiber regenerating agent consisting of chondroitinase ABC derived from Proteus vulgaris and a therapeutic agent for keloids and / or hypertrophic scars comprising the regenerating agent as an active ingredient are provided.
Owner:SUZUKI SHIGEHIKO +1
Agent for neuropathic pain
ActiveUS20130230509A1Increase painFunction increaseNervous disorderOrganic chemistryMechanism of actionMedicine
Owner:NAGOYA UNIVERSITY +1
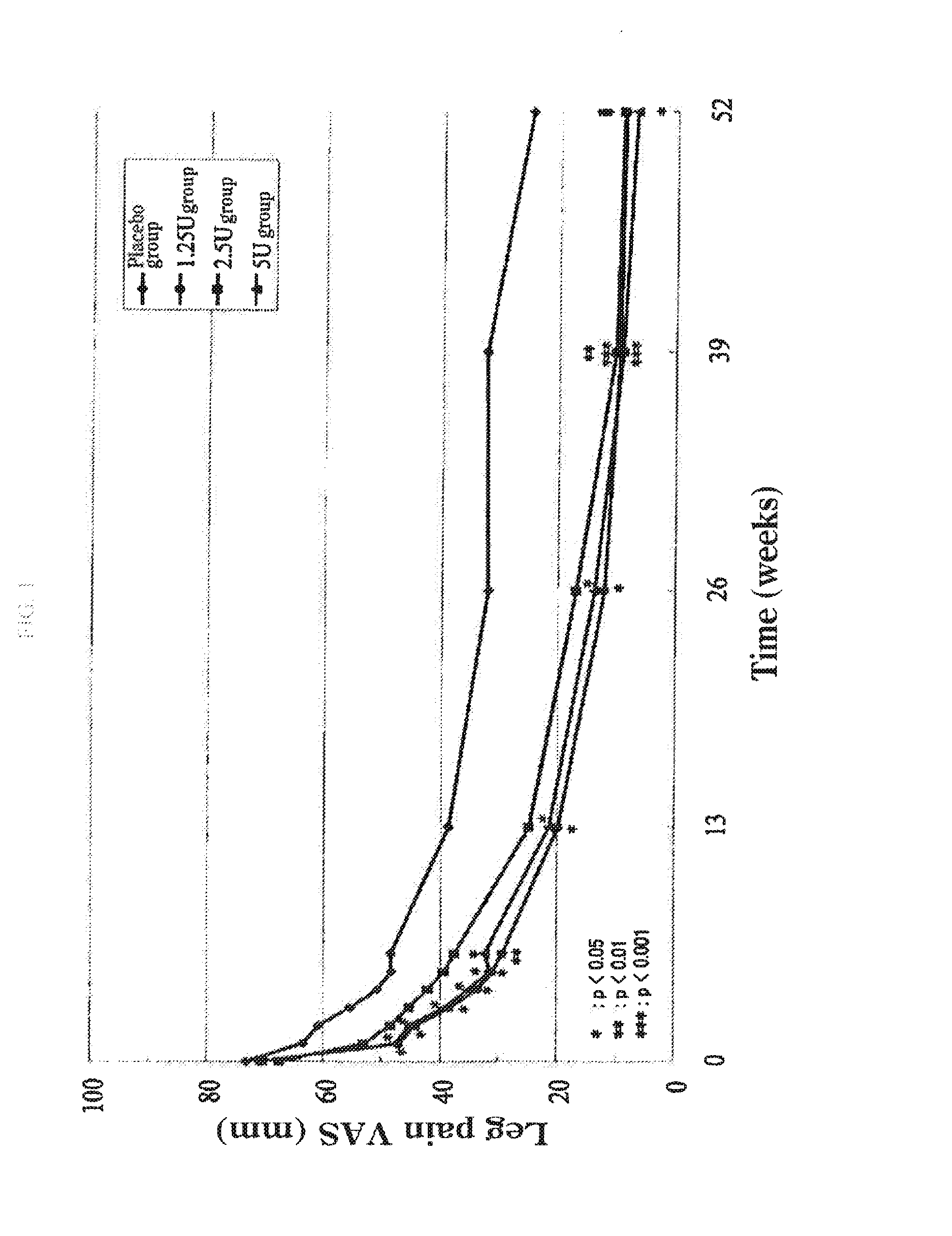
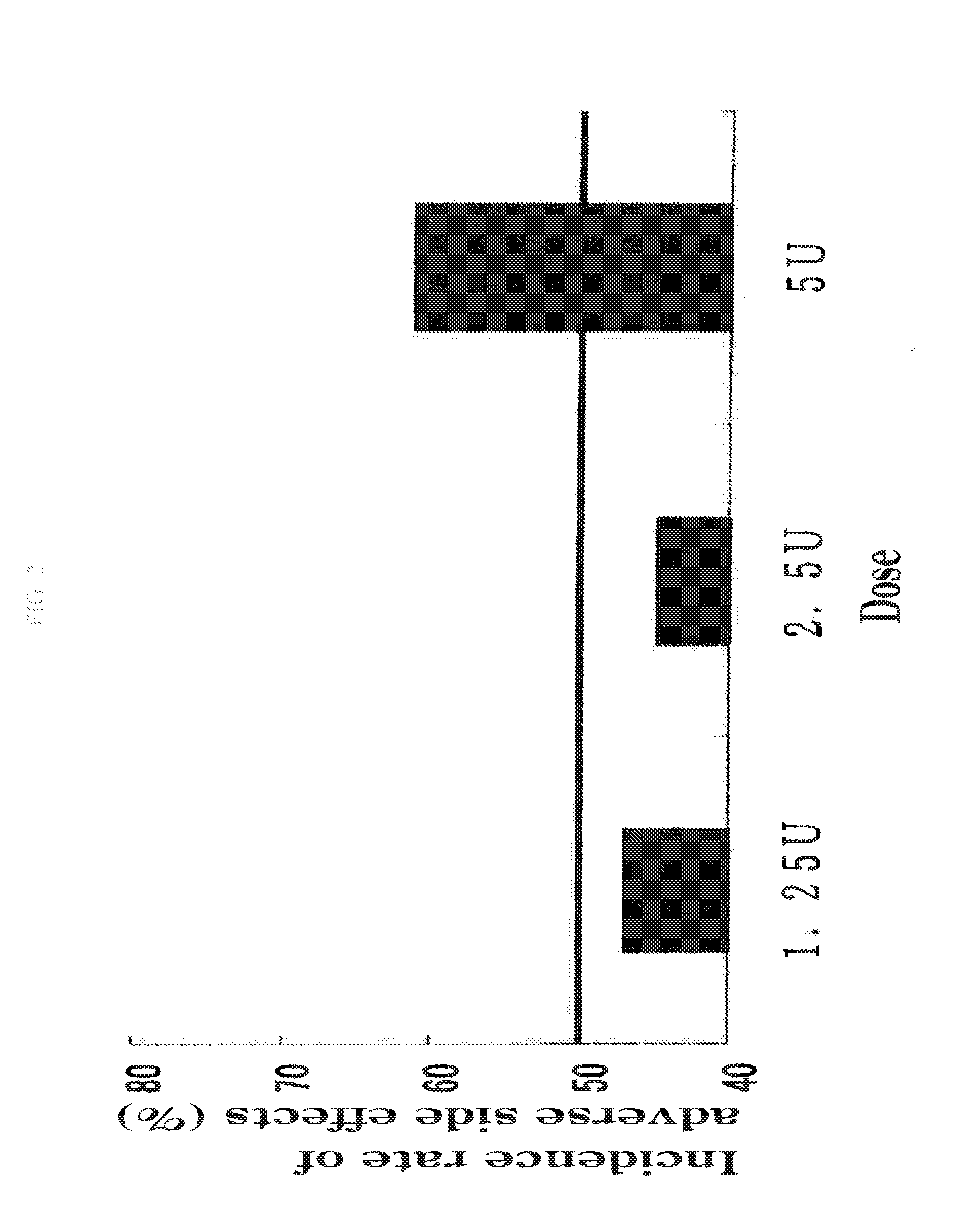
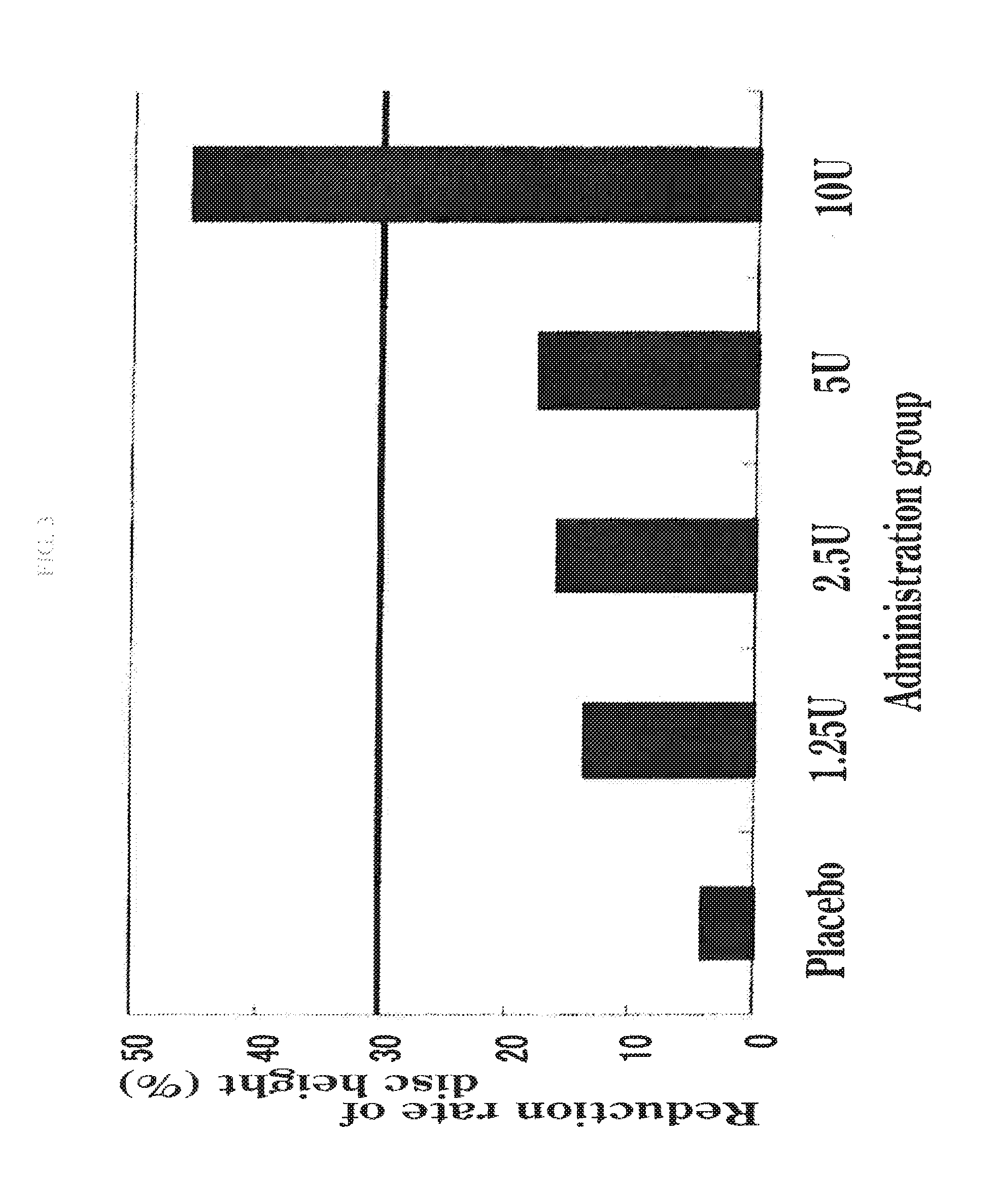
Therapeutic agent for disc herniation
InactiveUS20130266555A1Less side effectsHigh usefulnessNervous disorderPeptide/protein ingredientsSide effectAdditive ingredient
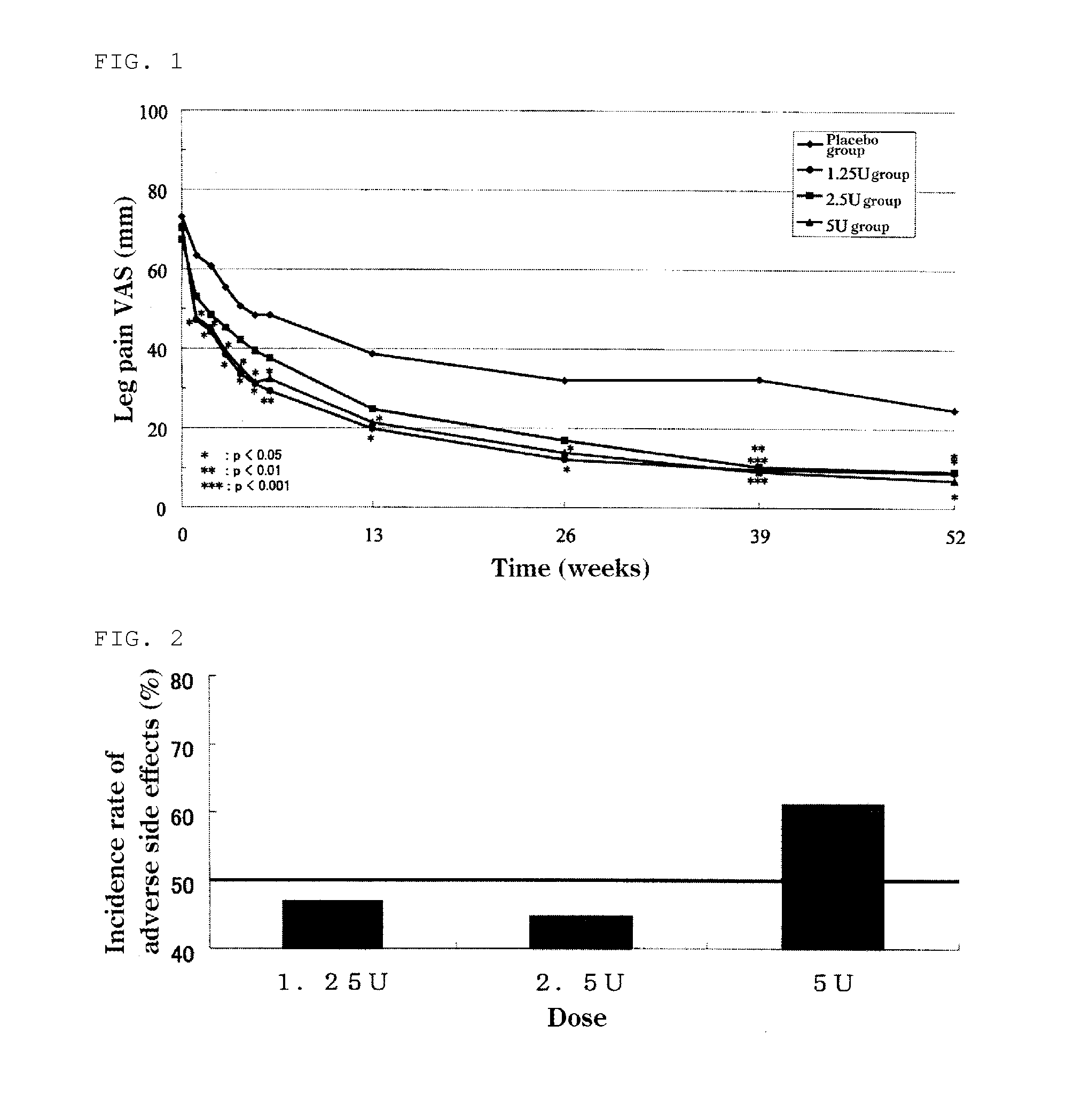
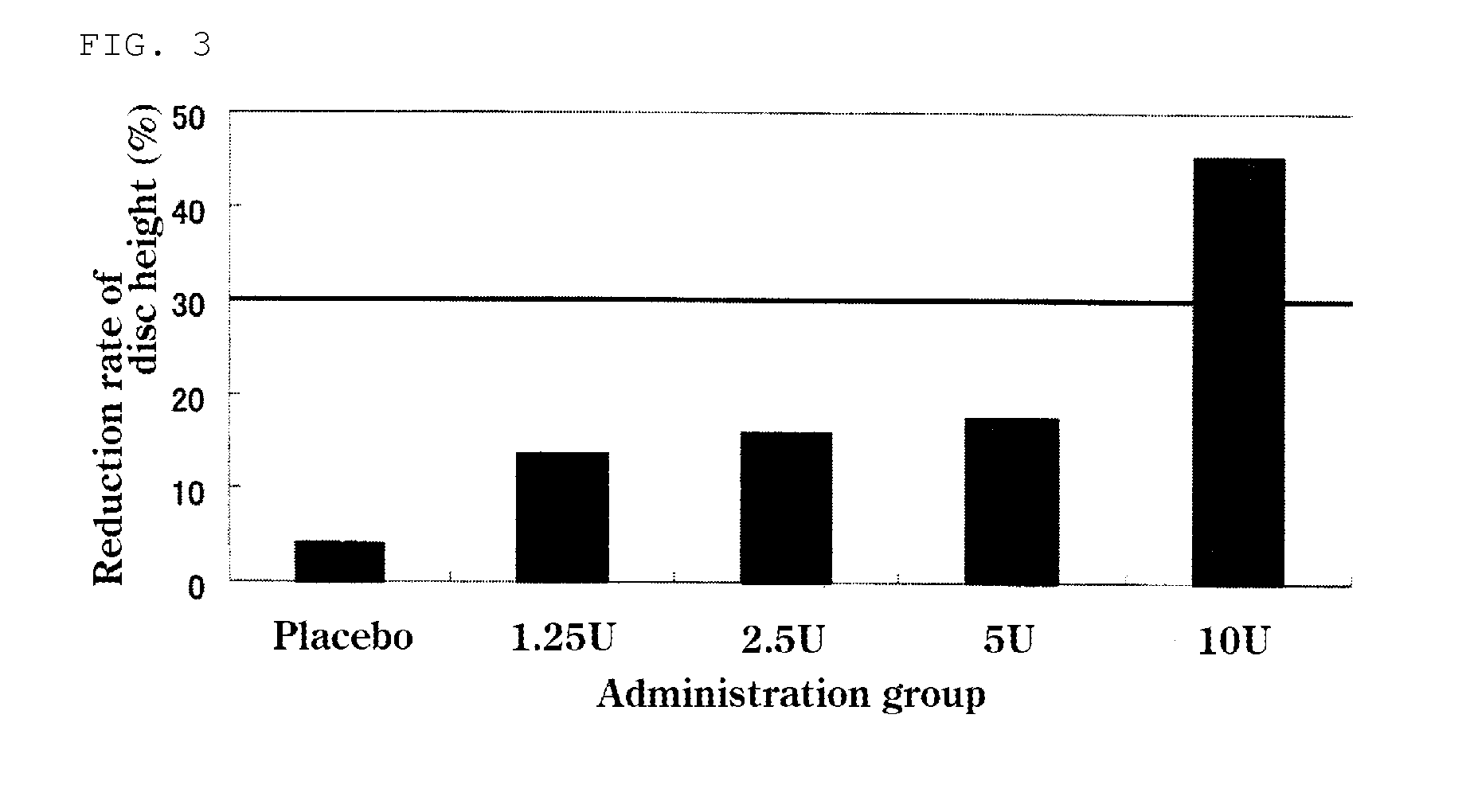
The present invention provides a therapeutic agent for disc herniation, which has extremely few adverse side effects, can achieve a prolonged pain-ameliorating effect when administered in only a single dose, and can exhibit a high therapeutic effect and high safety in clinical applications. The present invention relates to a therapeutic agent for disc herniation, which is characterized by containing chondroitinase ABC as an active ingredient and being administered in such a manner that the ingredient can be administered into a human disk in an amount of 1-8 units per disk.
Owner:SEIKAGAKU KOGYO CO LTD
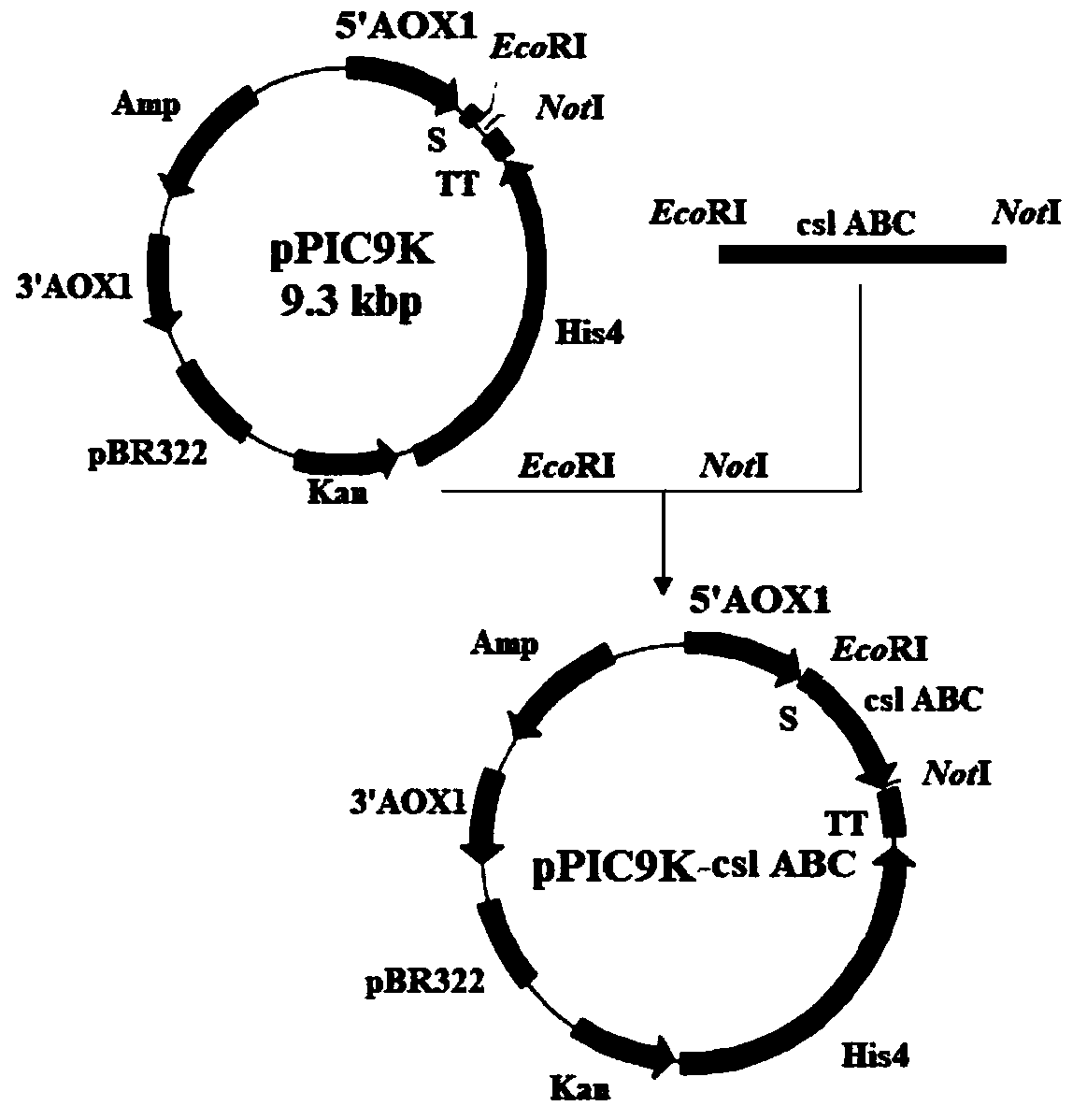
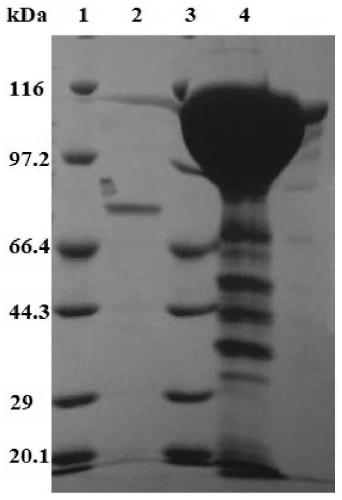
Chondroitinase ABC producing recombinant yeast strain and structuring and applying methods thereof
ActiveCN109988723AMeet health careFulfil requirementsBioreactor/fermenter combinationsFungiPichia pastorisBiotechnology
The invention discloses a chondroitinase ABC producing recombinant yeast strain and application thereof and belongs to the technical field of bioengineering. According to the chondroitinase ABC producing recombinant yeast strain, chondroitinase ABC from Proteus vulgaris ATCC33420 is subjected to heterogeneous expression, and with pPIC9K carriers and through induction of methanol, secretory expression of the chondroitinase ABC in pichia pastoris GS115 can be achieve; by means of a designed chondroitinase ABC-enzymatic membrane reactor, efficient and continuous production of small-molecular chondroitin sulfate can be achieved, the chondroitinase ABC can be recycled and reutilized, and the production cost can be greatly reduced. By taking food-grade yeast as host strains, the chondroitinase ABC producing recombinant yeast strain can be safe and reliable, provide effective references for industrialized and green production of small-molecular chondroitin sulfate A, B and C, and meanwhile, save energy, reduce emission and achieve significant economic and social benefits.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
Production of high purity chondroitinase ABC
ActiveUS9796970B1Solid sorbent liquid separationCarbon-oxygen lyasesPresent methodChondroitinase ABC
The present invention provides a method for purifying Chondroitinase ABC (ChABC). The present method includes using a heparin-immobilized affinity chromatography column, and through chromatography method obtaining a purified ChABC from a matrix containing the ChABC. The present method is capable of obtaining ChABC in high purity with the advantages of simplicity in preparation and high yield.
Owner:ADVANTEK SERUM LAB
Method for promoting axonal re-growth and behavior recovery in spinal cord injury
A method for promoting axon re-growth and behavior recovery in a subject suffering from a nerve injury is described. The method includes administering a pharmaceutical composition comprising a safe and effective amount of a chondroitinase ABC (CHABC) to an injury site of the nerve injury in the subject.
Owner:HENRICH CHENG
Low anticoagulant heparin and oligosaccharides thereof, and preparation methods and application of low anticoagulant heparin and oligosaccharides thereof in preparation of anti-Alzheimer's disease drugs
ActiveCN105399870ARich sourcesLow costOrganic active ingredientsNervous disorderDiseaseEthanol precipitation
The invention relates to low anticoagulant heparin and oligosaccharides thereof, and preparation methods and an application of the low anticoagulant heparin and the oligosaccharides thereof in preparation of anti-Alzheimer's disease drugs. A porcine small intestinal mucosa crude product heparin is treated by chondroitinase ABC to remove chondroitin sulfate and hyaluronic acid, subjected to hydrochloric acid precipitation to remove nucleic acid, subjected to ethanol precipitation and subjected to anion exchange resin purification, and finally the high-purity low anticoagulant heparin is obtained; furthermore, the low anticoagulant heparin is degraded to obtain the oligosaccharides with different molecular weights by heparinase or a beta-removal method; the obtained low anticoagulant heparin has the molecular weight of 10-20 kDa, the oligosaccharides have the molecular weight of 1-7 kDa, and each disaccharide unit averagely contains 1.5-2.5 sulfates. The prepared low anticoagulant heparin and the oligosaccharides thereof can significantly inhibit the BACE1 activity through experimental evidence and can be used for preparation of the anti-Alzheimer's disease drugs.
Owner:OCEAN UNIV OF CHINA
Therapeutic agent for disc herniation
ActiveUS20150071896A1Less side effectsHigh usefulnessNervous disorderPeptide/protein ingredientsSide effectChondroitinase ABC
The present invention provides a therapeutic agent for disc herniation, which has extremely few adverse side effects, can achieve a prolonged pain-ameliorating effect when administered in only a single dose, and can exhibit a high therapeutic effect and high safety in clinical applications. The present invention relates to a therapeutic agent for disc herniation, which is characterized by containing chondroitinase ABC as an active ingredient and being administered in such a manner that the ingredient can be administered into a human disk in an amount of 1-8 units per disk.
Owner:SEIKAGAKU KOGYO CO LTD
Radical therapeutic agent for keloid and hypertrophic scar
Disclosed is an effective therapeutic agent for keloid and / or hypertrophic scar. Specifically disclosed are: an elastic fiber-regenerating agent, which comprises chondroitinase ABC derived from Proteus vulgaris; and a therapeutic agent for keloid and / or hypertrophic scar, which comprises the regenerating agent as an active ingredient.
Owner:SEIKAGAKU KOGYO CO LTD +1
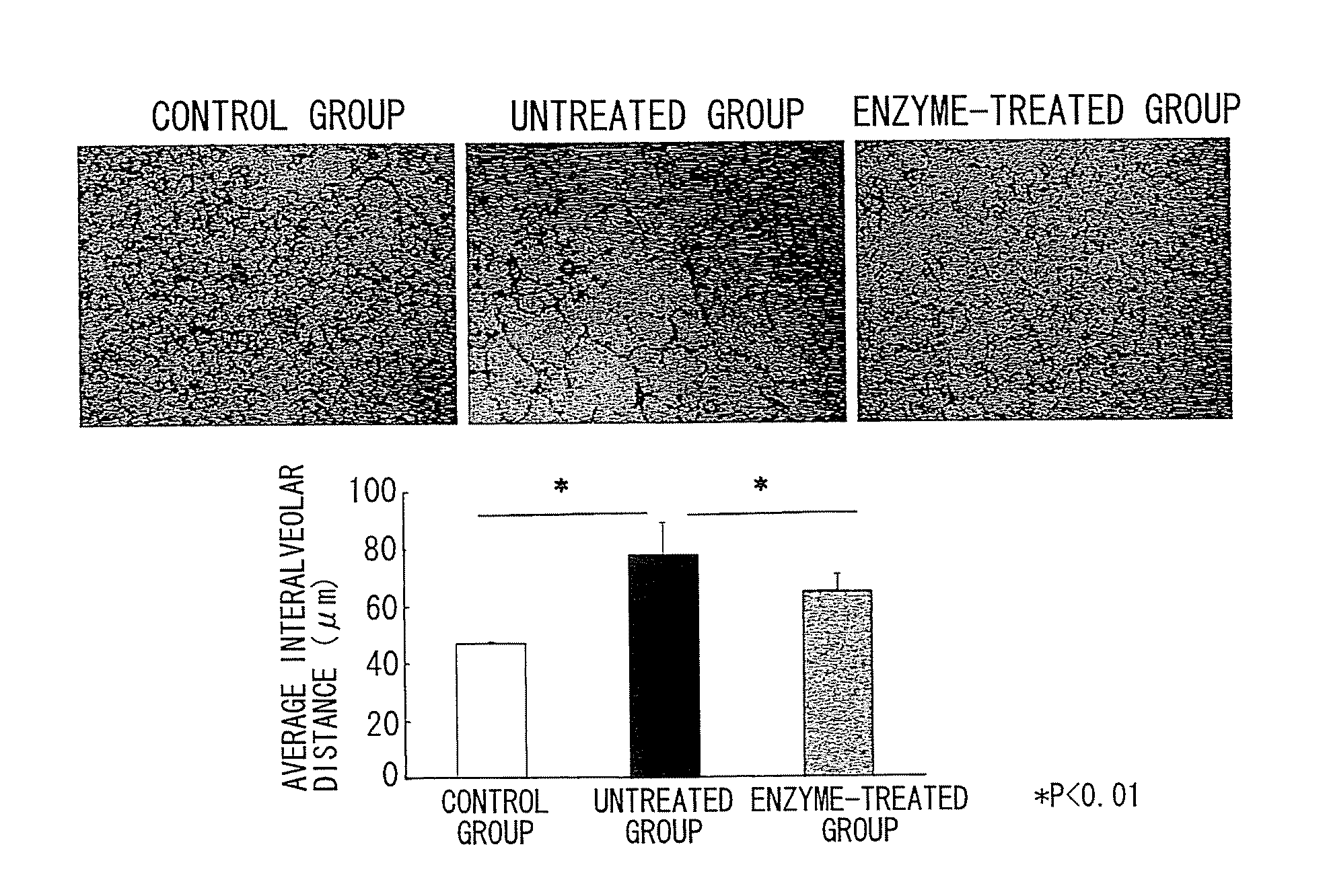
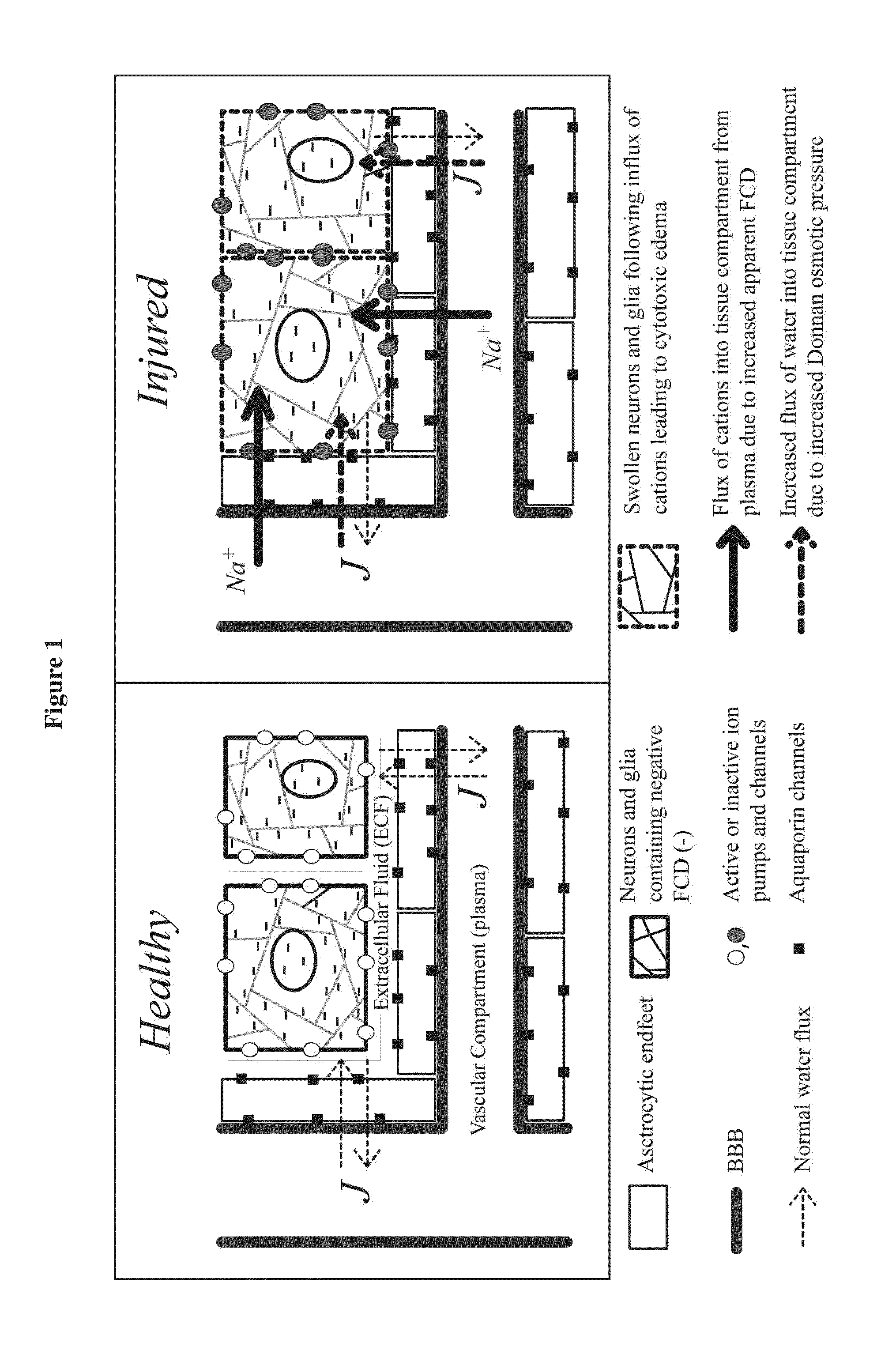
Enzyme combinations to reduce brain tissue swelling
InactiveUS20130259848A1Reduction in tissue swellingReduce edemaPeptide/protein ingredientsChondroitinase ABCWater entry
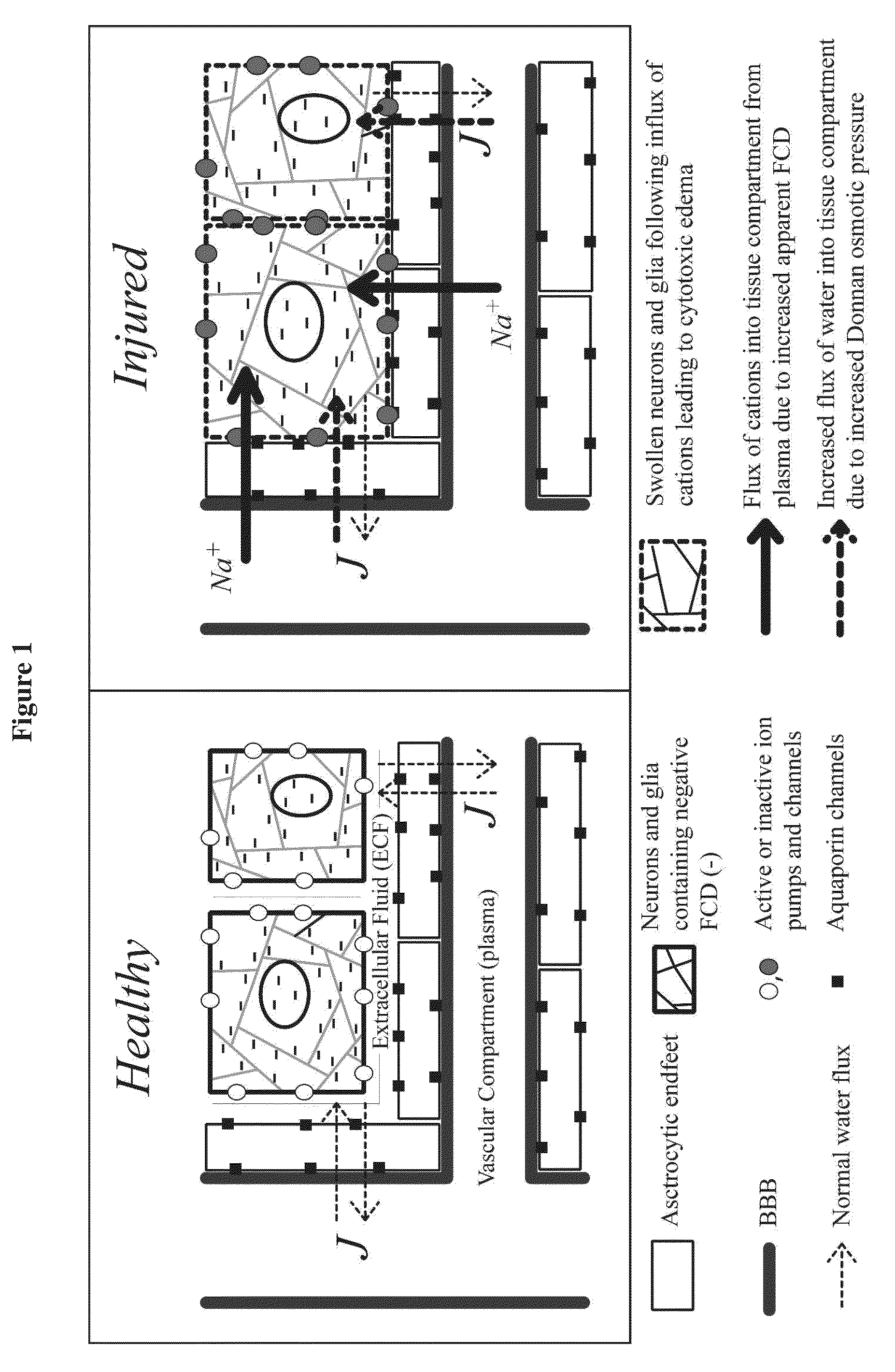
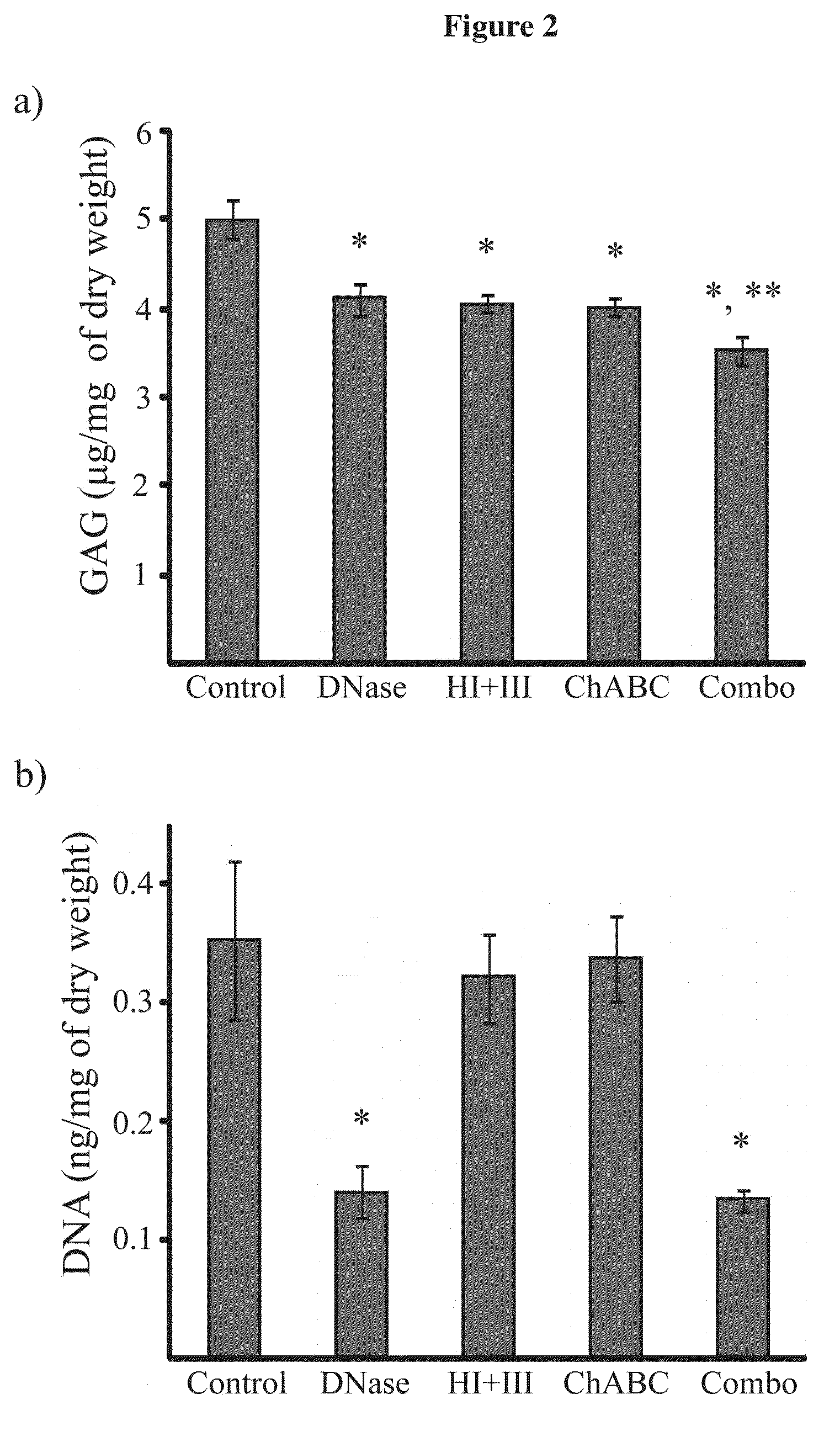
Tissue fixed charge density (FCD) is identified as another potential therapeutic target for reducing brain tissue swelling. Reduction of the FCD could reduce the thermodynamic force driving water entry into the brain. The present invention discloses chondroitinase ABC (ChABC) reduces tissue FCD and reduces tissue swelling, indicating that it may be an effective treatment to reduce edema and control intracranial pressure.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Preparation method of human stem cell for continuously secreting active chondroitinase ABC, kit and clinical use of human stem cell
InactiveCN105002216ATo achieve the effect of "two-pronged approach"Unknown materialsVector-based foreign material introductionDiseaseDual effect
The invention provides a preparation method of a human stem cell for continuously secreting active chondroitinase ABC. The preparation method comprises constructing an N-terminal signal peptide-free chondroitinase ABC gene, adding a gene segment for transcription and translation improvement to the end 3' of the gene, constructing the recombinant gene to a virus vector, and transfecting a human stem cell to obtain the human stem cell for continuously secreting active chondroitinase ABC. The human stem cell can effectively secrete chondroitinase ABC for a long time. The chondroitinase ABC has enzyme activity at a temperature of -20 DEG C for a long time. The invention provides a clinical use of the human stem cell continuously secreting active chondroitinase ABC. Through seed cells and a microenvironment going against nerve regeneration, the human stem cell can produce dual effects of treating chronic neurodegenerative diseases.
Owner:陈镇洲
Nanosphere/microsphere delivery system for the treatment of spinal cord injury
InactiveUS8765122B2Promote recoveryAvoid scaringNervous disorderPeptide/protein ingredientsMedicineMicrosphere
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Enzyme combinations to reduce brain tissue swelling
Tissue fixed charge density (FCD) is identified as another potential therapeutic target for reducing brain tissue swelling. Reduction of the FCD could reduce the thermodynamic force driving water entry into the brain. The present invention discloses chondroitinase ABC (ChABC) reduces tissue FCD and reduces tissue swelling, indicating that it may be an effective treatment to reduce edema and control intracranial pressure.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Production of high purity chondroitinase ABC
ActiveUS10036004B1Solid sorbent liquid separationCarbon-oxygen lyasesPresent methodChondroitinase ABC
The present invention provides a method for purifying Chondroitinase ABC (ChABC). The present method includes using a heparin-immobilized affinity chromatography column, and through chromatography method obtaining a purified ChABC from a matrix containing said ChABC. The present method is capable of obtaining ChABC in high purity with the advantages of simplicity in preparation and high yield.
Owner:ADVANTEK SERUM LAB LTD
Application of chondroitin sulfatase in preparation of medicine for treating pain
PendingCN114668835ASpontaneous pain suppressionThermal hyperalgesia suppressionNervous disorderPeptide/protein ingredientsCarrageenanChondroitinase ABC
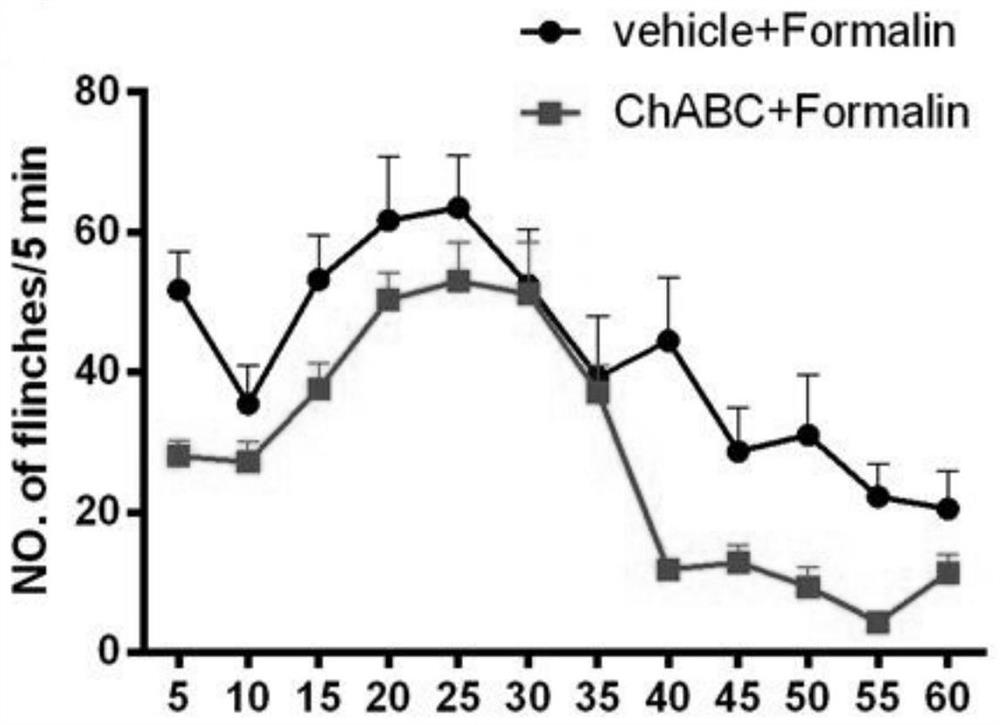
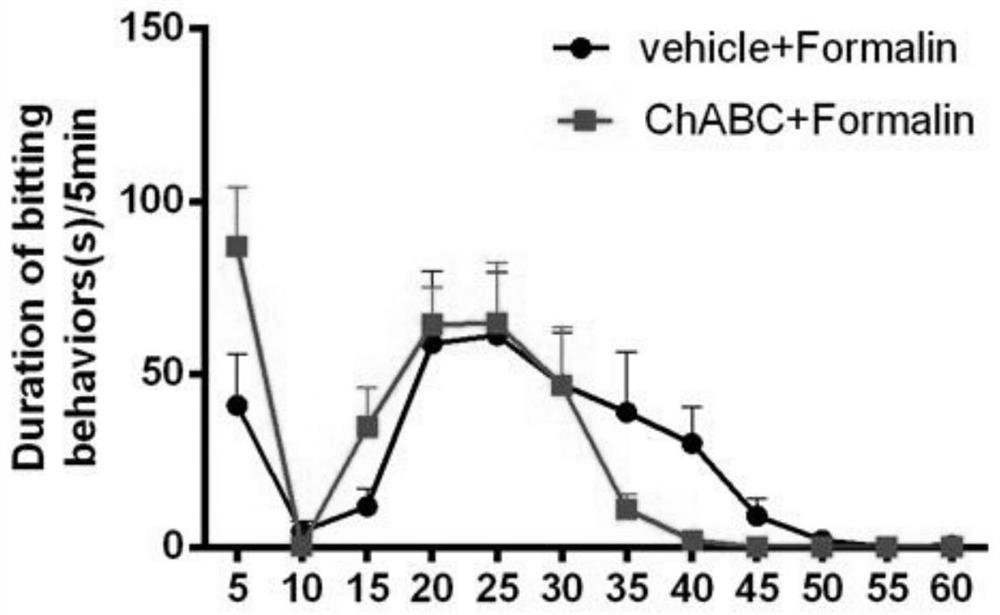
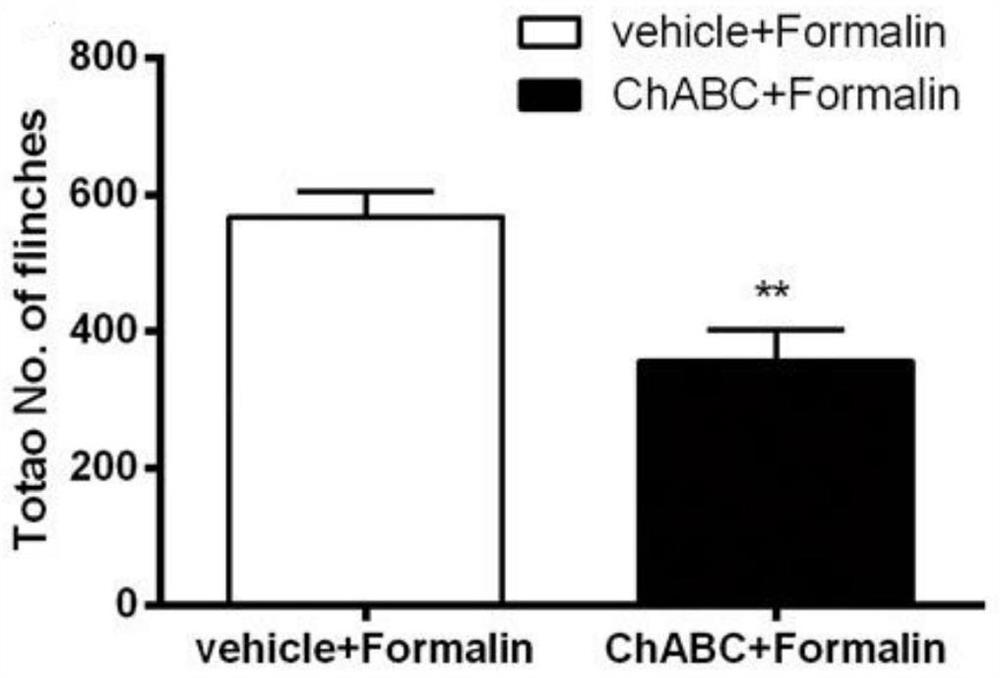
The invention discloses an application of chondrosulphatase in preparation of a medicine for treating pain. By adopting formalin and carrageenan painful animal models and simulating a BmK I mirror image pain model constructed by natural scorpion jellyfish, clinical common symptoms of spontaneous pain, heat pain sensitivity and mechanical pain sensitivity are simulated, so that the chondroitinase ABC has an inhibition effect on the spontaneous pain, the heat pain sensitivity and the mechanical pain sensitivity, and can be used as a new drug target for treating pain.
Owner:YANAN UNIV +1
A bacterium and the chondroitinsulfate enzyme abc it produces
ActiveCN105802875BIncrease vitalityImprove stabilityBacteriaMicroorganism based processesBiotechnologyNutrition
The invention belongs to the field of biotechnology and provides a bacterium and the chondroitinase ABC produced by it. The present invention relates to a kind of Acinetobacter ( Acinetobacter sp.C26), the strain has the characteristics of simple nutritional conditions, easy cultivation, and short generation time; the use of the strain of the present invention to produce chondroitin sulfate has the characteristics of high activity, good stability, and low cost, which can realize large-scale cultivation and meet the requirements of industrial applications.
Owner:OCEAN UNIV OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com