Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98 results about "Thiazolidines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reduced (protonated) form of THIAZOLES. They can be oxidized to THIAZOLIDINEDIONES.
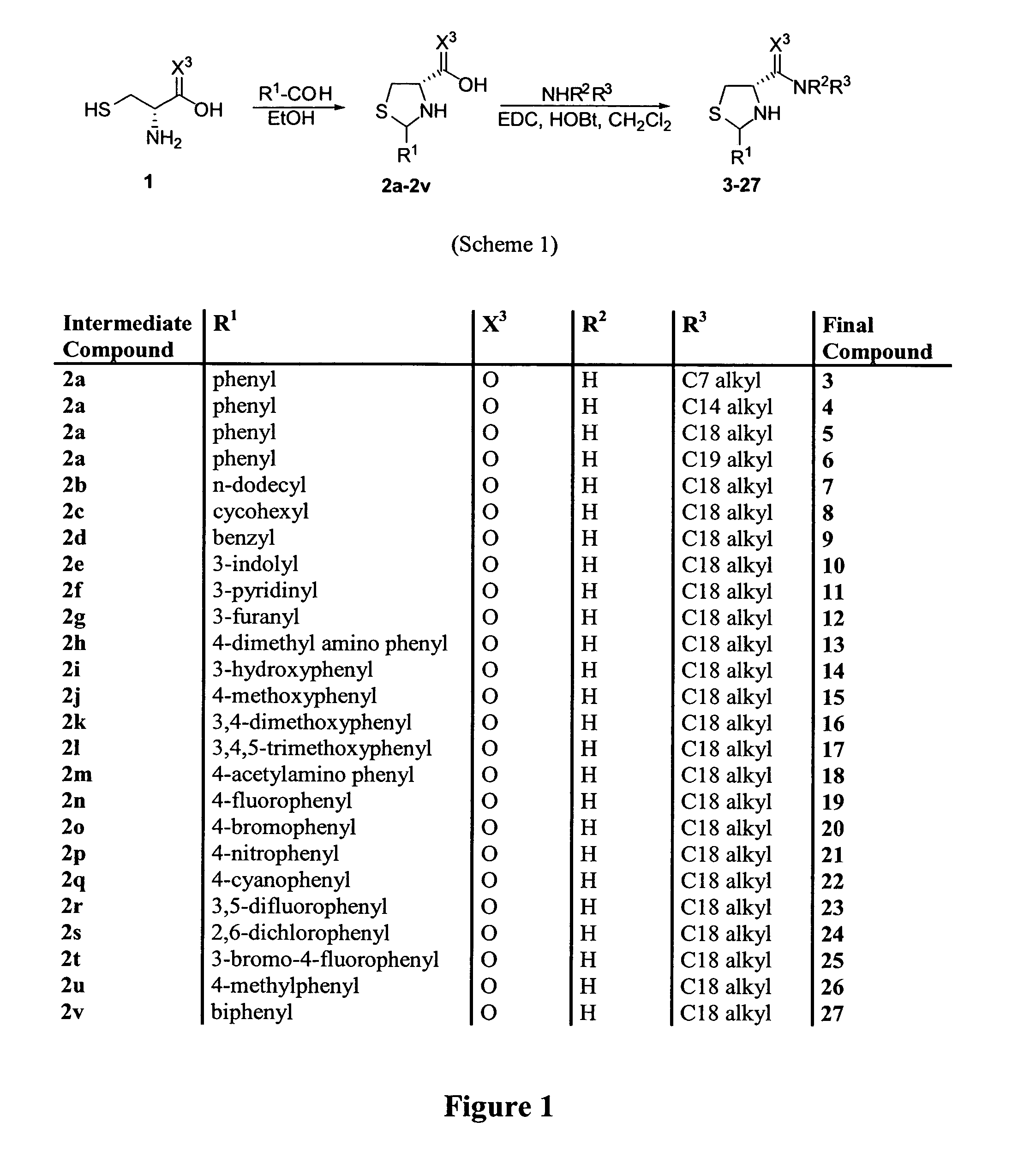
N-(substituted glycyl)-thiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV
The invention discloses certain N-(substituted glycyl)-thiazolidines, pharmaceutical compositions containing said compounds as an active ingredient thereof, and the use of said compounds in inhibiting dipeptidyl peptidase-IV.
Owner:NOVARTIS AG
Fragrance pro-accords and aldehyde and ketone fragrance libraries
InactiveUS7018978B2Extend your lifeEasy to prepareCosmetic preparationsOrganic chemistryFlavorOxazolidine E
The present invention relates to novel heterocyclic pro-fragrances, preferably oxazolidines, tertahydro-1,3-oxazines, thiazolidines, or tetrahydro-1,3-thiazines, more preferably oxazolidines, or tertahydro-1,3-oxazines, most preferably oxazolidines, which are capable of sustained release of fragrance raw material ketones and aldehydes and to fragrance delivery systems which comprise said pro-fragrances.
Owner:THE PROCTER & GAMBLE COMPANY
Dipeptidyl peptidase-IV inhibitors
The present invention relates generally to pyrrolidine and thiazolidine DPP-IV inhibitor compounds. The present invention also provides synthetic methods for preparation of such compounds, methods of inhibiting DPP-IV using such compounds and pharmaceutical formulations containing them for treatment of DPP-IV mediated diseases, in particular, Type-2 diabetes.
Owner:ALANTOS PHARMA HLDG INC
Therapeutic agent for keratoconjunctival disorder
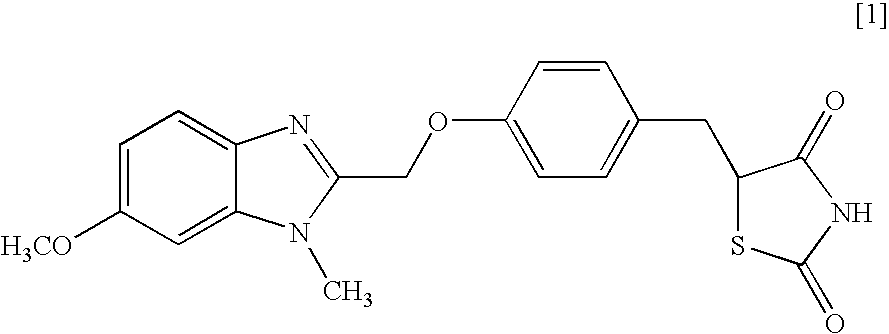
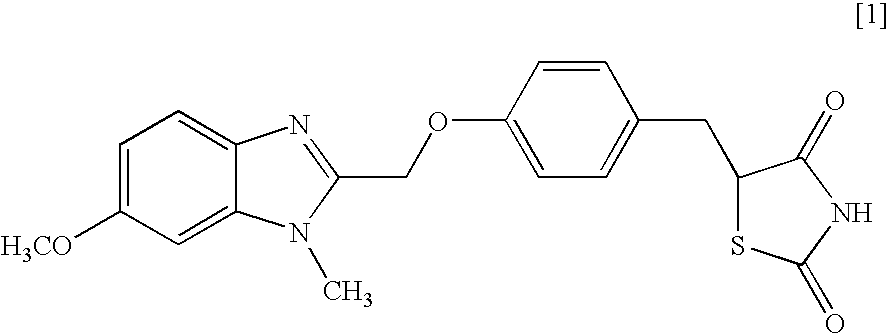
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
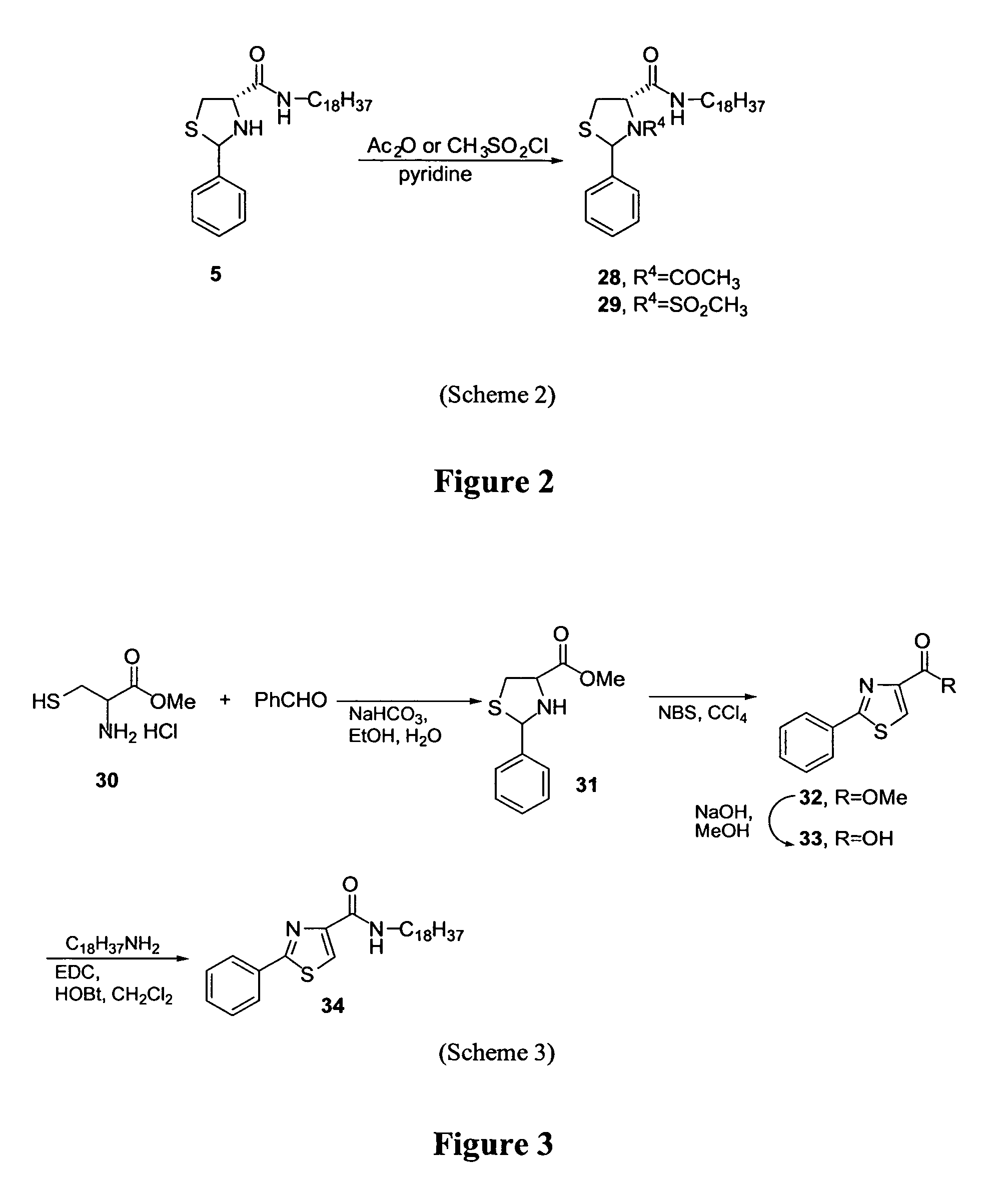
Thiazolidine derivatives and methods for the preparation thereof
The present invention relates to novel 2-carbonyl-3-acyl-1,3-thiazolidines having a β-amino group on the acyl chain, in free, prodrug form or pharmaceutically acceptable salt thereof, including their enantiomers, diastereomers and racemates, as efficient inhibitors against DPP-IV. The invention further relates to the pharmaceutical compositions comprising the disclosed compounds. The present invention also relates to methods for preparing the disclosed compounds and for treating DPP-IV-mediated diseases.
Owner:KAINOS MEDICINE INC +2
Cosmetic compositions and methods for using same to improve the aesthetic appearance of skin
InactiveUS20040067245A1Treating and preventing and ameliorating sign of dermatological agingImprove aestheticsCosmetic preparationsSalicyclic acid active ingredientsPerilla oilCarboxylic acid
The present invention relates to compositions useful in treating hyperpigmentation and the various signs of dermatological aging in human skin. The present invention also relates to cosmetic compositions and methods of using such compositions that improve the aesthetic appearance of skin. Further, the present invention relates to methods of applying the compositions to the skin to effect treatment and to improve the aesthetic appearance of skin, particularly, by providing anti-aging benefits to the skin. These and other objects of the present invention are achieved by a method and composition that comprises (a) a de-pigmenting agent or anti-aging agent in an amount effective to prevent, treat and / or ameliorate pigmentation or the various signs of aging at an area of skin to which it is applied, and (b) a cosmetically or pharmaceutically acceptable vehicle. Suitable de-pigmenting agents include 3,3'-thiodipropionic acid, thiazolidine-2-carboxylic acid, Kaempferol-7-glucoside, perilla oil, and clofibrate and clofibrate analogs and / or derivatives, as well as those set forth below. Suitable anti-aging agents include 3,3'-thiodipropionic acid and / or its derivatives.
Owner:AVON PROD INC
Thiazolidinone amides, thiazolidine carboxylic acid amides, and serine amides, including polyamine conjugates thereof, as selective Anti-cancer agents
Owner:UNIV OF TENNESSEE RES FOUND +1
Synthesis method of pidotimod
InactiveCN102167727AThe synthesis process is simple and safeEasy to operatePeptidesSynthesis methodsThiazolidine carboxylate
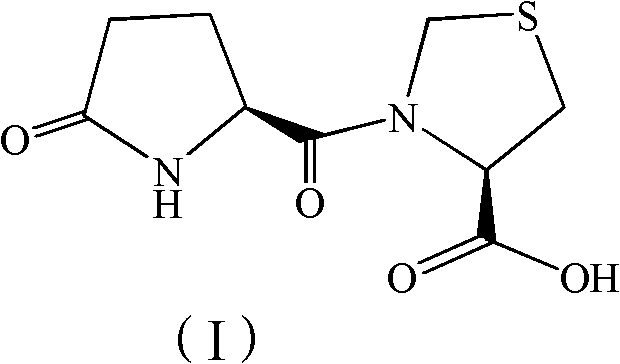
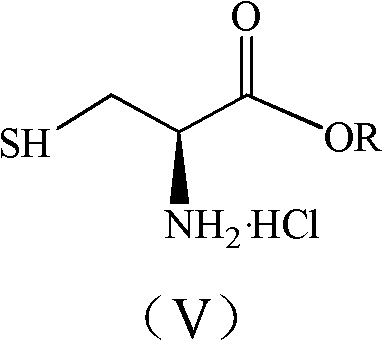
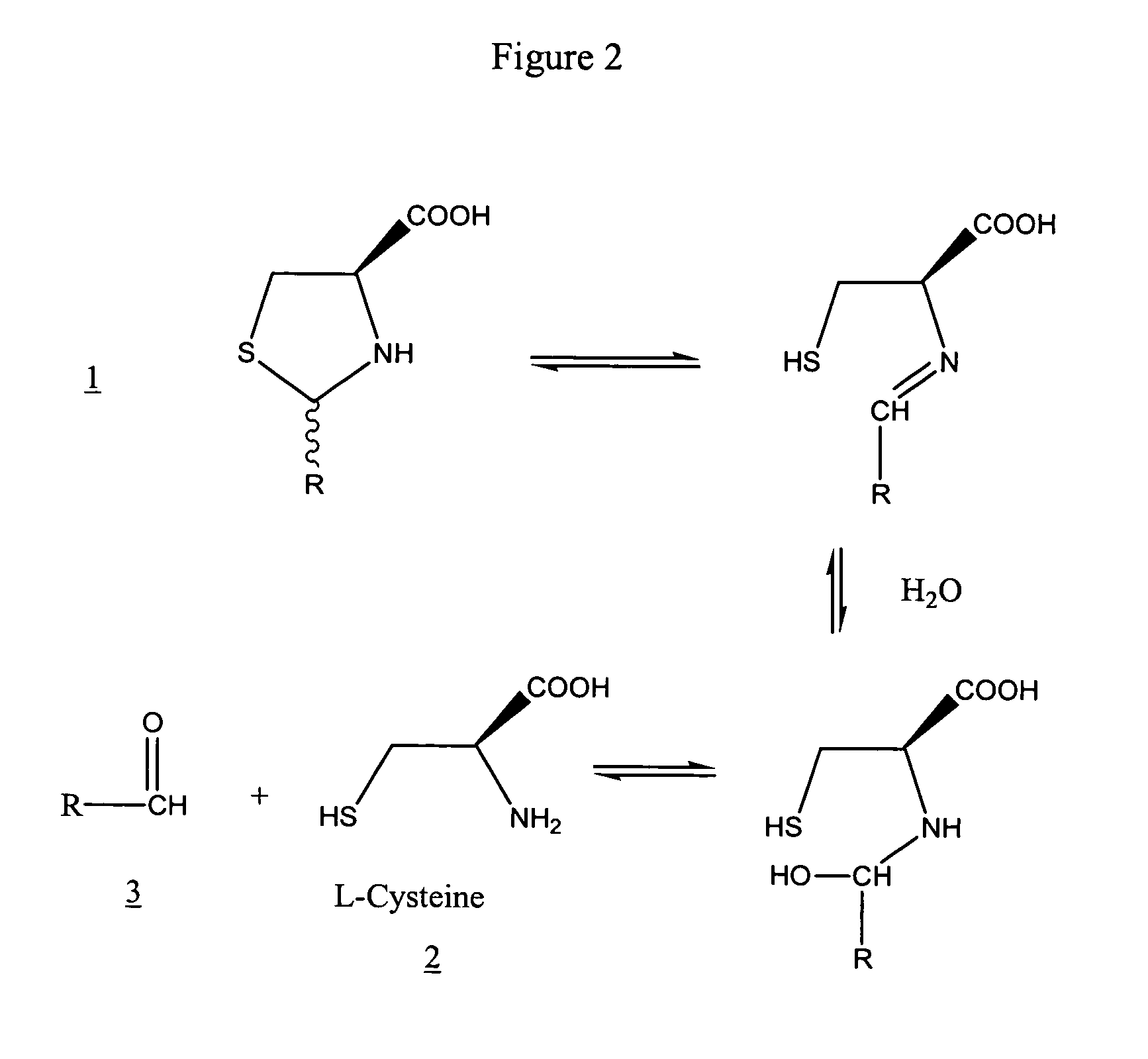
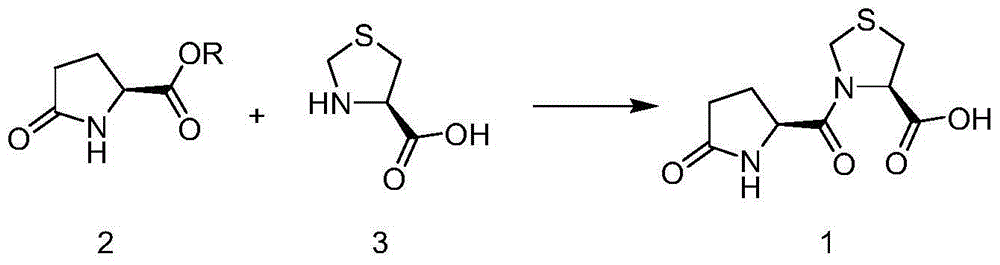
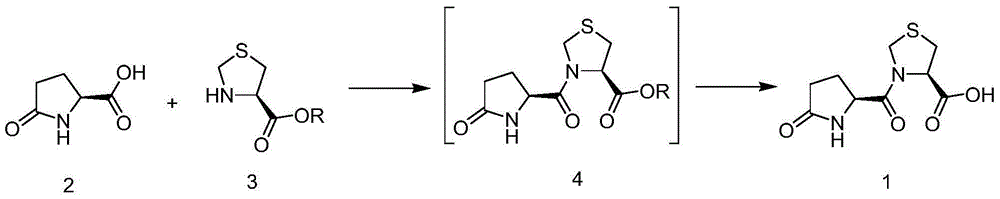
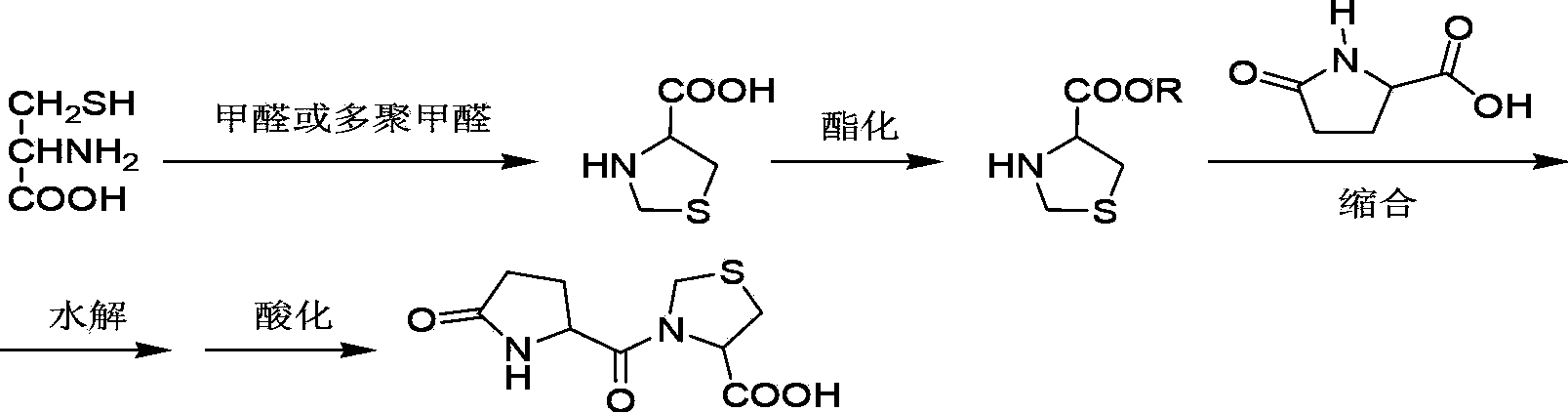
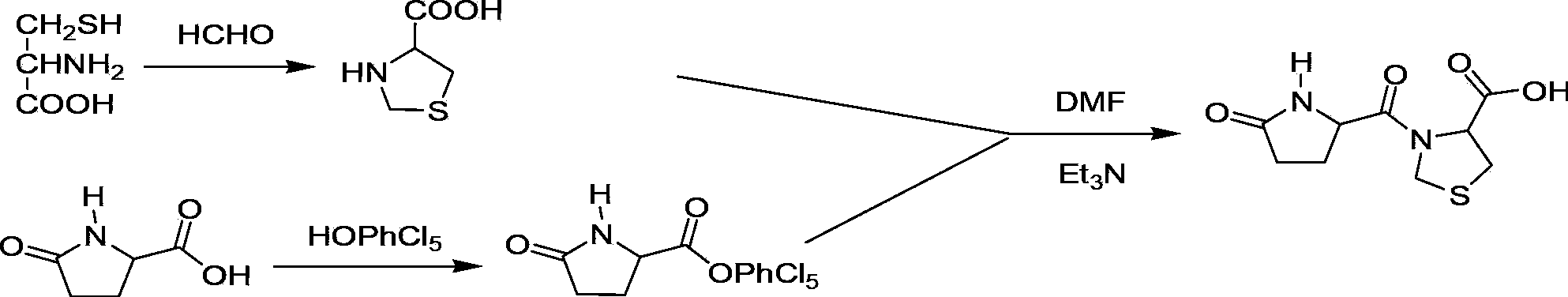
The invention discloses a synthesis method of pidotimod. The synthesis method comprises the following steps: reacting L-cysteine with paraformaldehyde or formaldehyde to generate L-thiazolidine-4-carboxylic acid, performing esterification to generate L-thiazolidine-4-carboxylate, or reacting L-cysteine ester hydrochloride with paraformaldehyde or formaldehyde to generate L-thiazolidine-4-carboxylate; and then carrying out condensation reaction on L-thiazolidine-4-carboxylate and L-pyroglutamic acid to generate (4R)-3-[[(2S)-5-oxo-2-pyrrolidinyl]carbonyl]-4-thiazolidine carboxylate, and then synthesizing pidotimod through hydrolysis reaction. Compared with the prior art, the technology in the invention is simple, is easy to operate, has high product yield and low production cost, is environmentally friendly and is suitable for industrial production.
Owner:ZHEJIANG KINGLYUAN PHARMA
Compositions and methods for treating hyperpigmentation
InactiveUS6562321B2Reduce retention timeImprove shipping rateCosmetic preparationsBiocidePerilla oilCarboxylic acid
There is provided a topical composition for treating, preventing or ameliorating hyperpigmentation in human skin. The composition has a de-pigmenting agent in an amount effect to reduce or diminish pigmentation at an area of skin to which it is applied, and a cosmetically or pharmaceutically acceptable vehicle. Suitable de-pigmenting agents include 3,3'-thiodipropionic acid, thiazolidine-2-carboxylic acid, kaempferol-7-glucoside, perilla oil, and clofibrate and clofibrate analogs and derivatives. There is also provided methods for treating, preventing or ameliorating hyperpigmentation in human skin.
Owner:AVON PROD INC
3-(2-amino-ethyl)-5-(3-cyclohexyl-propylidene)-thiazolidine-2,4-dione and its derivatives as multiple signaling pathway inhibitors and for the treatment of cancer
3-(2-amino-ethyl)-5-(3-cyclohexyl-propylidene)-thiazolidine-2,4-dione and derivatives thereof are provided for use as dual inhibitors of the Raf / MEK / ERK and PI3K / Akt pathways and for use in the treatment of cancer.
Owner:ZHANG SHIJUN +1
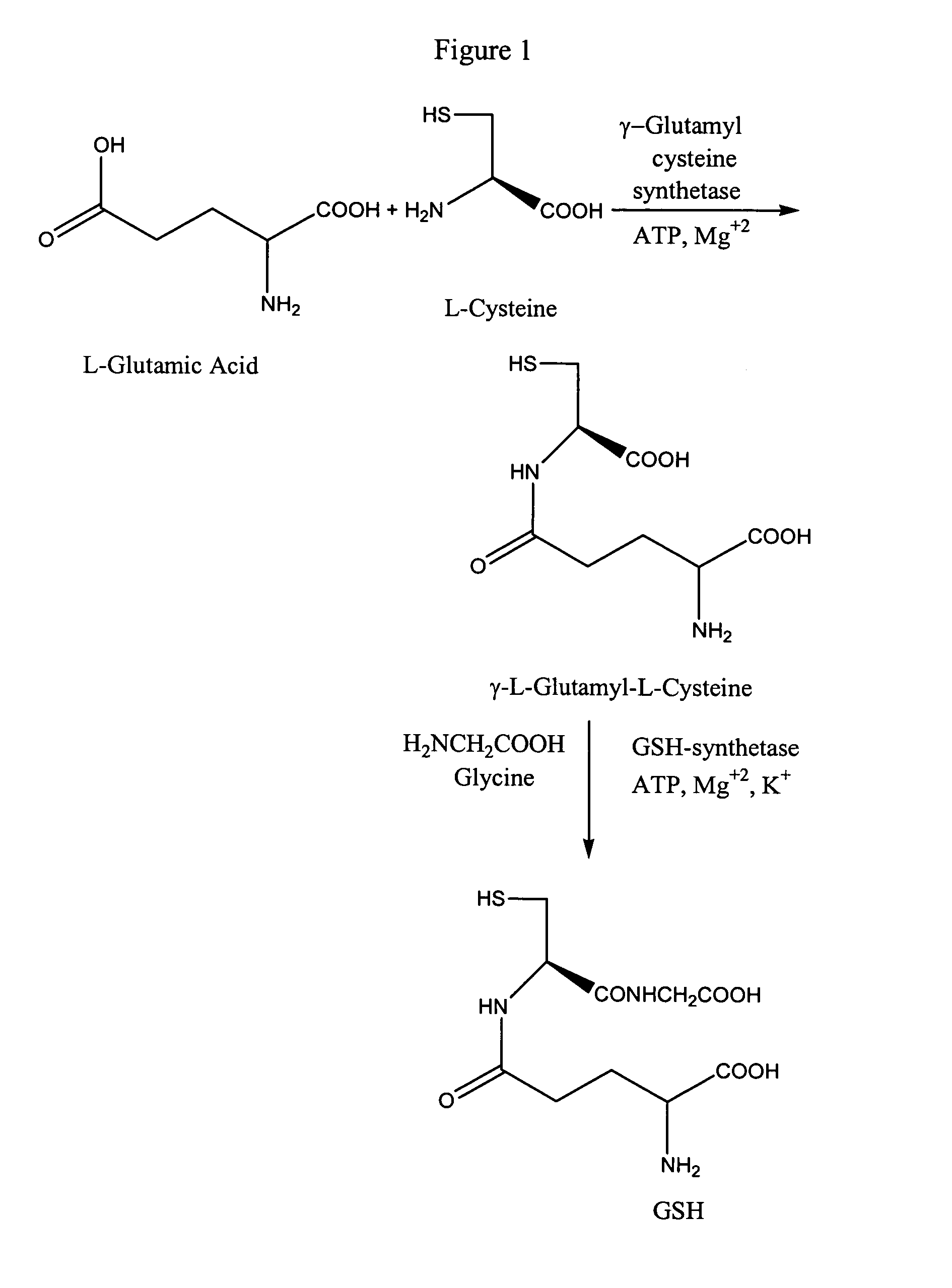
Method to enhance delivery of glutathione and ATP levels in cells
A therapeutic method is provided comprising treating a mammal subject to hypoxia with an amount of 2(R,S)-D-ribo-(1′,2′,3′,4′-tetrahydroxybutyl)thiazolidine-4(R)-carboxylic acid (RibCys) or a pharmaceutically acceptable salt thereof effective to both maintain, restore or increase both the ATP levels and the glutathione (GSH) levels in said tissue.
Owner:MAX INT LLC
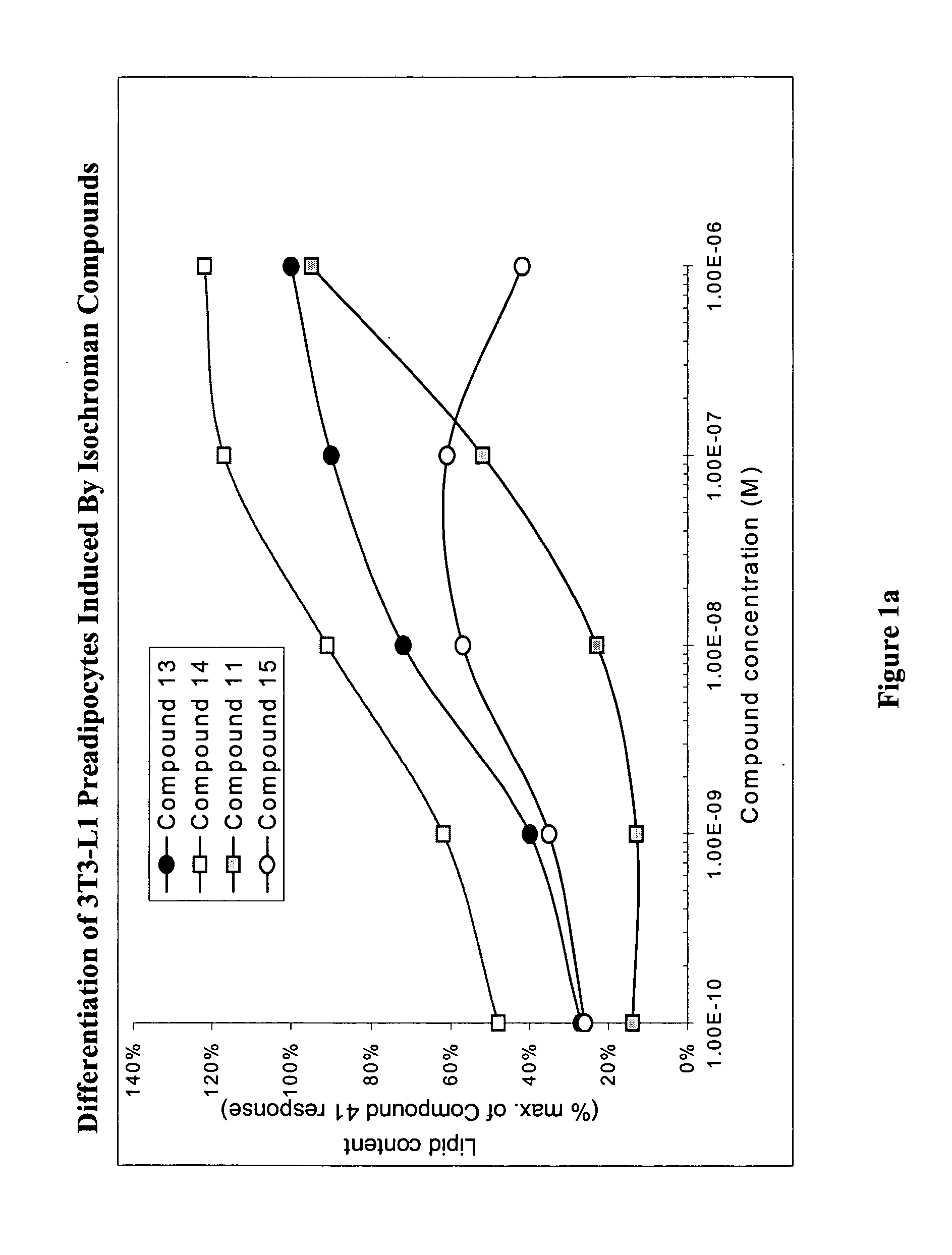
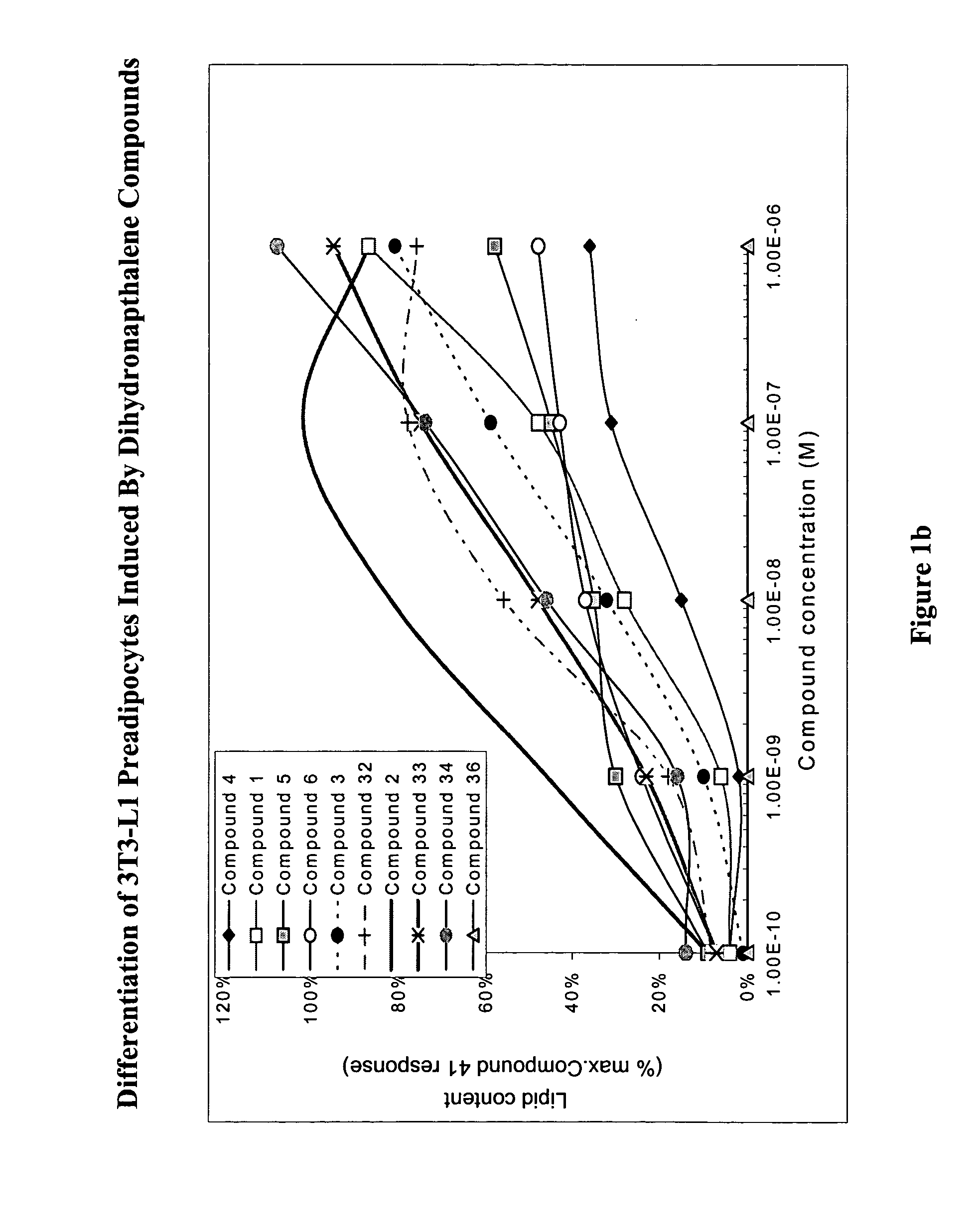
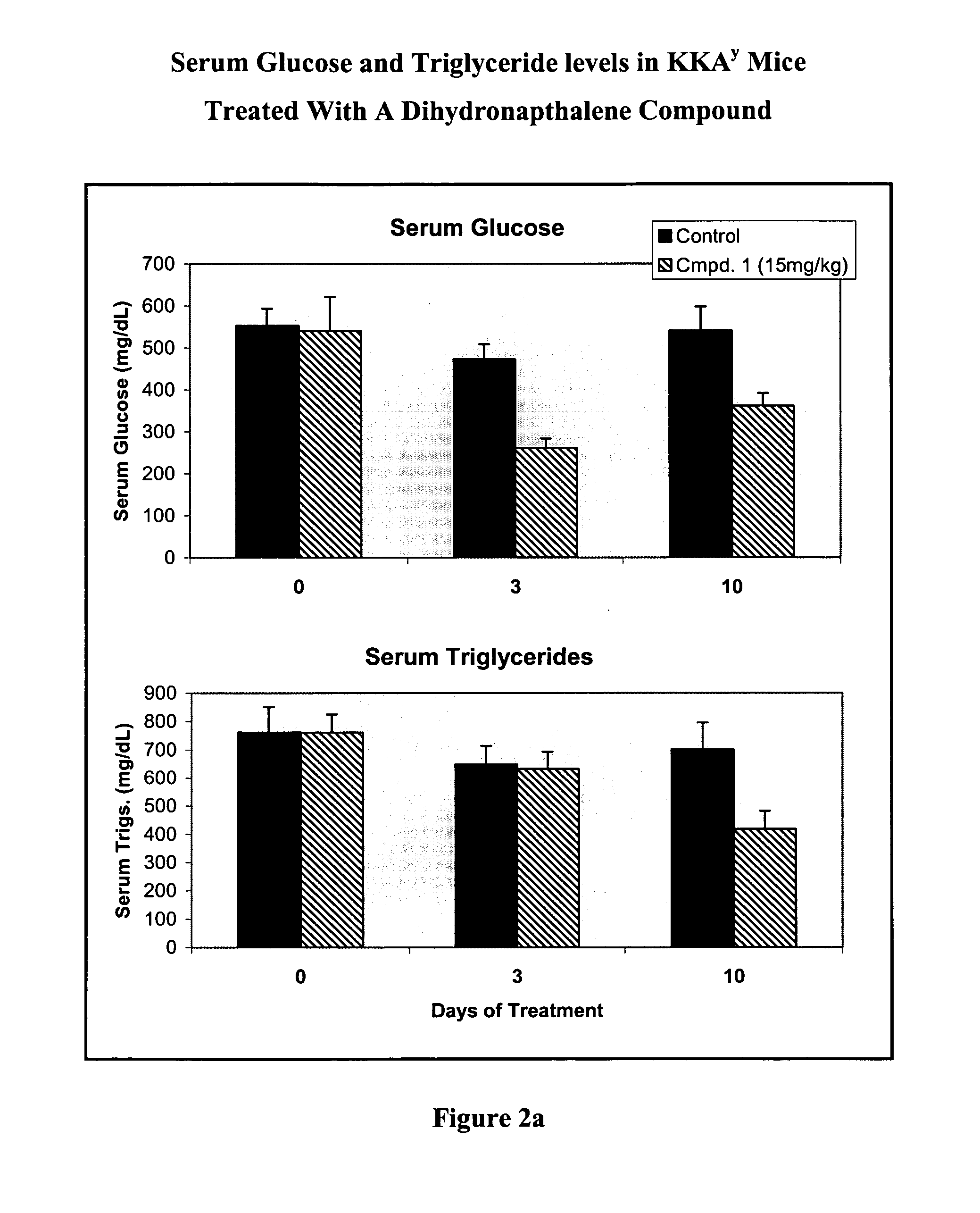
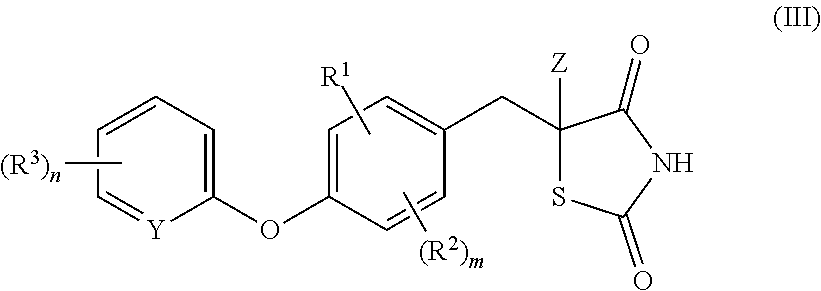
Substituted dihydronaphthalene and isochroman compounds for the treatment of metabolic disorders, cancer and other diseases
InactiveUS20050038098A1Superior physical property and bioavailabilityLower Level RequirementsBiocideOrganic chemistryThiazolidinedioneDrug biological activity
The invention relates to novel heterocyclic compounds having the structure illustrated by Formula (I) wherein the Ar1 radicals are substituted dihydronapthalene or isochroman radicals, the Ar2 radicals are aryl or heteroaryl radicals; and HAr is a 2,4-thiazolidinedione, 2-thioxo-thiazolidine-4-one, 2,4-imidazolidinedione or 2-thioxo-imidazolidine-4-one radical. The compounds of Formula (I) can have biological activity for advantageously regulating carbohydrate metabolism, including serum glucose level, and lipid metabolism, and can be useful for the treatment of hyperlipidemia and / or hypercholesterolemia, and Type II diabetes. The compounds of Formula (I) can also have utility in the treatment of diseases of uncontrolled proliferation, including cancer.
Owner:INCYTE CORP
Thiazolidinone amides, thiazolidine carboxylic acid amides, methods of making, and uses thereof
InactiveUS20060040998A1Improve effectivenessHigh selectivityOrganic active ingredientsBiocideCarboxylic acidThiazolidine
Substituted thiazolidinone carboxylic acid amides and substituted thiazolidine carboxylic acid amides according to formulae (I) and (II) are disclosed where the various substituent groups are as defined in the specification. Methods of making these compounds, pharmaceutical compositions containing the compounds, and their use, particularly for treating or preventing cancer, are also disclosed.
Owner:UNIV OF TENNESSEE RES FOUND +1
Thiazolidinone amides, thiazolidine carboxylic acid amides, and serine amides, including polyamine conjugates thereof, as selective Anti-cancer agents
InactiveUS20080255213A1Improve effectivenessHigh selectivityBiocideOrganic active ingredientsAnticarcinogenCarboxylic acid
Substituted thiazolidinone carboxylic acid amides and substituted thiazolidine carboxylic acid amides having a structurewhere the various substituent groups are as defined in the specification. Methods of making these compounds, pharmaceutical compositions containing the compounds, and their use, particularly for treating or preventing cancer, are also disclosed.
Owner:THE OHIO STATE UNIV RES FOUND +1
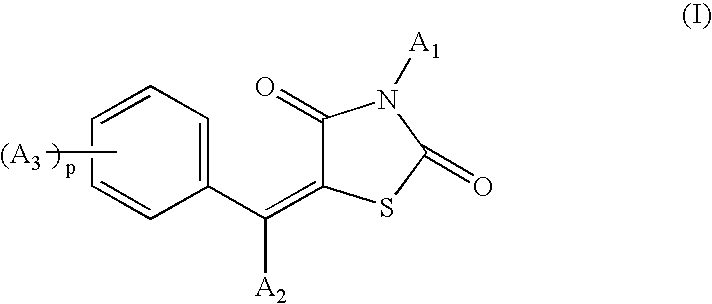
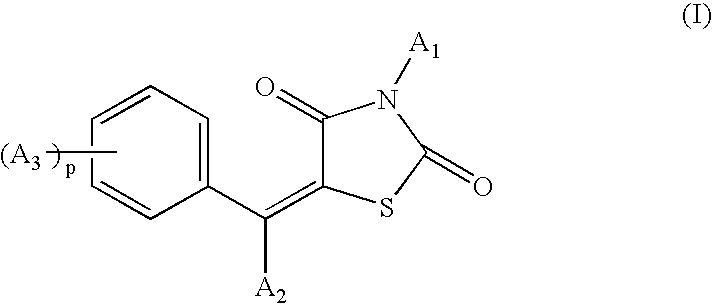
Benzylidene-1,3-thiazolidine-2,4-dione compounds for stimulating or inducing the growth and/or for reducing the loss and/or for increasing the density of keratin fibers
InactiveUS20070059265A1Effect hair growthHigh activityCosmetic preparationsBiocideActive agentWhiskers
The benzylidene-1,3-thiazolidine-2,4-dione compounds having the structural formula (I), or salt and / or solvate and / or isomer thereof: are useful active agents for inducing and / or stimulating the growth of mammalian keratin fibers and / or reducing the loss and / or increasing the density thereof, particularly of human head hair, beard hair, moustache hair, eyelashes and eyebrows, and advantageously are active agents for caring for or making up the hair or the eyelashes.
Owner:LOREAL SA
Compositions and methods for treating hyperpigmentation
InactiveUS20020141953A1Reduce retention timeImprove shipping rateBiocideCosmetic preparationsPerilla oilCarboxylic acid
There is provided a topical composition for treating, preventing or ameliorating hyperpigmentation in human skin. The composition has a de-pigmenting agent in an amount effect to reduce or diminish pigmentation at an area of skin to which it is applied, and a cosmetically or pharmaceutically acceptable vehicle. Suitable de-pigmenting agents include 3,3'-thiodipropionic acid, thiazolidine-2-carboxylic acid, kaempferol-7-glucoside, perilla oil, and clofibrate and clofibrate analogs and derivatives. There is also provided methods for treating, preventing or ameliorating hyperpigmentation in human skin.
Owner:AVON PROD INC
Process for producing pidotimod
InactiveCN104447947AReduce complexityReduce labor intensityPeptidesL-Pyroglutamic AcidCondensation reaction
The invention discloses a process for producing pidotimod and belongs to the technical field of medicines. A pidotimod crude product is prepared by a one-pot method which mainly comprises the following steps: (1) in the presence of a condensing agent as a catalyst, carrying out condensation reaction on L-pyroglutamic acid and L-4-thiazolidine formate to produce pidotimod ester and after the reaction is completed, filtering solid to obtain a mother liquid; and (2) adding an appropriate amount of acid into the mother liquid for hydrolyzing pidotimod ester, after the reaction is completed, separating liquid, retaining an aqueous phase, precipitating solids in an ice bath and filtering to obtain pidotimod. The yield is greatly increased, the process is simple, no special device is needed and pidotimod is suitable for large-scale production. According to the preparation process of pidotimod, by preferably selecting the process parameters, the yield and purity are greatly improved. Compared with the prior art, the yield of the pharmaceutical-grade pidotimod pure product is increased by 10-20%.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Thiazolidine derivatives and medicinal use thereof
InactiveUS20070259880A1Improve stabilityInhibitory activityBiocideOrganic chemistryThiazolidinesDiabetes mellitus
A thiazolidine derivative represented by the formula (I) wherein each symbol is as defined in the specification, and a pharmaceutically acceptable salt thereof exhibit a potent DPP-IV inhibitory activity, and can be provided as an agent for the prophylaxis or treatment of diabetes, an agent for the prophylaxis or treatment of obesity and the like.
Owner:MITSUBISHI TANABE PHARMA CORP
2,4-thiazolidinedione derivatives in the treatment of central nervous system disorders
ActiveUS9782395B2Improve oral bioavailabilityLow systemic plasma clearanceOrganic active ingredientsNervous disorderDiseaseNervous system
The present invention provides 5-(4-(2-(5-(1-hydroxyethyl)pyridine-2-yl)ethoxy) benzyl)thiazolidine-2,4-dione and novel stereoisomers of said compound for use in the treatment of central nervous system (NS) disorders.
Owner:MINORYX THERAPEUTICS
Effectors of dipeptidyl peptidase IV for topical use
InactiveUS20050209159A1Organic active ingredientsCosmetic preparationsAutoimmune conditionSide chain
The invention relates to compounds for topically influencing the activity of dipeptidyl peptidase of the general formula wherein A is an amino acid having at least one functional group in the side chain; B is a chemical compound covalently bound to a functional group of the side chain of A, chosen from the group consisting of (a) oligopeptides having a chain length of up to 20 amino acids, (b) homopolymers of glycine consisting of up to 6 glycine monomers, and (c) polyethylene glycols having molar masses of up to 20 000 g / mol; and C is a group amide-bonded to A chosen from the group consisting of thiazolidine, pyrrolidine, cyanopyrrolidine, hydroxyproline, dehydroproline or piperidine. The invention further relates to the use of said compounds for targeted intervention in local immunological processes (chemotaxis, inflammatory processes, autoimmune diseases), as well as effective and targeted treatment of pathophysiological and physiological processes related thereto (psoriasis, periodontitis, arthritis, allergies, inflammation), inter alia.
Owner:VIVORYON THERAPEUTICS NV
Novel compositions
An oral dosage form that provides controlled release of an active pharmaceutical agent, 5 -[4-[2-(N-methyl-N-(2 pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione (hereinafter ‘Compound A’) or a pharmaceutically acceptable salt or solvate thereof in different body environments, a process for the preparation of such an oral dosage form, and the use of such a dosage form in medicine.
Owner:SMITHKLINE BEECHAM (CORK) LTD
Heterocyclic amide derivatives for the treatment of diabetes and other diseases
InactiveUS7102000B2Useful in treatmentDecrease level of serum serum serum cholesterolBiocideOrganic chemistryLipid formationDisease
The present invention relates to certain substituted heterocycles of Formula (200),wherein B, H, I, J and K together with the Ar5 form a ring containing at least one amide residue, and W, X, Y and Z together form a 2,4-thiazolidinedione, 2-thioxo-thiazolidine-4-one, 2,4-imidazolidinedione or 2-thioxo-imidazolidine-4-one residue; or a pharmaceutically acceptable salt thereof. The compounds are useful in the treatment of diseases such as type 2 diabetes, and related disorders of lipid and carbohydrate metabolism, including atherosclerosis. The compounds are also useful for treating diseases of uncontrolled proliferation, such as cancers in general, including breast cancer.
Owner:ORTHO MCNEIL PHARM INC
Preparation method of pidotimod
ActiveCN103897025ALow priceEasy to buyPeptide preparation methodsEthyl chloroformateL-Pyroglutamic Acid
The invention provides an improved preparation method of pidotimod. According to the improved preparation method, a mixed anhydride method is adopted, and the improved preparation method comprises the following steps: under alkaline conditions, enabling L-pyroglutamic acid, ethyl chloroformate and L-thiazolidine-4-carboxylic acid to react, then acidifying, refining and devitrifying to prepare pidotimod. The method has the advantages of simplicity and convenience in operation, high yield and high product purity, and is more suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
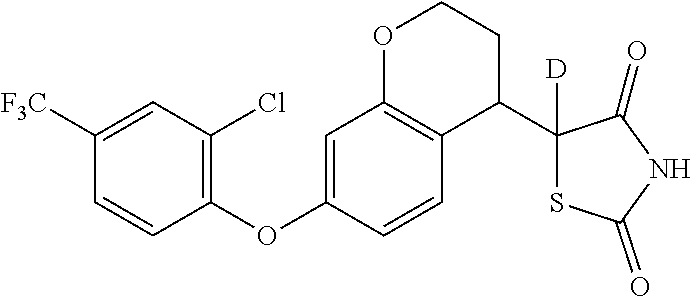
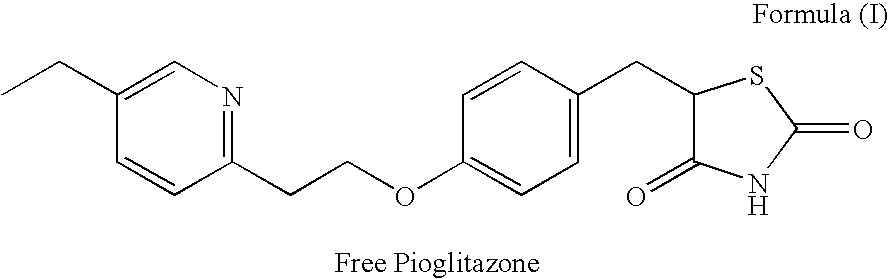
5-deutero-thiazolidinyldione compounds and methods of treating medical disorders using same
InactiveUS20180118730A1Isotope introduction to heterocyclic compoundsMetabolism disorderDiseaseMedical disorder
The invention provides deuterium-enriched thiazolidine-2,4-dione compounds (i.e., deuterium-enriched glitazone compounds), enantiopure forms of deuterium-enriched glitazone compounds, pharmaceutical compositions, and methods of treating medical disorders, such as a metabolic disorder, neurological disorders, cancer, or other disorder using deuterium-enriched glitazone compounds, which may be in enantiopure form.
Owner:POXEL
Stabilized pharmaceutical composition comprising antidiabetic agent
InactiveUS20060089387A1Process stabilityStabilize antidiabetic agentBiocidePowder deliveryDiabetes mellitusMedicine
This invention discloses a stabilized pharmaceutical composition comprising an antidiabetic agent and a stabilizer. The preferred stabilizers are selected from the group consisting of ascorbic acid, malic acid, maleic acid, tartaric acid, furmaric acid, citric acid, or combinations thereof. The antidiabetic agent is selected from the group consisting of [(±)5-[[2-(5-ethyl-2-pyridinyl)ethoxyl]phenyl]methyl]-thiazolidine-2,4-dione and (±)5-[4-[2-(N-methyl-N-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione. This invention also discloses amorphous forms of said antidiabetic agents and a process of preparation thereof, and a method for medical treatment of diabetic mellitus using said pharmaceutical composition.
Owner:MAI DE
Method for preparing hydrobromic acid teneligliptin
InactiveCN106349237ALow costHigh yieldOrganic chemistryBulk chemical productionSodium bicarbonateTert-Butyloxycarbonyl protecting group
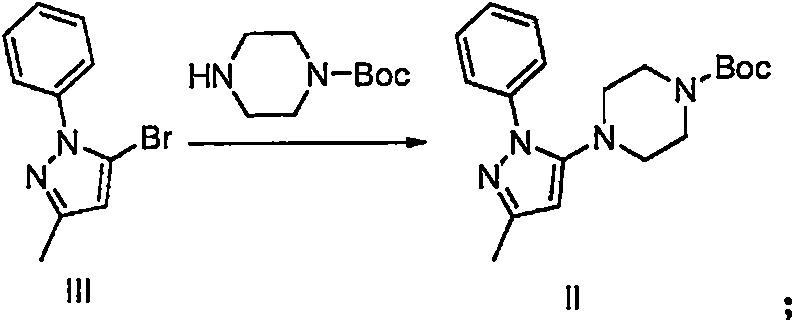
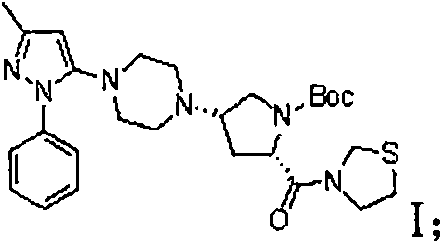
The invention provides a method for preparing hydrobromic acid teneligliptin. The method includes steps of preparing L-hydroxyproline; mixing the L-hydroxyproline and sodium bicarbonate with each other to obtain mixtures, dissolving the mixtures in water, adding acetone into the water, dropping di-tert-butyl dicarbonate into the water, carrying out room-temperature reaction overnight and then treating reaction products to obtain t-butyloxycarboryl-N-hydroxyproline; preparing t-butyloxycarboryl-N-4-oxo-proline from the t-butyloxycarboryl-N-hydroxyproline; preparing (2S)-4-oxo-2-(3-thiazolidine carbonyl)-1-pyrrolidine carboxylic acid tert-butyl ester from the t-butyloxycarboryl-N-4-oxo-proline; preparing compounds III from compounds IV; preparing compounds II from the compounds III; preparing compounds 1-(3-methyl-1-phenyl-1H-pyrazole-5-base) piperazine from the compounds II; preparing intermediates I; preparing the hydrobromic acid teneligliptin from the intermediates I. The method has the advantages that the method is low in cost, and the cost of the method is only two-thirds of the cost of an existing method in the prior art; the yield of the hydrobromic acid teneligliptin is higher than 95%, and the purity of the hydrobromic acid teneligliptin is higher than 98%.
Owner:南通普悦生物医药有限公司
Novel effectors of dipeptidyl peptidase IV
Dipeptide compounds and compounds analogous to dipeptide compounds that are formed from an amino acid and a thiazolidine or pyrrolidine group, and salts thereof used in the treatment of impaired glucose tolerance, glycosuria, hyperlipidaemia, metabolic acidoses, diabetes mellitus, diabetic neuropathy and nephropathy and also of sequelae of diabetes mellitus in mammals.
Owner:PROSIDION LIMITED
Therapeutic agent for keratoconjunctival disorder
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy) benzyl] thiazolidine-2, 4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy) benzyl] thiazolidine-2, 4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
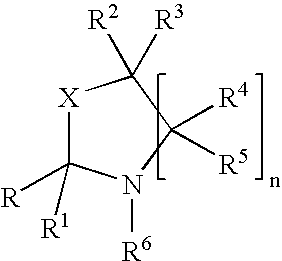
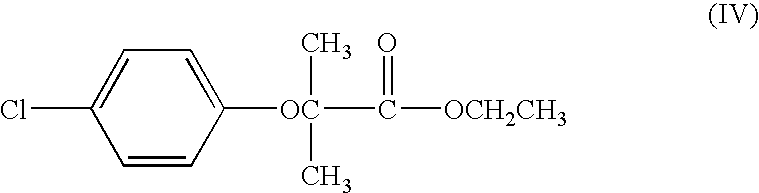
Process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl) amino] ethoxy] phenyl methyl] thiazolidine-2, 4-dione maleate
InactiveUS20050043539A1Cost effectiveCheaper & easily available raw-materialsOrganic chemistryN dimethylformamideBenzaldehyde
The present invention discloses a process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]phenyl methyl]thiazolidine-2,4-dione maleate (VI) comprising the steps of Coupling 2-[N-methyl-N-(2-pyridyl)amino]ethanol (I) and 4-fluorobenzaldehyde (II) in N,N-dimethylformamide, isolating the coupled product 4[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]benzaldehyde (III), converting said isolated benzaldehyde compound (III) to 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]benzylidene]thiazolidine-2,4-dione (IV) and purifying the same, reducing 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]benzylidene]thiazolidine-2,4-dione, by a novel reduction method for making 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]phenyl methyl]thiazolidine-2,4-dione (V). This reduction method involves reacting the compound (IV) with a novel metal legand complex and a reducing agent, purifying the product (V) obtained by a new method reported in the present invention and converting the said thiazolidine-2,4-dione compound (V) into a pharmaceutically acceptable salt.
Owner:USV LTD
Hydrates of proline derivative salt and production method thereof
The invention provides hydrates of a proline derivative salt, namely, 2.5-3.5 hydrate crystals of 3-{(2s,4s)-4-4[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidine-2-ylcarbonyl}thiazolidine 2.5 hydrobromide serving as dipeptidyl peptidases (i.e. DPP-IV) inhibitors. The crystals have the advantages of excellent stability and solubility as well as reproducibility and the invention also provides a production method of the crystals.
Owner:NANJING HUAWE MEDICINE TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





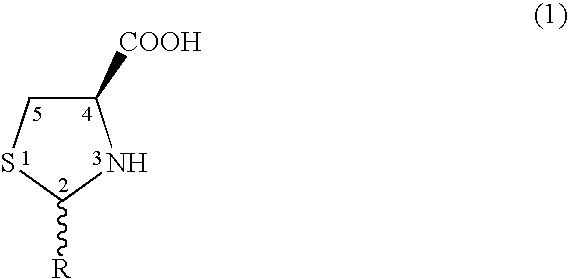















![Process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl) amino] ethoxy] phenyl methyl] thiazolidine-2, 4-dione maleate Process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl) amino] ethoxy] phenyl methyl] thiazolidine-2, 4-dione maleate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c18dcf06-a4a8-4073-9c43-844b563778e7/US20050043539A1-20050224-C00001.png)