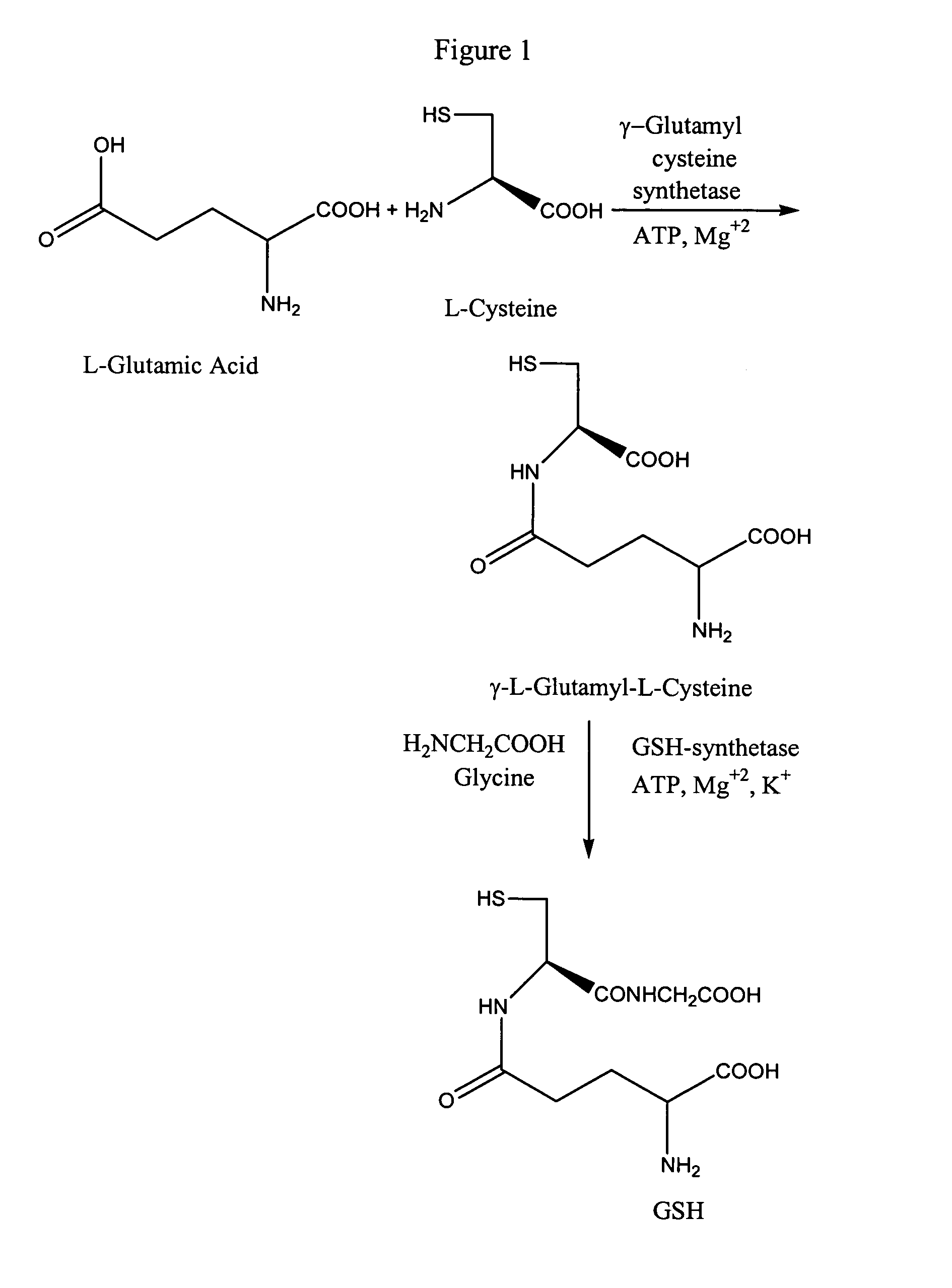
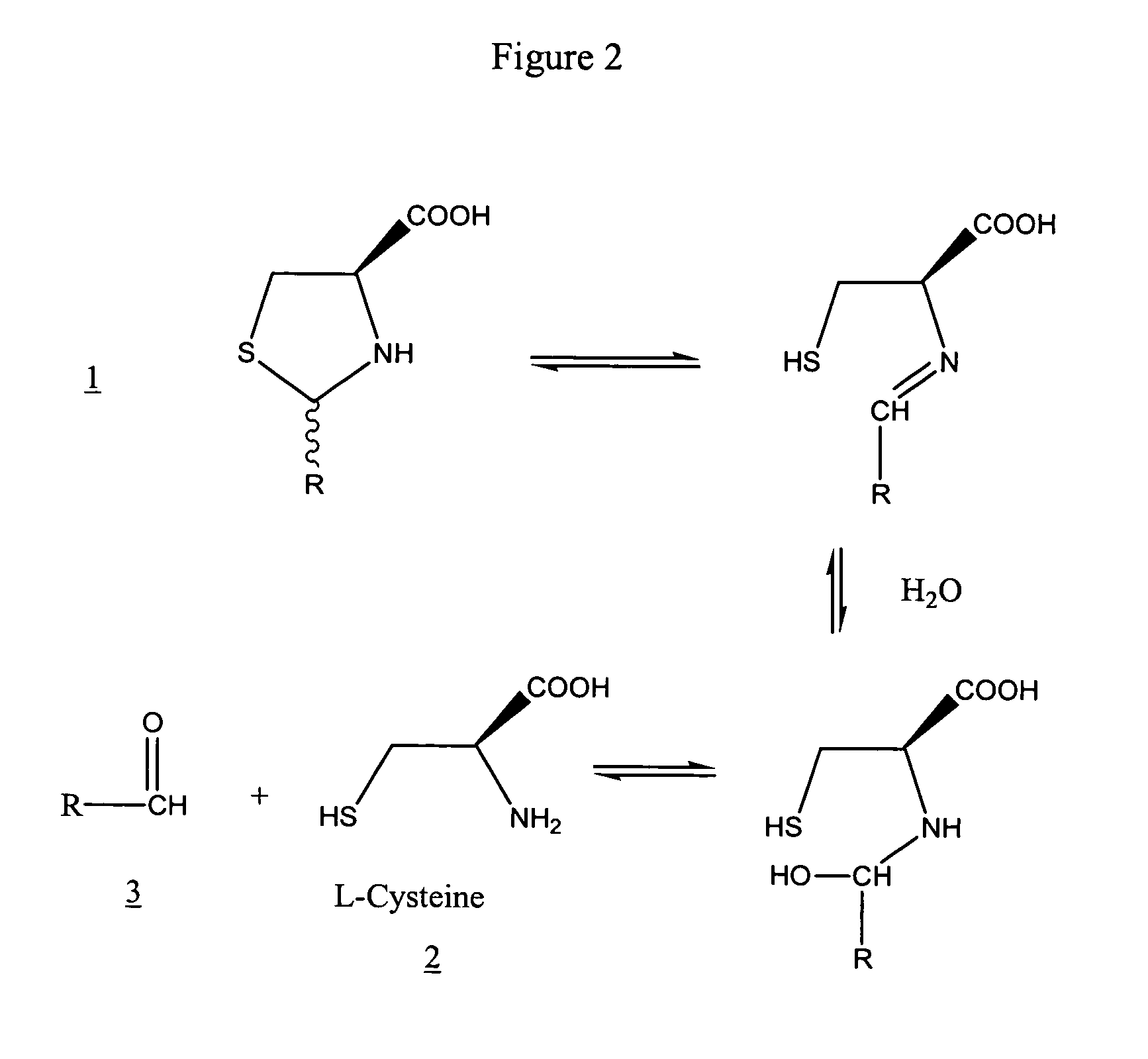
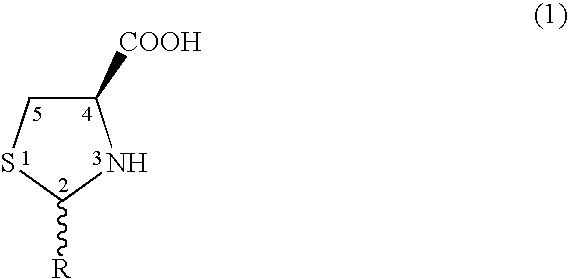Method to enhance delivery of glutathione and ATP levels in cells
a technology of atp and glutathione, which is applied in the direction of biocide, drug composition, genetically modified cells, etc., can solve the problem that simply administering a gsh precursor such as cysteine will not be as effectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2(R,S)-D-ribo-1′,2′,3′,4′-Tetrahydroxybutylthiazolidine-4(R)-carboxylic Acid (RibCys)
[0025] This compound was synthesized using ribose (Rib) as described by R. Bognar et al., Z. Liebigs Ann. Chem., 738, 68 (1970), the disclosure of which is incorporated by reference herein. The product was collected to give 4.71 g (92.2% yield) of pale yellow material, mp 149°-151° C. dec. [α]D25 −103.1° (c=0.52, H2O); IR (KBr) ν3220 (br, OH, COO−), 1610 cm−1 (COO−).
example 2
Stimulation of Glutathione Biosynthesis in Isolated Rat Hepatocytes by L-Cysteine Prodrugs and Inhibition by Buthionine Sulfoximine (BSO)
[0026] Rat hepatocytes were isolated following the methods of P. O. Seglen, Exper. Cell Res., 74, 450 (1972). After final plating, the hepatocytes were maintained in culture for 24 hr prior to use. Only primary cultures were used throughout the studies. The hepatocytes were incubated with cysteine prodrugs NAC and (Ia) for a 4-hr period, and after removal of media by aspiration, the cells were rinsed with cold phosphate-buffered saline and deproteinized with 5% sulfosalicylic acid. Total GSH content (GSH+GSSG) was determined by a modification of the DTNB [5,5′-dithiobis(2-nitrobenzoic acid)] glutathione reductase recycling method of F. Tietze, Anal. Biochem., 27, 502 (1969). The GSH concentration in the sample was quantified by determining the cycling rate (ΔOD at 412 nm / min) of the sample. For the inhibition studies with BSO, the cells were pre-e...
example 3
RibCys Elevates GSH in Heart and Muscle Tissue
[0030] As reported by J. C. Roberts et al., Toxicol. Lett., 59, 245 (1991), RibCys successfully elevated glutathione (GSH) levels in numerous organs of tumor-bearing CDF1 mice. GSH content was assayed 1, 2, 4, 8 and 16 h after RibCys administration (8 mmol / kg, i.p.); various organs achieved maximal GSH content at different time points. GSH in the liver was elevated 1.5-fold compared to untreated controls at the 16-h time point. Kidney GSH also was maximal at 16 h and achieved 1.6-times control values. GSH in muscle achieved 2.5 times the levels in control animals, while the bladder was elevated 2.1-fold, and the heart 1.8-fold. Other tissues tested (spleen, pancreas, lung) showed a 1.1 - to 1.2-fold increase in GSH content. GSH in implanted L1210 tumors was also elevated only 1.2-fold.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com