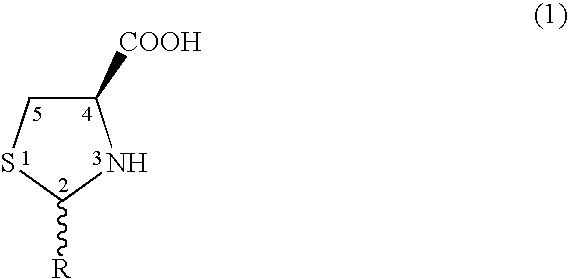Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Atp level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
55 ATP Levels in RBCs and T Cells and the Effects of Age on Mitochondrial Potential respectively. For red cells, the average level of ATP per 10 6 cells was 360 ± 64 nM. For T cells, the average level of ATP was 8500 ± 1400 nM per 106 cells. Pathological conditions may have an influence on the ATP content.
Compositions and methods for tissue preservation
ActiveUS20100151435A1Maintain functional integrityRecovery functionMicrobiological testing/measurementDead animal preservationPotassiumApoptosis
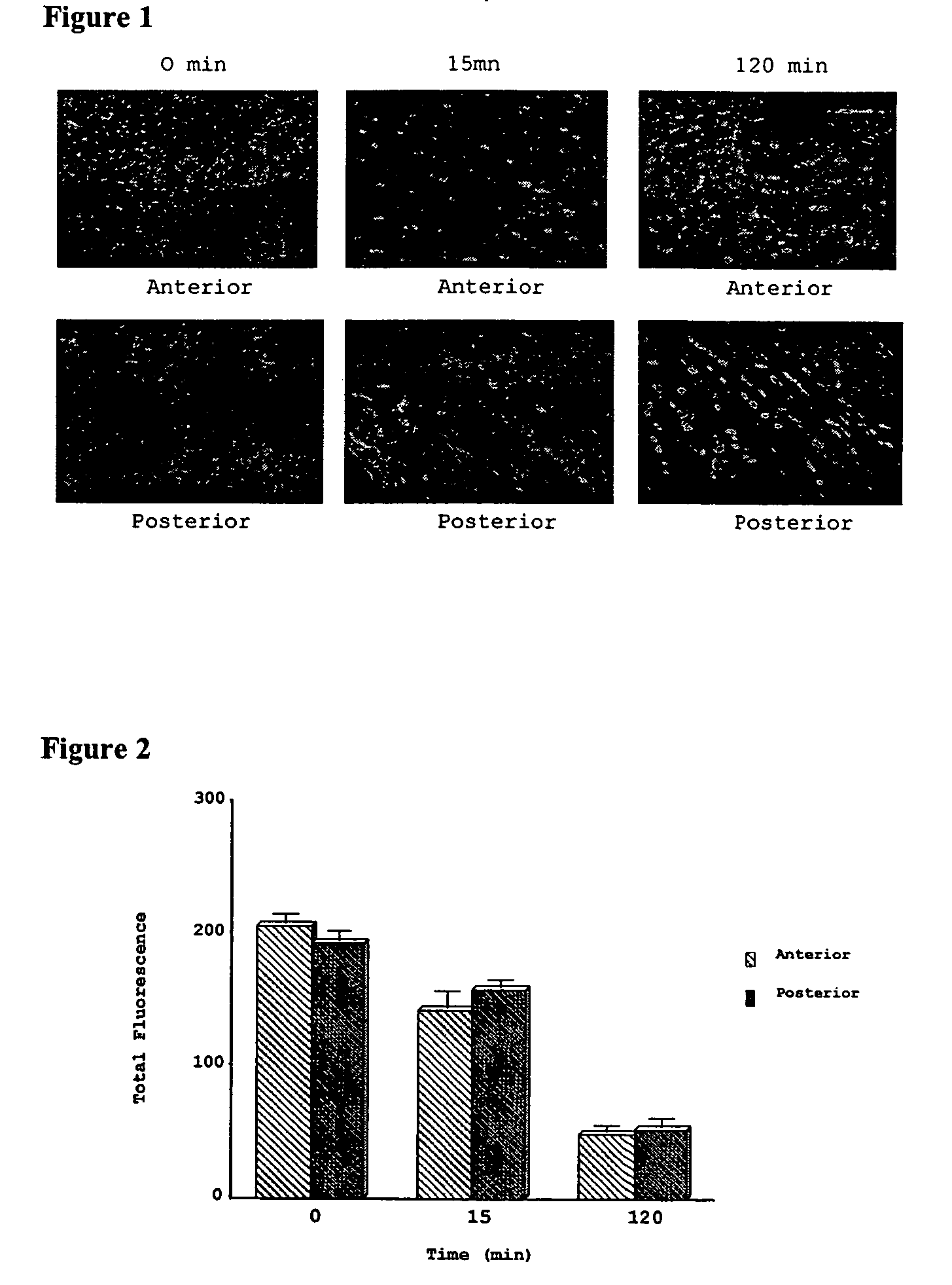
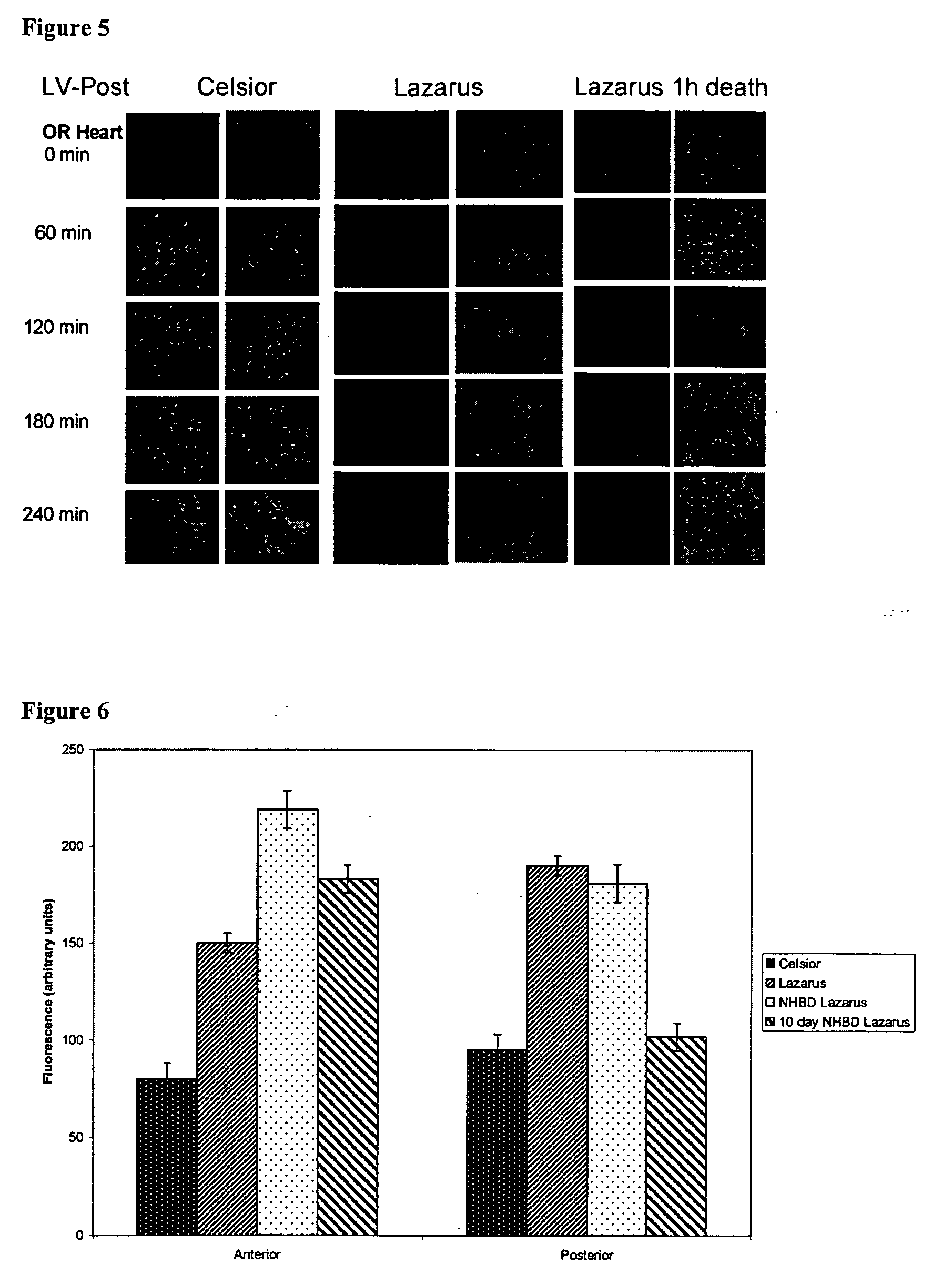
Methods and compositions for resuscitating, storing, and preserving functional integrity of organs and tissues. Metabolic function is maintained by sustaining ATP levels, mitochondrial function, cardiomyocyte contractility, prevention of acidosis, inhibition of induction of apoptosis, maintaining ionontrophy and lusiotrophy by regulating calcium, sodium, potassium and chloride ions.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Nonsymbiotic plant hemoglobins to maintain cell energy status
InactiveUS6936749B1Decrease in levelLower Level RequirementsSugar derivativesBacteriaSubstrate-level phosphorylationProgenitor
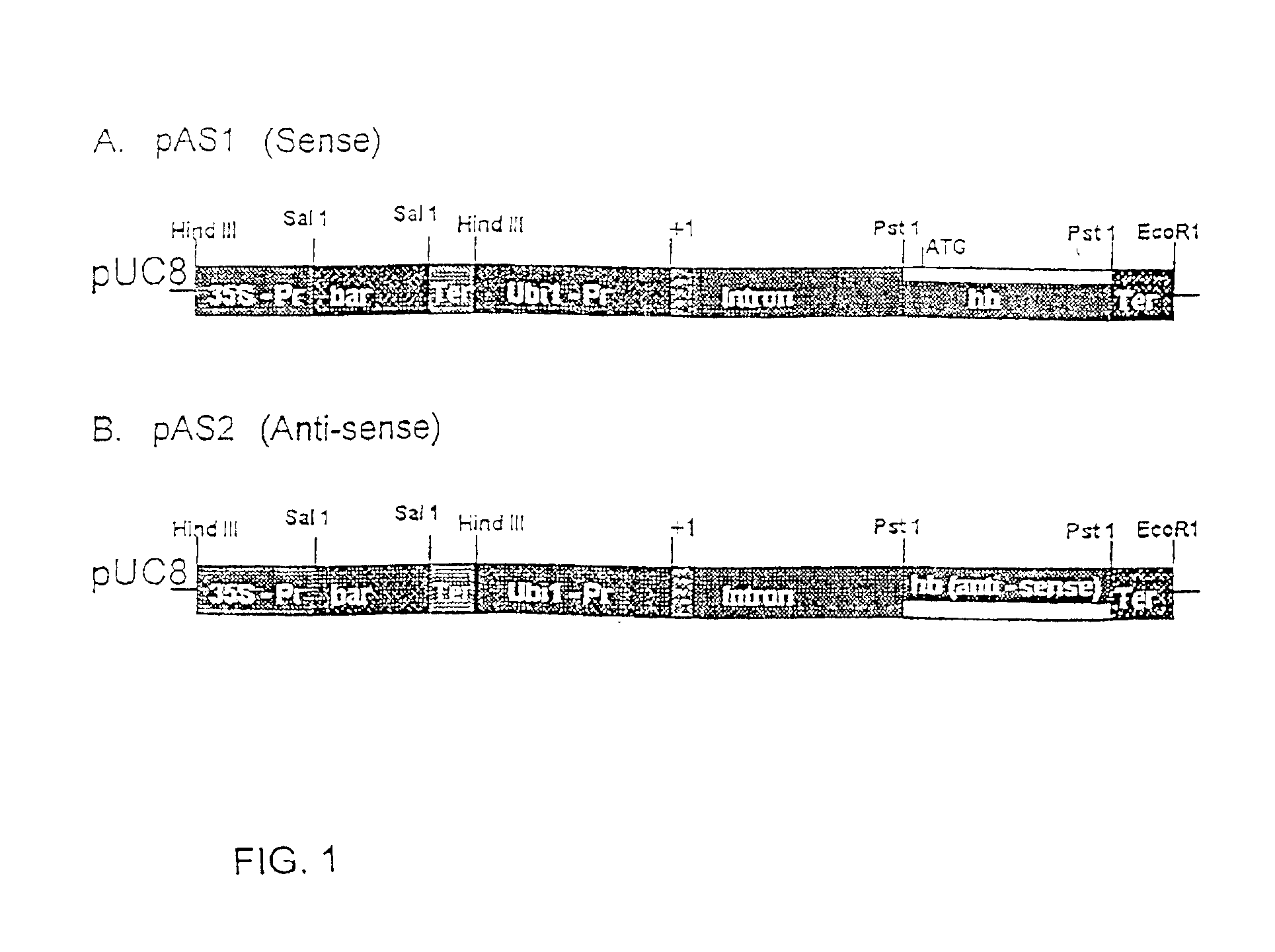
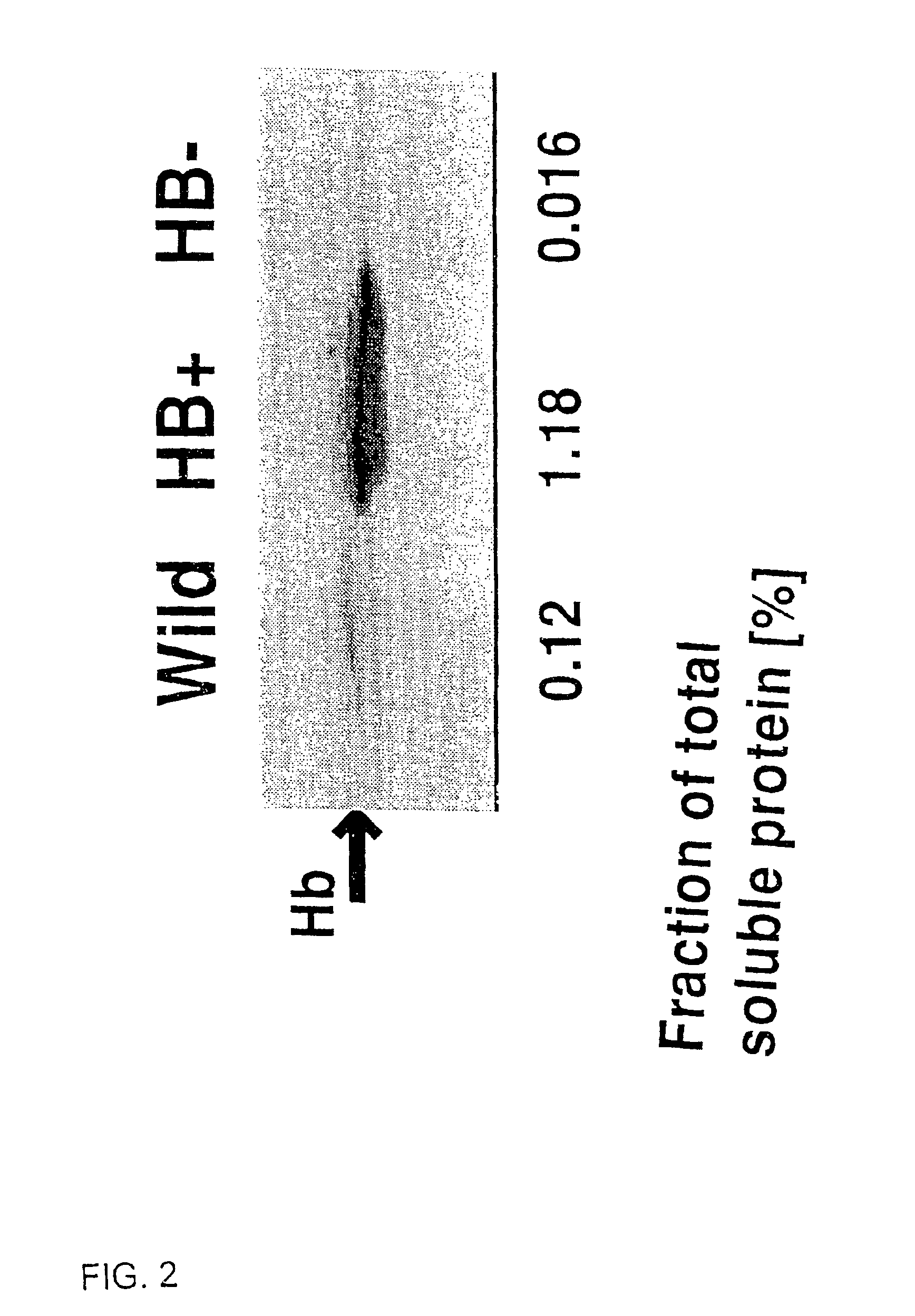
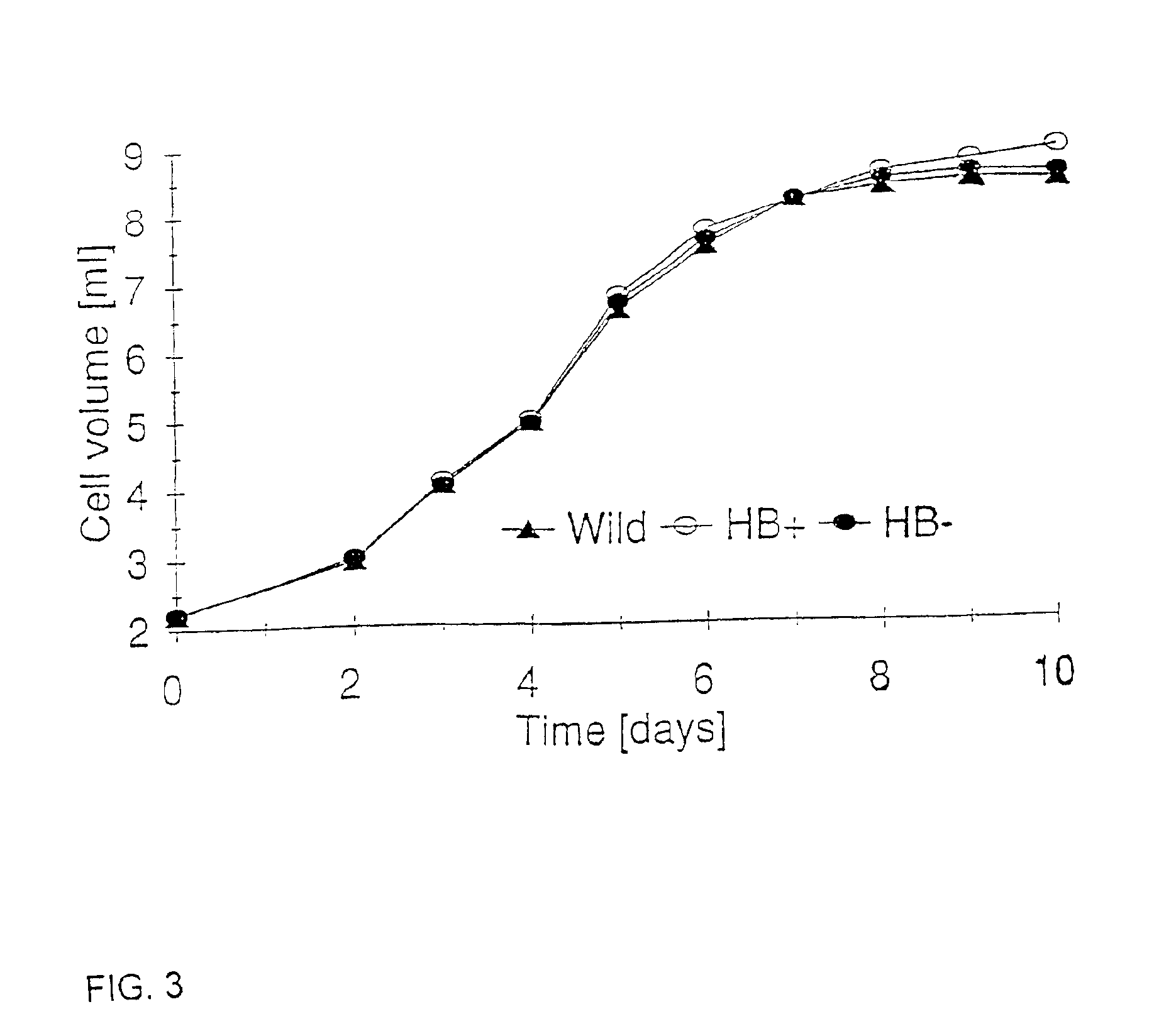
Nonsymbiotic hemoglobins are broadly present across evolution; however, the function of these proteins is unknown. Cultured maize cells have been transformed to constitutively express a barley hemoglobin gene in either the sense (HB+) or antisense (HB−) orientation. Hemoglobin protein in the transformed cell lines was correspondingly higher or lower than in wild type cells under normal atmospheric conditions. Limiting oxygen availability, by placing the cells in a nitrogen atmosphere for 12 hours, had little effect on the energy status of cells constitutively expressing hemoglobin, but had a pronounced effect on both wild type and HB− cells, where ATP levels declined by 27% and 61% respectively. Energy charge was relatively unaffected by the treatment in HB+ and wild type cells, but was reduced from 0.91 to 0.73 in HB− cells suggesting that the latter were incapable of maintaining their energy status under the low oxygen regime. Similar results were observed with P. aeruginosa cells transformed with an Hb expression vector. It is suggested that nonsymbiotic hemoglobins act to maintain the energy status of cells in low oxygen environments and that they accomplish this effect by promoting glycolytic flux through NADH oxidation, resulting in increased substrate level phosphorylation. Nonsymbiotic hemoglobins are likely ancestors of an early form of hemoglobin that sequestered oxygen in low oxygen environments, providing a source of oxygen to oxidize NADH to provide ATP for cell growth and development. This in turn suggests that cells containing increased levels of Hb protein will survive longer under low oxygen tension or high energy demand.
Owner:UNIVERSITY OF MANITOBA
Compositions and methods for tissue preservation
ActiveUS8211628B2Maintain functional integrityRecovery functionMicrobiological testing/measurementDead animal preservationPotassiumApoptosis
Methods and compositions for resuscitating, storing, and preserving functional integrity of organs and tissues. Metabolic function is maintained by sustaining ATP levels, mitochondrial function, cardiomyocyte contractility, prevention of acidosis, inhibition of induction of apoptosis, maintaining ionontrophy and lusiotrophy by regulating calcium, sodium, potassium and chloride ions.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Neuroprotection of retinal ganglion cells
InactiveUS20090220516A1Increase pressureReduce releaseBiocidePeptide/protein ingredientsExtracellularGlaucoma
This invention relates to the neuroprotection of the optic nerve and the treatment of glaucoma, more specifically, the invention is directed to a method of preventing, inhibiting, decreasing incidence and suppressing death in ganglion cells by manipulating the P2X7 and A3 receptors on ganglion cells, by reducing levels of ATP released into the extracellular space of the retina and enhancing the conversion of released extracellular ATP into adenosine.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
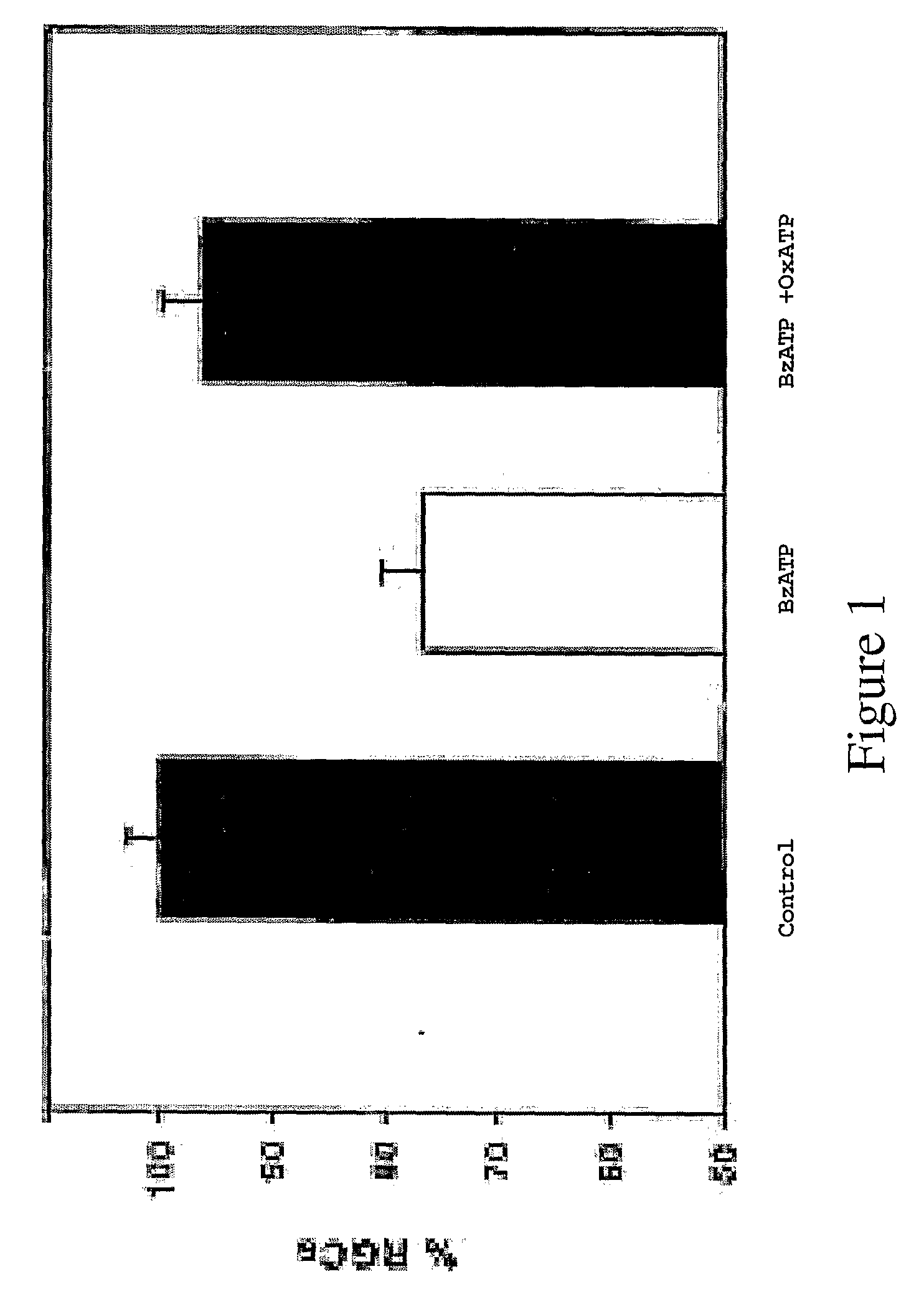
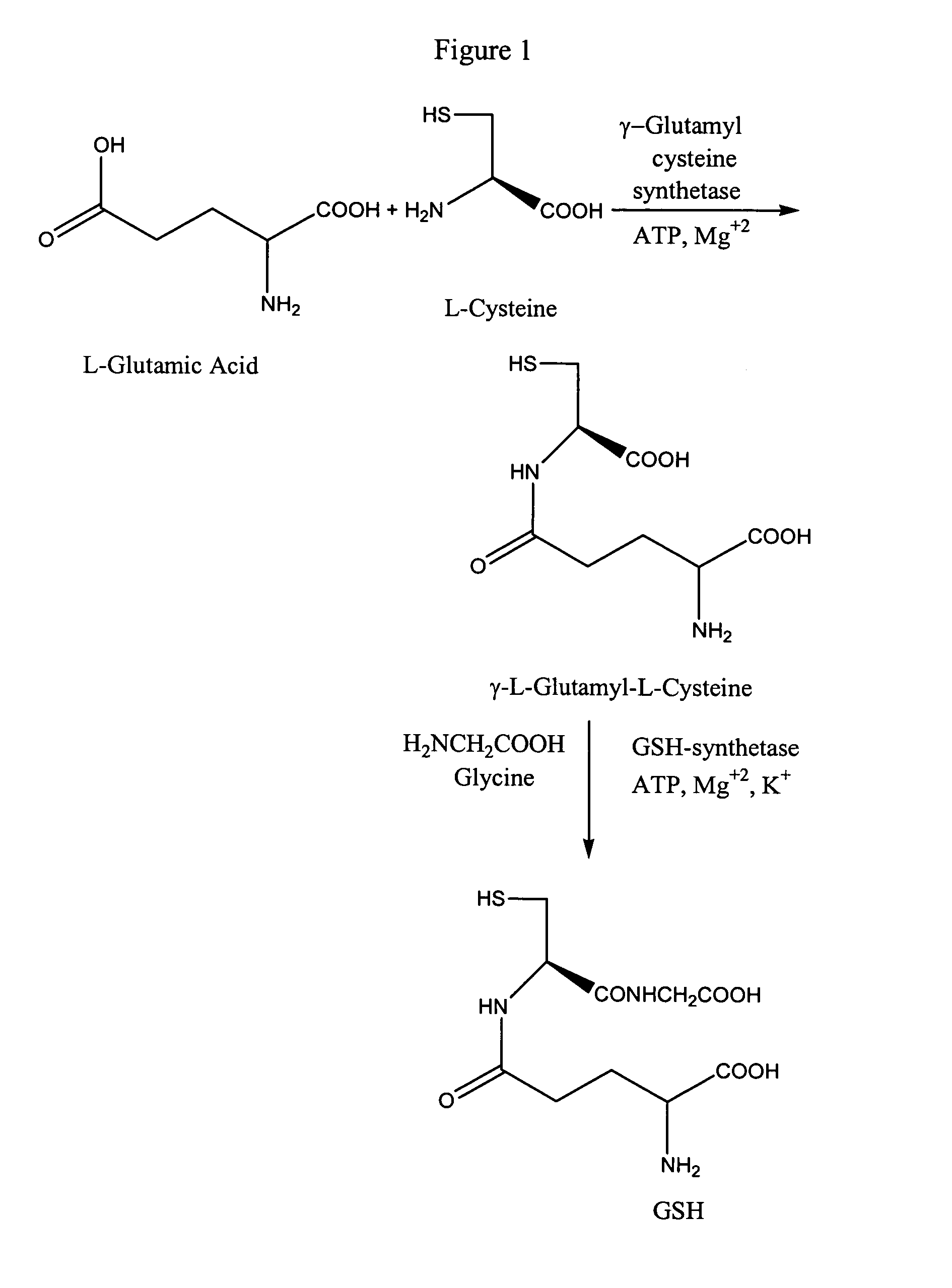
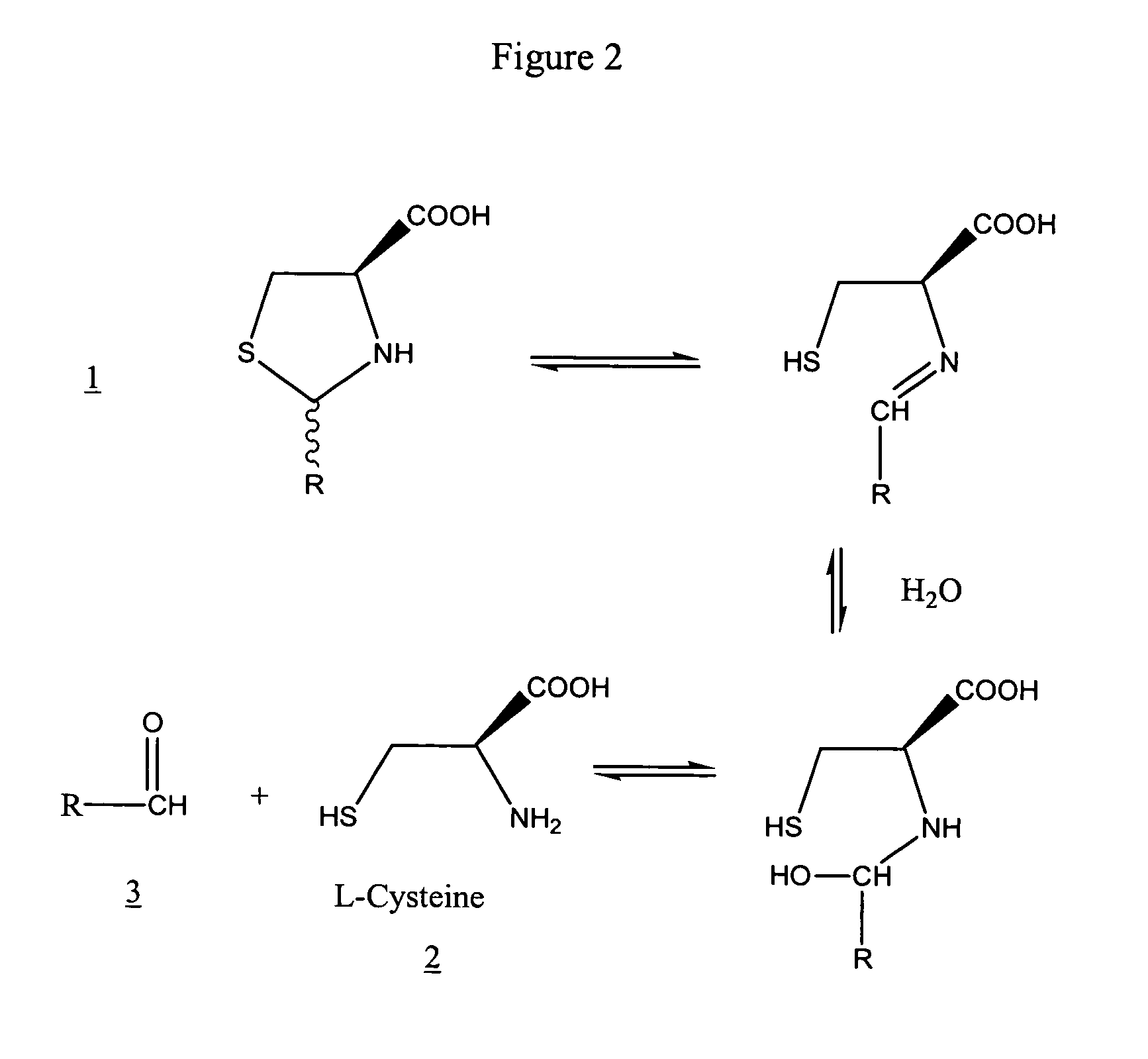
Method to enhance delivery of glutathione and ATP levels in cells
A therapeutic method is provided comprising treating a mammal subject to hypoxia with an amount of 2(R,S)-D-ribo-(1′,2′,3′,4′-tetrahydroxybutyl)thiazolidine-4(R)-carboxylic acid (RibCys) or a pharmaceutically acceptable salt thereof effective to both maintain, restore or increase both the ATP levels and the glutathione (GSH) levels in said tissue.
Owner:MAX INT LLC
Transgenic mice over-expressing amyloid-beta alcohol dehydrogenase (ABAD) in brain as model of alzheimer's disease and uses thereof
InactiveUS6891081B1Prevent and reduce disease progressionRelieve symptomsVectorsOther foreign material introduction processesAmyloid betaA-DNA
The present invention provides for a transgenic non-human animal whose cells contain a recombinant DNA sequence comprising a nerve tissue specific promoter operatively linked to a DNA sequence which encodes amyloid-beta peptide alcohol dehydrogenase (ABAD), and exhibits at least one phenotype from the group consisting of: overexpression of ABAD, elevated levels of basal ATP; protection from metabolic or ischemic stress.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Method to enhance delivery of glutathione and atp levels in cells
Owner:MAX INT LLC
Applications of compound SS-31 in preparing drugs or preparations used for treating atherosclerosis and related diseases
InactiveCN107041946AReduce formationReduce ROS levelsTetrapeptide ingredientsCardiovascular disorderCoronary heart diseaseAtheroma
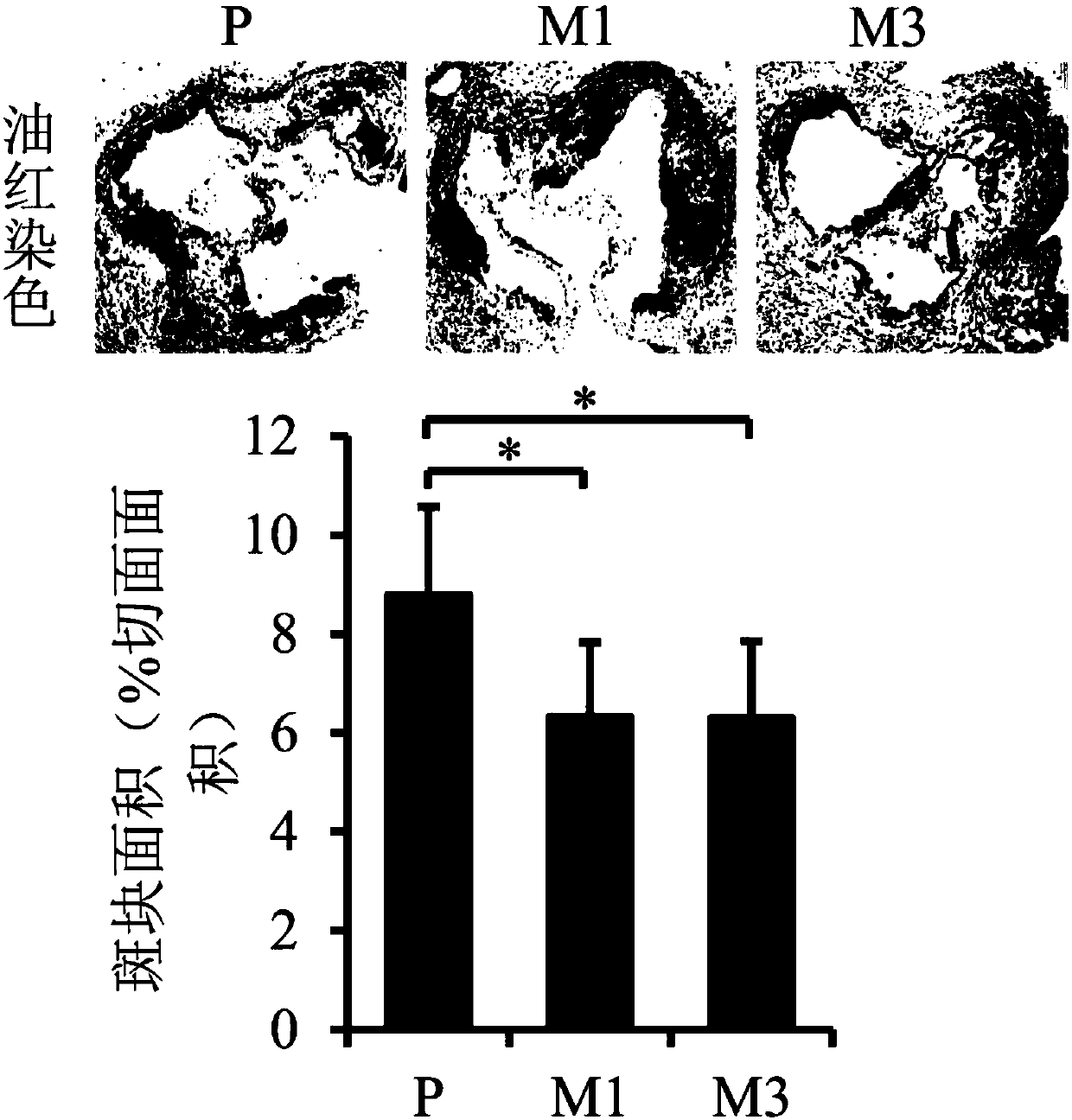
The invention provides drugs used for treating atherosclerotic diseases such as carotid artery stenosis, lower extremity atherosclerotic occlusive disease, and coronary heart disease, and more specifically discloses applications of compound Szeto-Schiller-31 (SS-31) in preparing drugs or preparations used for treating atherosclerosis and related diseases, and belongs to the field of medicine, and more specifically belongs to the field of novel pharmaceutical applications of compounds. It is found via experiments that SS-31 is capable of reducing atherosclerosis degree, reducing mouse arterial ROS level obviously, reducing oxidative damage, increasing ATP level, reducing the content of macrophages at atherosclerotic plaques obviously, increasing the content of smooth muscle cells and the content of collagen at atherosclerotic plaques obviously, stabilizing atherosclerotic plaques, improving inflammation level obviously, and reducing expression of lipid intake protein at atherosclerotic plaques; and it is shown by results that SS-31 is capable of treating atherosclerosis and related diseases.
Owner:NANJING UNIV
Technology for producing glutathione by fermenting saccharomyces cerevisiae
ActiveCN107043797AMitigate the effects of growthPromote accumulationMicroorganism based processesPeptidesBiotechnologyMicroorganism
The invention discloses a technology for producing glutathione by fermenting saccharomyces cerevisiae and belongs to the field of industrial microbial fermentation. The technology for producing glutathione by fermenting saccharomyces cerevisiae comprises the following steps: utilizing an improved O-media fermentation medium to ferment for 16h, and then adding molasses and corn liquor at constant speed of 1.5g / L / h and 0.4g / L / h; after the cell density reaches a certain degree, adding a certain amount of KMnO4 slow-released grains and sodium citrate into the fermentation liquor; fermenting for 120h so that the cell wet weight in the fermentation liquor reaches up to 121.4g / L and the GSH accumulated volume reaches up to 5.78g / L. According to the invention, the cell density of the fermentation liquor is promoted through the improvement for the O-media fermentation medium and the flow adding of the molasses and corn liquor; the oxidative stress for yeast cells is maintained and meanwhile the influence thereof on the yeast growth is relieved through the addition of oxidized stimulant KMnO4 slow-released grains; intracellular ATP level is improved through the addition of the energy auxiliary matter sodium citrate, and finally, the accumulated volume of glutathione is obviously improved and the production cost is lowered.
Owner:盐城仁越生物科技有限公司
Personalized assay for the identification of an effective therapy for cancer
InactiveUS20080113390A1Decreased ATP levelReduce productionMicrobiological testing/measurementMaterial analysisCancer cellAnticarcinogen
Effective anticancer agents for use in treatment of particular cancers can be predicted by testing whether particular anticancer agents are effective in reducing the lactic acid production by sample cells removed from the particular cancerous tissue to be treated. Similarly, effective anticancer agents can be predicted by testing whether particular anticancer agents are effective in reducing ATP levels in sample cells removed from the particular cancerous tissue. A method of predicting anticancer agents that will be effective for use in treating a particular cancer includes the steps of obtaining a plurality of samples of the cancerous tissue to be treated and determining the capacity of a sample to produce lactic acid in both the presence and absence of a particular anticancer agent. Anticancer agents that most reduce the lactic acid production should be effective treatment agents. Similarly, anticancer agents that most reduce the presence of ATP in the sample cancer cells should be effective treatment agents.
Owner:HAN IN SUK +1
Method for improving acid stress resistance of candida glabrata
ActiveCN104789600AImprove growth performanceImprove acid stress resistanceFungiStable introduction of DNAAcetic acidCandida glabrata
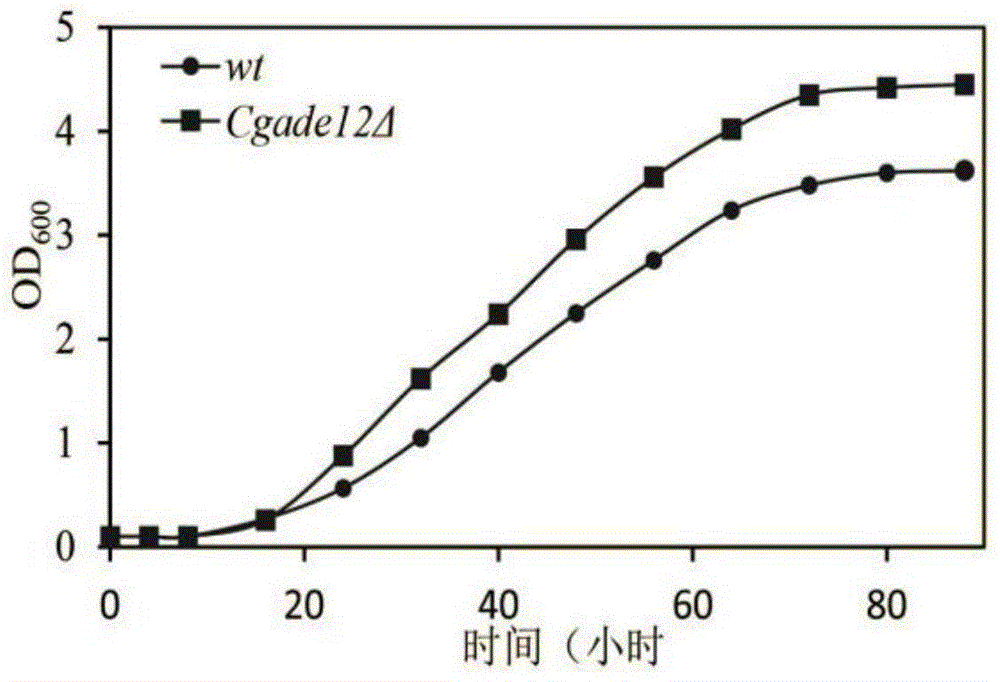
The invention discloses a method for improving the acid stress resistance of candida glabrata, and belongs to the field of bioengineering. The method comprises the following steps: improving the candida glabrata, constructing a gene-deleted mutant strain Cgade12Delta, measuring the intracellular ATP level of the constructed strain, and determining the organic acid tolerance of the strain by a plate growth experiment and cellular activity analysis. After the deletion of the gene CgADE12 is found out, the intracellular ATP level of the strain under the stress of 0.2 percent acetic acid is lowered, the growth capability under the stress of acetic acid is improved, and the acetic acid tolerance is improved.
Owner:JIANGNAN UNIV
Method for Assessing Skin Condition
InactiveUS20120251456A1Microbiological testing/measurementDiagnostic recording/measuringPathologySkin cleansing
A method for evaluating the efficacy of a skin-cleansing composition comprises measuring an ATP level on a skin site after cleansing. A method for evaluating the condition of living skin comprises measuring an ATP level on a skin site. The ATP level may be monitored over time to determine a rate of change in the ATP level on the skin site.
Owner:THE PROCTER & GAMBLE COMPANY
Method for preparing hydrogen gas
InactiveCN101294167AEasy to understandMicroorganism based processesFermentationBiotechnologyPhosphate ion
The invention discloses a method for producing hydrogen by culturing hydrogen-producing microbes in a culture medium containing phosphate oils. Phosphate is added in a cellular environment to simultaneously promote rapid growth and rapid recovery of ATP, thus controlling the ATP level and oxidization-reduction potential in cells, and accelerating consumption of carbon source and the production efficiency and hydrogen. Additionally, the phosphate can be recycled in the culture system without contaminating the environment. By using the method of the invention, the hydrogen production rate is improved by more than three times, the sugar utilization rate is improved by five times, and the hydrogen transformation efficiency is remarkably improved.
Owner:TSINGHUA UNIV
Transcription factors in neuronal dendrites-dendritic protein synthesis and cell death
ActiveUS20090227531A1Improve survivabilityLower Level RequirementsBiocidePeptide/protein ingredientsMitochondrial membrane permeability transitionDendrite
The present invention relates to methods of altering the competence of a dendrite and / or the viability of a neuron. The present invention also provides methods of altering the ATP levels in a neuron, methods of isolating at least one protein of a mitochondrial permeability transition pore complex, methods of introducing an RNA into a neuron, methods of translating an RNA in a dendrite, methods of monitoring risk of neurodegeneration of a neuron, and methods of treatment for neurodegenerative diseases.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
A method for improving the quality and efficiency of in vitro maturation of an oocyte
PendingCN109182253AQuality improvementImprove efficiencyCell culture active agentsGerm cellsMitophagyBiology
The invention provides a method for improving the quality and efficiency of in vitro maturation of an oocyte, which includes the step of changing an in vitro maturation fluid at least once during in vitro maturation, and further comprising adding an amount of FGF to the in vitro maturation fluid. The oocyte prepared by the method of the invention has remarkably improved nuclear maturation efficiency and cytoplasmic maturation efficiency, the MII oocyte is up to 95%, the proportion of oocytes with normal distribution of cortical granules and mitochondria, the levels of glutathione and ATP are significantly higher than those of the normal maturation group; Adding a certain amount of FGF to IVM can further improve the maturation quality and efficiency of oocytes. After in vitro fertilization,the in vitro fertilization efficiency of the oocytes prepared by the method of the invention is significantly higher than that of the normal maturation group, which is embodied in higher cleavage rate, blastocyst rate and more blastocyst cells, and has great application value.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Pharmaceutical and/or cosmetic composition containing an active principle activator of cytochrome C
ActiveUS8304392B2High expressionIncrease ATP synthesisCosmetic preparationsNervous disorderBULK ACTIVE INGREDIENTMitophagy
A peptide of formula: R1-X1-X2-X3-X4-X5-R2, wherein X1 is T; S2 is L; X3 is K; X4 is T, S, K, R or no amino acid; X5 is V, A, Y, M or no amino acid; R1 represents a primary amine function of the N-terminal amino acid; and R2 represents a hydroxyl function of the C-terminal amino acid, is capable of activating cytochrome C. The peptide can be included in cosmetic and pharmaceutical compositions intended to stimulate the functions of the mitochondria and increase intracellular ATP levels. Such compositions are useful to help protect the skin from external aggression, reduce and treat skin damage caused by UV radiation and combat cutaneous signs of aging.
Owner:ISP INVESTMENTS LLC
Improvement of ethanol yield with reduction of biomass accumulation in the recombinant strain of saccharomyces cerevisiae overexpressing alkaline phosphate
Described herein is a method to increase ethanol yield during alcoholic fermentation by expression a truncated versions of the Saccharomyces cerevisiae PHO8 gene coding for vacuolar or cytosolic form of alkaline phosphatase, which expression lowers biomass accumulation while diverting greater carbon to ethanol production. Also described are as nucleic acid sequences and vectors for expression of the PHO8 gene and strains carrying the same. Strains containing intact PHO8 gene encoding vacuolar form of alkaline phosphatase, had slightly lower intracellular ATP levels with insignificant changes in biomass accumulation and up to 13% increase in ethanol production.
Owner:ARCHER DANIELS MIDLAND CO
Method for testing the severtiy of an illness
InactiveUS20130071866A1Accurately deducedHigh risk of mortalityMicrobiological testing/measurementDisease diagnosisSeverity levelKetone
An object of the present invention is directed to a method for assaying the severity of an illness in real time and is to provide a testing method capable of assessing the severity of an illness in more detail than the conventional APACHE II and SOFA scores. The established method can accurately measure an ATP level in a sample, thereby accurately and quickly deducing the “state of intracellular energy required for living organisms” from the ATP level, and by extension, determining the severity of an illness. The present invention further provides a novel biomarker ATP-lactate energy risk score (A-LES) value that is capable of determining the severity of an illness by the reevaluation, with the ATP concentration as an index (specifically, on the basis of a lactic acid level (mM) / ATP concentration (mM) ratio), of the level of lactic acid that accumulates in the sample due to the breakdown of in vivo metabolic balance accompanied by the increased severity of the illness. The present invention also provides a novel biomarker ATP-ketone energy risk score (A-KES) value that is capable of determining the severity of an illness by the reevaluation of a ketone body level in the sample with the ATP concentration as an index (specifically, on the basis of a ketone body level (mM) / ATP concentration (mM) ratio).
Owner:UNIVERSITY OF TOKUSHIMA
Compositions and methods for tissue preservation at ambient or subnormothermic temperatures
InactiveCN108366552AReduce incidenceImprove overall lifespanOrganic active ingredientsPeptide/protein ingredientsPotassiumFunctional integrity
Provided herein are methods and compositions for resuscitating, storing, and preserving the functional integrity of organs and tissues at ambient temperatures for up to 24 hours. Metabolic function ismaintained by sustaining ATP levels, mitochondrial function, prevention of edema, and by regulating calcium, sodium, potassium, magnesium, and chloride ions.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Use of composition in prevention or treatment of neurodegenerative diseases
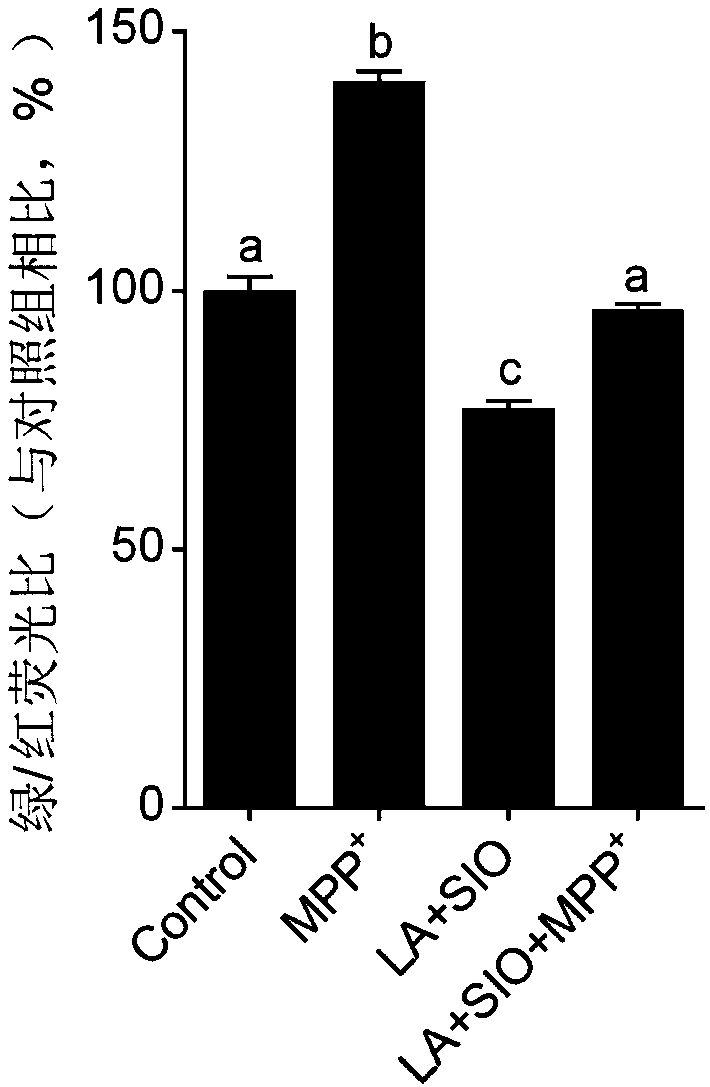
ActiveCN111467385AImprove survival rateReduce apoptosisOrganic active ingredientsNervous disorderPharmaceutical drugMitochondrion membrane
The invention provides a pharmaceutical composition and application of the composition in prevention or treatment of neurodegenerative diseases. The pharmaceutical composition comprises sacha inchi oil and / or lipoic acid, and is used for at least one of the following functions: preventing and / or treating neurodegenerative diseases, increasing the survival rate of nerve cells, reducing apoptosis ofnerve cells, increasing the mitochondrial membrane potential of nerve cells, protecting mitochondrial functions and increasing the ATP level in nerve cells. The pharmaceutical composition is good ineffect of preventing and / or treating neurodegenerative diseases, is small in side effect and is capable of effectively increasing the survival rate of nerve cells, effectively protecting mitochondrialmembrane potential of the nerve cells and effectively protecting the energy metabolism level of the nerve cells.
Owner:BGI INST OF APPLIED AGRI
Preparation method of compound PGHG in medicine for treating parkinson's disease
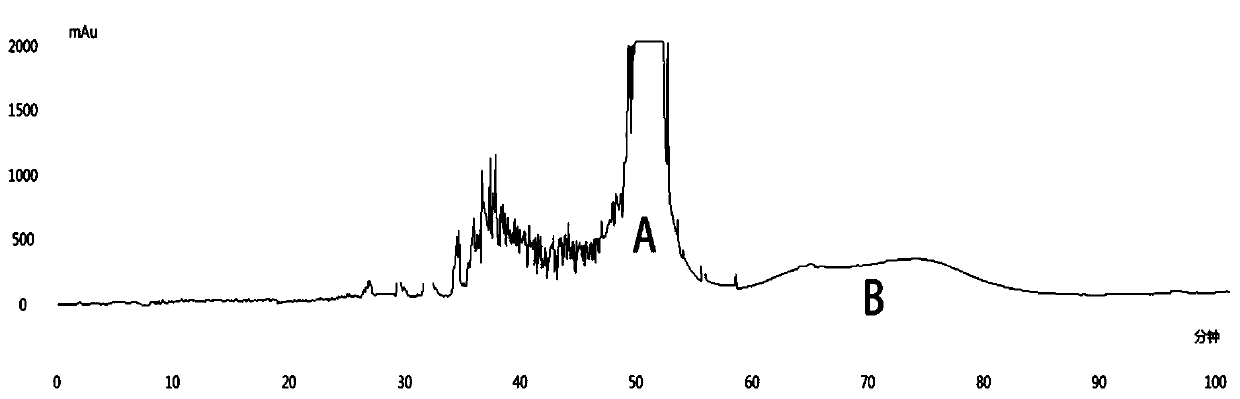
ActiveCN110229204AIncrease vitalityLower levelNervous disorderSugar derivativesEthyl acetatePolyphenol
The invention discloses a preparation method of a compound PGHG in a medicine for treating parkinson's disease. The method comprises the following steps: performing ultrasonic extraction on Penthorumchinense Pursh. stem dry powder by using 70 percent of ethanol (1: 25), sequentially performing extraction by using petroleum ether and ethyl acetate to obtain extracting solutions; performing decompressing concentration and lyophilization on the extracting solutions to obtain Penthorum chinense Pursh. polyphenol fragments (PSE); separating the PSE through high-speed countercurrent chromatographyto obtain flow fractions A and B; further purifying the flow fraction A by adopting a preparative HPLC (High Performance Liquid Chromatography); manually collecting effluent liquid according to a chromatographic peak; performing decompressing concentration to obtain a monomer compound of which the purity is up to 99 percent; and the monomer compound is identified as PGHG through mass spectrometryand nuclear magnetic resonance spectroscopy. Due to the PSE and the PGHG, reduction of ATP level of 6-hydroxydopamine induced SH-SY5Y cells can be obviously suppressed, and the cell activity is improved. The PGHG can modulate and control expression of apoptosis protein and inflammatory protein through activating Nrf2 / HO-1 antioxidant pathway, and neuroprotection has an obvious effect on preventionand treatment of the parkinson's disease.
Owner:SICHUAN UNIV
Treatment of cancer by reduction of intracellular energy and pyrimidines
This invention provides a method for treating a cancer subject comprising administering to the subject a combination of ATP-depleting agents at concentrations which deplete the ATP level to at least 15% of normal in cancer cells, a pyrimidine antagonist, and an anticancer agent to which the treated cancer is sensitive. This invention also provides a composition comprising a combination of ATP-depleting agents at concentrations which deplete the ATP level to at least 15% of normal in cancer cells, a pyrimidine antagonist, and an anticancer agent to which the treated cancer is sensitive. Finally this invention provides a pharmaceutical composition comprising the above composition or a combination thereof and a pharmaceutically acceptable carrier.
Owner:SLOAN KETTERING INST FOR CANCER RES
In-vivo energy depleting strategies for killing drug-resistant cancer cells
This invention also provides a method for treating a cancer subject comprising administering to the subject a combination of ATP-depleting agents at concentrations which deplete the ATP level to, or close to, at least 15% of normal in cancer cells wherein at least one of the ATP-depleting agents is a mitochondrial ATP-inhibitor, a methylthioadenosine phosphorylase inhibitor or an inhibitor of De Novo purine synthesis other than 6-Methylmercaptopurine riboside, wherein said composition produces a substantially better effect than a composition without at least one of the ATP-depleting agents: a mitochondrial ATP-inhibitor, a glycolytic inhibitor, a methylthioadenosine phosphorylase inhibitor and an inhibitor of De Novo purine synthesis other than 6-Methylmercaptopurine riboside.
Owner:SLOAN KETTERING INST FOR CANCER RES
Preparation method of ganoderma spore composition for treating Alzheimer disease
InactiveCN110393729ASimple production processEasy to operateNervous disorderPlant ingredientsSporeAlzheimer's disease
The invention belongs to the technical field of ganoderma spore compositions, and discloses a preparation method of a ganoderma spore composition for treating an Alzheimer disease. The preparation method comprises the following steps of picking fresh ganoderma spores, removing impurities and no-medicinal parts of the fresh ganoderma spores, the cleaning the fresh ganoderma spores, removing dust onthe upper parts of the ganoderma spores, and drying the fresh ganoderma spores; putting the dried ganoderma spores into a low temperature supersonic speed airflow energy grender to conduct grinding treatment, and ganoderma spore powder is formed; and taking the dried ganoderma spore powder, and through a 120 mesh screen, removing impurities. According to the ganoderma spore composition, the production technology is simple, operation is convenient, the ROS levels in blood platelets of an AD patient can be reduced by taking the ganoderma spore composition, plasma Abeta42 of the AD patient can be stabilized at a certain level, influences on the ATP levels of the blood platelets, mitochondrion COX activity of the blood platelets and the LEH level of the plasma of the AD patient are not generated, and swelling of mitochondrial of the blood platelets of the AD patient can be relaxed certainly.
Owner:钟志强
Application of rhodotorula glutinis deacetylase protein 2 for improving mitochondrial functions
ActiveCN110879295AImprove the ability to resist agingEasy to shapePeptide/protein ingredientsAntinoxious agentsCell phenotypeProtein DRP1
The invention discloses application of sirtuin2 protein for improving mitochondrial functions and an action mechanism, which belong to the technical field of biology. The rhodotorula glutinis sirtuin2gene with the functions of reconstructing a mitochondrial network and improving the phenotype of mammalian senescent cells is recombined and expressed in mammalian cells. Experimental results show that the expression levels of fusion proteins Mfn1, Mfn2 and the division protein Drp1 can be improved, the SA-beta-gal activity level of mammalian cells is reduced, the expression level of CK19 and beta1 integrin is enhanced, the intracellular ROS level is reduced, the intracellular ATP level is obviously improved, the mitochondrial morphology is improved, the mitochondrial network is improved, theanti-aging capability of the cells is improved, and a new strategy is provided for treating mitochondrial dysfunction and preventing aging by using the sirtuin2 protein.
Owner:广州弘润生物科技有限公司
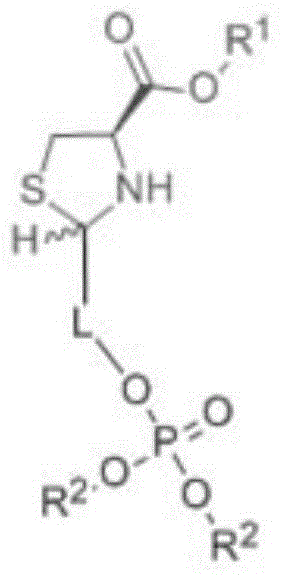
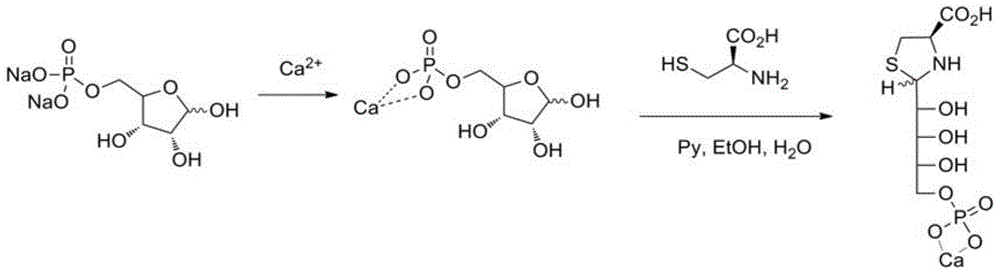
Sugar alcohol phosphate thiazolidine-4-carboxy compound and application thereof
InactiveCN104059107AImprove anti-inflammatory and detoxification abilityIncrease ATP levelsOrganic active ingredientsAntipyreticPhosphatePhosphoric acid
The invention relates to a sugar alcohol phosphate thiazolidine-4-carboxy compound with the structural formula as the specification. The compound is formed by integrating three groups and can release cysteine and ribose phosphate after being absorbed in vivo. Cysteine can be used for increasing the concentration of glutathione (GSH) in cells, improving the oxidation resistance of the cells and improving the inflammation diminishing and detoxifying capabilities of a human body from inside to outside. Ribose phosphate can be used for replenishing the energy loss caused by wounds or strenuous exercise while rapidly and effectively improving the ATP (Adenosine Triphosphate) level in tissues. Bodybuilding and brain-strengthening effects can be synchronously realized through the combination of carboxylate and phosphate groups and different trace elements.
Owner:魏景芬
Method for uncoupling yield and productivity of non-catabolic compounds produced by host cells
The present invention provides compositions and methods for uncoupling the yield and productivity of an isoprenoid compound produced in a host cell. In some embodiments, yield and productivity are uncoupled by genetically modifying host cells to reduce flux through citric acid circulation (TCA). In other embodiments, yield and productivity are uncoupled by reducing ATP levels in host cells.
Owner:AMYRIS INC
Applications of inhibition of Dcf1 gene expression in preparing drugs used for treating obesity
ActiveCN106913875ALose weightCompounds screening/testingMetabolism disorderEnergy balancingWild type
The invention relates to applications of inhibition of Dcf1 gene expression in preparing drugs used for treating obesity. According to the invention, behavior tests of wild type mice (as a reference) and dcf1 knock-out mice are carried out, it is detected that the weight and food intake of mice are reduced because of dcf1 deletion, and NPY protein down-regulation is detected in Westernblotting and immunohistochemical detection, so that it is confirmed that dcf1 is at the upstream of NPY. ATP kit is used for detecting that after dcf1 knockout, mice hypothalamus tissue and primary culture cell ATP level increases. Expression of dcf1 and expression of NPY in 293T cells are both capable of reducing cell ATP level, so that dcf1 possesses an important effect in energy balance, and a novel method used for treating obesity is provided.
Owner:SHANGHAI UNIV
Ethanol yield with reduction of biomass accumulation in the recombinant strain of Saccharomyces cerevisiae overexpressing alkaline phosphate
Described herein is a method to increase ethanol yield during alcoholic fermentation by expression a truncated versions of the Saccharomyces cerevisiae PHO8 gene coding for vacuolar or cytosolic form of alkaline phosphatase, which expression lowers biomass accumulation while diverting greater carbon to ethanol production. Also described are as nucleic acid sequences and vectors for expression of the PHO8 gene and strains carrying the same. Strains containing intact PHO8 gene encoding vacuolar form of alkaline phosphatase, had slightly lower intracellular ATP levels with insignificant changes in biomass accumulation and up to 13% increase in ethanol production.
Owner:ARCHER DANIELS MIDLAND CO
A kind of preparation method of phosphoric sugar alcohol tetrahydrothiazole-4-carboxylic acid compound
The invention relates to a preparation method of a sugar alcohol phosphate thiazolidine-4-carboxy compound with the structural formula as the specification. The compound is formed by integrating three groups and can release cysteine and ribose phosphate after being absorbed in vivo. Cysteine can be used for increasing the concentration of glutathione (GSH) in cells, improving the oxidation resistance of the cells and improving the inflammation diminishing and detoxifying capabilities of a human body from inside to outside. Ribose phosphate can be used for replenishing the energy loss caused by wounds or strenuous exercise while rapidly and effectively improving the ATP (Adenosine Triphosphate) level in tissues. Bodybuilding and brain-strengthening effects can be synchronously realized through the combination of carboxylate and phosphate groups and different trace elements.
Owner:魏景芬
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com