Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
190 results about "Ethyl chloroformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethyl chloroformate is the ethyl ester of chloroformic acid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group and for the formation of carboxylic anhydrides.
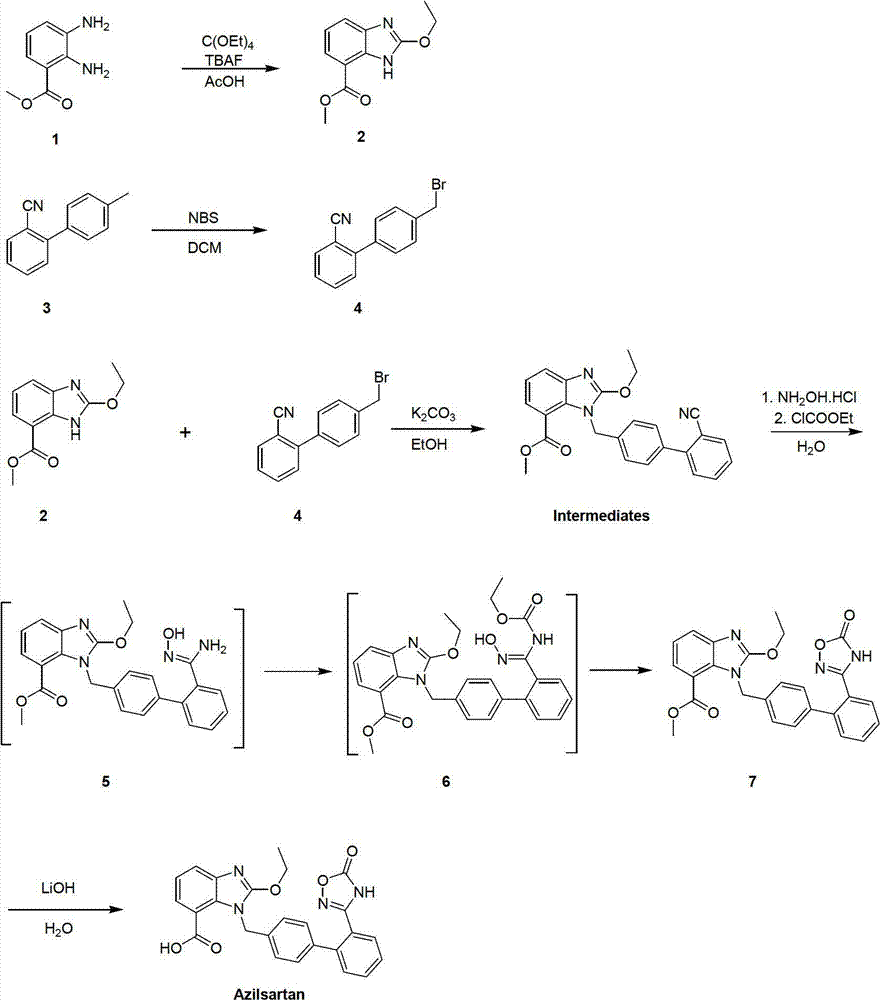
Preparation method of azilsartan
InactiveCN102766138AReduce usageEmission reductionOrganic chemistryEthyl chloroformateCarboxylic salt
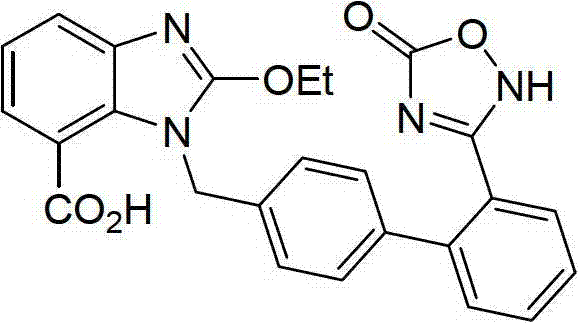
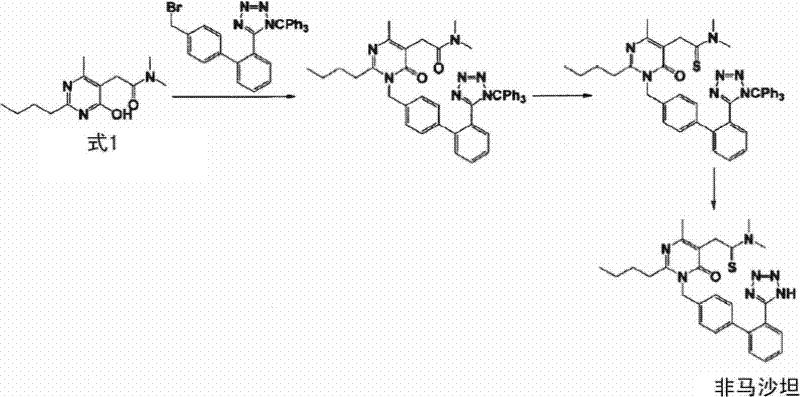
The invention relates to a preparation method of azilsartan, comprising the following steps of: (1) preparing ethoxybenzimidazole-7-methyl carboxylate; (2) preparing 2-cyan-4'-bromomethyl biphenyl; (3) dissolving the ethoxybenzimidazole-7-methyl carboxylate and the 2-cyan-4'-bromomethyl biphenyl into ethanol; adding potassium carbonate to react to obtain 1-[(2'-cyan diphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-methyl carboxylate; (4) suspending the 1-[(2'-cyan diphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-methyl carboxylate in water; adding hydroxylamine hydrochloride, sodium hydroxide and tetrabutylammonium fluoride; heating and reflowing, and then cooling; adding the sodium hydroxide and ethyl chloroformate, heating and reflowing to obtain azilsartan methyl ester; and (5) hydrolyzing the azilsartan methyl ester to obtain a product. The preparation method of the azilsartan, disclosed by the invention, has the advantages of being short in process route, high in yield, and safe and reliable; and the purity of the azilsartan obtained by using the method is high.
Owner:WENZHOU PEOPLES HOSPITAL
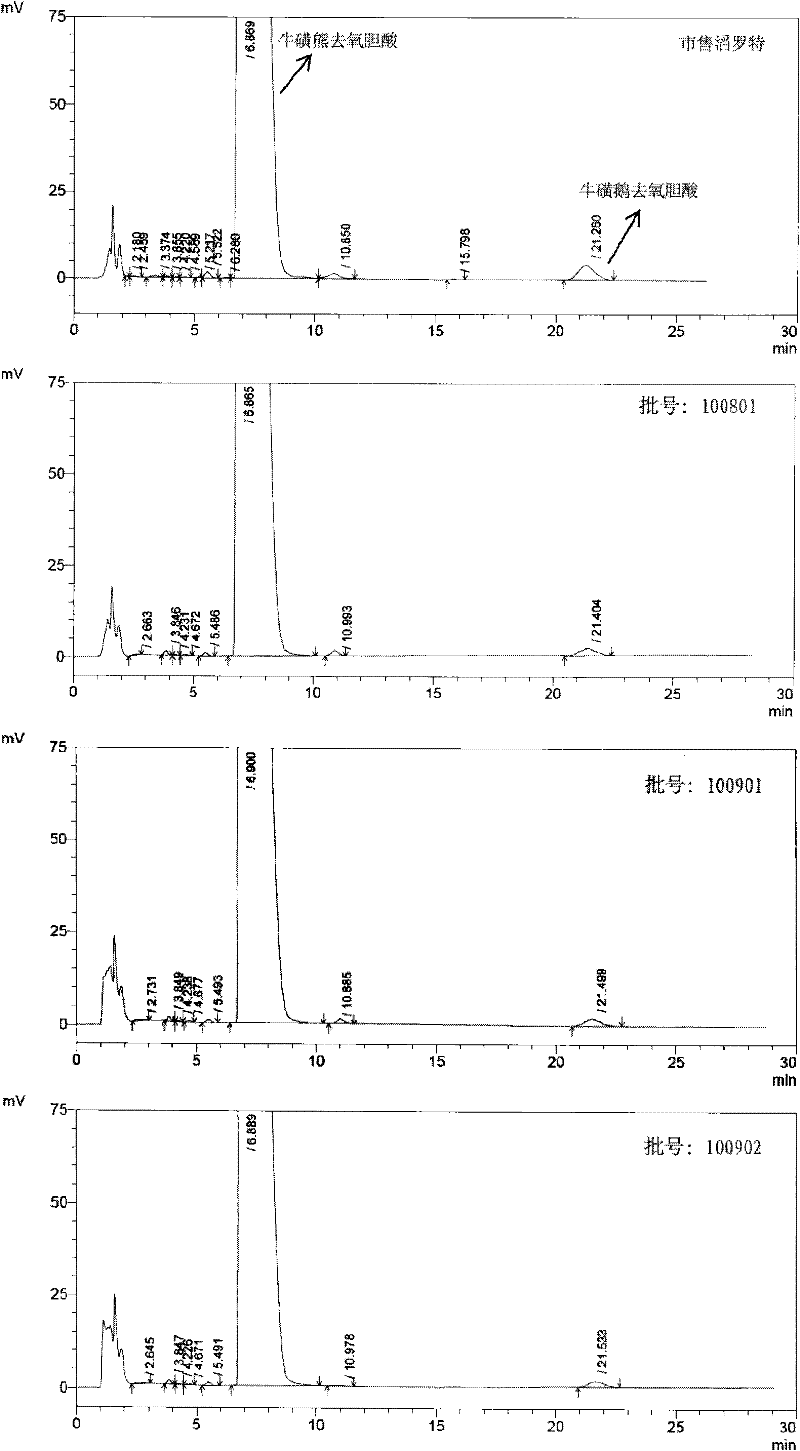
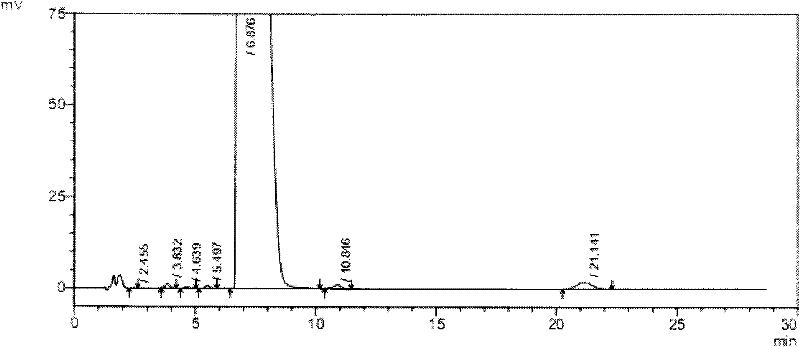
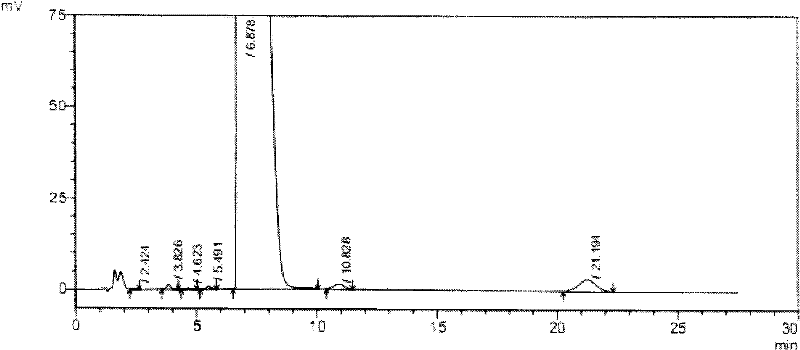
High-purity tauro ursodesoxy cholic acid and preparation method thereof
ActiveCN102477059AHigh purityImprove securityOrganic active ingredientsDigestive systemEthyl chloroformateCholic acid
The invention relates to high-purity tauro ursodesoxy cholic acid and a preparation method thereof. The content of taurochenodeoxycholic acid in the tauro ursodesoxy cholic acid is less than 0.7%. The tauro ursodesoxy cholic acid is safe and effective and does not have toxic and side effects in clinical application. The invention further provides a mixed anhydride reaction of ursodesoxycholic acid and ethyl chloroformate by taking acetone as a solvent. By means of control of a reaction condition and a reaction solvent, the tauro ursodesoxy cholic acid has the advantages of simple process, low cost, environmental friendlessness and industrial production; furthermore, the high-purity tauro ursodesoxy cholic acid can be obtained.
Owner:CHENGDU GUOHONG PHARMA
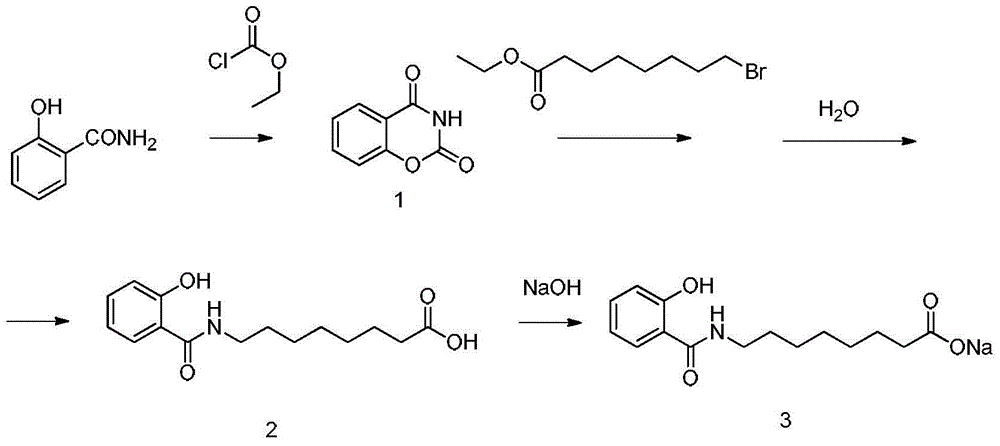
Method for preparing sodium, 8-(2-hydroxybenzamido)octanoate
InactiveCN104974060AGood choiceHigh yieldOrganic compound preparationCarboxylic acid amides preparationEthyl chloroformateOctanoic Acids
The invention discloses a method for preparing sodium, 8-(2-hydroxybenzamido)octanoate which is a drug intermediate. The method comprises the following steps of reacting salicylamide, which serves as a raw material, with ethyl chloroformate to produce an intermediate 2H-benzo[e][1,3]oxazine-2,4(3H)-dione, reacting the intermediate with 8-bromooctanoic acid ethyl ester, carrying out hydrolysis so as to obtain 8-(2-hydroxybenzamido)octanoic acid, and then, reacting 8-(2-hydroxybenzamido)octanoic acid with sodium hydroxide so as to obtain the final product, namely sodium, 8-(2-hydroxybenzamido)octanoate. According to the method, the raw material reacts with ethyl chloroformate to produce the intermediate 2H-benzo[e][1,3]oxazin-2,4(3H)-dione, so that the yield of the reaction is greatly increased, the selectivity of reaction is greatly improved, the production cost is reduced, the production of byproducts is lowered, industrial production is facilitated; and the method is environment-friendly.
Owner:CHEMSOON CO LTD
Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate
ActiveCN108689876AReduce usageLow toxicityOrganic compound preparationCarboxylic acid amide separation/purificationEthyl chloroformateCarbonyldiimidazole
The invention discloses a preparation method of a pharmaceutical intermediate, i.e., sodium 8-[(2-hydroxybenzoyl) amino] octanoate. The preparation method comprises the steps of enabling salicylamide,used as a raw material, to react with N' N-carbonyl diimidazole so as to generate an intermediate, i.e., 2H-benzo[e][1,3]oxazine-2, 4 (3H)-dione; enabling the intermediate to react with 8-ethyl bromooctanoate to obtain 8-(2, 4-dicarbonyl-2H-benzo[e][1, 3]oxazine-3(4H)-yl) ethyl caprylate; hydrolyzing by using sodium hydroxide, and then acidizing to obtain 8-[(2-hydroxybenzoyl) amino] caprylic acid; then, enabling the 8-[(2-hydroxybenzoyl) amino] caprylic acid to react with sodium hydroxide to obtain the final product, i.e., the sodium 8-[(2-hydroxybenzoyl) amino] octanoate. The preparation method avoids the use of a genotoxic raw material-ethyl chloroformate, is low in reaction energy consumption, less in by-products and high in yield, greatly lowers the production cost, and is simple inprocess and suitable for industrial production.
Owner:江苏东南纳米材料有限公司
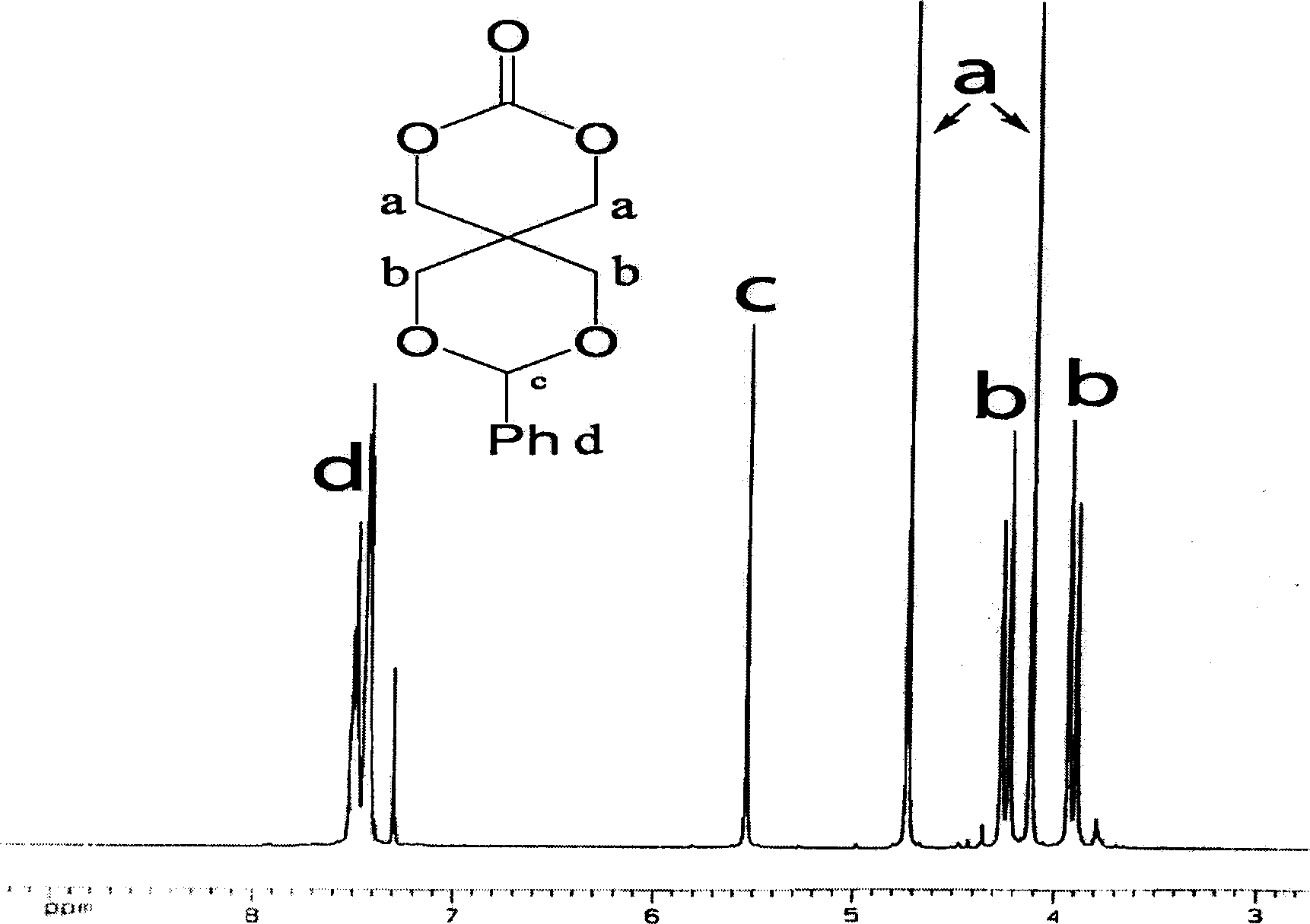
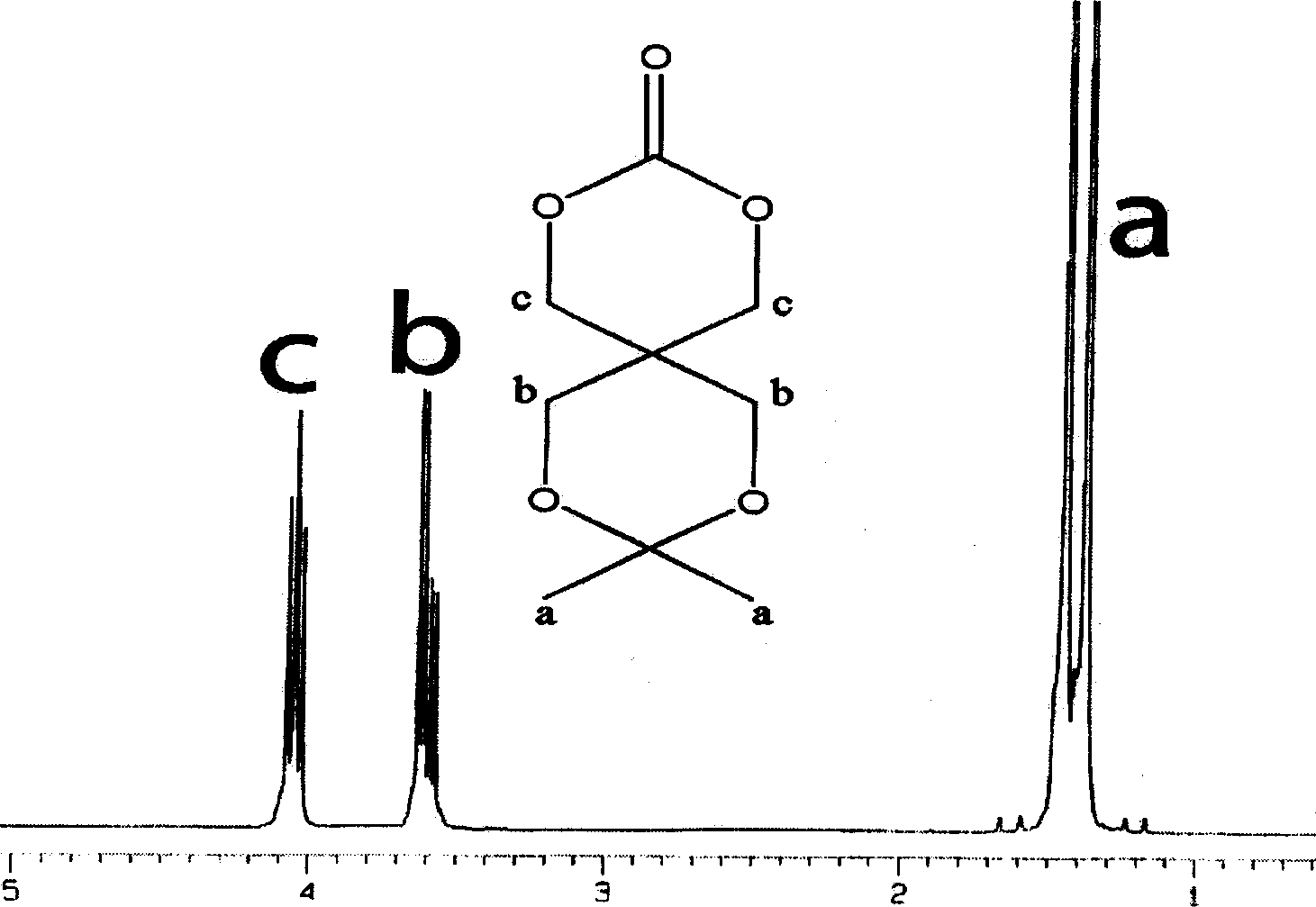
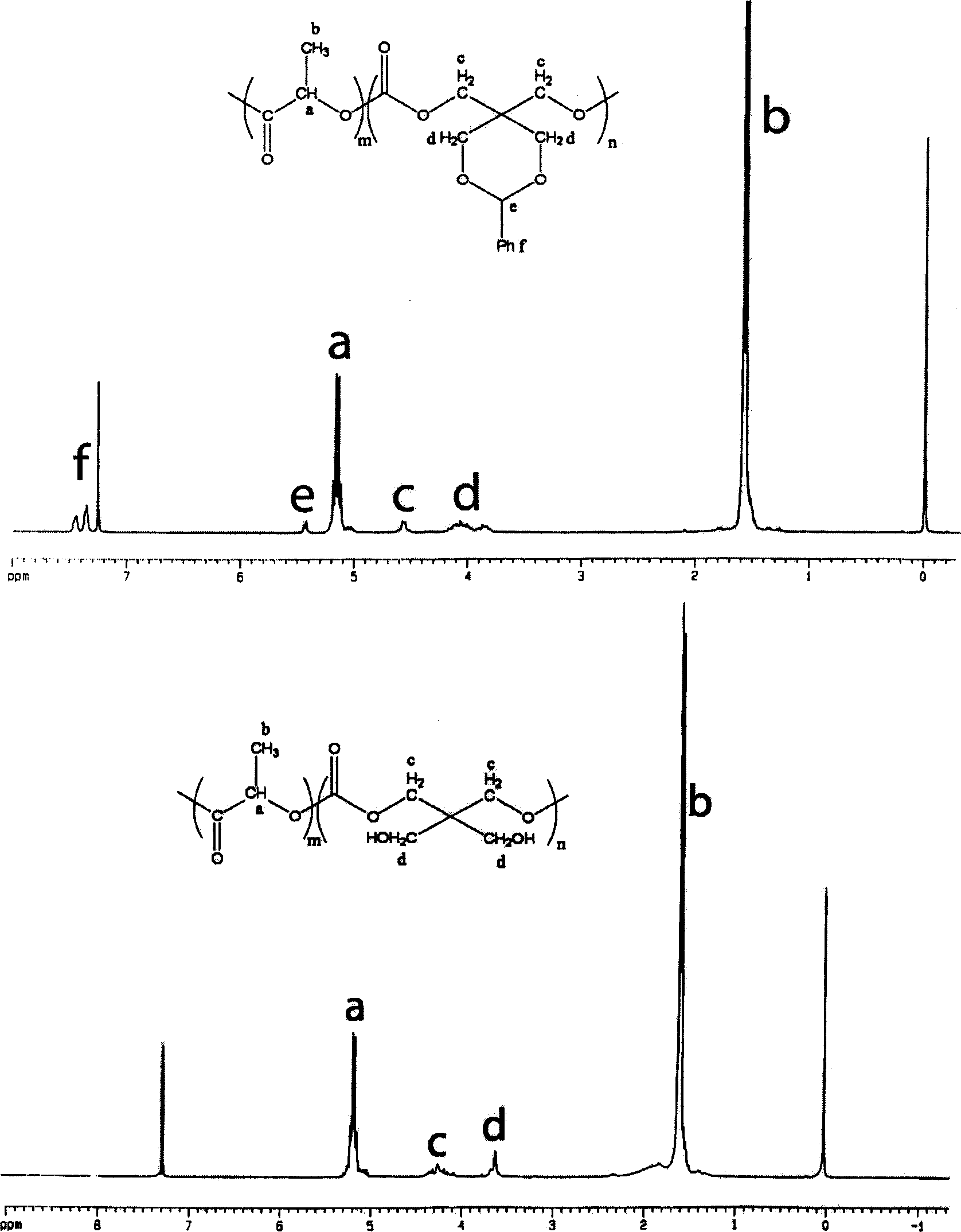
Cyclic aliphatic acid ester carbonate, its polymer, synthesis method and uses thereof
The provided preparation methdo for cyclic aliphatic carbonate comprises: protecting two hydroxyl groups of the pentaerythritol to let another couple of hydroxyl react with ethyl chloroformate; further, ring-opening polymerizing or copolymerizing with aliphatic cyclic ester, removing the protection, and obtaining the product. This cyclic esters is fit to biodegradation without toxin, can connect the drug and active short peptide to improve the polymer bio- compatibility and bio-compatibility, and has wide application.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of zopiclone
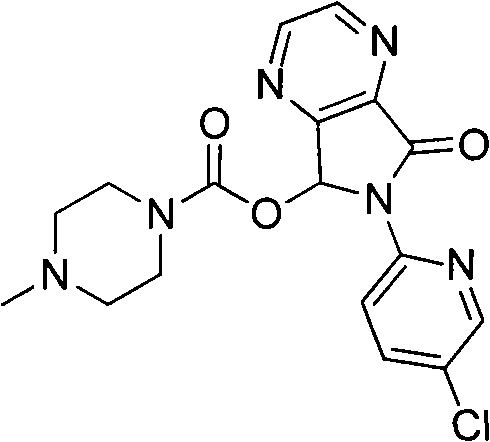
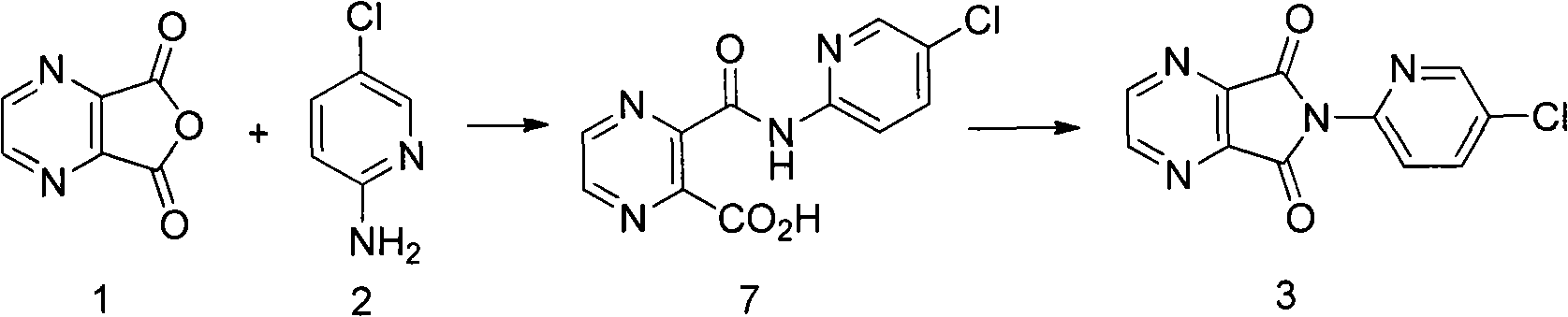
ActiveCN103664952AReduce usageEmission reductionOrganic chemistryEthyl chloroformateAcetic anhydride
The invention relates to a preparation method of zopiclone for improving sleeping, belonging to the field of medicines. In the preparation process of 6-(5-chlro-2-pyridyl)-5, 7-dioxo-5, 6-dihydropyrrolo[3, 4-b] pyrazine, namely a compound 3, by taking DMAP (dimethylaminopyridine) as a catalyst, in the presence of triethylamine, cyclization is directly carried out to synthesize an intermediate 3. The crude product yield is 85%, the yield is improved, the operation is simplified, and irritant reagents such as acetic anhydride, thionyl chloride, ethyl chloroformate and the like are not used, thereby facilitating production, facilitating recovery of xylene as a solvent and reducing emission of three wastes. Zopiclone is further synthesized by the compound 3. The method is concise in whole line, simple and convenient to operate and more suitable for industrialized production.
Owner:迪嘉药业集团股份有限公司
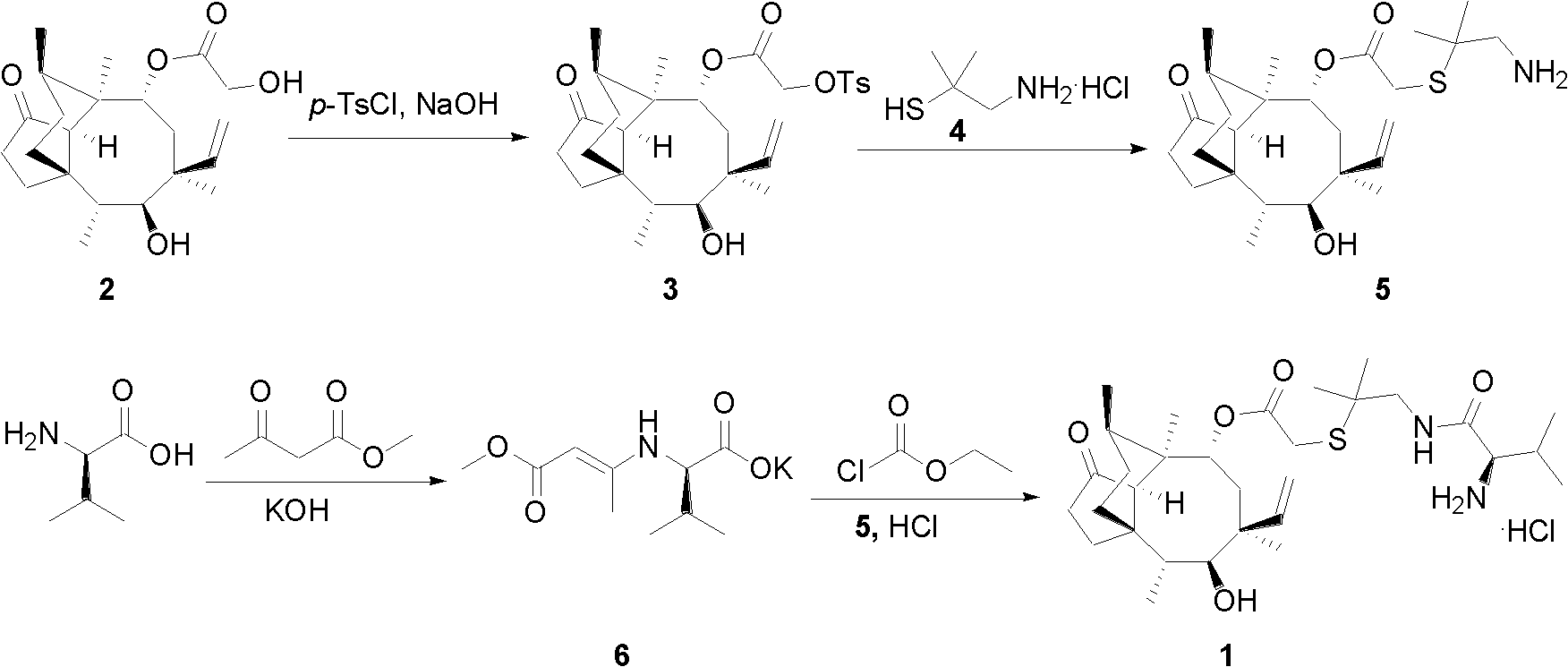
Chemical synthesis method of valnemulin hydrochloride
The invention discloses a chemical synthesis method of valnemulin hydrochloride, which comprises the following steps of: taking refined pleuromutilin as raw material, carrying out sulfonation by paratoluensulfonyl chloride, and reacting with dimethyl cysteamine hcl, to obtain the pleuromutilin dimethyl cysteamine substitute; reacting D-valine, methyl acetoacetate and potassium hydroxide to obtain (R)-2-(1-methoxycarbonyl group-2-allyl) amino-3-methyl potassium butyrate, activating by ethyl chloroformate and reacting with the pleuromutilin dimethyl cysteamine substitute, adjusting PH value, carrying out reverse phase extraction, and carrying out freeze-drying to obtain the valnemulin hydrochloride. The method has the advantages that due to the refining of the raw material pleuromutilin, the impurities in the product can be effectively removed, and the purifying process can be simplified from the source; the carboxyl of D-valine can be activated by the ethyl chloroformate, so that the reaction is easier to carry out; and due to the pH adjustment, the reverse phase extraction, and the freeze-drying, the product can be obtained, so that the product is stable in quality, and high in purity.
Owner:武汉回盛生物科技股份有限公司
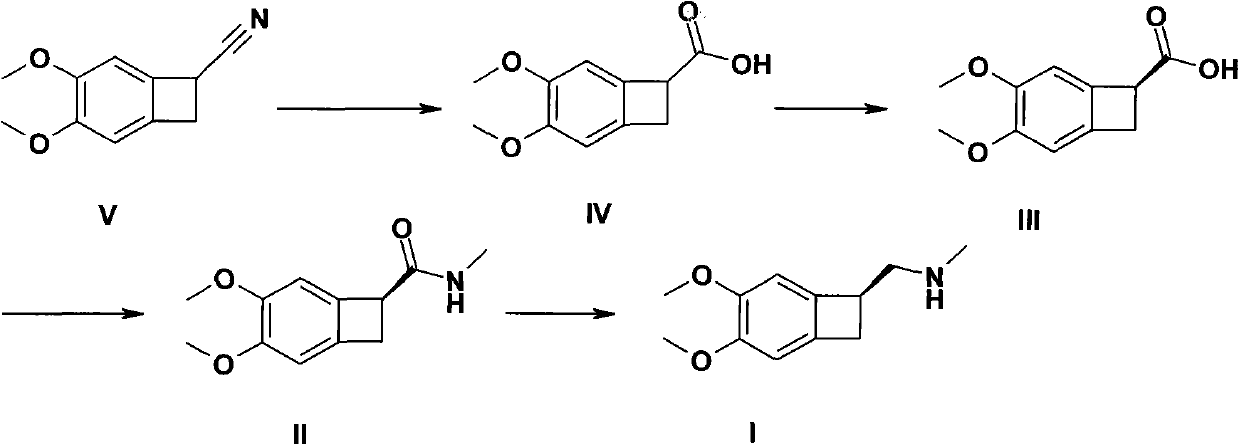
Preparation method of 1-(S)-4, 5-dimethyamino-1-methylaminomethyl-benzocyclobutane
InactiveCN102249937ALow costReduce manufacturing costOrganic compound preparationAmino-hyroxy compound preparationEthyl chloroformateCondensation reaction
The invention provides a preparation method of 1-(S)-4, 5-dimethyamino-1-methylaminomethyl-benzocyclobutane (compound I). The preparation method comprises the following steps that (S)-4, 5-dimethoxy benzocyclobutyl-1-carboxylic acid (compound III) is prepared through the split of 4, 5-dimethoxy-1-carboxyl benzocyclobutane (compound IV); then compound III obtained from the previous step undergoes a condensation reaction to form 4, 5-dimethoxy-1-methylaminoformyl benzocyclobutane (compound II); and compound II obtained from the previous step undergoes a reduction reaction to form 1-(S)-4, 5-dimethyamino-1-methylaminomethyl-benzocyclobutane (compound I). The preparation method has the advantages of less reduction steps, no need of reagents such as ethyl chloroformate and the like, simple and convenient processes, low production cost and great application values.
Owner:SHANGHAI JINGXIN BIOLOGICAL MEDICAL
Novel preparation method for azilsartan and intermediate thereof
InactiveCN105669495AConvenient sourceReduce one step reactionOrganic chemistryEthyl chloroformateCarboxylic salt
The invention relates to an intermediate for preparing azilsartan and a novel preparation method for the azilsartan. The method includes the following steps: 1) reducing 2-cyano-4'-bromomethylbiphenyl with hydroxylamine hydrochloride to generate (Z)-4'-bromomethyl-(N')-hydroxy-[1,1'-biphenyl]-2-amidine; 2) performing esterification with ethyl chloroformate to generate (Z)-4'-bromomethyl-(N')-((ethoxycarbonyl)oxyl)-[1,1'-biphenyl]-2-amidine; 3) performing ring closing to prepare 3-(4'-(bromomethyl)-[1,1'-biphenyl]-2-yl)-1,2,4-oxadiazole-5-(4H)-one; 4) performing a condensation reaction to 2-ethoxy-1H-benzimidazole-7-methyl carboxylate and the 3-(4'-(bromomethyl)-[1,1'-biphenyl]-2-yl)-1,2,4-oxadiazole-5-(4H)-one to prepare 2-ethoxy-1-((2'-(2,5-dihydro-5-oxy-1,2,4-oxadiazole-3-yl)biphenyl-4-yl)methyl)benzimidazole-7-methyl carboxylate; and 5) finally performing hydrolysis to prepare the azilsartan. The method employs the raw material being easy to obtain, is short in synthetic route, is low in device cost, is less in side products, is low in toxicity and pollution, is environment-friendly, is high in product purity and is suitable for industrial production.
Owner:CHONGQING LAND TOWER PHARMA
Synthetic method of urethane artificial antigen
InactiveCN102718861AReduced shieldingAccurate dosageOvalbuminSerum albuminEthyl chloroformateChemical synthesis
The invention provides a synthetic method of urethane artificial antigen. The urethane artificial antigen is prepared by the following steps of: 1, preparation of beta-alanine and ethyl chloroformate; 2, synthesis of hapten; 3, purification of hapten; 4, preparation of 1-(3-dimethylamino propyl)-3-ethyl carbodiimide solution; 5, synthesis of artificial antigen; 6, dialysis and detection of artificial antigen; and 7, calculation of quantity of different used carrier proteins. According to the synthetic method, the shielding effect of the protein to the hapten obtained by chemical synthesis is low, therefore, high-titer polyclonal antibody can be generated in an immune animal, and the molecular weight can be detected during synthesizing the artificial antigen, and the coupling ratio can be calculated, and the quantity of used raw materials for synthesizing is precise.
Owner:ZHEJIANG FORESTRY UNIVERSITY
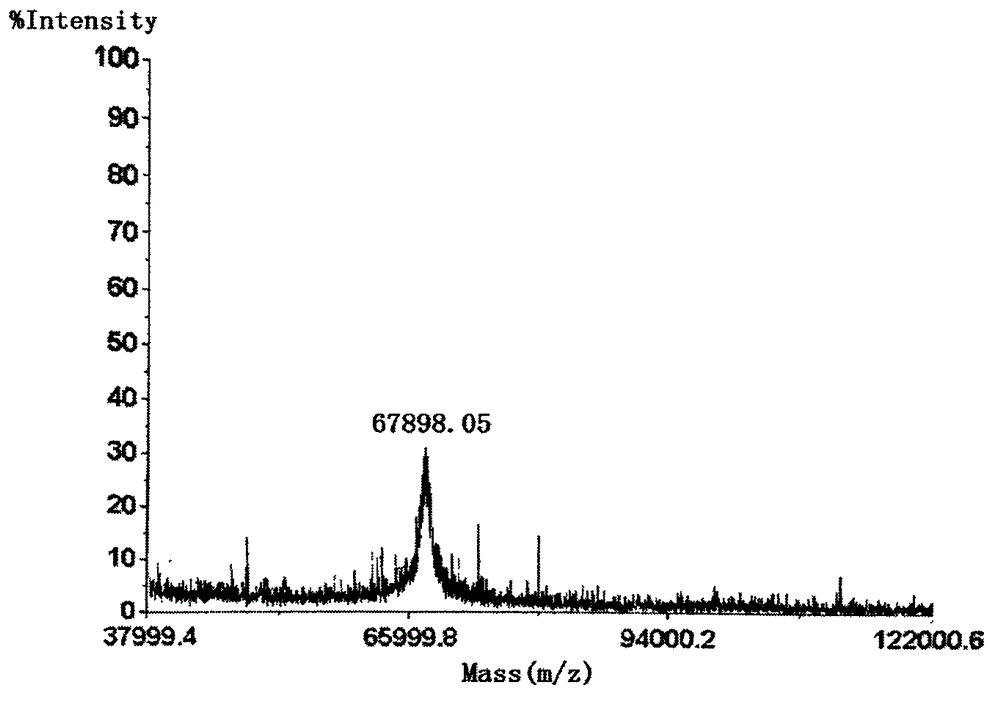
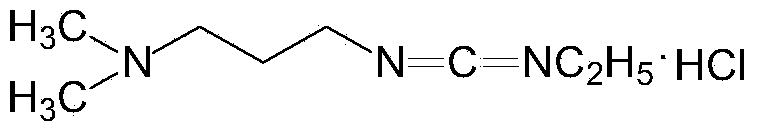
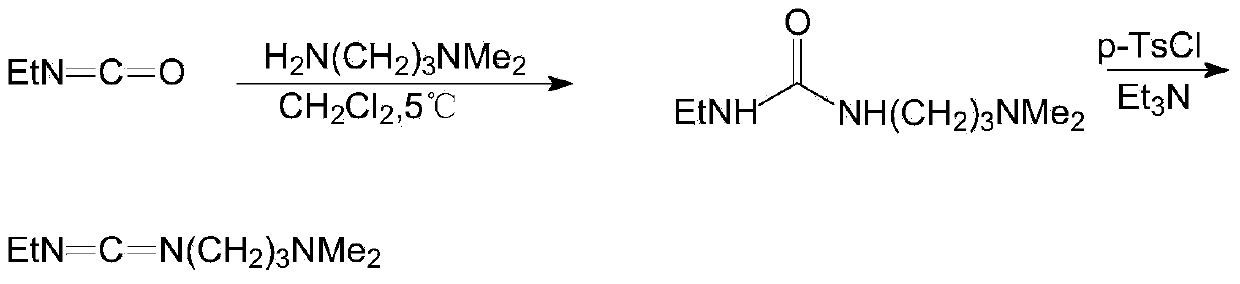
Preparation method of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
ActiveCN104193654AHigh yieldThe total yield is 80%; this process has a high conversion rateOrganic chemistryEthyl chloroformatePtru catalyst
The invention belongs to the field of organic chemical industry, and particularly relates to a preparation method of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride. The method comprises the following steps: enabling N,N'-dimethyl-1,3-propanediamine and carbon disulfide as raw materials to react in an organic solvent to generate an intermediate 1; enabling the intermediate 1 and ethyl chloroformate to react in the organic solvent, and preparing an intermediate 2 from triethylamine as an acid-binding agent; enabling the intermediate 2 and ethylamine to react in the organic solvent, so as to prepare an intermediate 3; adding a catalyst to the intermediate 3, oxidizing one time with an oxidant, so as to obtain a crude product 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide, extracting and separating, so as to obtain an intermediate 4; and carrying out salt exchange reaction on the intermediate 4 and hydrochloride, so as to prepare the product 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride. The method has the advantages of relatively high conversion rate and relatively high total recovery rate and is simple to operate and suitable for industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
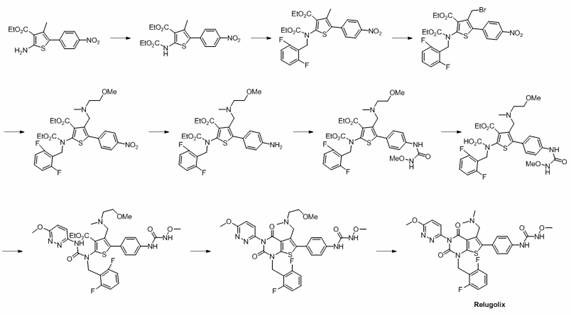
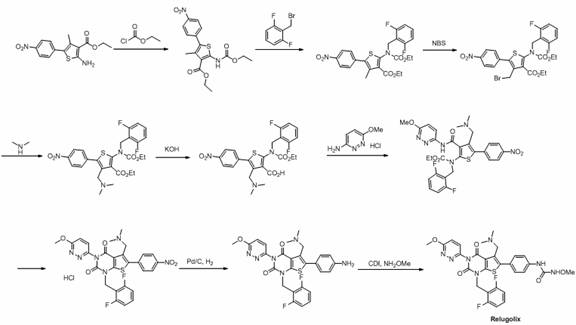
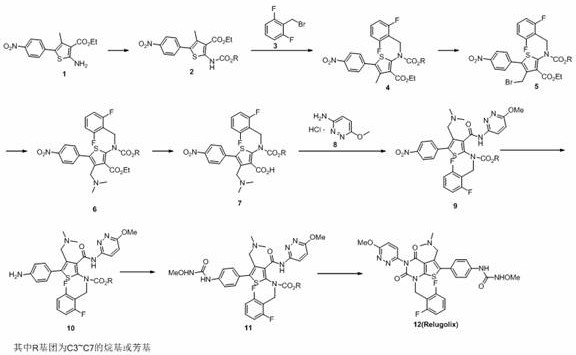
Preparation method of relugolix drug intermediate
The invention provides a relugolix intermediate and a synthesis scheme of relugolix, the synthesis scheme adopted by the invention effectively avoids the use of a high-toxicity substance methyl chloroformate or ethyl chloroformate, and other types of chloroformate which is low in toxicity and convenient to use are adopted, so that the use risk in the production process of crude drugs can be reduced, and the production cost is reduced. Operation is easy, the process is safer, and industrial production is facilitated.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
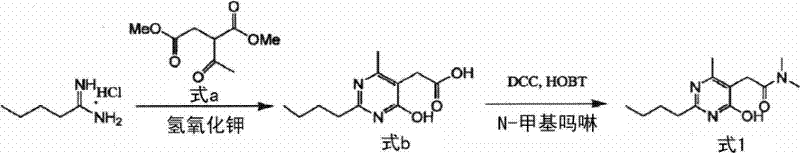
New preparation of 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidine-5-yl)-n,n-dimethylacetamide
ActiveCN102666496AInhibitionHigh yieldOrganic compound preparationCarboxylic acid amides preparationEthyl chloroformateAcetic acid
PURPOSE: A novel method for preparing 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidine-5-yl)-N,N-dimethylacetamide is provided to suppress urea by-product production and t improve production yield. CONSTITUTION: A method for preparing 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidine-5-yl)-N,N-dimethylacetamide of chemical formula 1 comprises: a step of reacting a compound of chemical formula 3 with 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidine-5-yl)-acetic acid of chemical formula 2 under the presence of base; and a step of reacting with dimethylacmine. The base is triethyl amine, trimethyl amine, triisopropyl amine or diisopropyl ethyl amine. The compound of chemical formula 3 is ethyl chloroformate.
Owner:BORYUNG PHARMA CO LTD
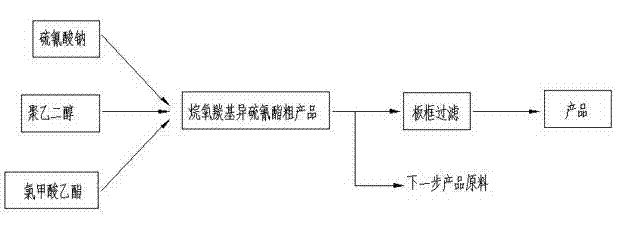
Synthesis process for ethoxy carbonyl isothiocyanate
ActiveCN104761479AReduce dosageReduce pollutionOrganic chemistryEthyl chloroformateChemical synthesis
Belonging to the technical field of chemical product synthesis and beneficiation reagent production, the invention in particular relates to a synthesis process for ethoxy carbonyl isothiocyanate. The process includes: dissolving 45 parts of 99% sodium thiocyanate in 80 parts of water, adding 0.55 part of polyethylene glycol with an average molecular weight of 200 into the solution, lowering the temperature of the solution to less than 10DEG C, adding 54.8 parts of 99% ethyl chloroformate slowly into the reaction system under stirring, controlling the reaction temperature not higher than 15DEG C, and carrying out stirring reaction for 3h, at the end of the reaction, adding 30 parts of water, performing stirring for 10min, then conducting standing for 1h, separating a lower layer water phase so as to obtain an upper layer oily liquid, i.e. N-ethoxy carbonyl isothiocyanate. The ethyl chloroformate based product yield is 83.42%, the environmental pollution is reduced, energy consumption is also lowered, and the catalyst dosage is reduced.
Owner:SHENYANG YOUYAN MINERAL CHEM CO LTD
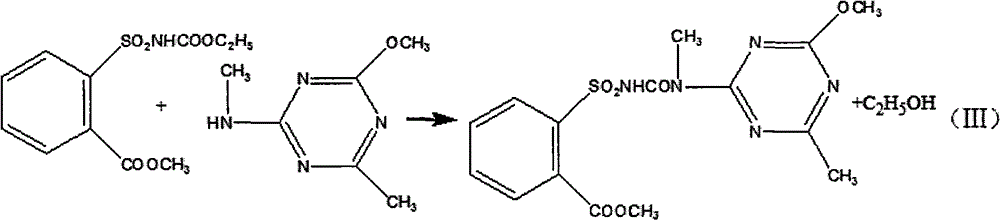
Method for preparing tribenuron-methyl
InactiveCN102718723AEmission reductionEasy to operateOrganic chemistryEthyl chloroformateReaction temperature
The invention discloses a method for preparing tribenuron-methyl, which comprises the following steps: ortho carbomethoxyl group benzene sulfonamide and ethyl chloroformate are reacted in a solvent, filtered and dried to obtain an ortho carbomethoxyl group benzene sulfonamide ethyl formate solid, the ortho carbomethoxyl group benzene sulfonamide ethyl formate solid is dissolved in the solvent, a 2-methylamino-4-methoxy-6-methyl-s-triazine solution is added drop by drop, stirred and heated, and reacted to obtain the tribenuron-methyl, wherein the purity is greater than or equal to 95%. According to the invention, ethyl chloroformate is used as an amide agent, the reaction temperature can be controlled by ice bath, and the speed for adding ethyl chloroformate drop by drop can be controlled, thereby the ethyl chloroformate is reacted with ortho carbomethoxyl group benzene sulfonamide in the solvent to obtain the ortho carbomethoxyl group benzene sulfonamide ethyl formate, and then reacted with s-triazine to obtain tribenuron-methyl. The present invention adopts ethyl chloroformate as a raw material, compared with phosgene, triphosgene and oxalyl chloride, and the method for preparing the tribenuron-methyl has the advantages of simple operation, high production security, low cost and less generation amount of three wastes.
Owner:HEFEI JIUYI AGRI DEV
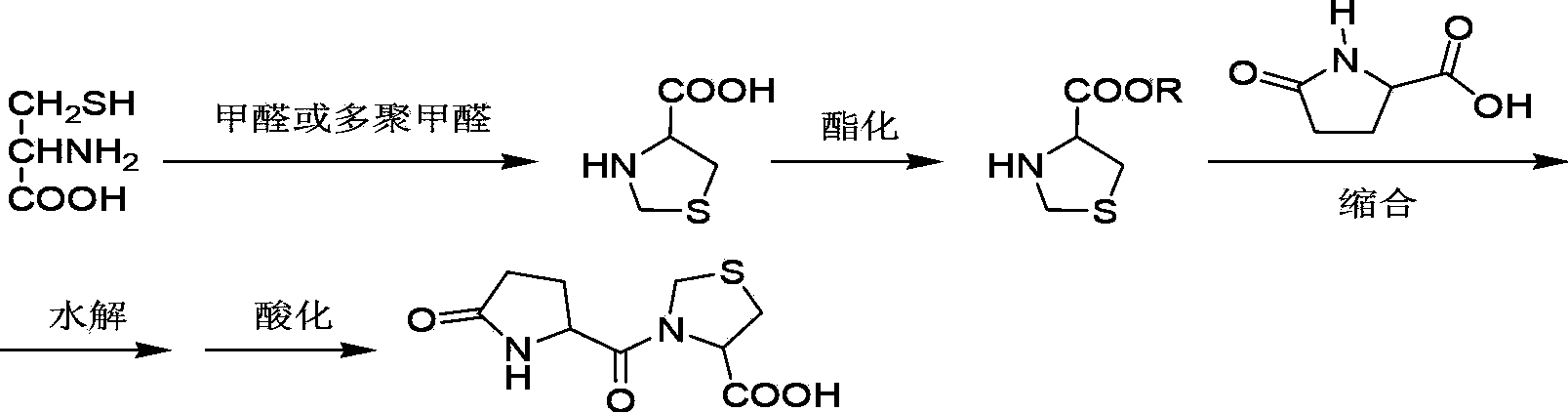
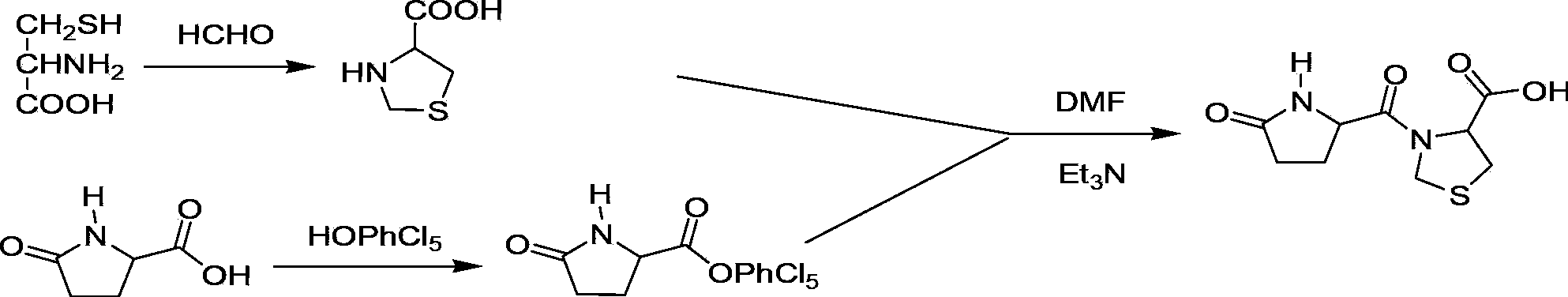
Preparation method of pidotimod
ActiveCN103897025ALow priceEasy to buyPeptide preparation methodsEthyl chloroformateL-Pyroglutamic Acid
The invention provides an improved preparation method of pidotimod. According to the improved preparation method, a mixed anhydride method is adopted, and the improved preparation method comprises the following steps: under alkaline conditions, enabling L-pyroglutamic acid, ethyl chloroformate and L-thiazolidine-4-carboxylic acid to react, then acidifying, refining and devitrifying to prepare pidotimod. The method has the advantages of simplicity and convenience in operation, high yield and high product purity, and is more suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
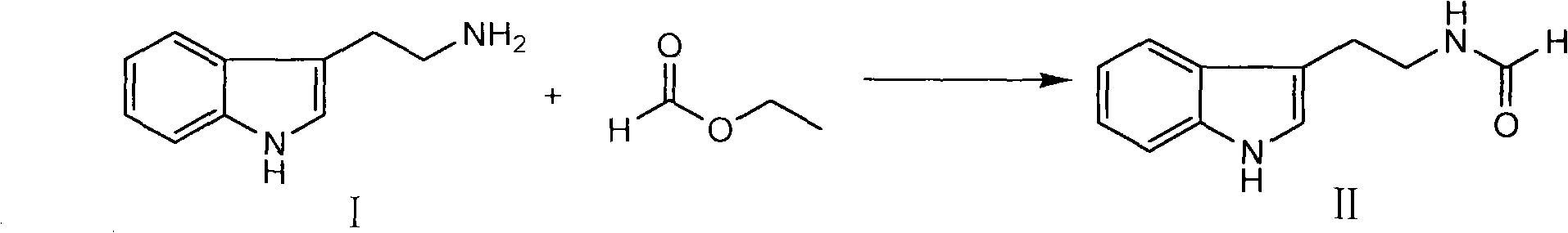
Method for synthesizing evodiamine
The invention discloses an organic chemical synthesis method, in particular a method for chemically synthesizing evodiamine. Indole acetonitrile is taken as a raw material, and the method comprises the following steps of: performing catalytic hydrogenation on palladium carbon to prepare tryptamine; mixing with ethyl formate to form solution for a formylation reaction, and dissolving the reactants in solution of dichloromethane; adding trifluoroacetic acid for cyclization, and preparing another intermediate in a reflux state from N-methylanthranilic acid and excessive ethyl chloroformate; and finally, subjecting two intermediates to condensation reaction in a non-polar solvent system. The method for chemically synthesizing the evodiamine has the advantages of rich raw material source, low price of the raw material, capacities of improving the yield and fulfilling the aim of reducing the production cost by optimizing reaction conditions, environmental friendliness and suitability for large-scale industrial production. The product can be widely applied to pharmaceutical industry.
Owner:HANGZHOU FST PHARMA
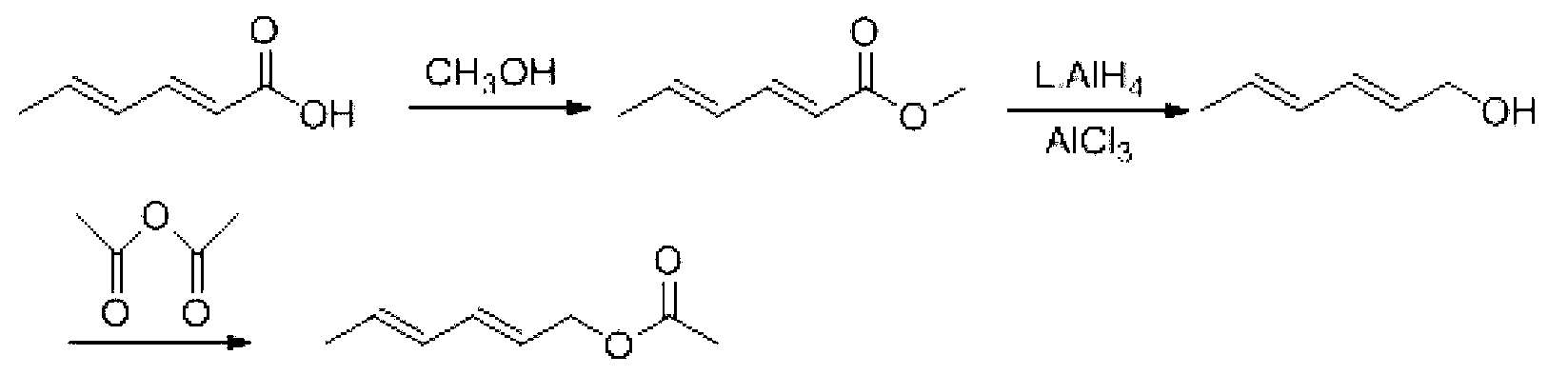
Preparation method for trans, trans-2,4-hexadiene acetate
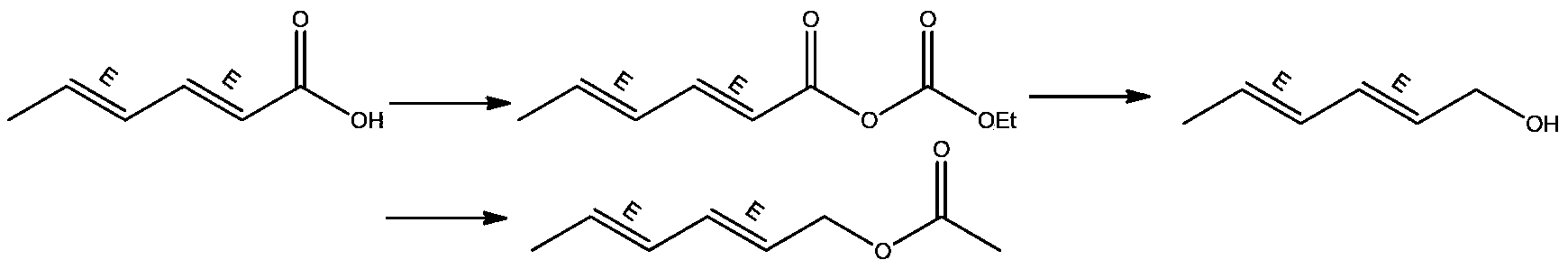
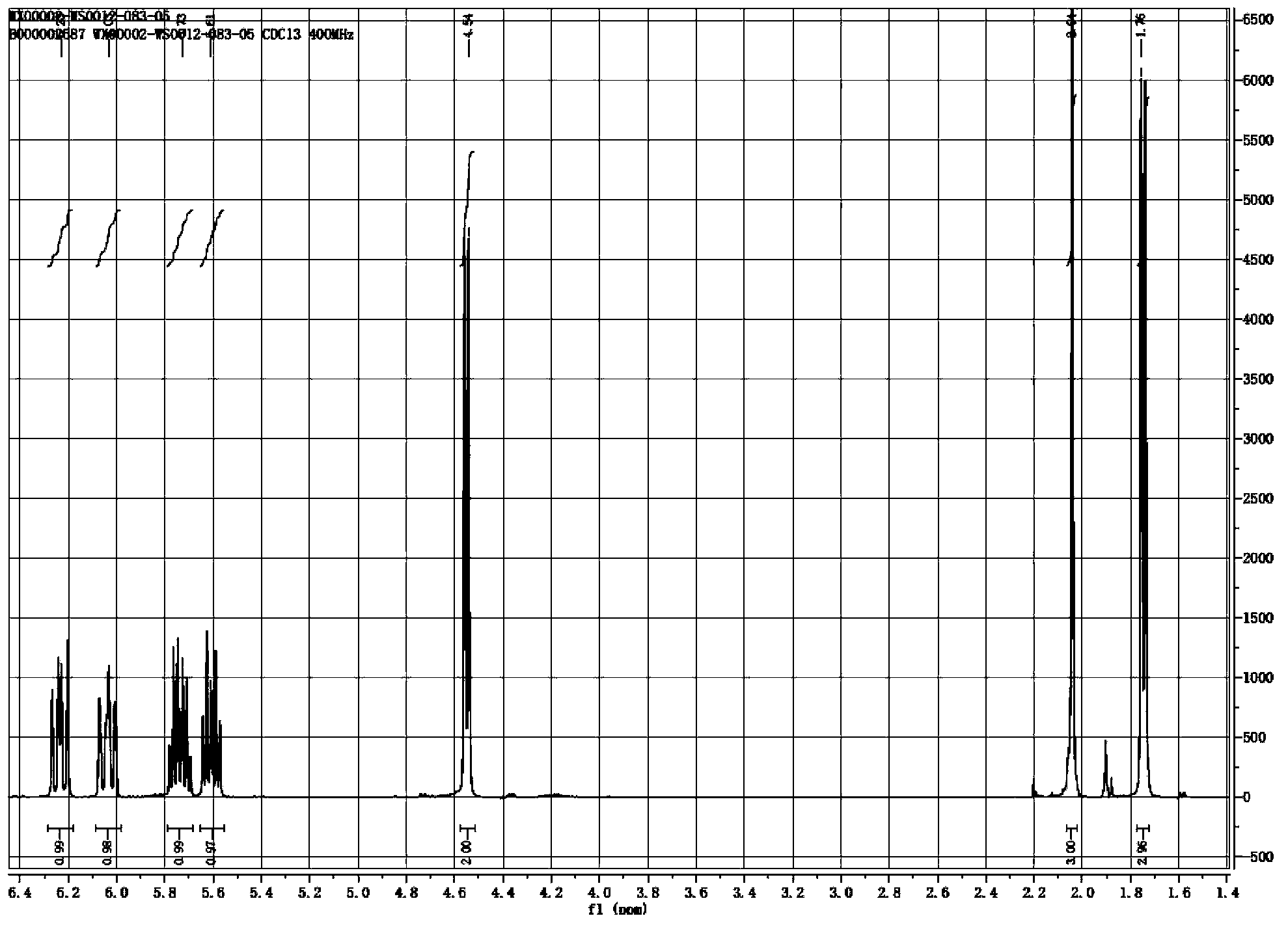
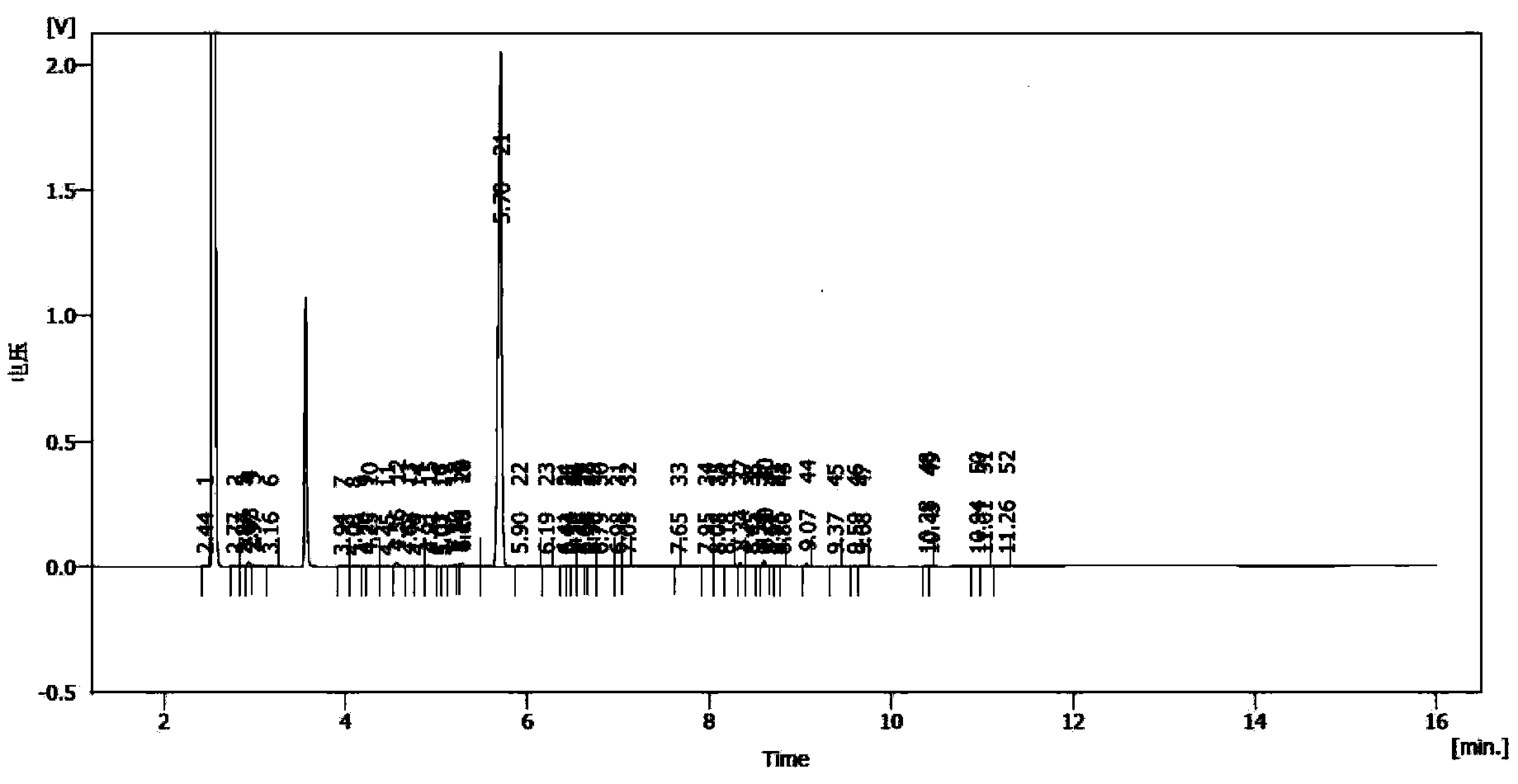
InactiveCN103254069ASufficient supply in the marketLow costOrganic compound preparationCarboxylic acid esters preparationEthyl chloroformateAcetic anhydride
The invention relates to a preparation method for trans, trans-2,4-hexadiene acetate. The preparation method comprises the following steps of: reacting sorbic acid with ethyl chloroformate in a solvent under an alkaline condition to obtain sorbic acid mixed anhydride; performing reduction reaction on the sorbic acid mixed anhydride and sodium borohydride to obtain trans, trans-2,4-hexadienol; and performing acetylation reaction on the trans, trans-2,4-hexadienol and acetic anhydride to obtain the trans, trans-2,4-hexadiene acetate. According to the preparation method, cheap sorbic acid is used as a raw material, so that market supply is adequate and the cost is low; and the trans, trans-2,4-hexadienol is obtained by reduction of sodium borohydride, so that the reaction safety is high, and industrialized production is easy to perform.
Owner:SHANDONG ACADEMY OF PESTICIDE SCI +1
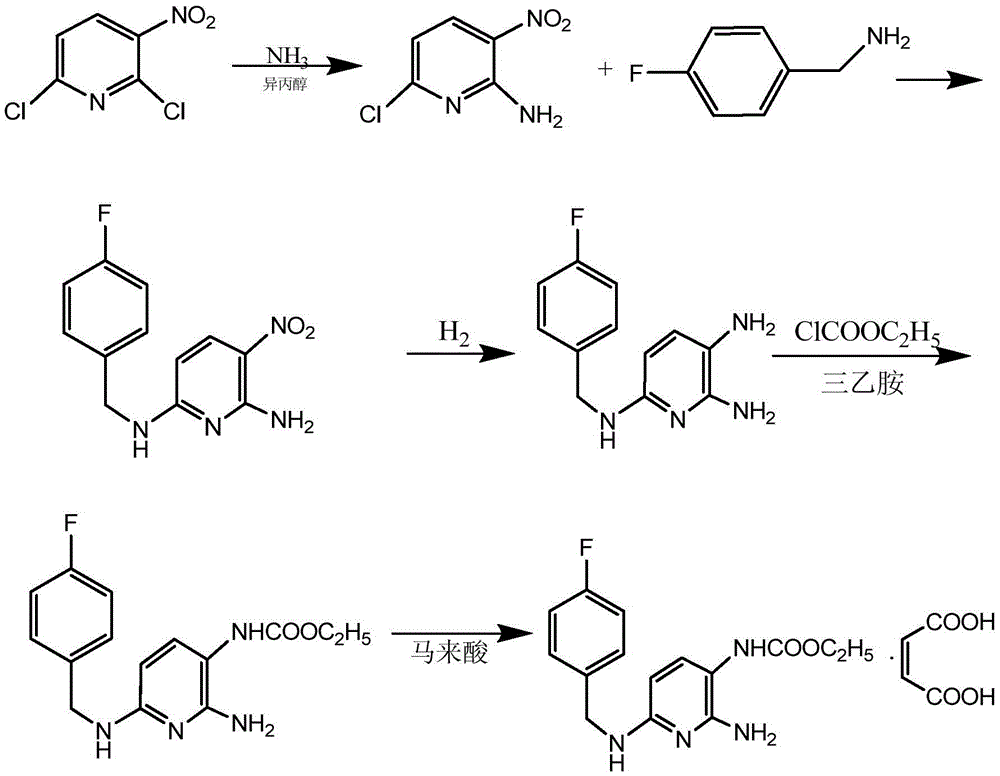
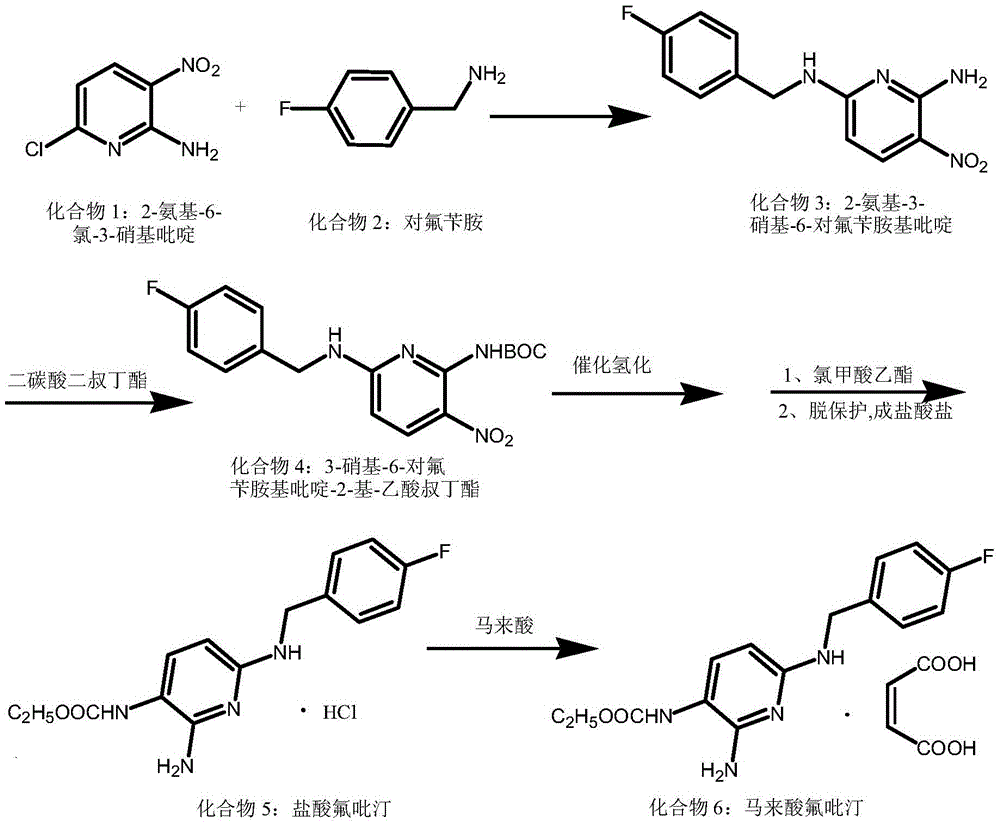
Synthesis method for flupirtine maleate compound
ActiveCN105541705AReduce manufacturing costReduce the possibilityOrganic chemistryEthyl chloroformateSynthesis methods
The invention discloses a synthesis method for a flupirtine maleate compound. The method comprises the steps that 2-amino-3-nitro-6-chloropyridine (a first compound) is used as a starting material to react with fluorobenzylamine (a second compound) to generate 2-amino-3-nitro-6-p-fluorobenzylamine pyridine (a third compound), the third compound is processed through di-tert-butyl dicarbonate ester protection to obtain 2-amino-3-nitro-6-p-fluorobenzylamine pyridine-3-based-tert-butyl acetate (a fourth compound), the fourth compound is processed through hydrogenation reduction and then react with ethyl chloroformate, after reacting is finished, deprotection is performed to obtain flupirtine hydrochloride (a fifth compound), and the fifth compound is salified with maleic acid to obtain flupirtine maleate (the sixth compound). According to the synthesis method, the starting material is cheap and easy to obtain, byproduct generation is avoided through amino protection, therefore, the impurity content is decreased, and the product quality is improved; catalytic reduction is performed by adopting palladium chloride, the reaction condition is mild, the reaction process is easy to operate, and the method is suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
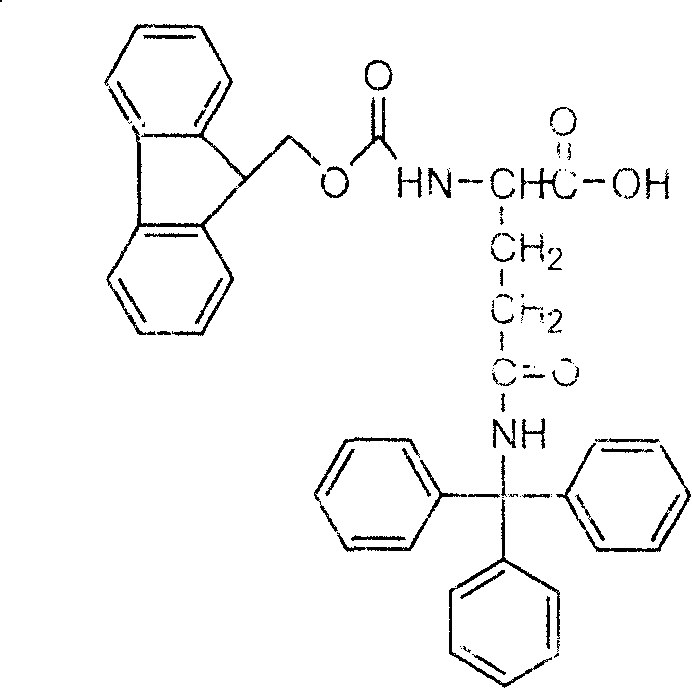
Method for synthesizing N-fluorene methoxycarbonyl-N-trityl-D-glutamine
ActiveCN101219970AReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationEthyl chloroformateN dimethylformamide
The invention relates to a synthetic method of N-fluorenylmethoxycarbonyl-N- triphenylmethyl-D-glutamine to solve the current problems of the shortage of D-Gln raw materials and high cost of synthesizing products from the D-Gln. The synthesis includes the following steps: a. D-glutamate and a carbobenzoxy chloride are reacted to get the N-carbobenzoxy-D-glutamate; in an organic solvent N and N-dimethylformamide with the existence of a triethylamine and bromomethyl-benzene, the N-carbobenzoxy-D-glutamate selectively protects an Alpha-carboxyl to get an N-benzyloxycarbonyl-D-benzyl L-glutamate; b. the triethylamine, ethyl chloroformate and ammonia are added to N- benzyloxycarbonyl-D- benzyl L-glutamate organic solvent with a molar ratio of 1:1:2 to 6; after reacted for 6 to 24 hours under a temperature of -20 to 20 DEG C, an N- benzyloxycarbonyl-D-glutaminebenzylester is gotten; c. the product of b, acetate liquor is reacted with a triphenylmethanol under a catalysis of concentrated sulfuric acid, and N- benzyloxycarbonyl-N-triphenylmethyl-D-glutaminebenzylester can be gotten after reacted for 8 to 24 hours under a temperature of 40 to 60 DEG C; d. benzyloxycarbonyl and benzyl are detracted from the product of c to get the N-triphenylmethyl-D-glutamine; e. the product of d is protected by an Fmoc group to get the N-fluorenylmethoxycarbonyl-N-triphenylmethyl-D- glutamine.
Owner:GL BIOCHEM SHANGHAI
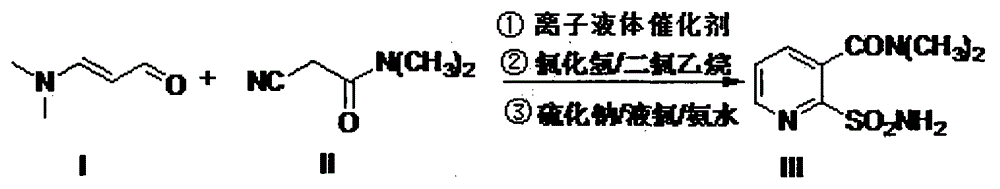
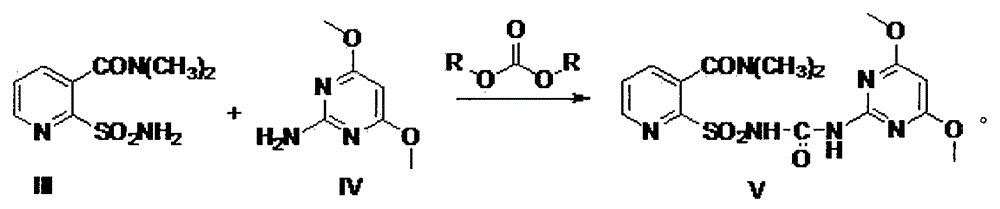
Method for synthesizing 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide
ActiveCN102382049ASimple and thorough separationHigh yieldOrganic chemistryEthyl chloroformateAqueous acetone
The invention relates to a novel method for synthesizing 2-ethoxycarbonylaminosulfonyl-N, N-dimethyl nicotinamide. 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide is prepared by the following steps of taking liquid alkali as an acid binding agent; performing reaction of 2-aminosulfamide-N,N-dimethyl nicotinamide and ethyl chloroformate; adding 2-aminosulfamide-N,N-dimethyl nicotinamide into an acetone aqueous solution, adding ethyl chloroformate and liquid alkali into acetone aqueous solution while stirring; preserving heat till the completion of the reaction, distilling and recyclingacetone; filtering; separating; and drying to obtain the finished product. The product prepared by the method saves energy and reduces consumption and achieves the environmental safety; no pollutantssuch as carbon dioxide and salt-containing waste water are generated; and the yield and the content of the 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide is high.
Owner:ANHUI FENGLE AGROCHEM
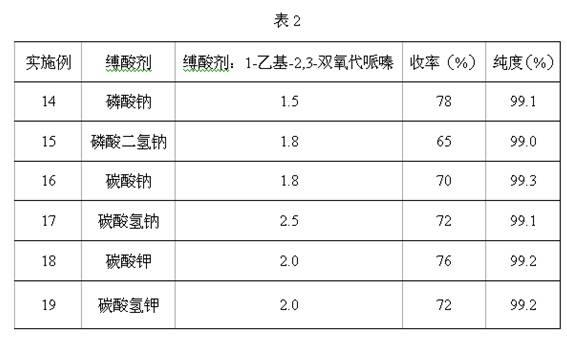
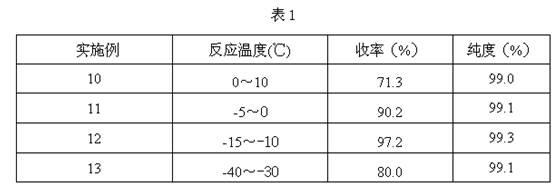
Method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate
The invention discloses a method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate. In the method, 1-ethyl-2,3-dioxypiperazine and chloro-formate are used as raw materials and reacted in an organic solvent system in the presence of an acid binding agent to form 4-ethyl-2,3-dioxypiperazine-1-formate, wherein the molar ratio of the 1-ethyl-2,3-dioxypiperazine to the acid binding agent to the chloro-formate is 1:1.0-3.0:1.0-2.0; the chloro-formate is methyl chloroformate or ethyl chloroformate; and the 4-ethyl-2,3-dioxypiperazine-1-formate is 4-ethyl-2,3-dioxypiperazine-1-methyl formate or 4-ethyl-2,3-dioxypiperazine-1-ethyl formate. The method greatly reduces cost, simplifies process, reduces byproducts, improves product purity and reduces solvent separation processes; and the prepared product can be used as an intermediate for piperacillin and cefoperazone and is suitable for industrial production.
Owner:山东艾孚特科技有限公司
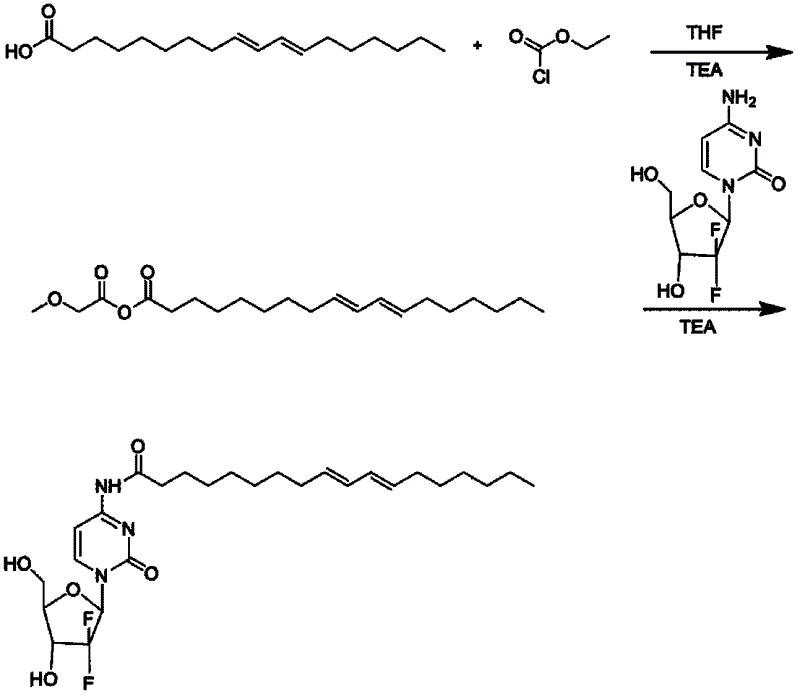
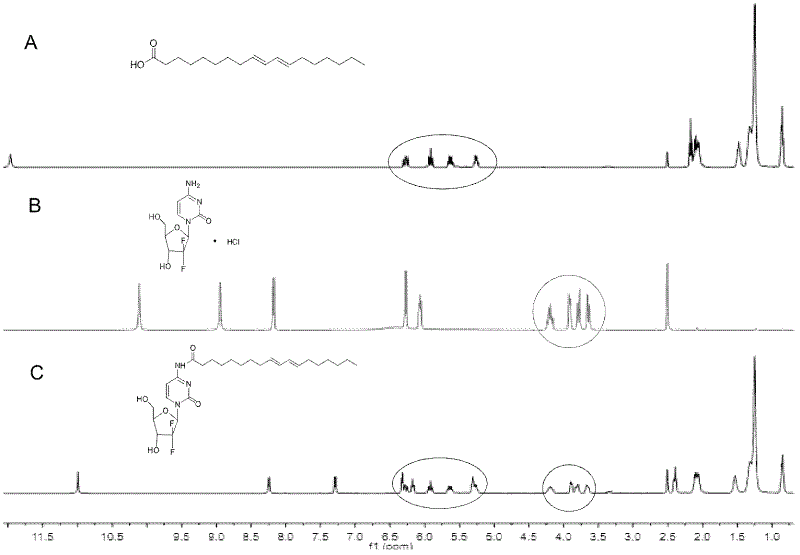
Preparation method and application of connected conjugated linoleic acid and gemcitabine prodrug
ActiveCN102617679AImprove anti-tumor efficacyLow toxicityOrganic active ingredientsSugar derivativesEthyl chloroformateNitrogen gas
The invention relates to a preparation method and application of a connected conjugated linoleic acid and gemcitabine prodrug. The conjugated linoleic acid is connected with the gemcitabine via an amide bond. The preparation method comprises the following steps that: under the protection of nitrogen, the conjugated linoleic acid is dissolved in a solvent, and an activator is added at a low temperature of -15 DEG C under stirring to activate the conjugated linoleic acid to form a highly reactive mixed acid anhydride; the gemcitabine is dissolved in a solvent to be added dropwise to the mixed acid anhydride, and the reaction is undergone under stirring at room temperature; the solvent refers to one or a mixed solution of triethylamine, tetrahydrofuran, N,N-dimethyl formamide, and the activator is ethyl chloroformate; the reaction time is 2-72 hours, and the feed molar ratio of the gemcitabine to the conjugated linoleic acid is 1:(0.5-5); and the molar ratio of the activator to the conjugated linoleic acid is 1:(0.5-5). The object of the invention is to increase the anti-tumor efficacy of the gemcitabine.
Owner:PEKING UNIV
Method for preparing nicosulfuron
The invention discloses a method for preparing nicosulfuron. The method adopts dimethylamino acrolein and 2-oxopropionitrile dimethylamine as raw materials to generate 2-sulfonylamino-N,N-dimethylnicotinamide through a multi-unit continuous operation method, and the generated 2-sulfonylamino-N,N-dimethylnicotinamide, 4,6-dimethoxy pyrilamine and alkyl carbonate are condensed to prepare nicosulfuron. According to the method for preparing nicosulfuron, the defect of dangerous operation of using highly toxic gas phosgene or polymers thereof in an isocyanate method to cause larger potential safety hazards is overcome, meanwhile the defect of using a highly toxic reagent, namely, ethyl chloroformate, and a thionyl chloride reagent with larger harm on environment in a chlorine carbonyl amine method is also overcome, and the method provided by the invention has the characteristics of less pollution, safety and environmental protection, and is suitable for large scale industrialized production.
Owner:HEFEI JIUYI AGRI DEV
Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative
InactiveCN102167680AHigh yieldSuccessful realization of mass productionOrganic chemistryBulk chemical productionEthyl chloroformateCarboxylic acid
The invention discloses a preparation method of an octahydrocyclopenta[c]pyrrole carboxylic acid derivative. The method comprises the following steps: octahydrocyclopenta[c]pyrrole protected by N is used as raw material, one of tetrahydrofuran, methyl tert-butyl ether and dioxane is used as solvent, chiral organic ligand is added to react with lithium alkylide for 2-3 hours at -50 DEG C to -78 DEG C, then the reaction product reacts with carbon dioxide or ethyl chloroformate to obtain the octahydrocyclopenta[c]pyrrole carboxylic acid derivative. The preparation method of the octahydrocyclopenta[c]pyrrole carboxylic acid derivative has short synthetic route and high yield and can be used to realize mass production successfully.
Owner:ACME BIOPHARMA
New crystal form of mianserin hydrochloride, detection method and applications thereof
InactiveCN105012864ADefinite curative effectLittle side effectsNervous disorderOrganic chemistryBenzaldehydeTherapeutic effect
The present invention provides a new crystal form of mianserin hydrochloride, a detection method and applications, and relates to a traditional Chinese medicine and Western medicine preparation for depression, especially to applications of a compound drug prepared from a Western medicine mianserin hydrochloride adopted as the main component and Chinese herbs in treatment of depression. According to the mianserin hydrochloride, benzaldehyde and ethanolamine are adopted as starting raw materials and are subjected to chemical combination to obtain an intermediate I, the intermediate I reacts with styrene oxide to obtain an intermediate II, the intermediate II and thionyl chloride are subjected to chemical combination under an alkaline condition to obtain an intermediate III, the intermediate III, o-aminobenzyl alcohol and fumaric acid are subjected to chemical combination to obtain an intermediate IV, an intermediate V is obtained from the intermediate IV under the action of concentrated sulfuric acid and sodium carbonate, the intermediate V reacts with ethyl chloroformate under an alkaline condition to obtain an intermediate VI, the intermediate VI reacts with formaldehyde and formic acid to obtain an intermediate VII, and the intermediate VII is acidified with hydrochloric acid-ethyl acetate to obtain the mianserin hydrochloride. The new crystal form of the mianserin hydrochloride of the present invention has characteristics of significant treatment effect, symptom and root cause treatment, and wide application prospets.
Owner:仁和堂药业有限公司
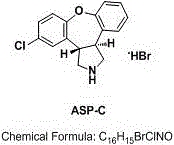
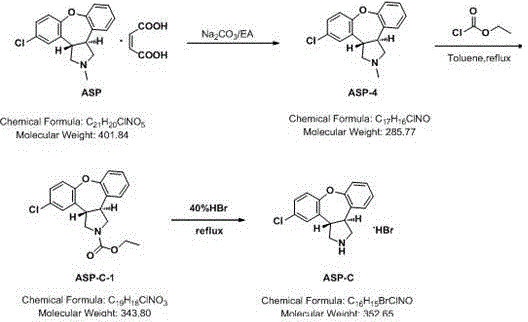
Novel method for removing methyl impurity in preparation of asenapine
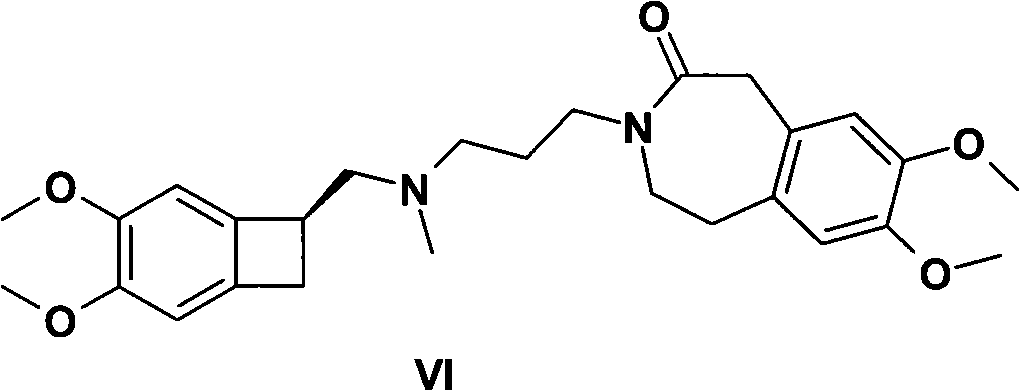
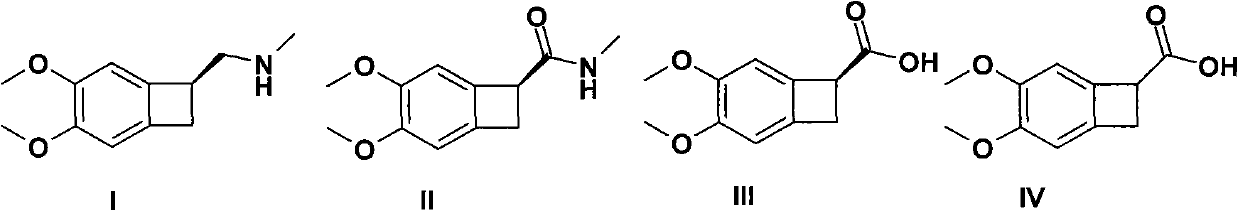
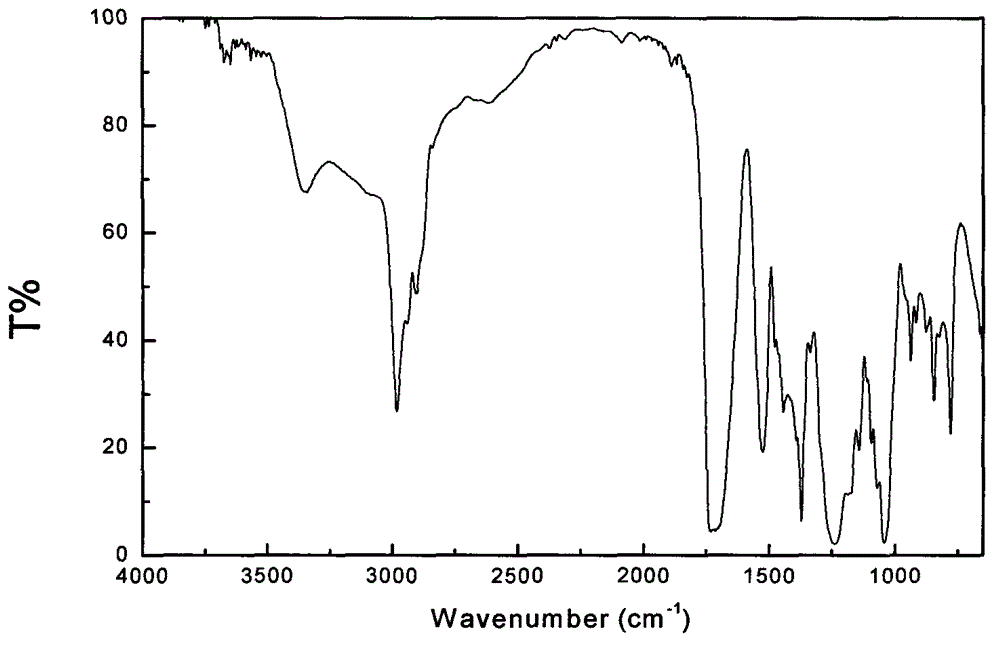
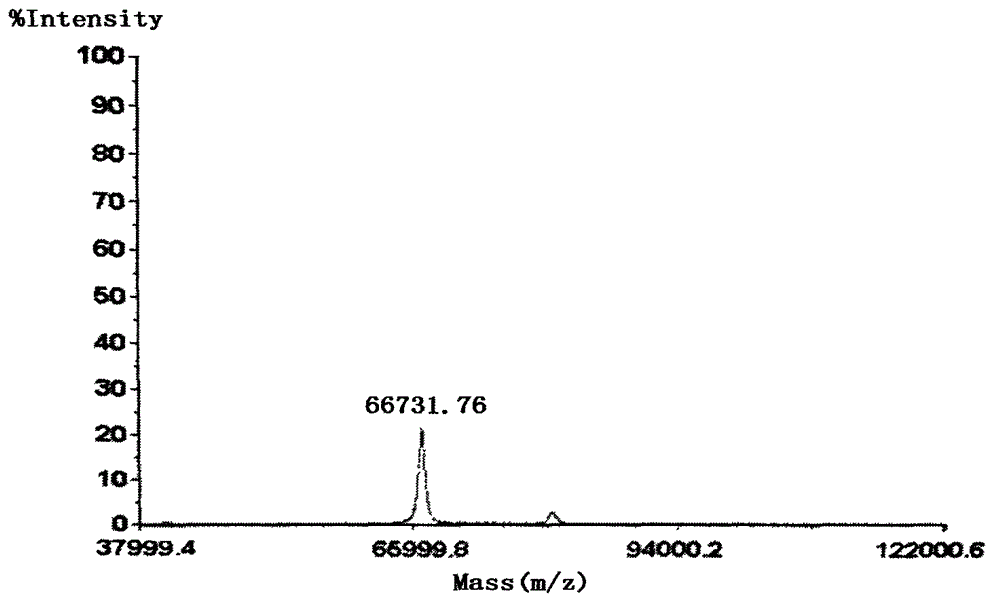
InactiveCN105566336ARaw materials are easy to getSimple processOrganic chemistryEthyl chloroformateEthyl ester
Owner:AVENTIS PHARMA HAINAN
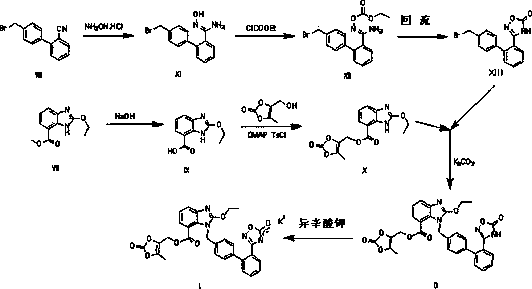
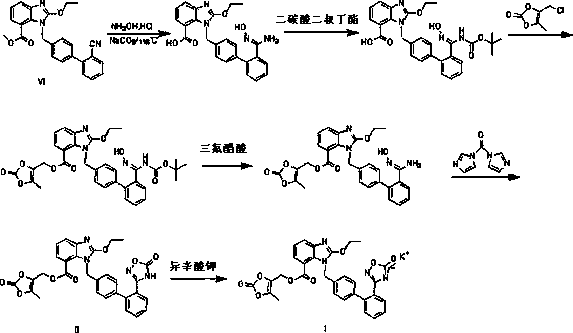
Novel preparation method of azilsartan medoxomil sylvite and its intermediate
InactiveCN105622595AConvenient sourceLong reaction stepsOrganic chemistryEthyl chloroformateSide chain
The invention relates to a novel preparation method of azilsartan medoxomil sylvite and its intermediate. The method comprises the following steps: hydrolyzing a starting material VII to obtain an intermediate IX, then performing esterification with a side chain IV to obtain the intermediate X; reducing the other starting material VIII by hydroxylamine hydrochloride to obtain the intermediate XI, then performing esterification with ethyl chloroformate to obtain the intermediate XII, and closing ring to obtain the intermediate XIII; performing condensation of the intermediate X and the intermediate XIII to obtain the intermediate II, and finally performing reaction with potassium isooctanoate to obtain the azilsartan medoxomil sylvite. The method has the advantages of easy acquisition of raw materials, short synthesis route, less equipment investment, less by-product, low toxicity, little pollution, environment protection, and high product purity, and is suitable for industrial production.
Owner:CHONGQING LAND TOWER PHARMA
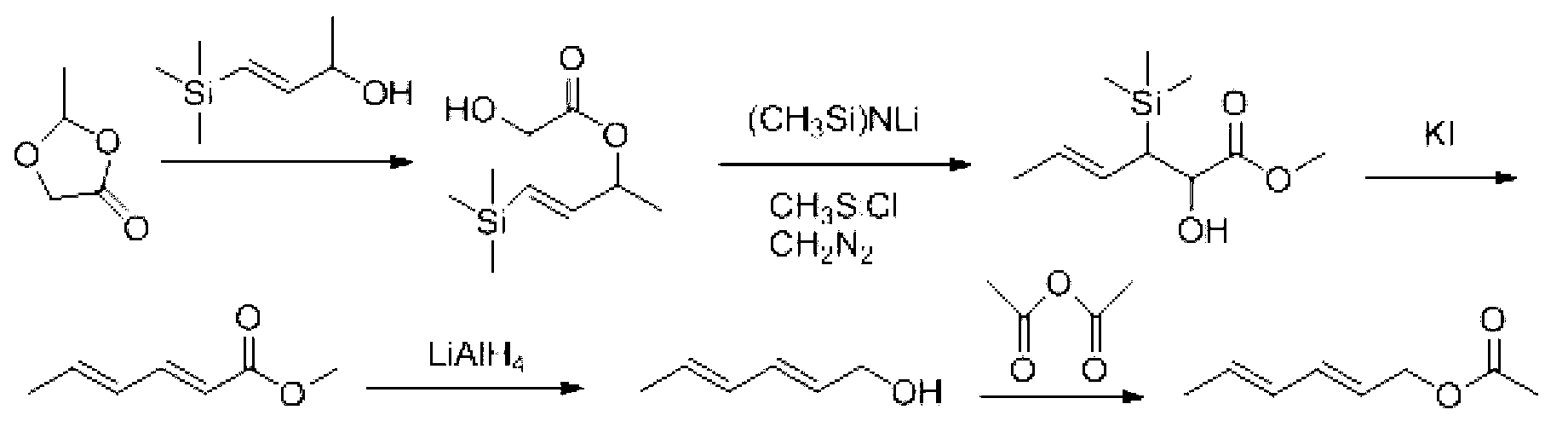
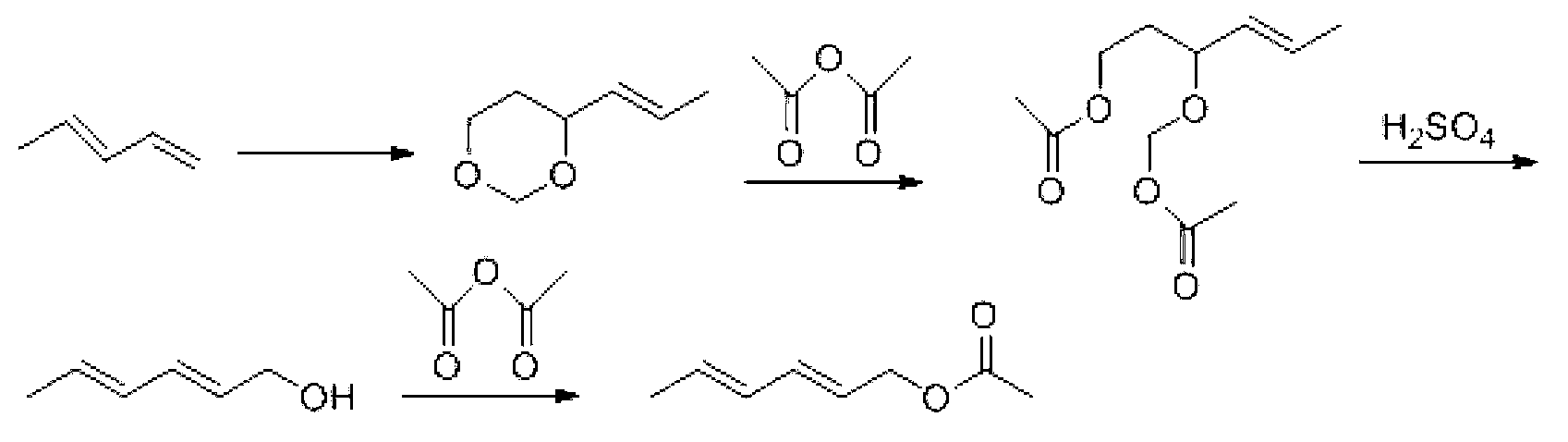
Synthesis method of laspeyresia pomonella sex pheromone intermediate (2E, 4E)-2,4-hexadienol acetate
InactiveCN103360248AImprove conversion rateAvoid the defects of incomplete conventional esterification reactionOrganic compound preparationCarboxylic acid esters preparationChemical synthesisEthyl chloroformate
The invention relates to the field of chemical synthesis of an insect sex pheromone intermediate, and particularly relates to a synthesis method of a laspeyresia pomonella sex pheromone intermediate (2E, 4E)-2,4-hexadienol acetate. According to the method provided by the invention, sorbic acid is used as a start material and generates an active acid anhydride with ethyl chloroformate; the active acid anhydride generates (2E, 4E)-hexadiene-1-ol in a reducing system of hydroboron anion reducing agent / inorganic aqueous alkali; and (2E, 4E)-2,4-hexadienol acetate is finally obtained through an esterification reaction. The synthesis method provided by the invention has the advantages of easily available raw materials, mild reaction conditions, convenience in operation, low requirements for equipment pipelines, high product yield and simple after-treatment, and is environment-friendly and suitable for large-scale industrial production.
Owner:WATERSTONE PHARMA WUHAN
Environment-friendly novel technology for synthesizing high content nicosulfuron
InactiveCN103483318ADoes not affect product qualityThe reaction device is simpleOrganic chemistryEthyl chloroformatePtru catalyst
The invention provides an environment-friendly novel technology for synthesizing high content nicosulfuron. According to the invention, phenyl chloroformate or diphenyl carbonate is adopted to substitute ethyl chloroformate or methyl clhlorofonmate, which is a toxic substance; organic catalyst or inorganic catalyst is adopted; dichloromethane, dichloroethane, or acetonitrile is used as a solvent; N,N-dimethyl-2-amidosulfonyl-picolinamide, 2-amidogen-4,6,-dimethoxy-pyrimidine, catalyst I, and the solvent are added into a reaction kettle as whole; adding phenyl chloroformate or diphenyl carbonate and catalyst II sequentially at room temperature; performing heating reflux to obtain the product. The yield is above 90%, and the content is above 98%.
Owner:ANHUI FENGLE AGROCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000011.png)
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000021.png)
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000022.png)





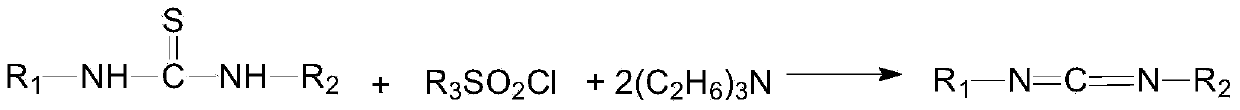

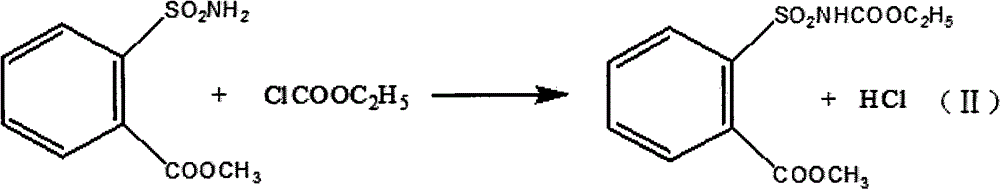

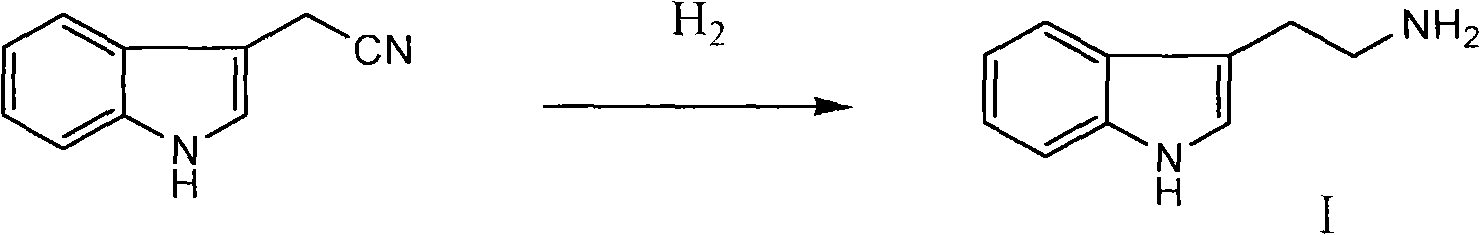


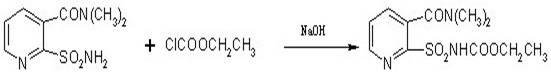


![Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1abd3be9-33b0-41fd-9db2-5d5f11496b13/2011100700397100001DEST_PATH_IMAGE001.png)
![Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1abd3be9-33b0-41fd-9db2-5d5f11496b13/84408DEST_PATH_IMAGE001.png)
![Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative Preparation method of octahydrocyclopenta[c]pyrrole carboxylic acid derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1abd3be9-33b0-41fd-9db2-5d5f11496b13/653722DEST_PATH_IMAGE003.png)