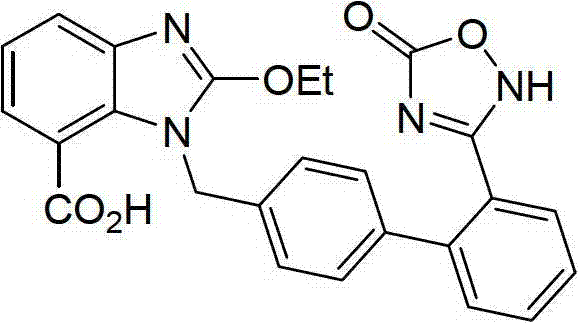
Preparation method of azilsartan
A technology of molar ratio, methyl carboxylate, applied in the field of preparation of drugs for treating hypertension, can solve the problems of increased risk, unpublished, and high synthesis cost, and achieves reduction of production cost and cycle, emission and treatment cost, and process. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
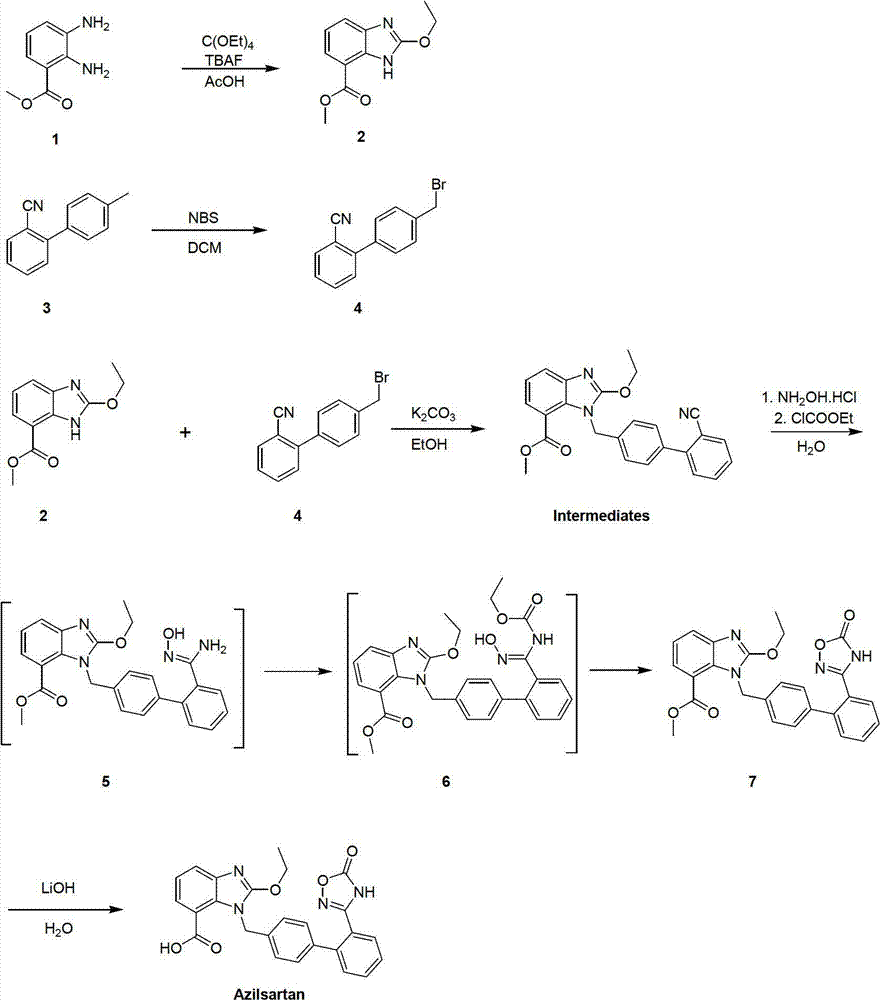
[0047] (1) Methyl ethoxybenzimidazole-7-carboxylate
[0048] Dissolve methyl 2,3-diaminobenzoate (166.18g, 1mol) and tetraethyl orthocarbonate (192.3g, 1mol) in acetic acid (2L), add tetrabutylammonium fluoride (13g, 5%mol), heated up and refluxed for 10 hours, cooled to room temperature, evaporated solvent acetic acid, and the thick product was filtered with water, and the solid was recrystallized with ethanol, and dried to obtain methyl ethoxybenzimidazole-7-carboxylate (202.6 g, yield 92%);
[0049] (2) Synthesis of 2-cyano-4'-bromomethylbiphenyl
[0050] Dissolve 2-cyano-4'-methylbiphenyl (193g, 1mol) in dichloromethane (2L), add N-bromosuccinimide (178g, 1mol) in batches at room temperature, and stir at room temperature for 4 Hours, after the reaction was over, washing with water, distillation, beating with petroleum ether, filtering, and drying to obtain 2-cyano-4'-bromomethylbiphenyl, the crude product did not need to be further purified (243.3g, yield 89.4%);
[0051]...
Embodiment 2
[0056] (1) Methyl ethoxybenzimidazole-7-carboxylate
[0057] Dissolve methyl 2,3-diaminobenzoate (166.18g, 1mol) and tetraethyl orthocarbonate (384.6g, 2mol) in acetic acid (2L), add tetrabutylammonium fluoride (13g, 5%mol), heated up and refluxed for 10 hours, cooled to room temperature, evaporated solvent acetic acid, and the thick product was filtered with water, and the solid was recrystallized with ethanol, and dried to obtain methyl ethoxybenzimidazole-7-carboxylate (204.8 g, yield 93%);
[0058] (2) Synthesis of 2-cyano-4'-bromomethylbiphenyl
[0059] Dissolve 2-cyano-4'-methylbiphenyl (193g, 1mol) in dichloromethane (2L), add N-bromosuccinimide (356g, 2mol) in batches at room temperature, and stir at room temperature for 4 Hours, after the reaction was over, washing with water, distillation, beating with petroleum ether, filtration, and drying to obtain 2-cyano-4'-bromomethylbiphenyl, the crude product did not need to be further purified (244.9g, yield 90%);
[0060...
Embodiment 3
[0065] (1) Methyl ethoxybenzimidazole-7-carboxylate
[0066] Dissolve methyl 2,3-diaminobenzoate (166.18g, 1mol) and tetraethyl orthocarbonate (384.6g, 2mol) in acetic acid (2L), add tetrabutylammonium fluoride (13g, 5%mol), heated up and refluxed for 16 hours, cooled to room temperature, evaporated solvent acetic acid, the thick product was filtered with water, and the solid was recrystallized with ethanol, dried to obtain methyl ethoxybenzimidazole-7-carboxylate (211.4 g, yield 96%);
[0067] (2) Synthesis of 2-cyano-4'-bromomethylbiphenyl
[0068] Dissolve 2-cyano-4'-methylbiphenyl (193g, 1mol) in dichloromethane (2L), add N-bromosuccinimide (356g, 2mol) in batches at room temperature, and stir at room temperature for 8 Hours, after the reaction was over, washing with water, distillation, beating with petroleum ether, filtration, and drying to obtain 2-cyano-4'-bromomethylbiphenyl, the crude product did not need to be further purified (255.8g, yield 94%);
[0069] (3) Sy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com