Preparation method of 1-(S)-4, 5-dimethyamino-1-methylaminomethyl-benzocyclobutane
A technology of dimethoxybenzene and methylaminomethyl, applied in the field of pharmaceutical preparation, can solve the problems of difficult industrialization, difficult reaction control, complicated post-processing, etc., and achieves removal of reagents and steps, low production cost, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
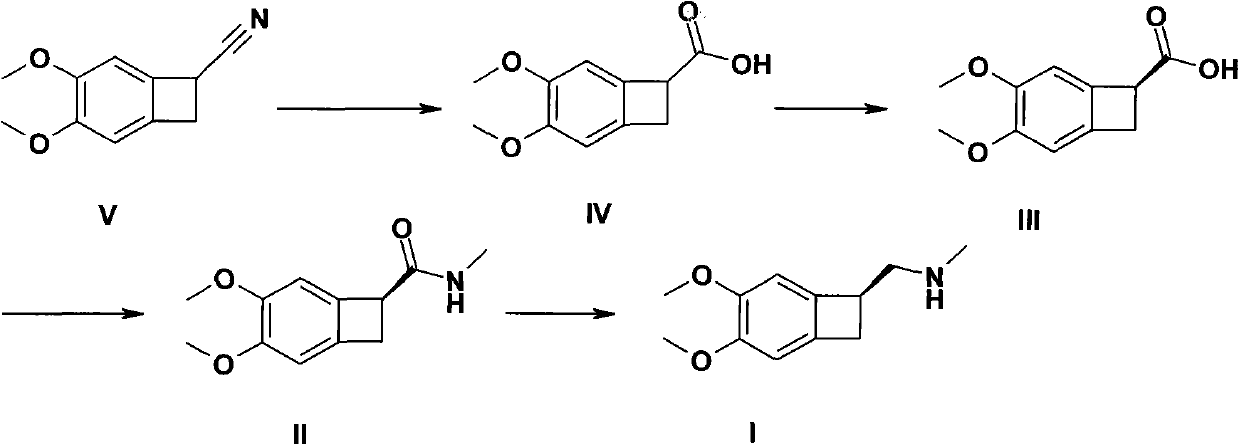
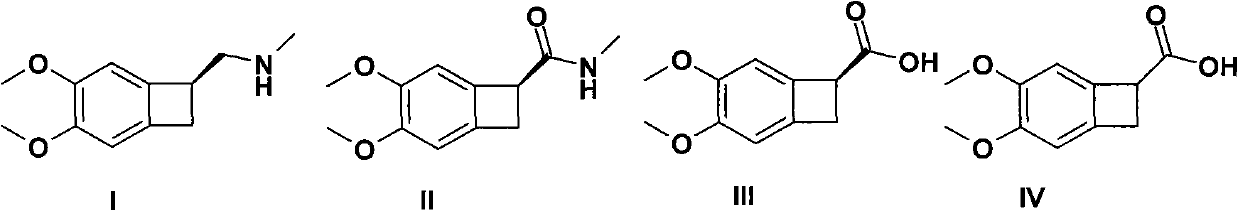
[0029] Embodiment 1: the synthesis of 4,5-dimethoxy-1-carboxybenzocyclobutane (compound IV)
[0030] 6 grams of 4,5-dimethoxy-1-cyanobenzocyclobutane (compound V) was added to 24 milliliters of potassium hydroxide in ethanol (2 mol / liter), and the temperature was controlled at 10-25 ℃, stirred overnight, added 8 ml of water, and refluxed for 3 hours. After cooling to 0-5°C with an ice-water bath, pour into 200 ml of water. Add 10 ml of concentrated hydrochloric acid, adjust the pH to 4-5, stir for 2 hours, filter, wash the filter residue with 100 ml of toluene, and vacuum-dry at 50°C for 24 hours. 6.0 g of a white solid (Compound IV) was obtained with a yield of 90%.
Embodiment 2
[0032] Add 20 grams of 4,5-dimethoxy-1-carboxybenzocyclobutane and 12 grams of (S)-(-)-α-phenethylamine into 200 ml of ethanol, stir, heat, and reflux for 2 hours , stop stirring, stop heating, cool down to 10-25°C, let stand for 5-10 hours, filter, take the solid, add 150 ml of ethanol, heat, reflux for 0.5 hours, stop stirring, stop heating, cool down to 10-25°C, Stand still for 5-10 hours, filter, adjust the solid to pH = 1.0 with dilute hydrochloric acid, and filter to obtain 8.2 g of white solid (Compound III), yield 41%;
[0033] e.e value: >99%
[0034] MS (ESI): 209 (M+1)
[0035] 1 HNMR (CDCl 3 ): δ 3.38 (2H, m), 3.83 (6H, s), 4.22 (2H, t, J=4Hz), 6.69 (s, 1H), 6.75 (s, 1H).
Embodiment 3
[0037] 20 grams of 4,5-dimethoxy-1-carboxybenzocyclobutane and 30 grams of cinchonidine were added to 300 milliliters of ethanol, stirred, heated, refluxed for 2 hours, stopped stirring, stopped heating, and cooled to 10-25°C, stand still for 5-10 hours, filter, take the solid, add 200 ml of ethanol, heat, reflux for 0.5 hours, stop stirring, stop heating, cool down to 10-25°C, stand still for 5-10 hours, filter, The solid was adjusted to pH = 1.0 with dilute hydrochloric acid, and filtered to obtain 6.7 g of white solid (Compound III), with a yield of 34%;
[0038] e.e value: >95%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com