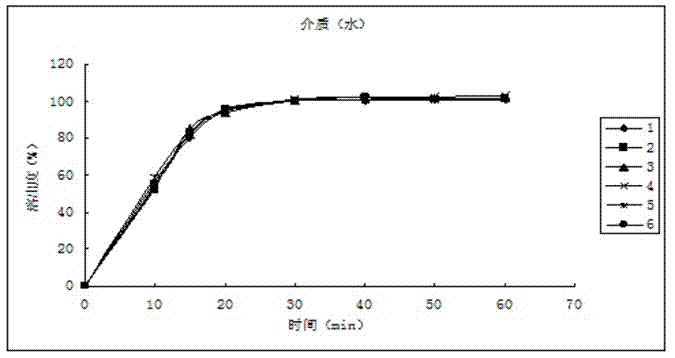
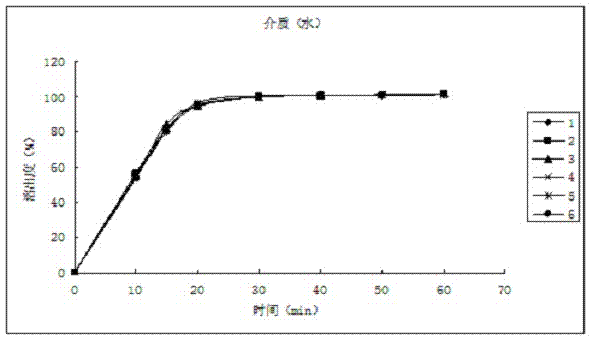
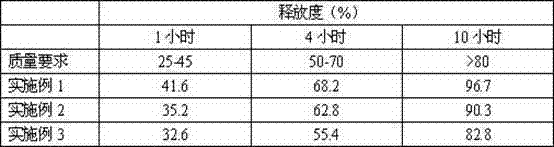
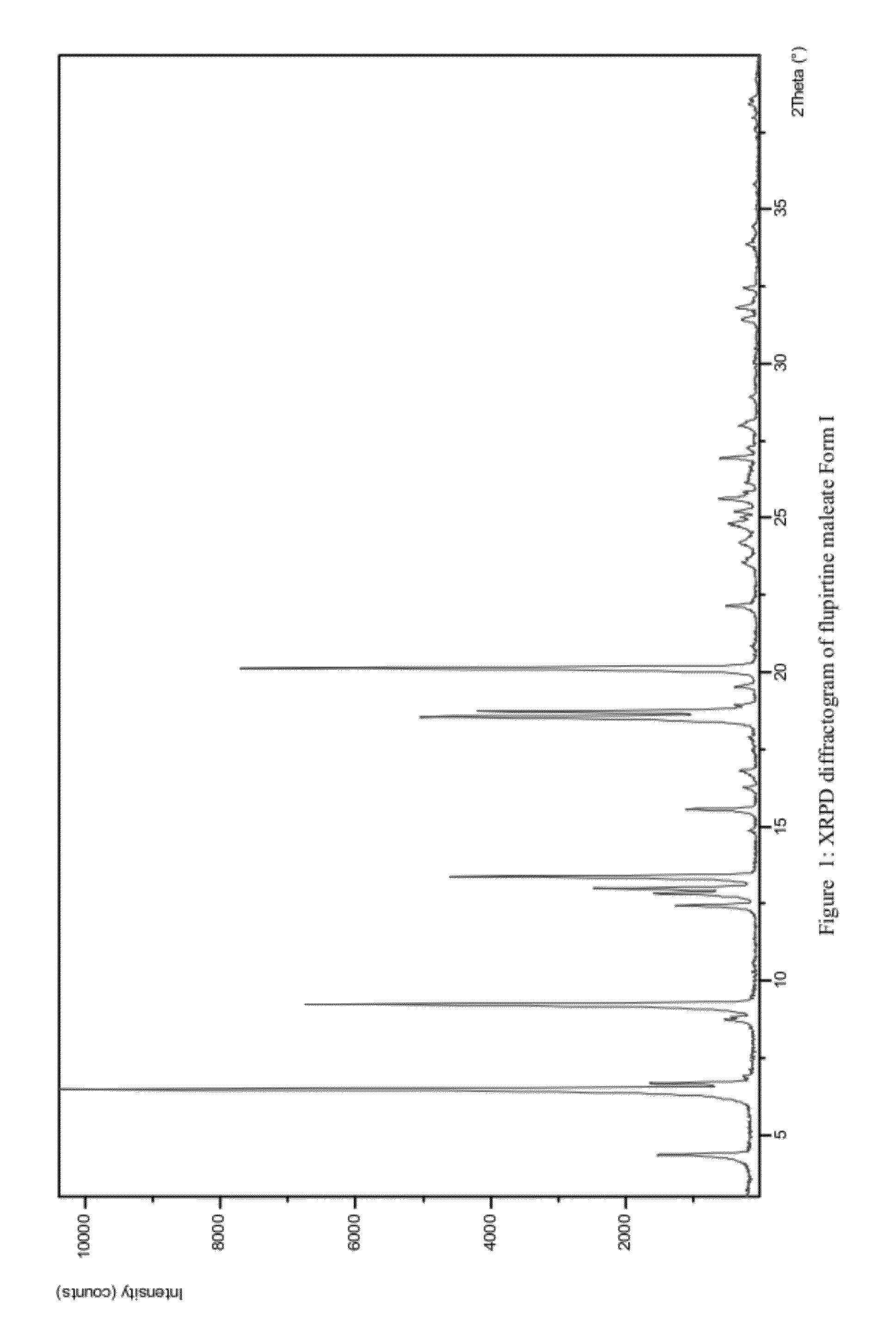
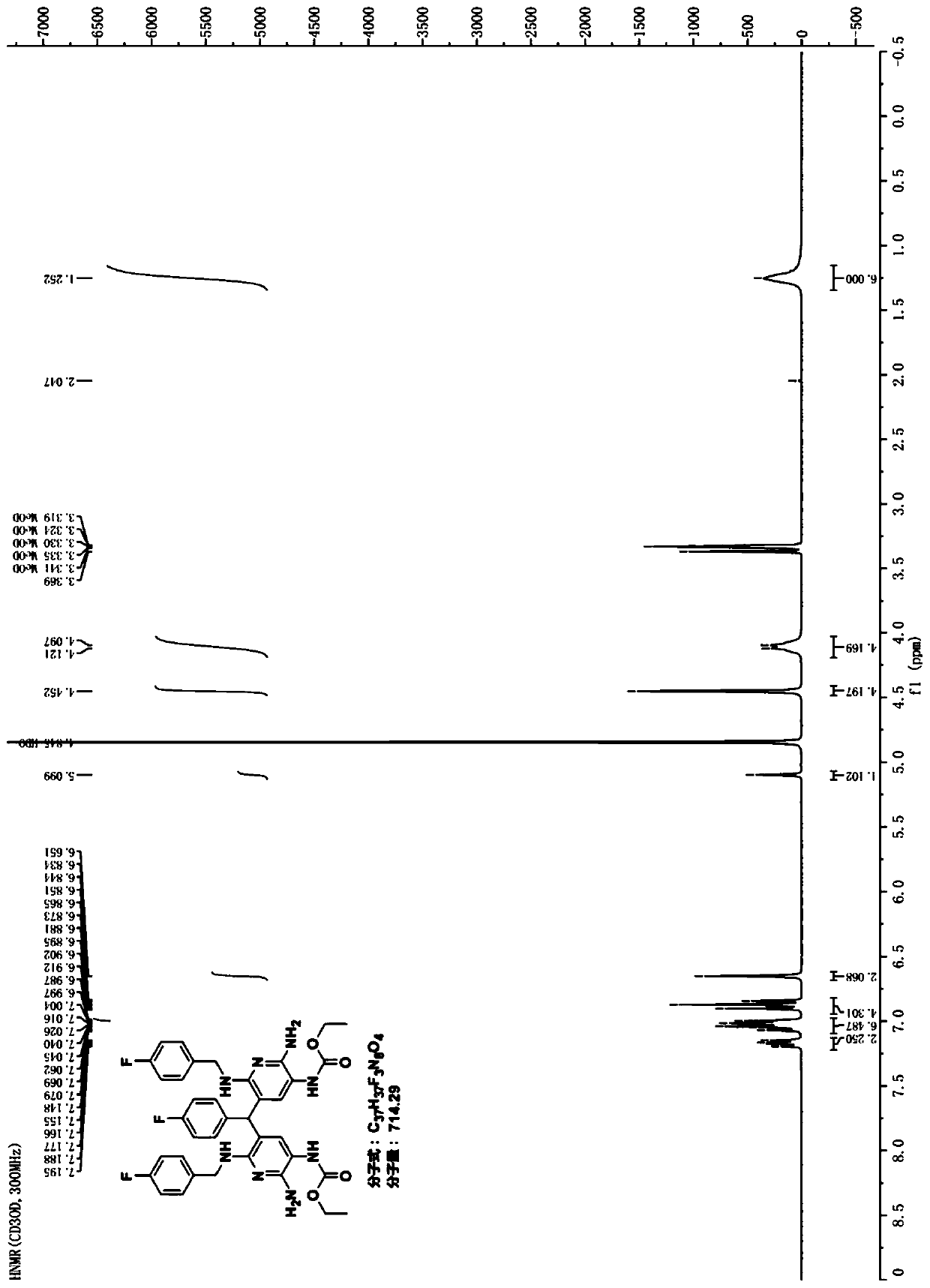
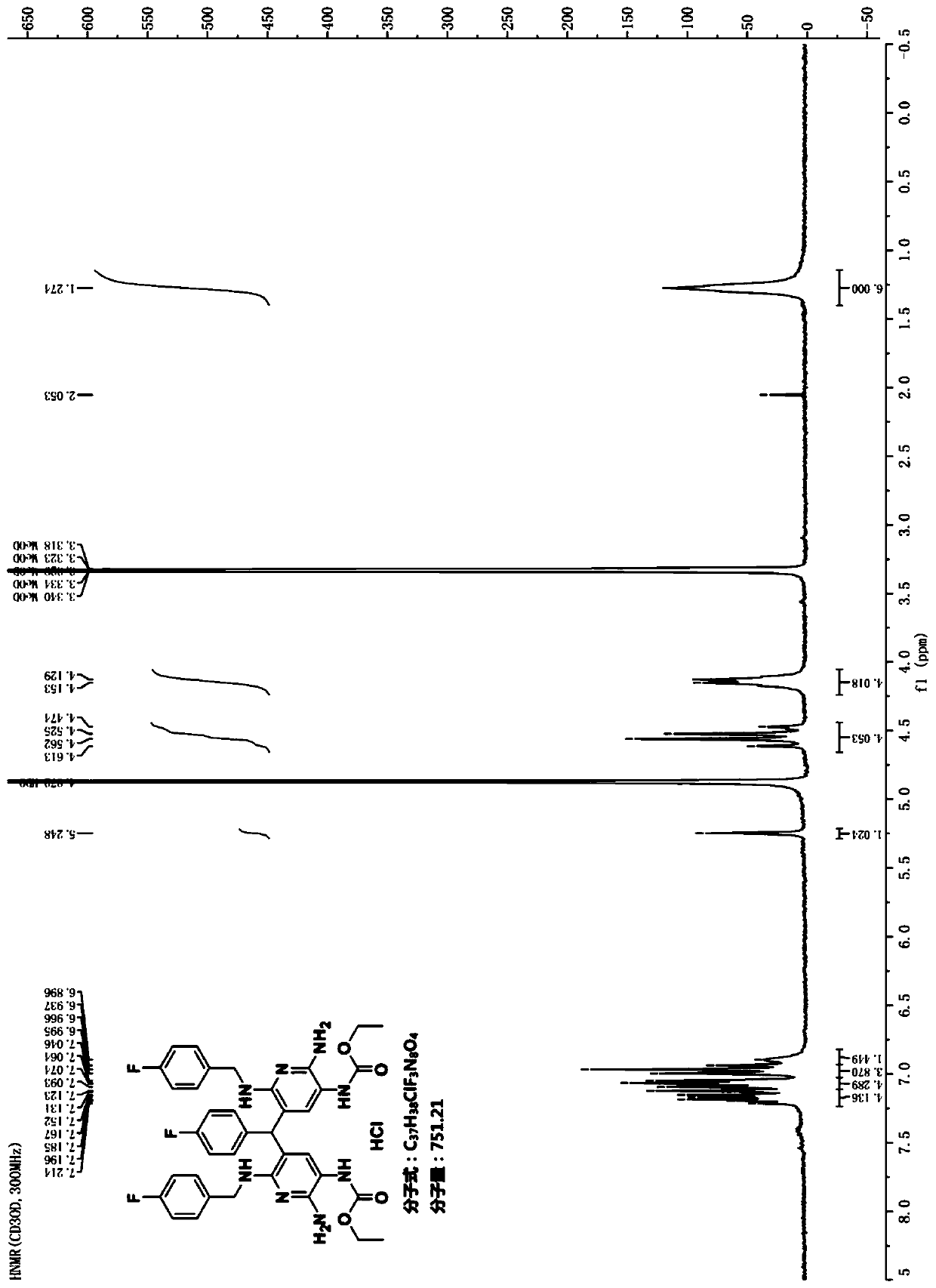
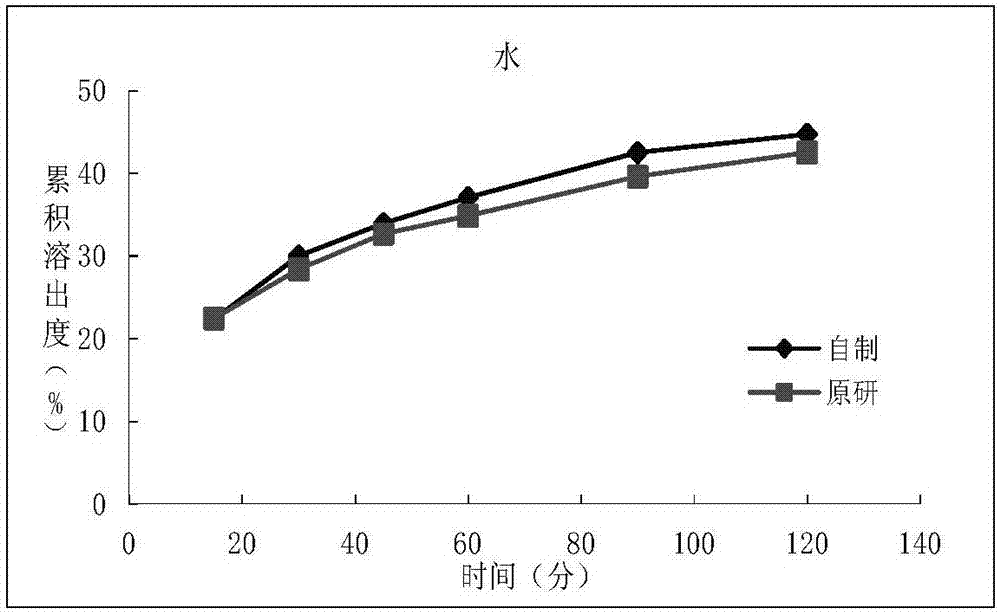
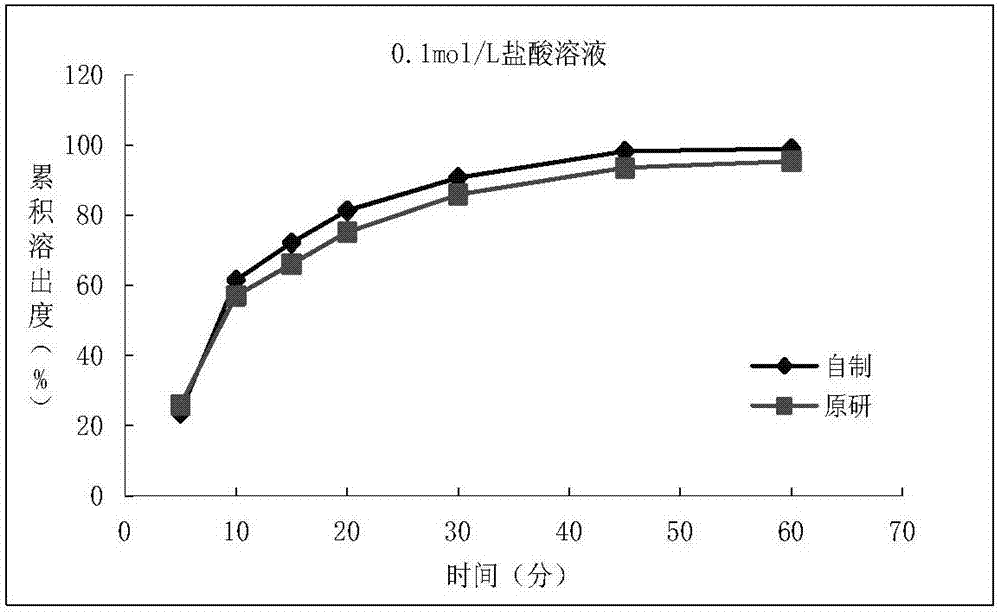
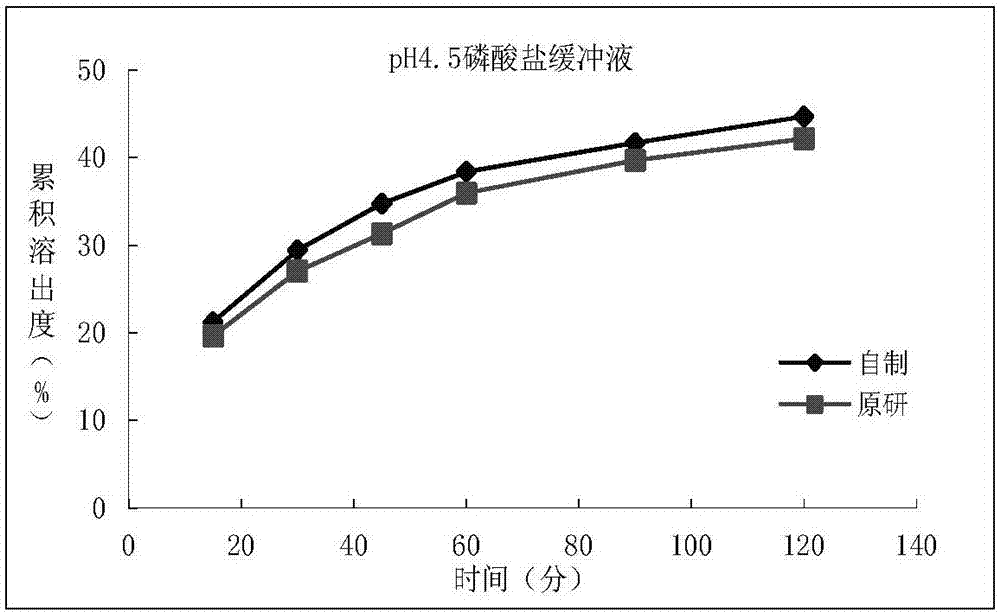
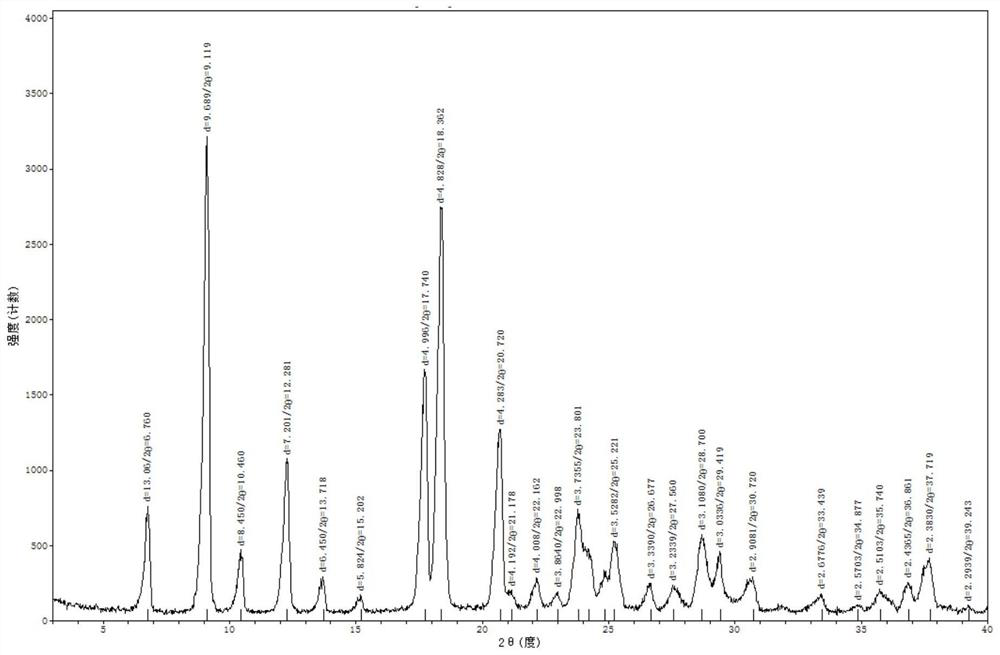
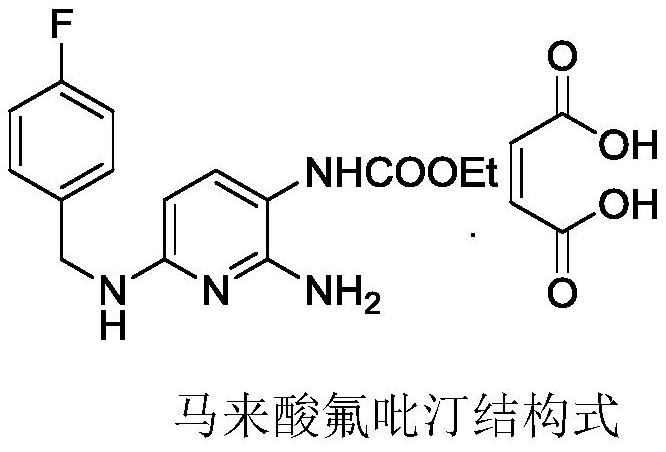
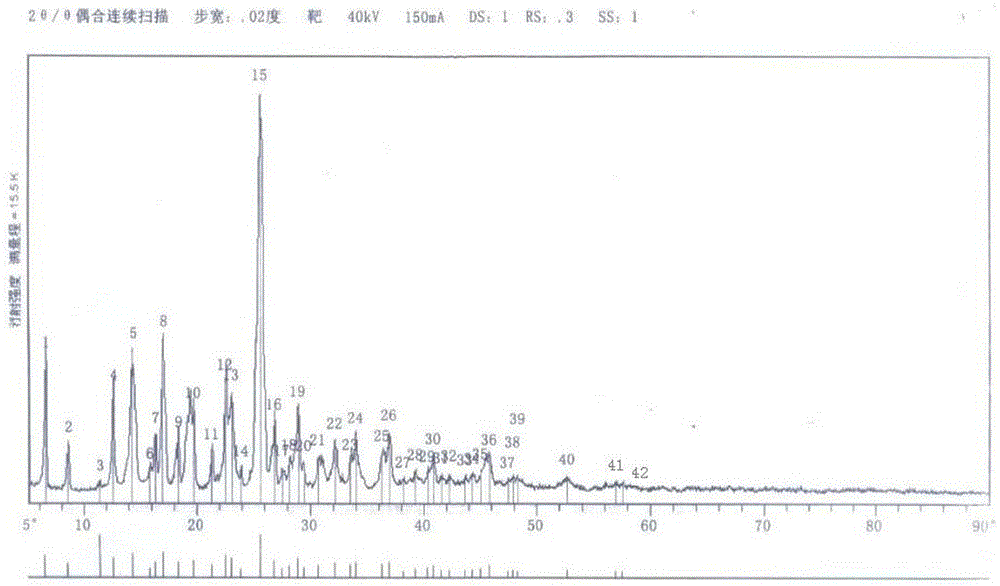
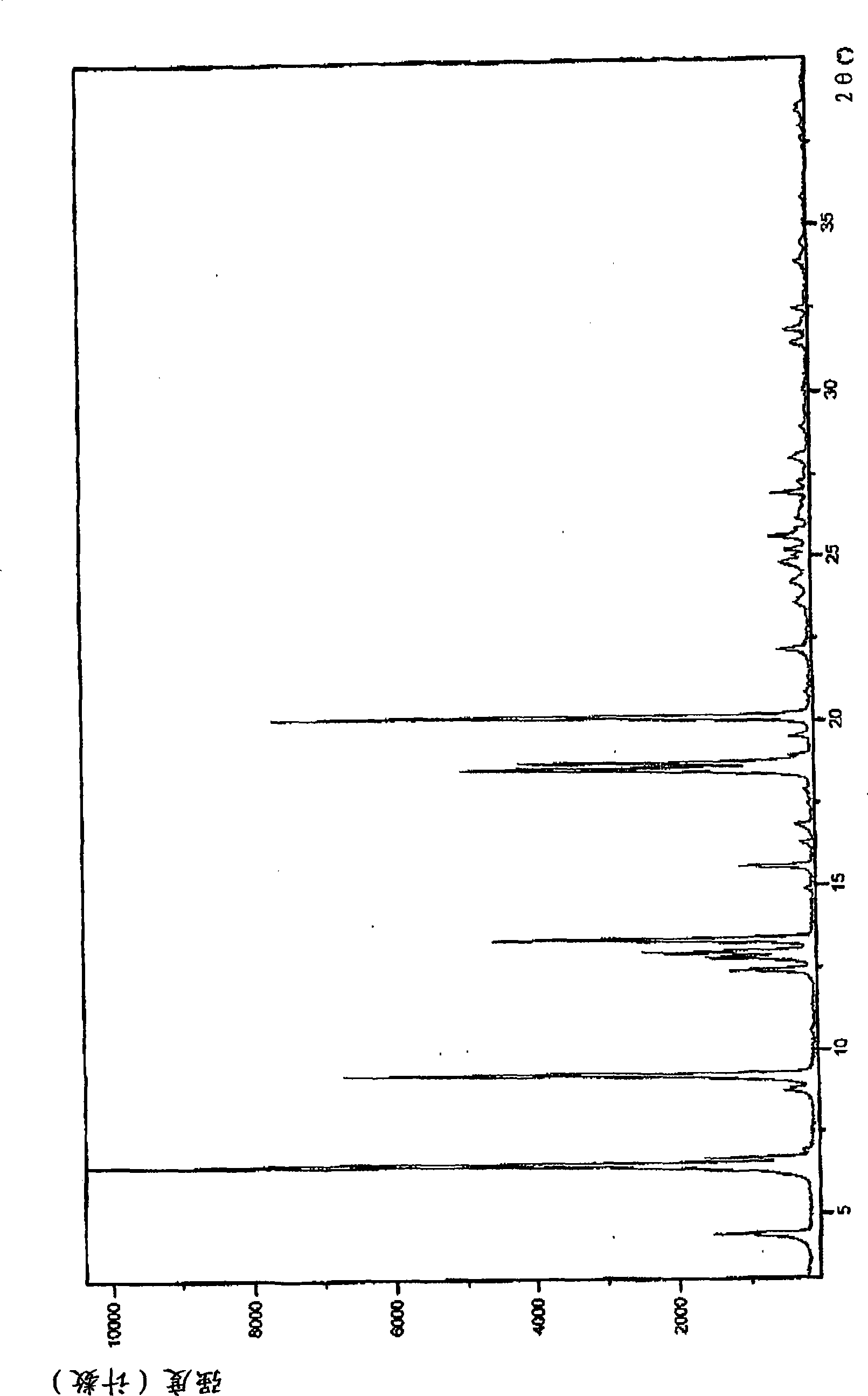
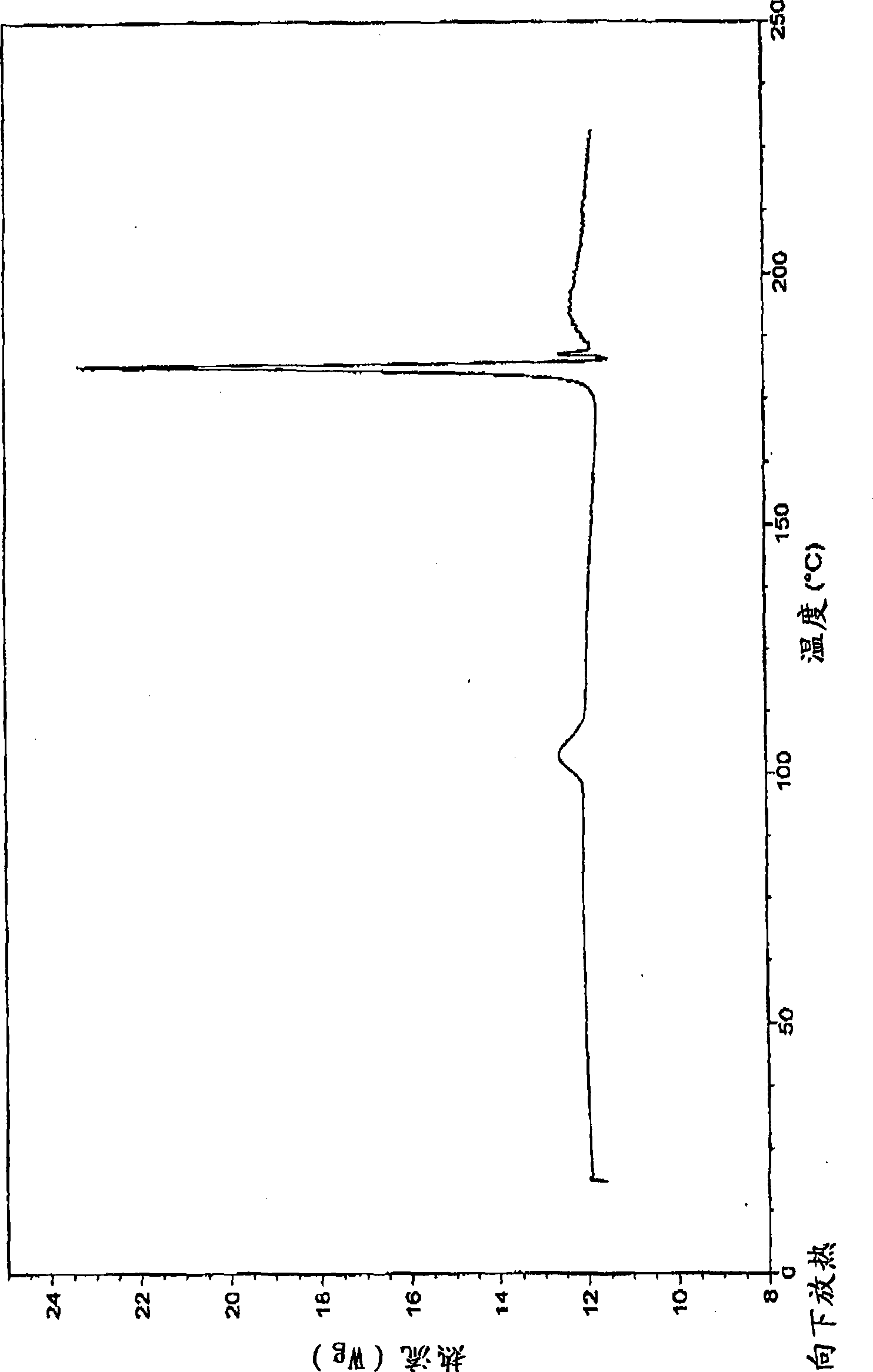
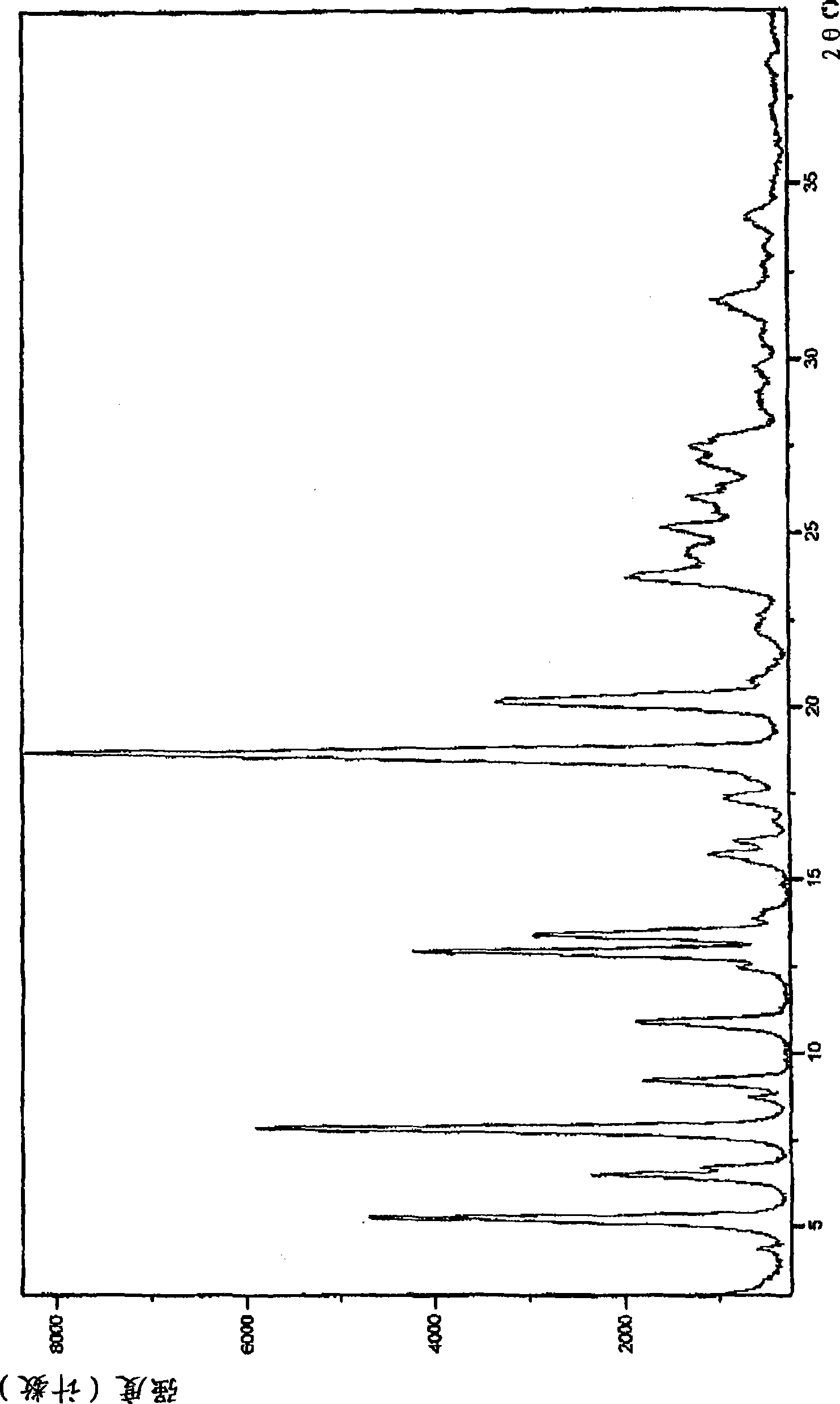
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
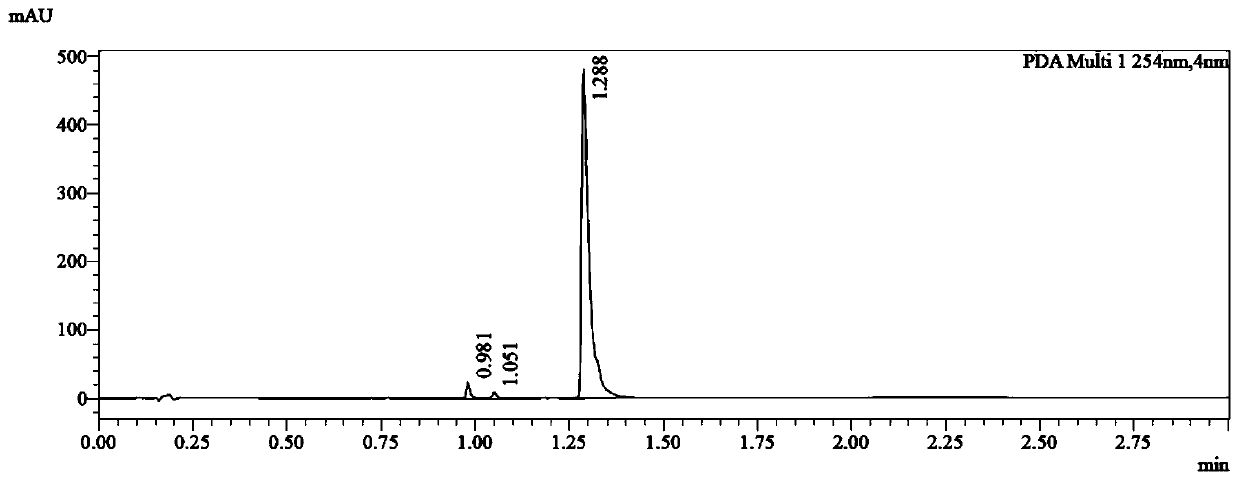
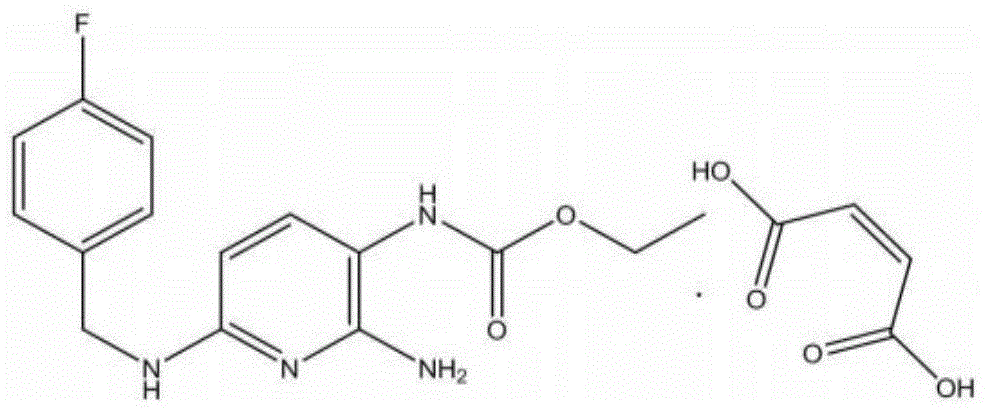
30 results about "FLUPIRTINE MALEATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis process of flupirtine maleate
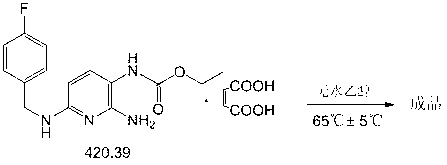
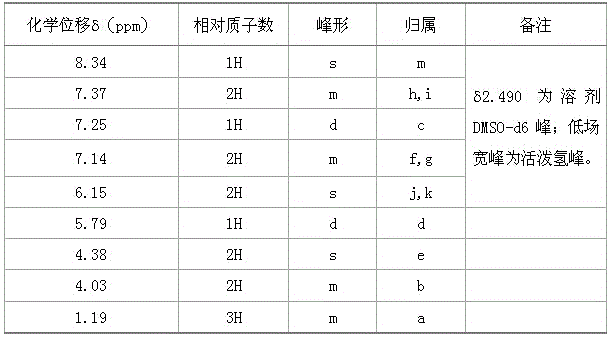
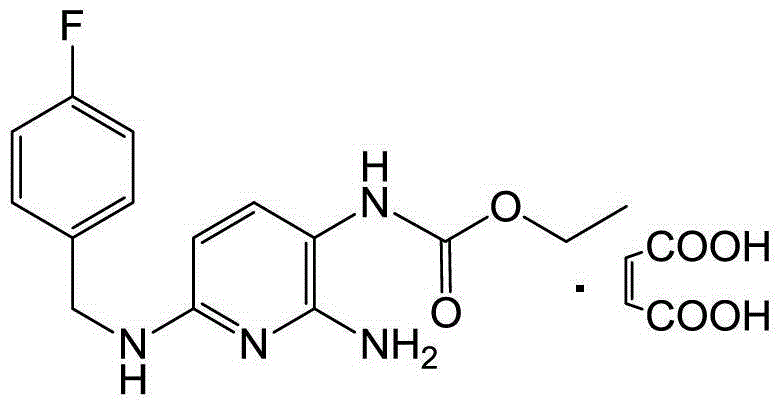
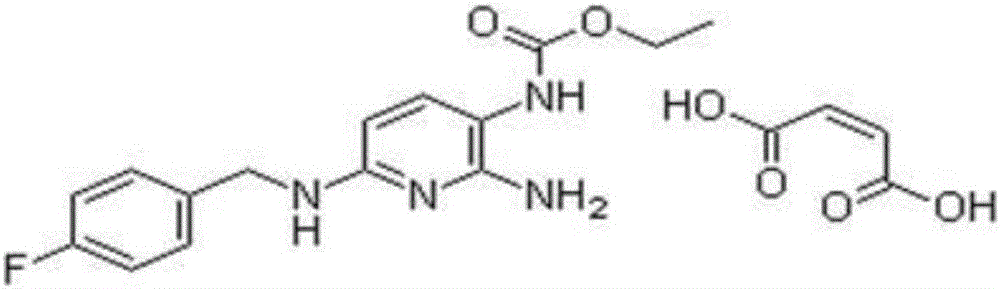
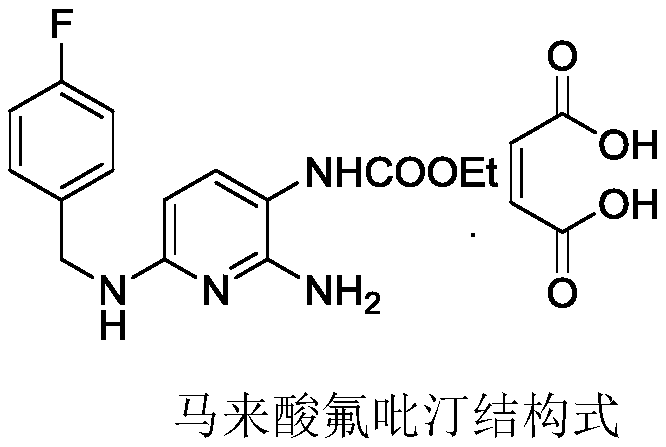
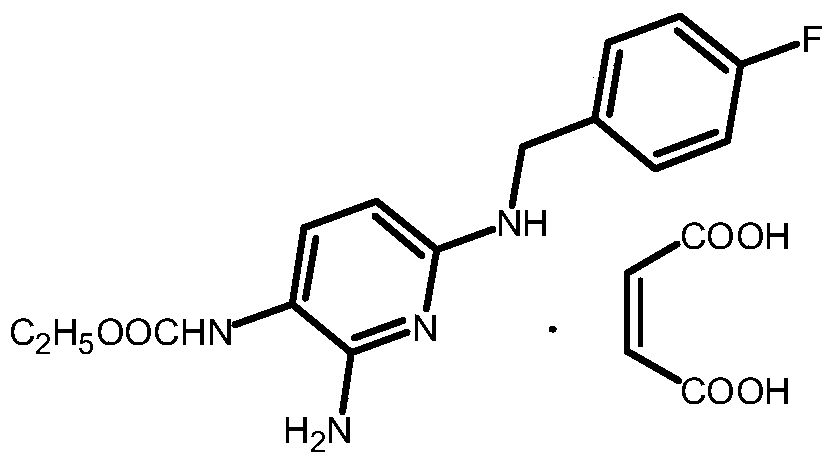
The invention relates to a preparation method of flupirtine maleate. The method comprises the following steps of: 1, synthesizing 2-amino-3-nitro-6-chloropyridine; 2, synthesizing 2-amino-3-nitro-6-[(4-luorobenzyl)amino] pyridine; 3, synthesizing 2,3-diamino-6-[(4-luorobenzyl)amino] pyridine; 4, synthesizing 2-amino-3-nitro-ethyl carbamate-6-[(4-luorobenzyl)amino] pyridine; 5, synthesizing 2-amino-6-[(4-luorobenzyl)amino] pyridine-3-ethyl carbamate maleate; and 6, refining flupirtine maleate.
Owner:北京华睿鼎信科技有限公司
Preparation method of flupirtine maleate
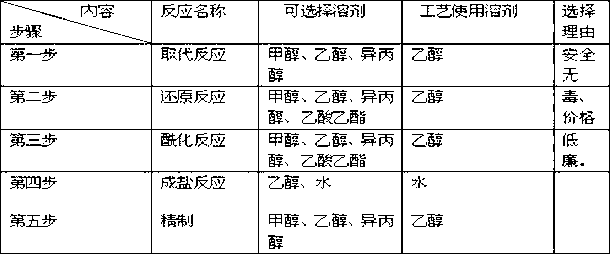
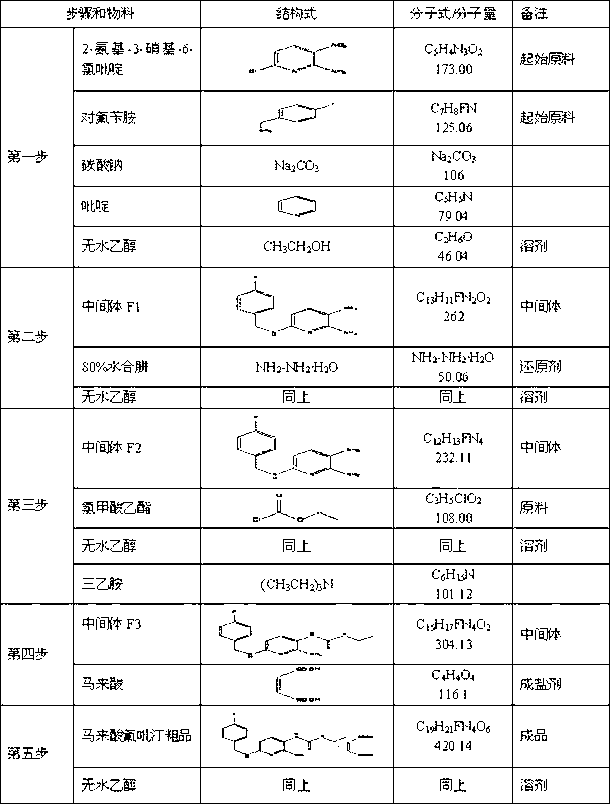
The invention provides a novel preparation method of flupirtine maleate; the method mainly comprises steps of substitution reaction, reduction reaction, acylation reaction, salt forming reaction and refining. It is proved by small and medium scale tests that a novel process route and reaction process are achievable, technical parameters are stable, reproducibility is good and product quality is stable.
Owner:SUZHOU ERYE PHARMA CO LTD
Synthesis method of flupirtine maleate
ActiveCN104086481AWhite appearanceHigh purityCarboxylic acid salt preparationCarboxylic compound separation/purificationSynthesis methodsReaction temperature
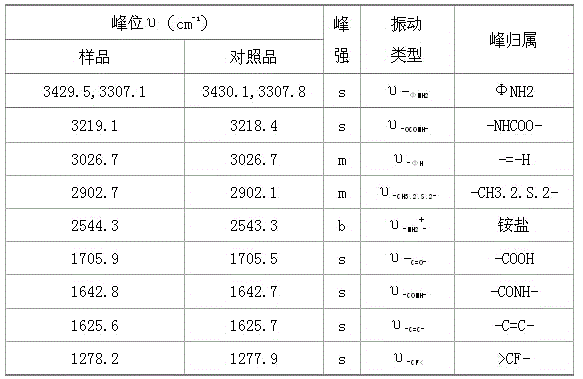
The invention provides a synthesis method of flupirtine maleate. Recrystallization by use of methanol is carried out in the refining step of the crude product of the flupirtine maleate so that the product is white in appearance and high in purity, and the crystal form of the product is pure A crystal and same as the crystal form of the commercial products. The optimal reaction solvent, reaction time and reaction temperature are explored and found out by use of a simplified process flow, and a method for preparing the flupirtine maleate in the pure A crystal form, which is high in yield, low in cost and simple to operate, uses easily available raw materials and is applicable to the industrial production is found.
Owner:SICHUAN XINSIDUN PHARMA
Synthesis method for flupirtine maleate compound
ActiveCN105541705AReduce manufacturing costReduce the possibilityOrganic chemistryEthyl chloroformateSynthesis methods
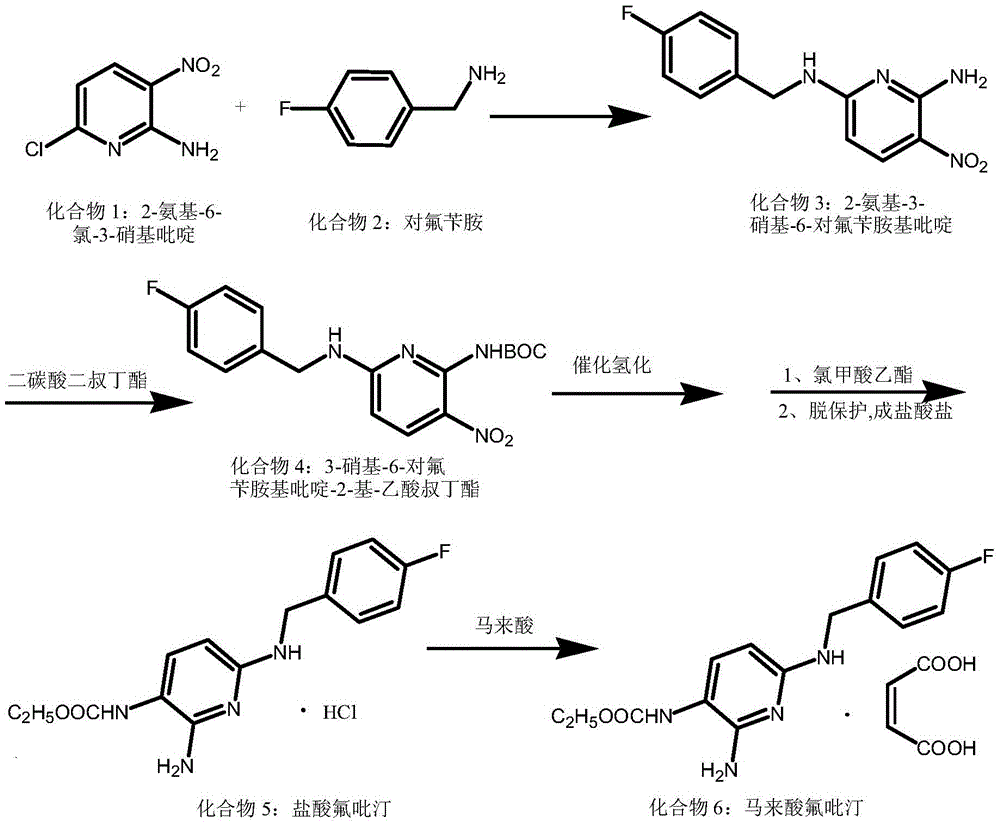
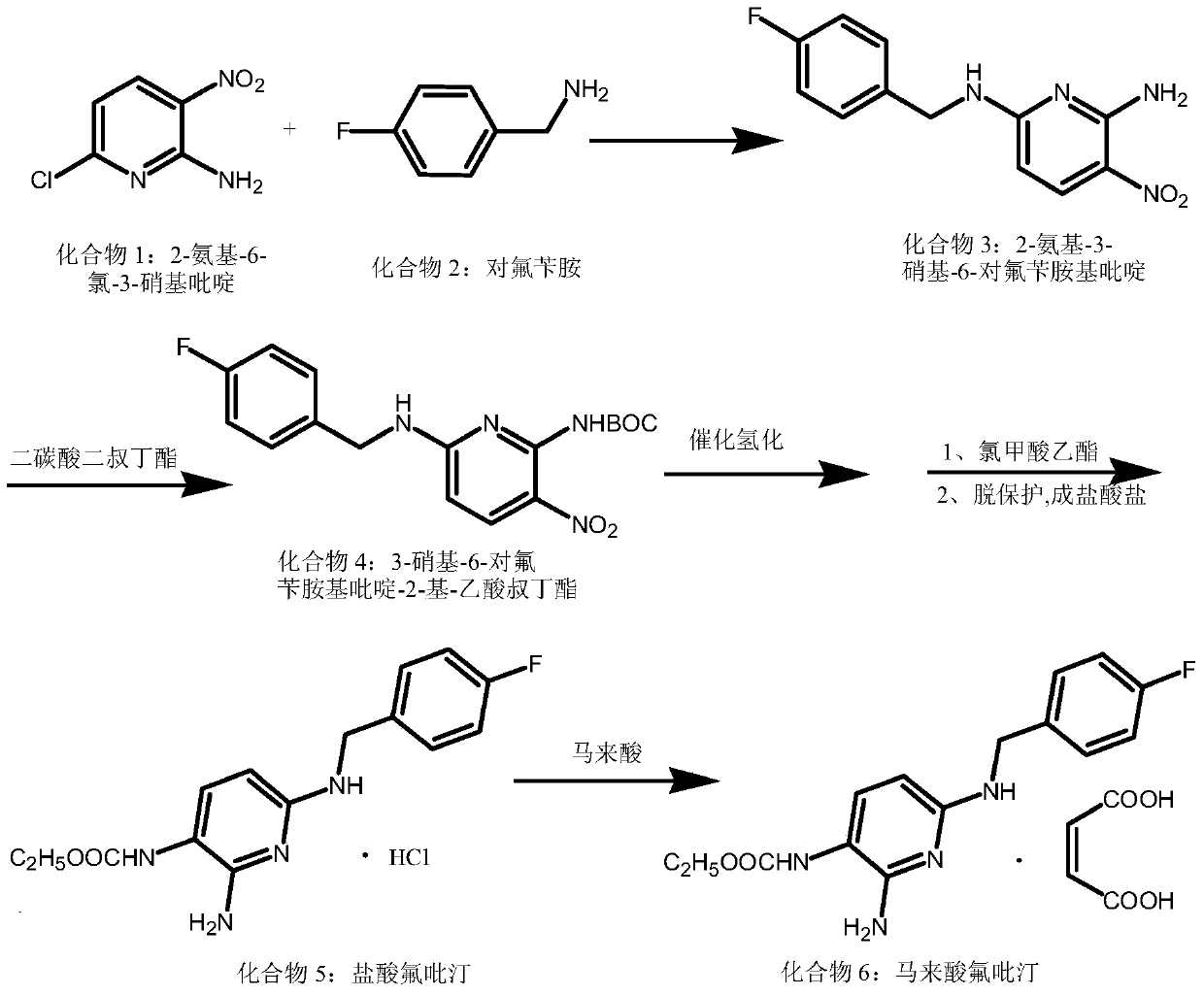
The invention discloses a synthesis method for a flupirtine maleate compound. The method comprises the steps that 2-amino-3-nitro-6-chloropyridine (a first compound) is used as a starting material to react with fluorobenzylamine (a second compound) to generate 2-amino-3-nitro-6-p-fluorobenzylamine pyridine (a third compound), the third compound is processed through di-tert-butyl dicarbonate ester protection to obtain 2-amino-3-nitro-6-p-fluorobenzylamine pyridine-3-based-tert-butyl acetate (a fourth compound), the fourth compound is processed through hydrogenation reduction and then react with ethyl chloroformate, after reacting is finished, deprotection is performed to obtain flupirtine hydrochloride (a fifth compound), and the fifth compound is salified with maleic acid to obtain flupirtine maleate (the sixth compound). According to the synthesis method, the starting material is cheap and easy to obtain, byproduct generation is avoided through amino protection, therefore, the impurity content is decreased, and the product quality is improved; catalytic reduction is performed by adopting palladium chloride, the reaction condition is mild, the reaction process is easy to operate, and the method is suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Method for preparing flupirtine maleate by one-pot method
ActiveCN103333103AAvoid generatingProcess stabilityCarboxylic acid salt preparationEthyl chloroformate4-fluorobenzylamine
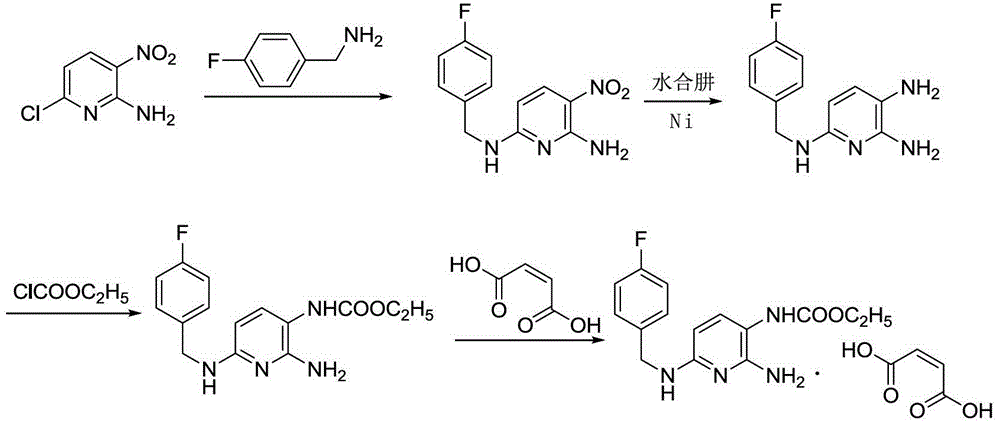
The invention discloses a method for preparing flupirtine maleate by a one-pot method. The method is characterized in that 2-amino-3-nitro-6-pyridine and 4-fluorobenzylamine serve as starting materials, and condensation, raney nickel reduction, ethyl chloroformate acylation and maleic acid salification are accomplished in a reactor without intermediate separation. The total yield of flupirtine maleate is increased from below 40% of a step-by-step method to above 70%, and the purity of a crude product is greater than 99%. According to the method, the technical flow is shortened, equipment is decreased, and the problem of high oxidation stain possibility of aminopyridine intermediates in a separation process is effectively solved. The feasibility and controllability of the one-pot method is proven by small-scale and pilot-scale studies, and the method is a handleable and environment-friendly manner to prepare flupirtine maleate, and is suitable for industrial production.
Owner:NANJING CHIA TAI TIANQING PHARMA
Flupirtine maleate sustained release tablet and preparation method thereof
InactiveCN104523634AHas pain reliefHave muscle relaxationNervous disorderAntipyreticPlastic packagingProlonged-release tablet
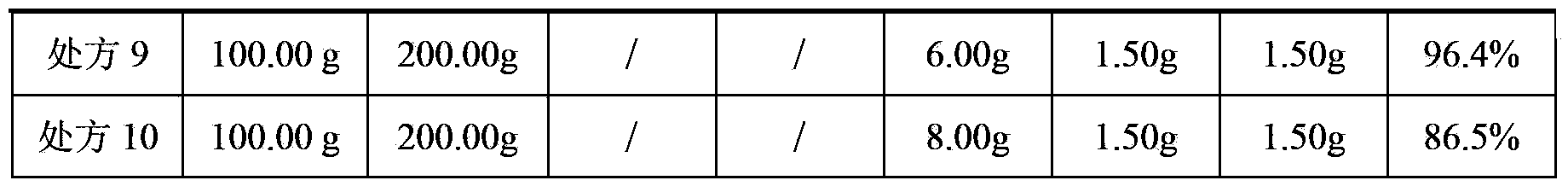
The invention discloses a flupirtine maleate sustained release tablet. The flupirtine maleate sustained release tablet is prepared from, by weight, 0.2 parts of flupirtine maleate, 70 to 190 parts of a sustained-release matrix material, and 10 to 20 parts of a lubricant. A preparation method of the flupirtine maleate sustained release tablet comprises following steps: material preparation, total blending, granulating, total blending, performing, and aluminum plastic packaging. The flupirtine maleate sustained release tablet is capable of relieving pain, relaxing muscle, and protecting nerve. According to the preparation method, novel sustained release preparations are adopted. According to the sustained release preparations, medicine releasing rate of medicines from the preparations is slowed, and absorption rate of the medicines in body is reduced, so that more stable treatment effects are achieved. Compared with oral liquid, the flupirtine maleate sustained release tablet is high in drug stability, and is convenient for packaging, transportation, and storing; and the preparation method is simple and convenient, and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
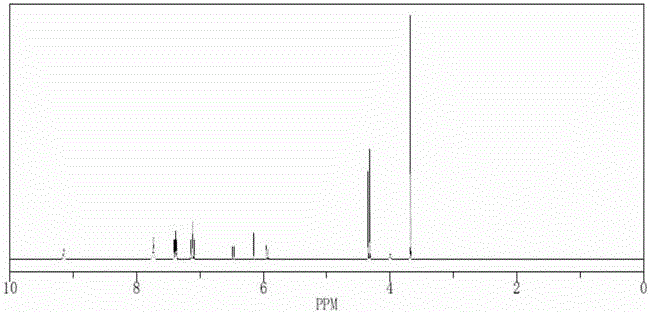
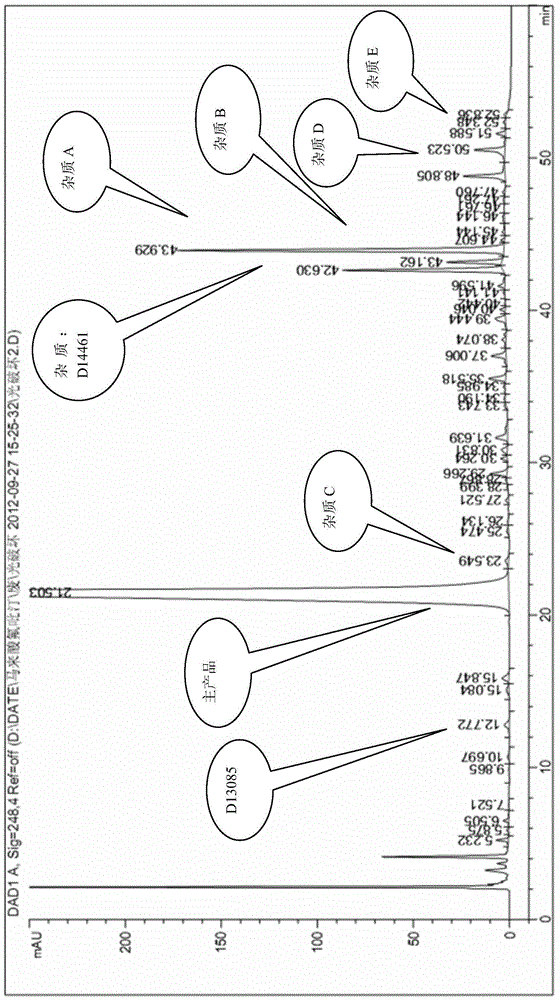
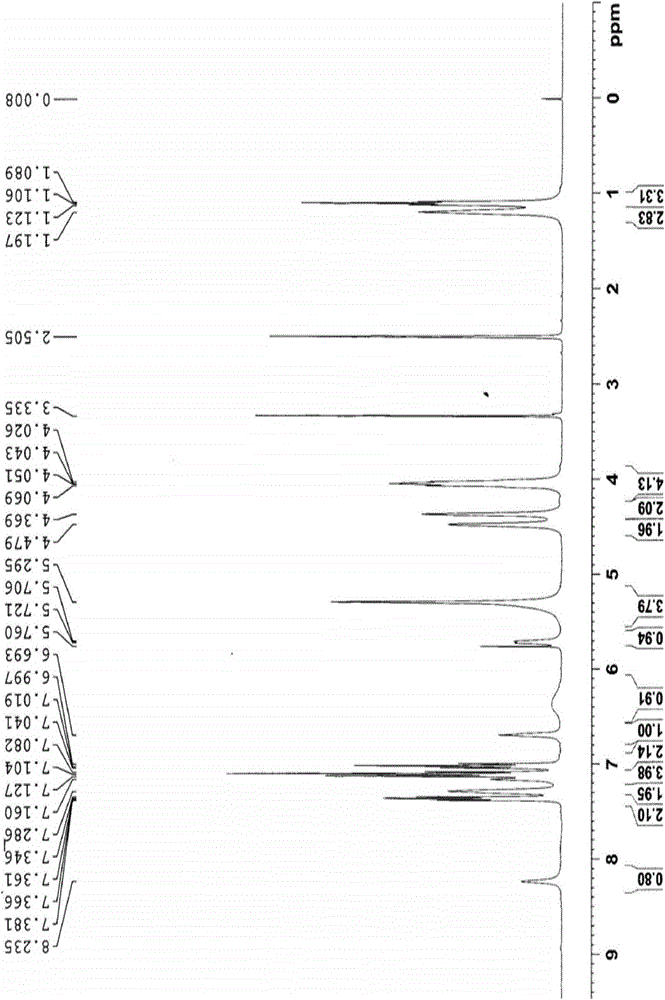
Reference compounds for flupirtine maleate analysis
ActiveCN103910674AHigh purityIncrease contentOrganic chemistryComponent separationCompound aMedicine
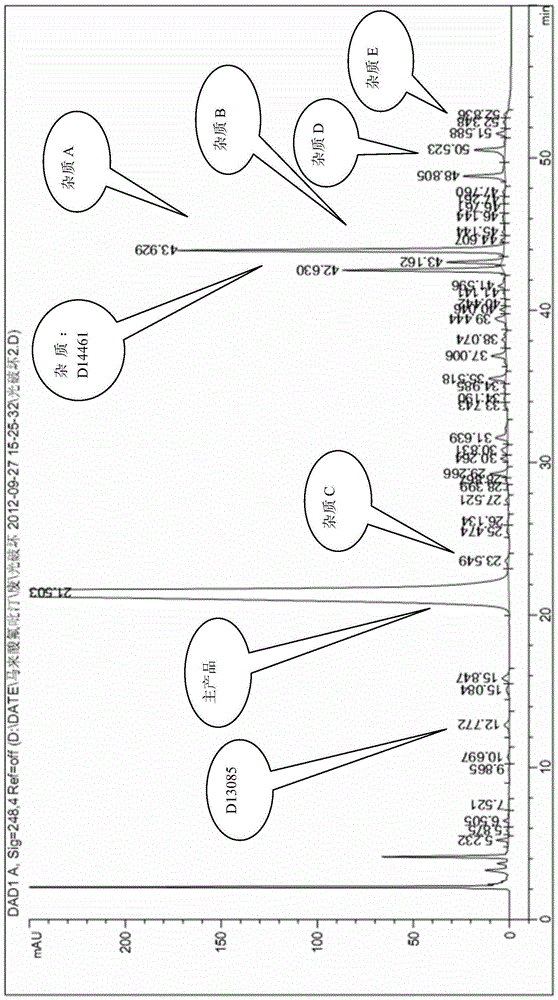
The invention belongs to the technical field of medicine and relates to impurity compounds A, B, C, D and E for flupirtine maleate analysis and a use of the impurity compounds in flupirtine maleate drug analysis.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Method for preparing flupirtine maleate capsule
ActiveCN102772388ALarge specific surface areaHigh dissolution rateNervous disorderMuscular disorderPyrrolidinonesMagnesium stearate
The invention discloses a method for preparing a flupirtine maleate capsule. The method comprises the following steps of: (A) weighting calcium hydrophosphate, crosslinking polyvinylpyrrolidone, lauryl sodium sulfate, magnesium stearate and superfine silica powder according to a formula and sieving through a sieve of 100 meshes; (B) performing superfine grinding on a flupirtine maleate raw material according to the formula until the particle size is 10 to 25 mu m, and uniformly mixing the treated flupirtine maleate raw material with the auxiliary materials in the step (A) according to an equivalent adding method; (C) granulating 50 percent of ethanol solution with the volume of 80 to 100 ml serving as an adhesive preparation soft material after sieving through a sieve of 20 meshes; (D) drying the wet particles prepared in the step (C) at the temperature of 55 to 65 DEG C for 2 to 4 hours, and arranging the particles after sieving through the sieve of 20 meshes; and (E) uniformly mixing the magnesium stearate and the superfine silica powder according to the formula, and filling to obtain the flupirtine maleate capsule. The method is ingenious in concept and simple in flow; the flupirtine maleate capsule is high in blood concentration, small in medicine amount and high in bioavailability; and the pain treating effect is obvious.
Owner:SICHUAN BAILI PHARM CO LTD
Flupirtine maleate sustained release tablet
ActiveCN102764257AQuick effectGuaranteed effective concentrationNervous disorderPharmaceutical delivery mechanismSustained Release TabletAdditive ingredient
The invention discloses a flupirtine maleate sustained release tablet which is composed of the following ingredients of: by weight, 90-110 parts of flupirtine maleate intermediate-release particles, 290-310 parts of flupirtine maleate sustained release particles, 25-35 parts of sodium carboxymethyl starch, 20-30 parts of aerosol, 3-8 parts of magnesium stearate and 15-25 parts of microcrystallinecellulose. Weight proportions of the flupirtine maleate intermediate-release particles and the flupirtine maleate sustained release particles are both weighed according to the weight of flupirtine maleate.
Owner:SICHUAN BAILI PHARM CO LTD
High efficient synthesis method of flupirtine maleate
InactiveCN106397313AHigh yieldHigh purityCarboxylic acid salt preparationCarboxylic compound separation/purificationEthyl chloroformateSynthesis methods
The invention provides a high efficient synthesis method of flupirtine maleate, and relates to the technical field of drug preparation. According to the synthesis method, flupirtine maleate is taken as the raw material; flupirtine maleate is obtained after steps of condensation reactions, reduction reactions (in the presence of a reducing agent), ethyl chloroformate acylation reaction, and maleic acid salt forming reactions; during the synthesis process, the yield of product can reach 93%, the product purity is high, the generation of byproducts is avoided, moreover, the technology route is simple, the reaction conditions are easy to control, better reaction conditions are provided for industrial production, the equipment requirements are largely reduced, and the synthesis method can be better applied to the industrial production.
Owner:合肥美利康医药技术股份有限公司
Method for synthesizing flupirtine maleate by use of one-pot process
ActiveCN104817494AOvercoming problems such as easy oxidation and discolorationReduce adverse effectsOrganic chemistryEthyl chloroformateFLUPIRTINE MALEATE
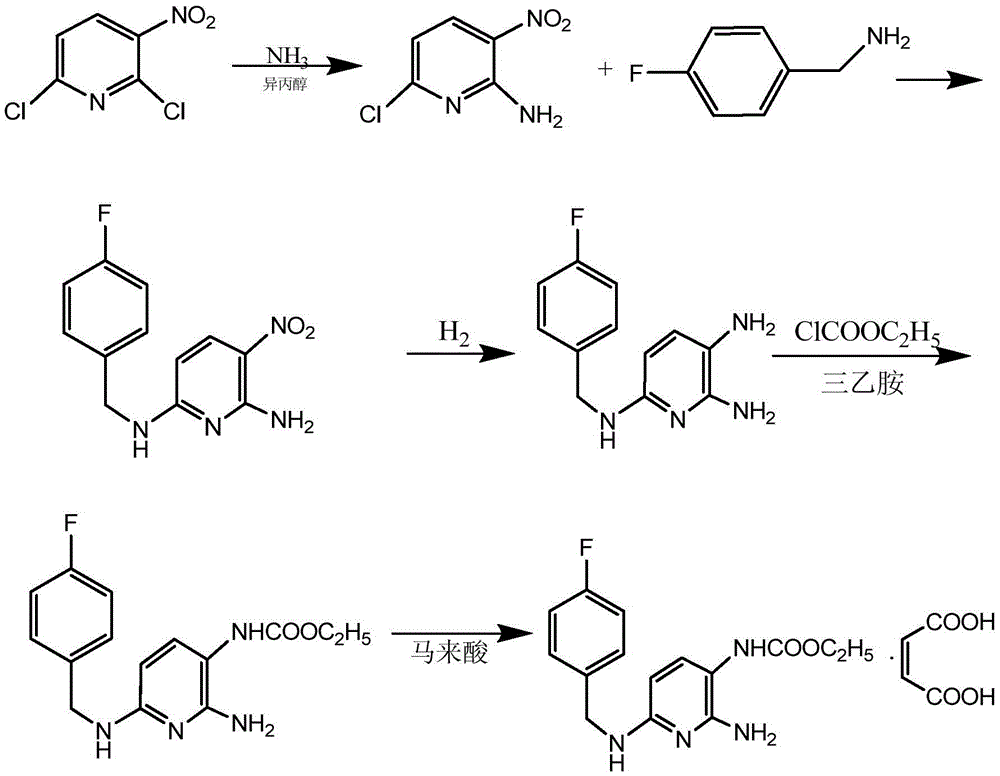
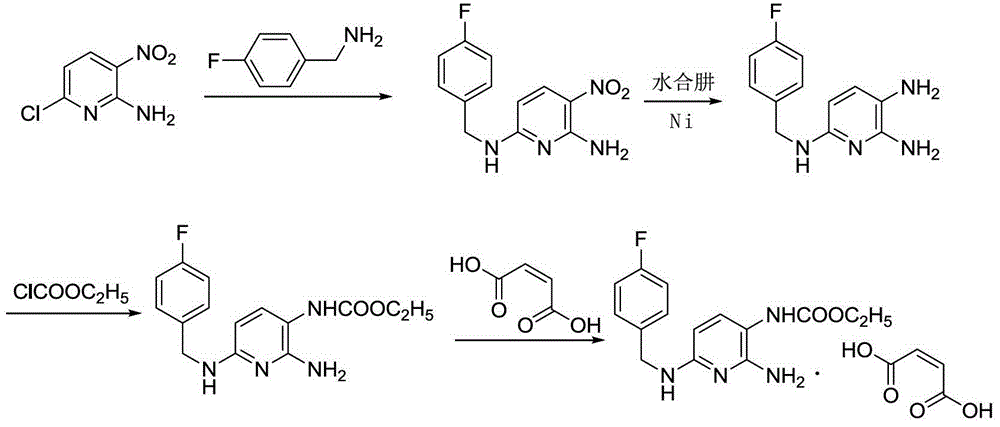
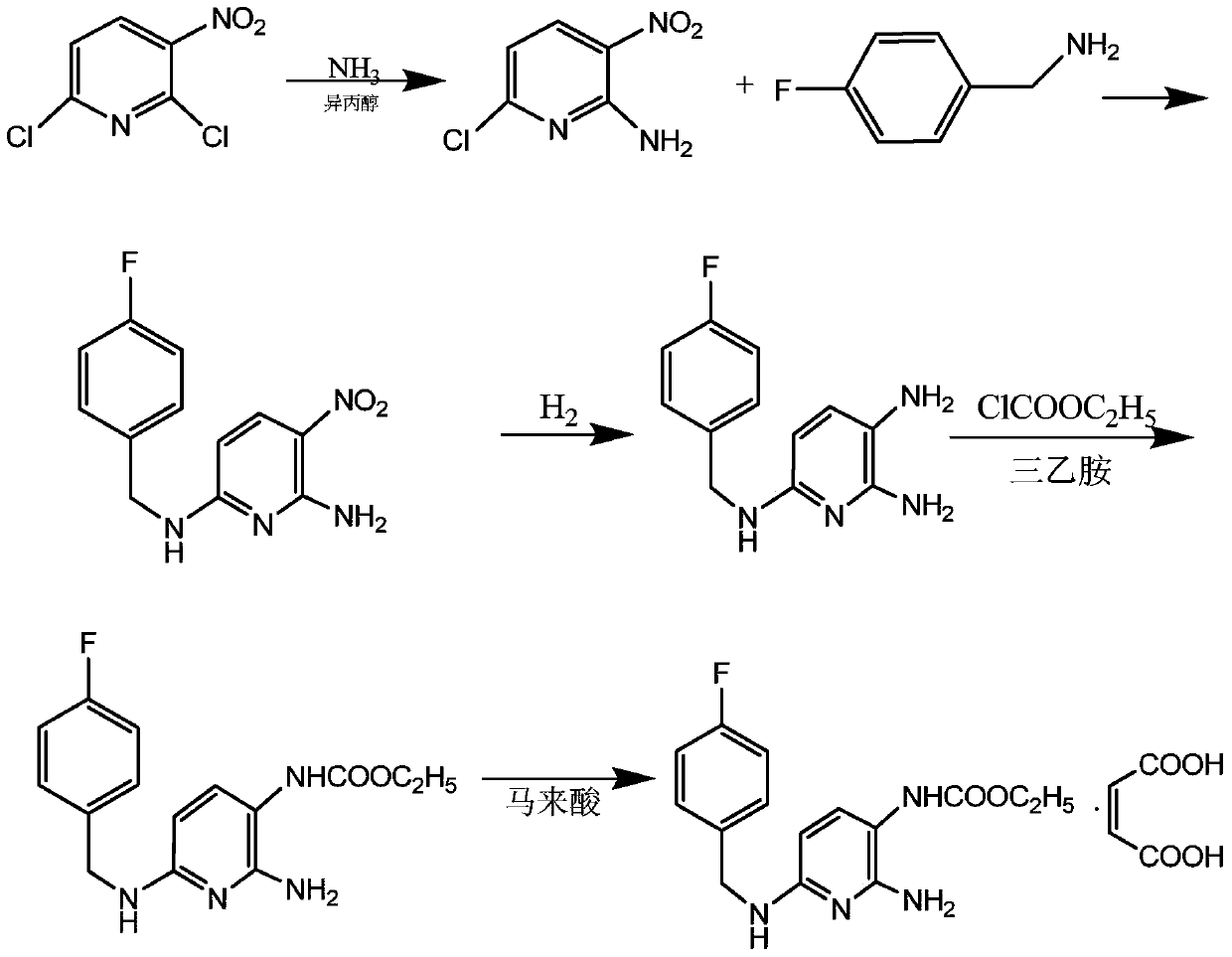
The invention discloses a method for synthesizing flupirtine maleate by use of a one-pot process. The method comprises the steps of taking 2,6-dichloro-3-nitropyridine as a starting material and performing ammonolysis to obtain a key intermediate 2,6-diamino-3-nitropyridine, and then condensing the key intermediate with p-fluorobenzaldehyde and performing Pd / C catalytic hydrogenation reduction, ethyl chloroformate acylation and maleic acid salifying to obtain the flupirtine maleate; the selected process route is simple and convenient to operate; the various steps are not separated and one-pot process production is realized; as a result, the production cost is effectively reduced, and the yield and the product purity are improved; in short, the method is suitable for industrial large-scale production.
Owner:江苏海岸药业有限公司
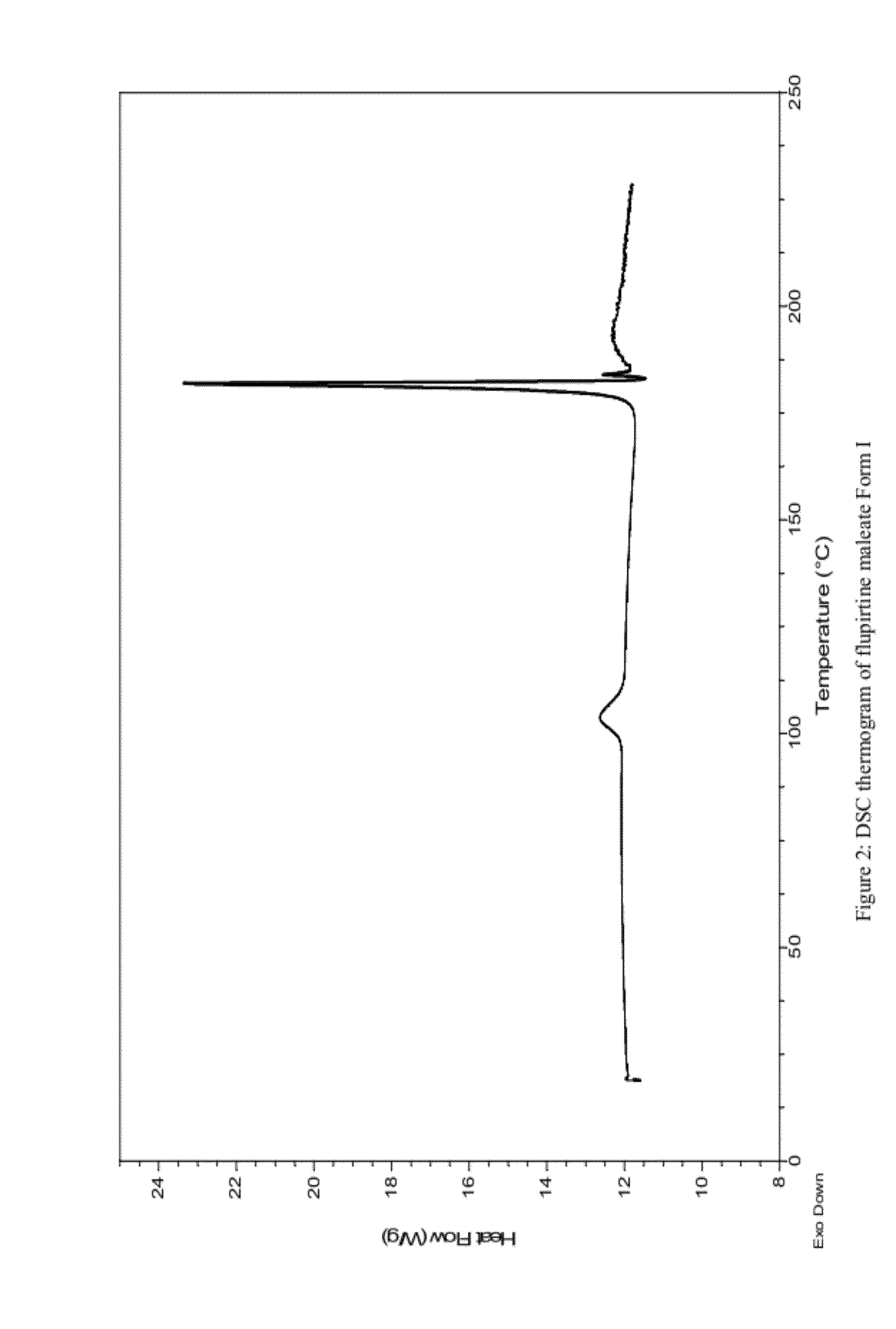
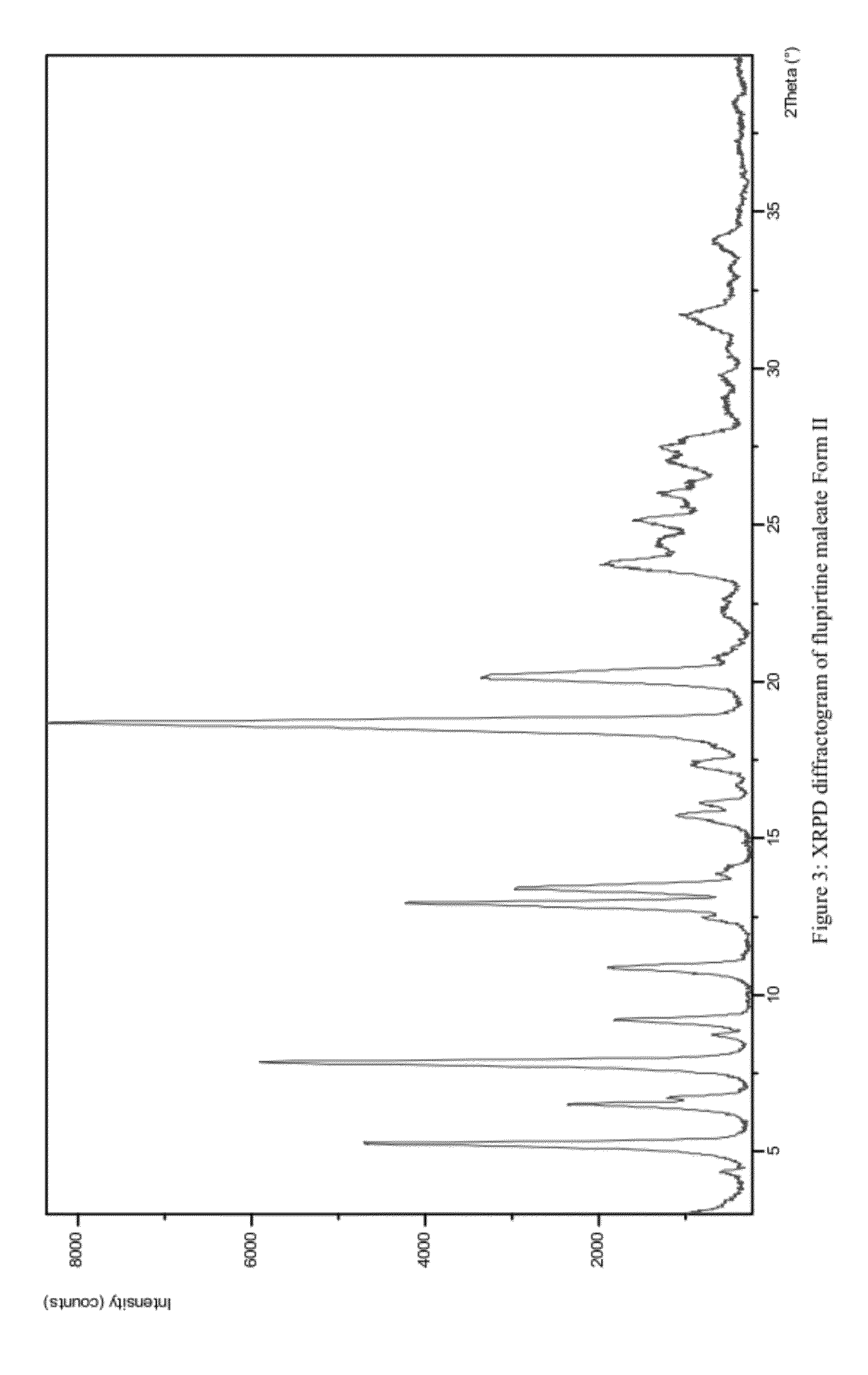
Pharmaceutically acceptable salt and polymorphic forms of flupirtine maleate
InactiveUS20120142743A1Maintain good propertiesPotent cyto and neuroprotective effectBiocideNervous disorderClinical psychologyFLUPIRTINE MALEATE
Owner:PLIVA HRVATSKA D O O
Flupirtine maleate capsule composition and preparation method thereof
ActiveCN104083335AEasy to handleReduced steps for ultrafine grindingNervous disorderMuscular disorderMedicineMedical prescription
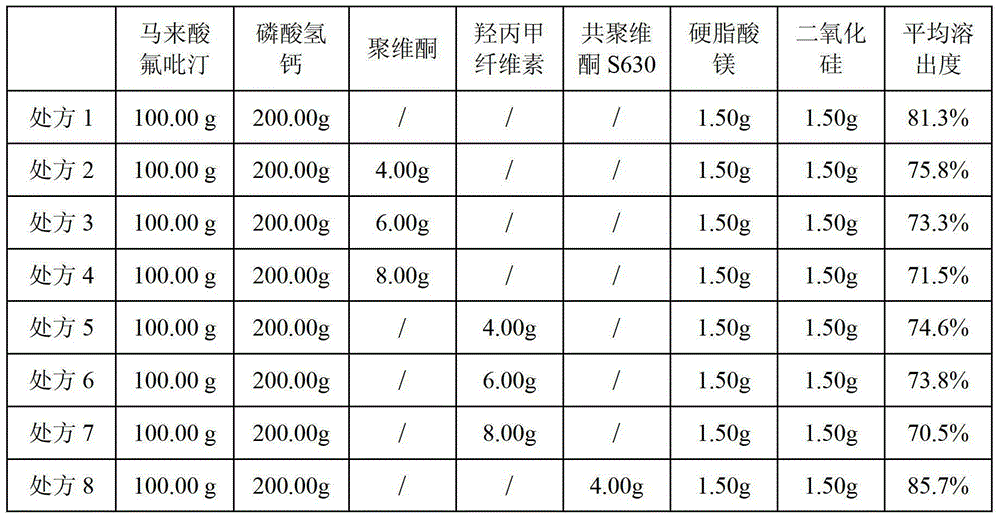
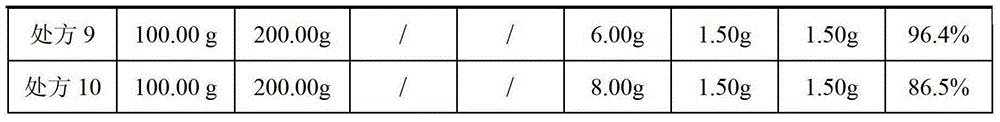
The invention provides a flupirtine maleate capsule composition. The flupirtine maleate capsule composition contains flupirtine maleate and a solubilizer. The solubilizer is copovidone S630. The invention also provides a flupirtine maleate capsule preparation method having simple processes and quality controllability. a flupirtine maleate raw material and calcium hydrophosphate are mixed and sieved by a sieve of 60 meshes so that raw material and auxiliary material uniform mixing is guaranteed, a yield is improved and a production cost is reduced. The preparation method utilizes a dry granulation technology so that granule bulk density is large, and compared with the existing granules having small bulk density, the same weight of the granules obtained by the preparation method can be put into a small-volume capsule. The products obtained by the preparation method and the formula have qualified quality and stable drug effects and are conducive to large-scale production realization.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Rivastigmine hydrogen tartrate preparation method and application thereof
InactiveCN106397314AMild reaction conditionsReduce manufacturing costCarboxylic acid salt preparationCarboxylic compound separation/purificationRivastigmine hydrogen tartrateFLUPIRTINE MALEATE
The invention relates to the technical field of medicinal chemistry production, in particular to a rivastigmine hydrogen tartrate preparation method and an application thereof. To be specific, rivastigmine hydrogen tartrate is prepared from 2-amino-3-nitro-6-chloropyridine and p-fluorobenzylamine used as raw materials, the reaction condition is mild, the production cost is low, and the method is simple to operate, adopts a preparation process with high controllability, has the low requirement for production equipment and is suitable for industrial production.
Owner:合肥美利康医药技术股份有限公司
Preparation method of flupirtine maleate
ActiveCN103274998APrevent oxidationAvoid it happening againCarboxylic acid salt preparationCarboxylic compound separation/purificationActivated carbonPurification methods
The invention relates to a preparation method of flupirtine maleate, which takes flupirtine as a raw material, and obtains flupirtine maleate pure products through salification and fine purification steps. By strictly controlling the use level and the reaction condition of maleic acid, the method greatly improves the yield of crude products, adopts a fine purification method integrating ultrafiltration and recrystallization, avoids product loss caused by adopting activated carbon, and greatly improves the purity and the yield of the flupirtine maleate pure products.
Owner:SUZHOU ERYE PHARMA CO LTD
Flupirtine maleate sustained release tablet
ActiveCN102764257BQuick effectGuaranteed effective concentrationNervous disorderPharmaceutical delivery mechanismSustained Release TabletStearic acid
The invention discloses a flupirtine maleate sustained release tablet which is composed of the following ingredients of: by weight, 90-110 parts of flupirtine maleate intermediate-release particles, 290-310 parts of flupirtine maleate sustained release particles, 25-35 parts of sodium carboxymethyl starch, 20-30 parts of aerosol, 3-8 parts of magnesium stearate and 15-25 parts of microcrystalline cellulose. Weight proportions of the flupirtine maleate intermediate-release particles and the flupirtine maleate sustained release particles are both weighed according to the weight of flupirtine maleate.
Owner:SICHUAN BAILI PHARM CO LTD
Method for preparing flupirtine maleate capsule
ActiveCN102772388BLarge specific surface areaHigh dissolution rateNervous disorderMuscular disorderPyrrolidinonesMagnesium stearate
The invention discloses a preparation method of flupirtine maleate capsules, comprising the following steps: A, taking the prescribed amount of calcium hydrogen phosphate, cross-linked polyvinylpyrrolidone, sodium lauryl sulfate, magnesium stearate, and micropowder silica gel , pass through 100 mesh sieves respectively; B, take the prescribed amount of flupirtine maleate raw material and carry out ultrafine pulverization treatment to 10-25um, then mix it with the auxiliary materials in step A according to the equal amount incremental method; C, take 80-100ml 50% ethanol solution is used as a binder to make soft materials, and granulated through a 20-mesh sieve; D. Dry the wet granules prepared in step C at a temperature of 55-65°C for 2-4 hours, and granulate through a 20-mesh sieve E. Add magnesium stearate and micropowder silica gel in the prescribed amount, mix well, and fill to obtain the required flupirtine maleate capsules. The invention has ingenious concept and simple process, and the prepared flupirtine maleate capsule has high blood drug concentration, small drug dose, high bioavailability and obvious pain treatment effect.
Owner:SICHUAN BAILI PHARM CO LTD
A kind of method of one-pot synthetic flupirtine maleate
ActiveCN104817494BOvercoming problems such as easy oxidation and discolorationReduce adverse effectsOrganic chemistryEthyl chloroformateFLUPIRTINE MALEATE
The invention discloses a method for synthesizing flupirtine maleate by use of a one-pot process. The method comprises the steps of taking 2,6-dichloro-3-nitropyridine as a starting material and performing ammonolysis to obtain a key intermediate 2,6-diamino-3-nitropyridine, and then condensing the key intermediate with p-fluorobenzaldehyde and performing Pd / C catalytic hydrogenation reduction, ethyl chloroformate acylation and maleic acid salifying to obtain the flupirtine maleate; the selected process route is simple and convenient to operate; the various steps are not separated and one-pot process production is realized; as a result, the production cost is effectively reduced, and the yield and the product purity are improved; in short, the method is suitable for industrial large-scale production.
Owner:江苏海岸药业有限公司
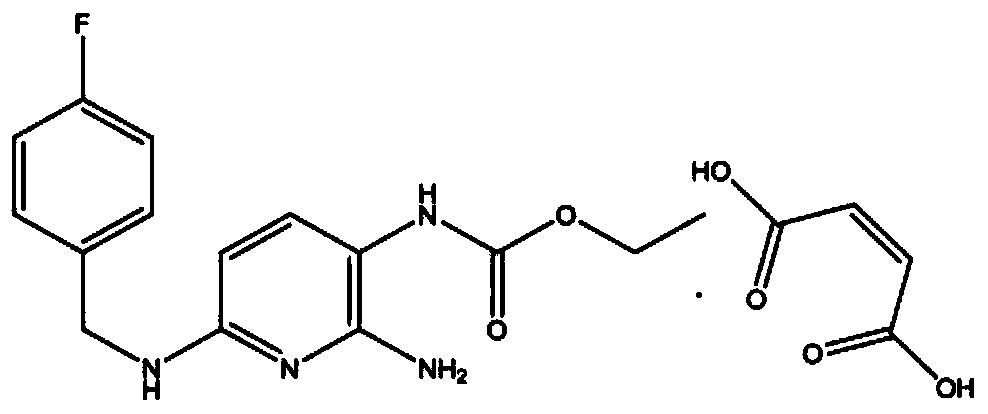
Preparation method of flupirtine derivative and preparation of inorganic acid salts of flupirtine derivative
The invention relates to a preparation method of a flupirtine derivative and a preparation of inorganic acid salts of the flupirtine derivative. The flupirtine derivative is shown in a chemical formula 1, wherein the flupirtine derivative is an important impurity produced in a flupirtine maleate synthesizing and / or storage process. By adopting the preparation methods, the flupirtine derivative andthe inorganic acid salts thereof can be prepared with high yields, and the reaction yield of each step can reach 55% or above.
Owner:西安都创医药科技有限公司
A kind of flupirtine maleate capsule composition and preparation method thereof
ActiveCN104083335BEasy to handleReduced steps for ultrafine grindingNervous disorderMuscular disorderMedicineSolvent
The invention provides a flupirtine maleate capsule composition. The flupirtine maleate capsule composition contains flupirtine maleate and a solubilizer. The solubilizer is copovidone S630. The invention also provides a flupirtine maleate capsule preparation method having simple processes and quality controllability. a flupirtine maleate raw material and calcium hydrophosphate are mixed and sieved by a sieve of 60 meshes so that raw material and auxiliary material uniform mixing is guaranteed, a yield is improved and a production cost is reduced. The preparation method utilizes a dry granulation technology so that granule bulk density is large, and compared with the existing granules having small bulk density, the same weight of the granules obtained by the preparation method can be put into a small-volume capsule. The products obtained by the preparation method and the formula have qualified quality and stable drug effects and are conducive to large-scale production realization.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
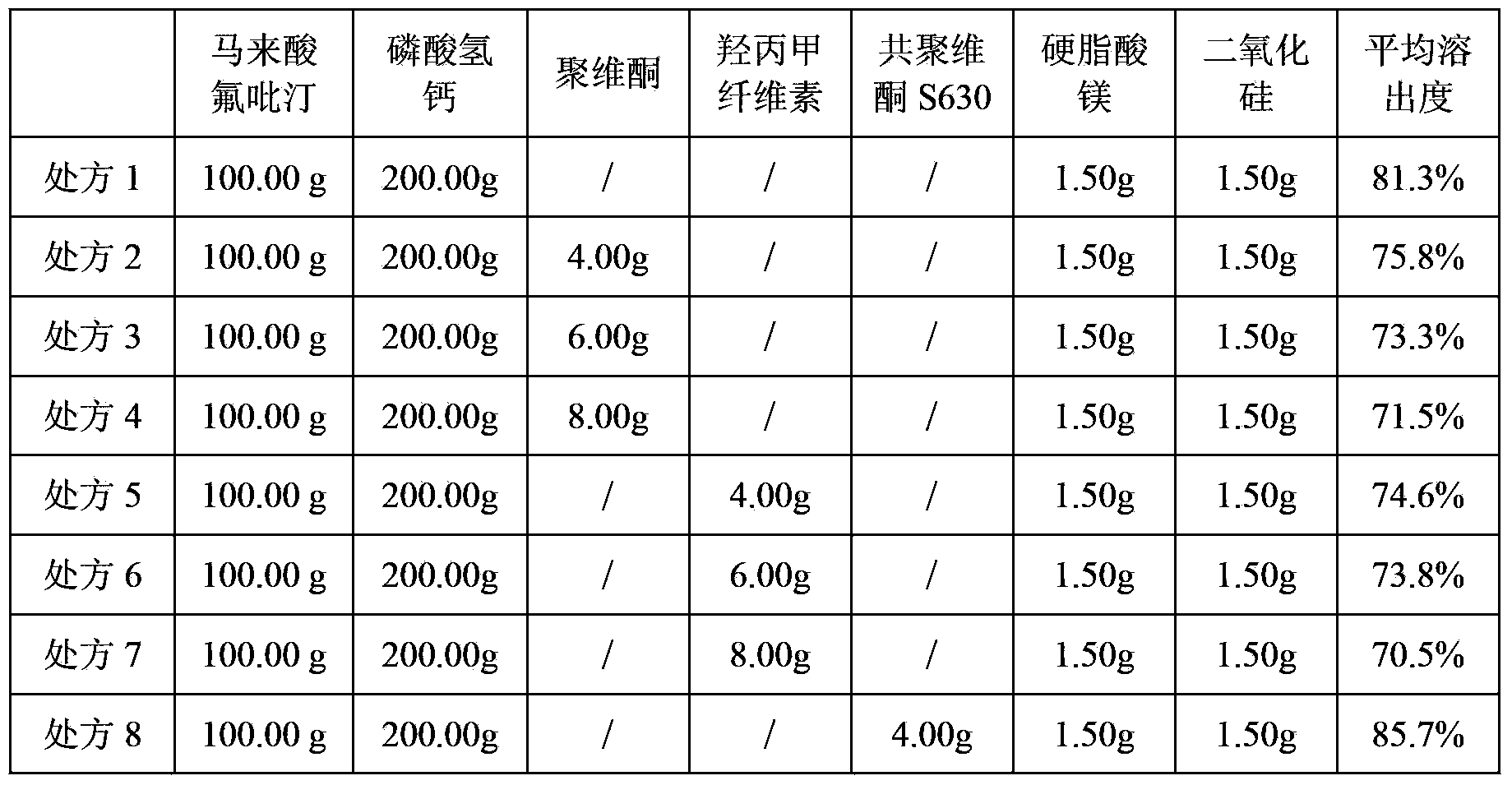
Composition containing flupirtine maleate and preparation method of composition
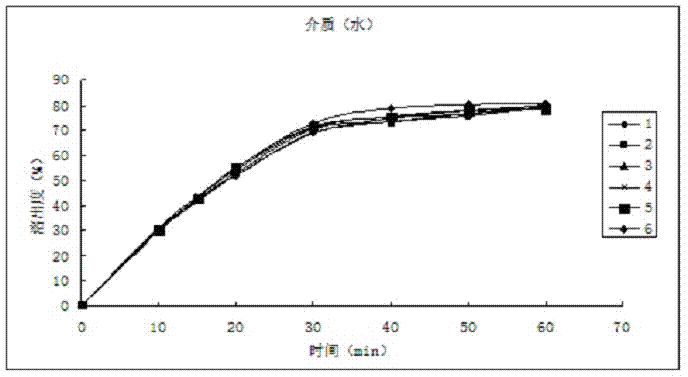
InactiveCN107412186APrevent unstableSimple processNervous disorderPharmaceutical non-active ingredientsClinical efficacyHigh humidity
The invention relates to composition containing flupirtine maleate and a preparation method of the composition. The composition is prepared from raw materials in percentage by weight as follows: 25%-35% of flupirtine maleate, 50%-70% of a diluent, 1.5%-4.5% of a disintegrating agent, 1.5%-4.5% of a flow aid, 0.5%-1.5% of a cosolvent and 0.5%-1.5% of a lubricant. The problem that the raw materials are unstable under illumination and high-humidity conditions is solved. The composition is stable in quality, the content of flupirtine maleate, related impurities and dissolution rate have no remarkable change, the in-vitro dissolution behavior is consistent with that of flupirtine maleate capsules from the German AWD company, the risk of clinical effect difference is reduced, the process is simple, and realization of industrial production is facilitated.
Owner:REYOUNG PHARMA
Preparation method of flupirtine maleate
ActiveCN103274998BPrevent oxidationAvoid it happening againCarboxylic acid salt preparationCarboxylic compound separation/purificationActivated carbonPurification methods
The invention relates to a preparation method of flupirtine maleate, which takes flupirtine as a raw material, and obtains flupirtine maleate pure products through salification and fine purification steps. By strictly controlling the use level and the reaction condition of maleic acid, the method greatly improves the yield of crude products, adopts a fine purification method integrating ultrafiltration and recrystallization, avoids product loss caused by adopting activated carbon, and greatly improves the purity and the yield of the flupirtine maleate pure products.
Owner:SUZHOU ERYE PHARMA CO LTD
A method for preparing flupirtine maleate with a crystal form high bulk density
ActiveCN109053562BHigh bulk densityCrystal content is stableOrganic chemistry methodsPhysical chemistryFLUPIRTINE MALEATE
Owner:SICHUAN QINGMU PHARMA CO LTD
Method for preparing polymorph-A flupirtine maleate with high bulk density
ActiveCN109053562AHigh bulk densityCrystal content is stableOrganic chemistry methodsSolventFLUPIRTINE MALEATE
The invention discloses a method for preparing polymorph-A flupirtine maleate with high bulk density. The method comprises the following steps: dissolving flupirtine by adopting a mixed solvent comprising DMSO and a solvent X, adding a maleic acid solution into flupirtine solution, stirring, after crystals are sufficiently and completely precipitated, filtering, washing, and drying, thus obtainingthe polymorph-A flupirtine maleate product with high bulk density. The prepared flupirtine maleate prepared in the method of the invention has high bulk density and stable crystal content, can meet the production requirement of high-quality preparations, and is high in process repetition, simple in process condition requirement, low in cost and more suitable for the industrialized production.
Owner:SICHUAN QINGMU PHARMA CO LTD
Synthesis process of flupirtine maleate
The invention relates to a preparation method of flupirtine maleate. The method comprises the following steps of: 1, synthesizing 2-amino-3-nitro-6-chloropyridine; 2, synthesizing 2-amino-3-nitro-6-[(4-luorobenzyl)amino] pyridine; 3, synthesizing 2,3-diamino-6-[(4-luorobenzyl)amino] pyridine; 4, synthesizing 2-amino-3-nitro-ethyl carbamate-6-[(4-luorobenzyl)amino] pyridine; 5, synthesizing 2-amino-6-[(4-luorobenzyl)amino] pyridine-3-ethyl carbamate maleate; and 6, refining flupirtine maleate.
Owner:北京华睿鼎信科技有限公司
Method for preparing flupirtine maleate by one-pot method
ActiveCN103333103BPrevent oxidationHigh purityCarboxylic acid salt preparationEthyl chloroformate4-fluorobenzylamine
Owner:NANJING CHIA TAI TIANQING PHARMA
Reference compound used in the analysis of flupirtine maleate
ActiveCN103910674BHigh purityIncrease contentOrganic chemistryComponent separationCompound aMedicine
The invention belongs to the technical field of medicine and relates to impurity compounds A, B, C, D and E for flupirtine maleate analysis and a use of the impurity compounds in flupirtine maleate drug analysis.
Owner:TIANJIN CHASE SUN PHARM CO LTD
A kind of synthetic method of flupirtine maleate
ActiveCN104086481BMild reaction conditionsEasy to operateCarboxylic acid salt preparationCarboxylic compound separation/purificationSynthesis methodsReaction temperature
The invention provides a synthesis method of flupirtine maleate. Recrystallization by use of methanol is carried out in the refining step of the crude product of the flupirtine maleate so that the product is white in appearance and high in purity, and the crystal form of the product is pure A crystal and same as the crystal form of the commercial products. The optimal reaction solvent, reaction time and reaction temperature are explored and found out by use of a simplified process flow, and a method for preparing the flupirtine maleate in the pure A crystal form, which is high in yield, low in cost and simple to operate, uses easily available raw materials and is applicable to the industrial production is found.
Owner:SICHUAN XINSIDUN PHARMA
Pharmaceutically acceptable salt and polymorphic forms of Flupirtine maleate
InactiveCN101506169APrevent muscle tensionPrevent muscle crampsNervous disorderOrganic chemistryMedicinePharmaceutical drug
The present invention is concerned with new polymorphic forms of flupirtine maleate, processes for preparing the new polymorphic forms, pharmaceutical compositions containing them, therapeutic uses thereof and methods of treatment employing them.
Owner:普利瓦·赫尔瓦茨卡有限责任公司
A kind of synthetic method of flupirtine maleate compound
ActiveCN105541705BReduce manufacturing costReduce the possibilityOrganic chemistryEthyl chloroformateSynthesis methods
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com