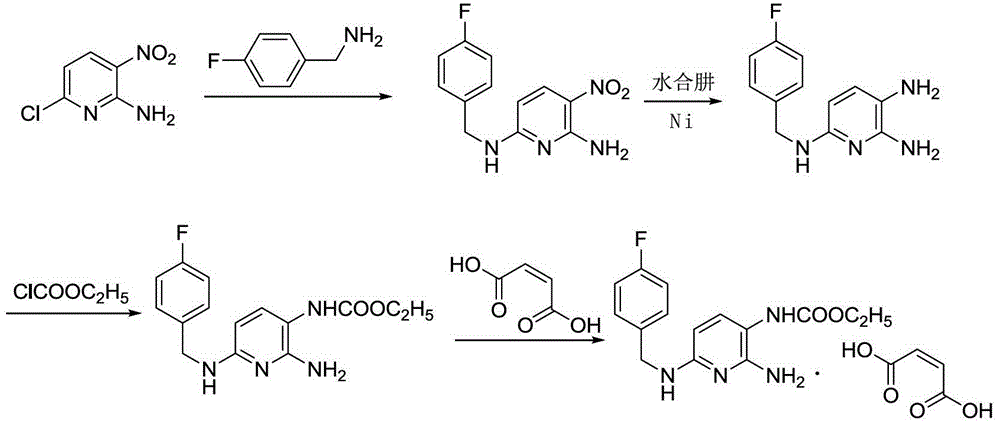
Method for preparing flupirtine maleate by one-pot method
A technology of flupirtine maleate and fluorobenzylaminopyridine, which is applied in the field of medicine and chemical industry, can solve the problems of easy oxidation and discoloration of fluorobenzylaminopyridine and flupirtine, achieve improved yield, product purity, and convenient operation , The effect of simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
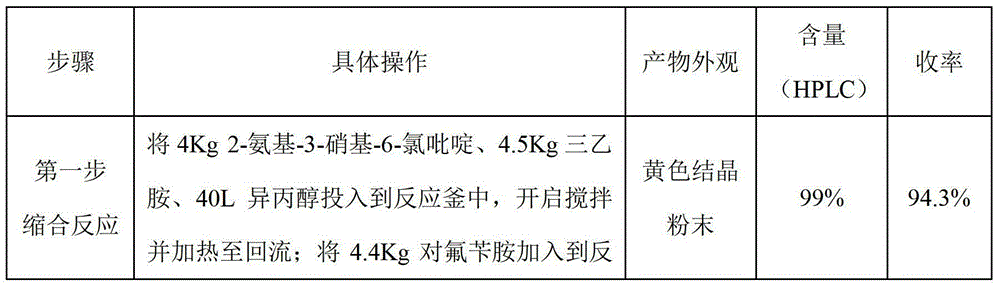
[0027] Put 4Kg of 2-amino-3-nitro-6-chloropyridine, 4Kg of ethylenediamine, and 40L of isopropanol into the reactor, start stirring and heat to reflux; add 3.6Kg of p-fluorobenzylamine into the reactor, The reaction was carried out under reflux for 3 hours. After the heating was stopped, 40 L of purified water was added to the reaction solution, a large amount of yellow solid precipitated and filtered, and the obtained wet product was kept in the reaction kettle. Add 1.2Kg Raney nickel and 40L isopropanol to the reaction kettle, start stirring and heat to reflux, drop 8Kg80% hydrazine hydrate, reflux reaction for 2 hours, after the reaction is complete, drop to room temperature under nitrogen protection, quickly add 4Kg chloroformic acid Ethyl ester was reacted at room temperature for 3 hours. Add 3.2Kg concentrated ammonia water, free for 1 hour, filter, add the filtrate to 6Kg / 120L maleic acid isopropanol solution, cool down and crystallize to obtain off-white solid, blow d...
Embodiment 2
[0029] Put 4Kg of 2-amino-3-nitro-6-chloropyridine, 3Kg of triethylamine, and 50L of absolute ethanol into the reaction kettle, start stirring and heat to reflux; add 4Kg of p-fluorobenzylamine into the reaction kettle, and reflux reaction conditions for 2 hours. After the heating was stopped, 50 L of purified water was added to the reaction solution, a large amount of yellow solid precipitated and filtered, and the obtained wet product was kept in the reaction kettle. Add 1Kg Raney nickel and 50L absolute ethanol to the reaction kettle, start stirring and heat to reflux, add 8.8Kg80% hydrazine hydrate dropwise, reflux reaction for 2 hours, after the reaction is complete, drop to room temperature under nitrogen protection, and quickly add 4.5Kg chlorine Ethyl formate was reacted at room temperature for 2 hours. Add 3Kg triethylamine, free for 1 hour, filter, add the filtrate to 6Kg / 180L maleic acid ethanol solution, cool down and crystallize to obtain off-white solid, blow dr...
Embodiment 3
[0031] Put 4Kg of 2-amino-3-nitro-6-chloropyridine, 3Kg of pyridine, and 20L of methanol into the reactor, start stirring and heat to reflux; add 4.4Kg of p-fluorobenzylamine into the reactor, and react under reflux conditions 4 hours. After the heating was stopped, 20 L of purified water was added to the reaction solution, a large amount of yellow solid precipitated and filtered, and the obtained wet product was kept in the reaction kettle. Add 500g of Raney nickel and 20L of methanol to the reaction kettle, start stirring and heat to reflux, dropwise add 7Kg80% hydrazine hydrate, reflux reaction for 4 hours, after the reaction is complete, drop to room temperature under nitrogen protection, quickly add 3.6Kg ethyl chloroformate , reacted at room temperature for 3 hours. Add 3.6Kg concentrated ammonia water, free for 2 hours, filter, add the filtrate to 5Kg / 50L maleic acid methanol solution, cool down and crystallize to obtain off-white solid, blow dry at 60°C, net weight 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com