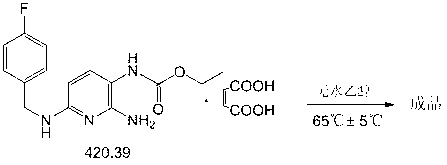
Preparation method of flupirtine maleate
A technology of flupirtine maleate and clopyridine, which is applied in the field of preparation of flupirtine maleate, and can solve problems such as no optimization of industrialized large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1——Preparation experiment of flupirtine maleate
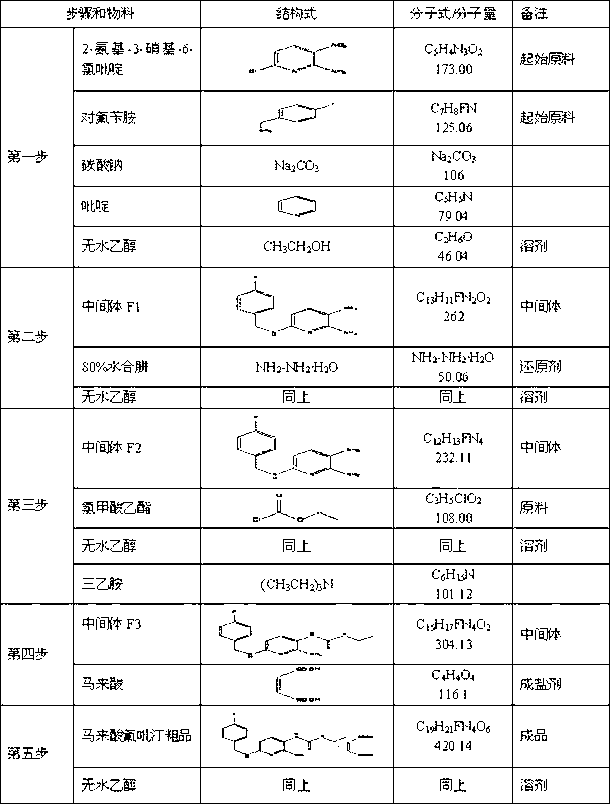
[0096] (1) Substitution reaction - synthesis of intermediate F1
[0097] I. Feeding amount and ratio
[0098] name kg mol 2-Amino-3-nitro-6-chloropyridine 5.0 28.9 Absolute ethanol 14.0 -- p-Fluorobenzylamine 4.3 34.4 Sodium carbonate 6.02 -- pyridine 0.85 -- purified water 34.0 --
[0099] II. Experimental process
[0100] Put 2-amino-3-nitro-6-chloropyridine, sodium carbonate, absolute ethanol, and pyridine into a dry 211# reactor, start stirring and heat to about 80°C (80°C±5°C), Obvious reflux; slowly drop p-fluorobenzylamine into the reaction kettle, after the dropwise addition, stir and reflux at about 80°C (80°C±5°C) for about 4 hours.
[0101] Stop heating, add 34.0kg of purified water, stir and cool down to room temperature (25°C±5°C), a large amount of yellow crystals precipitated.
[0102] Filter, collect the filter cake, wash the filte...
Embodiment 2
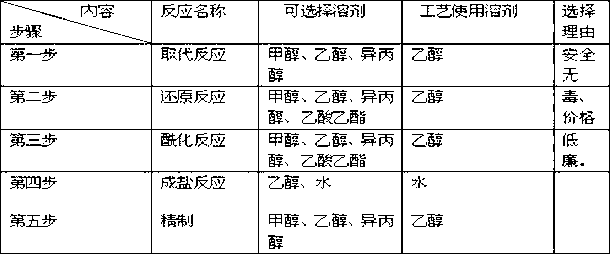
[0141] Embodiment 2 - the control of key steps and the refinement of product
[0142] The production of this product is divided into five steps: substitution reaction, reduction reaction, acylation reaction, salt formation, and refining. Among them, the substitution reaction can obtain solid products, and the products of reduction reaction and acylation reaction are not easy to obtain solids during operation, and the products are easy to produce. The crude product obtained by oxidation and salt formation is not easy to be refined after drying, and the small amount of water contained in the crude product has no obvious influence on the quality of the finished product. During the experiment, we focused on the reaction temperature, material feeding ratio, and reaction time as the research objects, and gradually explored the corresponding parameters.
[0143]
[0144] In the synthesis process, the product of the substitution reaction is the intermediate F1 and can be separated...
Embodiment 3
[0150] Embodiment 3——Multi-batch process research and data summary thereof
[0151] According to the above-mentioned preparation method of flupirtine maleate, multiple batches and different batch sizes of trial production were carried out, and the yield and product quality were studied, and the data are shown in the table below. The data show that the synthetic method of the present invention is stable and reliable, can obtain high-purity products, and at the same time, the content of related substances and the largest single impurity is very low, the process parameters are stable, the reproducibility is good, and the product quality is stable.
[0152]
[0153]
[0154]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com