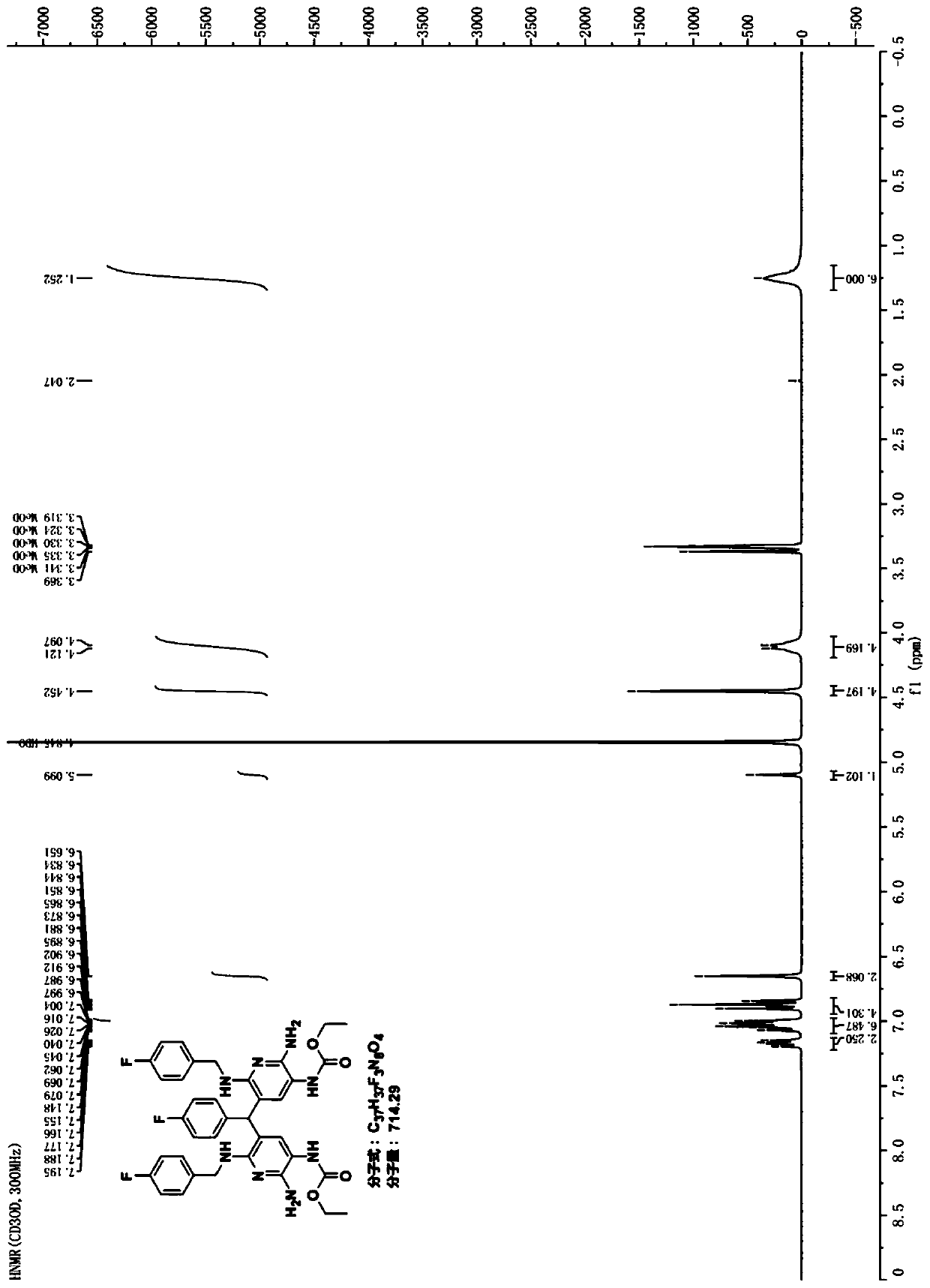
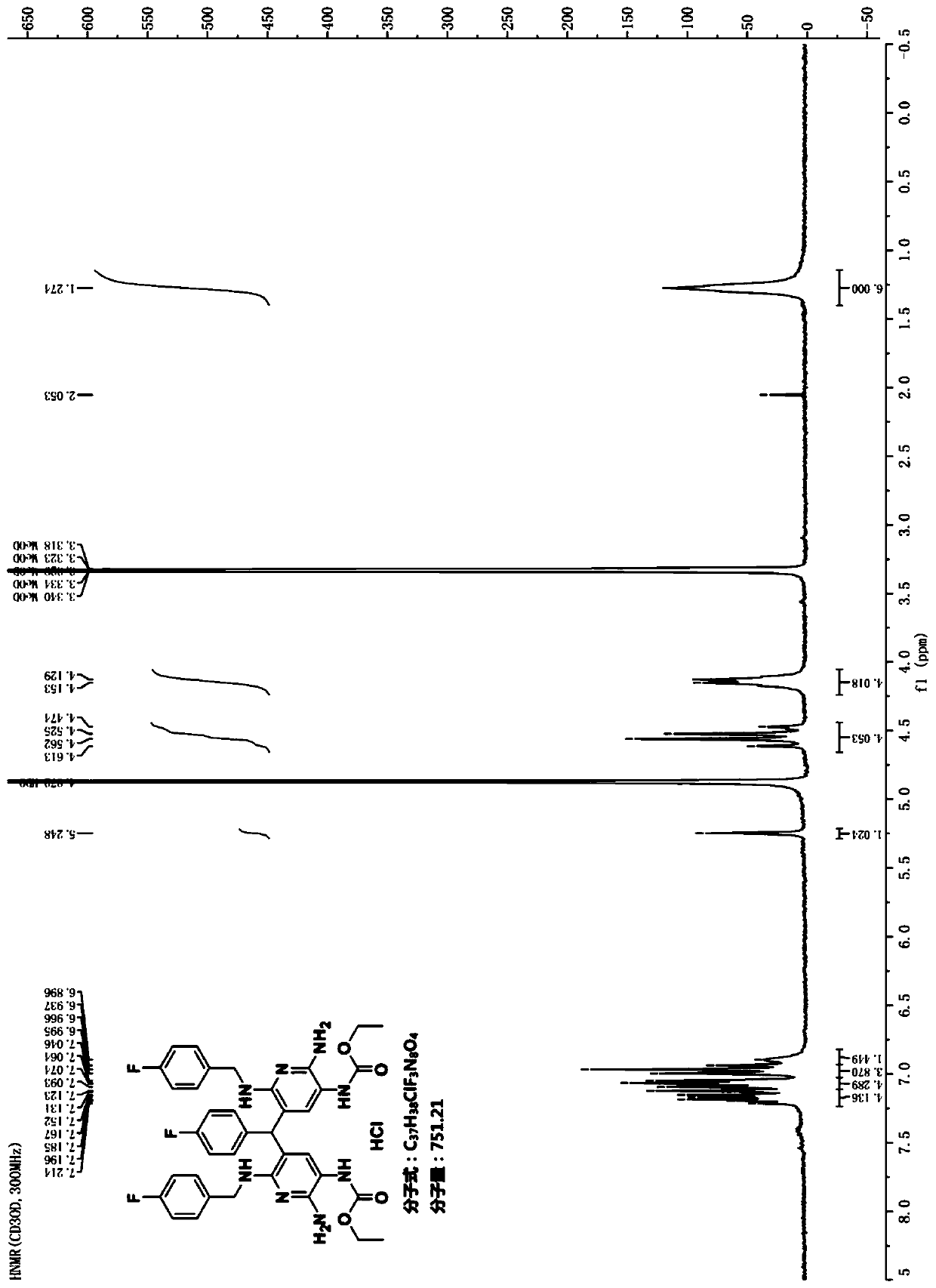
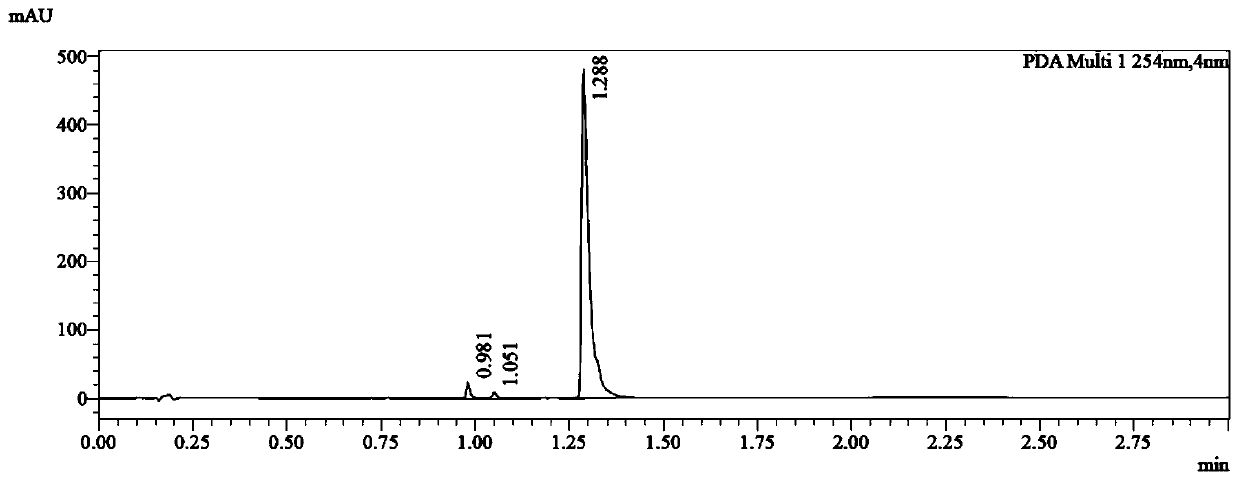
Preparation method of flupirtine derivative and preparation of inorganic acid salts of flupirtine derivative
A technology of inorganic acid salts and derivatives, applied in the field of preparation of flupirtine derivatives and inorganic acid salts thereof, can solve the problems of reduced activity, influence on drug stability, low drug content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (a) Substituting compound 1 with p-fluorobenzylamine to prepare compound 2:
[0056] 2-Amino-3-nitro-6-chloropyridine (compound 1) (50.0 g, 288 mmol, 1.00 eq) was dissolved in isopropanol (500 mL), p-fluorobenzylamine (39.7 g, 317 mmol, 1.10 eq ) and TEA (32.0g, 317mmol, 1.10eq), heated to reflux for 10h. Thereafter, the reaction solution was poured into water (1.5 L), slowly cooled to 5° C. and stirred for 30 minutes. Filter and wash the filter cake with water (500 mL). The filter cake was collected and dried to obtain 60 g of a yellow solid (Compound 2), with a yield of 79.4%.
[0057] (b) Compound 2 is subjected to a reduction reaction to prepare compound 3:
[0058] Compound 2 (60 g, 229 mmol, 1.00 eq) was dissolved in methanol (600 mL), and Pd / C (6.0 g) was added under nitrogen protection. The reactor was replaced with hydrogen for 3 times, and then the reaction was stirred at room temperature under hydrogen (30 psi) for 5 h. Afterwards, the reaction solut...
Embodiment 2
[0066] (a) Substituting compound 1 with p-fluorobenzylamine to prepare compound 2:
[0067] 2-Amino-3-nitro-6-chloropyridine (compound 1) (50.0g, 288mmol, 1.00eq) was dissolved in N,N-dimethylformamide (500mL), and p-fluorobenzylamine (39.7 g, 317mmol, 1.10eq) and K 2 CO 3 (43.8g, 317mmol, 1.10eq), heated at 100°C for 6h. Thereafter, the reaction solution was poured into water (1.5 L), slowly cooled to 5° C. and stirred for 30 minutes. Filter and wash the filter cake with water (500 mL). The filter cake was collected and dried to obtain 55.2 g of a yellow solid (Compound 2), with a yield of 73.1%.
[0068] (b) Compound 2 is subjected to a reduction reaction to prepare compound 3:
[0069] Compound 2 (55.2g, 210mmol, 1.00eq) was dissolved in methanol (552mL), and Pd(OH) was added under nitrogen protection 2 / C (2.76g). The reactor was replaced with hydrogen for 3 times, and then the reaction was stirred at room temperature under hydrogen (30 psi) for 8 h. Afterwards...
Embodiment 3
[0077] (a) Substituting compound 1 with p-fluorobenzylamine to prepare compound 2:
[0078] 2-Amino-3-nitro-6-chloropyridine (compound 1) (50.0g, 288mmol, 1.00eq) was dissolved in methanol (500mL), p-fluorobenzylamine (39.7g, 317mmol, 1.10eq) was added and Pyridine (25.1g, 317mmol, 1.10eq), heated to reflux for 12h. Thereafter, the reaction solution was poured into water (1.5 L), slowly cooled to 5° C. and stirred for 30 minutes. Filter and wash the filter cake with water (500 mL). The filter cake was collected and dried to obtain 37.9 g of a yellow solid (Compound 2), with a yield of 50.1%.
[0079] (b) Compound 2 is subjected to a reduction reaction to prepare compound 3:
[0080] Compound 2 (37.9 g, 145 mmol, 1.00 eq) was dissolved in ethanol (380 mL), and Pd / C (6.0 g) was added under nitrogen protection. The reactor was replaced with hydrogen for 3 times, and then the reaction was stirred at room temperature under hydrogen (30 psi) for 8 h. Afterwards, the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com