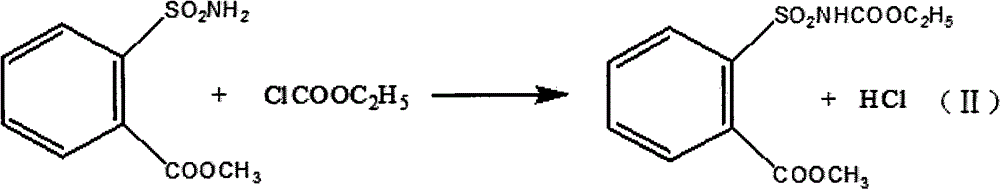Method for preparing tribenuron-methyl
A technology for trisulfuron-methyl and oxycarbonylbenzenesulfonamide is applied in the field of preparation of trisulfuron-methyl, which can solve the problems of high cost, low production safety and high product cost, and achieves small amount of three wastes, high production safety and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 5000-liter reactor, add 21.4 kilograms of potassium carbonate, water, 43.2 liters of acetone, and 13.6 kilograms of o-methoxycarbonylbenzenesulfonamide, stir to form a paste and lower the temperature to 5°C, add 8.463 kilograms of ethyl chloroformate dropwise, and heat up to 20°C, react for more than 3 hours, filter and dry to obtain ethyl o-methoxycarbonylbenzenesulfonamide formate as a solid;
[0023] 8.736 kg of 2-methylamino-4-methoxy-6-methyl-s-triazine was dissolved in toluene to obtain 2-methylamino-4-methoxy-6-methyl-s-triazine solution;
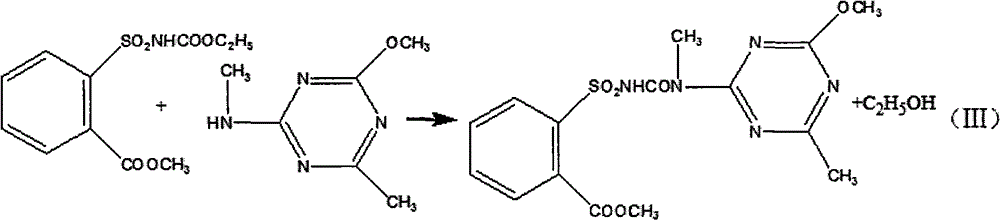
[0024] Dissolve the solid ethyl o-methoxycarbonylbenzenesulfonamide formate obtained above in toluene, add 2-methylamino-4-methoxy-6-methyl-s-triazine solution dropwise at 50°C, and control the dropping time for 1h , kept at this temperature for 8h, then distilled, filtered, and dried to obtain 18.8 kg of the original drug of tribenuron-methyl, the calculated yield was 79.32%, and the determined content of tribenuron-meth...
Embodiment 2
[0026] In a 5000-liter reactor, add 21.4 kilograms of pyridine, water, 43.2 liters of acetone, and 13.6 kilograms of o-methoxycarbonylbenzenesulfonamide, stir to form a paste and lower the temperature to 3°C, add 9.114 kilograms of ethyl chloroformate dropwise, and heat up to 18 °C, react for more than 3 hours, filter and dry to obtain ethyl o-methoxycarbonylbenzenesulfonamide formate as a solid;
[0027] 8.82 kg of 2-methylamino-4-methoxy-6-methyl-s-triazine was dissolved in toluene to obtain 2-methylamino-4-methoxy-6-methyl-s-triazine solution;
[0028] Dissolve the solid ethyl o-methoxycarbonylbenzenesulfonamide formate obtained above in toluene, add 2-methylamino-4-methoxy-6-methyl-s-triazine solution dropwise at 50°C, and control the dropping time for 1h. Insulate at this temperature for 8 hours, then distill, filter, and dry to obtain 19.4 kg of the original drug of tribenuron-methyl, the calculated yield is 81.86%, and the content of tribenuron-methyl is determined to b...
Embodiment 3
[0030] In a 5,000-liter reactor, add 16.4 kilograms of sodium hydroxide, water, 43.2 liters of ethanol, and 13.6 kilograms of o-methoxycarbonylbenzenesulfonamide, stir to form a paste and lower the temperature to 2°C, add 8.463 kilograms of ethyl chloroformate dropwise, and heat up To 13°C, react for more than 3 hours, filter and dry to obtain ethyl o-methoxycarbonylbenzenesulfonamide formate as a solid;
[0031] 8.736 kg of 2-methylamino-4-methoxy-6-methyl-s-triazine was dissolved in toluene to obtain 2-methylamino-4-methoxy-6-methyl-s-triazine solution;
[0032] Dissolve the solid ethyl o-methoxycarbonylbenzenesulfonamide formate obtained above in toluene, add 2-methylamino-4-methoxy-6-methyl-s-triazine solution dropwise at 50°C, and control the dropping time for 1h. Keep it warm at this temperature for 8 hours, then distill, filter, and dry to obtain 18.97 kg of the original drug of tribenuron-methyl, the calculated yield is 79.97%, and the determined content of tribenuron-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com