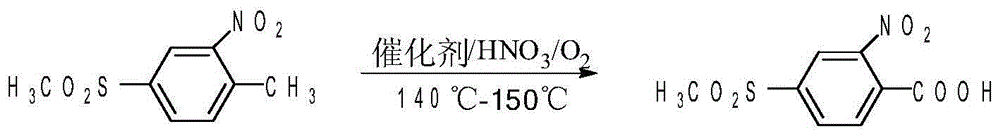
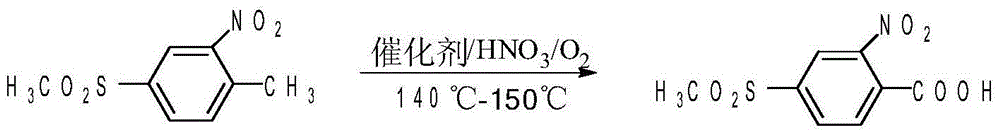
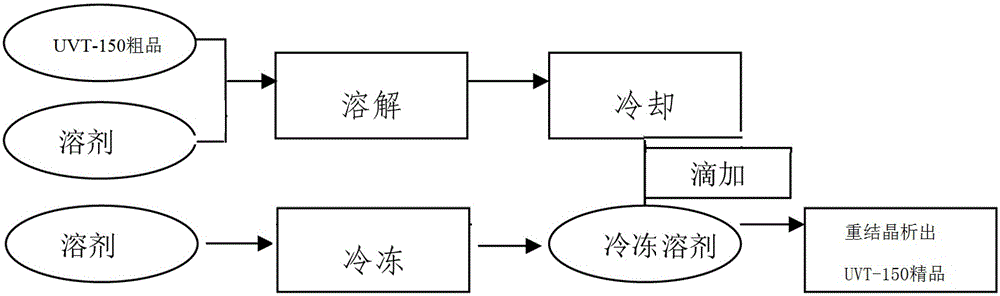
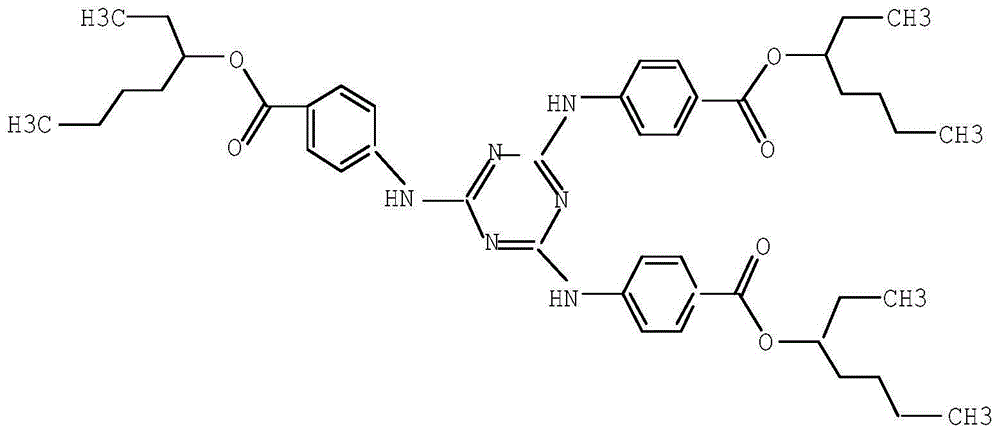
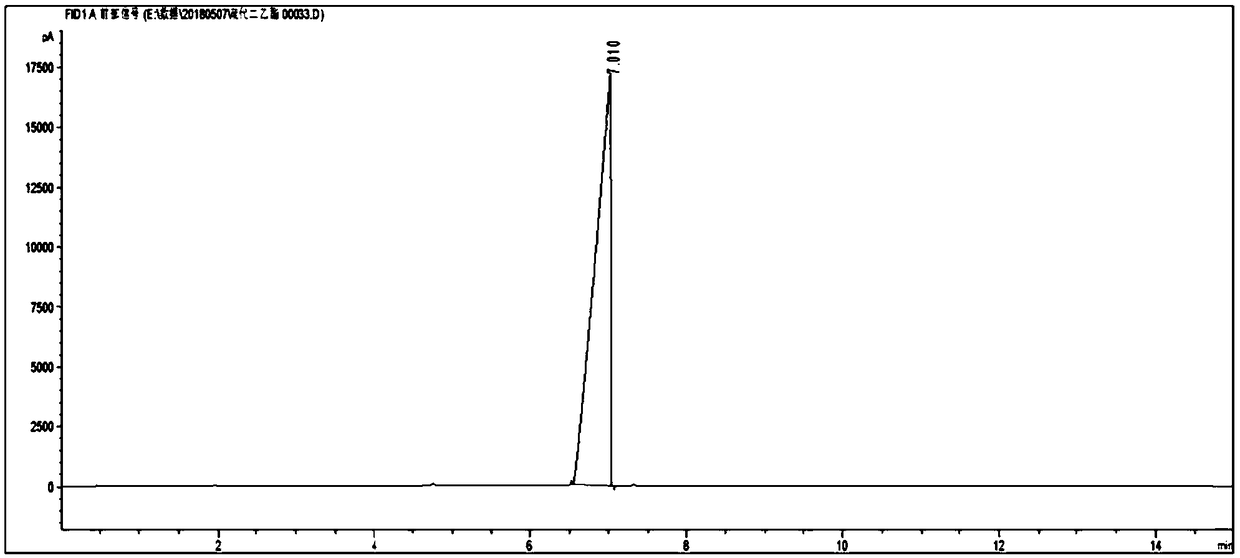
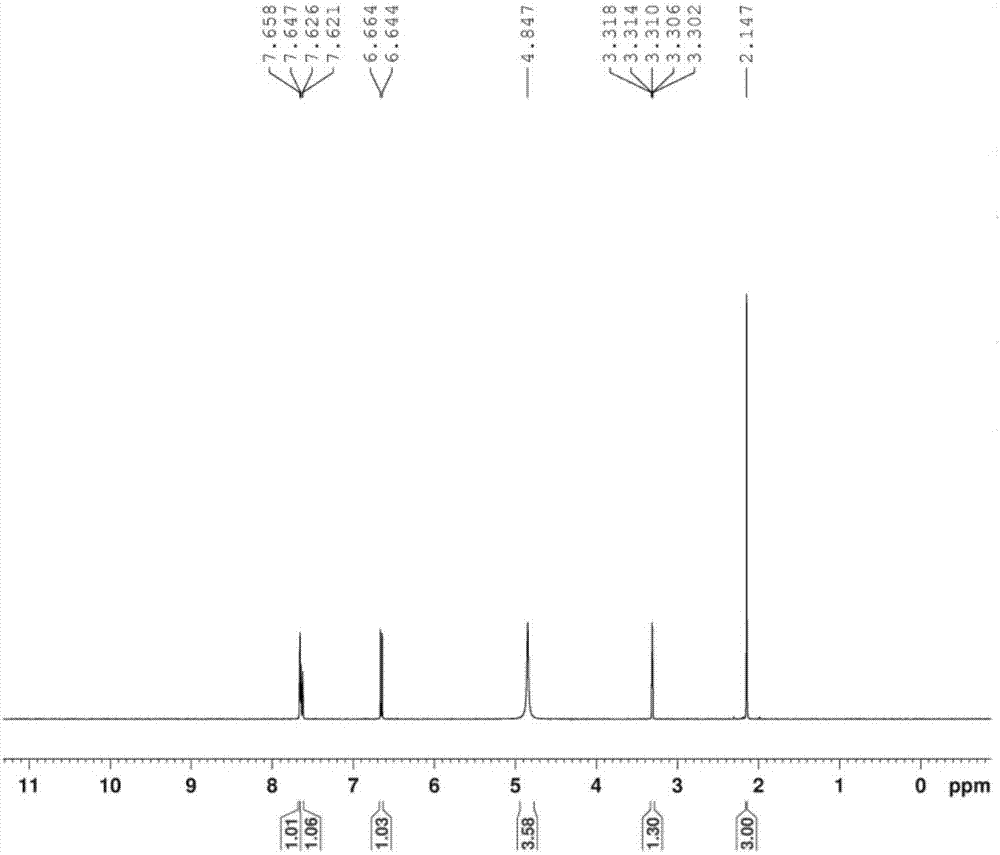
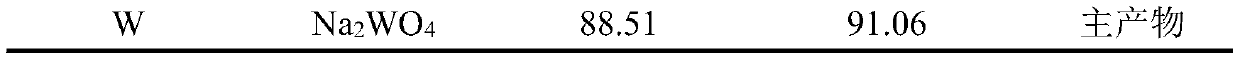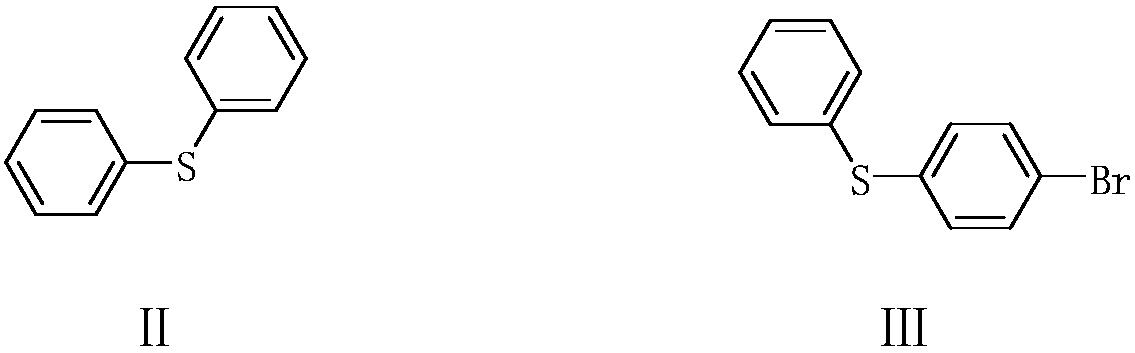Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81results about How to "The amount of three wastes is less" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
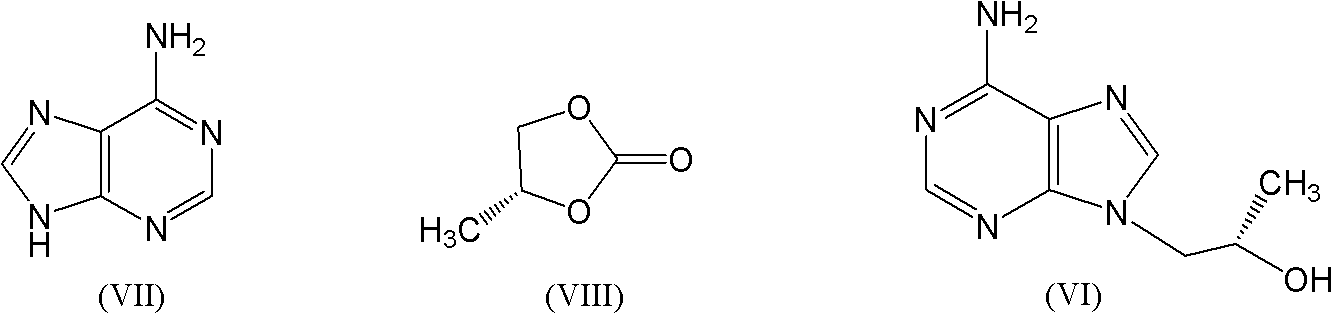
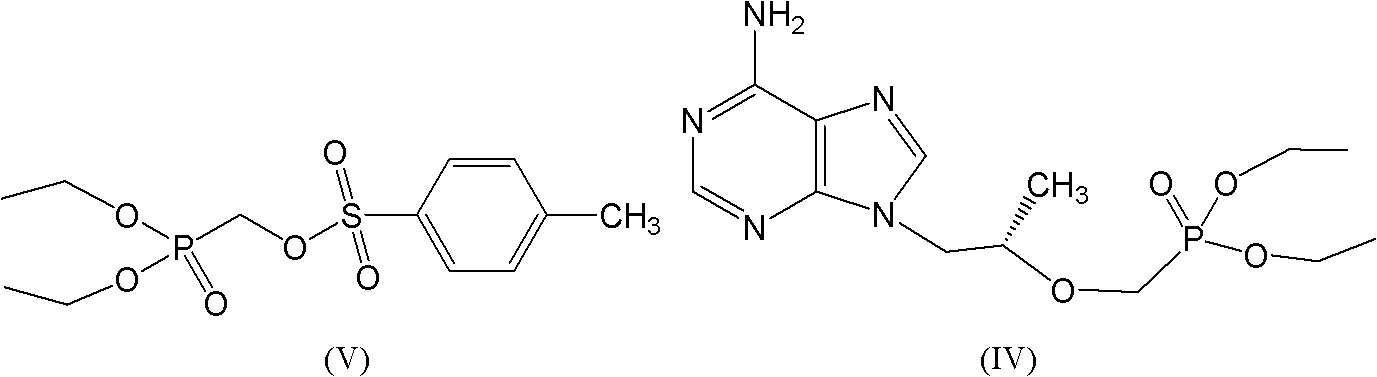
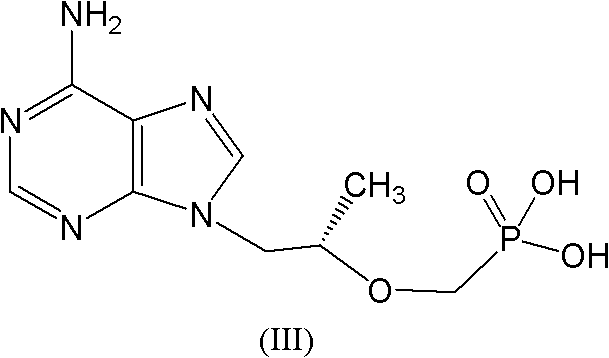
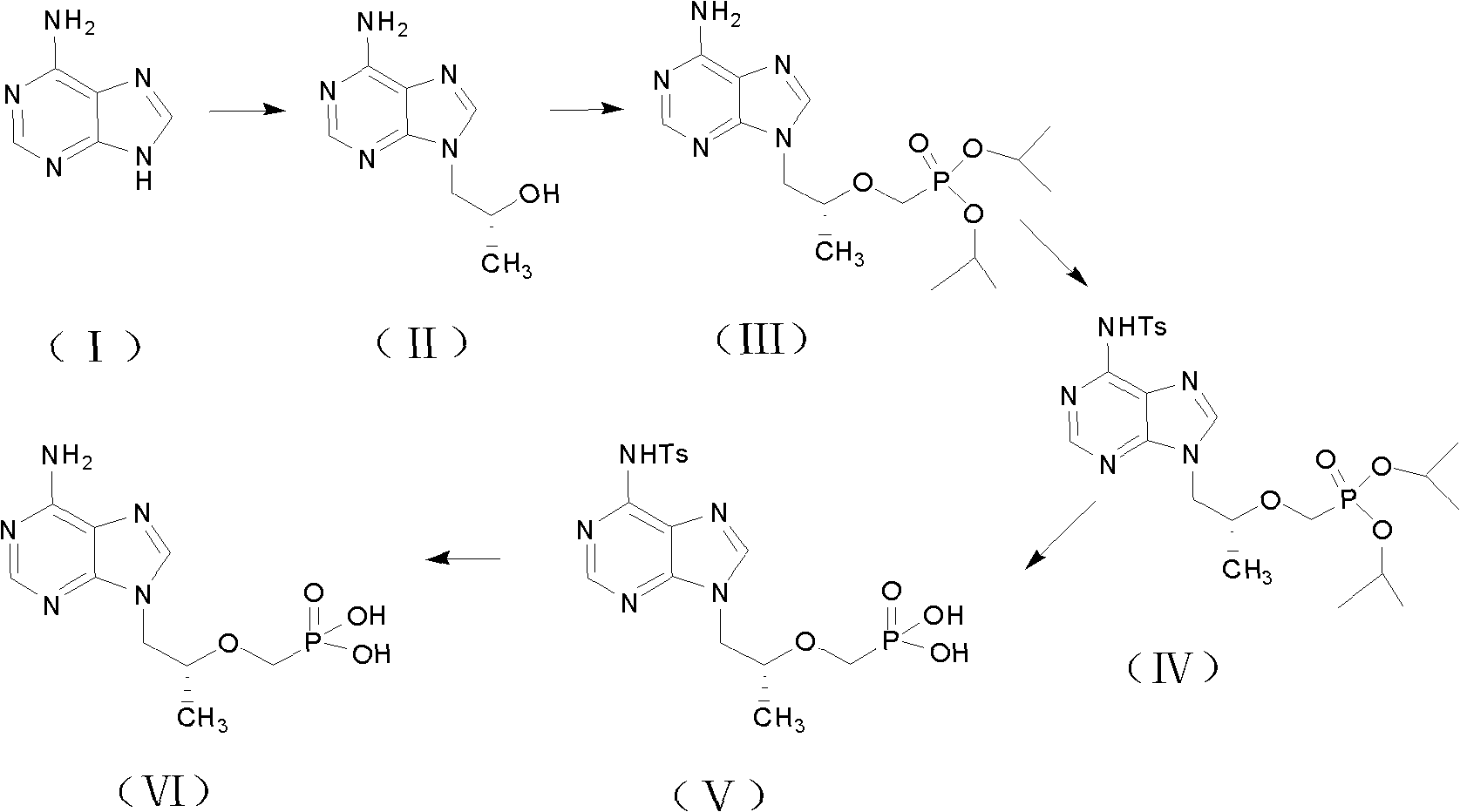
Industrial production process for tenofovir disoproxil fumarate
InactiveCN101870713AImprove securitySolve the problem of excessive contentGroup 5/15 element organic compoundsAlcoholPropylene carbonate
The invention discloses an industrial production process for tenofovir disoproxil fumarate represented by a structural formula (II). The production process comprises the following steps of: (1) preparing R-9-(2-hydroxypropyl)adenine by taking adenine and R-propylene carbonate as initial raw materials; (2) performing a condensation reaction on the obtained R-9-(2-hydroxypropyl)adenine and diethyl(tosyloxy)methylphosphonate under the catalytic action of magnesium alkoxide to prepare R-9[2-(diethyl-phosphonic acid methoxy)propyl]adenine; (3) hydrolyzing R-9[2-(diethyl-phosphonic acid methoxy)propyl]adenine to obtain tenofovir; and (4) performing condensation on the tenofovir and the chloromethyl isopropyl carbonate under the catalytic action of triethylamine to prepare the tenofovir disoproxil fumarate. The process of the invention has the characteristics of low cost, safe process, high product quality and suitability for industrial production.
Owner:杭州和素企业管理有限公司
Preparation method for tenofovir
ActiveCN102060876AReduce energy consumptionImprove securityGroup 5/15 element organic compoundsBenzeneAlcohol
The invention discloses a preparation method for tenofovir disoproxil fumarate, which comprises the following steps of: A, performing condensation reaction adenine and (R)-propylene carbonate which serve as raw materials to generate (R)-9-(2-hydroxypropyl) adenine; B, performing condensation reaction on the (R)-9-(2-hydroxypropyl) adenine and p-methylphenyl mesyloxy diethyl phosphonate under the catalysis of potassium alcoholate to prepare (R)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine; C, reacting the (R)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine obtained by the step B with para-toluenesulfonate acyl chloride to protect an amino group at bit four to prepare (R)-4-(p-toluenesulfonyl)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine; D, hydrolyzing the (R)-4-(p-toluenesulfonyl)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine obtained by the step C under a strong acid condition to obtain (R)-4-(p-toluenesulfonyl)-9-[2-(dihydroxy phosphonyl methoxy) propyl] adenine; and E, reacting the (R)-4-(p-toluenesulfonyl)-9-[2-(dihydroxy phosphonyl methoxy) propyl] adenine obtained by the step D with mercapto-benzene under a weak alkaline condition to remove a para-toluenesulfonate group to obtain the tenofovir. The invention aims to provide the preparation method for the tenofovir, which is low in cost, safe in process and good in product quality, and is suitable for industrialization.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method of preparing 2-nitro-4-methylsulfonyl benzoic acid
ActiveCN104557639AReduce usageHigh reaction yieldOrganic chemistryOrganic compound preparationBenzoic acidOxygen
The invention discloses a novel method of preparing 2-nitro-4-methylsulfonyl benzoic acid. The target product which is 2-nitro-4-methylsulfonyl benzoic acid is prepared by carrying out reaction by adopting an oxidation reaction device with a self-priming stirrer, wherein 2-nitro-4-methylsulfonyltoluene is used as the raw material, sulfuric acid is used as a reaction medium, a transition metal oxide is used as the catalyst, and oxygen is introduced in the course of reaction. The method provided by the invention has the advantage that the reaction condition is easy to control, the production cost is low, and the amount of the generated three wastes is reduced.
Owner:SHENYANG RES INST OF CHEM IND
Preparation method of 2,5-dichlorophenol
InactiveCN104591973AThe synthesis process is simpleShort routeOrganic compound preparationCarboxylic acid esters preparationHydrolysisPeroxide
The invention discloses a preparation method of 2,5-dichlorophenol and relates to the technical field of pesticide intermediate synthesis. The preparation method comprises the following steps: with p-dichlorobenzene as a start raw material, performing a Friedel-Crafts acylation reaction between the p-dichlorobenzene and acetyl chloride in the presence of aluminum trichloride to obtain 2,5-dichloroacetophenone; performing a Baeyer-Villiger oxidation reaction between the 2,5-dichloroacetophenone and a peroxide in the presence of a catalyst at room temperature to obtain 2,5-dichlorobenzene acetate; and performing a hydrolysis reaction between the 2,5-dichlorobenzene acetate and inorganic aqueous alkali in a reflux condition to obtain 2,5-dichlorophenol. The preparation method disclosed by the invention has the characteristics of simple synthesis process, short line, low production cost and high yield; and moreover, with less quantity of generated three wastes and high environmental protection property, the preparation method is more suitable for large-scale industrial production.
Owner:ANHUI XUELANG BIOTECHNOLOGY CO LTD
Method for synthesizing dinitrile ethyl tertiary amine from aliphatic primary amine by one-step method
ActiveCN113372241AWeak corrosiveNo need for increased investmentCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystDistillation
The invention provides a method for synthesizing dinitrile ethyl tertiary amine from aliphatic primary amine by a one-step method, which comprises the steps of adding aliphatic primary amine into acrylonitrile by using a glycollic acid aqueous solution as a catalyst, synthesizing a dinitrile ethyl tertiary amine compound by a one-step method under heating reflux, and after the reaction is finished, carrying out reduced pressure distillation treatment to remove low-boiling-point components, and obtaining the dinitrile ethyl tertiary amine compound with the yield being higher than 95%. The process provided by the invention has the following advantages: 1) single nitrile ethylation reaction and double nitrile ethylation reaction are carried out at the same time, so that the reaction efficiency is improved; 2) the glycollic acid is weak in acidity and basically has no corrosion to reaction equipment, so that the equipment investment is reduced; and 3) the reaction mother liquor does not need to be neutralized by adding alkali, so that a large amount of salt-containing wastewater is avoided, and the post-treatment process is simplified.
Owner:WANHUA CHEM GRP CO LTD
Synthesis method of UV absorbent ethylhexyl triazone
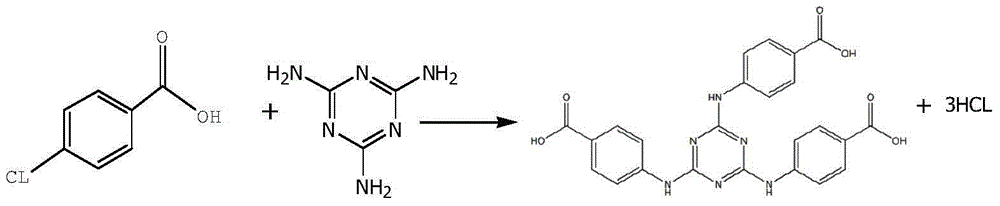
ActiveCN105061345ASimple process routeRaw materials are easy to getOrganic chemistrySynthesis methodsDistillation
The invention discloses a synthesis method of a UV absorbent ethylhexyl triazone. The synthesis method comprises that melamine and 4-chlorobenzoic acid as initial raw materials undergo a trisubstitution reaction to produce 2,4,6-tris[(p-carboxyphenyl)amino]-1,3,5-triazine (H3TATAB), 2,4,6-tris[(p-carboxyphenyl)amino]-1,3,5-triazine (H3TATAB) and isooctanol undergo an esterification reaction to produce an ethylhexyl triazone crude product, the ethylhexyl triazone crude product is subjected to distillation desolvation and crystallization and the crystals are dried to form an ethylhexyl triazone finished product. The synthesis method has the characteristics of simple production route, easily available raw materials, mild reaction conditions, unique crystallization method, less three wastes and high product purity and is suitable for industrial production.
Owner:宜都市华阳化工有限责任公司
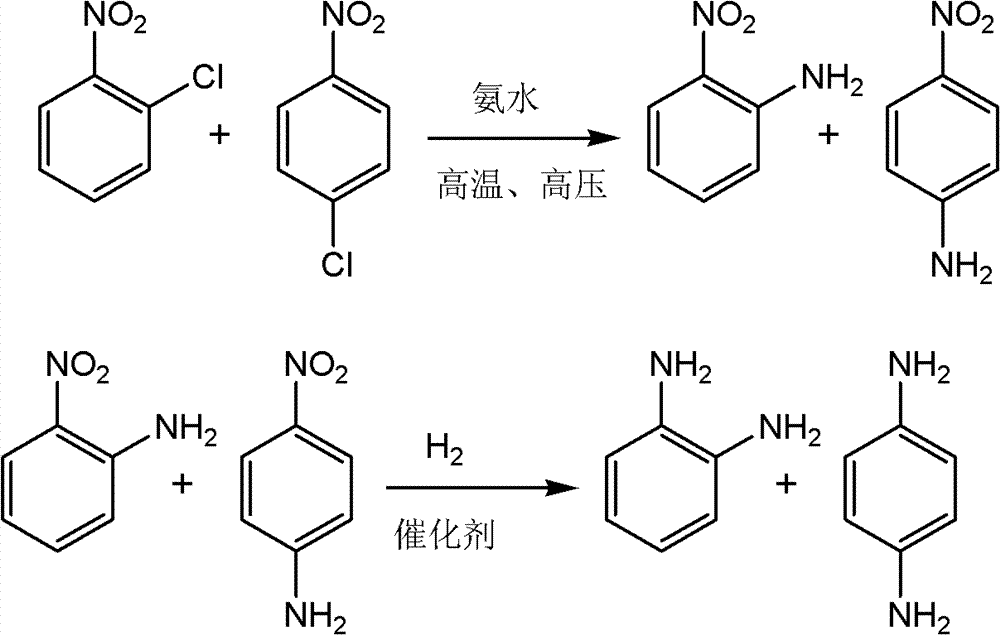
Method for preparing aromatic amine from mixed nitrochlorobenzene
ActiveCN103086895ALow pre-processing requirementsReduce energy consumptionOrganic compound preparationChemical recyclingP-NitroanilineSolvent
The invention provides a method for preparing aromatic amine by using mixed nitrochlorobenzene as a raw material. The method comprises the steps that: mixed nitrochlorobenzene is subjected to ammonolysis, deamination stripping, cooling crystallization, and centrifugal separation, such that a material comprising mixed nitroanilide is obtained; the material can be subjected to catalytic hydrogenation in an alcohol solvent, and p-phenylenediamine and o-phenylenediamine products can be prepared through separation; or before catalytic hydrogenation, part of p-nitroanilide is separated; and hydrogenation is carried out, such that p-phenylenediamine and o-phenylenediamine products can be prepared. According to the aromatic amine preparation method, the mixed nitrochlorobenzene raw material can be used in phenylenediamine production without separation, such that a product material separation process of a traditional production process is simplified, and energy consumption during production process is reduced. The method is economical and highly efficient, and the product has high purity.
Owner:ZHEJIANG HONGSHENG CHEM IND +1
Post-treatment method of L-glufosinase hydrolysate
PendingCN111574559AThe amount of three wastes is lessLow costGroup 5/15 element organic compoundsOrganic acidEnzymatic hydrolysis
The invention discloses a post-treatment method of an enzymatic hydrolysate of L-glufosinate-ammonium. The enzymatic hydrolysate of L-glufosinate-ammonium prepared by a biological enzyme resolution method is treated by four steps of pretreatment, extraction, desalination and crystallization; impurities such as unreacted D-glufosinate-ammonium derivatives, generated organic acids, ammonium salts and sodium salts are removed, and L-glufosinase of which the yield is greater than 98%, the content is greater than 98%, and the ee value is greater than 99% is obtained. According to the method, a traditional cation exchange resin passing mode is replaced with an extraction mode so that the method is simpler, more convenient and feasible, the purification efficiency is high, the extraction agent can be recycled, and the yield of three wastes is greatly reduced.
Owner:HEBEI VEYONG BIO CHEM
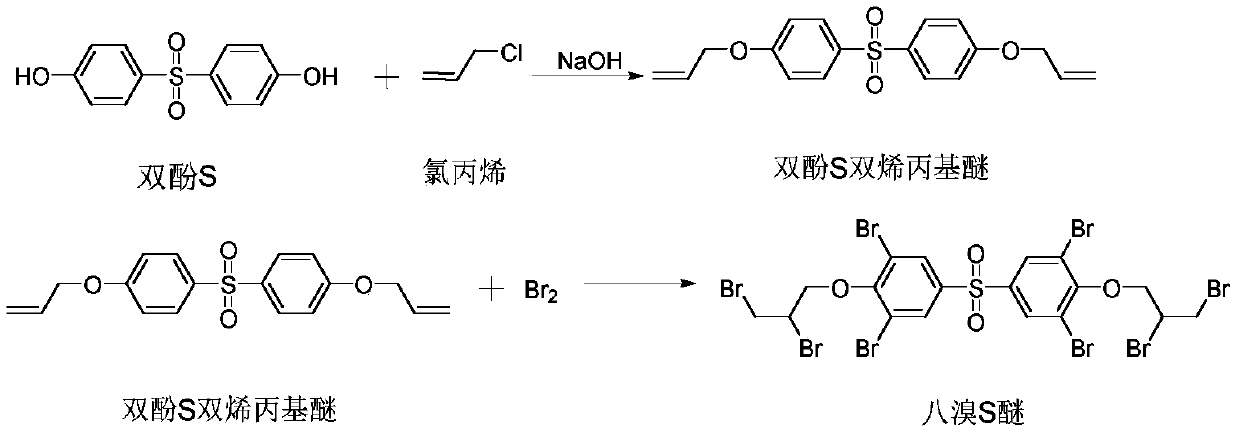
Preparation method of tetrabromobisphenol S bis(2,3-dibromopropyl ether) flame retardant
ActiveCN110981767AShort process routeEasy to operateOrganic chemistryOrganic compound preparationPolymer scienceSide chain
The invention provides a preparation method of a tetrabromobisphenol S bis(2,3-dibromopropyl ether) flame retardant. Bisphenol S used as a raw material and chloropropene undergo a substitution reaction to obtain a bisphenol S diallyl ether intermediate, and the bisphenol S diallyl ether and liquid bromine undergo addition and substitution reactions to obtain tetrabromobisphenol S bis(2,3-dibromopropyl ether). One-time bromination is adopted to introduce all eight bromine atoms into an aromatic ring and a side chain at the same time, so that the process route is shortened, the operation is simple, the safety is high, and the production cost is low. A solvent can be recycled in the process of preparing the bisphenol S diallyl ether intermediate, and the output of three wastes is greatly reduced, so the pollution to the environment is reduced, and the production cost is saved. The tetrabromobisphenol S bis(2,3-dibromopropyl ether) flame retardant prepared in the invention has high productpurity and improved application quality.
Owner:山东旭锐新材股份有限公司
L-carnitine production process
PendingCN113603601AIncrease profitHigh reaction yieldOrganic compound preparationOrganic chemistry methodsPtru catalystSodium cyanide
The invention discloses an L-carnitine production process which comprises the raw materials: epichlorohydrin, water, a catalyst, trimethylamine hydrochloride, a 30% sodium cyanide solution, a 30% hydrochloric acid solution and a 20% ammonia water solution. The L-carnitine production process has the following beneficial effects that the raw material epichlorohydrin is firstly split, by-products can be sold, and the reaction yield is high; the product is low in total cost and easy to refine, and an epichlorohydrin synthesis method of a route of splitting, quaternary ammoniation and cyaniding is selected; the production process has the advantages of short synthesis route, high yield, high resource utilization rate, low cost and small three-waste generation amount, ammonia water and hydrochloric acid are used for reacting to adjust the pH value, and excessive alkaline substances are prevented; impurities such as epichlorohydrin, hydrogen chloride, ethanol and isopropanol are injected into water generated by drying, so that pollution caused by leakage is prevented, the generation of wastewater is reduced; and when the temperature is raised to 90 DEG C for centrifugal desalting, a part of heat is provided, so that the heat is recycled.
Owner:宁夏坤正生物科技有限公司
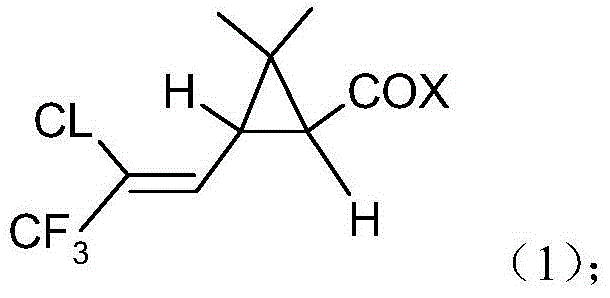
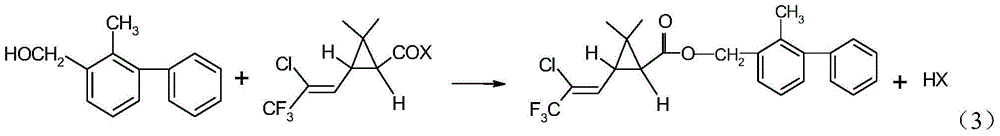
Method for producing bifenthrin with clean synthesizing process
InactiveCN104628569AHigh synthetic conversion rateHigh product contentPreparation from carboxylic acid halidesHalogenSolvent
The invention provides a method for producing bifenthrin with a clean synthesizing process. According to the method, 3-(2-chloro-3,3,3-trifluoro-1-allyl)-2,2-dimethylcyclopropane formyl halide and 2-methyl-3-biphenylmethanol are adopted to directly react in one solvent or mixed solvent of more than two solvents in favor of overflow of halogen hydride, halogen hydride generated in the reaction is removed from tail gas in time by means of negative pressure, and the product is directly cooled and crystallized to obtain bifenthrin. The reaction equation is as shown in a formula (3), wherein X is Cl or Br. The production method has the characteristics of high product content, simple process flow, small output of the 'Three Wastes' and the like, and accords with the requirement for clean production.
Owner:JIANGSU YANGNONG CHEM +1
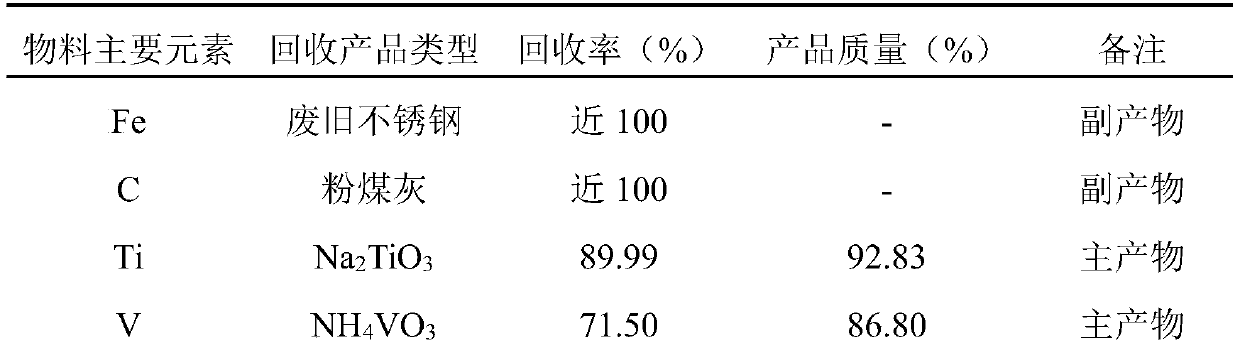
Treatment process of ship tail gas denitration waste catalyst
InactiveCN110747339ASolving RecyclingImprove leaching rateProcess efficiency improvementPtru catalystProcess engineering
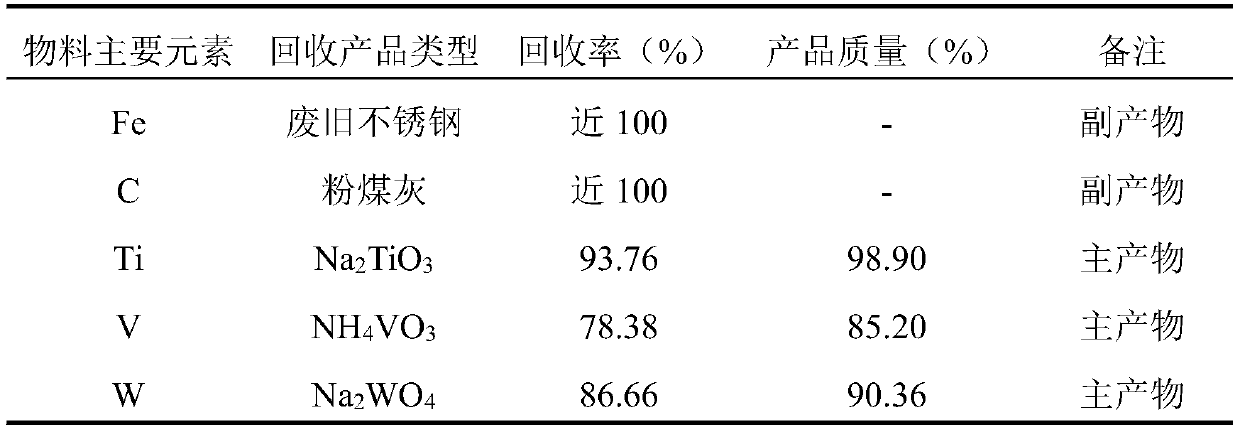
The invention relates to the technical field of resource regeneration and recycling, and particularly discloses a treatment process of a ship tail gas denitration waste catalyst. The process mainly comprises the following steps of pretreatment of the ship tail gas denitration waste catalyst, titanium recovery, vanadium recovery and tungsten recovery. By adopting the process to treat the ship tailgas denitration waste catalyst, high-added-value sodium titanate, ammonium metavanadate and sodium tungstate can be efficiently enriched and recovered. Meanwhile, the whole process of the treatment process is a closed cycle, so that the resource waste is avoided, and the utilization rate of resources is improved; and the treatment process is small in three-waste generation amount theoretically, sothat the pollution to the environment is reduced, and the process is a clean recovery process.
Owner:广东诚一环保科技有限公司
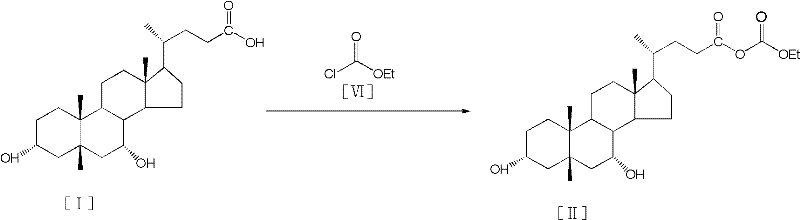
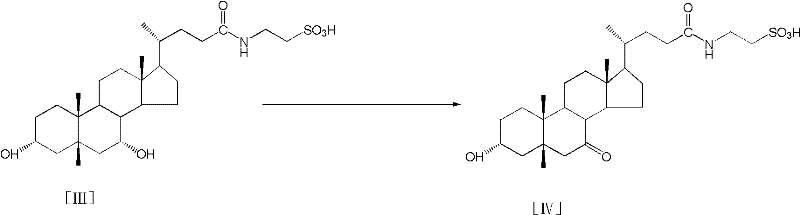
Process for preparing tauroursodeoxycholic acid hydrate
InactiveCN102558268AEfficient waySolve the problem of chiral isomersSteroidsScavengerChenodeoxycholic acid
The invention discloses a process for preparing a tauroursodeoxycholic acid hydrate, which comprises the steps of reacting chenodeoxycholic acid with acyl chloride compound to obtain chenodeoxycholic acid mixed anhydride in an anhydrous organic solvent with ketone groups and in presence of an acid scavenger; reacting the chenodeoxycholic acid mixed anhydride with igepon with water under an alkaling condition to obtain taurine chenodeoxycholic acid; reacting the taurine chenodeoxycholic acid with an oxidizing agent in presence of water under an alkaling condition to obtain taurine-7-ketone group gallstone acid; leading hydrogen into the taurine-7- ketone group gallstone acid under the condition of chiral catalyst and alkalinity and maintaining a certain pressure and temperature for a hydrogenation reduction reaction to obtain the tauroursodeoxycholic acid; mingling the tauroursodeoxycholic acid with the mixed organic solvent to obtain the tauroursodeoxycholic acid hydrate. The tauroursodeoxycholic acid hydrate has the advantages of being low in cost, safe in process, good in production quality, and applicable to industrialization.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
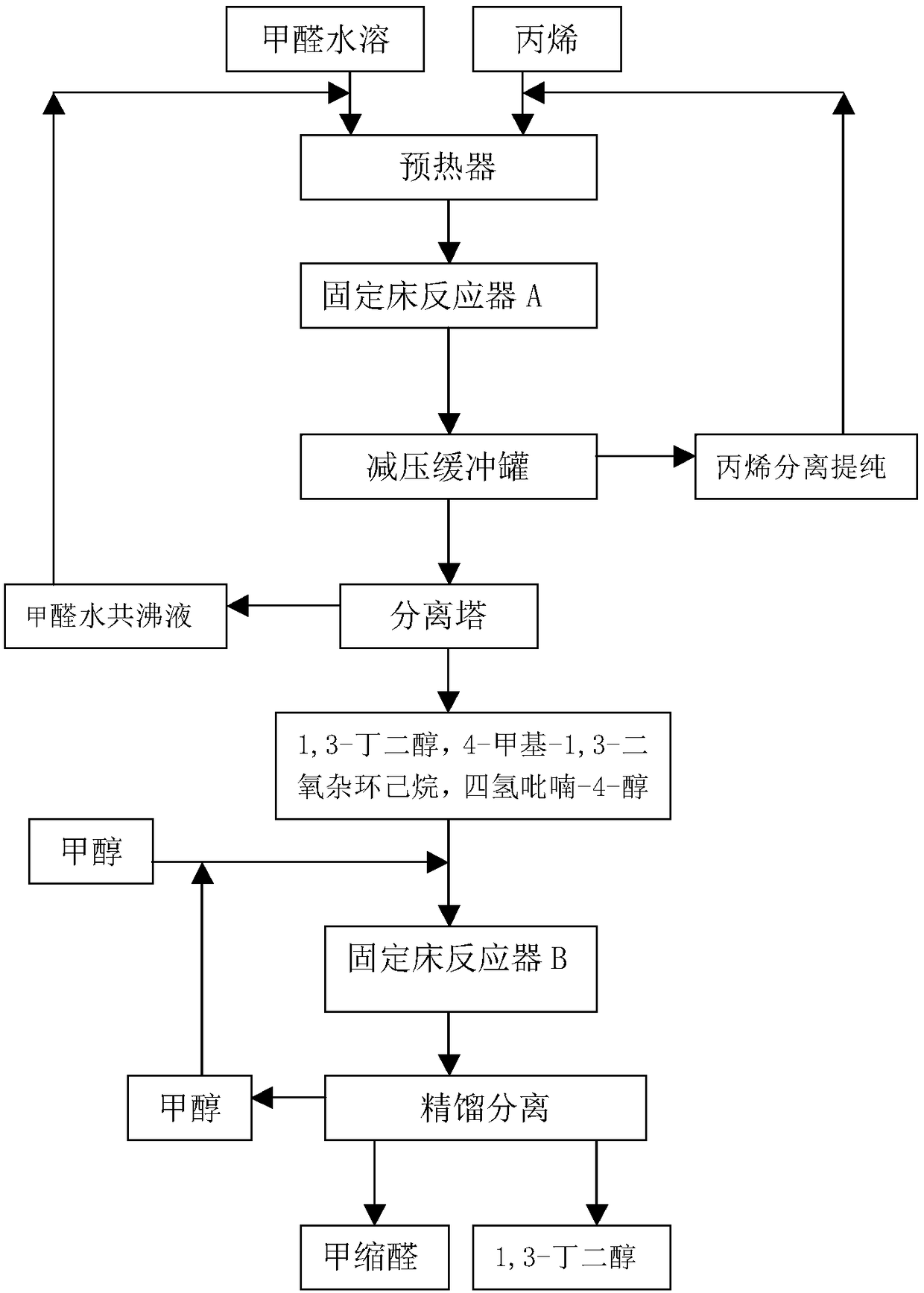
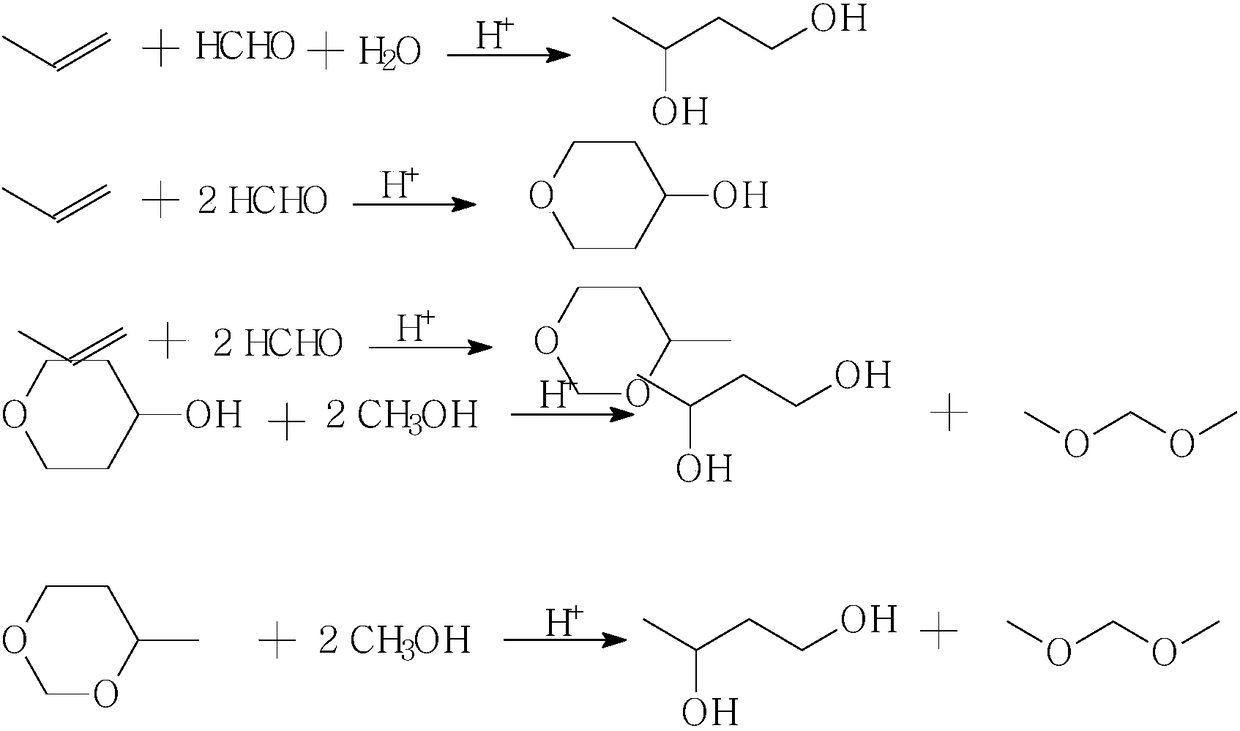
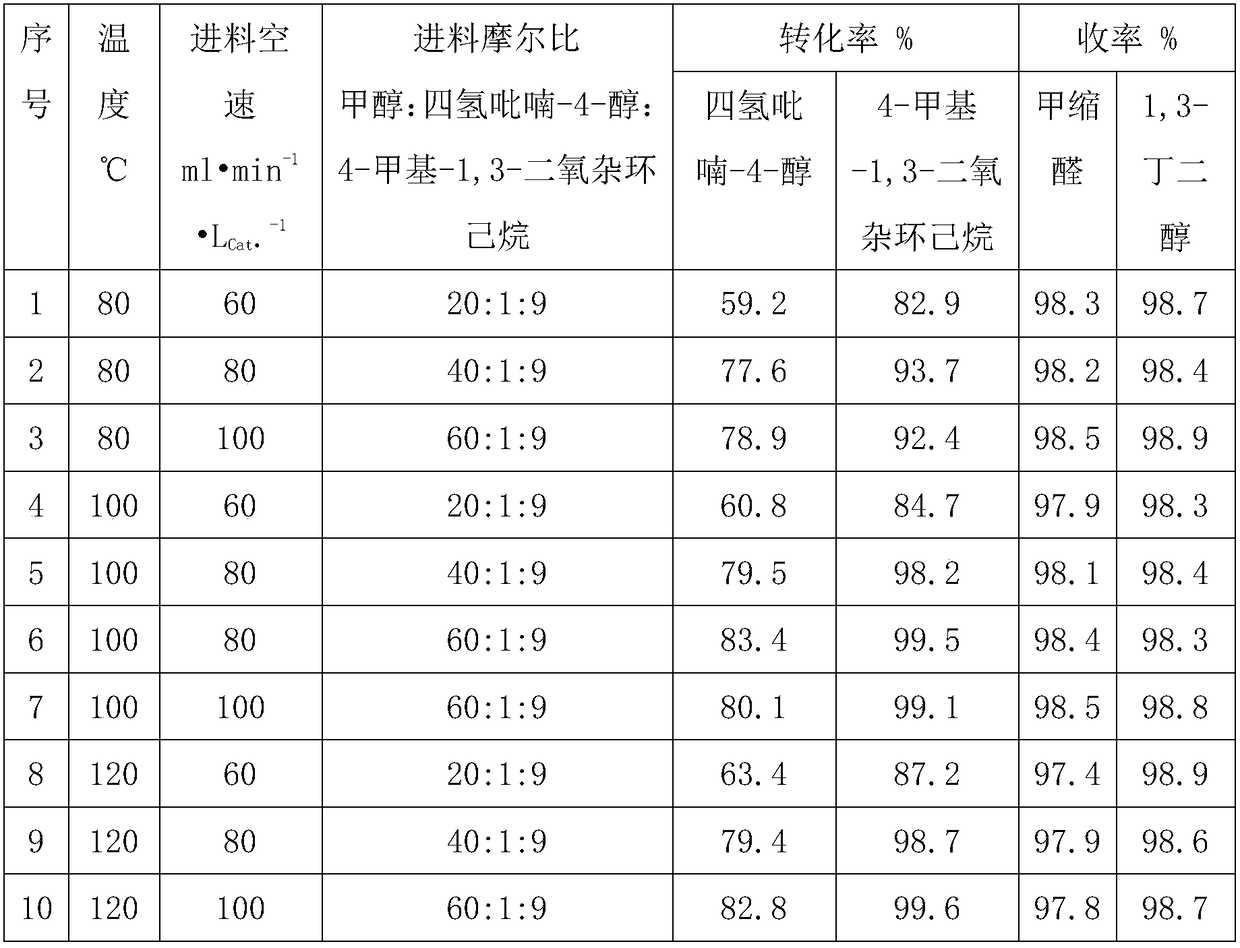
Production method of 1,3-butanediol
InactiveCN108586197AReduce manufacturing costThe amount of three wastes is lessOrganic compound preparationPreparation by alcoholysisSolid acidButanediol
The invention relates to a production method of 1,3-butanediol, in particular to a production process for synthesizing 1,3-butanediol by using propylene and formaldehyde as starting raw materials, andbelongs to the technical field of chemical engineering. The process is characterized in that formaldehyde and the propylene are used as the starting raw materials; prins condensation reaction is performed under the effect of solid acid catalysts; then, the product and methanol are subjected to dehydration reaction to prepare the 1,3-butanediol. The process has the advantages that the raw materialresources are sufficient; the process is suitable for large-scale continuous production; the environment-friendly effect is achieved; three wastes (waste water, waste air and waste solid) are almostnot generated; the comprehensive production cost is low.
Owner:ZHEJIANG XINHUA CHEM
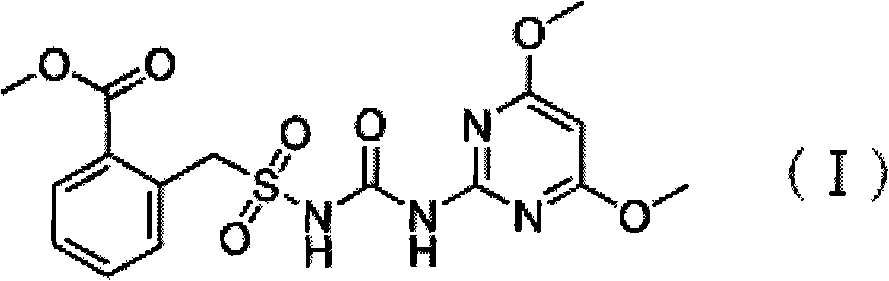
Preparation method of bensulfuron
InactiveCN102702112AHigh yieldImprove production safetyOrganic chemistryEthyl chloroformateOrganic solvent
The invention discloses a new method for preparing bensulfuron. The preparation method comprises the following steps of: reacting o-methyl formate benzene benzyl sulfamide and ethyl chloroformate in a dried organic solvent to obtain o-methyl formate benzene benzyl sulfamide ethyl formate; and then adding 2-amino-4, 6-dimethoxy pyrimidine and a few amount of catalyst to obtain the bensulfuron. According to the invention, the ethyl chloroformate is used as the amide agent to control the reaction temperature and the speed of dropping the ethyl chloroformate, so that the ethyl chloroformate is reacted with the o-methyl formate benzene benzyl sulfamide in the dried organic solvent to obtain the o-methyl formate benzene benzyl sulfamide ethyl formate; then the o-methyl formate benzene benzyl sulfamide ethyl formate is further reacted with 2-amino-4, 6-dimethoxy pyrimidine under the action of the catalyst to obtain bensulfuron; and the purity of bensulfuron is greater than or equal to 95%. With the adoption of the ethyl chloroformate as the raw material, the preparation method of bensulfuron provided by the invention has the advantages of high output, high producing safety, low cost, and small producing amount of the three wastes compared with the traditional isocyanate method and sodium cyanate method.
Owner:HEFEI JIUYI AGRI DEV
Method for co-producing and preparing methylthio acetate and diethyl thioglycolate
ActiveCN108774160AHigh yieldEasy to separate and purifySulfide preparationChemical synthesisHomoserine
The invention relates to the technical field of chemical synthesis and in particular relates to a method for co-producing and preparing methylthio acetate and diethyl thioglycolate by taking methionine as a raw material. The method for co-producing and preparing the methylthio acetate and the diethyl thioglycolate, provided by the invention, takes the methionine and haloacetic acid as raw materials to prepare three chemical products including homoserine lactone hydrohalide (IV), the methylthio acetate (I) and the diethyl thioglycolate (III) at the same time; all the products are easy to separate and purify.
Owner:SHANDONG ACADEMY OF PESTICIDE SCI
Synthetic method of 2,5-dichlorophenol
InactiveCN105801334AIncrease profitAvoid wastingOrganic compound preparationHalogenated hydrocarbon preparationChlorobenzeneEngineering
The invention relates to a synthetic method of 2,5-dichlorophenol, and belongs to the technical field of dicamba key intermediate synthesis. 1,2,4-trichlorobenzene generated by pure benzene chlorination can be easily separated from 1,4-dichlorobenzene to avoid high production cost and low production efficiency caused by difficulty in separation. Since the 1,2,4-trichlorobenzene and the 1,4-dichlorobenzene can independently obtain the 2,5-dichlorophenol, the use ratio of raw materials is high, the waste of a by-product is avoided, and therefore, the production cost is greatly lowered. In addition, the 1,4-dichlorobenzene can obtain the 2,5-dichlorophenol through two different methods. The synthetic method has the advantages of simple synthetic process, low production cost, high raw material use ratio, less process byproducts and small three-waste output and is more suitable for large-scale industrial production.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
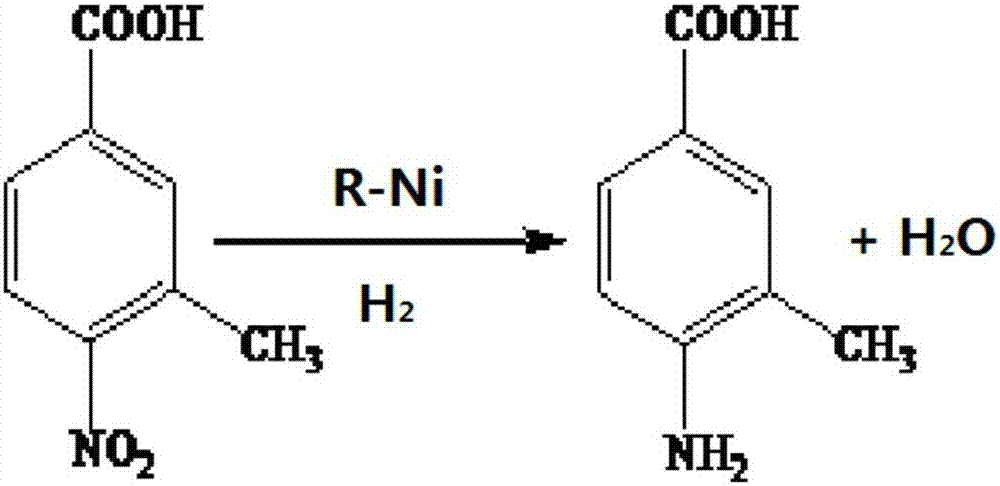
Method for preparing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation
InactiveCN107501106ARaw materials are easy to getEasy to operateOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisP-Aminobenzoic acid
The invention provides a method for preparing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation, belonging to the technical field of chemical synthesis. The method comprises the following steps: adding 3-methyl-4-nitrobenzoic acid and a solvent into a reaction vessel so as to obtain a 3-methyl-4-nitrobenzoic acid solution, and adjusting the pH value of the solution to be alkaline; pouring the obtained alkaline aqueous solution into an autoclave, adding a catalyst, respectively carrying out replacement with nitrogen and hydrogen for three times, introducing ammonia gas for 2 minutes, carrying out pressurizing with hydrogen to 3.45 MPa to 4.50 MPa, and carrying out a reaction under the pressure of 4.50 to 2.50 MPa at 100 to 160 DEG C so as to obtain a reaction solution; and subjecting the reaction solution to post-processing so as to obtain the 3-methyl-4-aminobenzoic acid. The method provided by the invention has the advantages of easily-available raw materials, simple operation, low production amount of three wastes, environmental friendliness, capability of reaching a mole yield up to 90%, and more applicability to industrial production.
Owner:中涛新材料有限公司
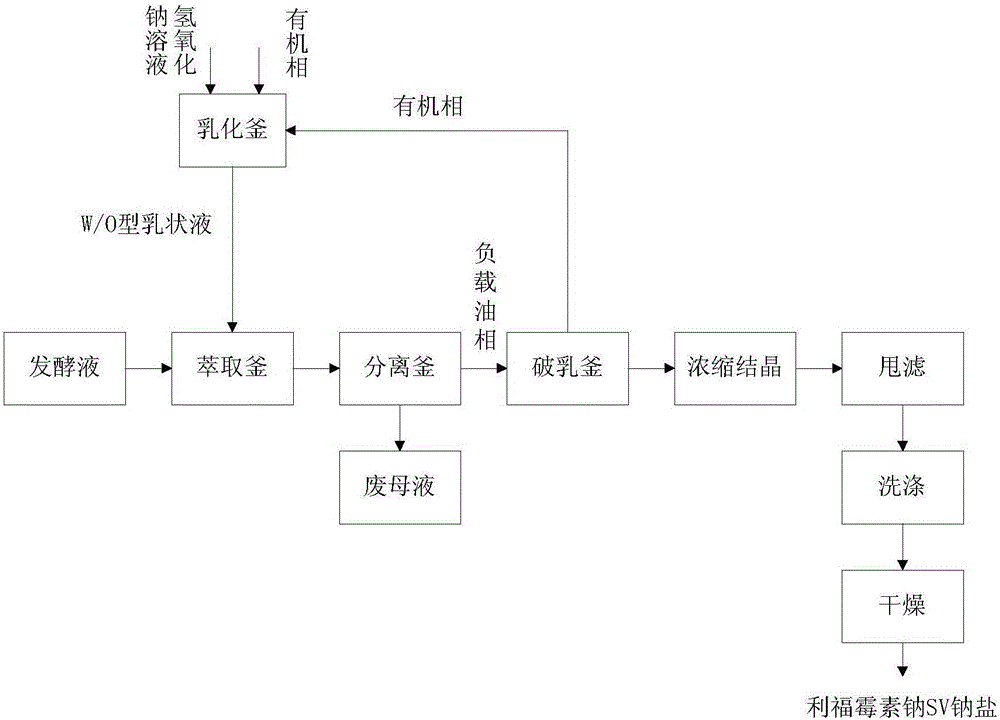
Novel rifamycin SV sodium salt production technology
ActiveCN105713010AQuality assuranceHigh yieldOrganic chemistryEmulsion liquid membraneRifamycin SV Sodium
The invention relates to a bulk pharmaceutical chemical production technology, in particular to a novel rifamycin SV sodium salt production technology.A W / O emulsion liquid membrane is extracted from fermentation broth, and rifamycin SV sodium is directly generated.The content of the product obtained by means of the production technology is 90-97%, and water content is 2.6-5%.The technological process is short, three-waste output is low, raw material consumption is low, the yield of rifamycin SV sodium is effectively increased, and the problems of existing technologies that the technological process is long, impurity content is high and yield is low are solved.The novel rifamycin SV sodium salt production technology has great significance in promoting industrial development and has broad industrialized application prospects.
Owner:SHENYANG RES INST OF CHEM IND +1
Preparation method of tetraimidazole free alkali
ActiveCN111377949AHigh activityShort synthetic stepsOrganic chemistryThioureaBenzylamine hydrochloride
The invention discloses a preparation method of tetraimidazole free alkali. Alpha-[[(2-hydroxyethyl)amino]methyl]benzyl alcohol reacts with thionyl chloride, then water is added for heating dissolution, after activated carbon thermal filtration, N-(2-chloroethyl)-alpha-(chloromethyl)-benzylamine hydrochloride is obtained through cooling crystallization, then N-(2-chloroethyl)-alpha-(chloromethyl)-benzylamine hydrochloride and thiourea are subjected to direct cyclization, and tetraimidazole free alkali is generated. The method is simple in production process, mild in reaction condition and highin total yield, the production cost is reduced, the generation amount of three wastes is small, the double contradiction between economy and environment in the development process of modern enterprises is well solved, and the production process has great competitiveness and good industrial prospects.
Owner:SHANDONG GUOBANG PHARMA +1
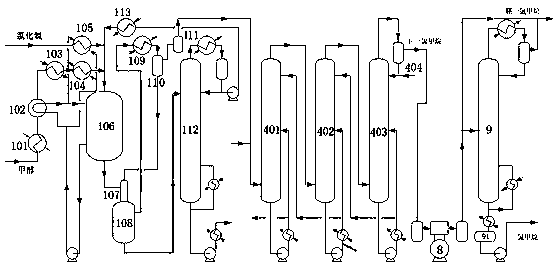
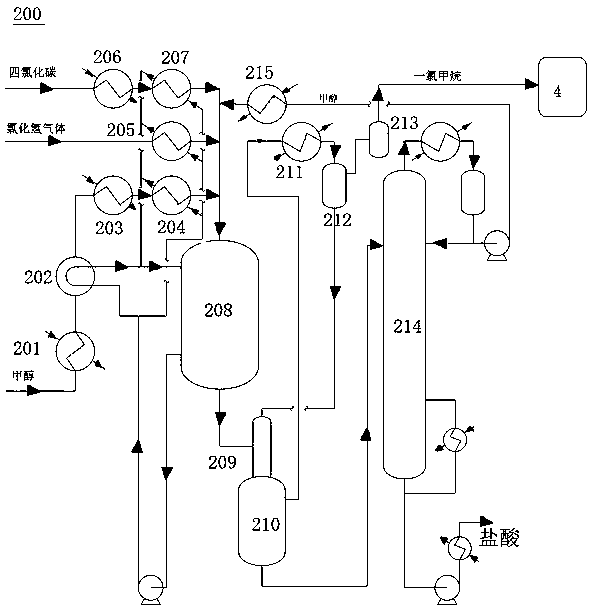
Ultra-pure methane chloride production system and process
InactiveCN111333484AReduce energy consumptionShort processHalogenated hydrocarbon preparationDehydrogenationCarbon Chloride
The invention discloses an ultra-pure methane chloride production system which comprises a reaction system for producing methane chloride by a methanol gas-phase hydrochlorination dry method and a carbon tetrachloride conversion reaction system. The two systems are sequentially connected with a sulfuric acid drying system and a methane chloride rectification system to obtain a methane chloride finished product; the sulfuric acid drying system is further connected with a compressor, and a gas outlet of the compressor is connected with a top gas outlet of the methane chloride rectification system to be mixed into a reaction system for producing methane chloride from crude methane chloride to methane chloride through a gas-phase thermal chlorination method. With a reaction system for producing methane chloride through the methane chloride gas phase thermal chlorination method, obtained mixed gas obtained after methane chloride and chlorine react sequentially enters a steam generation system, a chiller III, a condensation separation system, a dehydrogenation system, a dichloromethane refining system, a trichloromethane refining system and a carbon tetrachloride refining system. The system provided by the invention realizes full-process coupling energy conservation, energy conservation and emission reduction, the condition of single product proportion is changed, and free adjustmentof the proportion of the whole series of products and composite dehydration of the whole system are realized.
Owner:高永宝
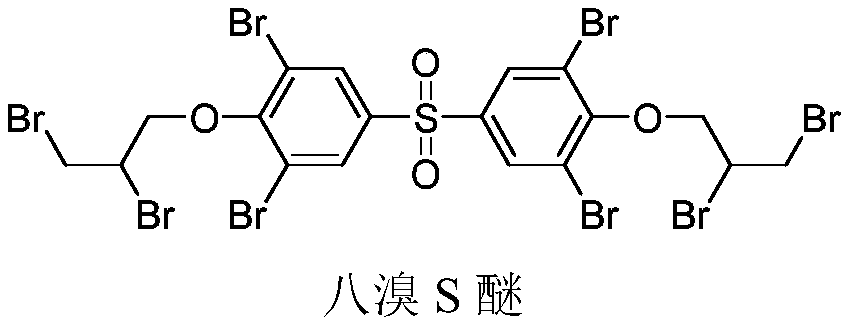
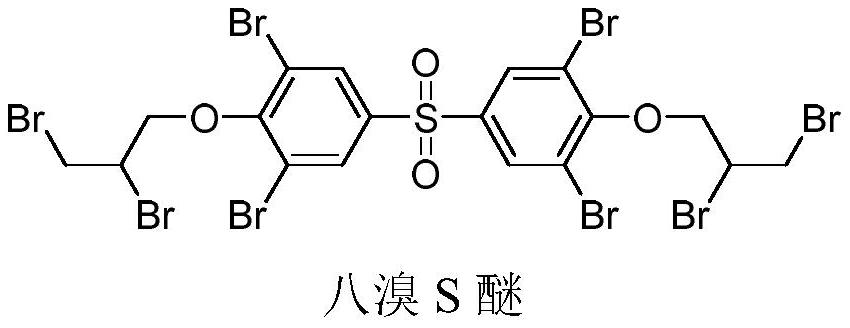
A kind of preparation method of octabromo S ether flame retardant
ActiveCN110981767BShort process routeEasy to operateOrganic chemistryOrganic compound preparationSide chainPropyl ether
Owner:山东旭锐新材股份有限公司
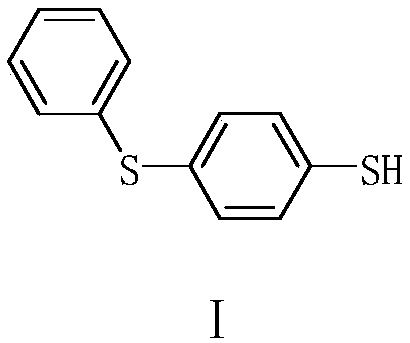
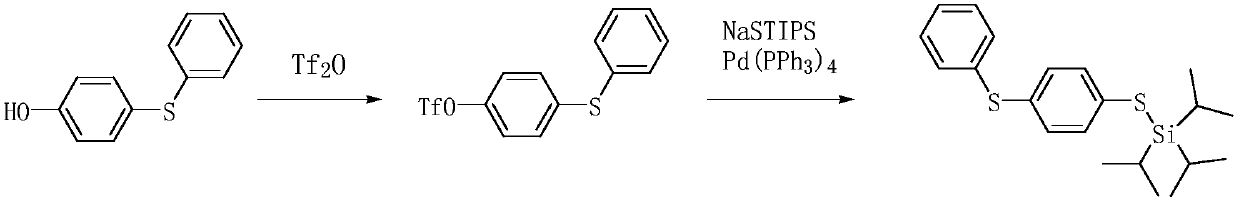
Preparation method of 4-phenylmercaptothiophenol
ActiveCN111302993AReduce productionAtom economy is highOrganic compound preparationSulfide preparationGrignard reagentBiochemical engineering
The invention provides a preparation method of 4-phenylthiolthiophenol. The preparation method comprises the following steps: performing a bromination reaction on diphenyl sulfide and a bromination reagent to prepare 4-bromodiphenyl sulfide, performing a Grignard reaction on the obtained 4-bromodiphenyl sulfide to obtain a corresponding Grignard reagent, reacting the Grignard reagent with sulfur,and acidifying to prepare the 4-phenylthiolthiophenol. The method has the advantages of cheap and accessible raw materials, simple operation technique and low cost, can easily implement the reaction conditions, and is low in wastewater generation, safe, environment-friendly, high in atom economy, few in byproducts, high in yield and purity and suitable for industrial production.
Owner:XINFA PHARMA
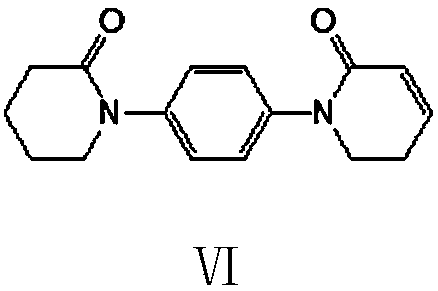
Preparation method of 5,6-dihydropyridine-2 (1H)-one derivative
The invention provides a preparation method of a 5,6-dihydropyridine-2 (1H)-one derivative, and particularly relates to a 1-(piperidine-2-keto-1-yl)-4-(5,6-dihydro-3-R substituent pyridine-2 (1H)-keto-1-yl) benzene preparation method, wherein the R substituent is chlorine or morpholine-4-yl. According to the invention, p-acetamido aniline is used as a raw material, and amidation with delta-valerolactone, halogenation with a halogenation reagent or sulfonylation with sulfonyl chloride, condensation, deacetylation, amidation with 2,2-dichloro-delta-valerolactone, halogenation with a halogenationreagent or sulfonylation with sulfonyl chloride, condensation elimination or condensation elimination substitution in the presence of morpholine are performed to obtain the target product. Accordingto the invention, the raw materials used in the preparation method are cheap, easy to obtain and low in cost; the process operation is simple, reaction conditions are easy to realize, the wastewater yield is low, and safety and greenness are realized; and the reaction selectivity of each step is high, the product yield and purity are high, and industrial production is facilitated.
Owner:XINFA PHARMA
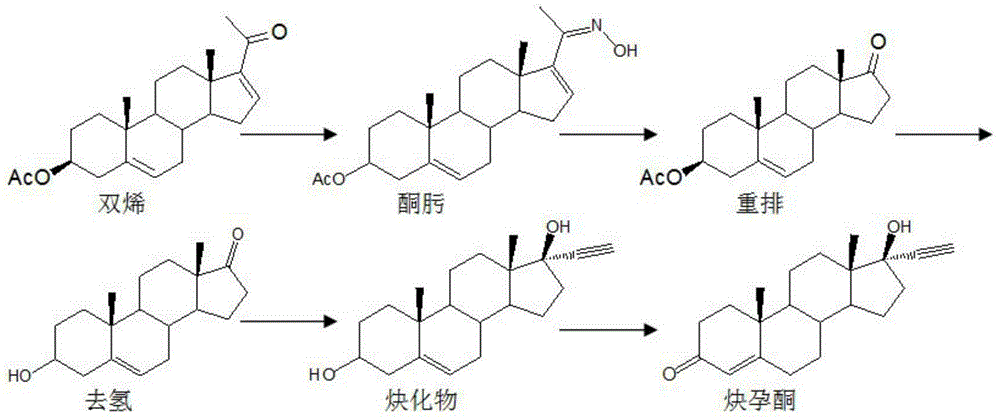
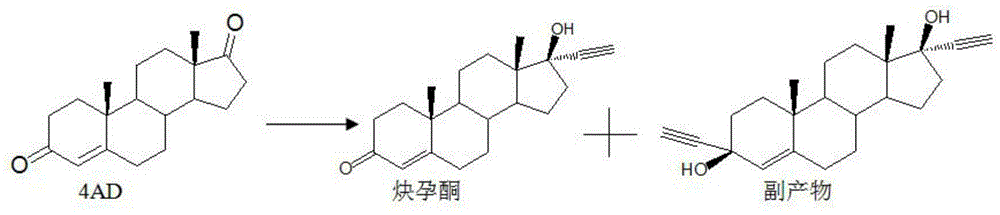
Synthesis method of ethisterone
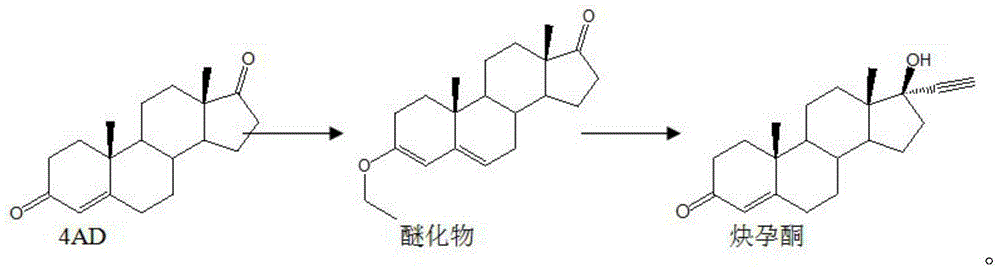
The invention discloses a synthesis method of ethisterone which is prepared by adopting 4AD namely soybean oil tailings as a raw material, carrying out etherification protection as well as two-step reaction, namely, ethynylation and hydrolysis, and then refining. According to the invention, the raw material is replaced, that is the industrial tailing fermentation product 4AD of the soybean oil is used to replace diene; the raw material is wide in supply, low in cost and little in pollution; the reaction route is short; the process is simple; the auxiliary materials are commonly used chemical products; the generation amount of waste gas, waste water and waste solid is small and is a quarter of that generated in the traditional process.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +1
Buparvaquone preparation method
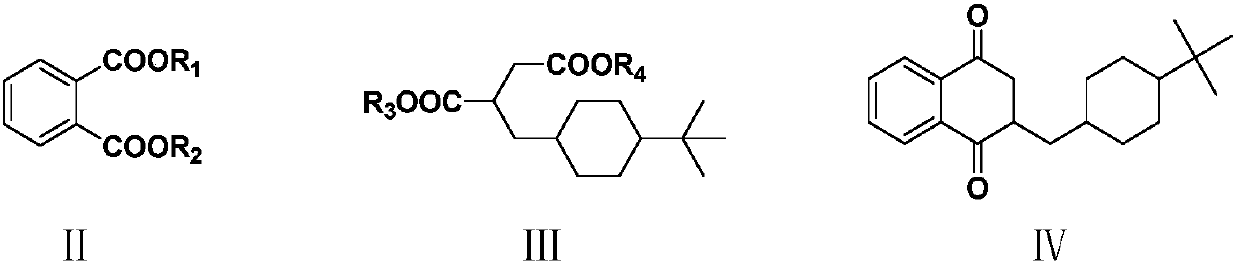
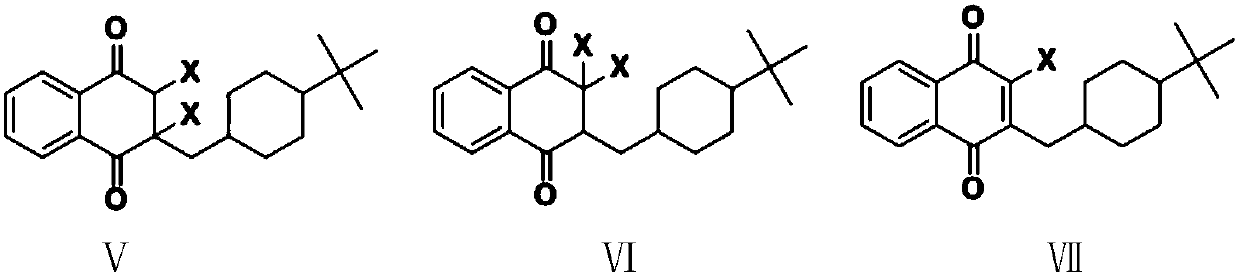
ActiveCN110734368AHigh purityHigh yieldOrganic compound preparationQuinone separation/purificationHydrogen halideButanedioic acid
The invention provides a buparvaquone preparation method, which comprises: carrying out condensation, hydrolysis and decarboxylation by using o-phthalic acid diester and 2-(4-tert-butylcyclohexylmethyl)succinic acid diester as a raw material to prepare 2-[(4-tert-butylcyclohexyl)methyl]-2,3-dihydro-1,4-naphthalenedione, carrying out a substitution reaction on the 2-[(4-tert-butylcyclohexyl)methyl]-2,3-dihydro-1,4-naphthalenedione and a halogenating reagent to obtain a dihalogenated compound mixture, removing halogen hydride through an elimination reaction to obtain 2-[(4-tert-butylcyclohexyl)methyl]-3-halo-1,4-naphthalenedione, and finally carrying out a hydrolysis reaction to obtain the buparvaquone (I). According to the invention, the method has advantages of cheap and easily available raw materials, safe and simple process operation, low cost, little wastewater generation, safety and environmental protection, easily achieved reaction conditions, stable reaction intermediate, high reaction activity, high selectivity and few side reactions, and the prepared buparvaquone is few in impurity and high in purity and yield.
Owner:XINFA PHARMA
Preparation method of amiodarone hydrochloride intermittent
InactiveCN109053652AMild conditionsHigh reaction conversion rateOrganic chemistryMethyl groupAmiodarone Hydrochloride
The invention belongs to the field of medicine synthesis, and relates to a preparation method of an amiodarone hydrochloride intermittent. The method is characterized by including the following stepsthat 1, under an alkaline condition, in the presence of a phase transfer catalyst, a compound 1 and a compound 2 are subjected to nucleophilic substitution reaction to obtain a compound 3; 2, under analkaline condition, the compound 3 is hydrolyzed to generate a compound 4; 3, the compound 4 is subjected to intramolecular aldol condensation, decarboxylation and dehydration to obtain a compound 5;4, a compound 6 and thionyl chloride are subjected to heating reaction to obtain a compound 7; 5, under the presence of lewis acid, the compound 5 and the compound 7 are subjected to friede-crafts acylation reaction to obtain a compound 8; 6, under the presence of lewis acid, the compound 8 is subjected to demethylation to generate a compound 9, namely the amiodarone hydrochloride intermittent 2-butyl-3-(4-hydroxybenzoyl)benzofuran. The preparation method of the amiodarone hydrochloride intermittent has the advantages of being short in reaction time, high in product purity and high in yield,and the amiodarone hydrochloride intermittent is suitable for large-scale industrial production.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
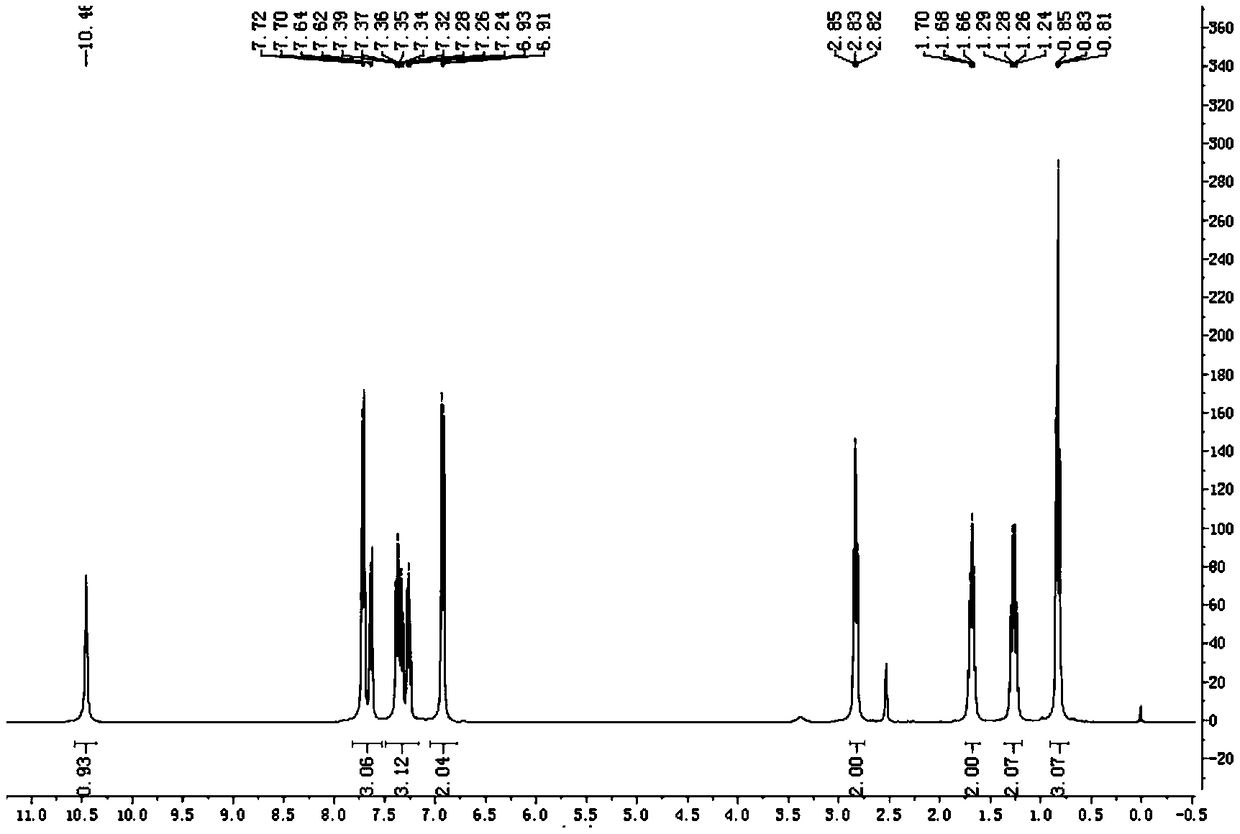
Intermediate compound, carbamazepine and derivative thereof as well as preparation method of oxcarbazepine and derivative thereof
The invention provides an intermediate compound, carbamazepine and a derivative thereof as well as a preparation method of oxcarbazepine and a derivative thereof. 2-substituted aminophenylacetate or 2-substituted aminophenylacetonitrile and 2-halobenzonitrile are used as raw materials, substitution reaction, intramolecular condensation reaction, hydrolysis and hydrochloric acid acidification are carried out to obtain the oxcarbazepine and the derivative 5-substituent-10-oxa-10, 11-dihydro-5H-dibenzo [b, f] aza thereof, and the derivative of the oxcarbazepine can be used as a raw material to prepare the carbamazepine and the derivative 5-substituted iminostilbene thereof, an intermediate compound iminostilbene and intermediate compounds 5-substituted-10-methoxyiminostilbene and 10-methoxyiminostilbene. The raw materials used in the method are cheap and easy to obtain, and the cost is low; the preparation method is simple, conditions are easy to realize, the method is simple, convenientand safe to operate, and the process flow is short; the production amount of three wastes is small, and thus, the method is environmentally friendly; and a target product has high yield and purity, and is suitable for industrial production.
Owner:XINFA PHARMA
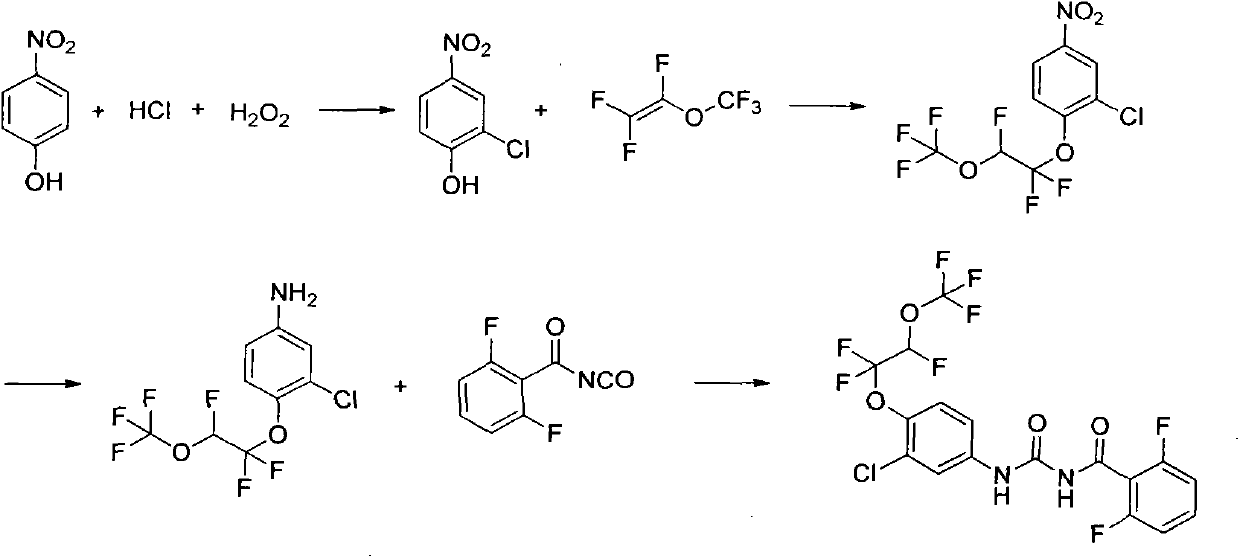
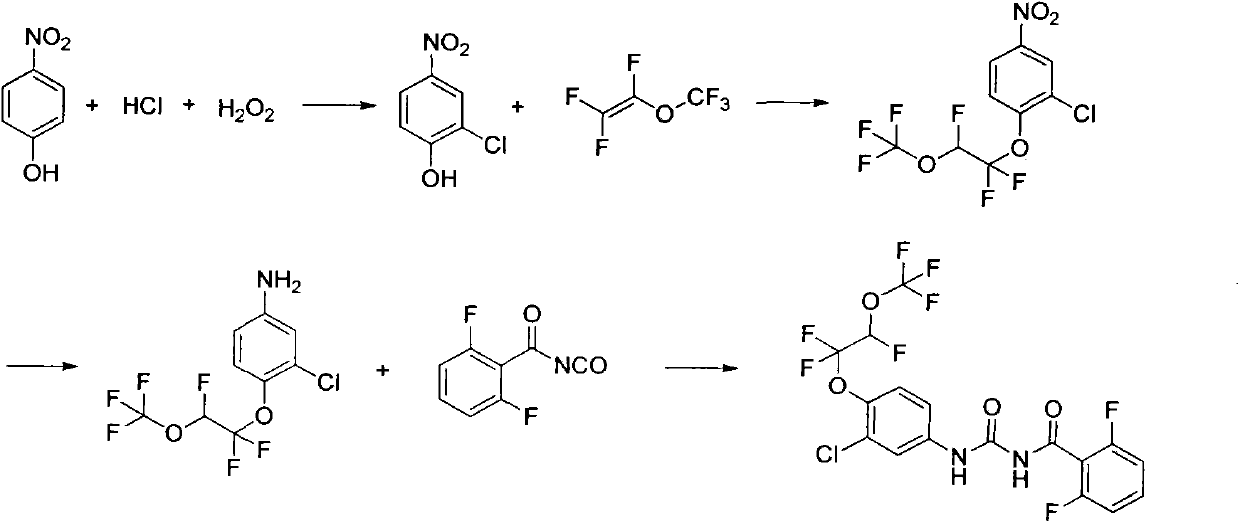
Synthesis method of novaluron
ActiveCN103724233AAvoid side effectsThe amount of three wastes is lessUrea derivatives preparationOrganic compound preparationNovaluronBenzene
The invention discloses a synthesis method of novaluron. P-nitrophenol is taken as a starting material, and hydrogen peroxide and hydrochloric acid chloro are used for preparing 2-chloro-4-nitrophenol; an inorganic base catalyst and perfluorinated vinyl methyl ether are used for addition reaction, 2-chloro-4-nitro-1-(1, 1, 2-trifluoro-2-(trifluoro methoxy) ethoxy) benzene is obtained, then nitro is reduced to obtain 3-chloro-4-(1, 1, 2-trifluoro-2-(trifluoro methoxy) ethoxy) aniline, and finally, 3-chloro-4-(1, 1, 2-trifluoro-2-(trifluoro methoxy) ethoxy) aniline reacts with 2, 6-difluorobenzoyl isocyanate to obtain novaluron. According to the method, raw materials are low in cost and easy to obtain, the operation is simple and convenient, the cost is low, the side reaction is few, and the method is suitable for industrial production.
Owner:JIANGSU JIANNONG PLANT PROTECTION CO LTD
Simple preparation method of 5,6-dihydropyridine-2 (1H)-one derivative
The invention provides a simple and convenient preparation method of a 5,6-dihydropyridine-2(1H)-one derivative. The preparation method comprises the following steps: amidating p-phenylenediamine as araw material and delta-valerolactone, and reacting and condensing the hydroxyl of the obtained amidation product and a halogenating reagent 1 or sulfonyl chloride to obtain 1,4-bis(piperidine-2-keto-1-yl)benzene; carrying out carbonyl ortho-halogenation and elimination to obtain 1-(piperidine-2-keto-1-yl)-4-(5,6-dihydropyridine-2(1H)-keto-1-yl)benzene, and carrying out addition and elimination with a halogenating reagent 3 or carrying out elimination substitution in the presence of morpholine to obtain the 5,6-dihydropyridine-2(1H)-one derivative (I). The preparation method has the advantagesof cheap and easily available raw materials, short steps and low cost; the process operation is simple, reaction conditions are easy to realize, the wastewater yield is low, and safety and greennessare realized; and the reaction selectivity of each step is high, the product yield and purity are high, and industrial production is facilitated.
Owner:XINFA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com