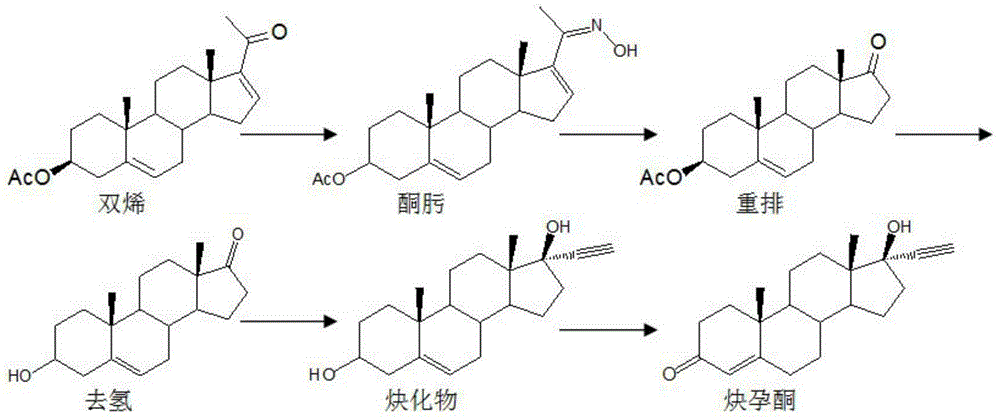
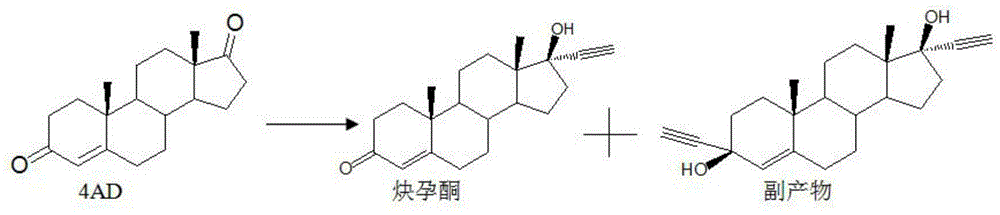
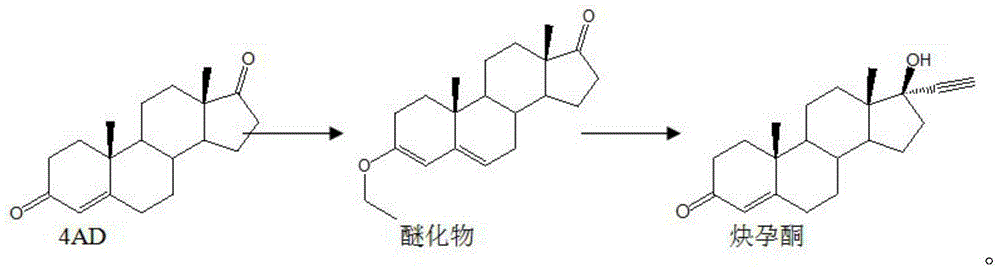
Synthesis method of ethisterone
A synthesis method and a technology for ethinylprogesterone, which are applied in the field of pharmaceutical preparation, can solve the problems of low degree of reaction specificity, high proportion of side reactions, shortage of raw material resources and the like, and achieve the effects of low cost, short reaction route and wide supply of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 10 grams of 4AD, 40 milliliters of ethanol, 20 milliliters of dichloromethane, and 0.02 grams of PTS into the reaction bottle, stir, cool down to 10°C, add 9 milliliters of triethyl orthoformate, control the temperature at 18-20°C and keep the reaction for 120 minutes. Spot the plate (developer: petroleum ether: acetone = 5:2) the raw material spots basically disappear, add 50 ml of ice water to terminate the reaction, stir for 30-40 minutes, add triethylamine dropwise to adjust the pH to 8, and reduce the pressure below 50 °C Concentrate until the remaining amount of ethanol is 1W, then add 50 ml of water to cool down to about 20°C, stir for 2 hours, filter, and drain. Dry at 40°C. Control moisture at 0.2%. Yield: 110%.
[0037] Put 130 grams of toluene isobutanol, 55 grams of isobutanol, and 10 grams of potassium hydroxide into the reaction flask, stir and heat to reflux, control the distillation speed and atmospheric pressure distillation dehydration for 6 hour...
Embodiment 2
[0042] Add 10 grams of 4AD, 60 milliliters of methanol, 20 milliliters of dichloromethane, and 0.02 grams of PTS into the reaction flask, stir, cool down to 20°C, add 10 milliliters of triethyl orthoformate, control the temperature at 35°C for 180 minutes, and spot the plate (Developer: Petroleum ether: Acetone = 5:2) The raw material point basically disappears, add 50 ml of ice water to stop the reaction, stir for 30-40 minutes, add triethylamine dropwise to adjust pH = 8, and concentrate under reduced pressure below 50°C to The remaining amount of methanol is 2W, add 50 ml of water to cool down to about 25°C, stir for 2 hours, filter, and drain. Dry at 60°C. Control moisture at 0.2%. Yield: 110%.
[0043] Put 150 grams of toluene isobutanol, 65 grams of isobutanol, and 10 grams of potassium hydroxide into the reaction flask, stir and heat to reflux, control the distillation speed and atmospheric pressure distillation dehydration for 6 hours, and steam half of the toluene i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com