Synthesis method of UV absorbent ethylhexyl triazone
A technology of ethylhexyl triazone and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as crystallization (solidification) method defects, inability to form crystallized products, complicated crystallization operation, etc., and achieve good crystal form and high production efficiency , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
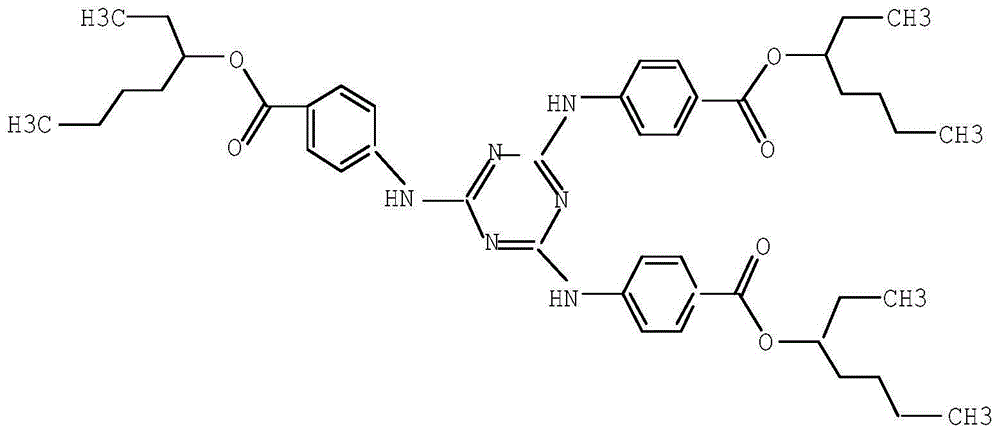
[0037] Example 1: H 3 Synthesis of TATAB
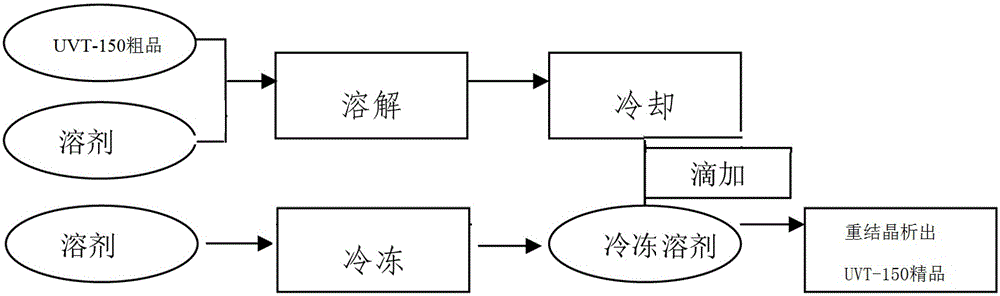
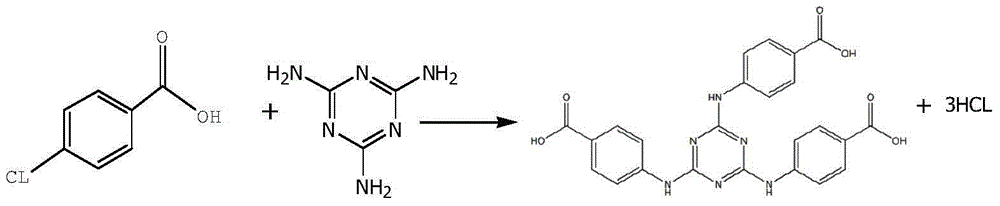
[0038]In a 2000ML four-necked flask, drop melamine 63.10g (0.50mol), 1,4-dioxane 300ml, water 300g, sodium carbonate 86.90g (0.82mol), stir at room temperature; 246.60g (1.58mol ) p-chlorobenzoic acid, dissolved in 300ml of 1,4-dioxane, and then dropped into the reactor at room temperature; during the dropwise addition, the temperature gradually increased to reflux, the temperature was 100-105°C, and the dropwise addition was completed and the insulation reaction was 5h. Sampling and detection of 0.03% melamine residue (HPLC); adding 500 g of water to dilute, freezing at 5°C for suction filtration, washing the filter residue with water until neutral, draining, and drying; 233.54 g (0.48 mol) of white solid H was obtained. 3 TATAB, yield 96.02%.
example 2
[0039] Example 2: H 3 Synthesis of TATAB
[0040] In a 5000ML four-necked flask, drop melamine 126.10g (1.0mol), 1,4-dioxane 600ml, water 600g, sodium carbonate 180.20g (1.70mol), stir at room temperature; 513.35g (3.30mol ) p-chlorobenzoic acid, dissolved in 600ml of acetone, then drop into the reactor at normal temperature; during the dropwise addition, the temperature gradually rises to reflux, the temperature is 56~60°C, after the dropwise addition, the insulation reaction is 5h, and the residual amount of melamine is detected by sampling for 0.05% (HPLC); dilute with 1000g of water, freeze at 5°C for suction filtration, wash the filter residue with water until neutral, drain, and dry; obtain 470.64g (0.97mol) of white solid H 3 TATAB, yield 96.79%.
example 3
[0041] Example 3: H 3 Synthesis of TATAB
[0042] Drop into melamine 63.10g (0.50mol), acetone 300ml, water 300g, sodium carbonate 90.10g (0.85mol) in a 2000ML four-necked flask, stir at room temperature; 246.60g (1.58mol) p-chlorobenzoic acid, dissolved Add 300ml of acetone, and then drop it into the reactor at room temperature; during the dropping process, the temperature gradually rises to reflux, the temperature is 100-105°C, after the dropping is completed, the reaction is kept for 5 hours, and the residual amount of melamine is detected by sampling at 0.05% (HPLC); add 500g of water to dilute , refrigerated at 5°C and suction filtered, the filter residue was washed with water until neutral, drained, and dried; 235.54g (0.48mol) of white solid H was obtained. 3 TATAB, yield 96.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com