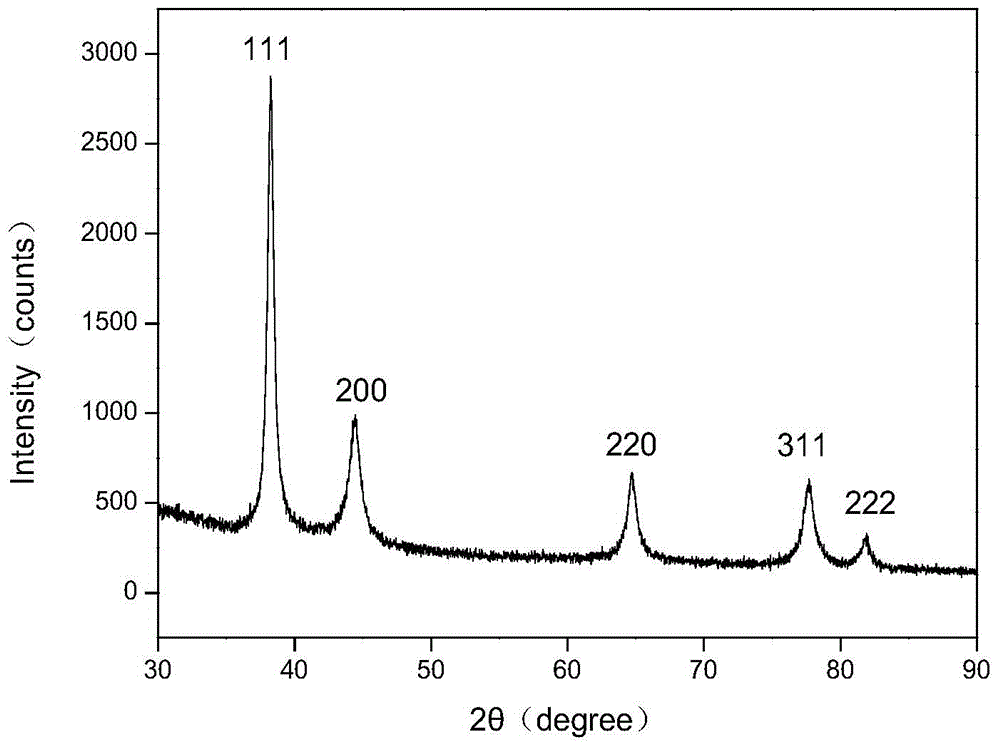
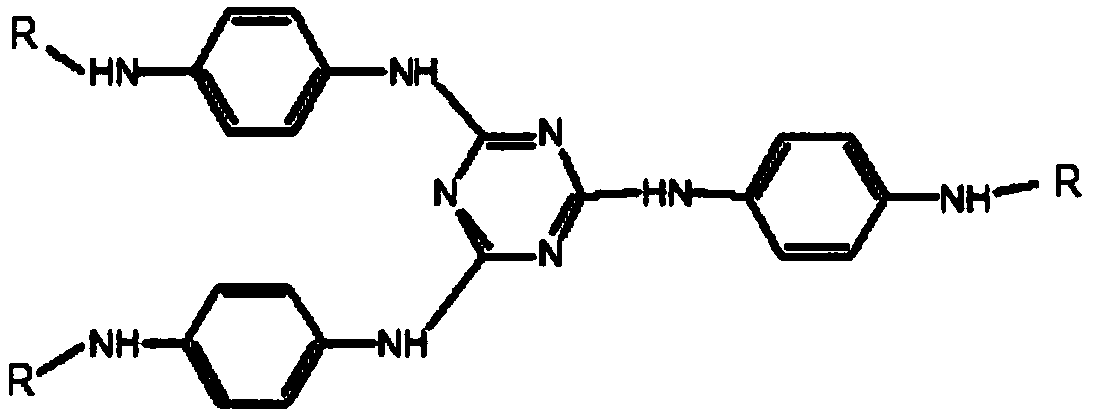
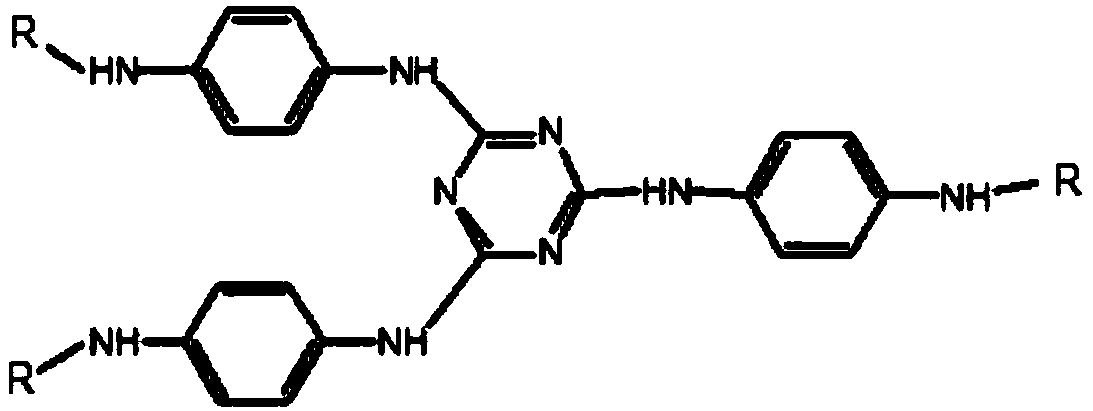
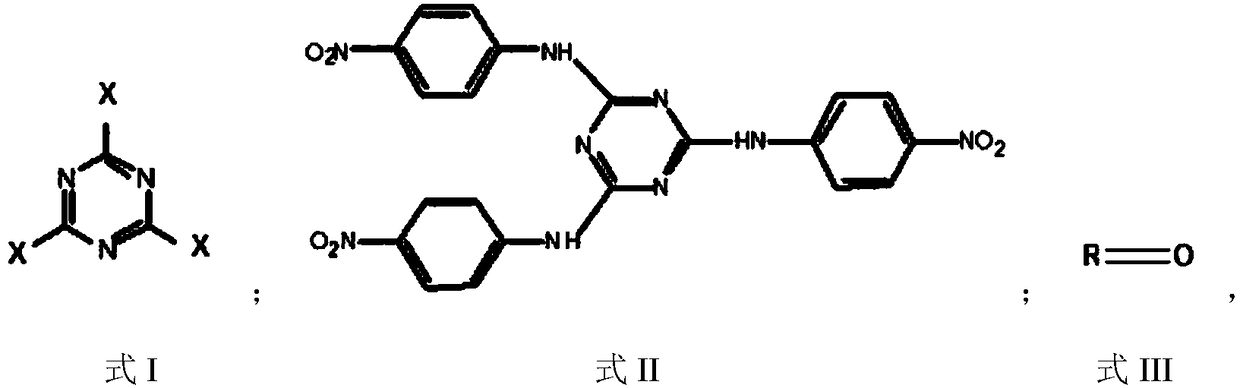
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
264 results about "P-Nitroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment for wastewater in para-nitraniline production and method for resource recovery and use
InactiveCN101244878AImprove adsorption capacityAchieve recyclingOrganic chemistryMultistage water/sewage treatmentAbsorption capacityPhosphate
The invention discloses a method for nitroaniline wastewater treatment and recycling, comprising a plurality of steps: cool p-nitroaniline mother liquor wastewater and crystals are allowed to be separated out; filter the wastewater after crystal precipitation and remove the free ammonia in the filtrate; filter the effluent ever treated at the procedure B and change PH value to acidity or weak alkalinity; use the viscose-based active carbon fiber made by phosphate-impregnated high-temperature steam activation to absorb the p-nitroaniline wherein; evaporate and concentrate the absorbed water; precipitate crystal and filter out ammonium chloride crystal. The active carbon fiber has large absorption capacity and fast absorption speed for p-nitroaniline and can be utilize repeatedly, which makes the recovery rate of p-nitroaniline near 100%. The method has the advantages that the method can fully recover p-nitroaniline and free ammonia in the p-nitroaniline wastewater and the byproduct of ammonium chloride, realizing the unification of wastewater treatment and resources recovery; the method is of great economic and practical value in the treatment of p-nitroaniline wastewater.
Owner:NANJING UNIV
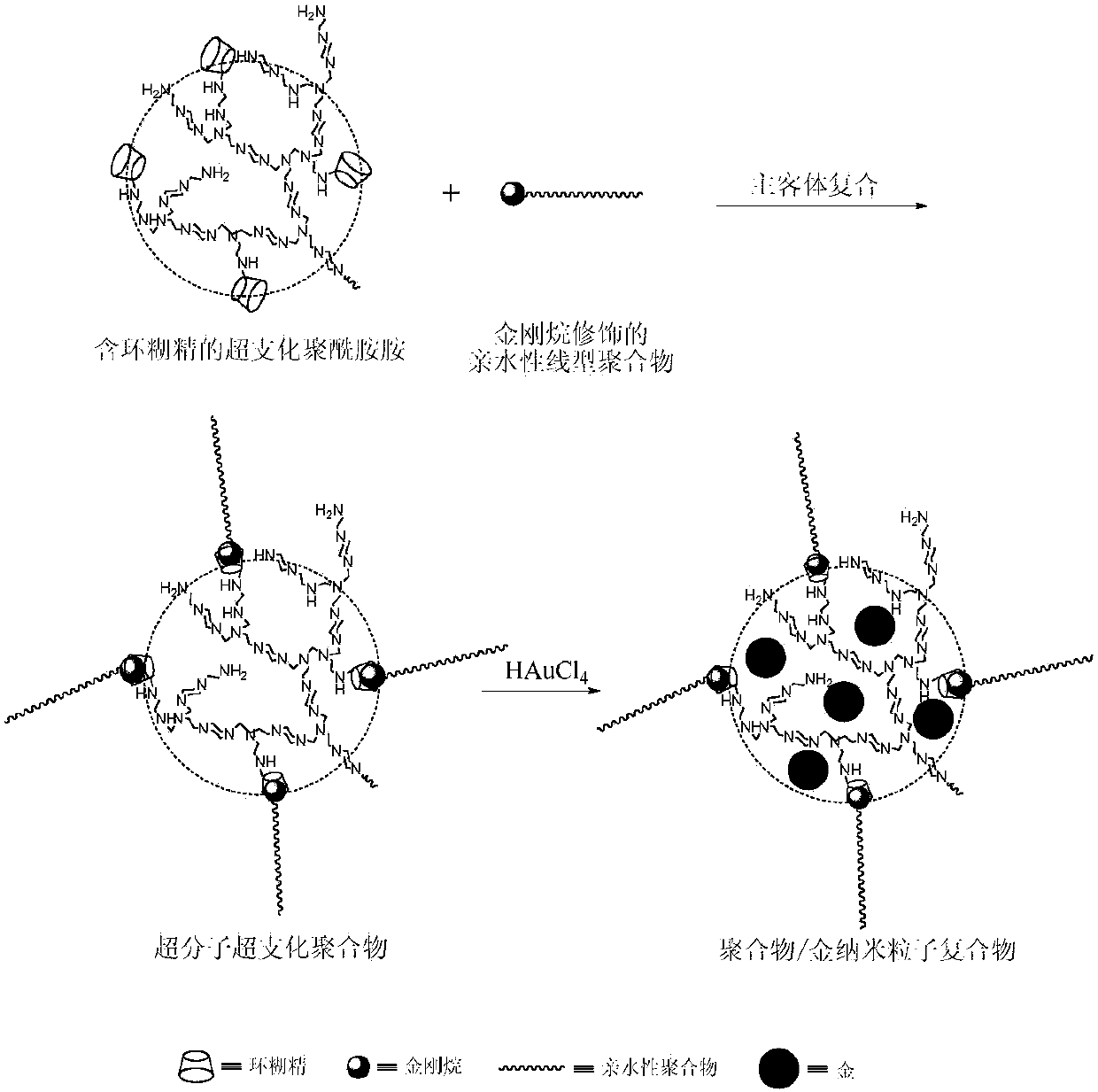
Method for preparing gold nanoparticles based on polyamidoamine amine supermolecular hyperbranched polymer
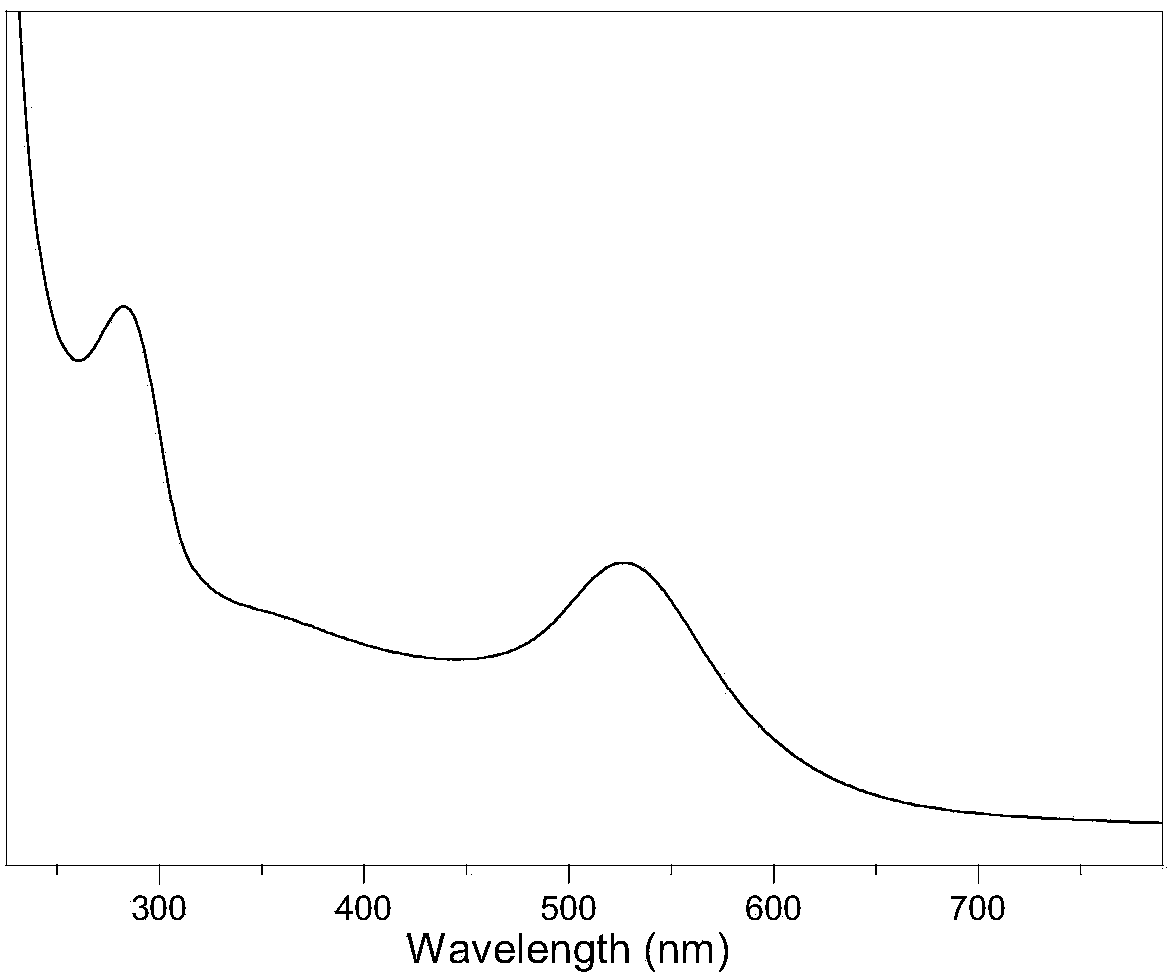
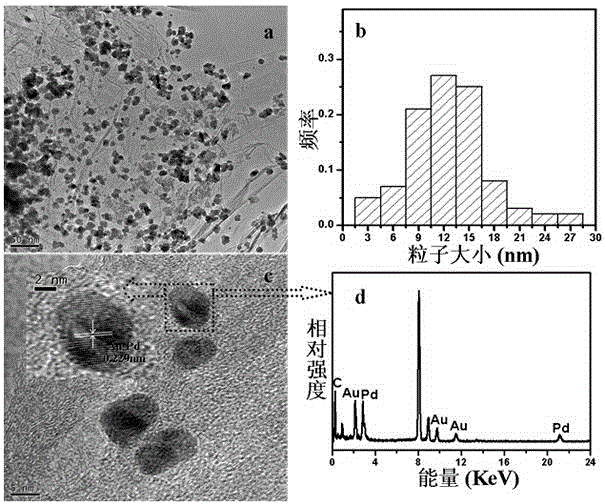
The invention discloses a method for preparing gold nanoparticles based on a polyamidoamine amine supermolecular hyperbranched polymer. The method comprises the steps of slowly dripping an HAuCl4 aqueous solution into an aqueous solution of the polyamidoamine amine supermolecular hyperbranched polymer, and performing stirring reaction under a shading condition to obtain gold nanoparticle aqueous dispersion liquid. According to the preparation method, the polyamidoamine amine supermolecular hyperbranched polymer is simultaneously used as a reducing agent and a stabilizing agent; under a mild reaction condition, the gold nanoparticles which are high in dispersivity and stability, are uniform in particle size distribution and are extremely high in catalysis activity to reduction of nitroaniline are prepared; particularly, the method can be used for effectively controlling the particle size of the gold nanoparticles by controlling the reaction condition and is easy to operate and feasible.
Owner:CENT SOUTH UNIV
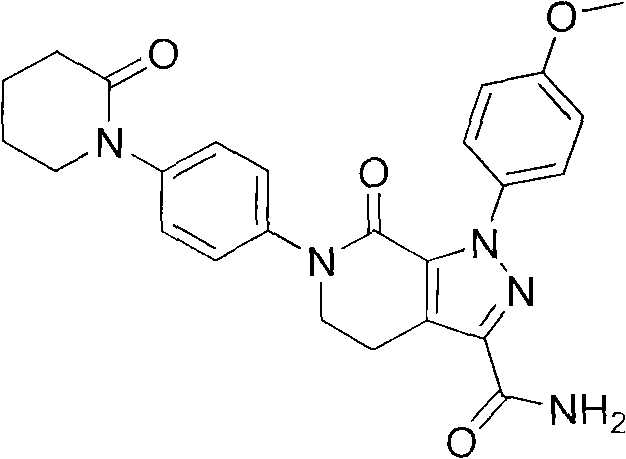
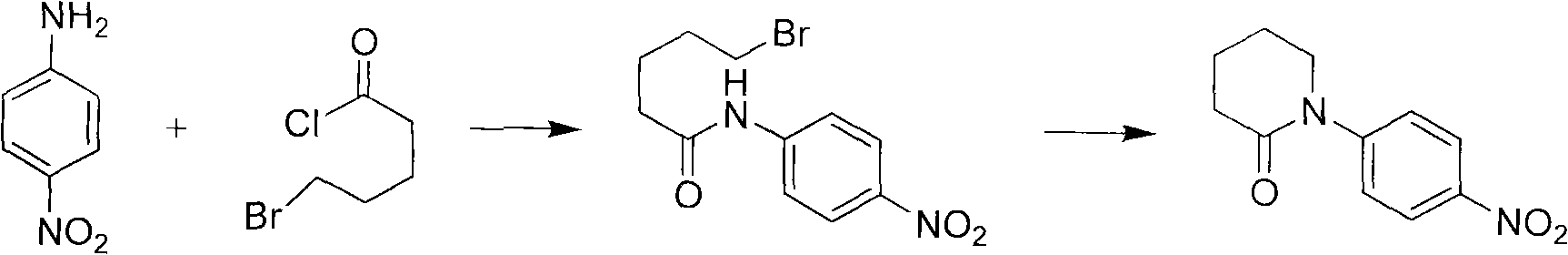
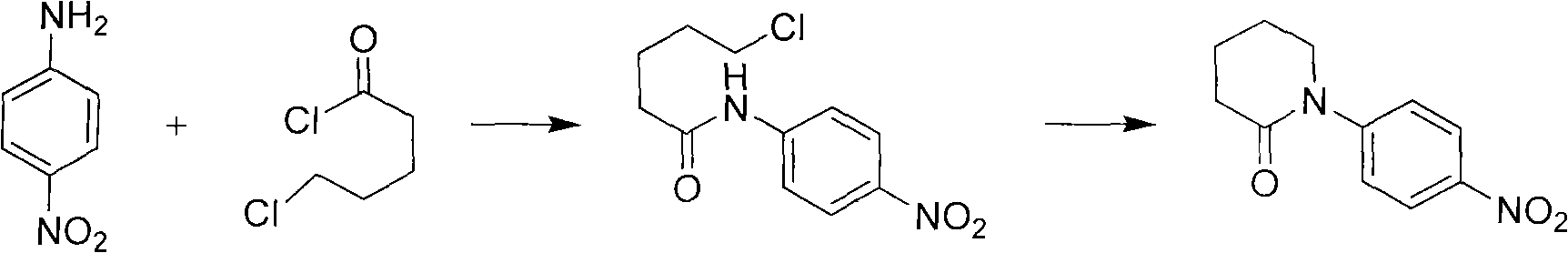
Synthetic method of apixaban intermediate 1-(4-nitrobenzophenone)-2-piperidone
InactiveCN103159670AReasonable process conditionsEasy to operateOrganic chemistryP-NitroanilineChloride
The invention discloses a synthetic method of an apixaban intermediate 1-(4-nitrobenzophenone)-2-piperidone. The 1-(4-nitrobenzophenone)-2-piperidone is formed through an integrated reaction of paranitroaniline and halogenate valeryl chloride under the condition that phase transfer catalyst exists and in a mixed system of inorganic base aqueous solution and aprotic solvent. The synthetic method of the apixaban intermediate 1-(4-nitrobenzophenone)-2-piperidone is reasonable in process condition, easy to operate, high in reaction yield, low in production cost and almost free of discharging of the three wastes.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for producing p-p-phenylene diamine by p-nitroaniline hydrogenation
The invention provides a novel process for synthesizing p-phenylene diamine which comprises, using a catalyst and a solvent, producing p-p-phenylene diamine by p-nitroaniline hydrogenation, disintegrating catalyst after end of reaction, and obtaining p-phenylene through disintegrating solvent from the solution by crystallization or distillation.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
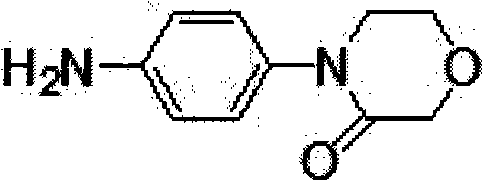
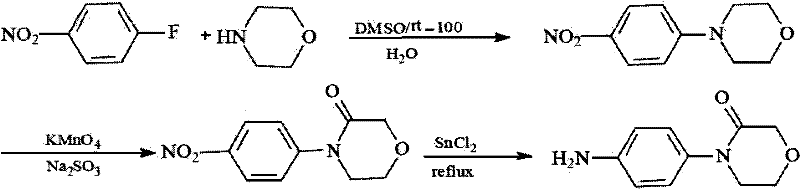
Synthesis method of 4-(4-aminophenyl)-3-morpholone
The invention provides a synthesis method of an important intermediate 4-(4-aminophenyl)-3-morpholone of a new anticoagulant rivaroxaban, belonging to the field of chemical synthesis. The method comprises the following steps: 1) cyclizing nitroaniline and di-halogen aether to obtain 4-(4-nitrophenyl)-morpholine; 2) reacting 4-(4-nitrophenyl)-morpholine and organic hydroperoxide under the action of a catalyst to generate 4-(4-nitrophenyl)-morpholone; and 3) reducing the 4-(4-nitrophenyl)-morpholone into the under the action of a catalyst, wherein the mol yield of the three steps is higher than 49% (on the basis of nitroaniline). The synthesis method provided by the invention has the characteristics of accessible raw materials, low price, simple operation steps, mild reaction environment and the like, and is suitable for industrialized mass production; and the three wastes are easy to process.
Owner:BEIJING GUANHONG TECH
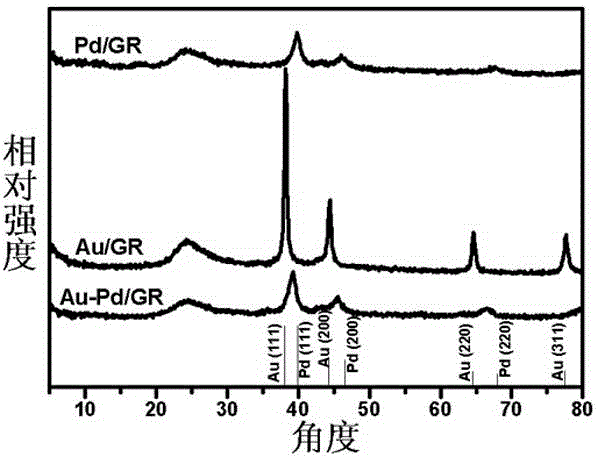
Au-Pd/graphene catalyst and preparation method and application thereof
ActiveCN104307515AHigh selectivityHigh catalytic efficiencyOrganic compound preparationCarbonyl compound preparationRefluxSimple Organic Compounds
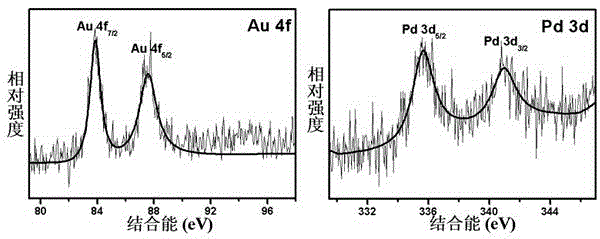
The invention provides an Au-Pd / graphene catalyst and a preparation method and application thereof, and the Au-Pd bimetallic alloy supported graphene catalyst is prepared from graphene oxide, HAuCl4, H2PdCl4 and NaBH4 as raw materials through a one-step reflux. For the first time, the Au-Pd / graphene catalyst is used for solvothermal-free selective oxidation of phemethylol and catalytic reduction of paranitroaniline, and catalytic efficiency and selectivity are high. The catalyst has the advantages of simple preparation method, uses low temperature heat energy as driving energy, is used for the selective oxidation reduction of organic compounds, and is helpful for the sustainable development of the environment and energy.
Owner:MINNAN NORMAL UNIV
Preparation method of apixaban
ActiveCN105732622AAdaptable to conditionsFast aminolysisOrganic chemistryP-NitroanilineNitro reduction
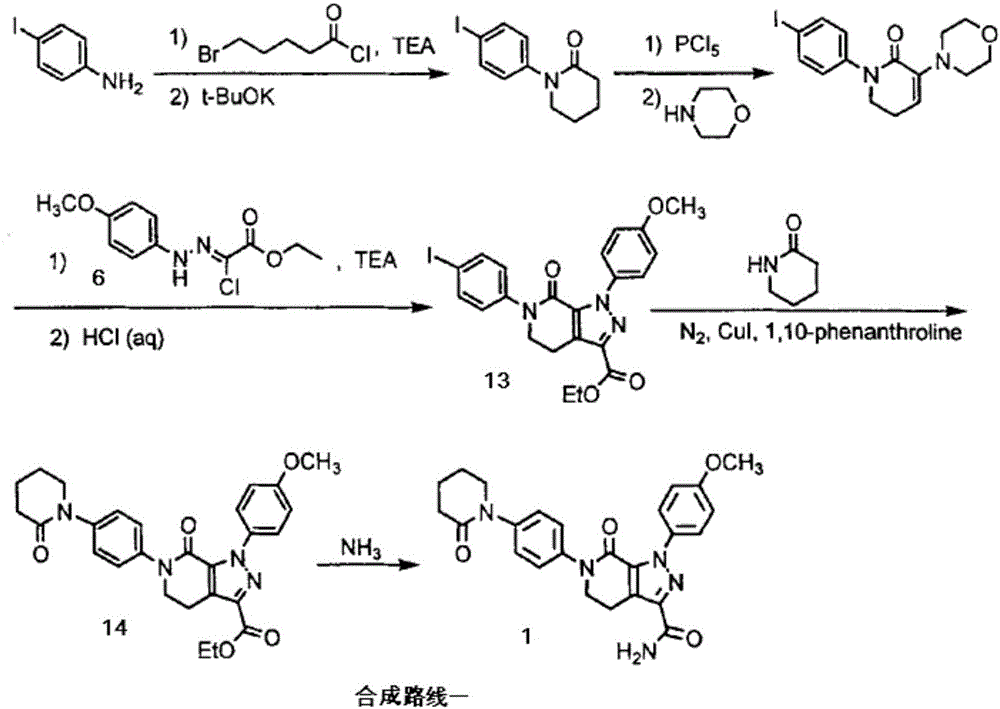
The invention discloses a preparation method of apixaban.The method comprises the steps that paranitroaniline serves as the raw material, paranitroaniline and delta-valerolactone are subjected to amidation ring-opening, substituting and ring-closing reactions under the action of AlMe3, and a compound 8 is obtained; the compound 8 is subjected to alpha-position dichloro substituting and condensation-elimination reactions, and a compound 7 is obtained; the compound 7 and a compound 6 are subjected to [3+2] cyclization-elimination and nitro reduction, and a compound 4 is obtained; the compound 4 sequentially reacts with delta-valerolactone and ammonium chloride under the action of AlMe3, and a compound 3 is obtained; the compound 3 is subjected to substituting and ring closing, and apixaban is obtained.According to the preparation method of apixaban, paranitroaniline and delta-valerolactone which are low in price are adopted to serve as the raw materials, operation of the whole route is simple, conditions of each reaction are mild, and the synthesizing method is easy to operate, high in yield and purity and suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for preparing 2,6-dichloro-4-nitroaniline through direct chlorination of chlorine gas and oxidative chlorination of hydrogen peroxide
ActiveCN104610071AThe concentration of hydrochloric acid does not changeThe reaction process is stable and easy to controlOrganic compound preparationAmino compound preparationP-NitroanilineReaction system
The invention relates to a method for preparing 2,6-dichloro-4-nitroaniline through direct chlorination of chlorine gas and oxidative chlorination of hydrogen peroxide. The method comprises the following steps: (1) adding paranitroaniline into hydrochloric acid at the concentration of 5-35wt%, heating to 40-80 DEG C, stirring for uniformly mixing, slowly inflating the chlorine gas within 0.5-5h, slowly adding hydrogen peroxide dropwise, and after adding the chlorine gas and the hydrogen peroxide, continuing to preserve heat and react for 0.3-1.5h; (2) filtering a product obtained in the step (1), washing a filter cake to be neutral, and drying to obtain the 2,6-dichloro-4-nitroaniline. By regulating a dosage ratio of the chlorine gas to the hydrogen peroxide, the concentration of the hydrochloric acid in a reaction system can be kept unchanged, the reaction process is steady and easily controlled, and the product is high in yield and purity.
Owner:昌邑新澳化工有限公司
Method for synthesizing rivaroxaban intermediate 4-(4-aminophenyl)-3-molindone
ActiveCN103524447AReduce pollutionRaw materials are cheap and easy to getOrganic chemistryIron powderEthylene oxide
The invention discloses a method for synthesizing a rivaroxaban intermediate 4-(4-aminophenyl)-3-molindone. The method comprises the following steps: reacting paranitroaniline with ethylene oxide to obtain a compound II; performing a cyclization reaction on the compound II and bromoacetyl bromide to obtain a compound III; adding the compound III into iron powder to perform a reduction reaction to obtain the 4-(4-aminophenyl)-3-molindone. According to the method, the raw materials are low in cost and easily obtained, the cost is low, the synthesis operation is simple, industrial nitration is avoided, environmental pollution is greatly reduced, and the method is suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Preparation method of paranitroaniline
ActiveCN102617361APromote safe productionIncrease production capacityOrganic compound preparationAmino compound preparationP-NitroanilineReaction temperature
Owner:苏州市罗森助剂有限公司
Method for continuously preparing N, N'-bis(1,4-dimethylpentyl)-p-phenylenediamine
InactiveCN103467305AReduce stepsReduce labor intensityPreparation by reductive alkylationPtru catalystVapor–liquid separator
The invention belongs to the technical field of fine chemical engineering, and relates to a method for continuously preparing N, N'-bis(1,4-dimethylpentyl)-p-phenylenediamine, and the method helps to realize continuous and low-pressure production of N'-bis(1,4-dimethylpentyl)-p-phenylenediamine, so that cost is saved and economical benefit is raised. The method comprises: mixing p-phenylenediamine (or p-nitroaniline ) and 5-methyl-2-hexanone according to a certain ratio, continuously conveying to a preheater, keeping the preheater at 110-150 DEG C, then enabling preheated materials to enter into a narrow and long reactor loaded with a solid catalyst via a pipeline, and keeping the reactor at 150-200 DEG C, at the same time continuously introducing hydrogen with a pressure of 0.5-2.5 MPa into the reactor, after the materials are reacted, collecting the product into a gas-liquid separator via a cooler, moving out the reaction solution and performing reduced pressure distillation to obtain N, N'-bis(1,4-dimethylpentyl)-p-phenylenediamine. The conversion rate of p-nitroaniline is 99% or more and selectivity is 95% or more.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing antithrombotic medicament apixaban
InactiveCN101967145BReasonable designThe reaction steps are simpleOrganic chemistryPhysical/chemical process catalystsMorpholineP-Nitroaniline
The invention discloses a method for preparing antithrombotic medicament apixaban. The method comprises the following steps of: obtaining a compound V by performing an amidation-cyclization two-step one-pot reaction on paranitroaniline serving as a raw material and a general purpose reagent 5-chlorine valeryl chloride under an alkaline condition; performing di-chlorination on the alpha-hydrogen of the V by using phosphorus pentachloride; performing a condensation-elimination reaction with excess morpholine to obtain a compound VI; reducing the VI into a compound VII by using sodium sulfide; performing the amidation-cyclization two-step one-pot reaction on the VII and the 5-chlorine valeryl chloride to obtain a key intermediate III; obtaining II by performing a [3+2] cyclization-elimination reaction on the III and another intermediate IV; and finally, obtaining I by performing aminolysis on the II. The method has the advantages that: process design is reasonable, expensive reagent is not used, reaction yield is high, raw material cost is low, and the method is simple and convenient to operate, has no harsh reaction condition, and is easy to perform large-scale production.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Continuous synthetic method of high-purity p-nitroaniline
InactiveCN107619373AReduce energy consumptionImprove product qualityOrganic compound preparationChemical recyclingFixed bedP-Nitroaniline
The invention provides a new process for synthesizing p-nitroaniline. The process comprises: taking p-nitrochlorobenzene and ammonia water as raw materials, in a fixed bed reactor or shell-and-tube reactor and in the presence of a catalyst, carrying out an ammonification reaction to prepare a p-nitroaniline crude product, performing separation recycling to the crude product to prepare p-nitroaniline and obtain ammonium chloride as a by-product, and recycling excess ammonia for cyclic application. According to the process, the p-nitroaniline conversion rate can reach 100%, and the purity of p-nitroaniline can reach 99.9%. The process is green, eco-friendly and new.
Owner:CHINA PETROLEUM & CHEM CORP +1
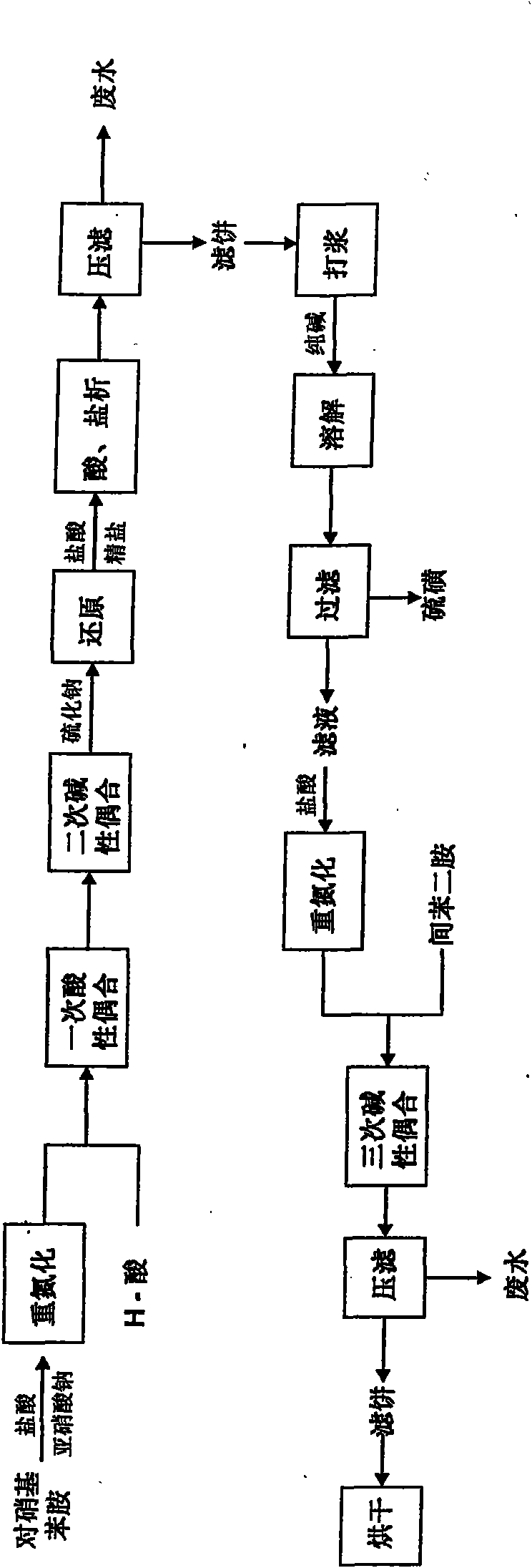
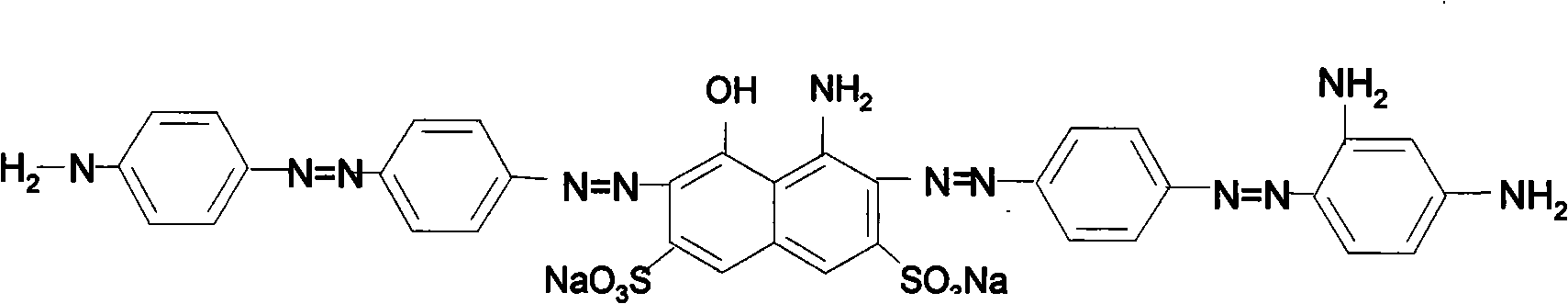
Pollution-free production technology of direct fast black G
The invention relates to a pollution-free production technology of direct fast black G, comprising the following implementation steps: firstly, primary acid coupling is carried out on nitroaniline after diazotization and H-acid; after the full reaction of the H-acid is detected, sodium carbonate is added and pH is adjusted to 8 to 8.2 to carry out secondary basic coupling; the coupling product is added into a sodium sulfide solution for reduction, and the temperature gradually raises to t of 38 DEG C to 40 DEG C in the reaction process; after the sodium carbonate is added into the reduction product after acidification to adjust the pH of a medium to 7.5 to 8, the mixture is adsorbed by activated carbon and filtered, and ternary coupling is carried out on the obtained filter liquor after diazotization and lentine; and after the reaction is detected to be end point, the materials are directly delivered to a drying tower for spray drying. In the invention, the reduced reaction solution does not need the processes of salting out, acidification and filtration, activated carbon adsorption is adopted instead. In the process, no waste liquor is generated, and environment pollution caused in the production process of dye is greatly reduced. Simultaneously, wastewater processing is not needed, and the cost is saved. The optimized technology directly transfers the reaction solution after ternary coupling into the drying tower for spray drying; and the yield and the coloring intensity of the dye are effectively increased, energy is saved, the consumption is reduced, and the effect is obvious.
Owner:INNER MONGOLIA XINYA CHEM
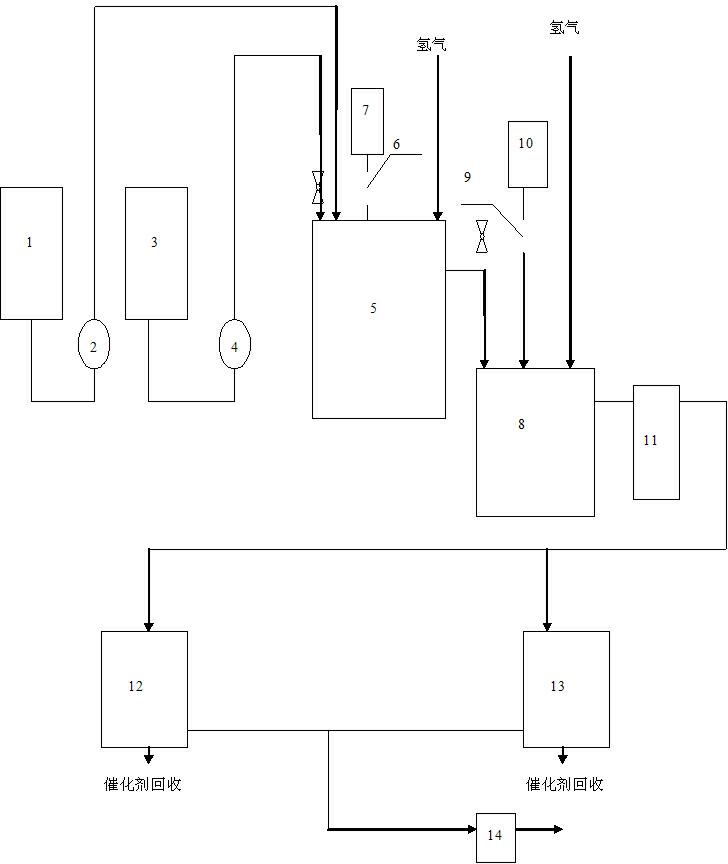
Method and device for producing p-phenylenediamine by using liquid phase continuous hydrogenation method
InactiveCN102276479AIncrease exposureReduce contentOrganic compound preparationChemical recyclingPtru catalystFluid phase
The invention discloses a method and device for producing p-phenylenediamine by using a liquid phase continuous hydrogenation method. The reaction is carried out in first-stage and second-stage hydrogenation reaction kettles which are connected in series. The method comprises the following steps of: adding methanol which is 50-70% of the volume of the reaction kettles into the first-stage and the second-stage hydrogenation reaction kettles, adding a catalyst which is 0.5-1.5% of the methanol, and continuously introducing hydrogen into the first-stage and the second-stage hydrogenation reaction kettles in a stirring state; after reaching a certain pressure in the first-stage and the second-stage hydrogenation reaction kettles, continuously adding methanol and paranitroaniline into the first-stage hydrogenation reaction kettle, continuously keeping the pressure in the first-stage and the second-stage hydrogenation reaction kettles, meanwhile, discharging from the first-stage hydrogenation reaction kettle to the second-stage hydrogenation reaction kettle, and continuously discharging from the second-stage hydrogenation reaction kettle; recycling the catalyst from the reaction liquid from the second-stage hydrogenation reaction kettle through a settling tank; and removing methanol and water by rectifying the reaction liquid after the catalyst is recycled so as to obtain the p-phenylenediamine. The method and the device disclosed by the invention have the advantages of low cost, high yield, good safety and environment friendliness.
Owner:JIANGSU KESHENG CHEM MACHINERY
Preparation method and application of pyrite inhibitor
InactiveCN101972707AEnhanced inhibitory effectInhibition does not occurSulfonic acid amide preparationFlotationPyriteWater soluble
The invention discloses a method for preparing a pyrite inhibitor, which comprises the following steps of: adding 14 to 16g of paranitroaniline into 120 to 150ml of 20 volume percent sulfuric acid solution, heating for dissolution, cooling to the temperature of 10 DEG C in an ice bath, and slowly adding 25 to 30 ml of 25 mass percent solution of sodium nitrite to prepare a product 1 at the temperature of 10 DEG C; preparing 12 to 15g of 4,4'-diamidobenzene sulphonamide and 15 to 20ml of 1mol / L hydrochloric acid into solution and cooling to the temperature of 10 DEG C in the ice bath, and coupling with the product 1 under the condition of a pH value of 9 to 10 so as to prepare a product 2; and coupling the product 2 with 15 to 20ml of 1mol / L m-phenylenediamine under the condition of a pH value of 9 to 10 so as to prepare black water-soluble powder, namely the pyrite inhibitor. The inhibitor has good inhibitory action on pyrite, no influence on golden or silver ore in lead ore, low consumption and no toxin or pollution.
Owner:GUANGXI UNIV
Method for producing 2,6-dichloro p-nitroaniline and special reactor thereof
InactiveCN101423480ALow costIncrease productionOrganic compound preparationAmino compound preparationP-NitroanilineTower
The invention relates to a method for producing 2, 6-dichloro-p-nitroaniline and a special reaction kettle thereof. The method is as follows: nitroaniline and chlorine gas are subjected to substitution reaction in a medium of concentrated hydrochloric acid to generate the 2, 6-dichloro-p-nitroaniline, in particular, the substitution reaction is carried out in a large-volume reaction kettle connected with a tail gas absorption tower, the large-volume reaction kettle comprises a kettle body cast by cement and ceramic tiles lined on the inner surface of the kettle body, and the volume of the reaction kettle is more than or equal to 15m<3>. The adopted large-volume reaction kettle can improve yield, reduce production cost, and reduce environmental pollution.
Owner:苏州市罗森助剂有限公司
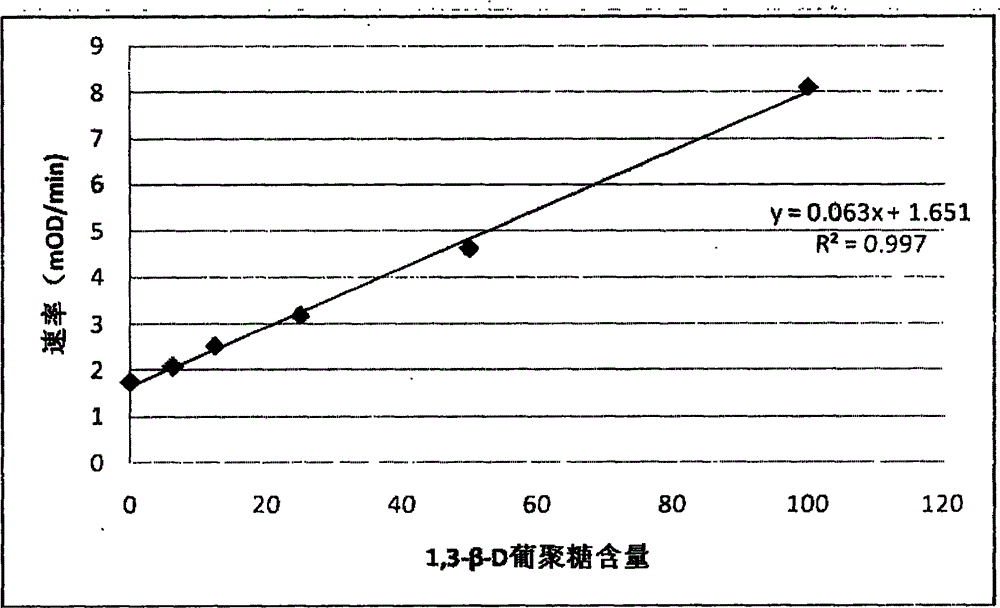
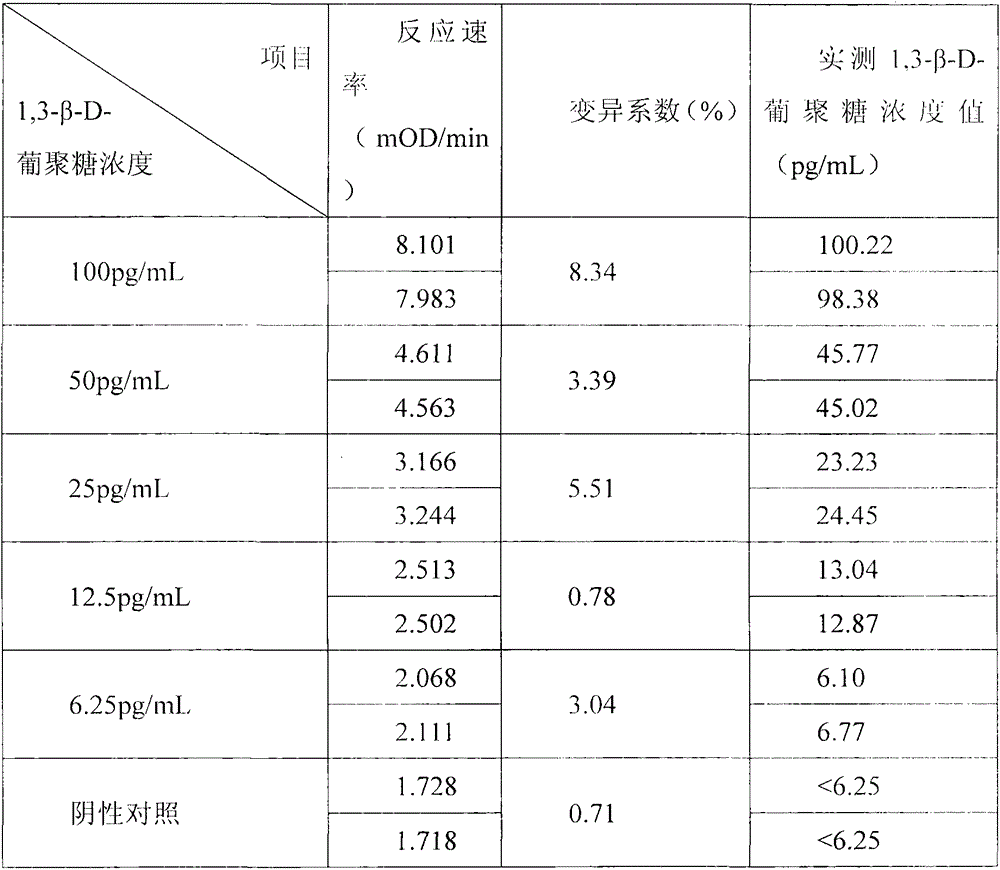
Developing-process fungus 1,3-beta-D-glucan detection kit for human body fluid
ActiveCN105021817AReduce false positive rateLess susceptible to interferenceColor/spectral properties measurementsBiological testingZymogenEnzyme digestion
The invention relates to a developing-process fungus 1,3-beta-D-glucan detection kit for human body fluid. The developing-process fungus 1,3-beta-D-glucan detection kit comprises a reaction main agent, a main agent compound solution, a sample treatment solution, heat-source-free water, a standard product and a quality control product, wherein the reaction main agent takes horseshoe crab blood cells as a main raw material and contains G factors, coagulase, coagulase zymogen and a polypeptide developing substrate; the polypeptide developing substrate is synthesized tripeptide or tetrapeptide with a Gly-Arg tail end connected with a PNA; the polypeptide developing substrate is subjected to enzyme digestion by adopting the coagulase; after the free paranitroaniline (PNA) is generated, a microplate reader is used for directly detecting so that a detection route is shortened and the cost is reduced; the microplate reader is used for carrying out a velocity-method enzyme kinetics detection method so that the sensitivity is relatively high when being compared with a nephelometry detection method; and the reaction main agent is not easily interfered by protein in a body fluid sample and medicines to generate non-specific turbidity, so that the probability of a false positive detection result is reduced and the detection accuracy is relatively high.
Owner:DYNAMIKER BIOTECH TIANJIN
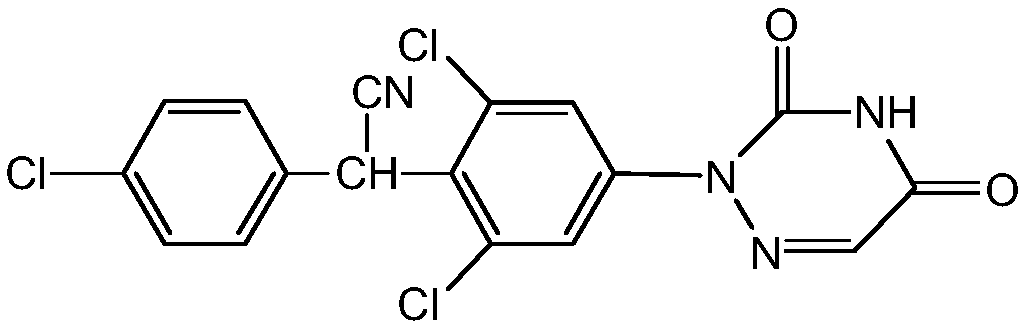
Preparation method of anticoccidial drug Diclazuril
ActiveCN107746390ASolve the environmental protection problems caused by the strong smell of thioglycolic acidHigh yieldOrganic chemistryP-NitroanilineHydrolysis
The invention discloses a preparation method of an anticoccidial drug Diclazuril. The method takes 3,4,5-trichloronitrobenzene as a raw material and the 3,4,5-trichloronitrobenzene and 4-chlorobenzylcyanide are subjected to condensation reaction to generate 2,6-dichloro-alpha-(4-chlorobenzyl)-4-nitrophenylacetonitrile; enabling a condensate and hydrazine hydrate to be subjected to reduction to generate 2,6-dichloro-alpha-(4-chlorobenzyl)-4-aminophenylacetonitrile, enabling a reduzate and malonyl ethyl dicarbamate to be subjected to diazo, coupling, cyclization, hydrolysis and decarboxylationone-pot reaction to generate the Diclazuril. The method disclosed by the invention has a simple technology and is easy to operate; the environment protection problem caused by the fact that the odor of thioglycolic acid is great in a decarboxylation process of the Diclazuril is solved; meanwhile, the yield is improved and the total yield of the Diclazuril synthesized by taking 2,6-dichloro p-nitroaniline as the raw material is 43.8 percent, so that the preparation method is suitable for industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
Method for synthesizing shewanella halitios into god nanoparticles and application of gold nanoparticles
ActiveCN104588677ASimple methodMild conditionsNanotechnologyMetal/metal-oxides/metal-hydroxide catalystsSodium lactateHydrogen
The invention discloses a method for synthesizing shewanella halitios into god nanoparticles and application of the gold nanoparticles. The method comprises the following steps that firstly, shewanella halitios Z4 cultivated to a stable period is centrifugally collected, the shewanella halitios Z4 is cleaned by deionized water, and then the shewanella halitios Z4 is prepared into a bacterium suspension; secondly, the bacterium suspension in the step one is added into a chloroauric acid solution, sodium lactate is added as an electron donor, a table concentrator carries out shake cultivation, and a nanogold solution is obtained through the reaction; thirdly, the nanogold solution in the step two is centrifugally collected, and is dried for 12 hours to 36 hours at the temperature of 60 DEG C to 90 DEG C to obtain the gold nanoparticles. According to a green synthesis method, no large type equipment is needed, the method is simple, the condition is moderate, hydrogen does not need to be added as the electron donor, cost is low, and the method is safe. The synthetic gold nanoparticle particles can be used as catalysts to catalyz degradation of nitroaniline, and the method can be applied to removal of environment pollutants.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of phase-change solar heat-absorption coating with high heat absorption rate
InactiveCN108976880AImprove thermal conductivityImprove efficiencyChemical industryPolyurea/polyurethane coatingsHigh energyPolyethylene glycol
The invention discloses a preparation method of phase-change solar heat-absorption coating with a high heat absorption rate and belongs to the technical field of preparation of coating. According to the preparation method, after an emulsifier OP-10, liquid paraffin and hydrochloric acid are acidified and dissolved, a mixture is homogenized and emulsified under a heating state to obtain oil-in-water emulsion; outer holes of organic dispersion carbon nanotubes are blocked through black absorbents including a manganese ferrite black spinel pigment, carbon black, copper oxide powder and the like;on one hand, added titanium white has a light extinction effect; on the other hand, titanium dioxide is used as a photo-thermal absorption catalyst and can be compounded with silicon dioxide in thermal phase-change microcapsules, so that adjustable phase change temperature, shaping and phase change energy storage, and high energy storage density of a material are realized; polyethylene glycol 10000 with high latent heat and the liquid paraffin which easily has phase change at room temperature are utilized, so that the energy storage efficiency of the phase change material is improved; the surface of a carbon nanotube material is modified by utilizing p-nitroaniline diazonium salt; the distribution of a phase change substance in the phase change material is more uniform through high heat conductivity and high specific surface area of the carbon nanotube material, and convection and heat dissipation of the coating are reduced, so that the preparation method has a wide application prospect.
Owner:FOSHAN TENGLI NEW ENERGY TECH CO LTD
Preparation method of triazine derivative
The invention provides a preparation method of a triazine derivative. The preparation method comprises the following steps: carrying out a substitution reaction on p-nitroaniline and a compound A to form an intermediate B; carrying out a hydrogenation reduction hydrocarbylation reaction on the intermediate B and a compound C to obtain the triazine derivative. According to the preparation method provided by the invention, the triazine derivative is prepared by two steps sequentially comprising substitution and hydrogenation reduction hydrocarbylation, so that not only is the product yield relatively high, but also the technical process is greatly shortened. Meanwhile, due to the adoption of the method, a disubstituted p-phenylenediamine byproduct generated in a traditional process is effectively avoided, it is easy to separate a product, the cost is greatly reduced, no pollution is caused, the quality of the product is high, and it is easy to realize industrialization.
Owner:JIANGSU SINORGCHEM TECH CO LTD
Preparation method of nicarbazin midbody 4,4'- binitro sym-diphenylurea
ActiveCN101914042ASimple processLow costUrea derivatives preparationOrganic compound preparationOrganic solventP-Nitroaniline
The invention relates to a preparation method of 4,4'-binitro sym-diphenylurea, comprising the following steps of: adding paranitroaniline, urea and concentrated hydrochloric acid to a reactor, and reacting for 1-5 hours at 180-200 DEG C under stirring, and purifying to obtain the 4,4'-binitro sym-diphenylurea. The invention has simple process, low cost and high yield, does not use an organic solvent in the reaction process and is suitable for industrial production.
Owner:MASTEAM BIO TECH
Preparation method of p-nitrophenol
ActiveCN104649911ARouting SecurityEasy to operateOrganic chemistryOrganic compound preparationNitrophenol productP-Nitroaniline
Owner:QUZHOU UNIV
Synthetic method of 2,6-dibromo-4-nitroaniline diazosalt
The invention relates to a synthetic method of 2,6-dibromo-4-nitroaniline diazosalt, solving the technical problems of simplified technique and operation, low requirement for production field and equipment, and energy consumption and 'three wastes' reduction. The invention comprises the following steps: using paranitroaniline as a raw material, pulping in sulphuric acid medium at the mass percentof 20% to 98%, adding brominated compound and oxidant for bromination, and directly diazotizing the mixture with the diazotizd agent after bromination to obtain product, wherein the molar ratio of paranitroaniline, sulphuric acid, brominated compound, oxidant and diazotized agent is 1:2.0 to 8.0:1.0 to 2.5:0.4 to 2.5:1.0 to 1.2.
Owner:HANGZHOU JIHUA JIANGDONG CHEMICAL CO LTD
Method for preparing o-chloro-p-nitroaniline diazosalt
ActiveCN101613305AAvoid emissionsReduce manufacturing costsOrganic chemistrySynthesis methodsP-Nitroaniline
The invention relates to a method for preparing an o-chloro-p-nitroaniline diazosalt and aims to solve a technical problem of extending a process for synthesizing o-chloro-p-nitroaniline into a diazotization process to obtain high-quality and low-cost products. The method comprises the following steps: using paranitroaniline as a raw material to pulp in a 5 to 30 mass percent acid medium; adding a chlorinating agent and a surfactant in an amount of 1 to 5 percent of the dosage of the paranitroaniline for chlorination; and after the chlorination is finished, directly adding a diazotizing agent to complete diazotization to obtain solution of the diazosalt. The molar ratio of the paranitroaniline to the acid medium to the chlorinating agent to the diazotizing agent is 1:2-10:1-1.3:1-1.3. The method has the advantages of simple synthesis method, high yield, small amount of three wastes, and the like.
Owner:HANGZHOU JIHUA JIANGDONG CHEMICAL CO LTD
Preparation method for 2-chloro-4-nitroaniline
ActiveCN101343232AImprove qualityLow costOrganic compound preparationAmino compound preparationWastewaterP-Nitroaniline
The invention relates to a preparation method of o-chloro-p-nitroaniline, which takes p-nitroaniline as a raw material, and prepares the o-chloro-p-nitroaniline by directly inputting chlorine gas for implementing the chlorination reaction in a diluted hydrochloric acid medium with a temperature of between -20 and 10 DEG C, wherein, the p-nitroaniline and the input chlorine gas are present in a molar ratio of 1:1-1.1. The preparation method can prepare the o-chloro-p-nitroaniline with best quality, and has advantages of simple process, high yield, low production cost and non wastewater discharge.
Owner:苏州市罗森助剂有限公司
Synthesis method of p-phenylenediame
ActiveCN101665436AEasy to makeEasy to operatePhysical/chemical process catalystsOrganic compound preparationHydrazine compoundSynthesis methods
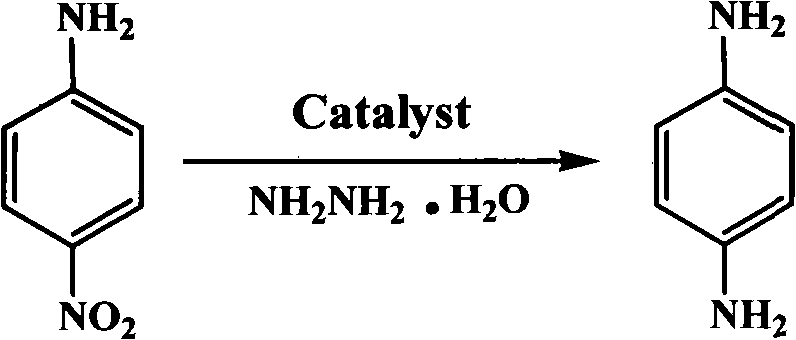
The invention discloses a synthesis method of p-phenylenediame. The method comprises the following steps: placing magnetic Mg / Al-hydrotalcite solid base catalyst and p-nitroaniline in a reaction container, heating, slowly dropping hydrazine hydrate to react at 0-100 DEG C for 0.5-20h, filtrating the hot reaction solution after the reaction, cooling, standing to precipitate white crystals, then filtrating the postcrystalline reaction solution, and drying to obtain the finished product. The invention adopts magnetic solid base as catalyst, uses hydrazine hydrate to reduce the nitryl group, reduces the emission of three wastes in the reaction process, simplifies the technology, increases the yield and purity of the product and ensures that the synthesis technology is suitable for industrialization.
Owner:CHIZHOU FANGDA SCI & TECH CO LTD +1
Continuous synthetic method of p-phenylenediamine
InactiveCN107619374ASimple process routeEasy to controlOrganic compound preparationAmino compound preparationBenzeneHydrogenation reaction
The invention provides a new process for producing p-phenylenediamine through a continuous method. The process comprises: taking p-nitroaniline, a solvent and hydrogen as main raw materials, in a fixed bed reactor or shell-and-tube reactor and in the presence of a catalyst, carrying out a hydrogenation reaction to prepare a p-phenylenediamine crude product, performing rectification recycling to the crude product to obtain high-purity p-phenylenediamine, and recycling excess hydrogen and solvent for cyclic application. According to the process, the p-phenylenediamine conversion rate can reach 100%, and the purity of p-phenylenediamine can reach no less than 99.9%. The process is green, eco-friendly and new.
Owner:CHINA PETROLEUM & CHEM CORP +1
Sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material and preparation method and application thereof
ActiveCN109621988AGood catalytic hydrogenation performancePhysical/chemical process catalystsOrganic compound preparationNitrostyrolP-Nitroaniline
The invention provides a sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material and a preparation method and application thereof. Copper nitrate trihydrateand nickel nitrate hexahydrate are dissolved into a mixing solvent of ethylene glycol and water, urea and polyvinylpyrrolidone are added, a hydrothermal reaction is performed, and the hollow sea urchin shaped precursor structure is formed; the precursor is dispersed into deionized water, a sodium hydrogen selenide solution is added into the precursor dispersing solution drowse, the precursor is adopted as a template, the hydrothermal reaction is performed, and the sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material is obtained. Compared with theprior art, the sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material is successfully prepared for the first time through the method which is mild in reaction condition and simple and easy to popularize, the prepared sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material can catalyze a hydrogenation reactionof p-nitrophenol, p-nitro-styrene and paranitroaniline, and the material has the good catalytic hydrogenation performance.
Owner:ANHUI NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com