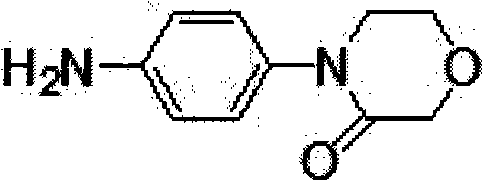
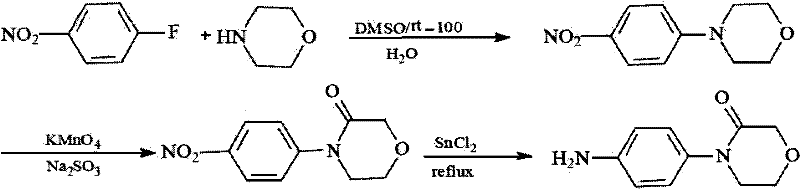
Synthesis method of 4-(4-aminophenyl)-3-morpholone
A technology of aminophenyl and morpholinone, which is applied in the field of synthesis of 4--3-morpholinone, an intermediate of rivaroxaban, can solve the problems of high raw material price, unsuitable for industrialized large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Add 13.8g (0.1mol) of p-nitroaniline and 17.7g (0.12mol) of dichloroethyl ether into the reactor, heat to reflux, and react for 2.5 hours. The solid was washed with acetone and dried to obtain 17.1 g of 4-(4-nitrophenyl)-morpholine with a yield of 82%.
[0033] Add 20.8g (0.1mol) of 4-(4-nitrophenyl)-morpholine, 22.5g (0.25mol) of tert-butyl hydroperoxide, 2.3g of ethyl acetoacetate manganese salt and appropriate amount of tetrahydrofuran into the reaction kettle, and heat Reflux and react for 4.5 hours. After the reaction is completed, add activated carbon, stir for 30 minutes, filter, remove the solids, wash the filtrate with saturated aqueous sodium carbonate, add anhydrous magnesium sulfate and let it stand for drying, filter to remove the solids, spin the filtrate, and put it on a chromatographic column. -The washing solution of (4-nitrophenyl)-morpholinone segment was rotary evaporated to obtain 18.4 g of 4-(4-nitrophenyl)-morpholinone solid, with a yield of 83%....
example 2
[0036] Add 13.8g (0.1mol) of p-nitroaniline and 25.7g (0.11mol) of dibromoethyl ether into the reactor, heat to reflux, and react for 3 hours. The solid was washed with acetone and dried to obtain 16.9 g of 4-(4-nitrophenyl)-morpholine with a yield of 81%.
[0037]Add 20.8g (0.1mol) of 4-(4-nitrophenyl)-morpholine, 22.8g (0.3mol) of isopropyl hydroperoxide, 2.1g of ethyl acetoacetate manganese salt and appropriate amount of tetrahydrofuran into the reaction kettle, and heat Reflux and react for 3.5 hours. After the reaction is completed, add activated carbon, stir for 15 minutes, filter, remove the solid, wash the filtrate with saturated aqueous sodium carbonate, add anhydrous magnesium sulfate and let it stand for drying, filter to remove the solid, and the filtrate is rotary evaporated, put on a chromatographic column, and collect 4 -The washing solution of (4-nitrophenyl)-morpholinone segment was rotary evaporated to obtain 18.9 g of 4-(4-nitrophenyl)-morpholinone as a sol...
example 3
[0040] Add 13.8g (0.1mol) of p-nitroaniline and 20.3g (0.14mol) of dichloroethyl ether into the reactor, heat to reflux, and react for 1 hour. The solid was washed with acetone and dried to obtain 17.9 g of 4-(4-nitrophenyl)-morpholine with a yield of 86%.
[0041] 20.8g (0.1mol) 4-(4-nitrophenyl)-morpholine, 16.7g (0.16mol) pentyl hydroperoxide and 5.7g 4-cyclohexylbutyric acid manganese salt and appropriate amount of 1,4- Dioxane was added into the reactor, heated to reflux, and reacted for 4 hours. After the reaction is completed, add activated carbon, stir for 15 minutes, filter, remove the solid, wash the filtrate with saturated aqueous sodium carbonate, add anhydrous magnesium sulfate and let it stand for drying, filter to remove the solid, and the filtrate is rotary evaporated, put on a chromatographic column, and collect 4 -The washing solution of (4-nitrophenyl)-morpholinone segment was rotary evaporated to obtain 18.5 g of 4-(4-nitrophenyl)-morpholinone solid, with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com