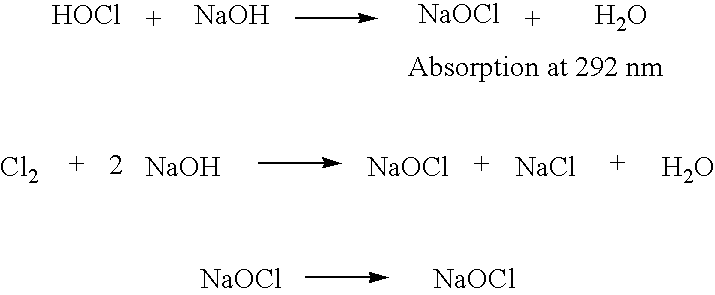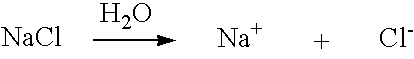Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3508 results about "Oxidation reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxidation and Reduction. Oxidation involves an increase in oxidation number, while reduction involves a decrease in oxidation number. Usually, the change in oxidation number is associated with a gain or loss of electrons, but there are some redox reactions (e.g., covalent bonding) that do not involve electron transfer.
Organic devices, organic electroluminescent devices, organic solar cells, organic FET structures and production method of organic devices
ActiveUS20050098207A1TransistorDischarge tube luminescnet screensElectronic transmissionOrganic solar cell
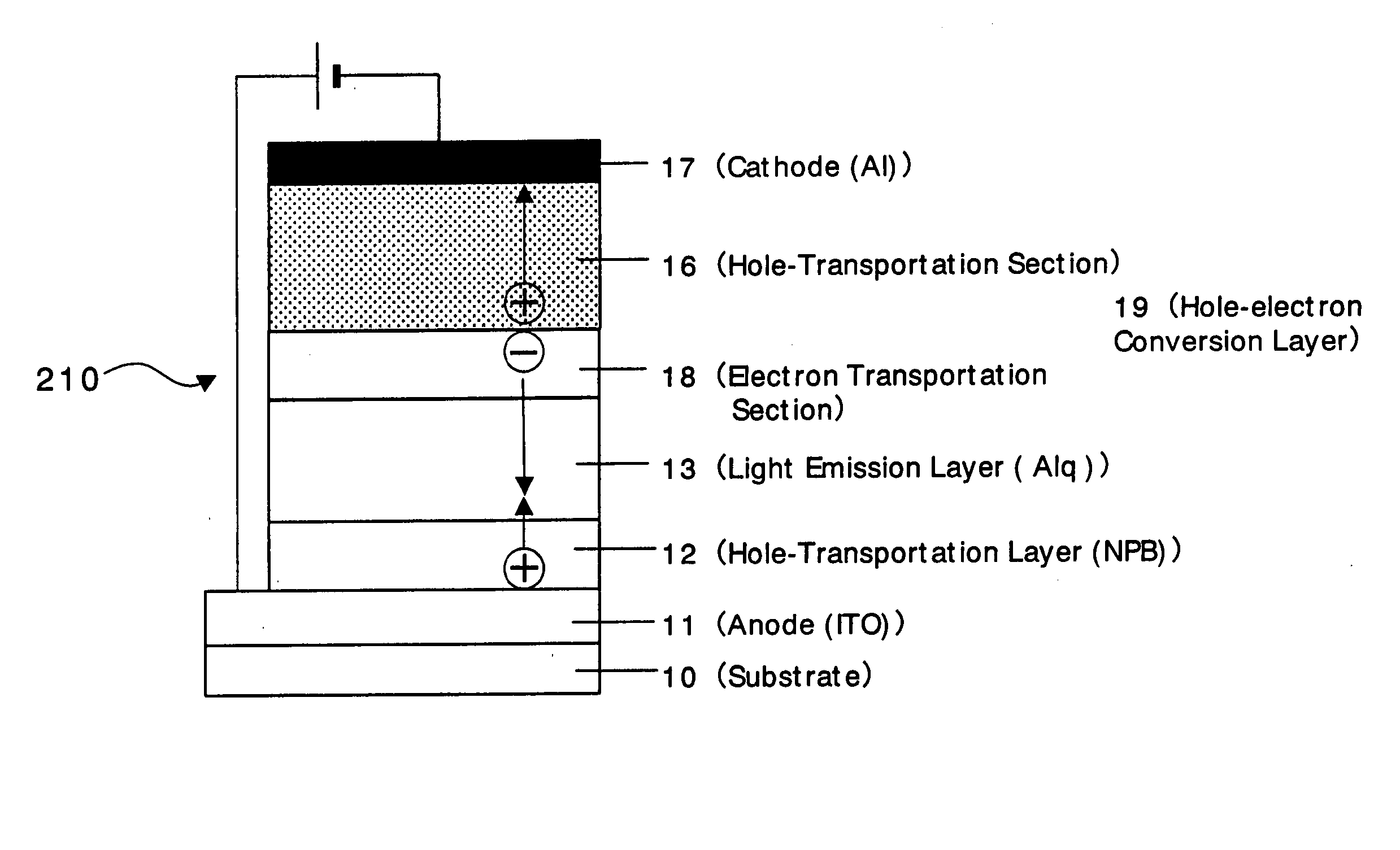
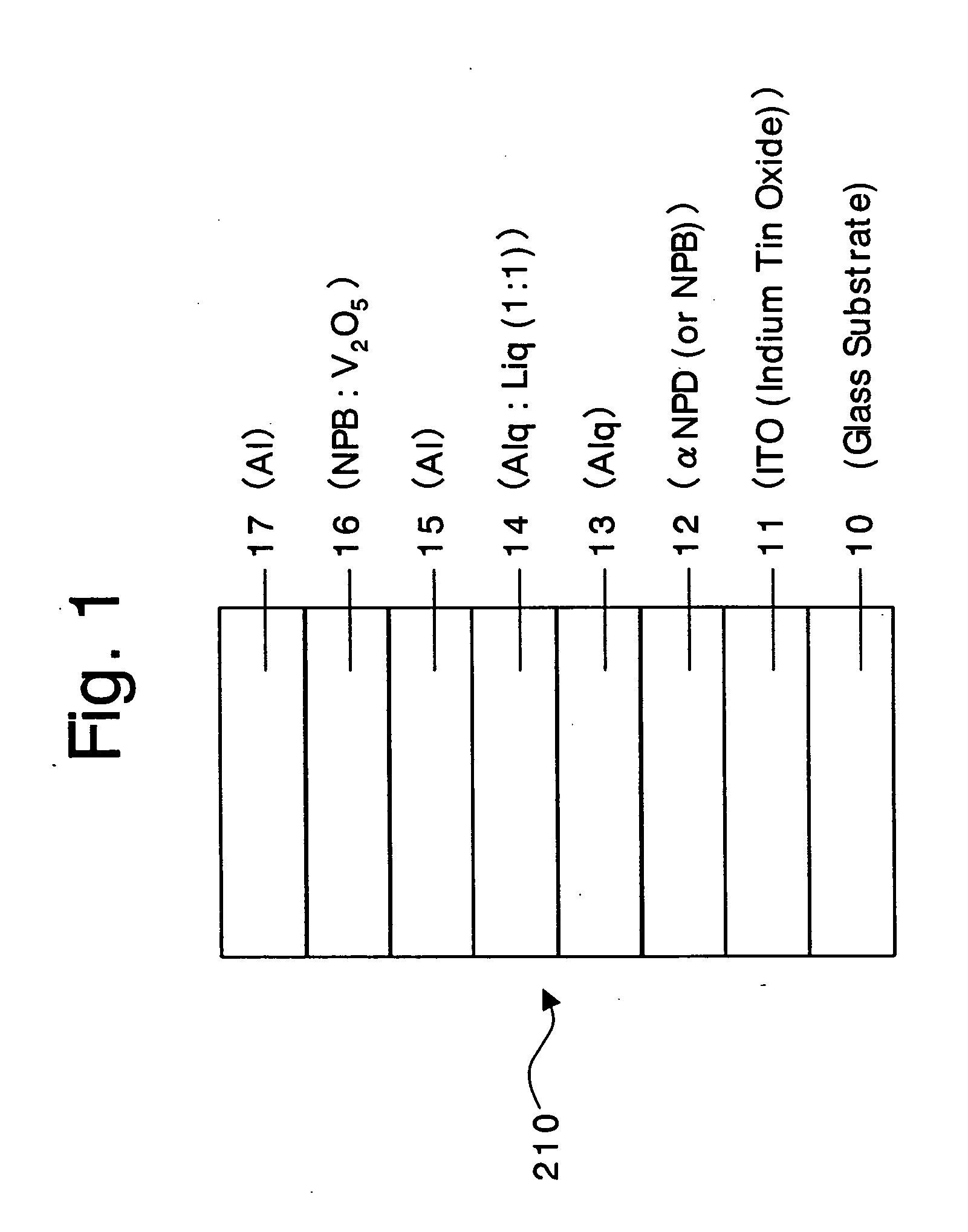
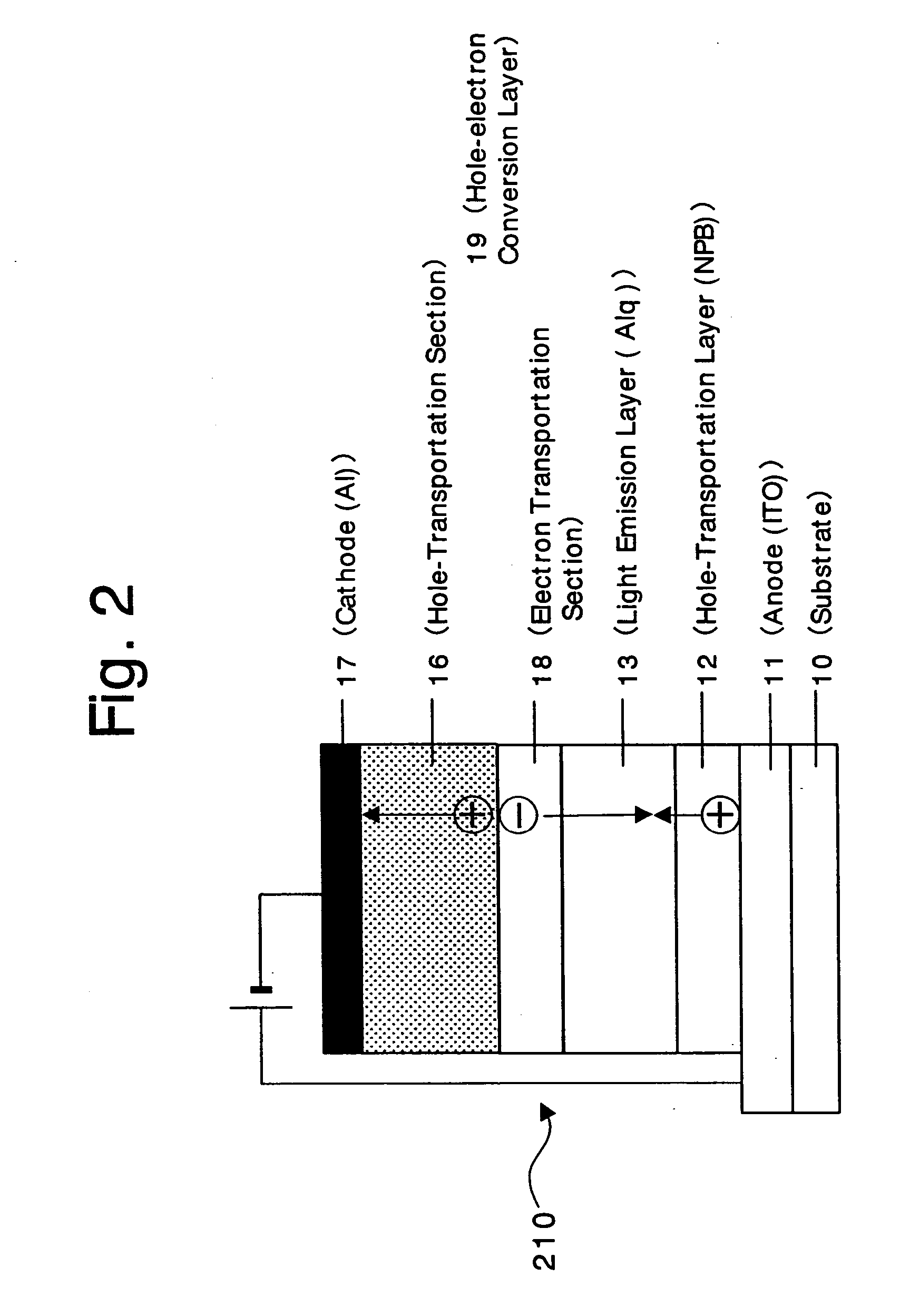
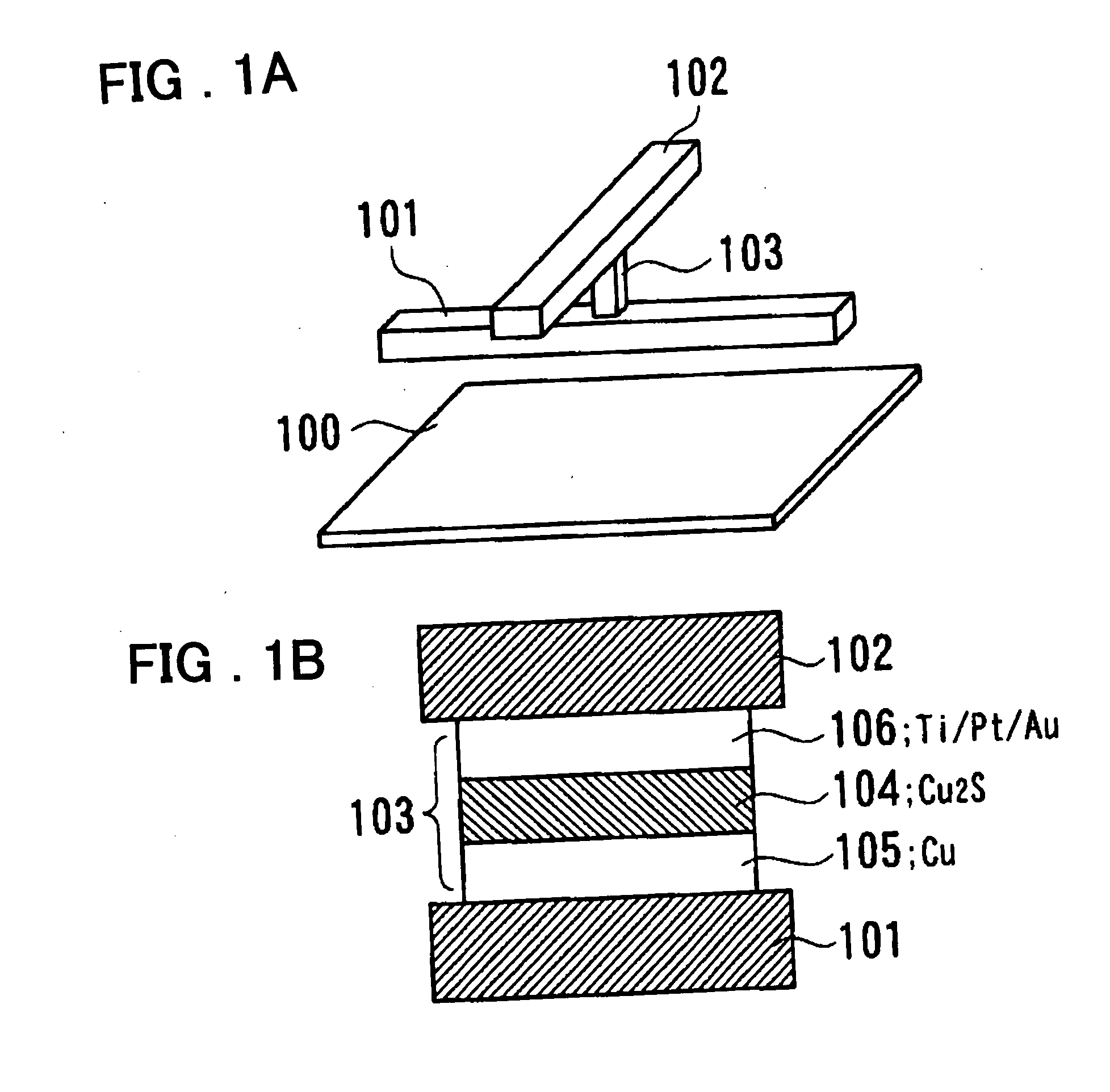
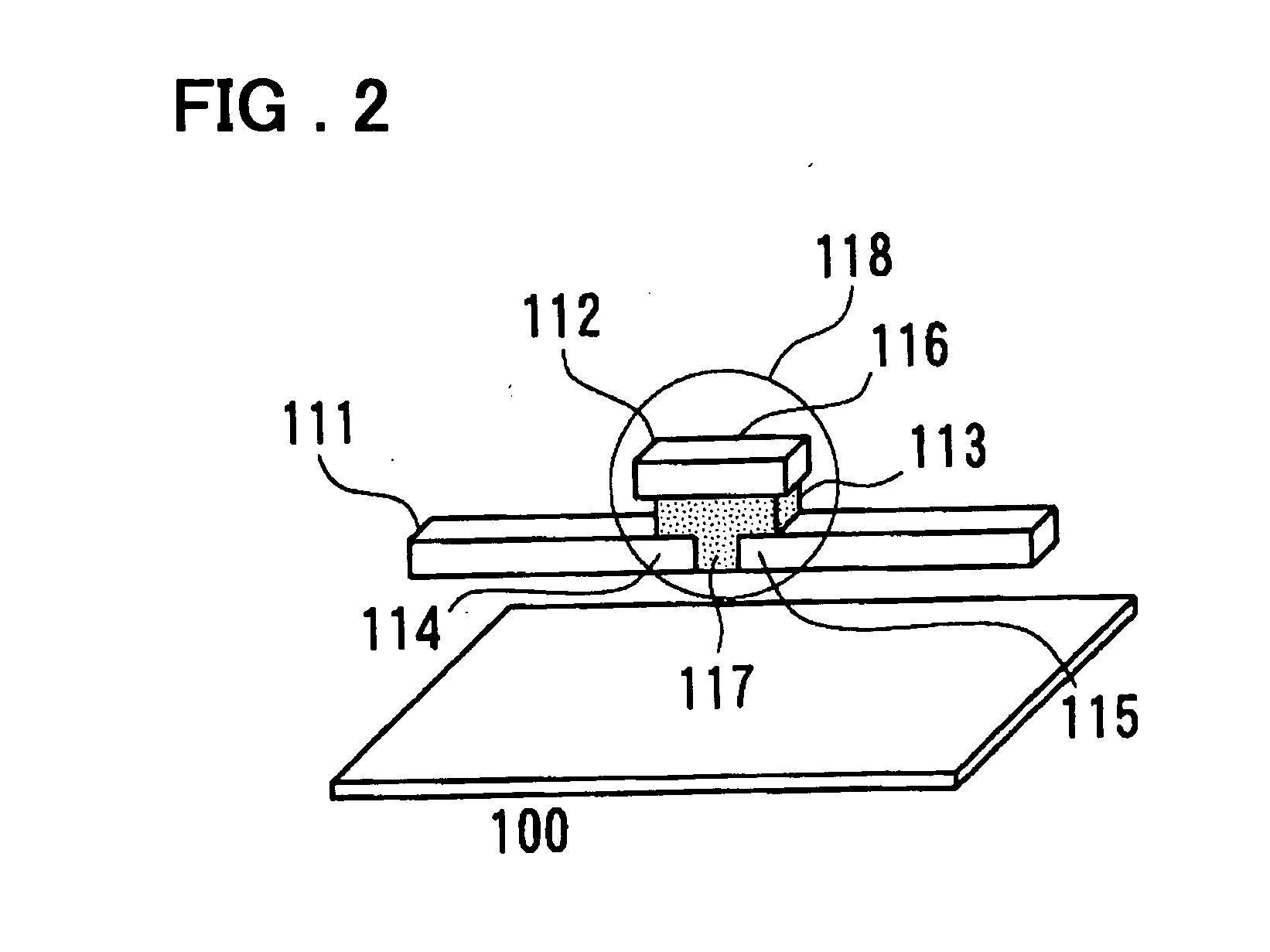
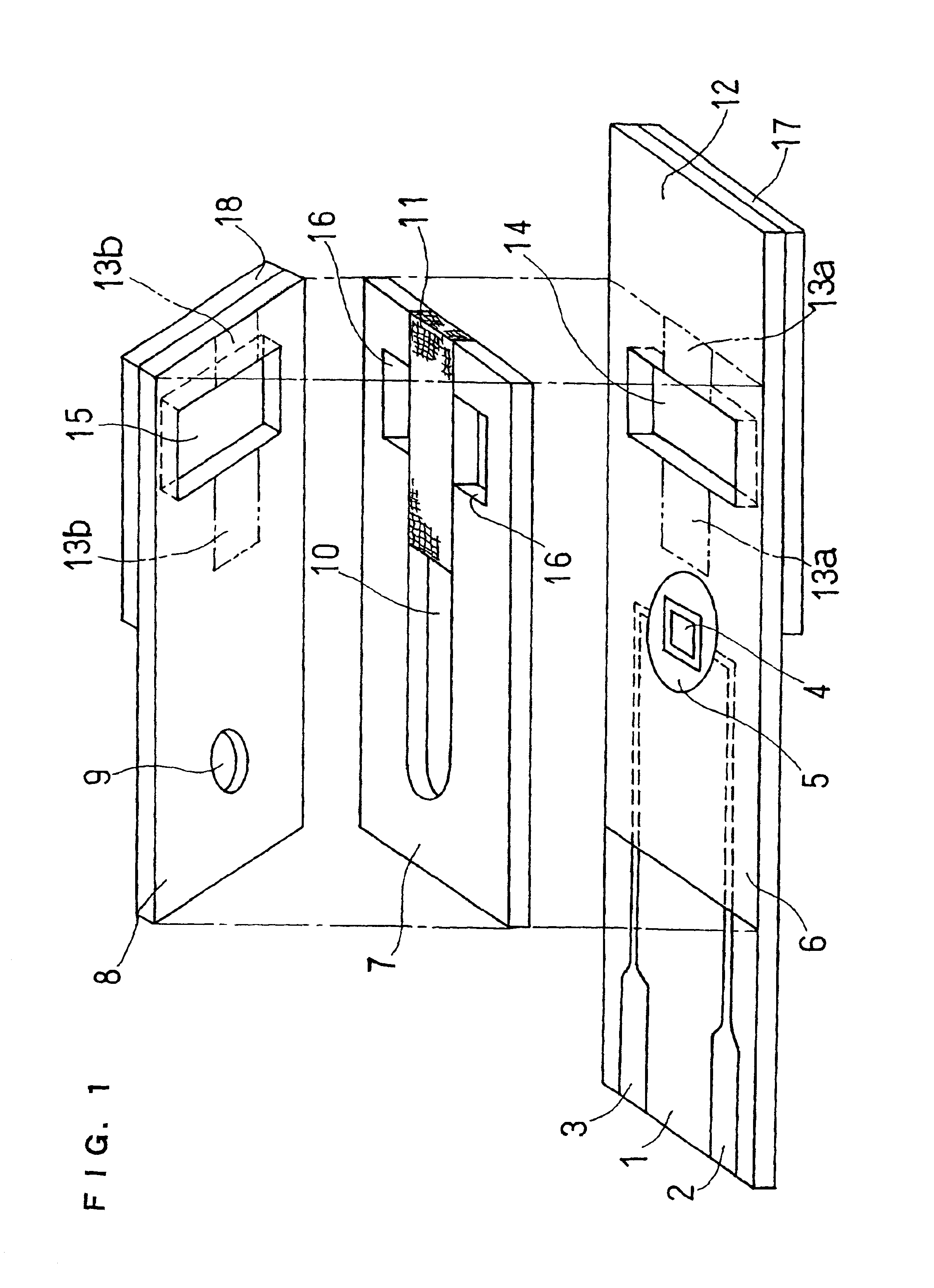
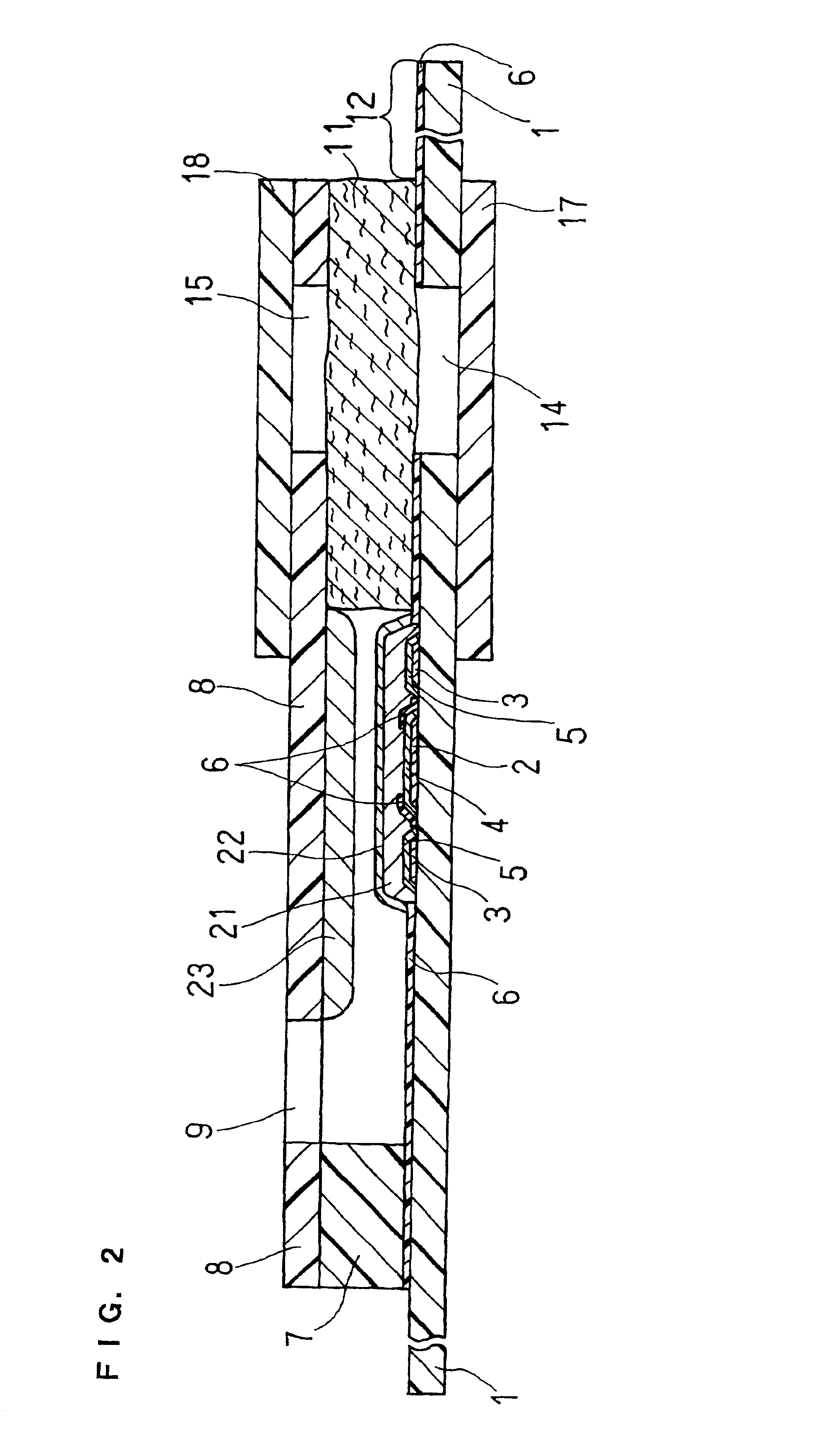
An organic device has a hole current-electron current conversion layer which comprises a laminate of an electron transportation section and a hole transportation section. The electron transportation section includes a charge transfer complex formed upon an oxidation-reduction reaction between a reduced low work function metal and an electron-accepting organic compound, the reduced metal being produced upon an in-situ thermal reduction reaction caused upon contact, through lamination or mixing by co-deposition, of an organic metal complex compound or an inorganic compound containing at least one metal ion selected from ions of low work function metals having a work function of not more than 4.0 eV, and a thermally reducible metal capable of reducing a metal ion contained in the organic metal complex compound or the inorganic compound in vacuum to the corresponding metal state, and the electron transportation section having the electron-accepting organic compound in the state of radical anions. The hole transportation section includes an organic compound having an ionization potential of less than 5.7 eV and an electron-donating property and an inorganic or organic substance capable of forming a charge transfer complex upon its oxidation-reduction reaction with the organic compound, the organic compound and the inorganic or organic substance being contacted through lamination or mixing, and the electron-donating organic compound is in the state of radical cations.
Owner:MITSUBISHI HEAVY IND LTD +1
Semiconductor device
InactiveUS20050045919A1Increase the areaFine granularitySemiconductor/solid-state device detailsSolid-state devicesDevice materialEngineering
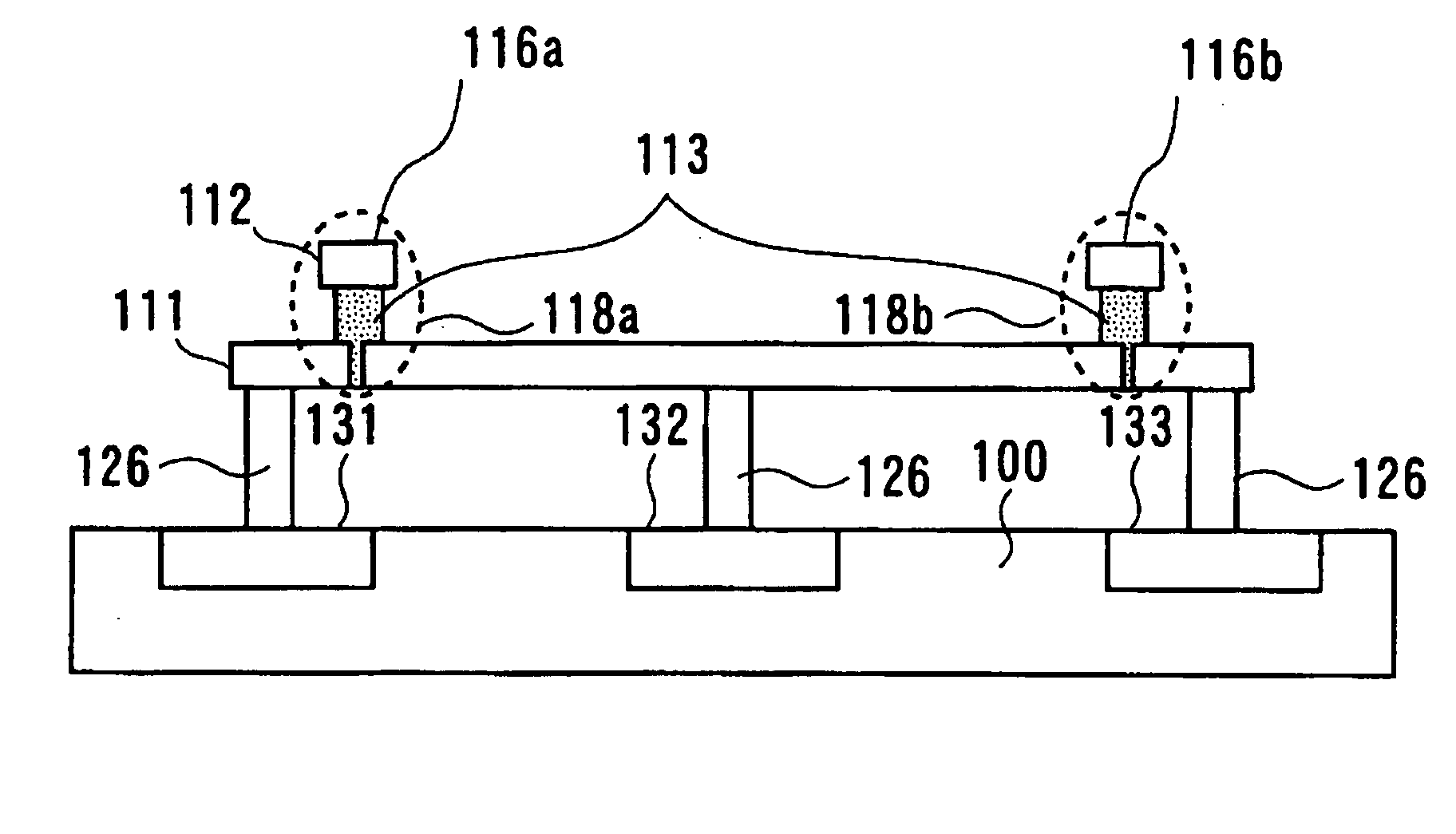
A programmable semiconductor device has a switch element in an interconnection layer, wherein in at least one of the inside of a via, interconnecting a wire of a first interconnection layer and a wire of a second interconnection layer, a contact part of the via with the wire of the first interconnection layer and a contact part of the via with the wire of the second interconnection layer, there is provided a variable electrical conductivity member, such as a member of an electrolyte material. The via is used as a variable electrical conductivity type switch element or as a variable resistance device having a contact part with the wire of the first interconnection layer as a first terminal and having a contact part with the wire of the second interconnection layer as a second terminal. By varying the electrical conductivity of the switch element, the state of connection of the via with the wire of the first interconnection layer and the state of connection of the via with the wire of the second interconnection layer may be variably set to a shorted state, an open-circuited state or to an intermediate state A two-state switch element includes an ion conductor for conducting metal ions interposed between the first and second electrodes. The second electrode is formed of a material lower in reactivity than the first electrode. The electrical conductivity across the first and second electrodes is changed by the oxidation-reduction reaction of the metal ions. There are provided first and second transistors of opposite polarities, connected to the first electrode, and third and fourth transistors of opposite polarities, connected to the second electrode.
Owner:NEC CORP
Device and method for determining the concentration of a substrate
InactiveUS20020043471A1Error in measurement resultExclude influenceImmobilised enzymesBioreactor/fermenter combinationsReaction layerElectricity
A method for determining the concentration of a substrate in a sample solution using an electrode system comprising a working electrode and a counter electrode, both being formed on an electrically insulating base plate, and a reaction layer which contains at least an oxidoreductase and an electron mediator and is formed on the electrode system to electrochemically measure a reduced amount of the electron mediator resulting from enzyme reaction in the reaction layer, wherein a third electrode is formed as an interfering substance detecting electrode somewhere apart from the reaction layer to detect supply of the sample solution on the basis of an electrical change between the counter electrode and the third electrode. A current flowing between the counter electrode and the third electrode is measured which is taken as a positive error. Subsequently, voltage application between the counter electrode and the third electrode is released and a voltage for oxidizing the reduced form electron mediator is applied between the working electrode and the counter electrode to measure a current flowing between the two electrodes. Influences of any interfering substance such as easy-to-oxidize substance are reduced, whereby a highly reliable value of substrate determination can be obtained.
Owner:PHC HLDG CORP
Method for synthesis of carbon-coated redox materials with controlled size
ActiveUS20040033360A1Low costReduce the numberMaterial nanotechnologyHybrid capacitorsCross-linkRedox
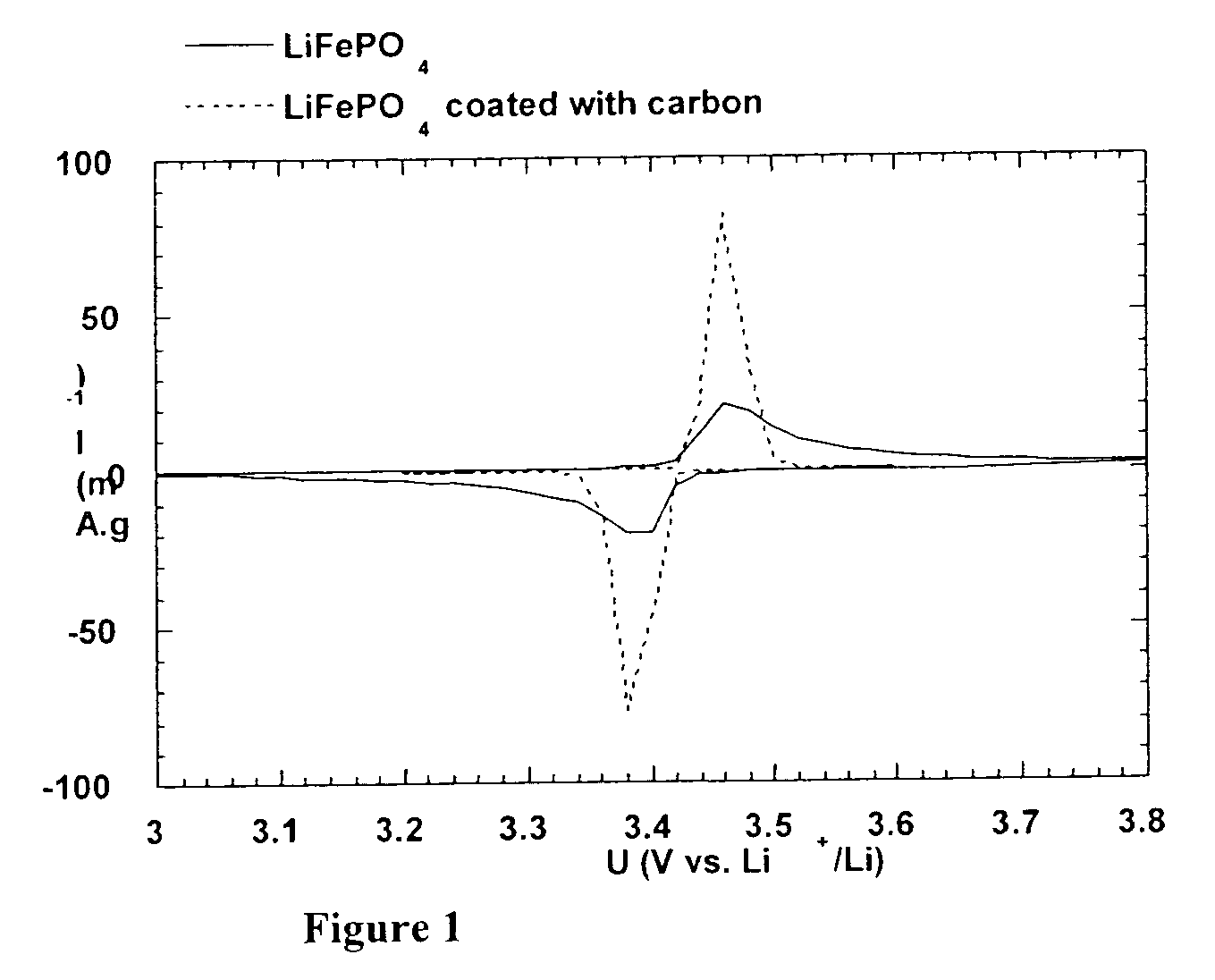
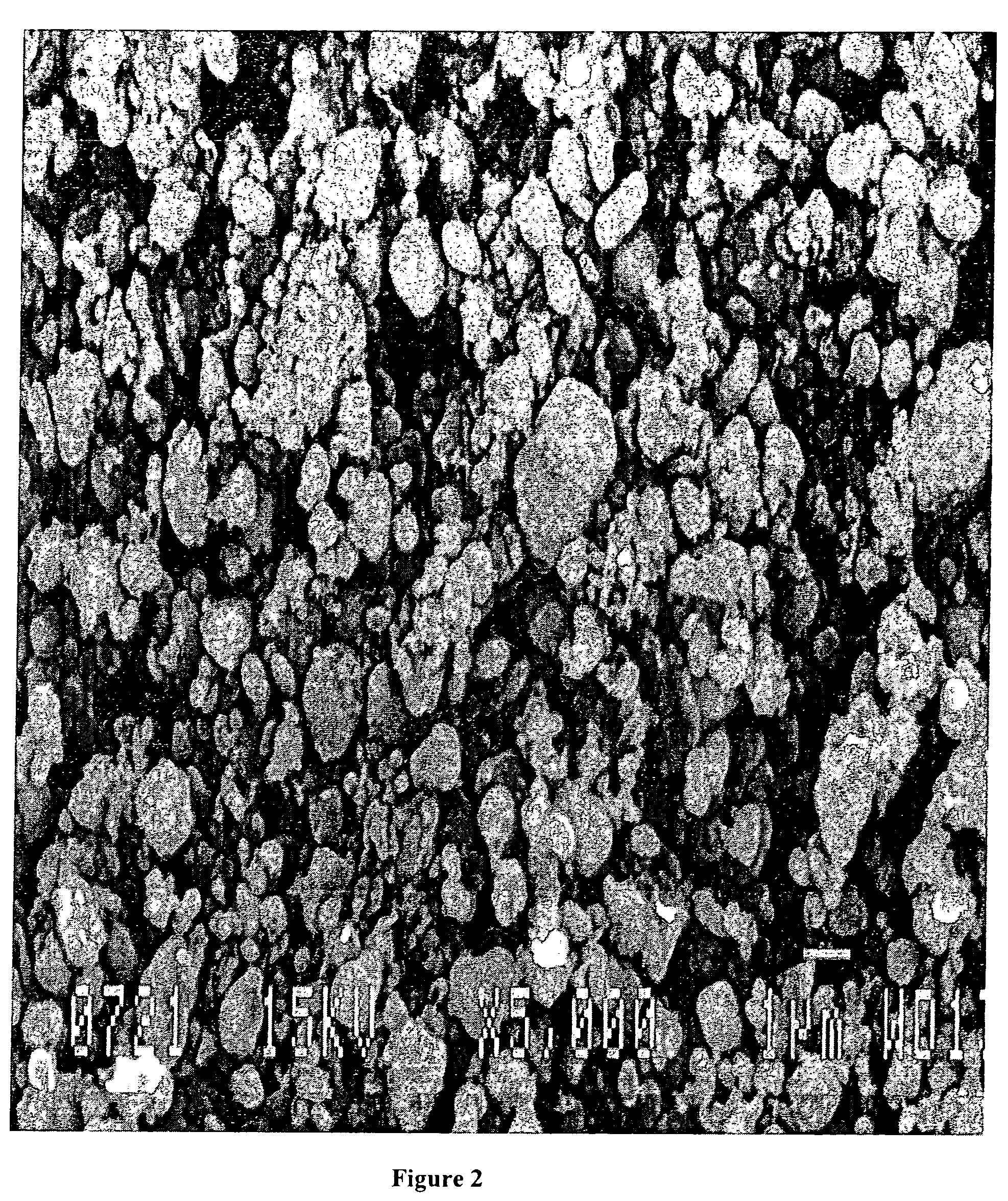
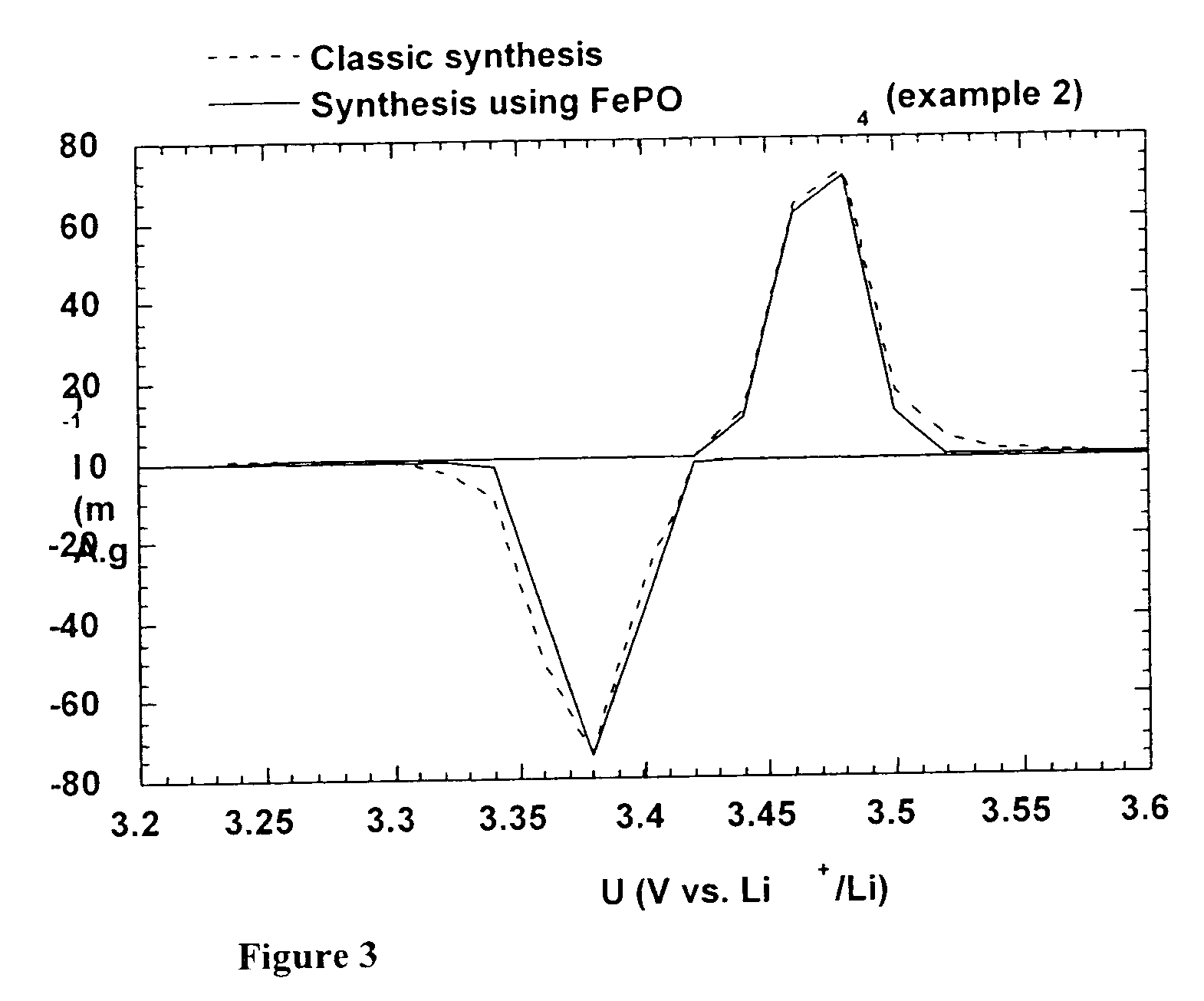
A method for the synthesis of compounds of the formula C-LixM1-yM'y(XO4)n, where C represents carbon cross-linked with the compound LixM1-yM'y(XO4)n, in which x, y and n are numbers such as 0<=x<=2, 0<=y<=0.6, and 1<=n<=1.5, M is a transition metal or a mixture of transition metals from the first period of the periodic table, M' is an element with fixed valency selected among Mg<2+>, Ca<2+>, Al<3+>, Zn<2+> or a combination of these same elements and X is chosen among S, P and Si, by bringing into equilibrium, in the required proportions, the mixture of precursors, with a gaseous atmosphere, the synthesis taking place by reaction and bringing into equilibrium, in the required proportions, the mixture of the precursors, the procedure comprising at least one pyrolysis step of the carbon source compound in such a way as to obtain a compound in which the electronic conductivity measured on a sample of powder compressed at a pressure of 3750 Kg.cm<-2 >is greater than 10<-8 >S.cm<-1>. The materials obtained have excellent electrical conductivity, as well a very improved chemical activity.
Owner:CENT NAT DE LA RECHERCHE SCI +2
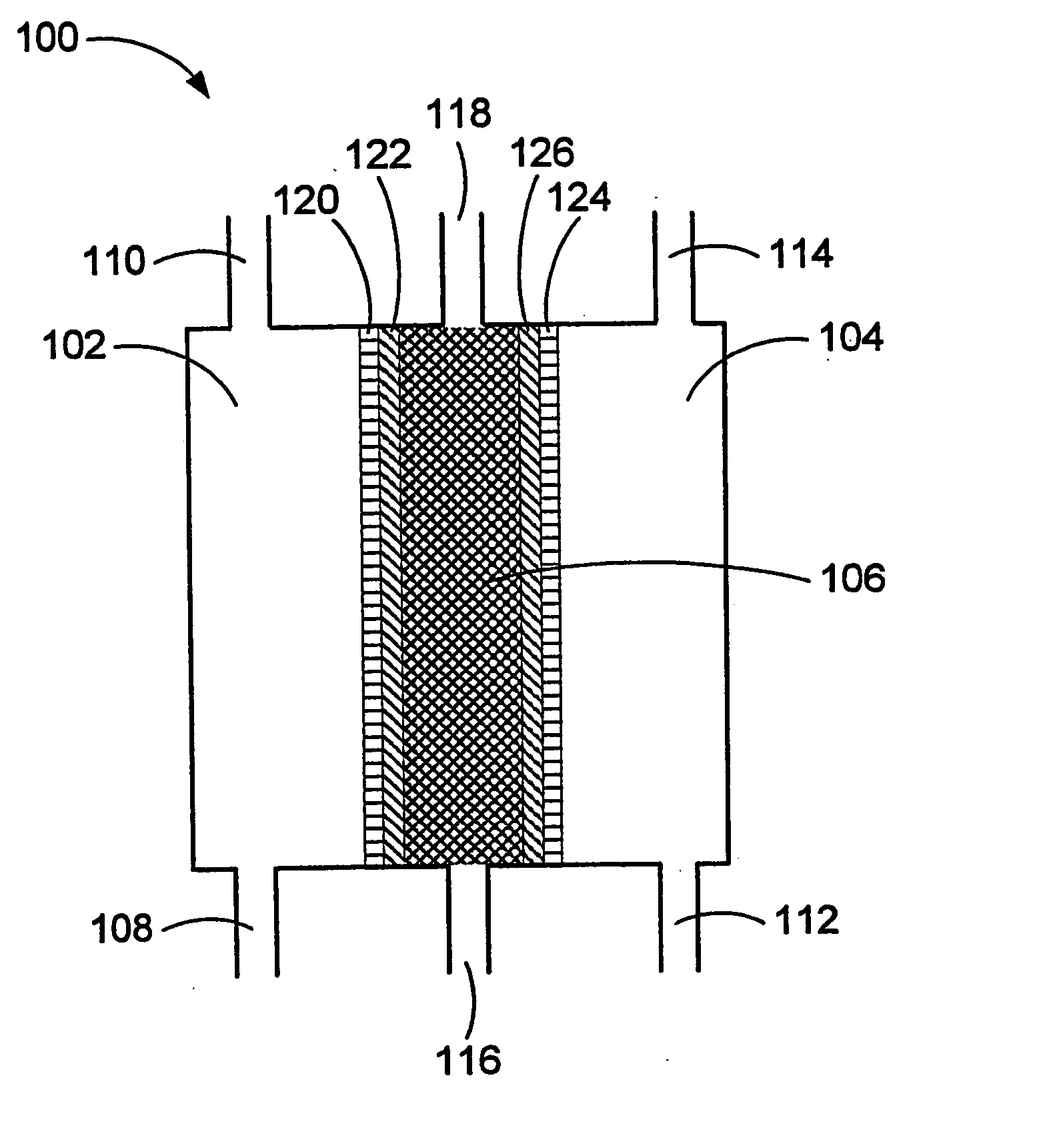
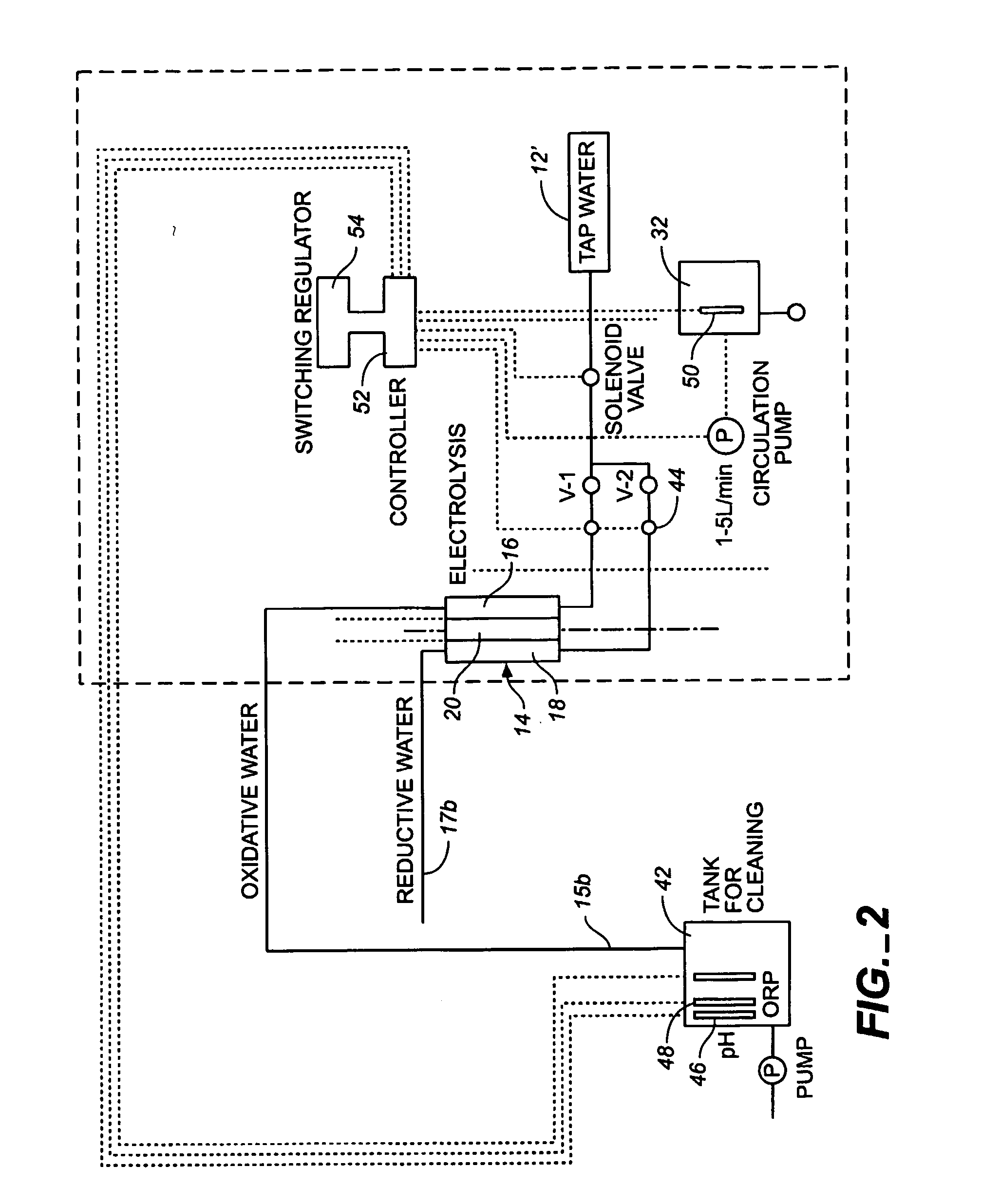
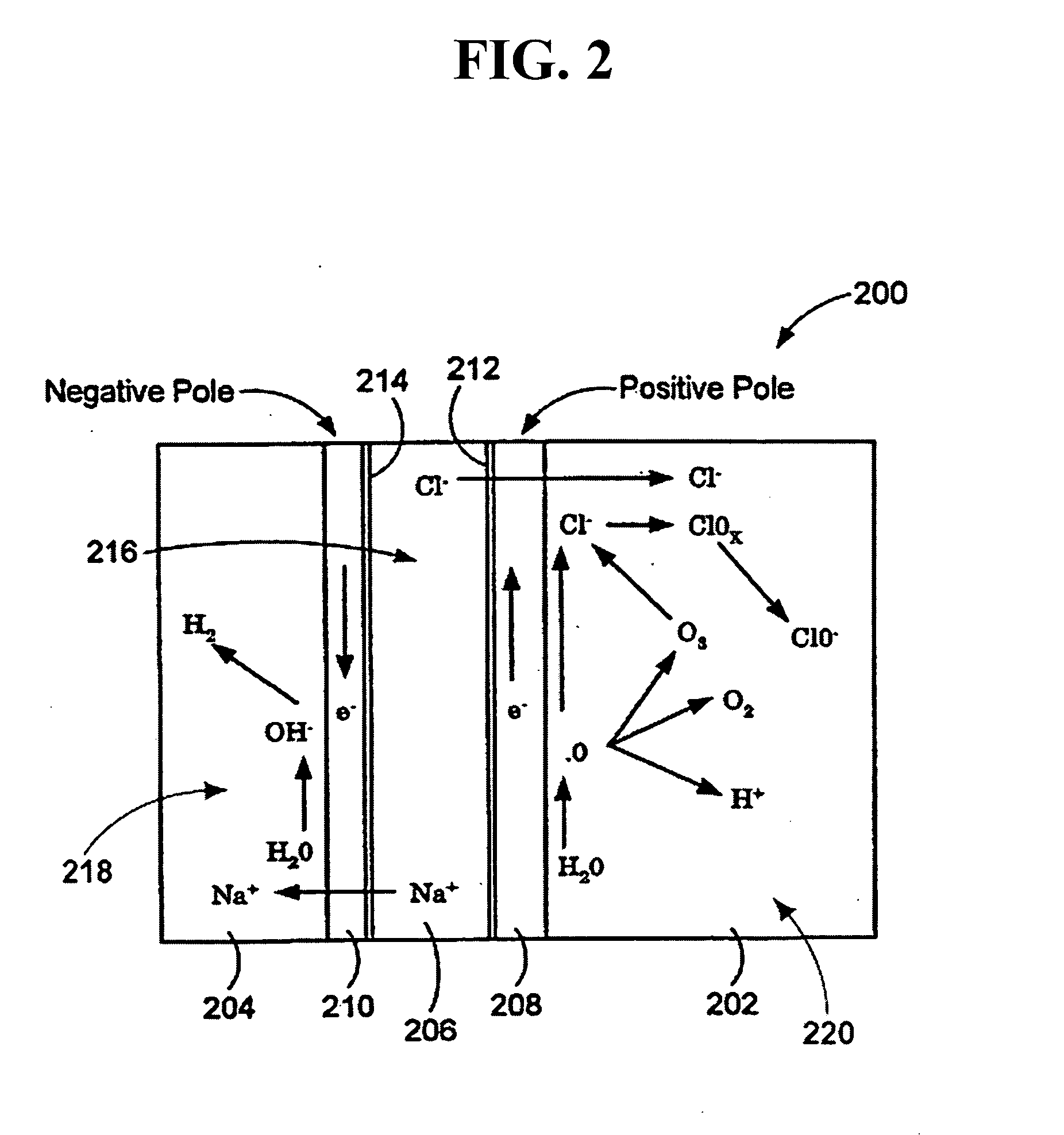
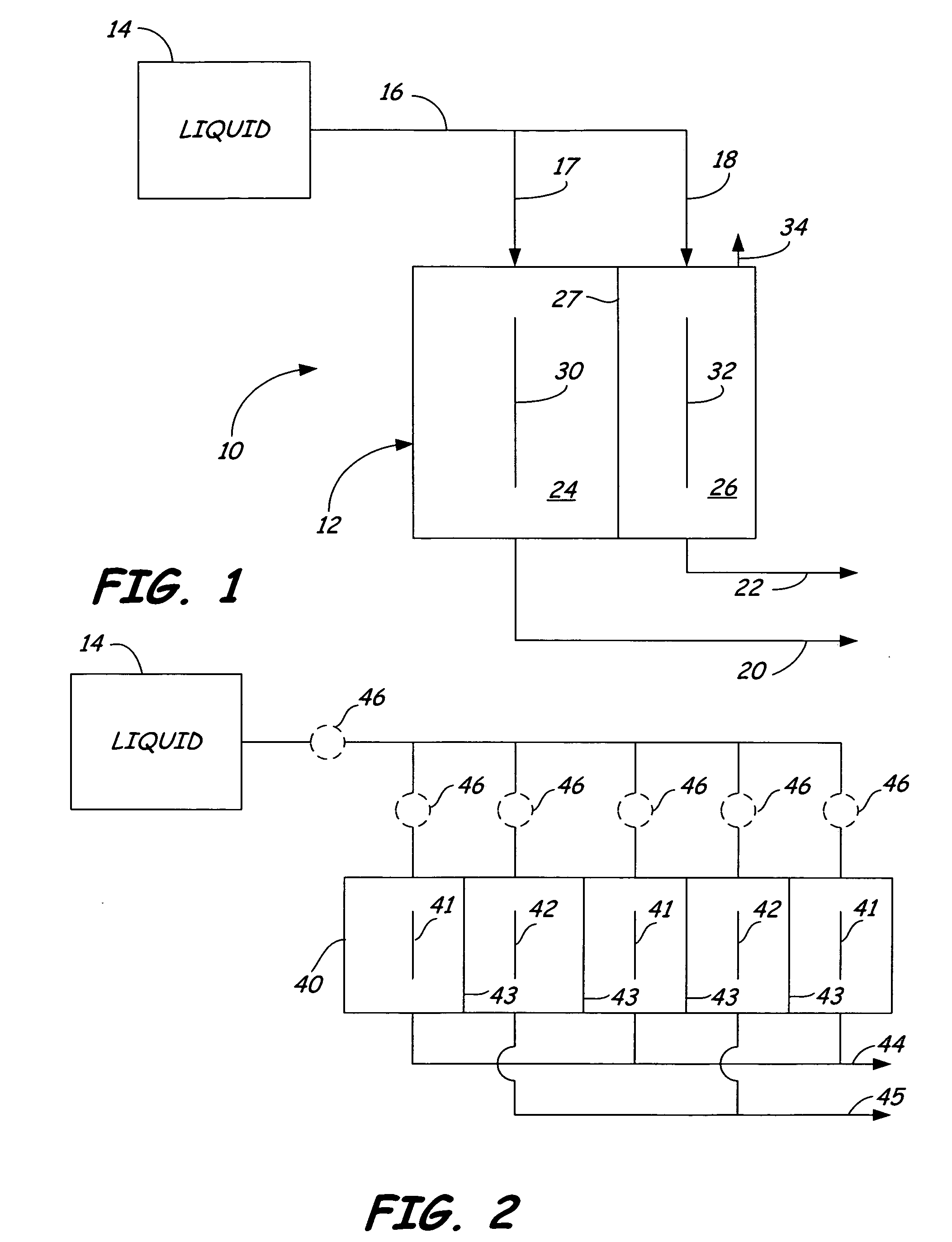
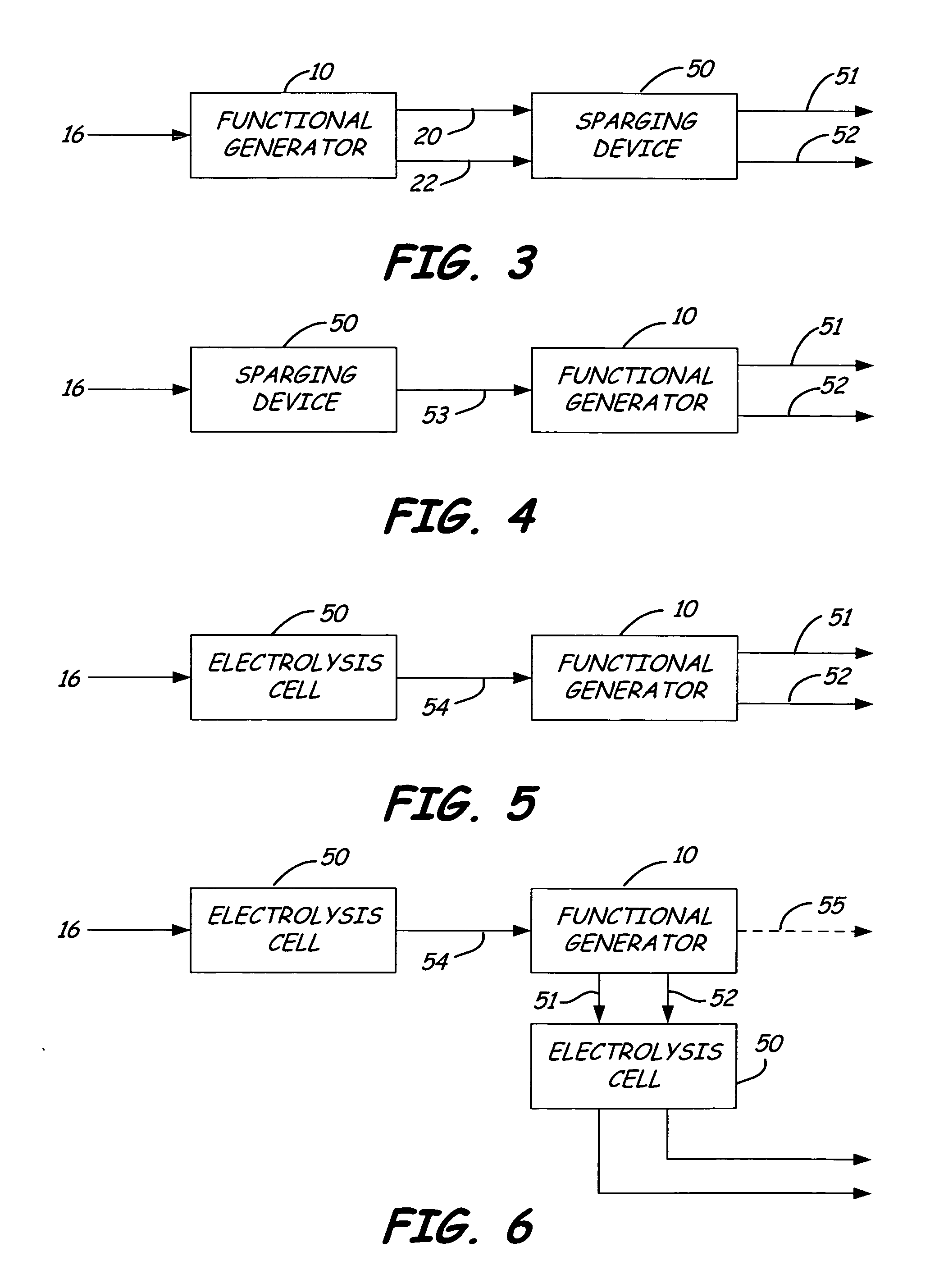
Oxidative reductive potential water solution and process for producing same
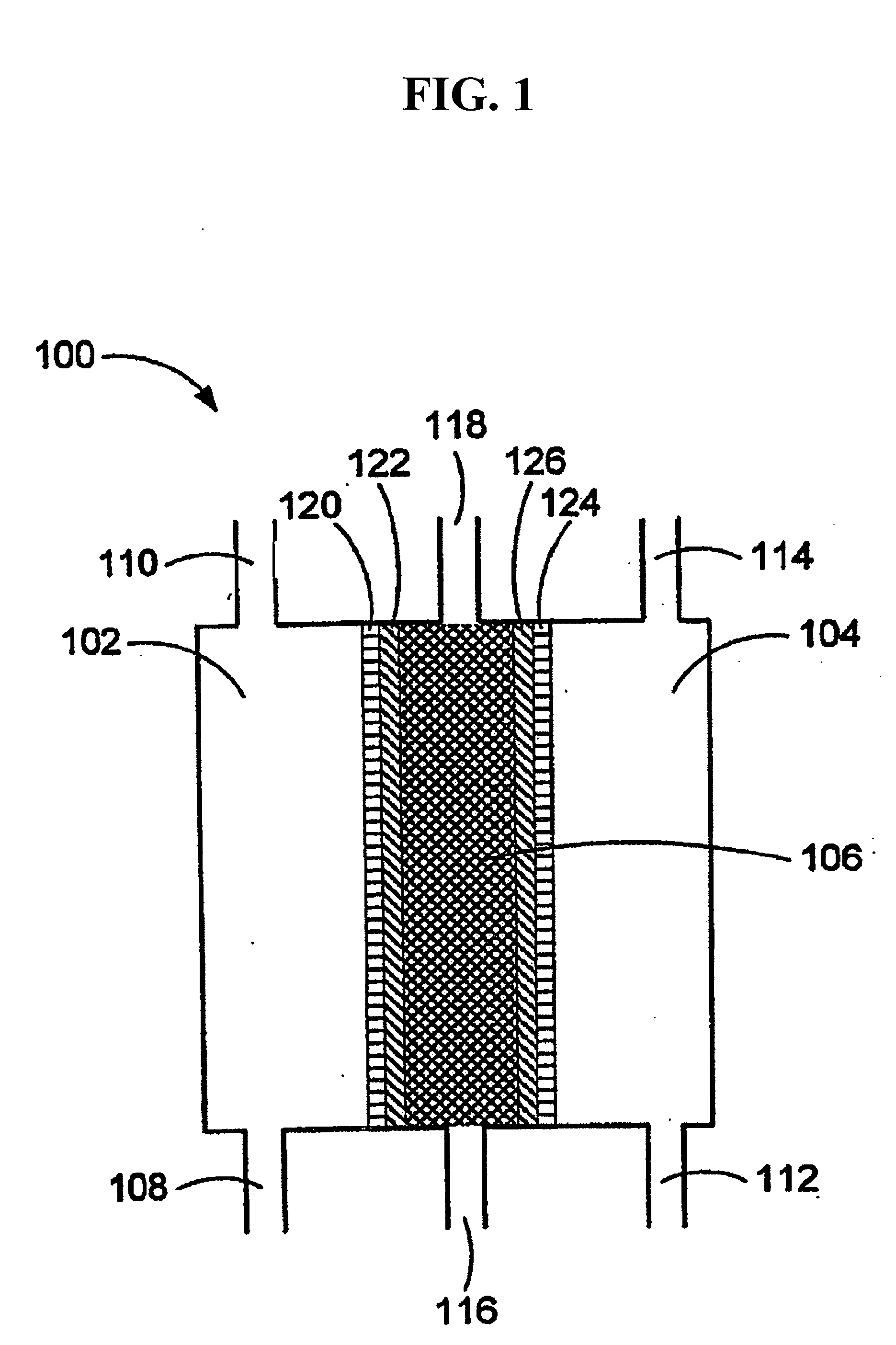
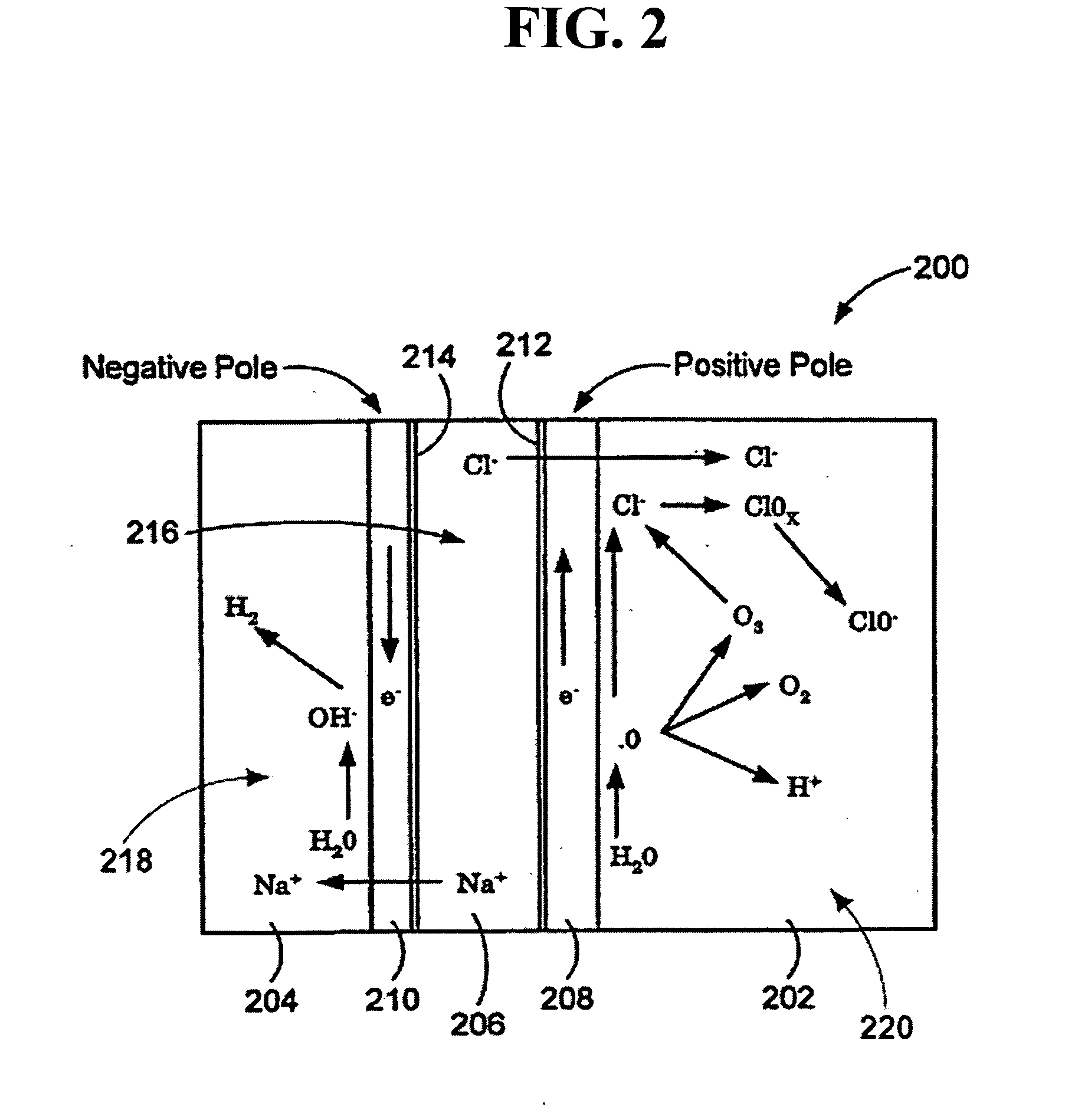
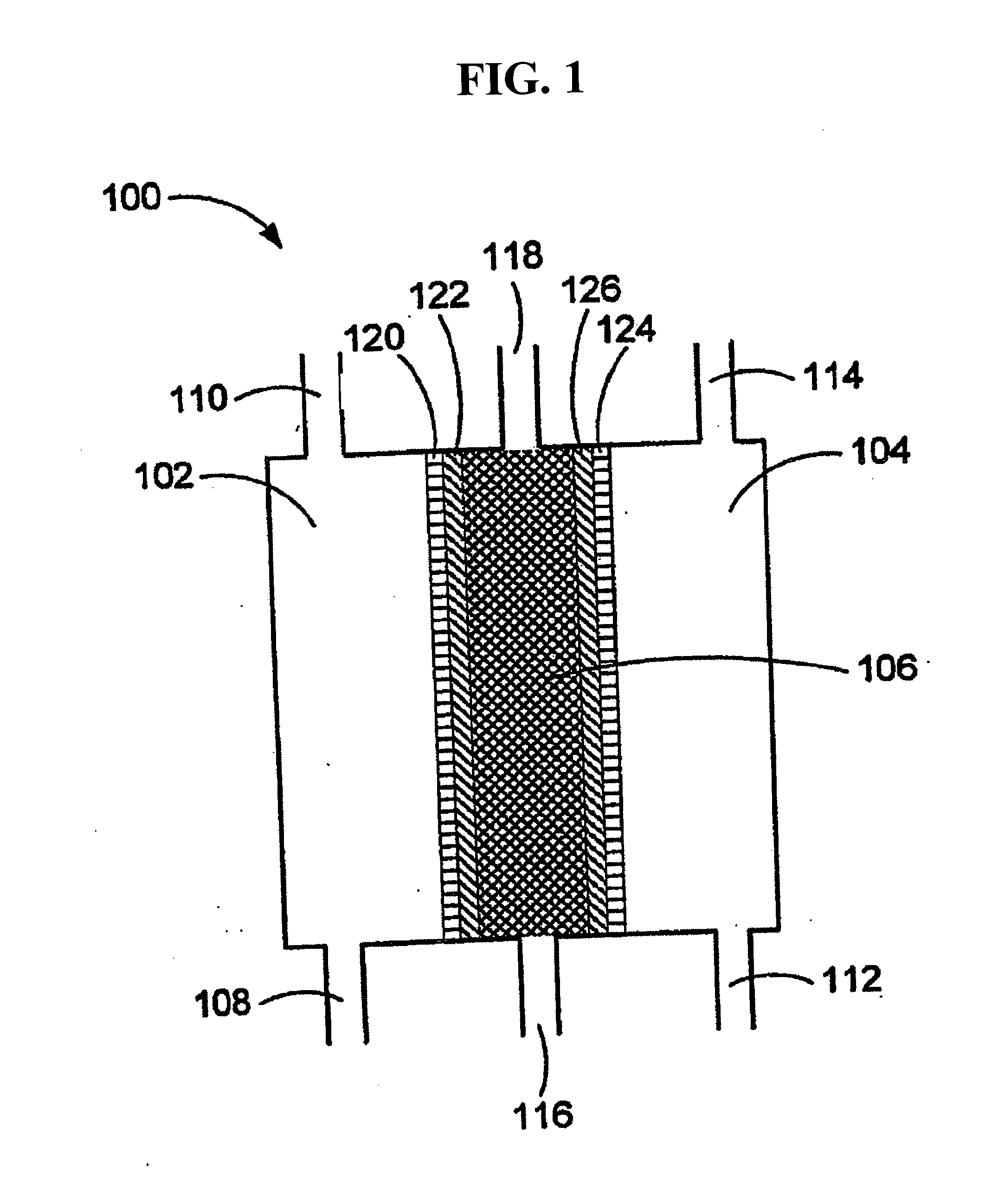
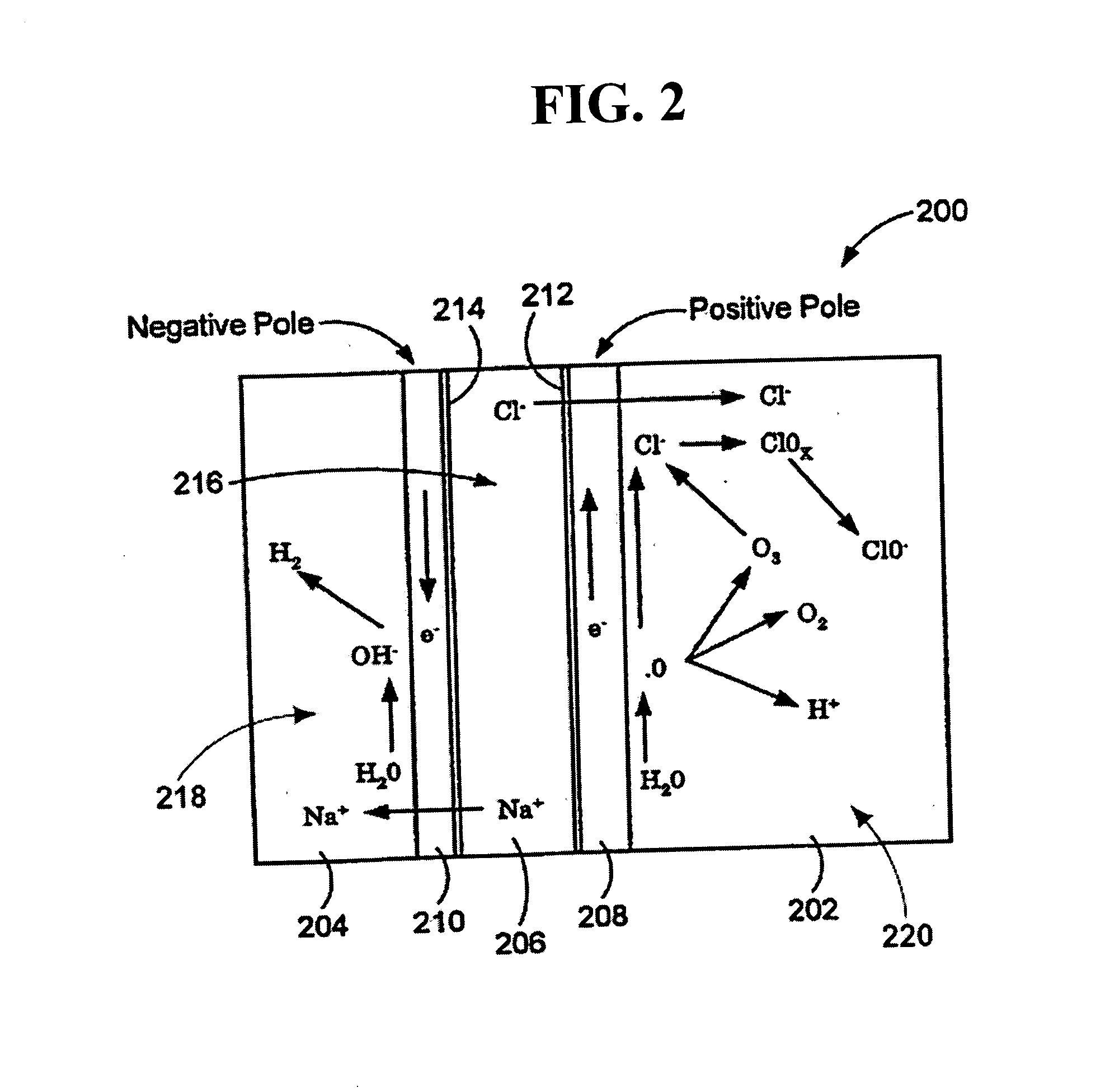
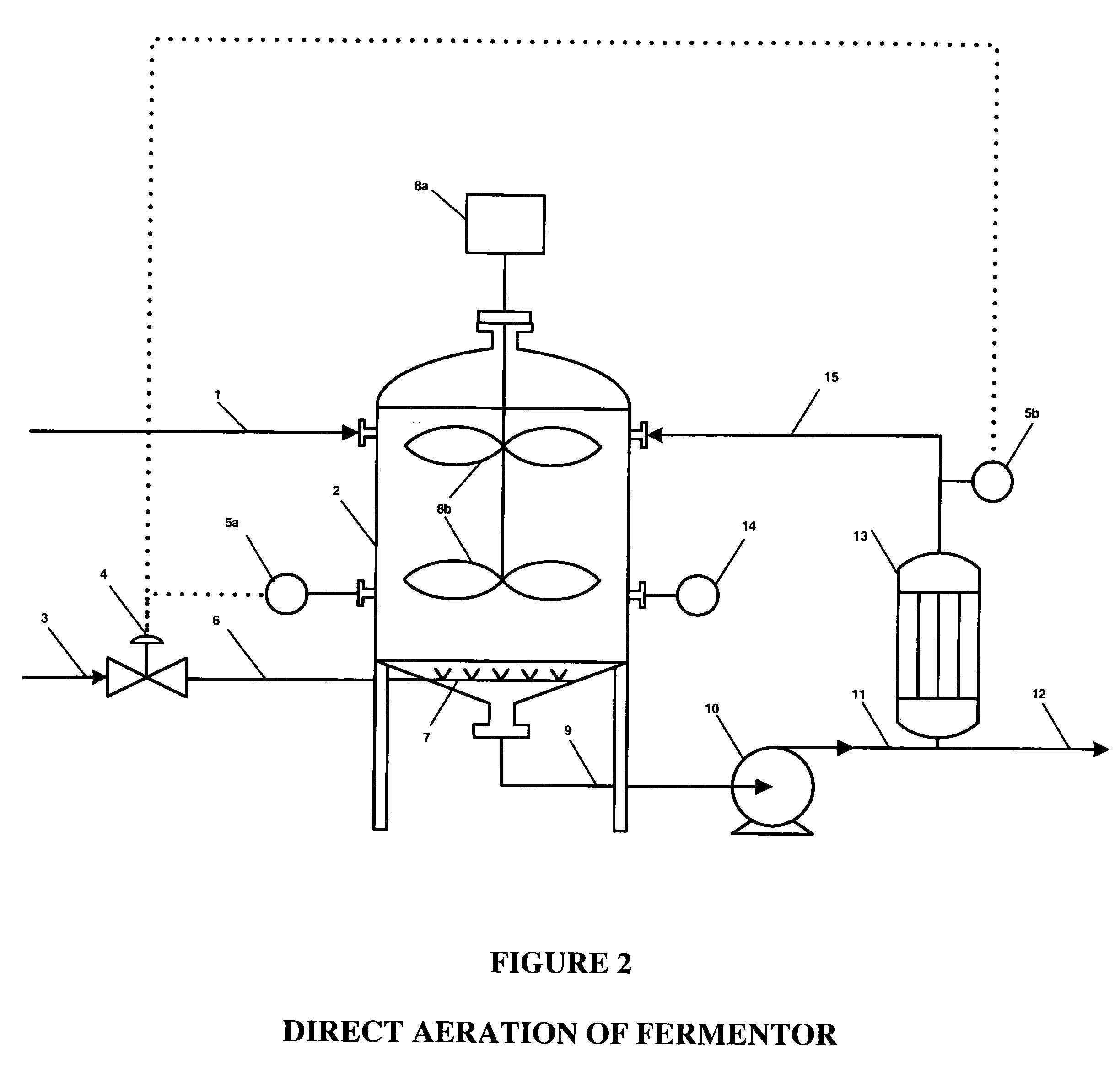
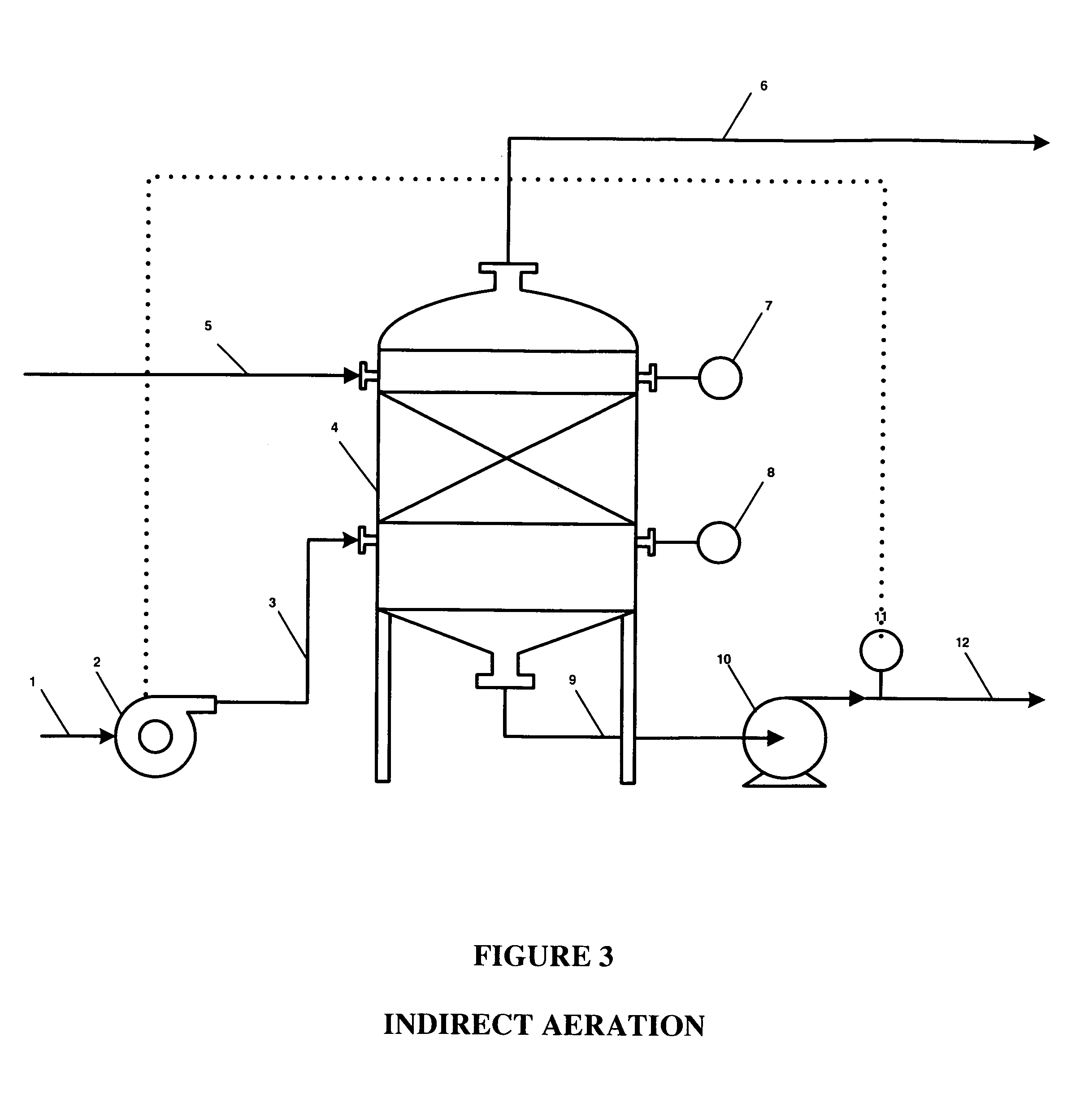
An oxidative reduction potential water solution that is stable for at least twenty-four hours. The invention also relates to an ORP water solution comprising anode water and cathode water. Another aspect of the invention is an apparatus for producing an ORP water solution comprising at least two electrolysis cells, wherein each cell comprises an anode chamber, cathode chamber and salt solution chamber located between the anode and cathode chambers, wherein the anode chamber is separated from the salt solution chamber by an anode electrode and a first membrane, and the cathode chamber is separated from the salt solution chamber by a cathode electrode and a second membrane.
Owner:SONOMA PHARMA INC
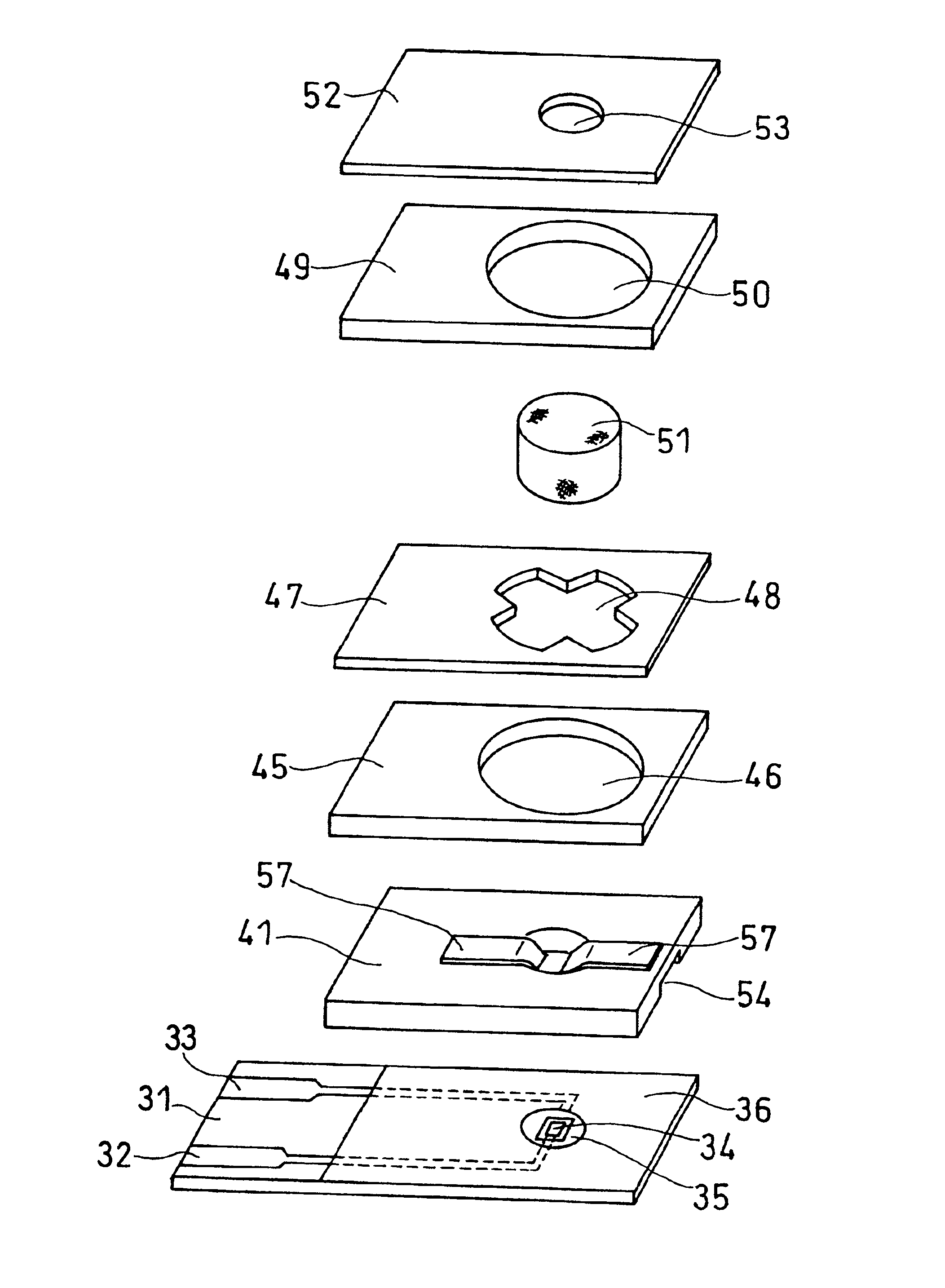
Biosensor
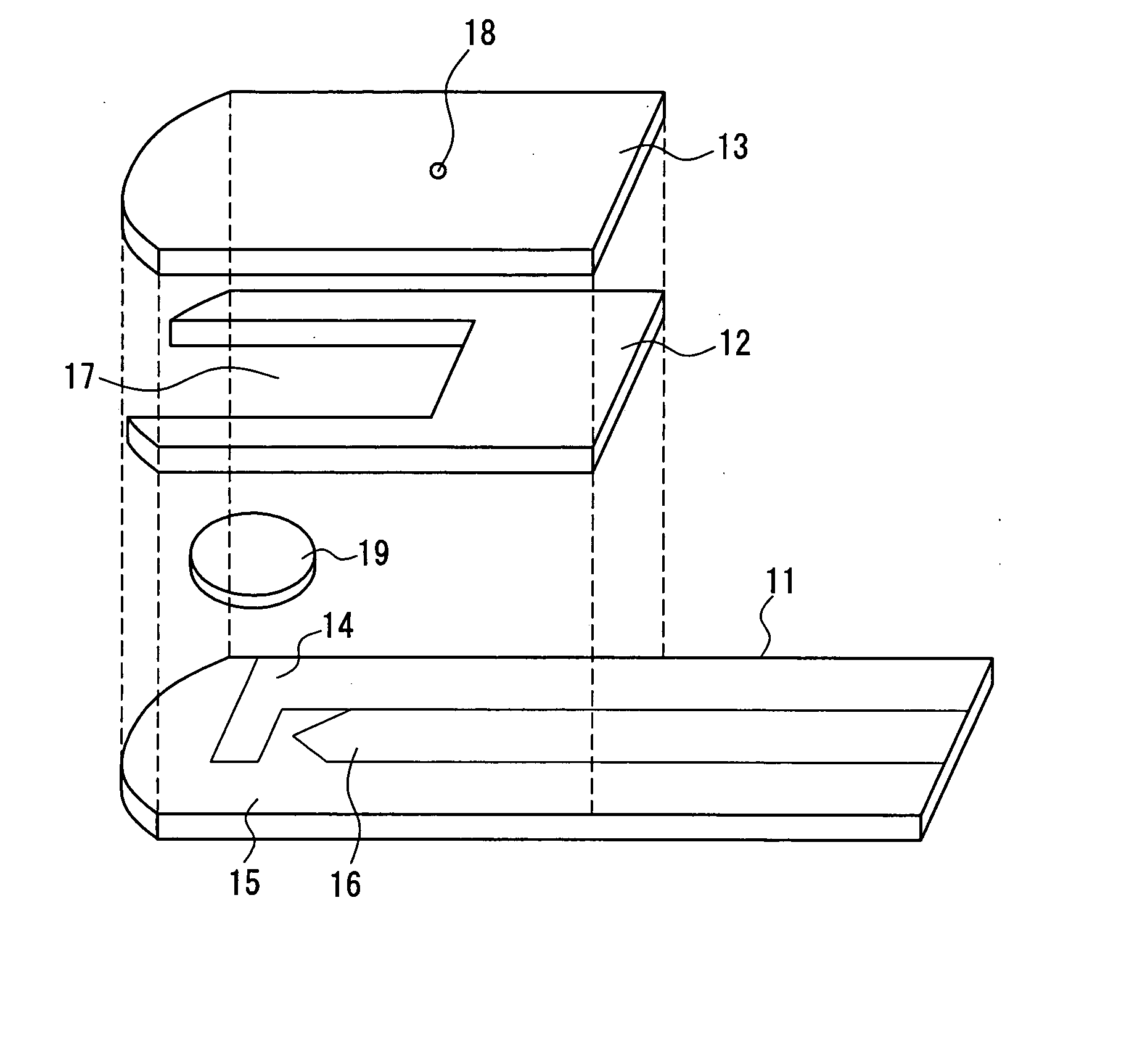
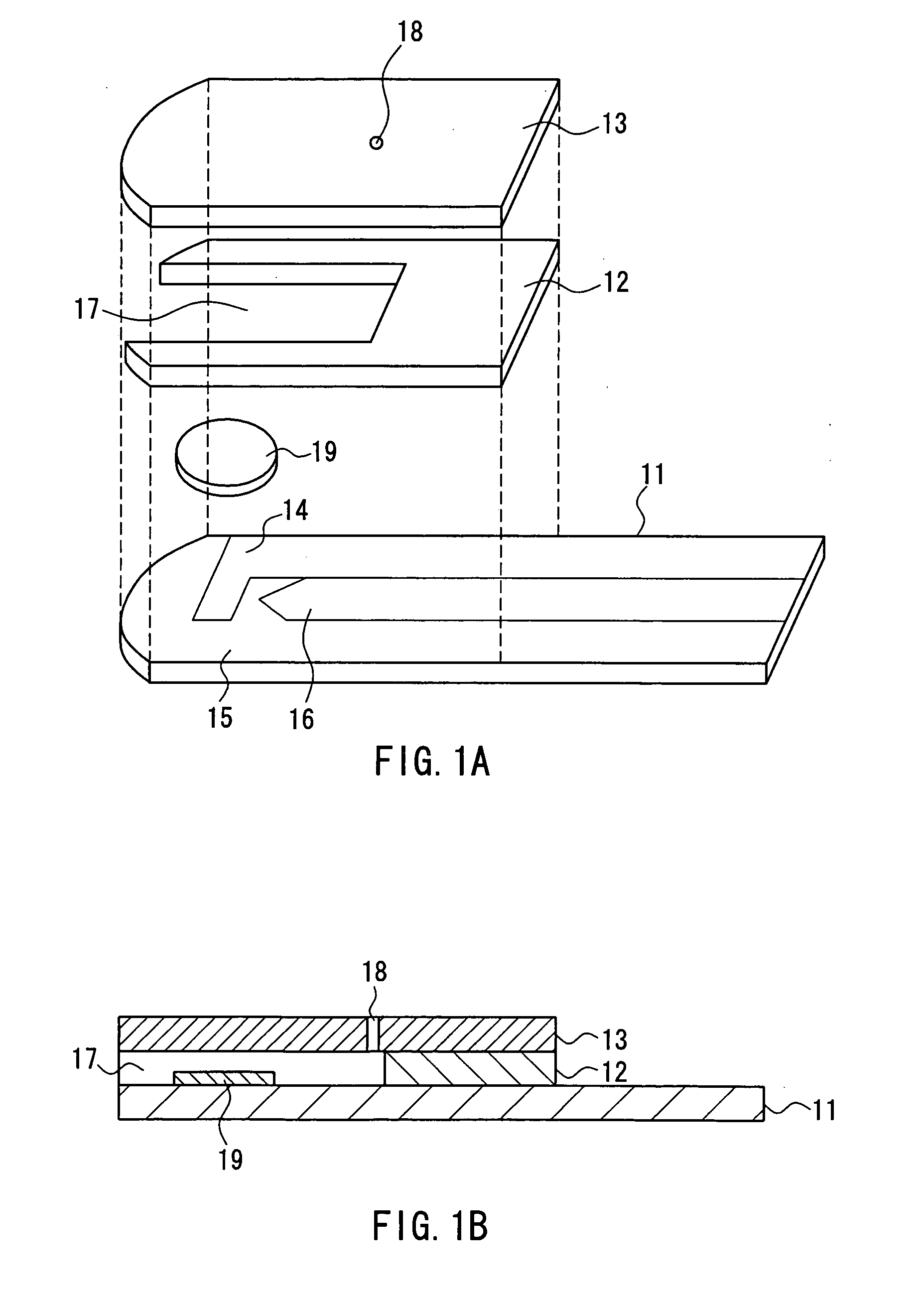
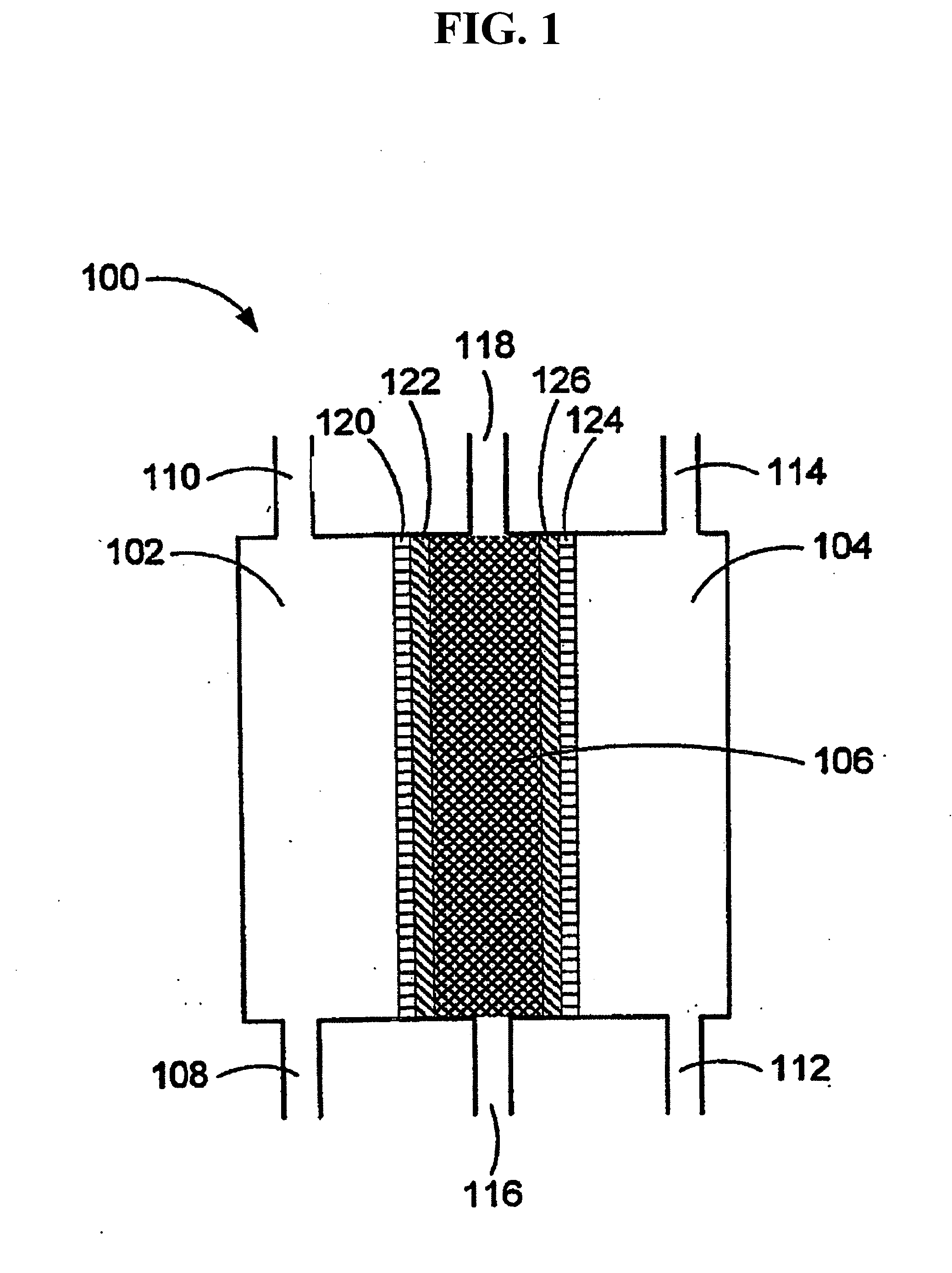
The present invention provides a biosensor that enables highly-accurate measurement of a sample solution including a solid component like hemocytes and has a little variation in response. The biosensor includes: an insulating base plate, an electrode system having at least a working electrode and a counter electrode provided on the base plate, a cover member that is combined with the base plate to define a sample solution supply pathway for leading a sample solution from a sample supply unit to the electrode system, a reaction reagent system including at least an oxidation-reduction enzyme and an electron mediator, and a filter disposed between the electrode system and the sample supply unit in the sample solution supply pathway. The biosensor has a space that encircles surface of the filter in an area from one end of the filter close to the sample supply unit to the other end of the filter close to the electrode system. This arrangement effectively prevents the solid component like hemocytes from flowing into the electrode system without being filtered out by the filter.
Owner:PANASONIC HEALTHCARE HLDG CO LTD
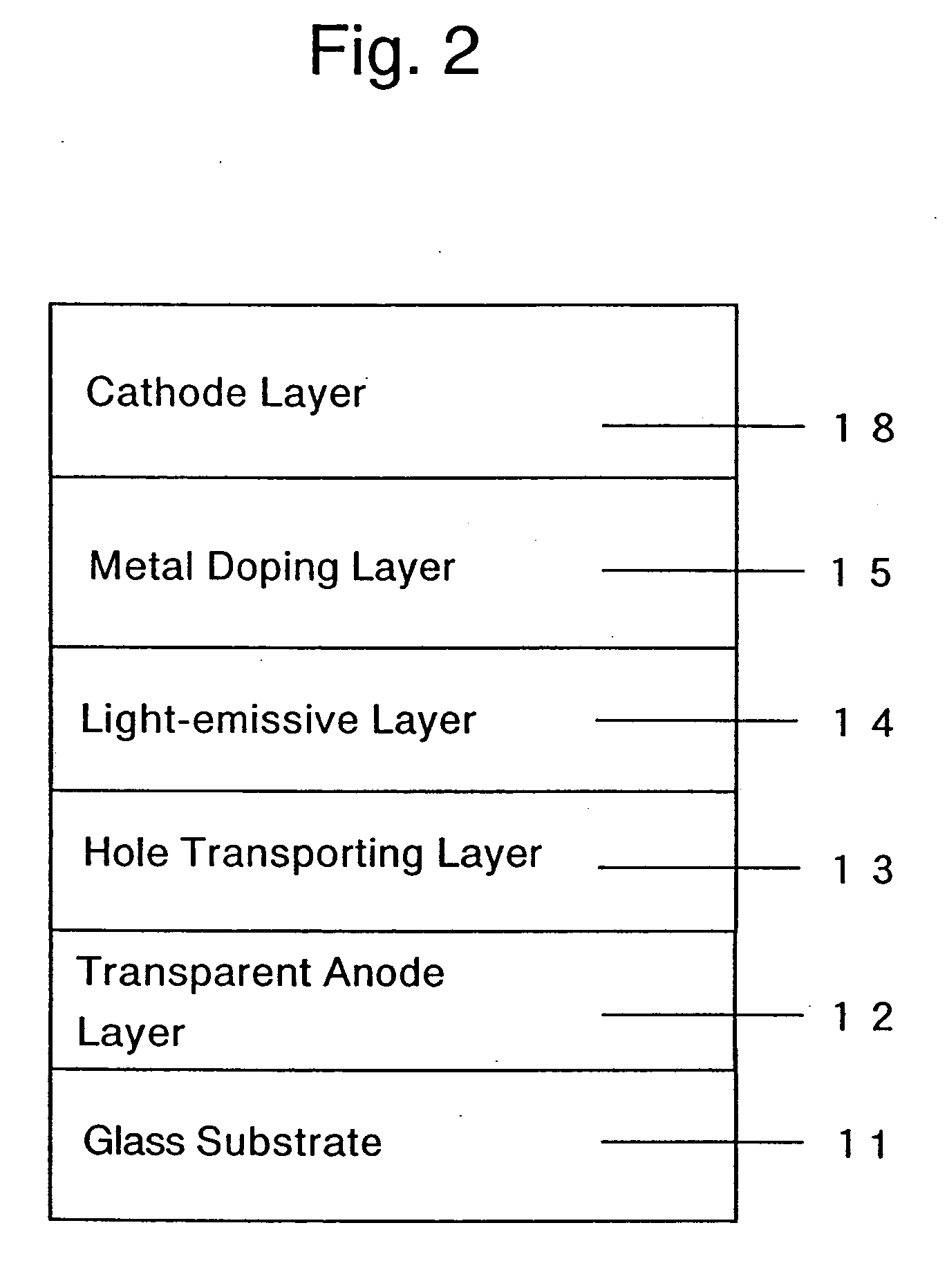
Organic electroluminescent device and production process thereof
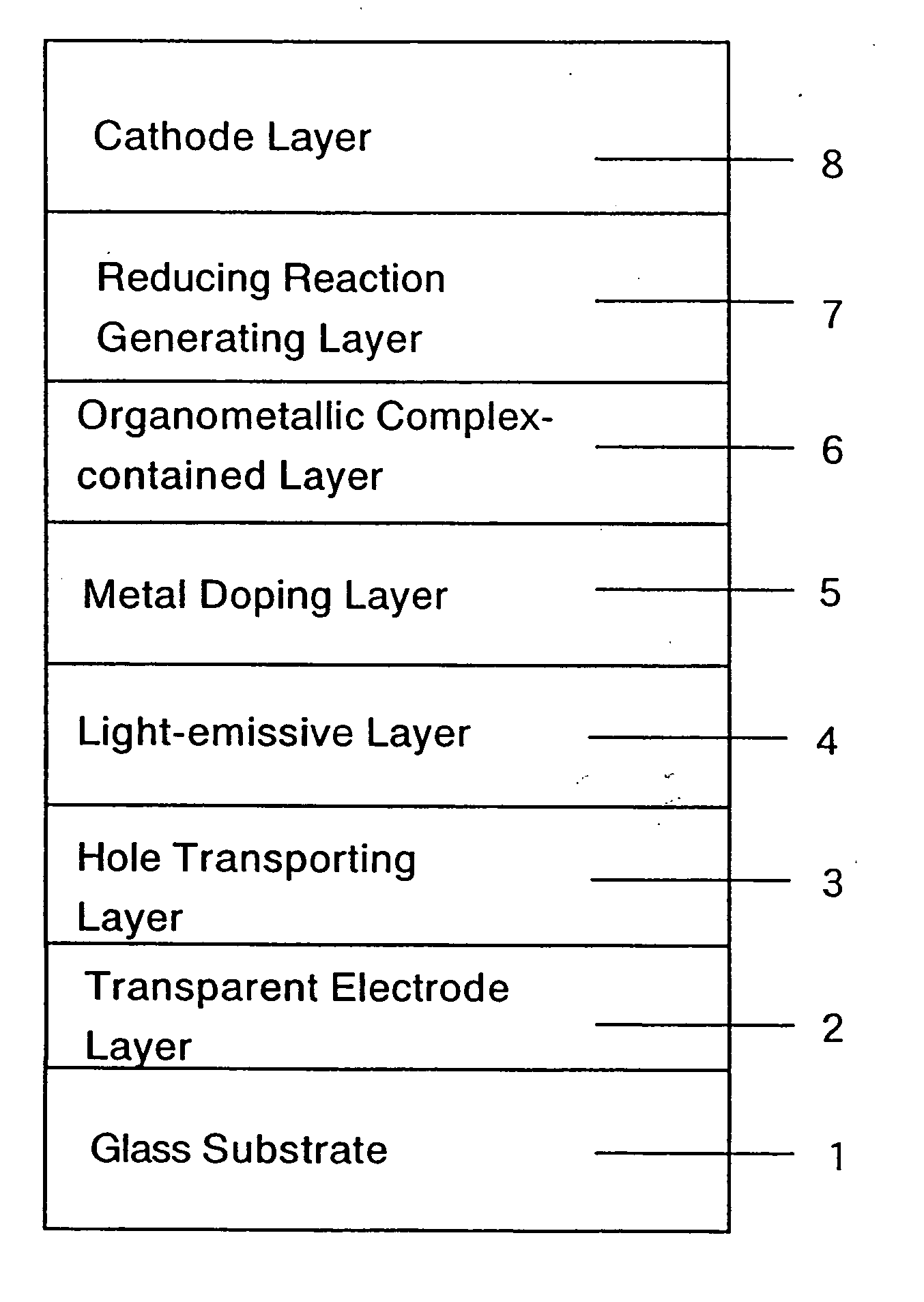
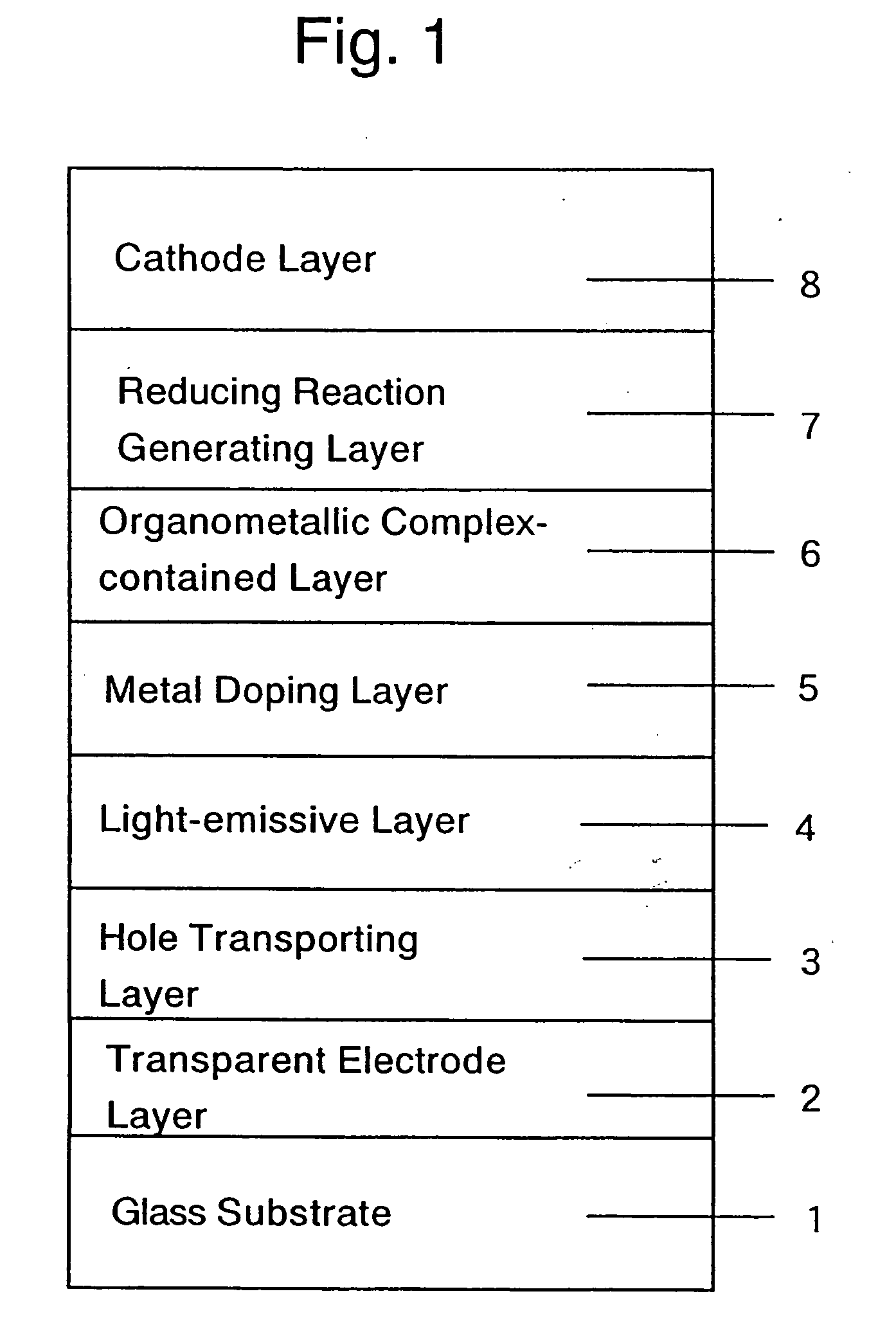
ActiveUS20050084713A1Stable device propertyIncrease the driving voltageDischarge tube luminescnet screensElectroluminescent light sourcesOrganic structureAlkaline earth metal
Owner:ROHM CO LTD +1
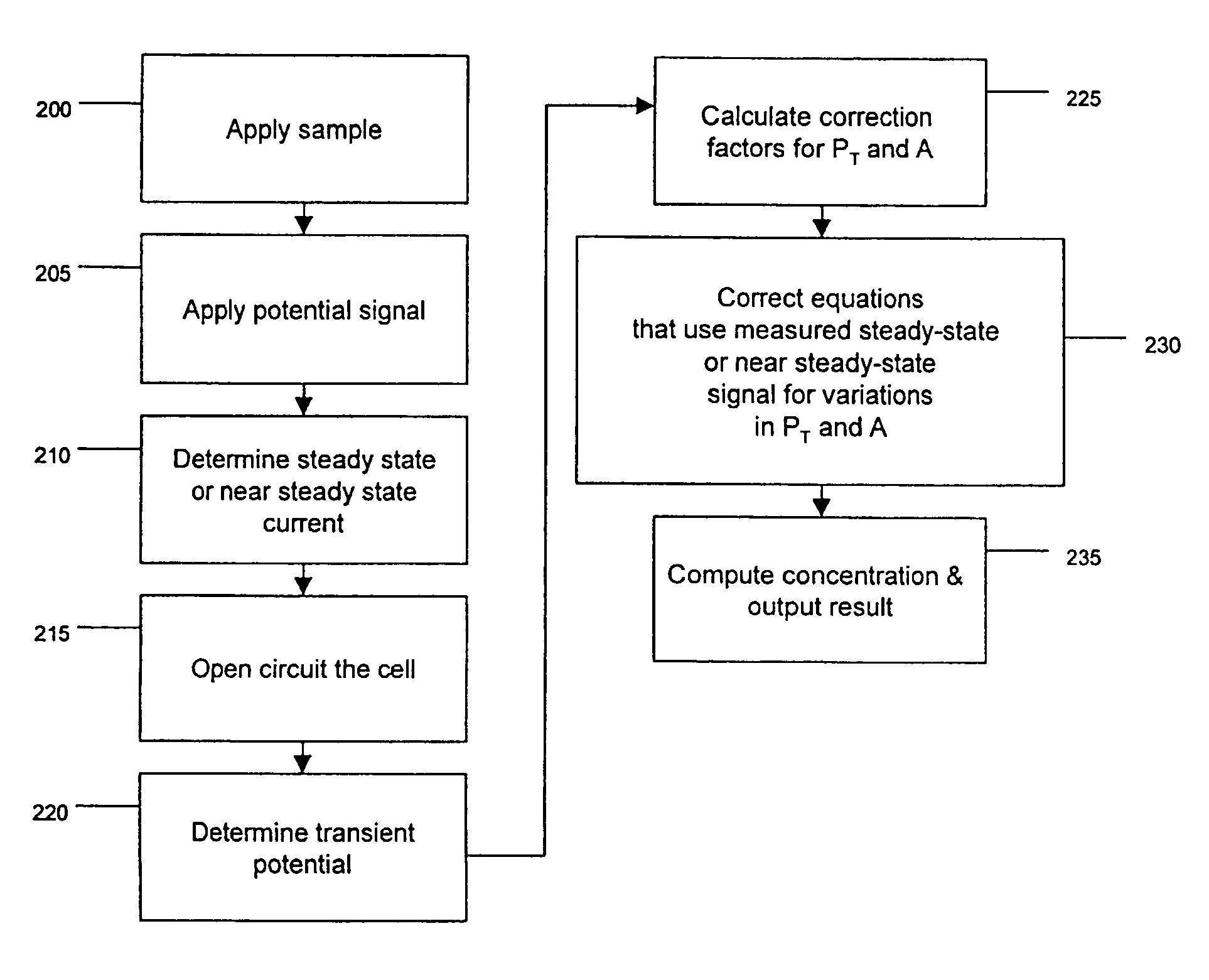
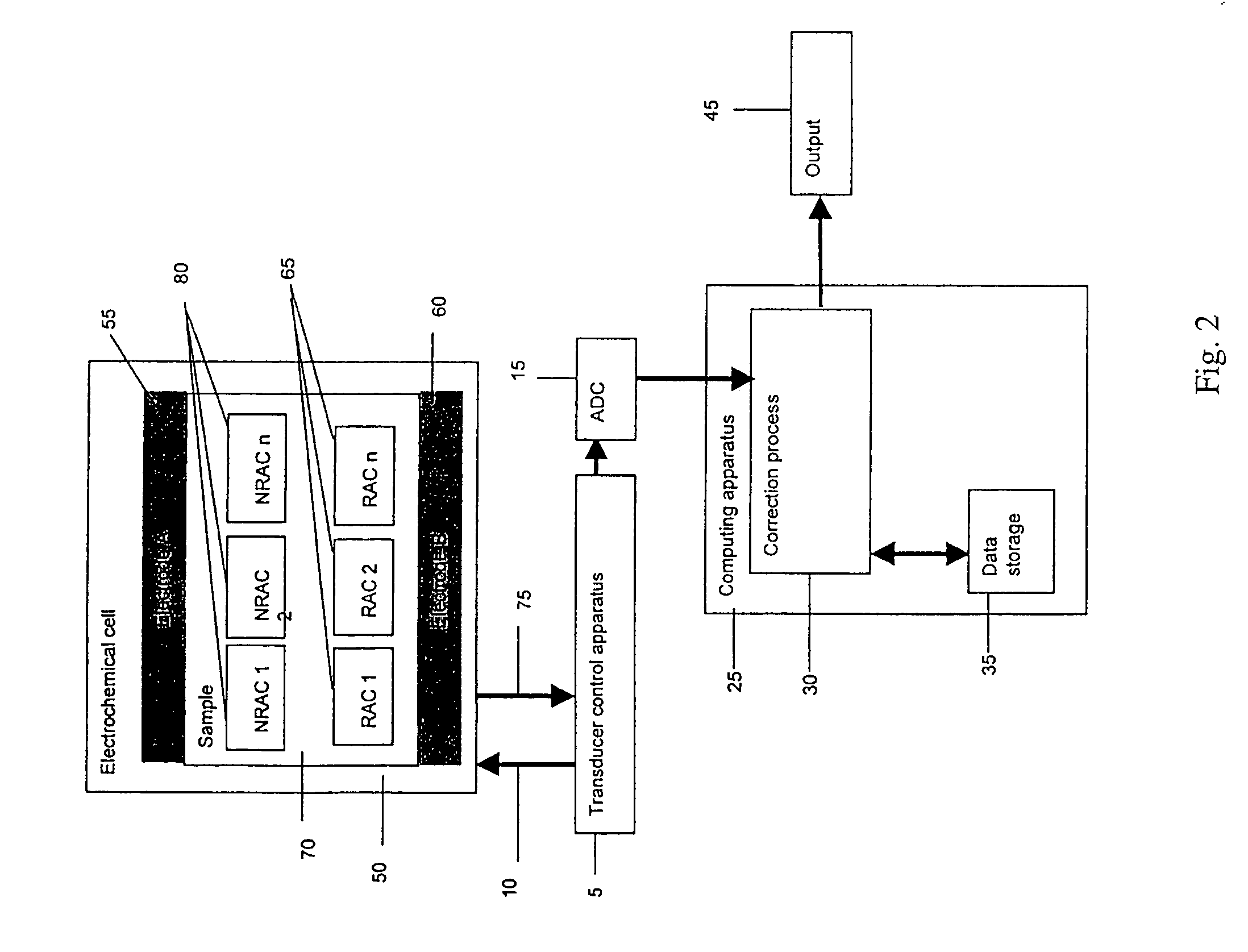
Method and apparatus for assay of electrochemical properties
ActiveUS7501052B2Easy to measureImprove accuracy and precisionImmobilised enzymesBioreactor/fermenter combinationsHand heldApplied potential
The presence of a select analyte in the sample is evaluated in an an electrochemical system using a conduction cell-type apparatus. A potential or current is generated between the two electrodes of the cell sufficient to bring about oxidation or reduction of the analyte or of a mediator in an analyte-detection redox system, thereby forming a chemical potential gradient of the analyte or mediator between the two electrodes After the gradient is established, the applied potential or current is discontinued and an analyte-independent signal is obtained from the relaxation of the chemical potential gradient. The analyte-independent signal is used to correct the analyte-dependent signal obtained during application of the potential or current. This correction allows an improved measurement of analyte concentration because it corrects for device-specific and test specific factors such as transport (mobility) of analyte and / or mediator, effective electrode area, and electrode spacing (and as a result, sample volume), without need for separate calibration values. The analysis can be performed using disposable test strips in a hand held meter, for example for glucose testing.
Owner:AGAMATRIX INC
Method of measuring blood component and sensor used in the method
ActiveUS20050145490A1Great HctConvenient amountImmobilised enzymesBioreactor/fermenter combinationsRed blood cellOxidoreductase
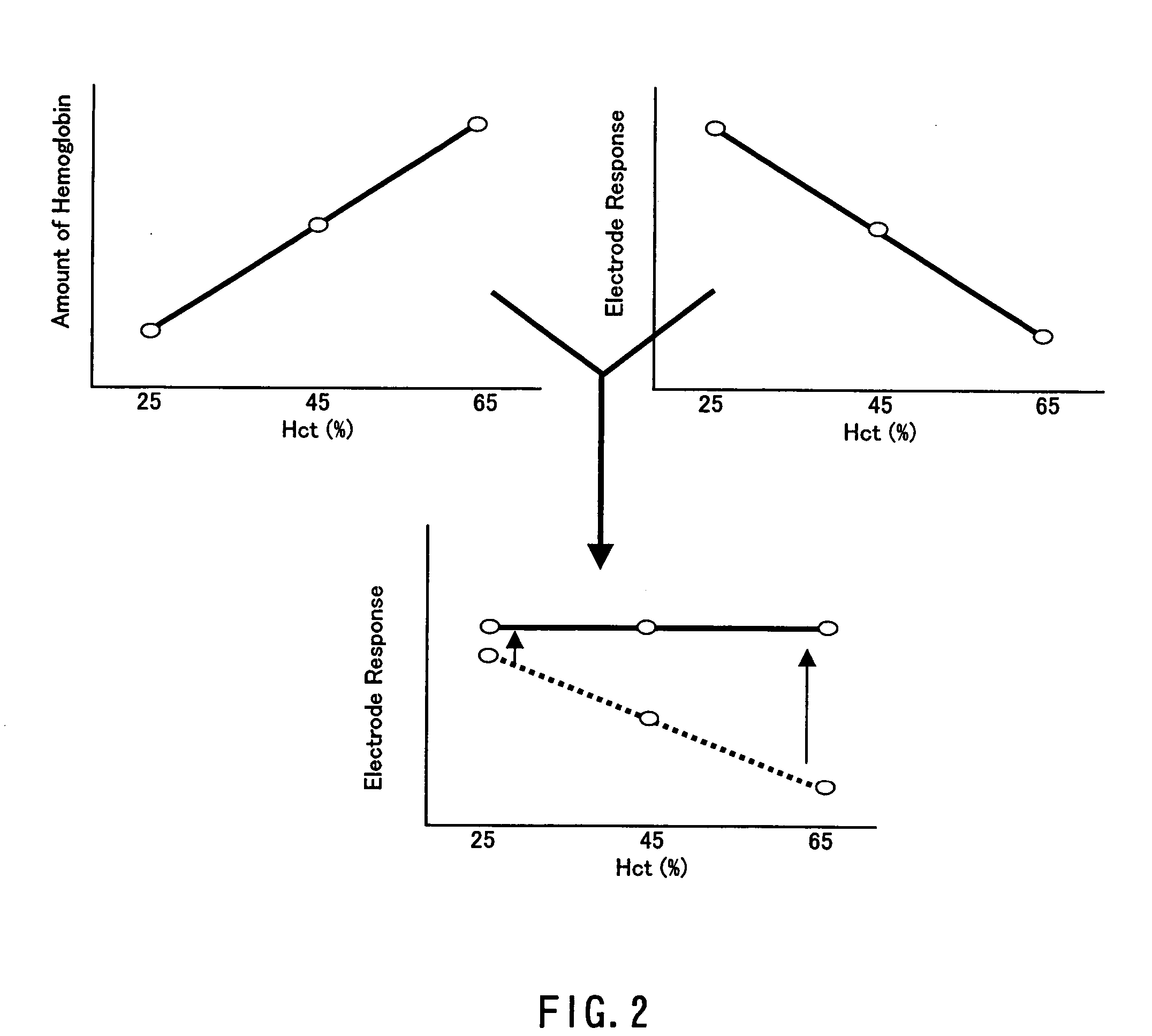
A sensor for blood component analysis that can correct the effect of a hematocrit easily is provided. The sensor includes an analysis portion including a working electrode, a counter electrode, and a reagent portion. The reagent portion includes an oxidoreductase that reacts with the blood component and a mediator, and the blood component is measured by causing a redox reaction between the blood component and the oxidoreductase in the presence of the mediator and detecting a redox current generated by the redox reaction by the working electrode and the counter electrode. In this sensor, the reagent portion further includes a hemolyzing agent (e.g., sodium cholate) for hemolyzing an erythrocyte, and when detecting the redox current, the erythrocyte is hemolyzed with the hemolyzing agent so as to cause hemoglobin released to an outside of the erythrocyte to react with the mediator and a current generated by this reaction also is detected to correct an effect of a hematocrit.
Owner:PHC HLDG CORP
Methods of treating or preventing inflammation and hypersensitivity with oxidative reductive potential water solution
InactiveUS20070196357A1Potent anti-inflammatory activityFree them from any toxicityAntibacterial agentsBiocideMedicineReduction potential
Provided is a method for preventing or treating inflammation and associated states (e.g. infection, hypersensitivity, pain) by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers and can be administered in conjunction with one or more additional therapeutic agents.
Owner:SONOMA PHARMA INC
Flue gas control system of coal combustion boiler and operating method thereof
ActiveUS8071060B2Reduce operating costsCombination devicesNitrogen compoundsAir preheaterParticulates
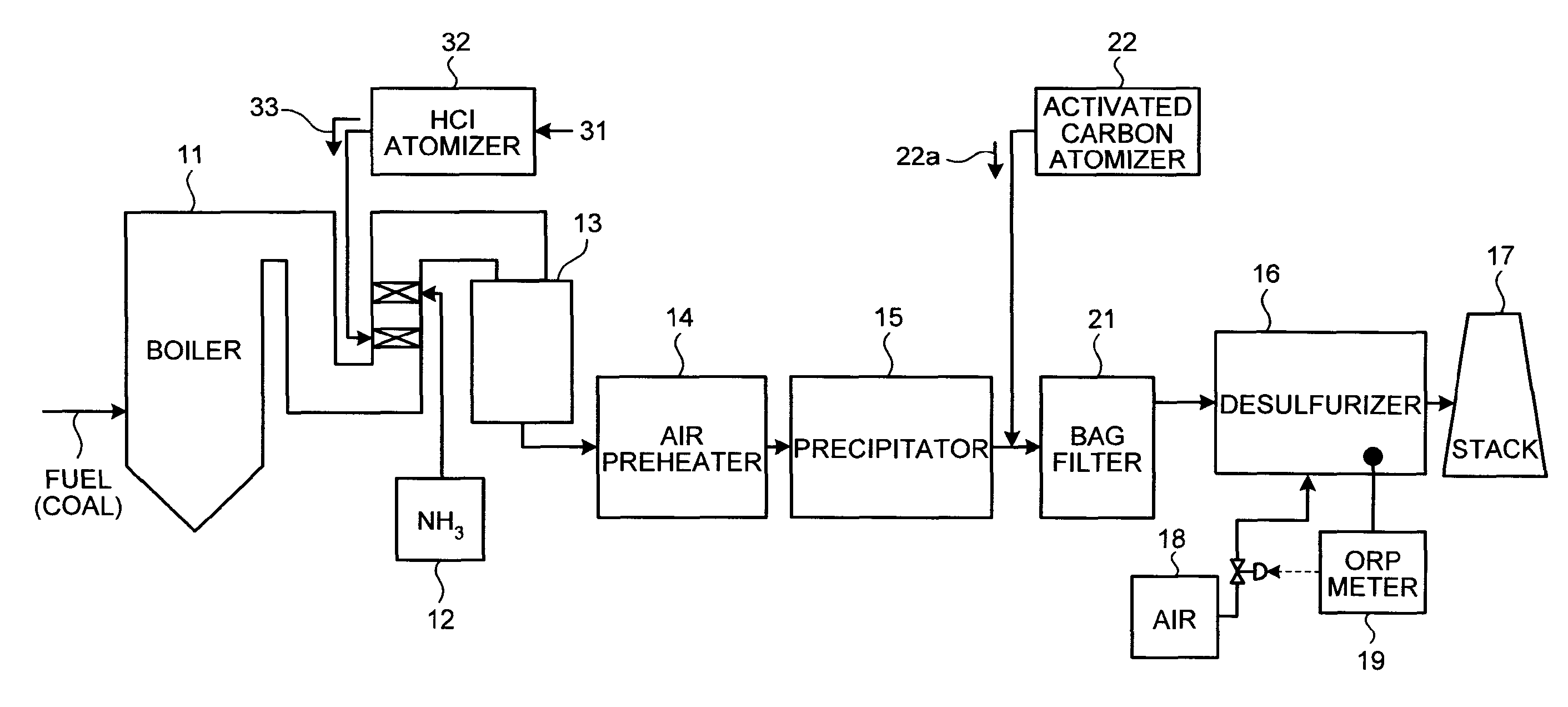
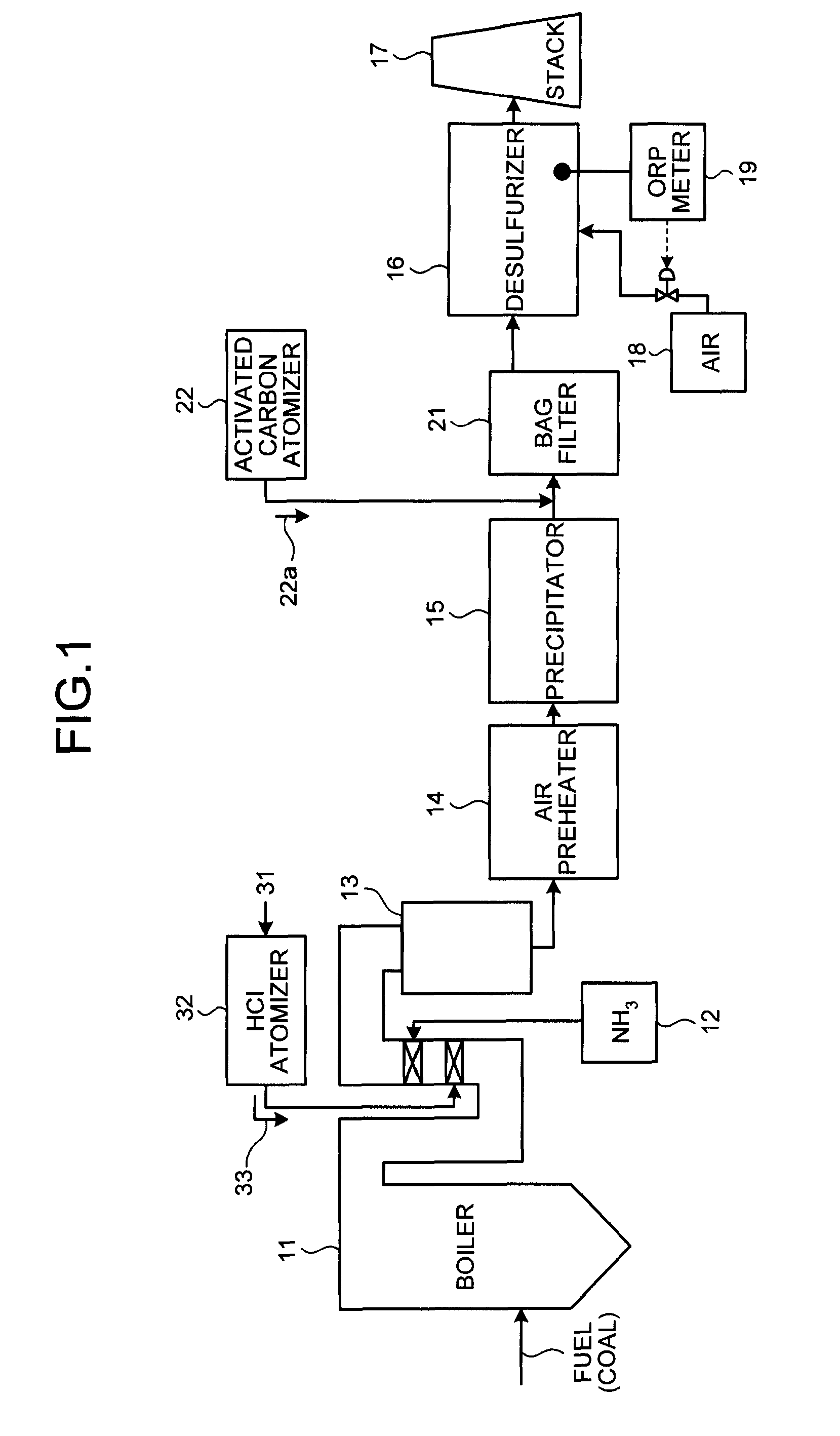
A flue gas control system of a coal combustion boiler comprises an HCl atomizer that sprays hydrogen chloride to flue gas from a coal combustion boiler that uses coal as a fuel; NOx removing apparatus that removes nitrogen oxides by ammonia denitration by adding ammonia to the flue gas after spraying hydrogen chloride and oxidizes mercury; an air preheater that recovers heat in the gas after removal of nitrogen oxides; a precipitator that removes particulates in the gas; an activated carbon atomizer that sprays activated carbon into the gas after particulate collection; a bag filter that collects activated carbon having adsorbed mercury; a desulfurizer that removes sulfur oxides in the flue gas after removal of activated carbon; a stack that discharges the gas which has undergone desulfurization to outside; and an ORP meter that measures an oxidation reduction potential for feeding air to a slurry absorbent in the desulfurizer.
Owner:MITSUBISHI HEAVY IND LTD
Method of treating skin ulcers using oxidative reductive potential water solution
ActiveUS20060235350A1Reduce inflammatory processReduce microbial loadAntibacterial agentsBiocideMedicineSkin ulcerations
Provided is a method of treating skin ulcers and related complications in patients by administering an oxidative reduction potential (ORP) water solution that is stable for at least twenty-four hours.
Owner:SONOMA PHARMA INC
Inorganic shaped bodies and methods for their production and use
InactiveUS6991803B2Overcome the lack of robustnessNovel featuresPowder deliveryDental implantsPorosityCellulose
Shaped, preferably porous, inorganic bodies are provided which are prepared from a reactive blend. In accordance with one preferred embodiment, the solution is absorbed into a porous sacrificial substrate such as a cellulose sponge. The solution-saturated substrate is heated and an oxidation-reduction reaction occurs thereby forming an inorganic solid. A shaped, inorganic body is formed in situ. Optional, but preferred additional thermal treatment of the shaped, inorganic body removes the organic substrate, leaving an inorganic body that faithfully mimics the porosity, shape, and other physical characteristics of the organic substrate. Inorganic substrates may also be used to good effect. Large varieties of shaped bodies can be prepared in accordance with other embodiments of the invention and such shapes find wide use in surgery, laboratory and industrial processes and otherwise. The invention also provides chemically and morphologically uniform powders, including those having uniformly small sizes.
Owner:ORTHOVITA INC
Electrochemical Analyte Detection Apparatus and Method
InactiveUS20090026075A1Easy to useLow costImmobilised enzymesBioreactor/fermenter combinationsRedox enzymesAnalyte
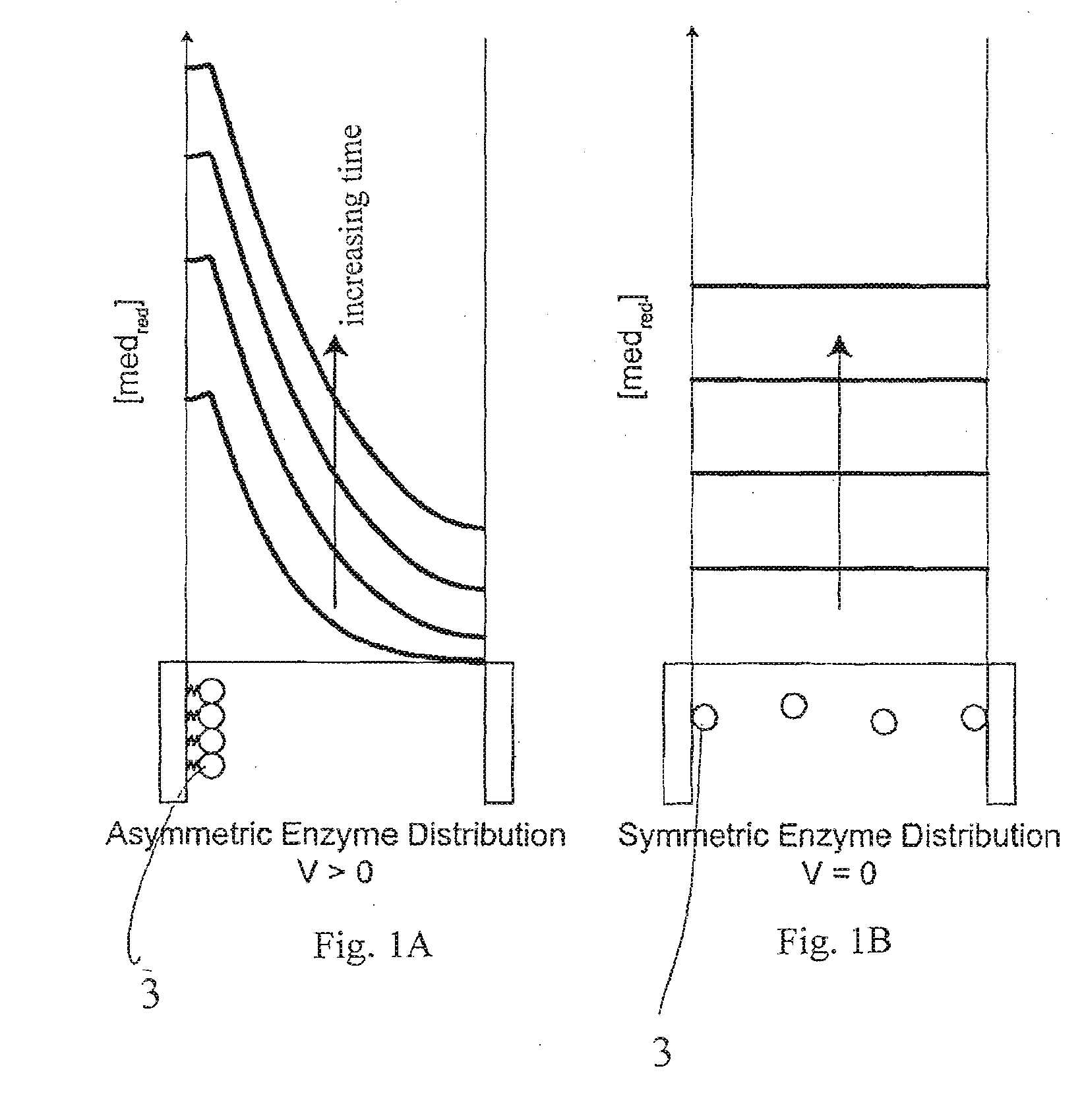
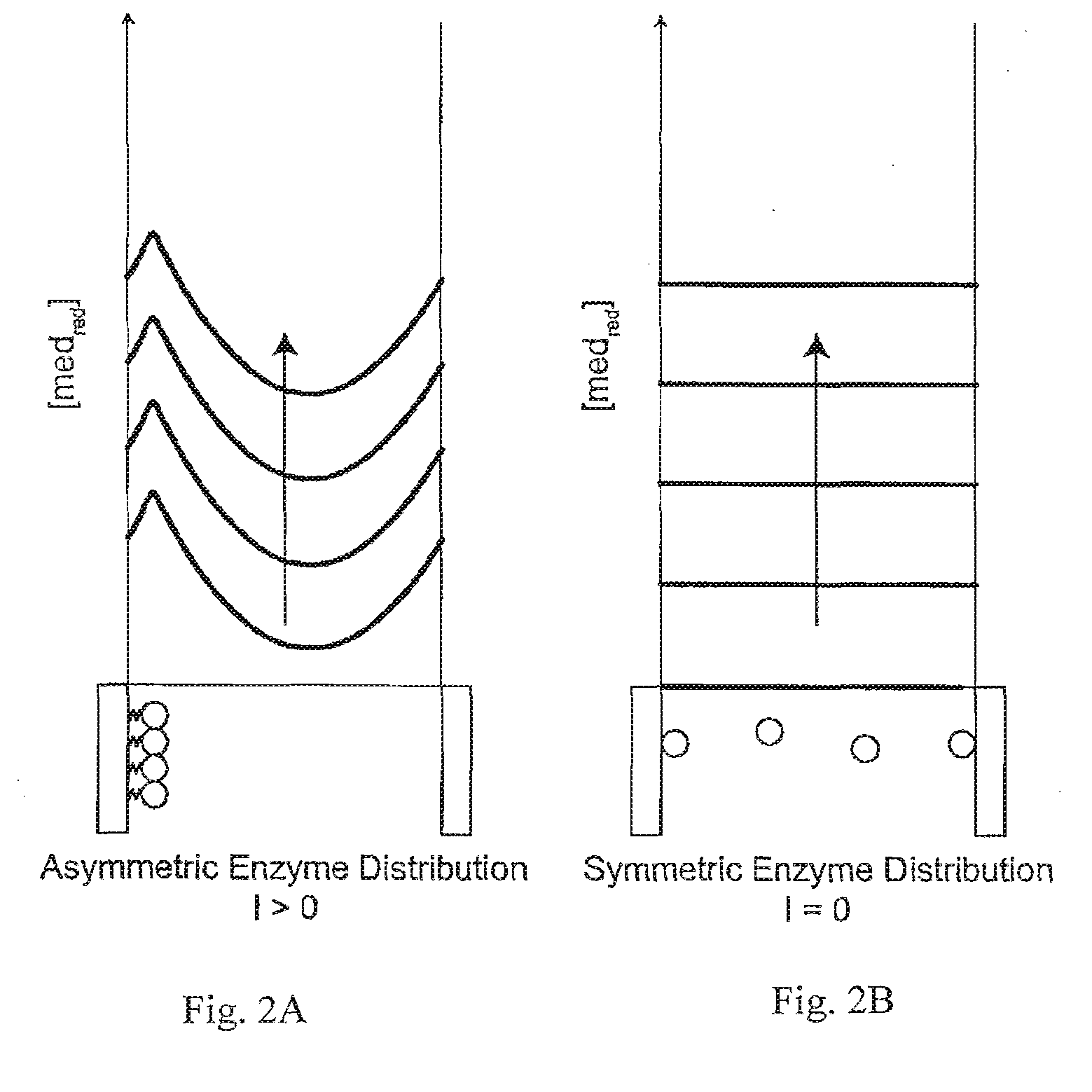
A method and apparatus for electrochemical detection of analyte in a sample makes use of a binding interaction and relies on the discovery that asymmetric distribution of a redox enzyme between two electrodes that occurs when a redox enzyme-containing reagent is immobilized at the surface of one electrode can be detected as a chemical potential gradient arising from an asymmetry, in the distribution of oxidized or reduced redox substrate. This chemical potential gradient can be detected potentiometrically by observing the potential difference between the electrodes in an open circuit, or amperometrically by observing the current flow between the electrodes when the circuit is closed. In both cases, the observation of asymmetry can be done without the application of an external potential or current to the electrodes.
Owner:AGAMATRIX INC
Method and apparatus for producting negative and positive oxidative reductive potential (orp) water
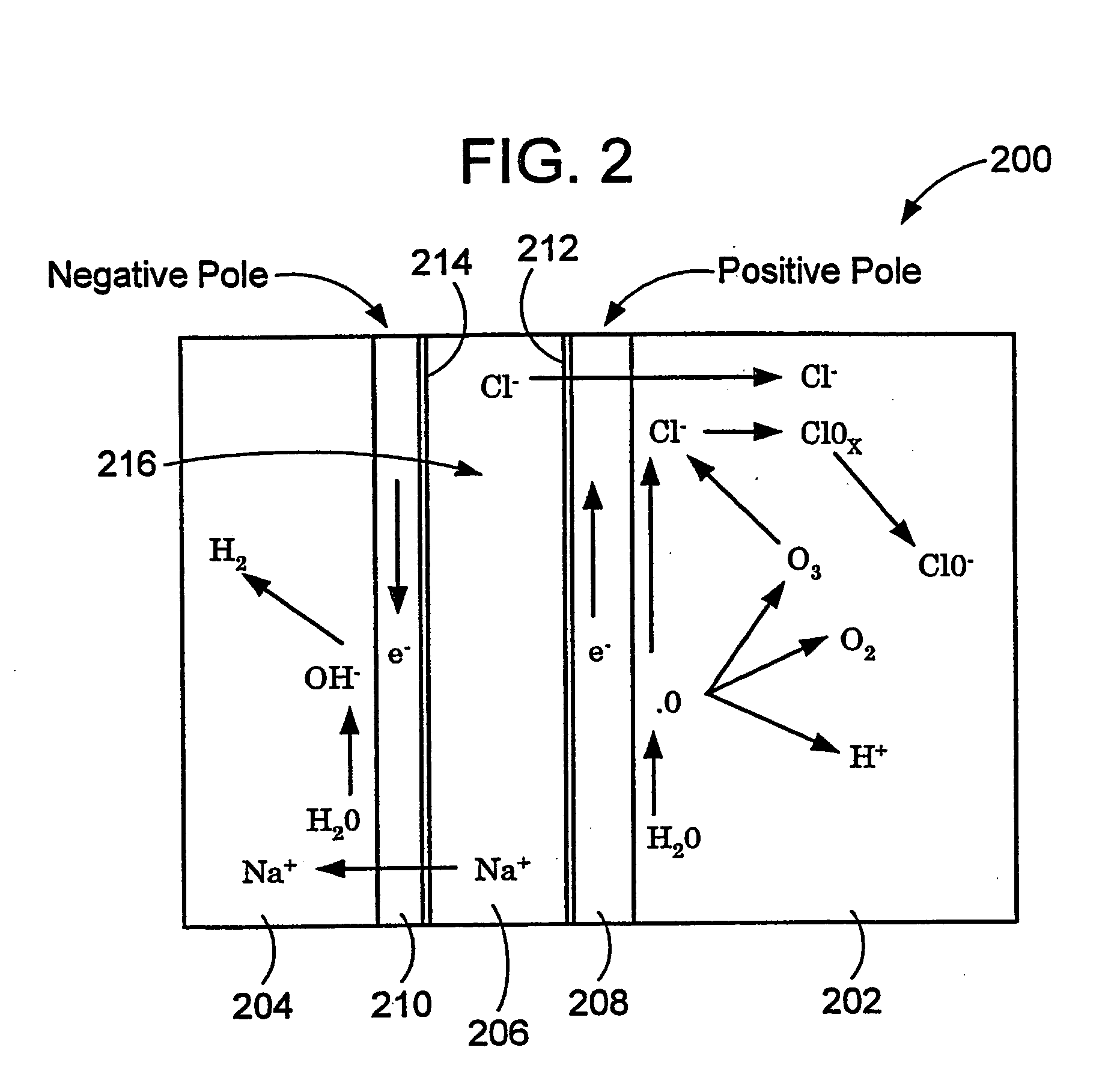
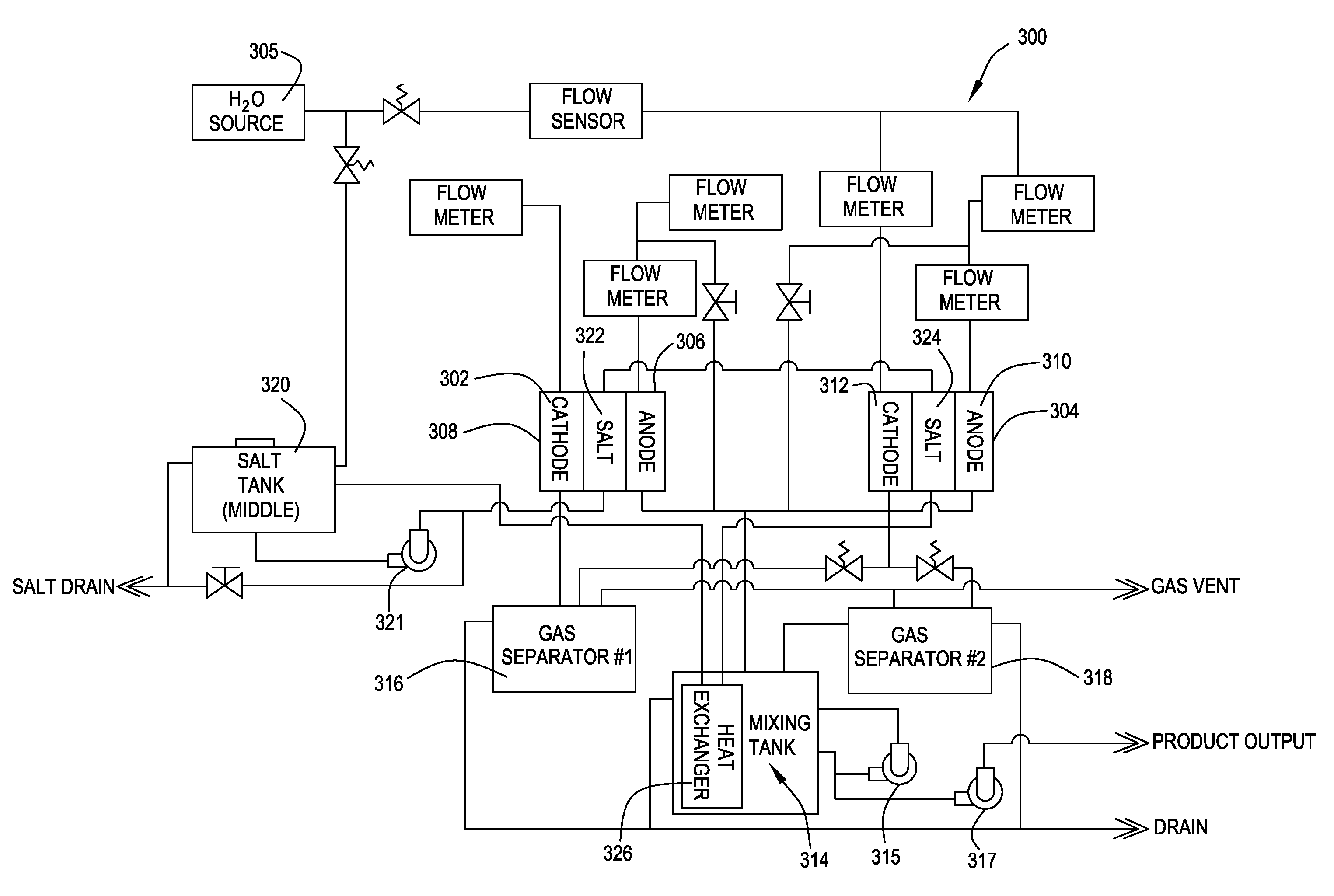
ActiveUS20050121334A1Effective and efficient and economicalCellsWater treatment parameter controlParticulatesElectrolysis
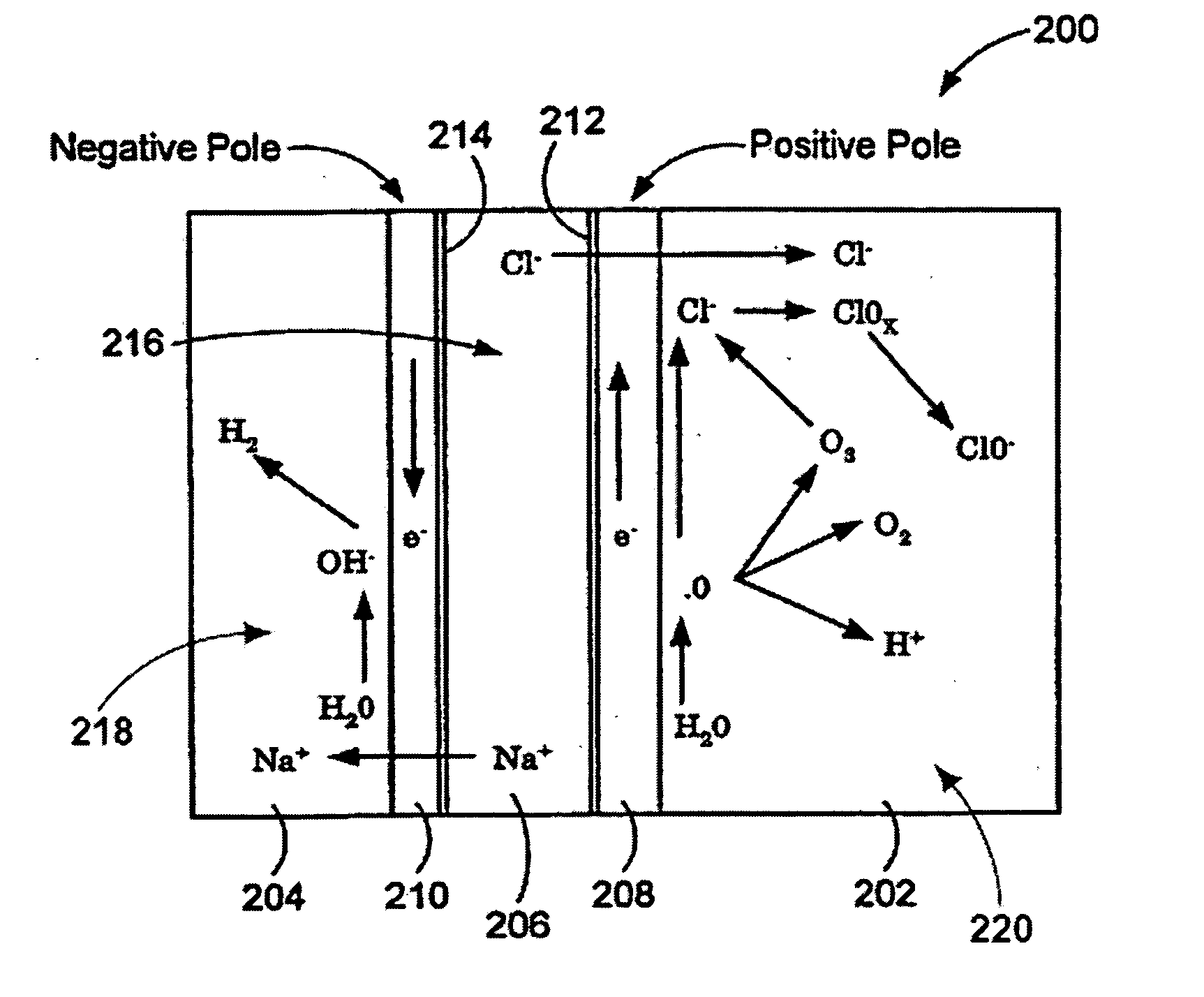
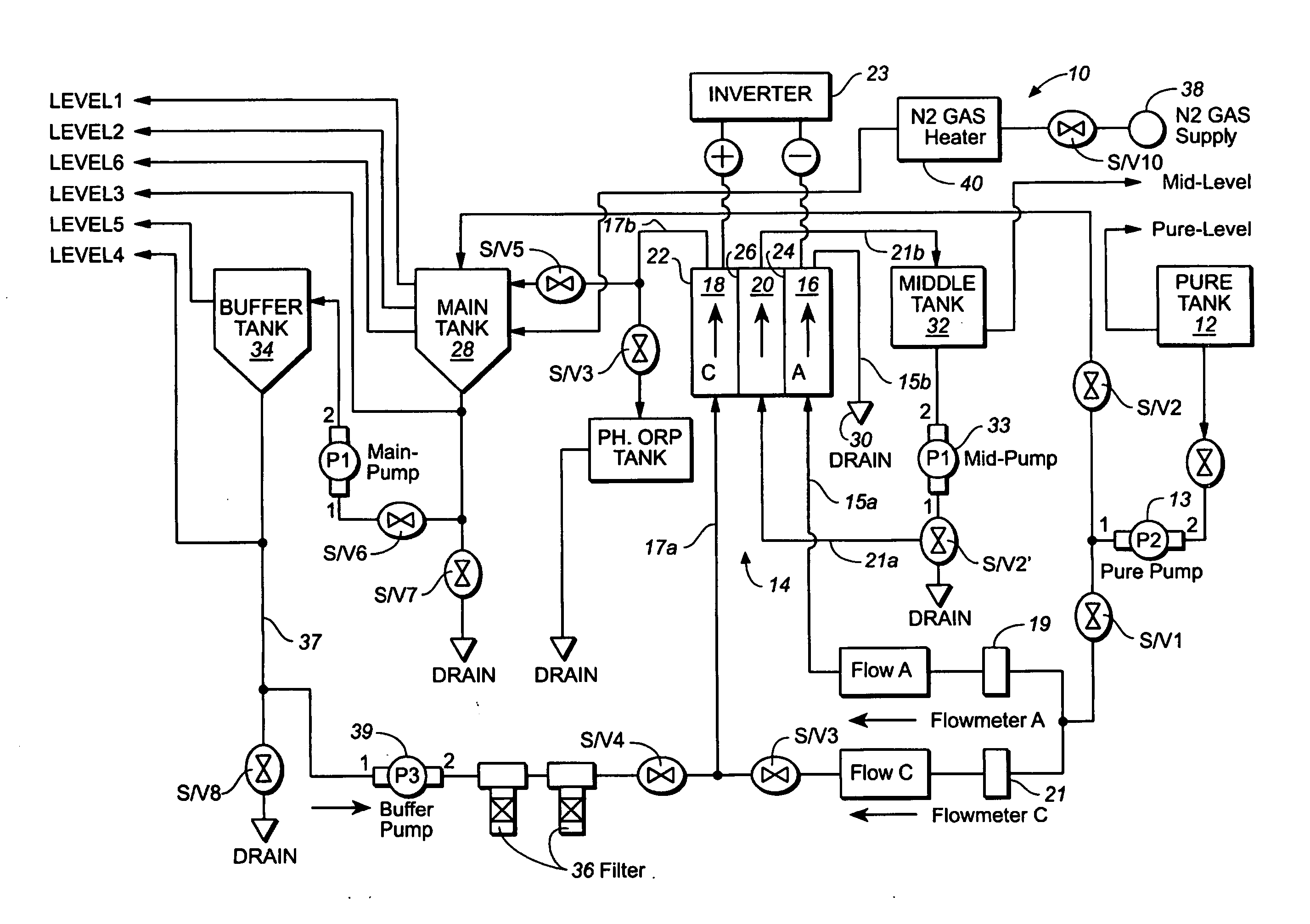
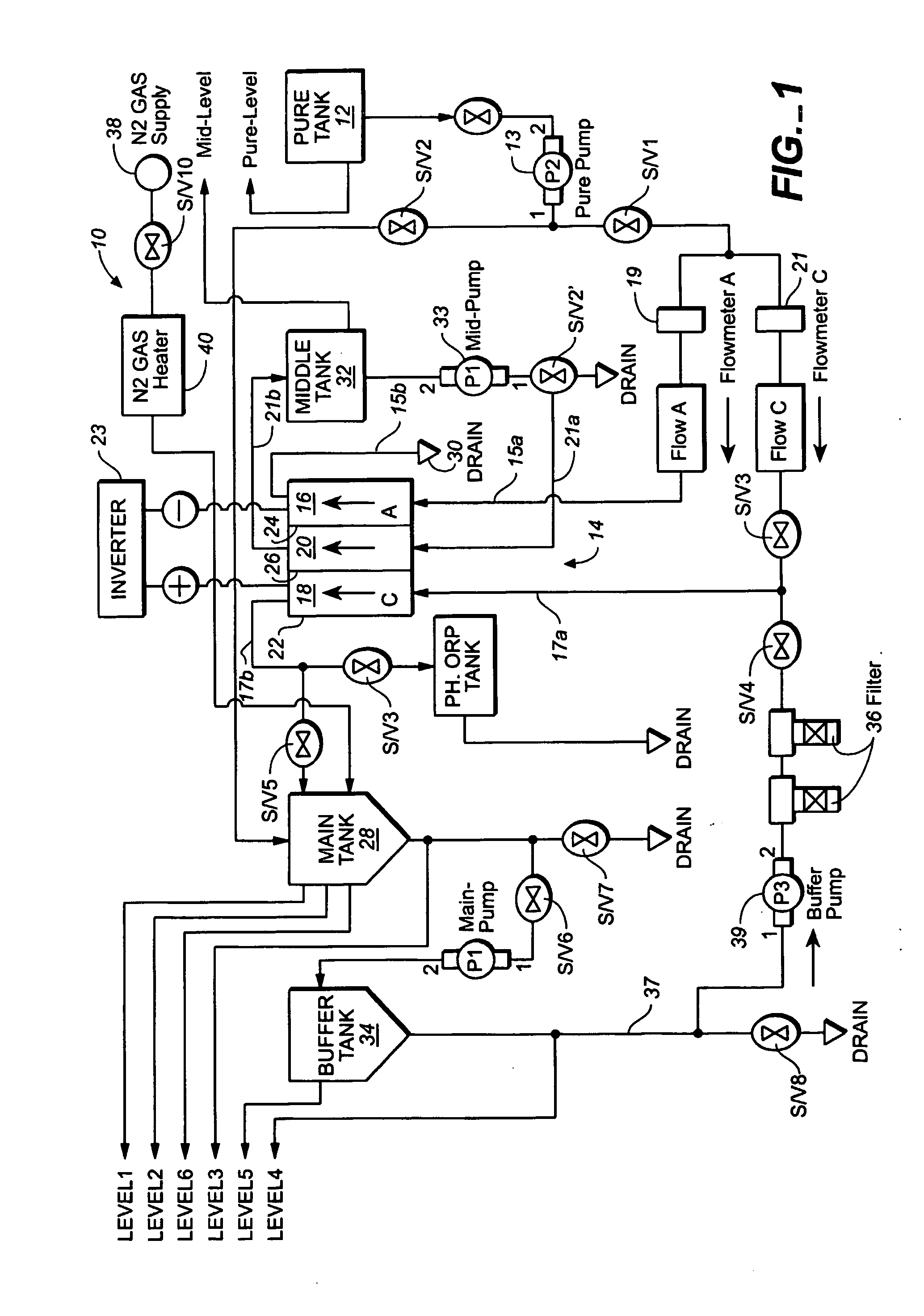
A method and apparatus for electrolytically producing oxidation reduction potential water from aqueous salt solutions for use in disinfection, sterilization, decontamination, wound cleansing. The apparatus includes an electrolysis unit having a three-compartment cell (22) comprising a cathode chamber (18), an anode chamber (16), and a saline solution chamber (20) interposed between the anode and cathod chambers. Two communicating (24, 26) membranes separate the three chambers. The center chamber includes a fluid flow inlet (21a) and outlet (21b) and contains insulative material that ensures direct voltage potential does not travel through the chamber. A supply of water flows through the cathode and anode chambers at the respective sides of the saline chamber. Saline solution flows through the center chamber, either by circulating a pre-prepared aqueous solution containing ionic species, or, alternatively, by circulating pure water or an aqueous solution of, e.g., aqueous hydrogen chloride and ammonium hydroxide, over particulate insulative material coated with a solid electrolyte. Electrical current is provided to the communicating membranes separating the chambers, thus causing an electrolytic reaction that produces both oxidative (positive) and reductive (negative) ORP water.
Owner:SONOMA PHARMA INC
Apparatus and process for mediated electrochemical oxidation of materials
A unique apparatus unique apparatus and process that uses mediated electrochemical oxidation (MEO) for: (1) Destruction of: a) nearly all organic solid, liquid, and gases materials, except fluorinated hydrocarbons; b) all biological solid, liquid, and gases materials; c) and / or dissolution and decontamination (such as cleaning equipment and containers, etc.) of nearly all inorganic solid, liquid, or gas where higher oxidation states exist which includes, but is not limited to, halogenated inorganic compounds (except fluorinated), inorganic pesticides and herbicides, inorganic fertilizers, carbon residues, inorganic carbon compounds, mineral formations, mining tailings, inorganic salts, metals and metal compounds, etc.); and d) combined materials (e.g. a mixture of any of the foregoing with each other); henceforth collectively referred to as materials. (2) Sterilization / disinfection of equipment, glassware, etc., by destroying all existing infectious materials. (3) Dissolution of transuranic / actinide materials and / or destruction of the oxidizable components in the hazardous waste portion of mixed waste. (4) Generation of hydrogen and oxygen from MEO of materials. (5) Alteration of organic, biological, and inorganic materials by MEO to produce other compounds from these materials. The materials are introduced into an apparatus for contacting the materials with an electrolyte containing the oxidized form of one or more reversible redox couples, at least one of which is produced electrochemically by anodic oxidation at the anode of an electrochemical cell. The oxidized forms of any other redox couples present are produced either by similar anodic oxidation or reaction with the oxidized form of other redox couples present and capable of affecting the required redox reaction. The oxidized species of the redox couples oxidize the materials molecules and are themselves converted to their reduced form, whereupon they are reoxidized by either of the aforementioned mechanisms and the redox cycle continues until all oxidizable material species, including intermediate reaction products, have undergone the desired degree of oxidation. The entire process takes place at temperatures between ambient and approximately 100° C. The oxidation process may be enhanced by the addition of reaction enhancements, such as: ultrasonic energy and / or ultraviolet radiation.
Owner:SCIMIST LNC
Physiologically balanced, ionized, acidic solution and methodology for use in wound healing
Described herein is a physiologically-balanced, acidic solution. Typically the solution is prepared by a chemical reactions or by the electrolysis of a solution comprising a mixture of an inorganic salt to form a physiologically balanced solution. This invention also relates to methods for use of the solutions, including a specialized bandage which may be used in combination with the solutions, or optionally with other topically applied materials. A mixture of inorganic salts and, optionally minerals, is used in order to mimic the electrolyte concentration and mixture of body fluid in an isotonic state. The solution typically comprises of one halide salt of lithium, sodium, potassium, calcium, and other cations. Typically the halide is fluoride, chloride, bromide, or iodide, and most typically chloride. A typical electrolyzed solution of the present invention has a pH within the range of about 2 to about 5, an oxidation reduction potential within the range of about +600 mV to about +1200 mV, and hypohalous acid concentration in the range of about 10 ppm to about 200 ppm. The solution has bactericidal, fungicidal, and sporicidal properties. The composition of the invention is nontoxic and has antibacterial properties, and is useful in any application in which antimicrobial properties are desirable.
Owner:NOVABAY PHARM INC
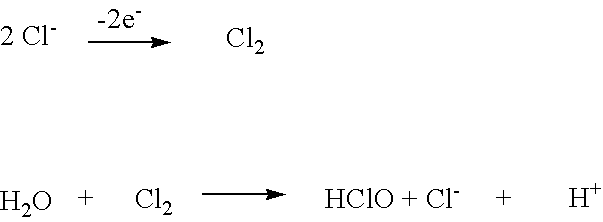
Wound and ulcer treatment with super-oxidized water
Super-oxidized water based on hypochlorous acid, such as is obtained by the electrochemical treatment of a saline solution, may be used in the treatment of leg ulcers or other open wounds. Preferably, the pH of the super-oxidized water is in a range of 4 to 7, and the water has a redox potential of >950 mV. Medicaments based on the super-oxidized water may be in liquid or gel form. The super-oxidized water is able to control the microbial population within the wound and at the same time permit cell proliferation.
Owner:STERILOX TECH INT +1
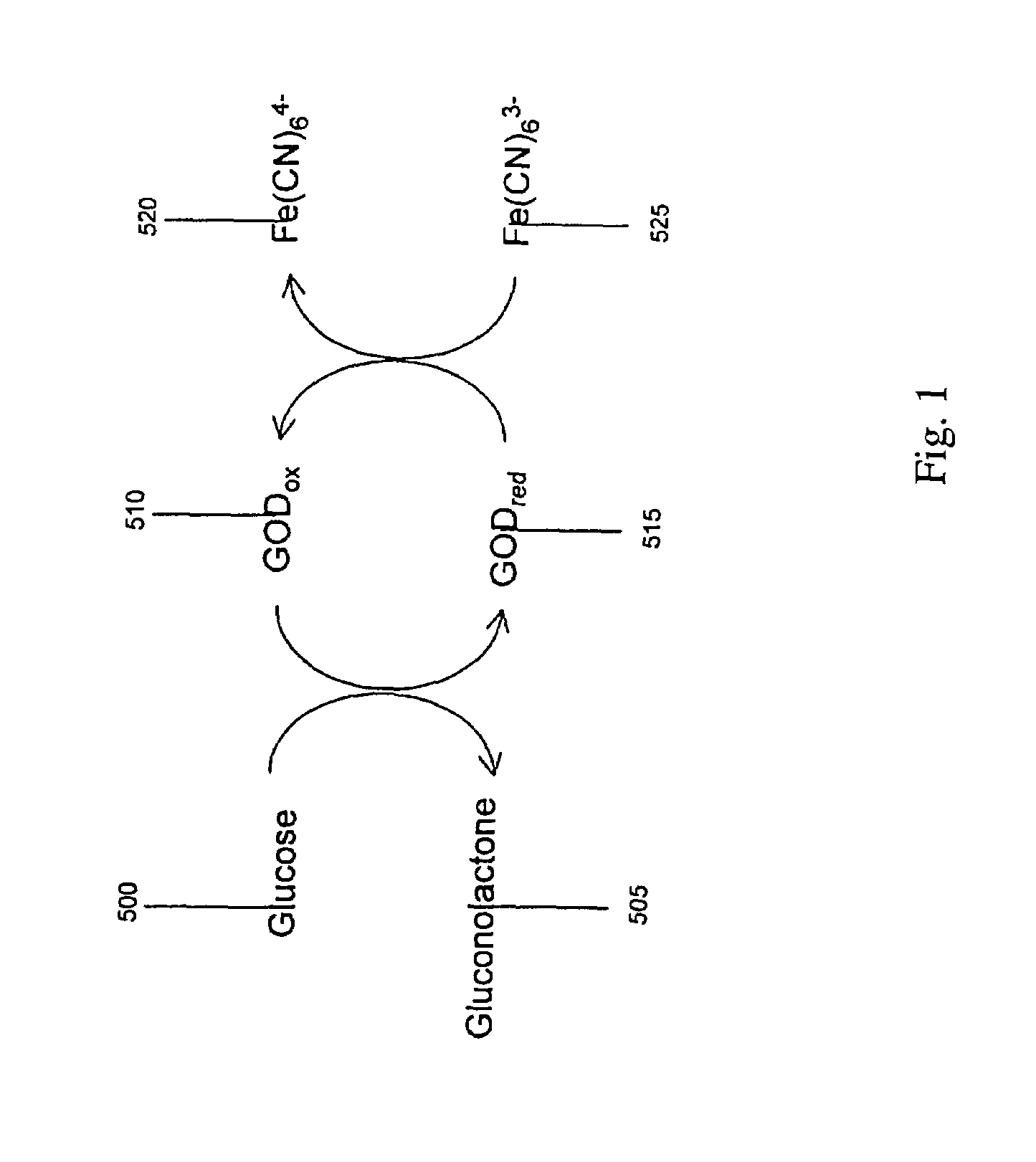
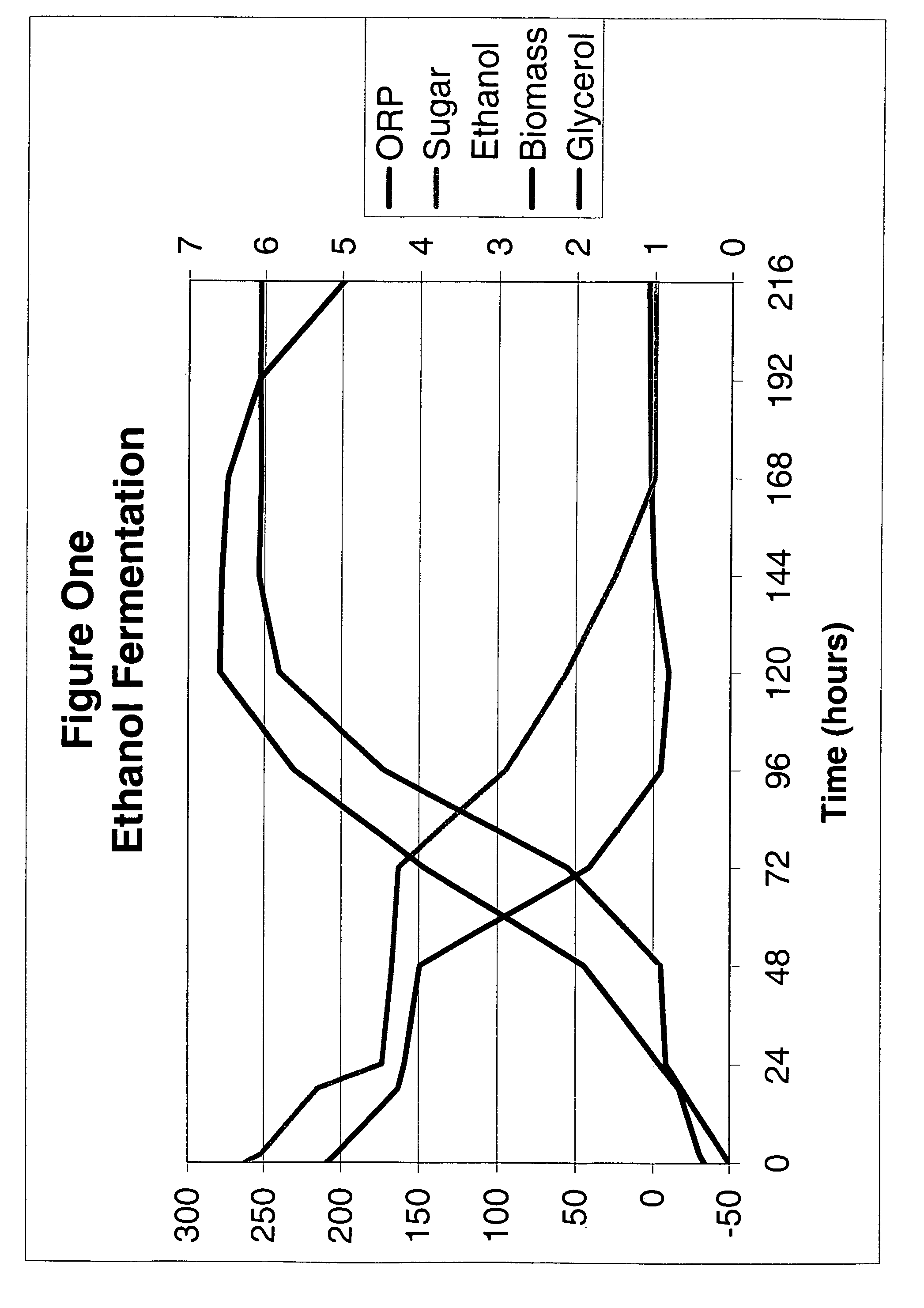
Ethanol fermentation using oxidation reduction potential
InactiveUS7078201B2Increased ethanol productionReduce formationMicrobiological testing/measurementBiofuelsOxidation-Reduction AgentRedox
A process to improve ethanol yield, decrease fermentation time and reduce byproduct formation by monitoring and controlling oxidation reduction potential (redox) of the fermentor is disclosed.
Owner:BURMASTER BRIAN M
Methods of preventing or treating sinusitis with oxidative reductive potential water solution
InactiveUS20070196434A1Treating and preventing sinusitisAntibacterial agentsBiocideSinusitisMedicine
Provided is a method for preventing or treating sinusitis by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers. The ORP water solution can be administered alone or, e.g., in combination with one or more additional therapeutic agents.
Owner:SONOMA PHARMA INC
Method for preparing grapheme through oxidation reduction
The invention relates to a method for preparing grapheme through oxidation reduction and belongs to grapheme preparation technique. The method specifically comprises the following steps: a modified Hummer method is used for preparing graphite oxide, and an oscillator is adopted for shaking and peeling off single layer graphite oxide softly. During the step of preparing grapheme through reduction, compared with kinds of conventional major reducing agents, the method has the advantages that the reduction procedures and conditions are optimized, and further the grapheme with excellent performance is obtained. In the invention, by optimizing the preparation technique and conditions, the grapheme, which is large in size, is single-layered, is excellent in electrical conductivity and has high quality is obtained, and the method has the characteristics of low cost, high product yield and easiness in industrialization.
Owner:NANJING UNIV OF TECH
Method and apparatus for generating, applying and neutralizing an electrochemically activated liquid
A method and apparatus are provided for receiving a cleaning liquid having a pH in a range between pH6-pH8 and an oxidation reduction potential (ORP) between ±50 mV. The liquid is converted into an anolyte liquid and a catholyte liquid having respective pHs outside of the range between pH6-pH8 and having respective ORPs outside the range between ±50 mV. The anolyte and catholyte liquids are applied to a surface, wherein the anolyte and catholyte liquids are, for example, in a combined state on the surface and substantially neutralize to a pH between pH6-pH8 and an ORP between ±50 mV within one minute of the time at which the anolyte and catholyte liquids are converted.
Owner:TENNANT COMPANY
Method of using oxidative reductive potential water solution in dental applications
Methods of using oxidative reduction potential (ORP) water solution that is stable for at least twenty-four hours in dental applications are provided. The ORP water solution can be administered to patients for the routine disinfection of the oral cavity as part of an on-going program of oral hygiene. The ORP water solution can further be used to irrigate and / or disinfect oral tissues and surfaces during dental procedures, oral surgery, or maxillo-facial surgery. Also, the ORP water solution can be administered to treat patients with damage to the oral tissues caused by disease or surgery.
Owner:SONOMA PHARMA INC
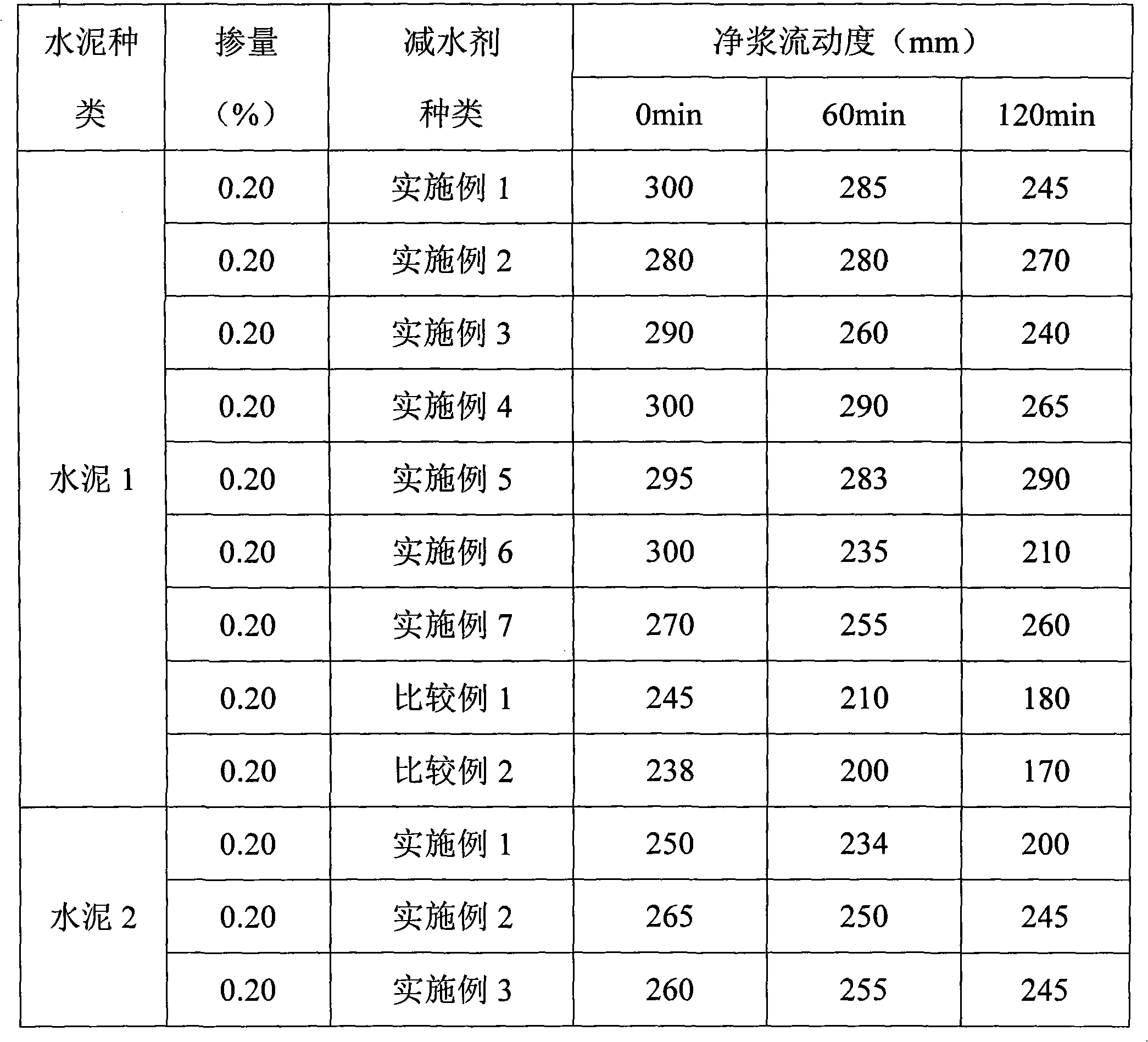
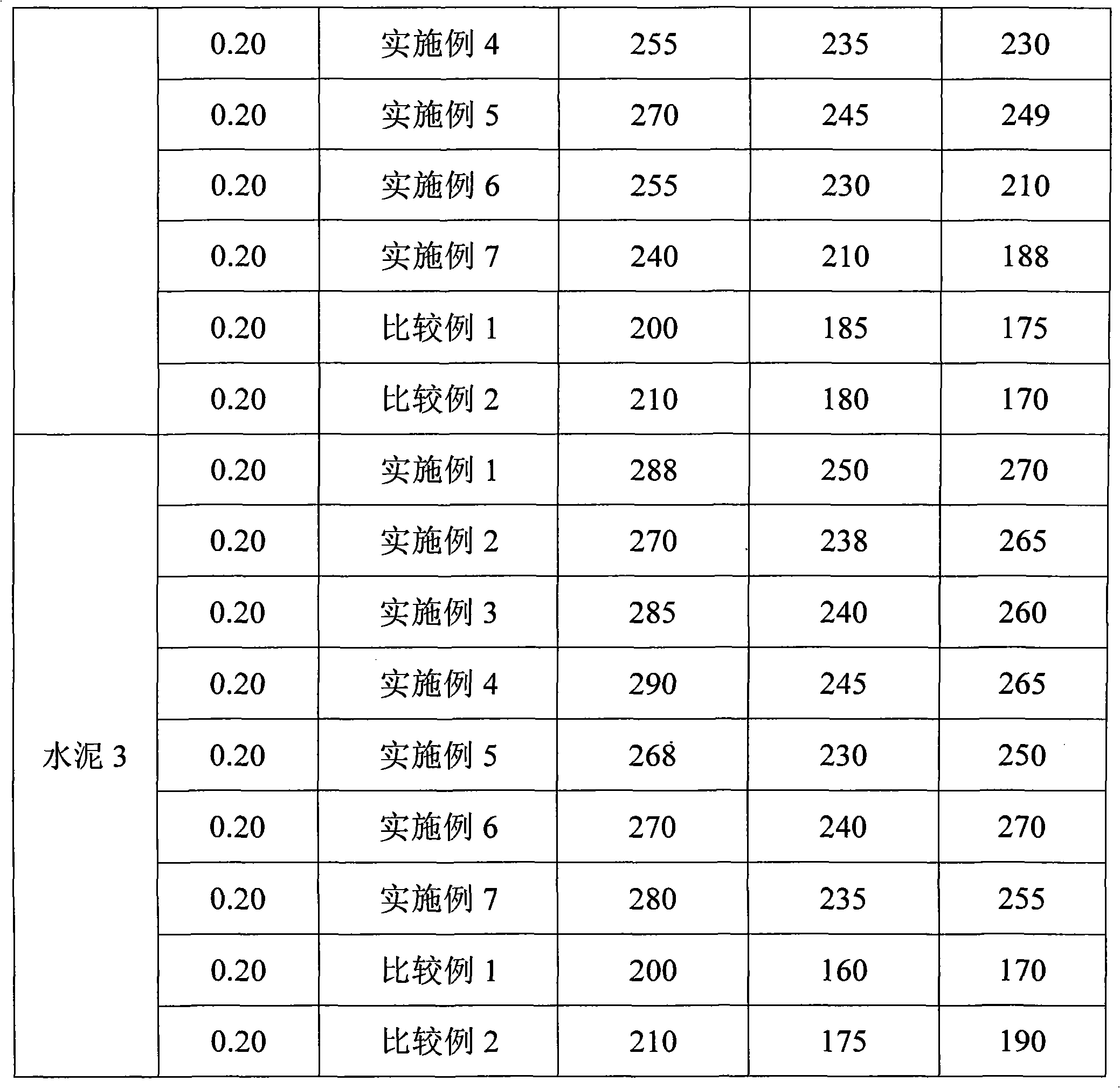
Normal-temperature synthesis method for polycarboxylic acid water-reducing agent
The invention discloses a normal-temperature synthesis method for a polycarboxylic acid water-reducing agent, and belongs to the field of cement concrete water-reducing agents. The water-reducing agent is prepared by the following steps of: copolymerizing polyoxyethylene ether monomer or polyoxyethylene ester monomer a containing unsaturated double bonds, unsaturated monocarboxylic acid and derivative monomer thereof b, unsaturated dicarboxylic acid c and unsaturated sulfonic acid or salt monomer thereof d in aqueous solution under the action of an oxidation reduction initiator, and finally neutralizing the solution by using alkali solution to obtain the water-reducing agent. The reaction can be performed at room temperature by adopting an oxidation reduction initiating system, and the appropriate reaction temperature is between 5 and 30 DEG C; and the synthesis process does not need heating, so energy is saved, and high-temperature side reaction is effectively controlled. The prepared polycarboxylic acid high-performance water-reducing agent has the characteristics of high water-reducing rate and good collapse protecting performance. The water-reducing agent has wide application range, and the method has low production process requirement and is suitable for industrialized large-scale production.
Owner:BEIJING UNIV OF TECH
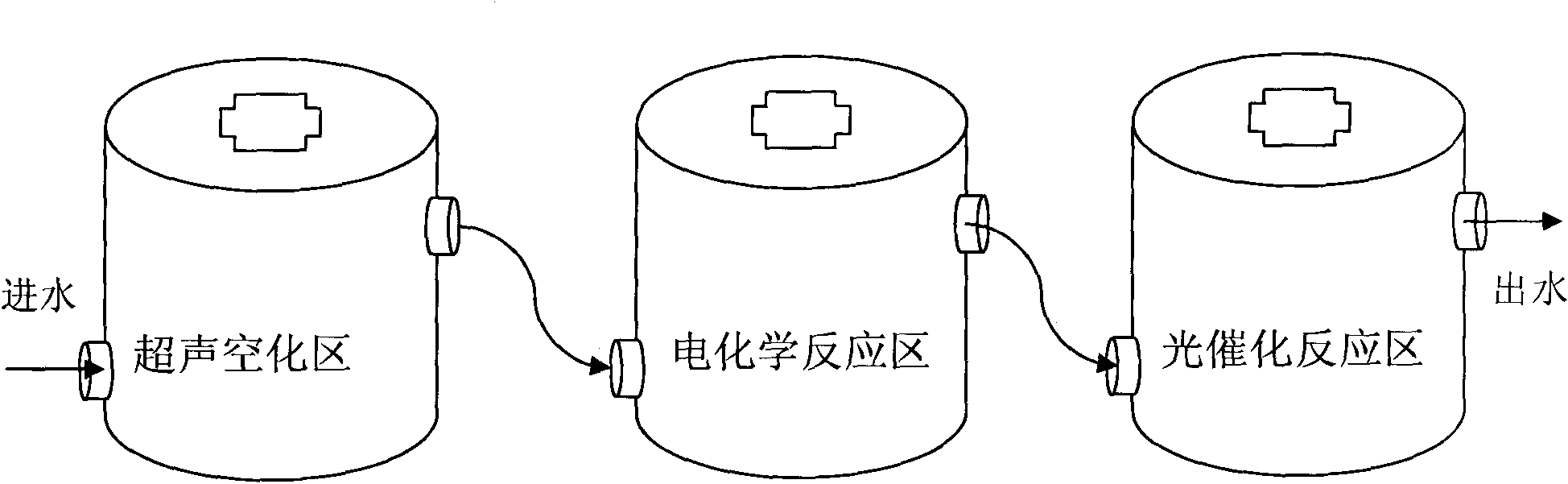
Process method for treating hardly-biodegradable organic wastewater
ActiveCN101786756AReduce dosageReduce generationWater/sewage treatment by irradiationWater/sewage treatment with mechanical oscillationsElectrochemical responseUltrasonic cavitation
The invention relates to a process method for treating hardly-biodegradable organic wastewater, and the method comprises the following steps: mainly utilizing the combination of ultraviolet light, electrochemistry, ultrasonic waves and oxidation-reduction chemical reaction for treating the hardly-biodegradable organic wastewater, and treating the wastewater by three reaction units of an ultrasonic cavitation zone, an electrochemical reaction zone and an ultraviolet light catalytic reaction zone, thereby realizing the high-efficient multi-stage deep wastewater oxidation reaction which combines the ultrasonic wastewater treatment method, the ultrasonic light and the collaborative Fenton reagent oxidation wastewater treatment, as well as the electrochemistry and the collaborative Fenton reagent oxidation wastewater treatment, effectively treating a variety of types of hardly-biodegradable organic wastewater and leading the COD removal rate of the wastewater after the treatment to be more than 90%.
Owner:GUANGXI BOSSCO ENVIRONMENTAL PROTECTION TECH
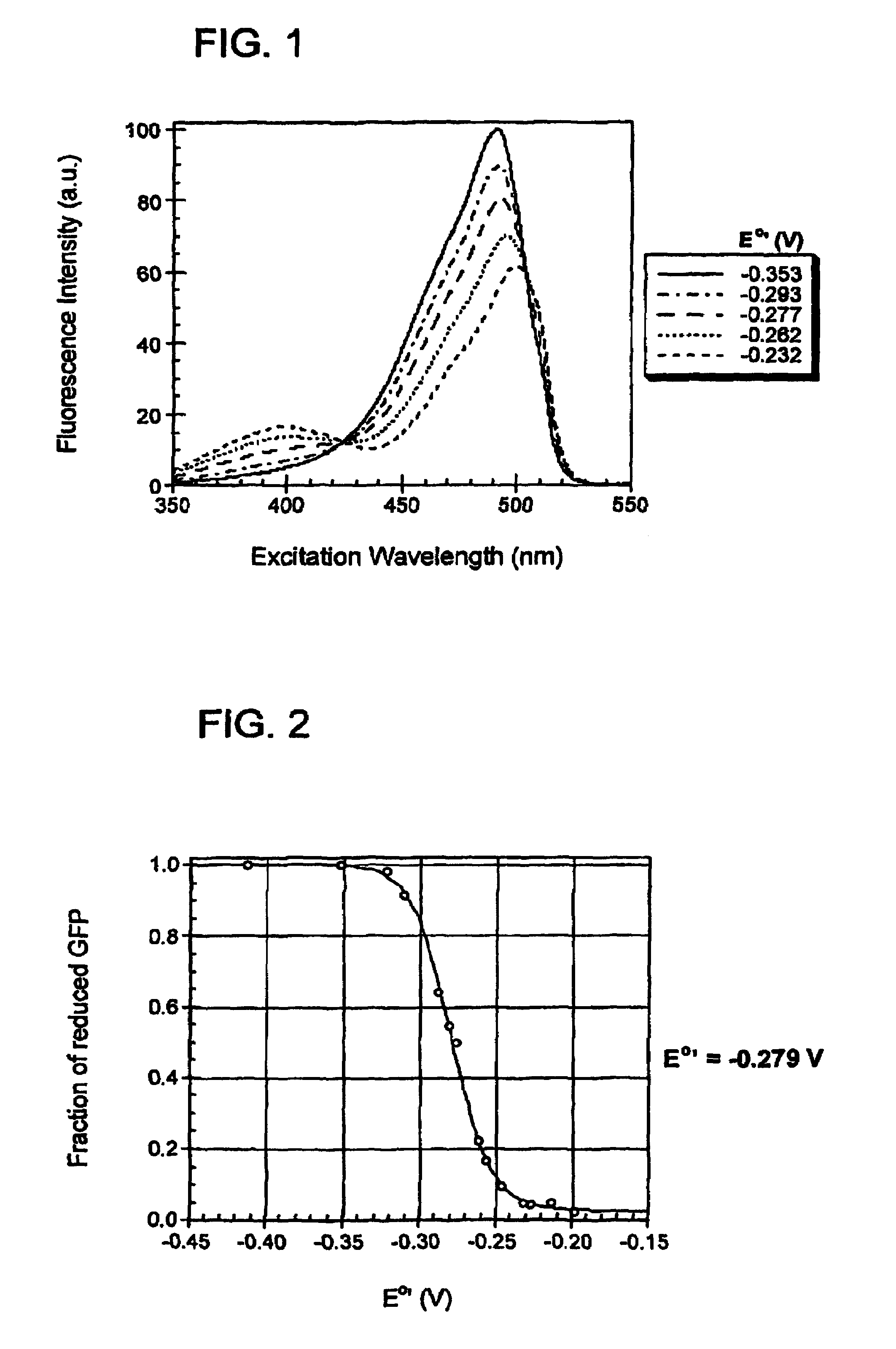
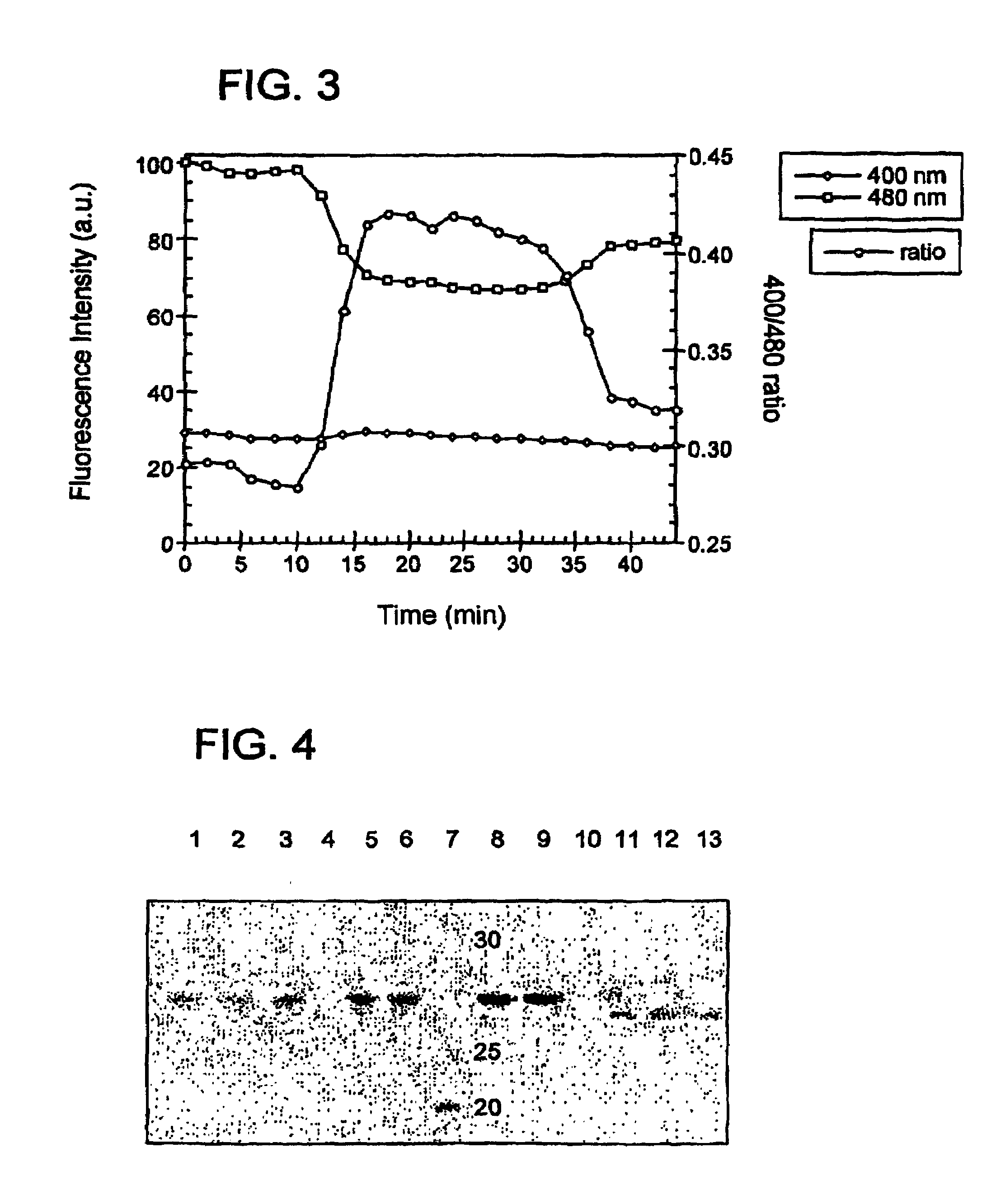
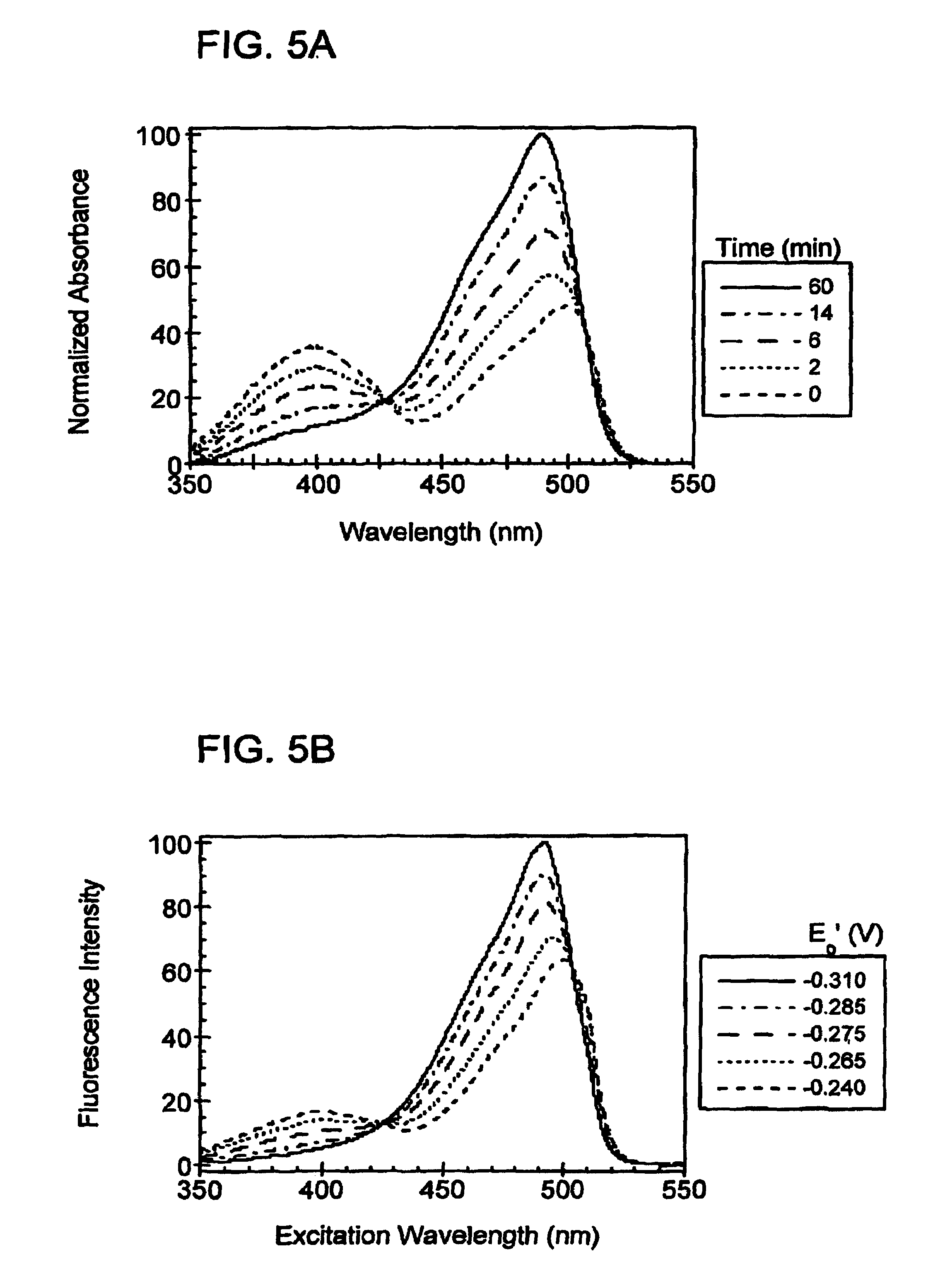
Oxidation reduction sensitive green fluorescent protein variants
The disclosure provides proteins that can be used to determine the redox status of an environment (such as the environment within a cell or subcellular compartment). These proteins are green fluorescent protein (GFP) variants (also referred to as redox sensitive GFP (rosGFP) mutants), which have been engineered to have two cysteine amino acids near the chromophore and within disulfide bonding distance of each other. Also provided are nucleic acid molecules that encode rosGFPs, vectors containing such encoding molecules, and cells transformed therewith. The disclosure further provides methods of using the rosGFPs (and encoding molecules) to analyze the redox status of an environment, such as a cell, or a subcellular compartment within a cell. In certain embodiments, both redox status and pH are analyzed concurrently.
Owner:OREGON HEALTH & SCI UNIV
Method for grafting polymer on inorganic material surface
The present invention discloses a method of grafting a polymer on the surface of the inorganic materials. The polyethylene oxide or polyethylene glycol which contains amino or hydroxyl at the chain-end by a covalent bond or an ionic bond, or a compound containing glucose units is firstly fixed at the surface of the inorganic materials, so as to have a reductive organic chemical group on the surface; then a high cerium salt and a polymerizable monomer are added, the present invention makes use of the high cerium salt and the reductive organic group on the surface of the inorganic materials to constitute an oxidation-reduction initiation system, so as to initiate the monomer polymerization under the acidic condition, further to graft the polymer on the surface of the inorganic materials. The method of the present invention can graft the polymer on the surface of the inorganic materials easily, which has the advantages of simple reaction process, mild reaction condition and high grafting rate, so the present invention is particularly applicable for the grafting of a water-soluble polymer or a polymer hydrogel thin layer on the surface of the inorganic materials.
Owner:ZHEJIANG UNIV
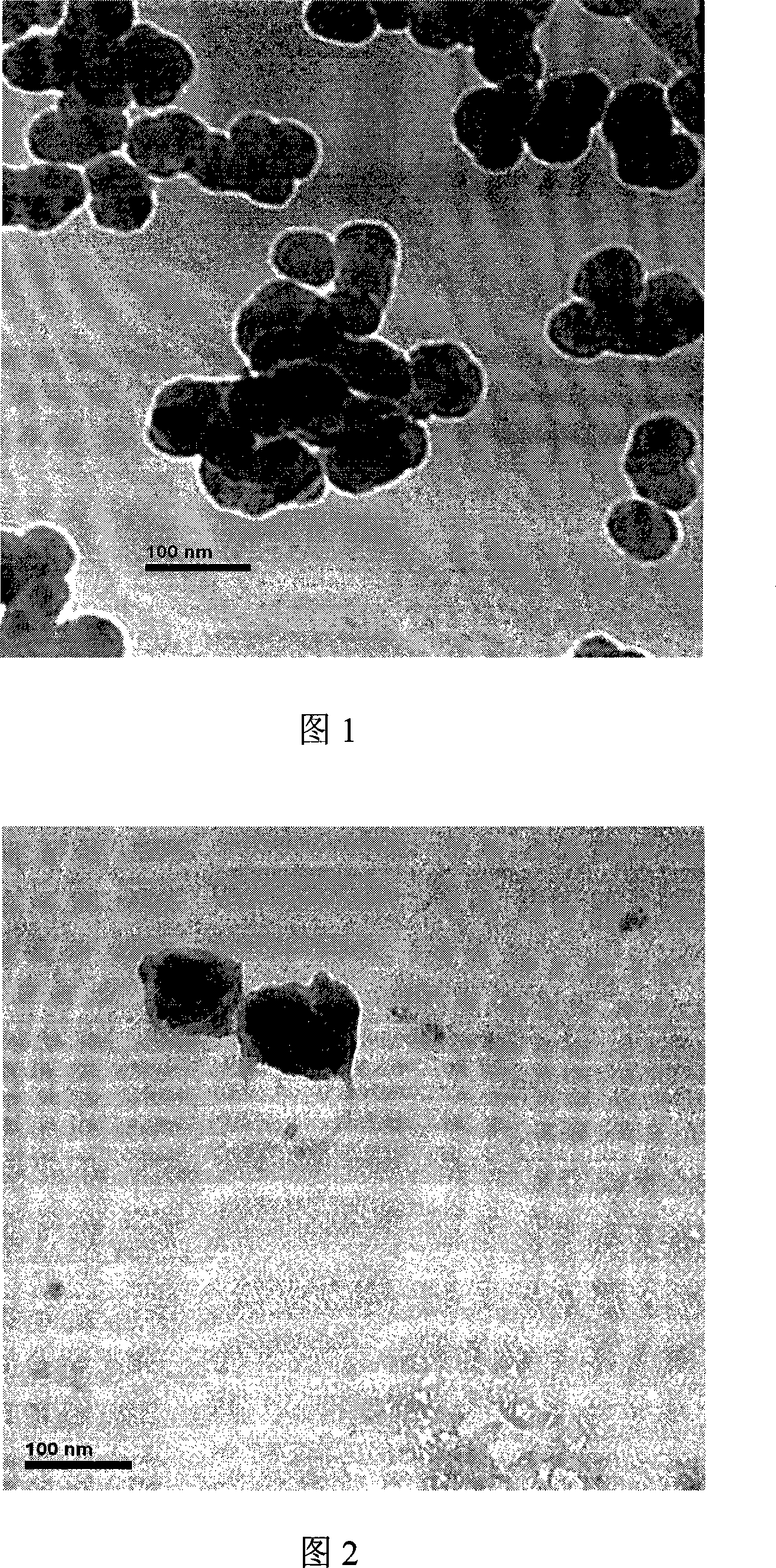
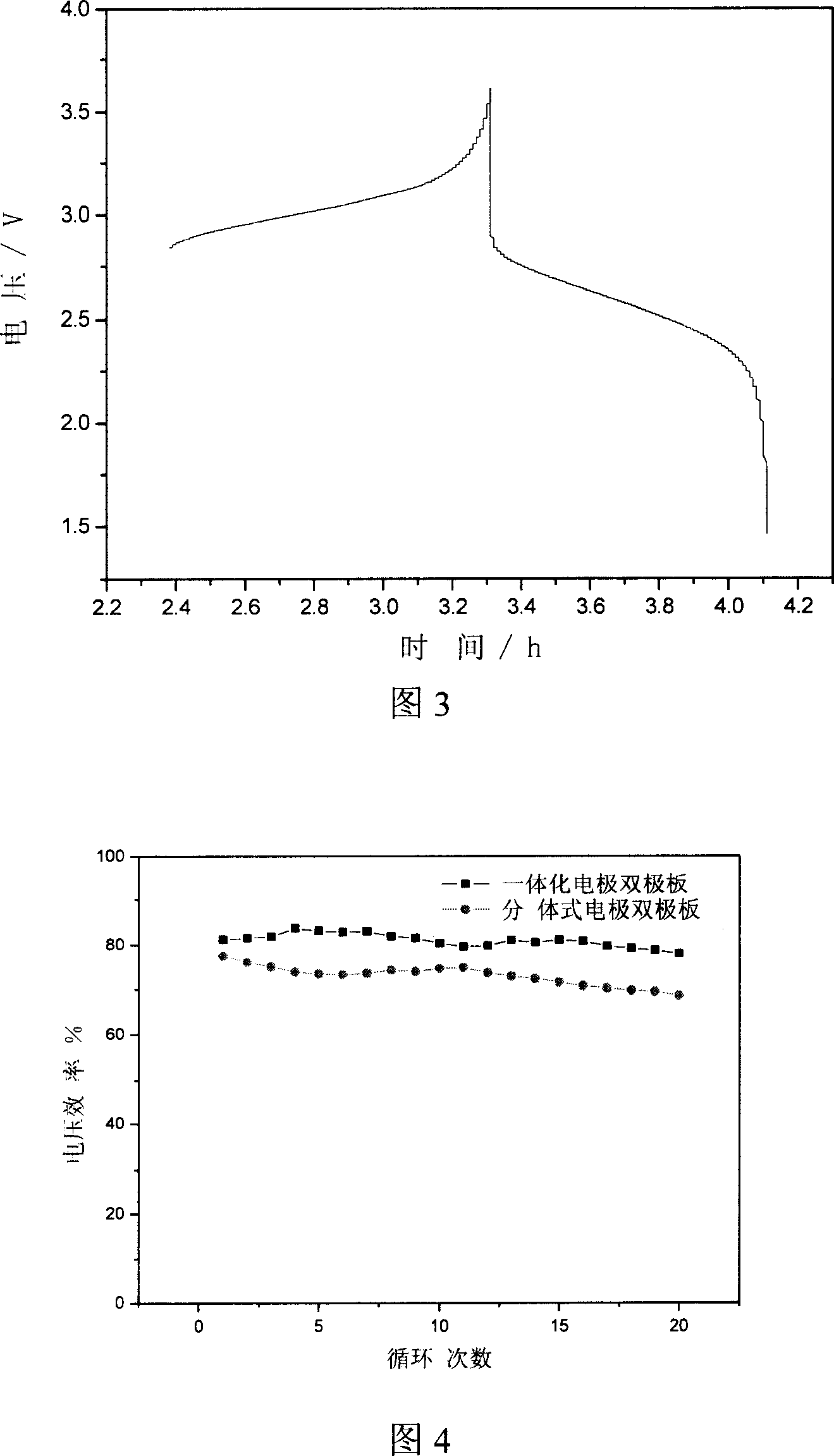
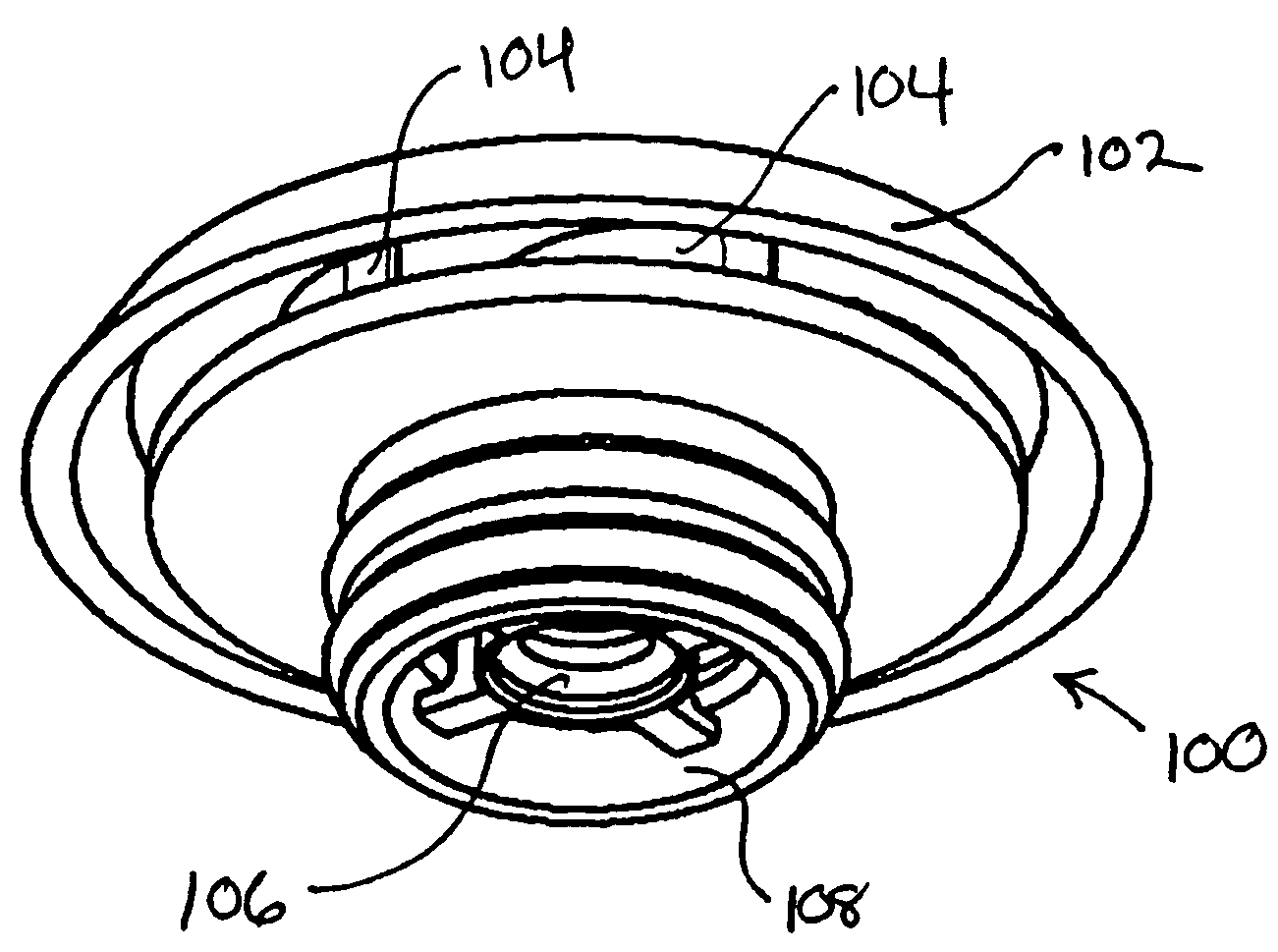
Integrated pole dual-pole board for oxidation deoxidization liquid energy-storing battery and its preparation
InactiveCN101009376AReduce contact resistanceImprove efficiencyElectrode manufacturing processesElectrode carriers/collectorsElectrical resistance and conductanceStored energy
The invention relates to the oxidation-reduction liquid stored-energy cell, especially integral electrode dipole plate and preparation, the integral electrode dipole plate includes: multiple-arch electrode and dipole plate, viscous conducting layer which is between the dipole plate and multiple-arch electrode, that is the multiple-arch electrode and dipole plate are felt to the dipole plate via the viscous conducting material. The advantages of the invention are: the integral electrode dipole plate can decrease the contact resistance between the multiple-arch electrode and dipole plate evidently, increase the voltage efficiency and energy efficiency of the oxidation-reduction liquid stored-energy cell, at the same time the distribution of the electrolyte in electrode is more even; the conductivity of the integral electrode dipole plate is perfect, the electrochemistry property is high, the chemical property is stable, it possesses high practicality value and wide application foreground.
Owner:DALIAN RONGKE POWER
Preparation method of noble metal modified titanium dioxide photocatalyst
InactiveCN101362087AMetal/metal-oxides/metal-hydroxide catalystsPhotocatalytic reactionPhotocatalytic degradation
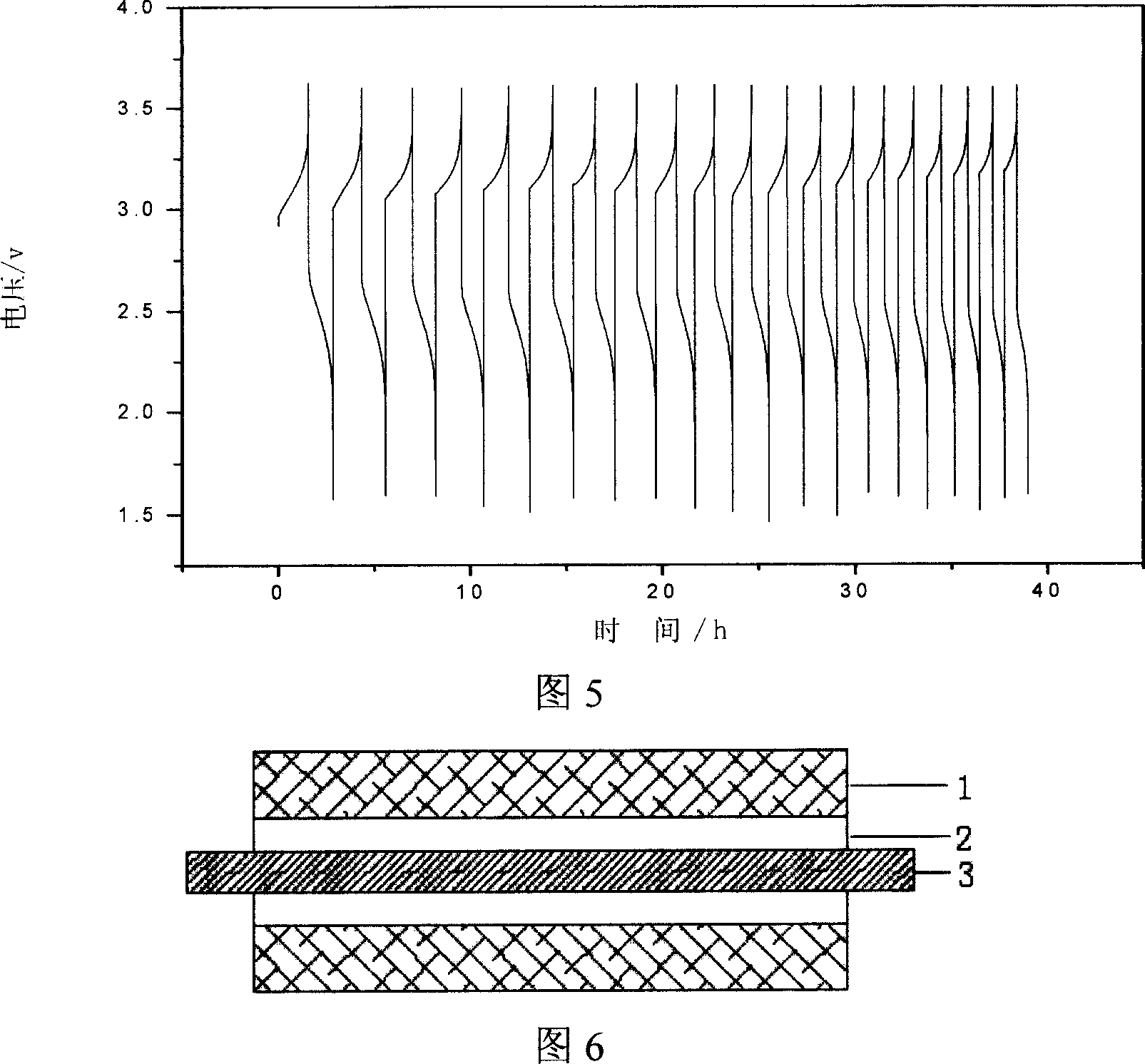
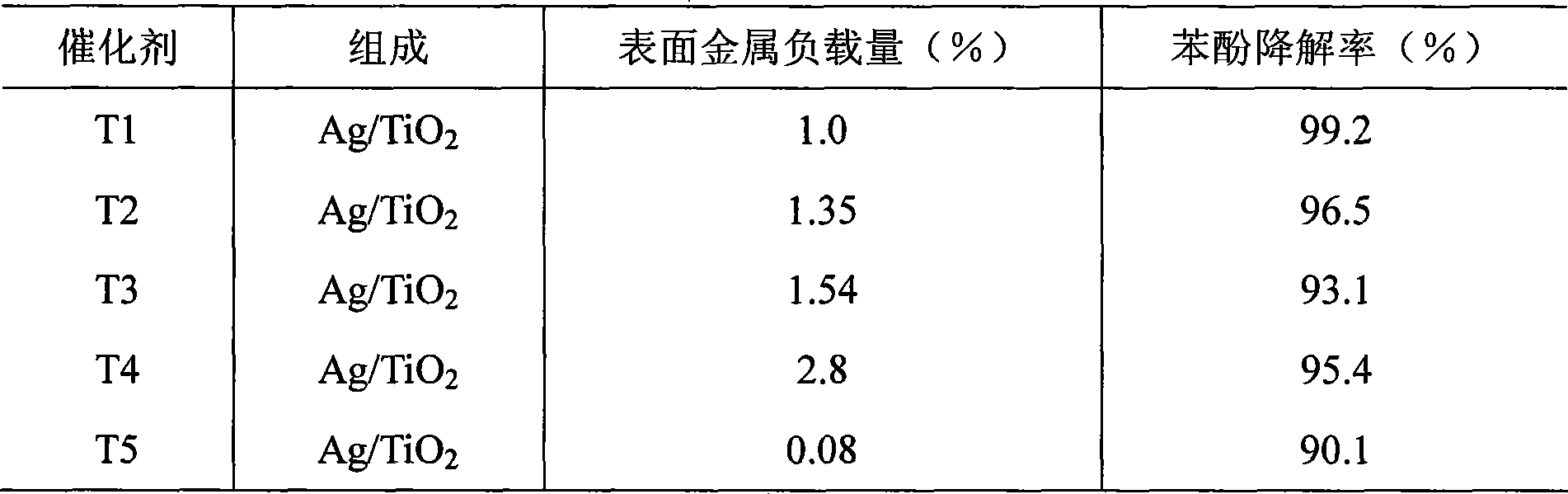
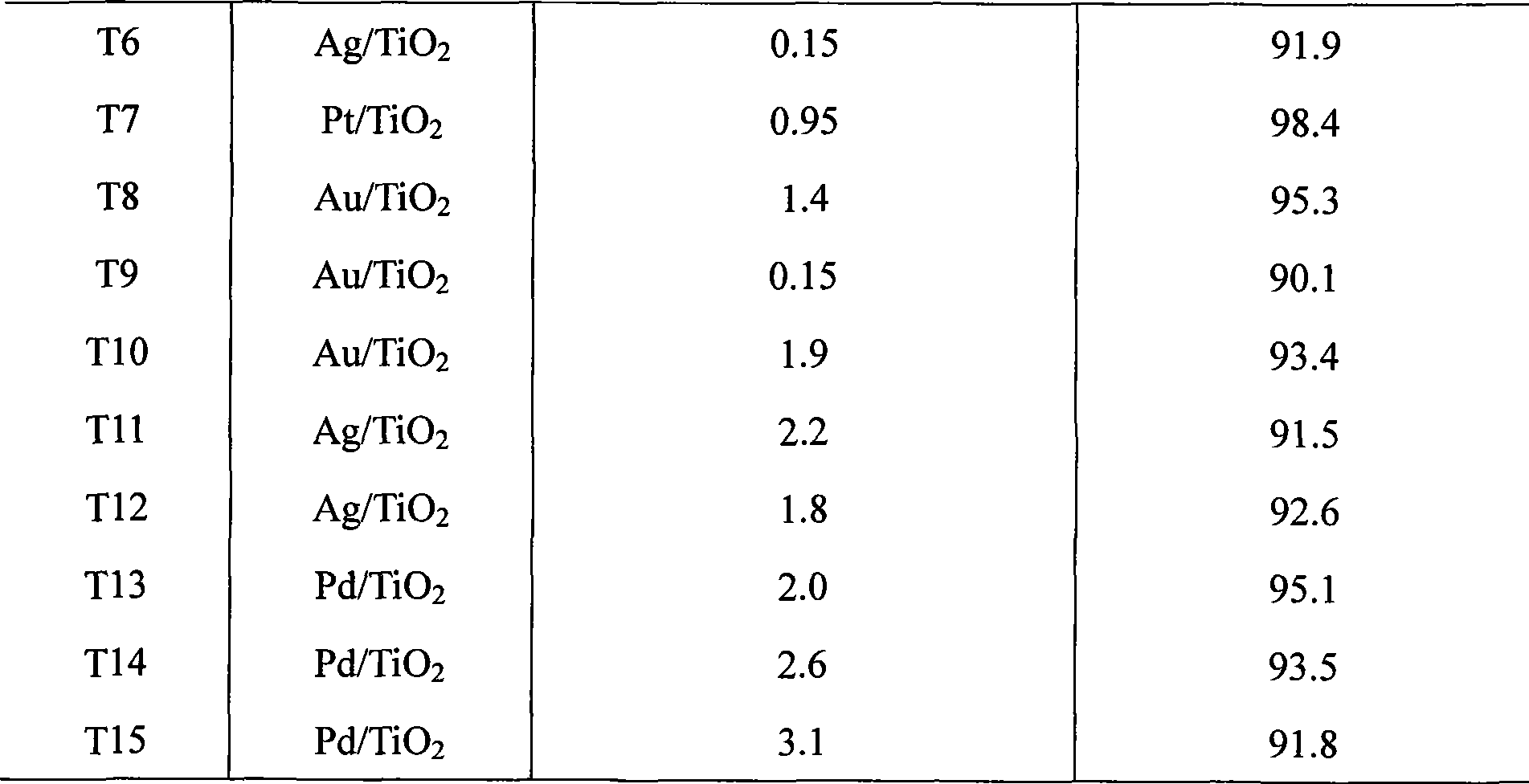
The invention relates to a preparation method of a noble metal modified titanium dioxide photocatalyst, which refers to a photo-catalytic oxidation-reduction coupling method realized in a photo-catalytic reactor: firstly, TiO2, noble-metal water soluble salt and organism are used for preparing a suspension which is then ultrasonically vibrated after the pH value is regulated to 1-12, put into a photo-catalytic reactor for reaction for 0.5h to 8h at 0 DEG C to 90 DEG C under the irradiation of an ultraviolet source with the power of 8W to 125W or a medium or high-voltage mercury lamp source with the power of 100W to 600W, and then separated, washed and dried to obtain the TiO2 photocatalyst modified by noble metal Ag, or Au, or Pt or Pd, wherein, the content of TiO2 is 90 percent to 99.99 percent, the content of the noble metal is 0.01 percent to 10 percent. The noble metal modified titanium dioxide photocatalyst has high photocatalytic degradation activity and strong surface hydrophobicity and the attached catabolites are easy to clean.
Owner:HEFEI UNIV OF TECH
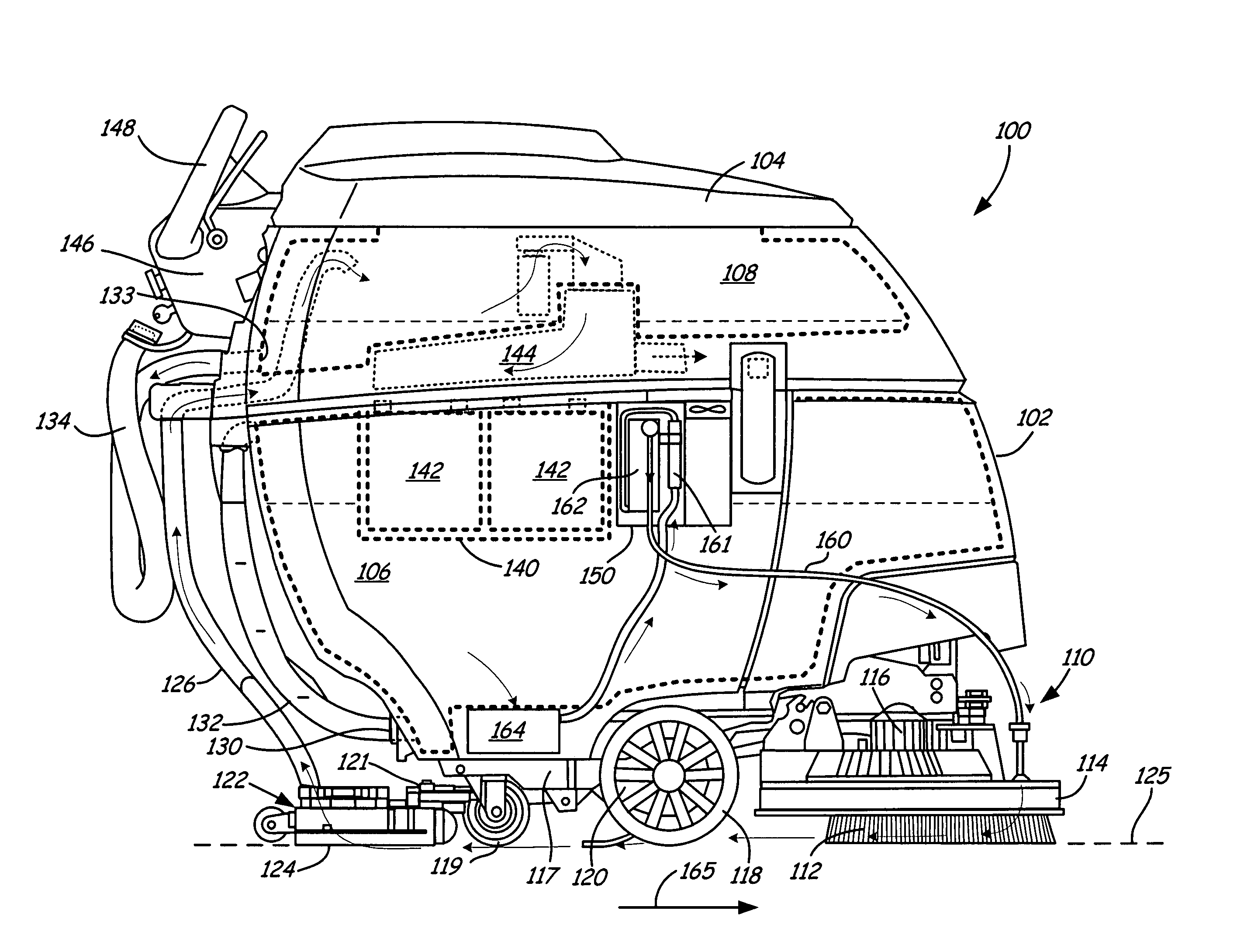
Sanitization system and system components
InactiveUS7767168B2Easy to adaptUsing liquid separation agentMixing methodsOzone generatorVapor–liquid separator
A multi-use sanitization system is disclosed which includes one or more containers in fluid communication with other system components. Components of the system include an ozone contacting device, such as a vortex-venturi or a sparger, for incorporating ozone into a liquid, an ozone generator to provide ozone to the vortex-venturi, a fluid transfer valve to allow simultaneous flow of liquid into and out of the container, and a pump to promote fluid flow through the system. Optionally, a gas-liquid separator with an optional integral gas release valve, an ozone destructor, an oxidation-reduction potential ozone sensor, or a pour-through type pre-filter may be incorporated into the system.
Owner:TERSANO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com