Preparation method of triazine derivative
A technology for triazine derivatives and compounds, applied in the field of preparation of triazine derivatives, can solve problems such as low yield and long route, and achieve the effects of high product quality, shortened process flow and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
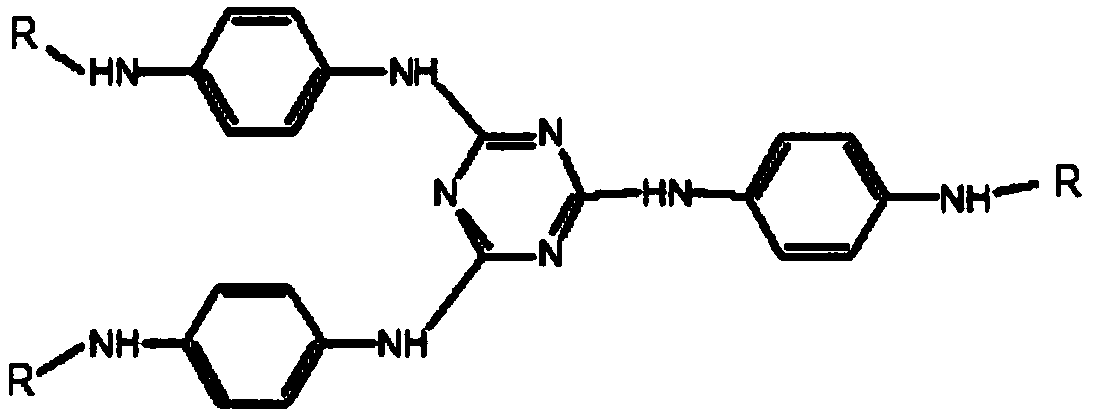
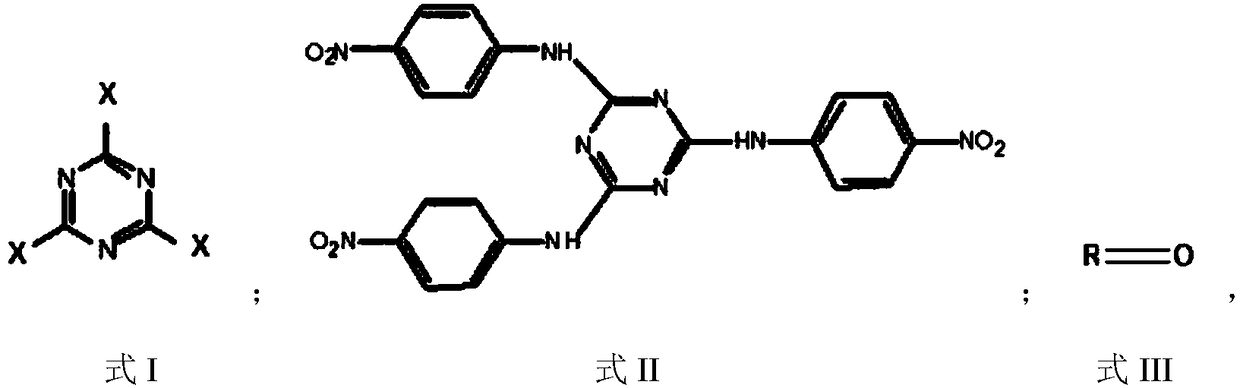
[0026] The preparation method comprises the following steps: performing substitution reaction on p-nitroaniline and compound A to form intermediate B; performing hydrogenation reduction alkylation reaction on intermediate B and compound C to obtain triazine derivatives; wherein, compound A has The structure shown in formula I, intermediate B has the structure shown in formula II, compound C has the structure shown in formula III, and formula I, formula II and formula III are as follows:
[0027]
[0028] Among them, the R base is C 3 ~C 10 An alkyl group; X is a halogen atom.
[0029] The above-mentioned preparation method provided by the present invention utilizes p-nitroaniline to perform a substitution reaction with compound A, and can replace X in compound A with the amino group in p-nitroaniline to form a trisubstituted triazine, namely intermediate B. Then use the ketone compound of compound C to carry out hydrogenation reduction alkylation reaction with the interme...
Embodiment 1
[0044] (a) Substitution reaction
[0045] Add 0.3mol p-nitroaniline and 225mL 1,4-dioxane into a 1000mL four-neck flask, cool down to 0-5°C, and slowly add cyanuric chloride solution (0.06mol cyanuric chloride dissolved in 180mL 1 , 4-dioxane), after the dropwise addition, the temperature was maintained to continue the reaction for 1h, and then heated to reflux for 1h, and then sodium bicarbonate was added for 2h. After the reaction, the feed solution was washed and filtered to obtain a yellow intermediate 2,4,6-tris-(4-nitroaniline)-1,3,5 triazine (intermediate B). The reaction conversion rate is 99.5%, the reaction selectivity is 98%, and the product purity is 98.5%.
[0046] (b) Hydrogenation reduction alkylation reaction
[0047] Intermediate 29g (0.06mol) 2,4,6-tri-(4-nitroaniline)-1,3,5-triazine, 405g (3.55mol) 5-methyl-2-hexanone, and 3 %Pt / C catalyst 3g (relative to the weight of p-nitroaniline) once drops in the 1L autoclave, respectively with N 2 、H 2 After thre...
Embodiment 2
[0052] (a) Substitution reaction
[0053] Add 0.12mol p-nitroaniline and 200mL N,N-dimethylformamide into a 1000mL four-necked flask, cool down to 0-5°C, and slowly add cyanuric chloride solution (0.04mol cyanuric chloride dissolved in 100mL N,N-dimethylformamide), after the dropwise addition was completed, the temperature was maintained to continue the reaction for 1h. Then heat and reflux for 1 hour, then add sodium bicarbonate to react for 2 hours, the reaction is completed, the material liquid is washed and filtered to obtain the yellow intermediate 2,4,6-tris-(4-nitroaniline)-1,3,5 triazine . The reaction conversion rate is 99%, the reaction selectivity is 98.6%, and the product purity is 97.5%.
[0054] (b) Hydrogenation reduction alkylation reaction
[0055] The intermediate 19.6g (0.04mol) 2,4,6-tri-(4-nitroaniline)-1,3,5 triazine, 270g (2.36mol) 5-methyl-2-hexanone and 3% Pd / C catalyst 6g (relative to the weight of p-nitroaniline) once drops in the 1L autoclave, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com