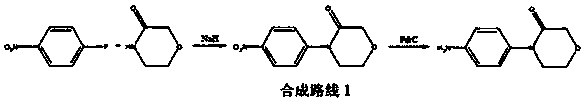
Method for synthesizing rivaroxaban intermediate 4-(4-aminophenyl)-3-molindone
A technology of aminophenyl and rivaroxaban, which is applied in the field of medicine, can solve problems such as low product yield, serious environmental pollution, and harsh reaction conditions, and achieve simple synthesis operation, reduced environmental pollution, and high total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1: Preparation of p-nitro-N-hydroxyethylaniline (II)
[0020] Put 3L of ethanol, 2.4L of purified water, 1kg of p-nitroaniline into the 10L reaction flask, be warmed up to 45-50 ℃, slowly dropwise add 690g of the ethanolic solution of 70% ethylene oxide, and finish adding dropwise in 2-3 hours. After the addition is completed, the reaction is kept at 45-50 °C for 15 hours, cooled to below 20 °C, 1.5 L of water is added, stirred for crystallization and filtration, the obtained product is added with 2 L of ethanol and 1.5 L of petroleum ether, heated and refluxed to dissolve, slowly cooled and filtered to obtain Compound (II) 1.055kg, yield 81%.
example 2
[0021] Example 2: Preparation of 4-(4-nitrophenyl)-3-morpholinone (III)
[0022] Put 100ml of isopropanol and 200ml of purified water into a 1000ml reaction flask, add 100g of p-nitro-N-hydroxyethylaniline (II), heat up to 50-55°C, and slowly add 277g of bromoacetyl bromide and 45% hydrogen Sodium oxide solution 250g, dripping for 1-1.5 hours, and maintaining the pH value in the range of 11-13 during the dripping process. After the dripping is completed, the reaction is incubated at 50-55 °C for 1.5 hours, cooled to below 20 °C, filtered, and the filter cake Washed with isopropanol and water respectively, and dried to obtain 109 g of compound III with a yield of 89.3%.
example 3
[0023] Example 3: Preparation of 4-(4-Aminophenyl)-3-morpholinone (Compound IV)
[0024] Put 600ml of water, 38.5g of reduced iron powder, and 15.6g of ammonium chloride in a 1000ml reaction flask, heat up to 70-75°C, slowly add 60g of compound III in batches, keep the system temperature not higher than 80°C, and finish the dose in 2-3 hours After casting, 75-80 ℃ insulation reaction for 3 hours, filter while hot, the filter cake is washed with sufficient hot water, the filtrate is cooled, 180 g of ammonium sulfate is added, extracted three times with 600 g of chloroform, the chloroform layers are combined, and 5 g of anhydrous magnesium sulfate is added. Dry, filter, and concentrate the filtrate under reduced pressure to obtain a solid, which is recrystallized by adding 200 ml of isopropanol and 400 ml of water to obtain 42.5 g of off-white crystalline powder with a yield of 81.8%, a melting point of 171.5-173.2°C, and a purity of 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com