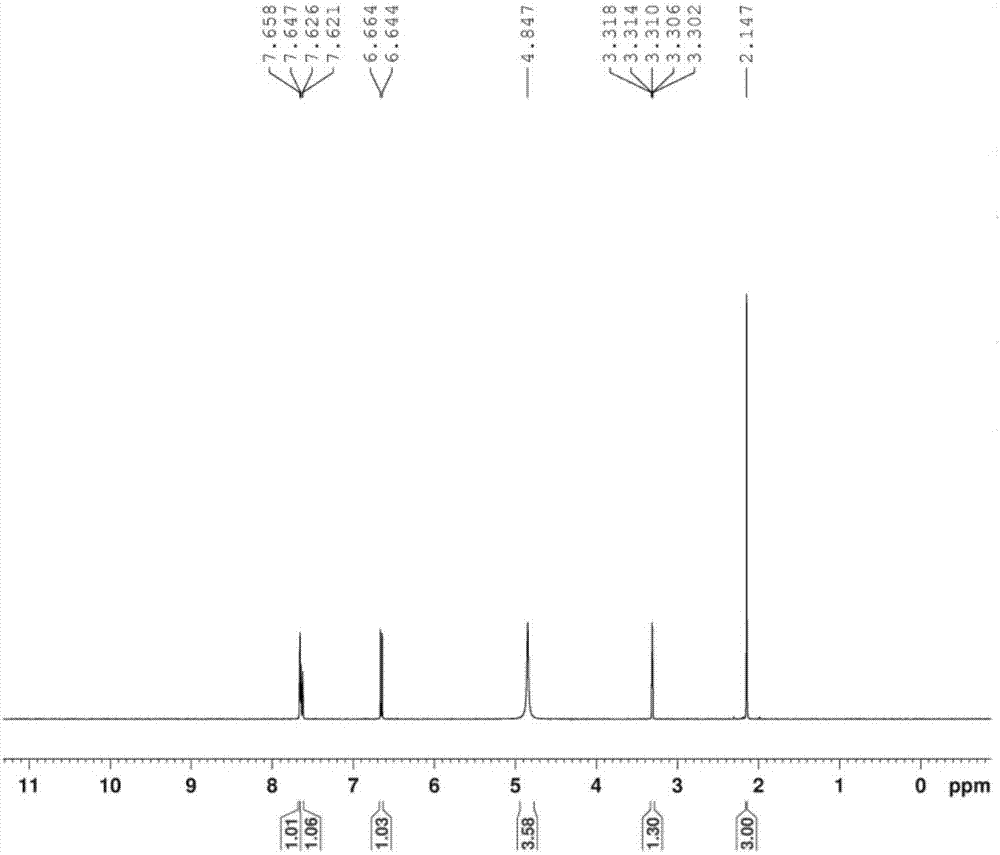
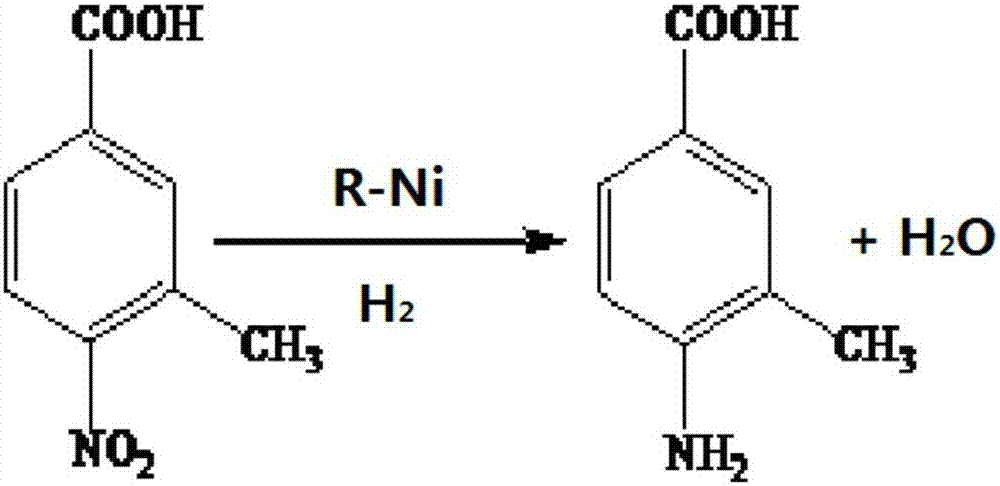
Method for preparing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation
An aminobenzoic acid, catalytic hydrogenation technology, applied in chemical instruments and methods, preparation of cyanide reaction, preparation of organic compounds, etc., can solve the problems of many by-products, complicated operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 1000ml beaker, add 36.2 grams (0.2mol) of 3-methyl-4-nitrobenzoic acid and 300ml of water, and adjust the pH with 25% sodium hydroxide under stirring until the reaction solution is alkaline;
[0029] Then the above solution is poured into a 1L autoclave, 3.0 g of Raney nickel catalyst is added, the autoclave is covered with a locking bolt, the stirring is started, nitrogen is charged into the autoclave to a pressure of 2.0 MPa in the autoclave, and the nitrogen inlet valve is closed. After 30 minutes, the pressure in the autoclave does not decrease, which proves that the autoclave is well sealed. Replace it with nitrogen and hydrogen three times respectively, fill it with ammonia for 2 minutes, and then pressurize it with hydrogen to 3.50MPa. Under the reaction for about 5h, until the hydrogen pressure no longer drops as the end point of the reaction, the reaction solution is obtained;
[0030] Cool to room temperature after reaction finishes, filter out catalyzer,...
Embodiment 2
[0034] In a 1000ml beaker, add 36.2 grams (0.2mol) of 3-methyl-4-nitrobenzoic acid and 300ml of water, and adjust the pH with 25% aqueous sodium carbonate solution under stirring until the reaction solution is alkaline;
[0035] Then the above solution is poured into a 1L autoclave, 2.0g of Raney nickel catalyst is added, the autoclave is covered with a locking bolt, the stirring is started, nitrogen is charged into the autoclave to a pressure of 2.0MPa in the autoclave, and the nitrogen inlet valve is closed. After 30 minutes, the pressure in the autoclave does not drop, which proves that the autoclave is well sealed. Replace it with nitrogen and hydrogen three times respectively, fill it with ammonia for 2 minutes, and then pressurize it with hydrogen to 4.50MPa. Under the reaction for about 1h, until the hydrogen pressure does not drop as the end point, the reaction solution is obtained;
[0036] Cool to room temperature after reaction finishes, filter out catalyzer, filtra...
Embodiment 3
[0039] In a 1000ml beaker, add 36.2 grams (0.2mol) of 3-methyl-4-nitrobenzoic acid and 300ml of water, and adjust the pH with 25% sodium hydroxide under stirring until the reaction solution is alkaline and clear;
[0040]Then the above solution is poured into a 1L autoclave, 1.5 g of Raney nickel catalyst is added, the autoclave is covered with a locking bolt, the stirring is started, nitrogen is charged into the autoclave to a pressure of 2.0 MPa in the autoclave, and the nitrogen inlet valve is closed. After 30 minutes, the pressure in the autoclave does not decrease, which proves that the autoclave is well sealed. Replace it with nitrogen and hydrogen three times respectively, fill it with ammonia for 2 minutes, and then pressurize it with hydrogen to 3.50MPa. The reaction was carried out for about 8 hours until the hydrogen pressure did not drop as the end point, and the reaction solution was obtained;
[0041] Cool to room temperature 5-30 ℃ after reaction finishes, filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com