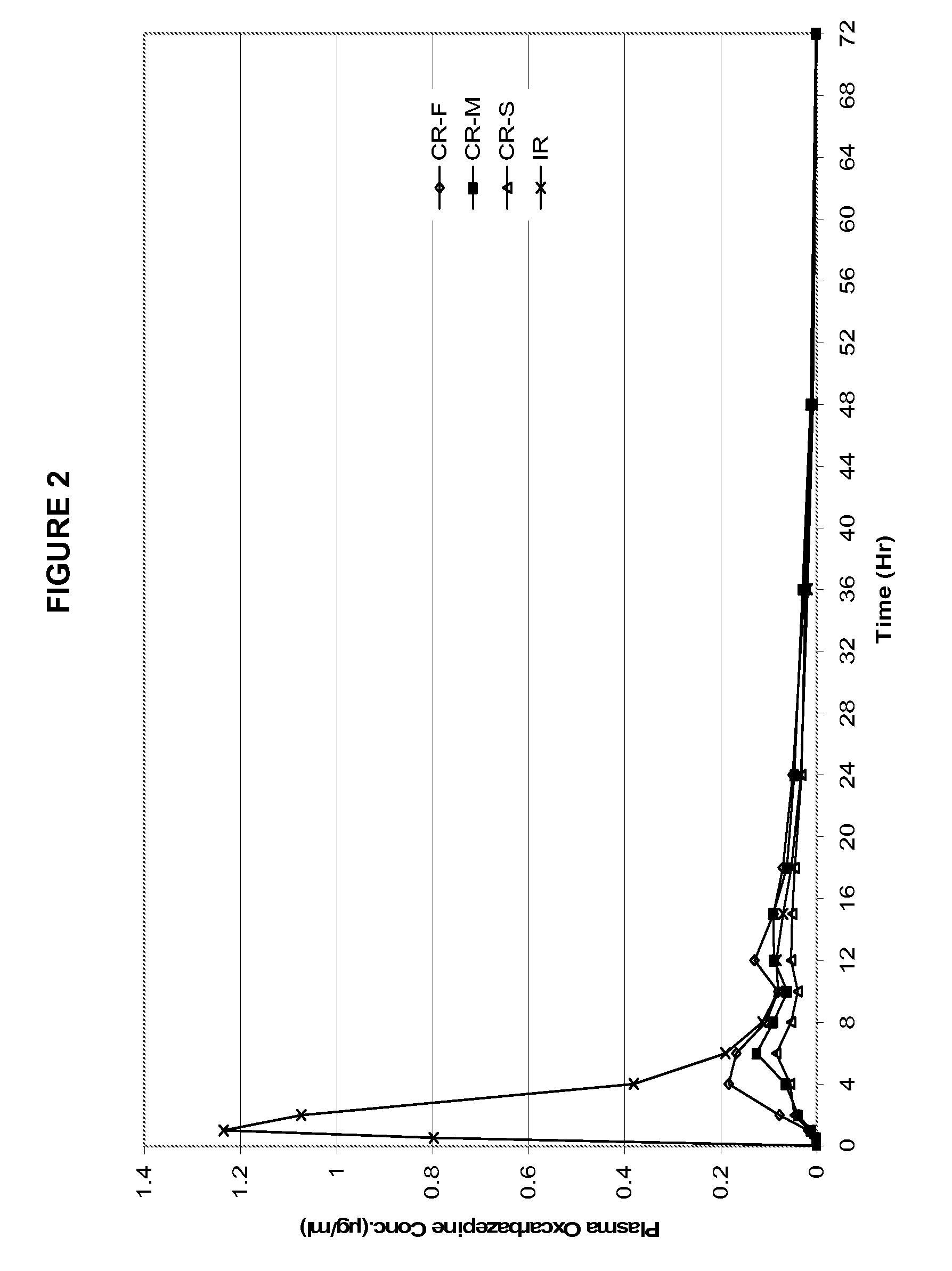
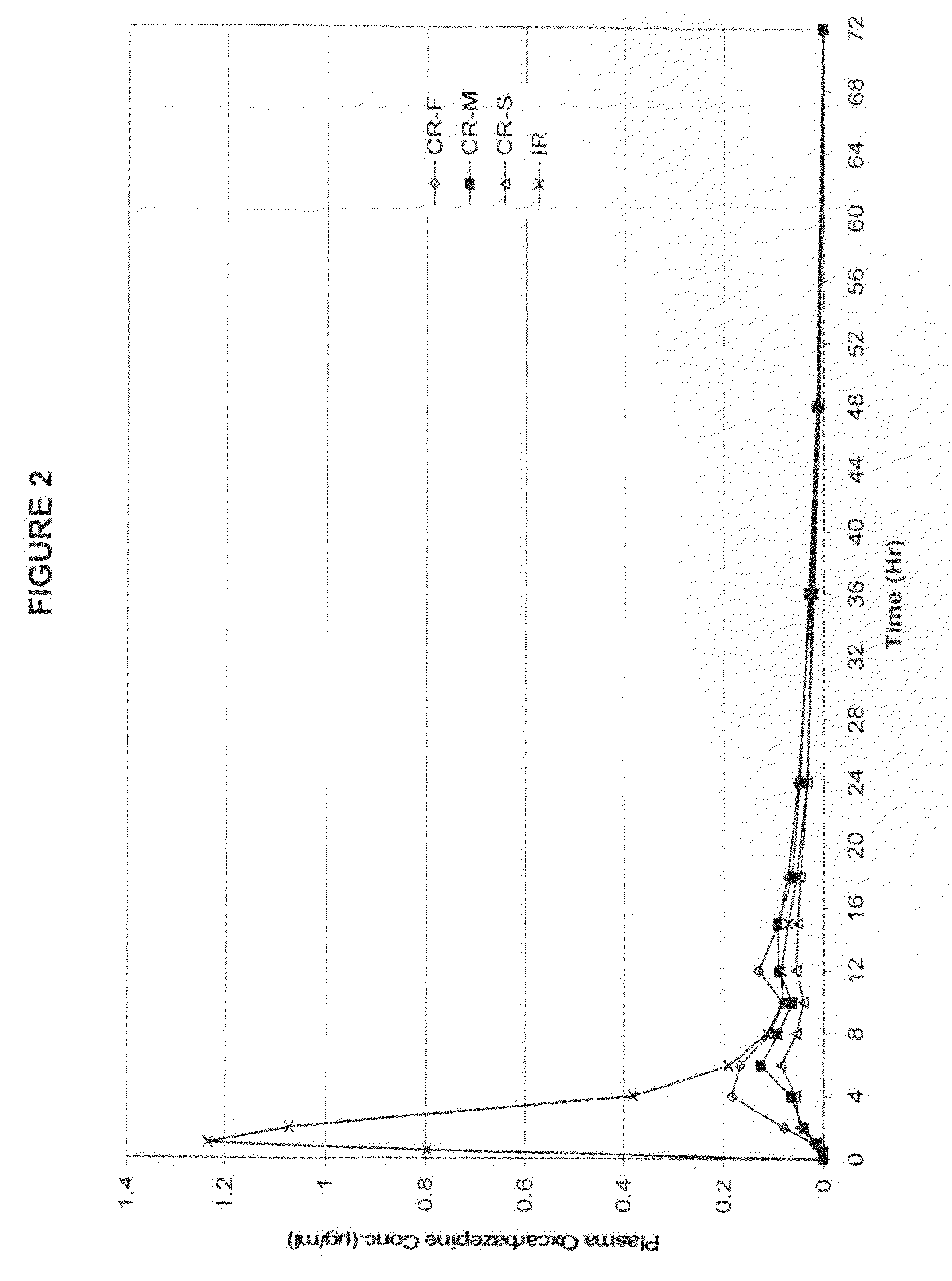
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Oxcarbazepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxcarbazepine is used alone or with other medications to treat seizure disorders (epilepsy)..
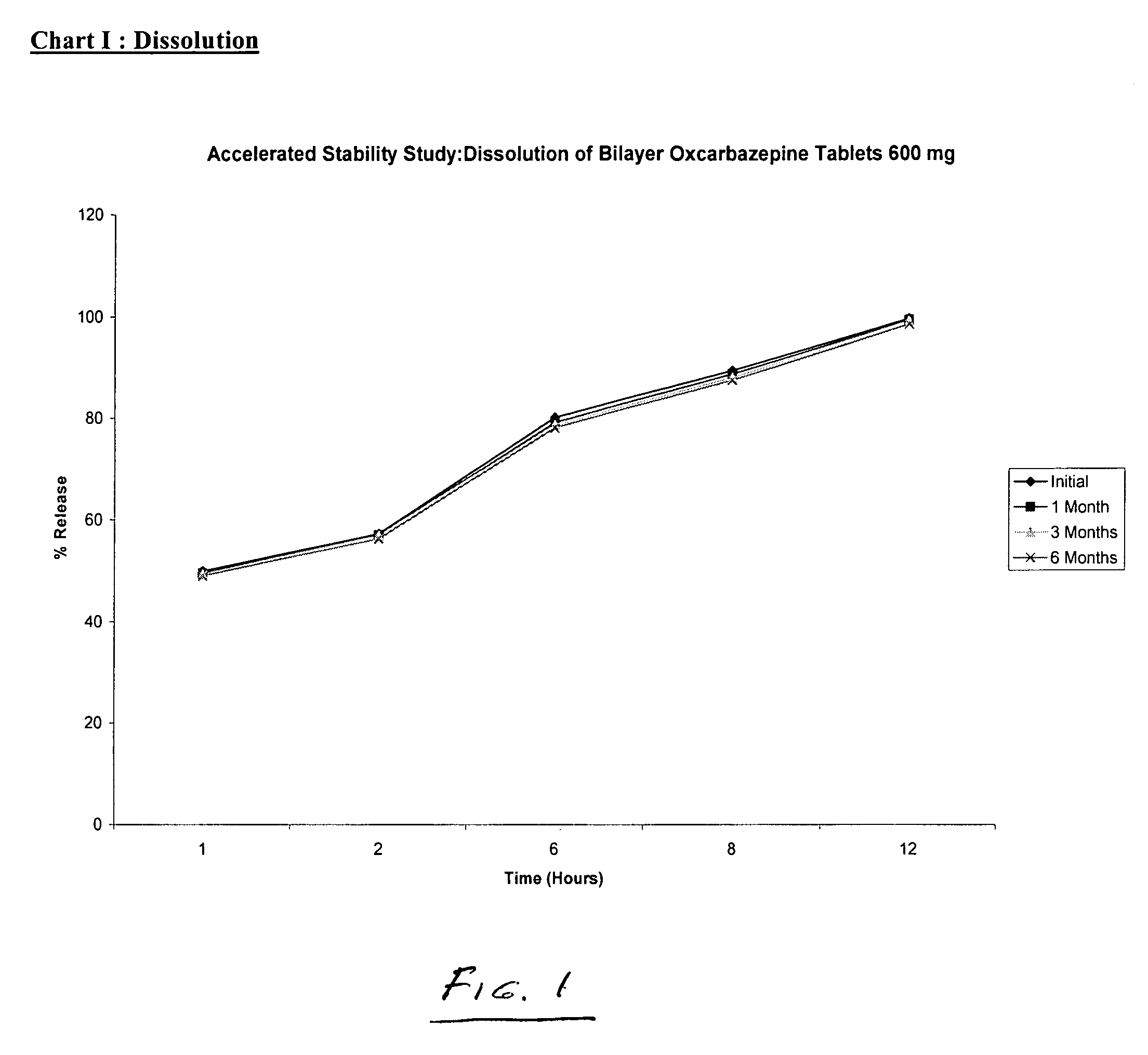
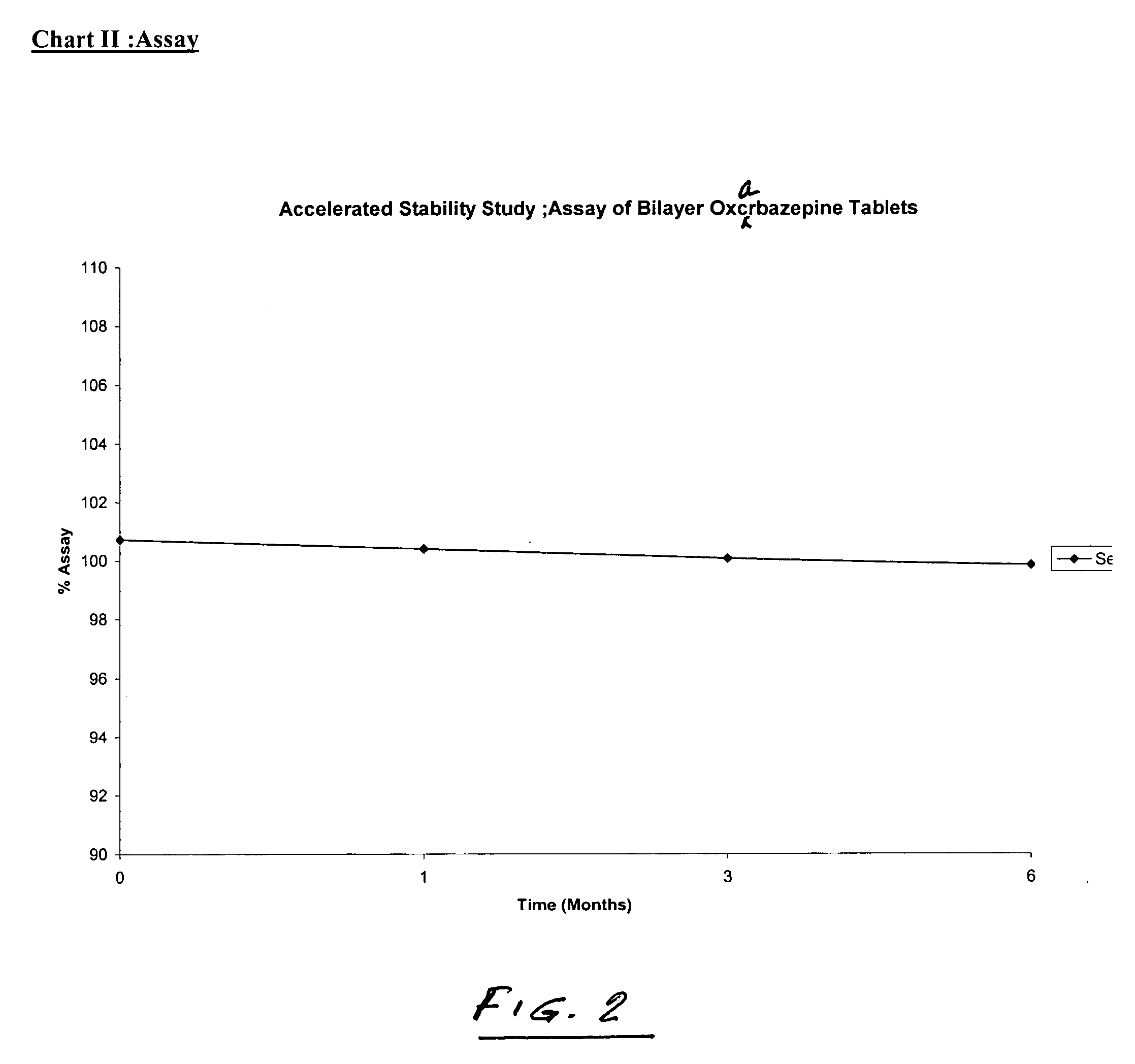
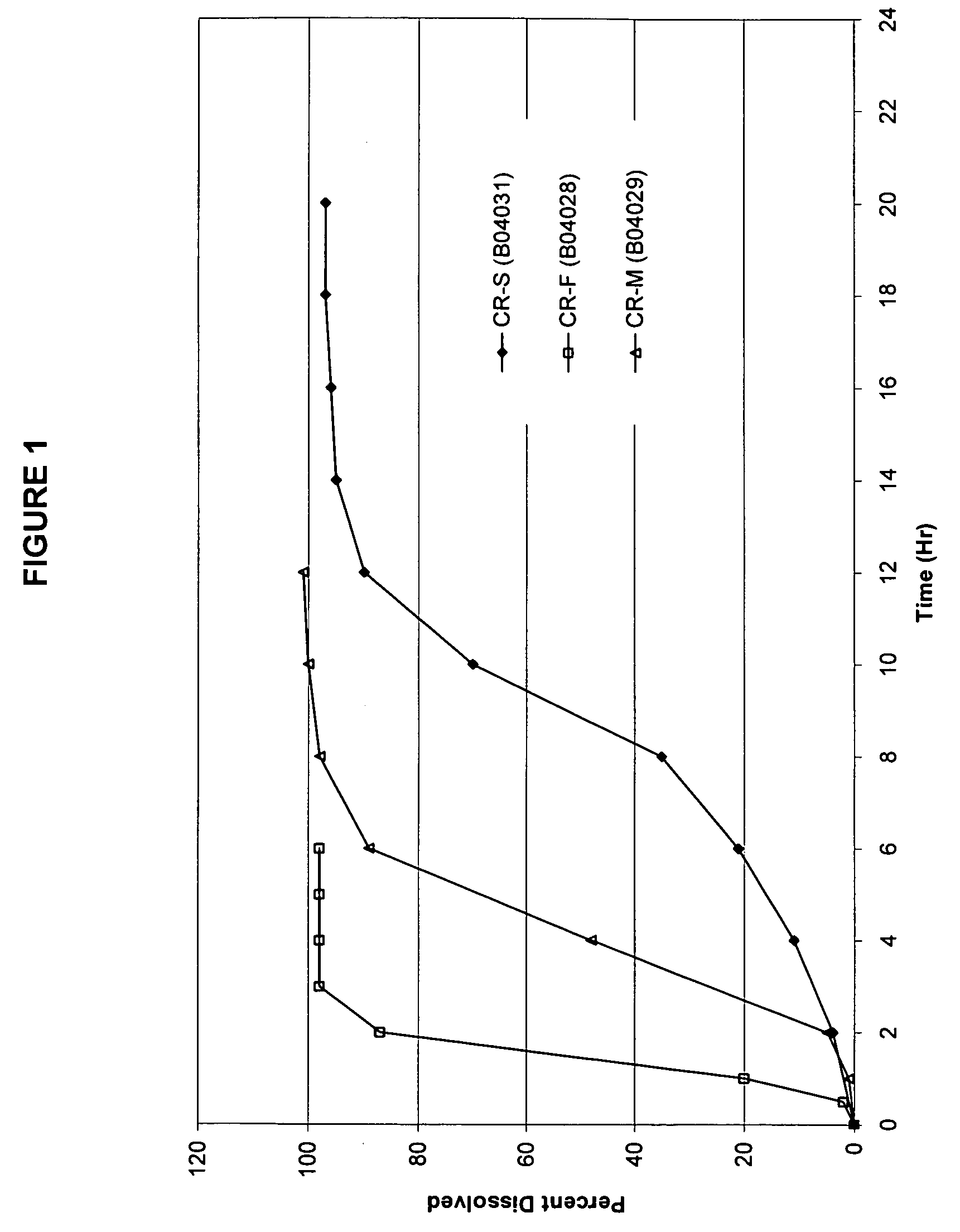
Bilayer tablets of oxcarbazepine for controlled delivery and a process of preparation thereof
InactiveUS20060141037A1Simple manufacturing processBiocidePill deliveryControlled releaseBlood concentration
Bilayer tablet comprising an immediate release first layer comprising an effective amount of oxcarbazepine and at least one pharmaceutically acceptable excipients and a controlled release second layer comprising an effective amount of oxcarbazepine and pharmaceutically acceptable excipients wherein the total amount of oxcarbazepine impurities is less than or equal to about 2% by weight. A process for preparation of controlled release bilayer tablets is capable of delivering oxcarbazepine from one layer immediately followed by a controlled delivery of oxcarbazepine from a matrix forming layer, and a process for preparation of oxcarbazepine bilayer tablets. Bilayer tablets of oxcarbazepine, which maintain a therapeutically effective blood concentration of oxcarbazepine with once a day administration.
Owner:J B CHEM & PHARMA
Modified-release preparations containing oxcarbazepine and derivatives thereof
ActiveUS20070254033A1Reduce volatilityBetter therapeutic profilePowder deliveryNervous disorderSolubilityOxcarbazepine
Controlled-release preparations of oxcarbazepine and derivatives thereof for once-a-day administration are disclosed. The inventive compositions comprise solubility-and / or release enhancing agents to provide tailored drug release profiles, preferably sigmoidal release profiles. Methods of treatment comprising the inventive compositions are also disclosed.
Owner:SUPERNUS PHARM INC
Rapidly dispersible dosage form of oxcarbazepine
ActiveUS9314429B2Easy to manageOvercome disadvantagesNervous disorderAdditive manufacturing apparatusDiseaseHigh doses
A high dose orodispersible dosage form of oxcarbazepine is provided. Drug-containing particles of oxcarbazepine are included within a porous bound matrix. The dosage form disperses in saliva or water in less than 15 sec and it has sufficient hardness to withstand handling and storage. It can be used to treat diseases or disorders that are therapeutically responsive to oxcarbazepine or a derivative thereof.
Owner:APRECIA PHARMA LLC
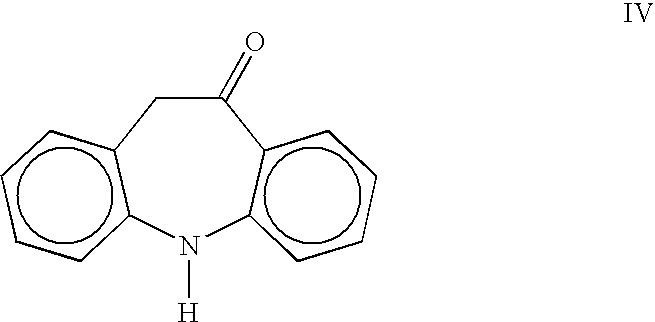
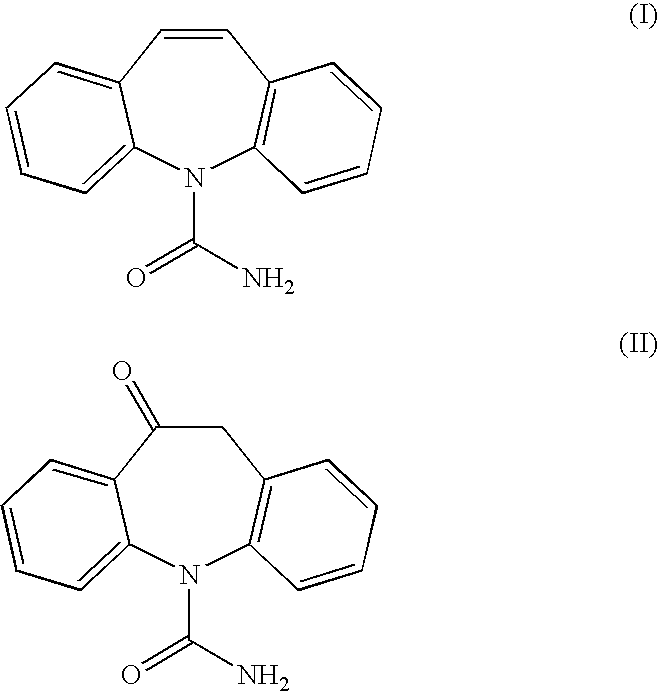
Process for the preparation of oxcarbazepine and related intermediates
InactiveUS20050282797A1High yieldSimple methodBiocideOrganic chemistryOrganic solventChlorosulfonyl isocyanate
A process for preparing Oxcarbazepine III comprising: a) reacting oximinostilbene IV with chlorosulfonyl isocyanate in an inert organic solvent and isolating compound V b) hydrolyzing compound V to form crude Oxcarbazepine III c) purifying oxcarbazepine.
Owner:APOTEX PHARMACHEN INC
Modified release preparations containing oxcarbazepine and derivatives thereof
ActiveUS20090004263A1Improve bioavailabilityMinimize fluctuationBiocidePowder deliverySolubilityDrug release
Controlled-release preparations of oxcarbazepine and derivatives thereof for once-a-day administration are disclosed. The inventive compositions comprise solubility- and / or release enhancing agents to provide tailored drug release profiles, preferably sigmoidal release profiles. Methods of treatment comprising the inventive compositions are also disclosed.
Owner:SUPERNUS PHARM INC
Controlled release formulation comprising Anti-epileptic drugs
InactiveUS20090196923A1Reduces fluctuation of blood levelControl releaseNervous disorderPill deliveryImmediate releaseAntiepileptic drug
The present invention relates to pharmaceutical formulation of antiepileptic drug preferably oxcarbazepine. The formulation comprises multiple tablets or pellets of immediate release or controlled release nature, which are filled, inside the capsule to provides drug effect for 24 hours and is suitable for once a day administration. The patent also provides process of preparation of the dosage form.
Owner:ASTRON RES LTD
Sustained release dosage forms of oxcarbazepine
This invention relates to a once a day sustained release dosage form suitable for oral administration of oxcarbazepine. The once a day sustained release dosage form of oxcarbazepine includes oxcarbazepine and hydroxypropyl methylcellulose (HPMC) having a viscosity of 11,000 to 25,000 cps.
Owner:RAMAKRISHNAN SANKAR +3
Method for simultaneously detecting seven sleep chemical medicines
The invention discloses a supplemental detection method of seven chemical medicines including carbamazepine, chlorpromazine hydrochloride, olanzapine, doxepin hydrochloride, quetiapine fumarate, oxcarbazepine and sulpiride which are illegally added into health-care food or Chinese patent medicine for improving sleep. After a sample is subjected to ultrasonic extraction with methyl alcohol, chromatogram column separation is conducted, a mobile phase is eluted, and DAN detector is used for detection. By means of a built detection method, methodological verification is conducted, and parameters of results are shown in the description. It is verified that the method is quick, high in specificity and suitable for detection of chemical medicine added to the health-care food or Chinese patent medicine for improving sleep.
Owner:SHANXI PROVINCE FOOD & DRUG INSPECTION INST
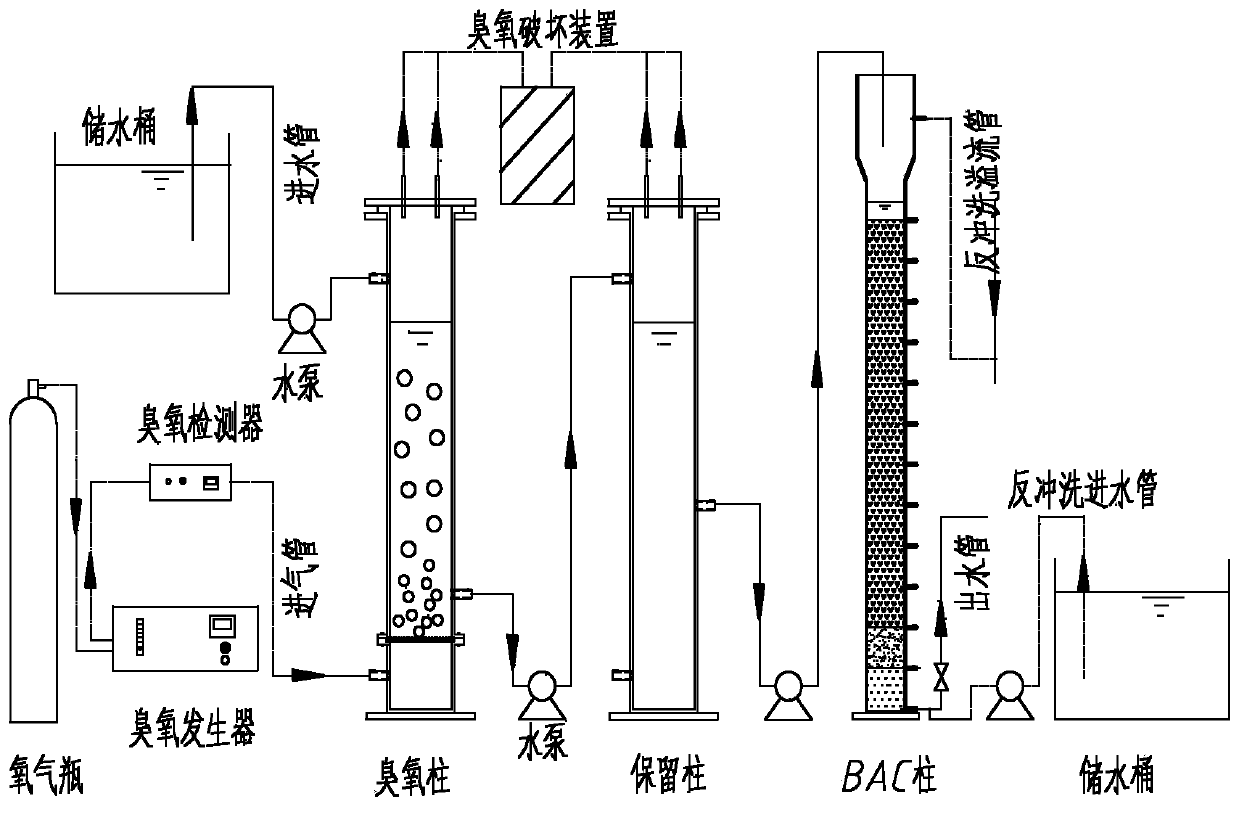
Method of removing PPCPs (Pharmaceutical and Personal Care Products) in drinking water by combination of ozone and activated charcoal
The invention discloses a method of removing PPCPs (Pharmaceutical and Personal Care Products) in drinking water by combination of ozone and activated charcoal. The method comprises the following steps: enabling ozone and aqueous liquor to be fully contacted by adopting a mode that gas and water are continuously and reversely contacted by means of ozone; then, carrying out further reaction through a retaining column, and then, enabling the obtained product to enter into a bio-activated charcoal column to be treated. The method disclosed by the invention can effectively remove PPCPs in drinking water, wherein the removal rate of carbamazepine, oxcarbazepine, progesterone and progesterone reaches over 99.9% while caffeine, antipyrine, aminopyrine and sulfamethoxazole are not detected in the effluent. Compared with existing drinking water treatment technologies, the method has higher chemical safety guarantee and meanwhile has a better removal effect on conventional pollutants in domestic drinking water. The effluent can stably reach the national sanitary standard (GB5749-2006) of domestic drinking water, so that the method has huge practical value and utilization potential.
Owner:JIANGNAN UNIV
Pharmaceutical formulations of oxcarbazepine and methods for its preparation
InactiveUS20070178164A1Improve solubilityEfficient deliveryBiocidePowder deliveryMedicineDissolution
The present invention provides a pharmaceutical composition comprising oxcarbazepine and at least one pharmaceutical excipient, wherein the oxcarbazepine in the composition has a broad particle size distribution and an enhanced oxcarbazepine dissolution rate. The broad particle size distribution of oxcarbazepine in the pharmaceutical composition is preferably a multi-modal oxcarbazepine particle size distribution.
Owner:TEVA PHARM USA INC
Oxcarbazepine and synthesizing process of its intermediate
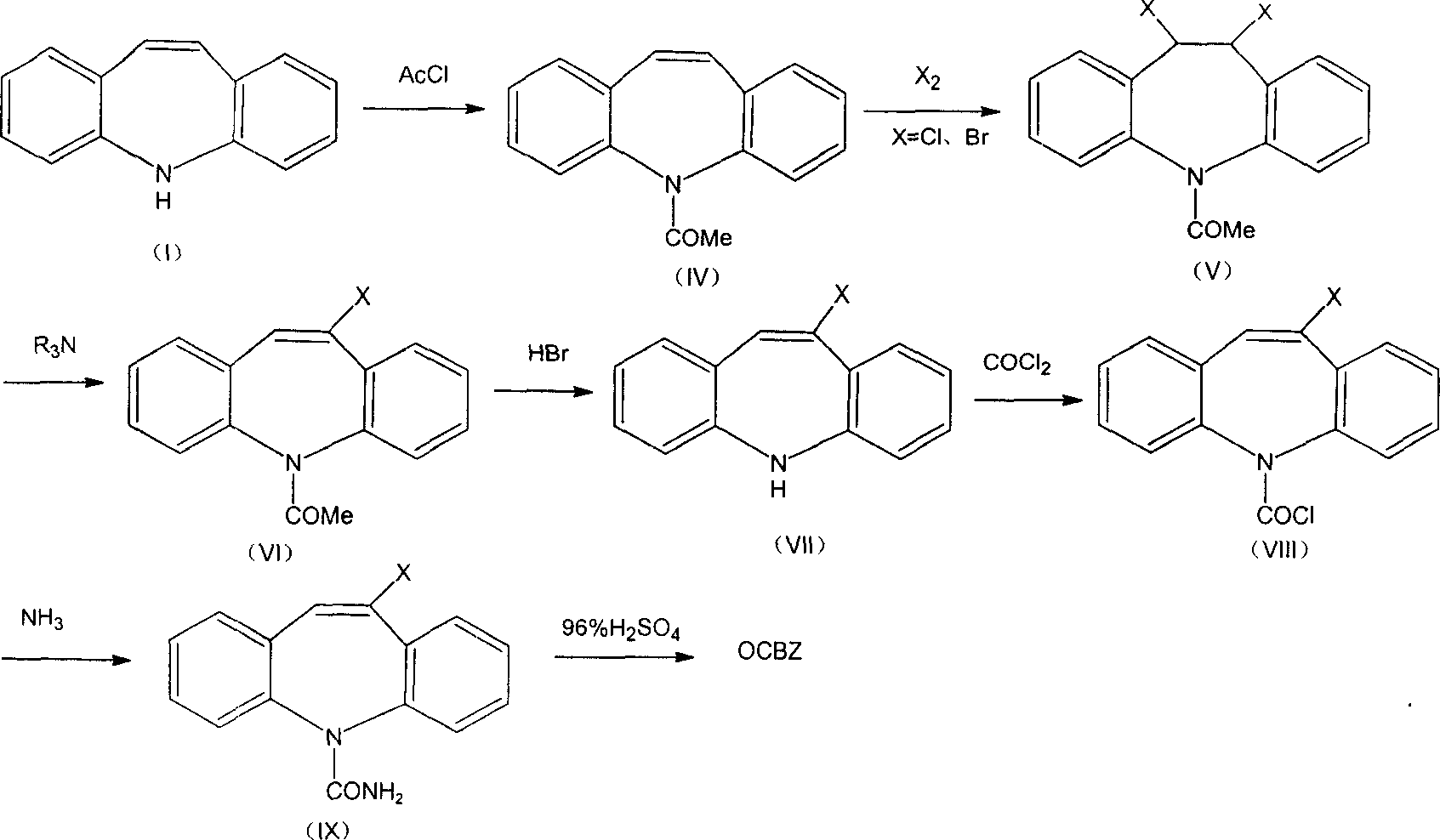
The invention relates to method of synthesizing OCBZ and its intermediates. It takes 5H - dibenzo [b, f] aza - 5- formyl chloride as raw materials, through the addition of halogenated, aminolysis, and hydrolysis reaction to obtain OCBZ, synthetic steps short, high yield and low cost.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Modified release preparations containing oxcarbazepine and derivatives thereof
ActiveUS20090005360A1Improve bioavailabilityMinimize fluctuationBiocidePowder deliverySolubilityMedicine
Controlled-release preparations of oxcarbazepine and derivatives thereof for once-a-day administration are disclosed. The inventive compositions comprise solubility- and / or release enhancing agents to provide tailored drug release profiles, preferably sigmoidal release profiles. Methods of treatment comprising the inventive compositions are also disclosed.
Owner:SUPERNUS PHARM INC
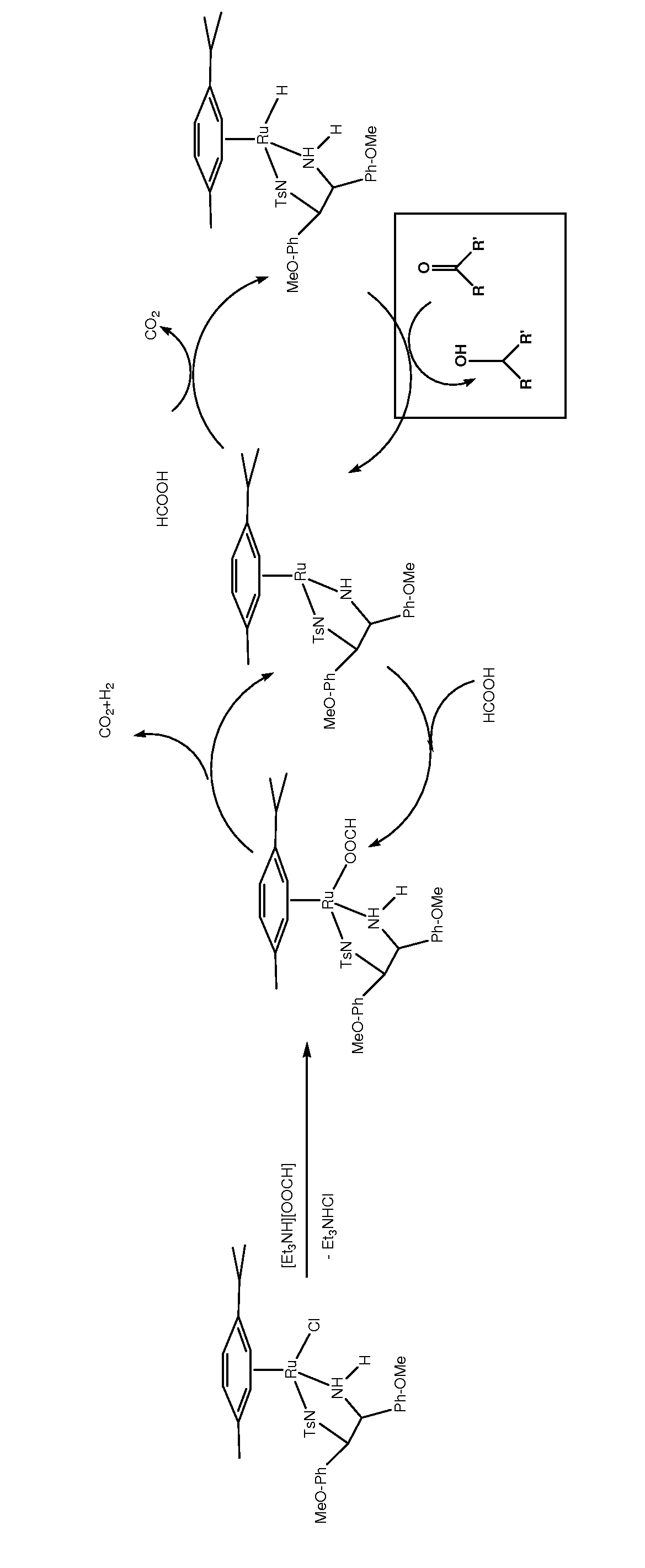
Asymmetric catalytic reduction of oxcarbazepine
ActiveUS8288532B2High substrate concentrationOptimize volumeAntibacterial agentsNervous disorderHydrogenAlkaline earth metal
A process for preparing (S)-(+)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide or (R)-(−)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide, by reduction of oxcarbazepine in the presence of a catalyst and a hydride source is disclosed. The catalyst is prepared from a combination of [RuX2(L)]2 wherein X is chlorine, bromine or iodine, and L is an aryl or aryl-aliphatic ligand, with a ligand of formula (A) or formula (B):wherein R1 is chosen from C1-6 alkoxy and C1-6 alkyl, n is a number from 0 to 5, and when n is a number from 2 to 5, R1 can be the same or different, and R2 is alkyl, substituted alkyl, aryl, substituted aryl, alkaryl or substituted alkaryl. The hydride source is either NR3R4R5 and formic acid, [R3R4R5NH][OOCH] and optionally formic acid, or [M][OOCH]x and formic acid, wherein R3, R4 and R5 are C1-6 alkyl, M is an alkali metal or alkaline earth metal and x is 1 or 2. A pH from 6.5 to 8 is maintained during the process.
Owner:BIAL PORTELA & CA SA
Chemical synthesis method for oxcarbazepine
ActiveCN101314590AReduce usageSimple processNervous disorderOrganic chemistryChemical synthesisNickel catalyst
The invention discloses a chemical synthesis method of oxcarbazepine, which comprises the steps as follows: adding 5-cyan-11-nitro-5H Dibenzoyl nitrogen heterocyclic shown in formula (III), Raney's nickel catalyst and organic solvent to a reactor, charging hydrogen till the pressure of the hydrogen reaches 2-20atm, performing the reduction reaction completely at 40-120 DEC C, filtering to obtain the filtrate, adding 20wt%-36.5wt% of hydrochloric acid for hydrolyzing completely, concentrating the reactant, and cooling for crystallization to obtain oxcarbazepine shown in formula (I). The reaction equation is described as follows. The synthetic route has the advantages of advanced technological line, simple and safe operation, higher product yield and purity and wide industrialized prospect.
Owner:ZHEJIANG UNIV OF TECH +1
Method for measuring density of anti-epileptic in blood
InactiveCN101093214ASimple and fast operationEasy to operateComponent separationColor/spectral properties measurementsUltraviolet detectorsAntiepileptic drug
A method for determining blood-medicine concentration of antiepileptic medicine includes pre-treating sample to be determined and separating pretreated sample by acidic mobile phase in chromatographic column then utilizing ultraviolet detector to simultaneously detect quantitative concentration of phenobarbital, lamotrigine, oxcarbazepin and adipomono- oxcarbazepin as antiepileptic medicine.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Methods of treating disorders associated with protein polymerization
ActiveUS20120129839A1Lower Level RequirementsIncreasing autophagyBiocideNervous disorderDiseaseMutated protein
The present invention relates to methods of treatment of clinical disorders associated with protein polymerization comprising administering, to a subject, an effective amount of carbamazepine, oxcarbazepine or another carbamazepine-like compound. It is based, at least in part, on the discovery that, in cells having a genetic defect in α1-antitrypsin, carbamazepine was able to decrease levels of the mutant protein. Furthermore, carbamazepine reduced the hepatic load of mutant α1-antitrypsin and the toxic effect of that mutant protein accumulation, hepatic fibrosis, in vivo using a mouse model of the disease. As patients having this defect in α1-antitrypsin exhibit toxic accumulations of the protein, treatment according to the invention may be used to ameliorate symptoms and signs of disease.
Owner:UNIVERSITY OF PITTSBURGH
Biomarker for forecasting severe drug-induced cutaneous adverse reaction of child patient and application
The invention discloses a biomarker for forecasting severe drug-induced cutaneous adverse reaction of a child patient and an application. The biomarker is capable of forecasting the risk of severe cutaneous adverse reaction of the child patient using beta-lactam antibiotics such as penicillin, cephalosporin, carbamazepine, lamotrigine, oxcarbazepine, phenytoin, allopurinol, nevirapine, abacavir, methazolamide and dapsone.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Oxcarbazepine pharmaceutical formulation and its method of preparation, wherein oxcarbazepine has a broad and multi-modal particle size distribution
The present invention provides a pharmaceutical composition comprising oxcarbazepine .and at least one pharmaceutical excipient, wherein the oxcarbazepine in the composition has a broad particle size distribution. The broad particle size distribution of oxcarbazepine in the pharmaceutical composition is preferably a multi-modal oxcarbazepine particle size distribution, preferably with an enhanced oxcarbazepine dissolution rate.
Owner:TEVA PHARMA IND LTD
Oxcarbazepine dry suspension and preparation method thereof
The invention discloses an oxcarbazepine dry suspension and a preparation method thereof. The oxcarbazepine dry suspension is prepared from oxcarbazepine, microcrystalline cellulose, xanthan gum, solubilizer, filler, a conventional amount of flavoring agent and aromatizer. According to the oxcarbazepine dry suspension prepared by adopting a spray drying method, time can be saved, the defects of agglomeration, high particle cracking rate, non-uniform shape and grain diameters and the like of a wet-process granulation method can be overcome, and the prepared granules are uniform, high in dissolving speed, relatively excellent in liquidity and excellent in taste, the production efficiency can be improved, and the cost for the dry suspension can be reduced. The oxcarbazepine dry suspension is suitable for industrial production.
Owner:AVENTIS PHARMA HAINAN
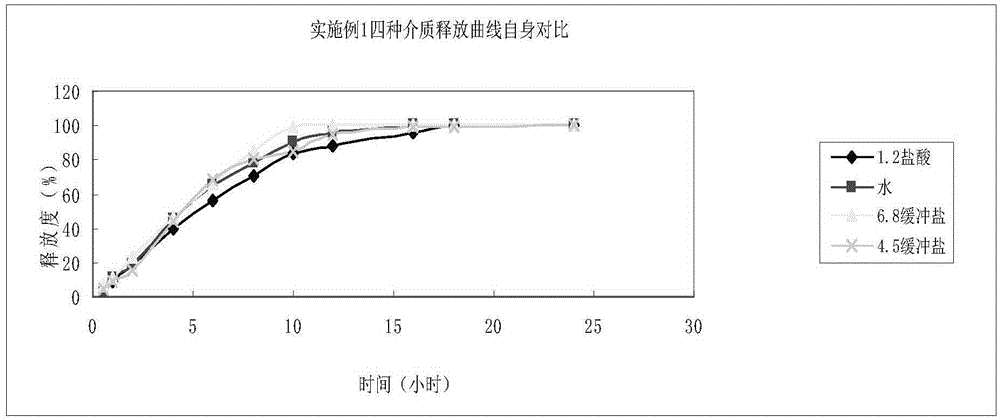
Oxcarbazepine sustained release tablet and preparation method thereof
InactiveCN106727391ASolve solubilityGuaranteed bioequivalenceNervous disorderPharmaceutical non-active ingredientsSustained Release TabletReference product
The invention relates to an oxcarbazepine sustained release tablet and a preparation method thereof. According to the method adopted by the invention, raw materials are specially treated together with an auxiliary material first to prepare oxcarbazepine composition, and the particle size of the oxcarbazepine composition is strictly controlled. KG-802 is added into an extra auxiliary material. According to the oxcarbazepine sustained release tablet prepared with the method provided by the invention, the use of a large amount of a cosolvent namely sodium lauryl sulfate in an original developed preparation is avoided, the toxicity of a preparation finished product to a human body after taking is reduced, and the pollution to the environment in a preparation process is reduced; and compared with a reference product on sale, release curves in release media with different pH values can keep similar, the bioequivalence of an in-vitro test is ensured, and the release curves of the release media with different pH values are more similar to one another in comparison with the preparation on sale. The oxcarbazepine sustained release tablet provided by the invention is prepared by adopting a conventional granulation and tabletting process, the process is simple, and the oxcarbazepine sustained release tablet is suitable for large-scale production.
Owner:浙江四维医药科技有限公司
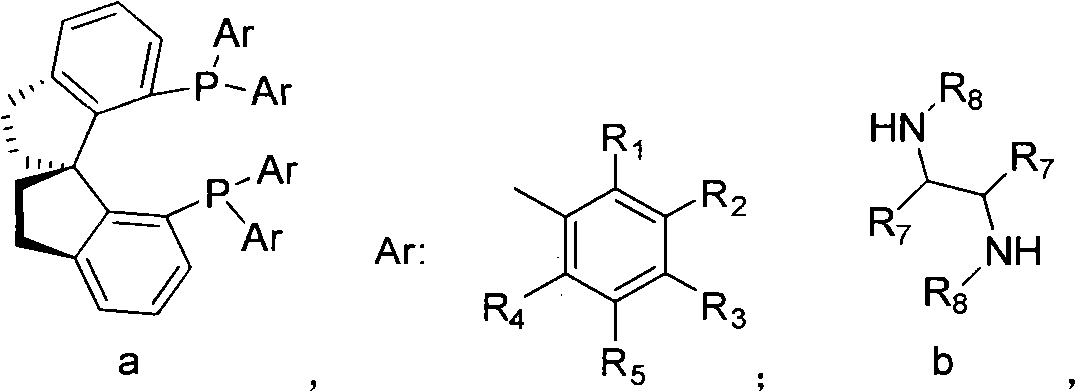
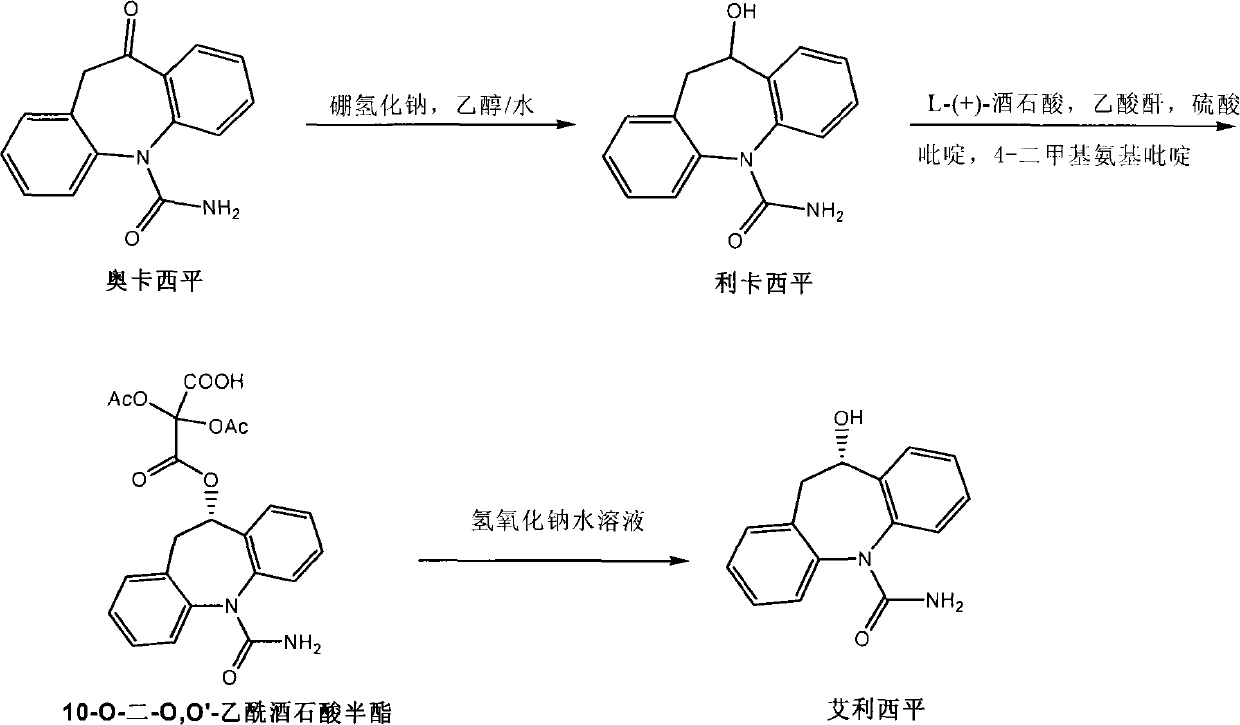
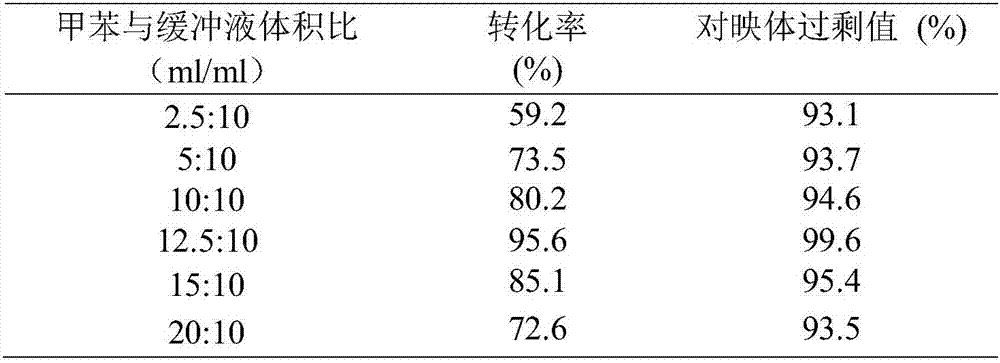
Preparation method of Eslicarbazepine
ActiveCN102250005AImprove recycling ratesWon't happenOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylHalogen
The invention relates to the field of chemical substance and medicament preparation, specifically to a preparation method of Eslicarbazepine. The preparation method provided by the invention is to perform a reaction of Oxcarbazepine in the presence of a chiral catalyst [MX2((S)-xyl-SDP)((R,R)-DPEN)] to obtain Eslicarbazepine. [MX2((S)-xyl-SDP)((R,R)-DPEN)] is mainly formed by the coordination combination of a chiral diphosphine ligand (a) compound, a chiral nitrogen ligand (b) compound and a metal salt catalyst (MX2(p-cymene)), wherein R1, R2, R3, R4 and R5 are C1-C6 alkyl group or C6-C10 aryl group or hydrogen or -OR6; R6 is C1-C6 alkyl group or C6-C10 aryl group or hydrogen; R7 is C6-C10 aryl group or C1-C6 alkyl group; R8 is C6-C10 aryl group or C1-C6 alkyl group or hydrogen; M is Ru, Rh, Ir, Fe, Co or Ni; and X is a halogen.
Owner:浙江瑞博制药有限公司
Rapidly Dispersible Dosage Form of Oxcarbazepine
ActiveUS20150366802A1Easy to manageOvercome disadvantagesBiocidePowder deliveryDiseasePharmaceutical drug
A high dose orodispersible dosage form of oxcarbazepine is provided. Drug-containing particles of oxcarbazepine are included within a porous bound matrix. The dosage form disperses in saliva or water in less than 15 sec and it has sufficient hardness to withstand handling and storage. It can be used to treat diseases or disorders that are therapeutically responsive to oxcarbazepine or a derivative thereof.
Owner:APRECIA PHARMA LLC
Preparation of interface self assembled carbonyl reductase and application thereof to S-eslicarbazepine synthesis
ActiveCN107475211AHigh catalytic efficiencyRealize subsequent separation and extractionMicroorganism based processesOxidoreductasesCentrifugationCatalytic function
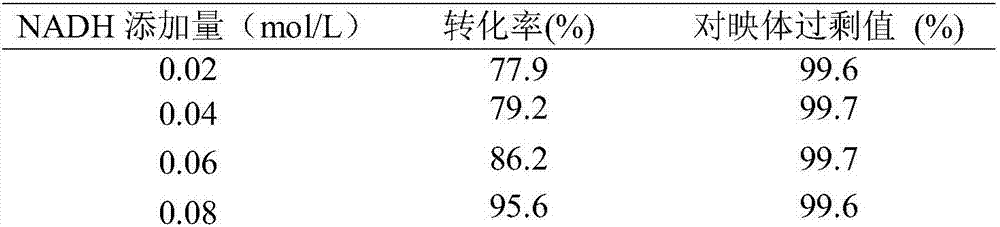
The invention discloses preparation of interface self assembled carbonyl reductase and application thereof to S-eslicarbazepine synthesis. The interface self assembled carbonyl reductase is prepared by the following method that carbonyl reductase is added into a buffer solution; then, a methylbenzene solution with dissolved polystyrene is added; oscillation reaction is performed for 2 hours in a shaking bed under the conditions of dark environment, 80r / min and 30 DEG C; centrifugation is performed; a reaction system with the upper layer being an organic phase, the interface self assembled carbonyl reductase distributed in the oil water interface, and the lower layer being a water phase is obtained; the interface self assembled carbonyl reductase is obtained; the interface self assembled carbonyl reductase has a good catalysis function in the oil and water two-phase interface; the oxcarbazepine can be converted into S-eslicarbazepine; the enantiometric excess value of the S-eslicarbazepine is greater than 98 percent. In the water phase, the coenzyme NADH can smoothly realize the in-situ regeneration; the substrate conversion rate can be favorably improved. The interface self assembled enzyme can realize the multi-time repeated utilization, the advantage is incomparable by free enzymes.
Owner:ZHEJIANG UNIV OF TECH
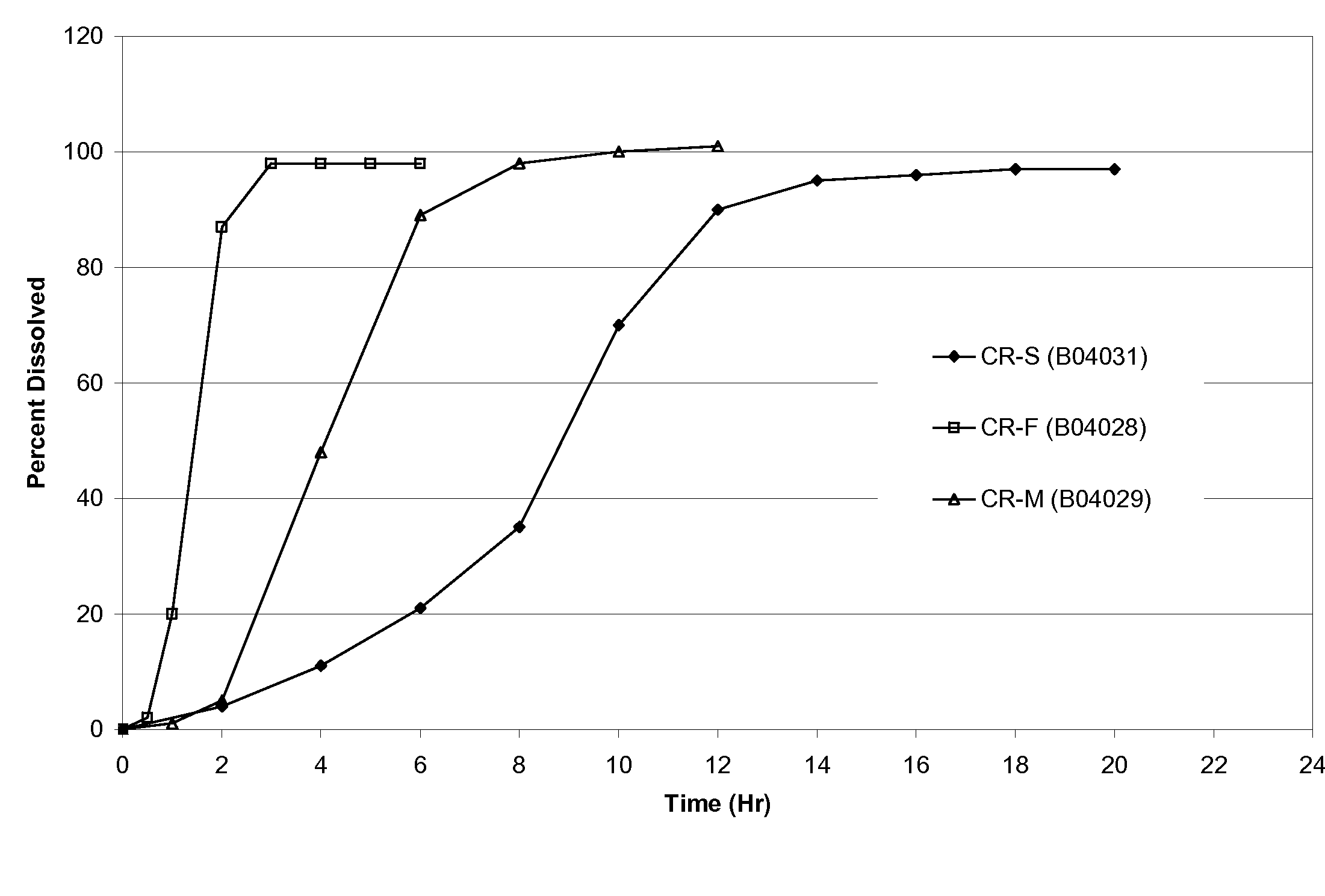
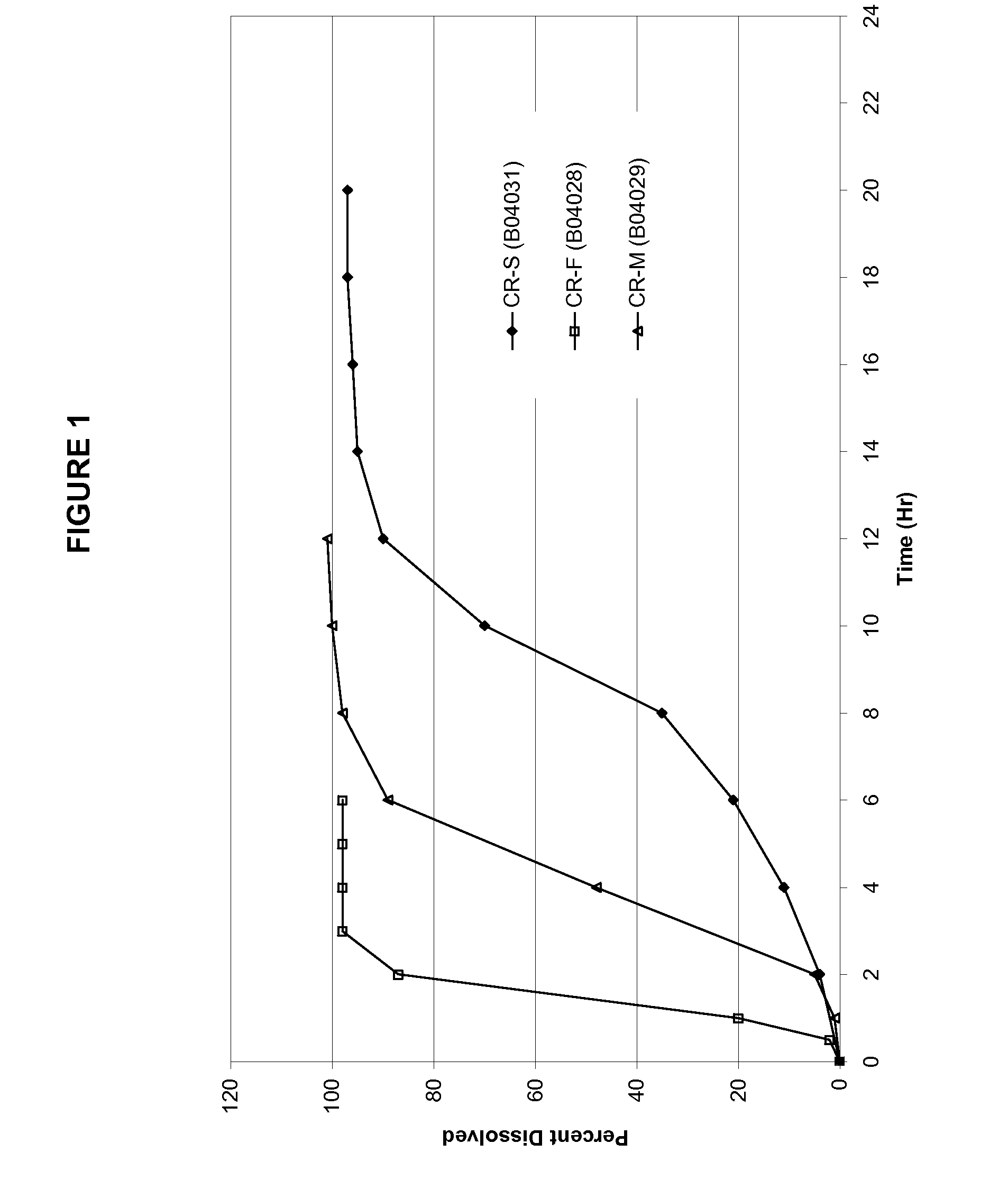
Pharmaceutical dosage forms of oxcarbazepine
A pharmaceutical dosage form comprising a mixture of a therapeutically effective amount of oxcarbazepine having median particle size ranging from about 15 μm to about 26 μm and one or more hydrophilic polymers, said mixture being formed by subjecting a suspension comprising said oxcarbazepine and a hydrophilic polymer in a solvent, to mixing in a homogenizer, optionally removing the solvent and converting the said mixture into a dosage form.
Owner:SUN PHARMA INDS
Methods and Compositions for the Treatment of Seizure-Related Disorders
ActiveUS20170189342A1Eliminate side effectsReduce releaseNervous disorderGranular deliveryVigabatrinDisease
Compositions and methods are provided for administering a pharmaceutical composition to a human patient. Compositions are administered to a human patient orally, once daily, at a therapeutically effective dose. The pharmaceutical compositions comprise a drug selected from the group consisting of brivaracetam, divalproex, lacosamide, levetiracetam, oxcarbazepine, vigabatrin, and pharmaceutically acceptable salts of any of the foregoing, and at least one excipient. At least one of said at least one excipients modifies the release of said drug to provide an extended release form. The pharmaceutical composition have pharmacokinetic properties recited in the claims.
Owner:ADAMAS PHARMA INC
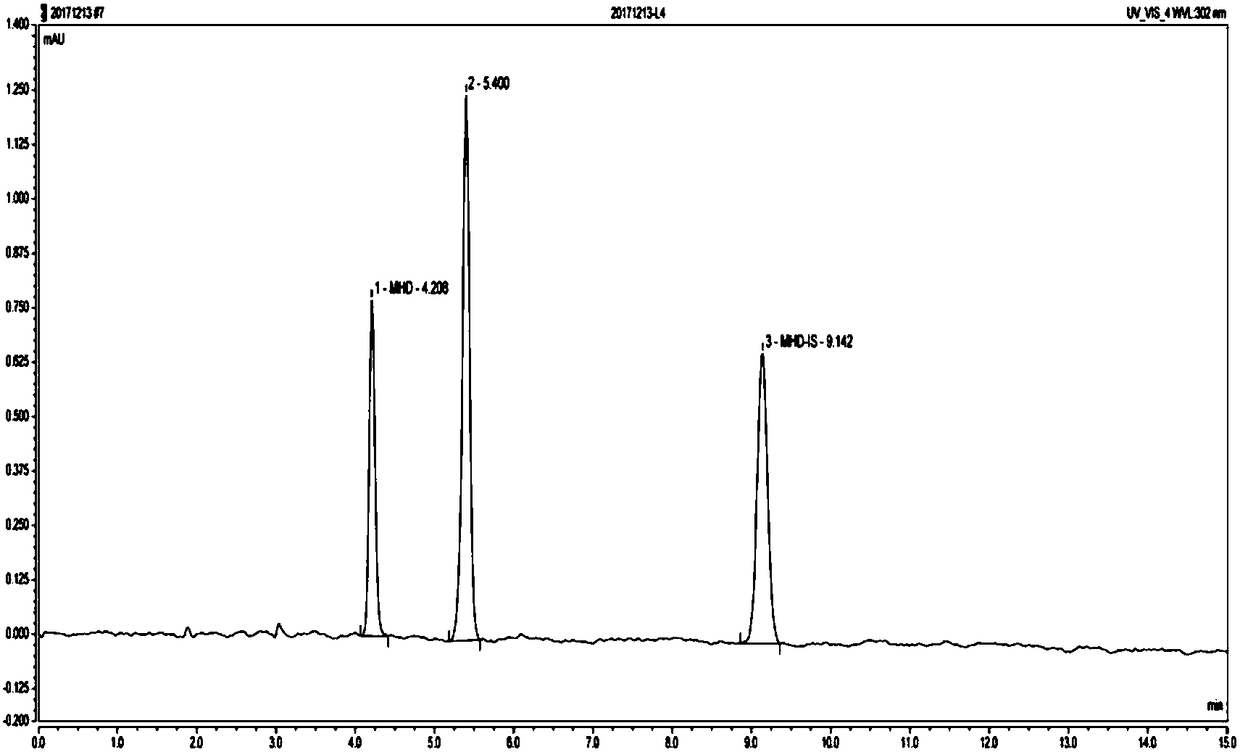
Method for detecting oxcarbazepine and 10, 11-dihydro-10-hydroxy carbamazepine in blood
InactiveCN110763800AShorten detection timeImprove accuracyComponent separationFluid phaseLiquid chromatography mass spectroscopy
The invention provides a method for detecting oxcarbazepine and 10, 11-dihydro-10-hydroxy carbamazepine in blood. The method comprises the following steps of preparing oxcarbazepine and 10, 11-dihydro-10-hydroxy-carbamazepine standard stock solution, preparing oxcarbazepine and 10, 11-dihydro-10-hydroxy-carbamazepine standard intermediate solution, preparing an internal standard working solution and preparing a standard solution, detecting the standard solution by utilizing a liquid chromatography-mass spectrometer, and fitting to obtain a standard curve equation corresponding to the oxcarbazepine and 10, 11-dihydro-10-hydroxy carbamazepine: y1=a*x1+b and y2=c*x2+d; treating the to-be-detected blood, detecting the to-be-detected blood by utilizing a liquid chromatography-mass spectrometer,and calculating the concentrations of oxcarbazepine and 10, 11-dihydro-10-hydroxy carbamazepine in the to-be-detected blood. The method disclosed by the invention is short in analysis time, small ininterference, quantitative and proper in internal standard, strong in specificity, and high in sensitivity.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
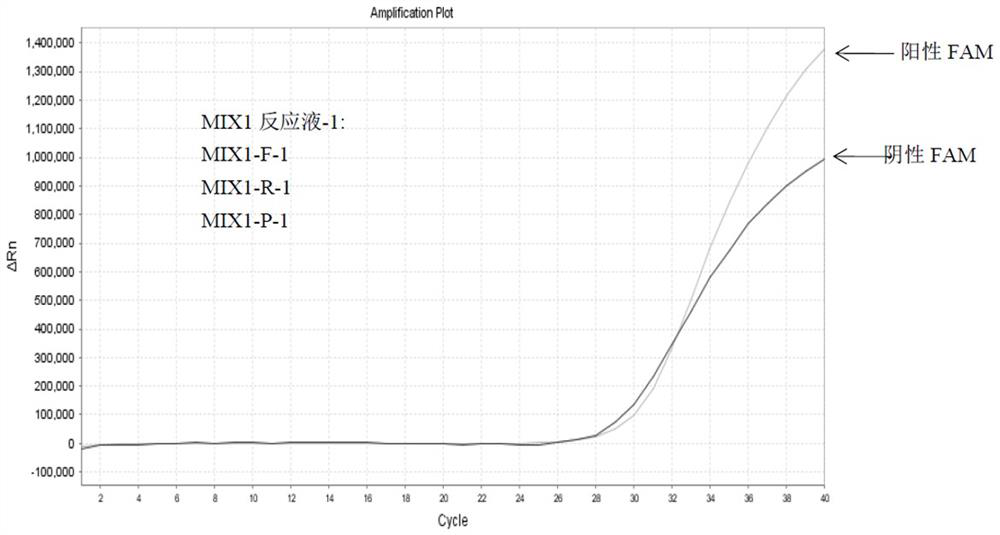
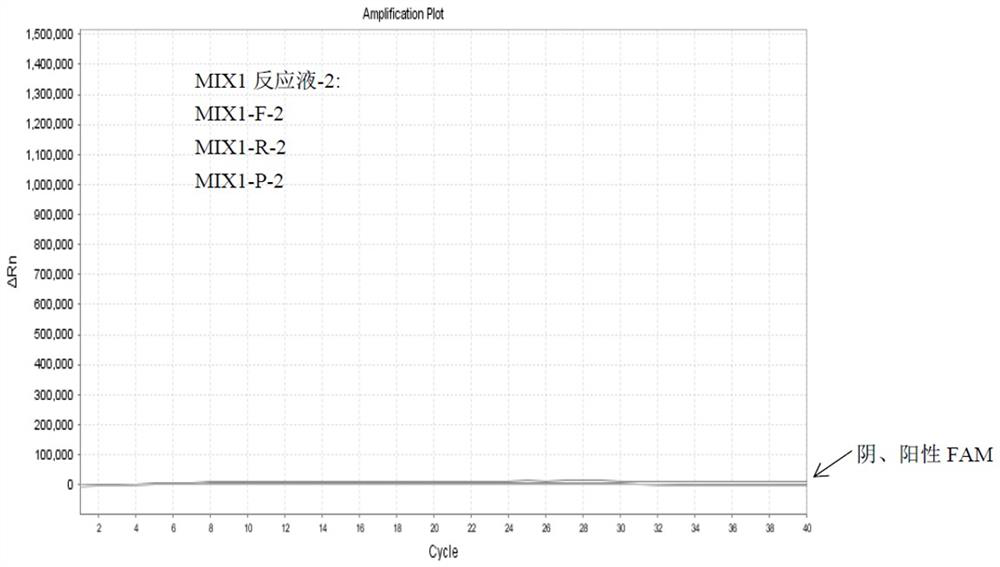
Detection method of HLA-B*1502 gene, detection kit and application thereof
The invention belongs to the technical field of biology, and provides a reagent for detecting human leukocyte antigen B site 1502 genotype (HLA-B*1502), application of the reagent in preparation of akit for guiding administration of antiepileptic drugs such as carbamazepine, oxcarbazepine, phenytoin and lamotrigine, a corresponding kit and a detection method of the kit. According to the HLA-B*1502 genotyping, three groups of MIX reaction solutions are detected by adopting a PCR-fluorescent probe method, and target nucleic acid molecules are circularly amplified through an amplification reaction, so that a fluorescence generation group is indirectly combined with an amplified target nucleotide sequence; the amount of fluorescence generated by the fluorescence generation group is determined, and the existence of the target nucleotide is determined. The detection reagent comprises a nucleic acid amplification system of three groups of MIX reaction solutions, wherein the nucleic acid amplification system comprises an upstream primer 1 and a downstream primer 1 which can be combined with target nucleotide, and an upstream primer 2 and a downstream primer 2 which can be combined with the target nucleotide; and three groups of probes 1 and probes 4 of a fluorescence detection system matched with the nucleic acid amplification system.
Owner:上海恩元生物科技有限公司
Oxcarbazepine controlled-release tablet and preparation method thereof
The invention aims at providing an oxcarbazepine controlled-release tablet which is capable of releasing medicines at a constant speed, and higher in medicine release stability and medicine use safety. The oxcarbazepine controlled-release tablet is characterized by consisting of curative dose of oxcarbazepine and physiologically acceptable pharmaceutical adjuvant; the medicine can be released at the constant speed in different release media. The controlled-release tablet disclosed by the invention has the characteristics of being convenient to dose, lasting in action, stable in curative effect, small in toxic and side effect, and the like.
Owner:QINGDAO CENT HOSPITAL
Liquid chromatographic analysis method for detecting plasma concentrations of oxcarbazepine and oxcarbazepine metabolite in blood
InactiveCN108195978AContent monitoringImprove accuracyComponent separationMetaboliteUltraviolet detectors
The invention discloses a liquid chromatographic analysis method for detecting the plasma concentrations of oxcarbazepine and an oxcarbazepine metabolite in blood. The method comprises the steps thata liquid chromatographic analysis instrument and an ultraviolet detector are used for calibrating a standard solution, and fitting is conducted to obtain a standard curve equation representing the concentration of oxcarbazepine and a standard curve equation representing the concentration of the oxcarbazepine metabolite separately, wherein the standard curve equation representing the concentrationof oxcarbazepine is that y1 is equal to the sum of b and the product of a and x1, and the standard curve equation representing the concentration of the oxcarbazepine metabolite is that y2 is equal tothe sum of d and the product of c and x2; a blood sample to be tested is taken, pretreated and detected by using the same liquid chromatographic analysis instrument and ultraviolet detector to obtainy1 and y2 values of the blood to be tested, the y1 and y2 values of the blood sample to be tested are substituted into the two standard curve equations respectively, the relative plasma concentrationsx1 and x2 of oxcarbazepine and the oxcarbazepine metabolite in the blood sample to be tested are obtained through calculation separately, the concentration of an internal standard substance working solution is known, and the plasma concentrations of oxcarbazepine and the oxcarbazepine metabolite in the blood sample to be tested can be obtained through calculation.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com