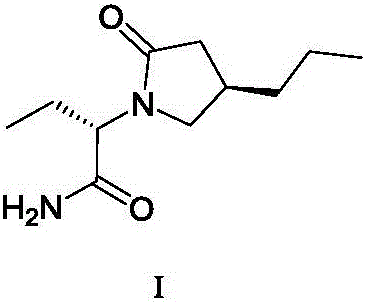
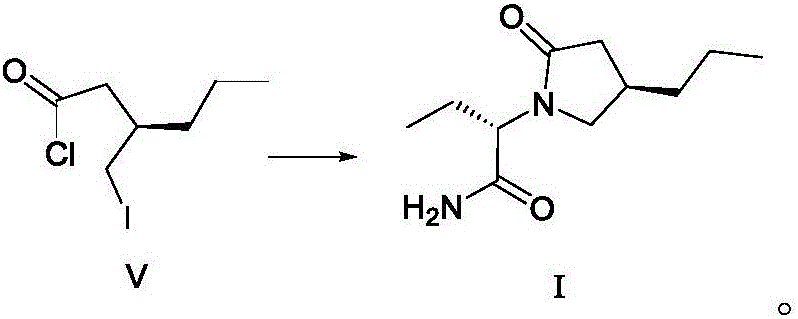
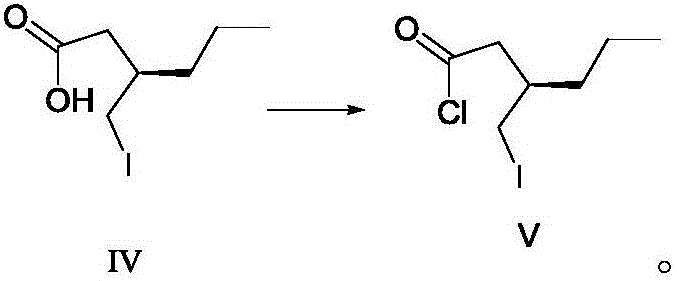
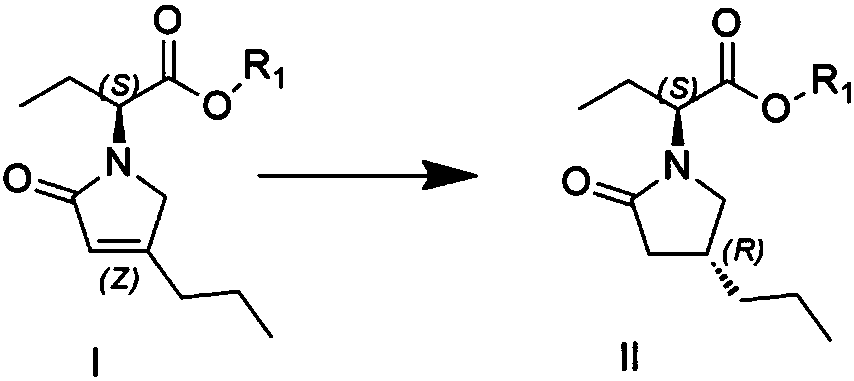
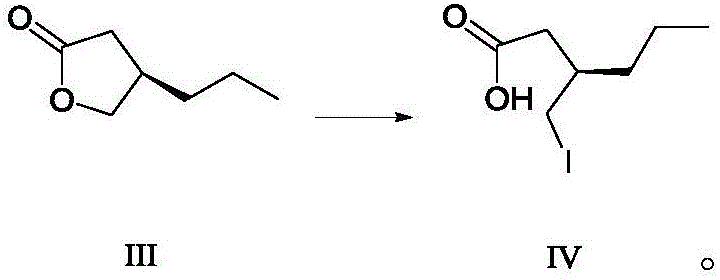
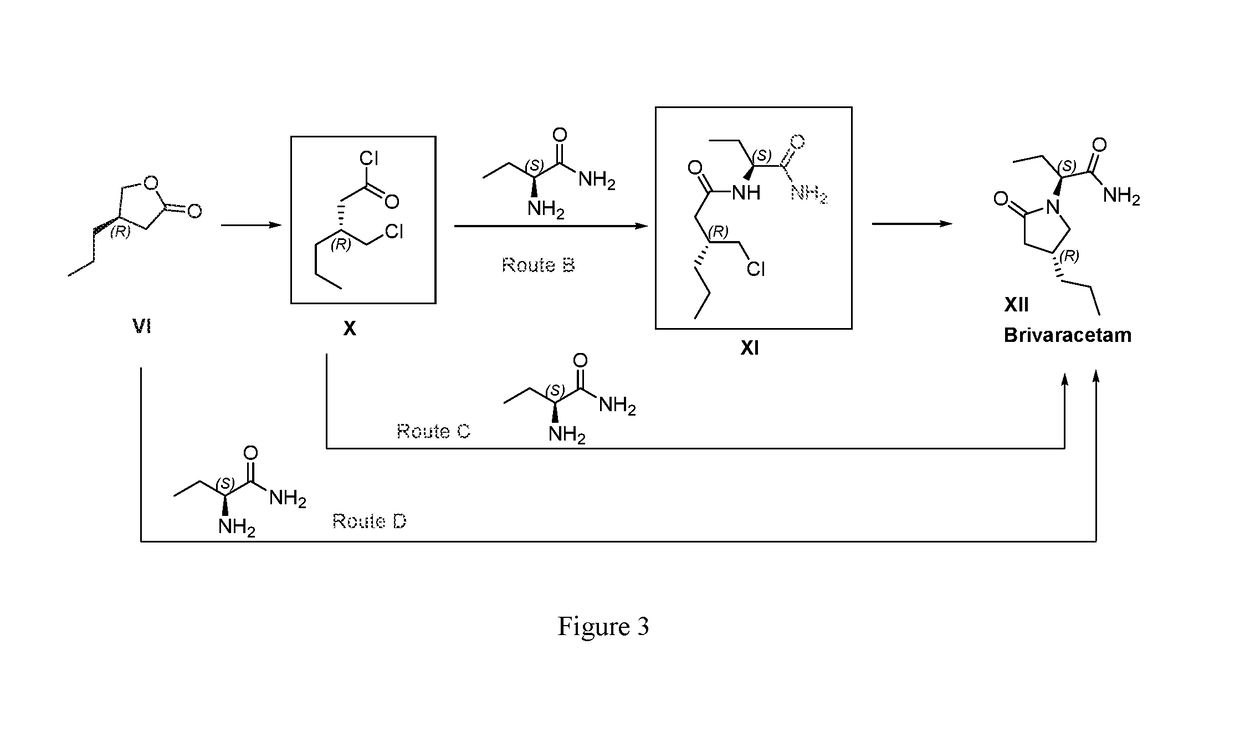
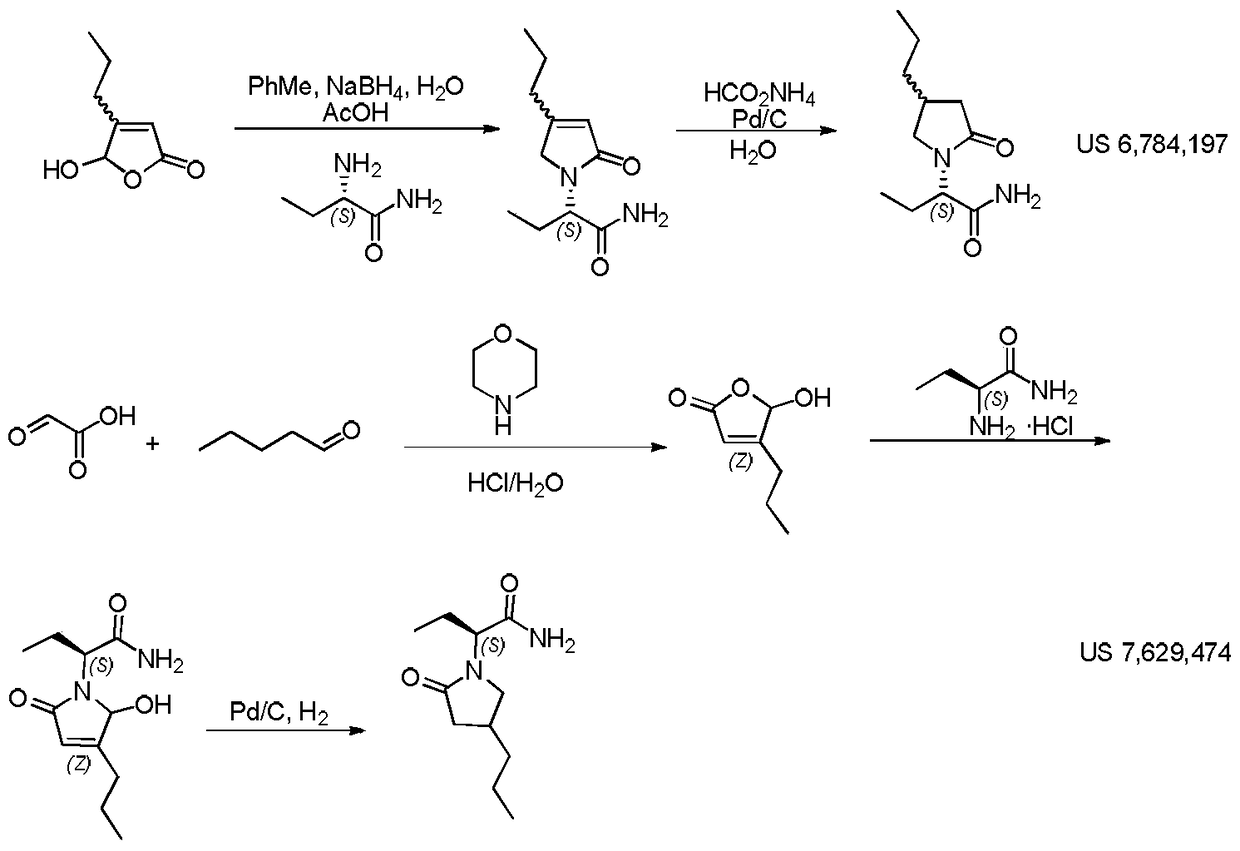
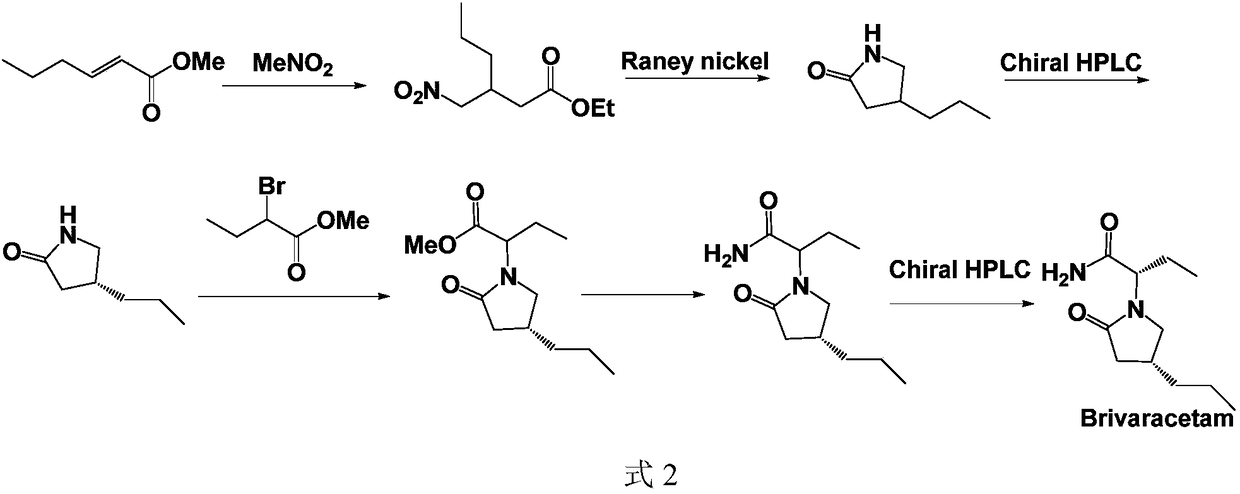
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Brivaracetam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
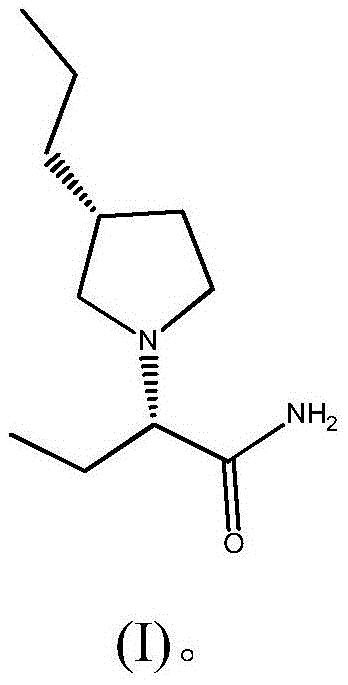
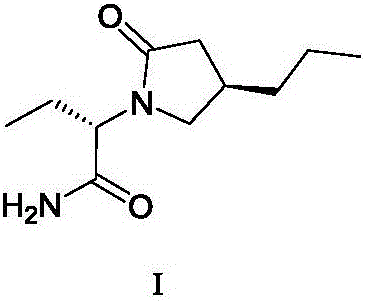
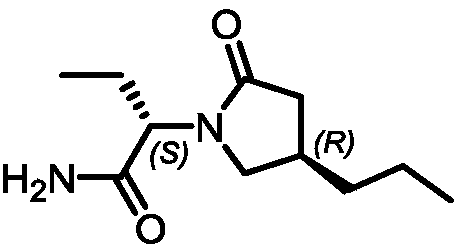
Brivaracetam is used to treat seizures (epilepsy)..
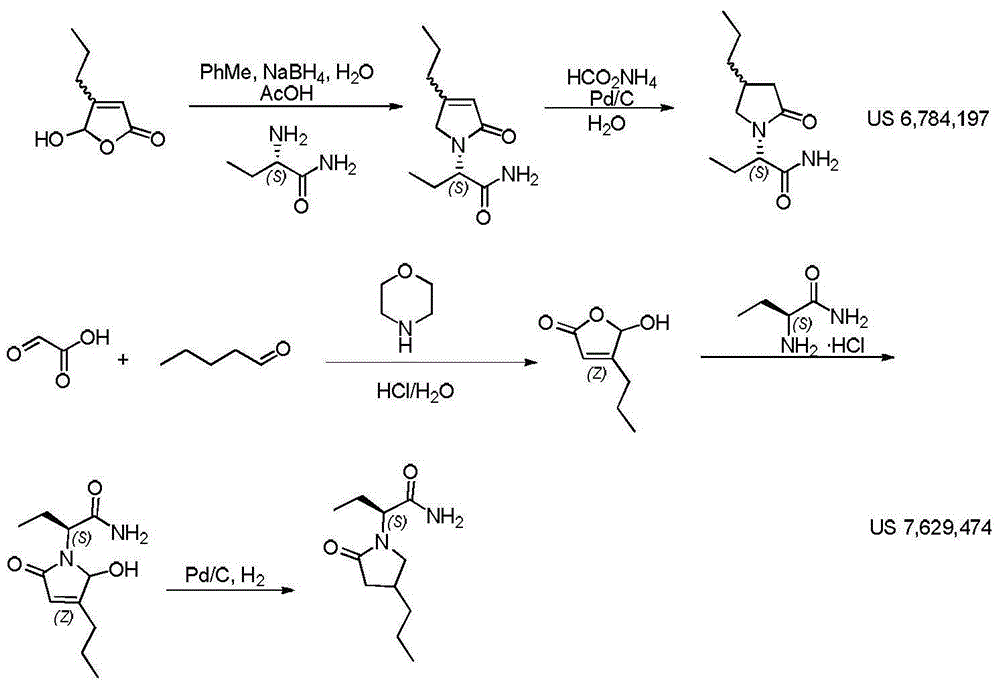
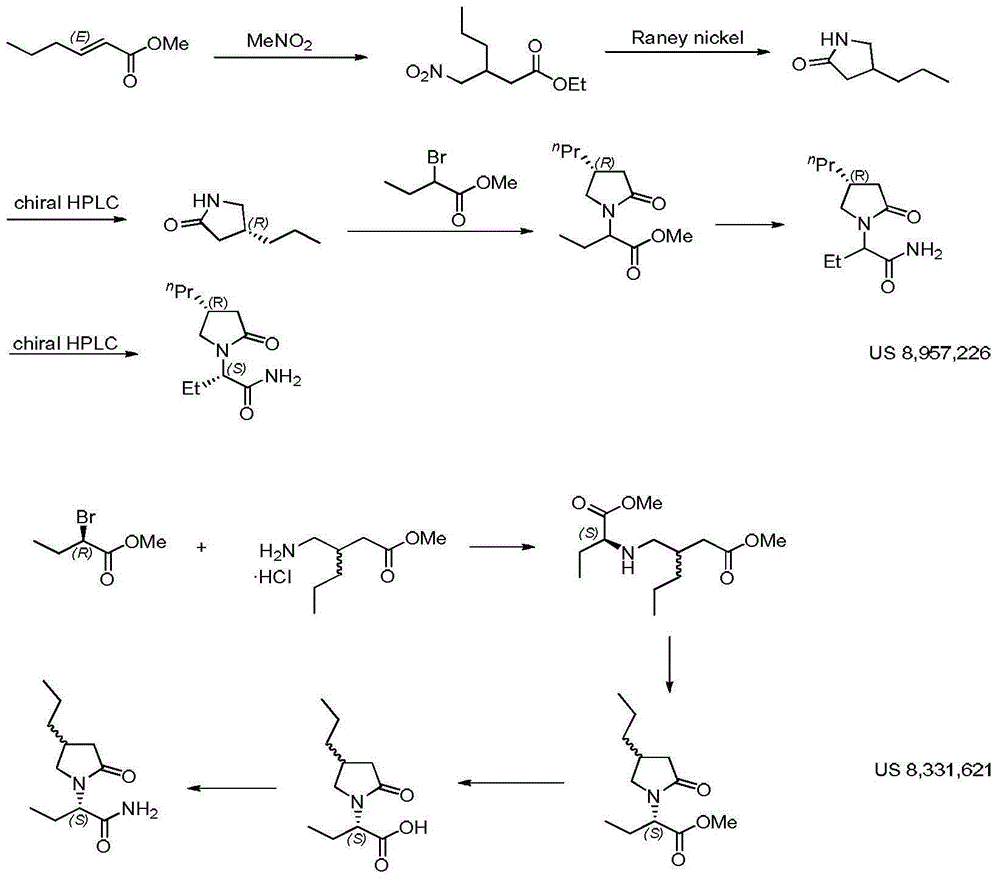
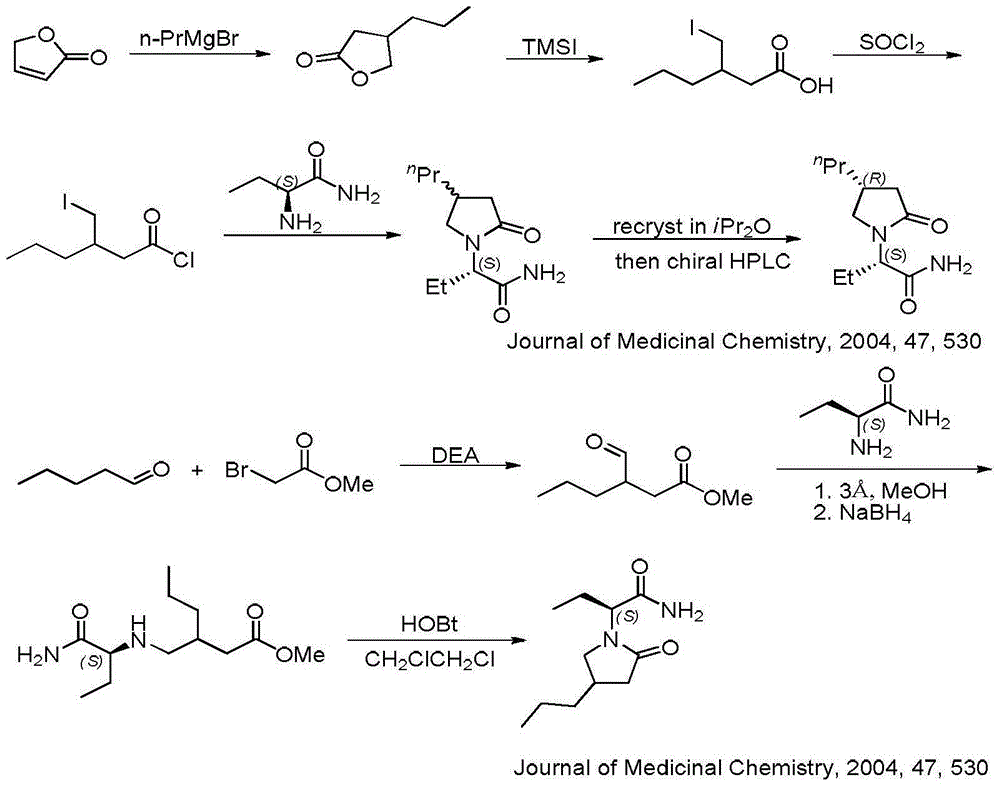
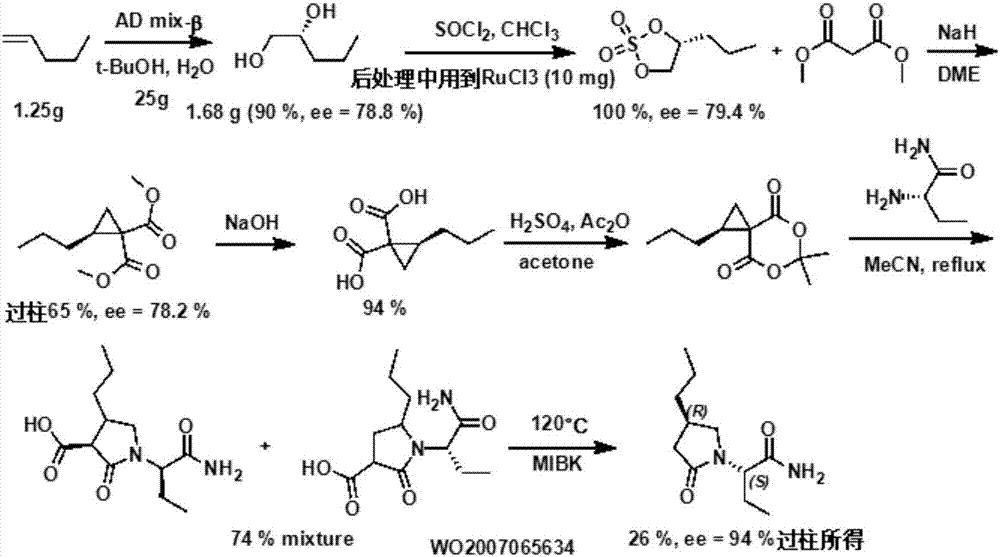
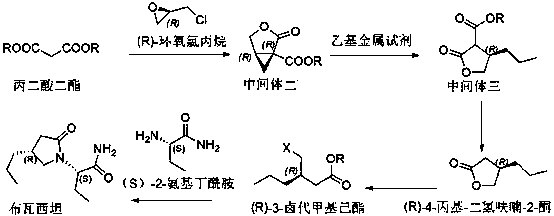
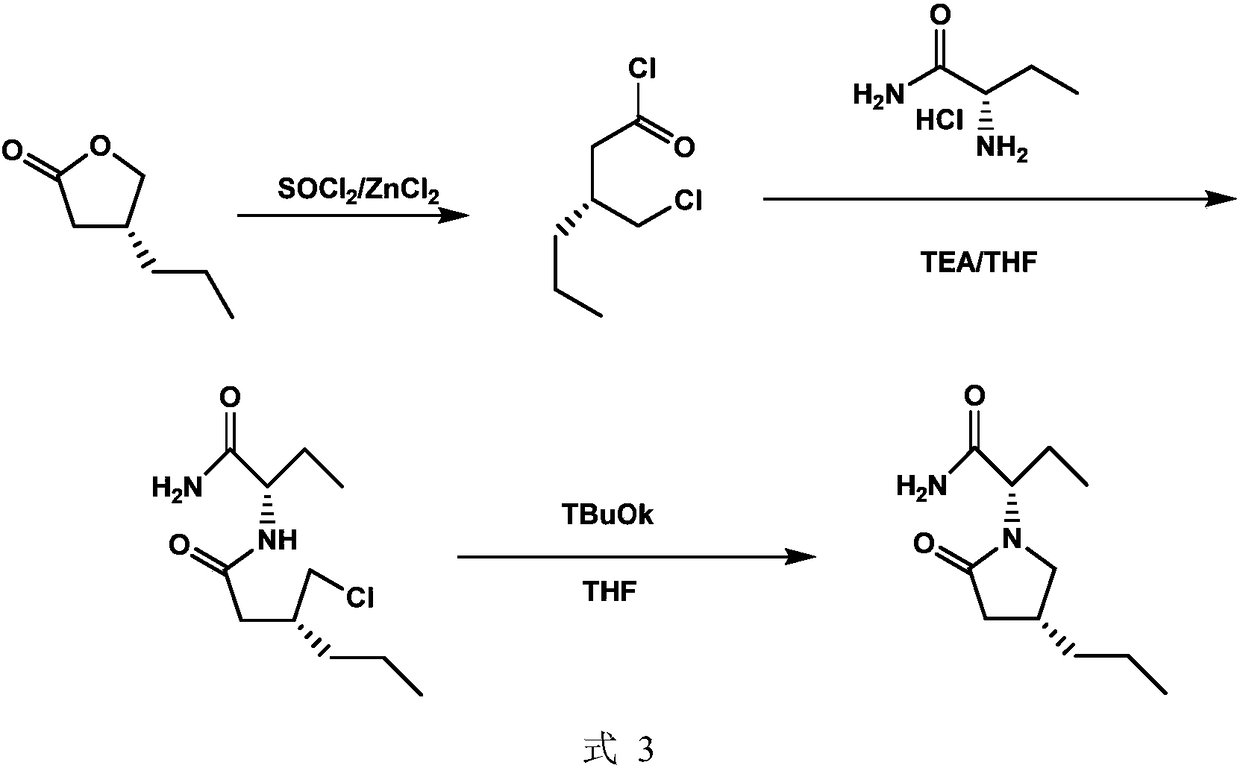
Preparation method of brivaracetam
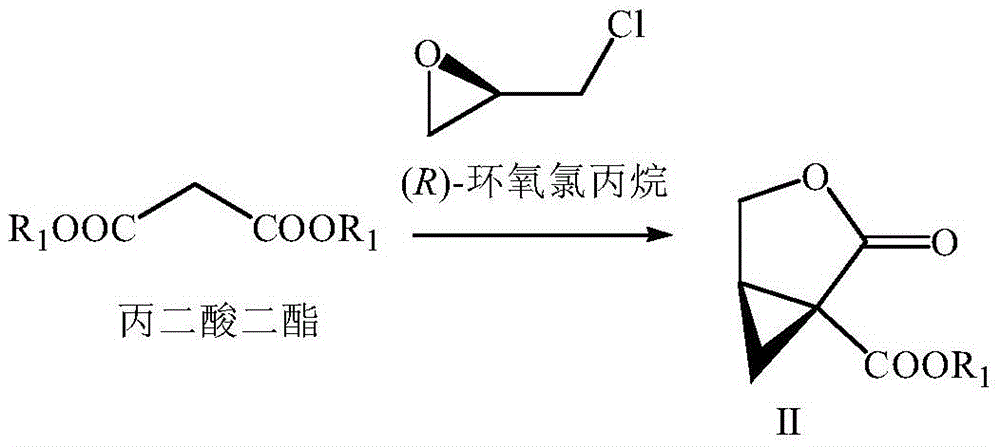
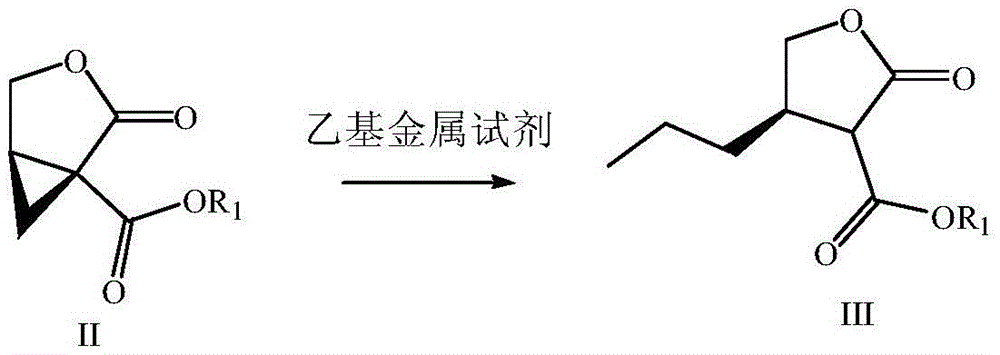
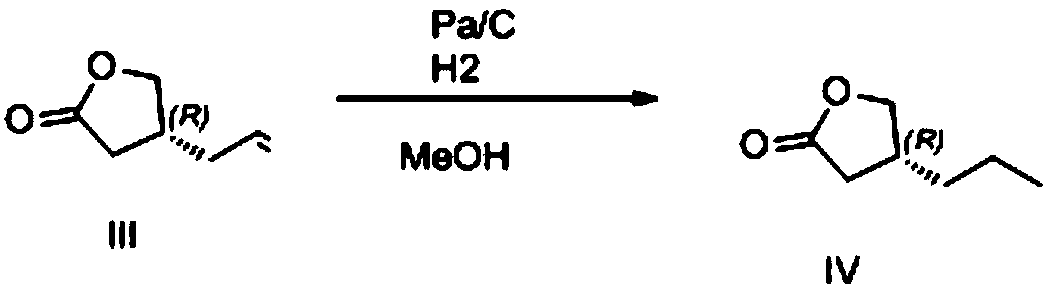
The invention provides a preparation method of brivaracetam. The preparation method of brivaracetam comprises the following steps of in an alkaline reagent, reacting malonate ester and (R)-epichlorohydrin to obtain lactone (II), reacting the lactone (II) and an ethyl metal-based reagent to obtain an intermediate (III), removing carboxylase to obtain (R)-4-propyl-dihydrofuran-2-ketone (IV), performing ring-opening reaction under the action of a halogenated ring-opening reagent to obtain (R)-3-halogenarated methyl hexanoate or (R)-3- halogenarated methyl hexyl acetate (V), and reacting with (S)-2-aminobutanamide or an acceptable salt, so as to obtain the brivaracetam. The preparation method has the advantages that the high-purity brivaracetam (HPLC (high performance liquid chromatography: greater than 96%) and stereo rotary brivaracetam (chirality HPLC: greater than 98%) can be directly prepared; the silicagel column separation and purification or the chirality preparation column separation and purification is not used, so that the complicated separation and purification step is not performed, the cost is saved, and the preparation method is more suitable for industrial production.
Owner:佛山市隆信医药科技有限公司
Compound and its preparation method and use in brivaracetam synthesis
ActiveCN106279074ARaw materials are easy to getLow priceOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention provides a compound shown in the formula I and a preparation method thereof. The invention also provides a use of the compound shown in the formula I in brivaracetam synthesis and a synthesis method of brivaracetam. The synthesis method utilizes cheap and easily available raw materials and can produce high optical purity brivaracetam.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
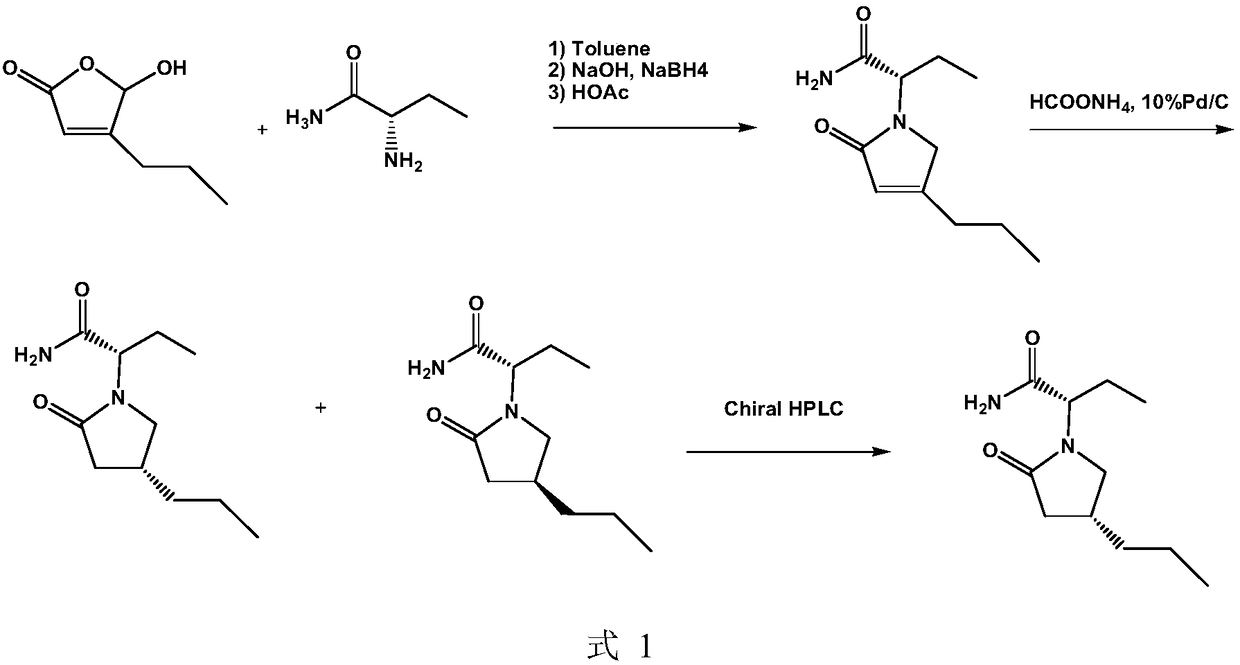
Brivaracetam and preparation method of intermediate thereof
The invention discloses a preparation method of a brivaracetam intermediate shown in B-VI. The preparation method comprises the following steps of dissolving B-IV and R-phenylethylamine into a solvent, crystallizing, filtering, and recrystallizing, so as to obtain B-V; then, converting into B-VI. The preparation method has the advantages that a chiral chromatographic column separation isomer is not needed in the preparation process, and only the simple steps of extraction, washing, drying, concentration and the like are performed, so as to separate effective ingredients; the separation process is simple, and the production cost of brivaracetam is greatly reduced. A formula is shown in the description.
Owner:宜宾市南溪区红光制药有限公司
Preparation method of brivaracetam
ActiveCN106432030ARaw materials are easy to getLow priceOrganic chemistryBrivaracetamColumn chromatography
The invention provides a method for synthesizing brivaracetam. Raw materials used in the method are available and inexpensive, a preparation process avoids appearance of chiral isomers difficult to separate, purification means such as column chromatography purification and the like adverse to industrial amplification production is avoided, a synthesis process is not involved with expensive and toxic heavy metal and chiral ligands, and a product brivaracetam with high quality and high optical purity can be obtained.
Owner:SUZHOU PENGXU PHARM TECH CO LTD +1
Compounds and preparation methods thereof, and uses of compounds in synthesis of brivaracetam
ActiveCN106365986AAvoid wastingHigh optical purityOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsBrivaracetam
The present invention provides compounds represented by formulas II and IV. The invention further provides uses of the compound represented by the formula II in synthesis of brivaracetam, and a synthesis method. According to the present invention, the used raw materials are easy to obtain and have low price, and the high optical-purity brivaracetam can be prepared. The formulas II and IV are defined in the specification.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Preparation method of brivaracetam and intermediate thereof
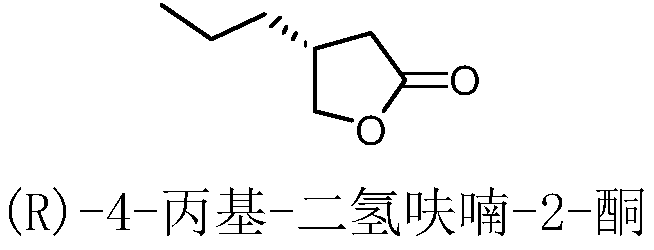
The invention relates to a preparation method of brivaracetam and an intermediate thereof, wherein the brivaracetam is prepared with high-optical-purity (R)-4-n-propyl-dihydrofuran-2-one as a raw material. The preparation method is low in requirement on production equipment and can be used for preparing a brivaracetam crude product at higher chemical and optical purities and higher yield. The method greatly reduces the cost of large-scale industrial production of the brivaracetam.
Owner:CHENGDU GUOHONG PHARMA
Method for preparing brivaracetam
ActiveCN106588741AHigh chiral puritySimple processing capacityOrganic chemistryPurification methodsOrganic solvent
The invention discloses a method for preparing brivaracetam. The method for preparing brivaracetam comprises the following step that in an organic solvent, under the condition of anhydrous and inert gas shielding, a compound V and L-2-aminobutanamide are subjected to a condensation reaction to obtain brivaracetam I. According to the method for preparing brivaracetam, brivaracetam is prepared through only four steps of reacting, the reaction steps are short, the total yield is high, aftertreatment steps and purifying methods are simple, a product with the de value larger than 99.80% can be prepared only through recrystallization, the grade API is reached, the production cost is low, and the method is suitable for industrial production. The formula is shown in the description.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Preparation method of high chiral purity lactam intermediate and brivaracetam
The invention provides a preparation method of a high chiral purity lactam intermediate. The preparation method of the high chiral purity lactam intermediate specifically includes the steps of: conducting hydrogenation reduction on a formula I compound under the conditions of a catalyst and a chiral inducer to generate a formula II compound with a de value of greater than 98%. The invention also discloses a preparation method of brivaracetam, and the formula II compound can produce a brivaracetam product under an ammoniation condition. The method provided by the invention has the advantages ofsimple operation of process route, low cost, high yield, high chiral purity of product, easy industrial production and the like.
Owner:YANGZHOU AORUITE PHARMA CO LTD +1
New synthesis method of brivaracetam
InactiveCN107216276AHigh yieldIncrease production capacityOrganic chemistrySynthesis methodsBrivaracetam
The invention provides a synthesis method of brivaracetam. The method adopts cheap and easily available pentanoic acid or valeryl halide as an initial raw material, and provides a brand new synthesis route of a brivaracetam medicine, and the whole reaction route has a high total yield. The method has the advantages of high productivity, low cost, and suitableness for large-scale industrial production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Synthesis method of brivaracetam intermediate and brivaracetam
InactiveCN109134406AAvoid chiral resolutionRaw materials are easy to getOrganic compound preparationOrganic chemistry methodsSynthesis methodsBrivaracetam
The invention discloses a synthesis method of a brivaracetam intermediate and brivaracetam. The synthesis method of the brivaracetam intermediate includes the steps of stripping carboxyl, triggering ahydrogenation reaction, and then conducting chiral resolution to obtain the brivaracetam intermediate which can be used for further synthesis of brivaracetam, wherein specific information is shown inthe description. According to the synthesis method of the brivaracetam intermediate, applied raw materials are easy to obtain, reaction operation is simple, and reaction conditions are easy to control. The synthesis method of brivaracetam is high in yield, the purity of synthesized brivaracetam can reach 99% or above, and the overall synthesis cost is lower.
Owner:LIVZON NEW NORTH RIVER PHARMA
New preparation method of brivaracetam
ActiveCN108503573AControl amountEffect of racemizationOrganic chemistry methodsChemical synthesisBrivaracetam
The invention relates to a new preparation method of brivaracetam, and belongs to the field of chemical synthesis. According to the present invention, optically pure (R)-4-n-propyl-dihydrofuran-2(3H)-one is used as a raw material, and ring opening, halogenation, condensation, ring closure and other steps are performed to obtain the high-purity brivaracetam; and the preparation method has advantages of easily available raw materials, low cost, high total yield, high optical purity of the obtained product, simple reaction conditions and simple operation process.
Owner:BEIJING ABLEPHARMTECH CO LTD
Preparation method of high chiral purity lactam intermediate and brivaracetam
InactiveCN108101823AEasy to separateLow costOrganic chemistry methodsChiral selectivityStructural formula
The invention discloses a preparation method of a high chiral purity lactam intermediate and brivaracetam. The preparation method of the lactam intermediate structural formula compound D includes thesteps of: in a solvent, by means of a heavy metal catalyst and a chiral inducer, conducting hydrogenation reduction on a compound C into the lactam intermediate D. The lactam intermediate structural formula compound D provided by the invention can be made into brivaracetam by only one step, the synthesis route is short, the reaction conditions are mild, the post-treatment is simple, the reaction yield is high, the chiral selectivity is good, and the production cost is low. In the reaction process, the conversion rate of the compound C reaches 81%, and the de value of the compound D reaches 99%or more, therefore the method is suitable for industrial production.
Owner:YANGZHOU AORUITE PHARMA CO LTD +1
Preparation method of brivaracetam intermediate
InactiveCN107604018ASettlement yieldSolve the costOxidoreductasesFermentationBuffer solutionBrivaracetam
The invention discloses a preparation method of a brivaracetam intermediate. The preparation method comprises the following steps: performing reaction on 4-n-propyl-2(5H)-furanone as a substrate in anaqueous buffer solution of which temperature is 25 to 30 DEG C and a pH value is 6.0 to 9.0 under effects of recombinant alkene reductase and coenzyme as well as a coenzyme regeneration system to generate an intermediate (R)-4-n-propyl-dihydrofuran-2-one. According to the preparation method disclosed by the invention, by adopting specific alkene reductase, the problems of long synthetic route, low yield and high cost of an existing brivaracetam intermediate (R)-4-n-propyl-dihydrofuran-2-one and large environment protection pressure caused by adopting a toxic and dangerous reagent are solved,and the brivaracetam intermediate in the prior art has important industrial application value.
Owner:重庆迪维斯生物科技有限公司 +3
Preparation method of brivaracetam
The invention discloses a preparation method of brivaracetam (formula IV). The preparation method comprises the following steps: carrying out ammonolysis on (R)-4-propyl-dihydrofuran-2-one and (S)-2-amino butyric acid as raw materials to obtain an intermediate II, then carrying out steps of esterification, chlorination and cyclization to obtain an intermediate III, and finally, carrying out an ammonolysis step to obtain the brivaracetam (IV), wherein the steps of esterification, chlorination and cyclization are operated in a combination mode. The preparation method provided by the invention ismild in condition, is simple and convenient to operate, has easily obtained raw materials, and is suitable for large-scale industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Brivaracetam chiral intermediate and preparing method thereof
InactiveCN108264495ALow costHigh chemical purityOrganic compound preparationOrganic chemistry methodsAntiepileptic drugUnit operation
The invention relates to an antiepileptic drug brivaracetam chiral intermediate and a preparing method thereof. The preparing method of a compound, shown in a formula 1, of the brivaracetam chiral intermediate comprises the steps of with S-epichlorohydrin (a compound shown in a formula 7 being an original raw material, conducting open-loop preparing on ethyl metal reagent under the effect of Lewisacid to obtain a compound shown in a formula 6; conducting preparing on the compound shown in the formula 6 in the presence of a metal catalyst through a substitution reaction to obtain a compound shown in a formula 5; conducting cyan alcoholysis on the compound shown in the formula 5 under the effect of acid to obtain a compound shown in a formula 4; preparing the compound shown in the formula 4through the effect of hydroxyl activity reagent to obtain a compound shown in a formula 3; conducting preparing on the compound shown in the formula 3 and nitromethane through an SN2 nucleophilic substitution effect to obtain a compound shown in a formula 2; conducting cyclization preparing on the compound shown in the formula 2 through the effect of concentrated sulfuric acid and an Nef reactionto obtain a compound shown in a formula 1, namely the brivaracetam chiral intermediate. By means of the preparing method, the compound high in optical purity and shown in the formula 1 can be prepared and solve the problem that currently produced brivaracetam needs complex unit operation high in cost like a column chromatography and a chiral preparation column.
Owner:CONSCI PHARMA
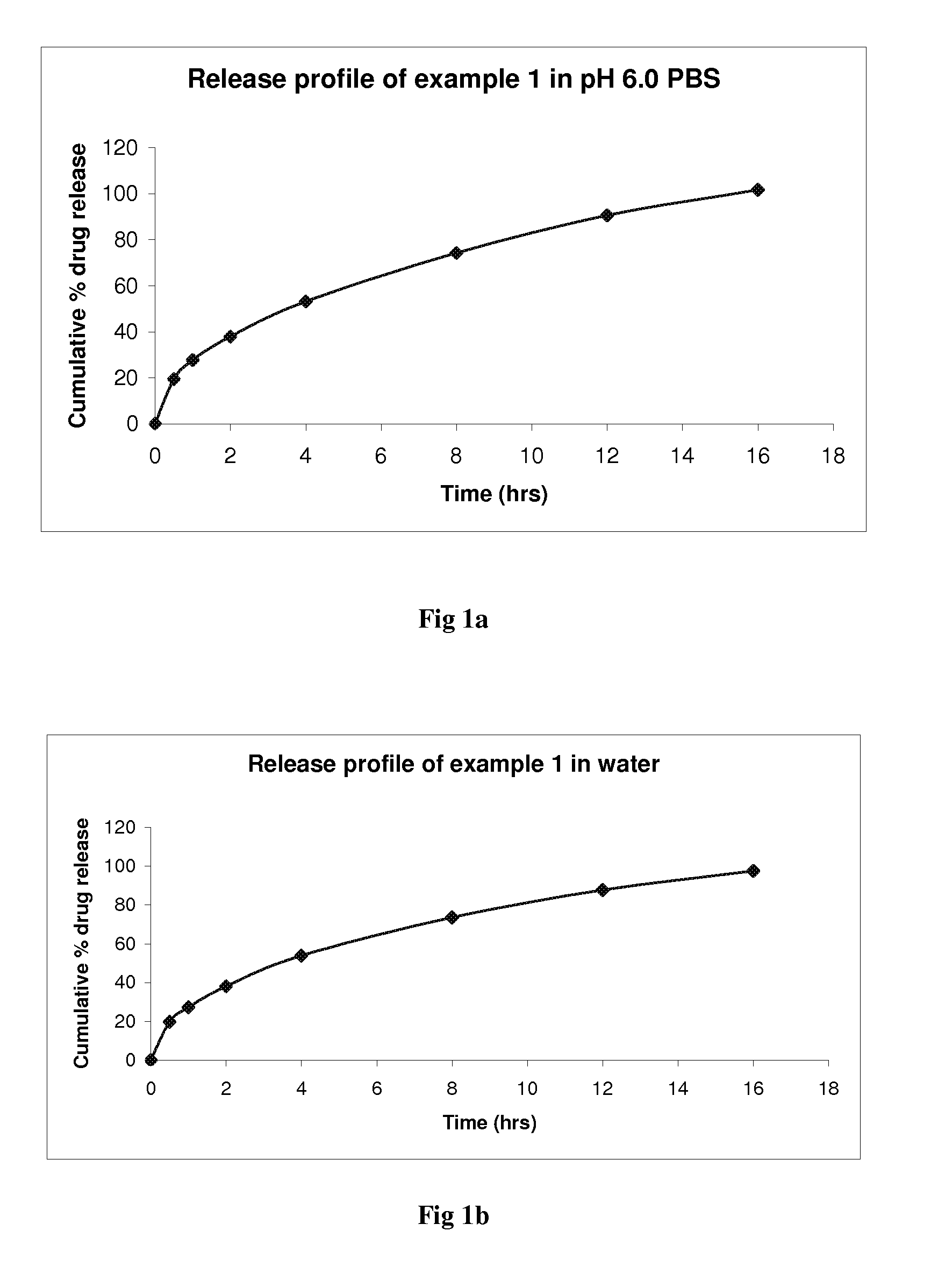
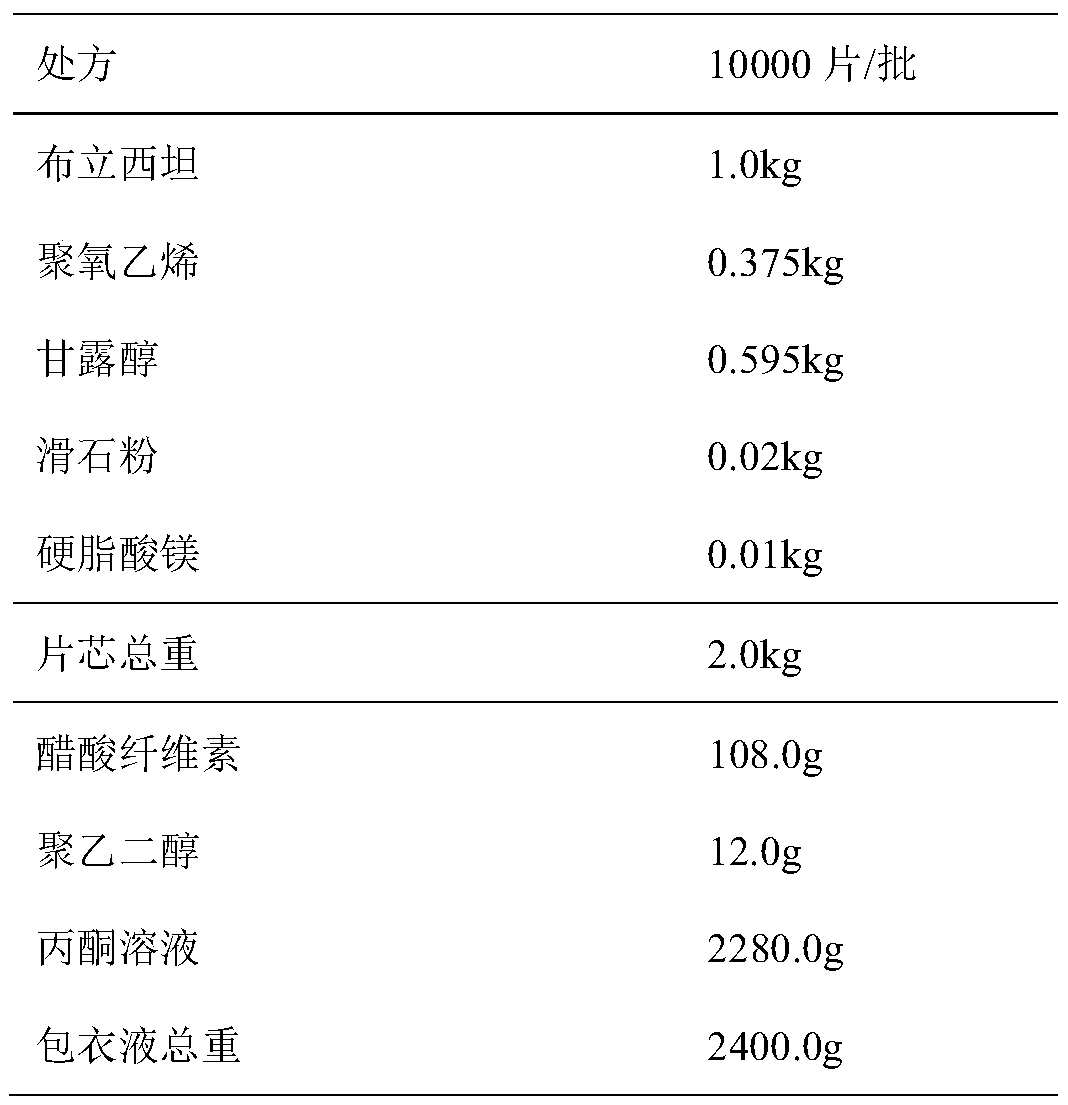
Controlled release pharmaceutical compositions of brivaracetam
The present invention relates to controlled release pharmaceutical compositions comprising Brivaracetam or its pharmaceutically acceptable derivatives thereof. Further disclosed is a controlled release pharmaceutical composition comprising a core and a coating surrounding the core, wherein the core comprises Brivaracetam or pharmaceutically acceptable derivative thereof and the coating comprises hydrophobic release controlling agent. The controlled release pharmaceutical composition comprises Brivaracetam or pharmaceutically acceptable derivatives thereof and hydrophobic release controlling agent, wherein said composition has dissolution of Brivaracetam at least 80% between about 7 to about 24 hours when measured in 900 ml of pH 6 phosphate buffer solution using USP apparatus type II, at 50 rpm and at 37° C. Also disclosed is a controlled release pharmaceutical composition useful for the treatment of epilepsy and treatment of symptomatic myoclonus comprises Brivaracetam or pharmaceutically acceptable derivative thereof and hydrophobic release controlling agent.
Owner:LUPIN LTD
Method for preparing furanone compounds
ActiveCN106588831AHigh chiral purityShort synthetic routeOrganic compound preparationPreparation from carboxylic acid esters/lactonesInorganic saltsSolvent
The invention discloses a method for preparing furanone compounds, and provides a method for preparing a furanone compound III. The method includes the following steps that in a solvent, in the presence of inorganic salt, a compound II and a reducing agent are subjected to a reduction reaction, and the furanone compound III is obtained; the solvent is a fatty alcohol solvent or a mixed solvent of a fatty alcohol solvent and water. The brivaracetam can be prepared with the furanone compound III only with the three steps, and the synthetic route is short; the ee value of the compound II is larger than 99.0%, racemization does not occur in the reaction process, and the de value of a brivaracetam I crude product is larger than 99.0%; the brivaracetam I crude product is further purified through a crystal instead of a chirality high-pressure-liquid-phase preparing column, and the chirality purity of brivaracetam I can be further increased to be the de value of 99.80% or above; meanwhile, the content of other individual impurities of the brivaracetam I is smaller than 0.1%, and reaches the API level, and the method is suitable for industrial production. The formula is defined in the description.
Owner:上海云晟研新生物科技有限公司
Method for preparing brivaracetam intermediate
ActiveCN109852644AMild reaction conditionsHigh yieldOrganic chemistryFermentationBrivaracetamOrganic chemistry
The invention provides a method for preparing a brivaracetam intermediate and particularly discloses preparation of the brivaracetam intermediate from part of alcohol dehydrogenase. The brivaracetam intermediate preparation method which is mild in reaction conditions, high in yield and low in cost is obtained on the basis.
Owner:ABIOCHEM BIOTECH CO LTD
Preparation method of brivaracetam isomer (2S,4S)
ActiveCN109593055AReduce usageRaw materials are cheap and easy to getOrganic compound preparationCarboxylic acid amides optical isomer preparationAlcoholSolvent
The invention discloses a preparation method of a brivaracetam isomer (2S,4S). The preparation method comprises the following step: in an alcohol solvent, carrying out a salt forming reaction as shownin a formula (shown in the specification) on a compound I and a resolving agent to obtain a compound as of a formula S, wherein the resolving agent is (+)-camphorsulfonic acid, D-(+)-camphanic acid,L-(-)-dibenzoyltartaric acid, D-(+)-dibenzoyltartaric acid, L-(-)-tartaric acid, L-(-)-phenylalaninol or S-(-)-phenylethylamine. The preparation method provided by the invention is low in price and easily available in raw material and mild in reaction condition. By adopting a chemical resolution method, a chromatographic column is not used, so that the preparation method is simple to operate, lowin cost and suitable for production on a large scale. The formula is as shown in the description.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Brivaracetam controlled-release preparation and preparation method thereof
InactiveCN111407738AReduce the number of daily dosesIdeal daily recommended dosageOrganic active ingredientsNervous disorderDigestive canalCoating system
The invention relates to a brivaracetam oral controlled-release preparation and a preparation method thereof. The controlled-release preparation is a single-chamber osmotic pump controlled-release preparation, and is composed of a drug tablet core, a membrane-controlled coating system and a drug-release hole. The drug tablet core comprises 30-60wt% of brivaracetam, and the membrane-controlled coating accounts for 3-8 (w / w, %) of the total preparation. The controlled-release preparation disclosed by the invention has an in-vitro zero-level constant-speed drug release curve, can form a sphericalpreparation after water absorption swelling, and is not prone to being adhered to the wall of a digestive tract to cause too high local drug concentration.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Brivaracetam intermediate and preparation method thereof
The invention provides a compound, 3-(((S)-1-amino-1-oxobutyl-2-yl)amino)methyl)hexanoic acid (I) and a preparation method thereof. The compound (I) comprises (R)-3-(((S)-1-amino-1-oxobutyl-2-yl)amino)methyl)hexanoic acid (I)-R, or (S)-3-(((S)-1-amino-1-oxobutyl-2-yl)amino)methyl)hexanoic acid (I)-S, or a mixture of (I)-R and (I)-S in any proportion. The compound shown in the formula (I) can be used for synthesizing brivaracetam, and a new thought and a new method are provided for designing a concise and efficient route for synthesizing brivaracetam.
Owner:SHANGHAI PUYI CHEM CO LTD
Ketene reductase and preparation method of brivaracetam intermediate
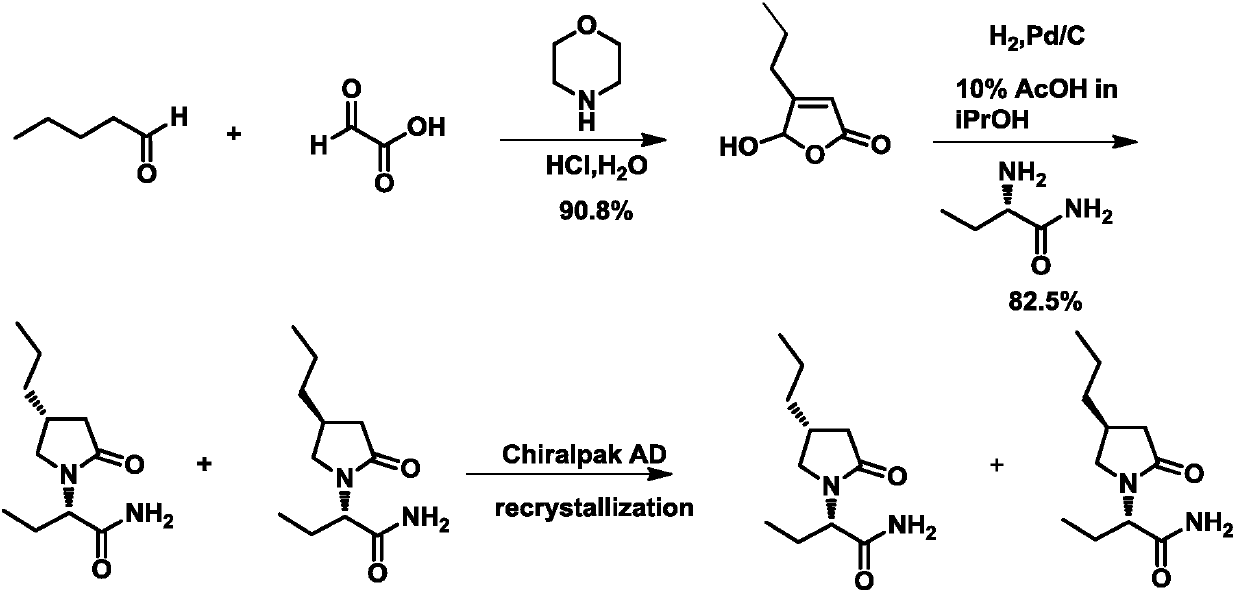
The invention relates to the technical field of medical intermediates, in particular to ketene reductase and a preparation method of a brivaracetam intermediate. The preparation method comprises the following steps of enabling valeraldehyde and glyoxylic acid to react under the catalysis of morpholine to generate 5-hydroxy-4-n-propyl-2(5H)-furanone; enabling the 5-hydroxy-4-n-propyl-2(5H)-furanoneto remove hydroxyl under the catalysis of sodium borohydride to generate 4-n-propyl-2(5H)-furanone; and in the presence of the ketene reductase, enabling the 4-n-propyl-2(5H)-furanone to have a reduction reaction to generate a target product namely the brivaracetam intermediate. The nucleotide sequence of the ketene reductase is as shown in SEQID NO.1, and the amino acid sequence of the ketene reductase is as shown in SEQID NO.2. According to the preparation method of the brivaracetam intermediate disclosed by the invention, only 3-step reactions are needed, the brivaracetam intermediate canbe prepared, additional chemical resolution is not needed, and the whole technology is environmentally-friendly, low in cost and easy to implement.
Owner:三明旻和医药科技有限公司
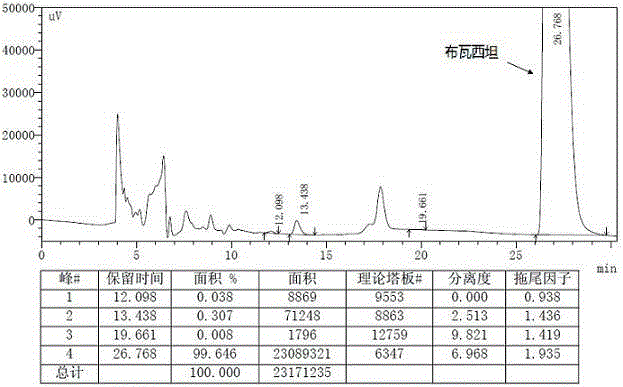
Brivaracetam isomer detection method
InactiveCN109932442AEasy to separateAccurate separationComponent separationCompound (substance)Active ingredient
The invention provides a brivaracetam isomer detection method. According to the detection method, high performance liquid chromatography is adopted. The operation process of the method includes the following steps of: (1) solution preparation; (2) detection; and (3) chromatogram analysis. According to the brivaracetam isomer detection method of the invention, specific chromatographic conditions are selected, and therefore, brivaracetam and the optical isomer impurities thereof can be separated out and detected simply and quickly, and therefore, the quality of basistatin and the preparations thereof can be controlled. With the method adopted, isomer content can be measured, and the development of a synthesis process can be guided, and the formulation of the quality standards of bulk pharmaceutical chemicals can be facilitated.
Owner:成都美域高制药有限公司
Detection method for brivaracetam and related substances thereof
ActiveCN110824093AQuality improvementImprove securityComponent separationBulk chemical productionGradient elutionBrivaracetam
The invention relates to a detection method for brivaracetam and related substances thereof. The detection method comprises a step of adopting high performance liquid chromatography to perform detection. The following liquid chromatography conditions are adopted: a reverse chromatographic column is adopted; a mobile phase A is a mixed solution of acetonitrile and a buffer solution in a volume ratio of (0-5):(95-100); a mobile phase B is a mixed solution of acetonitrile and a buffer solution in a volume ratio of (95-100):(0-5), and gradient elution is performed; the buffer solution contains 0.001 mol / L-0.1mol / L of a cationic ion pair reagent and 0.001 mol / L-0.1mol / L of buffer salt; and the pH value of the buffer solution is lower than 6. The detection method provided by the invention can realize full separation of brivaracetam and related substances thereof, is high in analysis sensitivity and accurate and reliable in result, can effectively improve the drug quality control level and safety of brivaracetam and reduces the risk of drug use.
Owner:北京海晶生物医药科技有限公司
Preparation method of (R)-3-propyl-gamma-butyrolactone
ActiveCN108530402ASimple process routeMild reaction conditionsOrganic chemistrySulfonateGrignard reagent
The invention discloses a preparation method of (R)-3-propyl-gamma-butyrolactone. The method comprises the following steps: with (S)-3-hydroxyl-gamma-butyrolactone as an initial raw material, activating hydroxyl by using sulfonate, reacting the hydroxyl-activated compound with a Grignard reagent in the presence of a copper catalyst, a cocatalyst and a nitrogen-containing compound to generate (R)-3-propyl-gamma-butyrolactone. The preparation method has the advantages that the process route is simple, the raw materials are cheap and easy to obtain, the synthesis route is short, the reaction conditions are mild, the operation is simple and convenient, the yield is high, and the stereoselectivity is good, the problems of the prior art that chiral separation and chiral column separation are needed in reaction, the yield is low and the chemical selectivity is poor are solved, and an important value is provided for the process research of brivaracetam.
Owner:ZHEJIANG UNIV OF TECH
Intermediate compounds for preparing brivaracetam, and preparation method and application thereof
ActiveCN108929289AImprove qualityHigh optical purityOrganic chemistry methodsMetal/metal-oxides/metal-hydroxide catalystsCompound aSilica gel
The invention provides intermediate compounds for preparing brivaracetam, and a preparation method and an application thereof. The invention also provides an intermediate compound A and an intermediate compound B and preparation methods thereof, and a synthetic route for preparing the brivaracetam by adopting the intermediate compound B. According to a technical scheme of the invention, the brivaracetam with high quality and high optical purity and intermediates thereof can be obtained; the ratio of the brivaracetam in four optical isomers is more than 99.5%; a silica gel column is not neededto be used for separation and purification; expensive chiral high-performance liquid chromatography is not needed to be used for resolution; complicated separation and purification steps are avoided;the waste of raw materials is avoided; the cost of production is reduced; and the preparation methods provided by the invention are more applicable to industrial production.
Owner:SHANGHAI BOC CHEM CO LTD
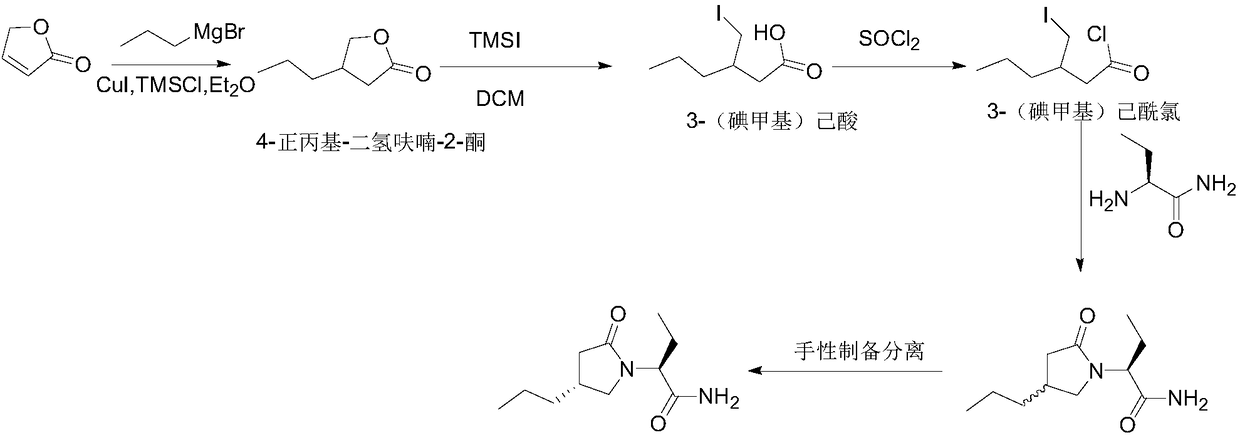
Preparing method for caproic acid derivative
ActiveCN106588740AHigh chiral purityShort synthetic routeOrganic compound preparationPreparation from carboxylic acid esters/lactonesTwo stepHigh pressure
The invention discloses a preparing method for a caproic acid derivative and provides a preparing method for a caproic acid derivative IV. The method comprises the following steps that in a polar nonprotic organic solvent, a compound III and an iodization reagent are subjected to a nucleophilic substitution reaction under inert gas shielding, and the caproic acid derivative IV is obtained. Brivaracetam can be prepared from the caproic acid derivative IV only through two steps, the synthetic route is short, the reaction condition is mild, posttreatment is simple, the reaction yield is high, and the production cost is low. Racemization does not happen in the reaction process, further purification is conducted through crystallization instead of a chiral high-pressure liquid phase preparing column, the chiral purity of the brivaracetam I can be improved till the de value is larger than 99.80%, meanwhile, the content of other single impurities of the brivaracetam I is smaller than 0.1%, the API level is reached, and the method is suitable for industrial production. The expression is shown in the description.
Owner:上海云晟研新生物科技有限公司
Processes to produce brivaracetam
ActiveUS20180155284A1High enantiomeric purityCost effectiveOrganic active ingredientsNervous disorderBrivaracetamRelated derivatives
The present invention provides a scalable synthesis of enantiomerically pure brivaracetam, and related derivatives.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
A compound, its preparation method and its use in the synthesis of Buvaracetam
ActiveCN106279074BRaw materials are easy to getLow priceOrganic chemistryCombinatorial chemistryBrivaracetam
The application provides a compound of formula I and a preparation method thereof. The application also provides the use of the compound of formula I for synthesizing buvaracetam and the synthesis method. The raw materials involved in the method described in this application are easy to obtain and cheap, and can prepare brivaracetam with high optical purity.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Novel brivaracetam intermediate as well as synthesis method thereof and application
InactiveCN108409557AHigh optical purityCheap and easy to getOrganic compound preparationOrganic chemistry methodsChromatographic separationSynthesis methods
The invention discloses a novel brivaracetam intermediate as well as a synthesis method thereof and application. A structural form of the intermediate is shown in the description. A reaction route forsynthesizing brivaracetam from the intermediate is shown in the description. The novel brivaracetam intermediate disclosed by the invention is used for synthesizing brivaracetam, and has the following beneficial effects: raw materials are cheap and easily available, the reaction route is short, the yield is high, and brivaracetam with high optical purity can be obtained without chiral resolutionor column chromatography separation. According to the brivaracetam synthesis process, the raw materials are cheap and easily available, the reaction route is short, the yield is high, and brivaracetamwith high optical purity can be obtained without chiral resolution or column chromatography separation.
Owner:LIVZON NEW NORTH RIVER PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com