Preparation method of brivaracetam isomer (2S,4S)
A volume, isopropanol technology, applied in the field of preparation of buvaracetam isomers, can solve the problems of high cost and high requirements for reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
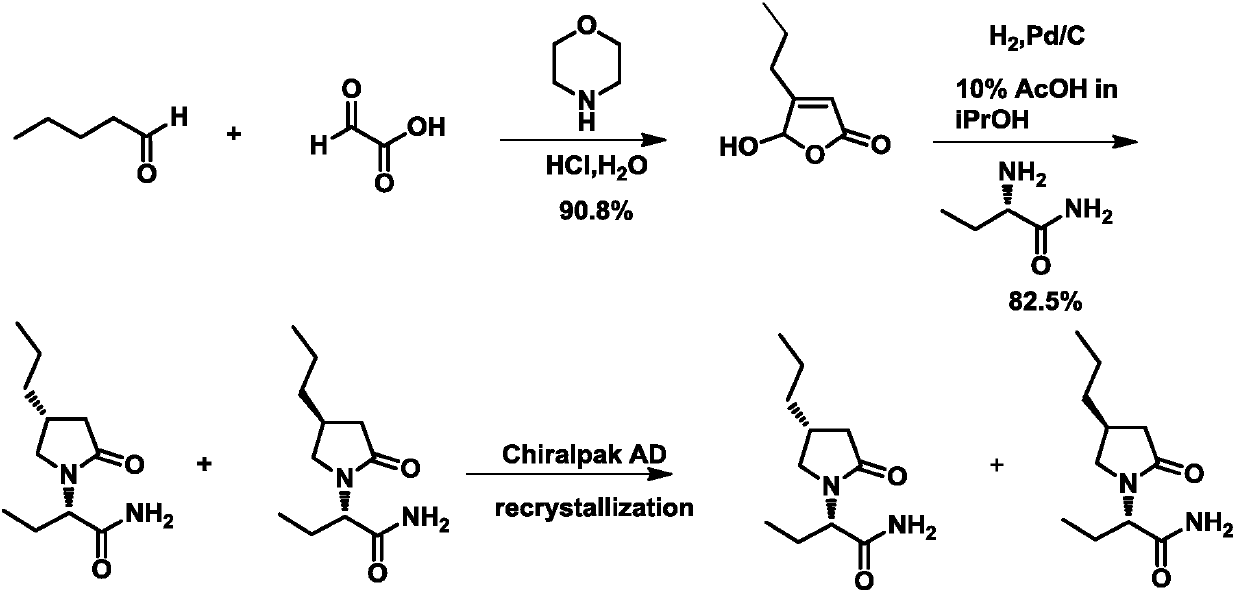
[0074] 1. Synthesis of 5-hydroxy-4-n-propyl-furan-2-one
[0075]
[0076] Add heptane (131mL) and morpholine (43mL) into a 500mL four-necked flask and stir mechanically. When t=0°C, add 50mL of glyoxylic acid and react at 20°C for 1h. 49.6 mL of n-valeraldehyde was added dropwise, followed by t=43°C for 20 h. After the reaction was completed, the reaction was quenched with 65 mL of concentrated hydrochloric acid, and stirred at room temperature for 2 h. The liquid was separated, and the aqueous phase was washed with heptane, extracted with isopropyl ether, washed with salt, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 51.8 g of brown oil with a yield of 83.1%. ESI-MS (m / z): 141 [M-H] - .
[0077] 2. Synthesis of (2S)-2-(2-oxo-4-n-propyl-5-hydroxyl-1-pyrrolidinyl)butanamide
[0078]
[0079] First dissociate an appropriate amount of 18g (S)-2-aminobutanamide hydrochloride with 100mL ammonia in iso...
Embodiment 2
[0106] 1. Synthesis of 5-hydroxy-4-n-propyl-furan-2-one
[0107]
[0108] Add heptane (65.6mL) and morpholine (21.3mL) into a 250mL four-neck flask and stir mechanically. When t=0°C, add 25mL of glyoxylic acid and react at 20°C for 1h. 24.8 mL of n-valeraldehyde was added dropwise, followed by t=43°C for 20 h. After the reaction was completed, the reaction was quenched with 33 mL of concentrated hydrochloric acid, and stirred at room temperature for 2 h. The liquid was separated, and the aqueous phase was washed with heptane, extracted with isopropyl ether, washed with salt, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 25.8 g of brown oil with a yield of 82.7%. ESI-MS (m / z): 141 [M-H] - .
[0109] 2. Synthesis of (2S)-2-(2-oxo-4-n-propyl-5-hydroxyl-1-pyrrolidinyl)butanamide
[0110]
[0111] First free an appropriate amount of (S)-2-aminobutyramide hydrochloride, and then react with 20 g of 5-hyd...
Embodiment 3
[0125] 1. Synthesis of 5-hydroxy-4-n-propyl-furan-2-one
[0126]
[0127]Add heptane (65.6mL) and morpholine (21.3mL) into a 250mL four-necked flask and stir mechanically. At t=0°C, add 25mL of glyoxylic acid and react at 20°C for 1 hour. Add 24.8mL of n-valeraldehyde dropwise, then t=0°C 43°C, 20h. After the reaction was completed, the reaction was quenched with 33 mL of concentrated hydrochloric acid, and stirred at room temperature for 2 h. The liquid was separated, and the aqueous phase was washed with heptane, extracted with isopropyl ether, washed with salt, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 25.6 g of brown oil with a yield of 82.1%. ESI-MS (m / z): 141 [M-H] - .
[0128] 2. Synthesis of (2S)-2-(2-oxo-4-n-propyl-5-hydroxyl-1-pyrrolidinyl)butanamide
[0129]
[0130] First free an appropriate amount of (S)-2-aminobutyramide hydrochloride, and then react with 20 g of 5-hydroxy-4-n-pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com