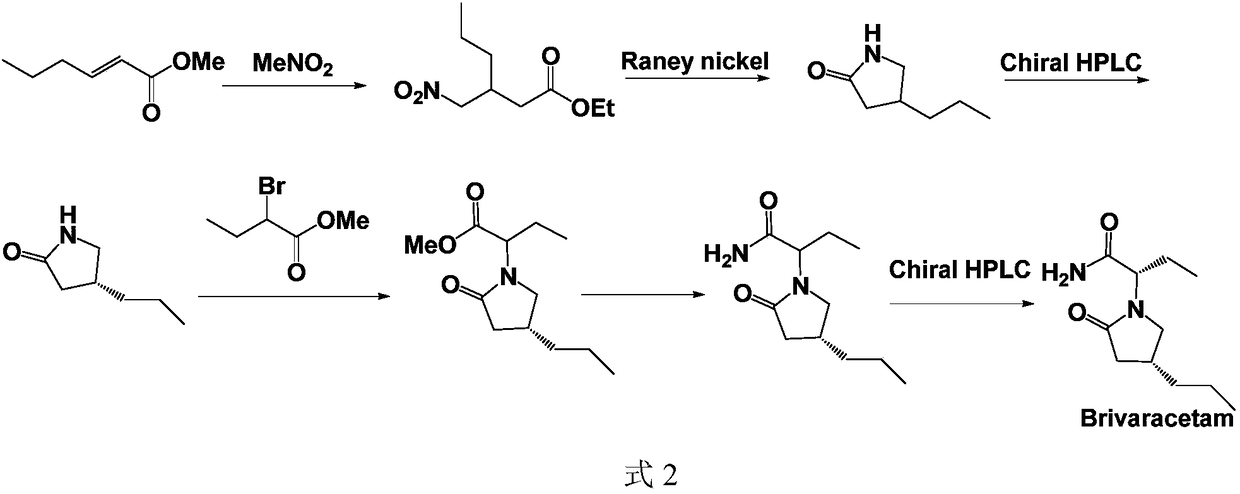
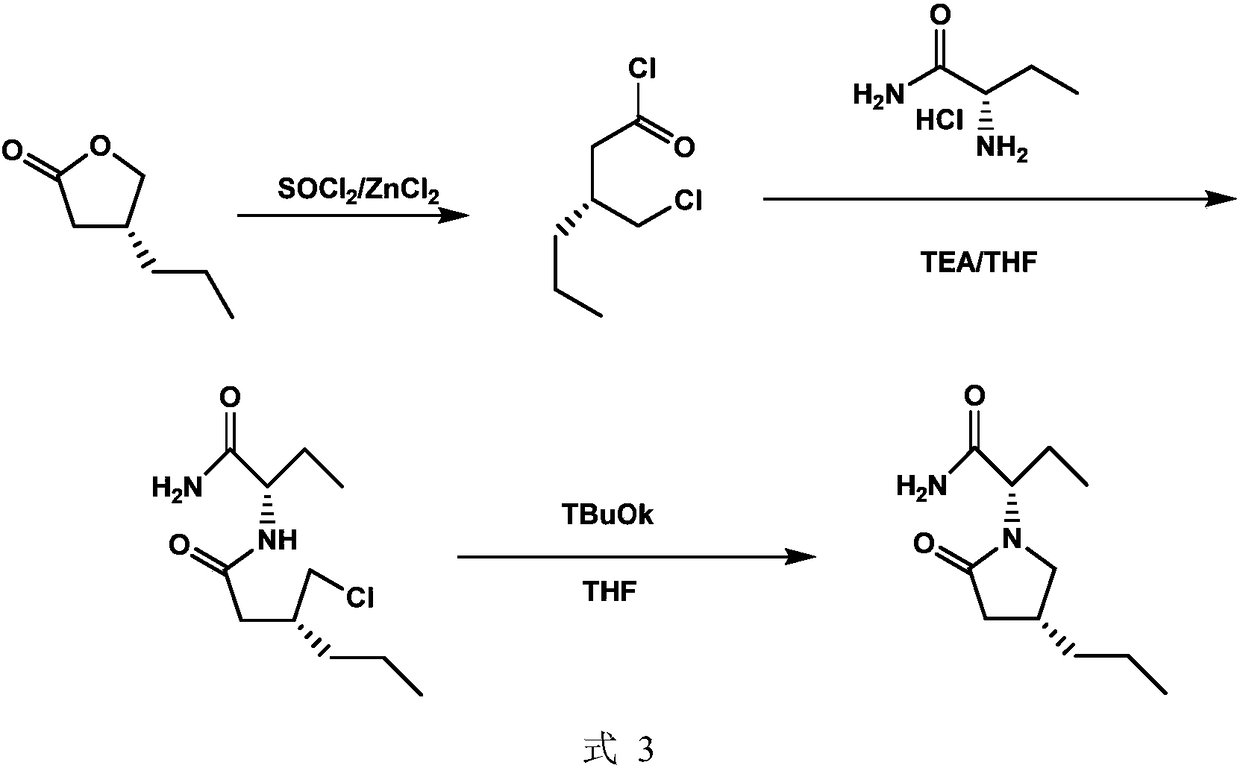
Novel brivaracetam intermediate as well as synthesis method thereof and application
A synthetic method and synthetic route technology, applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems that are not suitable for industrial production, the difficulty of amide condensation, and low yields, and achieve the goal of raw materials The effect of low price, low price, and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Compound of formula II Preparation of:
[0054] 1) Dissolve R-4-n-propyl-dihydrofuran-2-one (20g, 156mmol) in acetic acid, add 33% HBr in acetic acid solution (100ml) dropwise, and react at 55°C for 3h;
[0055] 2) Cool to room temperature, add toluene (120ml) and water (20ml), stir and separate the liquids, extract the water phase with toluene (2*60ml), combine the organic phases, wash with saturated brine, dry with anhydrous sodium sulfate, and filter , and concentrated in vacuo to obtain 14.7 g of light yellow oil, which is the compound of formula II.
[0056] Yield is 90%, HPLC purity > 97%, ee purity > 99%, 1 H NMR (300MHz, CDCl 3 ): δ11.0(1H,s), 3.20(1H,dd), 3.10(1H,dd), 2.32(1H,dd), 2.20(1H,dd), 1.25-1.33(4H,m), 0.96( 3H,t).
[0057] Compound of formula III Preparation of:
[0058] Take the compound of formula II (20.8g, 100mmol) and dissolve it in toluene (60ml), then add thionyl chloride (35.7g, 300mmol) dropwise, stir at room temperature for 6 hours, ...
Embodiment 2
[0066] Compound of formula II Preparation of:
[0067] 1) Dissolve R-4-n-propyl-dihydrofuran-2-one (40g, 312mmol) in acetic acid, add 33% HBr in acetic acid solution (200ml) dropwise, and react at 40-50°C for 4h;
[0068] 2) Cool to room temperature and add toluene (240ml) and water (40ml), stir and separate the liquids, extract the aqueous phase with toluene (2*120ml), combine the organic phases, wash with saturated brine, dry with anhydrous sodium sulfate, and filter , and concentrated in vacuo to obtain 29.7 g of light yellow oil, which is the compound of formula II.
[0069] Yield is 91%, HPLC purity > 97%, ee purity > 99%, 1 H NMR (300MHz, CDCl 3 ): δ11.0(1H,s), 3.20(1H,dd), 3.10(1H,dd), 2.32(1H,dd), 2.20(1H,dd), 1.25-1.33(4H,m), 0.96( 3H,t).
[0070] Compound of formula III Preparation of:
[0071] Dissolve the compound of formula II (20.8g, 100mmol) in dichloromethane (60ml), then add triphosgene (44.5g, 150mmol) dropwise, stir at room temperature for 6 hours, ...
Embodiment 3
[0079] Compound of formula II Preparation of:
[0080] 1) Dissolve R-4-n-propyl-dihydrofuran-2-one (5.0Kg, 39mol) in acetic acid, add 33% HBr in acetic acid solution (25L) dropwise, and react at 20°C for 6h;
[0081] 2) Cool to room temperature, add toluene (30L) and water (5L), stir and separate the liquids, extract the water phase with toluene (2*15L), combine the organic phases, wash with saturated brine, dry with anhydrous sodium sulfate, and filter , and concentrated in vacuo to obtain 3.8 Kg of light yellow oil, which is the compound of formula II.
[0082] Yield is 93%, HPLC purity > 97%, ee purity > 99%, 1 H NMR (300MHz, CDCl 3 ): δ11.0(1H,s), 3.20(1H,dd), 3.10(1H,dd), 2.32(1H,dd), 2.20(1H,dd), 1.25-1.33(4H,m), 0.96( 3H,t).
[0083] Compound of formula III Preparation of:
[0084] Take the compound of formula II (5.2Kg, 25mol) and dissolve it in toluene (15L), then add thionyl chloride (8.9kg, 75mol) dropwise, stir at room temperature for 6 hours, distill off ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com