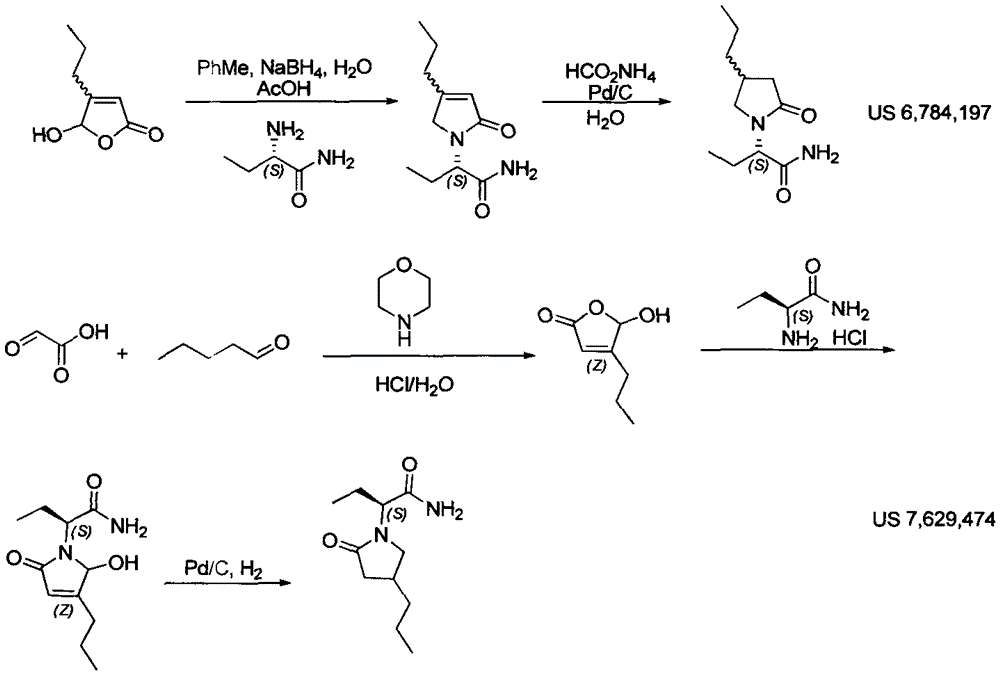
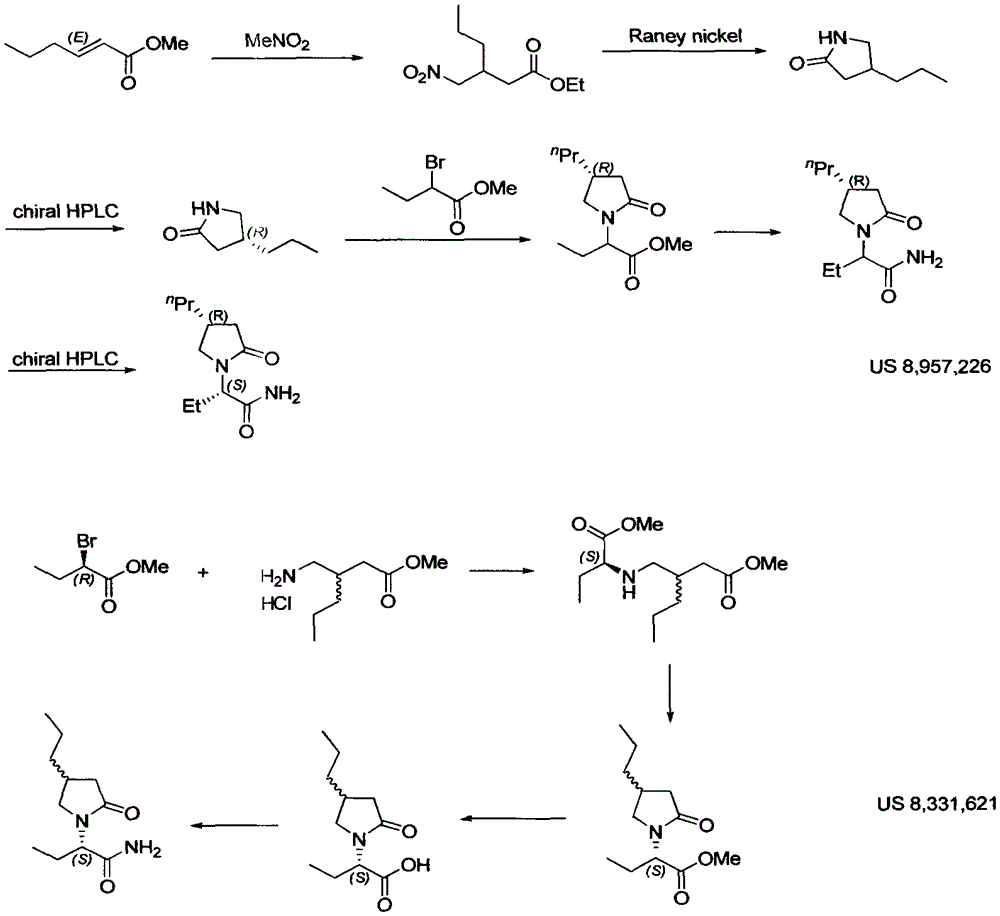
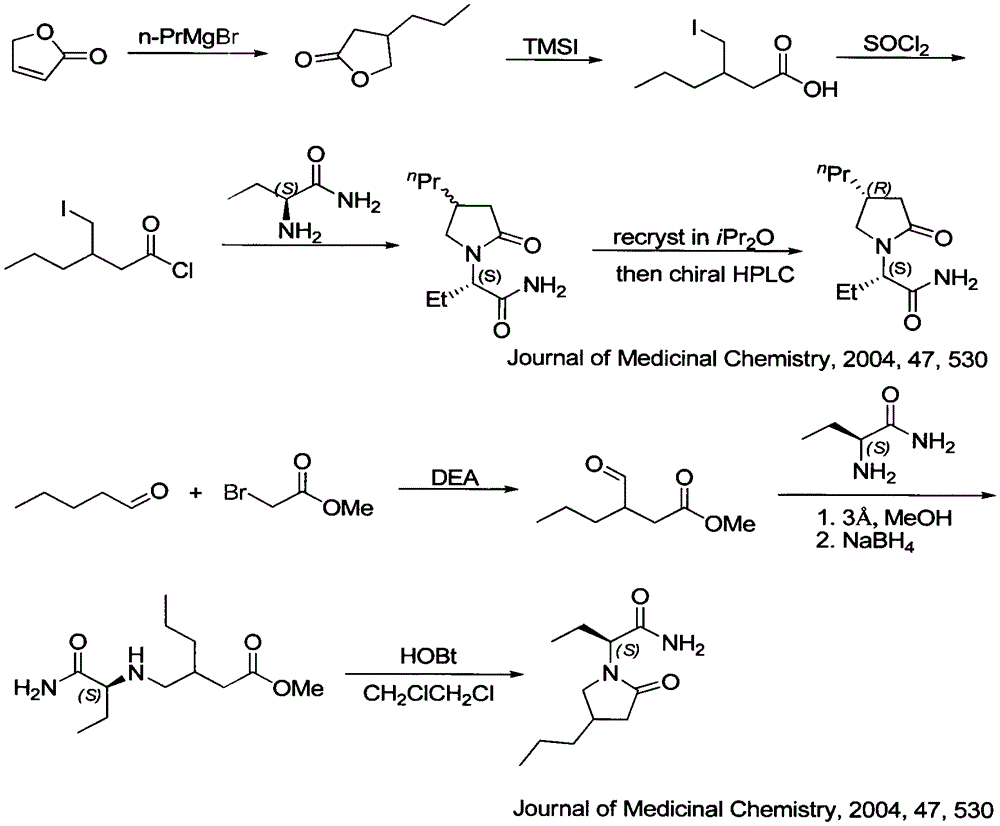
Preparation method of brivaracetam
A compound and equivalent technology, applied in the direction of organic chemistry, can solve the problems of uneconomical utilization of raw materials, no construction of butyrolactam, etc., and achieve the effects of low price, easy separation and purification, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1 prepares compound 3
[0057] Sodium methoxide (2.0kg, 37.03mol) was added into 44.5kg of absolute ethanol to dissolve completely, and diethyl malonate (6.0kg, 37.46mol) was added at an external temperature of 10°C. Stir at this temperature for 10 minutes, the system rises to room temperature, slowly add (R)-epichlorohydrin (ee>99%) (3.3kg, 35.67mol) (purchased from Anaiji Chemical) to the reaction system, add After completion, the system reacted under reflux for 2 hours, stopped the reaction, cooled the system to room temperature, spin-dried the solvent, added 19.03 kg of water, and extracted twice with 17.0 kg and 12 kg of ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, filtered after drying, the filtrate was spin-dried, and distilled under reduced pressure to obtain a colorless liquid. Compound 3 was obtained with a yield of 50%. Compound 3 chiral HPLC (ee 98.5%)
[0058] The NMR data of compound 3 are as follows: ...
Embodiment 2
[0059] Embodiment 2 prepares compound 4
[0060] Add a 2-methyltetrahydrofuran solution (1.29mol / kg, 2.44kg, 3.14mol) of ethyl Grignard reagent to the reaction kettle, control the internal temperature at -20--30°C, add CuI (108.3g, 0.57mol) The reaction system was stirred for 30 min, and then the dry 2-methyltetrahydrofuran solution of compound 3 (434 g, 2.55 mol) prepared by the method described in Example 1 was added dropwise to the reaction flask. After the dropwise addition was completed, after stirring at this temperature for 30 minutes, the reaction was quenched with saturated ammonium chloride, and the solution of compound 4 in 2-methyltetrahydrofuran was obtained by liquid separation, with a yield of 64%.
[0061] Purification by column chromatography (developing solvent polarity: petroleum ether / ethyl acetate=10 / 1) to obtain the nuclear magnetic data of the purified compound 4 is as follows: 1 H NMR (400MHz, CDCl 3 )δ4.52(1H, dd), 4.27(2H, q), 3.92(1H, dd), 3.23(1...
Embodiment 3
[0063] Embodiment 3 prepares compound 4
[0064] Add a solution of ethyl Grignard reagent in 2-methyltetrahydrofuran (1.29kg / mol, 63.51g, 81.93mmol) into the reaction flask, place the reaction flask in a low-temperature reaction bath, and control the internal temperature to -20--30°C, Add CuI (2.22g, 11.70mol) to the reaction flask and stir for 0.5 hours, then add dropwise the dry 2-methyl alcohol of compound 3 (10.0g, 58.52mmol) prepared as described in Example 1 to the reaction flask. THF solution. After the dropwise addition was completed, after stirring at this temperature for 30 minutes, the reaction was quenched with saturated ammonium chloride, and the solution of compound 4 in 2-methyltetrahydrofuran was obtained by liquid separation, with a yield of 87%.
[0065] Purification by column chromatography (developing solvent polarity: petroleum ether / ethyl acetate=10 / 1) to obtain the nuclear magnetic data of the purified compound 4 is as follows: 1 H NMR (400MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com