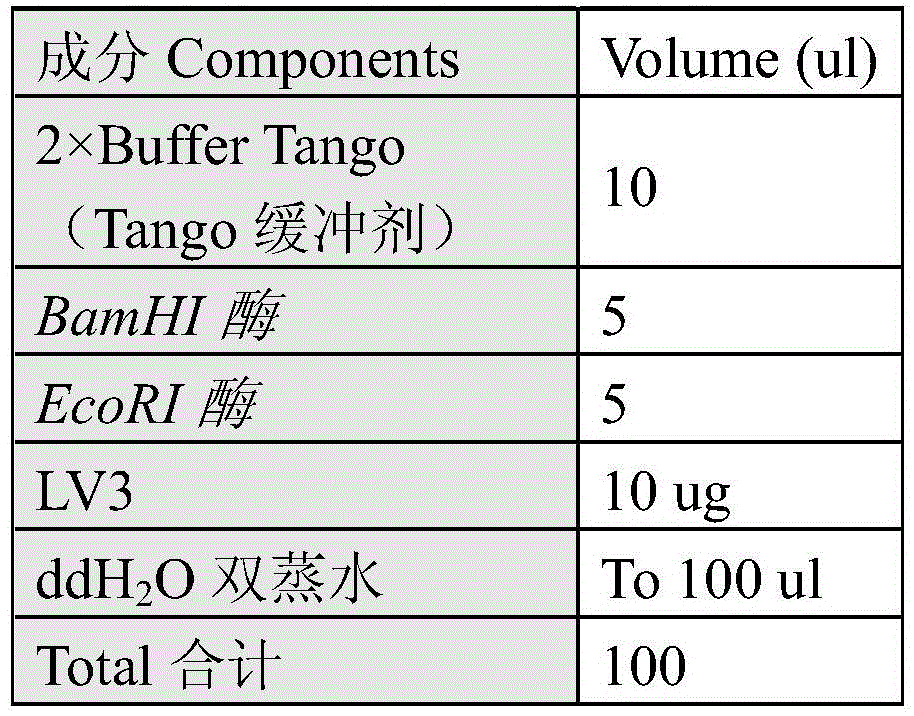
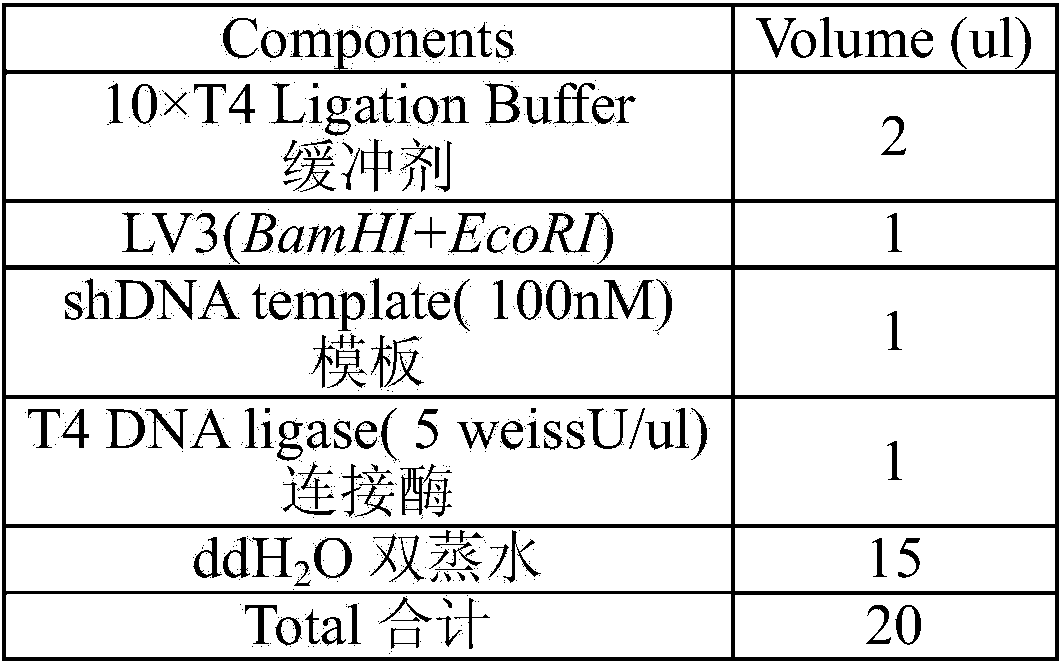
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Intractable epilepsy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ketogenic nutritional powder
ActiveCN102648749AEffective way of absorptionImprove immunityFood preparationAnimal proteinBifidus factor
The invention discloses ketogenic nutritional powder applied to ketogenic therapy on patients suffering from intractable epilepsy. The ketogenic nutritional powder comprises the following components as per parts by weight: 69.45 to 72 parts of fat, 7.2 to 10 parts of protein, 8 to 14 parts of carbohydrate, 4.5 to 5 parts of mineral salt and less than 2 parts of emulsifying agent, flavoring agent and thickening agent, wherein the fat comprises plant oil; the protein comprises plant protein, animal protein, soybean oligopeptide and immune globulin; the carbohydrate comprises soluble dietary fiber and bifidus factor; and the weight ratio of the fat to the sum of the protein and the carbohydrate is (3.2-4.0):1. Due to addition of the immune globulin, the soybean oligopeptide, the dietary fiber and the bifidus factor, the ketogenic nutritional powder can effectively adjust the intestine health of the patients, can enhance the immunity of the patients, and has a good absorption and utilization effect in the bodies of the patients.
Owner:广州金酮医疗科技有限公司
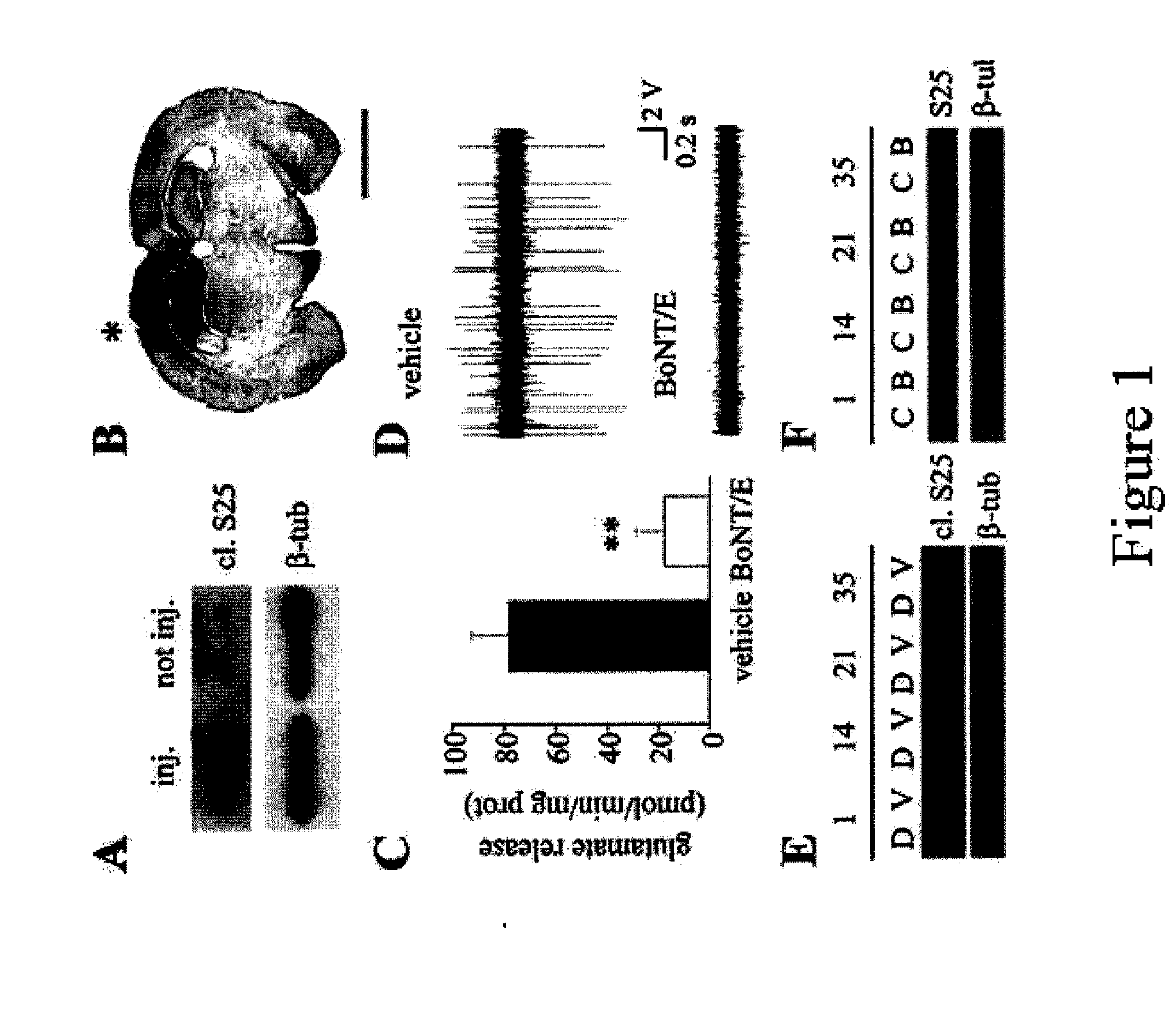
Method for the Mapping of the Epileptogenic Focus in the Pre-Surgical Evaluation of Patients with Intractable Epilepsy
InactiveUS20070218084A1Choose accuratelyGood choiceBacterial antigen ingredientsDisease diagnosisDiseasePre-operative evaluation
A method for functionally identifying an epileptogenic focus in pre-surgical evaluation in affected subjects with intractable epilepsy is described, the method including the delivery of an effective dose of a botulinum neurotoxin (BoNT) to a presumptive epileptogenic focus in the disease-compromised central nervous system of a mammal, under conditions whereby the effective dose of the botulinum neurotoxin interacts with the soluble N-ethylmaleimide-sensitive factor-attachment receptor (SNARE) proteins, thus impairing neurotransmission.
Owner:FOND PIERFRANCO E LUISA MARIANI ONLUS
Closed-loop system used for epilepsy treatment
InactiveCN104173044AImprove the quality of lifeReduce seizuresDiagnostic recording/measuringSensorsEpilepsy treatmentLife quality
The invention discloses a closed-loop system used for epilepsy treatment. The closed-loop system includes an electroencephalogram signal amplification module, a control unit, a stimulation module and a video module, wherein the electroencephalogram signal amplification module is used for collecting an electroencephalogram signal of a target to be detected; the state of the electroencephalogram signal is judged by the control unit, and the control unit controls the stimulation module to generate pulse current to stimulate the target to be detected to restrain epileptic seizure and further, closed loop regulation and control can be realized. The closed-loop system has the following functions: multichannel data with high sampling frequency can be obtained in a real-time manner through an amplifier, real-time drawing is carried out, epilepsy early warning is realized based on electroencephalogram signals, and epilepsy can be automatically restrained through electrostimulation with designated parameters after early warning. The invention provides a close-loop electrostimulation treatment system for patients suffering from intractable epilepsy, and the closed-loop system effectively reduces frequency of epileptic seizure, alleviates the intensity of epileptic seizure, and improves the living quality of patients.
Owner:SECOND AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Scorpion venom heat-resisting polypeptide, and preparing method and applications thereof
InactiveCN104341495AGood effectDefine pharmacodynamic activity and pharmacodynamic targetsNervous disorderPeptide/protein ingredientsDiseaseMedicine
The invention provides a scorpion venom heat-resisting polypeptide, and a preparing method and applications thereof, and belongs to the field of polypeptide and preparation thereof. The scorpion venom heat-resisting polypeptide which is safer is obtained by removing heat-resisting and heat-labile toxic components mainly from the scorpion venom of buthus martensii kirsch (BmK). The polypeptide is used for preventing and treating intractable epilepsy, Parkinson's disease (PD), Alzheimer's disease (AD), and other diseases, has a common function target, and has respective special medicine effects. Preparation of the polypeptide is stable in process, simple and controllable.
Owner:张万琴
Child tuberous sclerosis treatment drug
The present invention relates to the field of medicines, and in particular discloses a child tuberous sclerosis treatment drug including rapamycin, the child tuberous sclerosis includes child tuberous sclerosis combined intractable epilepsy, child tuberous sclerosis combined cardiac rhabdomyoma, child tuberous sclerosis combined depigmentation, facial steatadenoma, and child tuberous sclerosis combined renal angioleiomyolipoma, and the drug use method is as follows: the initial dose of the rapamycin is 1mg / (m2.d), and the blood drug concentration is maintained at 5-10ng / mL.
Owner:邹丽萍 +1
Ketogenic mix preparation suitable for treating intractable epilepsy
InactiveCN101269210APromote brain developmentPromote fat metabolismNervous disorderPeptide/protein ingredientsAdditive ingredientMedicine
The invention relate to a ketogenesis mixture preparation applicable for treating refractory epilepsy, which is characterized in that: (1) fat, protein and carbohydrate form the basic prescription, a small amount of essential vitamins, minerals and aminophenol promoting the growth and the development of the human body and brain and promoting the metabolism are added in the basic prescription; (2) in the basic preparation, the weight percentages of the components are respectively that: fat 70-80 percent, protein 15-20 percent, carbohydrate 5-10 percent; (3) the physical states of all the components in the basic preparation are powder, ointment, aqua, or solid state agent. The physical states of the ketogenesis mixture preparation are powder granule, electuary, liquid agent, tablet, capsule or other solid state agent. The ketogenesis mixture preparation aims at the special nutrition requirement of Chinese, nutritional ingredient promoting the development of the brain and the metabolism is added in, the curative efficiency on refractory epilepsy reaches 80 percent, which reaches or exceeds the curative effect of the equivalent product abroad.
Owner:邓荣光
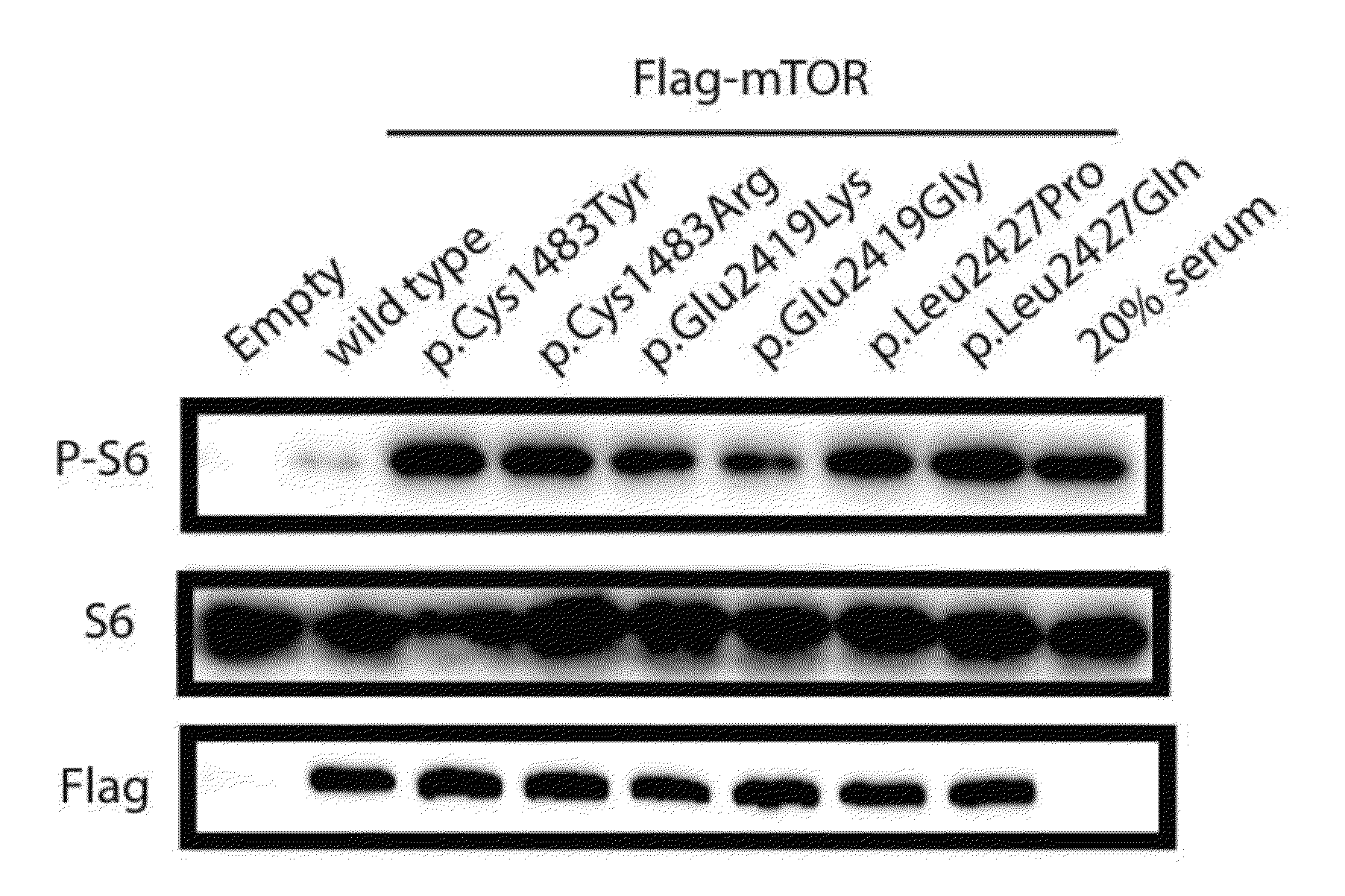
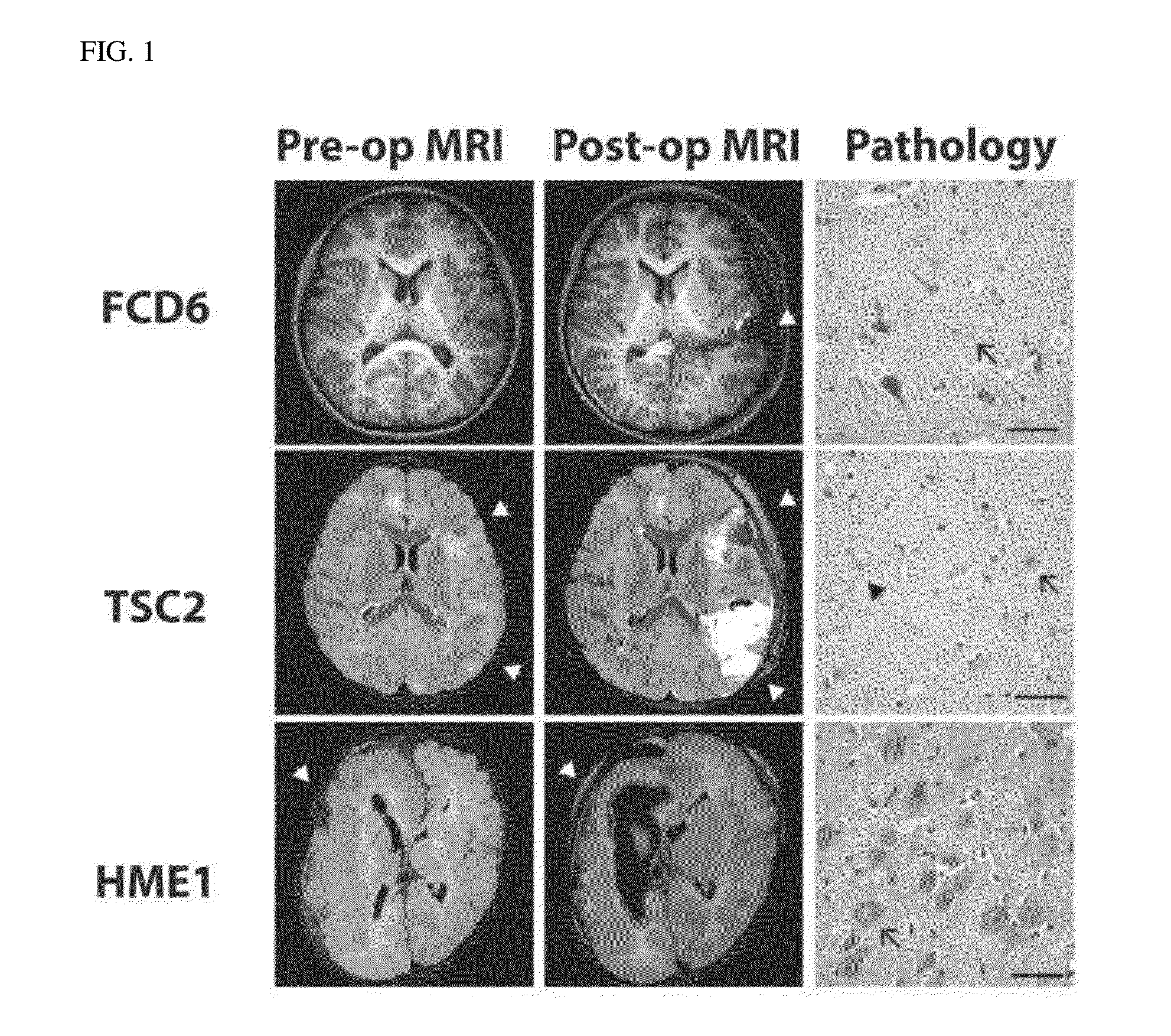
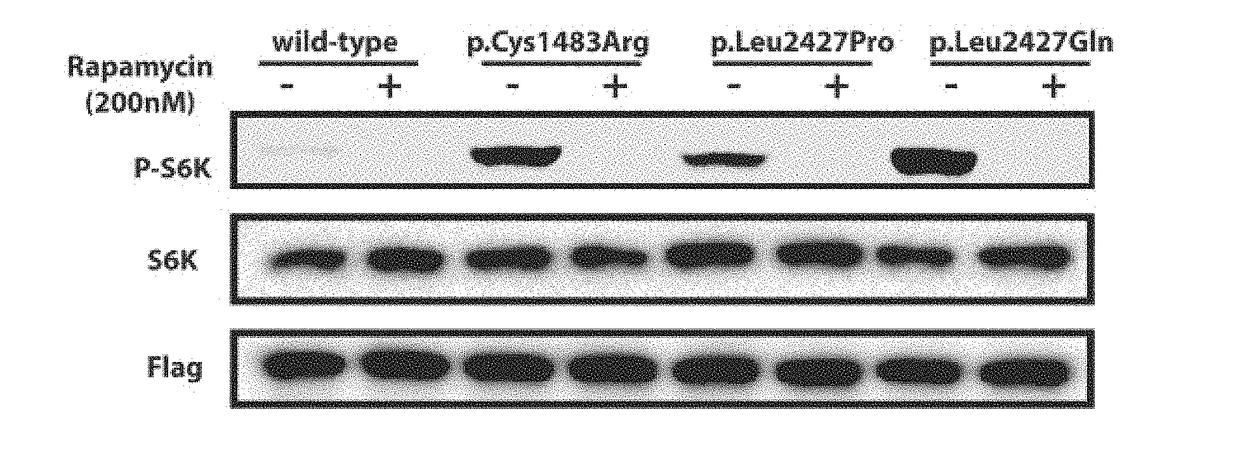
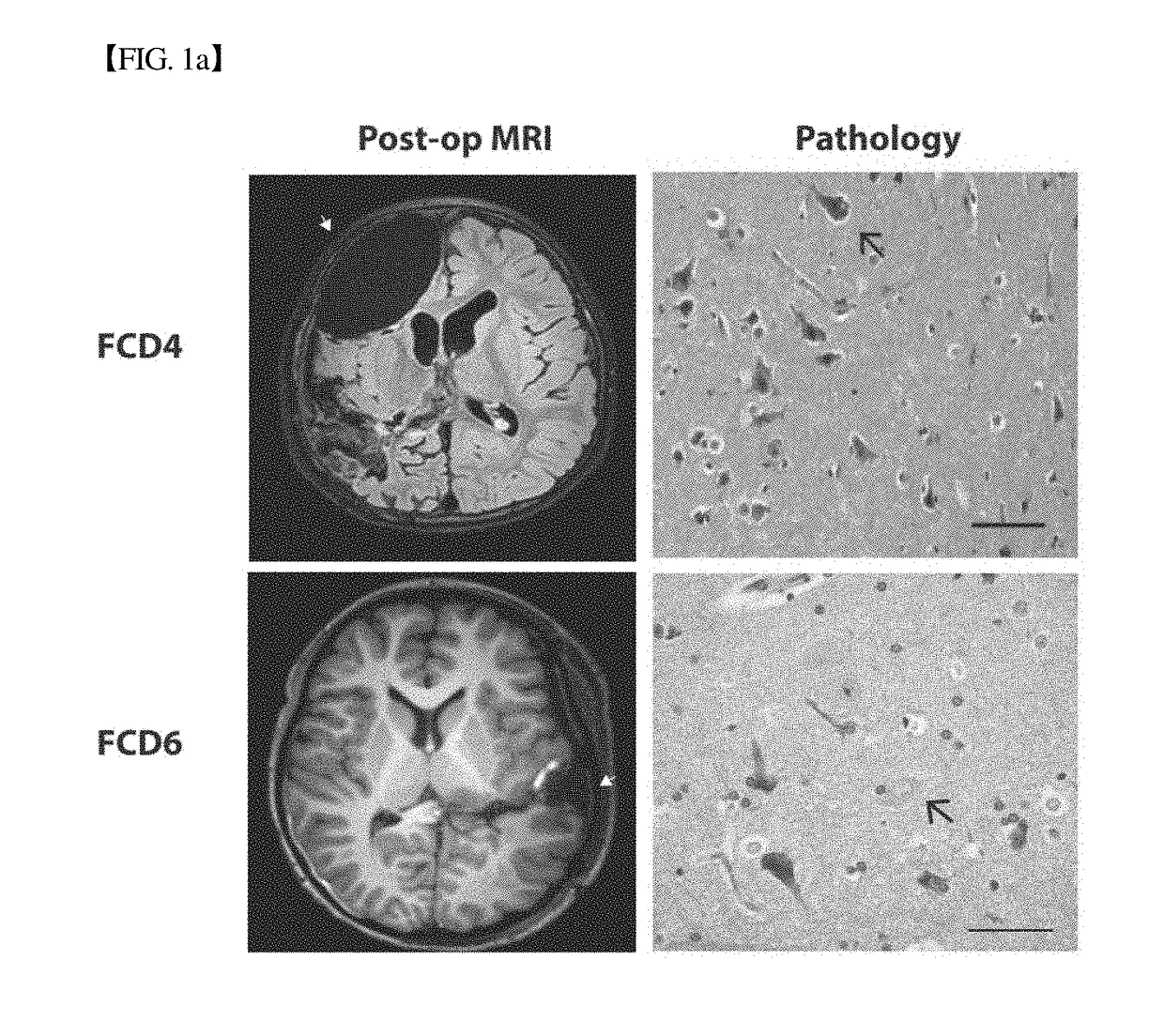
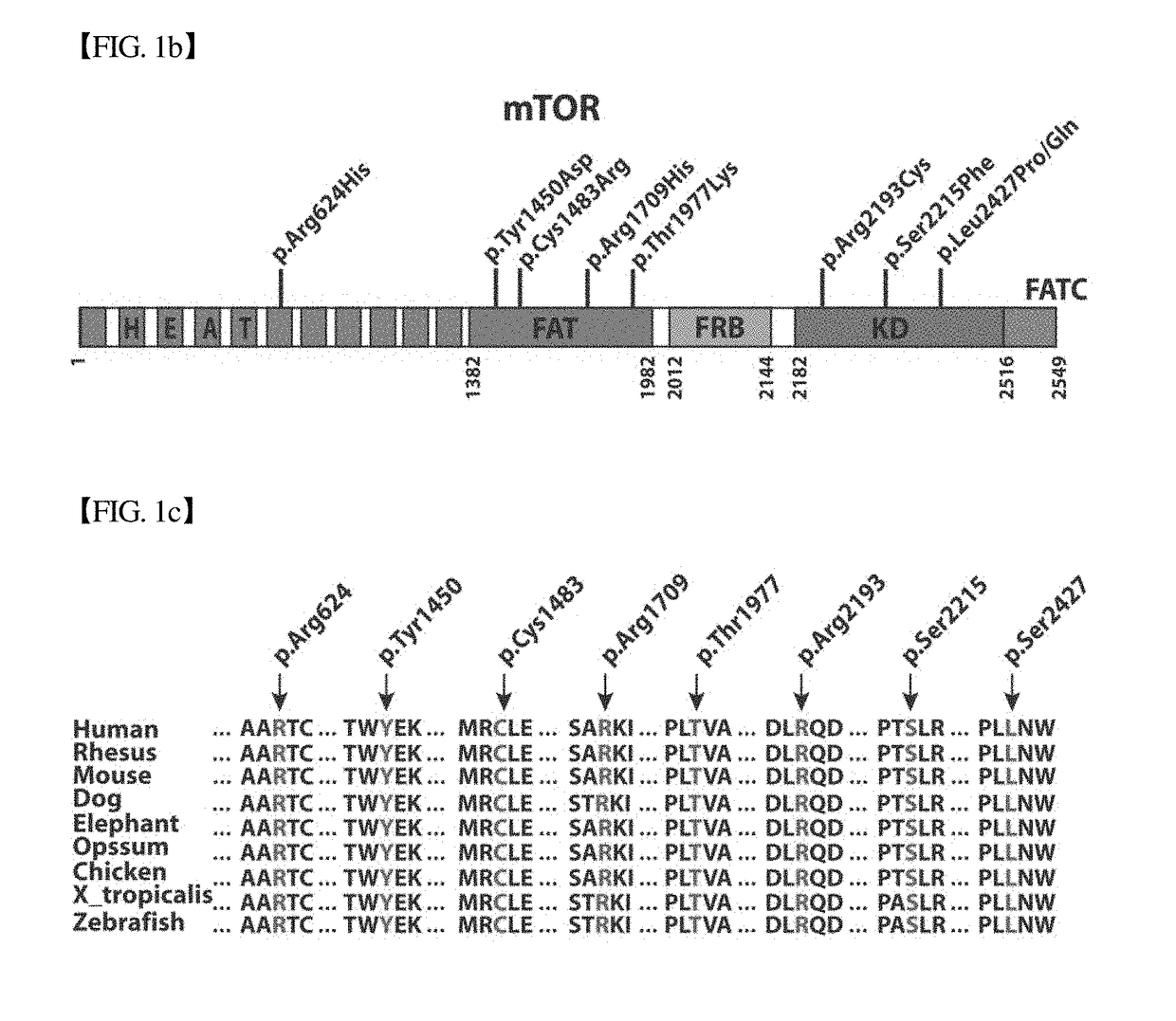
Brain somatic mutations associated to epilepsy and uses thereof
The present invention relates to epilepsy-inducing brain somatic mutations which are associated with intractable epilepsy caused by malformations of cortical development, and uses thereof. More particularly, the present invention relates to an mTOR (Mammalian target of rapamycin) gene having mutations in a nucleotide sequence or an mTOR protein having mutations in an amino acid sequence resulting from the mutations in the nucleotide sequence. Further, the present invention relates to a technique for diagnosing intractable epilepsy caused by malformations of cortical development using the gene or the protein.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
Scorpion venom heat-resistant synthetic peptide and application thereof
ActiveCN106220713AHas a promoting effectImprove chemotaxis abnormalitiesNervous disorderPeptide/protein ingredientsDiseaseChemical synthesis
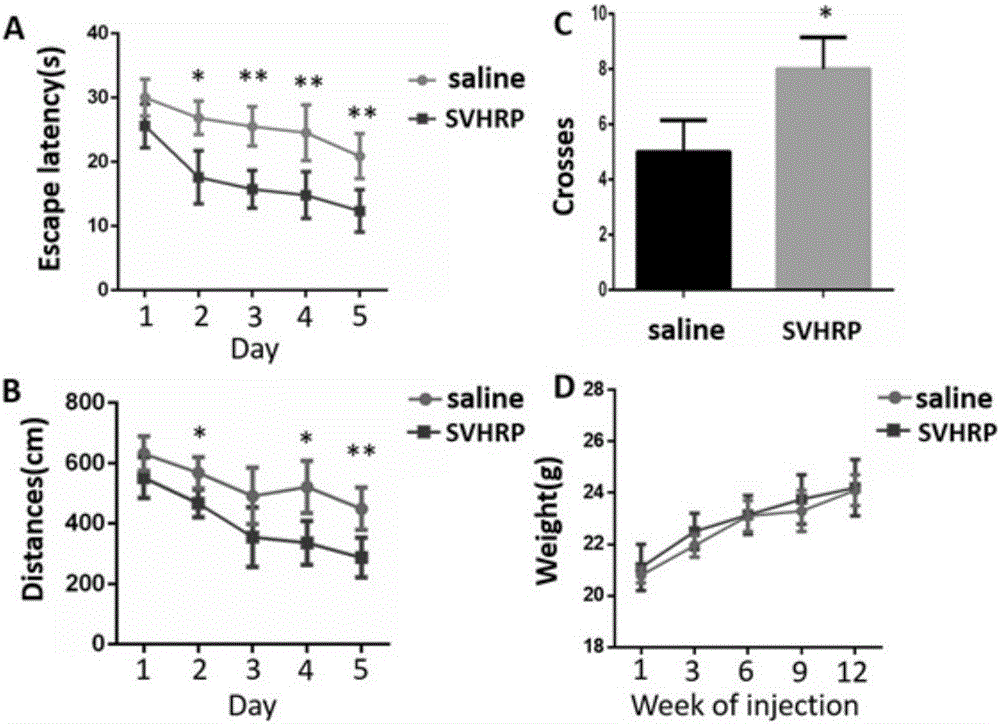
The invention belongs to the field of research and development of polypeptide medicines and discloses a scorpion venom heat-resistant synthetic peptide and application thereof. An amino acid sequence of an SVHRP (scorpion venom heat-resistant peptide) is detected out from BmK (Buthus martensii Karsch) which is a traditional Chinese medicine; according to animal experiment verification, a scorpion venom heat-resistant peptide extract liquid sample has pharmacological activity in prevention and treatment of intractable epilepsy, Parkinson's disease and Alzheimer's disease, the sample is subjected to LaGm composite and repeated fast magnetic separation prior to nanoLC-ESI-MS (nano-liter reversed-phase chromatography and electrospray ionization mass spectrometry) integrated mass spectrometry parallel experiment to detect out a polypeptide sequence formed by 15 amino acid residues. The scorpion venom heat-resistant synthetic peptide is prepared by solid-phase chemical synthesis, chromatography purification and mass spectrometry identification. An amino acid sequence of the scorpion venom heat-resistant synthetic peptide is as shown in SEQ ID NO.1 and keeps pharmacological activity and safety of the scorpion venom heat-resistant peptide, and the scorpion venom heat-resistant peptide also has a characteristic of biological activity in promotion of reverse differentiation of neuroglial cells into neural stem cells.
Owner:上海万锦医药科技有限公司
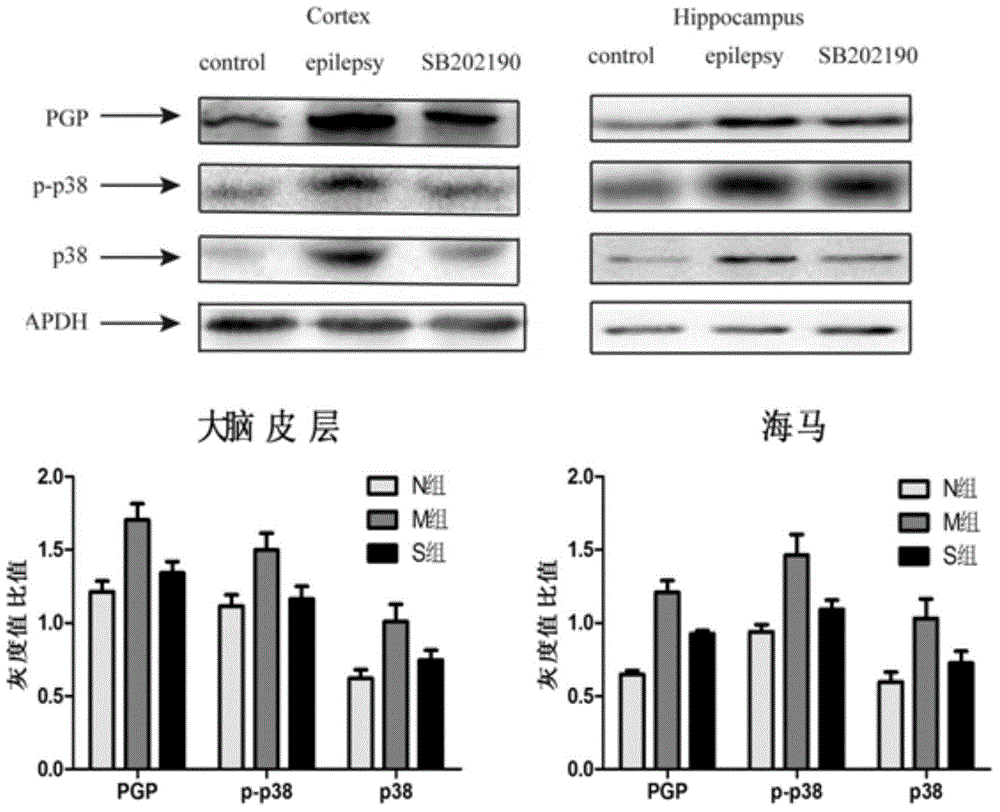
Application of SB202190 in preparation of medicines for treating intractable epilepsy
InactiveCN104606192AIncreased drug resistanceIncrease useOrganic active ingredientsNervous disorderAntiepileptic drugMultidrug transporter
The invention relates to application of SB202190 in preparation of medicines for treating intractable epilepsy. By utilizing an intractable epilepsy animal model, due to a series of researches, the SB202190 can reduce the expression of multidrug transporter-P glycoprotein (PGP) related to drug tolerance in the brain of an intractable epilepsy rat, the drug concentration of antiepileptic drugs (AEDs) in hippocampus cell extracellular fluid of the intractable epilepsy rat is improved, the AEDs is assisted to improve the electrophysiological activity of the rat, and the ignition post-attack classification is reduced, namely the drug tolerance of the intractable epilepsy can be improved by the SB202190. The application of the SB202190 is widened, and an effective way is provided for treatment of the intractable epilepsy.
Owner:JINSHAN HOSPITAL FUDAN UNIV
Pharmaceutical composition for targeting treatment of intractable epilepsy
InactiveCN102727895AIncreased frequency of suppressed seizuresExtended durationOrganic active ingredientsNervous disorderSeizure frequencyViral vector
The invention belongs to the biological medicine field, and relates to a pharmaceutical composition for targeting treatment of intractable epilepsy, the pharmaceutical composition of the present invention contains a micromolecule compound or a monoclonal antibody or virus vector carrying micromolecule interference RNA, the effective dosage of the micromolecule compound or the monoclonal antibody or the viral vector carrying micromolecule interference RNA in the composition on the P-glycoprotein expression inhibition efficiency can reach 30% or more than 30%, the animal experiment result shows that the effective dosage can perform the effects for inhibiting the epilepsy attack frequency and the attack duration. The corresponding brain wave presents the similar change with the epilepsy attack frequency and the attack duration.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL +1
Ketogenic nutritional powder
ActiveCN102648749BEffective way of absorptionImprove immunityFood preparationAnimal proteinBifidus factor
The invention discloses ketogenic nutritional powder applied to ketogenic therapy on patients suffering from intractable epilepsy. The ketogenic nutritional powder comprises the following components as per parts by weight: 69.45 to 72 parts of fat, 7.2 to 10 parts of protein, 8 to 14 parts of carbohydrate, 4.5 to 5 parts of mineral salt and less than 2 parts of emulsifying agent, flavoring agent and thickening agent, wherein the fat comprises plant oil; the protein comprises plant protein, animal protein, soybean oligopeptide and immune globulin; the carbohydrate comprises soluble dietary fiber and bifidus factor; and the weight ratio of the fat to the sum of the protein and the carbohydrate is (3.2-4.0):1. Due to addition of the immune globulin, the soybean oligopeptide, the dietary fiber and the bifidus factor, the ketogenic nutritional powder can effectively adjust the intestine health of the patients, can enhance the immunity of the patients, and has a good absorption and utilization effect in the bodies of the patients.
Owner:广州金酮医疗科技有限公司
COMPOSITION FOR PREVENTION OR TREATMENT OF INTRACTABLE EPILEPSY COMPRISING mTOR INHIBITOR
InactiveUS20180214452A1Reduce frequencyEffective diagnosisOrganic active ingredientsNervous disorderDiscovery and development of mTOR inhibitorsIntractable epilepsy
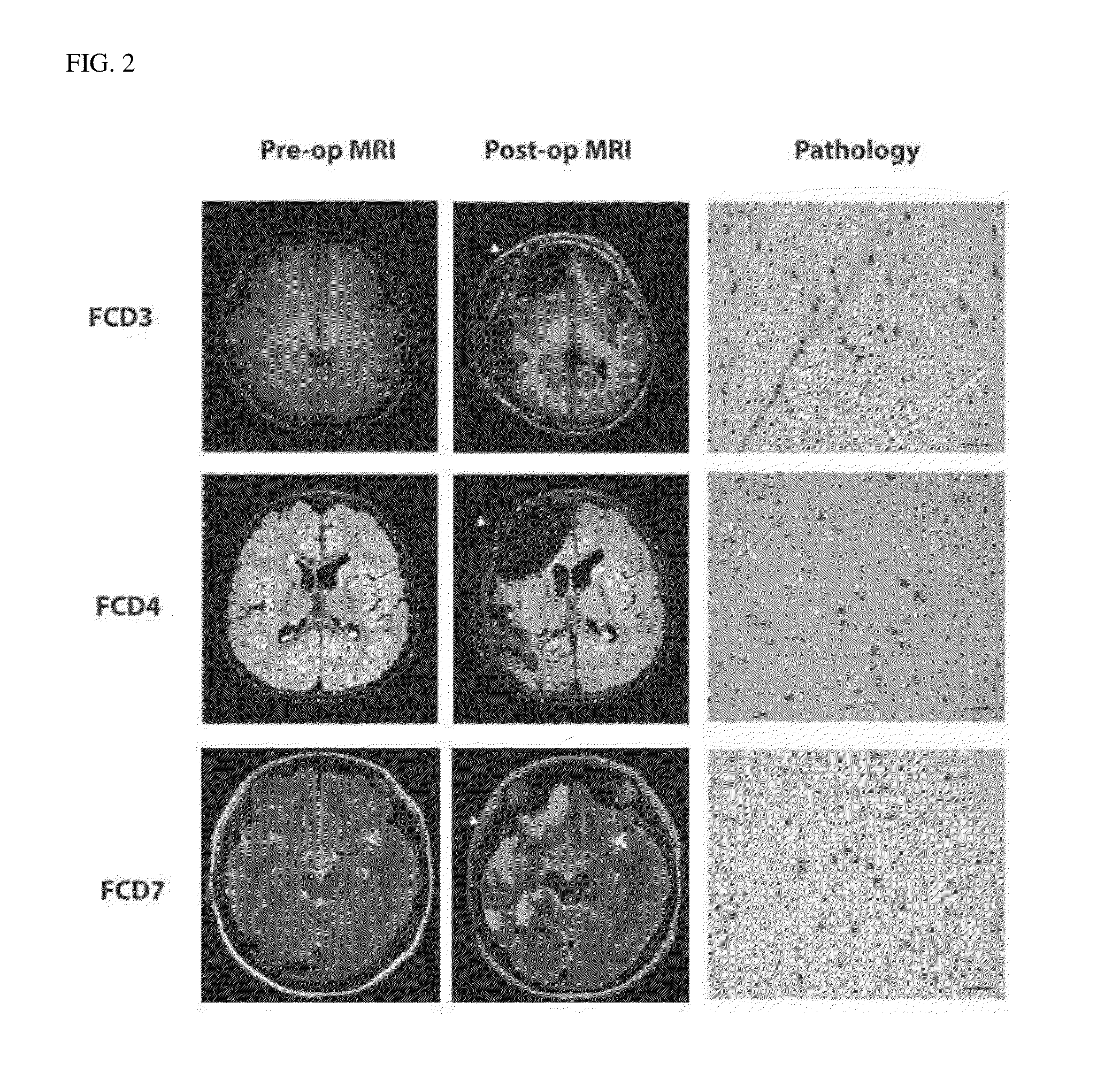
Provided is a use of the prophylaxis, amelioration or therapy of intractable epilepsy, for example, Focal Cortical Dysplasia (FCD).
Owner:KOREA ADVANCED INST OF SCI & TECH +1
Pharmaceutical composition for treating intractable epilepsy and application thereof
ActiveCN109966277AHigh dependenceSmall toxicityNervous disorderAnhydride/acid/halide active ingredientsSide effectActive component
The invention discloses a pharmaceutical composition for treating intractable epilepsy and application thereof. The active components of the pharmaceutical composition comprise sodium valproate or divalproex sodium and ethambutol or medicinal salt thereof. The pharmaceutical composition has the advantages that the fact that low-dose ethambutol hydrochloride has an anti-epileptic effect is discovered for the first time, the ethambutol hydrochloride is combined with the sodium valproate or divalproex sodium to treat the intractable epilepsy, the pharmaceutical composition is evident in anti-epileptic effect, and the toxic and side effects of the pharmaceutical composition are lowered evidently.
Owner:AFFILIATED HOSPITAL OF JINING MEDICAL UNIV
Parenteral ketogenic nutrient solution and preparation method thereof
InactiveCN109939135AAvoid seizuresRaise the ratioHydroxy compound active ingredientsMetabolism disorderDiseaseSodium acetate
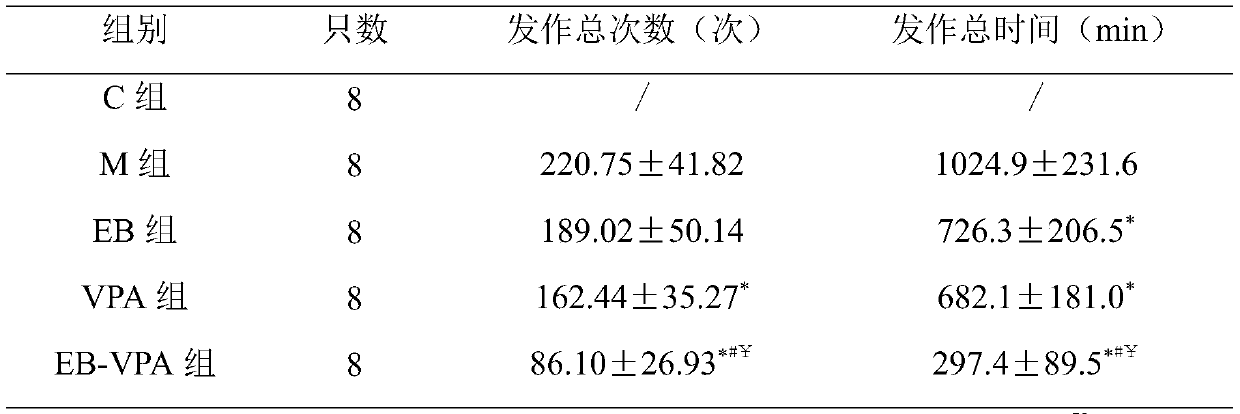
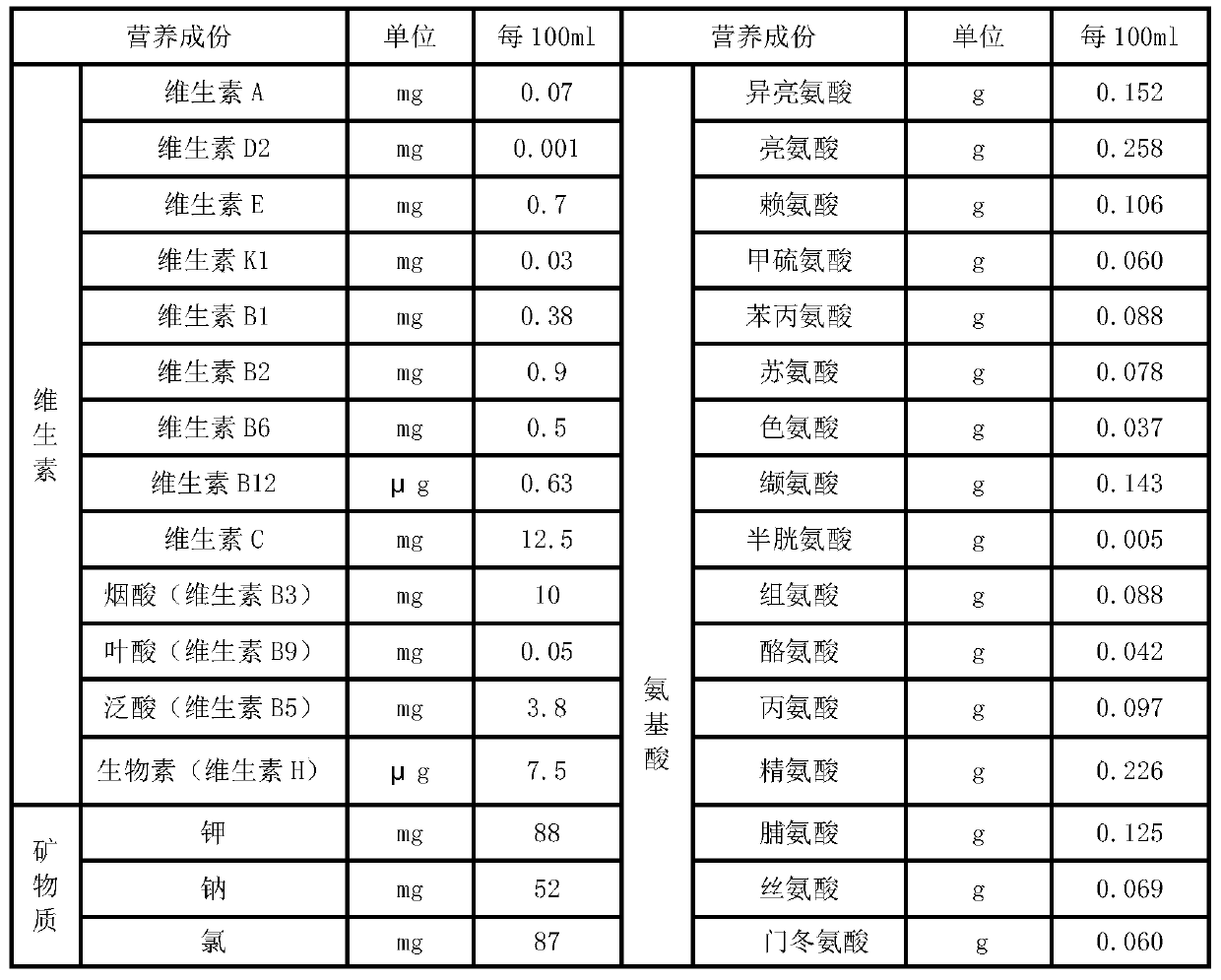
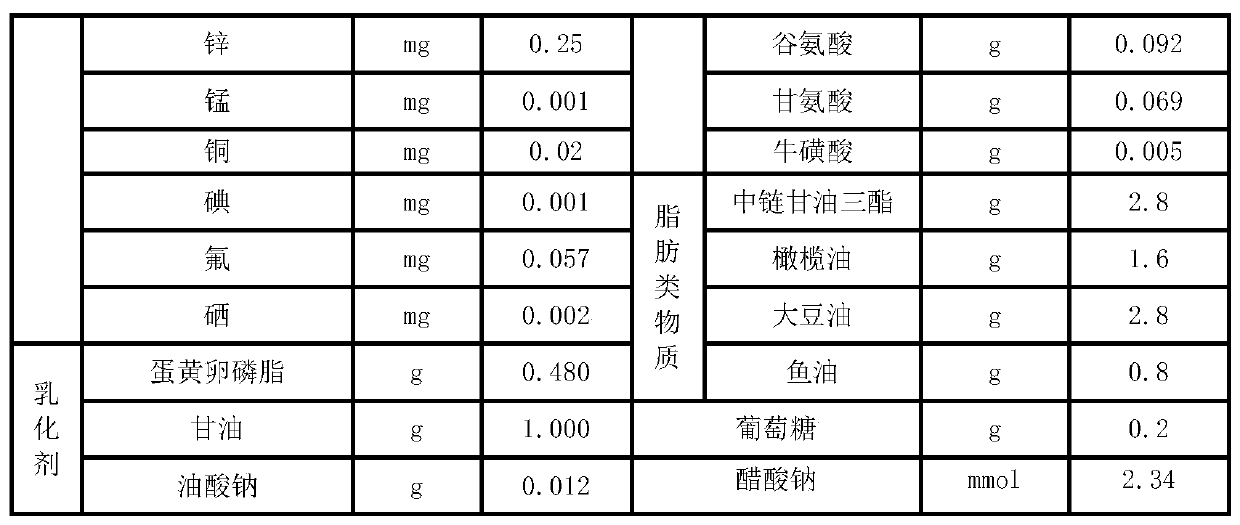
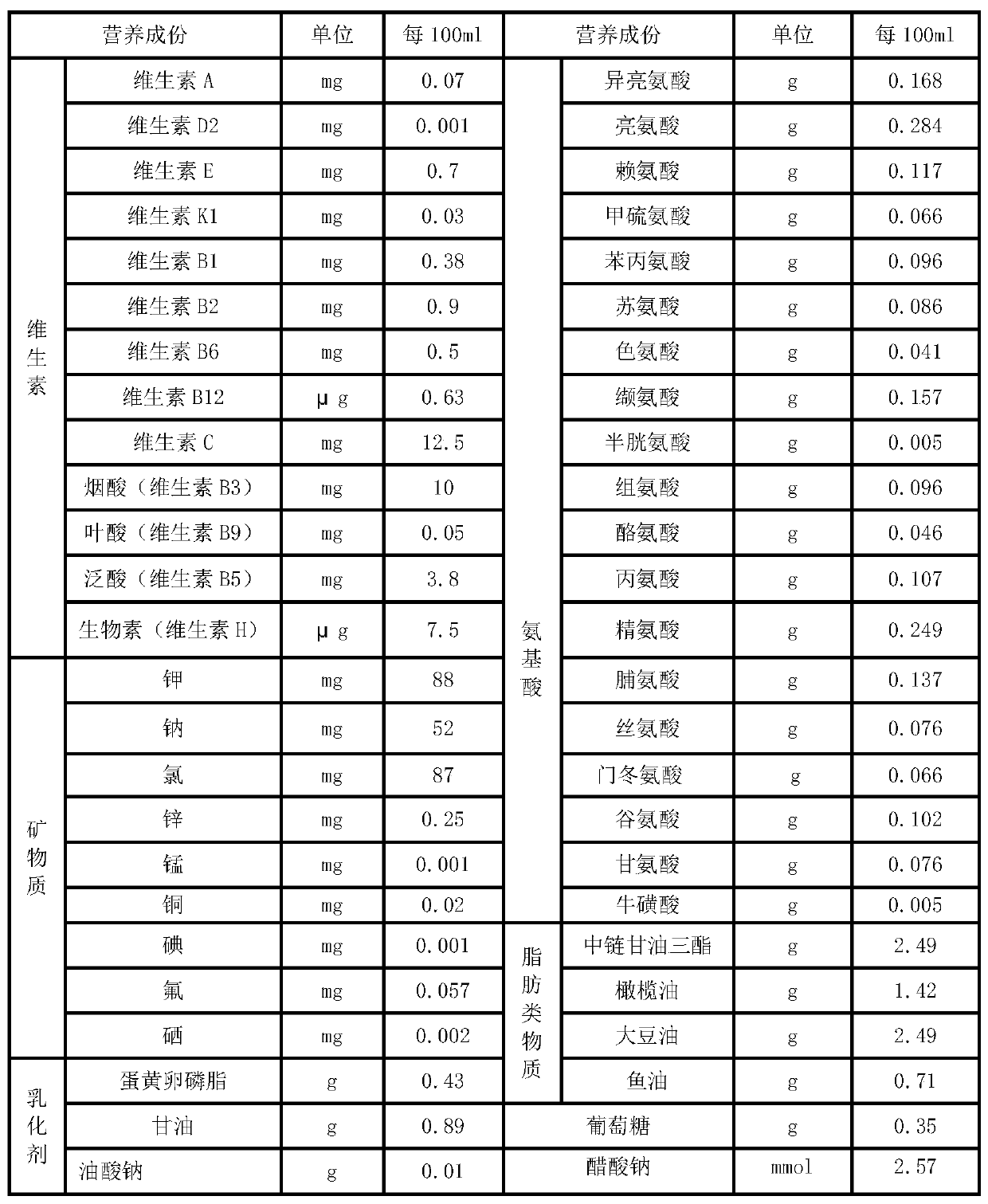
The invention relates to a parenteral ketogenic nutrient solution and a preparation method thereof. The nutrient solution is prepared from in per 100 mL of injection water, by mass, 1.80-3.3 g of amino acid, 6.5-8.0 g of fatty substances, 0.2-0.7 g of glucose, 28.93 mg of vitamins, 327.33 mg of mineral substances, 1.32-1.49 g of an emulgator and 2.34-4.29 mmol of sodium acetate, and all the substances are dissolved in the injection water. According to the formula of the high-fat low-sugar low-amino acid nutrient solution, the nutrient solution is used for well treating intractable epilepsy andother hereditary metabolic diseases such as glucose carrier deficiency disease, pyruvate dehydrogenase deficiency disease and part of tumors and fills the gap of a parenteral ketogenic nutrient solution technology.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Adriamycin-resistant U87 cell model and application thereof
InactiveCN104946593AIncrease concentrationIncreased chemosensitivityMicrobiological testing/measurementTumor/cancer cellsDead cellBiology
The invention relates to an adriamycin-resistant U87 cell model and an application thereof. U87 cells are intervened by use of the adriamycin, and then an adriamycin-free culture medium is adopted in stead; after the cells grow to 90% in 3-5 days under the circumstance of stable growth and full form of cells, no floating dead cells and 30% of passage density, normal passage is started and the concentration of the adriamycin is increased; the intervention time of the adriamycin each time is 24 hours; finally, the intervened U87 cells are detected, and consequently, the adriamycin-resistant U87 cell model is obtained; the collection number of the adriamycin-resistant U87 cell model is CCTCC NO: C201531. The cell model is strong in drug resistance, high in drug resistance coefficient, excellent in drug resistance stability, and can be applied to studying the drug resistance mechanism of the brain glioma, searching for the target spot of reversal of drug resistance of the brain glioma and screening drugs for treating the brain glioma or removing the drug resistance of the brain glioma, or to studying the mechanism of tumors causing intractable epilepsy, searching for the target spot of reversal of drug resistance of the epilepsy and screening drugs for treating the epilepsy or removing the drug resistance of the epilepsy.
Owner:JINSHAN HOSPITAL FUDAN UNIV
Method for screening sensitive type intractable epilepsy medicine
The invention belongs to the biological medicine field, and relates to a method for screening medicines, and concretely relates to a method for screening a sensitive type intractable epilepsy medicine. According to the invention, an epilepsy animal model capable of generating repeatedly spontaneous epileptic seizure is established, the medicine sensitive type and the medicine resistant subgroup are screened by providing the epilepsy animal model anti-epileptic medicine, and the sensitive type intractable epilepsy medicine can be screened according to the expression difference of the corresponding risk factors. The invention provides a novel treatment target and a solution method for solving the problems of drug effect reduction for intractable epilepsy presented in a present clinical application.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL +1
Drug for reversing drug resistance of intractable epilepsy
ActiveCN105483130AReduce pump rateReverse drug resistanceNervous disorderGene therapyLentivirusNucleotide
The invention discloses a nucleotide sequence shown in SEQ ID NO.1, and further discloses a recombinant virus containing the nucleotide sequence and application thereof. The recombinant lentivirus can inhibit expression of drug resistance protein Pgp and a drug-resistance related gene HIF-1alpha and also can decrease the pumping rate of a drug, can reverse the drug resistance of intractable epilepsy, can be jointly used with other drugs for treating epilepsy to treat the intractable epilepsy and is good in clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Application of 17-allylamine-17-demethoxygeldanamycin in preparation of medicine for treating epilepsy
The invention relates to an application of 17-allylamine-17-demethoxygeldanamycin in preparation of medicine for treating epilepsy, wherein preferably, epilepsy is intractable epilepsy, and more preferably is temporal lobe epilepsy which is ineffectively treated by medicines. According to the invention, 17-allylamine-17-demethoxygeldanamycin is realized by up regulation of G1t1 protein in brain tissue. 17-allylamine-17-demethoxygeldanamycin is employed for effectively treating temporal lobe epilepsy which is ineffectively treated by medicines, simultaneously, other physiological states of a subject can not be influenced during a treatment period.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
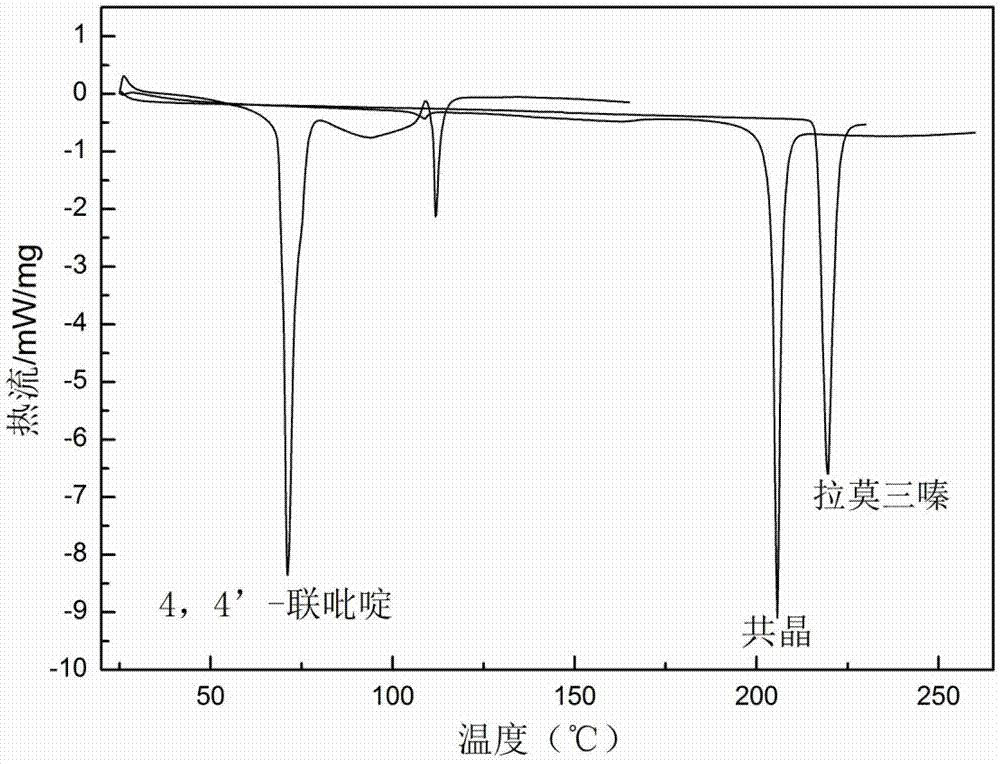
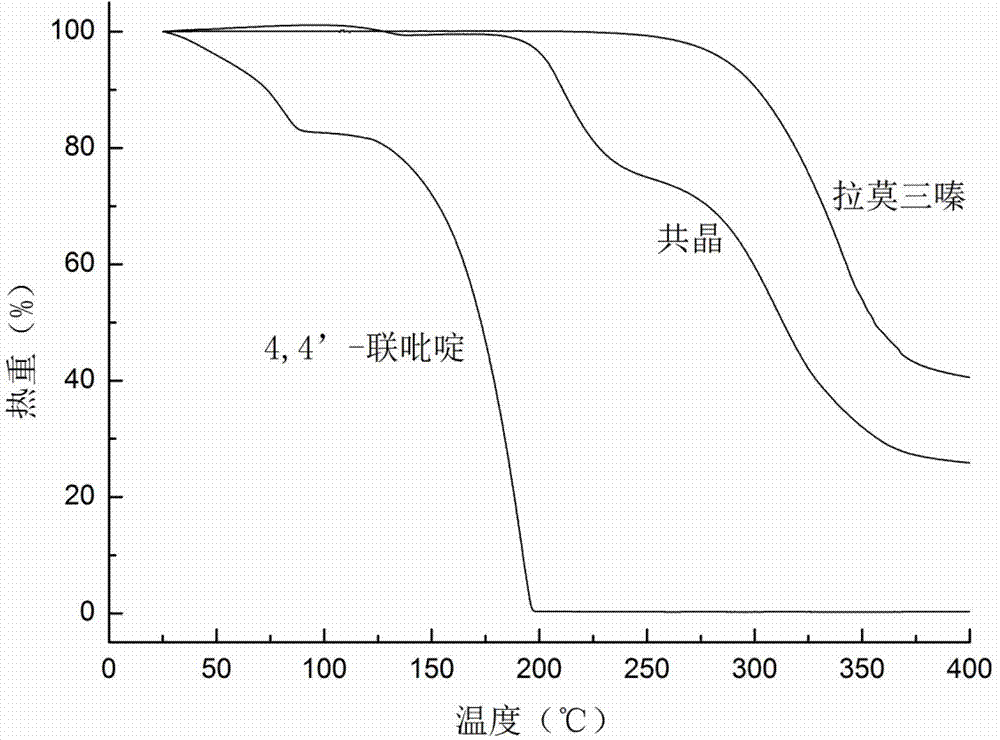
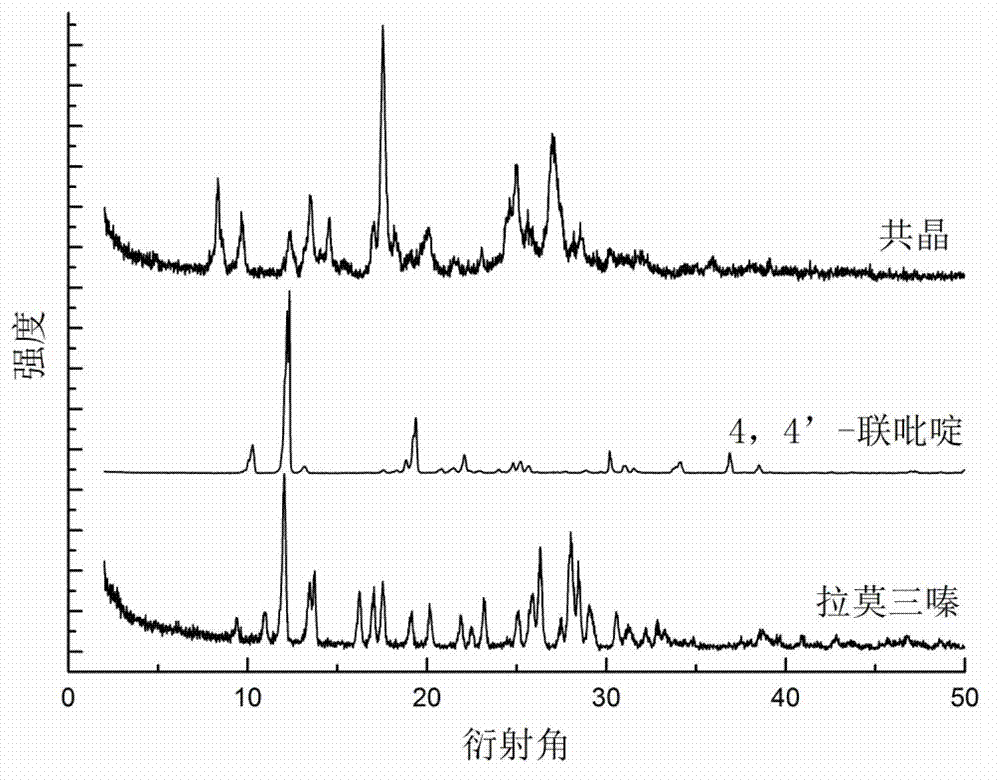
Novel lamotrigine pharmaceutical co-crystal and preparation method thereof
The invention relates to a novel lamotrigine pharmaceutical co-crystal and a preparation method thereof. A PXRD (Powder X Ray Diffractio) of the lamotrigine pharmaceutical co-crystal shows a series of characteristic peaks in 8.3+ / -0.2, 9.7+ / -0.2, 12.4+ / -0.2, 13.5+ / -0.2, 14.0+ / -0.2, 14.5+ / -0.2, 17.0+ / -0.2, 17.5+ / -0.2, 18.2+ / -0.2, 20.0+ / -0.2, 23.0+ / -0.2, 24.6+ / -0.2, 25.0+ / -0.2, 25.6+ / -0.2, 27.0+ / -0.2, and 28.5+ / -0.2. The lamotrigine pharmaceutical co-crystal is prepared through a solution mediate transformation or a grinding method. The prepared pharmaceutical co-crystal remains the characteristic of the traditional raw medicine on treating refractory epilepsy and also shows remarkable improvement on solubility, stability and bioavailability.
Owner:TIANJIN UNIV
Screening reagent box for intractable epilepsy
ActiveCN104762412ALow risk of developing treatment-resistant epilepsyReduce riskMicrobiological testing/measurementCrowdsCvd risk
The invention discloses a screening reagent box for intractable epilepsy. The screening reagent box for the intractable epilepsy comprises an optional reagent used for detecting the expression level of micro RNA-153. The invention further discloses an application of the optional reagent used for detecting the expression level of the micro RNA-153 in preparing a reagent used for screening the intractable epilepsy. According to the reagent box, the expression level of the micro RNA-153 is detected, the risk of suffering from the intractable epilepsy for crowds to be examined can be judged, the reagent box can be used for auxiliary diagnosis of clinical intractable epilepsy, an effective base is provided for a patient to take relevant treatment measures or decisions, and a good clinical application prospect is achieved.
Owner:SICHUAN CREDIT PHARMA
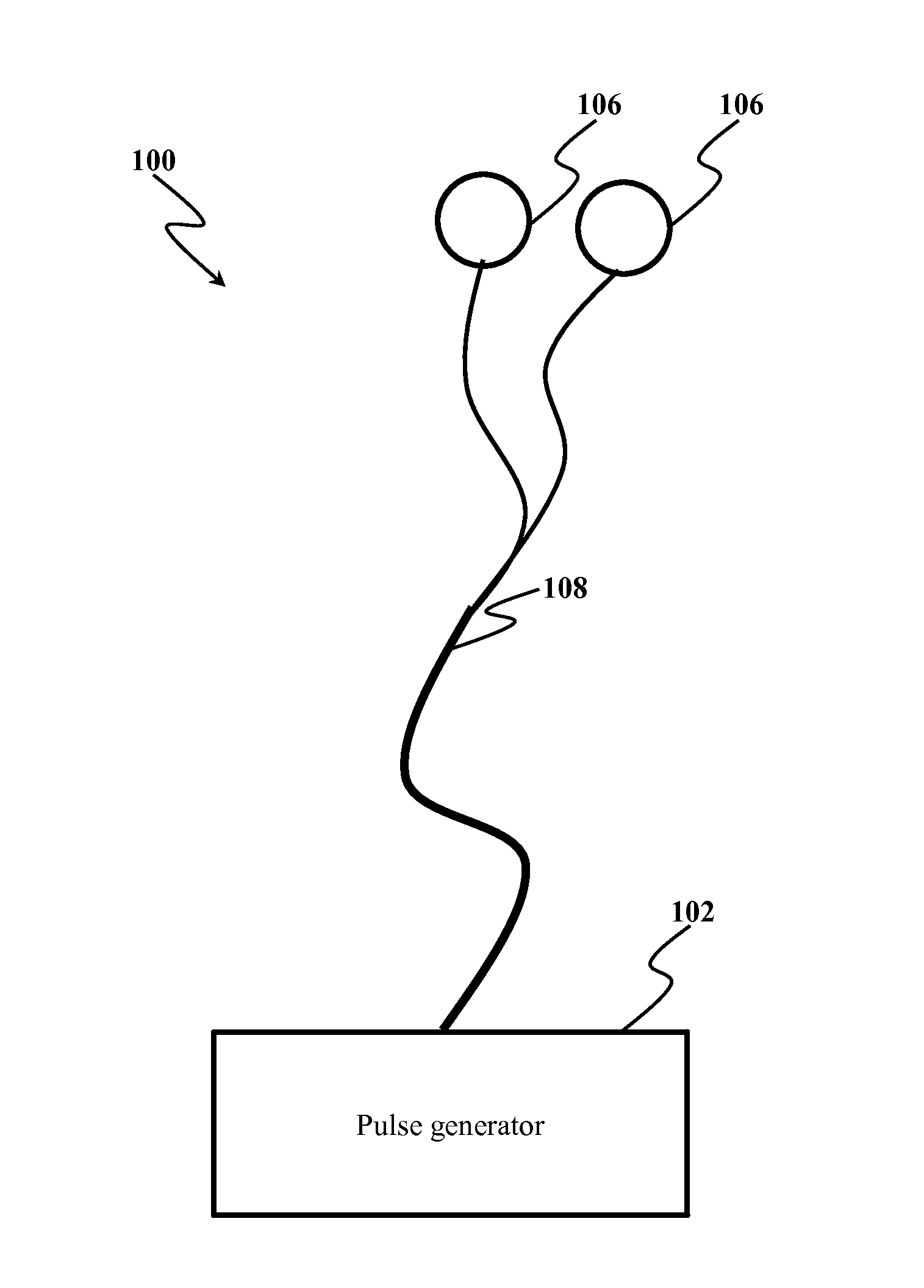
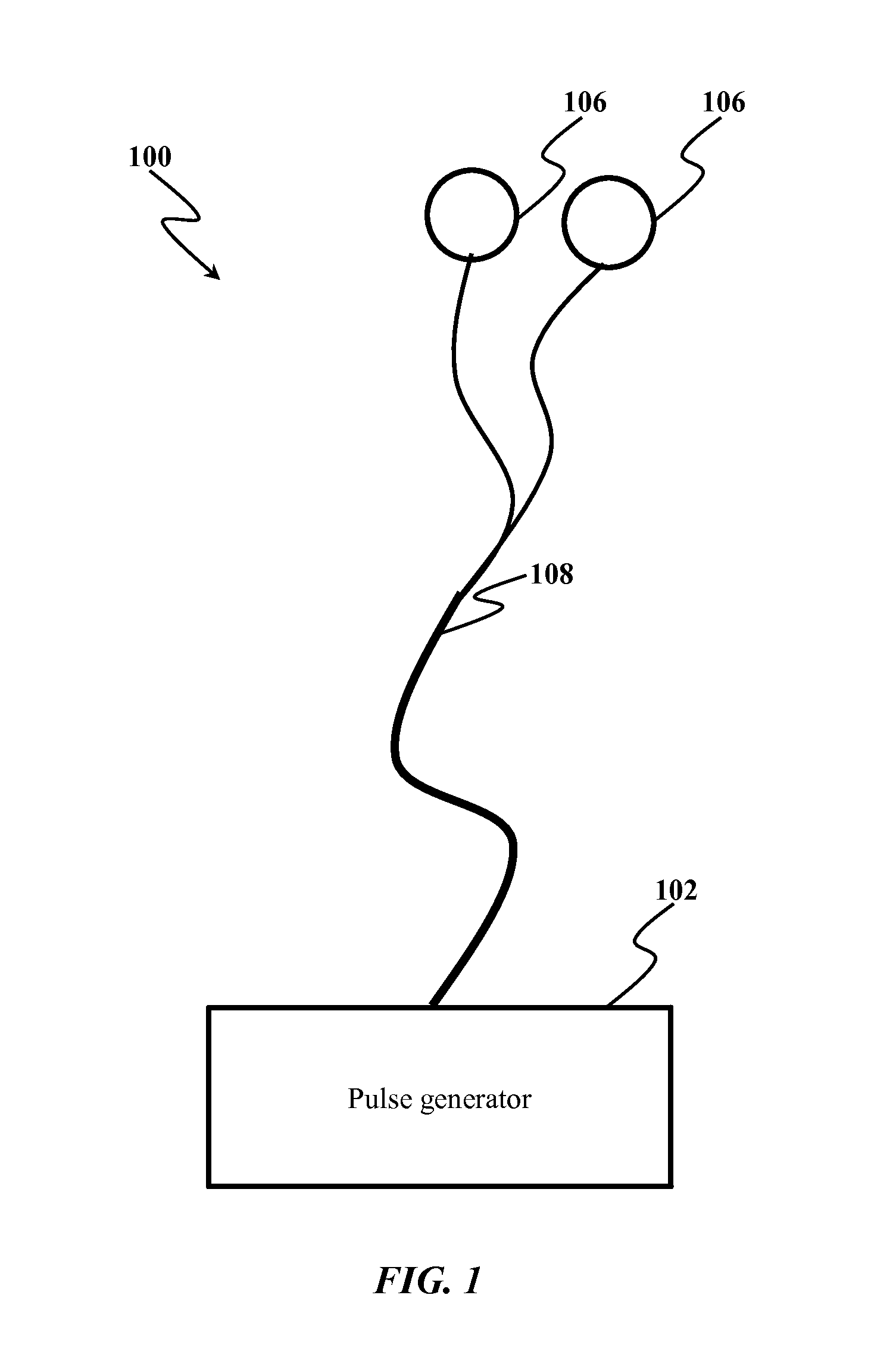
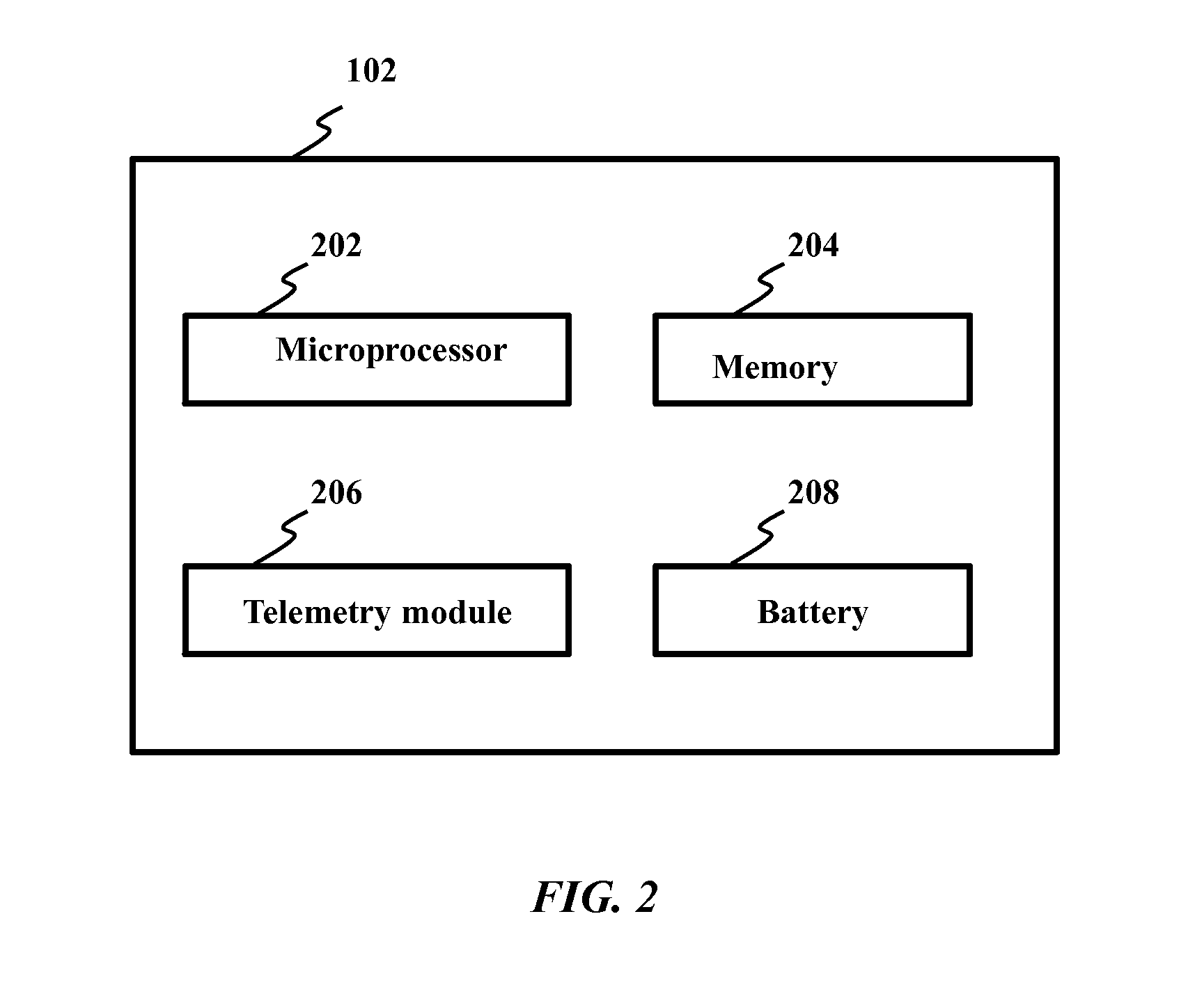
System and method of treating intractable epilepsy by stimulation
InactiveUS20140200624A1Suppress generationEnhance rapid eye movement (REM) sleepElectrotherapyArtificial respirationCholinergic cellsRapid eye movement sleep
System and method for treating intractable epilepsy are provided. The method includes implanting electrodes in pedunculopontine nucleus and delivering electrical pulses to the pedunculopontine nucleus, thereby inducing the stimulation of cholinergic neurons leading to the release of acetylcholine, thereby enhancing the genesis of rapid eye movement sleep, which reduces the occurrence of epileptic attacks and also chronically suppresses the epileptogenic process.
Owner:JASEJA HARINDER
Pharmaceutical preparation for treating intractable epilepsy
InactiveCN113018386AStrong targetingDefine Quantitative RequirementsNervous disorderHydroxy compound active ingredientsCannabis sativa plantDisease
The invention discloses a pharmaceutical preparation for treating intractable epilepsy, the preparation comprises a cannabis sativa extract, a gastrodia elata extract, succinylated protein, borneol and an auxiliary agent, the active ingredient of the cannabis sativa extract is a compound with an alkyl resorcinol structure, and the active ingredient of the gastrodia elata extract is a compound with an alkyl resorcinol structure. As an extraction substance which is extracted from a cannabis sativa plant and has the same biological activity, the pharmaceutical preparation has a great synergistic effect on playing a pharmacological effect on refractory epilepsy, and the pharmacological activity of the pharmaceutical preparation is effectively improved; the gastrodia elata extract can provide effective nutrition and repair nerves in the brain, and the borneol can be used as a channel guiding drug to enhance absorption of other drugs in a blood brain barrier and effectively relieve convulsion, spasm, unconsciousness, wandering and other clinical characteristics accompanied by epilepsy disease attack, the succinylated protein in the preparation can prevent the drug from being damaged in an acid environment, and the nano-emulsion preparation prepared by adding the succinylated protein can effectively improve the bioavailability and stability of the drug.
Owner:黑龙江汉普康制药有限公司
Medicine for treating intractable epilepsy and preparation method thereof
InactiveCN113018387APromote absorptionGood synergyNervous disorderHydroxy compound active ingredientsCannabisDisease
The invention discloses a medicine for treating intractable epilepsy and a preparation method thereof, the medicine comprises a cannabis sativa extract, a gastrodia elata extract and borneol, the effective component of the cannabis sativa extract is a compound which is separated and extracted from cannabis sativa and has an alkylresorcinol structure, the medicine has a great synergistic effect on playing a pharmacological effect on intractable epilepsy, and the pharmacological activity of the pharmaceutical composition is effectively improved; the gastrodia elata extract can provide effective nutrition and repair nerves in the brain, the borneol can be used as a channel guiding drug to enhance absorption of other drugs in a blood brain barrier and effectively relieve convulsion, spasm, unconsciousness, wandering and other clinical characteristics accompanied by epilepsy disease attack, and the three drug components are subjected to traditional Chinese medicine prescription compatibility, and the intractable epilepsy is effectively treated in a targeted manner from three aspects of pathological treatment of epilepsy diseases, nutrition and repair of damaged nerves in the brain and relieving of clinical characterization.
Owner:黑龙江汉普康制药有限公司
Application of benzodifuranone compound in treatment of intractable epilepsy
ActiveCN113679714AGood synergyNervous disorderAnhydride/acid/halide active ingredientsDiseasePharmaceutical drug
The invention discloses application of a benzodifuranone compound in preparation of medicines for treating intractable epilepsy, and belongs to the technical field of medicines. According to the application, the benzodifuranone compound is used for treating epilepsy for the first time, and particularly a new candidate drug is provided for treatment of intractable epilepsy. In addition, the invention further provides a compound medicine combining the benzodifuranone compound and sodium valproate, and the benzodifuranone compound and the sodium valproate have an obvious synergistic effect in treatment of intractable epilepsy diseases.
Owner:AFFILIATED HOSPITAL OF JINING MEDICAL UNIV
Application of ethambutol hydrochloride in the treatment of refractory epilepsy
ActiveCN109999016BAdvantages and Notable ImprovementsHas antiepileptic effectOrganic active ingredientsNervous disorderNew medicationsUse medication
The invention discloses the application of ethambutol or a pharmaceutically acceptable salt thereof as an active ingredient in the preparation of a drug for treating intractable epilepsy, and belongs to the technical field of medicine. Because the dosage of ethambutol hydrochloride is low, the toxic and side effects must be reduced, thereby reducing the toxic and side effects of the patient's medication. The new anti-refractory epilepsy drug has very significant social and economic significance.
Owner:浙江药苑生物科技有限公司
Stimulation of the ventral pallidum for the treatment of epilepsy
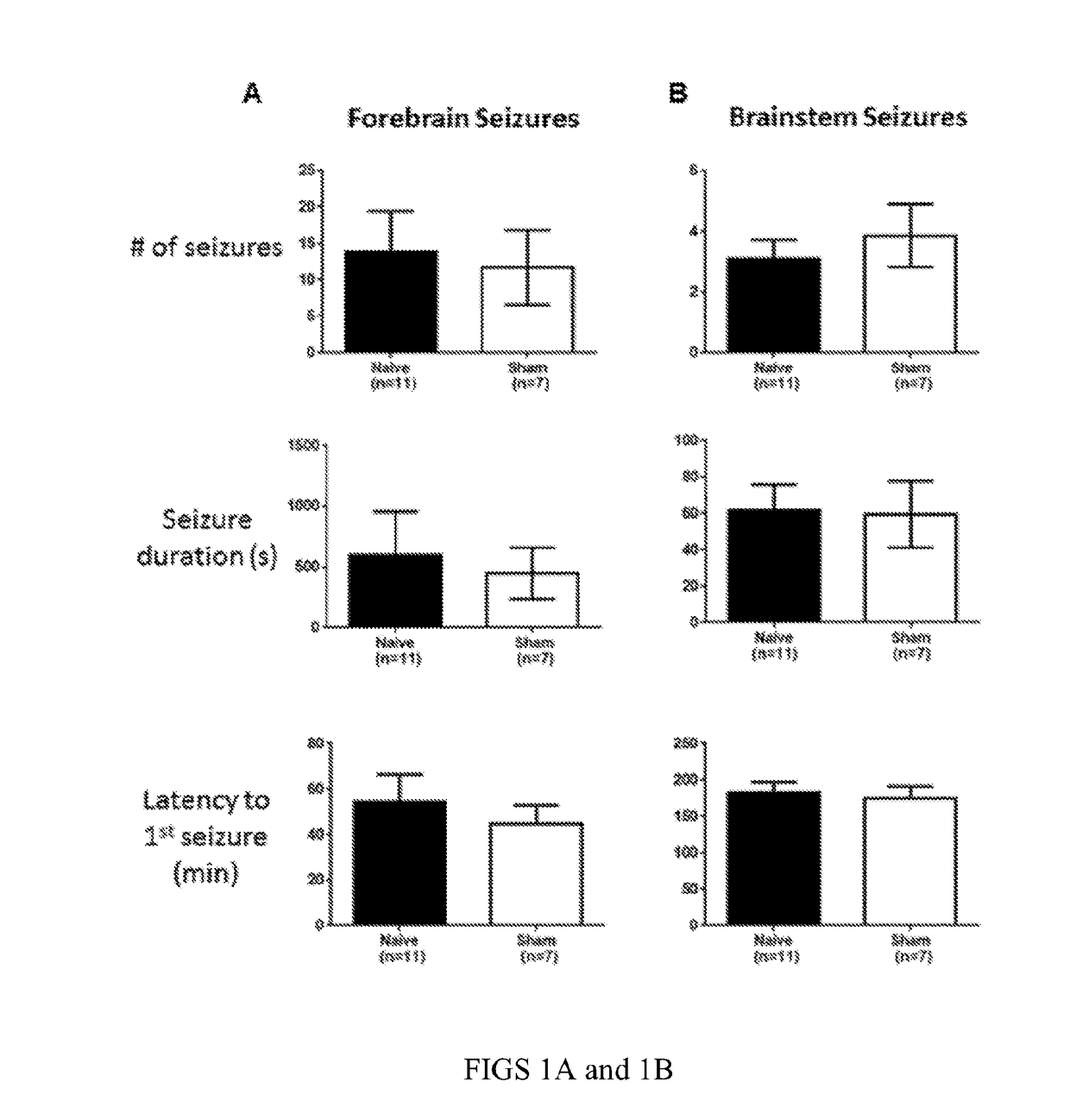
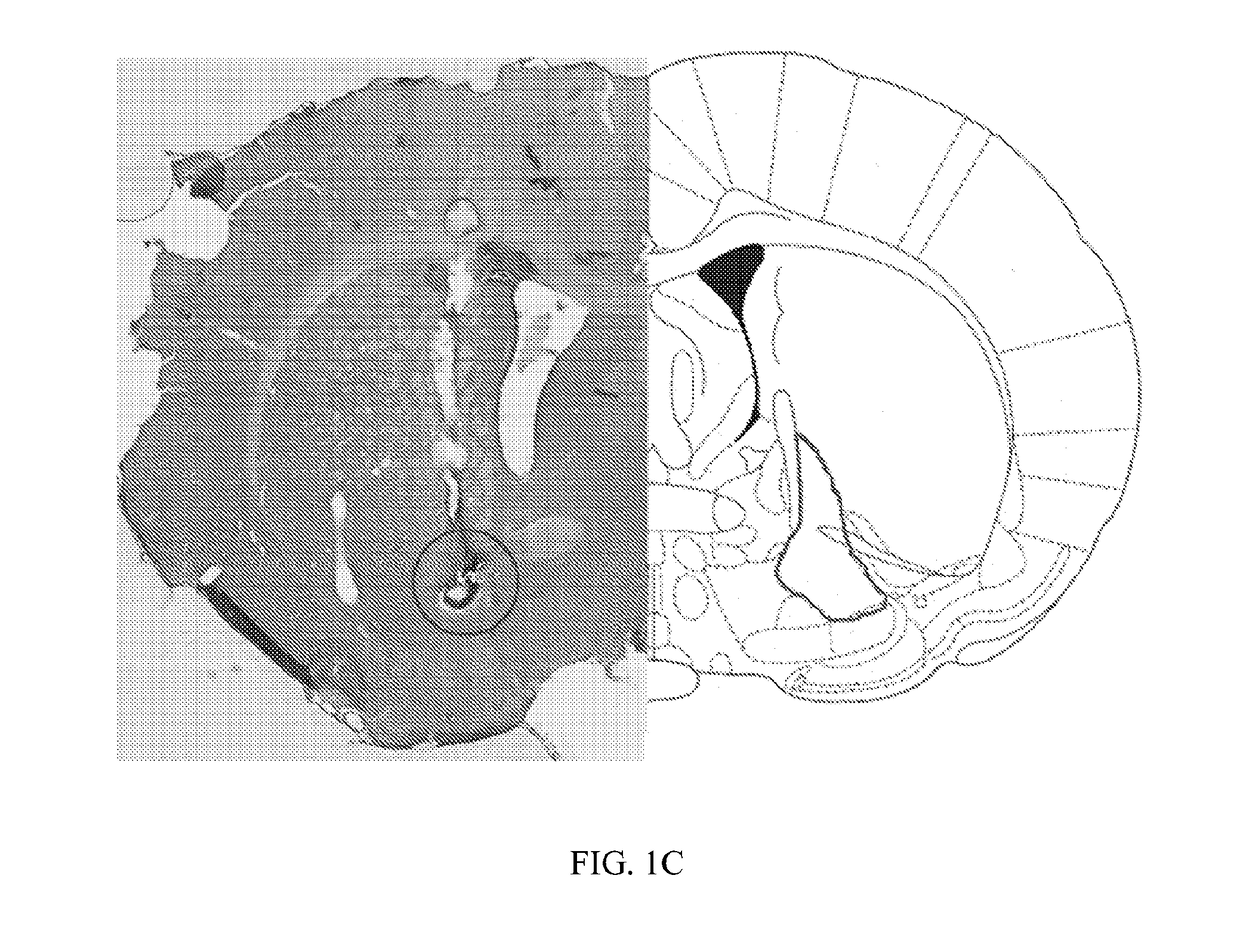
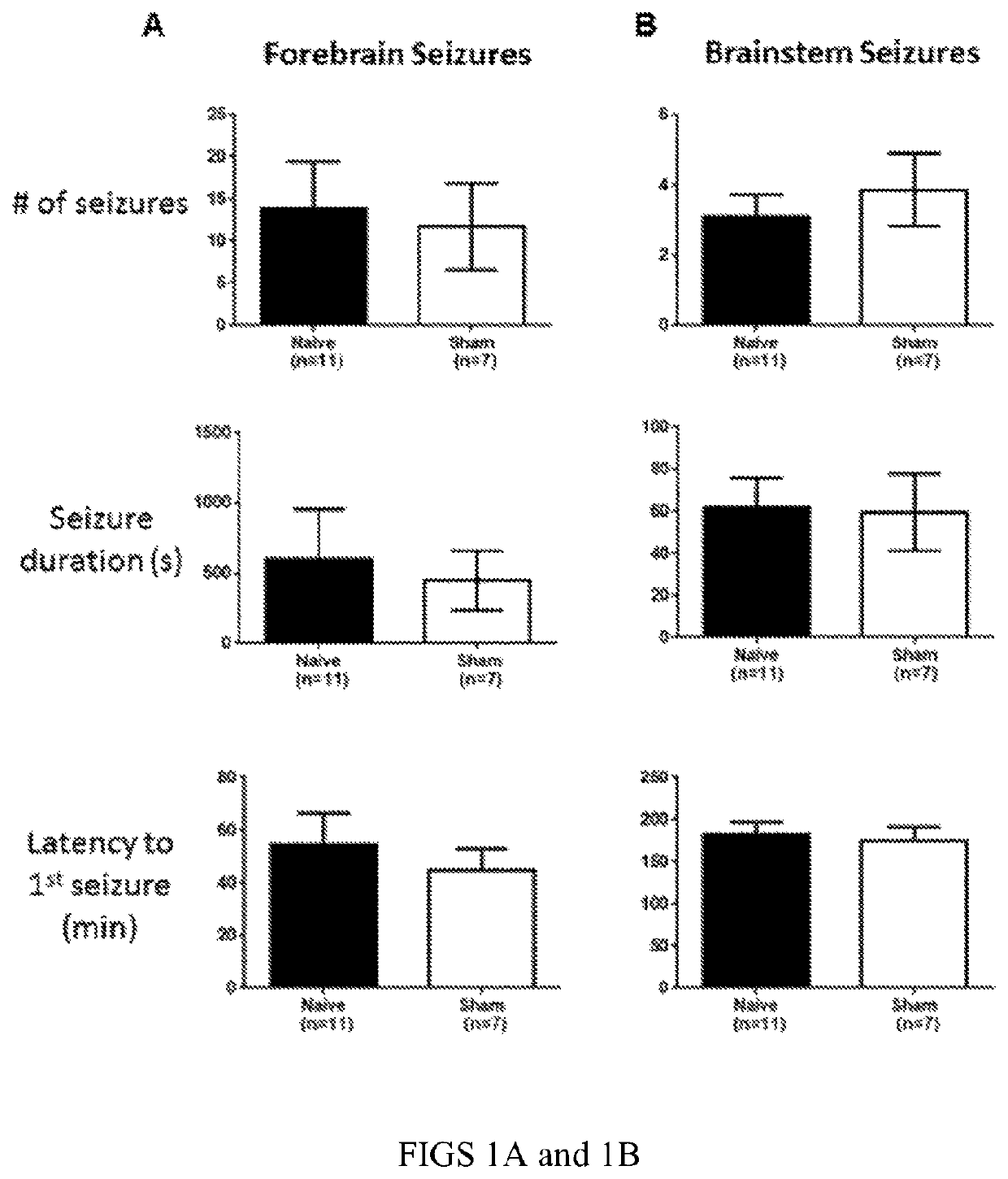
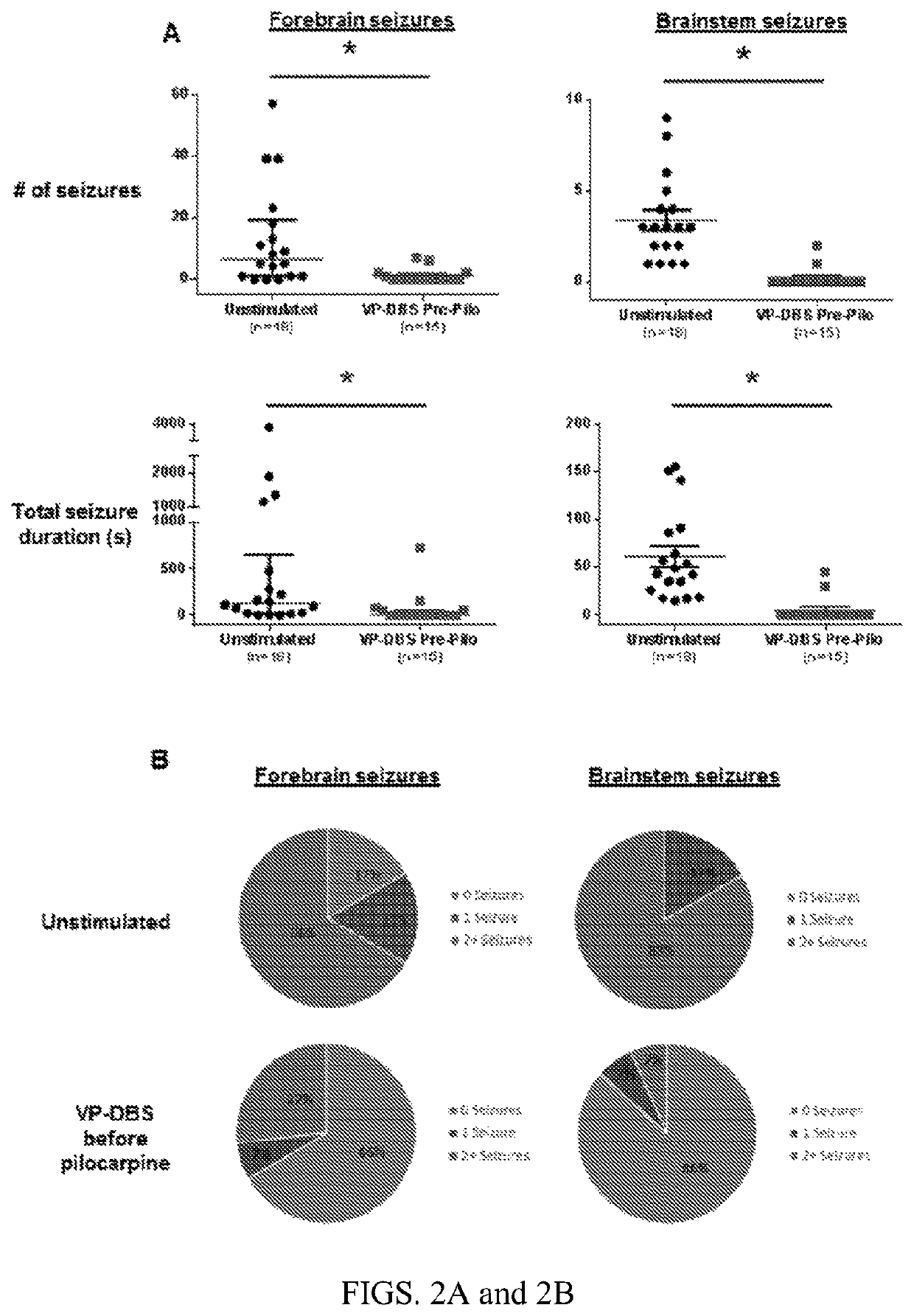
ActiveUS20180250512A1Reduce and prevent appearanceAvoid partialHead electrodesExternal electrodesGeneralized seizureForebrain
Deep brain stimulation of the ventral pallidum (VP-DBS) prevents or potently attenuates epileptiform activity and behavioral seizures. VP-DBS prior to pilocarpine prevented behavioral partial and generalized forebrain seizures and generalized brainstem seizures in most animals. VP-DBS after brainstem seizures emerged prevented or reduced the appearance of subsequent behavioral and electrographic brainstem seizures. Even if VP-DBS was turned on after partial forebrain seizures started, this timed approach could still reduce partial forebrain seizures but also prevented secondarily generalized forebrain seizures. Epileptiform activity in brainstem areas, especially in the nucleus of the solitary tract (NTS) which controls cardiovascular function, was prevented by VP-DBS. Altogether, VP-DBS is a therapeutic approach for individuals with intractable epilepsy with partial and / or generalized seizures and may potentially prevent or diminish sudden unexpected death in epilepsy (SUDEP) by preserving activity of brain stem neurons involved in cardio-respiratory function.
Owner:ALBANY MEDICAL COLLEGE
Pharmaceutical composition for treating epilepsy
ActiveCN113288958AAccelerated deathHigh riskNervous disorderAnthropod material medical ingredientsSide effectCognitive impairment
The invention discloses a pharmaceutical composition for treating epilepsy. The pharmaceutical composition is prepared from the following raw materials in parts by weight: 10-20 parts of radix bupleuri, 14-26 parts of rhizoma acori graminei, 21-39 parts of uncaria, 21-39 parts of fossil fragments, 21-39 parts of oyster, 21-39 parts of white paeony root, 7-13 parts of divaricate saposhnikovia root, 10-20 parts of fried stiff silkworm, 10-20 parts of earthworm and 3-7 parts of liquorice. The composition disclosed by the invention has a remarkable effect on treating epilepsy, also has the effects of improving symptoms and reducing or controlling attack on part of intractable epilepsy, and can play a synergistic effect with anti-epilepsy western medicines when being used for an epileptic state, and meanwhile, can promote a patient to wake up. Compared with western medicines, the composition disclosed by the invention is safer, has no obvious toxic and side effects in long-term clinical application, has no side effects such as cognitive impairment, language impairment, dizziness and the like, has no teratogenesis effect, and has a clinical application prospect.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
A drug that reverses drug resistance in refractory epilepsy
ActiveCN105483130BReduce pump rateReverse drug resistanceNervous disorderGene therapyLentivirusNucleotide sequencing
The invention discloses a nucleotide sequence shown in SEQ ID NO.1, and further discloses a recombinant virus containing the nucleotide sequence and application thereof. The recombinant lentivirus can inhibit expression of drug resistance protein Pgp and a drug-resistance related gene HIF-1alpha and also can decrease the pumping rate of a drug, can reverse the drug resistance of intractable epilepsy, can be jointly used with other drugs for treating epilepsy to treat the intractable epilepsy and is good in clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
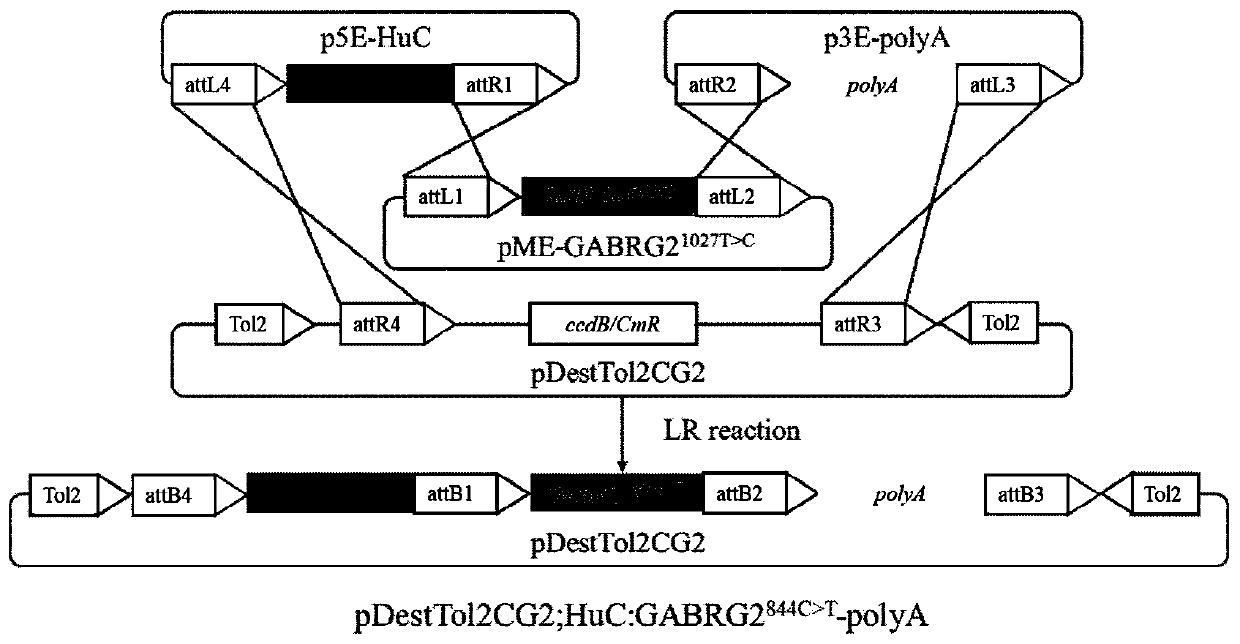
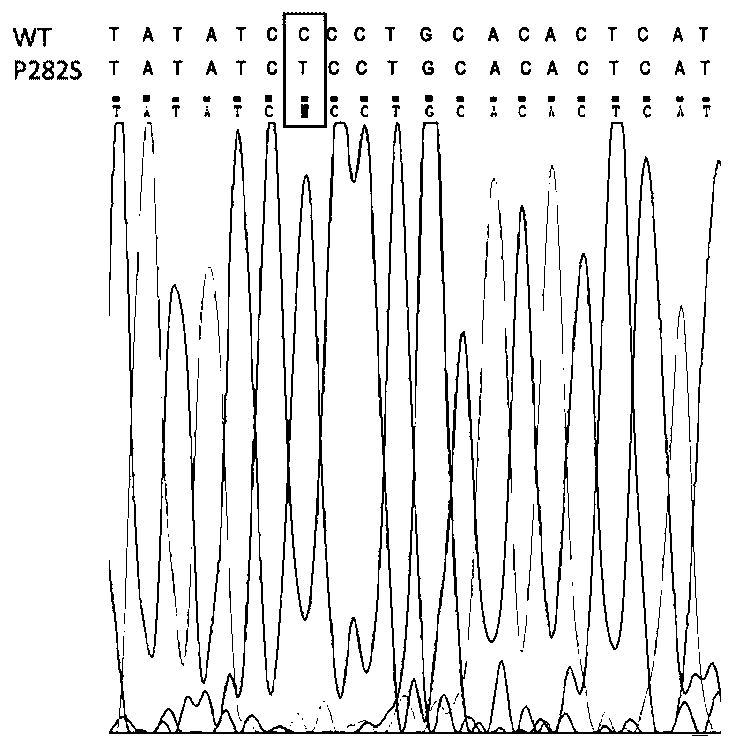
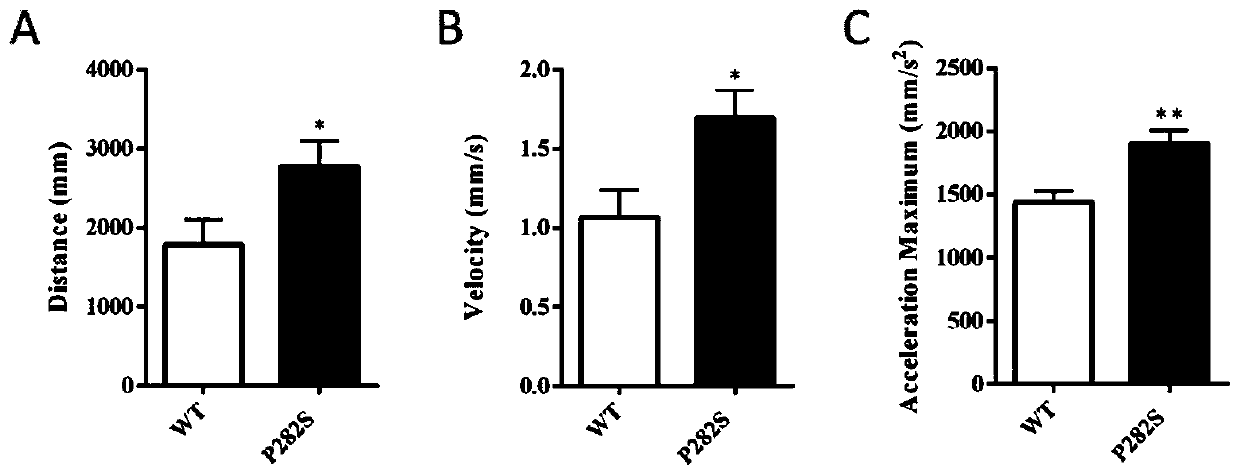
Construction method and application of zebra fish intractable epilepsy model
InactiveCN111304251ABig foundationGreat application value in clinical researchMicroinjection basedVector-based foreign material introductionGABRG2 geneAntiepileptic drug
The invention discloses a construction method of a zebra fish intractable epilepsy model. A mutant human GABRG2 gene of which the 282th proline codon is mutated into serine is transferred into a zebrafish, so that the zebra fish becomes a zebra fish line with intractable epilepsy phenotype. The method provides an effective method basis for construction of the zebra fish intractable epilepsy model, also provides an important tool for epilepsy pathology research and antiepileptic drug screening research, and has great foundation and clinical research application value.
Owner:NANTONG UNIVERSITY
Stimulation of the ventral pallidum for the treatment of epilepsy
ActiveUS10596376B2Reduce and prevent appearanceInhibition transitionHead electrodesExternal electrodesGeneralized seizureForebrain
Deep brain stimulation of the ventral pallidum (VP-DBS) prevents or potently attenuates epileptiform activity and behavioral seizures. VP-DBS prior to pilocarpine prevented behavioral partial and generalized forebrain seizures and generalized brainstem seizures in most animals. VP-DBS after brainstem seizures emerged prevented or reduced the appearance of subsequent behavioral and electrographic brainstem seizures. Even if VP-DBS was turned on after partial forebrain seizures started, this timed approach could still reduce partial forebrain seizures but also prevented secondarily generalized forebrain seizures. Epileptiform activity in brainstem areas, especially in the nucleus of the solitary tract (NTS) which controls cardiovascular function, was prevented by VP-DBS. Altogether, VP-DBS is a therapeutic approach for individuals with intractable epilepsy with partial and / or generalized seizures and may potentially prevent or diminish sudden unexpected death in epilepsy (SUDEP) by preserving activity of brain stem neurons involved in cardio-respiratory function.
Owner:ALBANY MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com