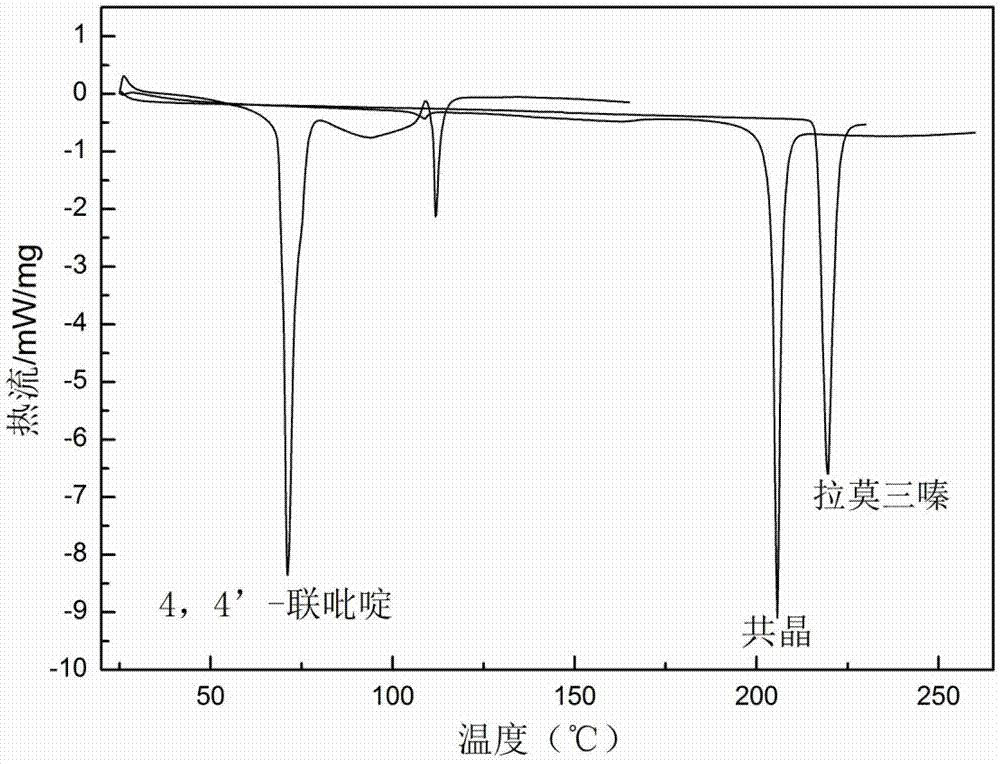
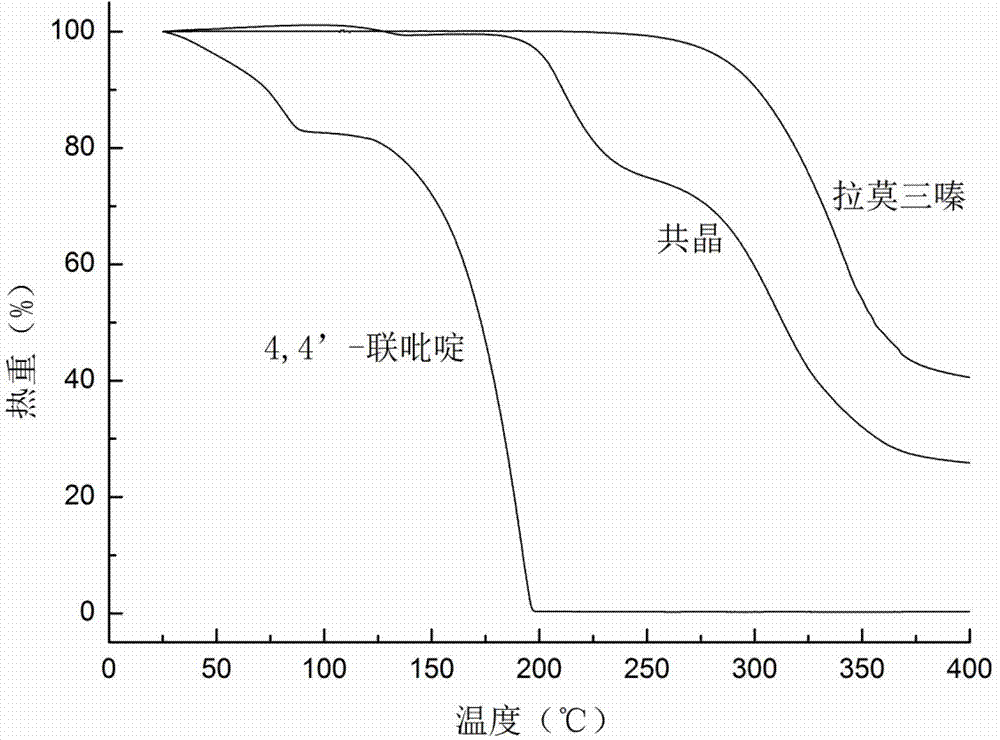
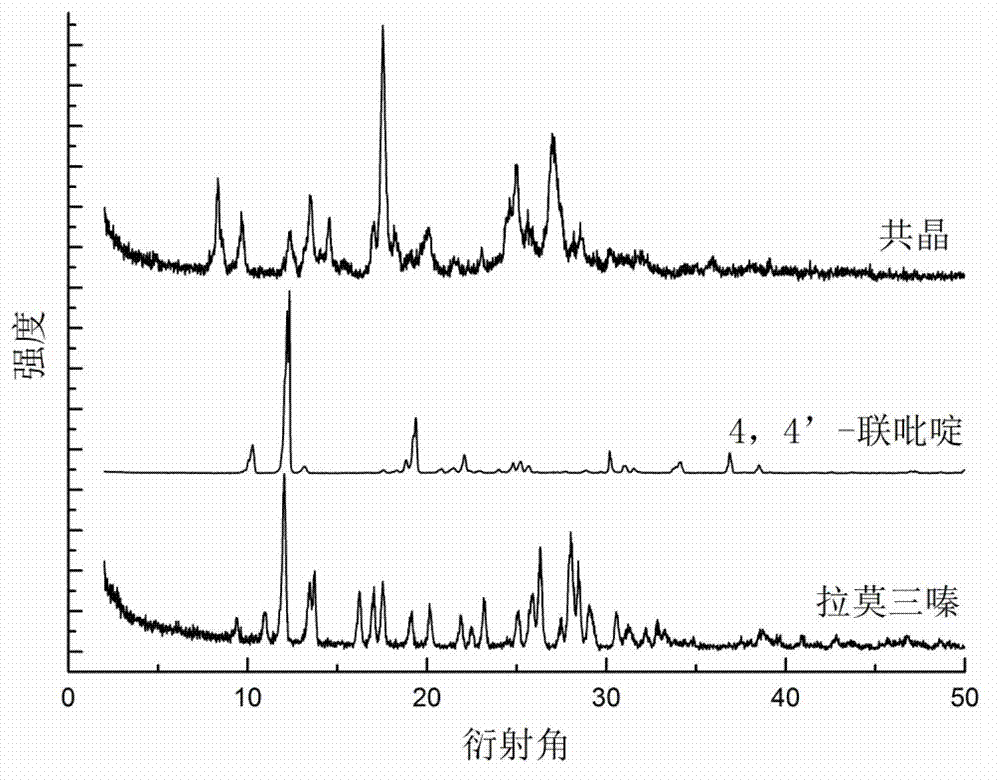
Novel lamotrigine pharmaceutical co-crystal and preparation method thereof
A technology of lamotrigine and medicine, applied in the field of lamotrigine drug co-crystal and its preparation, can solve the problem that the solvate is not an ideal dosage form, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Lamotrigine and 4,4'-bipyridine are synthesized into co-crystals by a solution-mediated transformation method, and the steps are as follows:
[0044] Weighing:
[0045] Reactant lamotrigine: 4,4'-bipyridine = 1:6.5 mass ratio feeding. Accurately weigh 0.3358g lamotrigine and 2.1693g 4,4'-bipyridyl in a 50mL crystallizer with an analytical balance.
[0046] Dissolution of API:
[0047] Accurately measure 28.8mL of ethanol in a 50mL crystallizer with a graduated cylinder.
[0048] Solution-mediated crystallization:
[0049] Connect the crystallizer in a constant temperature water bath, the reaction temperature is 25°C, turn on the magnetic stirrer, and react under stirring for 2-3 hours; after the stirring stops, filter the reaction solution, and dry the filtered product at room temperature, and the obtained product is Lamosan Oxyzine drug cocrystal.
Embodiment 2
[0051] Lamotrigine and 4,4'-bipyridine are synthesized into co-crystals by a solution-mediated transformation method, and the steps are as follows:
[0052] Weighing:
[0053] The reactant lamotrigine: 4,4'-bipyridine=1:5 mass ratio feeds. Accurately weigh 0.5781g of lamotrigine and 2.1695g of 4,4'-bipyridyl in a 50mL crystallizer with an analytical balance.
[0054] Dissolution of API:
[0055] Accurately measure 32.3mL of ethanol in a 50mL crystallizer with a graduated cylinder.
[0056] Solution-mediated crystallization:
[0057] Connect the crystallizer in a constant temperature water bath, the reaction temperature is 25°C, turn on the magnetic stirrer, and react under stirring for 2-3 hours; after the stirring stops, filter the reaction solution, and dry the filtered product at room temperature, and the obtained product is Lamosan Oxyzine drug cocrystal.
Embodiment 3
[0059] Lamotrigine and 4,4'-bipyridine are synthesized into co-crystals by a solution-mediated transformation method, and the steps are as follows:
[0060] Weighing:
[0061] Reactant lamotrigine: 4,4'-bipyridine = 1:4 mass ratio feeding. Accurately weigh 0.5109g of lamotrigine and 2.0436g of 4,4'-bipyridyl in a 50mL crystallizer with an analytical balance.
[0062] Dissolution of API:
[0063] Accurately measure 31.2mL of ethanol in a 50mL crystallizer with a graduated cylinder.
[0064] Solution-mediated crystallization:
[0065] Connect the crystallizer in a constant temperature water bath, the reaction temperature is 25°C, turn on the magnetic stirrer, and react under stirring for 2-3 hours; after the stirring stops, filter the reaction solution, and dry the filtered product at room temperature, and the obtained product is Lamosan Oxyzine drug cocrystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com