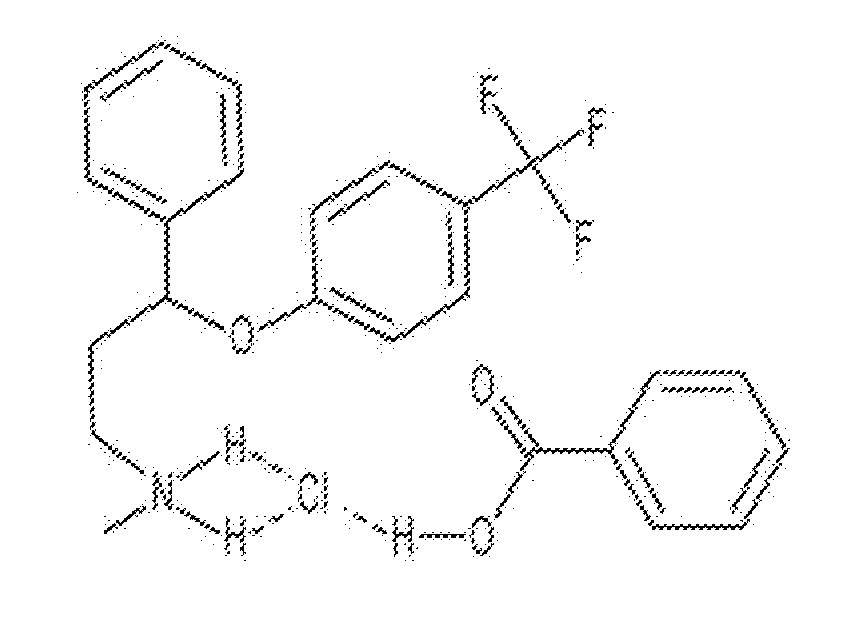
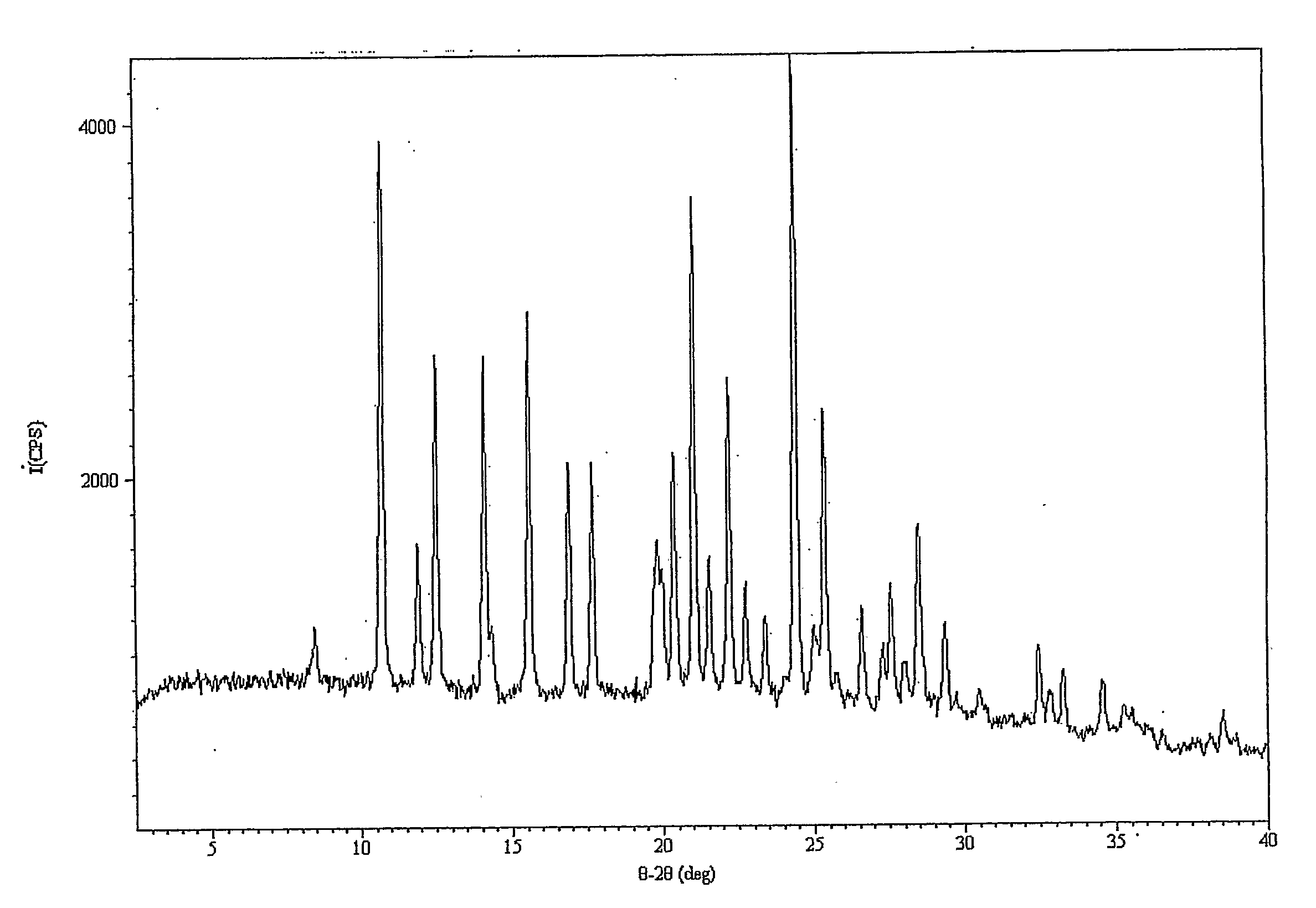
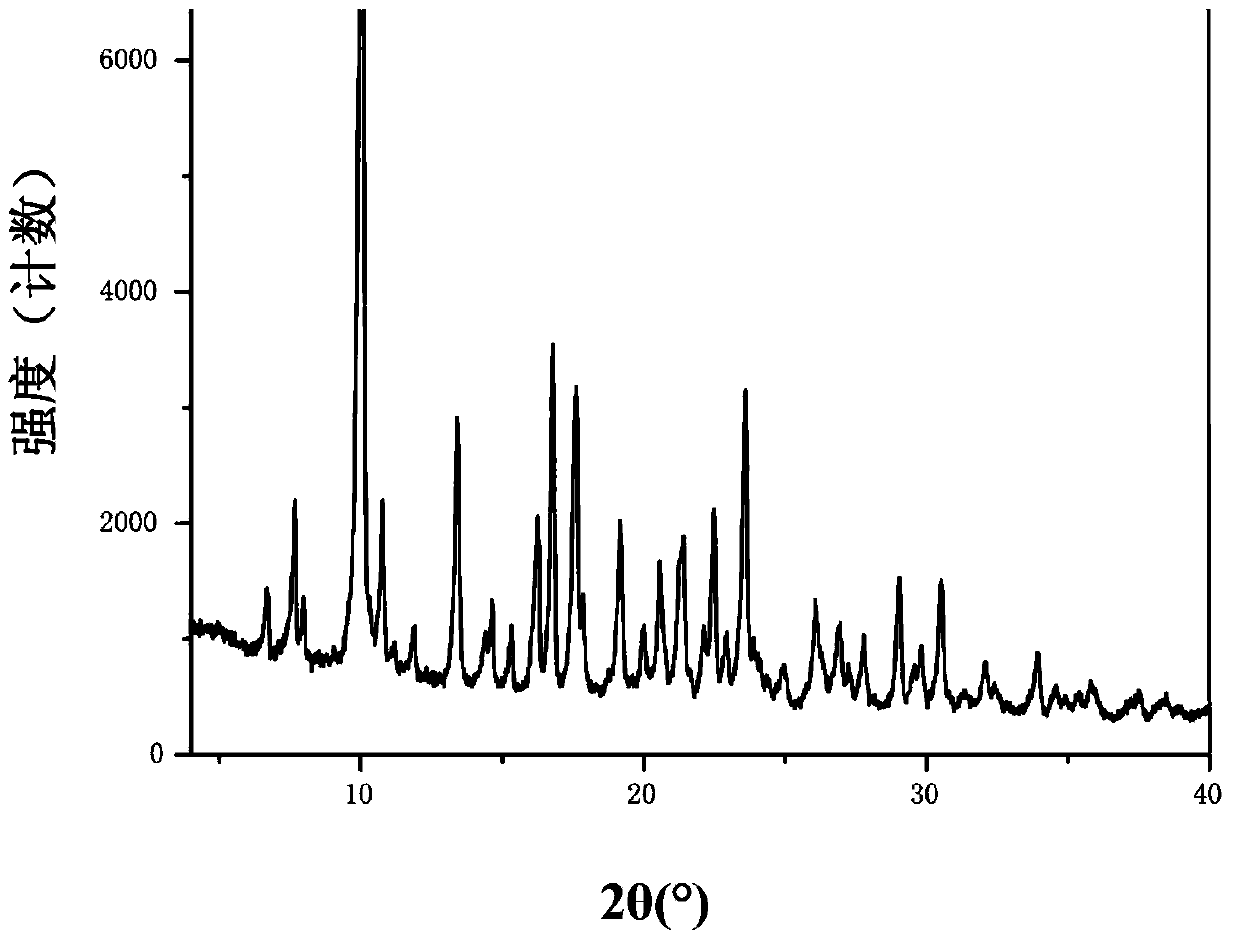
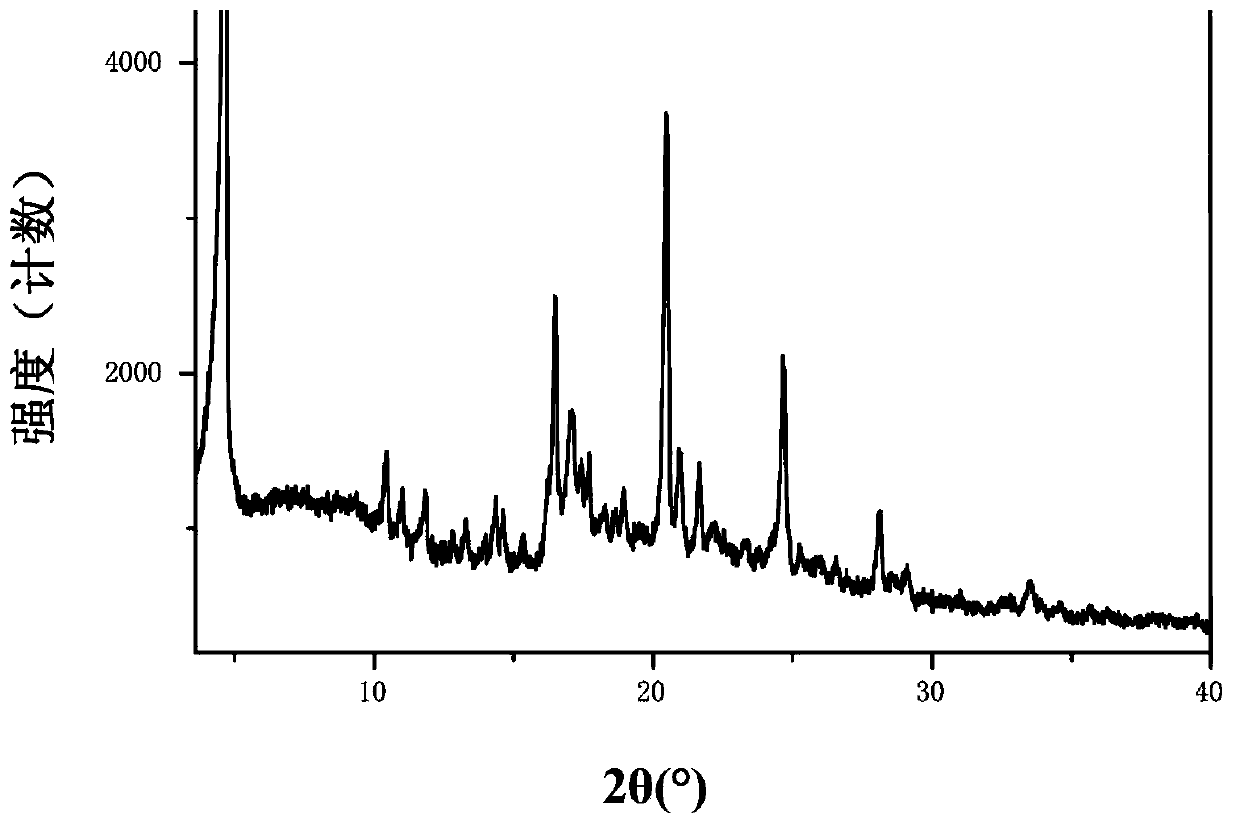
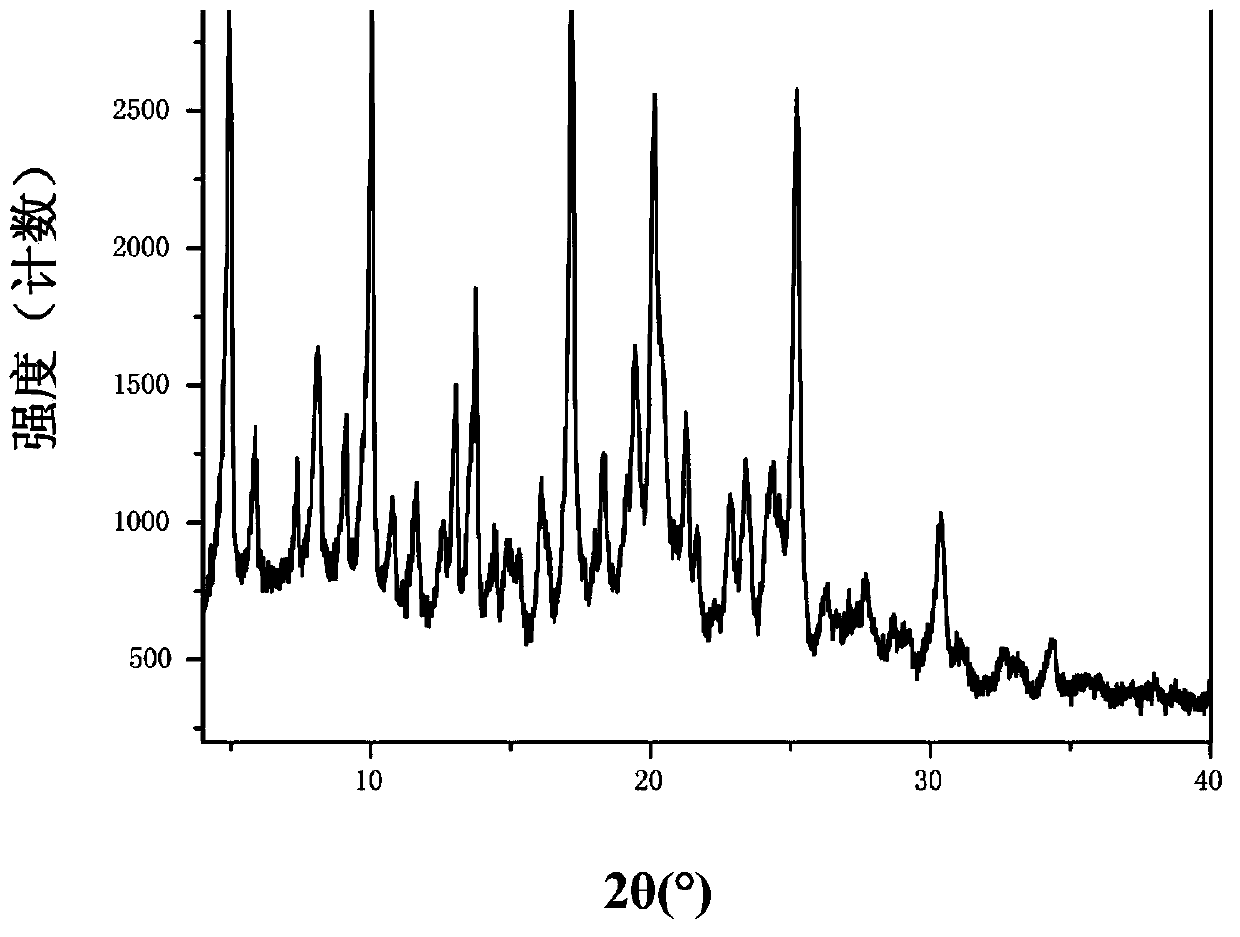
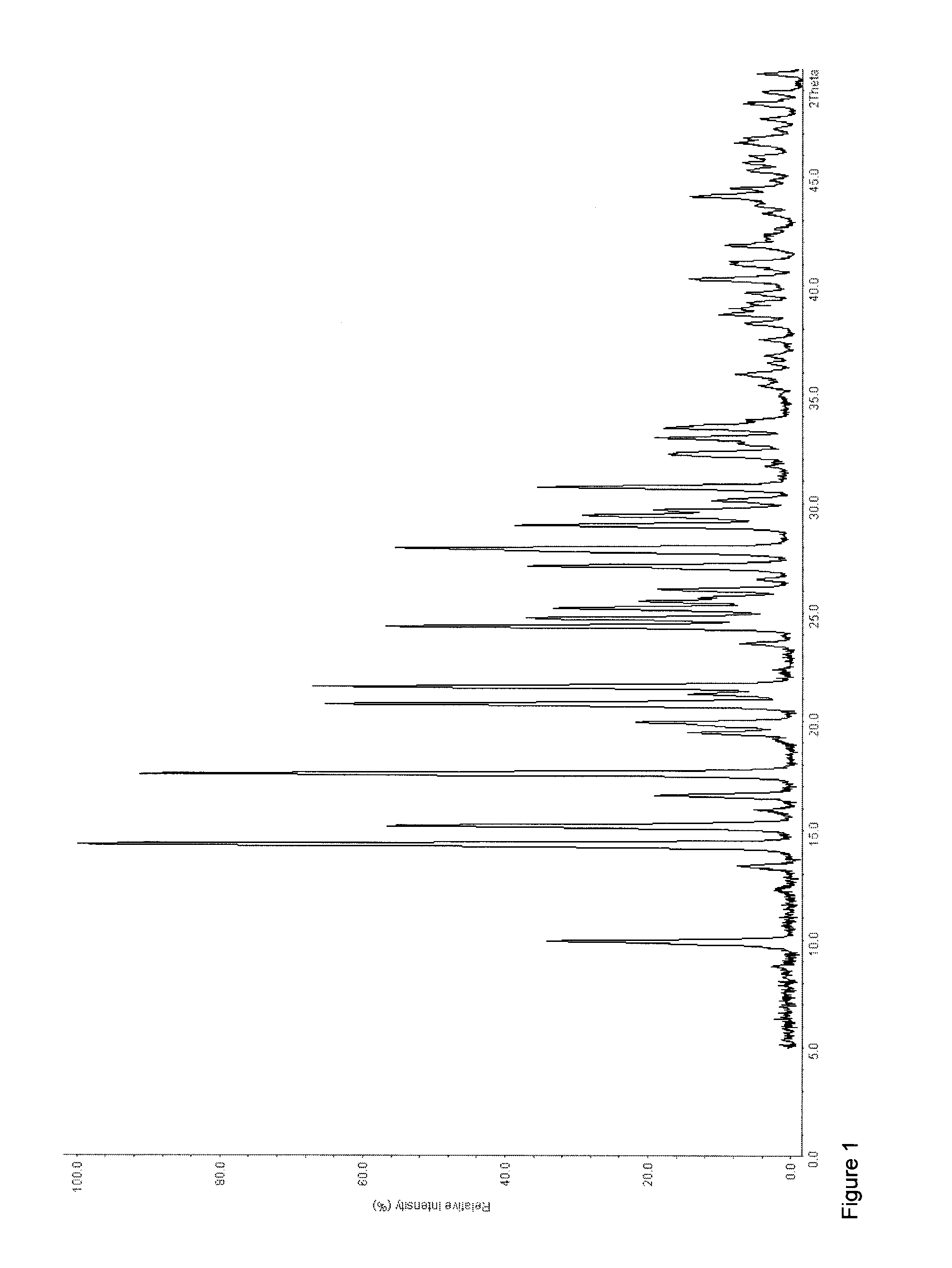
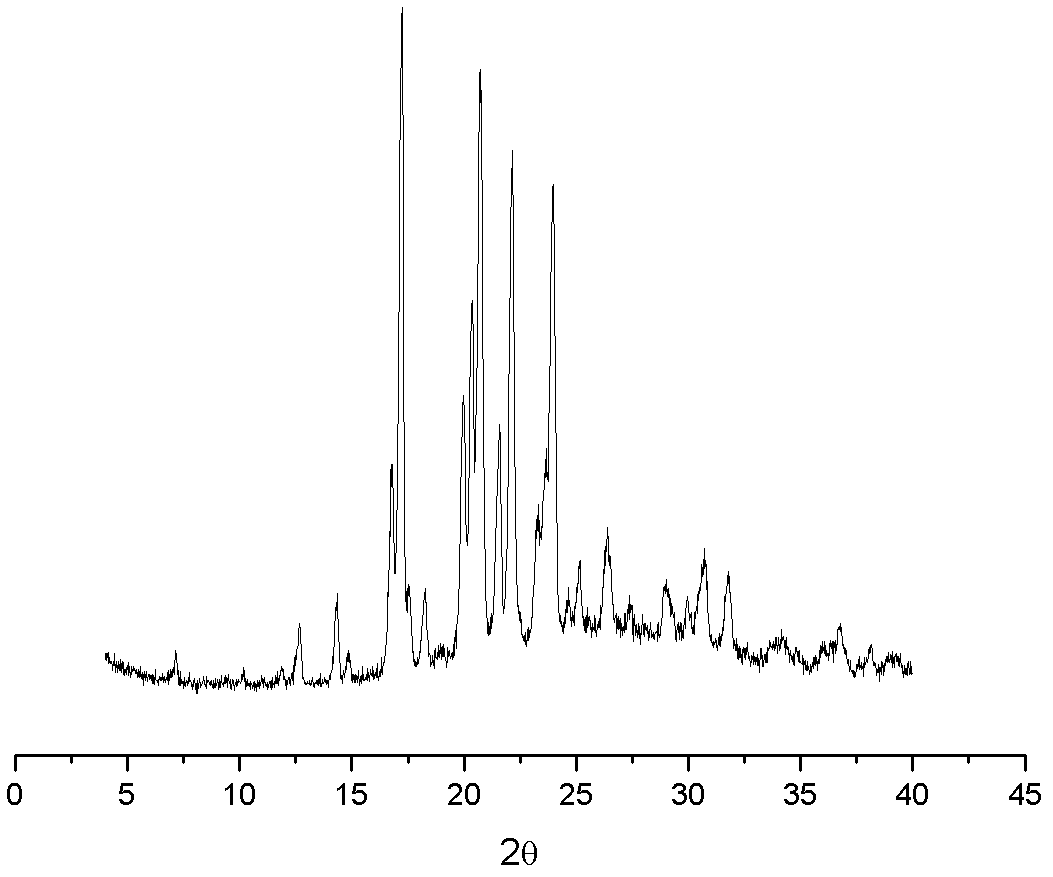
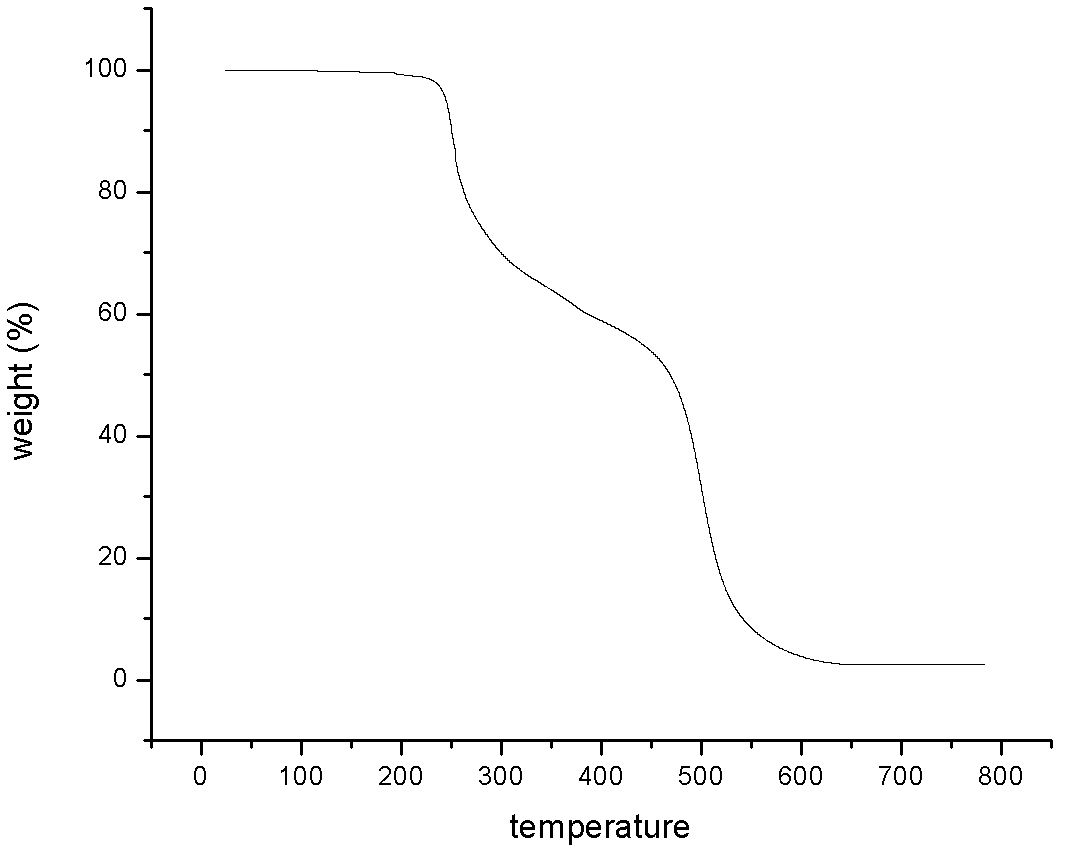
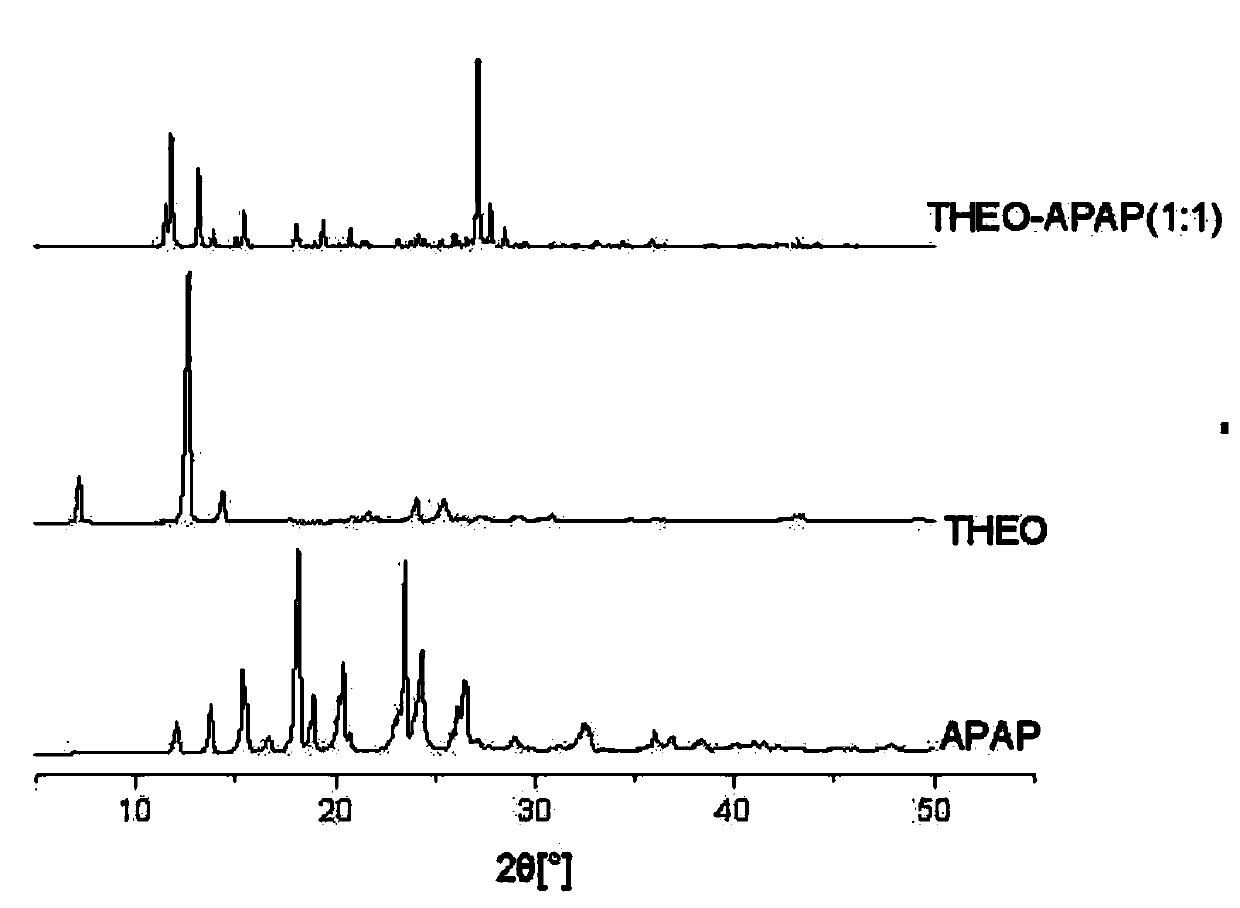
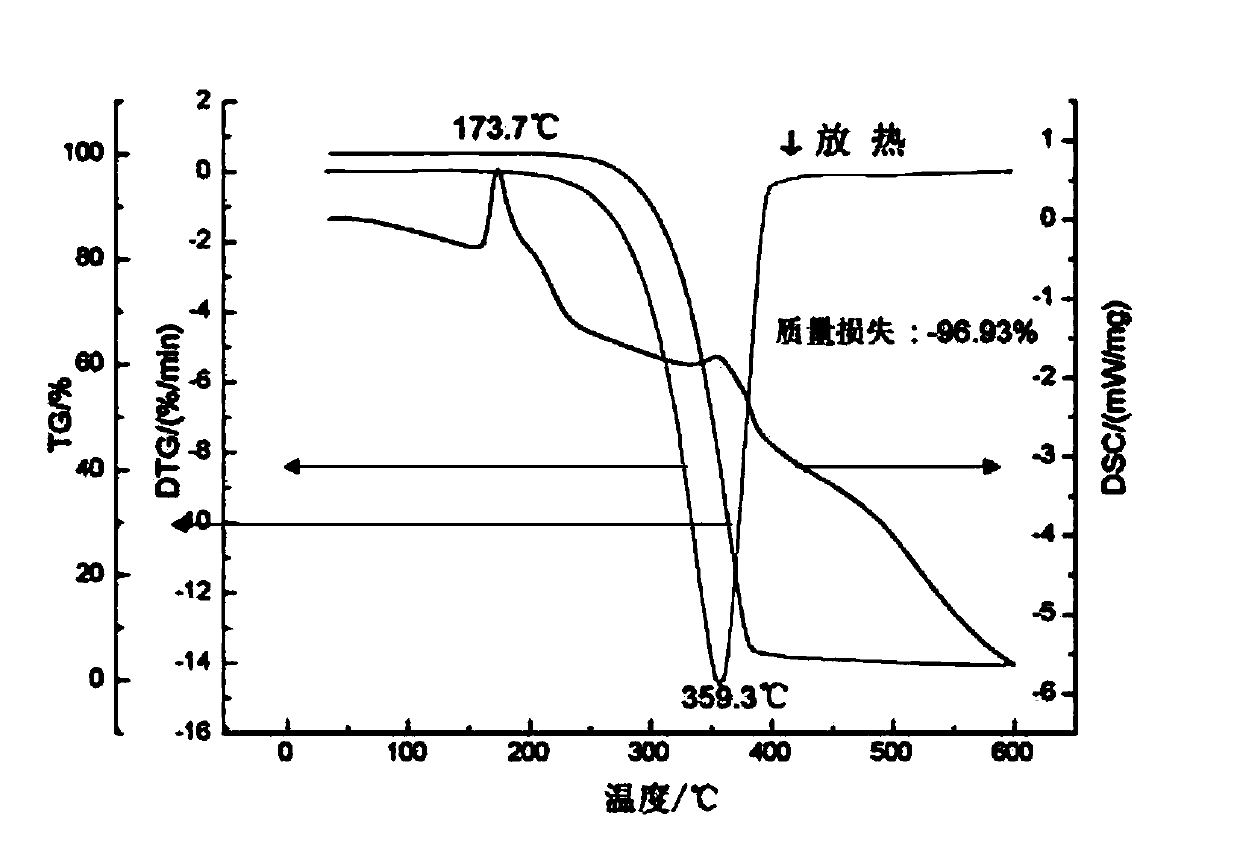
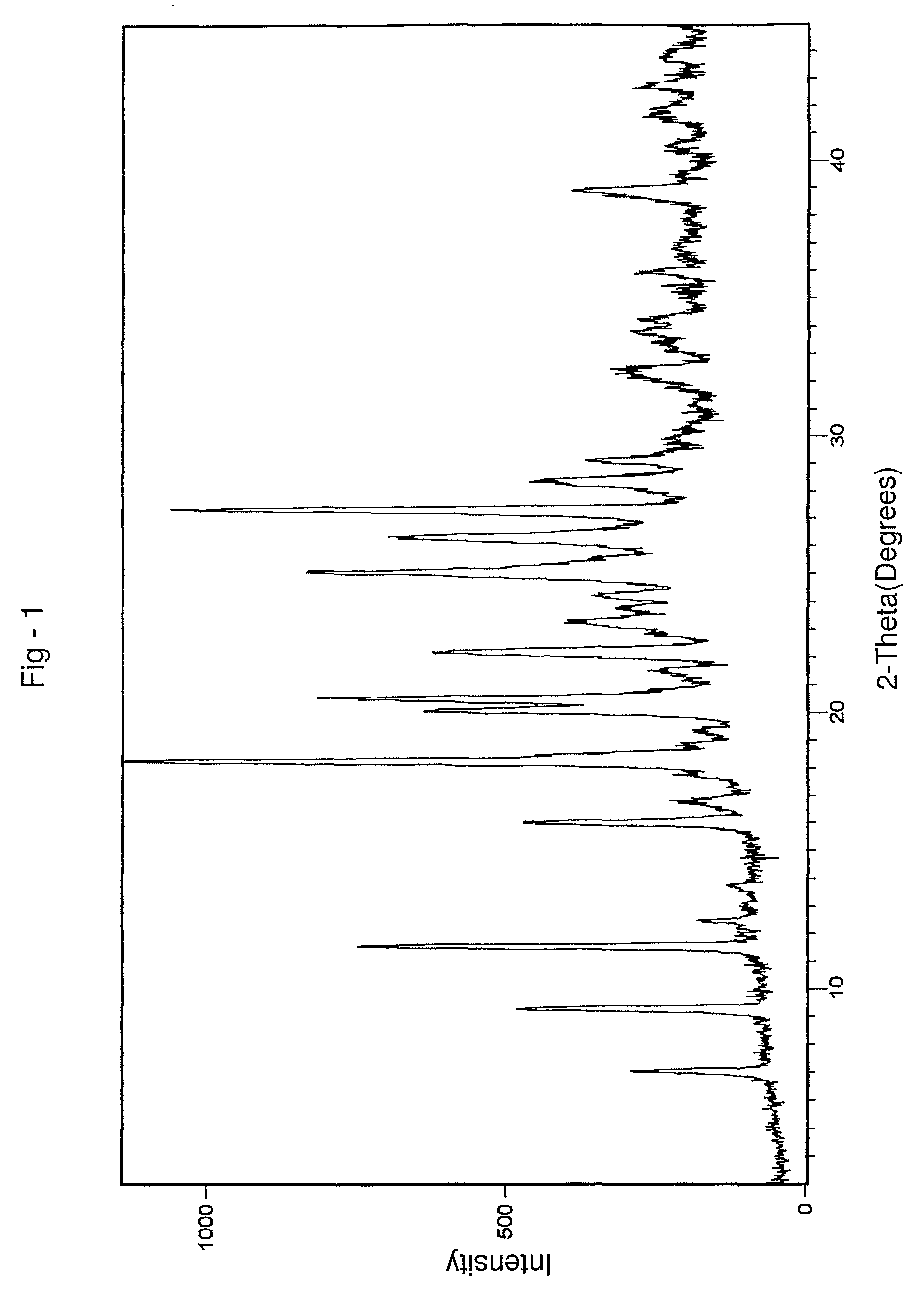
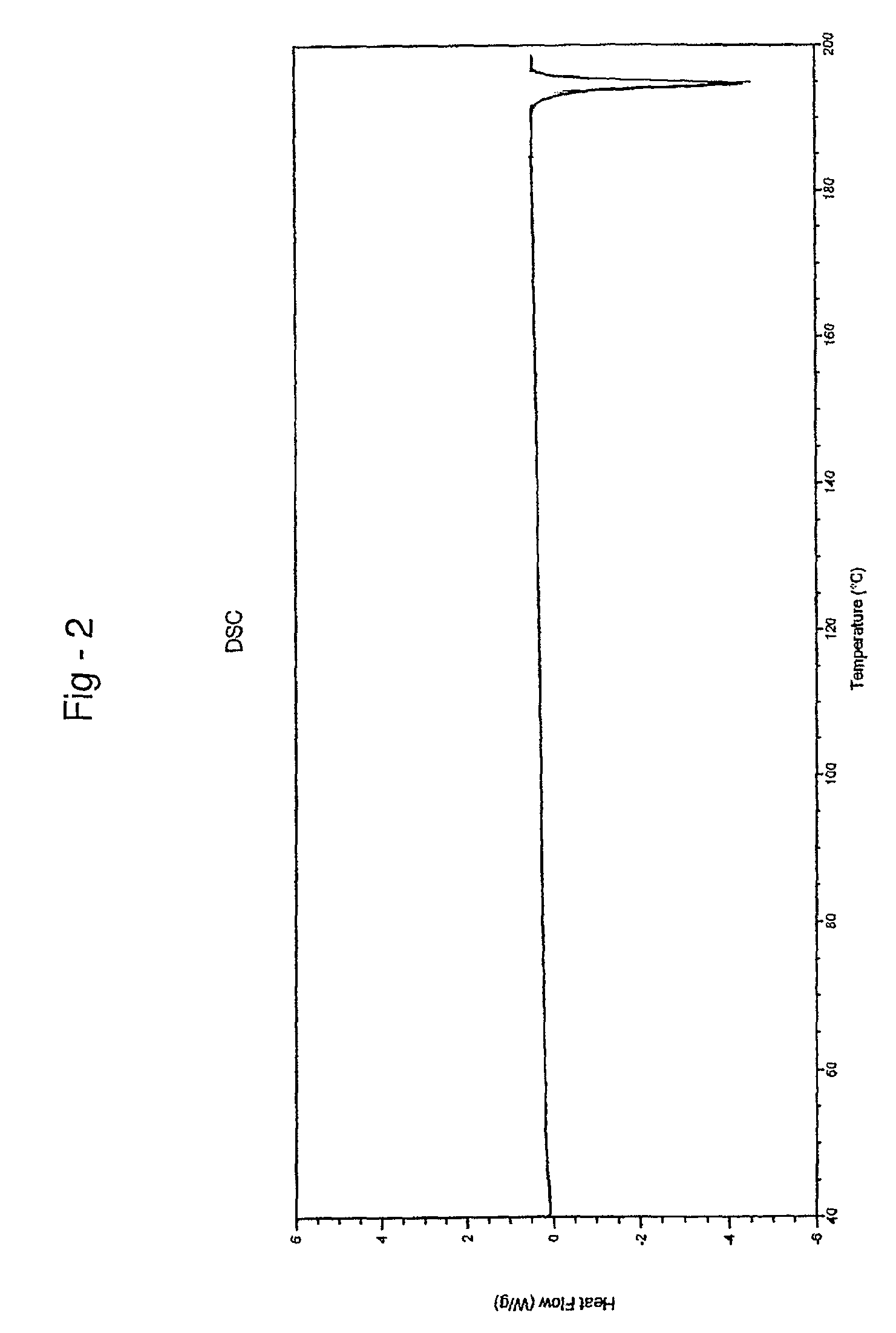
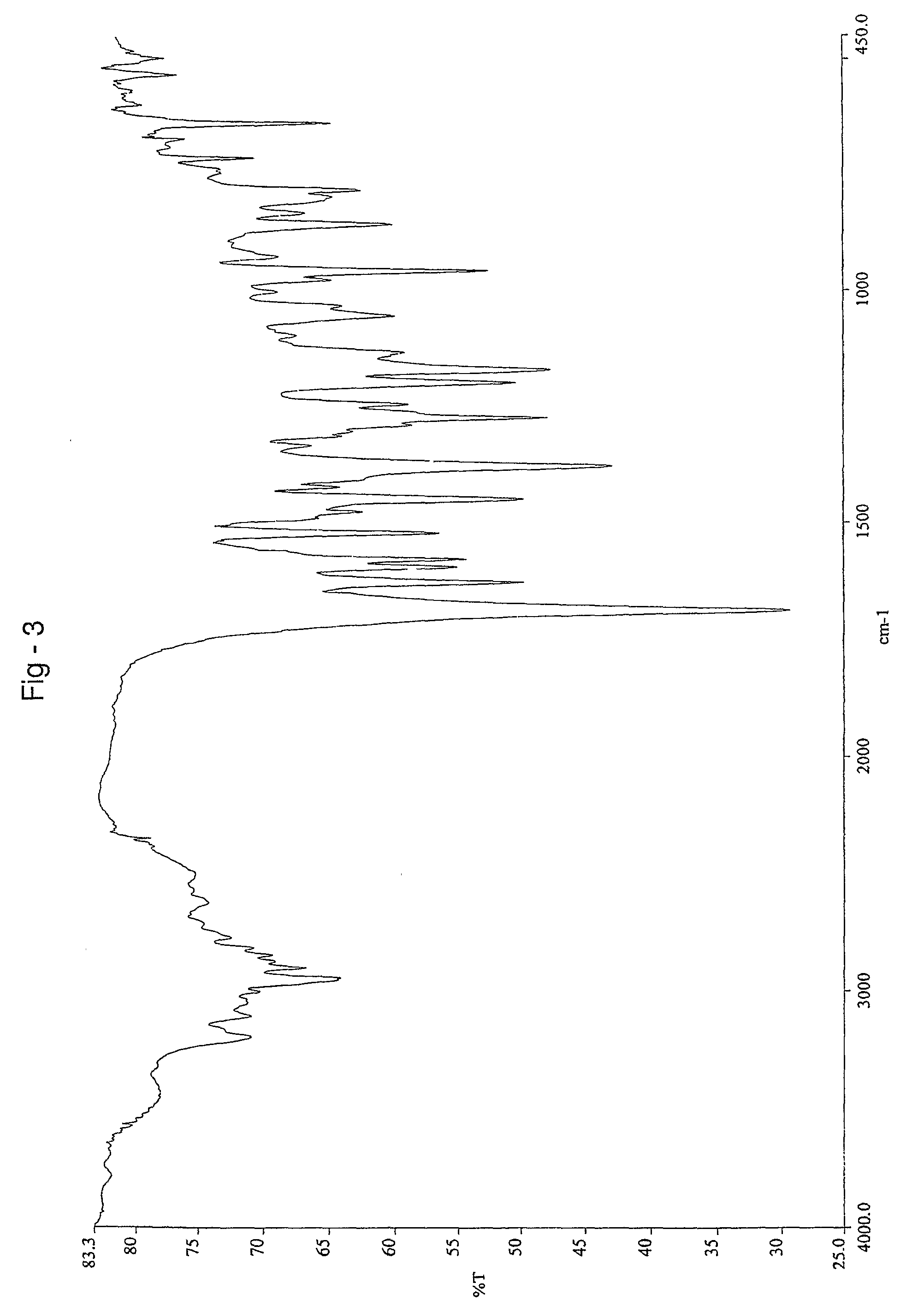
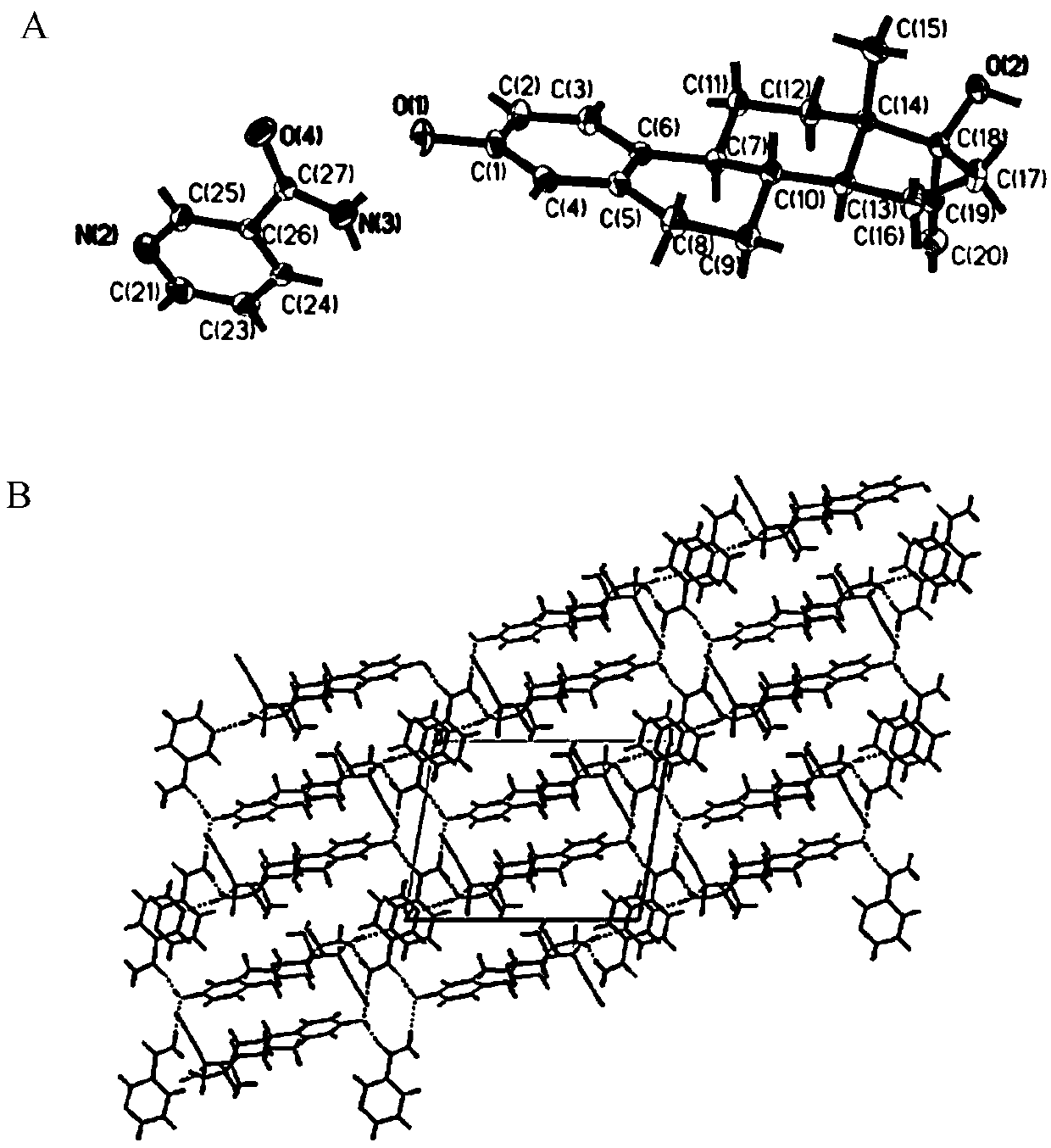
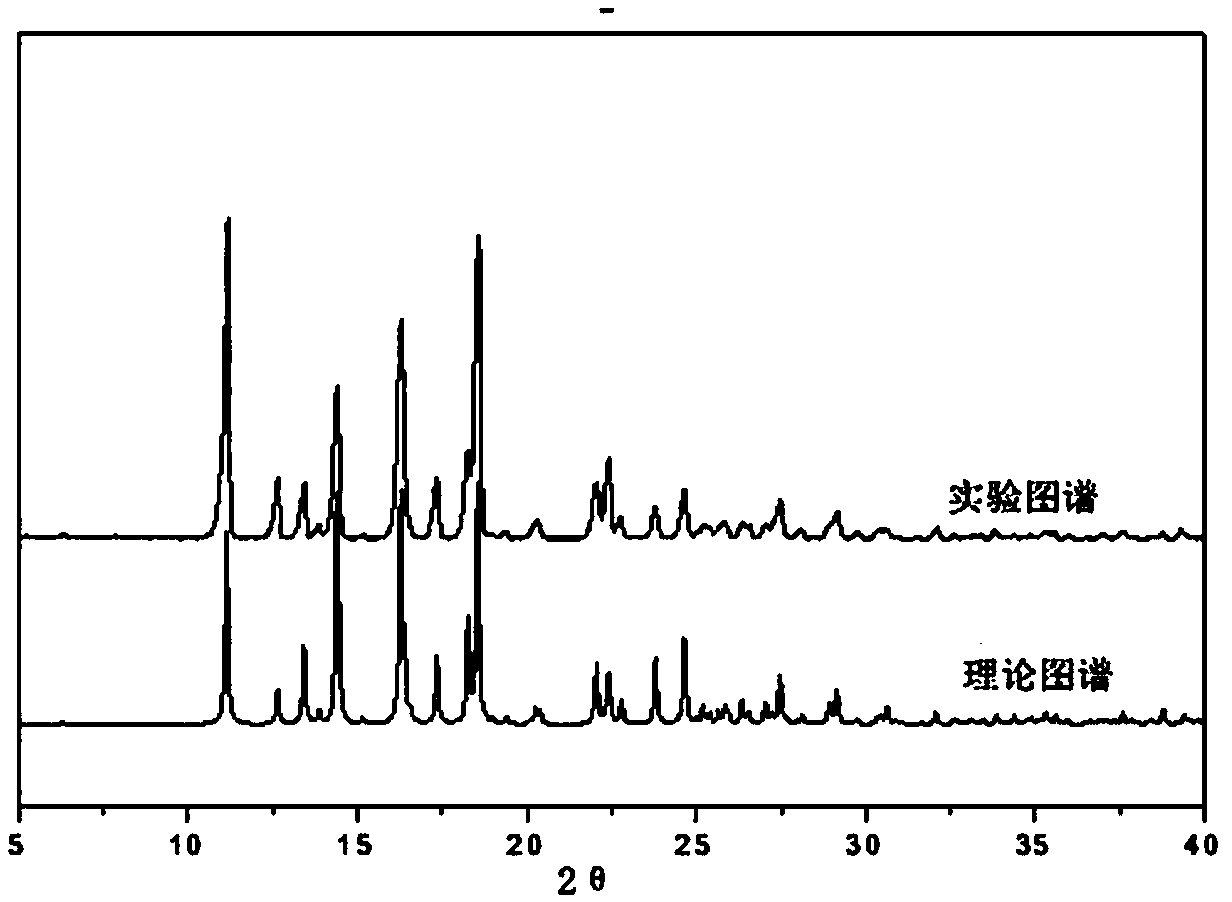
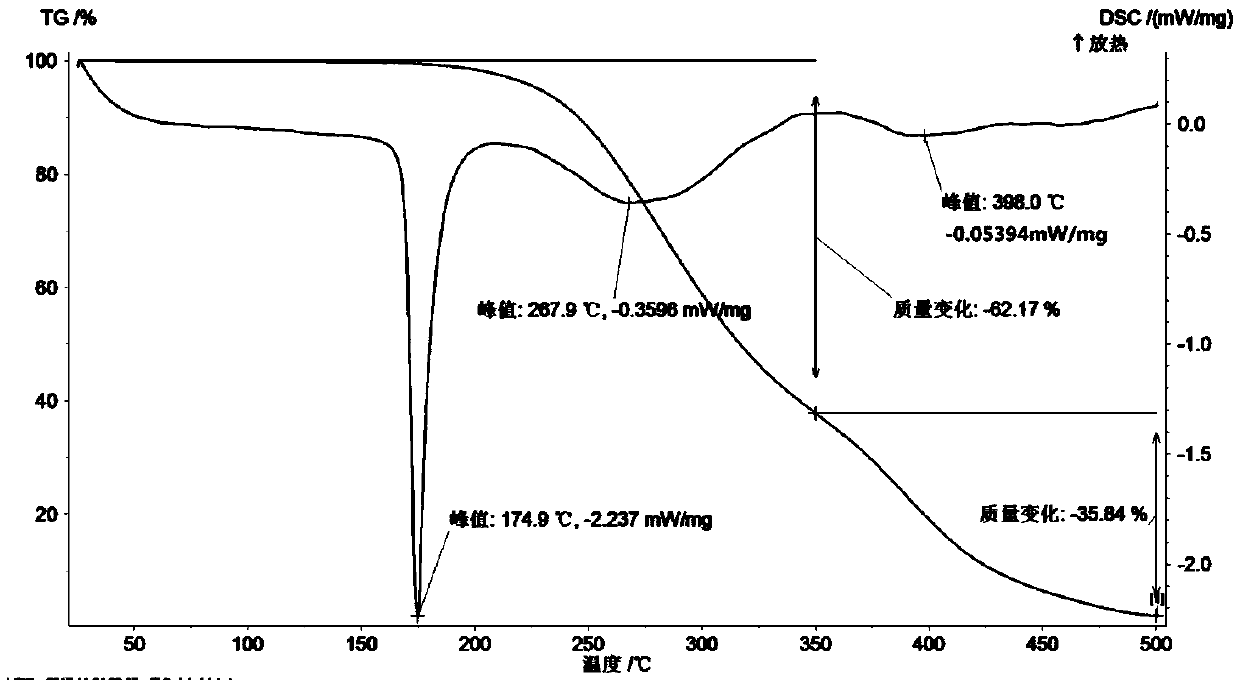
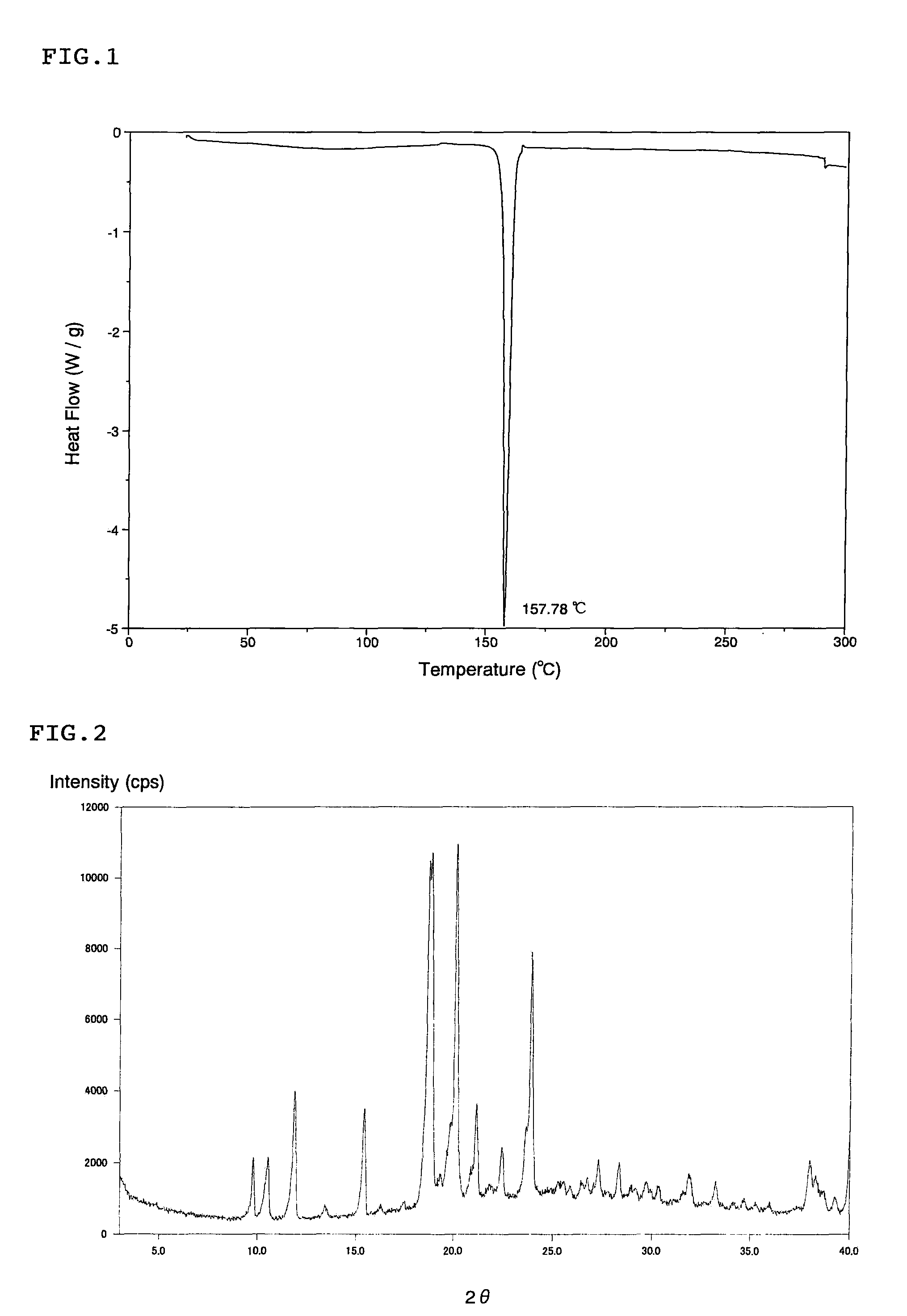
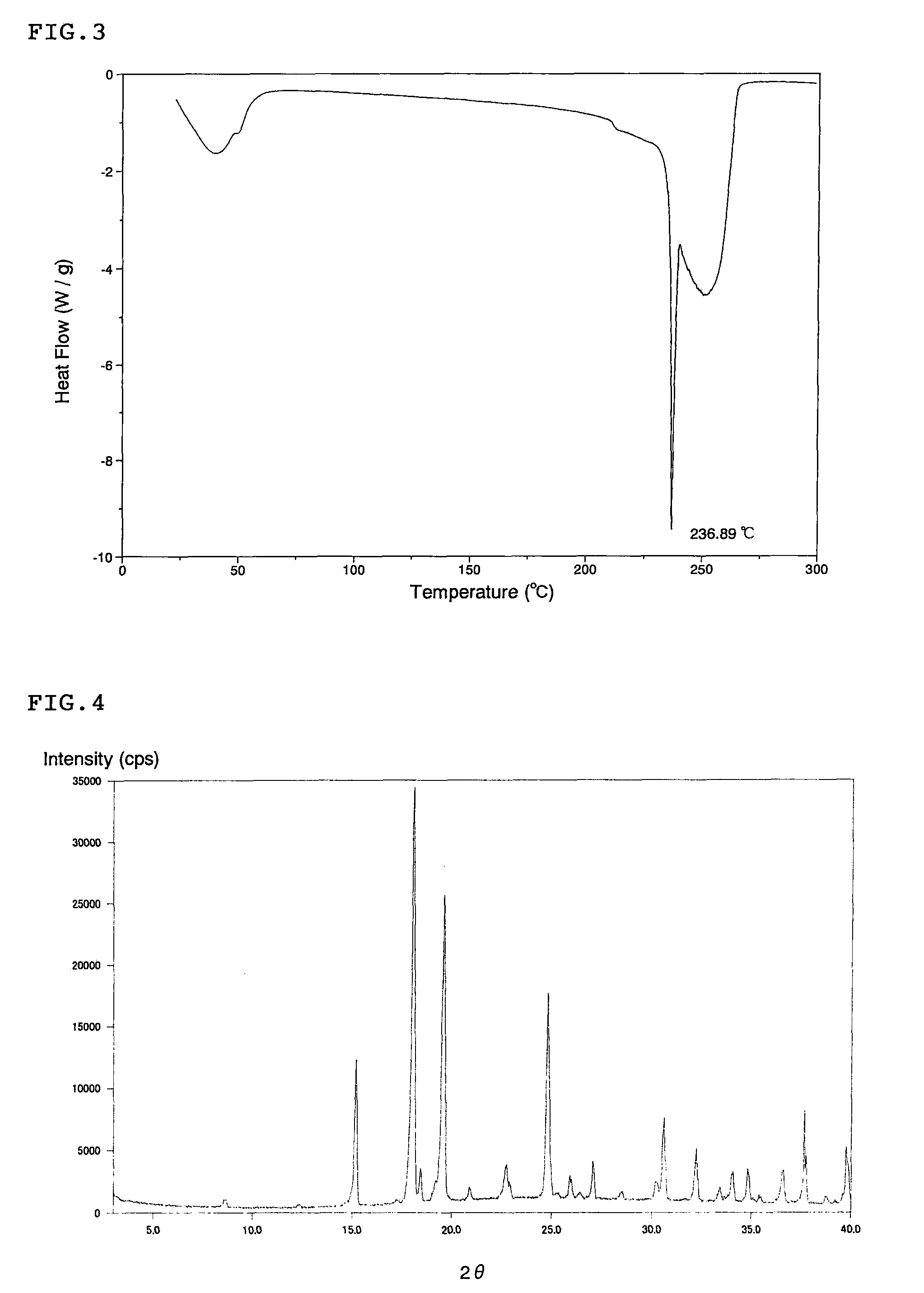
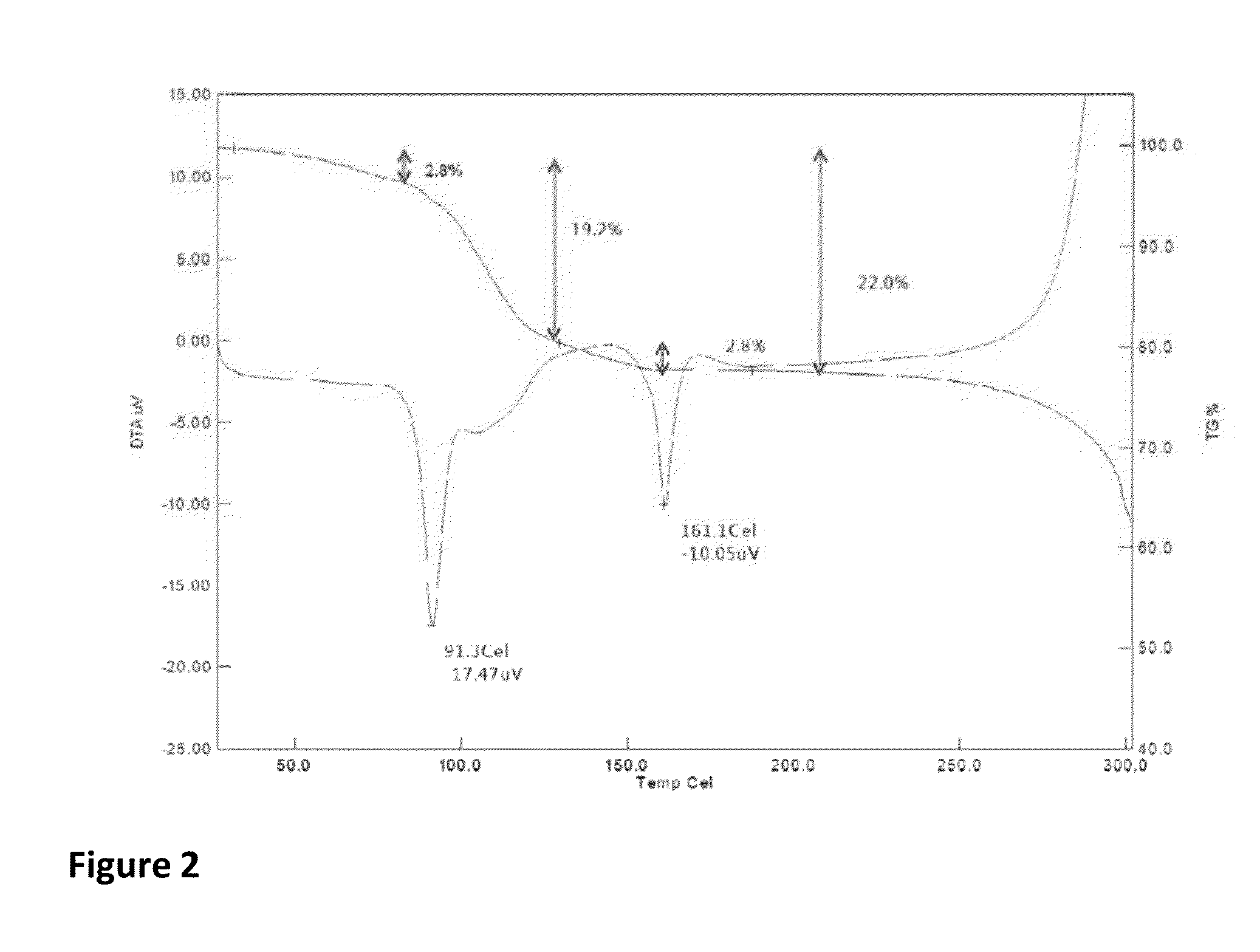
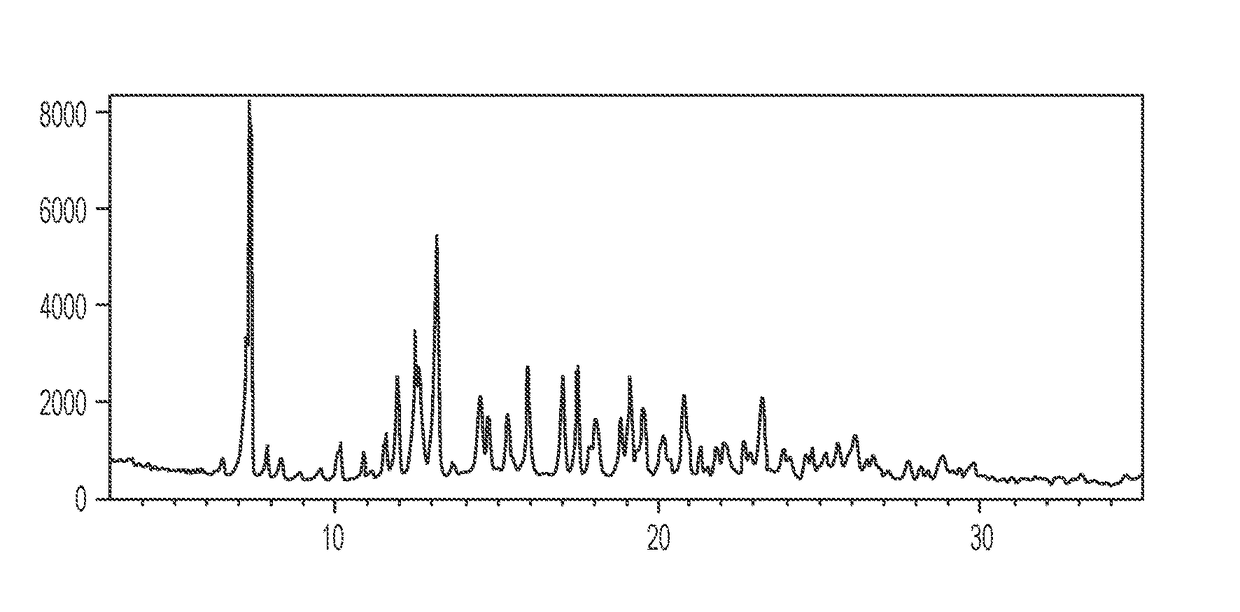
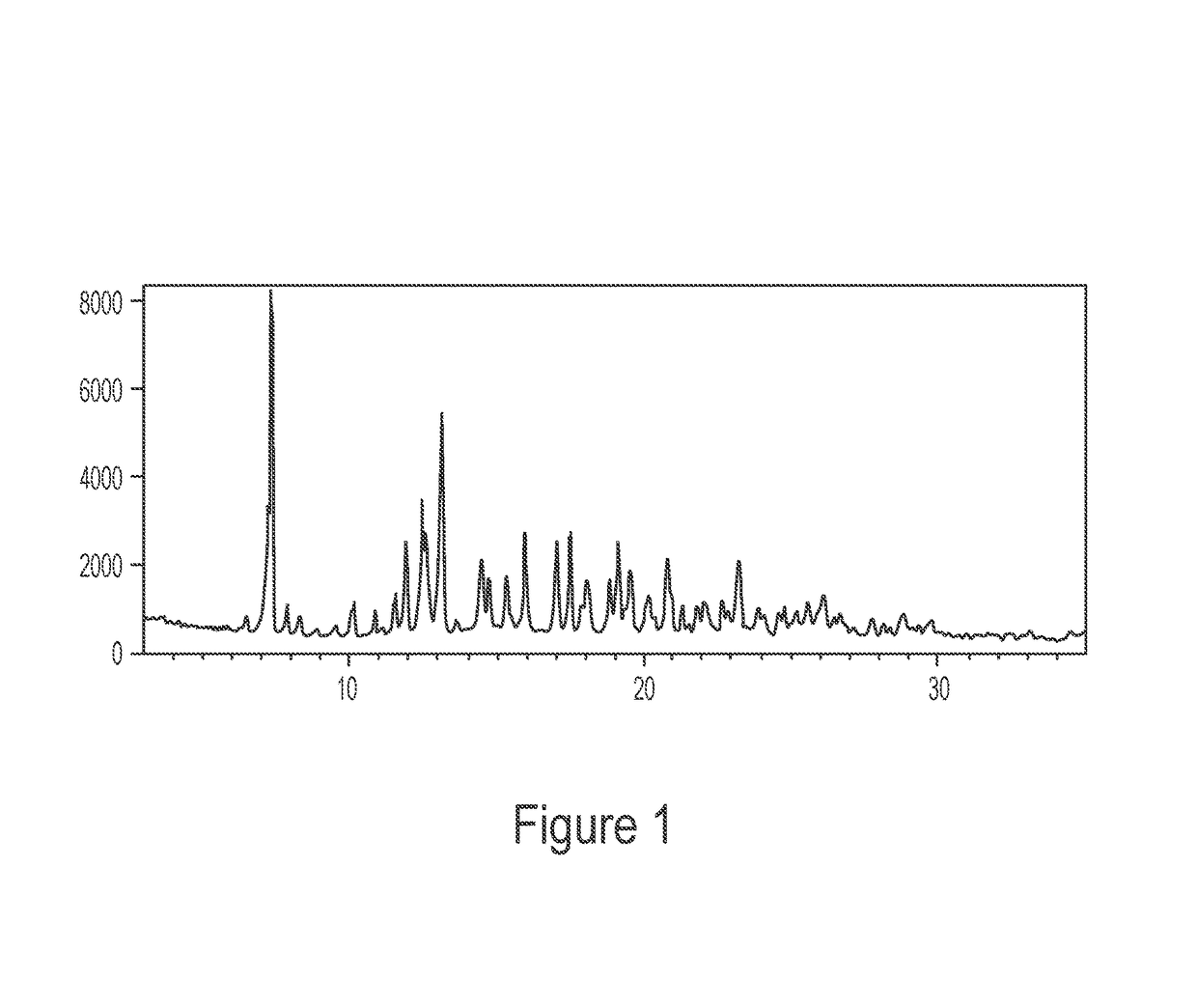
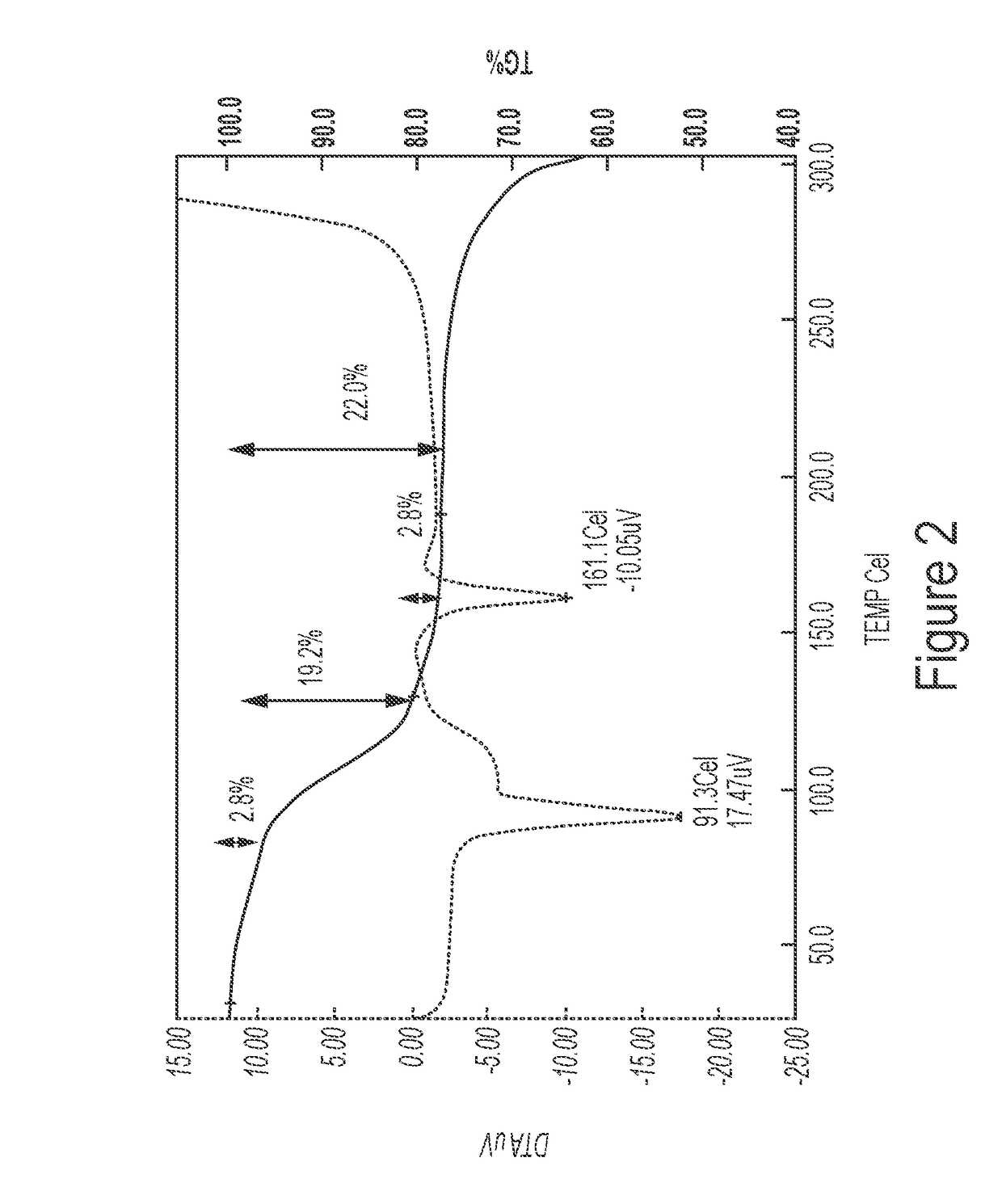
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
174 results about "Cocrystal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
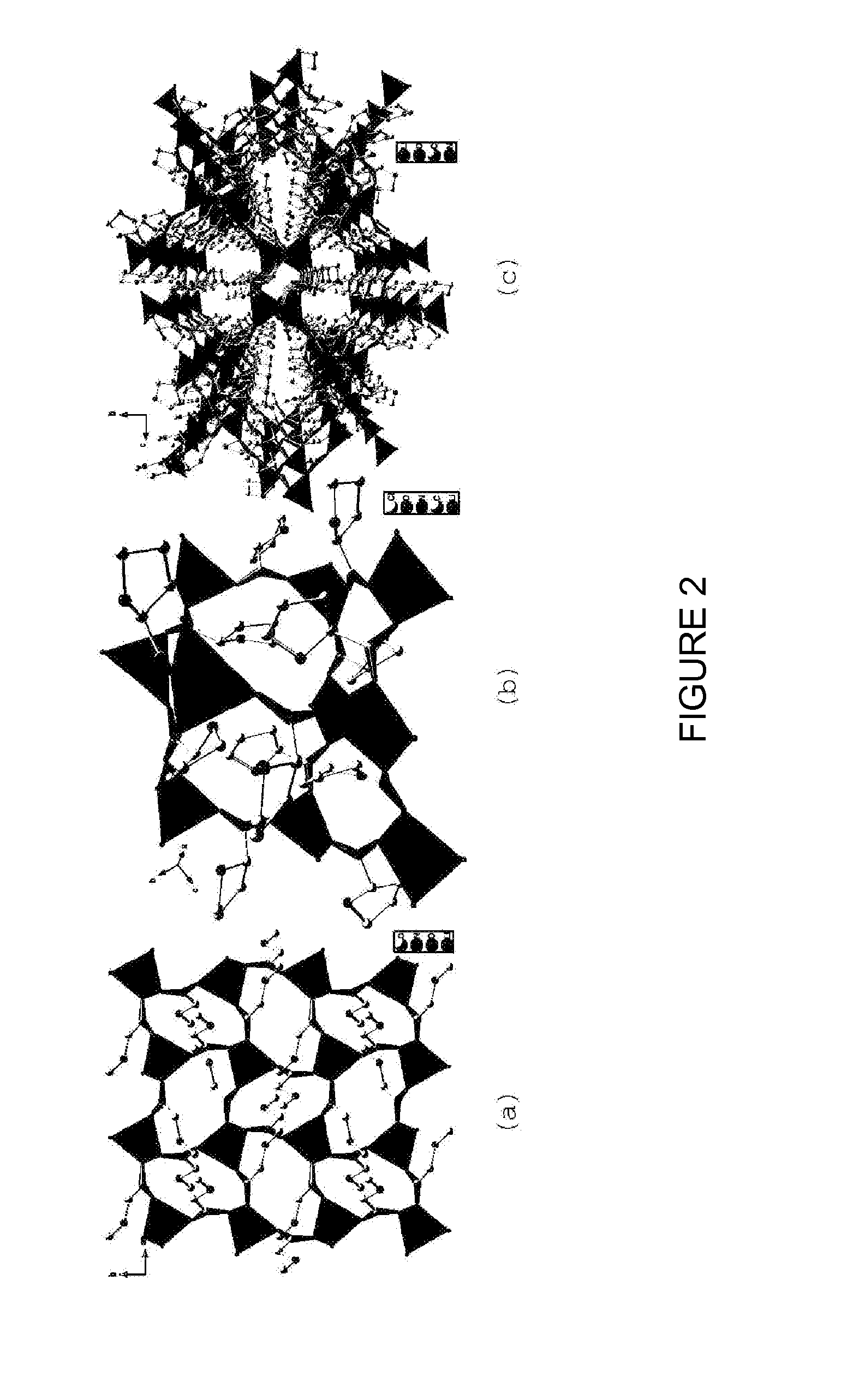
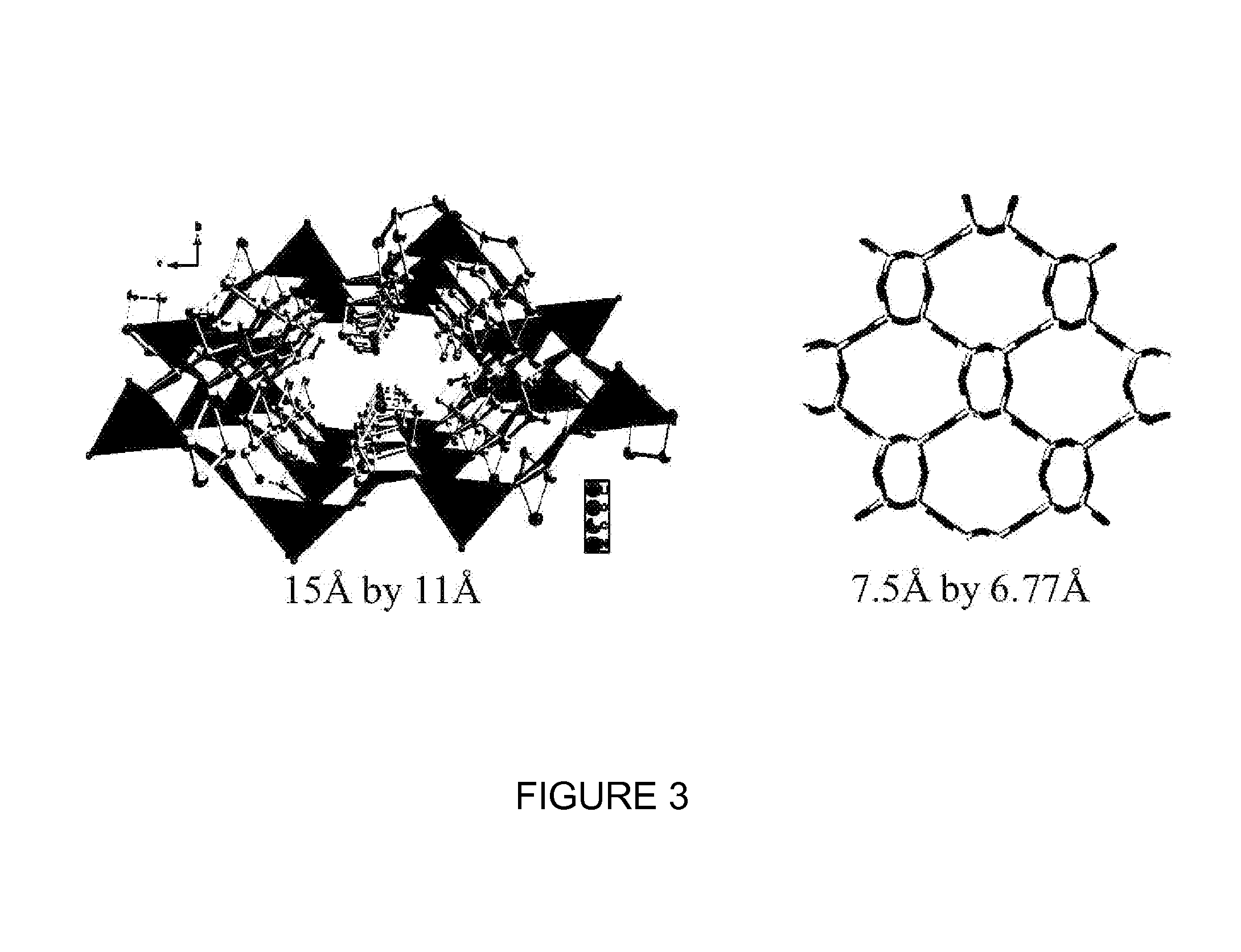
Cocrystals are "solids that are crystalline single phase materials composed of two or more different molecular or ionic compounds generally in a stoichiometric ratio which are neither solvates nor simple salts." A broader definition is that cocrystals "consist of two or more components that form a unique crystalline structure having unique properties." Several subclassifications of cocrystals exist.
Cocrystal of c-glycoside derivative and l-proline
InactiveUS20090143316A1Quality improvementGood storage stabilityBiocideSugar derivativesCompound aC-glycoside
A cocrystal of (1S)-1,5-anhydro-1-[3-(1-benzothien-2-ylmethyl)-4-fluorophenyl]-D-glucitol and L-proline. It is a cocrystal of known compound A, which has a constant quality, is superior in storage stability, has no moisture absorptivity, and is suitable as a crystal of a drug substance used for preparing pharmaceuticals.
Owner:ASTELLAS PHARMA INC +1
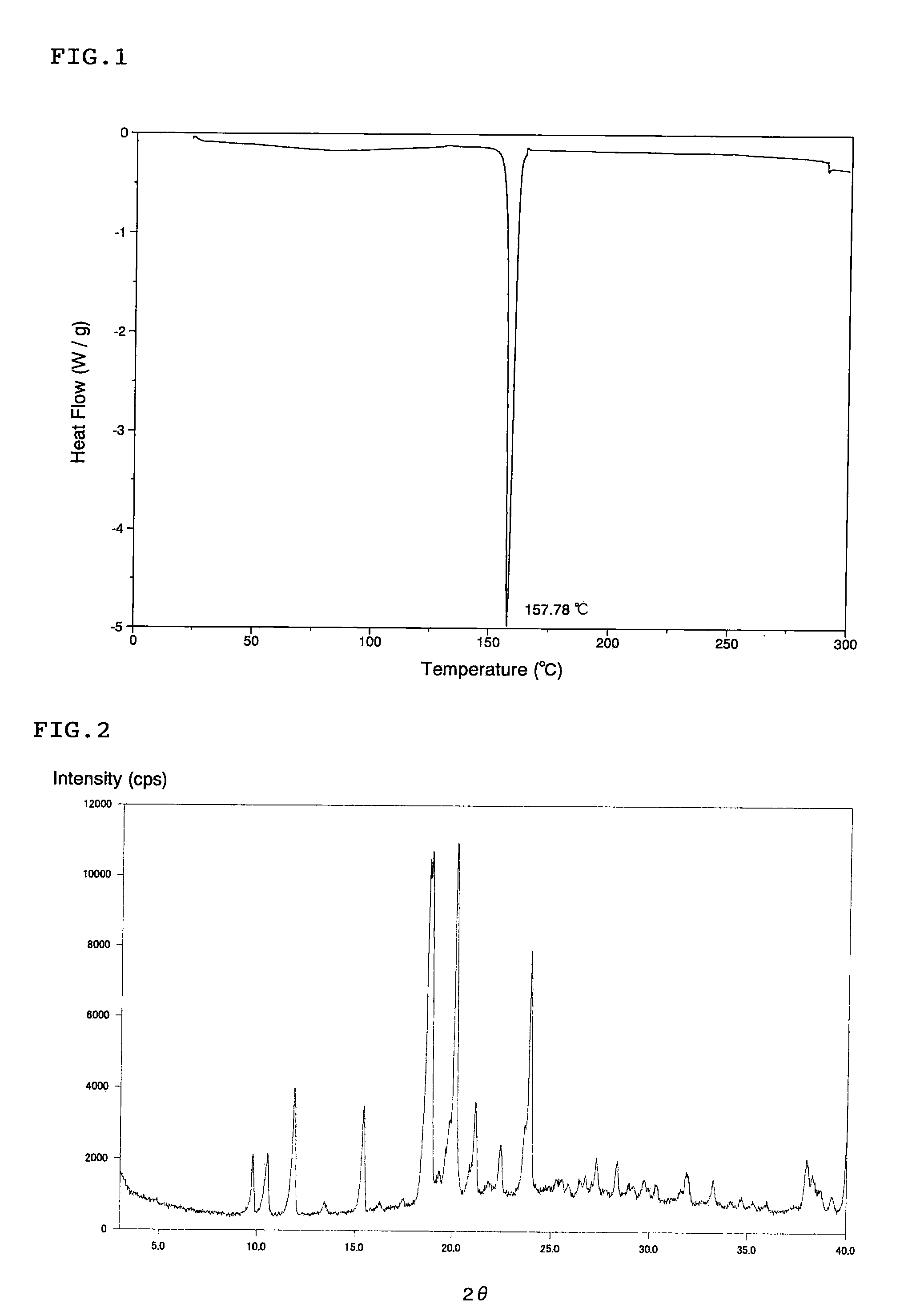
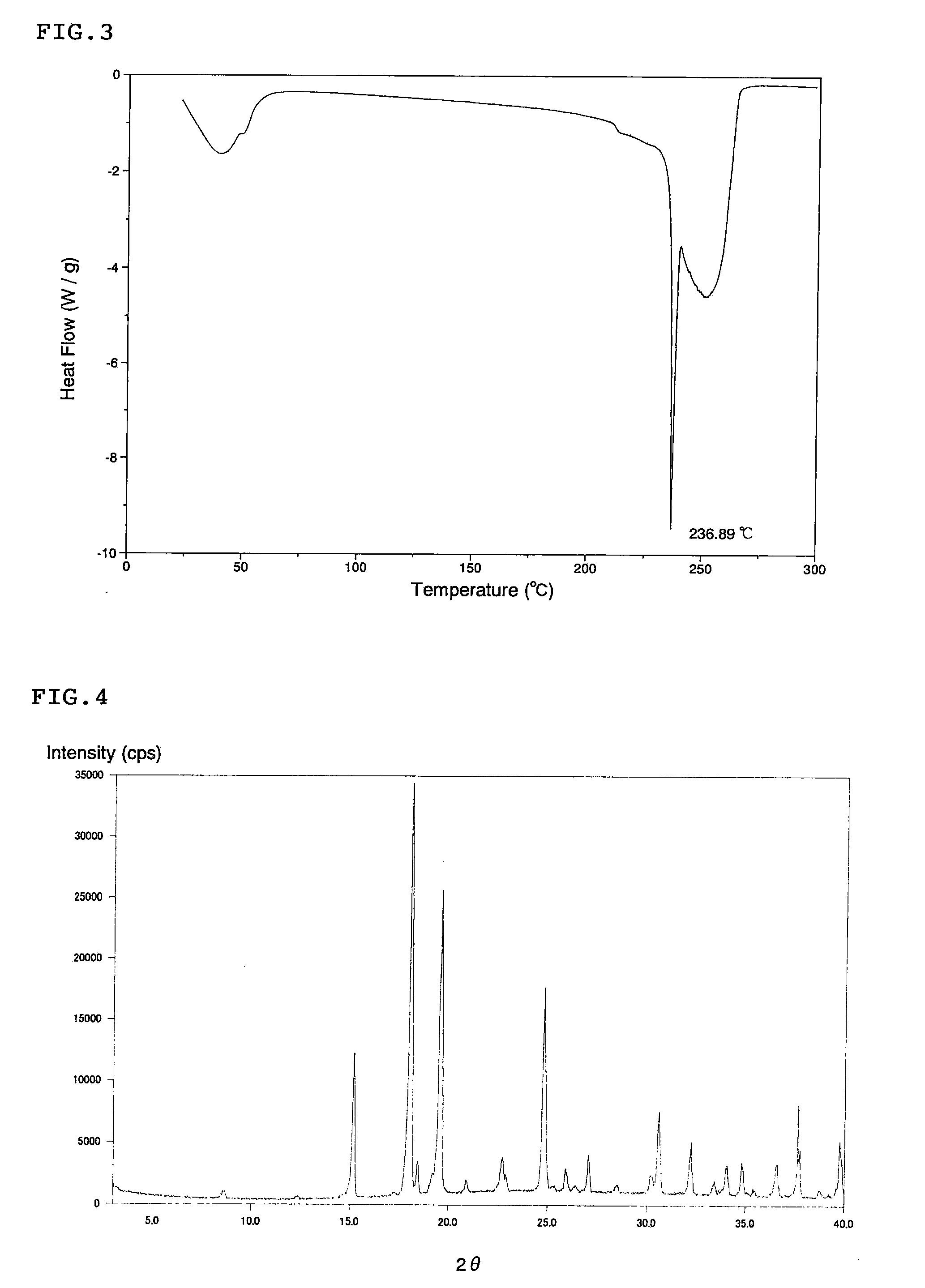
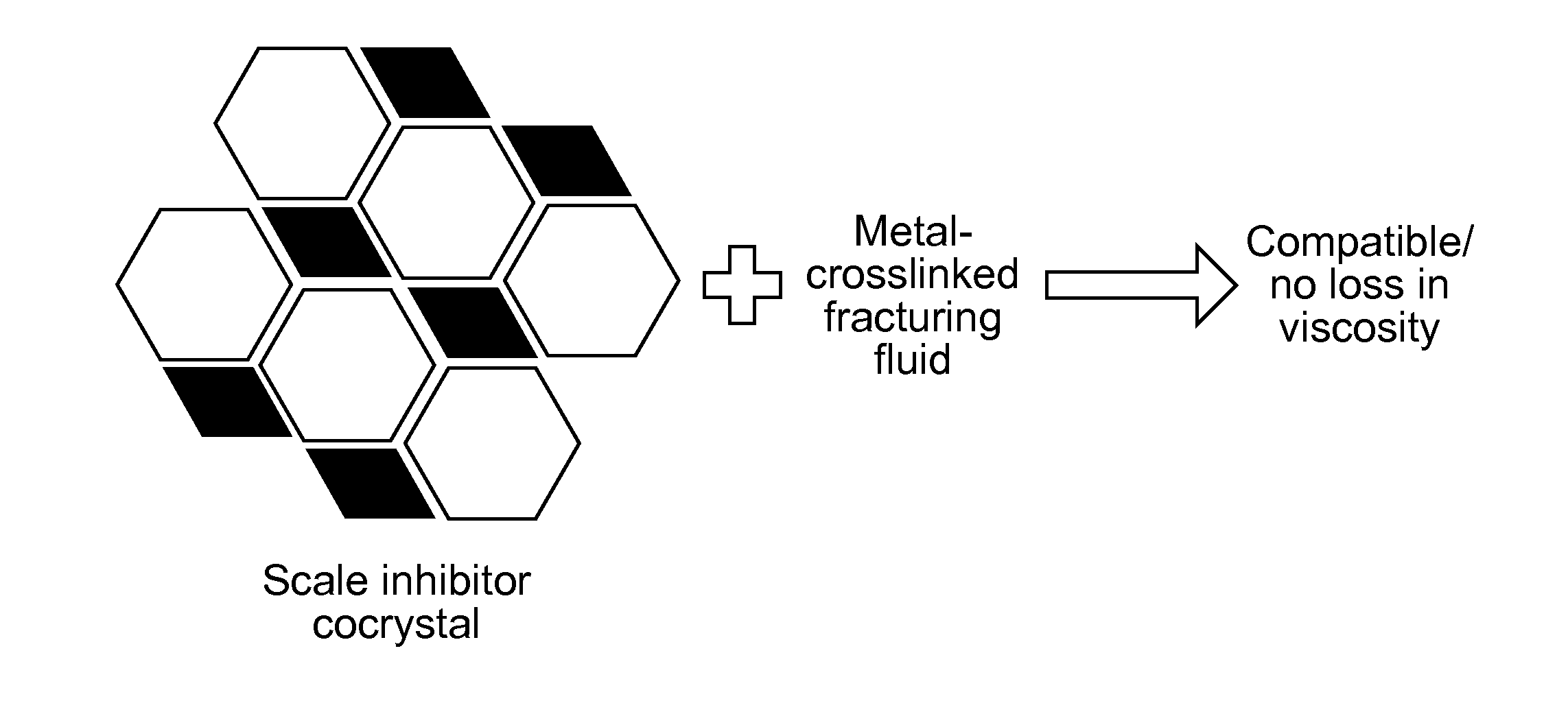
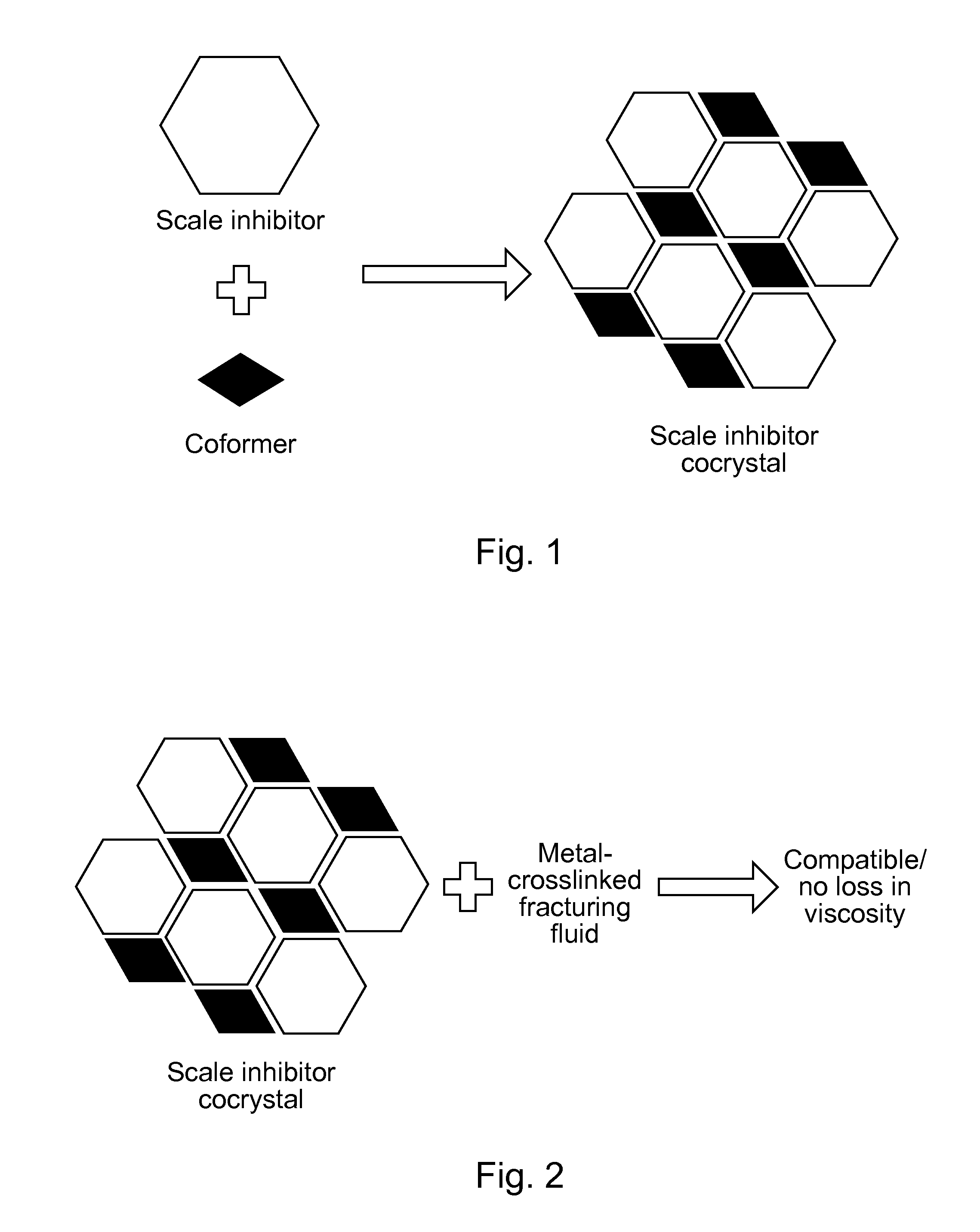
Scale-inhibiting cocrystals for treatment of a subterranean formation
InactiveUS20160130496A1Improve the environmentImprove effectivenessDrilling rodsSolid waste managementCrystalCocrystal
The present invention relates to cocrystals including a scale-inhibiting compound, and methods of using the cocrystals for treating a subterranean formation. In various embodiments, the present invention provides a method of treating a subterranean formation including obtaining or providing a composition including cocrystals. Each cocrystal independently includes a scale-inhibiting compound and a secondary material. The method also includes placing the composition in a subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Modafinil compositions
InactiveUS20070021510A1Improve solubilityHigh dissolution rateBiocideOrganic active ingredientsSolubilityMedicine
Co-crystals and solvates of racemic, enantiomerically pure, and enantiomerically mixed modafinil are formed and several important physical properties are modulated. The solubility, dissolution, bioavailability, dose response, and stability of modafinil can be modulated to improve efficacy in pharmaceutical compositions.
Owner:CEPHALON INC
Stable injectable composition of bivalirudin and process for its preparation
InactiveUS20170224789A1Peptide/protein ingredientsPharmaceutical delivery mechanismMedicineReady to use
The present invention relates to a non-aqueous, stable and ready-to-use injectable composition of bivalirudin or pharmaceutically acceptable salt(s) or co-crystals thereof; and processes for its preparation. It is not required to reconstitute the injectable composition of bivalirudin with water prior to administration, thereby rendering it an easy-to-use injectable composition.
Owner:PIRAMAL ENTERPRISES LTD
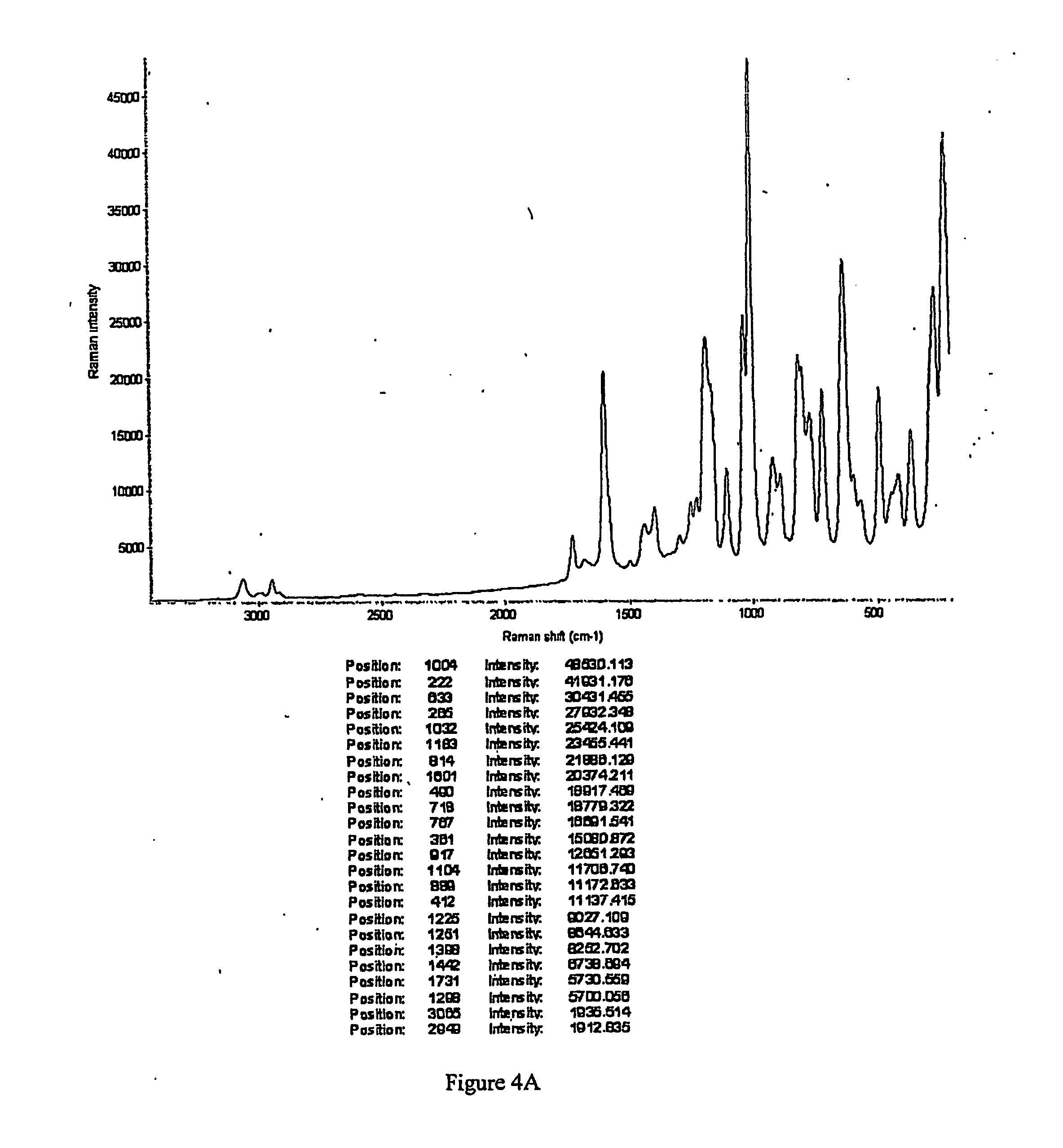
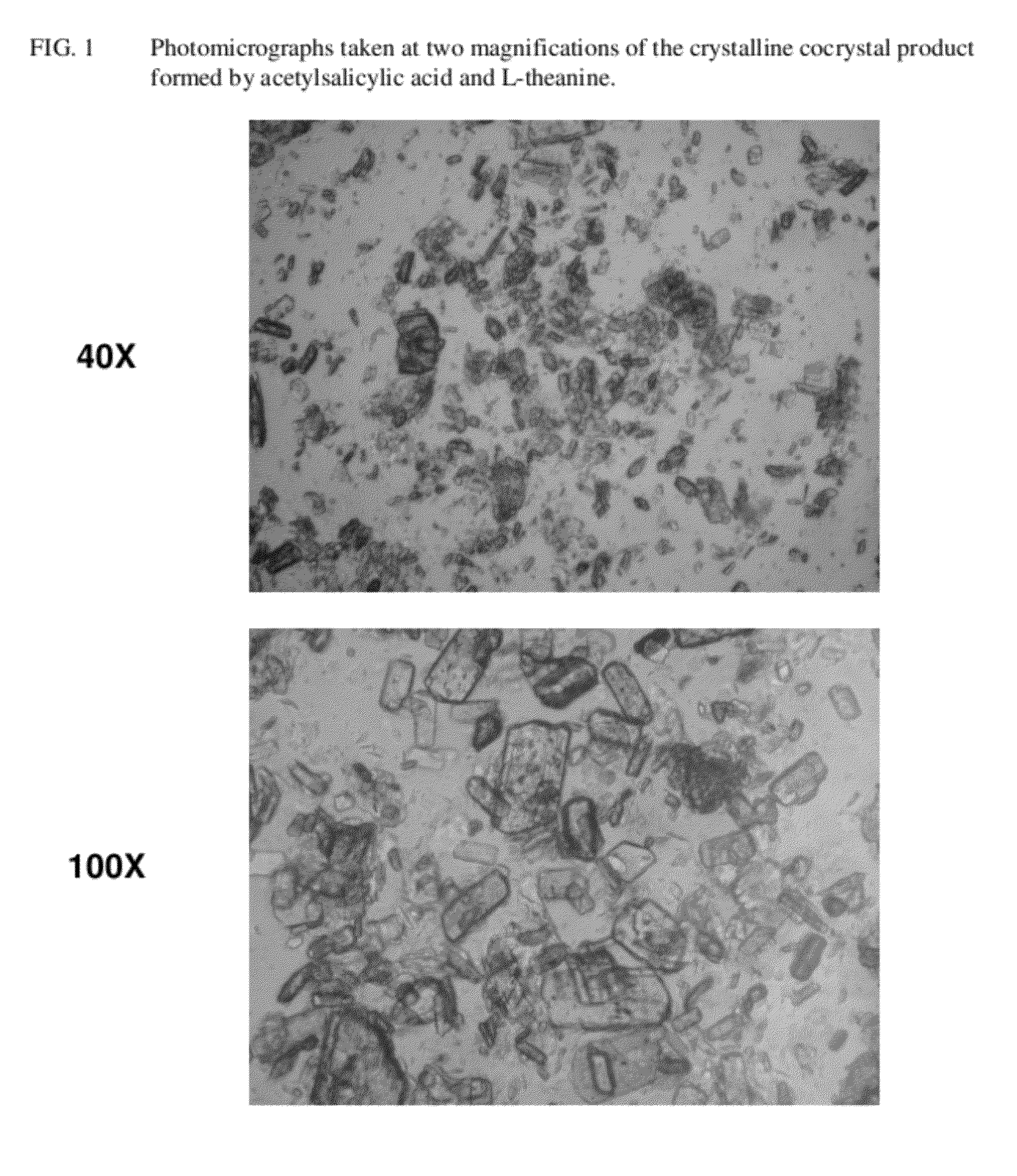
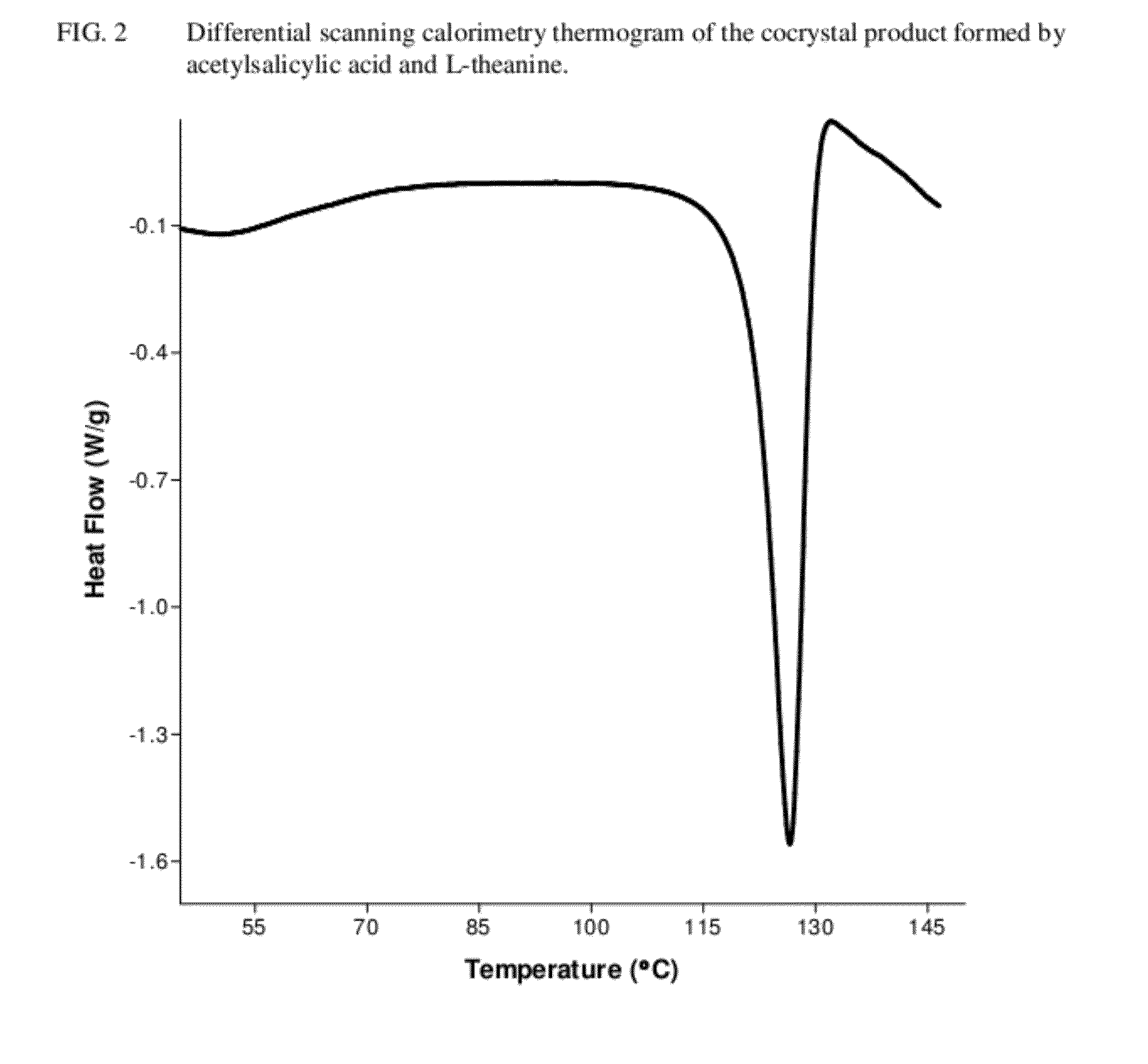
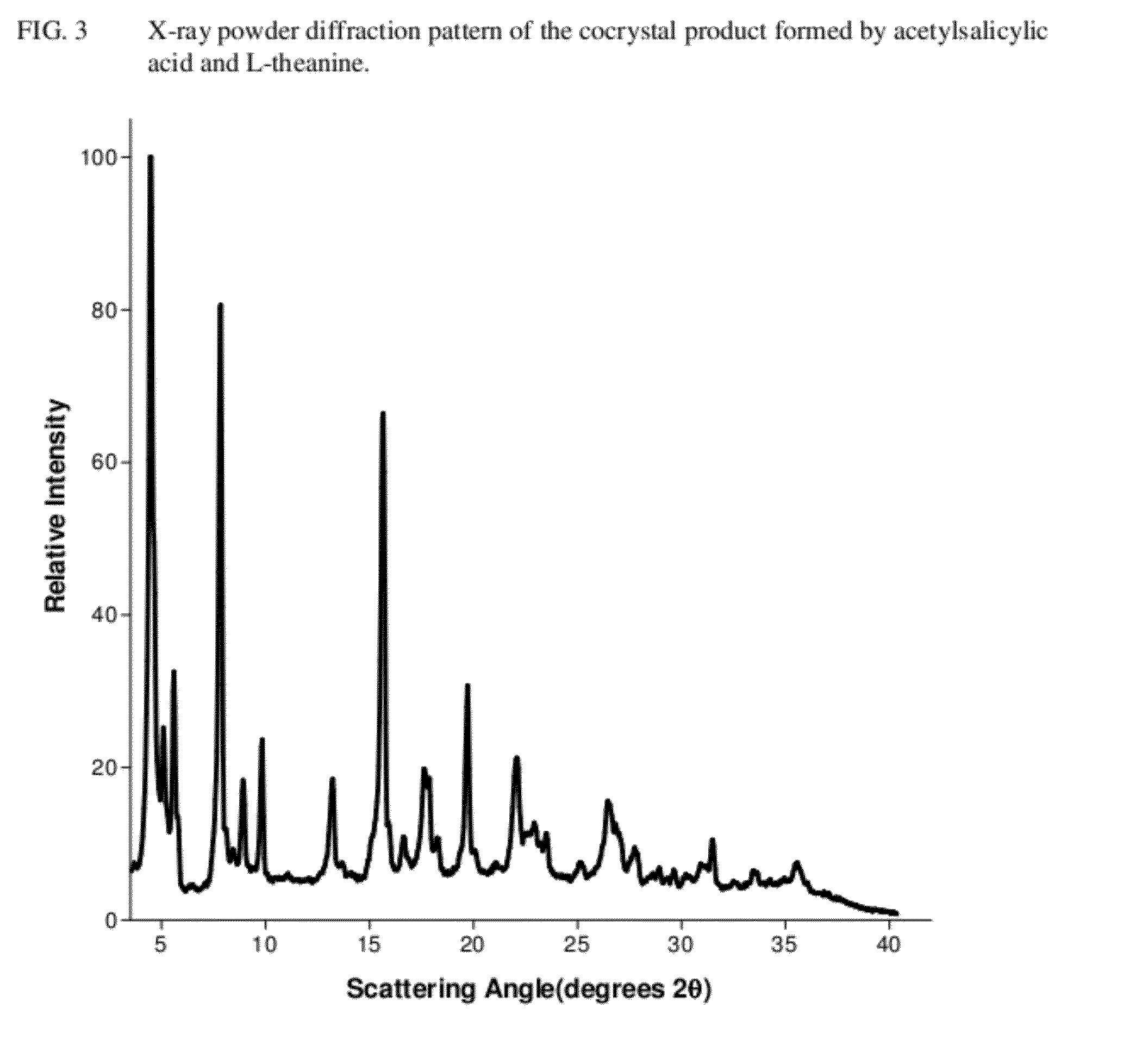
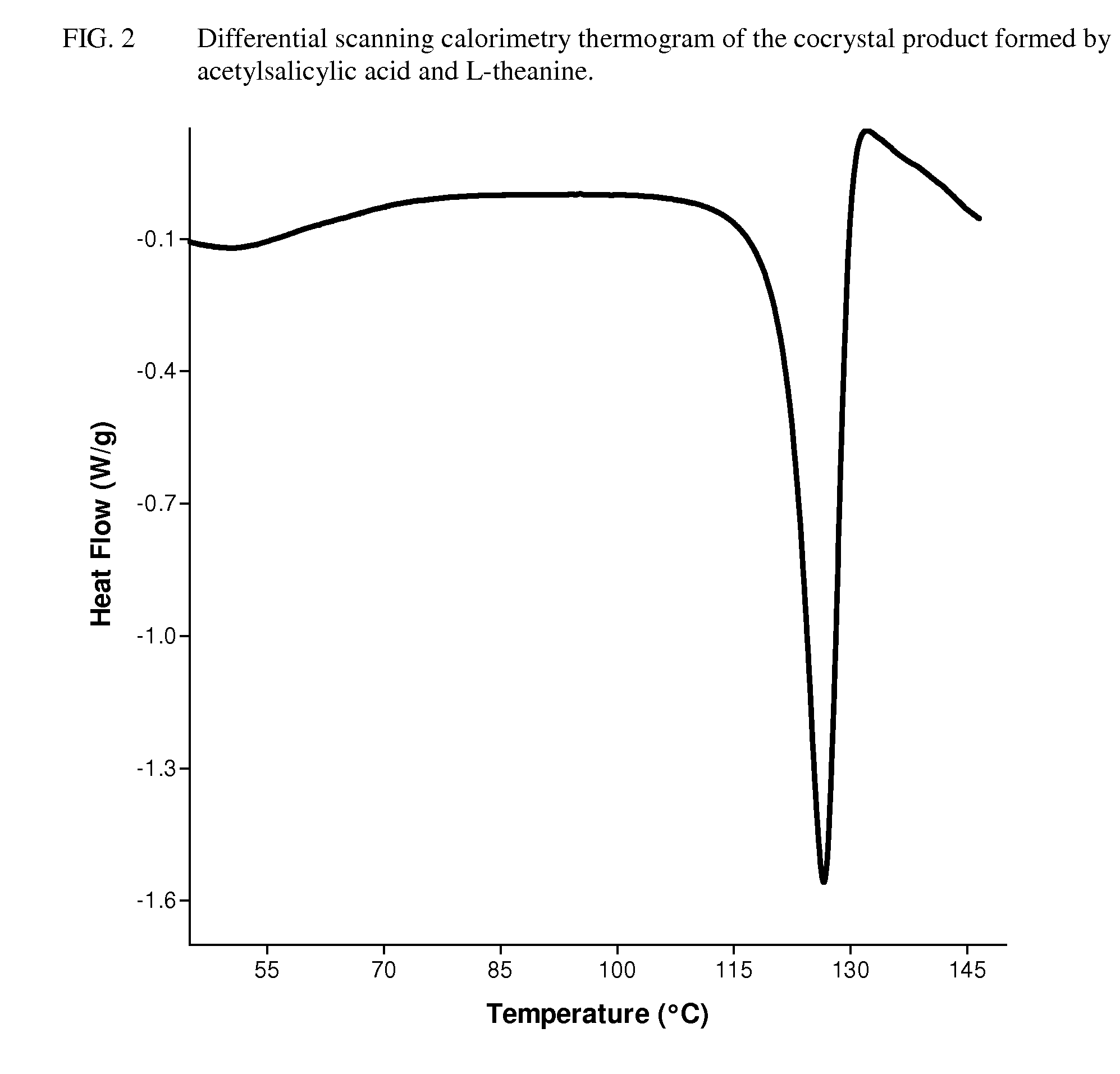
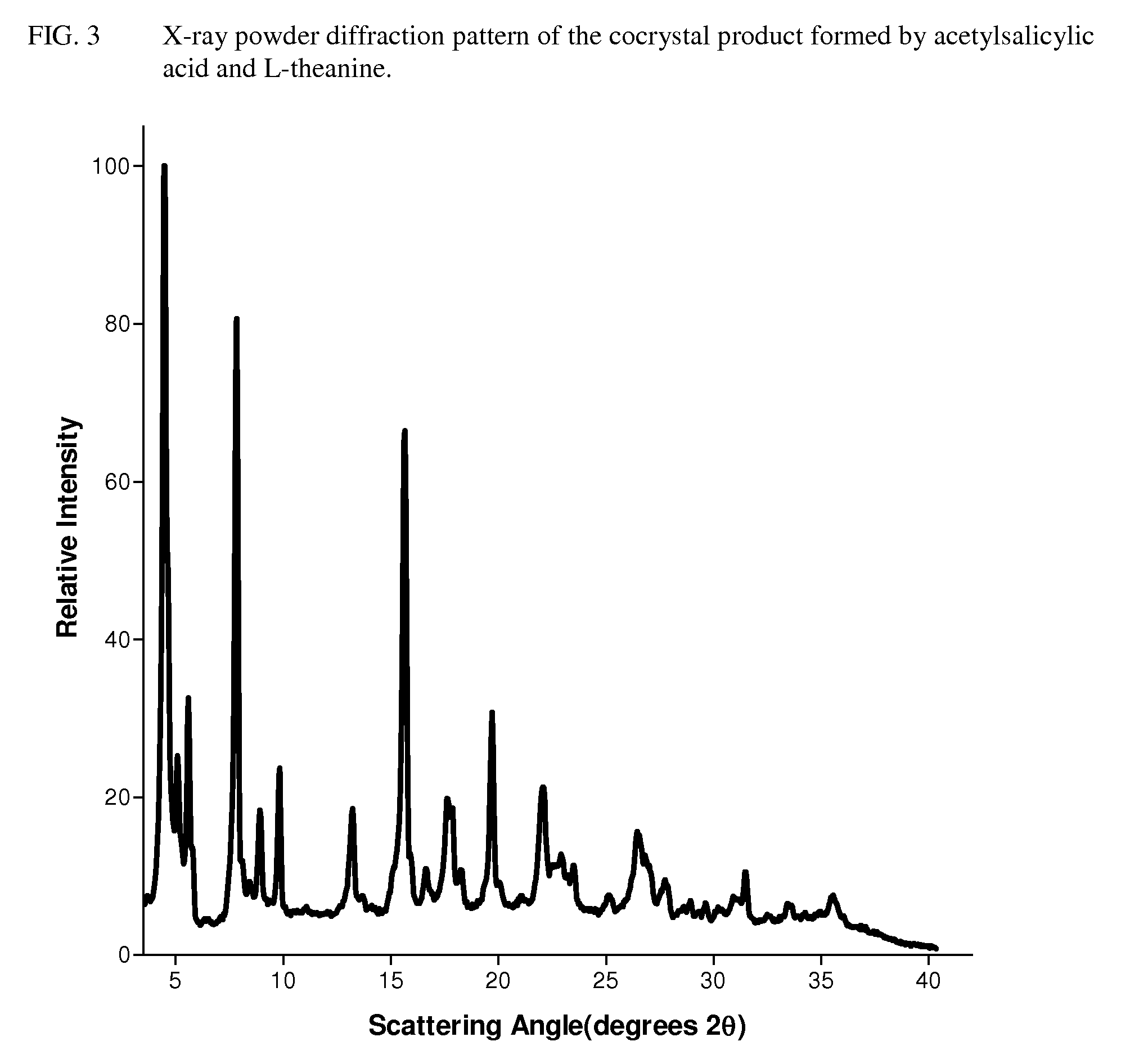
Intravenous formulation with water-soluble cocrystals of acetylsalicylic acid and theanine
A water-soluble aspirin-theanine cocrystal composition which includes a quantity of acetylsalicylic acid and a quantity of a theanine enantiomer associated with the quantity of acetylsalicylic acid. The composition may be created by a method including the steps of: (i) providing a quantity of acetylsalicylic acid; (ii) adding a quantity of a theanine enantiomer to the quantity of acetylsalicylic acid to form a mixture comprising the quantity of acetylsalicylic acid and the enantiomer of theanine; (iii) wetting the mixture; and (iv) grinding the mixture for a length of time sufficient to produce a dried crystalline mass. The water-soluble cocrystal composition is suitable for intravenous administration, preferably to humans.
Owner:THEAPRIN PHARMA
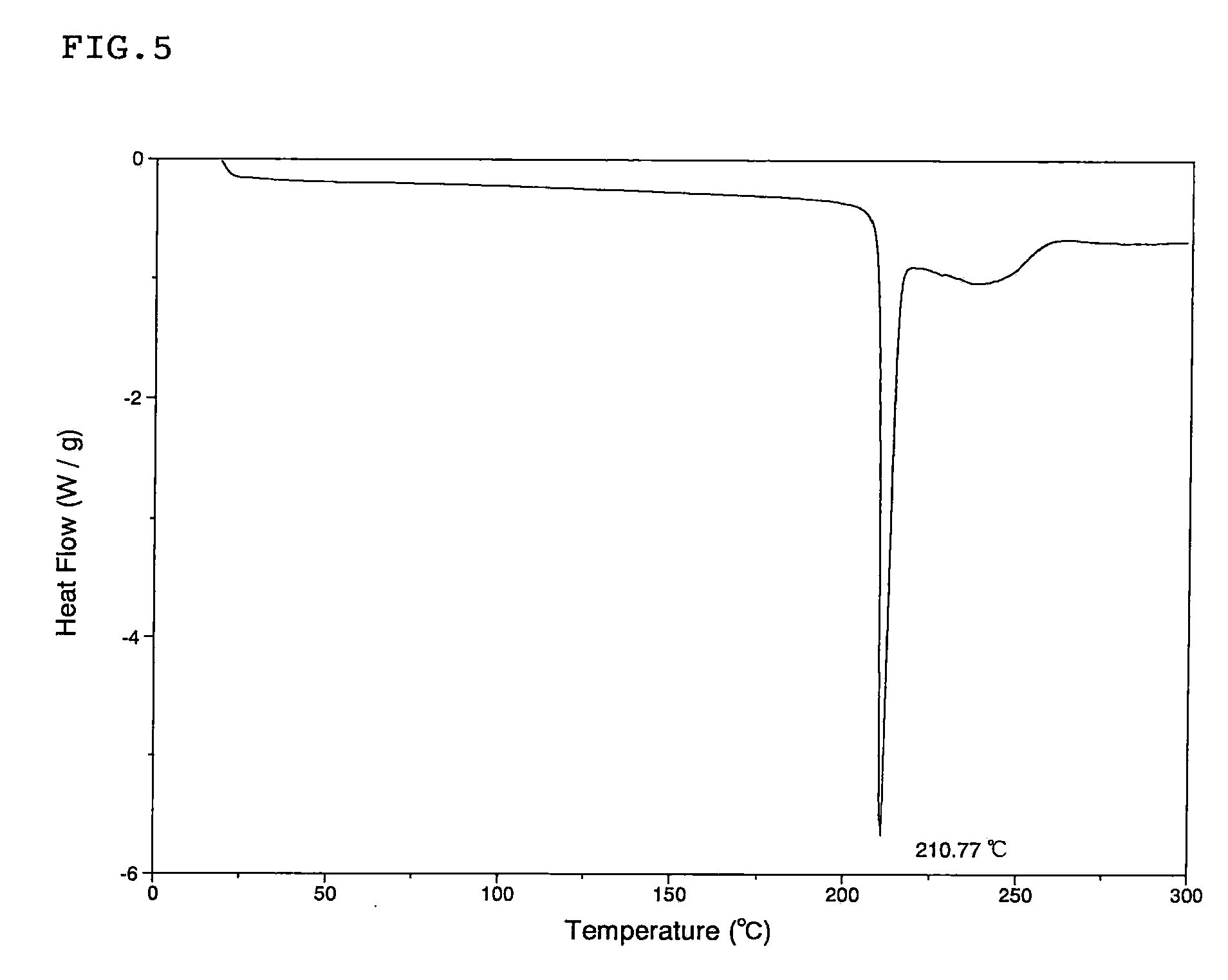
Energetic cocrystals for treatment of a subterranean formation
InactiveUS20160177698A1Well as to enableSafe handlingSurveyFluid removalChemistryHigh energy compound
The present invention relates to energetic cocrystals, and to methods for using the same for treatment of a subterranean formation. In various embodiments, the present invention provides a method of treating a subterranean formation, the method including obtaining or providing a composition including energetic cocrystals. Each energetic cocrystal independently includes an energetic compound and a secondary material. The method also includes placing the composition in a subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Screening for solid forms by ultrasound crystallization and cocrystallization using ultrasound
InactiveUS20110251426A1Raise the possibilitySignificant comprehensive benefitsCrystallization conditions screeningOrganic chemistryActive agentMedicine
The present disclosure relates to crystallizing a chemical substance(s) using ultrasound. Methods are provided for screening a chemical substance according to its solid forms by using ultrasound to generate new or unusual solid forms. Methods are also provided for crystallizing a chemical substance by novel techniques that include sonication. The present disclosure also relates to cocrystallization using ultrasound. Methods are provided for preparing cocrystals of an active agent and a guest by sonicating and crystallizing. Methods are also provided for screening a sample according to solid state phases (such as cocrystals and salts) and include generating a cocrystal from the sample using ultrasound.
Owner:AMRI SSCI
Screening For Solid Forms By Ultrasound Crystallization And Cocrystallization Using Ultrasound
InactiveUS20070287194A1Significant comprehensive benefitsRaise the possibilityCrystallization conditions screeningOrganic chemistrySonificationActive agent
The present disclosure relates to crystallizing a chemical substance(s) using ultrasound. Methods are provided for screening a chemical substance according to its solid forms by using ultrasound to generate new or unusual solid forms. Methods are also provided for crystallizing a chemical substance by novel techniques that include sonication. The present disclosure also relates to cocrystallization using ultrasound. Methods are provided for preparing cocrystals of an active agent and a guest by sonicating and crystallizing. Methods are also provided for screening a sample according to solid state phases (such as cocrystals and salts) and include generating a cocrystal from the sample using ultrasound.
Owner:AMRI SSCI
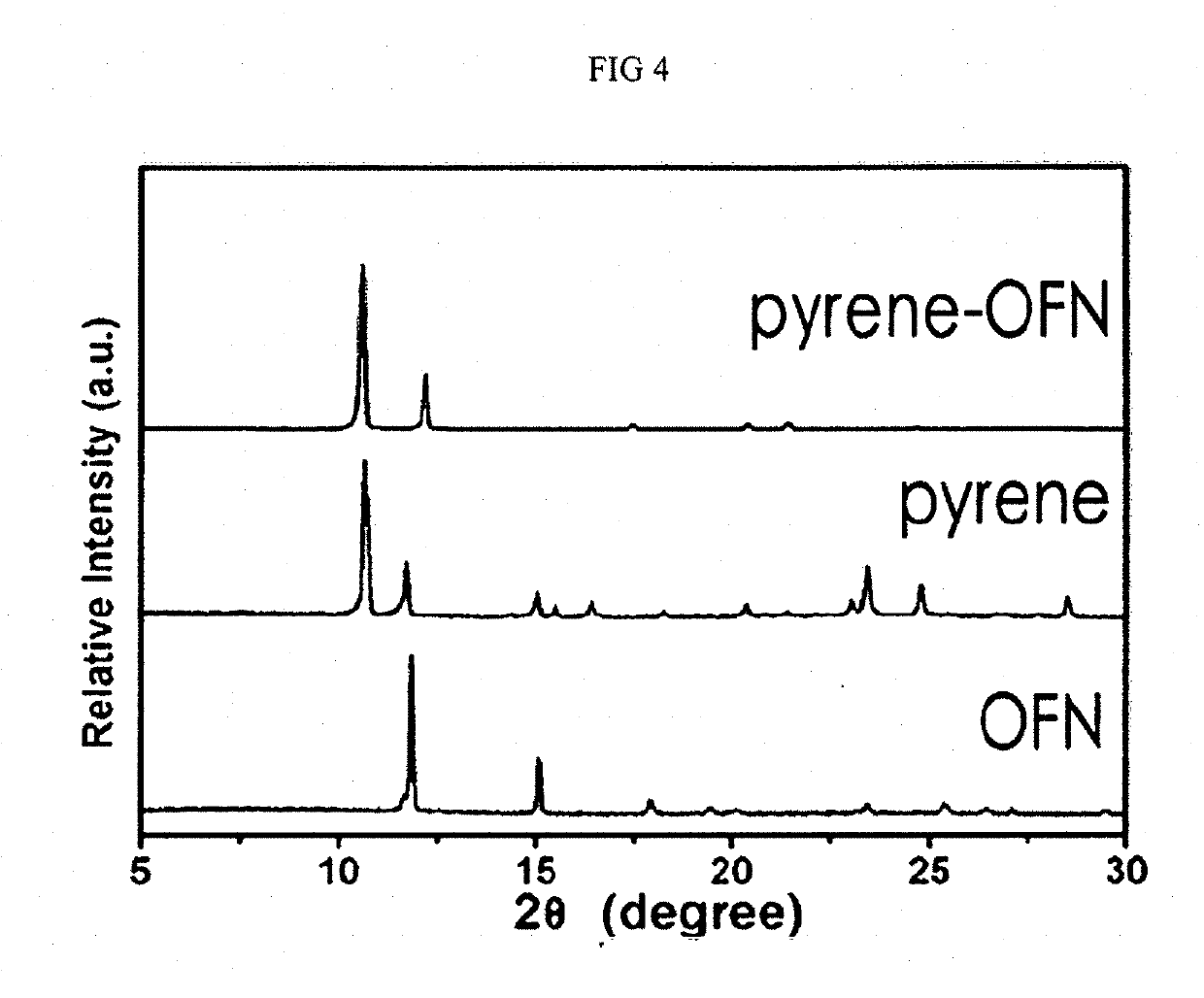
Coating solution containing cocrystals and or crystals of a charge-generation pigment or a mixture of charge-generation pigments
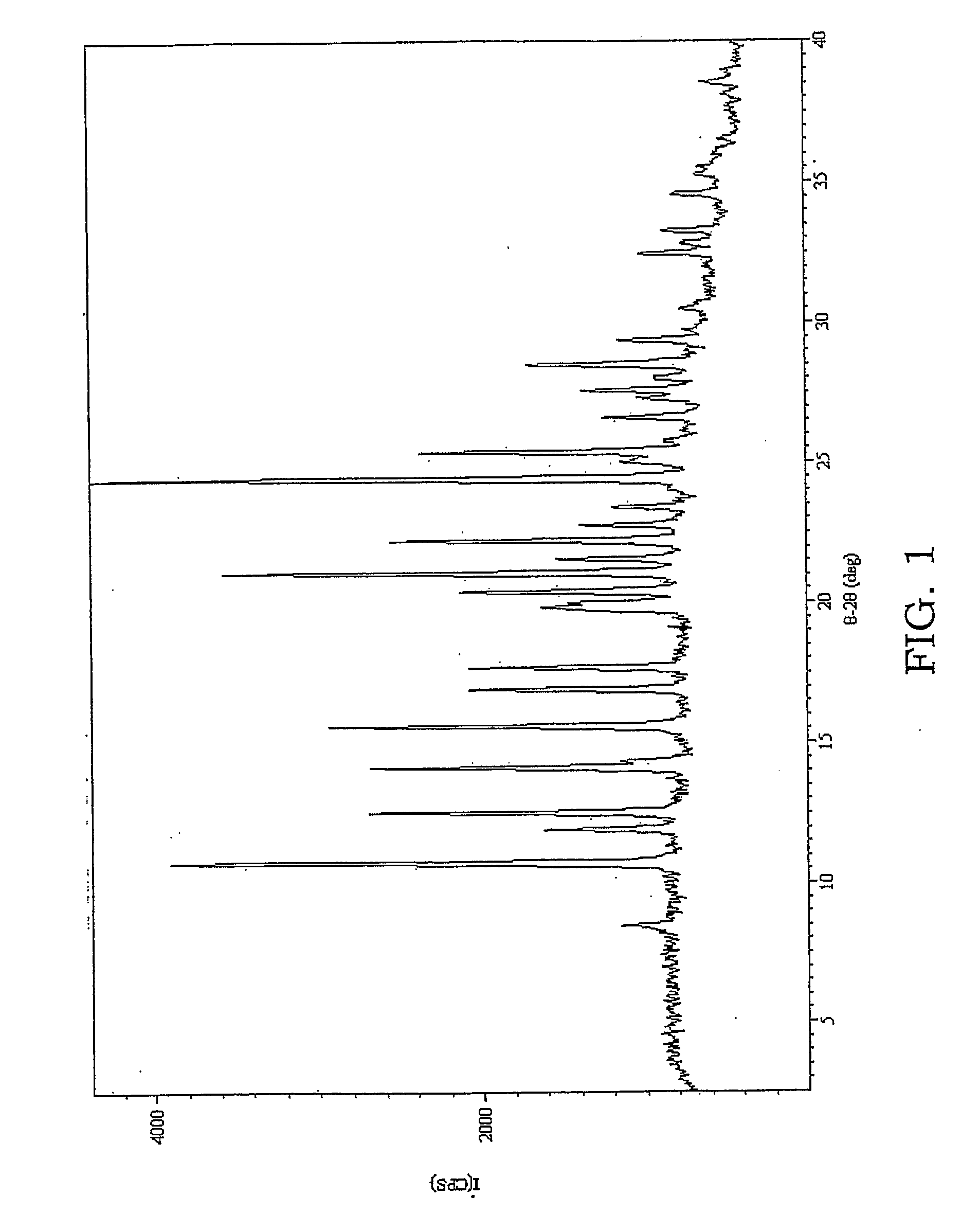
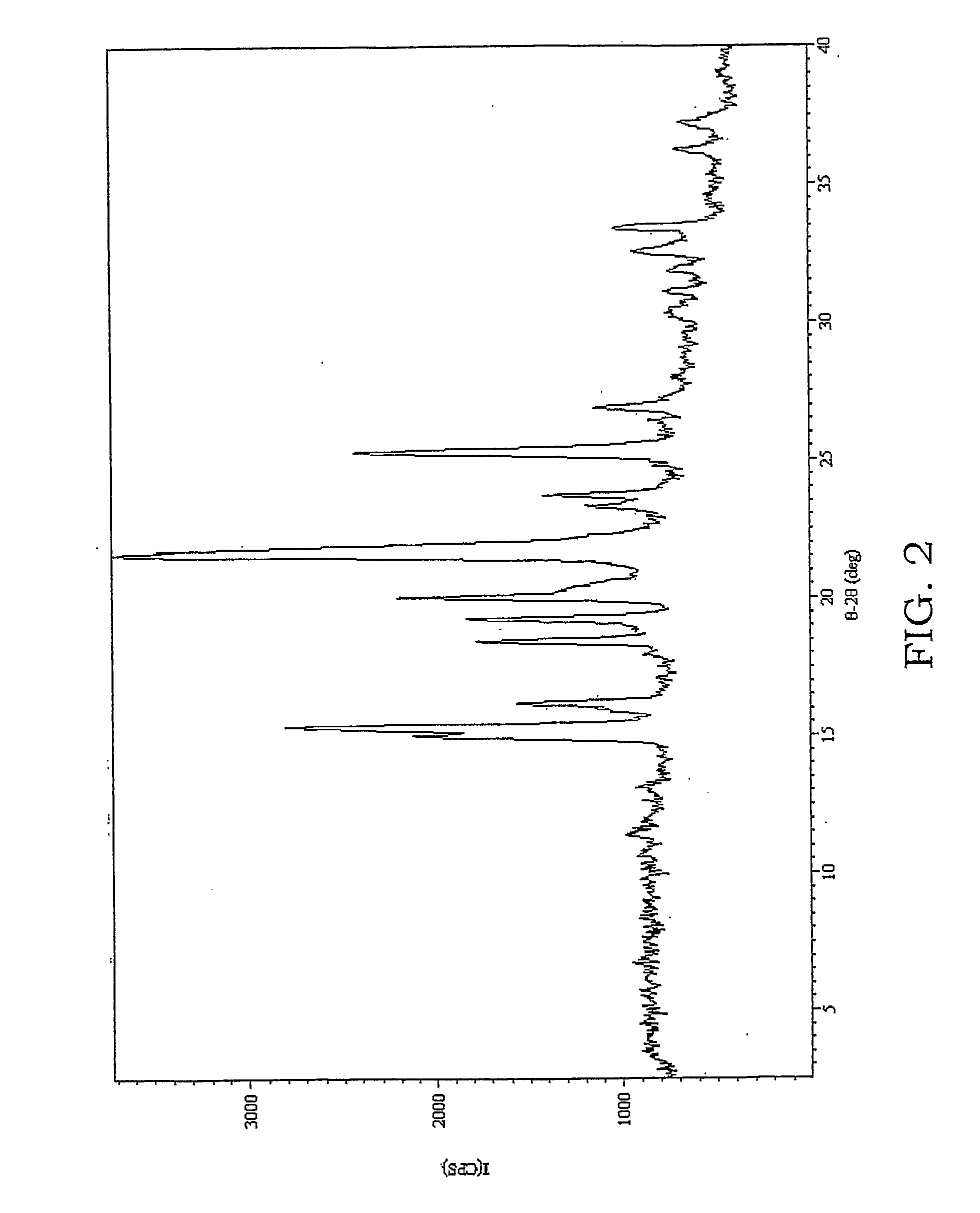
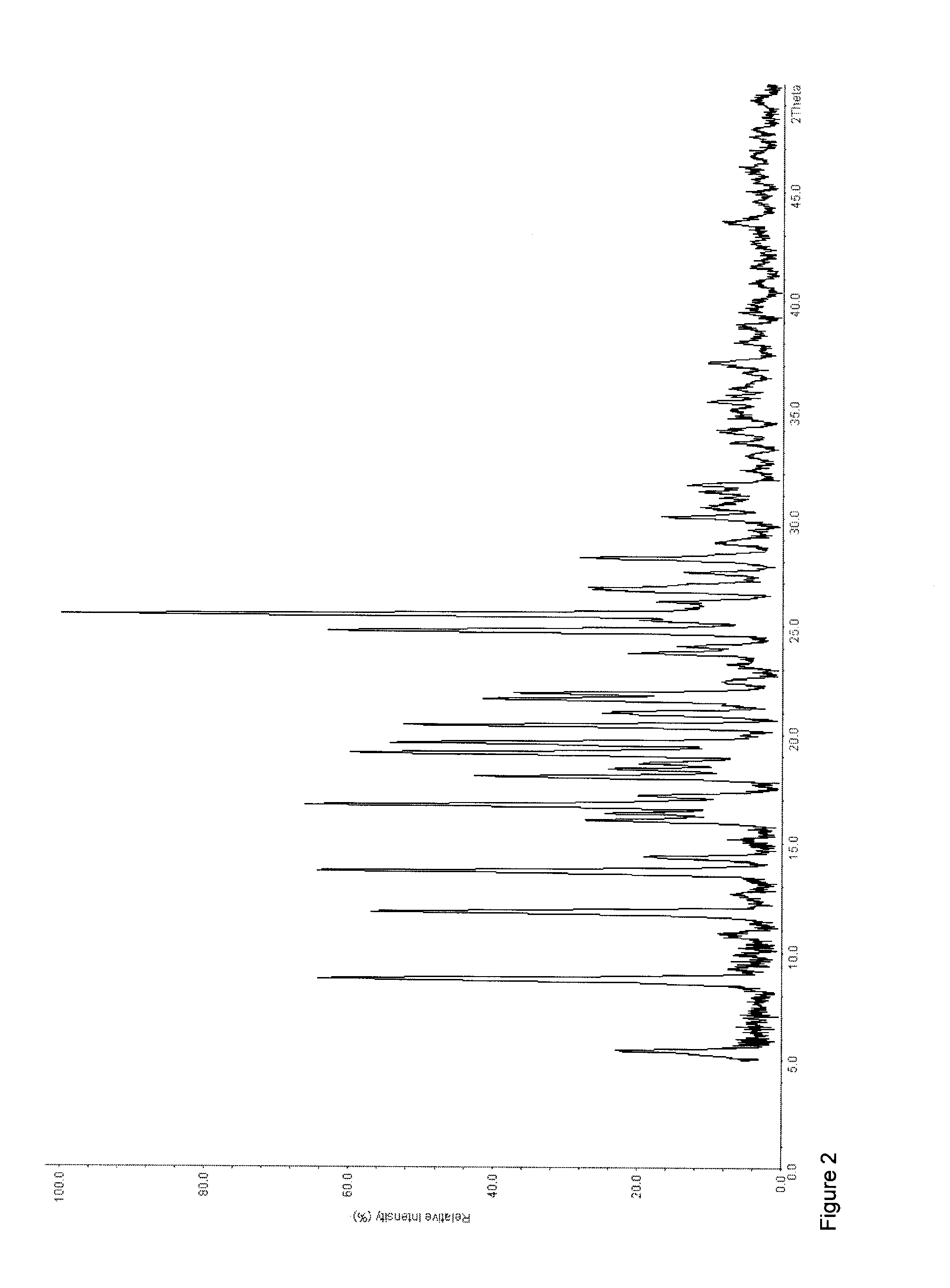
A method for preparing a coating solution containing a cocrystallized titanyl phthalocyanine-titanyl fluorophthalocyanine, the method comprising: dry milling a charge-generation pigment or mixtures of charge-generation pigments; increasing the amorphousness of the pigment mixture as determined by X-ray crystallography using X-radiation characteristic of Cu Kα at a wavelength of 1.541 Å of the Bragg angle 2θ to provide an amorphous pigment mixture; contacting the amorphous pigment mixture with a first organic solvent having a gammac hydrogen bonding parameter of less than 9, with or without the presence of a dispersant material, to produce a crystalline pigment of the charge-generation pigment prior to contacting the pigment with a second organic solvent having a gammac hydrogen bonding parameter greater than 9; mixing at least one of a second organic solvent, a dispersant and a binder with the crystalline pigment / first solvent mixture without isolating the crystalline pigment to produce a mixture; and, adjusting the concentrations of the first organic solvent, crystalline pigment, binder, dispersants and second organic solvent as required to produce the coating solution of a selected composition.
Owner:EASTMAN KODAK CO
Solid of tenofovir disoproxil, and preparation method and application thereof
ActiveCN103626803AEasy to prepareCrystal form controllableOrganic active ingredientsGroup 5/15 element organic compoundsMedicineHepatitis B virus
The invention relates to a solid of tenofovir disoproxil. The solid is (1) a tenofovir disoproxil compound represented by a formula IV or (2) a tenofovir disoproxil cocrystal or salt represented by a formula V. The invention further relates to a preparation method for the solid of tenofovir disoproxil, a pharmaceutical composition containing the solid and application of the solid in preparation of drugs used for preventing and / or treating virus infection, especially hepatitis b virus (HBV) and / or human immunodeficiency virus (HIV) infection.
Owner:SICHUAN HAISCO PHARMA CO LTD
Salts or Co-Crystals of 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol
A salt or cocrystal of 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol (component a) and at least one acid component (b1) or at least one acid component (b2), wherein the salt or cocrystal of component (a) and component (b2) is present in crystalline and / or amorphous form, a pharmaceutical composition comprising said salt or cocrystal, and a method of treating pain in a subject in need thereof by administering an effective amount of said salt or cocrystal.
Owner:GRUNENTHAL GMBH
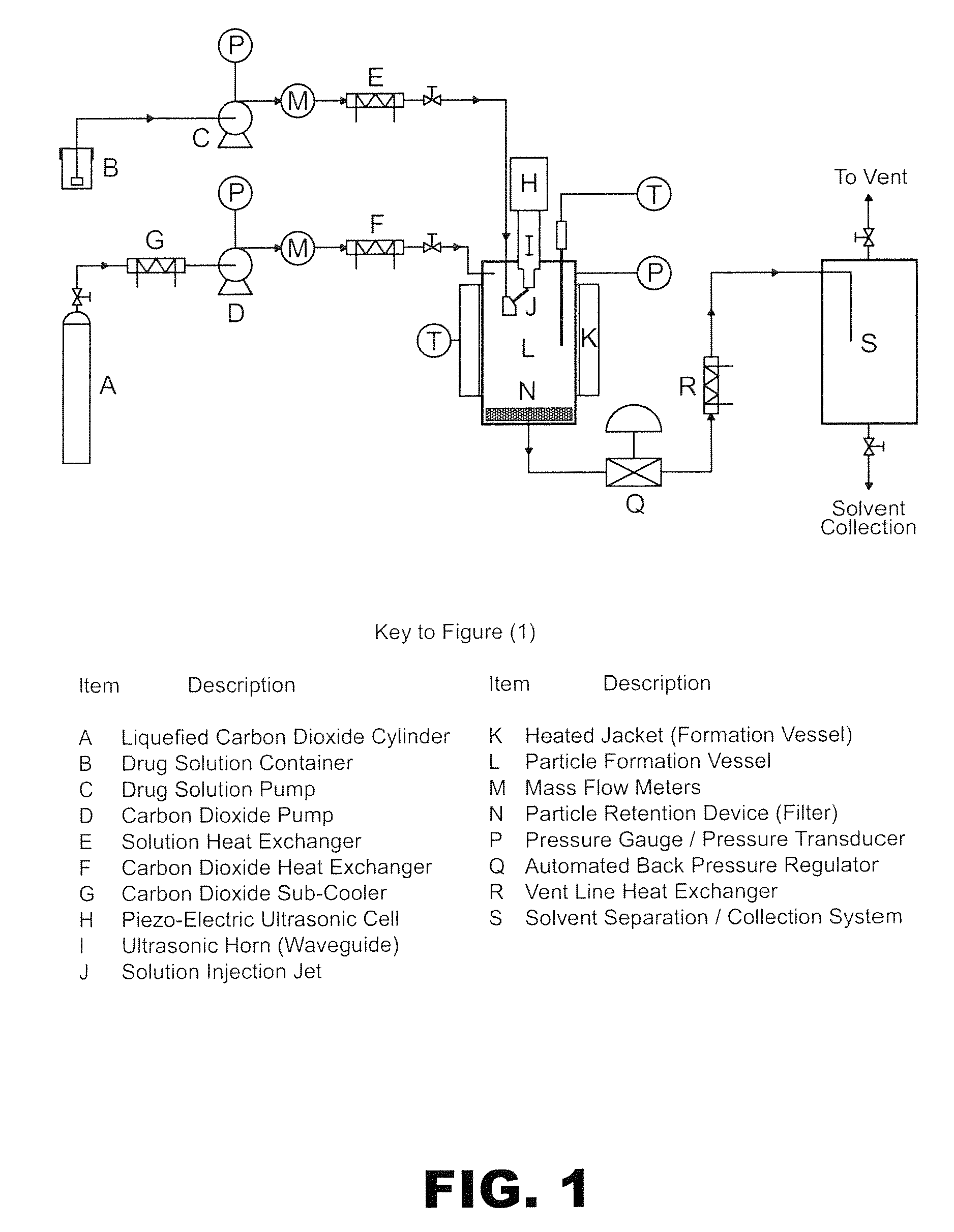
Method of creating crystalline substances
Process for preparing a cocrystal of an active substance and a cocrystal former, the process involving precipitating the active substance and the cocrystal former together from solution or suspension, in the presence of a supercritical or near-critical fluid, in particular using a GAS, SAS, SEDS or SAS-EM process. The invention also provides a cocrystal prepared using such a process, and its use as a seed crystal in a subsequent process for precipitating a cocrystal of an active substance and a cocrystal former.
Owner:THAR PHARMA
Method of creating crystalline substances
InactiveUS20080280858A1High crystallinityLower Level RequirementsBiocidePowder deliverySeed crystalCrystallization
Process for preparing a cocrystal of an active substance and a cocrystal former, the process involving precipitating the active substance and the cocrystal former together from solution or suspension, in the presence of a supercritical or near-critical fluid, in particular using a GAS, SAS, SEDS or SAS-EM process. The invention also provides a cocrystal prepared using such a process, and its use as a seed crystal in a subsequent process for precipitating a cocrystal of an active substance and a cocrystal former.
Owner:THAR PHARMA
A kind of novel iloperidone medicine co-crystal and preparation method thereof
ActiveCN102276594ASolubility changeImprove stabilityNervous disorderOrganic chemistrySolubilityCarboxylic acid
The invention belongs to the technical field of medicinal cocrystal, and particularly relates to a novel iloperidone medicinal cocrystal and a preparation method thereof. An N atom in a piperidine ring of iloperidone is used as a hydrogen bond donor, and an H atom on a carboxyl group in 3,5-pyridine dicarboxylic acid is used as a hydrogen bond receptor to form a hydrogen bond; an iloperidone molecule is combined with a 3,5-pyridine dicarboxylic acid molecule through the hydrogen bond to form a basic structural unit of the iloperidone medicinal cocrystal; and a space group of the medicinal cocrystal is a triclinic system. The preparation method of the iloperidone medicinal cocrystal is a reflux-room temperature volatilization method. The medicinal cocrystal prepared by using the preparation method disclosed by the invention has the characteristics of inheriting the conventional raw material medicament on treatment of schizophrenia; and the dissolubility, the stability and the bioavailability of the medicinal cocrystal are obviously improved.
Owner:JILIN UNIV
Novel choline cocrystal of epalrestat
The invention relates to a novel choline cocrystal of 5-[(lZ.2E)-2-methyl-3-phenylpropenylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid. The preparation and characterization of the novel choline cocrystal according to various embodiments of the invention is described. The invention also relates to pharmaceutical compositions containing the novel choline cocrystal and the therapeutic use of the novel choline cocrystal to treat and / or prevent various conditions, including treating and / or preventing diabetic complications, treating and / or preventing homocystinuria reducing levels of homocysteine in blood serum, inhibiting aldose reductase, and affording cardioprotection in non-diabetic patients.
Owner:BIONEVIA PHARMACEUTICALS INC
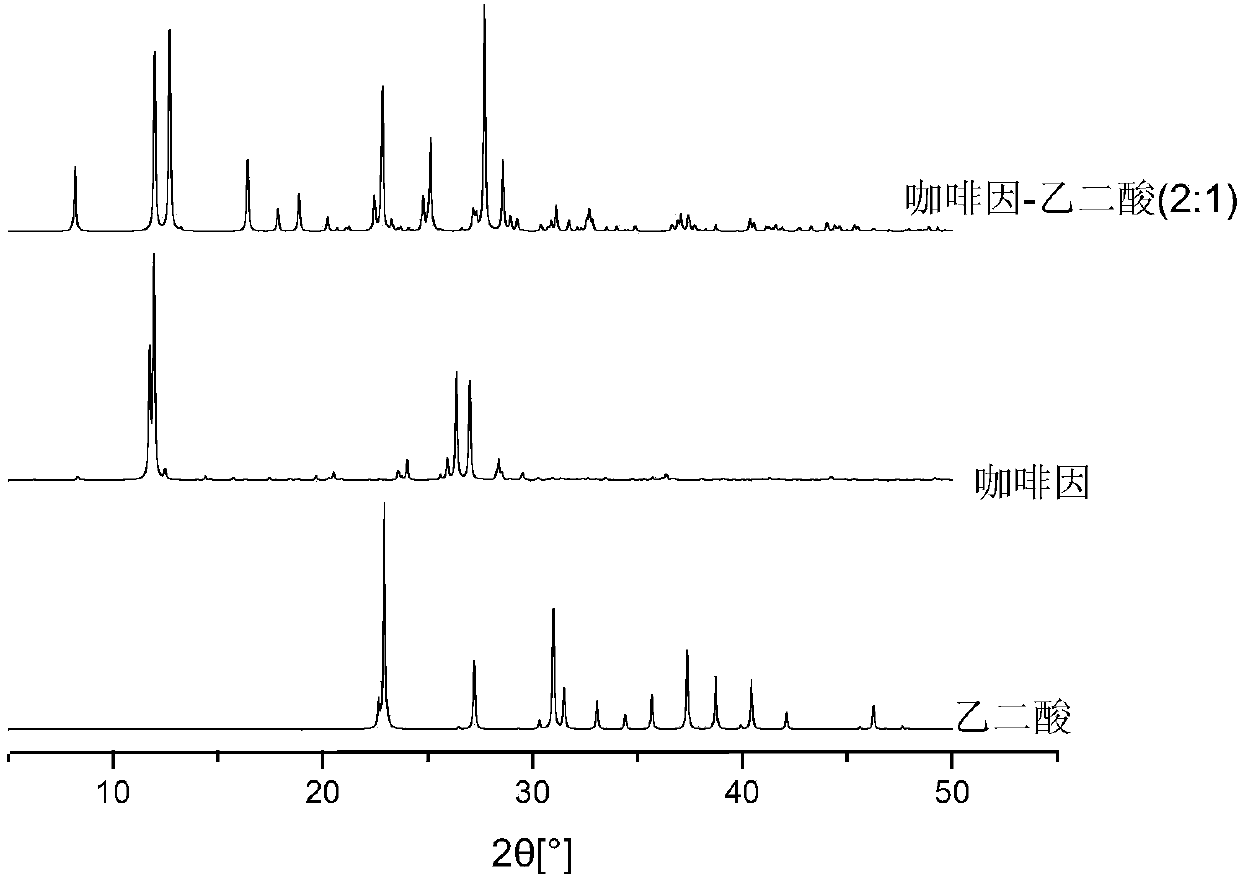
Method for preparing pharmaceutical cocrystals through suspension crystallization
InactiveCN103992320AEasy temperature controlNo energy consumptionCarboxylic acid amide separation/purificationCarboxylic compound separation/purificationFiltrationSolvent
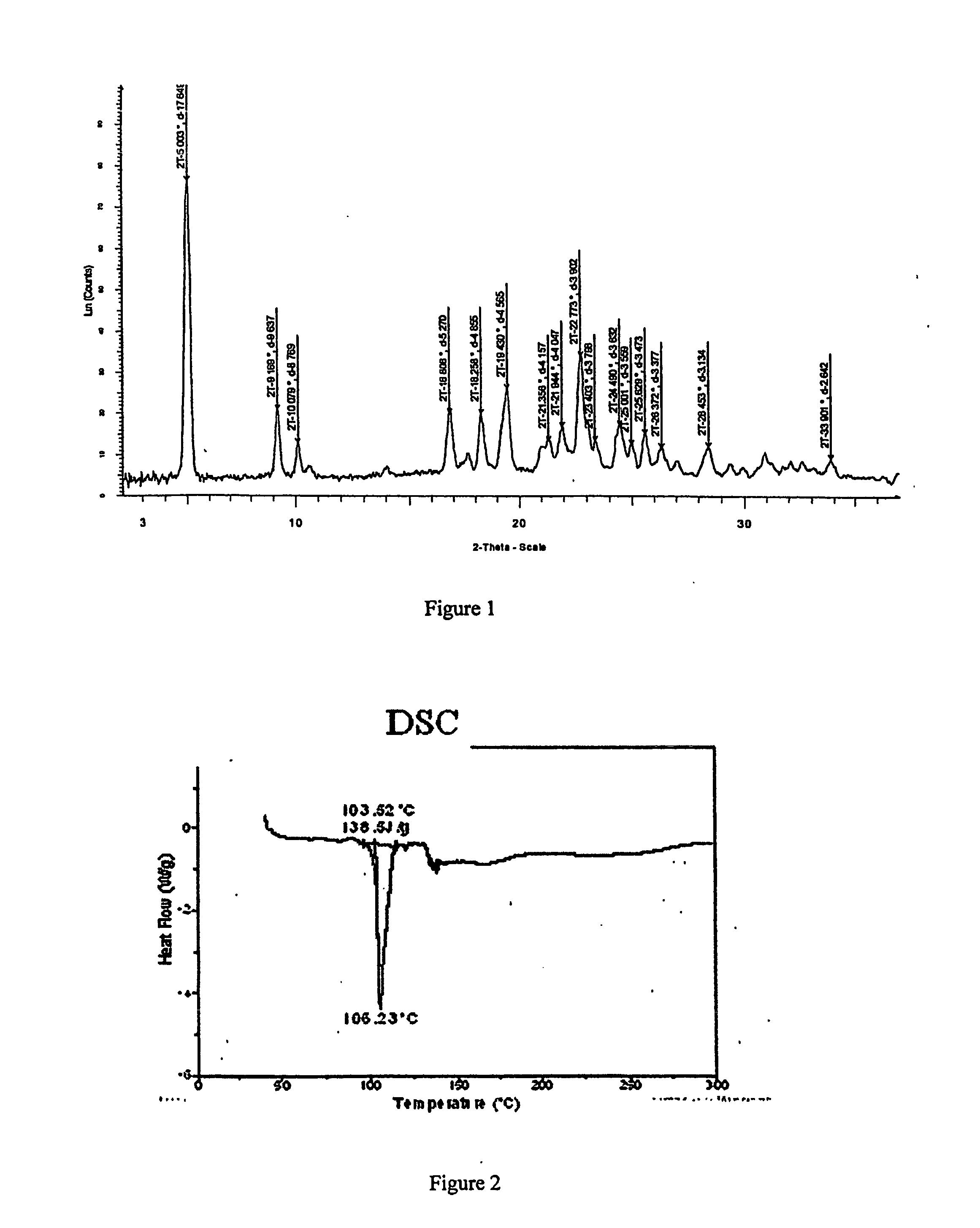
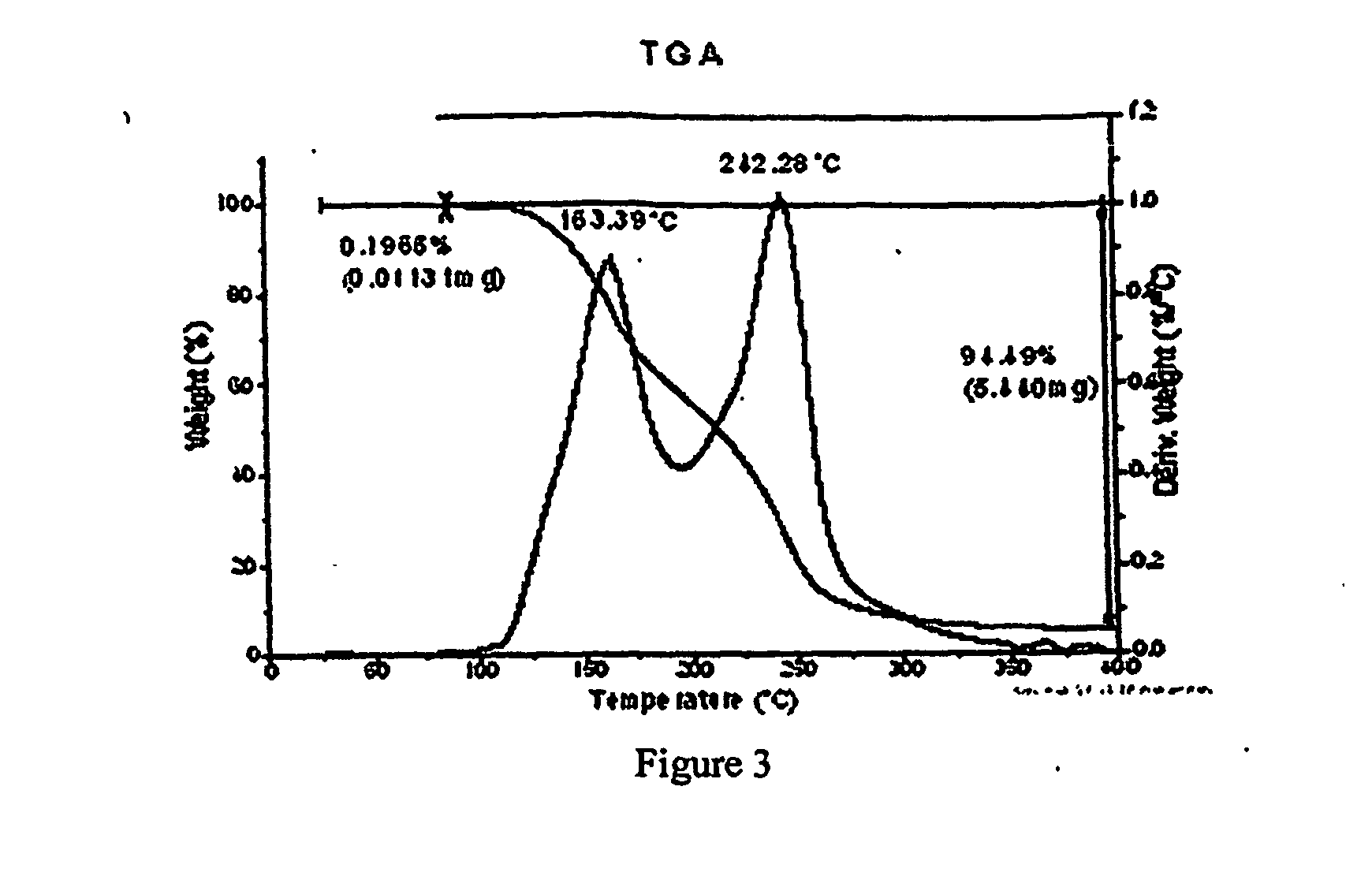
The invention relates to a method for preparing pharmaceutical cocrystals through suspension crystallization. The method concretely comprises the following steps: firstly screening solid forms of active pharmaceutical ingredients API and cocrystal formation CCF in different solvents, preparing a CCF saturation solution together with a solvent which cannot generate solvate together with the API and CCF; feeding the prepared CCF saturation solution into a vessel type stirrer, feeding the solid API and CCF according to a certain solid load rate, and deciding the ratio of molar weight of the API and CCF according to the cocrysal chemical metrological ratio; and sampling to do XRD or DSC testing after fully stirring and mixing, so as to prove that the cocrystal solid phase is pure, performing vacuum filtration to the obtained solid, and washing and drying the CCF saturation solution to obtain a solid, namely a final cocrystal product. Compared with other methods, the method is low in energy consumption, less in used solvent, low in cost, simple in technology, easy to amplify, fast and easy to obtain, high in the purity of products, and suitable for industrial large-batch synthesized pharmaceutical cocrystals.
Owner:NANJING UNIV OF TECH
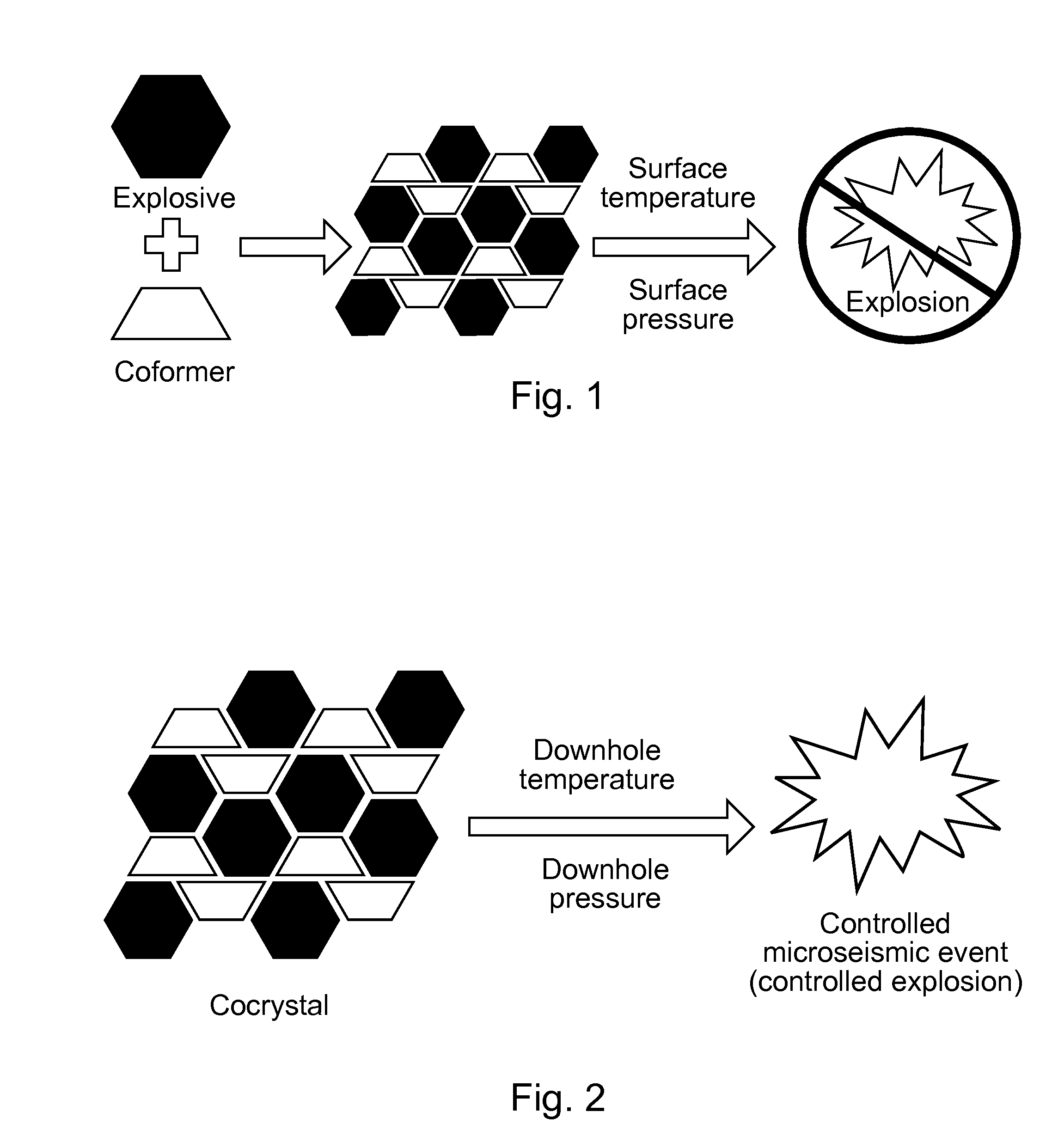
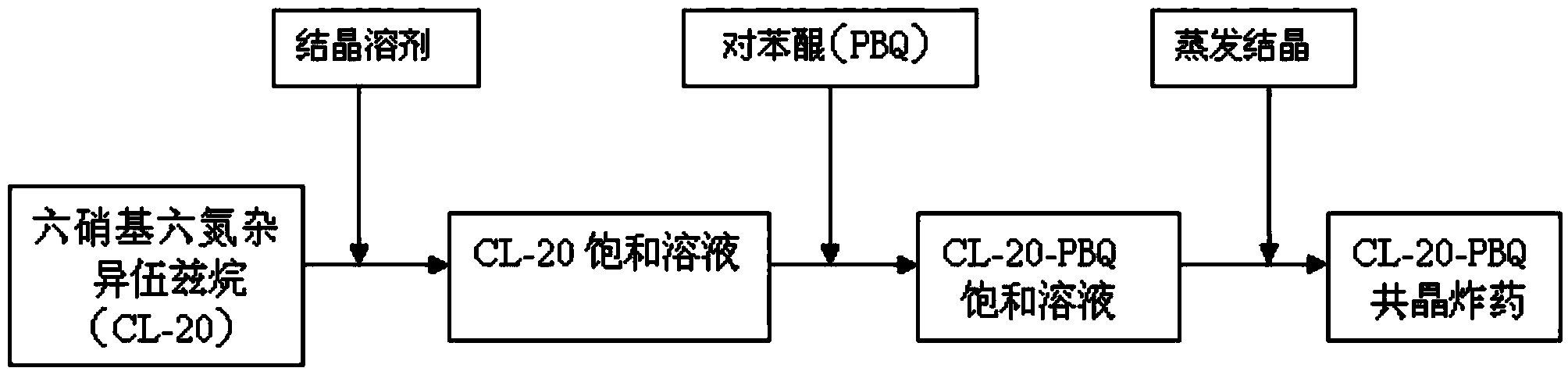
Preparation method of hexanitrohexaazaisowurtzitane/p-benzoquinone cocrystal explosive
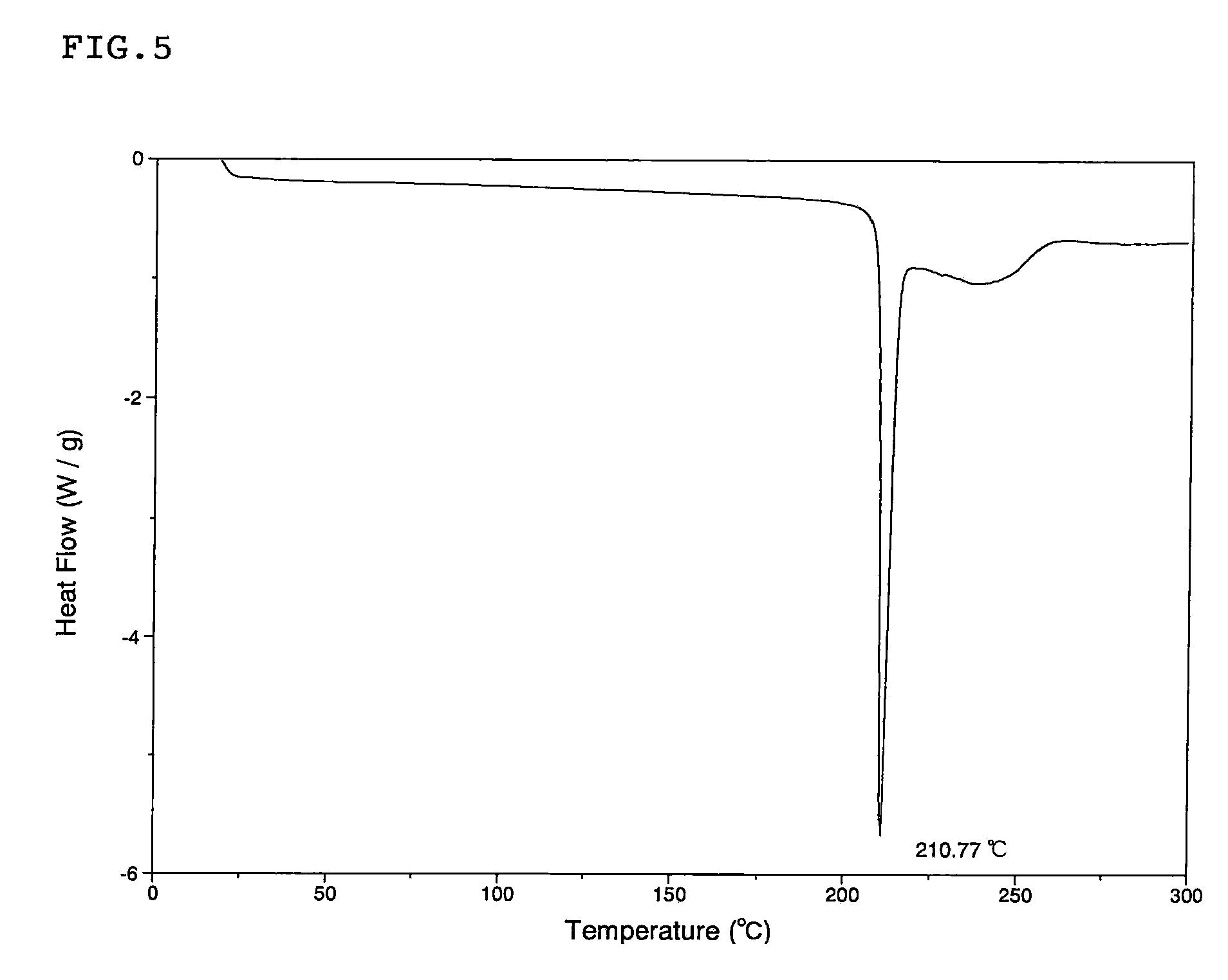
ActiveCN103435427ARegulation of Thermal Decomposition BehaviorImprove securityExplosive working-up apparatusNitroparaffin explosive compositionsDecompositionHigh energy
The invention discloses a preparation method of a hexanitrohexaazaisowurtzitane / p-benzoquinone cocrystal explosive. Firstly a saturated solution of hexanitrohexaazaisowurtzitane and a saturated solution of p-benzoquinone are prepared using a crystallization solvent, and then the solvent is allowed to evaporate through a constant-temperature incubator, followed by crystallization to prepare a hexanitrohexaazaisowurtzitane / p-benzoquinone cocrystal explosive. The invention has the benefits as follows: the crystal density of the hexanitrohexaazaisowurtzitane / p-benzoquinone cocrystal explosive is up to 1.737 g / cm3, the melting point of the cocrystal explosive is 132 DEG C and is greatly increased by 17 DEG C as compared with relatively-pure component PBQ, and the cocrystal decomposition temperature is lower than that of both CL-20 and BQ and 207 DEG C lower than that of PBQ. Therefore, by virtue of cocrystallization, the thermal decomposition behavior of the explosive can be obviously controlled, the high efficiency and desensitivity of cocrystallization of the explosive are achieved, the safety of the explosive is improved, and the explosive has a good application prospect in high-energy low-sensitivity ammunition.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Intravenous formulation with water-soluble cocrystals of acetylsalicylic acid and theanine
A water-soluble aspirin-theanine cocrystal composition which includes a quantity of acetylsalicylic acid and a quantity of a theanine enantiomer associated with the quantity of acetylsalicylic acid. The composition may be created by a method including the steps of: (i) providing a quantity of acetylsalicylic acid; (ii) adding a quantity of a theanine enantiomer to the quantity of acetylsalicylic acid to form a mixture comprising the quantity of acetylsalicylic acid and the enantiomer of theanine; (iii) wetting the mixture; and (iv) grinding the mixture for a length of time sufficient to produce a dried crystalline mass. The water-soluble cocrystal composition is suitable for intravenous administration, preferably to humans.
Owner:THEAPRIN PHARMA
Novel iloperidone pharmaceutical cocrystal and preparation method thereof
The invention belongs to the technical field of pharmaceutical cocrystals and in particular relates to a novel iloperidone pharmaceutical cocrystal and a preparation method thereof. The pharmaceutical cocrystal is characterized in that iloperidone is taken as the active pharmaceutical ingredient, 3,5-dihydroxy-benzoic acid is taken as the former, and an iloperidone molecule, a 3,5-dihydroxy-benzoic acid molecule and a water molecule jointly form a basic structural unit of the iloperidone pharmaceutical cocrystal through hydrogen bonds and deposition. The pharmaceutical cocrystal is prepared by taking ethanol as the solvent and adopting the method of reflux-room temperature diffusion and volatilization. As the selected organic solvent has lower boiling point, crystals are prepared in the process of solvent volatilization after reflux and filtration. The prepared pharmaceutical cocrystal carries forward the characteristic of the traditional active pharmaceutical ingredients in treating schizophrenia and the dissolubility, stability and bioavailability of the pharmaceutical cocrystal are also obviously improved.
Owner:CHANGCHUN LICHENG BICHENG NEW MEDICINE TECHDEV
Aripiprazole co-crystals
InactiveUS20090054455A1High dissolution rateImproved profileOrganic active ingredientsOrganic chemistry methodsAripiprazoleFumaric acid
Owner:DR REDDYS LAB LTD +1
Ethinyloestradiol pharmaceutical cocrystal and preparation method thereof
ActiveCN108794555AImprove stabilityImprove solubilityOrganic active ingredientsOrganic chemistry methodsSolubilityHigh humidity
The invention discloses an ethinyloestradiol novel crystal form. The crystal form adopts a nicotinamide co-crystal drug. The crystal form adopts a monoclinic crystal system, is stable in high-temperature, high-humidity and illumination conditions, has good solubility in 0.2 percent SDS and 0.5 percent SDS solutions, and is beneficial to the improvement of bioavailability of the drug.
Owner:CHINA RESOURCES ZIZHU PHARMA
Cocrystal of C-glycoside derivative and L-proline
InactiveUS8097592B2Quality improvementGood storage stabilityBiocideSugar derivativesCompound aC-glycoside
A cocrystal of (1S)-1,5-anhydro-1-[3-(1-benzothien-2-ylmethyl)-4-fluorophenyl]-D-glucitol and L-proline. It is a cocrystal of known compound A, which has a constant quality, is superior in storage stability, has no moisture absorptivity, and is suitable as a crystal of a drug substance used for preparing pharmaceuticals.
Owner:ASTELLAS PHARMA INC +1
Pharmaceutical nanosuspension
The present invention in general relates to a pharmaceutical suspension comprising nano-sized cocrystals of at least one active ingredient and at least one dicarboxylic acid. It in particular relates to a pharmaceutical suspension comprising nano-sized cocrystals of at least one anthelmintic drug and at least one dicarboxylic acid. The invention further relates to uses, methods for use and methods for manufacturing the pharmaceutical suspension according to this invention.
Owner:UNIV GENT
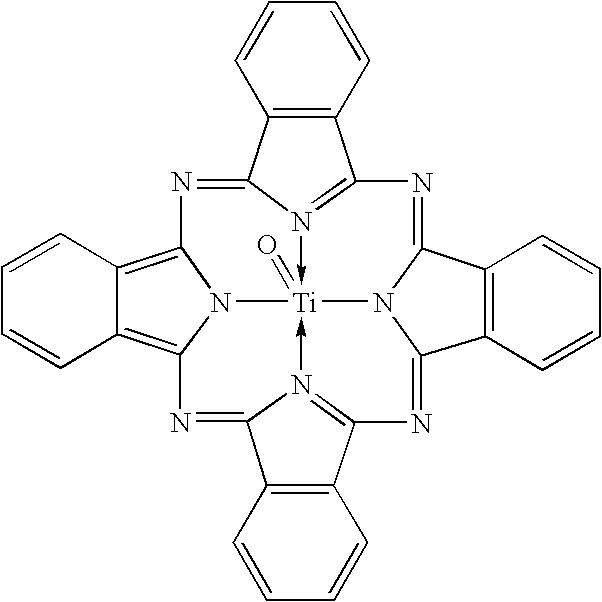
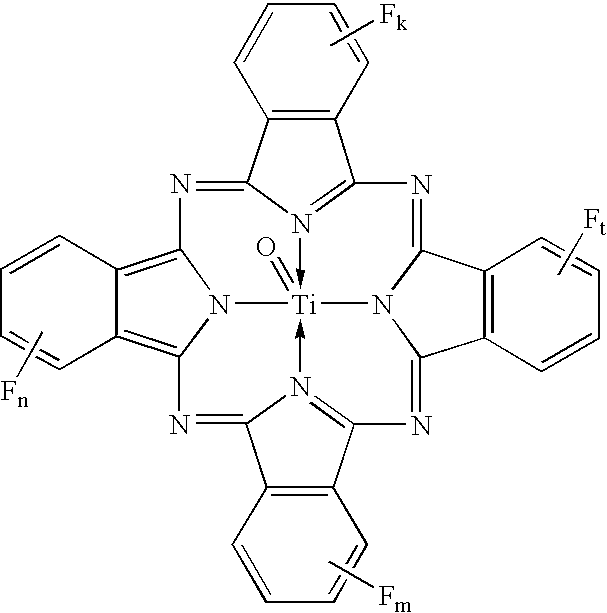
Uniform cocrystals of titanyl fluorophthalocyanine and titanyl phthalocyanine formed in trichloroethane, and charge generating layer containing same
In a process for forming a nanoparticulate crystalline titanium phthalocyanine pigment composition, a titanium phthalocyanine pigment is contacted with substantially pure 1,1,2-trichloroethane (TCE) under conditions effective to convert the titanium phthalocyanine pigment to the nanoparticulate crystalline composition.
Owner:EASTMAN KODAK CO
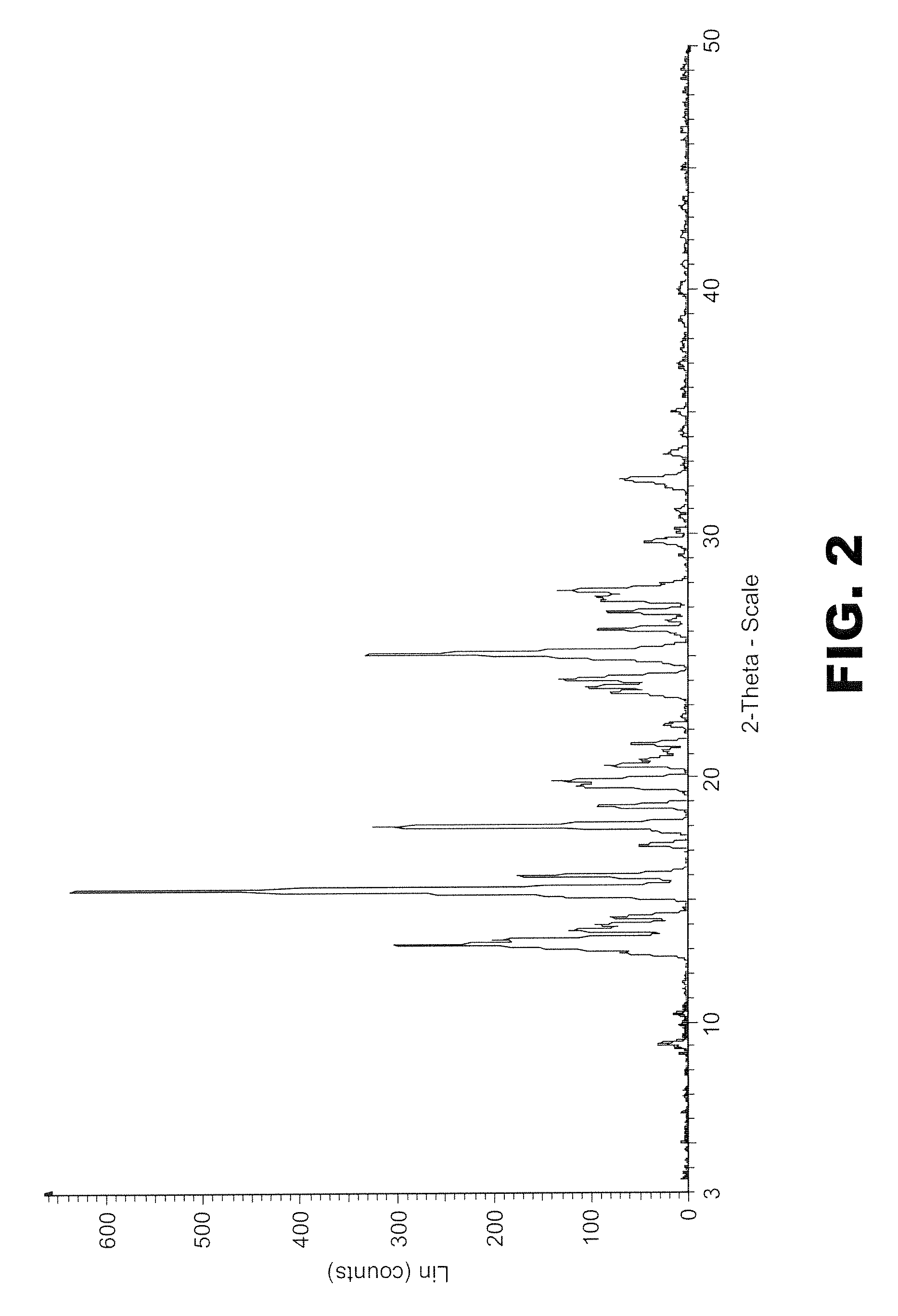
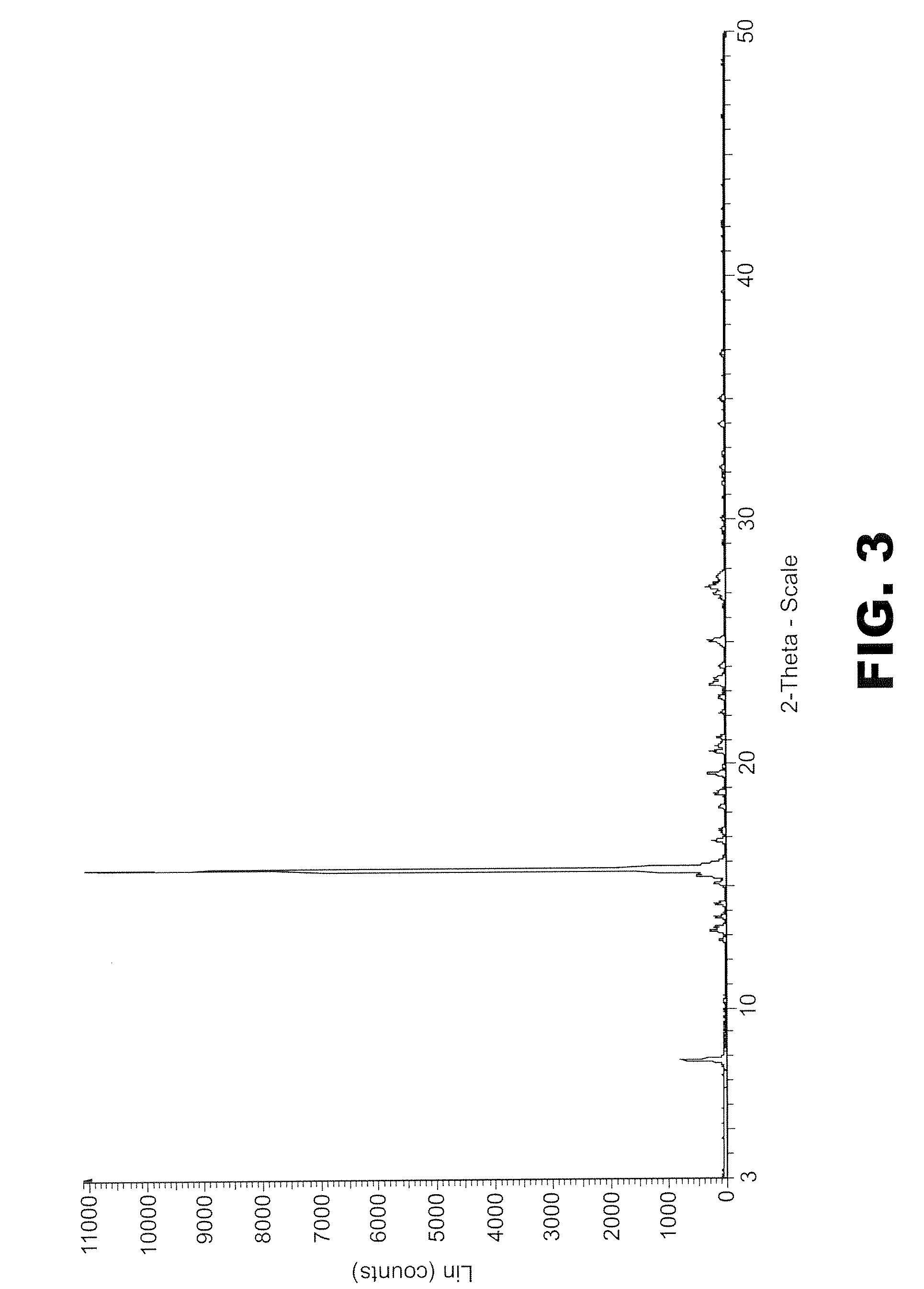
Olaparib and maleic acid cocrystal and preparation method thereof
ActiveCN111689905AImprove oral absorption efficiencyImprove apparent solubilityOrganic active ingredientsOrganic chemistry methodsSolubilityCombinatorial chemistry
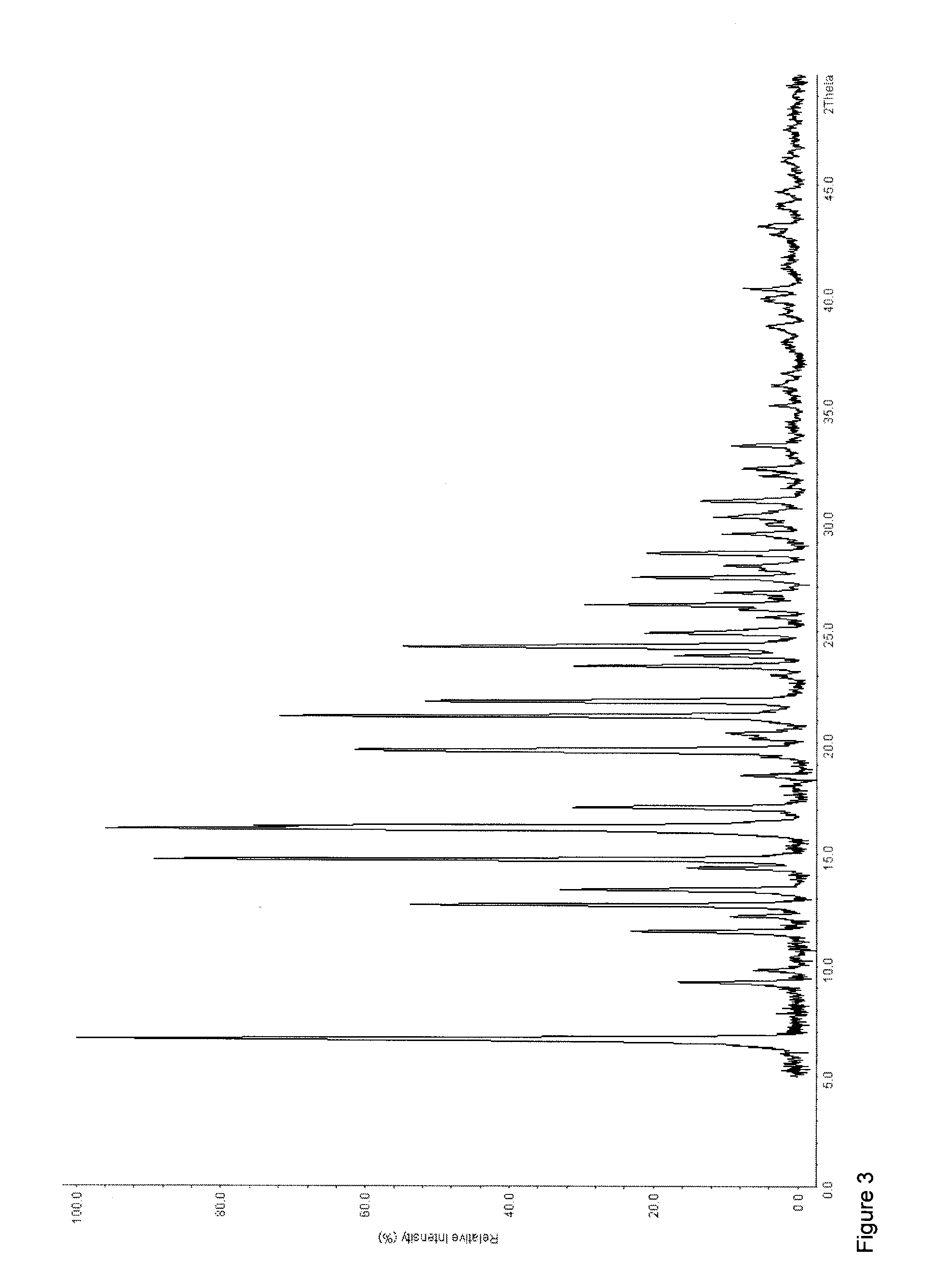
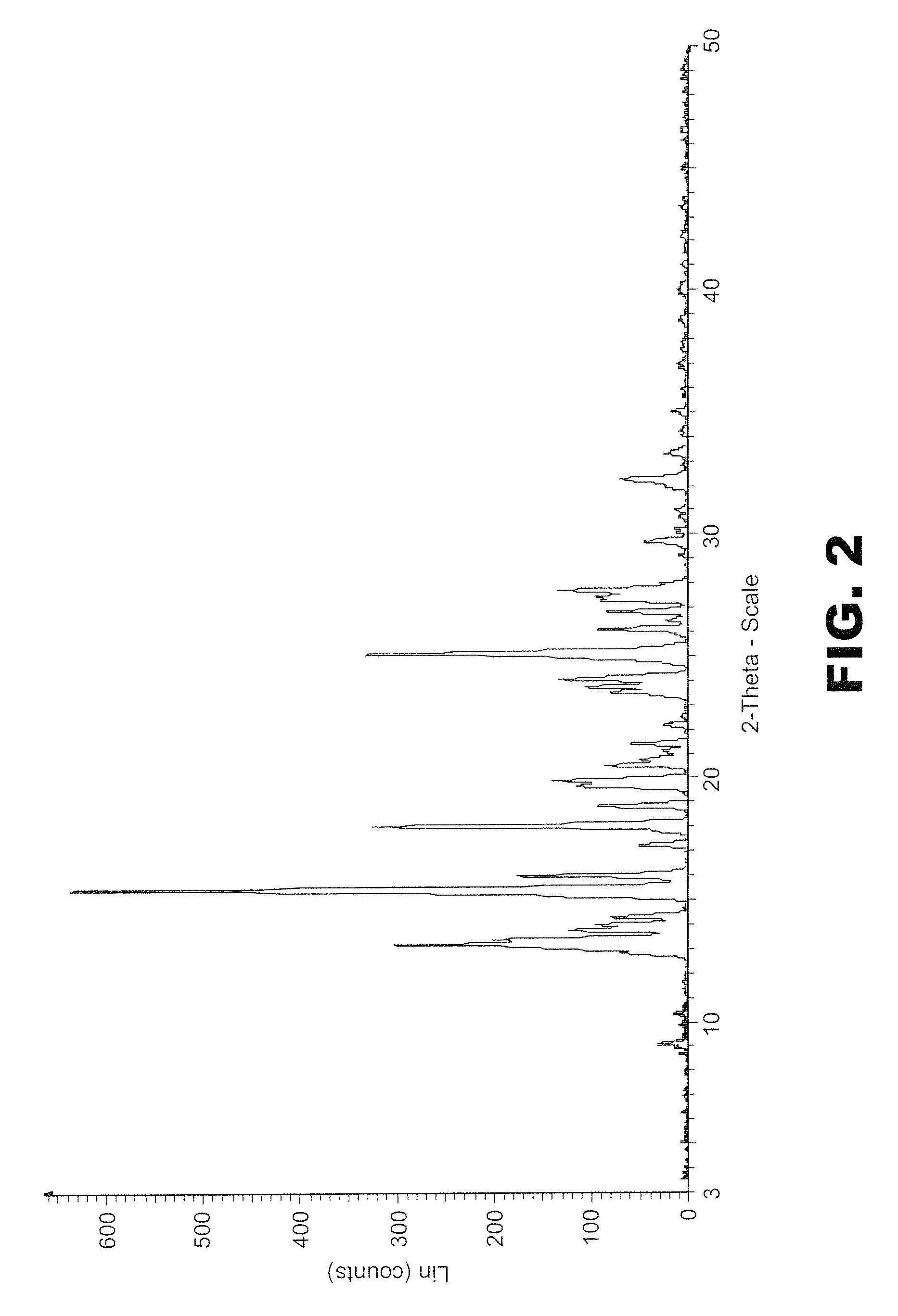
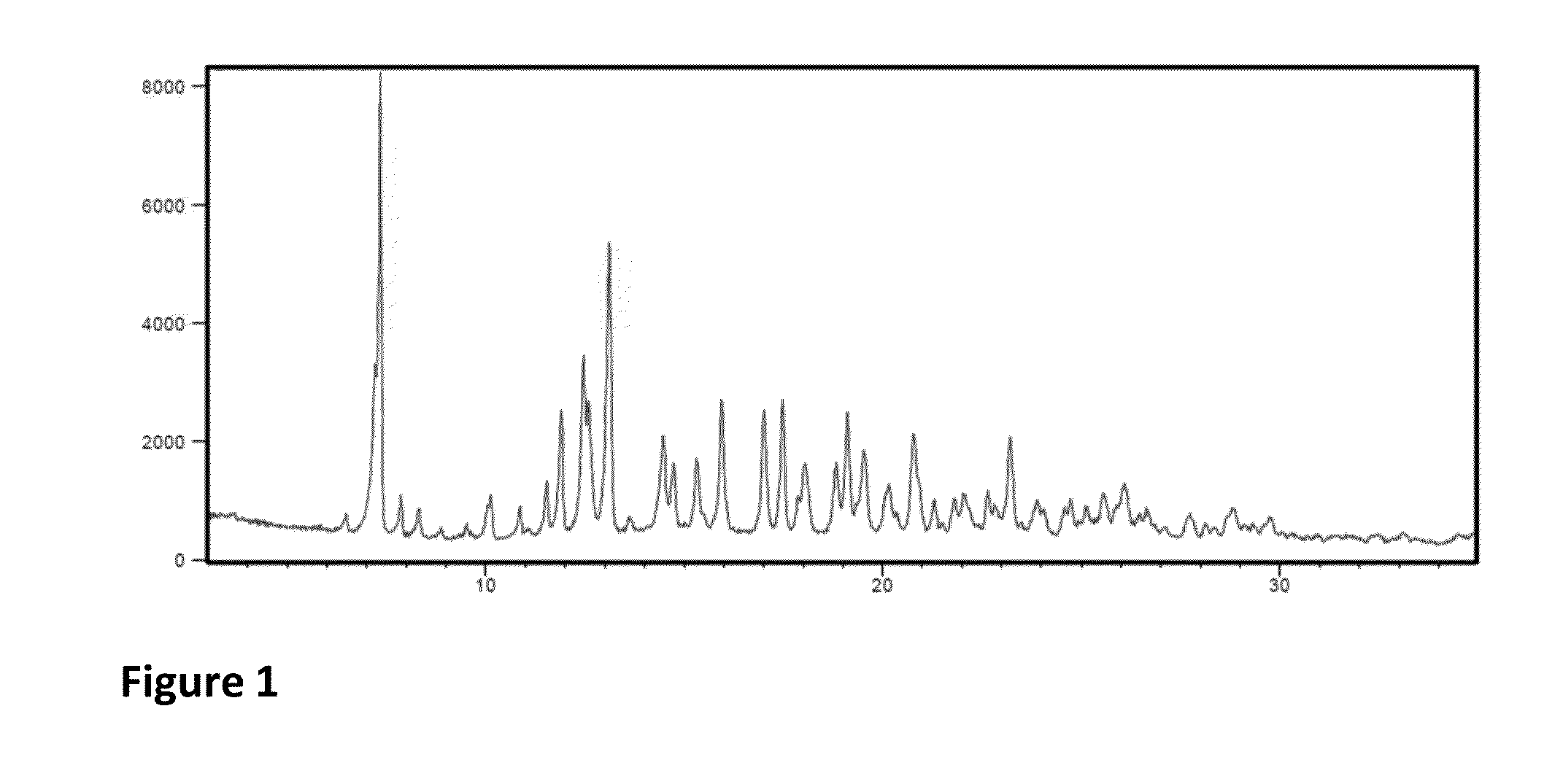
The invention discloses an olaparib and maleic acid cocrystal and a preparation method thereof. The molar ratio of olaparib to maleic acid in the cocrystal is 1:1, and an X-ray powder diffraction pattern of the cocrystal has characteristic peaks when the 2theta values are 5.1+ / -0.2 degrees, 9.8+ / -0.2 degrees, 13.7+ / -0.2 degrees, 16.0+ / -0.2 degrees, 17.7+ / -0.2 degrees and 20.0+ / -0.2 degrees. The cocrystal preparation method provided by the invention is simple in process, easy to control the crystallization process, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the cocrystal has higher apparent solubility, and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
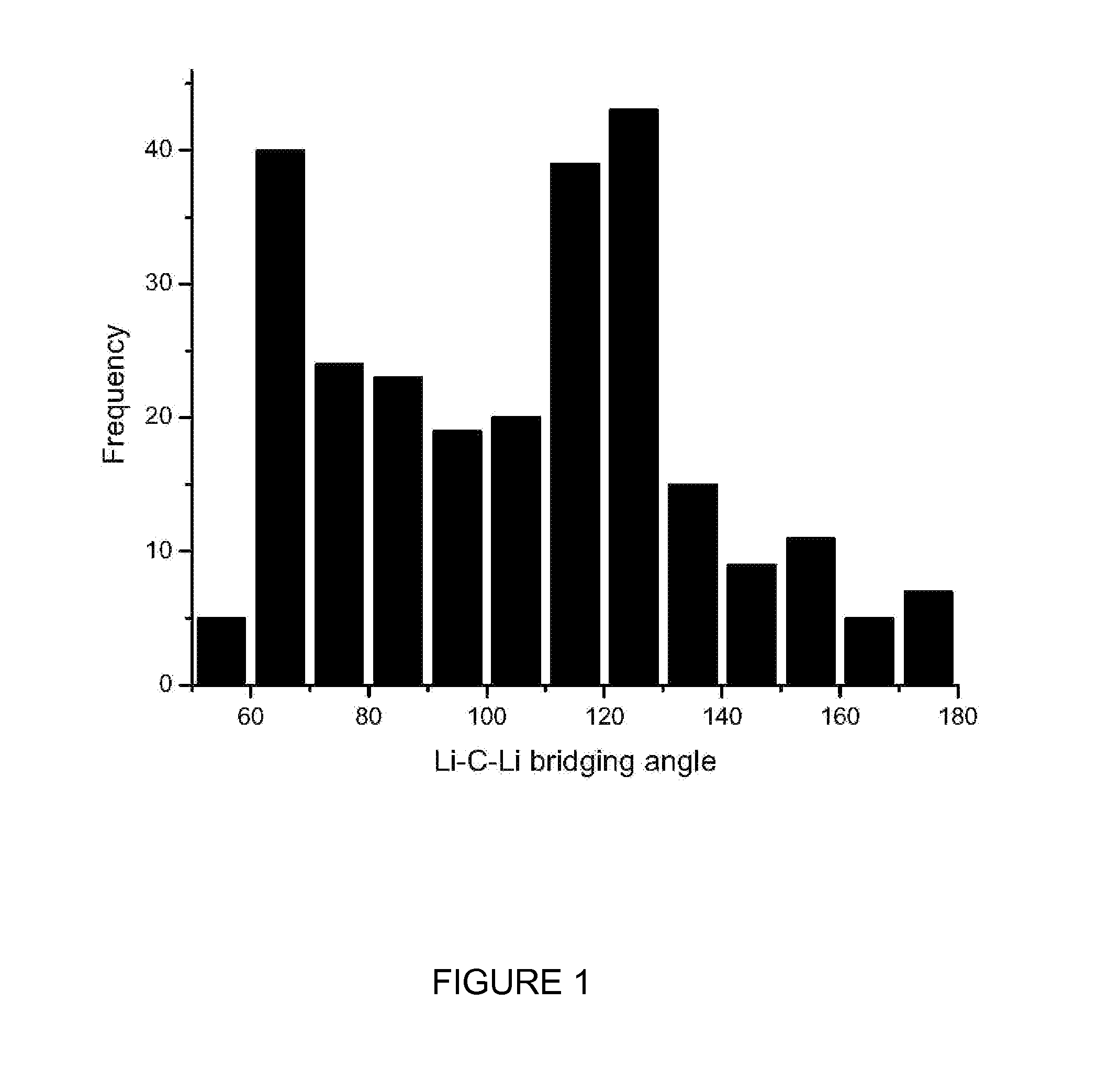
Lithium compositions
2:1 cocrystals of amino acids and Li+ salts crystallize from hot water to afford water stable cationic networks based upon tetrahedral lithium cations: bilayered square grids, a lithium zeolitic metal-organic material (LiZMOM) and several lithium diamondoid metal-organic materials (LiDMOMs). The compositions may be used as a pharmaceutical for the treatment of suicidality and other disorders that require lithium to penetrate the blood brain barrier and exert therapeutic effects in the CNS. Advantageously, the novel cocrystal forms described herein may be used to lower the oral dose required to achieve therapeutic concentrations of lithium in the brain, thus reducing the peripheral toxicity and potentially broadening the therapeutic index in comparison to conventional lithium forms.
Owner:UNIV OF SOUTH FLORIDA
4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal
A 4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal and its use as an opioid antagonist are disclosed. The invention also relates to a drug-in-adhesive transdermal patch containing the analgesic fentanyl, a mu opioid agonist, or an analog thereof and a 4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal, as an opioid antagonist. A transdermal patch of the invention is tamper-resistant and an abuse deterrent which protects against drug misuse or abuse. The invention also provides a method of treating pain, such as acute, chronic, or intermittent pain, by applying a drug-in-adhesive transdermal patch according to the invention to the skin of a patient in need thereof. Also disclosed is an improved transdermal patch for administering fentanyl or an analog thereof, or for administering a mu opioid agonist, the improvement wherein the transdermal patch contains a 4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal in an abuse limiting amount. The improved transdermal patch may be a drug-in-adhesive transdermal patch or a reservoir transdermal patch.
Owner:PAIN THERAPEUTICS INC
4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal
A 4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal and its use as an opioid antagonist are disclosed. The invention also relates to a drug-in-adhesive transdermal patch containing the analgesic fentanyl, a mu opioid agonist, or an analog thereof and a 4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal, as an opioid antagonist. A transdermal patch of the invention is tamper-resistant and an abuse deterrent which protects against drug misuse or abuse. The invention also provides a method of treating pain, such as acute, chronic, or intermittent pain, by applying a drug-in-adhesive transdermal patch according to the invention to the skin of a patient in need thereof. Also disclosed is an improved transdermal patch for administering fentanyl or an analog thereof, or for administering a mu opioid agonist, the improvement wherein the transdermal patch contains a 4:3 naltrexone: 5-methyl-2-furaldehyde cocrystal in an abuse limiting amount. The improved transdermal patch may be a drug-in-adhesive transdermal patch or a reservoir transdermal patch.
Owner:PAIN THERAPEUTICS INC
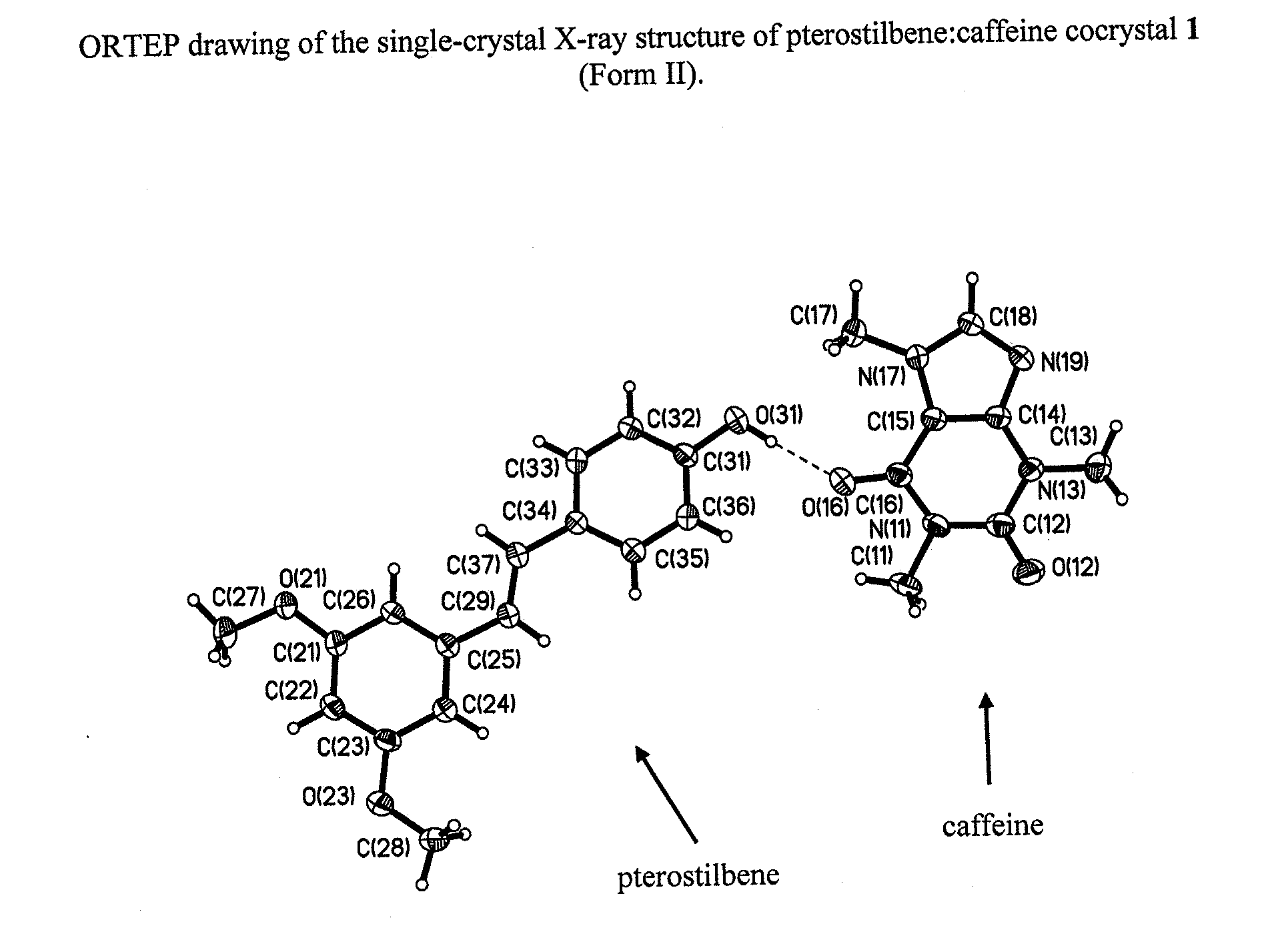
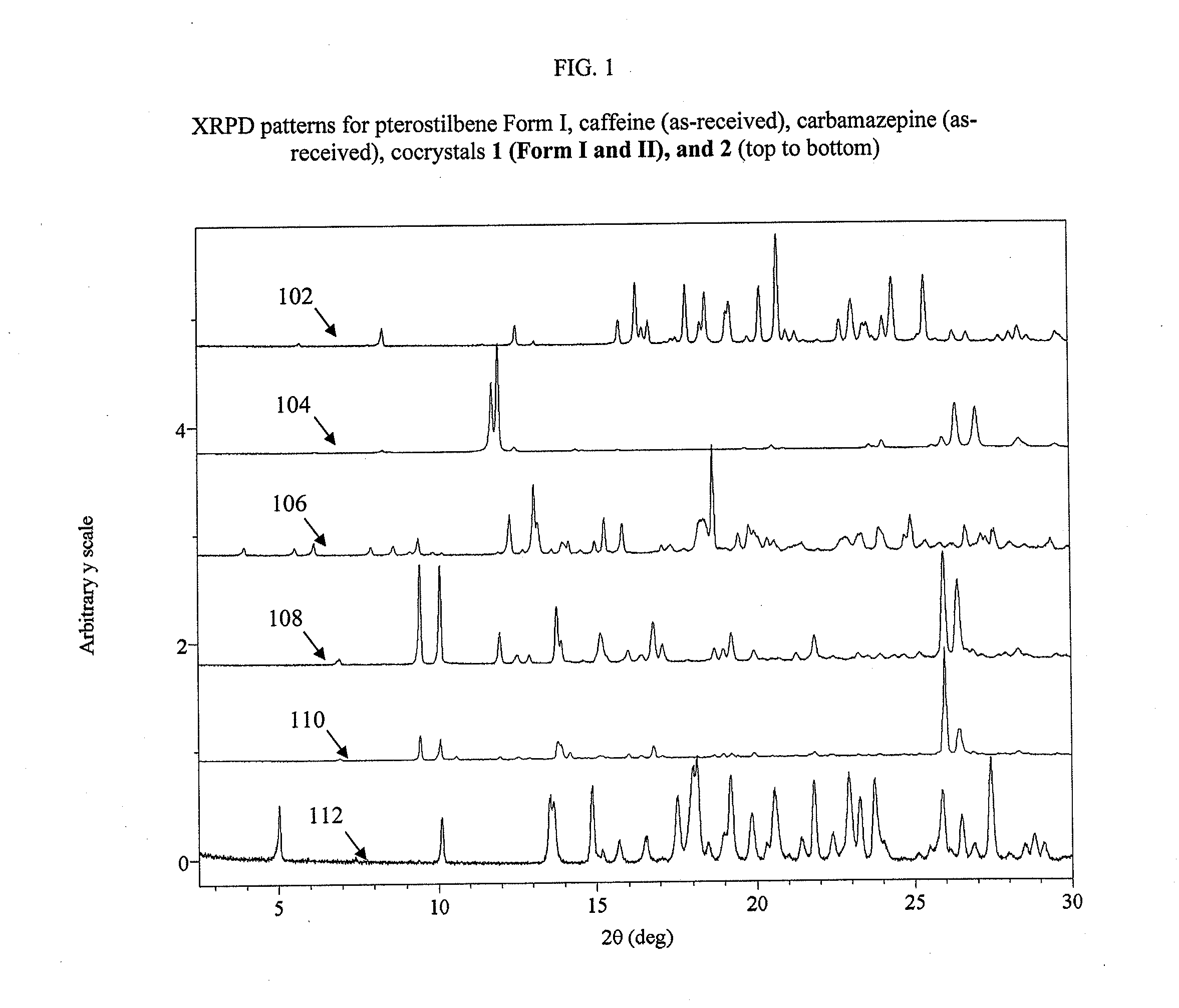
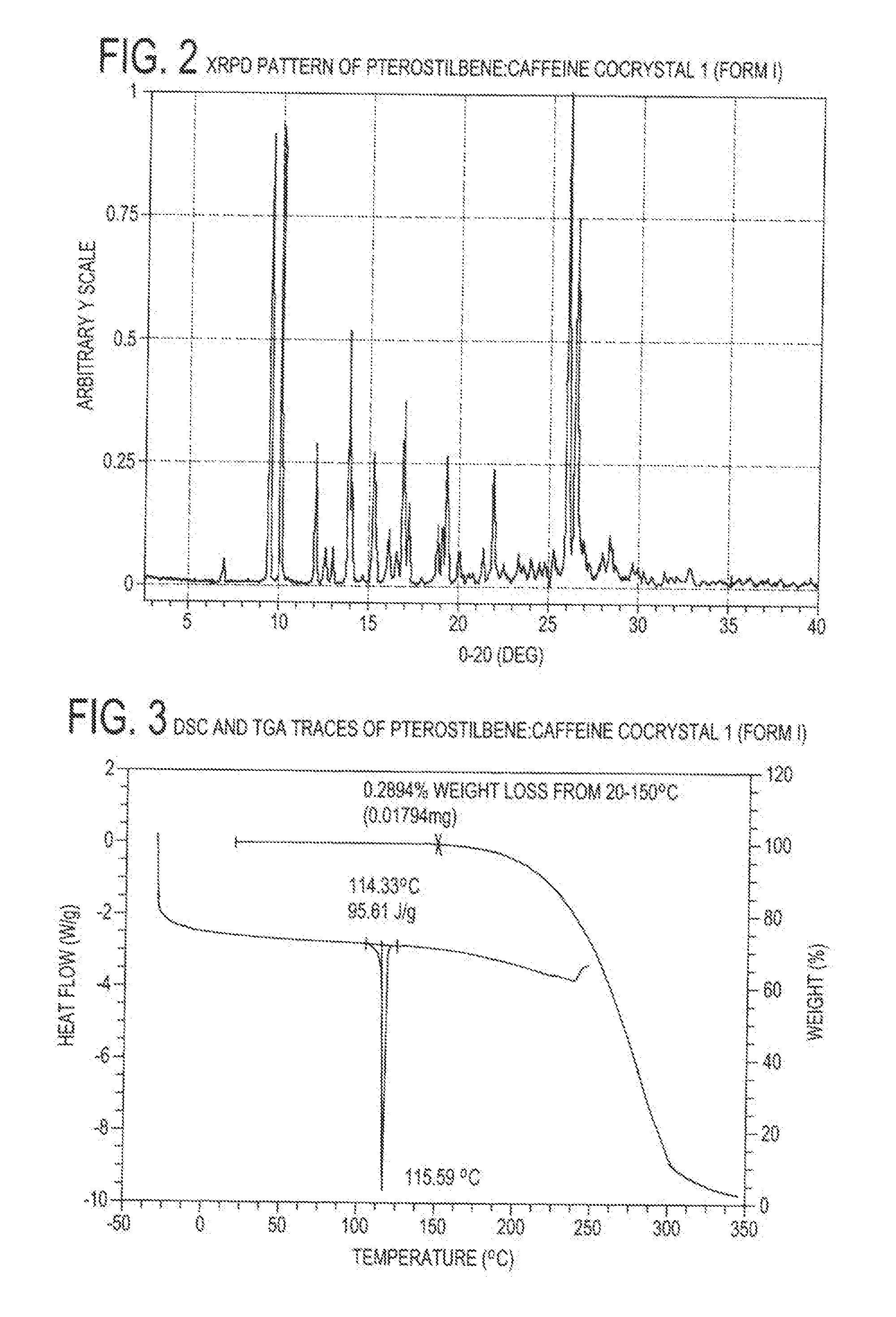
Pterostilbene cocrystals
ActiveUS20110189275A1Physical stabilityImprove physical stabilityBiocideOrganic chemistryGlutaric acidCarbamazepine
Cocrystals of pterostilbene are disclosed, including: pterostilbene:caffeine cocrystal, pterostilbene:carbamazepine cocrystal, pterostilbene:glutaric acid cocrystal, and pterostilbene:piperazine cocrystal. The pterostilbene:caffeine cocrystal is polymorphic. Forms I and II of the pterostilbene:caffeine cocrystal are disclosed. The therapeutic uses of the pterostilbene cocrystals and of pharmaceutical / nutraceutical compositions containing them are also disclosed. The disclosure sets out various methods of making and characterizing the pterostilbene cocrystals.
Owner:LAURUS LABS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com