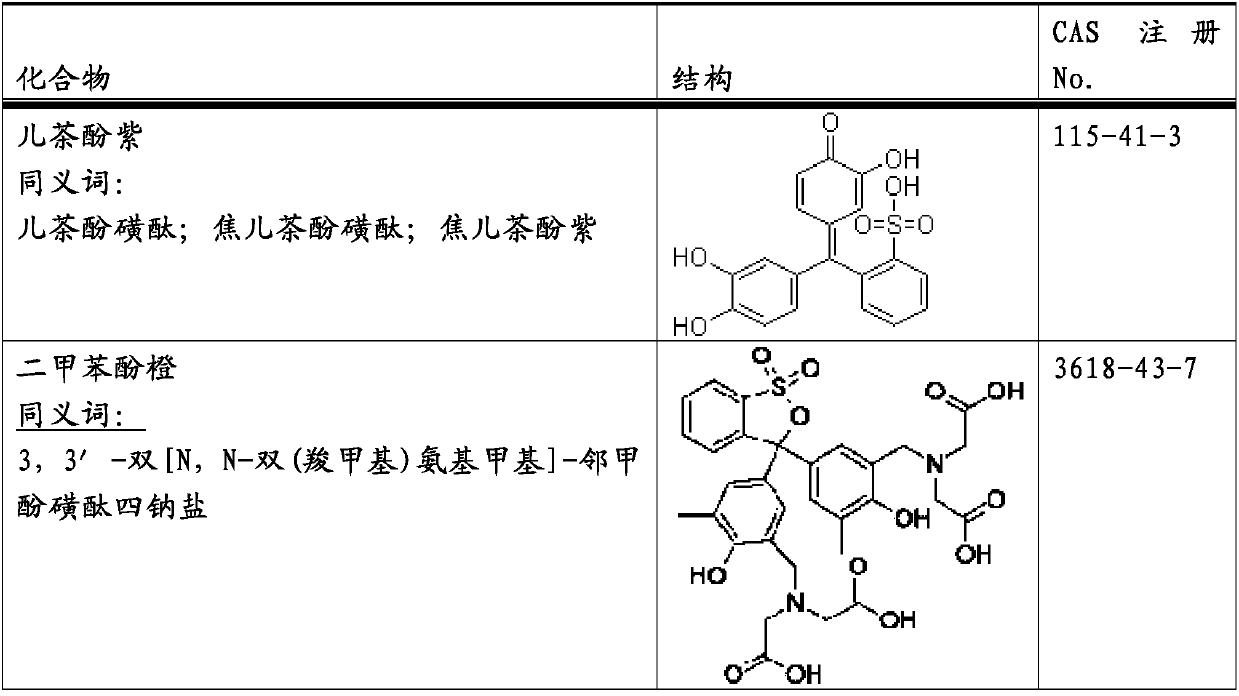
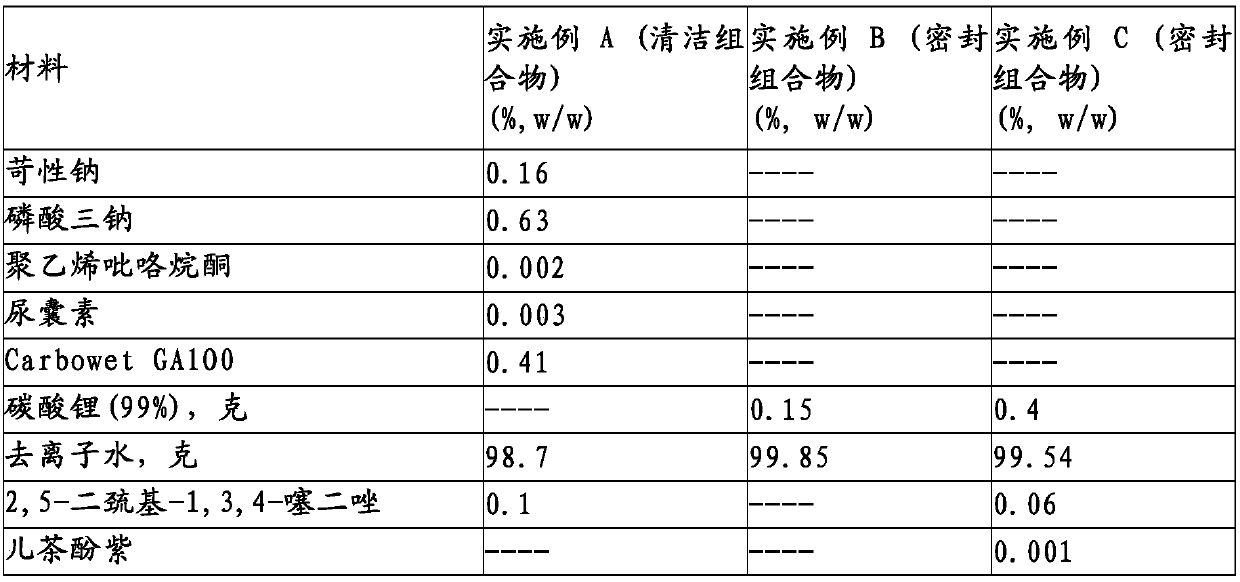
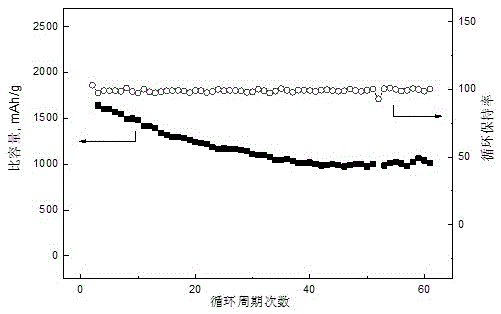
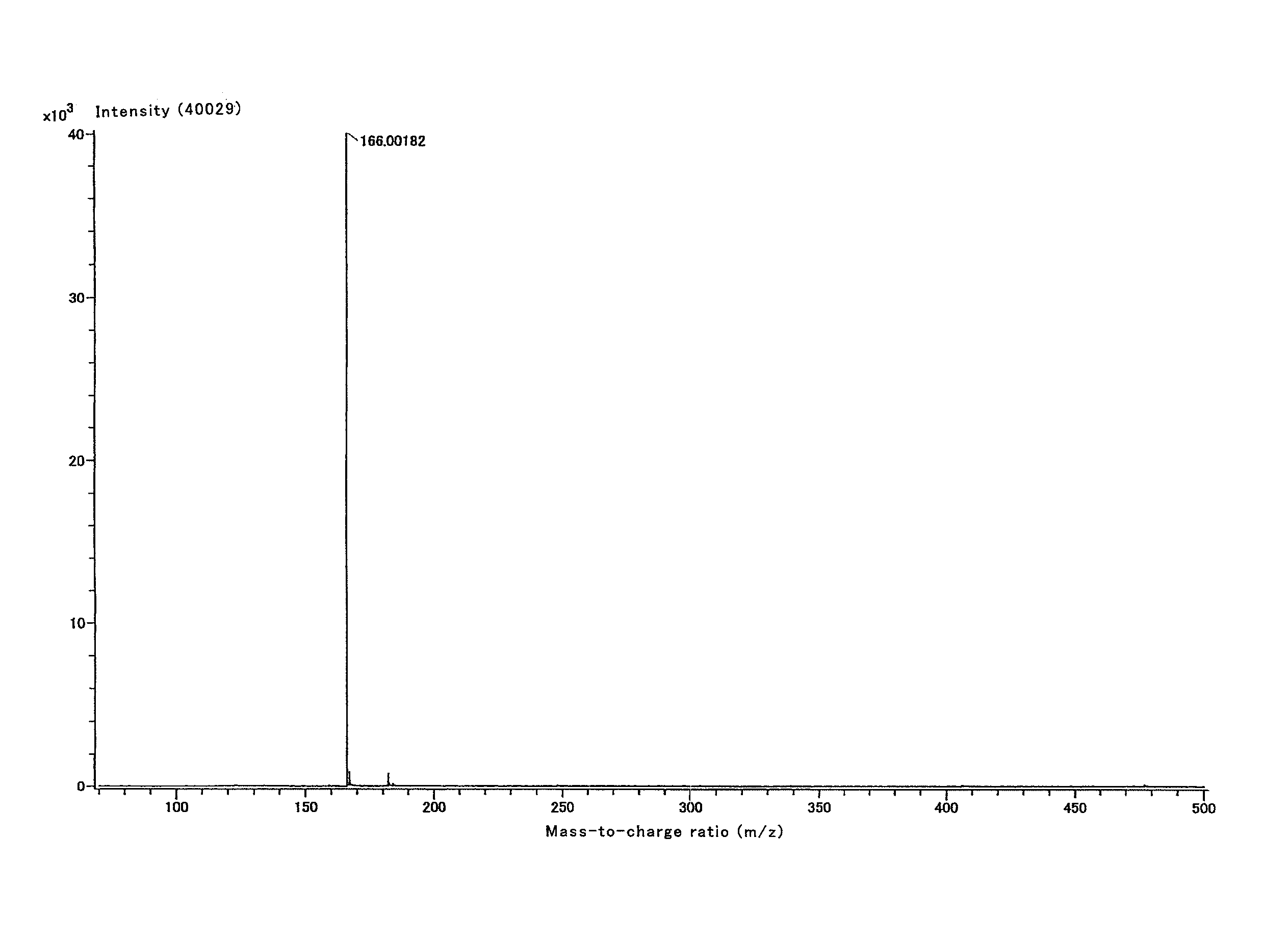
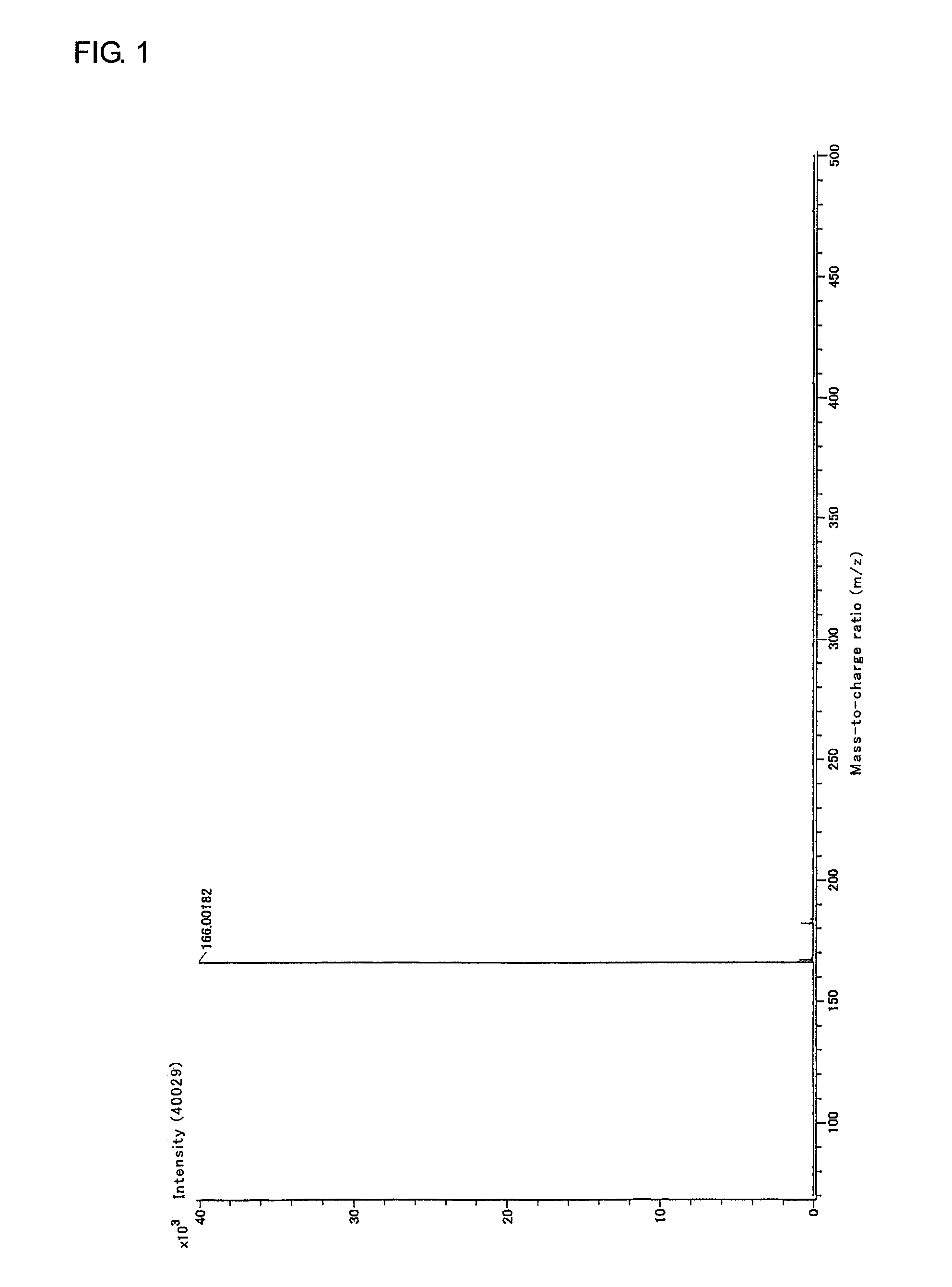
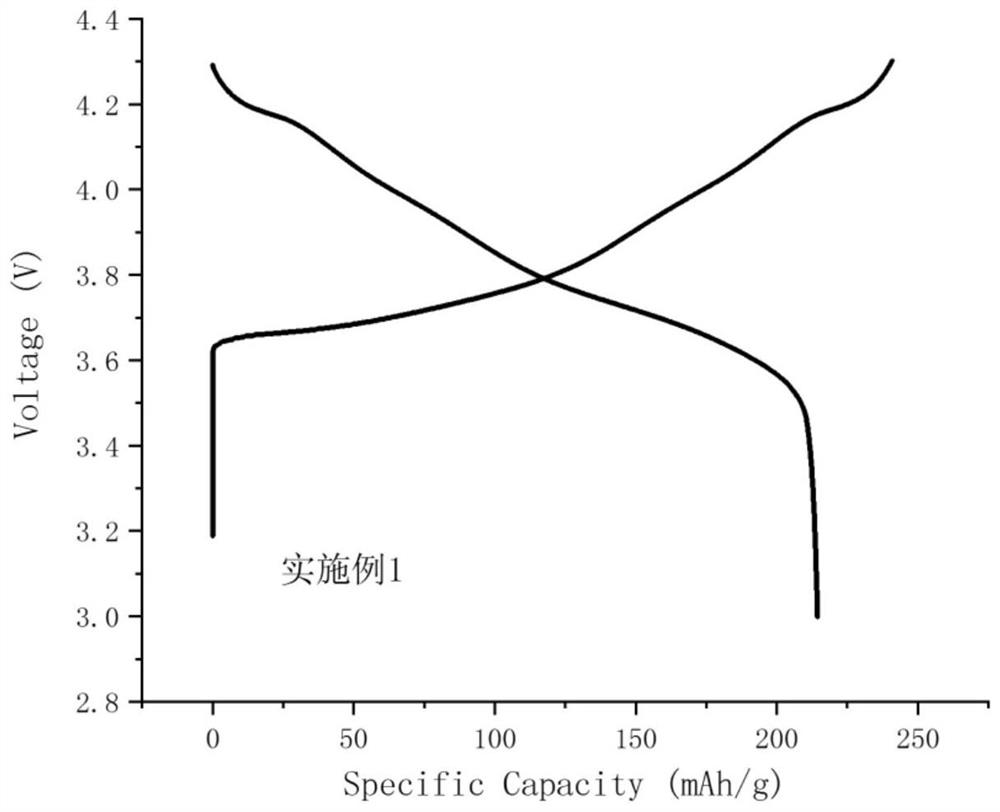
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Lithium Cation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A monovalent cation that is metabolized much like sodium and is important in many cellular functions inside or on the surface of cells.
Metal oxide structures, devices, and fabrication methods
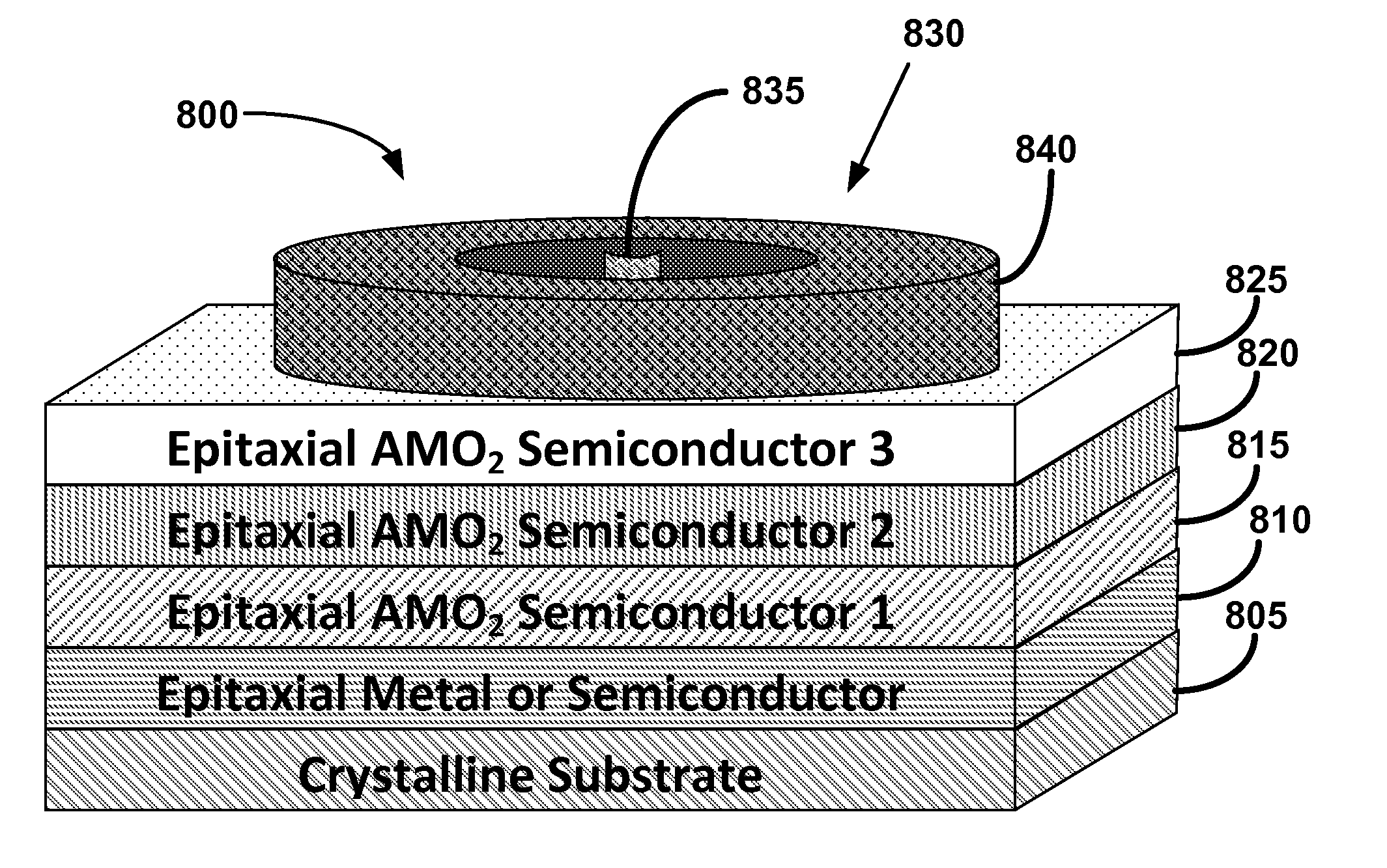
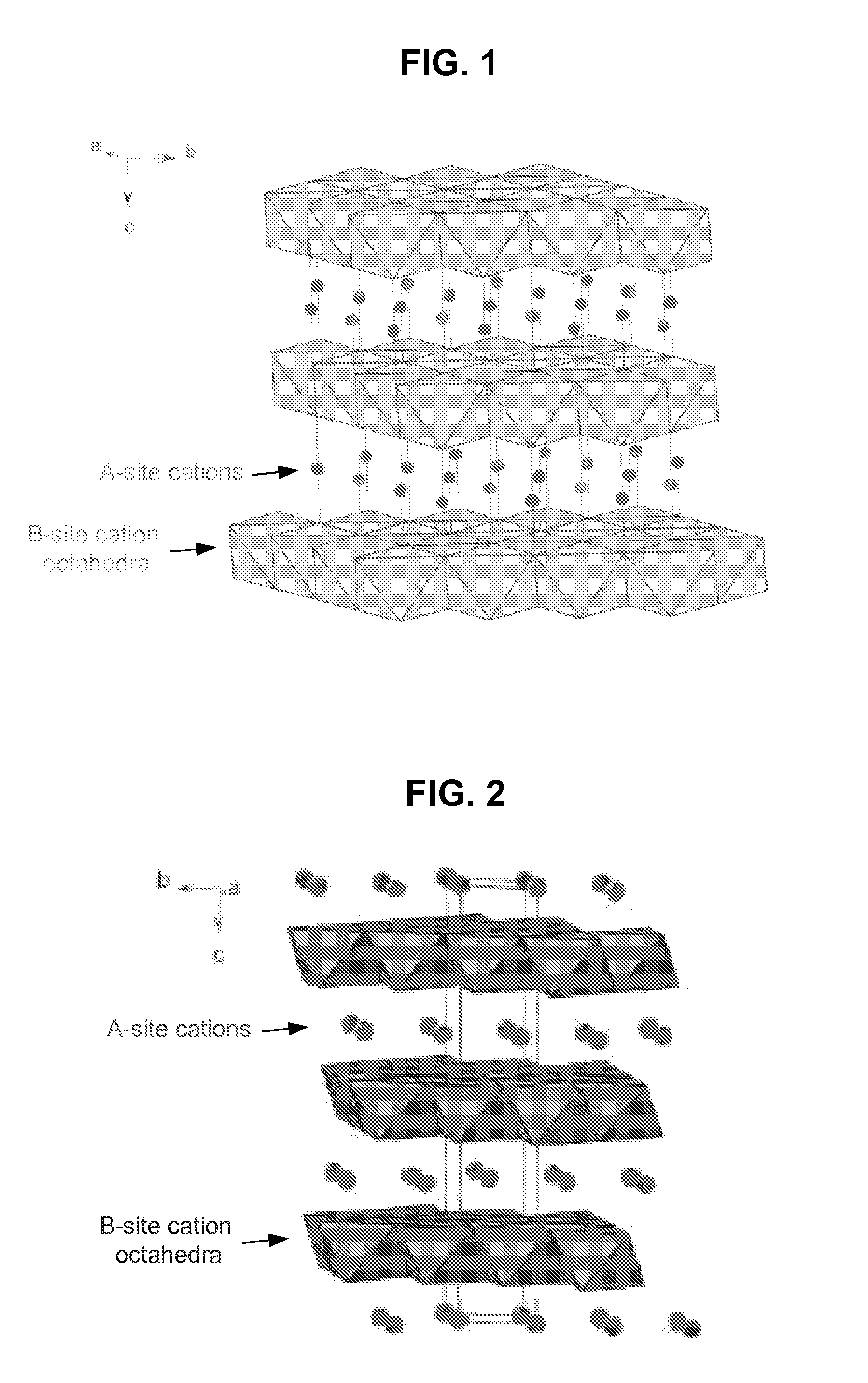
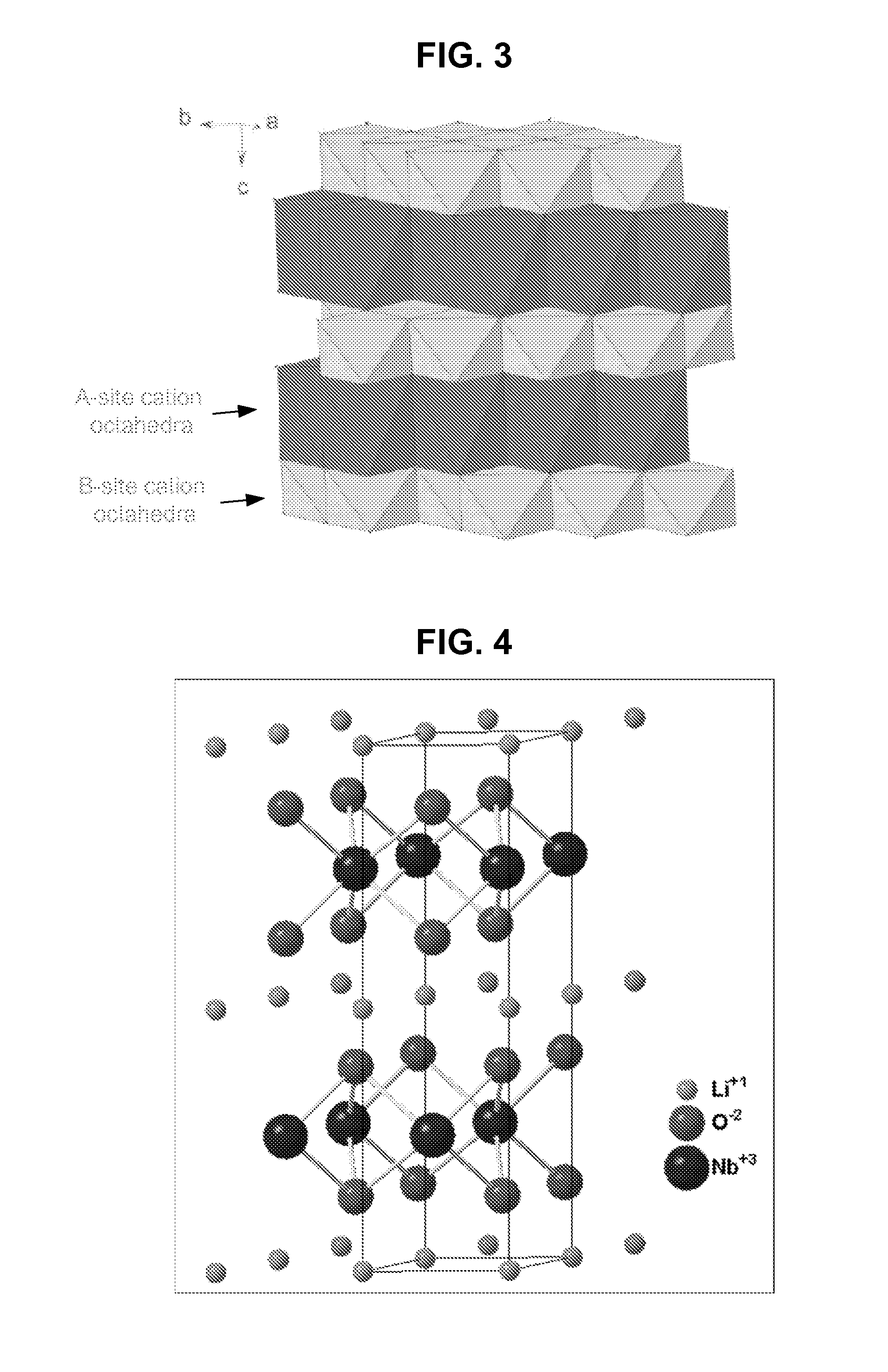
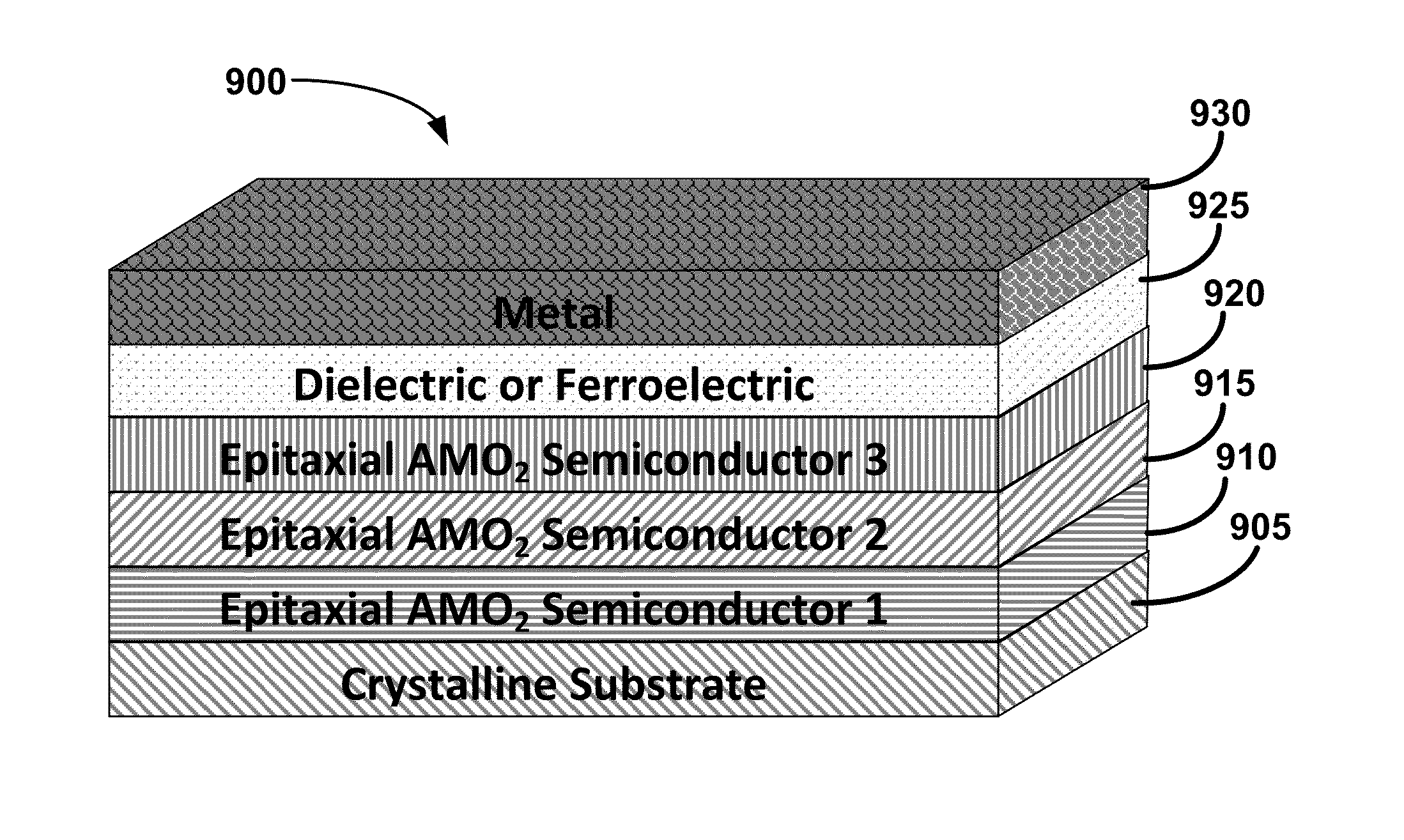
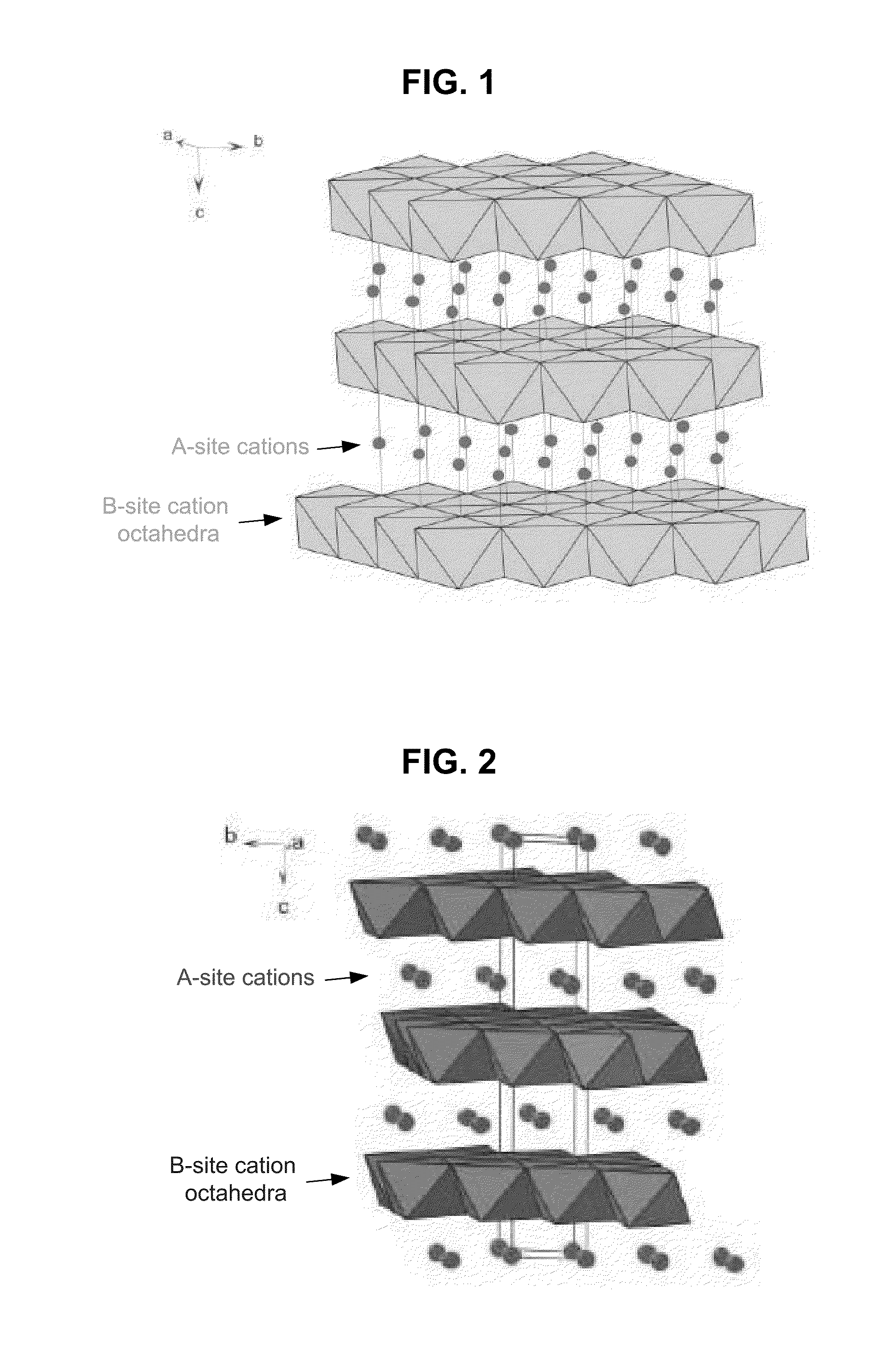
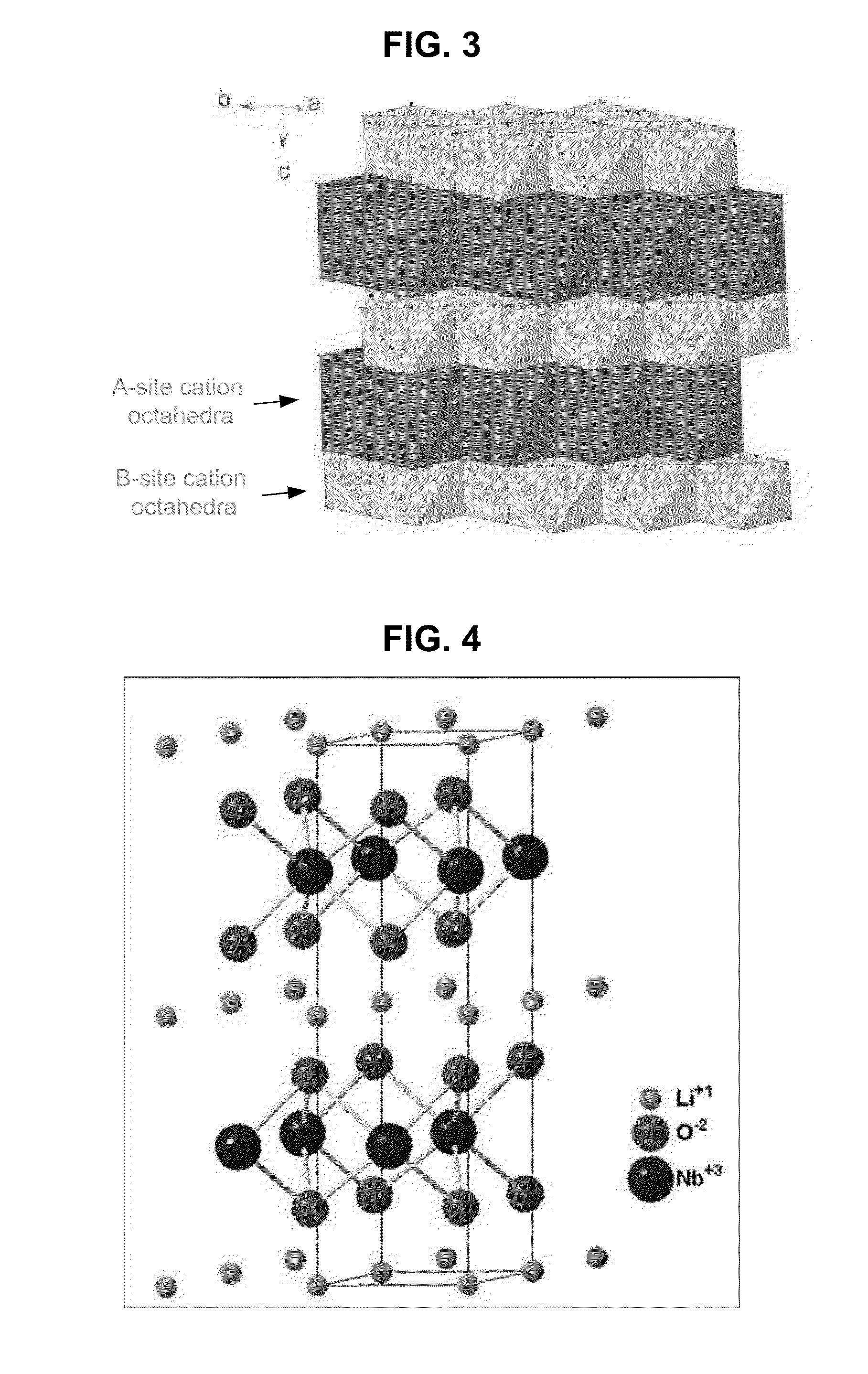
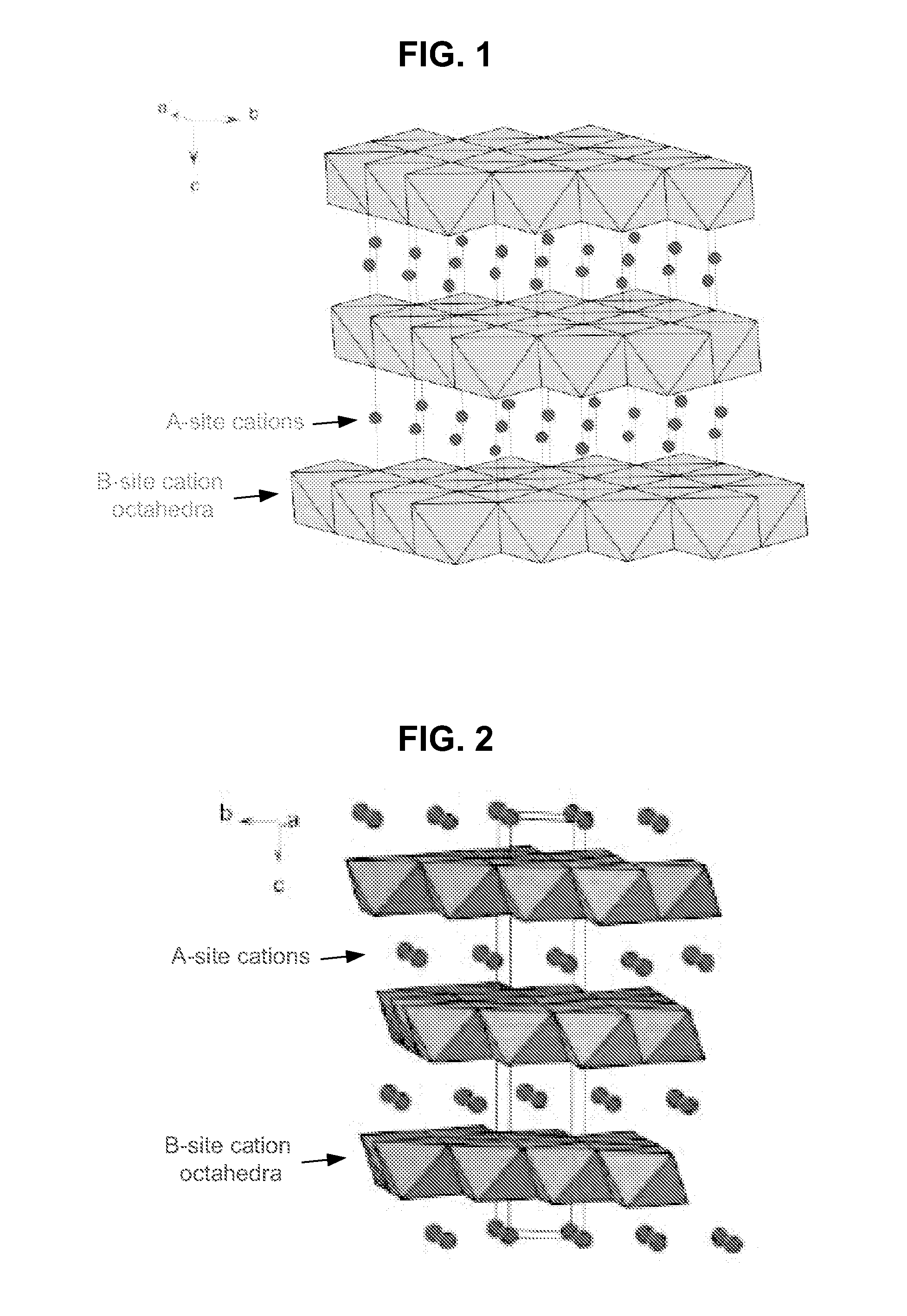
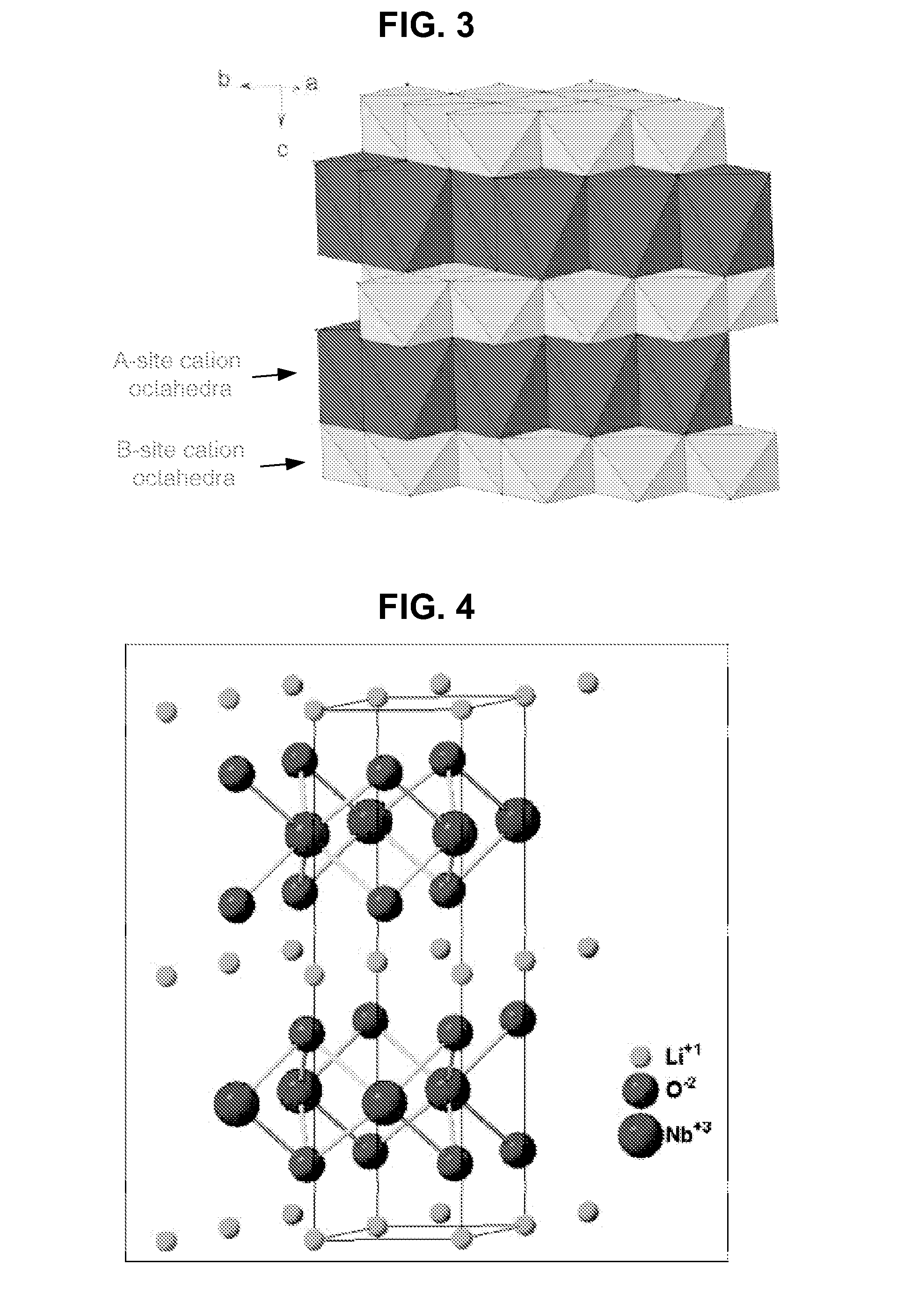
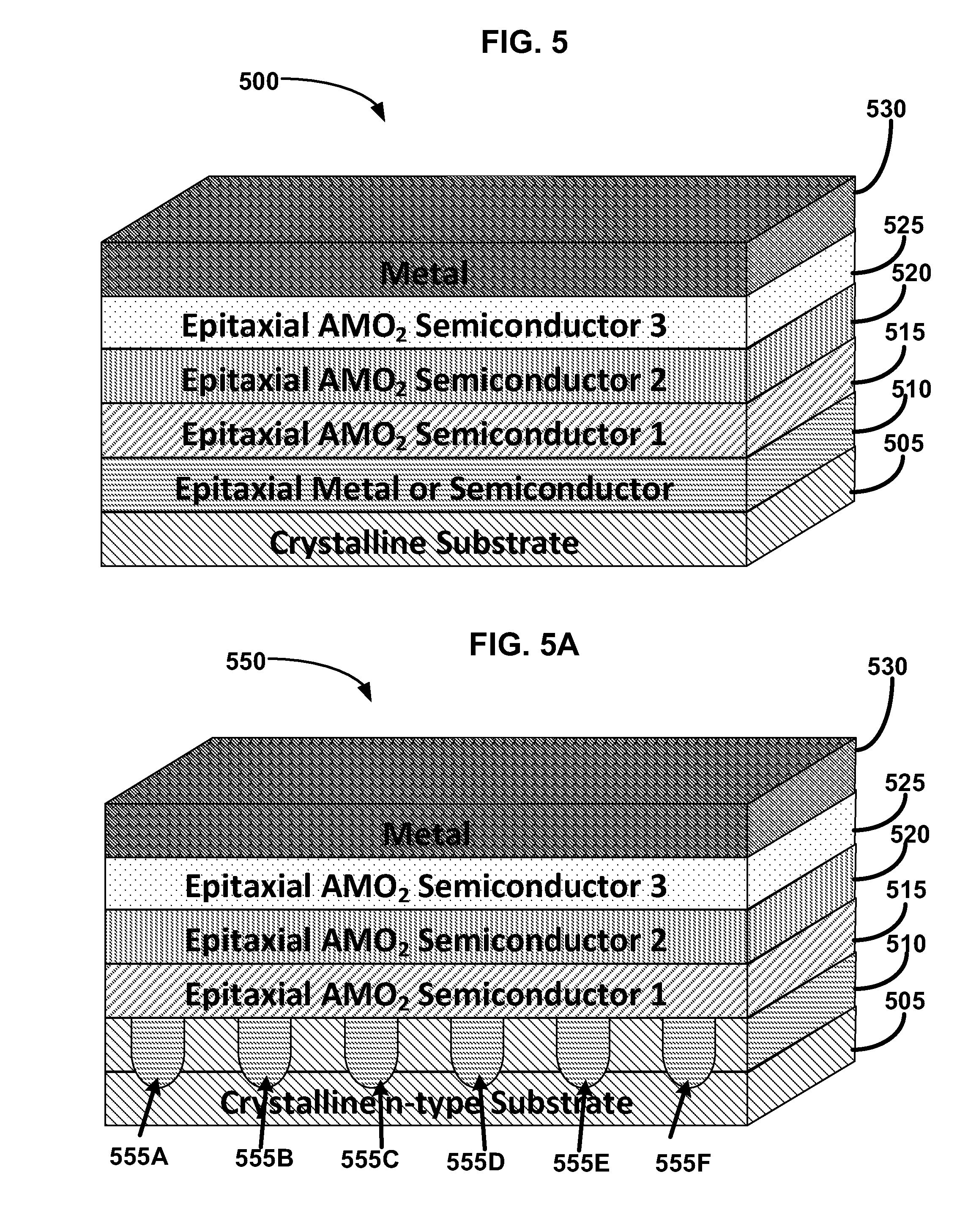
Metal oxide structures, devices, and fabrication methods are provided. In addition, applications of such structures, devices, and methods are provided. In some embodiments, an oxide material can include a substrate and a single-crystal epitaxial layer of an oxide composition disposed on a surface of the substrate, where the oxide composition is represented by ABO2 such that A is a lithium cation, B is a cation selected from the group consisting of trivalent transition metal cations, trivalent lanthanide cations, trivalent actinide cations, trivalent p-block cations, and combinations thereof, and O is an oxygen anion. The unit cell of crystal structure of the oxide composition can be characterized by first layer of a plane of lithium cations and a second layer of a plurality of edge-sharing octahedra having a B cation positioned in a center of each octahedron and an oxygen anion at each corner of each octahedron. The first layer and the second layer of the unit cell are alternatingly stacked along one axis of the unit cell. Other aspects, features, and embodiments are also claimed and described.
Owner:GEORGIA TECH RES CORP
Ion selective separator for lithium sulfur secondary battery as well as preparation method and application method thereof
ActiveCN103474603AOvercome efficiencyOvercome stabilityCell seperators/membranes/diaphragms/spacersLithium sulfurLithium Cation
The invention belongs to the technical field of ion selective separators, and in particular relates to an ion selective separator for a lithium sulfur secondary battery, and a preparation method and an application method of the ion selective separator. According to the ion selective separator provided by the invention, the separator of the lithium sulfur battery is made from an ion selective material or a compound structure of the ion selective material, and the shortcomings of quite low charging and discharging efficiency and quite poor battery performance stability caused by the dispersion of polysulfide of an original lithium sulfur battery between the anode and the cathode are conquered. As the selective transmission of the lithium cation is allowed to transmit and the transmission of the polysulfide anion is blocked in an electrolyte system of the lithium sulfur battery, the whole electrochemical performance of the battery system is improved. Targeted at that the original separator system only provides the single type function of electron insulation between the anode and the cathode, the ion selective separator provided by the invention can simultaneously achieve the two functions of electron insulation and ion selective transmission and has the potential to provide a new solution scheme for the technical obstacles of polysulfide migration inhibition and the like of the lithium sulfur battery, and the practicability of the sulfur in the lithium sulfur secondary battery can be promoted with a high-capacity anode material used in combination.
Owner:TSINGHUA UNIV
Thermal energy storage materials
InactiveUS20120216981A1Improve performanceHeat storage plantsHeat-exchange elementsThermal energyThermal energy storage
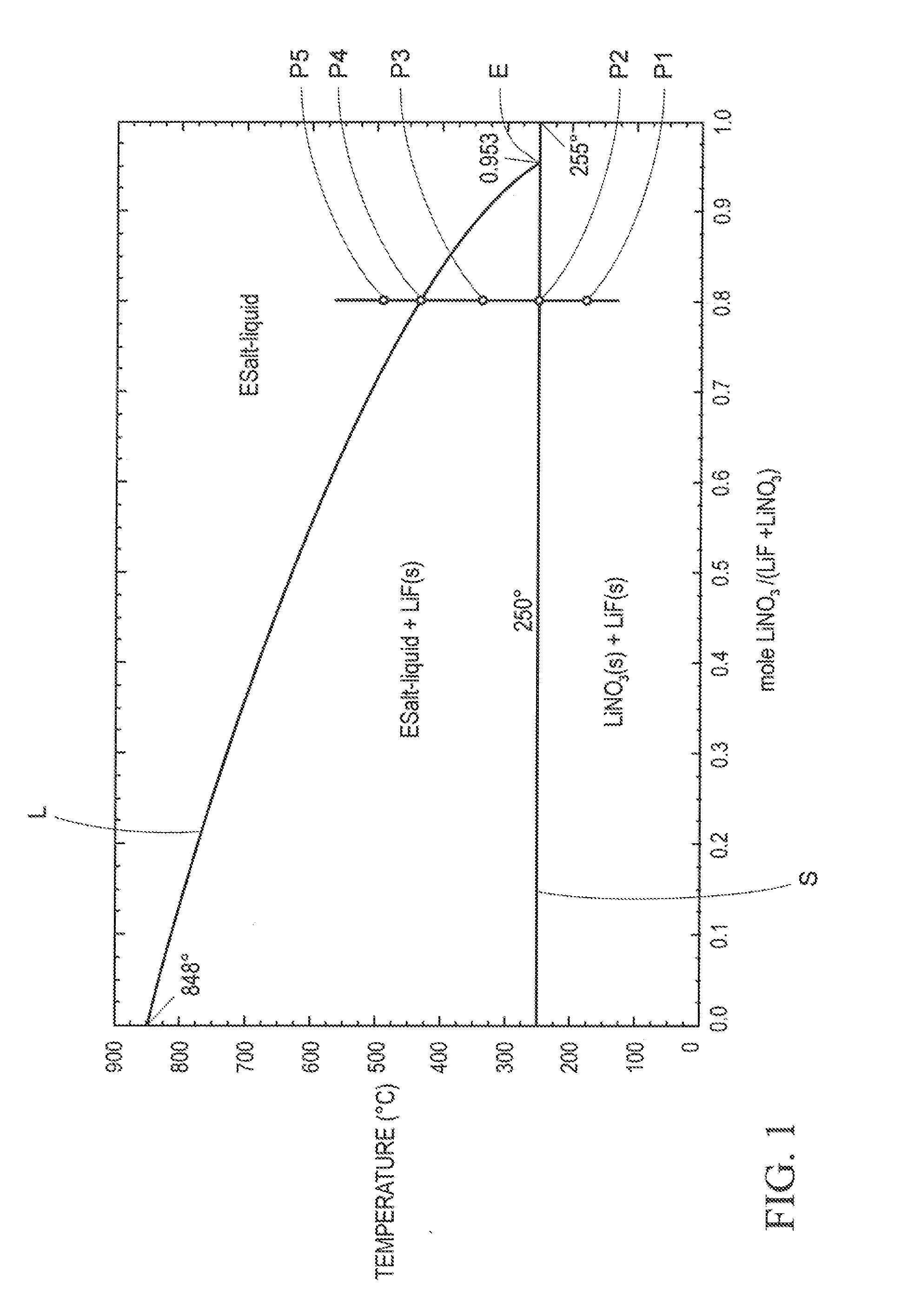
The present invention relates to a thermal energy storage material (TESM) system (and associated methods). The TESM system comprises i) a container having a wall surface; and ii) a TESM in at least partial contact with the wall surface. The TESM may include, consist essentially of, or consist of a metal containing compound comprising at least two different metal cations and one or more polyatomic anions. The at least two metal cations may include lithium cations. The TESM may have a liquidus temperature, TL, from about 100° C. to about 250° C. The TESM may exhibits a heat storage density from 300° C. to 80° C. of at least 1 MJ / l. The TESM system may be free of water. If any water is present in the TESM system, the water concentration preferably is less than about 10 wt. %. Preferably, the TESM system is generally resistant to corrosion at temperatures of about 300° C.
Owner:DOW GLOBAL TECH LLC
Heteroatomic polymer for more efficient solid polymer electrolytes for lithium batteries
InactiveUS6727343B2Solid electrolytesIon-exchanger regenerationPolymer electrolytesPolyethylene oxide
A new type of polymer is described that represents a new composition of matter. This polymer contains alternating electronegative group III-VI elements connected with hydrocarbon or fluorocarbon linkages to form a polyalkyl or polyfluoroalkyl heteroatomic polymer. These polymers can be combined with lithium salts to form a solid polymer electrolyte for use in electrochemical systems such as batteries. These new solid polymer electrolytes exhibit lithium cation diffusion and lithium cation transport numbers that are superior to similar solid polymer electrolytes composed of polyethylene oxide.
Owner:PHOENIX INNOVATIONS
Optionally reinforced polyamide composition containing ionomer
InactiveUS20130172470A1Excellent salt stress crack resistanceIncrease resistanceOrganic dyesCrack resistanceChloride salt
Disclosed is a composition comprising a polyamide composition comprising nylon-66 or nylon 66 / 6T, and optionally nylon-6, nylon-610, nylon-612, nylon-11, nylon-12, an ionomer composition comprising at least one ethylene acid copolymer wherein 30 to 90% of the total acid functionalities are neutralized to salts with a mixture of zinc cations and sodium or lithium cations, wherein the salts comprise from 20 to 90% equivalents of zinc; and optionally reinforcing filler. Articles prepared from the composition exhibit improved calcium chloride salt stress crack resistance over polyamide compositions not containing the ionomer.
Owner:EI DU PONT DE NEMOURS & CO
Preparation of ion exchanged polymer bound nitrogen adsorbent
ActiveUS7592284B2Increase the air concentrationIncrease oxygen concentrationGas treatmentMolecular sieve catalystsWater vaporSorbent
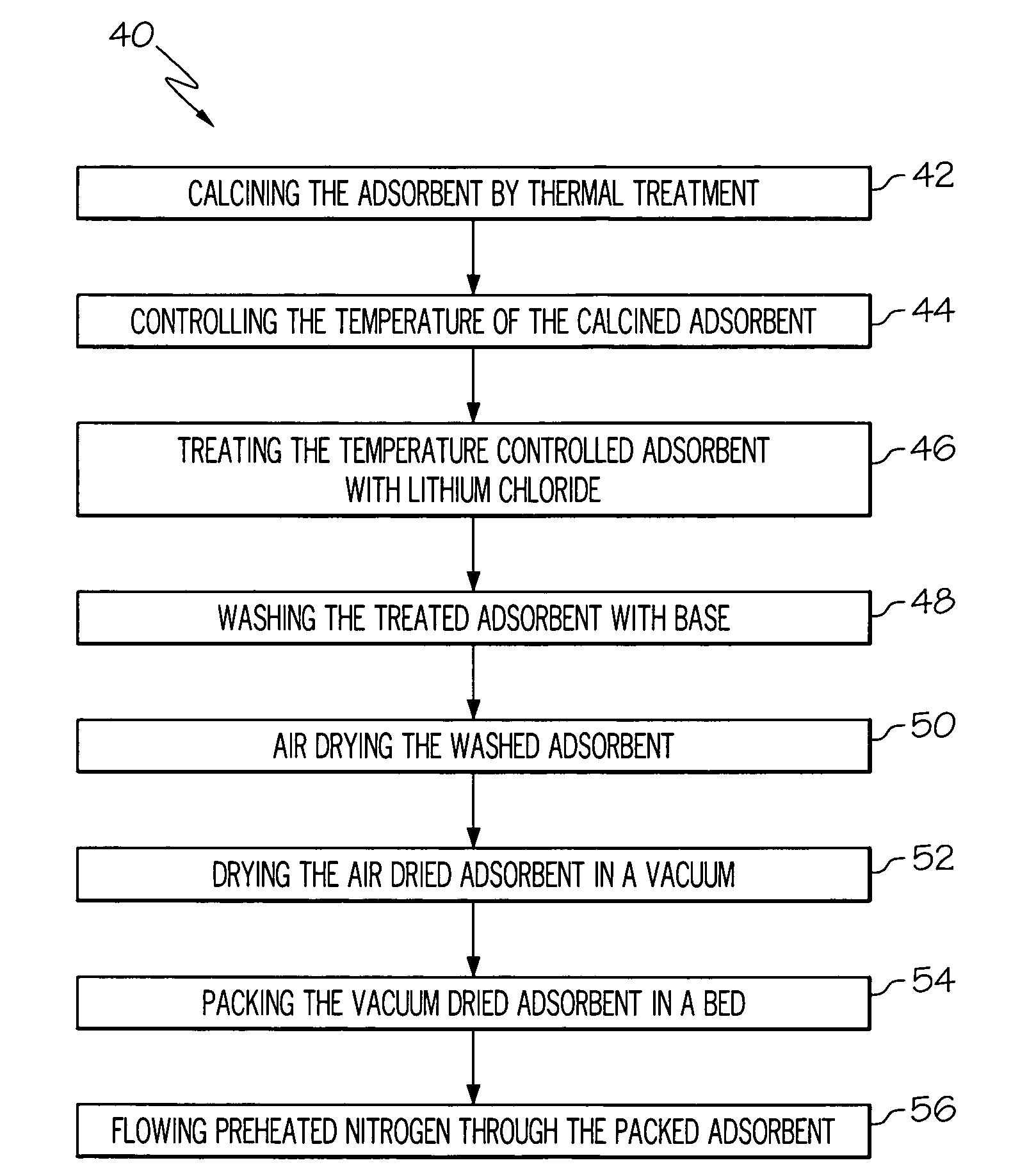
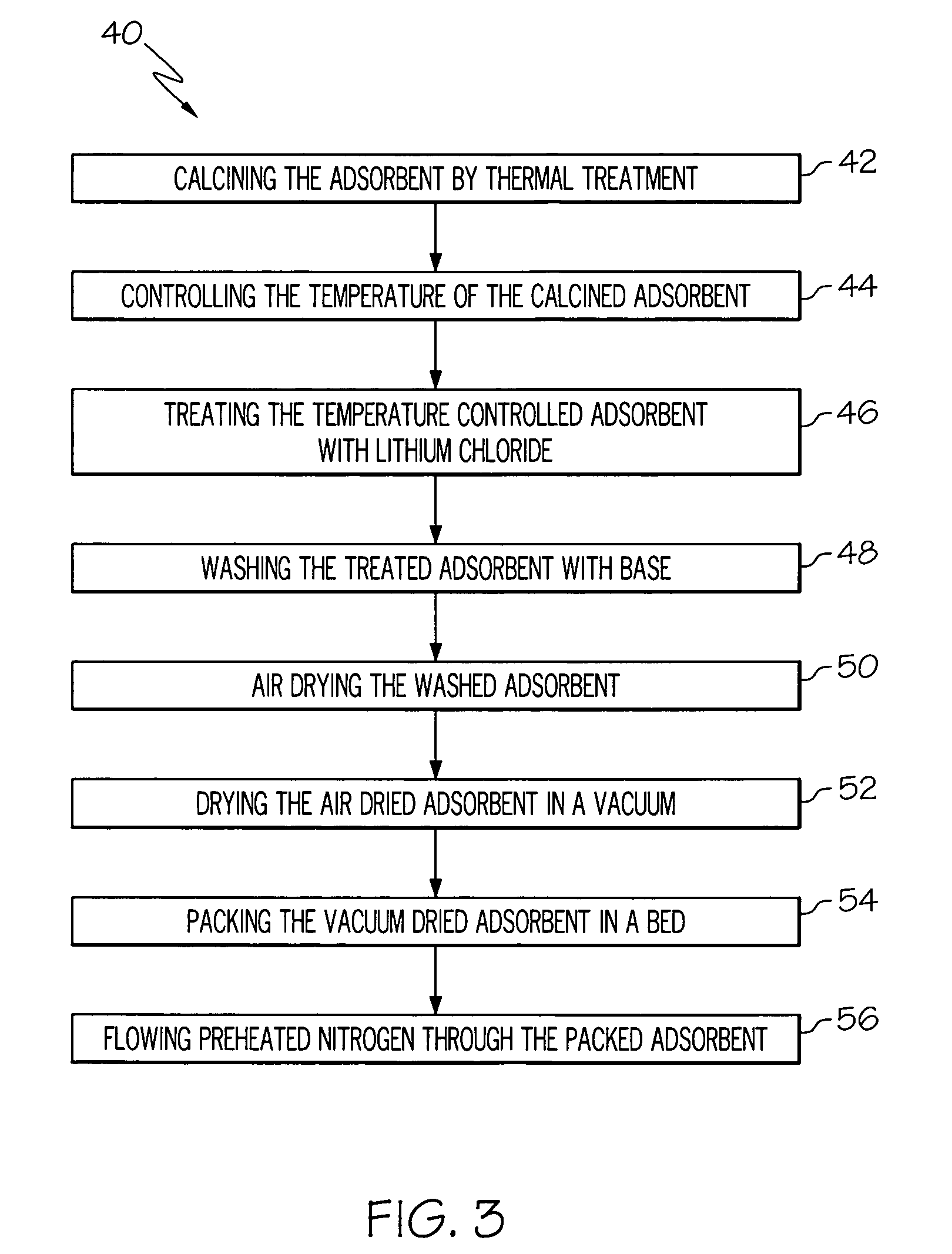
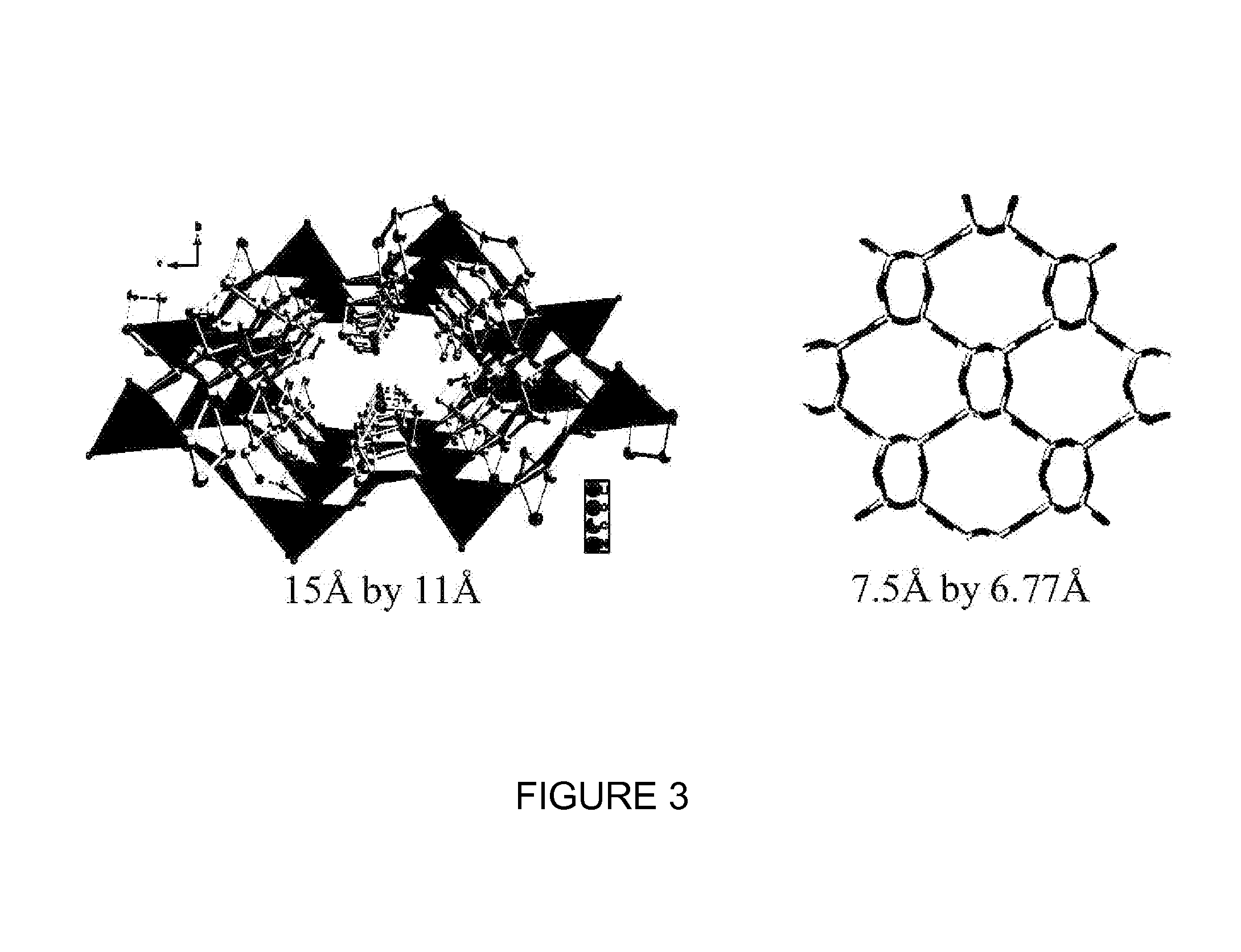
A high capacity adsorbent may be used for enriching oxygen concentration in an air stream. Such a high capacity adsorbent may be from about 2 to about 3 times lighter relative to the currently available technology. Furthermore, the high capacity adsorbent is readily capable of regeneration after deactivation by water vapor. Unlike current available immobilization technology in which clay binder was used to bind 13X zeolite and additional 10% organic binder was used to immobilize beads, the adsorbents of the present invention may be made using just an organic binder, thereby reducing pore spoilage caused by the clay binder. Further unlike conventional adsorbents, which may use sodium as its cation, the adsorbent of the present invention uses a lithium cation, thereby resulting in enhanced nitrogen adsorption performance.
Owner:HONEYWELL INT INC
Material for use as electrolyte, lithium secondary battery electrolyte, lithium secondary battery employing the same, and novel lithium salt
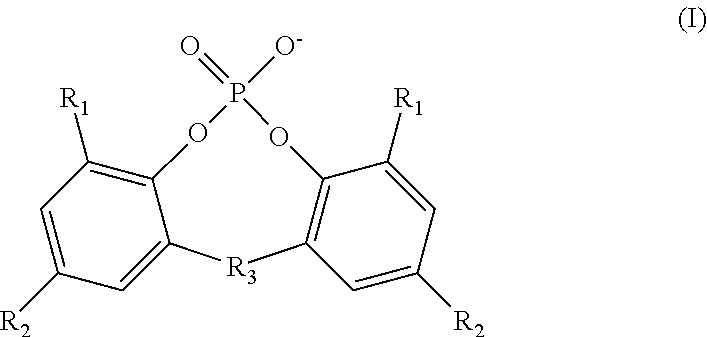
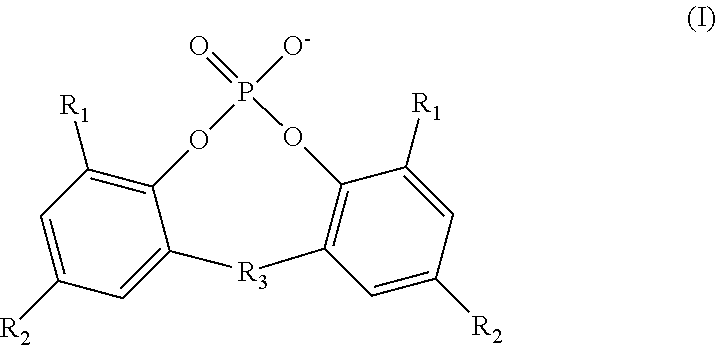
InactiveUS20130089777A1Improve electrochemical performanceImprove securityHybrid capacitor electrolytesPhosphorus halides/oxyhalidesLithium CationPhosphoric acid
An inventive electrolyte material contains a lithium salt comprising the following components (A1) and (B), or contains the following components (A1), (A2) and (B):(A1) a lithium cation;(A2) an organic cation; and(B) a cyanofluorophosphate anion represented by the following general formula (1):−P(CN)nF6-n (1)wherein n is an integer of 1 to 5. The inventive electrolyte material is excellent in electrochemical properties, i.e., has a higher electrical conductivity and a higher oxidation potential, and is capable of forming an electrode protection film, so that a highly safe lithium secondary battery can be provided.
Owner:THE NIPPON SYNTHETIC CHEM IND CO LTD
On-board oxygen production system for aircraft, in particular long-range aircraft
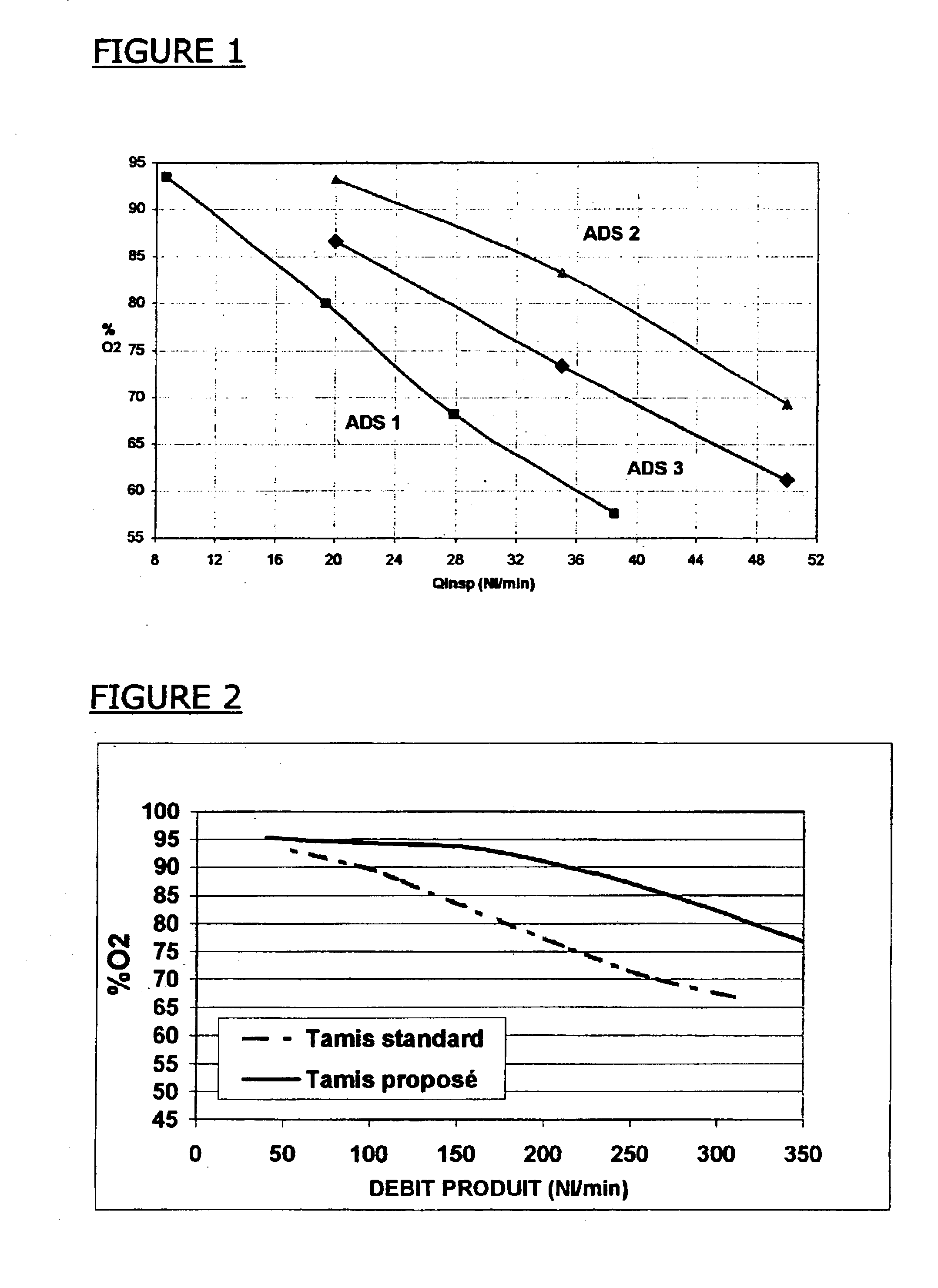
Method and system on board an aircraft for the production of an oxygen-enriched gas stream from an oxygen / nitrogen gas mixture, particularly air, comprising at least one adsorber containing at least one adsorbent for adsorbing at least some of the nitrogen molecules contained in the oxygen / nitrogen feed mixture, characterized in that the adsorbent comprises a faujasite-type zeolite, having a Si / Al ratio of 1 to 1.50, exchanged to at least 80% with lithium cations. Aircraft equipped with such a system, in particular an airliner, especially an airliner of the long-range, large-capacity type.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Lithium cationic single-ion conducting filler-containing composite polymer electrolyte for lithium secondary battery and method of manufacturing the same
ActiveUS7399556B2Excellent high rate discharge characteristicImprove ionic conductivityNon-metal conductorsSolid electrolytesHigh ratePolymer science
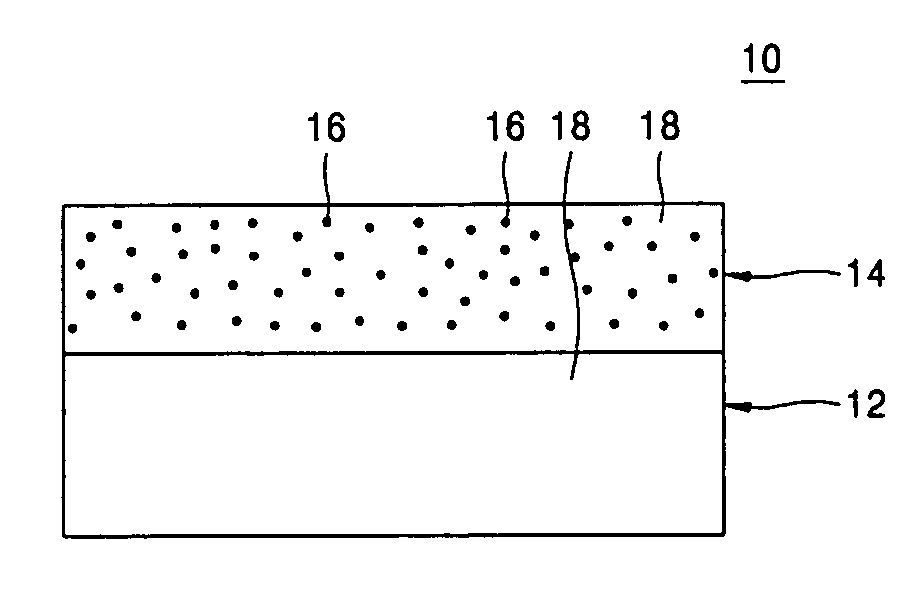
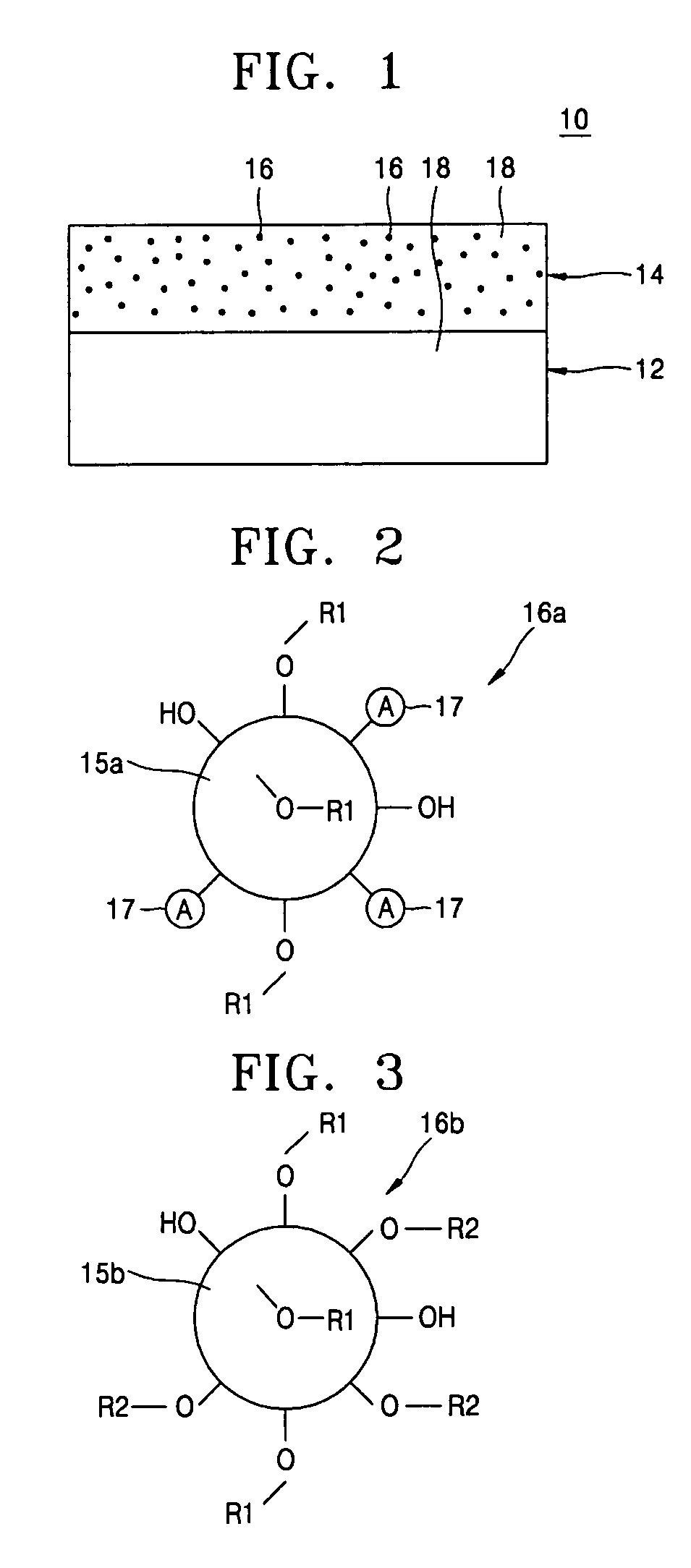
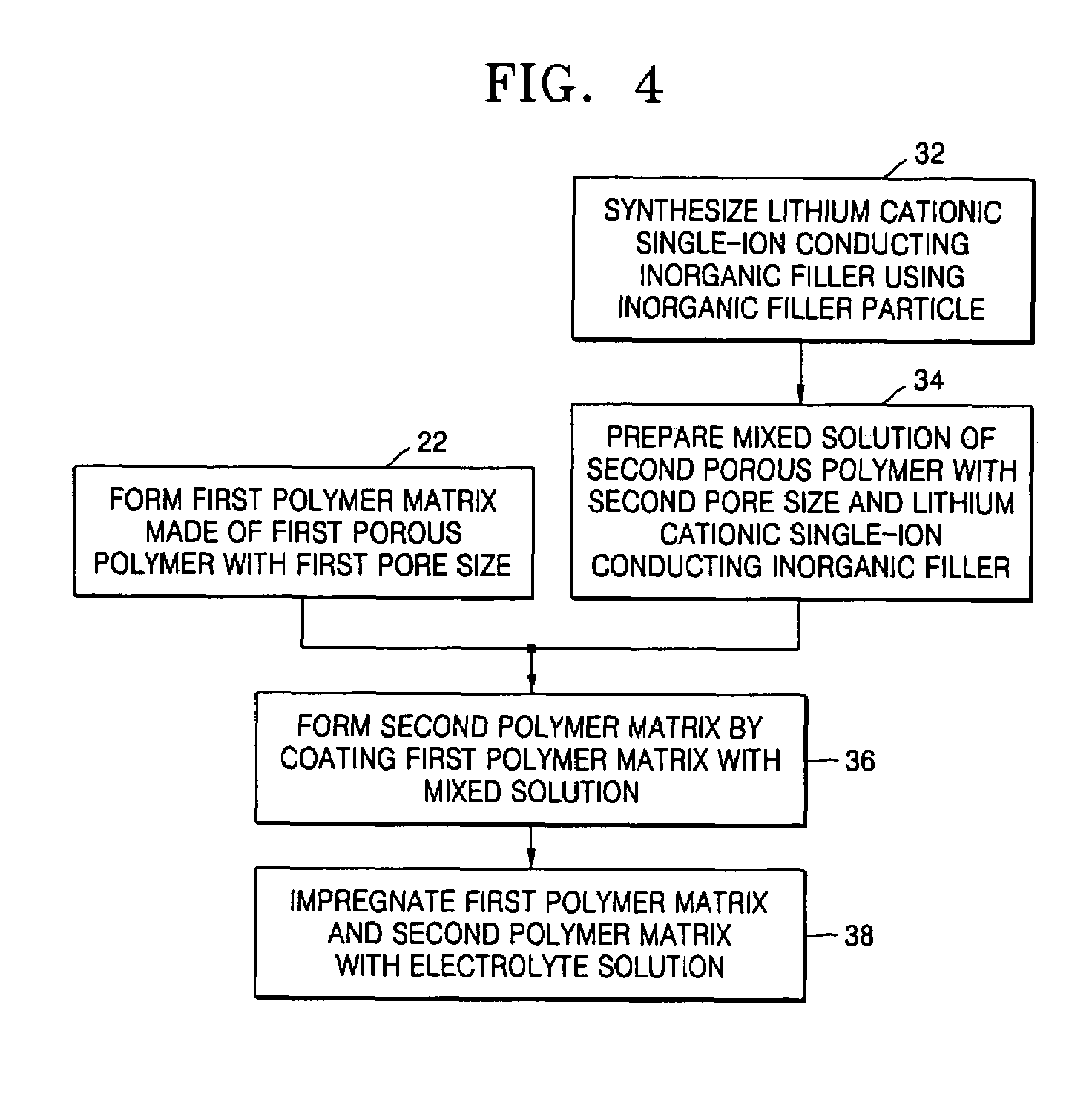
Provided are a composite polymer electrolyte for a lithium secondary battery in which a composite polymer matrix multi-layer structure composed of a plurality of polymer matrices with different pore sizes is impregnated with an electrolyte solution, and a method of manufacturing the same. Among the polymer matrices, a microporous polymer matrix with a smaller pore size contains a lithium cationic single-ion conducting inorganic filler, thereby enhancing ionic conductivity, the distribution uniformity of the impregnated electrolyte solution, and maintenance characteristics. The microporous polymer matrix containing the lithium cationic single-ion conducting inorganic filler is coated on a surface of a porous polymer matrix to form the composite polymer matrix multi-layer structure, which is then impregnated with the electrolyte solution, to manufacture the composite polymer electrolyte. The composite polymer electrolyte is used in a unit battery. The composite polymer matrix structure can increase mechanical properties. The introduction of the lithium cationic single-ion conducting inorganic filler can provide excellent ionic conductivity and high rate discharge characteristics.
Owner:ELECTRONICS & TELECOMM RES INST
Lithium sulfur secondary battery
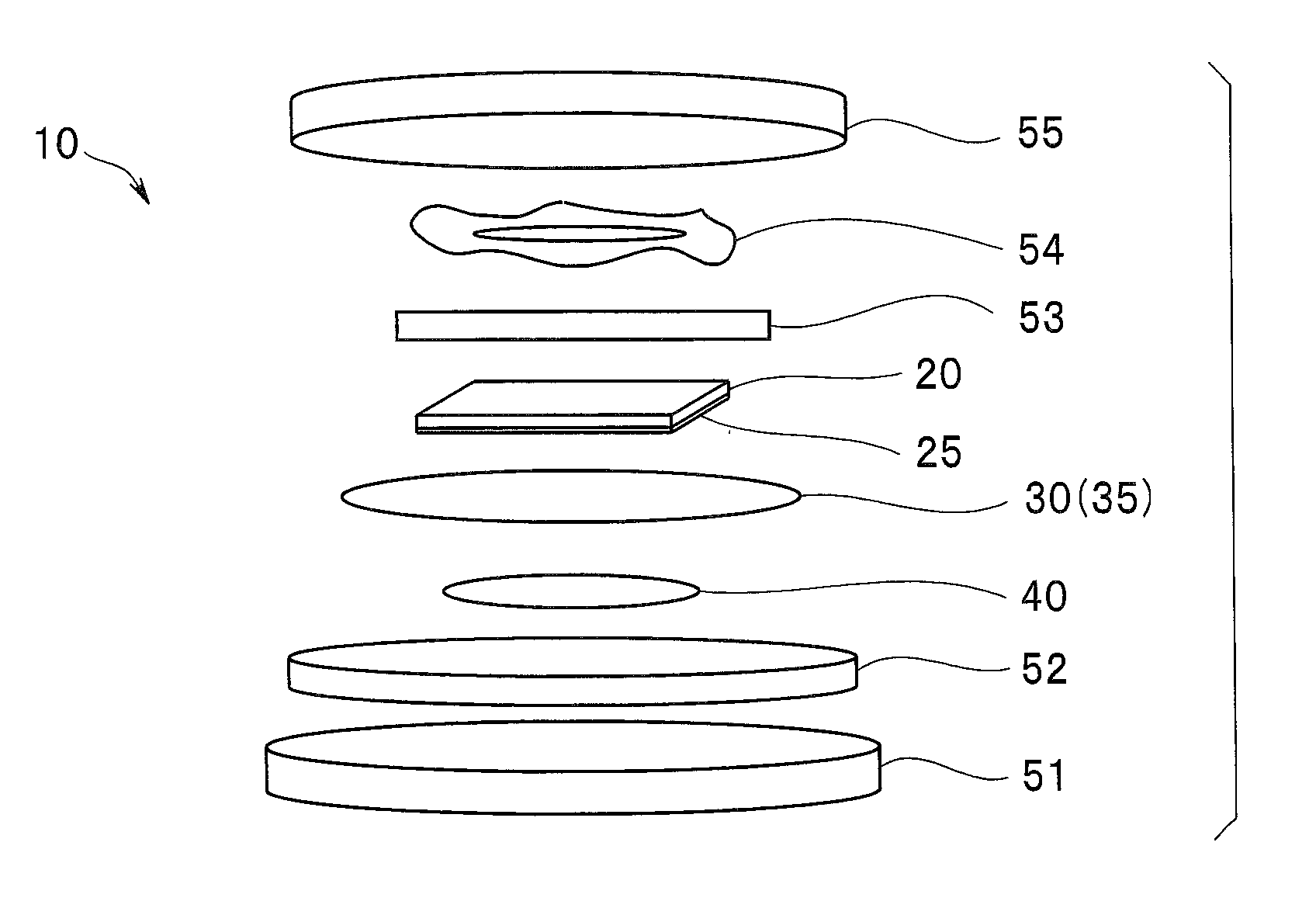
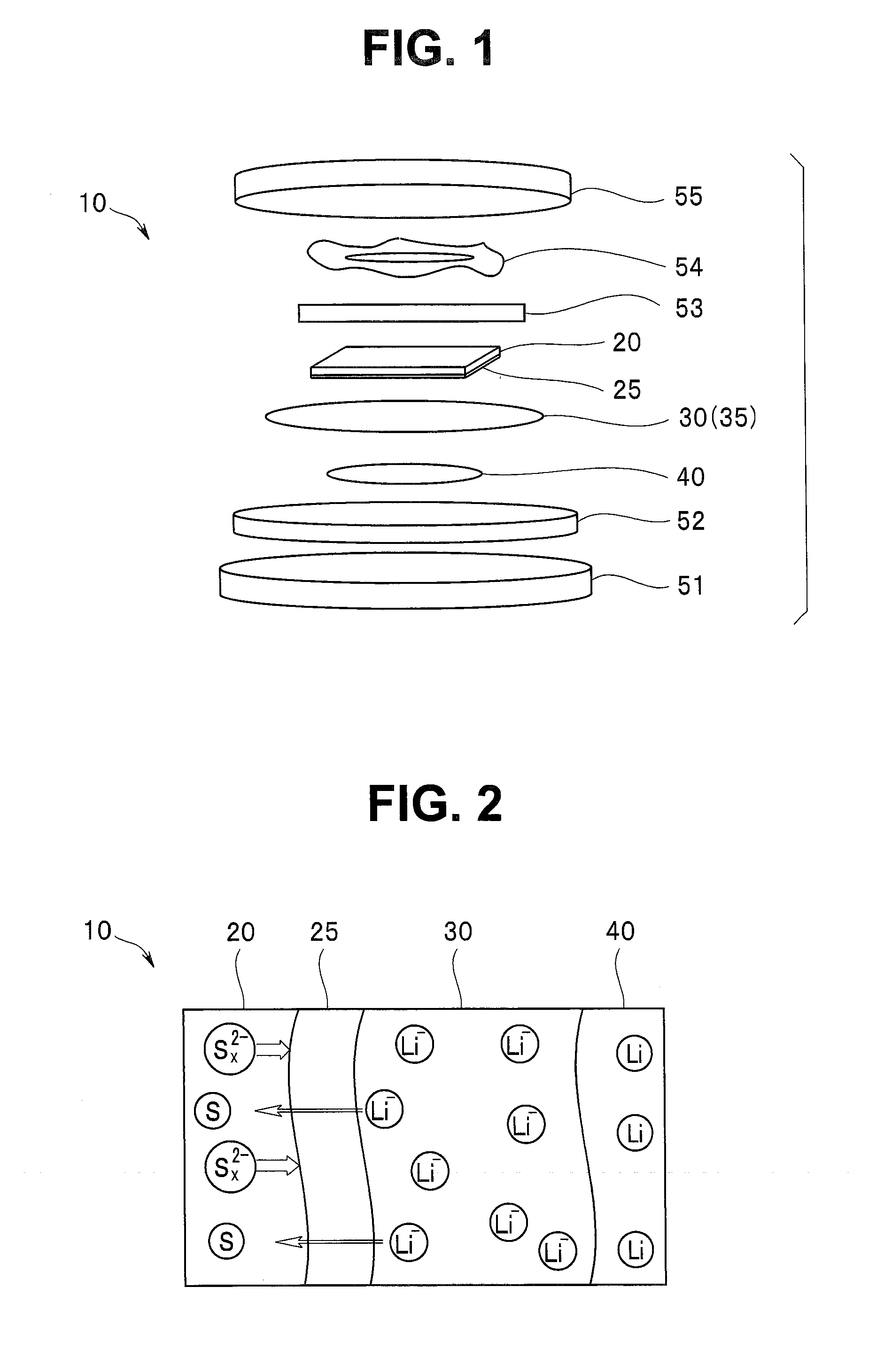
A lithium sulfur secondary battery includes a positive electrode containing a sulfur-based positive electrode active substance, an electrolytic solution, a negative electrode containing a negative electrode active substance that occludes and releases lithium, and a polymeric film that covers a surface of the positive electrode and allows lithium cations to pass but does not allow polysulfide anions to pass.
Owner:WASEDA UNIV
Metal oxide structures, devices & fabrication methods
Metal oxide structures, devices, and fabrication methods are provided. In addition, applications of such structures, devices, and methods are provided. In some embodiments, an oxide material can include a substrate and a single-crystal epitaxial layer of an oxide composition disposed on a surface of the substrate, where the oxide composition is represented by ABO2 such that A is a lithium cation, B is a cation selected from the group consisting of trivalent transition metal cations, trivalent lanthanide cations, trivalent actinide cations, trivalent p-block cations, and combinations thereof, and O is an oxygen anion. The unit cell of the crystal structure of the oxide composition can be characterized by first layer of a plane of lithium cations and a second layer of a plurality of edge-sharing octahedra having a B cation positioned in a center of each octahedron and an oxygen anion at each corner of each octahedron. The first layer and the second layer of the unit cell are alternatingly stacked along one axis of the unit cell. Other aspects, features, and embodiments are also claimed and described.
Owner:GEORGIA TECH RES CORP
Lithium compositions
2:1 cocrystals of amino acids and Li+ salts crystallize from hot water to afford water stable cationic networks based upon tetrahedral lithium cations: bilayered square grids, a lithium zeolitic metal-organic material (LiZMOM) and several lithium diamondoid metal-organic materials (LiDMOMs). The compositions may be used as a pharmaceutical for the treatment of suicidality and other disorders that require lithium to penetrate the blood brain barrier and exert therapeutic effects in the CNS. Advantageously, the novel cocrystal forms described herein may be used to lower the oral dose required to achieve therapeutic concentrations of lithium in the brain, thus reducing the peripheral toxicity and potentially broadening the therapeutic index in comparison to conventional lithium forms.
Owner:UNIV OF SOUTH FLORIDA
Preparation of ion exchanged polymer bound nitrogen adsorbent
ActiveUS20070209509A1Increase the air concentrationIncrease oxygen concentrationGas treatmentMolecular sieve catalystsWater vaporSorbent
A high capacity adsorbent may be used for enriching oxygen concentration in an air stream. Such a high capacity adsorbent may be from about 2 to about 3 times lighter relative to the currently available technology. Furthermore, the high capacity adsorbent is readily capable of regeneration after deactivation by water vapor. Unlike current available immobilization technology in which clay binder was used to bind 13X zeolite and additional 10% organic binder was used to immobilize beads, the adsorbents of the present invention may be made using just an organic binder, thereby reducing pore spoilage caused by the clay binder. Further unlike conventional adsorbents, which may use sodium as its cation, the adsorbent of the present invention uses a lithium cation, thereby resulting in enhanced nitrogen adsorption performance.
Owner:HONEYWELL INT INC
Lithium niobite compositions, syntheses, devices, and structures
Metal oxide structures, devices, and fabrication methods are provided. In addition, applications of such structures, devices, and methods are provided. In some embodiments, an oxide material can include a substrate and a single-crystal epitaxial layer of an oxide composition disposed on a surface of the substrate, where the oxide composition is represented by ABO2 such that A is a lithium cation, B is a cation selected from the group consisting of trivalent transition metal cations, trivalent lanthanide cations, trivalent actinide cations, trivalent p-block cations, and combinations thereof, and O is an oxygen anion. The ABO2 can be a high purity ABO2, with less than 1 atom % each of sodium, carbon, boron, and fluorine. The ABO2 can be prepared by a liquid phase electro-epitaxy using a molten solution of a metal oxide and LiBO2.
Owner:GEORGIA TECH RES CORP
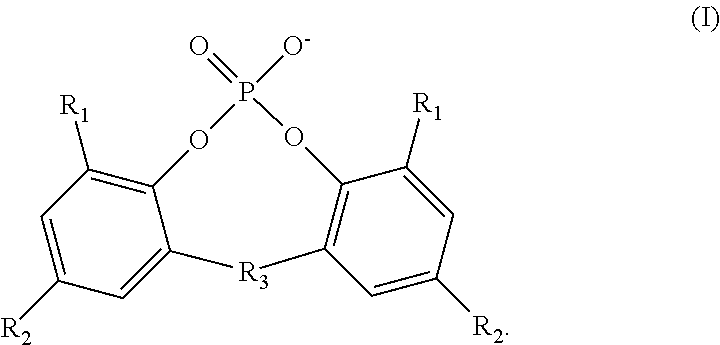
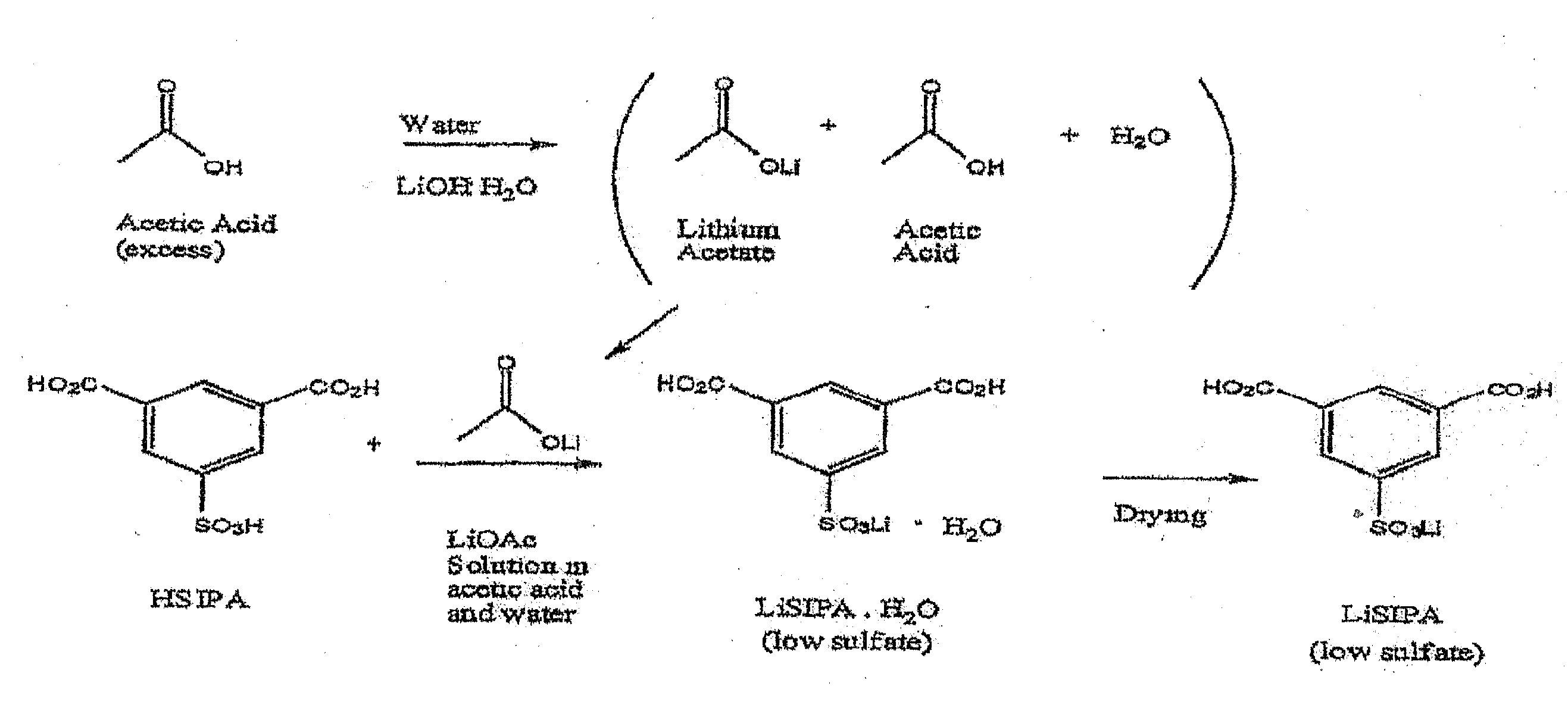
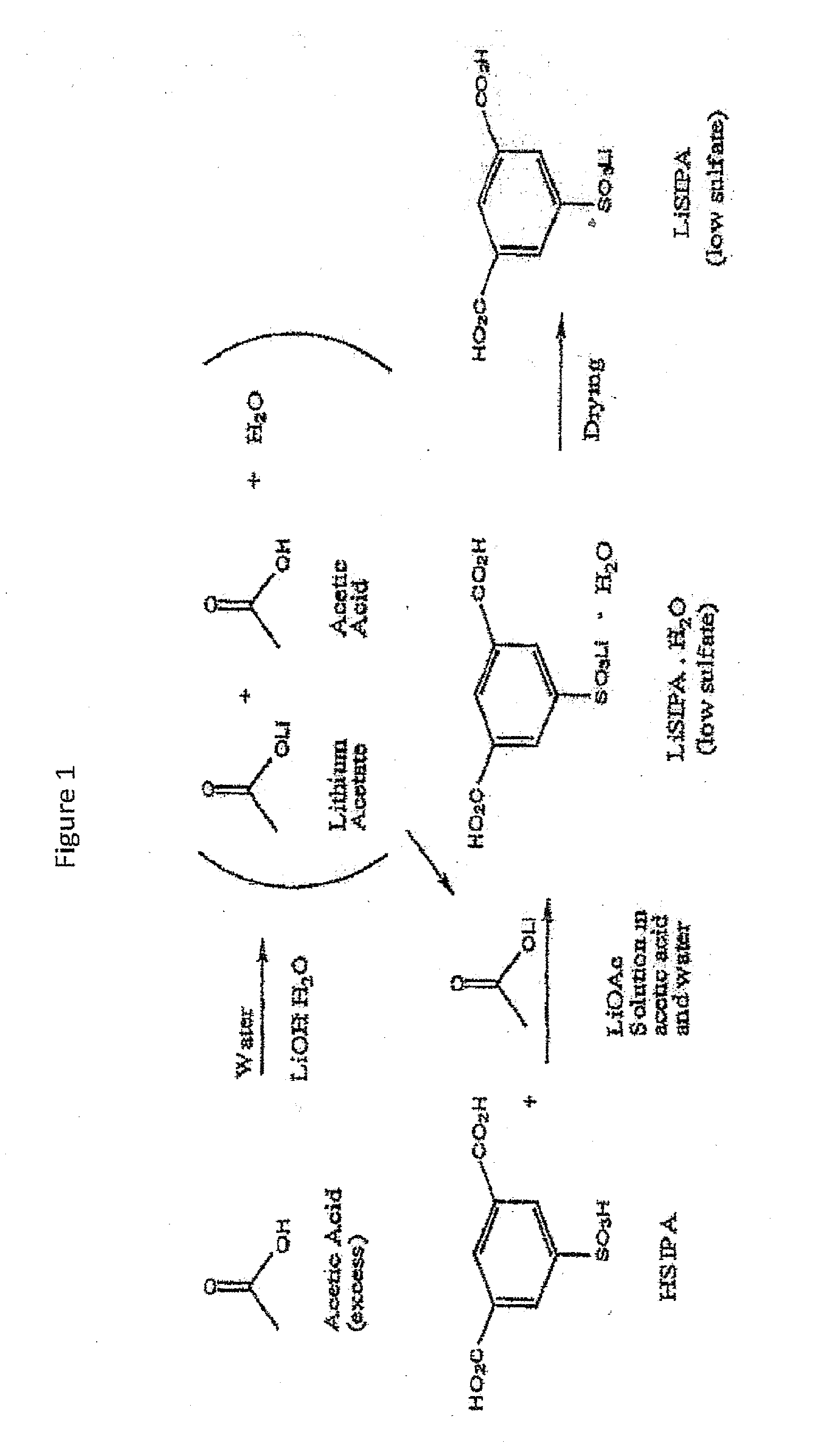
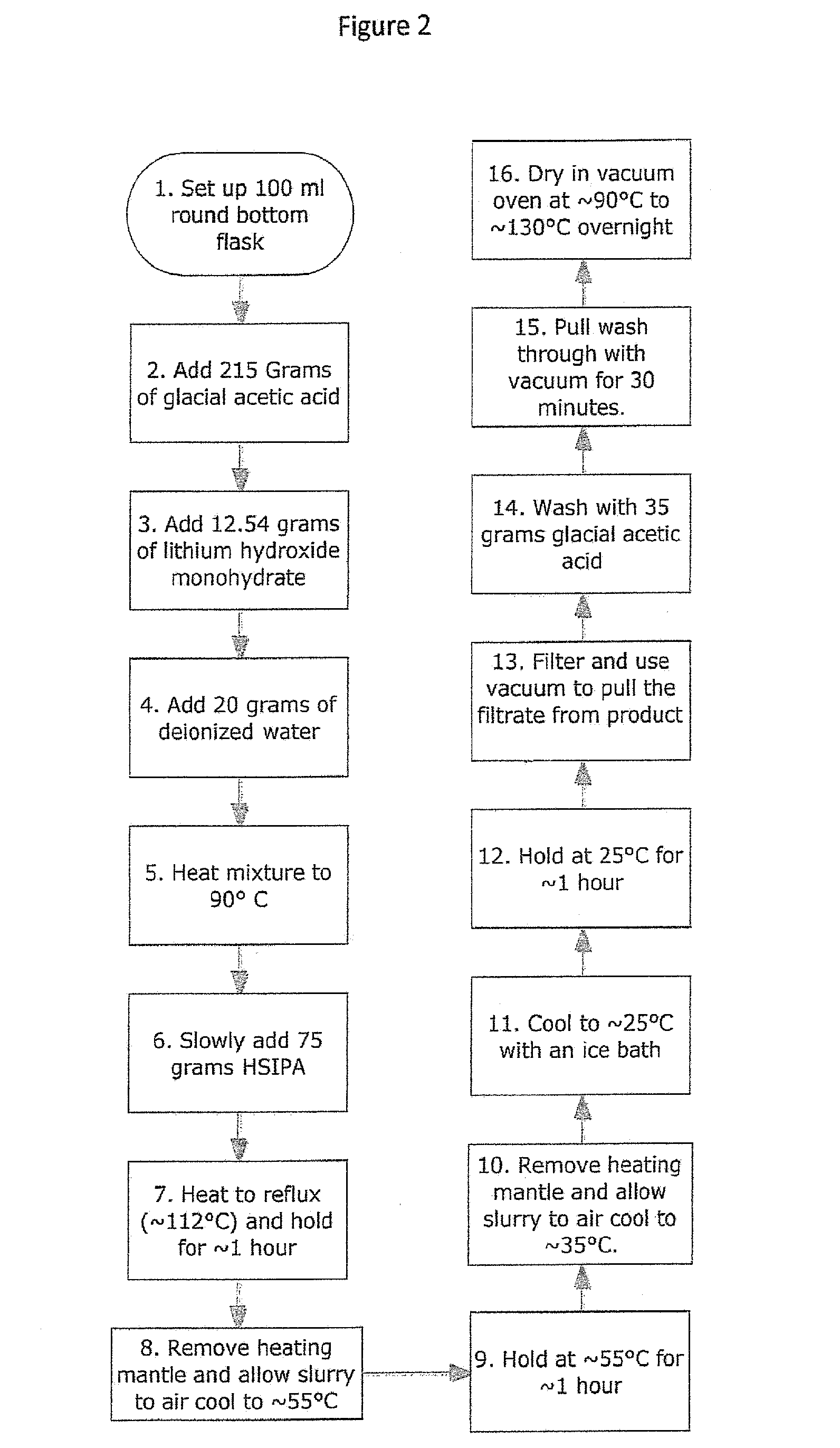
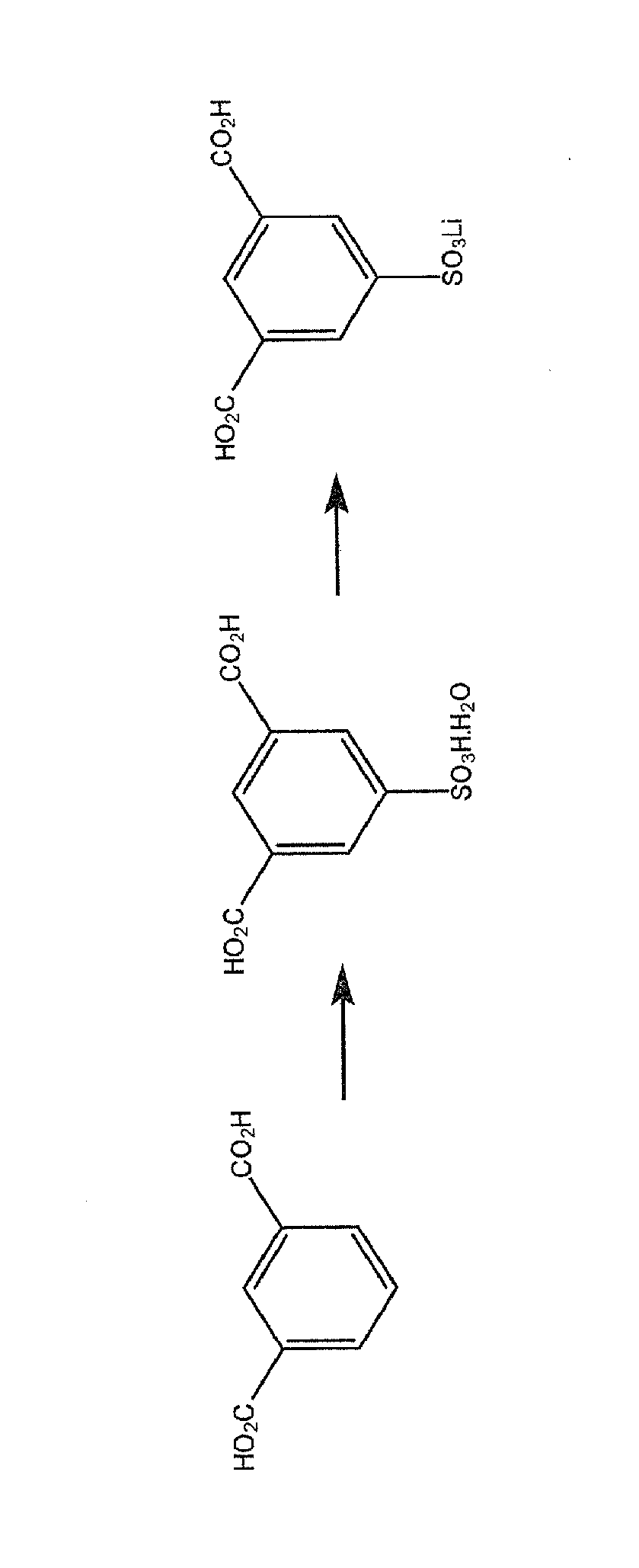
Use of an acetic acid wash to prepare low-sulfate 5-sulfoisophthalic acid, mono-lithium salt
There is disclosed a process for making a mono-lithium salt of 5-sulfoisophthalic acid (LiSIPA) having less than 500 ppm sulfate. The process uses a reaction mixture of water, a lithium cation producing compound, and 5-sulfoisophthalic acid. The reaction mixture is heated to reflux, cooled, filtered and washed with acetic acid to obtain a high quality LiSIPA having less than 500 ppm sulfate. Also disclosed is a high quality, non-purified reaction product containing a mono-lithium salt of 5-sulfoisophthalic acid and having less than 500 ppm sulfate.
Owner:FUTUREFUEL CHEM
Additive compositions and polymer compositions containing the same
An additive composition comprises phosphate ester anions conforming to a specified structure, aromatic carboxylate anions, optionally cycloaliphatic dicarboxylate anions, aluminum (III) cations, sodium cations, optionally calcium cations, optionally lithium cations, and optionally zinc (II) cations. The anions are present in the additive composition in specified molar percentages. The cations are also present in the additive composition in specified molar percentages.
Owner:MILLIKEN & CO
Use of an acetic acid/water solvent mixture for the preparation of low-sulfate 5-sulfoisophthalic acid, mono-lithium salt from 5-ulfoisophthalic acid
There is disclosed a process for making a mono-lithium salt of 5-sulfoisophthalic acid (LiSIPA) having less than 200 ppm sulfate. The process uses a reaction mixture of acetic acid, water, a lithium cation producing compound, and 5-sulfoisophthalic acid. The reaction mixture is heated to reflux, cooled, filtered and washed to obtain a high quality LiSIPA having less than 200 ppm sulfate. Also disclosed is a high quality mono-lithium salt of 5-sulfoisophthalic acid having less than 200 ppm sulfate.
Owner:FUTUREFUEL CHEM
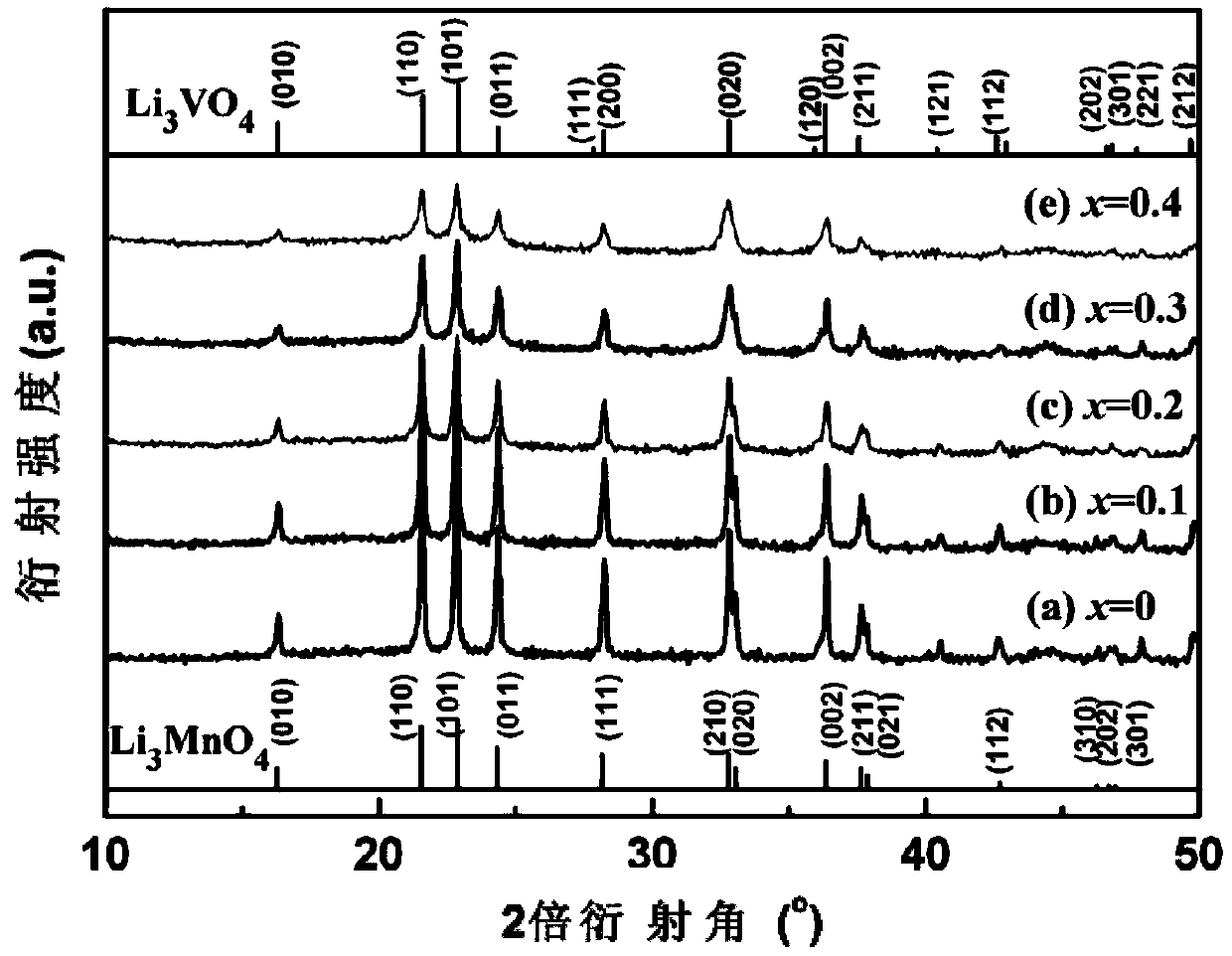
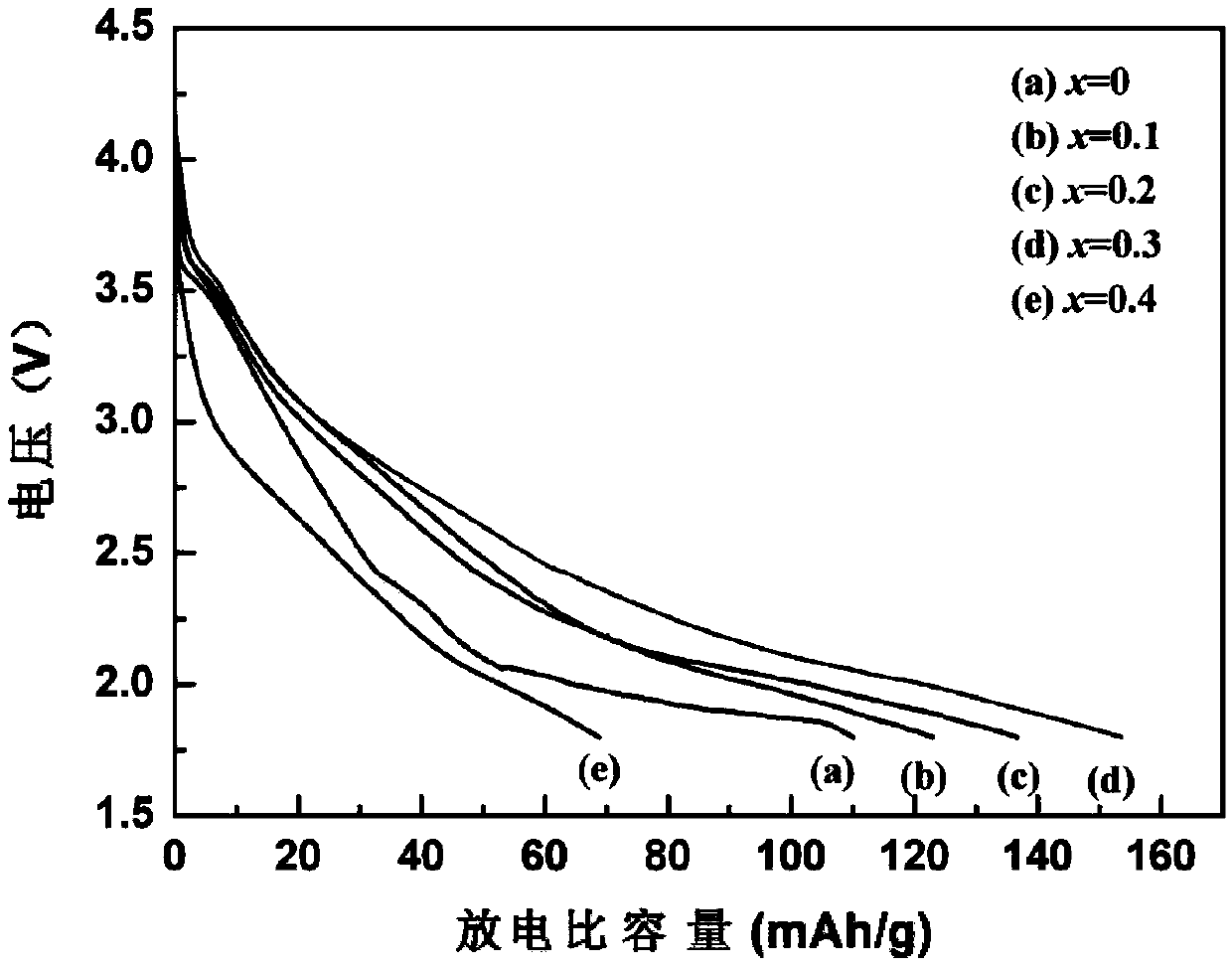
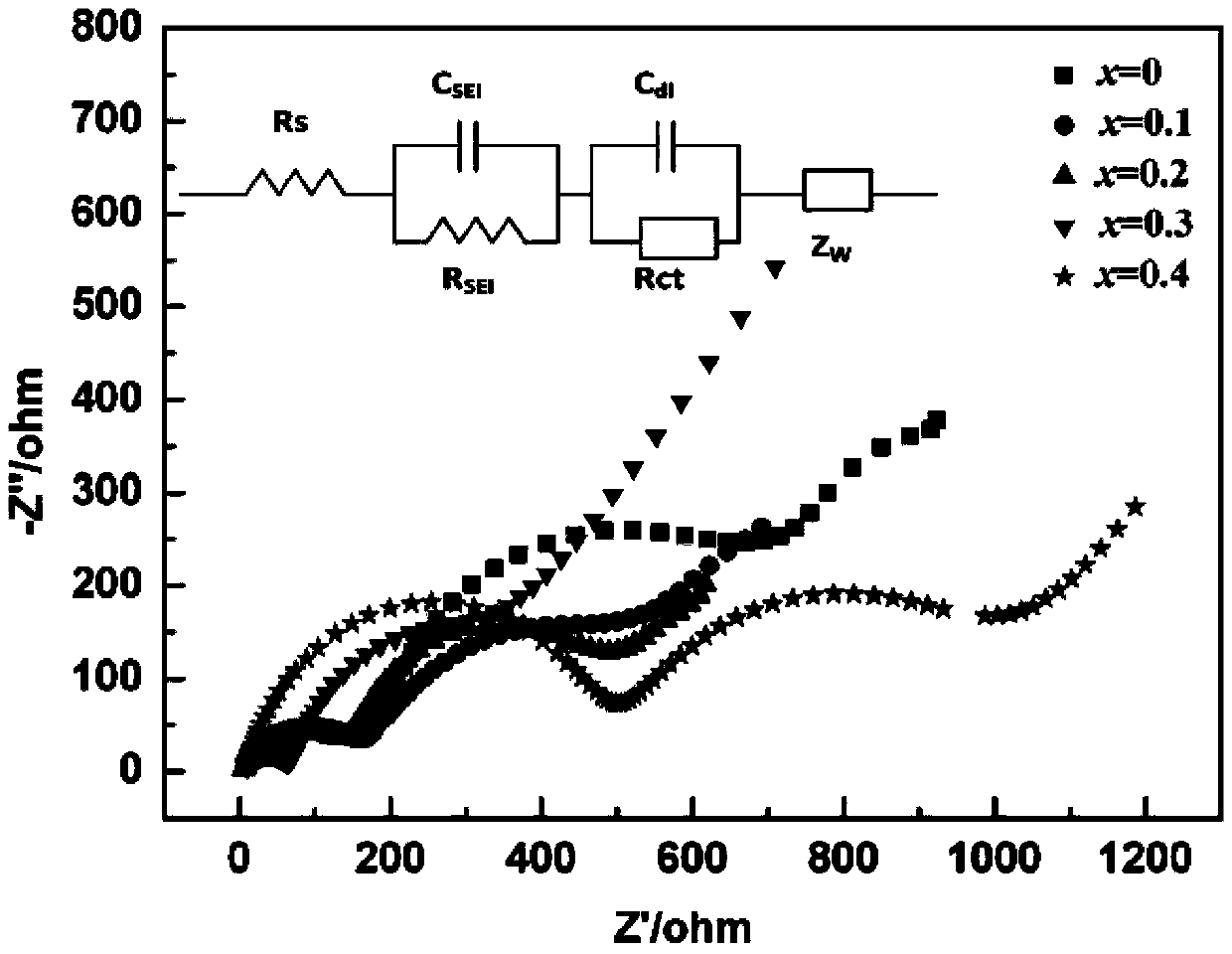
Lithium ion battery Li3MnO4 positive material doped with vanadium and preparation method thereof
InactiveCN103390747AImprove discharge performanceLower impedanceCell electrodesPhysical chemistryLithium Cation
The invention relates to a lithium ion battery Li3MnO4 positive material doped with vanadium and a preparation method thereof. The chemical formula of the lithium ion battery Li3MnO4 positive material is Li3Mn(1-x)VxO4, wherein x is more than or equal to 0.1 and smaller than or equal to 0.4. The preparation method comprises the following steps: 1) converting a KMnO4 solution into a LiMnO4 solution through lithium cation exchange resin, carrying out vacuum drying on the LiMnO4 solution to obtain LiMnO4.3H2O powder; 2) according to the stoichiometric ratio, weighing a lithium source, a manganese source and a vanadium source, fully mixing and grinding, calcining, grinding for one time at an interval being every 10DEG C in a range from 70 to 120DEG C, carrying out heat preservation for 1 hour after grinding at 125DEG C; raising the temperature to 170 DEG C and carrying out the heat preservation for 2.5 hours. The preparation method disclosed by the invention has the characteristics that (1) the preparation method has a simple process and a short reaction period, saves the cost and is easy to control; (2) through the doping of appropriate amount of the vanadium, the impedance is reduced to a certain extent, so that the discharge performance of the material is improved.
Owner:WUHAN UNIV OF TECH
Systems and methods for treating a metal substrate
PendingCN109563629AFilling pastesMetallic material coating processesPhysical chemistryLithium Cation
Disclosed is a conversion composition containing a trivalent chromium cation in an amount of 0.001 g / L to 20 g / L. Also disclosed is a system for treating a metal substrate that includes the conversioncomposition and a sealing composition comprising a lithium cation. Also disclosed is a method for treating a metal substrate that includes contacting at least a portion of a surface of the substratewith the conversion composition and then contacting at least a portion of the surface of the substrate with the sealing composition. Also disclosed is a substrate obtainable by treatment with the system and / or obtainable by the method of treating.
Owner:PRC DE SOTO INT INC
Preparation method of lithium/sulfur rechargeable battery electrolyte
The invention discloses a preparation method of a lithium / sulfur rechargeable battery electrolyte. An inorganic salt containing lithium cations or an organic salt is used as the electrolyte to be added into an organic solvent, constant-temperature stirring is performed for sufficient dissolution, the concentration of the electrolyte is controlled within the range of 0.01-6 mol / L, elemental sulfur and elemental lithium are added into the organic solvent, constant-temperature stirring is performed to achieve sufficient reaction and dissolution, standing is performed and then a supernate is taken to be used as the electrolyte. The electrolyte can stably exist between positive and negative electrodes of a battery, has the high lithium ion concentration and high conductivity, can sufficiently utilize positive and negative active materials, and can remarkably improve the cycle performance and the coulombic efficiency of the lithium / sulfur battery.
Owner:SHANDONG YUHUANG NEW ENERGY TECH
Large-particle single-crystal lithium cobalt oxide for lithium ion battery, and cation doping preparation method thereof
ActiveCN111663182ALarge particle sizeSmall specific surface areaPolycrystalline material growthFrom solid stateElectrolytic agentLithium Cation
The invention relates to large-particle single-crystal lithium cobalt oxide for a lithium ion battery, and a cation doping preparation method thereof. The preparation method comprises the following steps: mixing a precursor Co(OH)2 or a cation-doped precursor [Co1-xMx](OH)2 with a lithium source, sintering to form spinel phase Li2y[Co1-xMx]2O4 small-particle single crystals, mixing and sintering the small-particle single crystals and spinel-phase Co3O4 to promote fusion of the two spinel phase particles to prepare spinel phase Li2y-2m[Co1-x+nMx-n]2O4 large-particle single crystals, supplementing a lithium source, and continuously sintering to prepare the cation-doped large-particle single-crystal lithium cobalt oxide. Cationic doping or co-doping can effectively inhibit conversion from a hexagonal phase to a monoclinic phase under high voltage of lithium cobalt oxide, and formation of large-particle single crystals can reduce side reaction between an electrode and an electrolyte underhigh voltage of lithium cobalt oxide, so that the specific discharge capacity and the structural stability of lithium cobalt oxide are improved.
Owner:UNIV OF JINAN
Use of an acetic acid wash to prepare low-sulfate 5-sulfoisophthalic acid, mono-lithium salt
There is disclosed a process for making a mono-lithium salt of 5-sulfoisophthalic acid (LiSIPA) having less than 500 ppm sulfate. The process uses a reaction mixture of water, a lithium cation producing compound, and 5-sulfoisophthalic acid. The reaction mixture is heated to reflux, cooled, filtered and washed with acetic acid to obtain a high quality LiSIPA having less than 500 ppm sulfate. Also disclosed is a high quality, non-purified reaction product containing a mono-lithium salt of 5-sulfoisophthalic acid and having less than 500 ppm sulfate.
Owner:FUTUREFUEL CHEM
Improved rechargeable batteries and production thereof
PendingCN113169325AEasy to produceIncrease energy densityElectrolytesLi-accumulatorsElectrolytic agentElectrical battery
An electrochemical cell is disclosed comprising a carbonate : nitrile type solvent mixture based electrolyte. The electrolyte may comprise an alkali salt and / or at least one polymer additive. The alkali salt cation may be a lithium cation and / or the alkali salt anion may comprise an oxalato-borate group. The electrolyte may further comprise one or more electrolyte additives, which may be an SEI improving additive. The anode may comprise carbon. The anode may be an alkali metal anode.The operating voltage and energy density performance of the disclosed invention is at the same level as the performance of presently market- leading battery cells, thereby these disclosed improvements do not come at the expense of battery performance. The utility of the herein disclosed battery electrolyte allows stable cycling of advanced Li-ion battery electrodes, an extended operating temperature range and improves battery safety by making the electrolyte less reactive and volatile than conventional LiPF6 electrolyte salt in carbonate solvents.
Owner:BROADBIT BATTERIES OY
Material for use as electrolyte, lithium secondary battery electrolyte, lithium secondary battery employing the same, and novel lithium salt
InactiveUS8722243B2Improve electrochemical performanceImprove securityAlkaline accumulatorsHybrid capacitor electrolytesElectrical batteryLithium Cation
Owner:THE NIPPON SYNTHETIC CHEM IND CO LTD
High-nickel single-crystal ternary precursor and preparation method thereof
ActiveCN113373500AHigh crystallinityImprove cycle performancePolycrystalline material growthFrom normal temperature solutionsLithium CationPotassium
Provided are a high-nickel single-crystal ternary precursor and a preparation method thereof. The preparation method comprises the following steps of: preparing a mixed salt solution from Ni < 2 + >, Co < 2 + >, Mn < 2 + > and potassium trioxalato (III) acid according to the general formula, the molar ratio of Mn < 3 + > to Mn < 2 + > of the potassium trioxalato (III) acid is 2-4, primary particles of secondary particles are refined, and the specific surface area is increased. adding the mixed salt solution, a precipitant solution and a complexing agent solution into a reaction kettle, and reacting to generate high-nickel single-crystal ternary precursor slurry; and carrying out filter pressing, washing and drying to obtain the high-nickel single crystal ternary precursor. The primary particles are sheet-shaped, and the thickness is 50-110 nm. The technical problems of material capacity attenuation and inappropriate primary particle thickness caused by mixed arrangement of nickel and lithium cations in the subsequent mixed calcining process of the existing high-nickel single crystal ternary precursor and a lithium source are solved.
Owner:南通金通储能动力新材料有限公司
Additive compositions and polymer compositions containing the same
An additive composition comprises phosphate ester anions conforming to a specified structure, aromatic carboxylate anions, optionally cycloaliphatic dicarboxylate anions, aluminum (III) cations, sodium cations, optionally calcium cations, optionally lithium cations, and optionally zinc (II) cations. The anions are present in the additive composition in specified molar percentages. The cations are also present in the additive composition in specified molar percentages.
Owner:MILLIKEN & CO
Sealing composition
Disclosed is a method of treating a substrate. The surface is contacted with a sealing composition comprising a lithium cation; and optionally, with conversion composition comprising a cation of a lanthanide, a Group IIIB, and / or a Group IVB metal. The conversion composition is applied to provide a film on the substrate surface resulting in a level of the lanthanide, Group IIIB metal, and / or GroupIV metal thereon of at least 100 counts greater than on a surface of a substrate that does not have the film thereon as measured by X-ray fluorescence (measured using X-Met 7500, Oxford Instruments;operating parameters 60 second timed assay, 15Kv, 45 microamp, filter 3, T(p) = 1.5 microseconds for lanthanides, Group IIIB metals, and Group IVB metals except zirconium; operating parameters 60 second timed assay, 40Kv, 10 microamp, filter 4, T(p) = 1.5 microseconds for zirconium). A substrate obtainable by the methods also is disclosed.
Owner:PRC DE SOTO INT INC
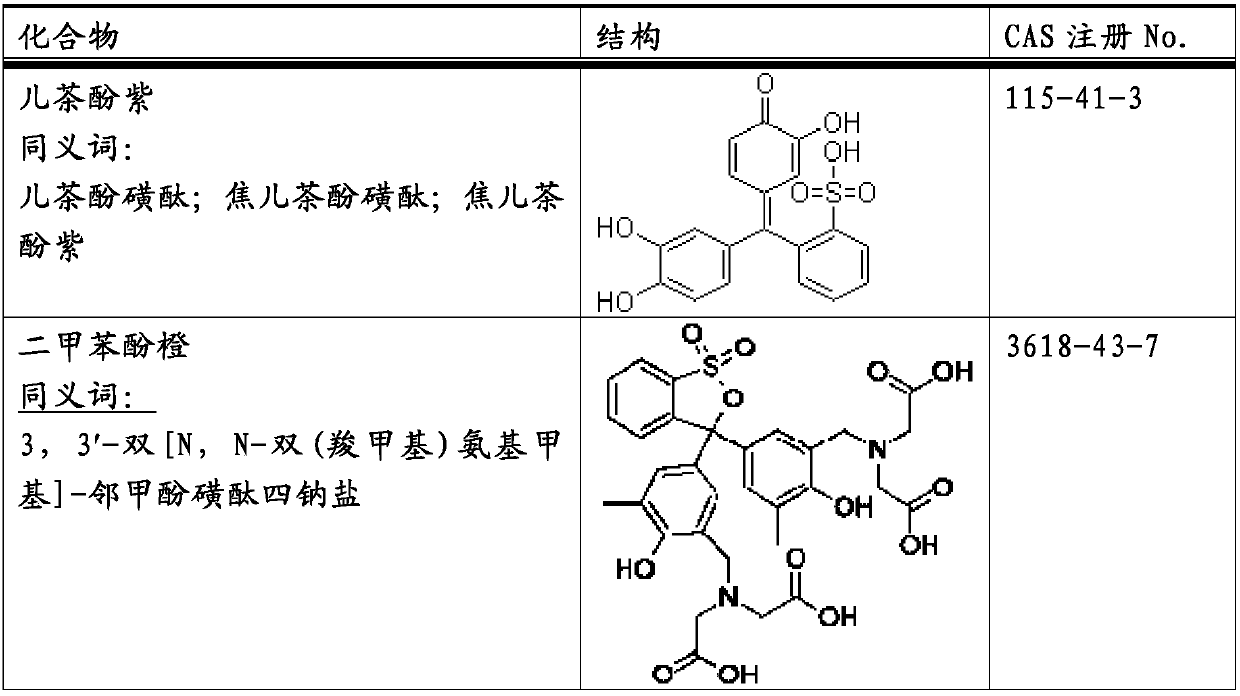
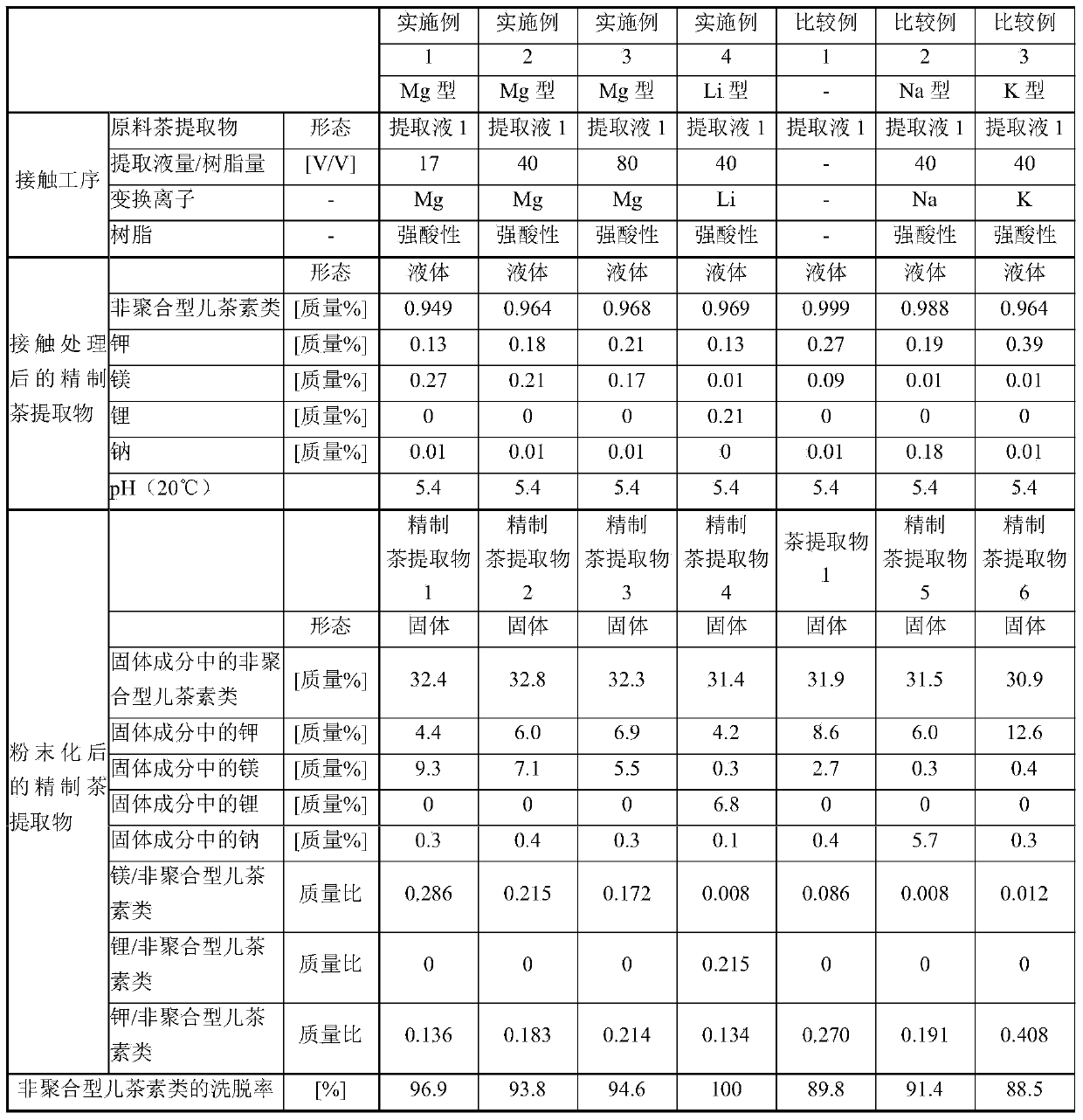
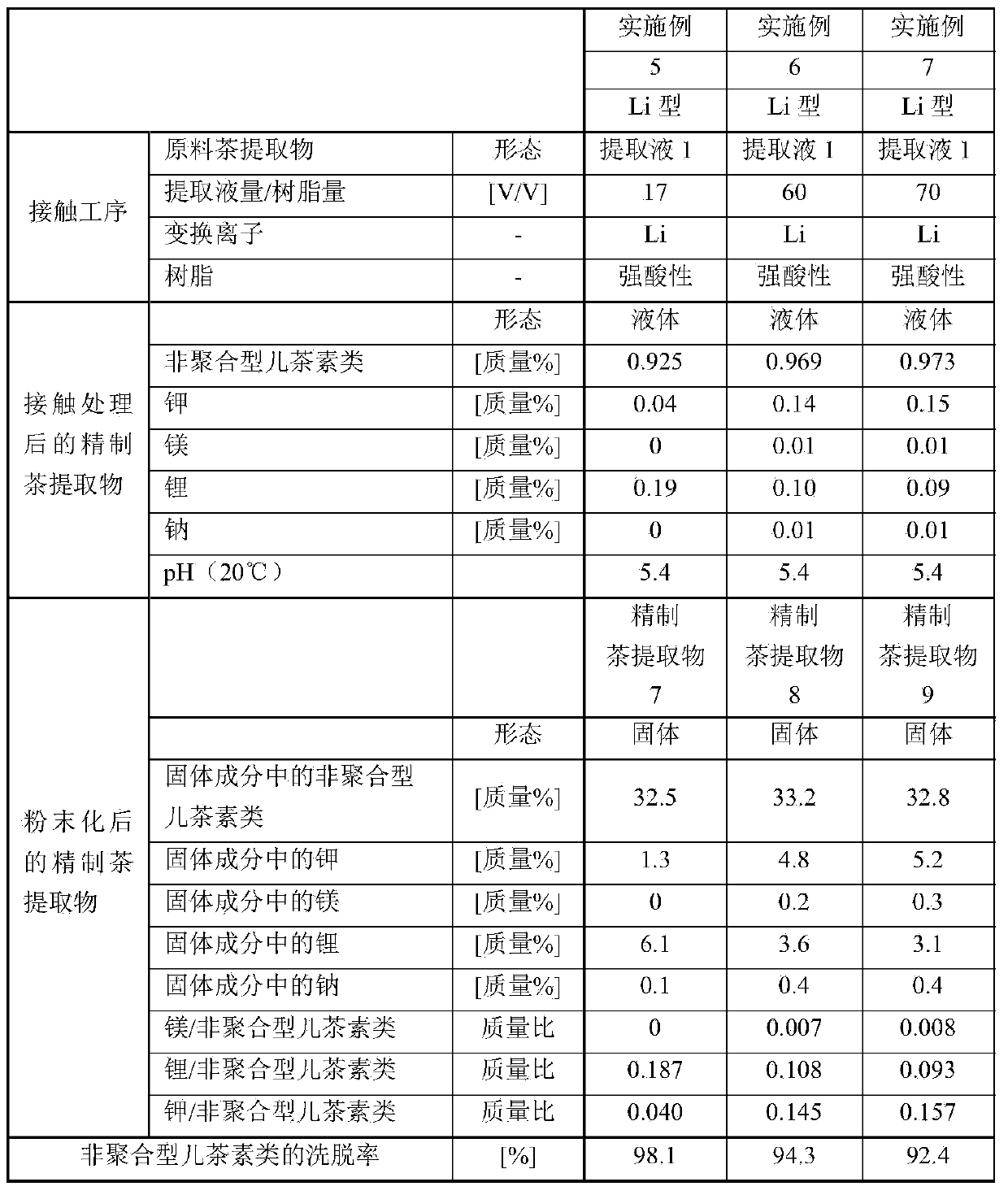
Method for producing purified tea extract
The present invention provides a method for producing a purified tea extract with which solubility of non-polymer catechins in solvents has been increased. This method for producing a purified tea extract involves bringing a tea extract into contact with a magnesium or lithium cation exchange resin.
Owner:KAO CORP
Improved rechargeable batteries and production thereof
PendingUS20220359914A1Increase energy densitySecondary cellsPositive electrodesElectrical batteryLithium Cation
An electrochemical cell is disclosed comprising a carbonate:nitrile type solvent mixture based electrolyte. The electrolyte may comprise an alkali salt and / or at least one polymer additive. The alkali salt cation may be a lithium cation and / or the alkali salt anion may comprise an oxalato-borate group. The electrolyte may further comprise one or more electrolyte additives, which may be an SEI improving additive. The anode may comprise carbon. The anode may be an alkali metal anode. The operating voltage and energy density performance of the disclosed invention is at the same level as the performance of presently market—leading battery cells, thereby these disclosed improvements do not come at the expense of battery performance. The utility of the herein disclosed battery electrolyte allows stable cycling of advanced Li-ion battery electrodes, an extended operating temperature range and improves battery safety by making the electrolyte less reactive and volatile than conventional LiPF6 electrolyte salt in carbonate solvents.
Owner:BROADBIT BATTERIES OY
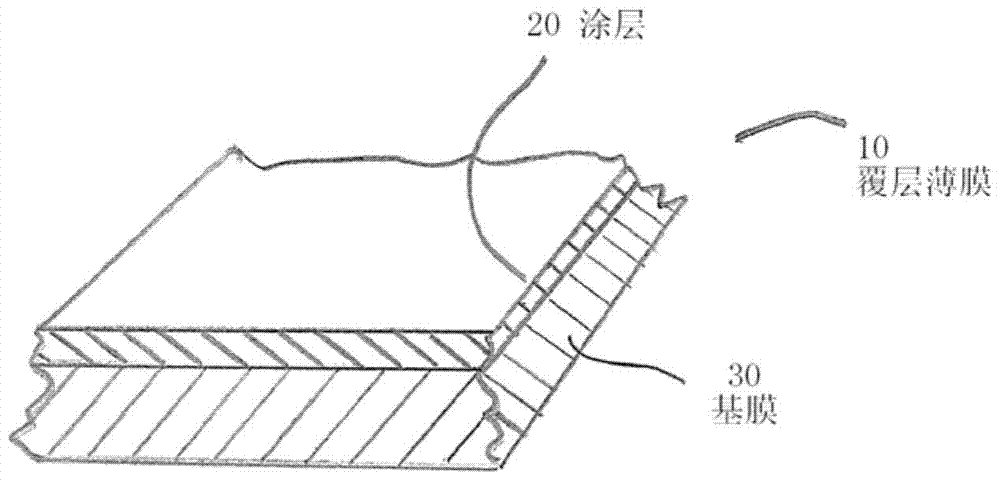
Barrier coatings for thin films and structures
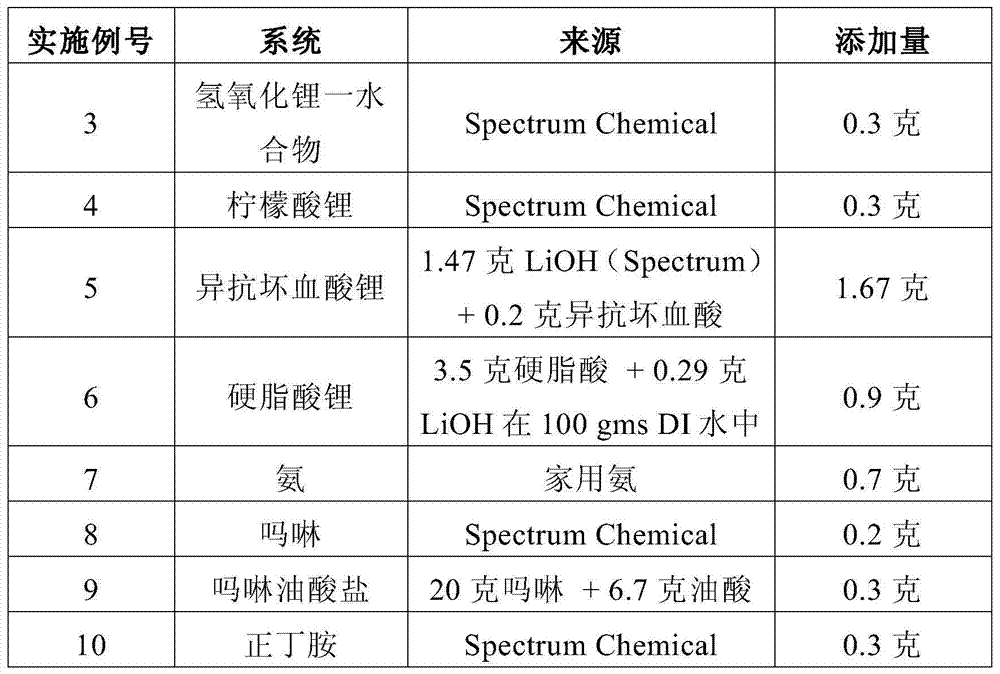
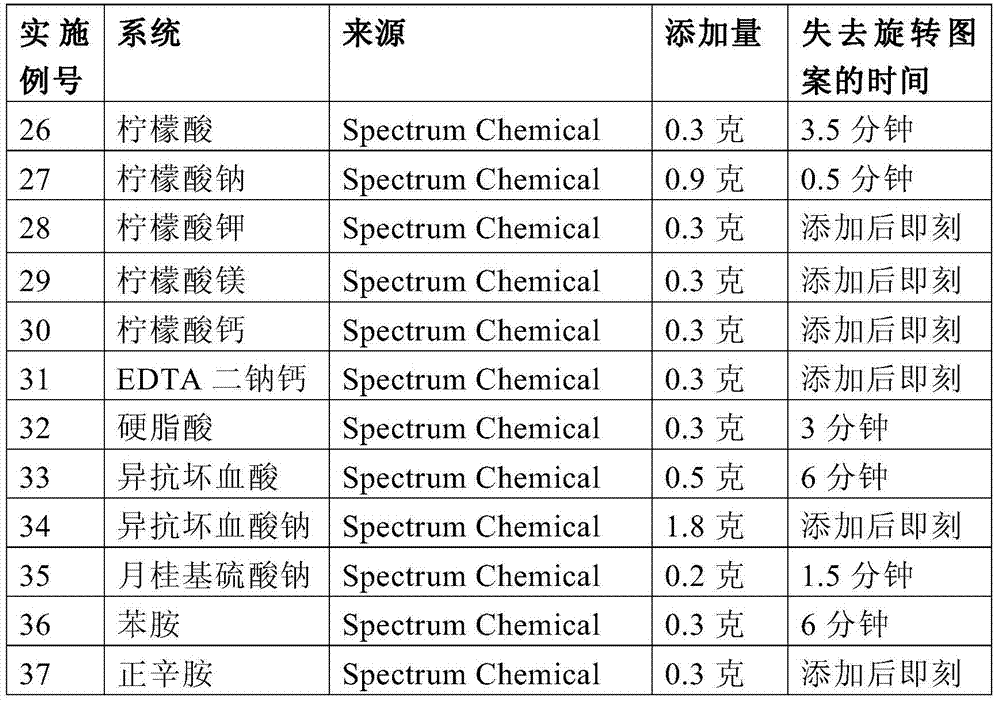
ActiveCN103717650BThe effect is not damagedCoatingsThin material handlingCross-linkLithium hydroxide
The present invention provides a coated substrate comprising a substrate and a barrier coating on at least one surface of the substrate. The barrier coating comprises: (i) vermiculite, (ii) a polymer capable of forming a thin film, (iii) a chemical stabilizer selected from the group consisting of lithium, alkyl C2-C6 ammonium, allyl ammonium, Materials with cationic functional groups of heterocyclic ammonium, morpholinium, ammonium and amino C3‑C6 alkylcarboxylic acids; in combination with anions selected from the group consisting of carboxylic acids, phosphoric acids, phosphonic acids, sulfonic acids and fatty acids, lithium chelating agents and lithium salts lithium cations; ammonia, C3‑C6 amines, heterocyclic amines, lithium hydroxide, morpholine and morpholine oleate; and (iv) crosslinkers. The invention also provides articles coated with such coatings, methods and compositions for making such coated substrates and articles.
Owner:NANOPACK
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com