Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1438results about "Niobium compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
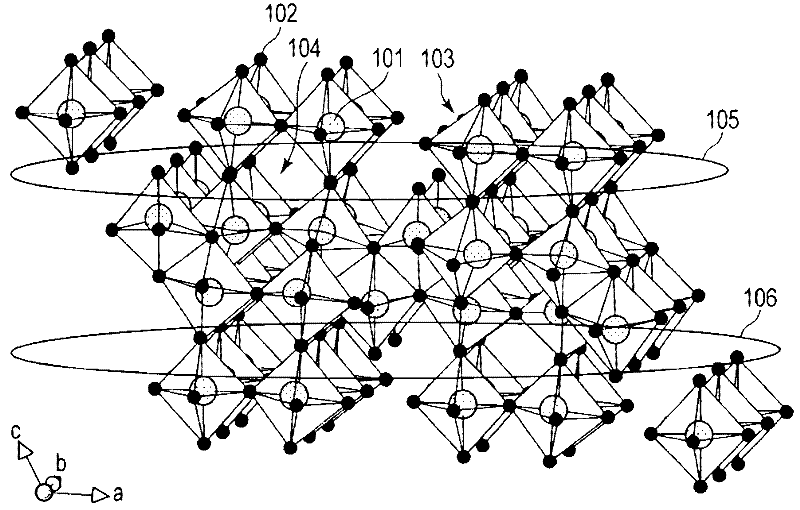
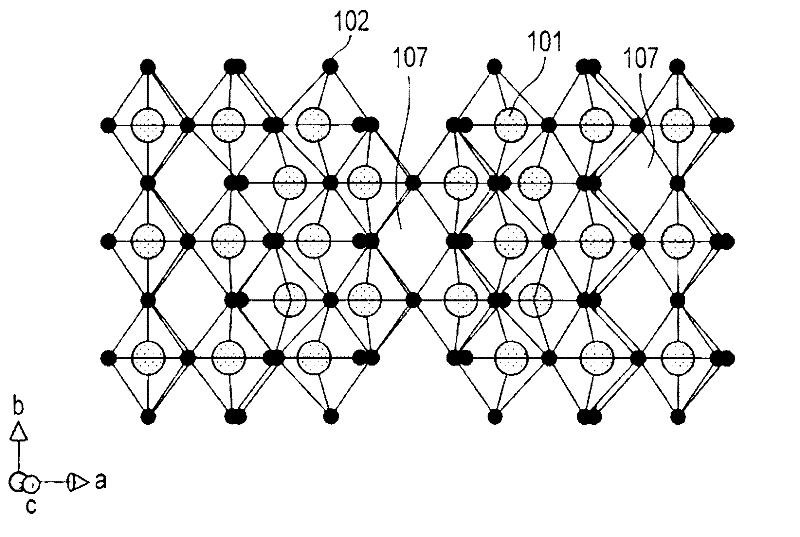
Modified oxygen reduced valve metal oxides
InactiveUS6639787B2Maintain good propertiesWear minimizationOxide/hydroxide preparationLiquid electrolytic capacitorsFluidized bedPhysical chemistry
Owner:GLOBAL ADVANCED METALS USA
Niobium suboxide powder
ActiveUS20050013765A1High currentReduce residual currentOxide/hydroxide preparationLiquid electrolytic capacitorsCapacitorTungsten
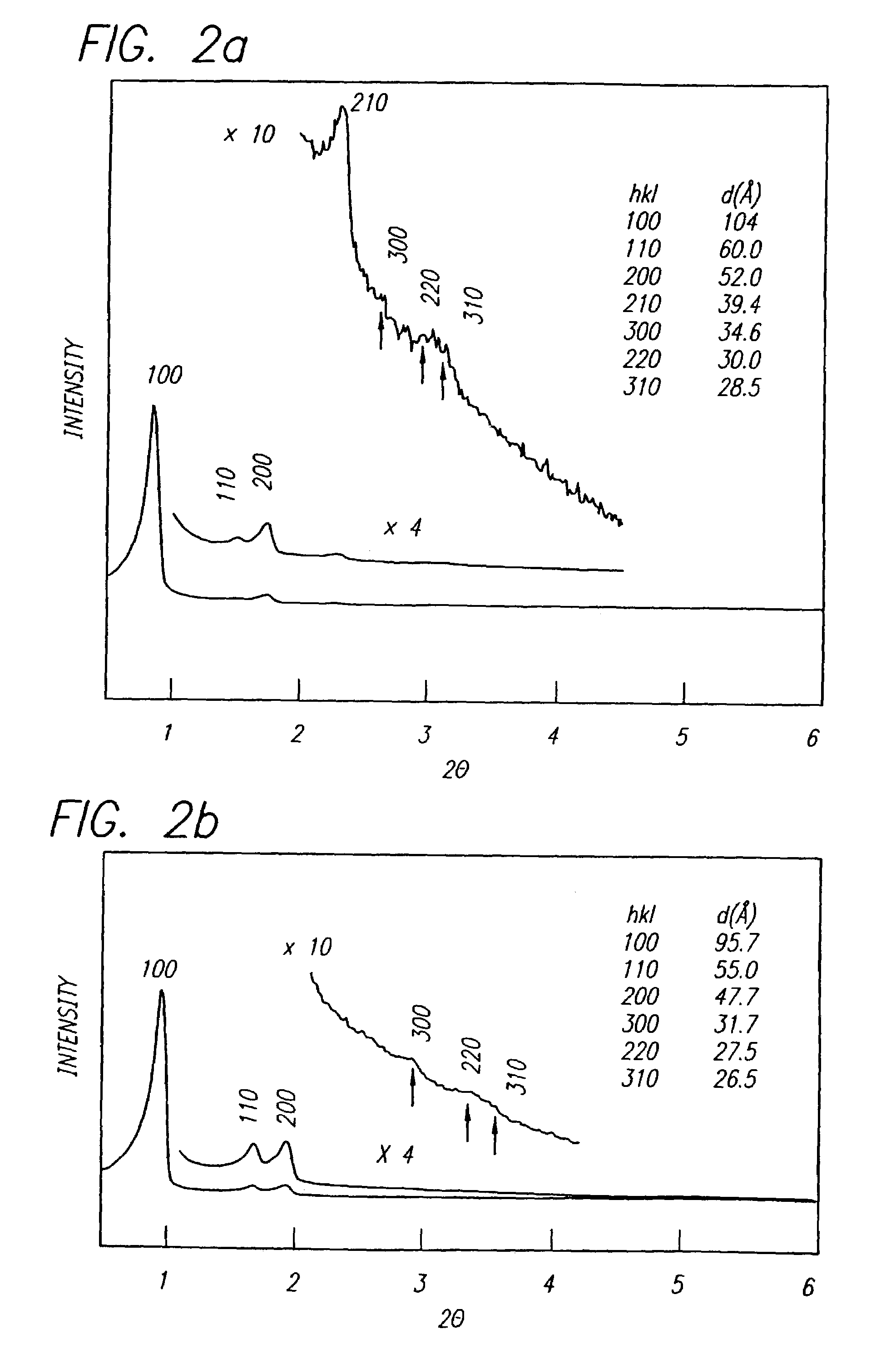
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Process for producing niobium suboxide
ActiveUS20050019581A1Increase capacityIncrease volumeLiquid electrolytic capacitorsTantalum compoundsHydrogenNiobium
A method is described for preparing a niobium suboxide represented by the formula, NbOx, in which 0.7<x<1.3. The method involves reacting NbOy (in which y<1.8<2.1) with a stoichiometric amount of niobium metal, in the presence of hydrogen. The niobium suboxide produced by such method may be used to fabricate anodes for solid electrolyte capacitors.
Owner:TANIOBIS GMBH
Niobium powder and a process for the production of niobium and/or tantalum powders
InactiveUS6136062APrevent local overheating effectStirring speed is fastElectrolytic capacitorsTantalum compoundsMischmetalRare earth
The process comprises the reduction of niobium and / or tantalum oxides by means of alkaline earth metals and / or rare earth metals, wherein the first reduction stage is carried out as far as an average composition corresponding to (Nb, Ta)Ox where x=0.5 to 1.5 and before the second stage the reduction product from the first stage is freed from alkaline earth oxides and / or rare earth metal oxides which are formed (and optionally from excess alkaline earth metal and / or rare earth metal) by washing with mineral acids.
Owner:H C STARCK TANTALUM & NIOBIUM GMBH
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS20080206124A1Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumPhysical chemistry
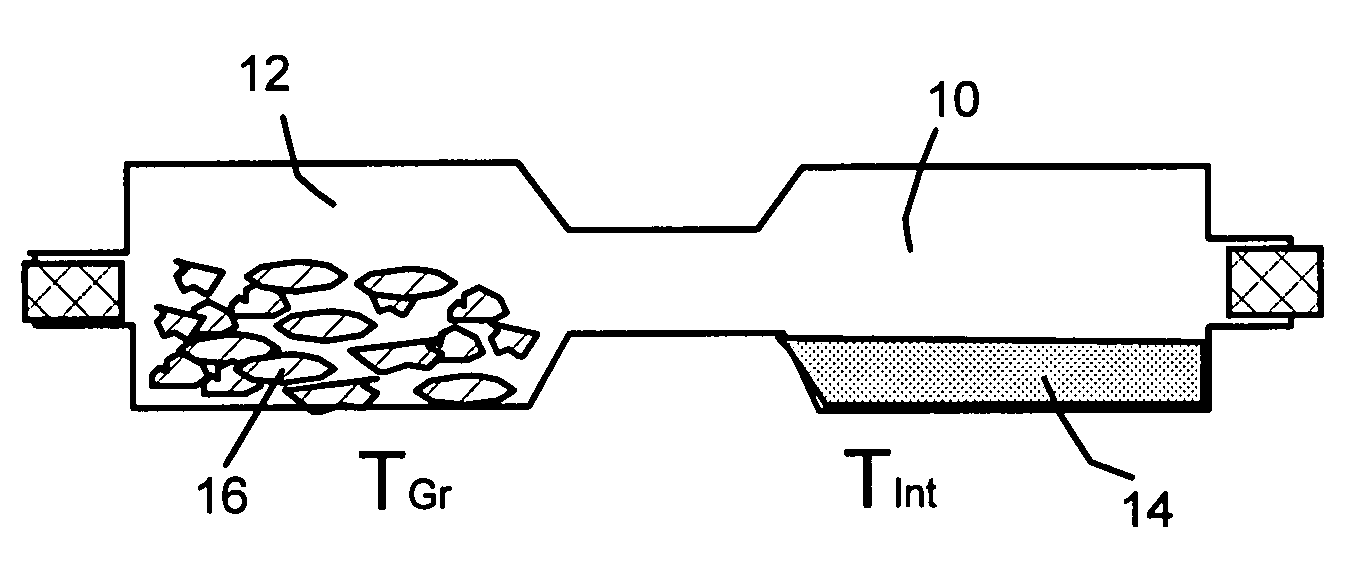
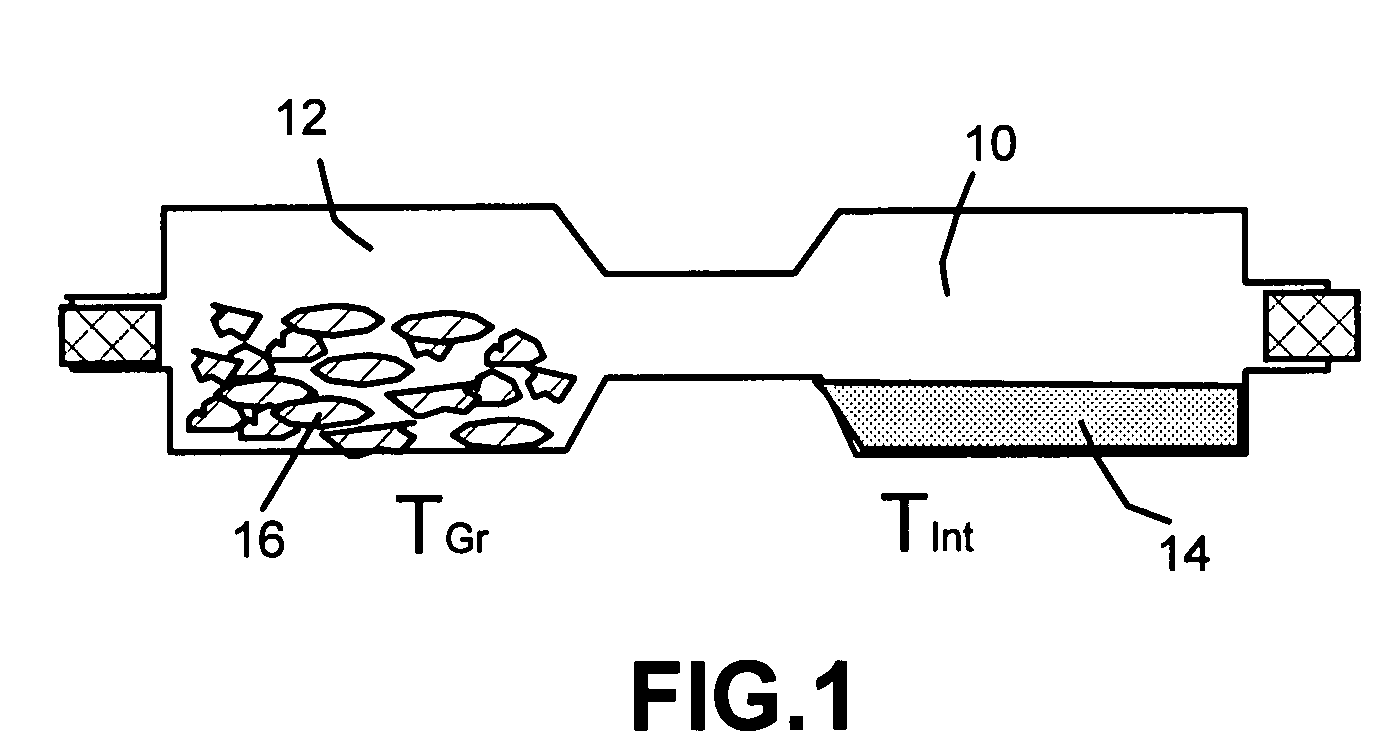
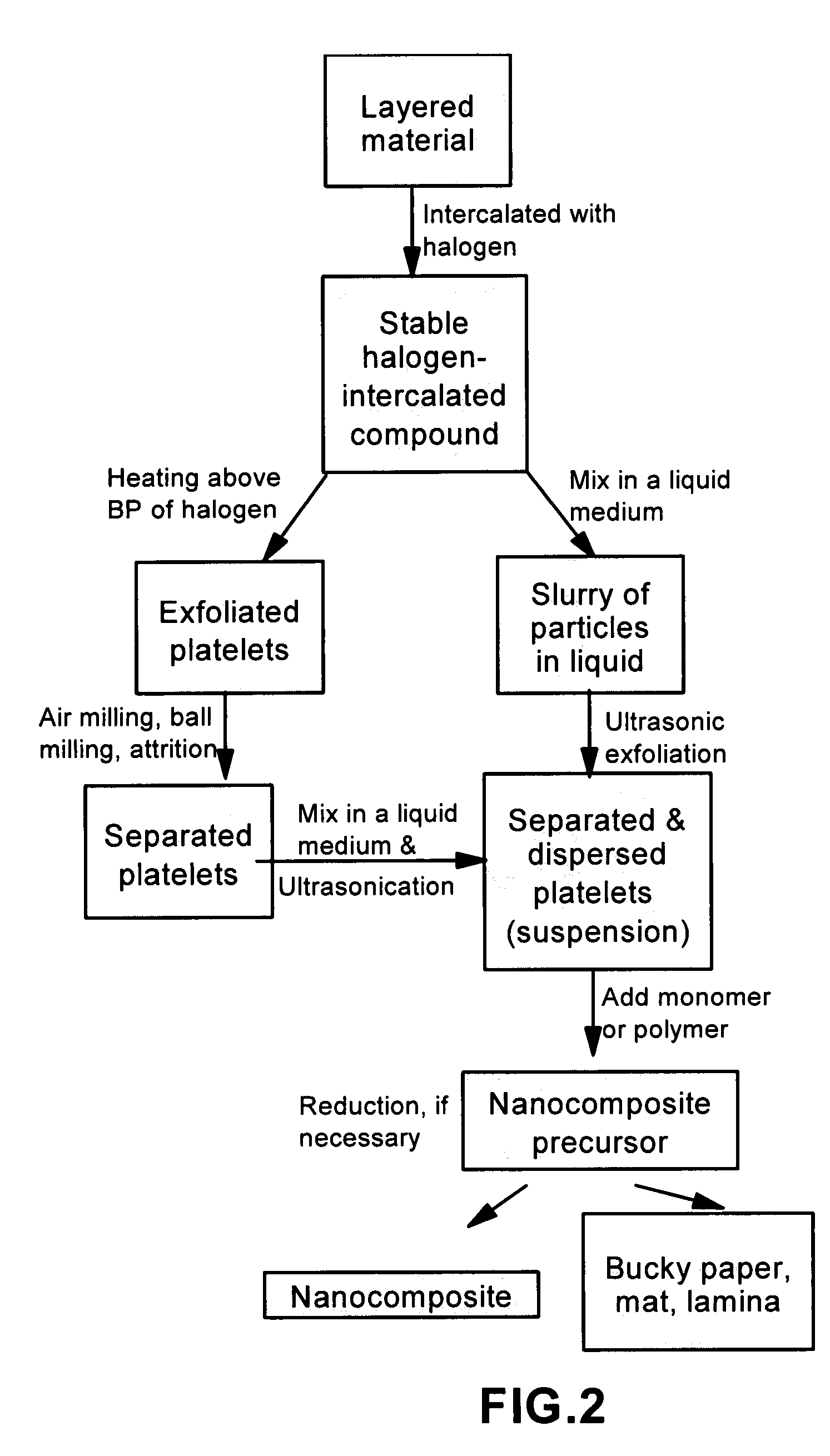
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
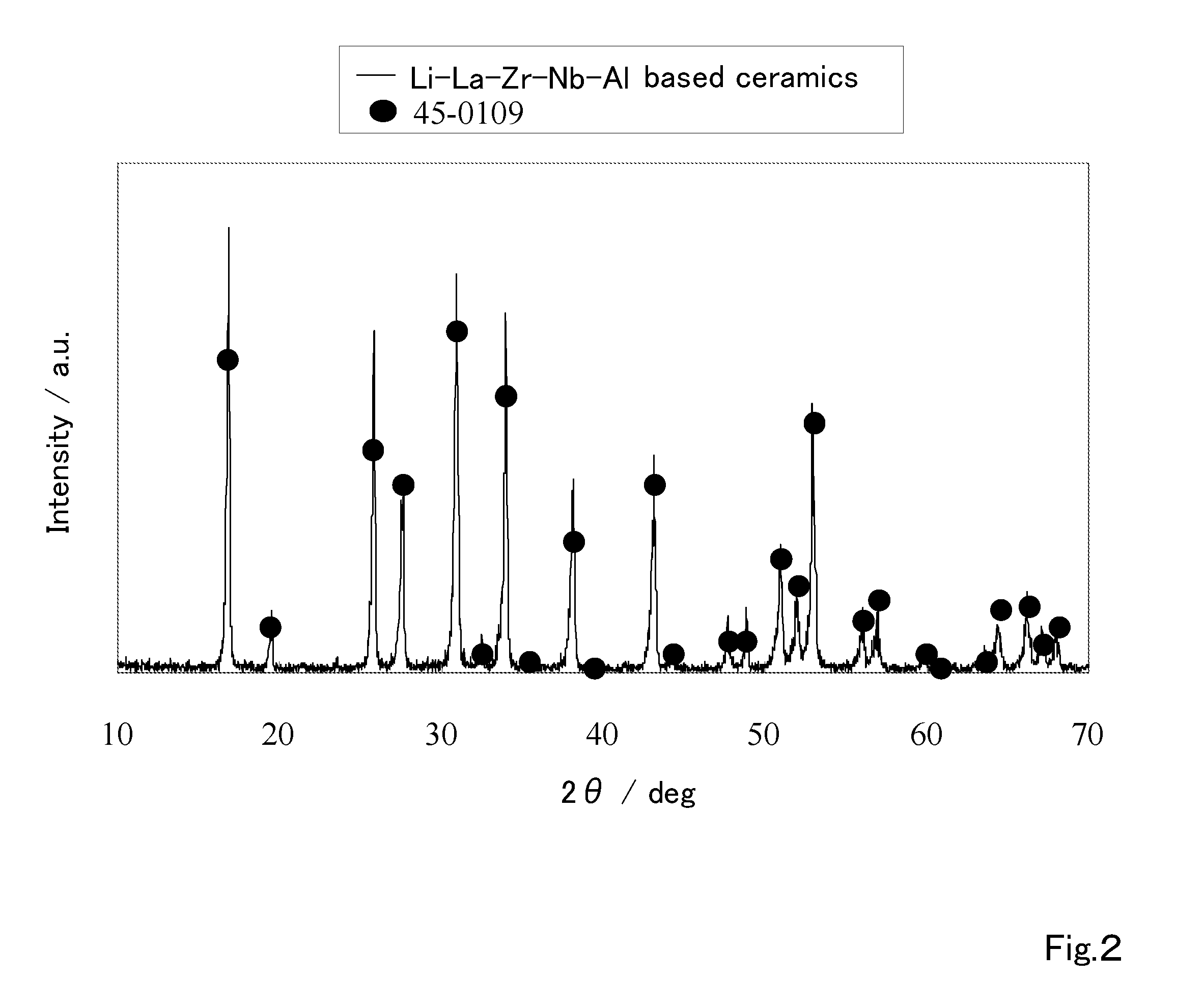
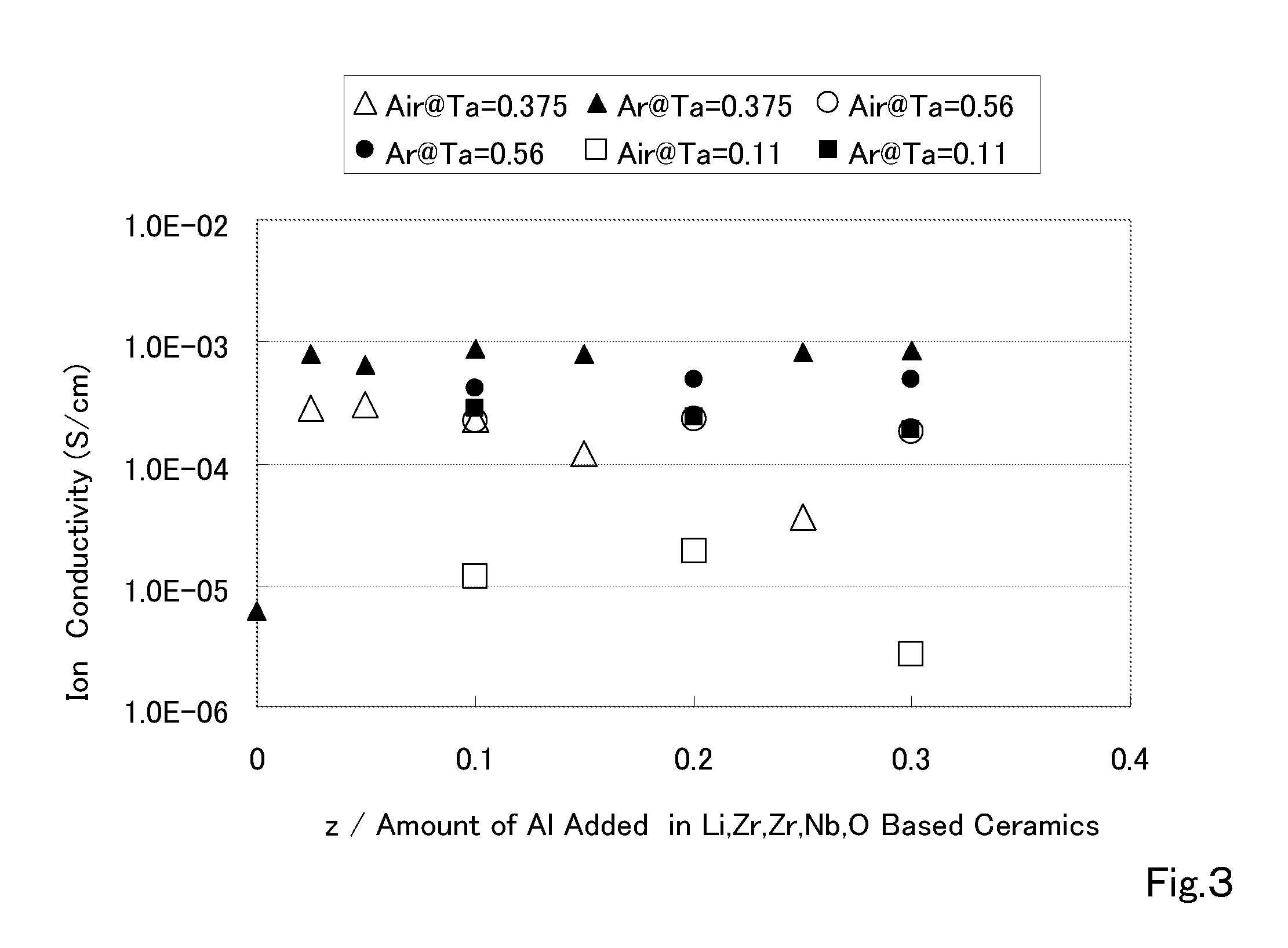
Ceramic material and preparation method therefor
ActiveUS20110053002A1Demonstrating compactnessDemonstrating conductivityFinal product manufactureTantalum compoundsSolid state electrolyteMetallurgy
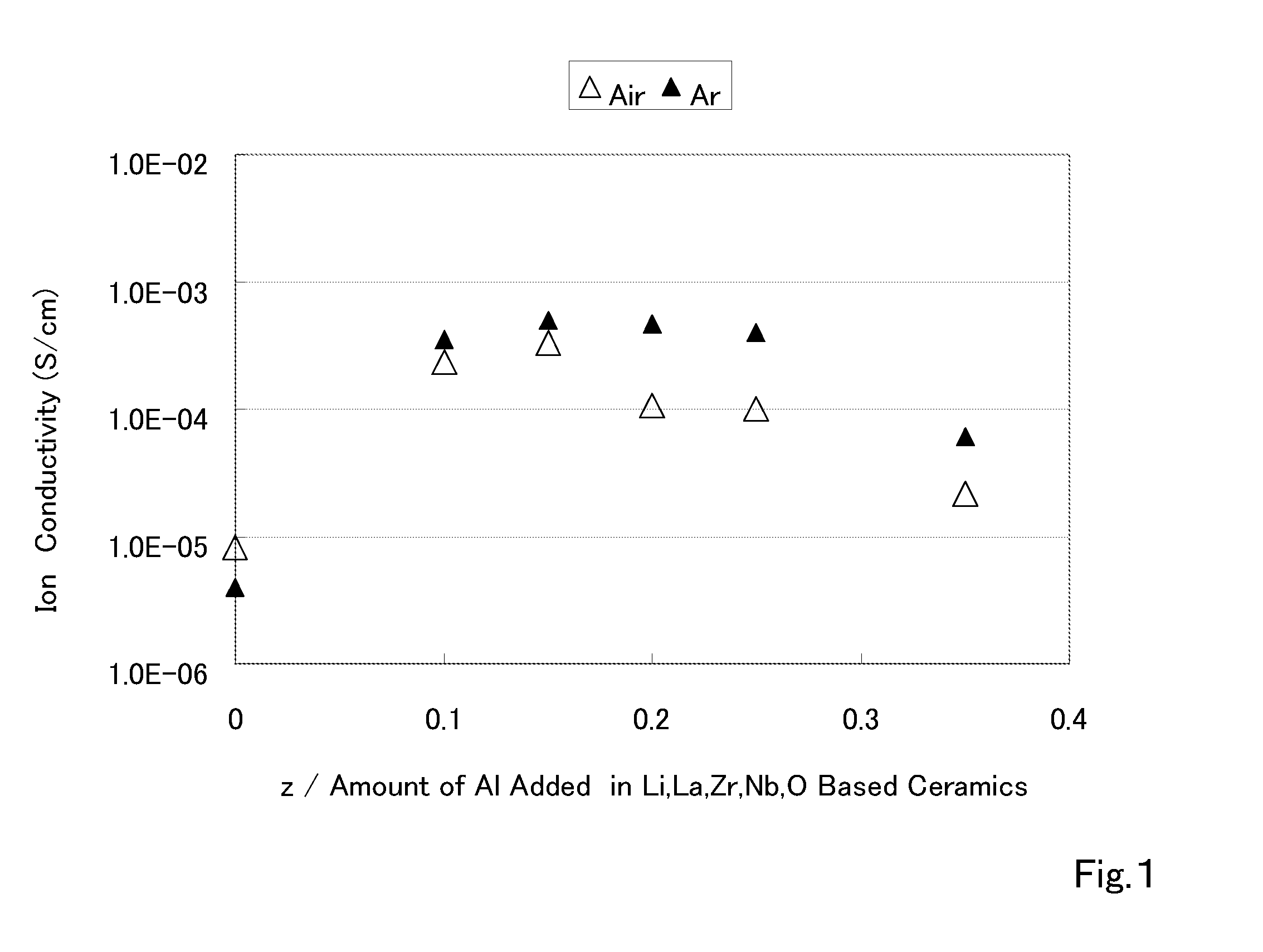
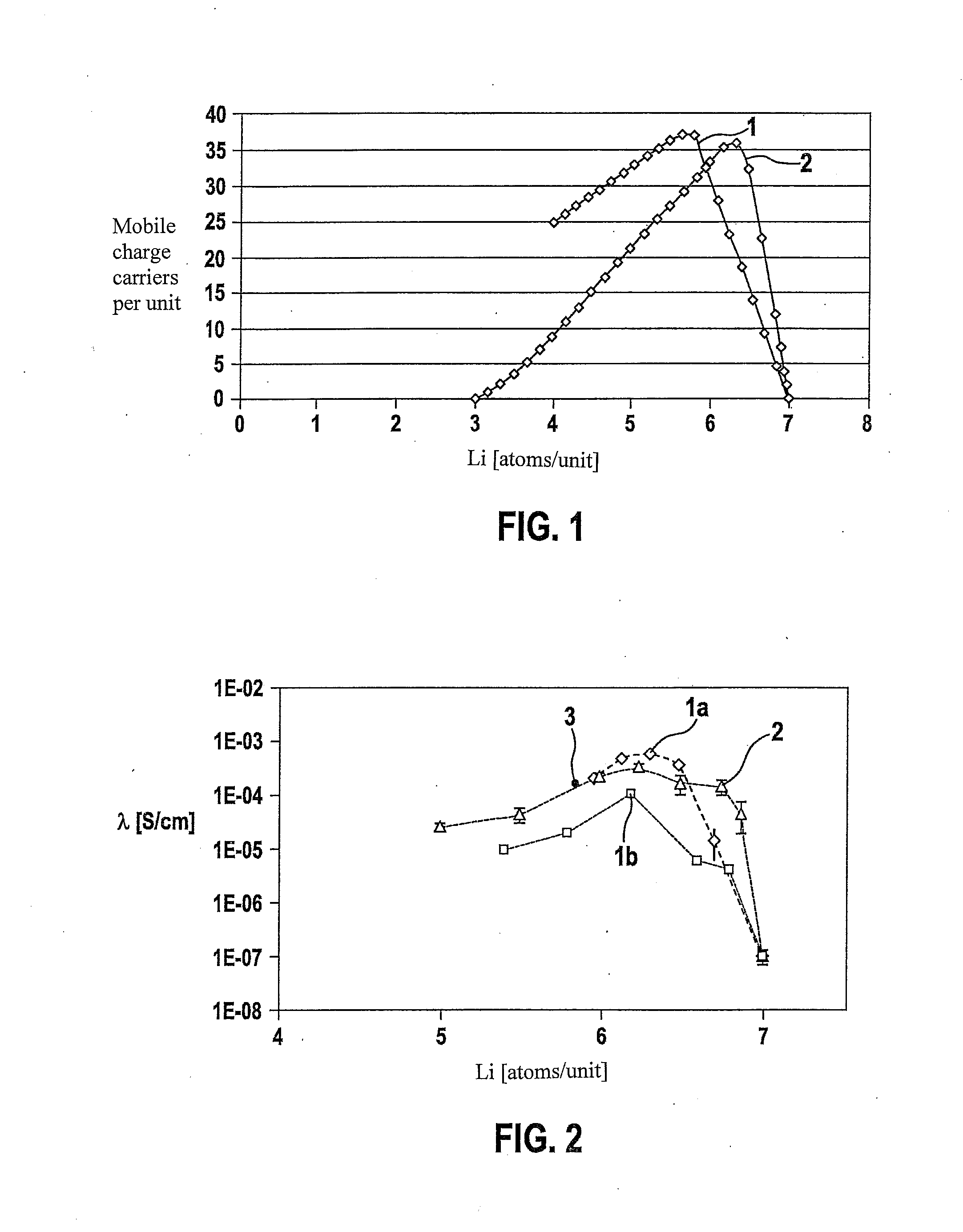
The present invention provides a ceramic material capable of demonstrating compactness and Li ion conductivity to an extent that enables the use of the ceramic material as a solid-state electrolyte material for a lithium secondary battery, or the like. A ceramic material containing Li, La, Zr, Nb and / or Ta, as well as O and having a garnet-type or garnet-like crystal structure is used.
Owner:NGK INSULATORS LTD
Surface acoustic wave device with KNb03 piezoelectric thin film, frequency filter, oscillator, electronic circuit, and electronic apparatus
InactiveUS6720846B2Polycrystalline material growthPiezoelectric/electrostriction/magnetostriction machinesTectorial membraneFrequency filtering
Surface acoustic wave device having a high k<2>, and a frequency filter, oscillator, electronic circuit and electronic device employing this surface acoustic wave device is provided, wherein a first oxide thin film layer comprising SrO or MgO and a second oxide thin film layer comprising SrTiO3 are sequentially formed on top of a (110) Si substrate, or a first oxide thin film layer comprising CeO2, ZrO2 or yttrium-stabilized zirconia and a second oxide thin film layer comprising SrTiO3 are sequentially formed on top of a (100) Si substrate, a KNbO3 piezoelectric thin film being then formed on top of either of these second oxide thin film layers, and then, a protective film comprising oxide or nitride is formed on top of the KNbO3 piezoelectric thin film, finally, at least one electrode is formed on top of this protective film, to form a surface acoustic wave device, which surface acoustic wave device is employed to form a frequency filter, oscillator, electronic circuit, or electronic device.
Owner:SEIKO EPSON CORP
Process for producing niobium suboxide
ActiveUS7341705B2Increase capacityLiquid electrolytic capacitorsTantalum compoundsNiobiumPhysical chemistry
A method is described for preparing a niobium suboxide represented by the formula, NbOx, in which 0.7<x<1.3. The method involves reacting NbOy (in which y<1.8<2.1) with a stoichiometric amount of niobium metal, in the presence of hydrogen. The niobium suboxide produced by such method may be used to fabricate anodes for solid electrolyte capacitors.
Owner:TANIOBIS GMBH
Niobium Oxide Compositions and Methods for Using Same
ActiveUS20120052401A1Alkaline accumulatorsElectric discharge heatingLithium-ion batteryCarbon coated
The disclosure relates a niobium oxide useful in anodes of secondary lithium ion batteries. Such niobium oxide has formula LixM1−yNbyNb2O7, wherein 0≦x≦3, 0≦y≦1, and M represents Ti or Zr. The niobium oxide may be in the form of particles, which may be carbon coated. The disclosure also relates to an electrode composition containing at least one or more niobium oxides of formula LixM1−yNbyNb2O7. The disclosure further relates to electrodes, such as anodes, and batteries containing at least one or more niobium oxides of formula LixM1−yNbyNb2O7. Furthermore, the disclosure relates to methods of forming the above.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Niobium suboxide powder
ActiveUS7381396B2Reduce residual currentReduce volatilityOxide/hydroxide preparationLiquid electrolytic capacitorsTungstenSuboxide
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Composite materials of nano-dispersed silicon and tin and methods of making the same
Composite compounds of tin and lithium, silicon and lithium, or tin, silicon, and lithium having tin and silicon nano-dispersed in a lithium-containing matrix may be used as electrode materials and particularly anode materials for use with rechargeable batteries. Methods of making the composite compounds include the oxidation of alloys, the reaction of stabilized lithium metal powder with tin and silicon oxides, and the reaction of inorganic salts of lithium with tin and silicon containing compounds.
Owner:LIVENT USA CORP
Sintered bodies based on niobium suboxide
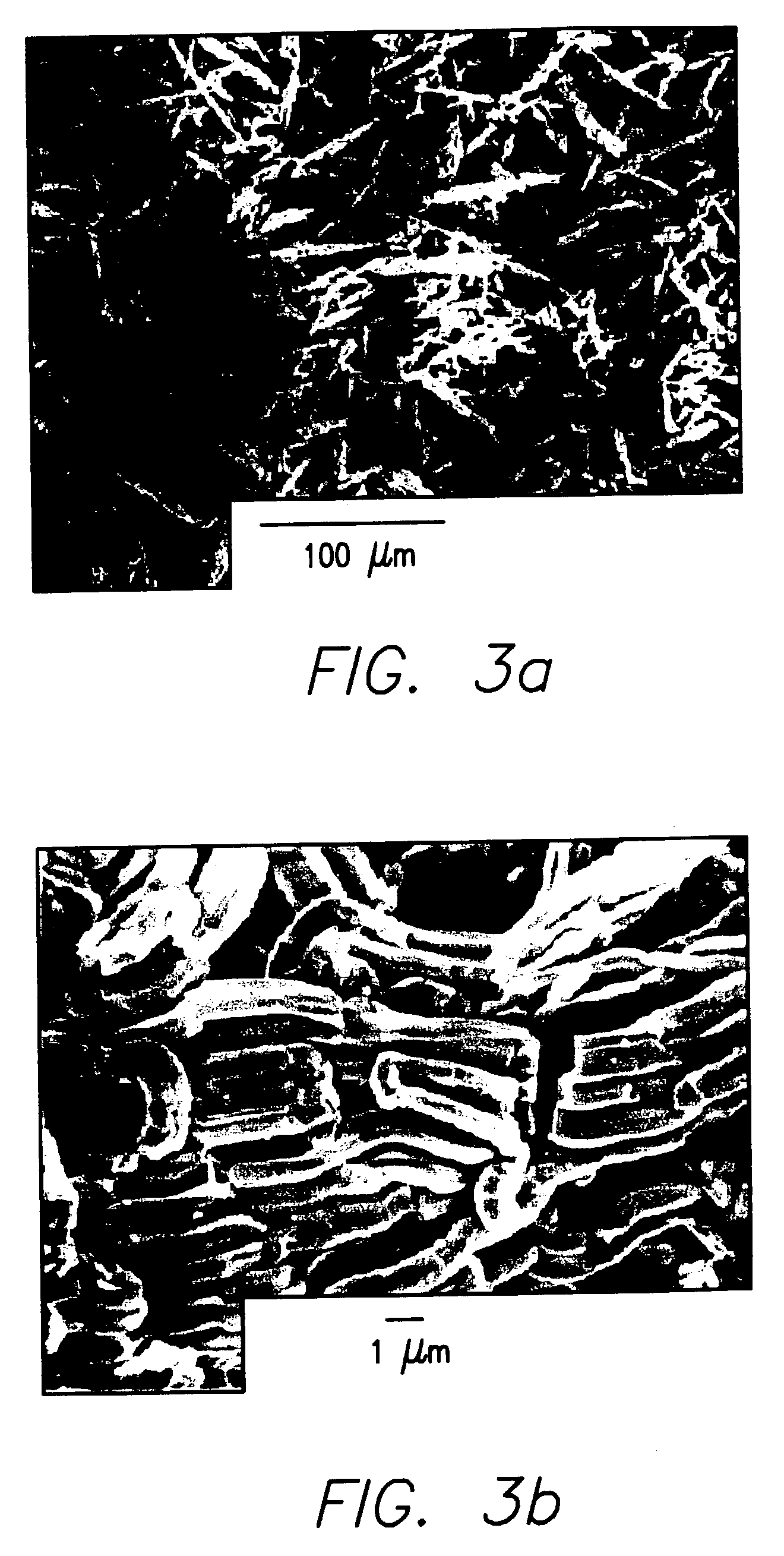
Disclosed are sintered bodies that include: (a) 30 to 100 mol % of NbOx, wherein 0.5<x<1.5; and (b) 0 to 70 mol % of MgO. The sintered bodies may be used as inert apparatuses in the production of niobium suboxide powder or niobium suboxide anodes, or as chemically resistant components in chemical apparatuses.
Owner:H C STARCK GMBH
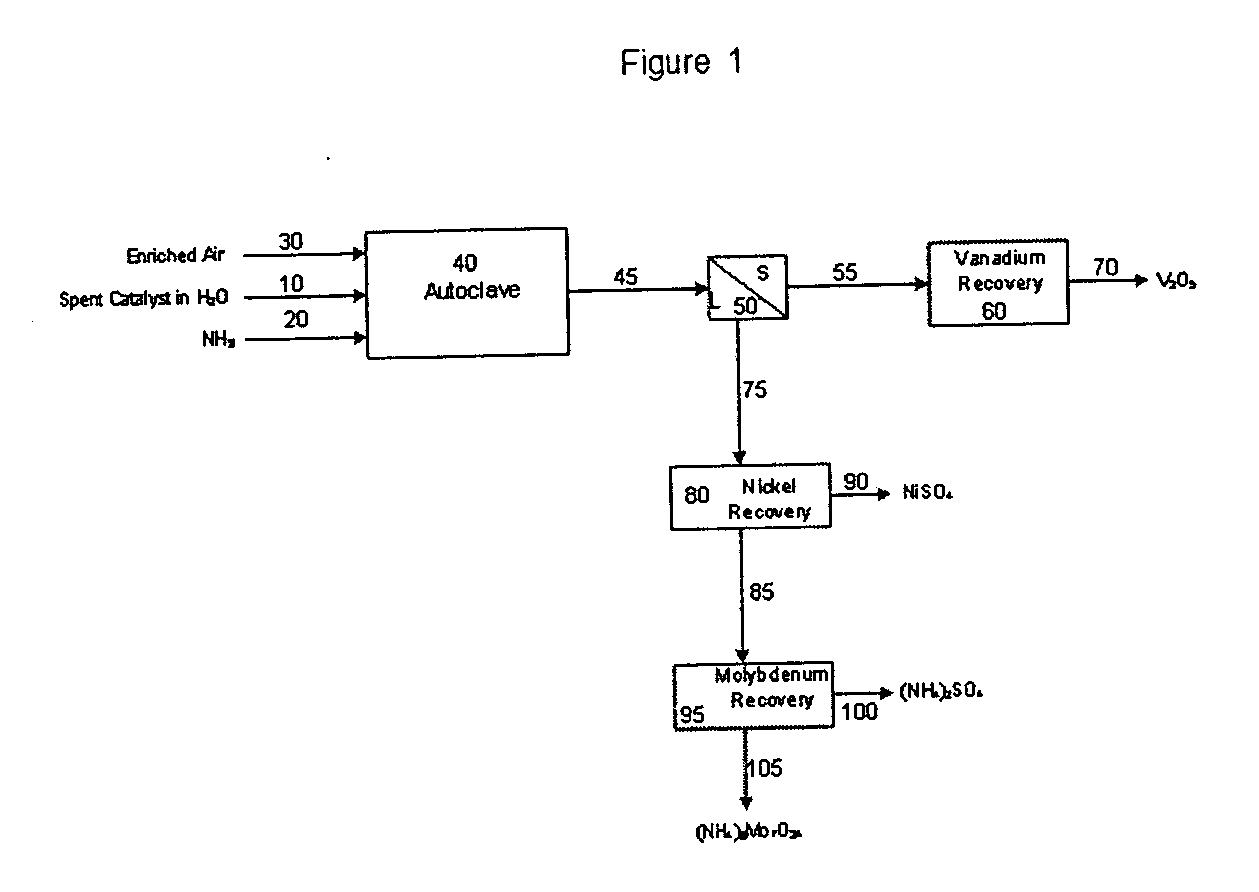
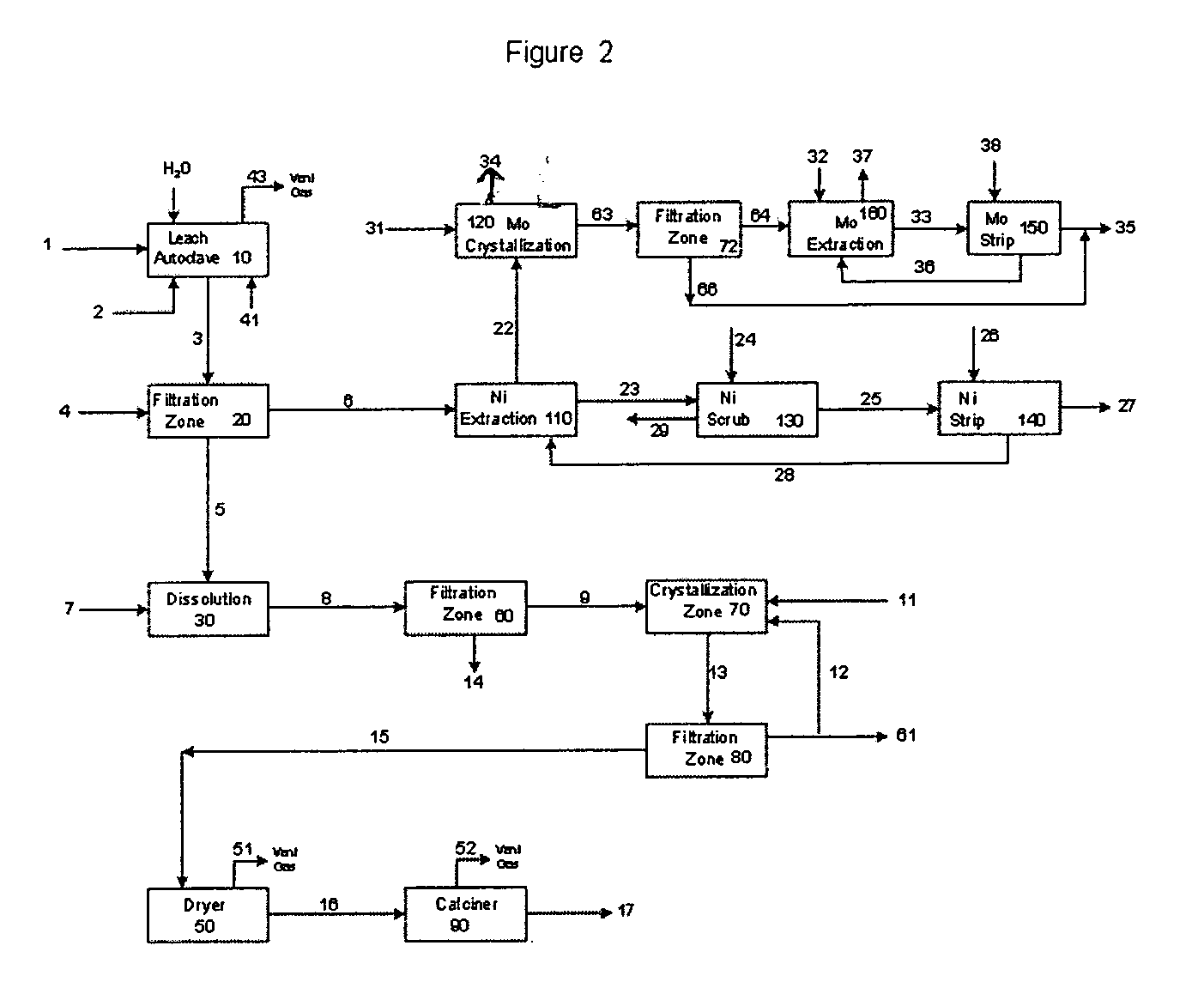
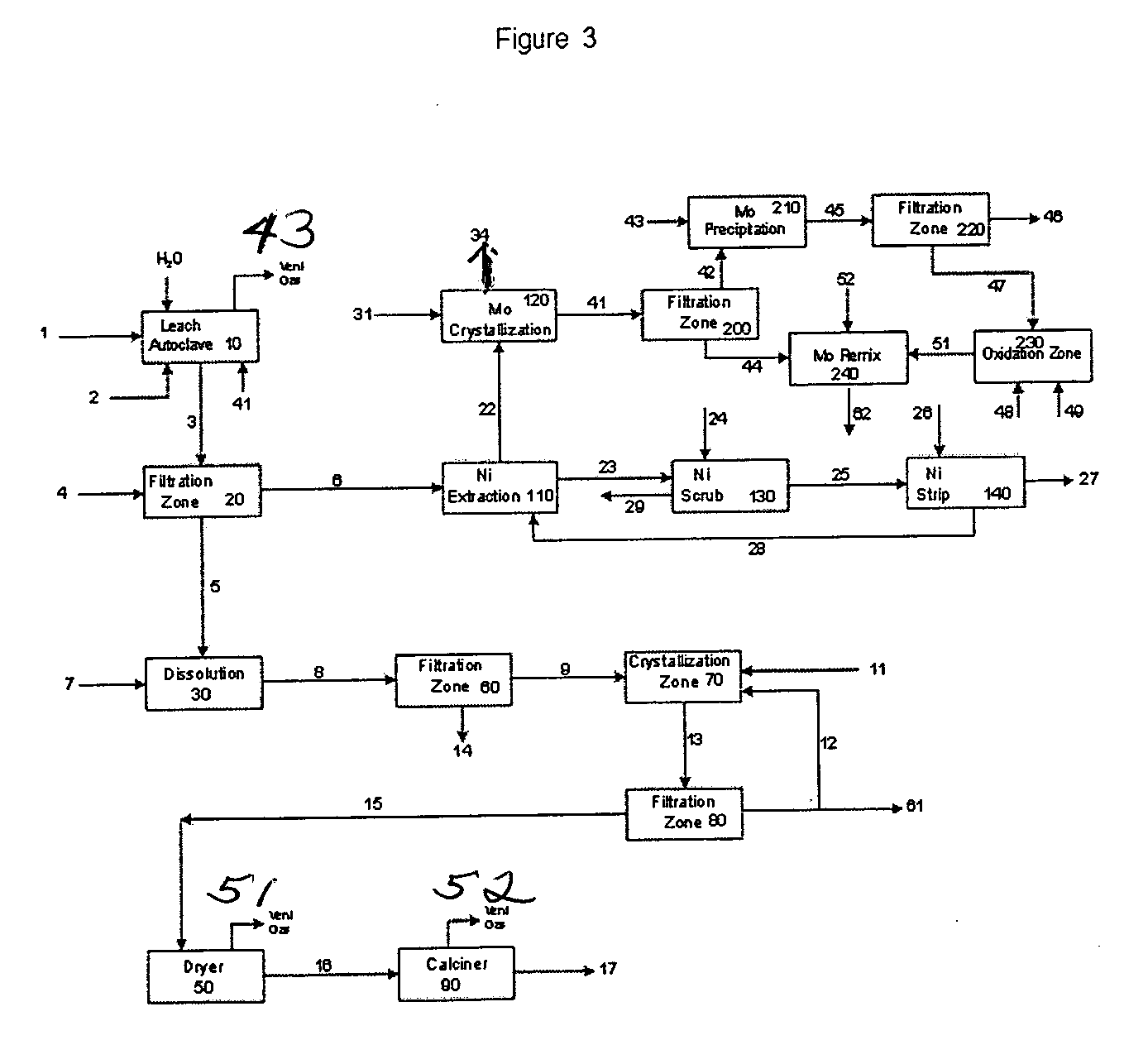
Process for metals recovery from spent catalyst
The process of this invention is directed to the removal of metals from an unsupported spent catalyst. The catalyst is subjected to leaching reactions. Vanadium is removed as a precipitate, while a solution comprising molybdenum and nickel is subjected to further extraction steps for the removal of these metals. Molybdenum may alternately be removed through precipitation.
Owner:CHEVROU USA INC
Hydromethanation of a carbonaceous feedstock with vanadium recovery
ActiveUS20110262323A1Easy to understandSolvent extractionOrganic compound preparationMaterials scienceHydromethanation
Owner:SURE CHAMPION INVESTMENT LTD
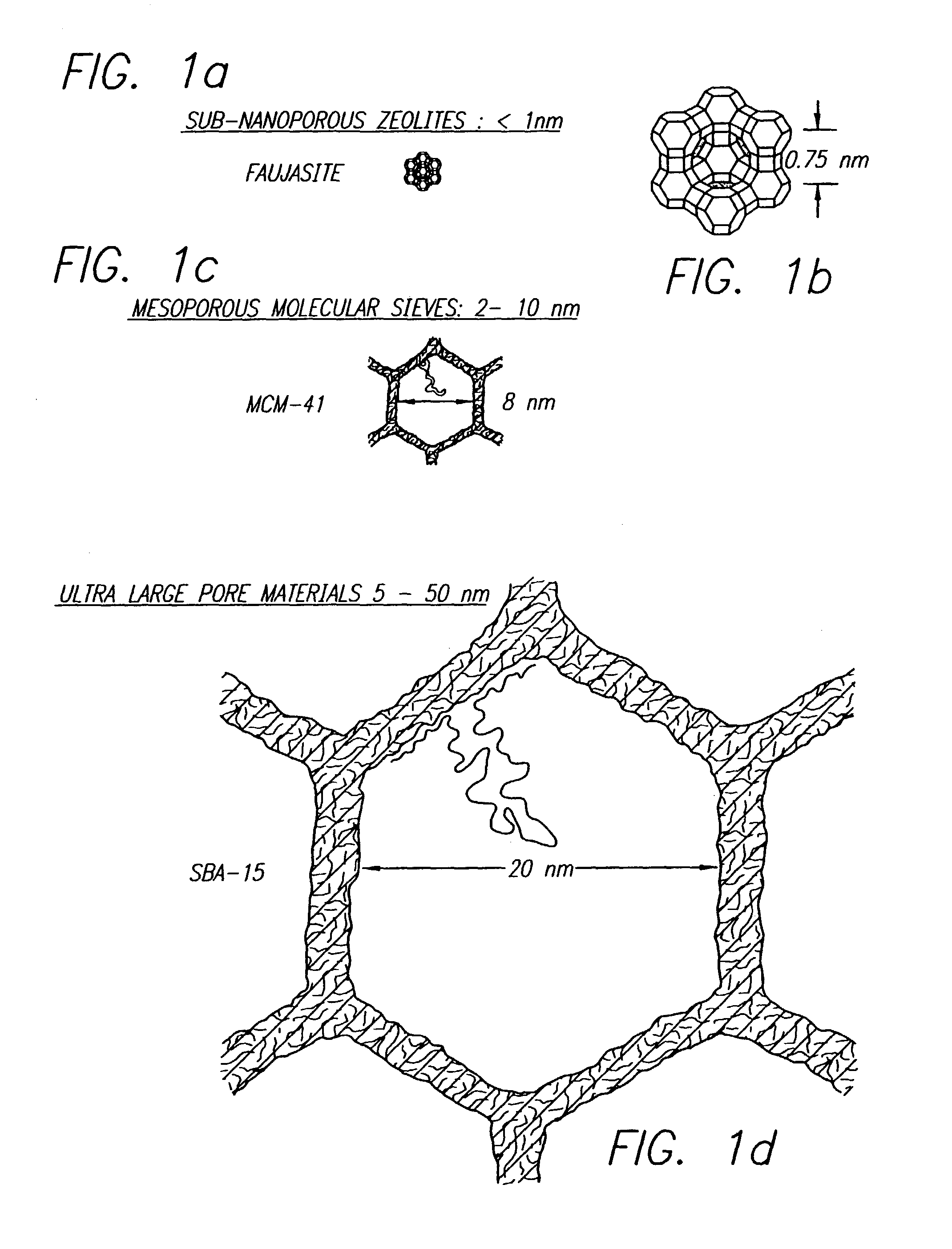
Block copolymer processing for mesostructured inorganic oxide materials
InactiveUS7176245B2High BET surface areaIncrease surface areaMolecular-sieve and base-exchange compoundsCation exchangersMesoporous materialCopolymer
Owner:SBA MATERIALS
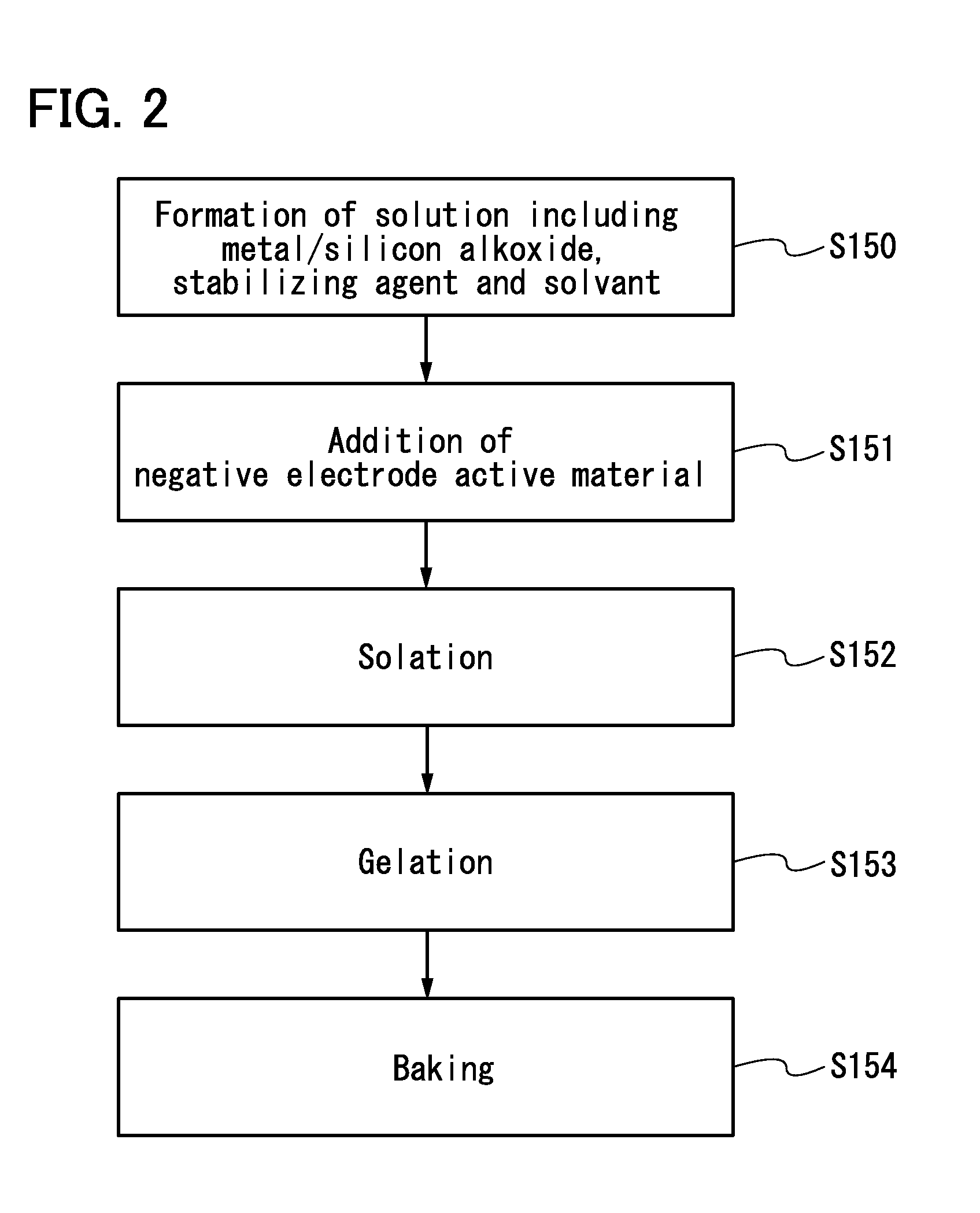
Negative electrode for power storage device, method for forming the same, and power storage device
ActiveUS20130266858A1Improve cycle performanceDecrease in capacity in chargeElectrode thermal treatmentGraphiteElectrical batteryEngineering
An object is to suppress electrochemical decomposition of an electrolyte solution and the like at a negative electrode in a lithium ion battery or a lithium ion capacitor; thus, irreversible capacity is reduced, cycle performance is improved, or operating temperature range is extended. A negative electrode for a power storage device including a negative electrode current collector, a negative electrode active material layer which is over the negative electrode current collector and includes a plurality of particles of a negative electrode active material, and a film covering part of the negative electrode active material. The film has an insulating property and lithium ion conductivity.
Owner:SEMICON ENERGY LAB CO LTD
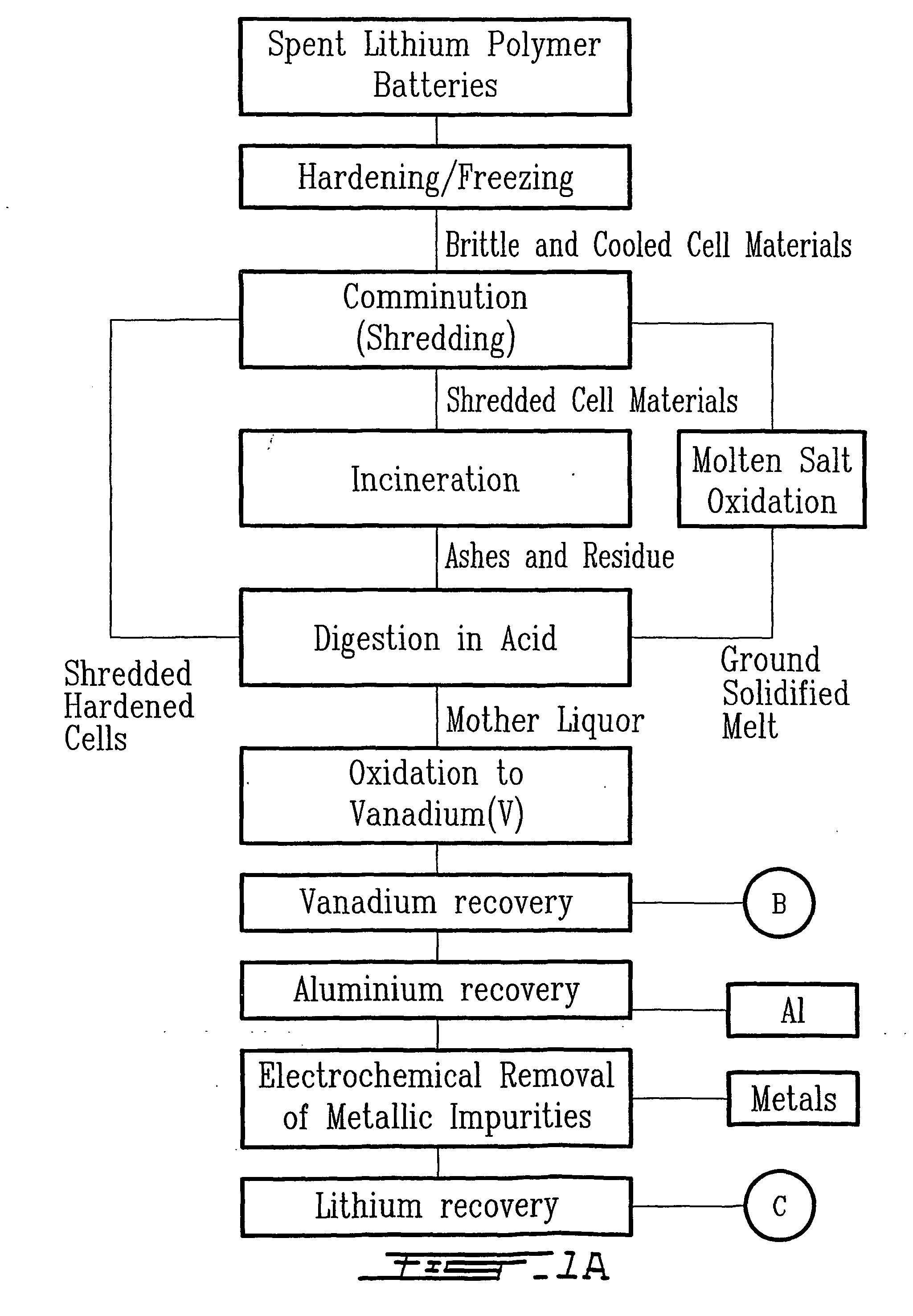
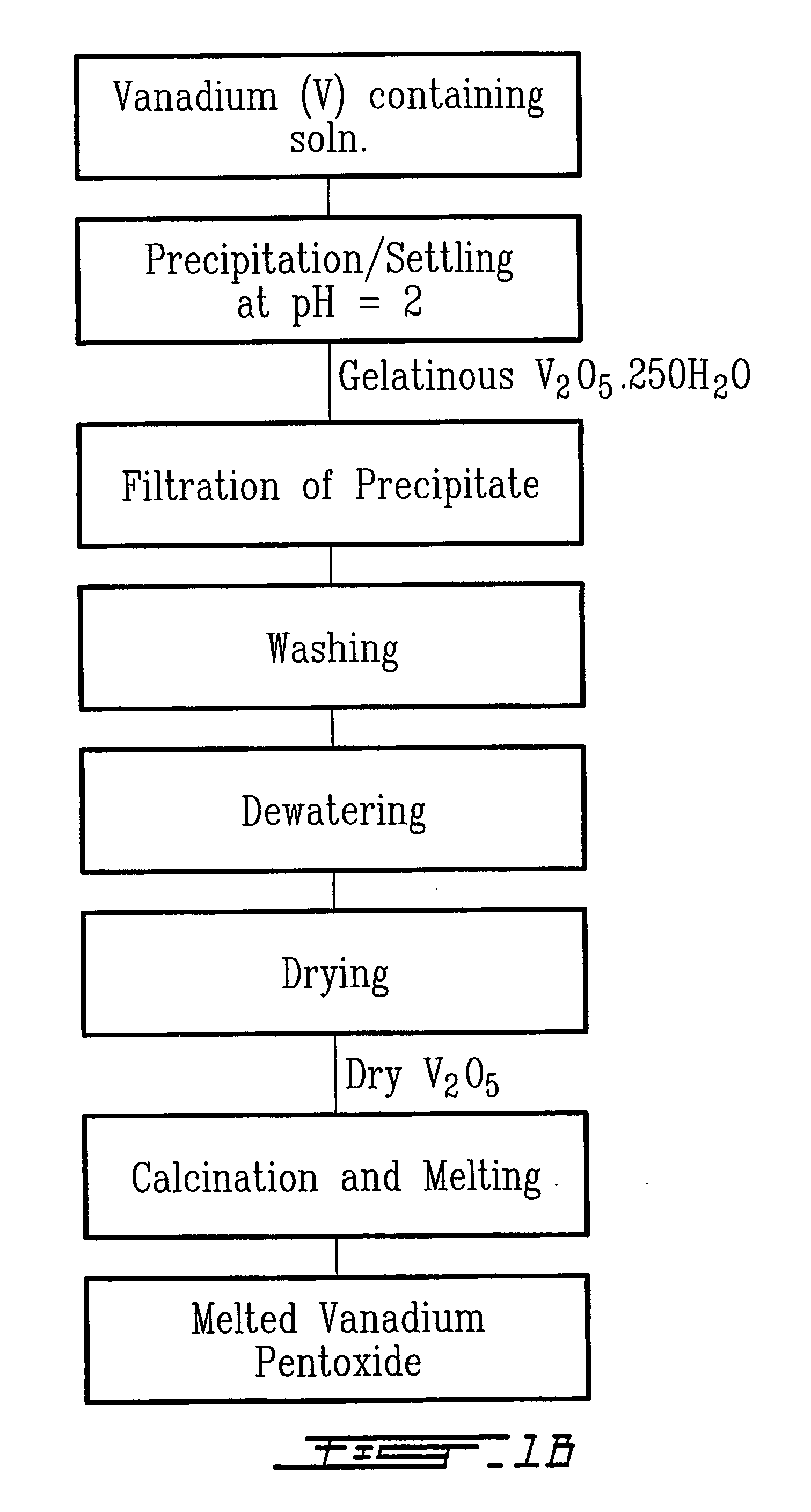
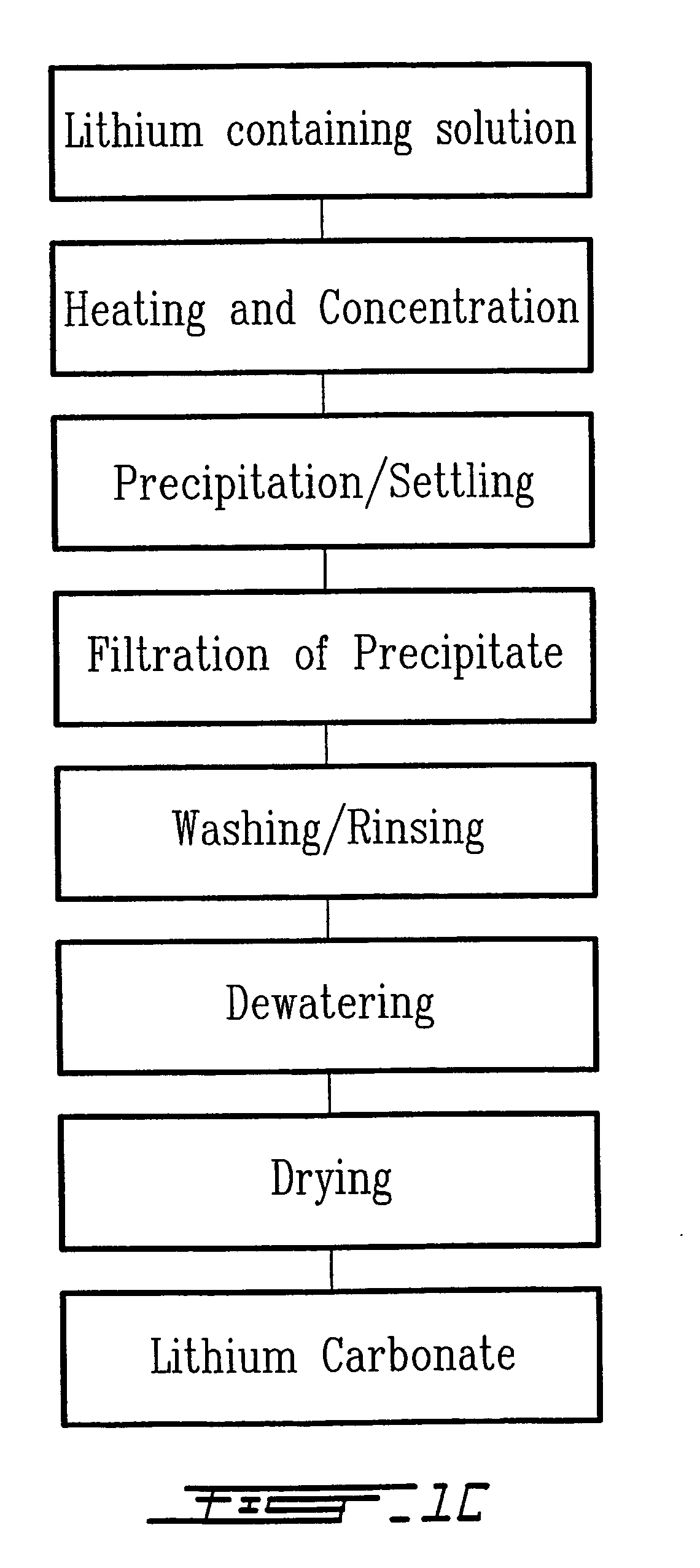
Method for recycling spent lithium metal polymer rechargeable batteries and related materials
The method relates to a pyrometallurgical and hydrometallurgical process for the recovery and recycling of lithium and vanadium compounds from a material comprising spent rechargeable lithium batteries, particularly lithium metal gel and solid polymer electrolyte rechargeable batteries. The method involves providing a mass of the material, hardening it by cooling at a temperature below room temperature, comminuting the mass of cooled and hardened material, digesting with an acid its ashes obtained by incineration, or its solidified salts obtained by molten salt oxidation, or the comminuted mass itself, to give a mother liquor, extracting vanadium compounds from the mother liquor, separating heavy metals and aluminium therefrom, and precipitating lithium carbonate from the remaining solution.
Owner:AVESTOR
Lithium ion-conducting garnet-like compounds
ActiveUS20140295287A1Loss can be compensatedImprove stabilityTantalum compoundsZirconium compoundsLithiumCompound a
A lithium ion-conducting compound, having a garnet-like crystal structure, and having the general formula: Lin[A(3-a′-a″)A′(a′)A″(a″)][B(2-b′-b″)B′(b′)B″(b″)][C′(c′)C″(c″)]O12, where A, A′, A″ stand for a dodecahedral position of the crystal structure, where A stands for La, Y, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm and / or Yb, A′ stands for Ca, Sr and / or Ba, A″ stands for Na and / or K, 0<a′<2 and 0<a″<1, where B, B′, B″ stand for an octahedral position of the crystal structure, where B stands for Zr, Hf and / or Sn, B′ stands for Ta, Nb, Sb and / or Bi, B″ stands for at least one element selected from the group including Te, W and Mo, 0<b′<2 and 0<b″<2, where C and C″ stand for a tetrahedral position of the crystal structure, where C stands for Al and Ga, C″ stands for Si and / or Ge, 0<c′<0.5 and 0<c″<0.4, and where n=7+a′+2·a″−b′−2·b″−3·c′−4·c″ and 5.5<n<6.875.
Owner:ROBERT BOSCH GMBH
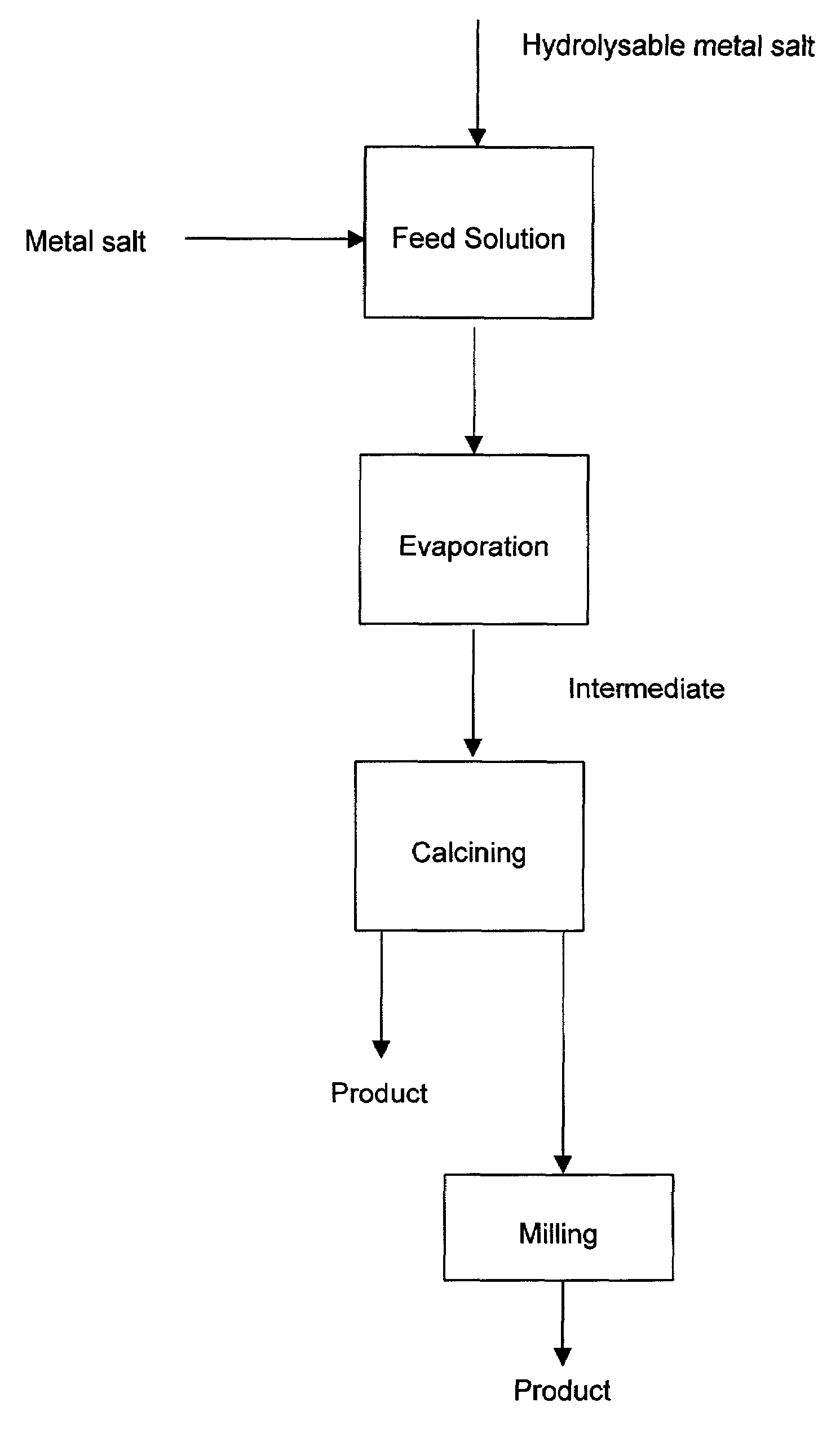
Method for producing mixed metal oxides and metal oxide compounds
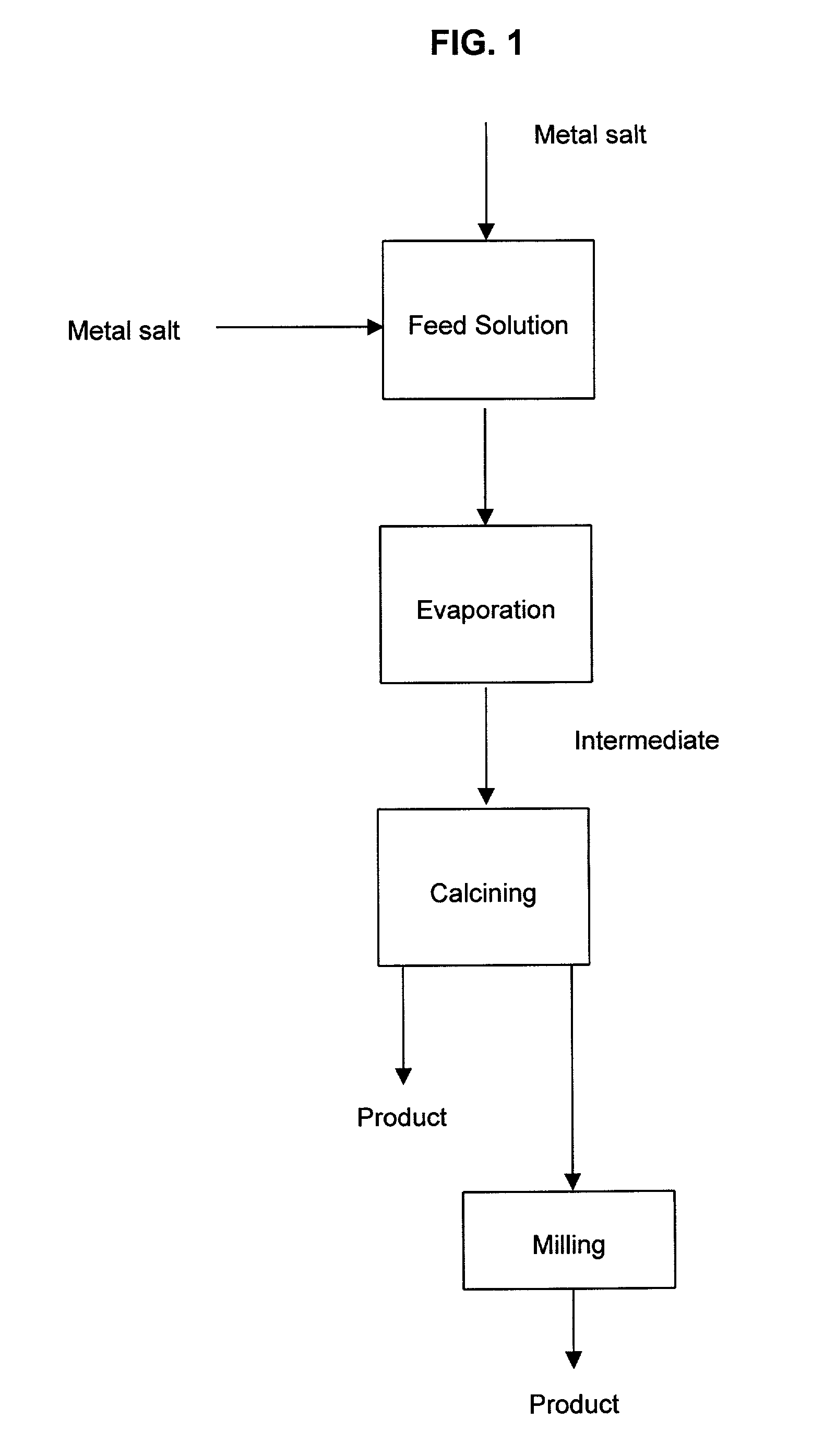
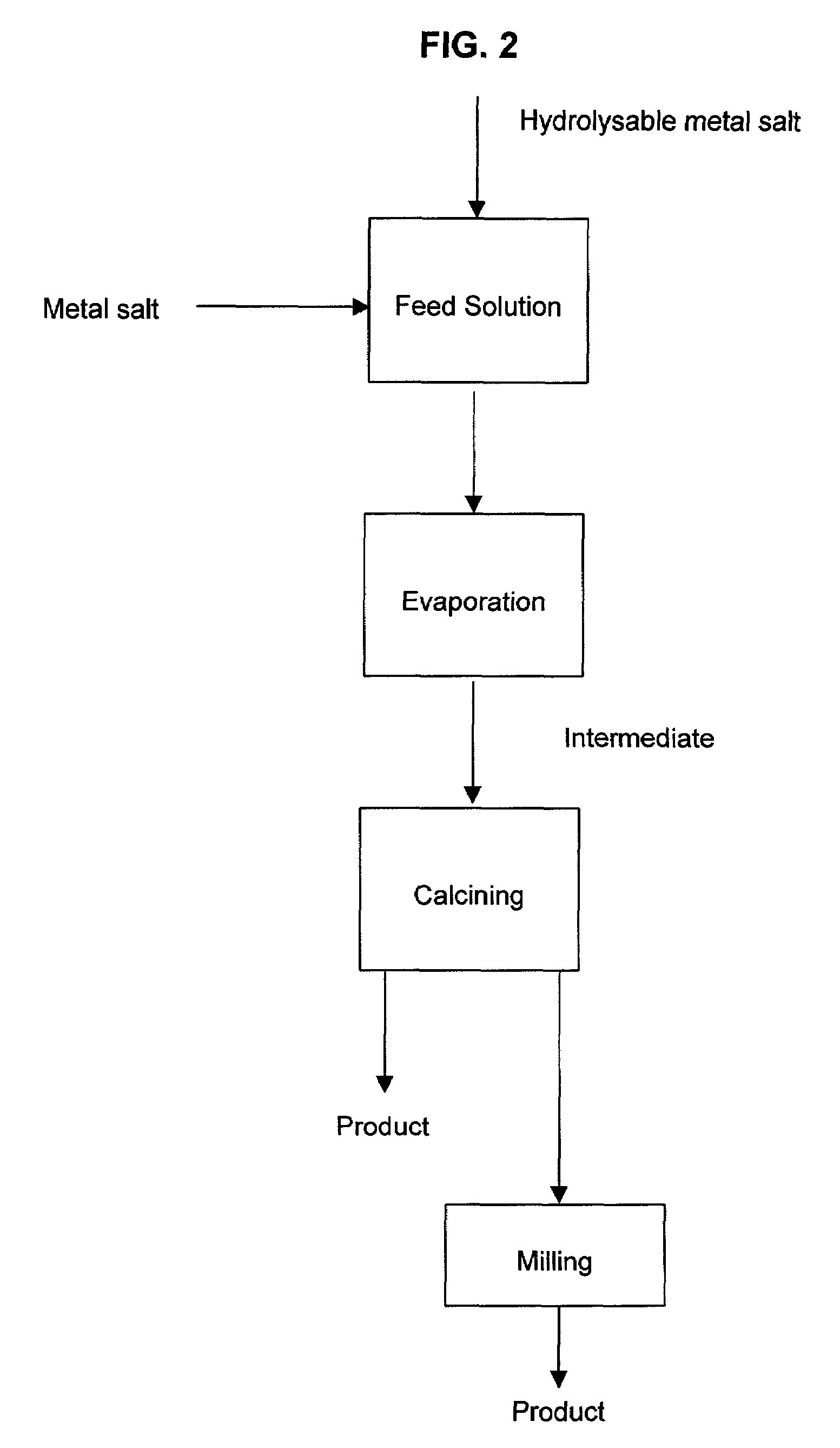
A process to produce mixed metal oxides and metal oxide compounds. The process includes evaporating a feed solution that contains at least two metal salts to form an intermediate. The evaporation is conducted at a temperature above the boiling point of the feed solution but below the temperature where there is significant crystal growth or below the calcination temperature of the intermediate. The intermediate is calcined, optionally in the presence of an oxidizing agent, to form the desired oxides. The calcined material can be milled and dispersed to yield individual particles of controllable size and narrow size distribution.
Owner:ALTAIR NANOMATERIALS INC
Nanoparticle production and corresponding structures
InactiveUS20060147369A1Material nanotechnologyCarbon compoundsProduction rateNanoparticle Production
Methods are described that have the capability of producing submicron / nanoscale particles, in some embodiments dispersible, at high production rates. In some embodiments, the methods result in the production of particles with an average diameter less than about 75 nanometers that are produced at a rate of at least about 35 grams per hour. In other embodiments, the particles are highly uniform. These methods can be used to form particle collections and / or powder coatings. Powder coatings and corresponding methods are described based on the deposition of highly uniform submicron / nanoscale particles.
Owner:NEOPHOTONICS CORP
Thermoelectric material and thermoelectric converting element using the same
Compounds are expressed by general formula of AxBC2-y where 0<=x<=2 and 0<=y<1, and have CdI2 analogous layer structures; A-site is occupied by at least one element selected from the group consisting of Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Zr, Nb, Mo, Ru, Rh, Pd, Ag, Cd, Hf, Ta, W, Re, Ir, Pt, Au, Sc, rare earth elements containing Y, B, Al, Ga, In, Tl, Sn, Pb and Bi; B-site is occupied by at least one element selected from the group consisting of Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, W, Ir, and Sn; C-site is occupied by at least one element selected from the group consisting of S, Se and Te; the compounds exhibit large figure of merit so as to be preferable for thermoelectric generator / refrigerator.
Owner:NEC CORP
Ceria-based mixed-metal oxide structure, including method of making and use
InactiveUS20030186805A1Increase surface areaSimple structureRare earth metal oxides/hydroxidesMaterial nanotechnologyPtru catalystCerium(IV) oxide
A homogeneous ceria-based mixed-metal oxide, useful as a catalyst support, a co-catalyst and / or a getter, is described. The mixed-metal oxide has a relatively large surface area per weight, typically exceeding 150 m<2> / g, a structure of nanocrystallites having diameters of less than 4 nm, and including pores larger than the nanocrystallites and having diameters in the range of 4 to about 9 nm. The ratio of the pore volumes, VP, to skeletal structure volumes, VS, is typically less than about 2.5, and the surface area per unit volume of the oxide material is greater than 320 m<2> / cm<3>, such that the structural morphology supports both a relatively low internal mass transfer resistance and large effective surface area for reaction activity of interest. The mixed metal oxide is made by co-precipitating a dilute metal salt solution containing the respective metals, which may include Zr, Hf, and / or other metal constituents in addition to Ce, replacing water in the co-precipitate with a water-miscible low surface-tension solvent, and relatively quickly drying and calcining the co-precipitate at moderate temperatures. A highly dispersive catalyst metal, such as Pt, may be loaded on the mixed metal oxide support from a catalyst-containing solution following a selected acid surface treatment of the oxide support. The mixed metal oxide, as catalyst support, co-catalyst or getter, is applied in various reactions, and particularly water gas shift and / or preferential oxidation reactions as associated with fuel processing systems, as for fuel cells and the like.
Owner:INT FUEL CELLS
Garnet-type ion conducting oxide, complex, lithium secondary battery, manufacturing method of garnet-type ion conducting oxide and manufacturing method of complex
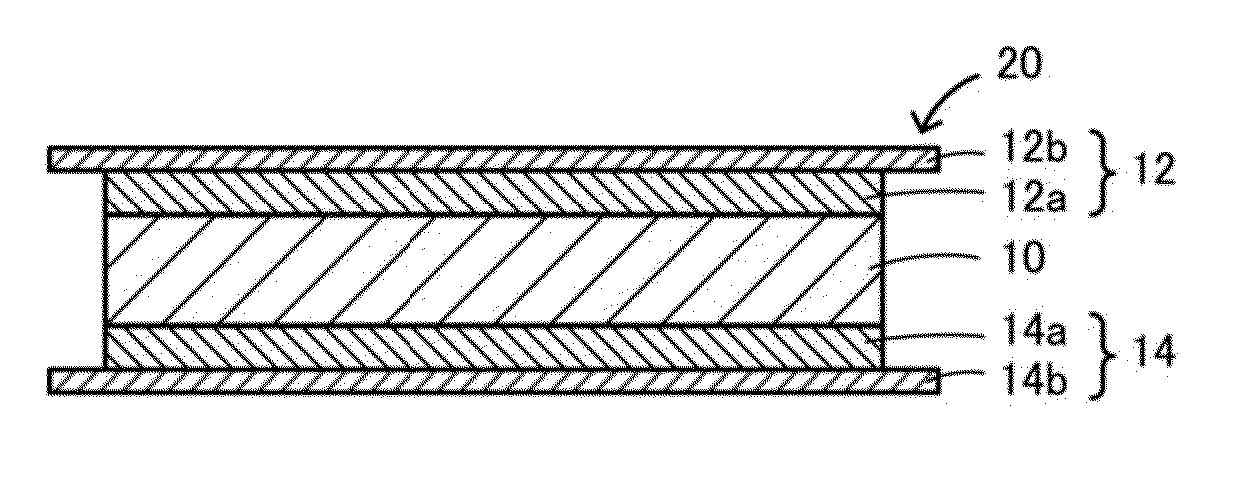
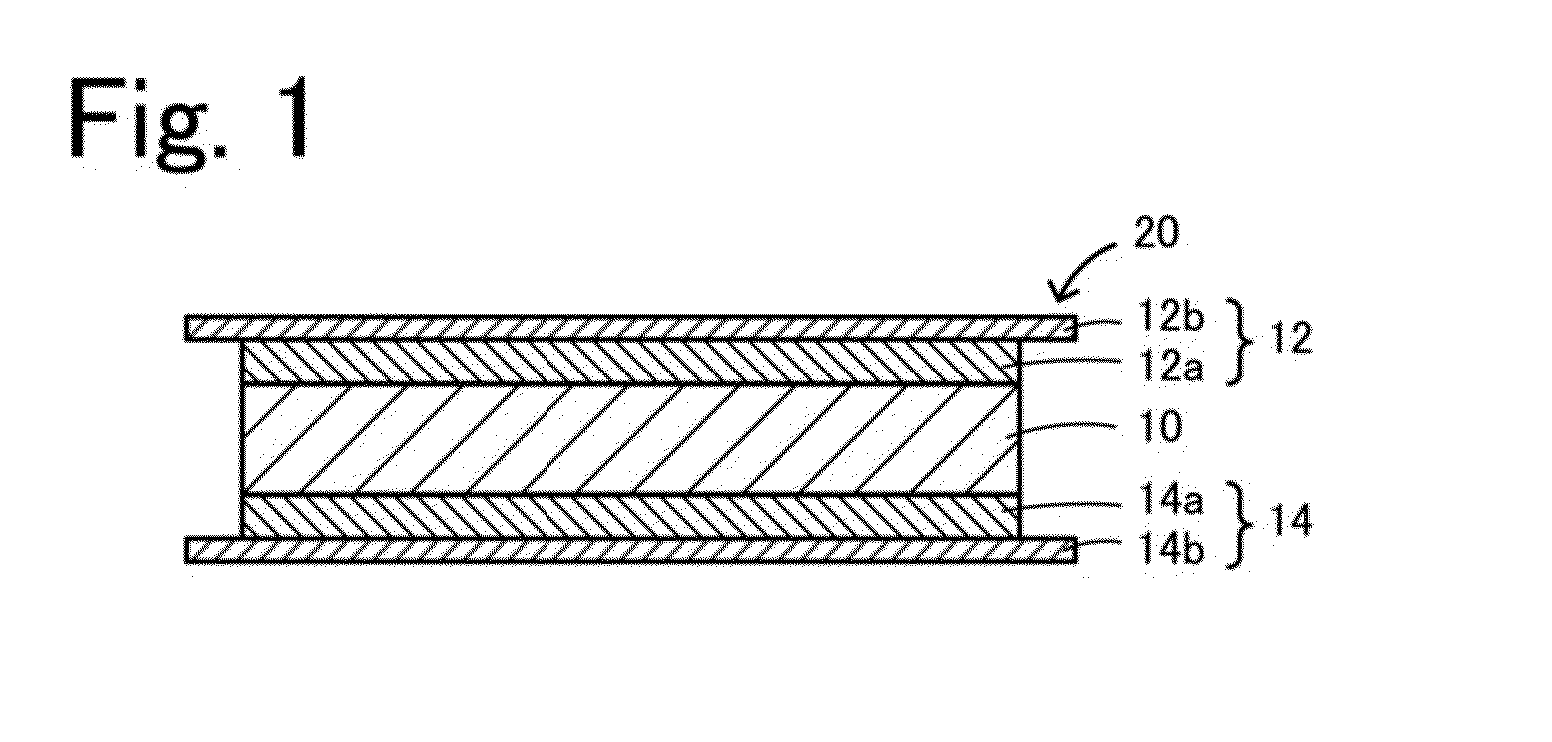
ActiveUS20150056519A1Reduce the temperatureAccelerates dissolution and diffusionElectrode thermal treatmentFinal product manufactureElectrolyteLithium borate
An all-solid lithium secondary battery 20 includes a solid electrolyte layer 10 composed of a garnet-type oxide, a positive electrode 12 formed on one surface of the solid electrolyte layer 10 and a negative electrode 14 formed on the other surface of the solid electrolyte layer 10. This all-solid lithium secondary battery 20 includes an integrally sintered complex of the solid electrolyte layer 10 and the positive electrode active material layer 12a. This complex is obtained by integrally sintering a stacked structure of an active material layer and a solid electrolyte layer. The solid electrolyte layer includes: abase material mainly including a fundamental composition of Li7+X−Y(La3−x,Ax) (Zr2−Y,TY)O12, wherein A is one or more of Sr and Ca, T is one or more of Nb and Ta, and 0≦X≦1.0 and 0≦Y<0.75 are satisfied, as a main component; and an additive component including lithium borate and aluminum oxide.
Owner:TOYOTA CENT RES & DEV LAB INC
Titanium comprising nanoparticles and related nanotechnology
ActiveUS7232556B2Increase volumeLow cost productionNitrogen compoundsGermanium dioxideNanoparticleTitanium metal
Owner:PPG IND OHIO INC
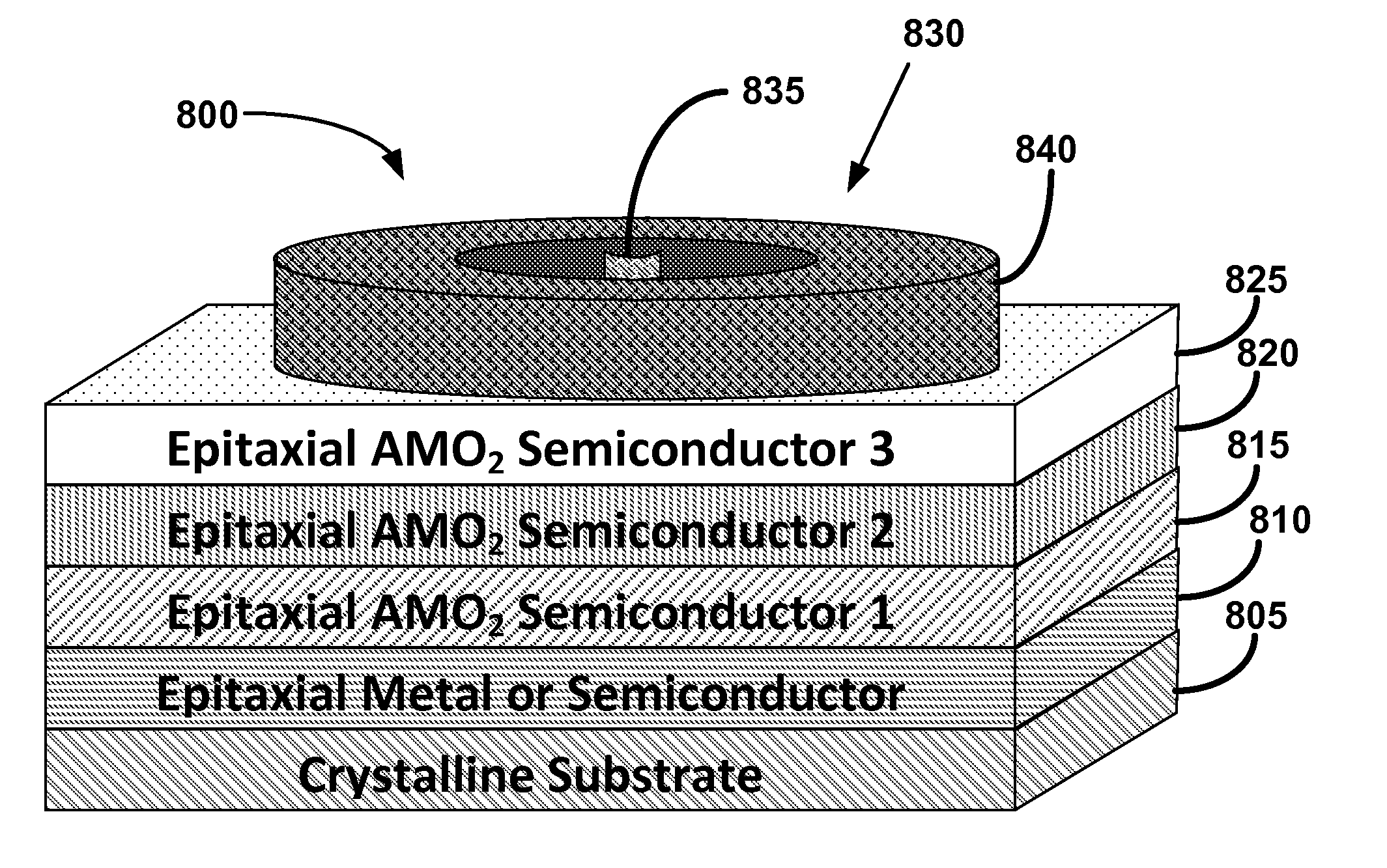
Metal oxide structures, devices, and fabrication methods
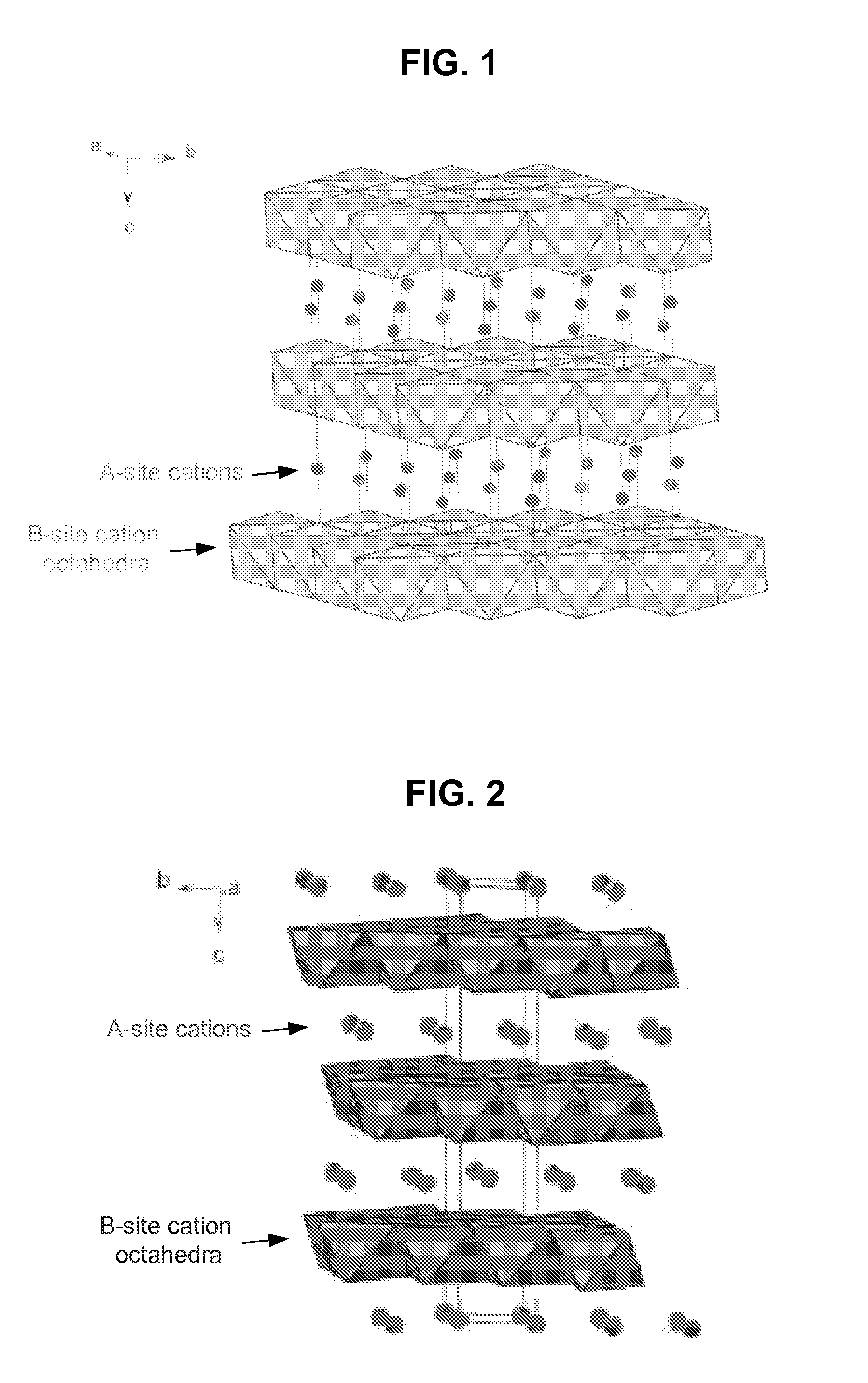
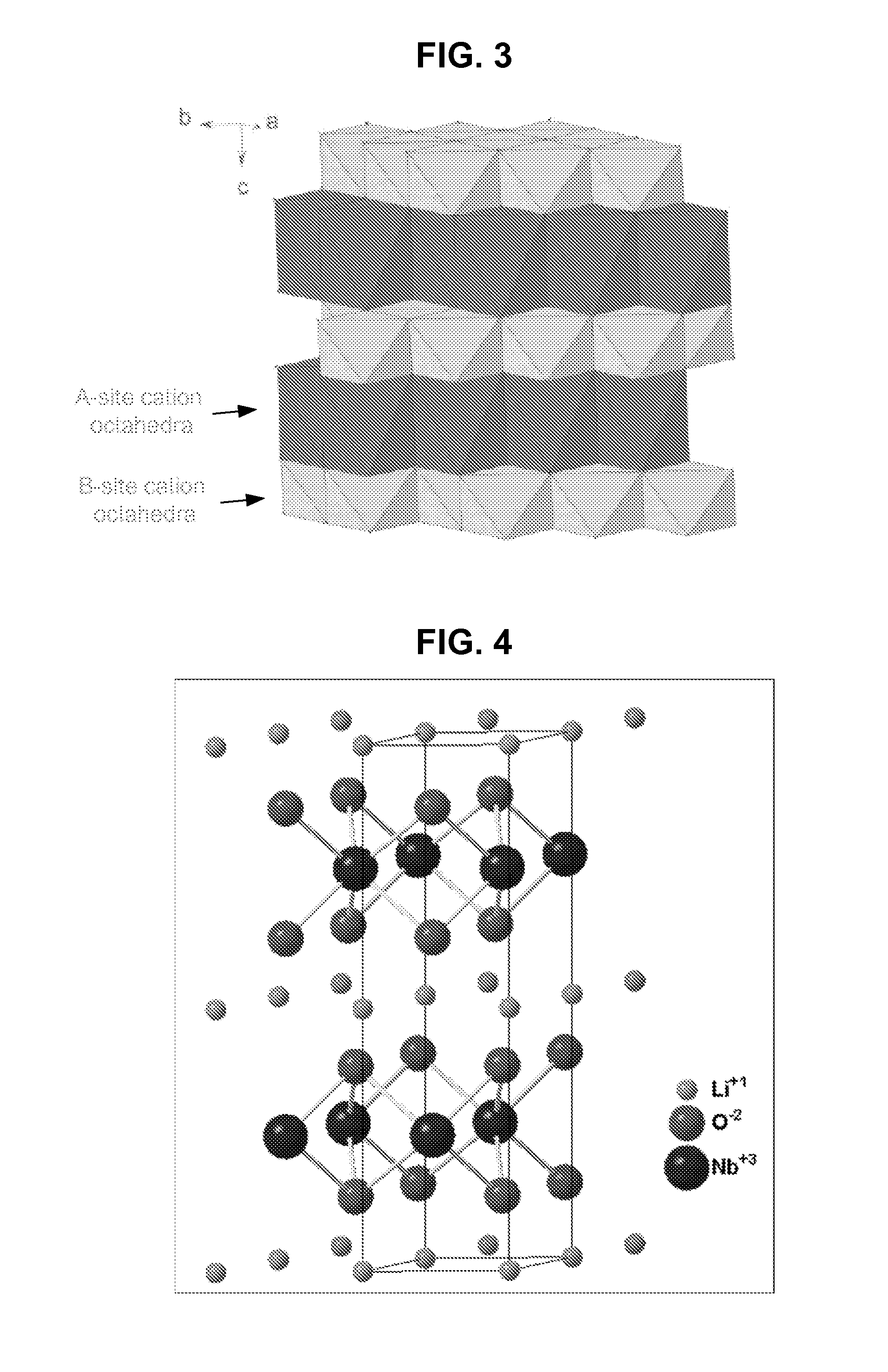
Metal oxide structures, devices, and fabrication methods are provided. In addition, applications of such structures, devices, and methods are provided. In some embodiments, an oxide material can include a substrate and a single-crystal epitaxial layer of an oxide composition disposed on a surface of the substrate, where the oxide composition is represented by ABO2 such that A is a lithium cation, B is a cation selected from the group consisting of trivalent transition metal cations, trivalent lanthanide cations, trivalent actinide cations, trivalent p-block cations, and combinations thereof, and O is an oxygen anion. The unit cell of crystal structure of the oxide composition can be characterized by first layer of a plane of lithium cations and a second layer of a plurality of edge-sharing octahedra having a B cation positioned in a center of each octahedron and an oxygen anion at each corner of each octahedron. The first layer and the second layer of the unit cell are alternatingly stacked along one axis of the unit cell. Other aspects, features, and embodiments are also claimed and described.
Owner:GEORGIA TECH RES CORP
All-solid-state cell
ActiveUS20150044576A1Transportation is highImprove cycle stabilityAlkali titanatesTantalum compoundsAll solid stateSolid state electrolyte
An all-solid-state cell, which includes a lithium-containing anode, a cathode and a lithium ions-conducting solid-state electrolyte separator situated between the anode and the cathode. To improve the safety and cycle stability of the cell, the cathode includes a composite material including at least one lithium titanate and at least one lithium ions-conducting solid-state electrolyte. Furthermore, the invention relates to a corresponding all-solid-state battery and a mobile or stationary system equipped with it.
Owner:ROBERT BOSCH GMBH
Ceria-based mixed-metal oxide structure, including method of making and use
InactiveUS20030235526A1Increased internal surface areaHigh catalytic activityRare earth metal oxides/hydroxidesMaterial nanotechnologyRheniumFuel cells
A homogeneous ceria-based mixed-metal oxide, useful as a catalyst support, a co-catalyst and / or a getter has a relatively large surface area per weight, typically exceeding 150 m<2> / g, a structure of nanocrystallites having diameters of less than 4 nm, and including pores larger than the nanocrystallites and having diameters in the range of 4 to about 9 nm. The ratio of pore volumes, VP, to skeletal structure volumes, VS, is typically less than about 2.5, and the surface area per unit volume of the oxide material is greater than 320 m<2> / cm<3>, for low internal mass transfer resistance and large effective surface area for reaction activity. The mixed metal oxide is ceria-based, includes Zr and or Hf, and is made by a novel co-precipitation process. A highly dispersed catalyst metal, typically a noble metal such as Pt, may be loaded on to the mixed metal oxide support from a catalyst metal-containing solution following a selected acid surface treatment of the oxide support. Appropriate ratioing of the Ce and other metal constituents of the oxide support contribute to it retaining in a cubic phase and enhancing catalytic performance. Rhenium is preferably further loaded on to the mixed-metal oxide support and passivated, to increase the activity of the catalyst. The metal-loaded mixed-metal oxide catalyst is applied particularly in water gas shift reactions as associated with fuel processing systems, as for fuel cells.
Owner:AUDI AG
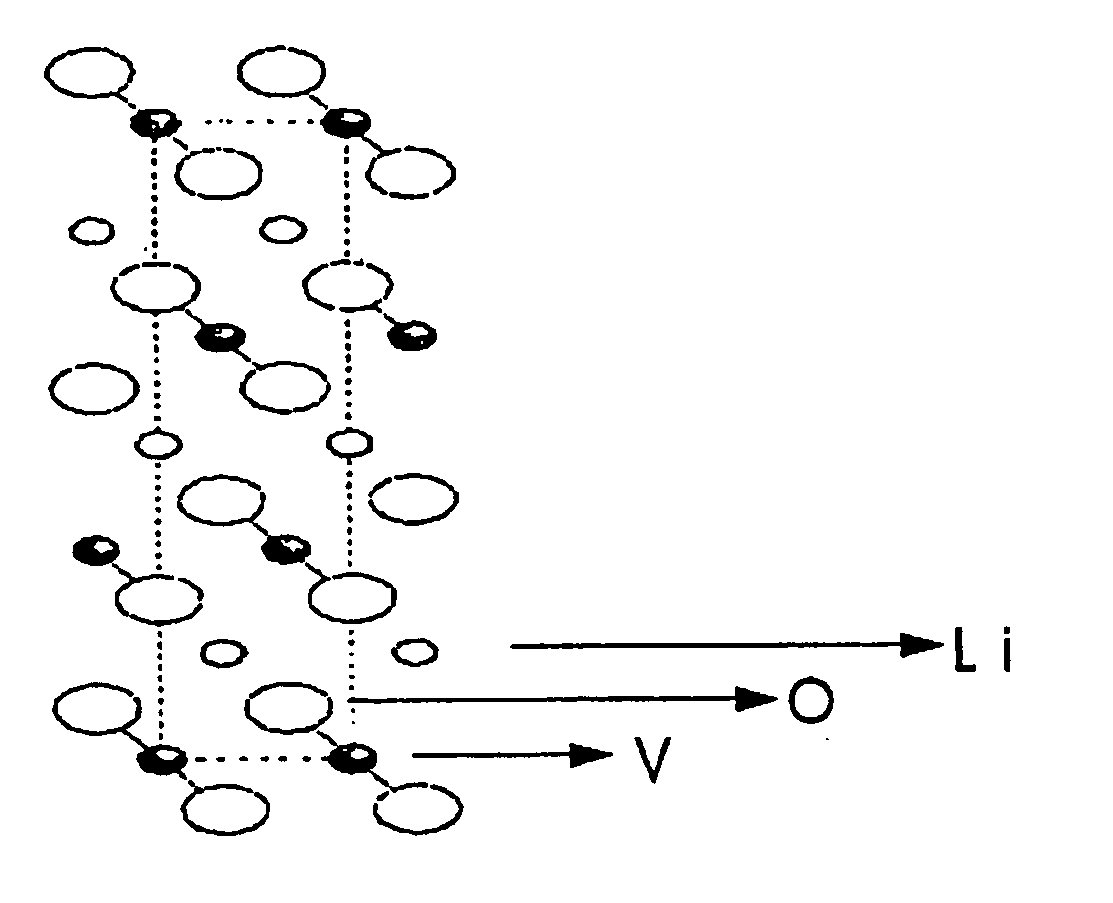
Battery active material, nonaqueous electrolyte battery and battery pack
The invention provides a battery active material. The battery active material includes monoclinic complex oxide represented by the formula Li x Ti 1-y M1 y Nb 2-z M2 z O 7+ Delta (0 <= x <= 5, 0 <= y <= 1, 0 <= z <= 2, -0.3 <= Delta <= 0.3). In the above formula, M1 is at least one element selected from the group consisting of Zr, Si and Sn, and M2 is at least one element selected from the group consisting of V, Ta and Bi.
Owner:KK TOSHIBA
Negative active material for non-aqueous electrolyte battery, method of preparing same, and non-aqueous electrolyte battery comprising same
InactiveUS20050079417A1Improve security featuresNon-aqueous electrolyte accumulatorsMolybdeum compoundsPhysical chemistryNon aqueous electrolytes
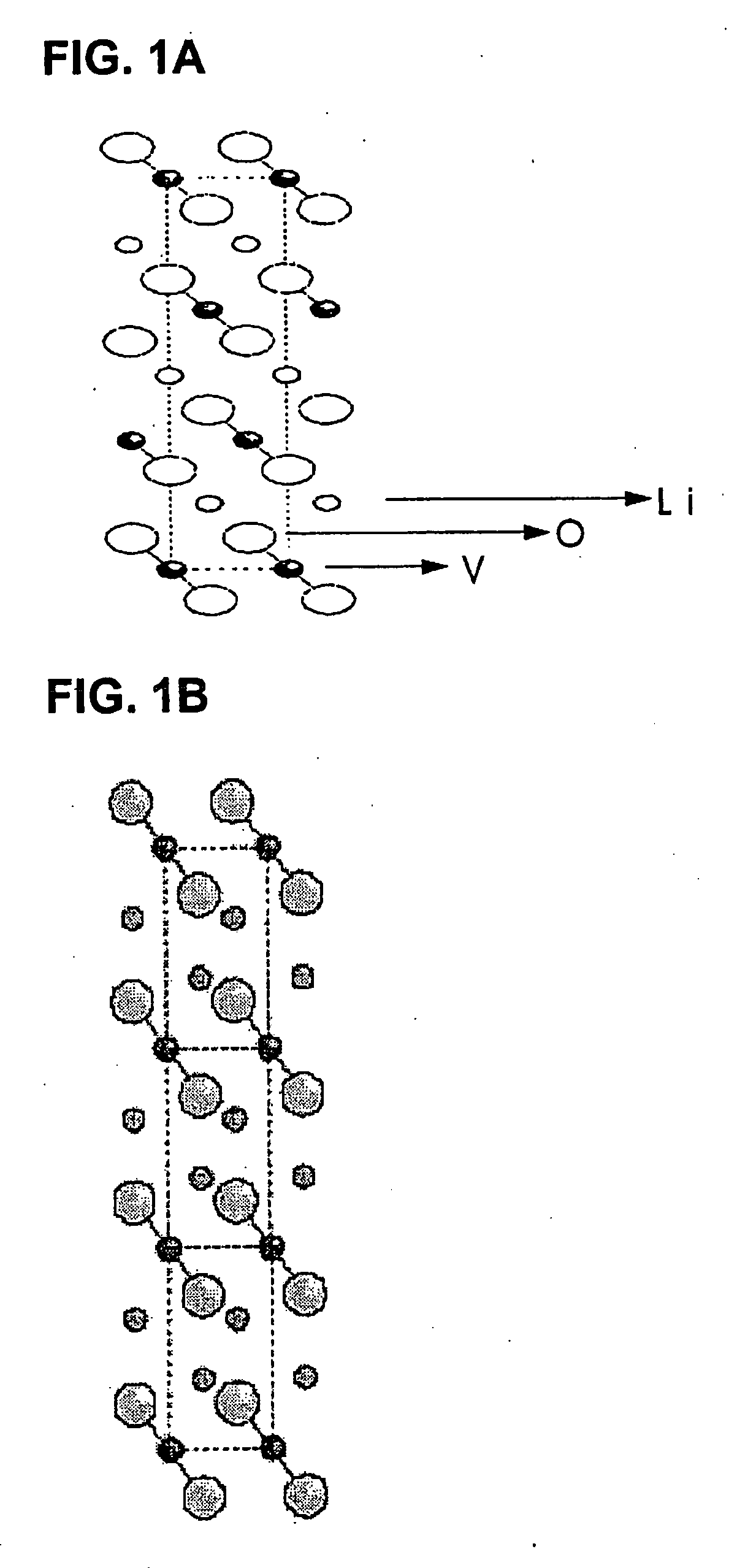
A negative active material of a non-aqueous electrolyte battery includes a compound represented by formula 1: LixMyVzO2+d (1) where 0.1≦x≦2.5, 0<y≦0.5, 0.5≦z≦1.5, 0≦d≦0.5, and M is at least one element selected from the group consisting of Al, Cr, Mo, Ti, W, and Zr.
Owner:SAMSUNG SDI CO LTD
Titanium comprising nanoparticles and related nanotechnology
ActiveUS20050191492A1Increase volumeLow cost productionNitrogen compoundsGermanium dioxideNanoparticleTitanium metal
Owner:PPG IND OHIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com