Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
772 results about "Cerium(IV) oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
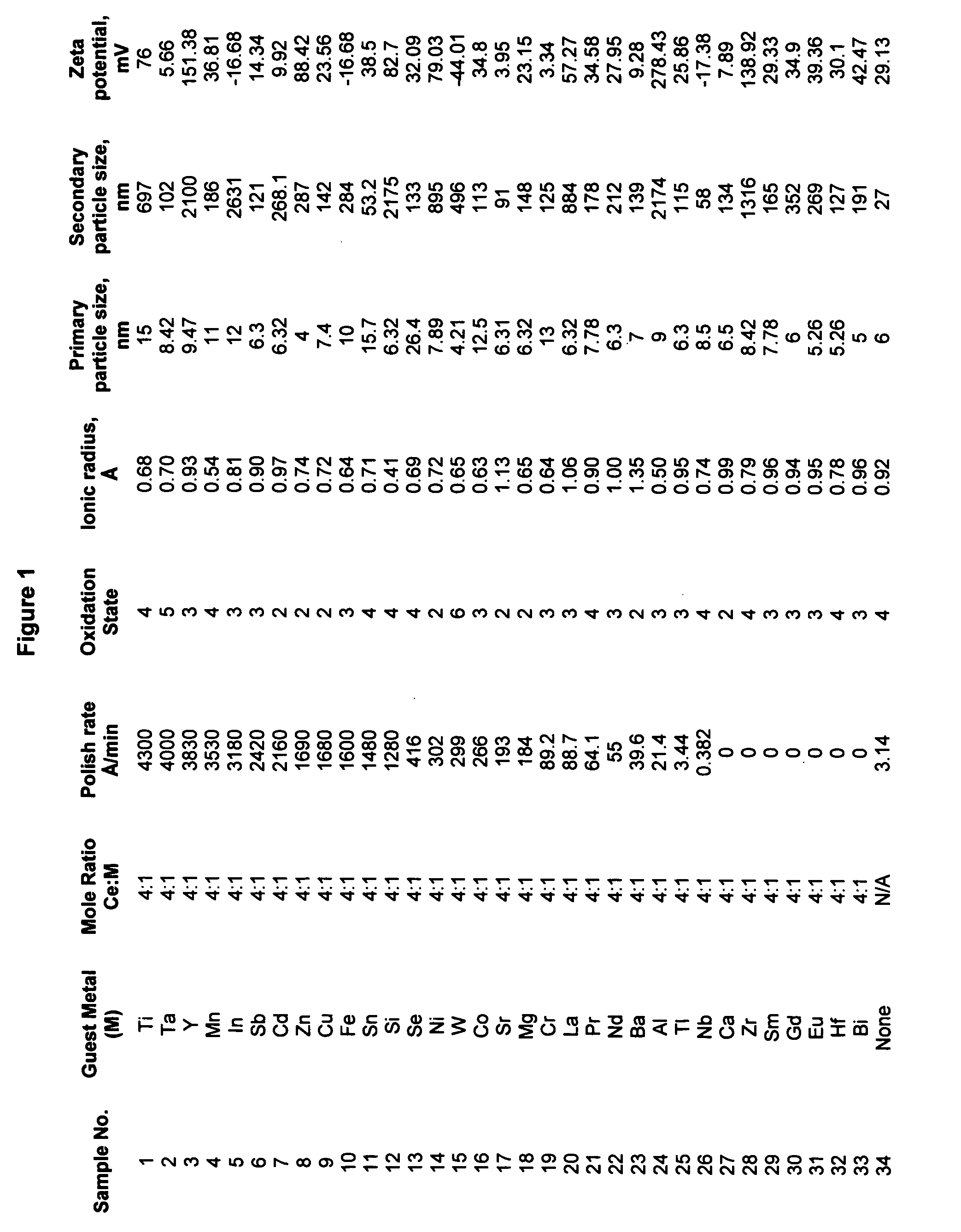
Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO₂. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a nonstoichiometric oxide.
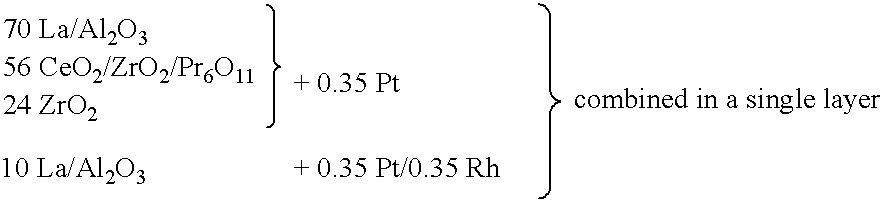
Layered noble metal-containing exhaust gas catalyst and its preparation
InactiveUS6294140B1Shorten recovery timeImprove conversion efficiencyOrganic chemistryNitrogen compoundsCerium(IV) oxideEngineering
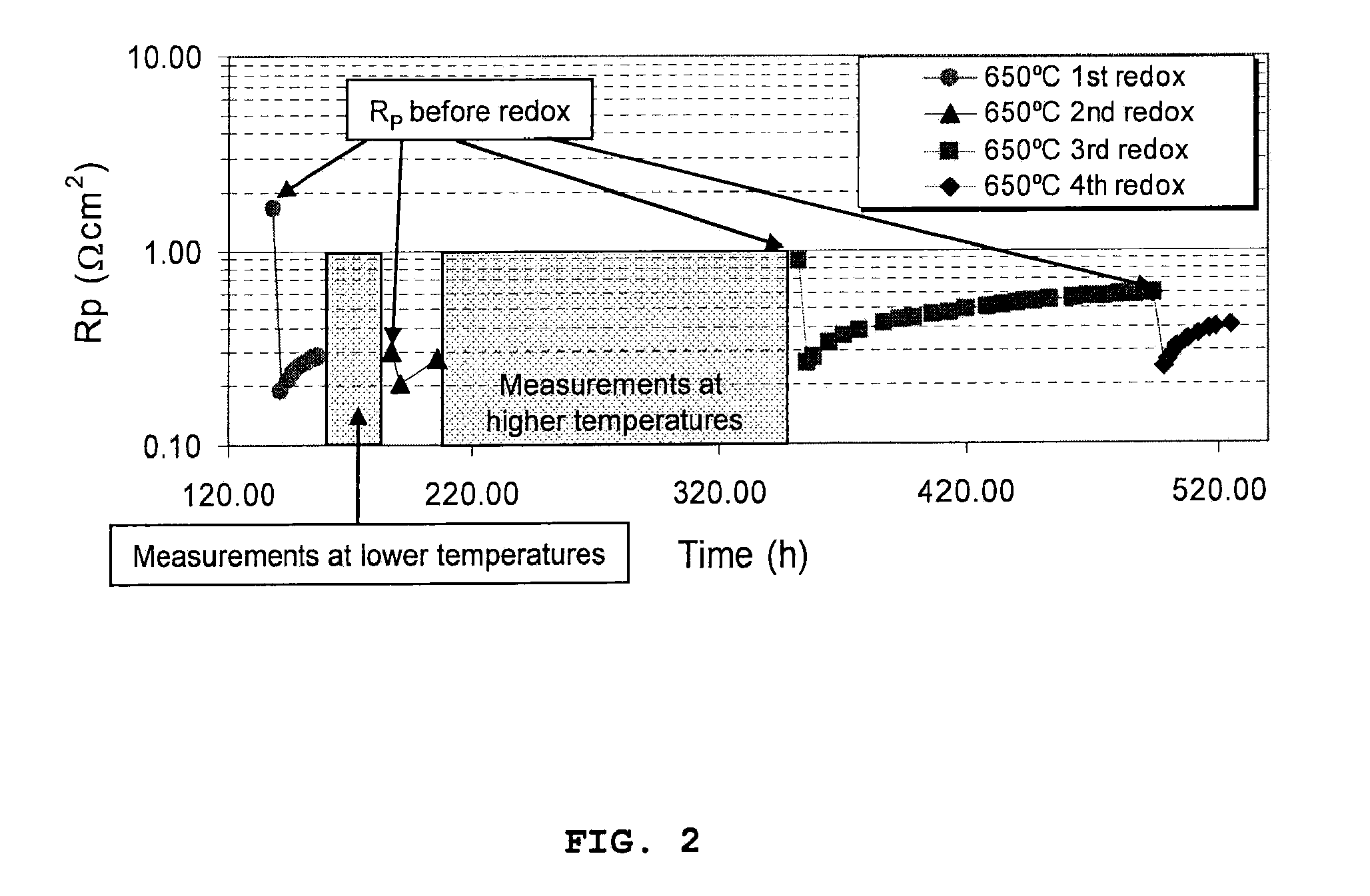
A catalyst for treating exhaust gas from an internal combustion engine includes a carrier body coated with an inner layer and an outer layer. The inner layer includes platinum deposited on a first support material and on a first oxygen storage component, and the outer layer includes platinum and rhodium deposited on a second support material and on a second oxygen storage component. The first and second support materials may be the same or different, and may be selected from the group of: silica, alumina, titania, zirconia, mixed oxides or mixtures thereof, and zirconia-rich zirconia / ceria mixed oxide. The first and second oxygen storage components may include ceria-rich ceria / zirconia mixed oxide compounds, optionally including praseodymia, yttria, neodymia, lanthana or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
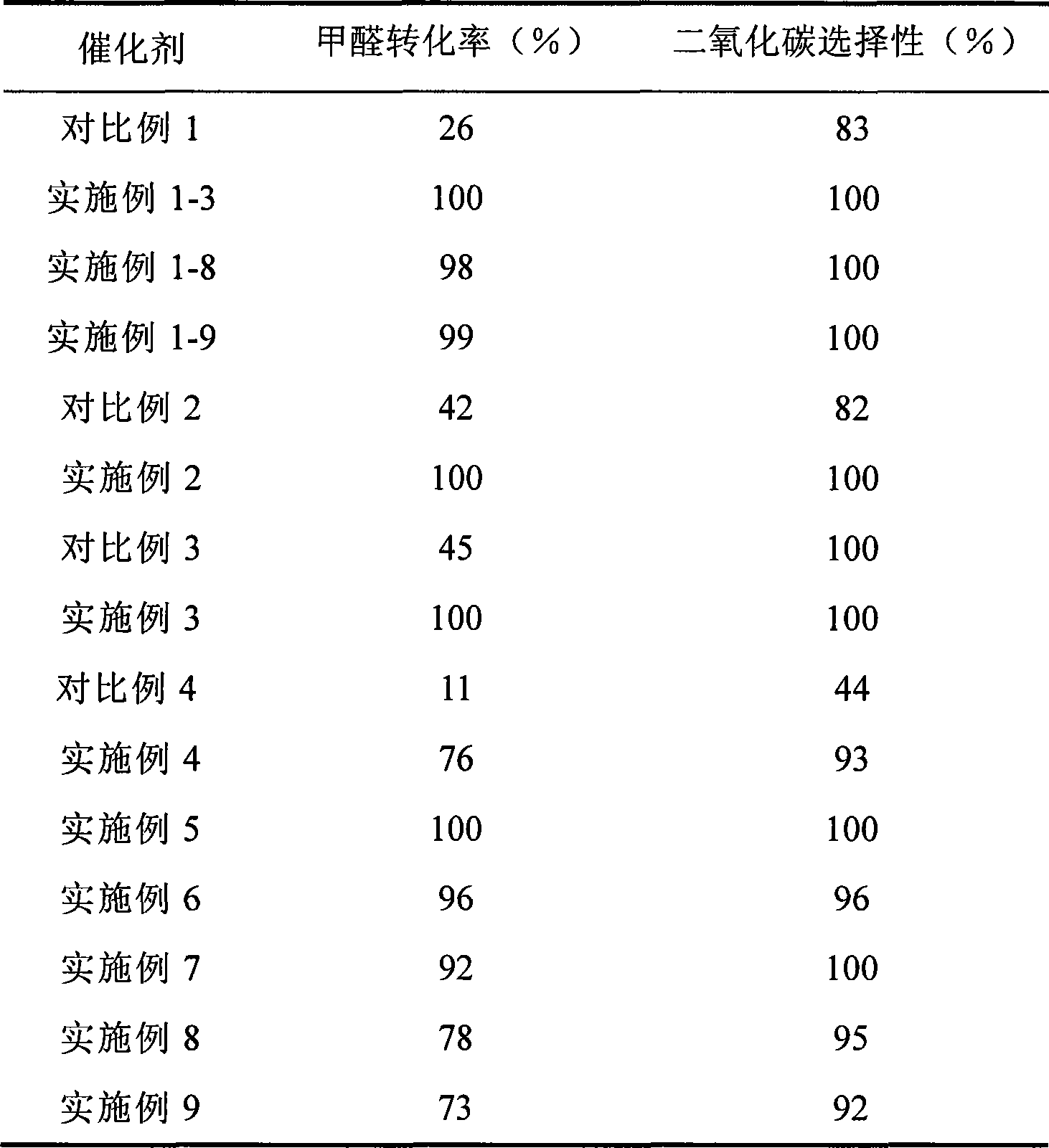
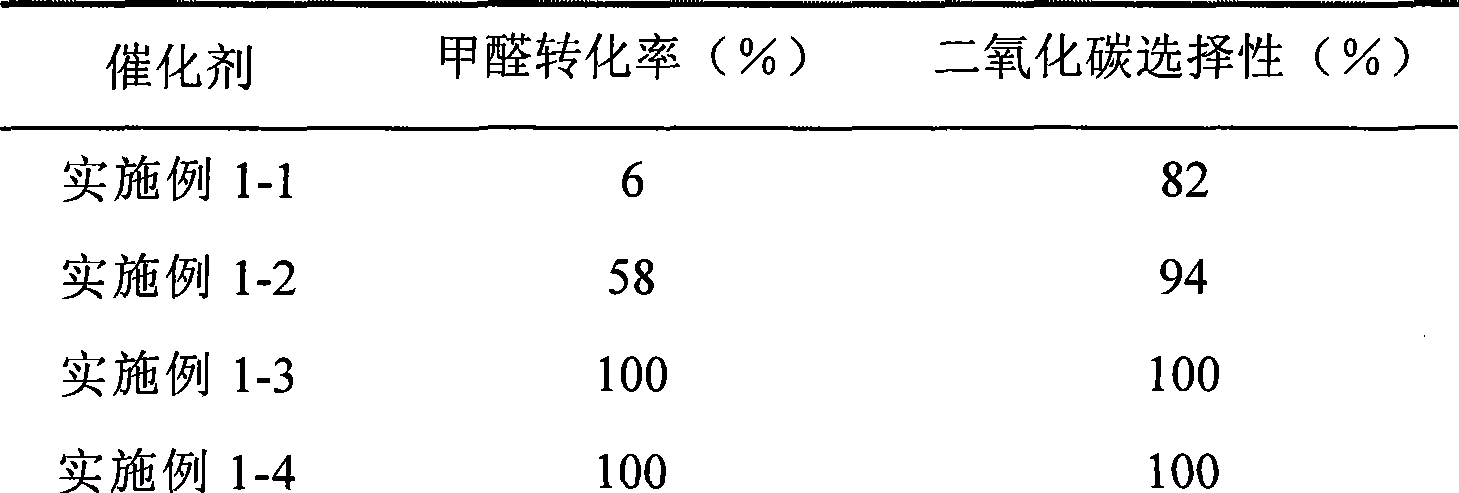
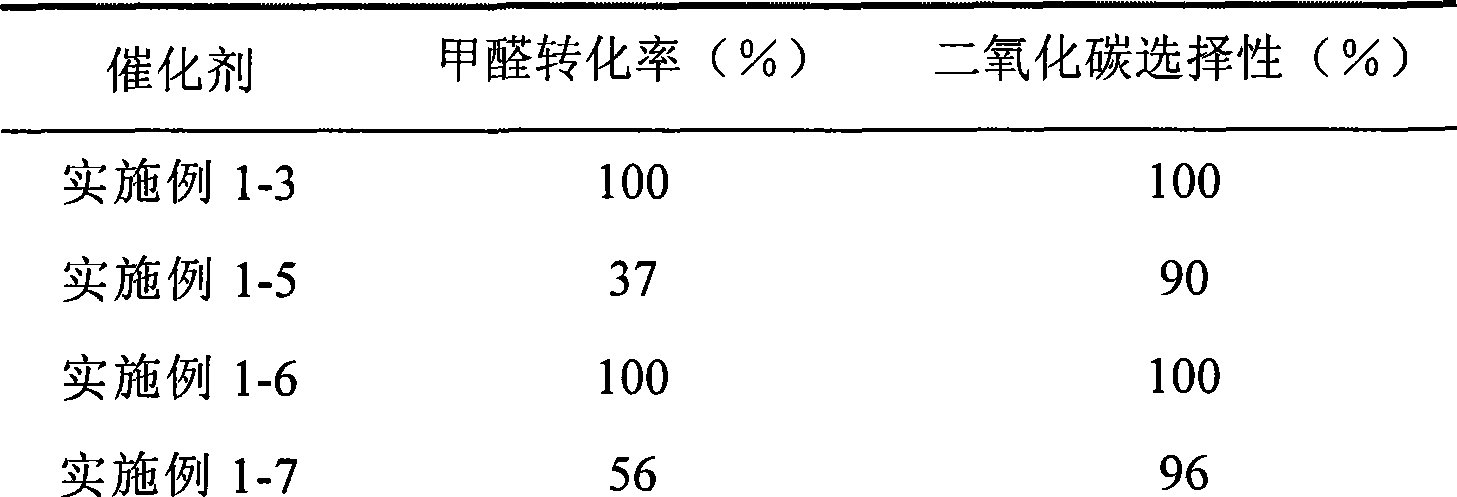
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
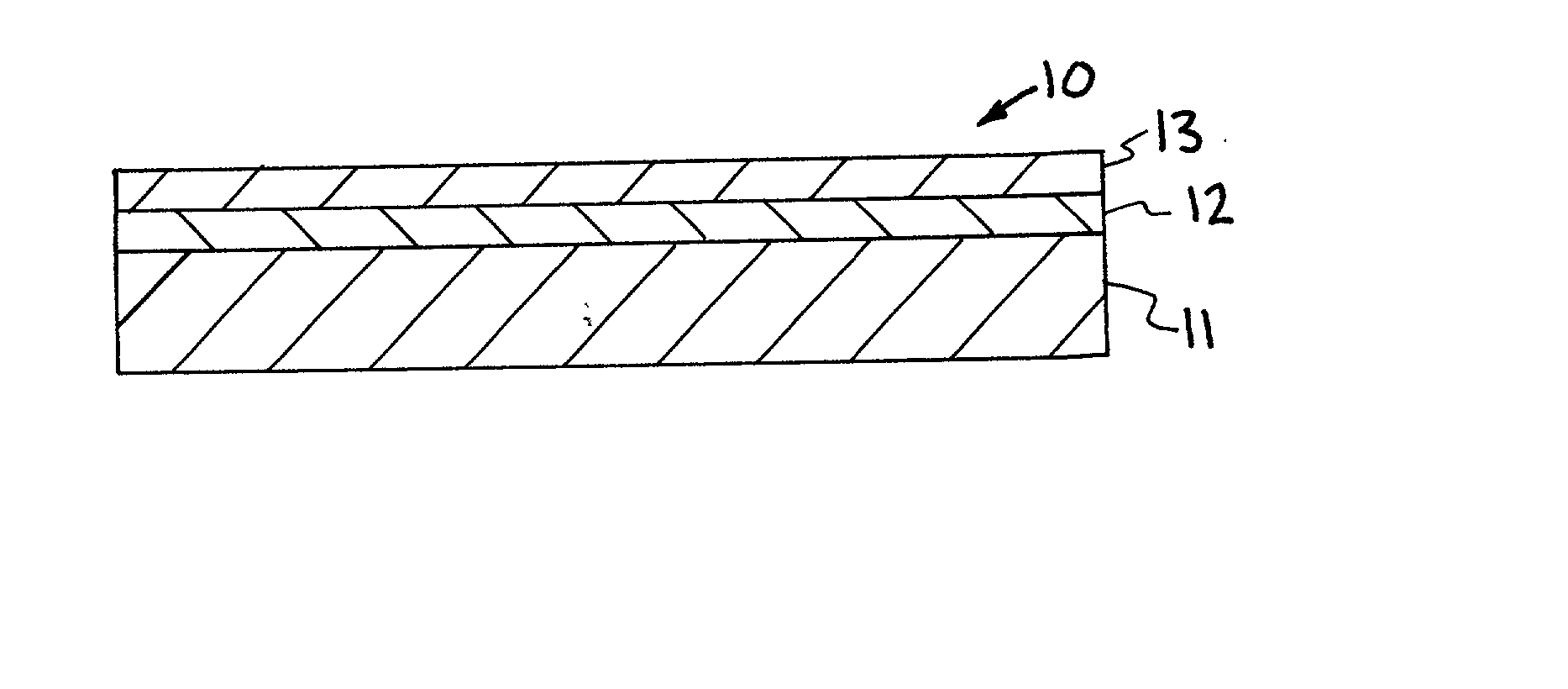
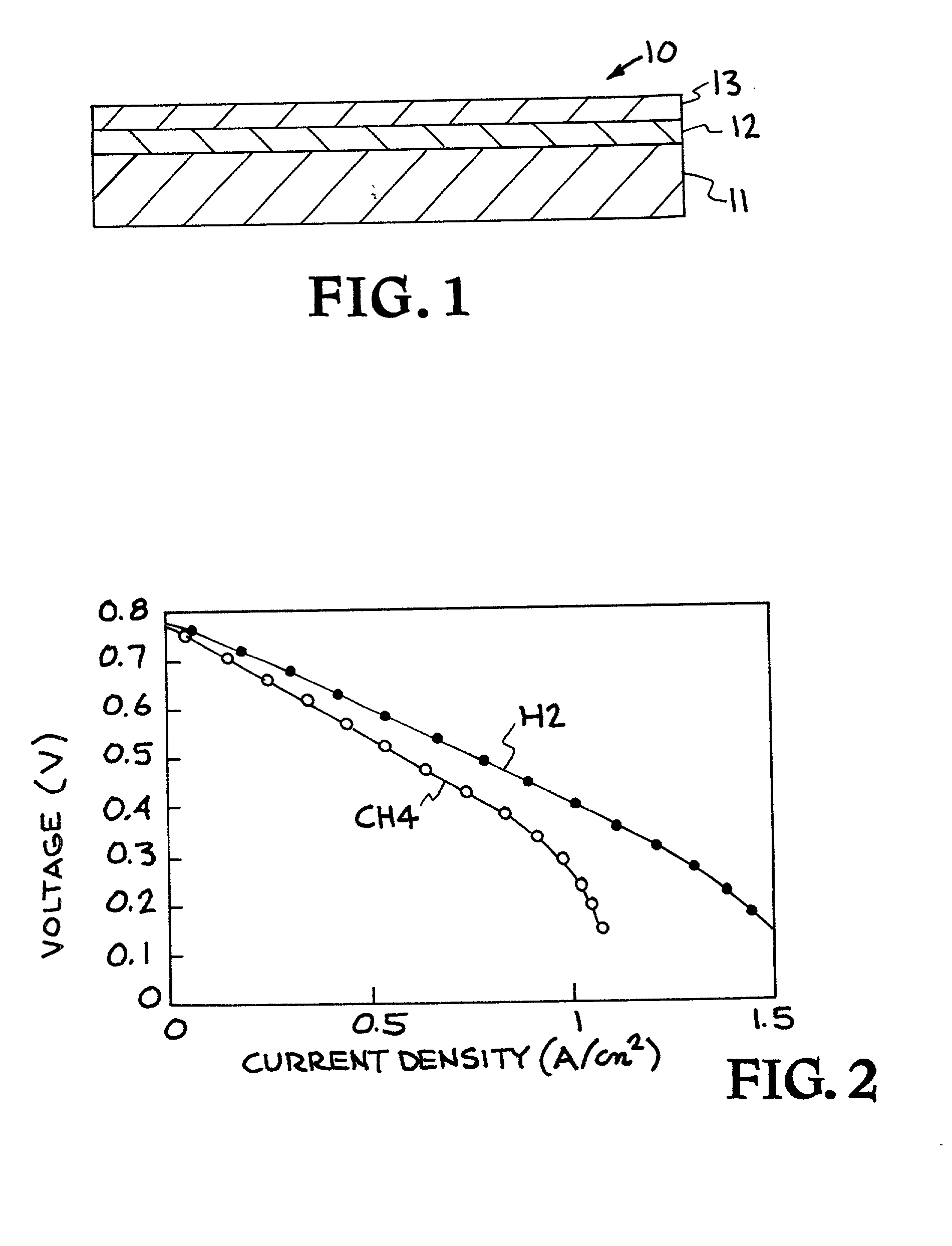
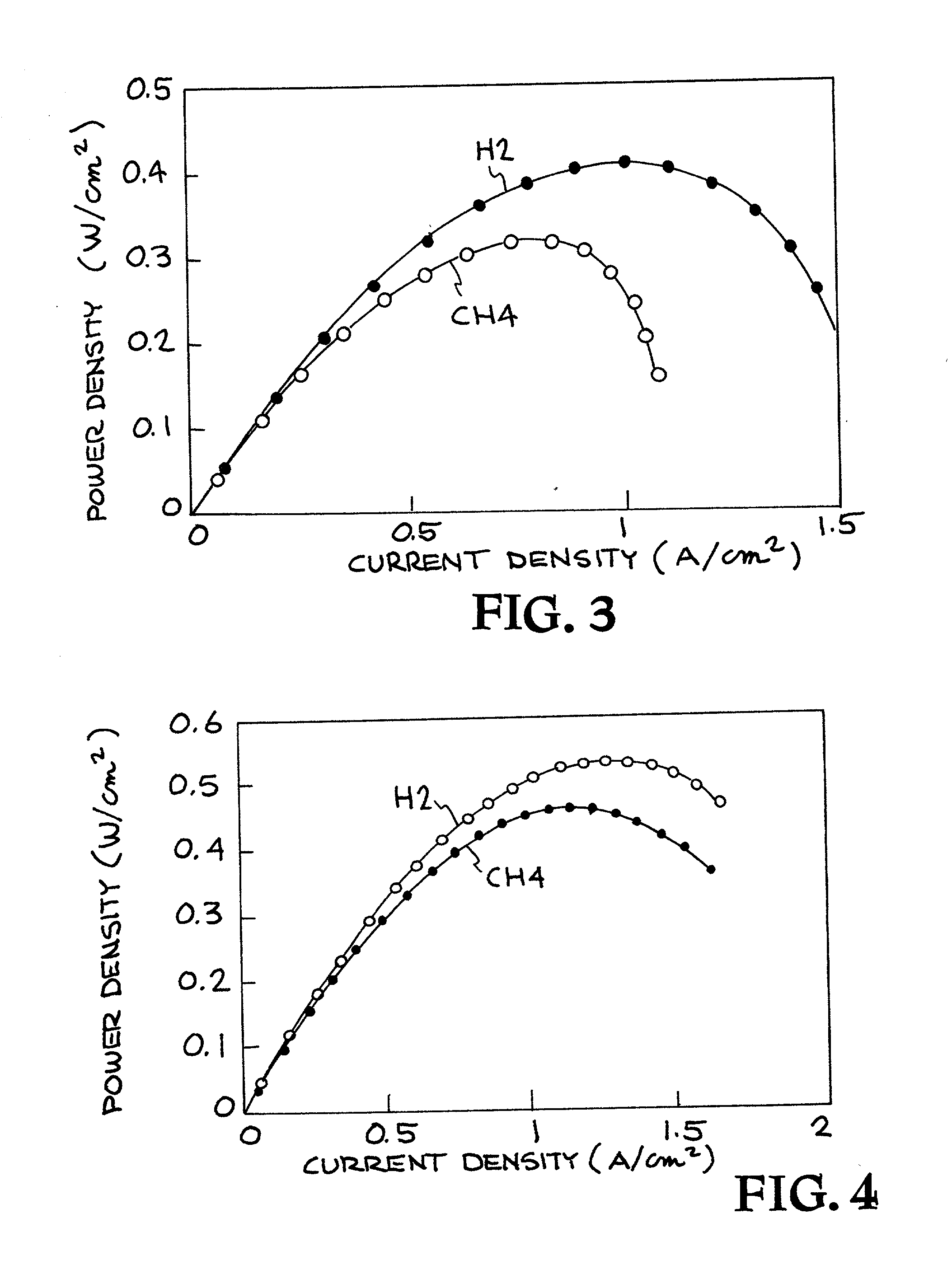
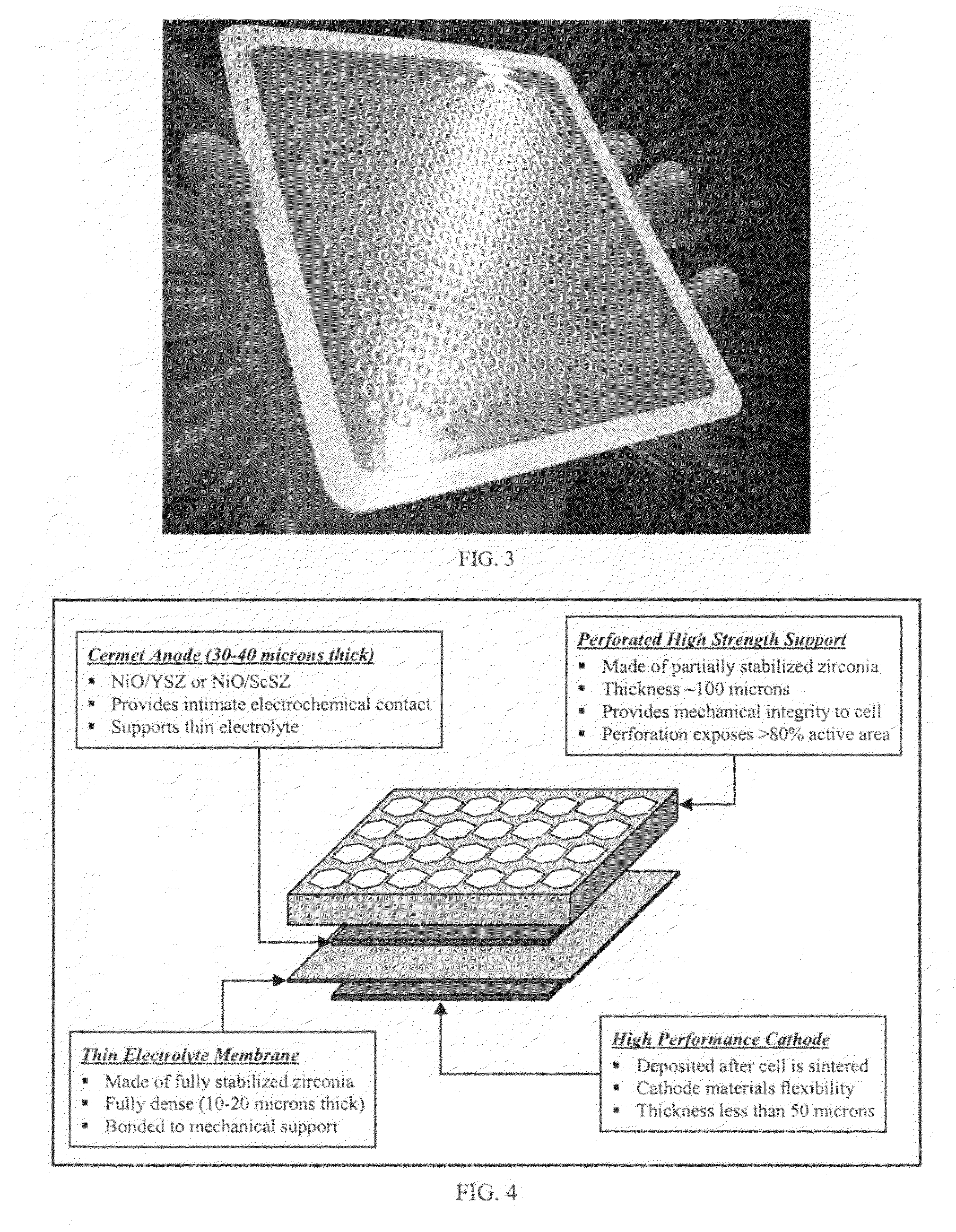
Ceria-based solid oxide fuel cells
InactiveUS20020127455A1Improve power densityMore option for sealingCell electrodesFuel cell auxillariesCerium(IV) oxideCobalt
High-performance intermediate temperature solid oxide fuel cells (SOFCs). The SOFCs are ceria-based structures that can achieve a power output of 300 mW / cm.sup.2 at an operating temperature below 600.degree. C. By way of example, the fuel cell as an anode made of NiO / doped-ceria, a thin film of doped-ceria and / or doped zirconia electrolyte is deposited on the anode, and a cathode of cobalt iron based material, such as (La, Sr)(Co, Fe)O.sub.3 or cobalt, ion, magnesium based material, is deposited on top of the electrolyte, and can operate at a temperature of 550.degree. C. The various layers may be deposited by colloidal spray deposition or aerosol spray casting.
Owner:RGT UNIV OF CALIFORNIA
Nanocomposite copper-ceria catalysts for low temperature or near-ambient temperature catalysis and methods for making such catalysts
InactiveUS6857431B2Reduce the amount requiredTobacco preparationNon-fibrous pulp additionPtru catalystHydrocotyle bowlesioides
Nanocomposite copper-ceria catalysts are provided, which comprise copper oxide nanoparticles, copper nanoparticles, or a mixture thereof combined with ceria nanoparticles. Methods for making such catalysts are also provided, which involve the steps of (i) combining ceria nanoparticles in an aqueous suspension with copper 2,4-pentanedionate to form a slurry; (ii) heating the slurry formed in step (i) under an inert gas atmosphere or an oxygen-argon atmosphere, at a temperature and for a time sufficient to cause decomposition of the copper 2,4-pentanedionate to form copper nanoparticles and / or copper oxide nanoparticles that are combined with the ceria nanoparticles; and (iii) optionally, subjecting the product formed in step (ii) to a heat treatment process under conditions effective to convert at least some of the copper nanoparticles to copper oxide nanoparticles. The nanocomposite copper-ceria catalysts are useful for low-temperature and near-ambient temperature catalysis, such as the oxidation of carbon monoxide, the reduction of nitric oxide and the conversion of hydrocarbons. The nanocomposite copper-ceria catalysts have a variety of potential applications, for example, in vehicle exhaust emission systems of automobiles and diesel engines, cold starting of automobile engine, fuel cells, lasers, hydrocarbon conversion reactors, air filters for the conversion of carbon monoxide and / or indoor volatile organic compounds, and smoking articles.
Owner:PHILIP MORRIS USA INC
Exhaust gas catalyst
InactiveUS6180075B1Favorable degree of conversionExceptional heat and aging resistanceNitrogen compoundsInternal combustion piston enginesZirconium hydrideCerium(IV) oxide
A single-layered three-way catalytic converter containing palladium as the only catalytically active noble metal, with high activity and heat resistance. The catalyst contains, in addition to finely divided, stabilized aluminum oxide, at least one finely divided cerium / zirconium mixed oxide and optionally finely divided nickel oxide as well as highly dispersed amounts of cerium oxide, zirconium oxide and barium oxide. The palladium is distributed largely uniformly throughout the entire catalyst.
Owner:DMC2 DEGUSSA METALS +1
Layered exhaust treatment catalyst
ActiveUS7795172B2Molecular sieve catalystsInternal combustion piston enginesCerium(IV) oxideMonitoring system
Owner:BASF CORP
Supported noble metal catalyst for low-temperature catalytic oxidation benzene series and preparation method thereof
The invention discloses a supported noble metal catalyst for low-temperature catalytically oxidizing benzene series (benzene, methylbenzene and dimethylbenzene) gas to CO2 and H2O. Active components of the noble metal catalyst are: at least one of Pd, Pt, Ag, Au and Rh; carrier is at least one of active carbon, red mud, molecular sieve, aluminum sesquioxide, titanium dioxide, manganese dioxide, zirconium dioxide, silicon dioxide, cerium dioxide, lanthanum sesquioxide, cobalt oxide, magnesium oxide, zinc oxide, calcium oxide and cupric oxide. Under general pressure, in atmosphere ambient, in fixed bed reactor, with a space velocity range from 10,000 to 100,000h<-1> and at a temperature range from 110 to 210 DEG C, the catalyst can be used for directly oxidizing 100 to 800ppm benzene series gas to CO2 and H2O without byproduct, which shows good low-temperature catalytic activity. The catalyst of the invention has the advantages of simple preparation, lower complete oxidation temperature, immunity to H2O, good stability and high practical value.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Base metal catalysts for the oxidation of carbon monoxide and volatile organic compounds
A method for oxidizing carbon monoxide (CO) and volatile organic compounds (VOCS) comprises contacting a gas containing water vapor and said CO and VOCs with a catalyst composition comprising at least one base metal promoter and at least one base metal catalyst supported on an oxide support material comprising one or more of alumina, silica, zirconia, ceria, and titania, wherein the VOCs comprise one or more of methyl acetate, methane, methyl bromide, benzene, methanol, methyl ethyl ketone, butane, and butene.
Owner:JOHNSON MATTHEY PLC
Ceria-based mixed-metal oxide structure, including method of making and use
InactiveUS20030186805A1Increase surface areaSimple structureRare earth metal oxides/hydroxidesMaterial nanotechnologyPtru catalystCerium(IV) oxide
A homogeneous ceria-based mixed-metal oxide, useful as a catalyst support, a co-catalyst and / or a getter, is described. The mixed-metal oxide has a relatively large surface area per weight, typically exceeding 150 m<2> / g, a structure of nanocrystallites having diameters of less than 4 nm, and including pores larger than the nanocrystallites and having diameters in the range of 4 to about 9 nm. The ratio of the pore volumes, VP, to skeletal structure volumes, VS, is typically less than about 2.5, and the surface area per unit volume of the oxide material is greater than 320 m<2> / cm<3>, such that the structural morphology supports both a relatively low internal mass transfer resistance and large effective surface area for reaction activity of interest. The mixed metal oxide is made by co-precipitating a dilute metal salt solution containing the respective metals, which may include Zr, Hf, and / or other metal constituents in addition to Ce, replacing water in the co-precipitate with a water-miscible low surface-tension solvent, and relatively quickly drying and calcining the co-precipitate at moderate temperatures. A highly dispersive catalyst metal, such as Pt, may be loaded on the mixed metal oxide support from a catalyst-containing solution following a selected acid surface treatment of the oxide support. The mixed metal oxide, as catalyst support, co-catalyst or getter, is applied in various reactions, and particularly water gas shift and / or preferential oxidation reactions as associated with fuel processing systems, as for fuel cells and the like.
Owner:INT FUEL CELLS
Metal oxide nanorod arrays on monolithic substrates
ActiveUS20140256534A1Improve thermal stabilitySulfur poisoningMolecular sieve catalystsLayered productsCerium(IV) oxideMetal particle
A metal oxide nanorod array structure according to embodiments disclosed herein includes a monolithic substrate having a surface and multiple channels, an interface layer bonded to the surface of the substrate, and a metal oxide nanorod array coupled to the substrate surface via the interface layer. The metal oxide can include ceria, zinc oxide, tin oxide, alumina, zirconia, cobalt oxide, and gallium oxide. The substrate can include a glass substrate, a plastic substrate, a silicon substrate, a ceramic monolith, and a stainless steel monolith. The ceramic can include cordierite, alumina, tin oxide, and titania. The nanorod array structure can include a perovskite shell, such as a lanthanum-based transition metal oxide, or a metal oxide shell, such as ceria, zinc oxide, tin oxide, alumina, zirconia, cobalt oxide, and gallium oxide, or a coating of metal particles, such as platinum, gold, palladium, rhodium, and ruthenium, over each metal oxide nanorod. Structures can be bonded to the surface of a substrate and resist erosion if exposed to high velocity flow rates.
Owner:UNIV OF CONNECTICUT
Single layer high performance catalyst
InactiveUS6524992B2Improve catalytic performanceLow production costInternal combustion piston enginesExhaust apparatusPlatinumMixed oxide
A single layer high performance catalyst containing on an inert carrier body a catalytic coating comprising platinum, rhodium and various oxide materials. The catalyst contains a catalytic coating having at least one first support material selected from the group having a first active alumina, a ceria rich ceria / zirconia mixed oxide and a zirconia component, said at least one first support material being catalyzed with a first part of the total platinum amount of the catalyst, and a second support material catalyzed with the second part of the total platinum amount and with rhodium said second support material being a second active alumina.
Owner:UMICORE AG & CO KG +1
NOx reduction composition for use in FCC processes
A composition for controlling NOx emissions during FCC processes comprises (i) an acidic oxide support, (ii) cerium oxide, (iii) a lanthanide oxide other than ceria such as praseodymium oxide, and (iv), optionally, an oxide of a metal from Groups Ib and IIb such as copper, silver and zinc.
Owner:ENGELHARD CORP
Ceria-based polish processes, and ceria-based slurries
InactiveUS7056192B2Minimize timeImprove throughputPigmenting treatmentOther chemical processesSilica particleGas phase
Owner:INT BUSINESS MASCH CORP
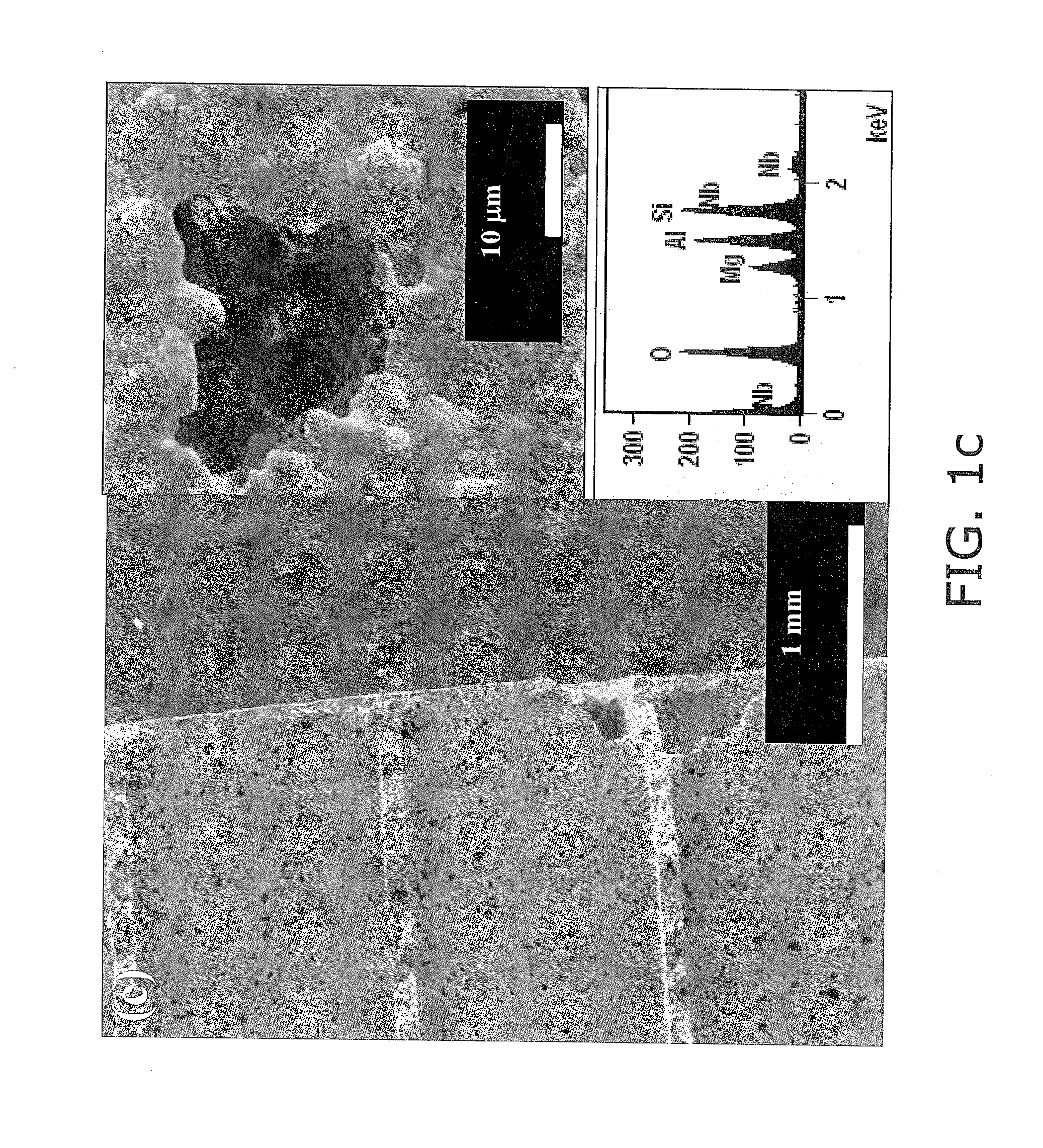
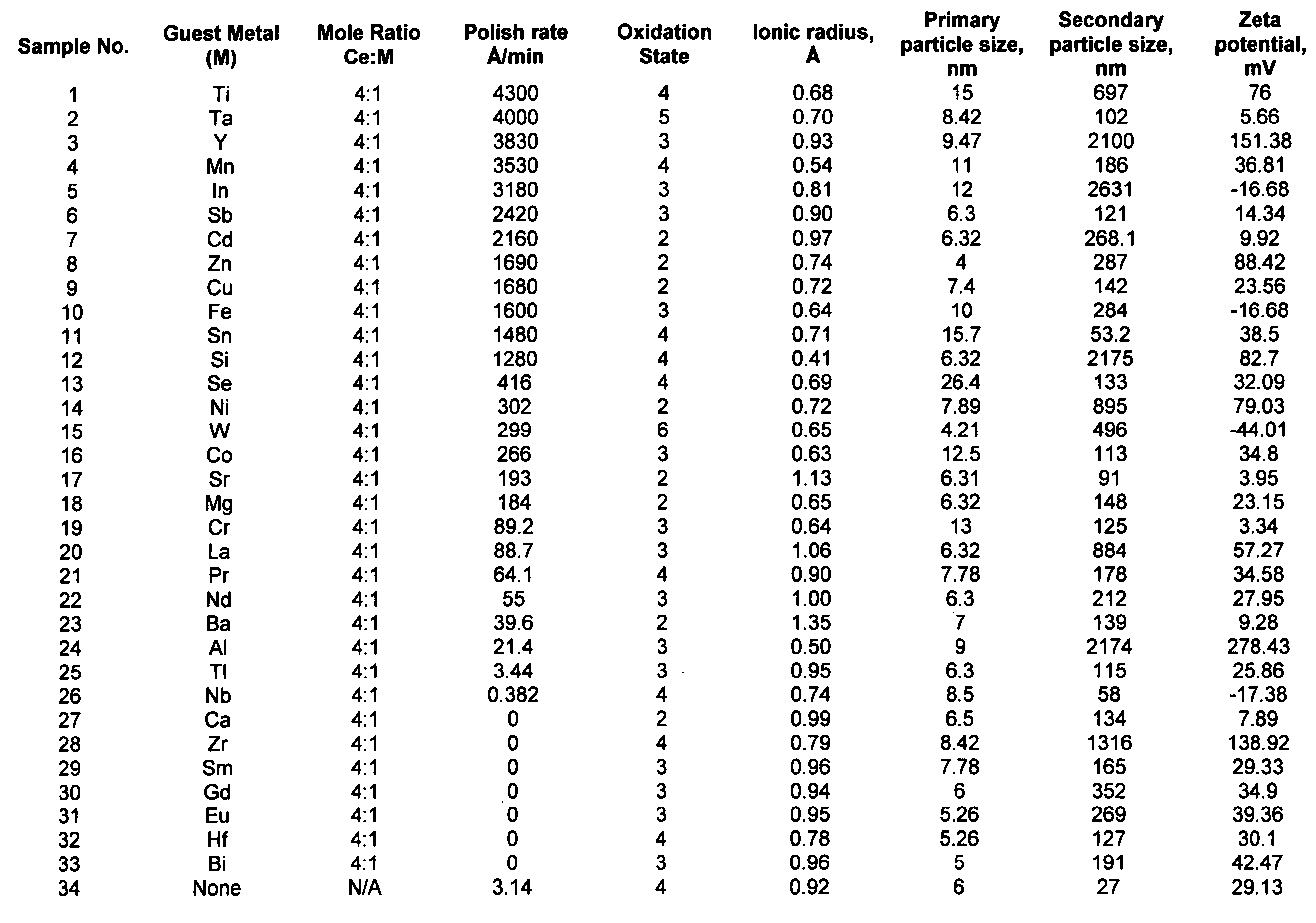
Synthesis of chemically reactive ceria composite nanoparticles and CMP applications thereof
InactiveUS20050003744A1Eliminate concernsReduce environmental impactPigmenting treatmentMaterial nanotechnologyChemical reactionCerium
The present invention provides a method of synthesizing nanosized abrasive particles and methods of using the same in chemical mechanical polishing slurry applications. The nanosized abrasive particles according to the invention are produced by hydrothermal synthesis. The crystallites of the particles include cerium atoms and atoms of metals other than cerium. In a preferred embodiment of the invention, the crystallites exhibit a cubic crystal lattice structure. The differences in electric potential between the cerium atoms and the atoms of metals other than cerium facilitate the polishing of films without the need for chemical oxidizers.
Owner:FERRO CORP
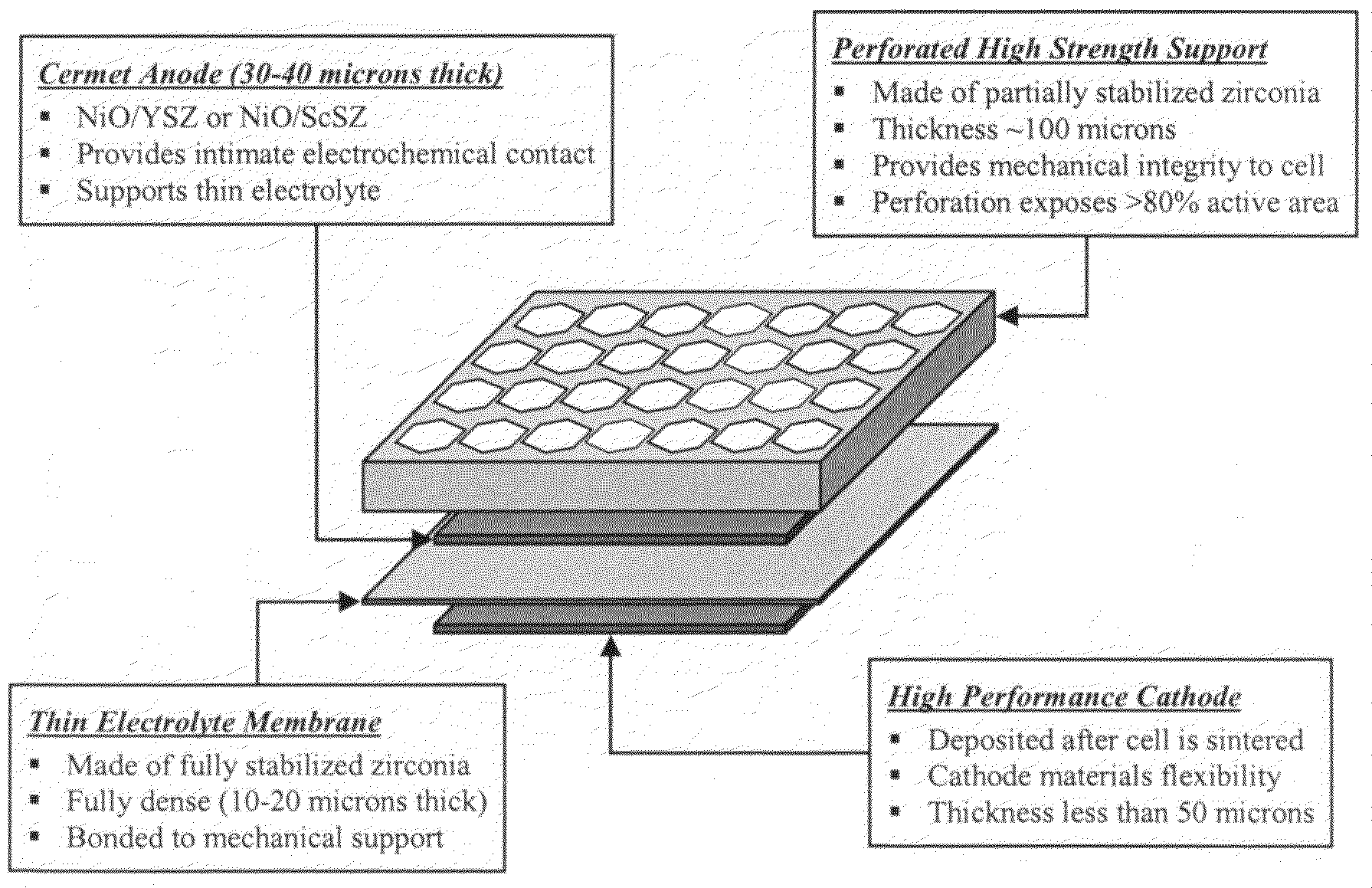
High performance multilayer electrodes for use in oxygen-containing gases
InactiveUS20090148743A1Final product manufactureNon-aqueous electrolyte accumulator electrodesCerium(IV) oxideLong term durability
Electrode materials systems for planar solid oxide fuel cells with high electrochemical performance including anode materials that provide exceptional long-term durability when used in reducing gases and cathode materials that provide exceptional long-term durability when used in oxygen-containing gases. The cathode materials comprise zinc-doped lanthanum strontium ferrite (LSZF) or an alternative ferrite, cobaltite or nickelate ceramic electrode material. The cathode material also may comprise a mixed-conducting ceria-based electrolyte material, a palladium dopant, or a combination of these. The cathode may have a bi-layer structure. A ceramic-based interfacial layer may be provided at the electrolyte / cathode interface. The multilayer cathode system and its palladium doped cathode material exhibit a high degree of tolerance to chromium contamination during operation with metallic interconnect materials.
Owner:NEXTECH MATERIALS
Thermal barrier coating system, components coated therewith and method for applying a thermal barrier coating system to components
A thermal barrier coating system on a base material includes a bond coat layer with a lower face in direct contact with the base material and an upper face, a first ceramic layer in direct contact with the upper face of the bond coating layer and a second ceramic layer disposed on an outermost surface of the coating system and configured to be exposed to hot gas. The first ceramic layer includes a layer, combination, mixture, alloy, blend or multilayer structure of at least one of yttria-stabilized zirconia with a yttria content in a range of 6-8 wt-%, YTaO4 doped zirconia, and titania doped zirconia. The second ceramic layer includes a layer, combination, mixture, alloy, blend or multilayer structure of at least one of YTaO4 doped zirconia, titania doped zirconia, scandia stabilized zirconia, ceria containing perovskite material, yttrium aluminium garnet material, Monazite material, and spinel material. A material of the second ceramic layer is different from a material of the first ceramic layer.
Owner:ANSALDO ENERGIA SWITZERLAND AG
Catalyst formulations containing Group 11 metals for hydrogen generation
ActiveUS7179442B2Maintain good propertiesExhaust apparatusElement comparisonCerium(IV) oxideSilicon dioxide
A method and catalysts and fuel processing apparatus for producing a hydrogen-rich gas, such as a hydrogen-rich syngas are disclosed. According to the method a CO-containing gas, such as a syngas, contacts a water gas shift (“WGS”) catalyst, in the presence of water, preferably at a temperature of less than about 450° C. to produce a hydrogen-rich gas, such as a hydrogen-rich syngas. Also disclosed is a water gas shift catalyst formulated from:a) at least one of Rh, Ni, Pt, their oxides and mixtures thereof,b) at least one of Cu, Ag, Au, their oxides and mixtures thereof; andc) at least one of K, Cs, Sc, Y, Ti, Zr, V, Mo, Re, Fe, Ru, Co, Ir, Pd, Cd, In, Ge, Sn, Pb, Sb, Te, La, Ce, Pr, Nd, Sm, Eu, their oxides and mixtures thereof. Another disclosed catalyst formulation comprises Rh, its oxides or mixtures thereof, Pt, its oxides or mixtures thereof and Ag, its oxides or mixtures thereof. The WGS catalyst may be supported on a carrier, such as any one member or a combination of alumina, zirconia, titania, ceria, magnesia, lanthania, niobia, zeolite, pervoskite, silica clay, yttria and iron oxide. Fuel processors containing such water gas shift catalysts are also disclosed.
Owner:HONDA MOTOR CO LTD +1
Method for preparing metal oxide particles and an exhaust gas purifying catalyst
ActiveUS7314846B2Improve heat resistanceImprove abilitiesMaterial nanotechnologyZirconium compoundsSurface layerCerium(IV) oxide
The present invention relates to metal oxide particles having cores comprising larger molar amounts of zirconia than of ceria, and surface layers comprising larger molar amounts of ceria than of zirconia. Further, the present invention relates to a method for preparing the particles. The method comprises preparing a solution comprising zirconia sol and ceria sol, adjusting the pH of the solution within ±0.5 on the basis of the isoelectric point of zirconia, and aggregating zirconia and then aggregating ceria around the aggregated zirconia from the solution to make aggregates. Furthermore, the present invention relates to an exhaust gas purifying catalyst comprising the metal oxide particles, and a noble metal carried by the metal oxide particles.
Owner:TOYOTA JIDOSHA KK
Platinum-ruthenium containing catalyst formulations for hydrogen generation
A method and catalysts for producing a hydrogen-rich syngas are disclosed. According to the method a CO-containing gas contacts a water gas shift (WGS) catalyst, optionally in the presence of water, preferably at a temperature of less than about 450° C. to produce a hydrogen-rich gas, such as a hydrogen-rich syngas. Also disclosed is a water gas shift catalyst formulated from:a) Pt, its oxides or mixtures thereof;b) Ru, its oxides or mixtures thereof; andc) at least one of Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, Sc, Y, Ti, Zr, V, Mo, Mn, Fe, Co, Rh, Ir, Ge, Sn, Sb, La, Ce, Pr, Sm, and Eu. Another disclosed catalyst formulation comprises Pt, its oxides or mixtures thereof; Ru, its oxides or mixtures thereof; Co, its oxides or mixtures thereof; and at least one of Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, Sc, Y, Ti, Zr, V, Mo, Mn, Fe, Rh, Ir, Ge, Sn, Sb, La, Ce, Pr, Sm, and Eu, their oxides and mixtures thereof. The WGS catalyst may be supported on a carrier, such as any one member or a combination of alumina, zirconia, titania, ceria, magnesia, lanthania, niobia, zeolite, perovskite, silica clay, yttria and iron oxide. Fuel processors containing such water gas shift catalysts are also disclosed.
Owner:FREESLATE +1
Exhaust gas purifying catalyst
InactiveUS20130143732A1Improve oxygen storage capacityReduce nitrogen oxide emissionsInternal combustion piston enginesDispersed particle separationCerium(IV) oxideRegular array
The object of the invention is to provide an exhaust gas purifying catalyst having a high oxygen storage capacity without changing the usage amount of the oxygen storage component. According to the present invention, the exhaust gas purifying catalyst containing (a) a ceria-zirconia composite oxide containing ceria in a higher amount than zirconia, (b) a ceria-zirconia composite oxide containing zirconia in a higher amount than ceria, and (c) a ceria-zirconia composite oxide having a pyrochlore-type regular array structure is provided. The exhaust gas purifying catalyst of the present invention has a higher oxygen storage capacity than conventional catalysts and is effective in reducing the amount of NOx emission.
Owner:TOYOTA JIDOSHA KK
Ceria and strontium titanate based electrodes
InactiveUS20090061284A1High BET surface areaSpeed up calcination procedureFuel cells groupingCell electrodesStrontium titanateVanadium doping
A ceramic anode structure obtainable by a process comprising the steps of: (a) providing a slurry by dispersing a powder of an electronically conductive phase and by adding a binder to the dispersion, in which said powder is selected from the group consisting of niobium-doped strontium titanate, vanadium-doped strontium titanate, tantalum-doped strontium titanate, and mixtures thereof, (b) sintering the slurry of step (a), (c) providing a precursor solution of ceria, said solution containing a solvent and a surfactant, (d) impregnating the resulting sintered structure of step (b) with the precursor solution of step (c), (e) subjecting the resulting structure of step (d) to calcination, and (f) conducting steps (d)-(e) at least once.
Owner:DANMARKS TEKNISKE UNIV
Catalyst containing little or no rhodium for purifying exhaust gases of internal combustion engine
InactiveUS20080045404A1Necessity for producingPromote oxidationInternal combustion piston enginesMolecular sieve catalystsCerium(IV) oxideInternal combustion engine
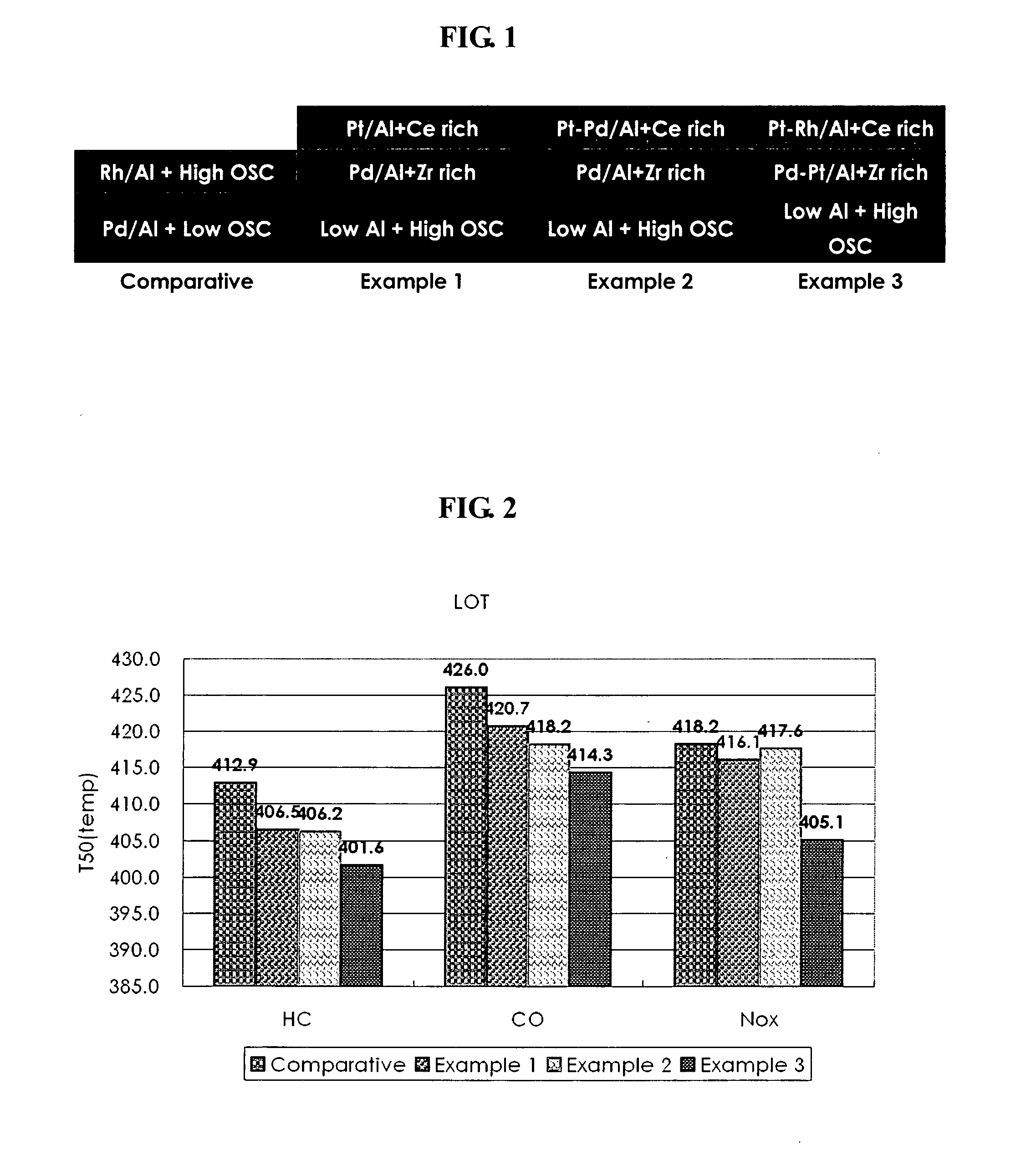
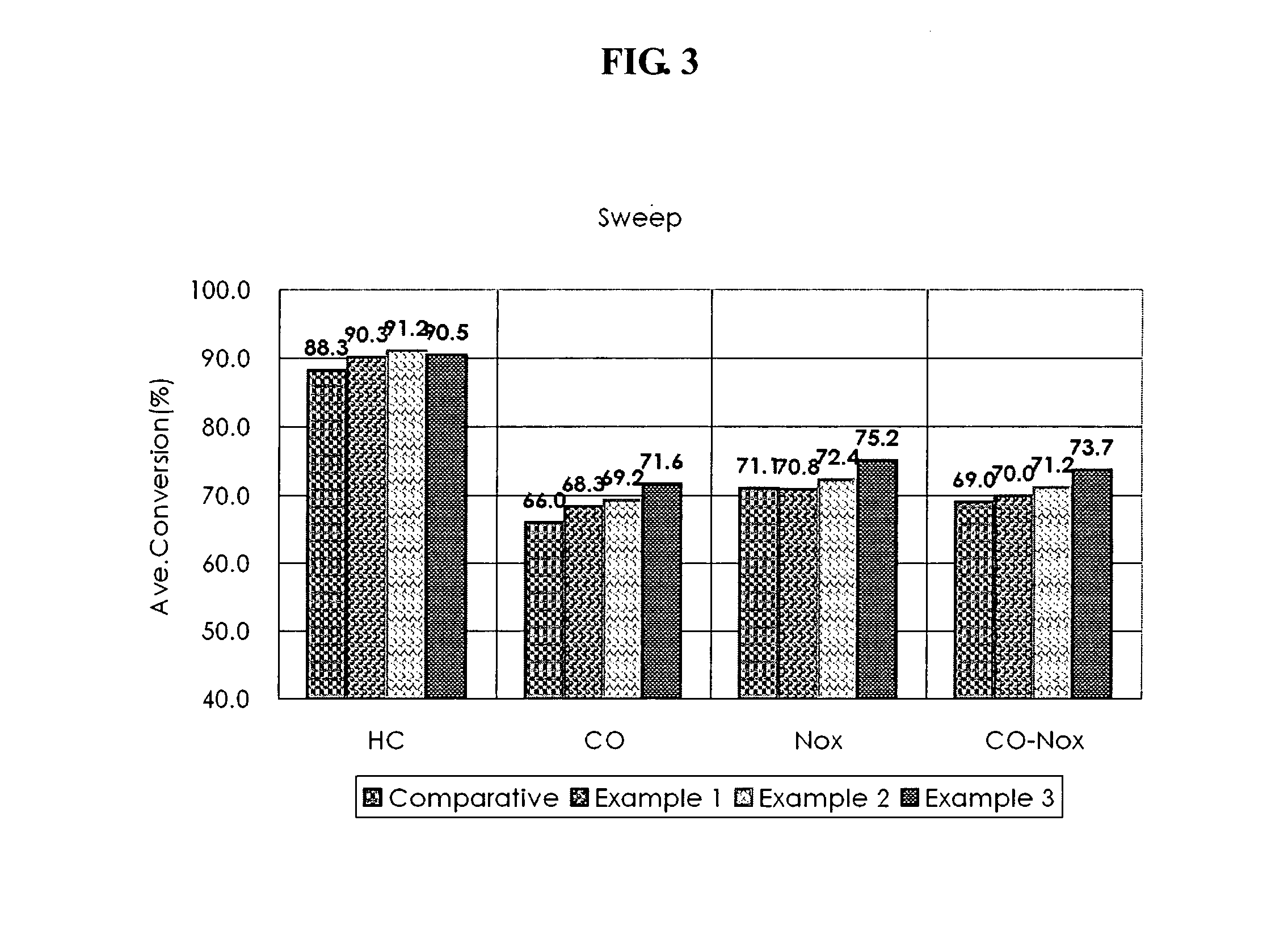
Disclosed herein is a three-way conversion (TWC) catalyst containing little or no rhodium for purifying exhaust gases of an internal combustion engine, having a multi-layers structure, including a lower layer including an alumina support and an oxygen storage material; an intermediate layer including alumina support impregnated only with palladium and a zirconia-rich oxygen storage material; and an upper layer including alumina support impregnated with platinum, minimum rhodium and platinum, or platinum-palladium and a ceria-rich oxygen storage material.
Owner:HEESUNG CATALYSTS CORP
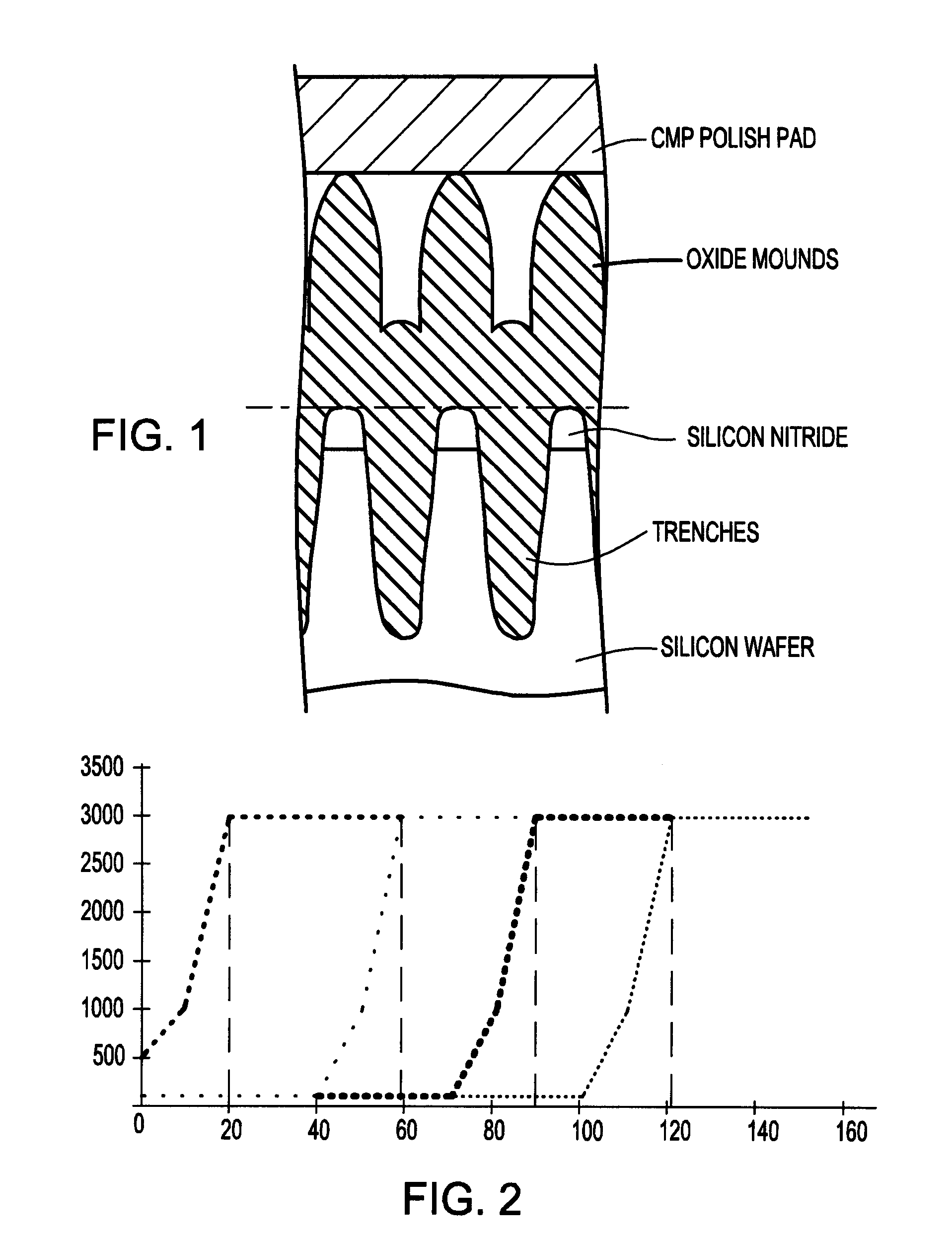
Chemical/mechanical polishing slurry, and chemical mechanical polishing process and shallow trench isolation process employing the same
InactiveUS6914001B2Improved removal selectivityHigh selectivityOther chemical processesDecorative surface effectsIonSilicon nitride
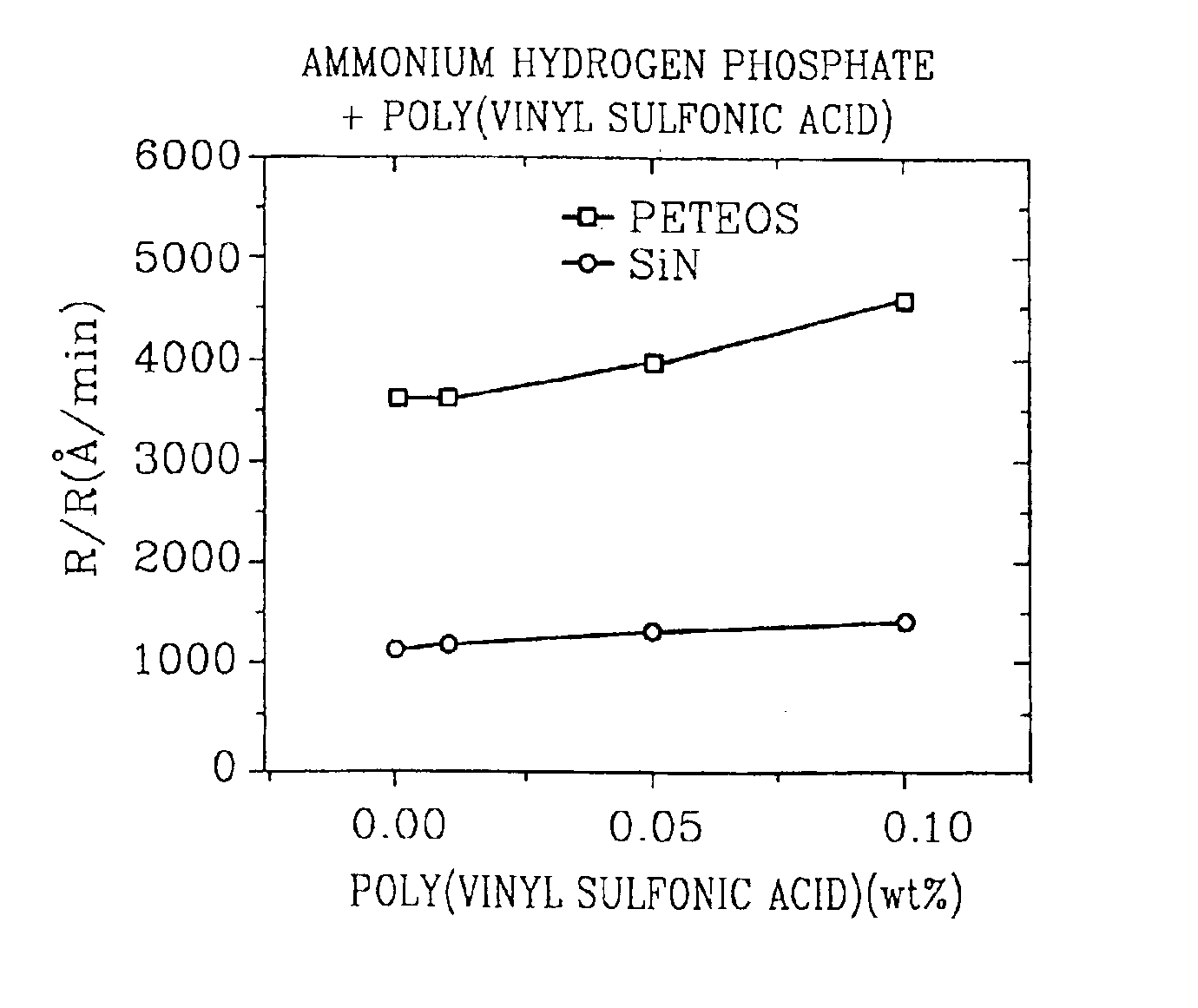
A CMP oxide slurry includes an aqueous solution containing abrasive particles and two or more different passivation agents. Preferably, the aqueous solution is made up of deionized water, and the abrasive particles are a metal oxide selected from the group consisting of ceria, silica, alumina, titania, zirconia and germania. Also, a first passivation agent may be an anionic, cationic or nonionic surfactant, and a second passivation agent may be a phthalic acid and its salts. In one example, the first passivation agent is poly-vinyl sulfonic acid, and the second passivation agent is potassium hydrogen phthalate. The slurry exhibits a high oxide to silicon nitride removal selectivity.
Owner:SAMSUNG ELECTRONICS CO LTD
Fuel additive containing lattice engineered cerium dioxide nanoparticles
ActiveUS20120124899A1Material nanotechnologyHeterogenous catalyst chemical elementsKinetic diameterNanoparticle
A process for making cerium dioxide nanoparticles containing at least one transition metal (M) utilizes a suspension of cerium hydroxide nanoparticles prepared by mechanical shearing of an aqueous mixture containing an oxidant in an amount effective to enable oxidation of cerous ion to ceric ion, thereby forming a product stream that contains transition metal-containing cerium dioxide nanoparticles, Ce1-xMxO2, wherein “x” has a value from about 0.3 to about 0.8. The nanoparticles thus obtained have a cubic fluorite structure, a mean hydrodynamic diameter in the range of about 1 nm to about 10 nm, and a geometric diameter of less than about 4 nm. The transition metal-containing crystalline cerium dioxide nanoparticles can be used to prepare a dispersion of the particles in a nonpolar medium.
Owner:CERION
Flame made metal oxides
ActiveUS20040126298A1Improve stabilitySmall crystal sizeMaterial nanotechnologyZirconium oxidesCarboxylic acidSolvent
Described is a method for the production of metal oxides, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Catalyst and Catalyst Structure for Reduction of Nitrogen Oxides, and Method for Catalytic Reduction of Nitrogen Oxides
InactiveUS20090084090A1Increased durabilityNo deteriorationGas treatmentMolecular sieve catalystsCobaltPt element
The invention provides a catalyst and a catalyst structure for catalytic reduction of nitrogen oxides contained in exhaust gas wherein fuel is supplied and subjected to combustion under periodic rich / lean conditions and the resulting exhaust gases are brought into contact therewith, which catalyst comprises:an outer catalyst layer comprising an outer catalyst component A which comprises at least one selected from a solid acid, and a solid acid supporting oxides and / or ions of at least one element selected from vanadium, tungsten, molybdenum, copper, iron, cobalt, nickel and manganese, andan inner catalyst layer comprising an inner catalyst component which comprises at least one noble metal catalyst component B selected from platinum, rhodium, palladium and an oxide thereof and a catalyst component C comprising ceria or praseodymium oxide or a mixture of oxides and / or a composite oxide of at least two elements selected from cerium, zirconium, praseodymium, neodymium, terbium, samarium, gadolinium and lanthanum. The invention further provides a method for catalytic reduction of nitrogen oxides by contacting the nitrogen oxides with the catalyst.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS +1
Process for producing ceria-zirconia-alumina composite oxides and applications thereof
InactiveUS20130108530A1Improved hydrocarbon conversionNitrogen compoundsInternal combustion piston enginesCombustionCerium(IV) oxide
A process for producing a ceria-zirconia-alumina composite oxide is disclosed. The process comprises combining a cerium (IV) compound and a zirconium (IV) compound with a slurry of aluminum oxide at a temperature greater than 40° C. to produce a reaction slurry, then contacting the reaction slurry with a precipitating agent to precipitate insoluble cerium and zirconium compounds onto the aluminum oxide and form cerium-zirconium-aluminum oxide particles, and calcining the cerium-zirconium-aluminum oxide particles to produce a ceria-zirconia-alumina composite oxide. The process to produce ceria-zirconia-alumina composite oxides provides a material having a high oxygen storage / release capacity that is suitable for a catalyst with enhanced cleaning of the exhaust gases from internal combustion engines.
Owner:JOHNSON MATTHEY PLC
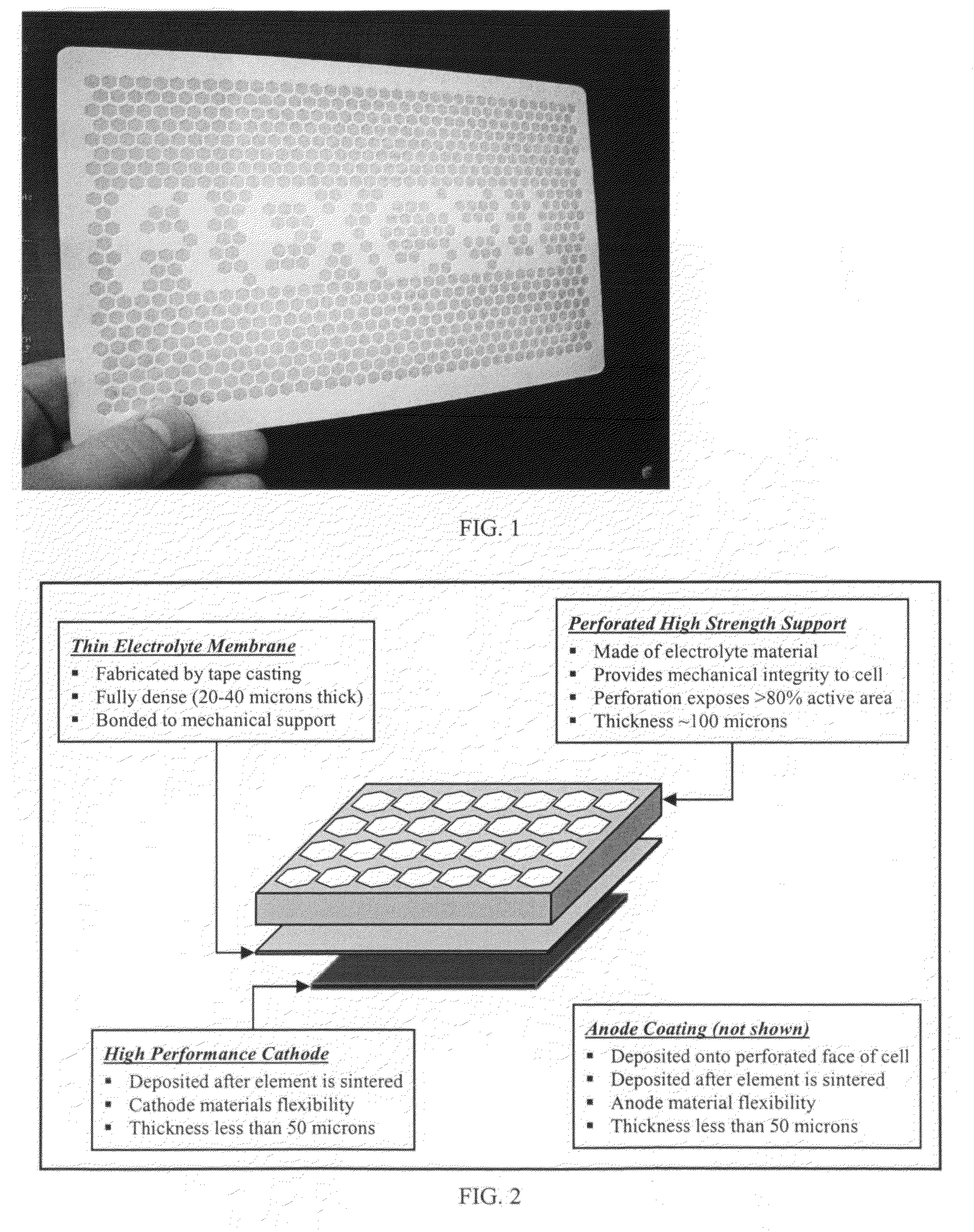
Phase stable doped zirconia electrolyte compositions with low degradation
ActiveUS8580456B2Final product manufactureSolid electrolyte fuel cellsCerium(IV) oxideElectrolyte composition
A solid oxide fuel cell (SOFC) includes a cathode electrode, a solid oxide electrolyte, and an anode electrode. The electrolyte and / or electrode composition includes zirconia stabilized with (i) scandia, (ii) ceria, and (iii) at least one of yttria and ytterbia. The composition does not experience a degradation of ionic conductivity of greater than 15% after 4000 hrs at a temperature of 850° C.
Owner:BLOOM ENERGY CORP
Ceria-zirconia base composite oxide, method for producing the same, and catalyst for purification of exhaust gas using the ceria-zirconia base composite oxide
ActiveUS20130029840A1High oxygen storage performanceImprove heat resistanceZirconium compoundsMetal/metal-oxides/metal-hydroxide catalystsCerium(IV) oxideX-ray
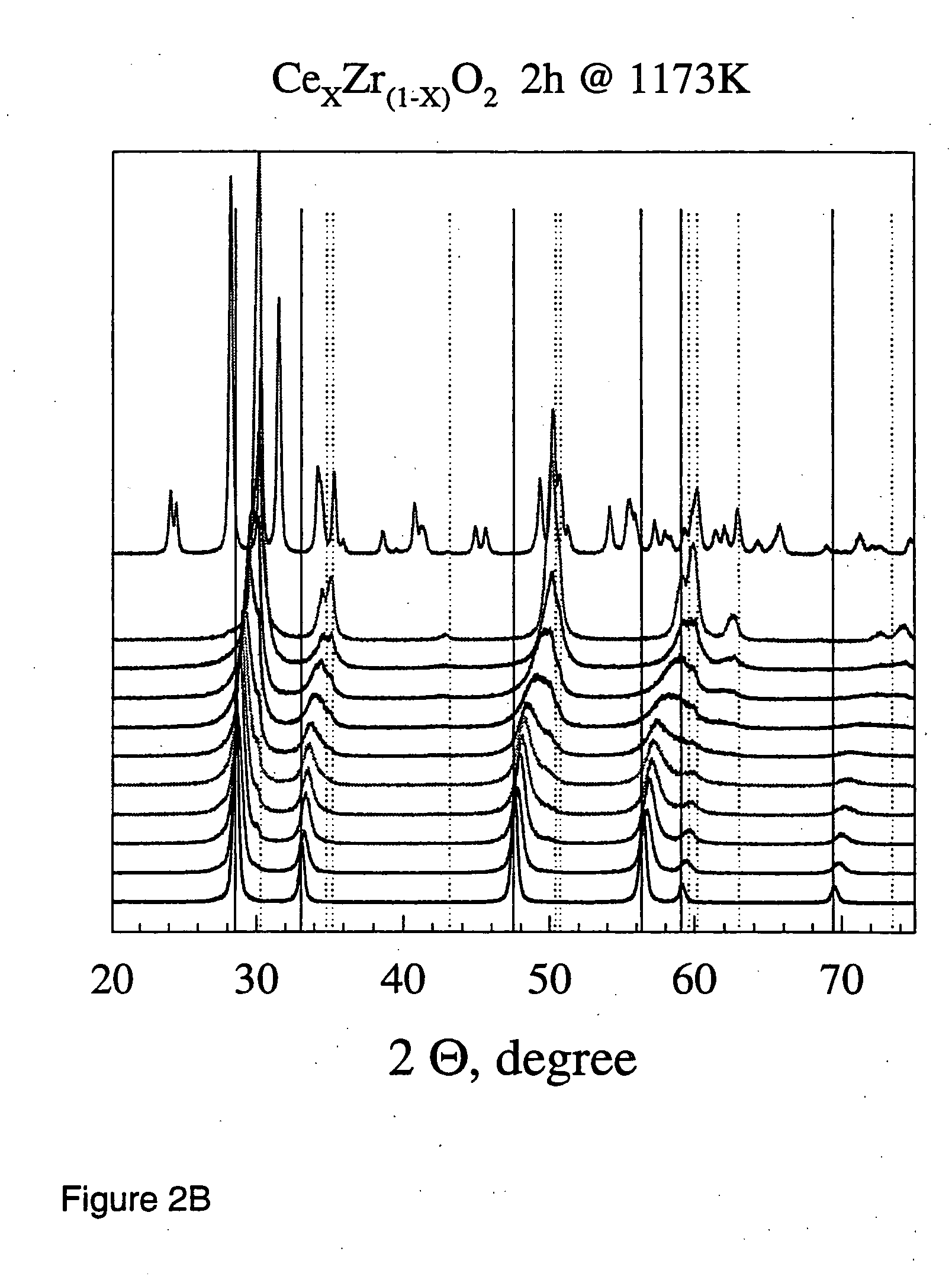
A ceria-zirconia base composite oxide contains a composite oxide of ceria and zirconia. In the ceria-zirconia base composite oxide, a content ratio between cerium and zirconium in the composite oxide is in a range from 43:57 to 48:52 in terms of molar ratio ([cerium]: [zirconium]). An intensity ratio of a diffraction line at 2θ=14.5° to a diffraction line at 2θ=29° {I(14 / 29) value} and an intensity ratio of a diffraction line at 2θ=28.5° to the diffraction line at 2θ=29° {I(28 / 29) value}, which are calculated from an X-ray diffraction pattern obtained by an X-ray diffraction measurement using CuKa after heating under a temperature condition of 1100° C. in air for 5 hours, respectively satisfy the following conditions:I(14 / 29)value≧0.015, and I(28 / 29)value≦0.08.
Owner:CATALER CORP +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com