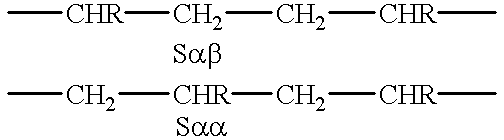Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
628 results about "Intensity ratio" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Capital intensity ratio of a company is a measure of the amount of capital needed per dollar of revenue. It is calculated by dividing total assets of a company by its sales. It is reciprocal of total asset turnover ratio.
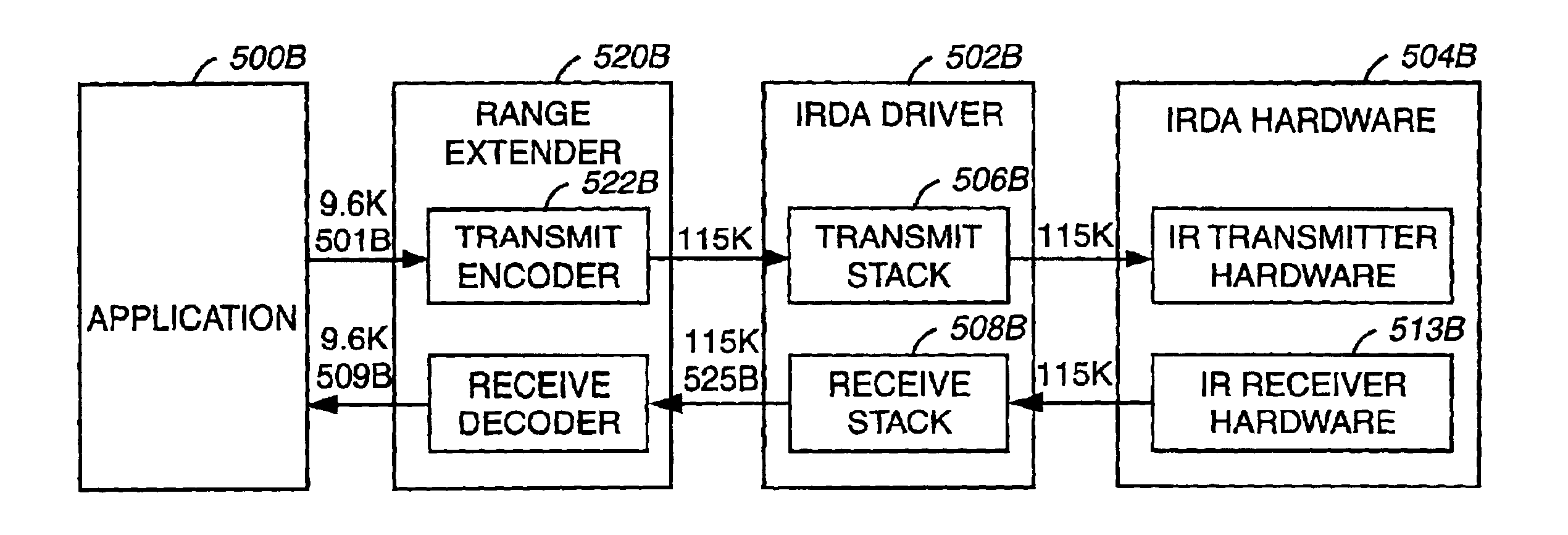
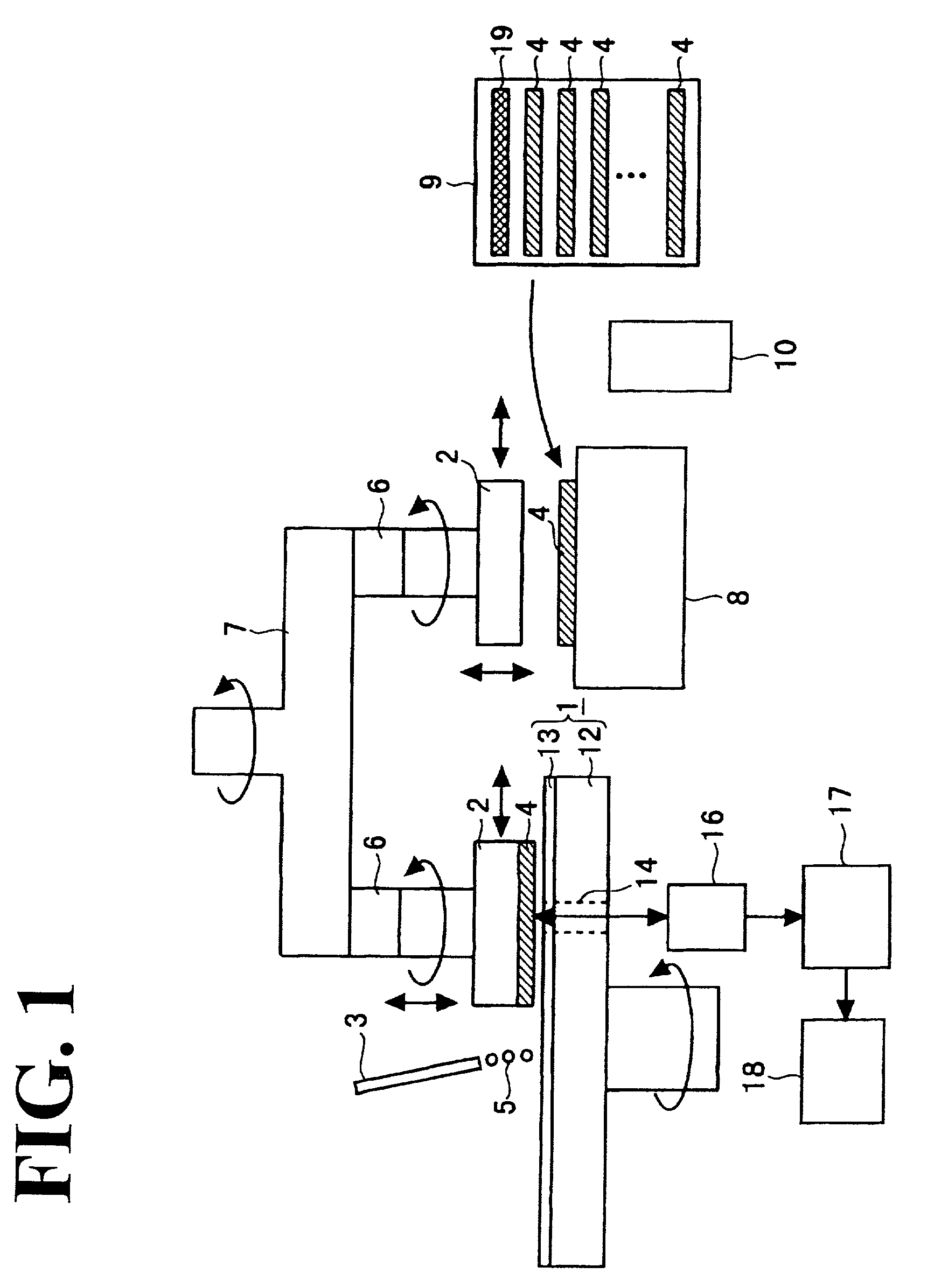
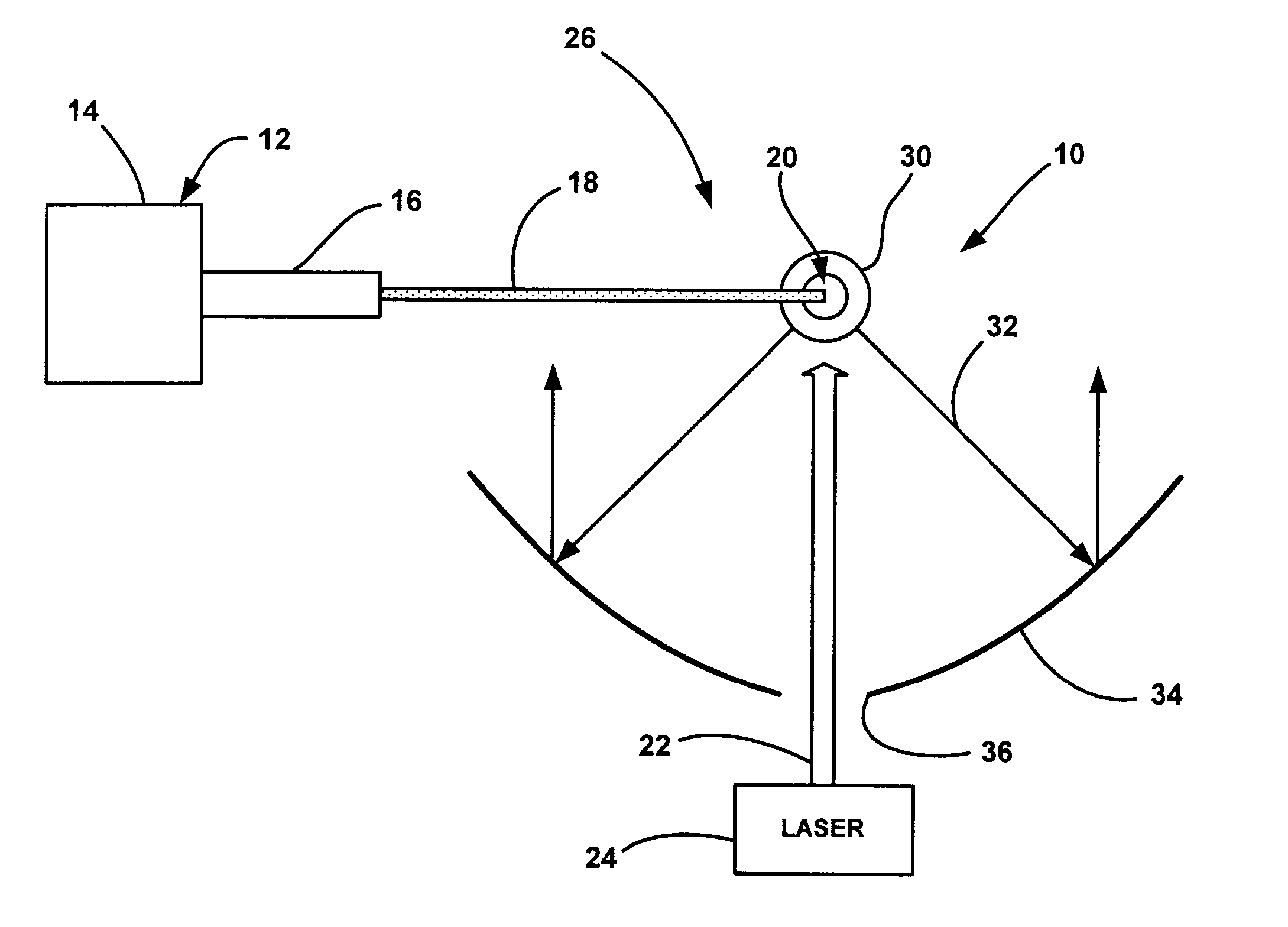
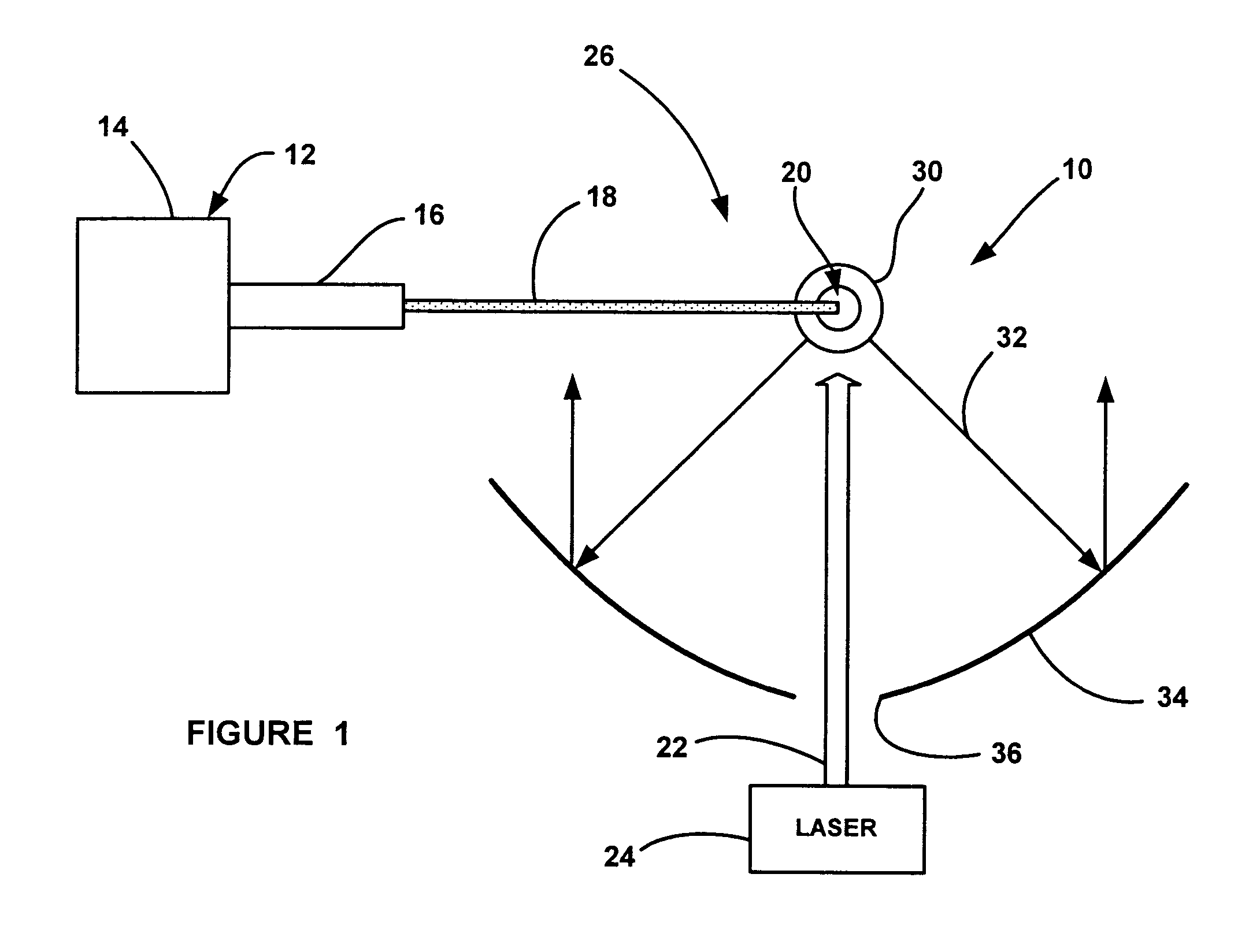
System and method for remote optical digital networking of computing devices
InactiveUS6920289B2Widely distributedIncrease rangeElectric signal transmission systemsFrequency/rate-modulated pulse demodulationData transmissionMobile device
This invention extends the range of optical data of mobile device by trading speed for distance as well as integrating a plurality of pulses over time to define a single bit of information. The present invention uses a number of integrated pulses to represent a single bit instead of utilizing a one to one correspondence between pulses and bits. The present invention executes a range extender application which executes on the mobile device without any hardware modification to the mobile device. The range extender application causes the optical transmitter to “stutter” or repetitively emanate the identical pulse representing a bit of information. Sufficient photons are thereby gathered at a receiver to reach a predetermined threshold. A tradeoff of the data transmission frequency in this invention is that a signal intensity drops by a factor of 100 when distance increases by a factor of 10 yielding a distance / intensity ratio of {fraction (1 / 10)}.
Owner:GLOBALFOUNDRIES US INC
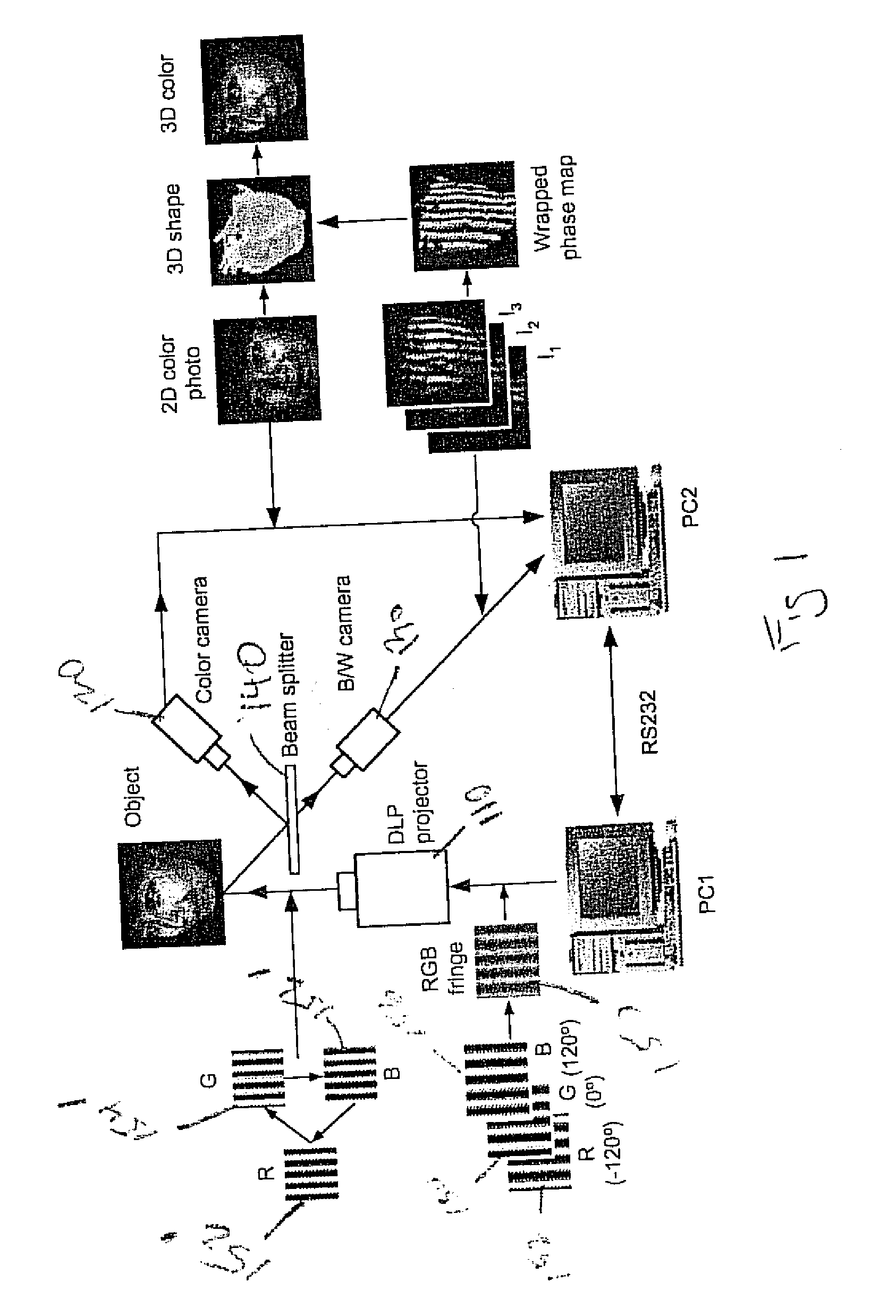
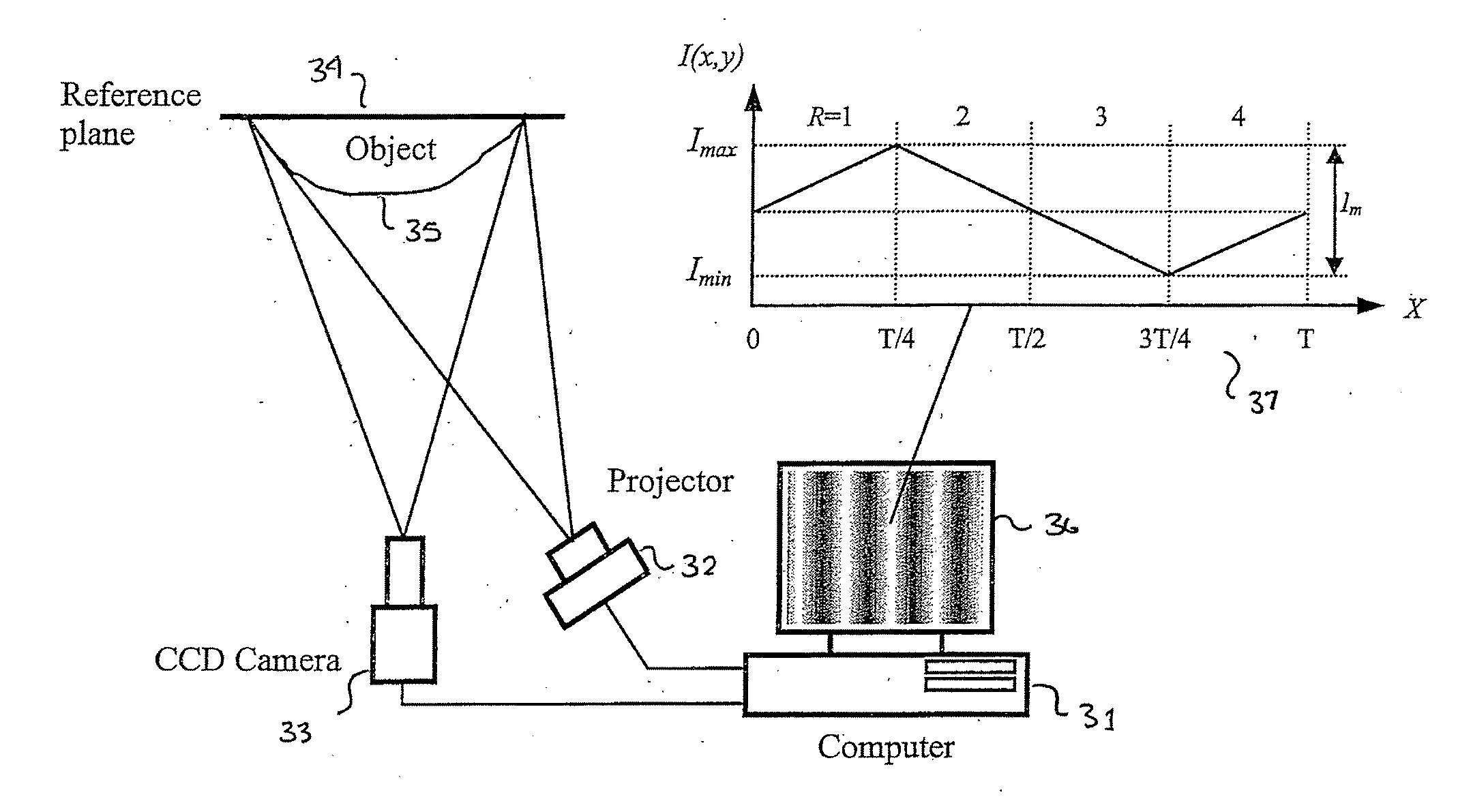
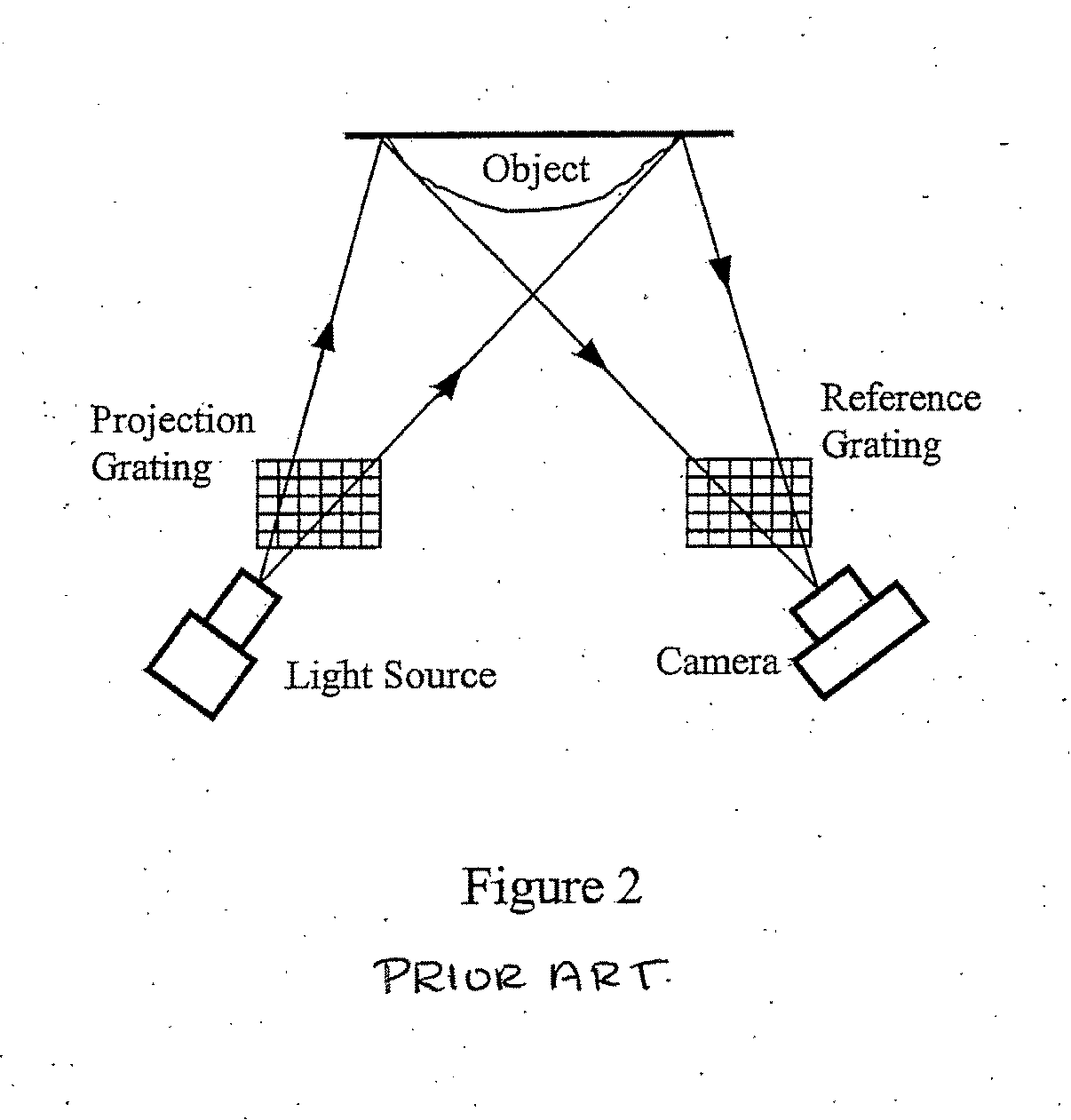
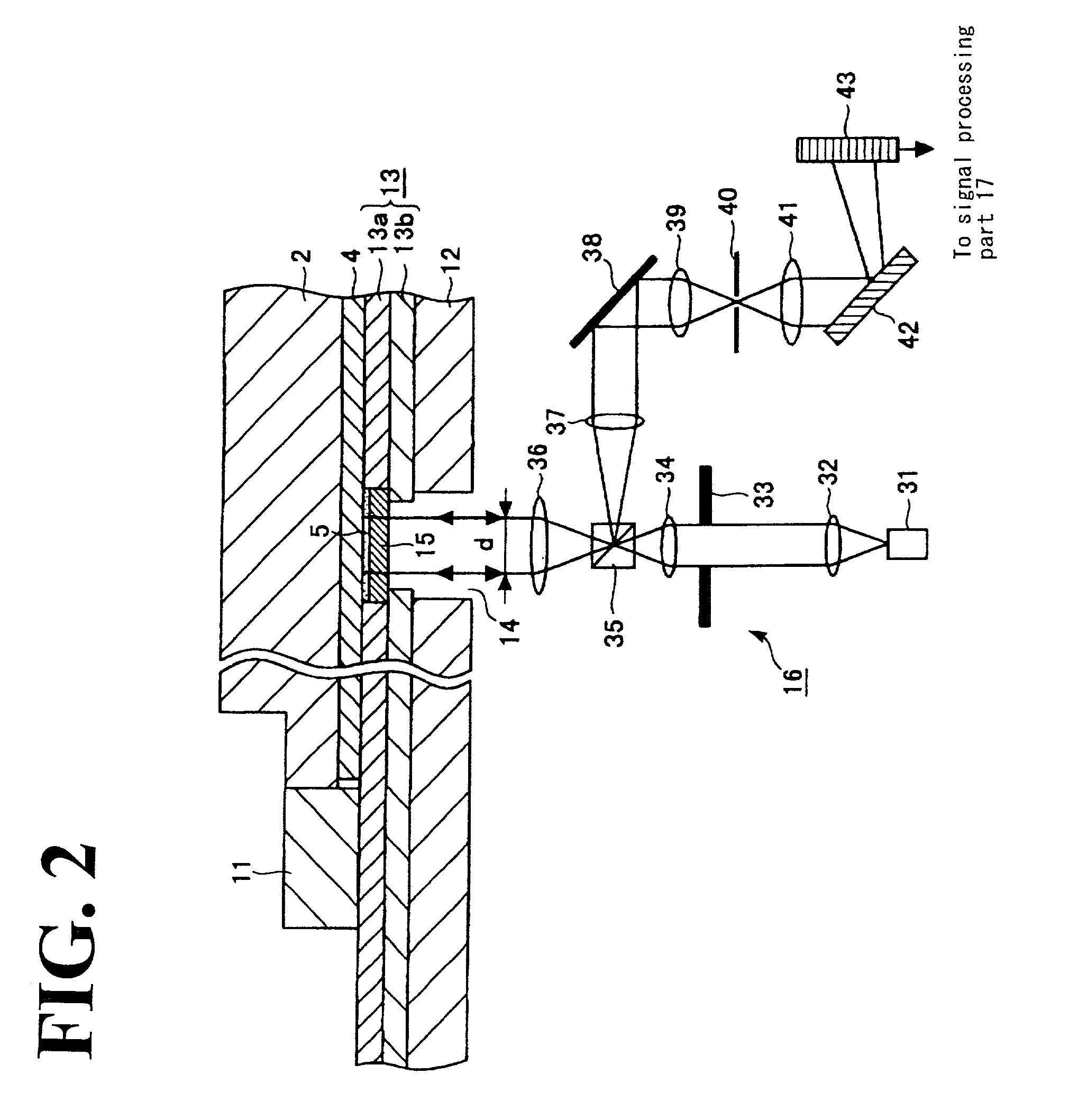
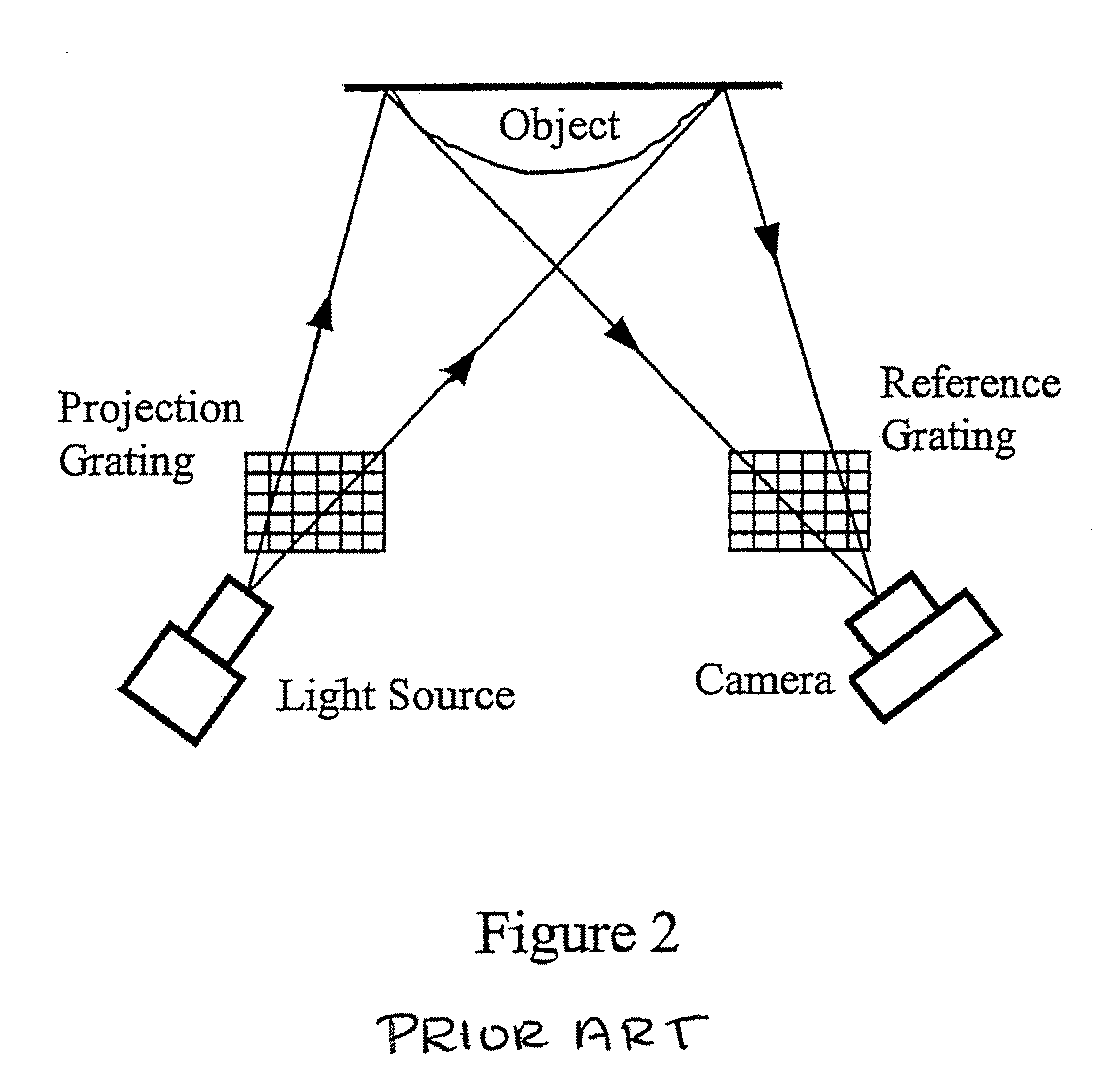
3D shape measurement system and method including fast three-step phase shifting, error compensation and calibration
InactiveUS20070115484A1Improve system speedFacilitates establishment of coordinate relationshipUsing optical means3d shapesPhase shifted
A structured light system for object ranging / measurement is disclosed that implements a trapezoidal-based phase-shifting function with intensity ratio modeling using sinusoidal intensity-varied fringe patterns to accommodate for defocus error. The structured light system includes a light projector constructed to project at least three sinusoidal intensity-varied fringe patterns onto an object that are each phase shifted with respect to the others, a camera for capturing the at least three intensity-varied phase-shifted fringe patterns as they are reflected from the object and a system processor in electrical communication with the light projector and camera for generating the at least three fringe patterns, shifting the patterns in phase and providing the patterns to the projector, wherein the projector projects the at least three phase-shifted fringe patterns sequentially, wherein the camera captures the patterns as reflected from the object and wherein the system processor processes the captured patterns to generate object coordinates.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
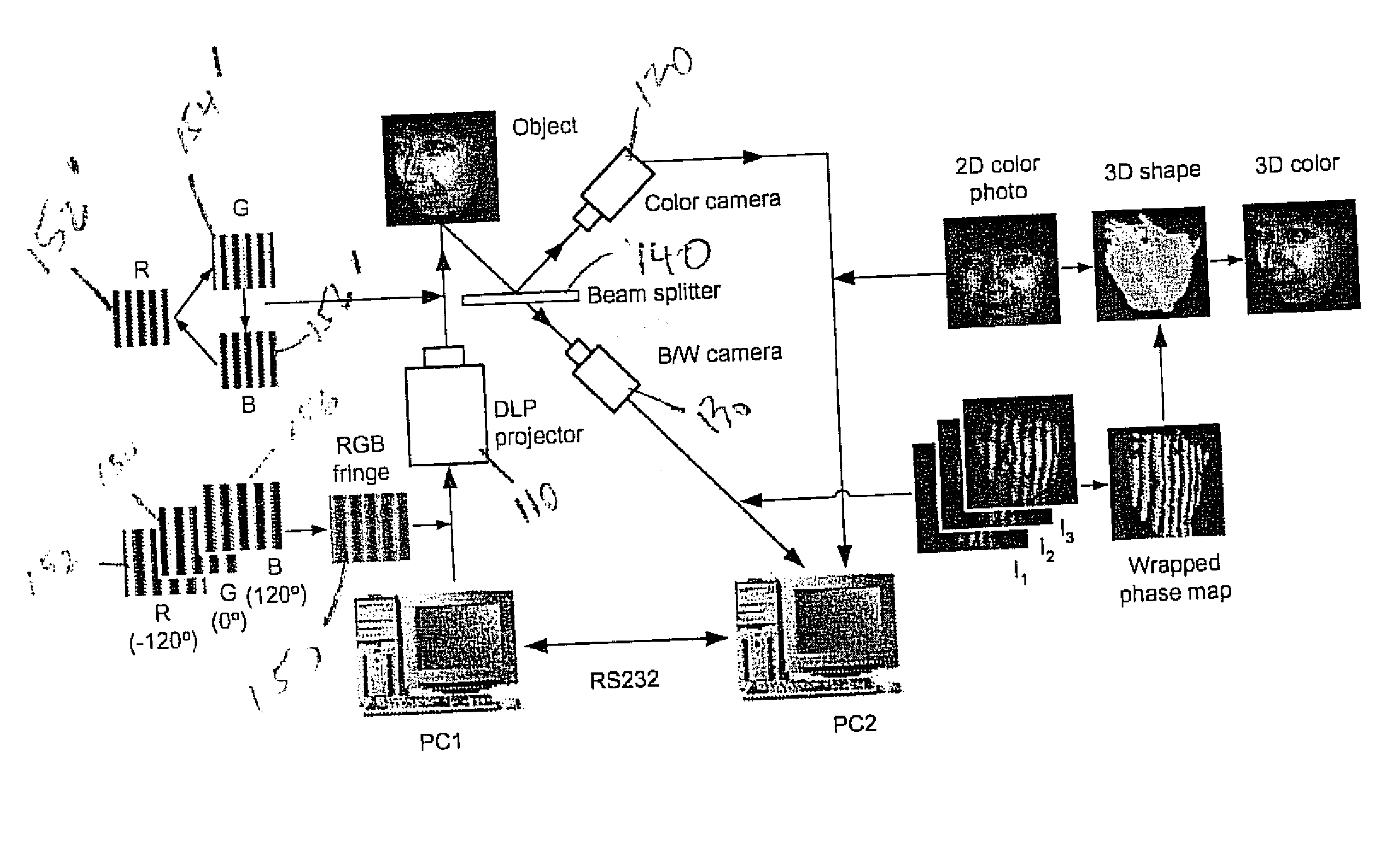
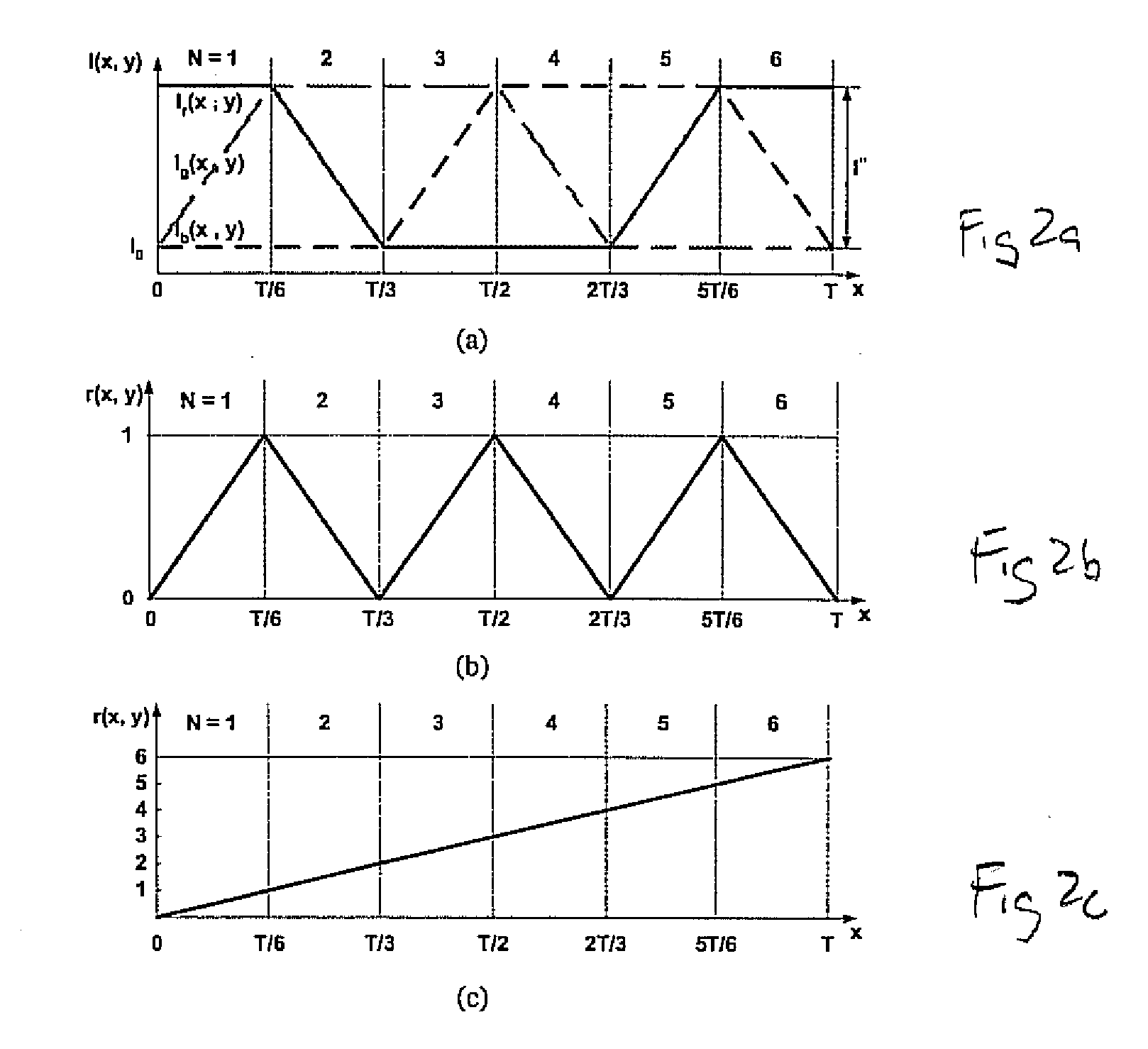
Full-field three-dimensional measurement method
InactiveUS20070206204A1Improve processing speedSimple calculationUsing optical meansTriangulationFull field
A method and system for full-field fringe-projection for 3-D surface-geometry measurement, referred to as “triangular-pattern phase-shifting” is disclosed. A triangular grey-scale-level-coded fringe pattern is computer generated, projected along a first direction onto an object or scene surface and distorted according to the surface geometry. The 3-D coordinates of points on the surface are calculated by triangulation from distorted triangular fringe-pattern images acquired by a CCD camera along a second direction and a triangular-shape intensity-ratio distribution is obtained from calculation of the captured distorted triangular fringe-pattern images. Removal of the triangular shape of the intensity ratio over each pattern pitch generates a wrapped intensity-ratio distribution obtained by removing the discontinuity of the wrapped image with a modified unwrapping method. Intensity ratio-to-height conversion is used to reconstruct the 3-D surface coordinates of the object. Intensity-ratio error compensation involves estimating intensity-ratio error in a simulation of the measurement process with both real and ideal captured triangular-pattern images obtained from real and ideal gamma non-linearity functions. A look-up table relating the measure intensity-ratio to the corresponding intensity-ratio error is constructed and used for intensity-ratio error compensation. The inventive system is based on two-step phase-shifting but can be extended for multiple-step phase-shifting.
Owner:UNIVERSITY OF WATERLOO
Magnetic tape device and magnetic reproducing method
ActiveUS20180286442A1Improve smoothnessImprove surface smoothnessMaterials with ironRecord information storageIn planeX-ray
The magnetic tape device includes a TMR head (reproducing head); and a magnetic tape including a magnetic layer including ferromagnetic hexagonal ferrite powder, a binding agent, and fatty acid ester, in which an XRD intensity ratio obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio is 0.65 to 1.00, Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, full widths at half maximum of spacing distribution measured by optical interferometry regarding the surface of the magnetic layer before and after performing a vacuum heating with respect to the magnetic tape are greater than 0 nm and equal to or smaller than 7.0 nm, and a difference between spacings before and after the vacuum heating is greater than 0 nm and equal to or smaller than 8.0 nm.
Owner:FUJIFILM CORP
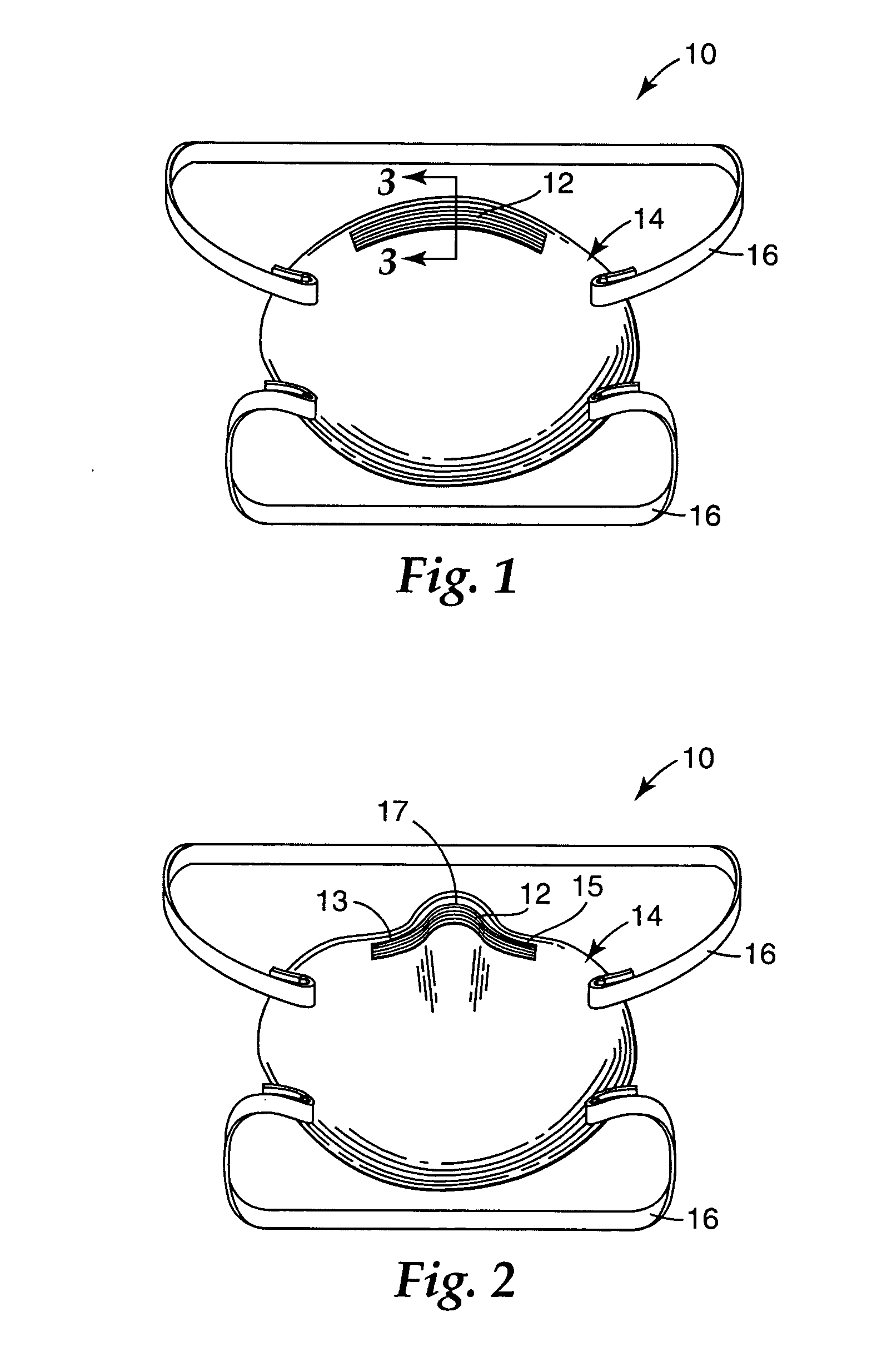
Respirator that uses a polymeric nose clip
InactiveUS20070068529A1Control flowWithout causing uncomfortable pressure pointsBreathing filtersBreathing masksRespiratorFilter media
A respirator 10 that has a mask body 14 and a malleable nose clip 12. The mask body 14 is adapted to fit at least over the nose and mouth of a person to define an interior gas space that is separate from the exterior gas space. The mask body 14 has the nose clip 12 secured to it and can include at least one layer of filter media 20. The malleable nose clip 12 comprises a semi-crystalline polymeric material that has an integrated diffraction intensity ratio of at least about 2.0. The nose clip 12 can be deformed into a desired configuration that enables the mask body 14 to maintain a snug fit over a person's nose when the respirator is worn for extended time periods. Because the nose clip 12 does not need to contain metal, the whole respirator 10 can be easily processed as waste in an incinerator when its service has ended.
Owner:3M INNOVATIVE PROPERTIES CO
Magnetic recording medium and magnetic recording and reproducing device
ActiveUS20190103133A1Excellent electromagnetic conversion characteristicAvoid it happening againMaterials with ironBase layers for recording layersIn planeX-ray
Provided are a magnetic recording medium, in which a magnetic layer includes a ferromagnetic hexagonal ferrite powder, a binding agent, an oxide abrasive, an intensity ratio Int(110) / Int(114) obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical squareness ratio is 0.65 to 1.00, one or more kinds of component selected from the group consisting of fatty acid and fatty acid amide is contained in a magnetic layer side portion on the non-magnetic support, a C—H derived C concentration of the magnetic layer is 45 atom % to 65 atom %, and an average particle diameter of the oxide abrasive obtained from a secondary ion image obtained by irradiating the surface of the magnetic layer with a focused ion beam is 0.04 μm to 0.08 μm, and a magnetic recording and reproducing device including this magnetic recording medium.
Owner:FUJIFILM CORP
Magnetic recording medium and magnetic recording and reproducing device
ActiveUS20190103134A1Excellent electromagnetic conversion characteristicAvoid it happening againMaterials with ironProtective coatings for layersIn planeX-ray
Provided are a magnetic recording medium, in which a magnetic layer includes ferromagnetic hexagonal ferrite powder, a binding agent, and an oxide abrasive, an intensity ratio Int(110) / Int(114) obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical squareness ratio of the magnetic recording medium is 0.65 to 1.00, a coefficient of friction measured regarding a base portion of a surface of the magnetic layer is equal to or smaller than 0.30, and an average particle diameter of the oxide abrasive obtained from a secondary ion image obtained by irradiating the surface of the magnetic layer with a focused ion beam is 0.04 μm to 0.08 μm, and a magnetic recording and reproducing device including this magnetic recording medium.
Owner:FUJIFILM CORP
Magnetic tape and magnetic tape device
ActiveUS20180374507A1Improve accuracyExact reproductionMaterials with ironAlignment for track following on tapesIn planeMagnetic force microscope
The magnetic tape includes a non-magnetic support; and a magnetic layer including ferromagnetic powder and a binding agent on the non-magnetic support, in which the magnetic layer includes a timing-based servo pattern, the ferromagnetic powder is ferromagnetic hexagonal ferrite powder having an activation volume equal to or smaller than 1,600 nm3, an XRD intensity ratio Int(110) / Int(114) obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, and an edge shape of the timing-based servo pattern specified by magnetic force microscope observation is a shape in which a difference (L99.9−L0.1) is equal to or smaller than 180 nm, and a magnetic tape device including the magnetic tape.
Owner:FUJIFILM CORP
Magnetic tape device and magnetic reproducing method
ActiveUS20180286450A1Improve smoothnessImprove surface smoothnessMaterials with ironRecord information storageIn planeX-ray
The magnetic tape device includes a magnetic tape including a magnetic layer; and a TMR head (reproducing head), in which an intensity ratio of a peak intensity of a diffraction peak of a (110) plane with respect to a peak intensity of a diffraction peak of a (114) plane of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, and a C—H derived C concentration calculated from a C—H peak area ratio of C1s spectra obtained by X-ray photoelectron spectroscopic analysis performed on the surface of the magnetic layer at a photoelectron take-off angle of 10 degrees is 45 to 65 atom %.
Owner:FUJIFILM CORP
Magnetic tape device and head tracking servo method
ActiveUS20180240481A1Lower the resistance valueAvoid it happening againAlignment for track following on tapesTape carriersX-rayEngineering
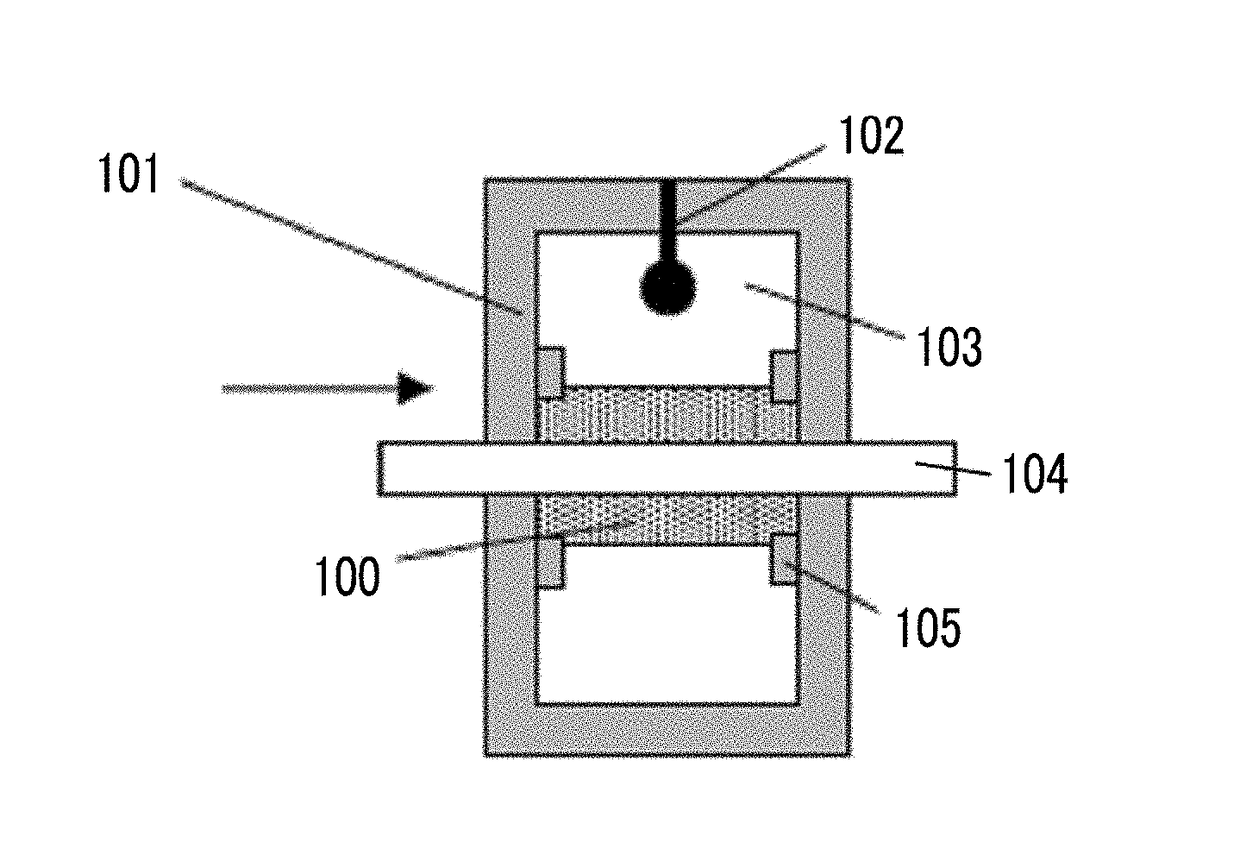
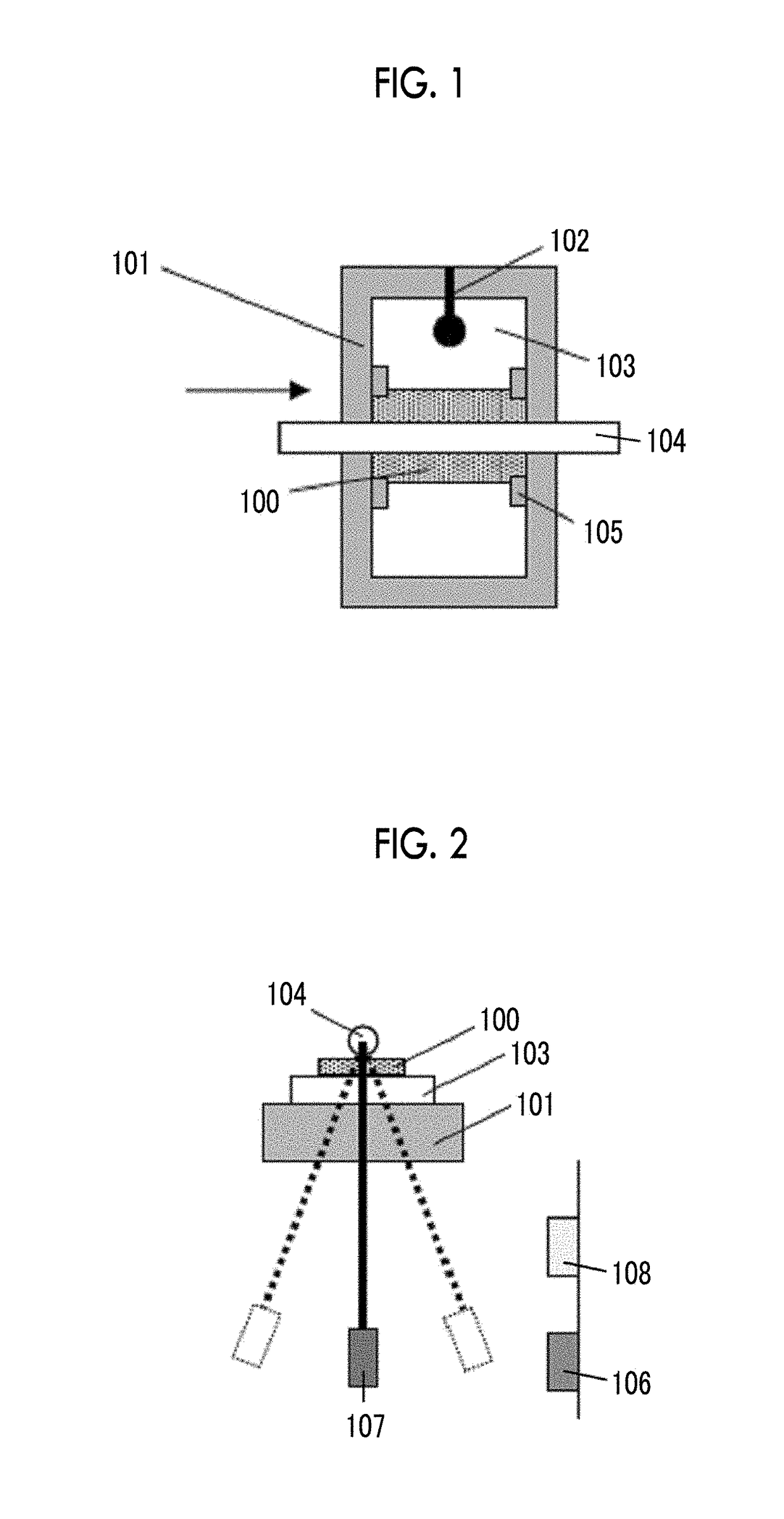
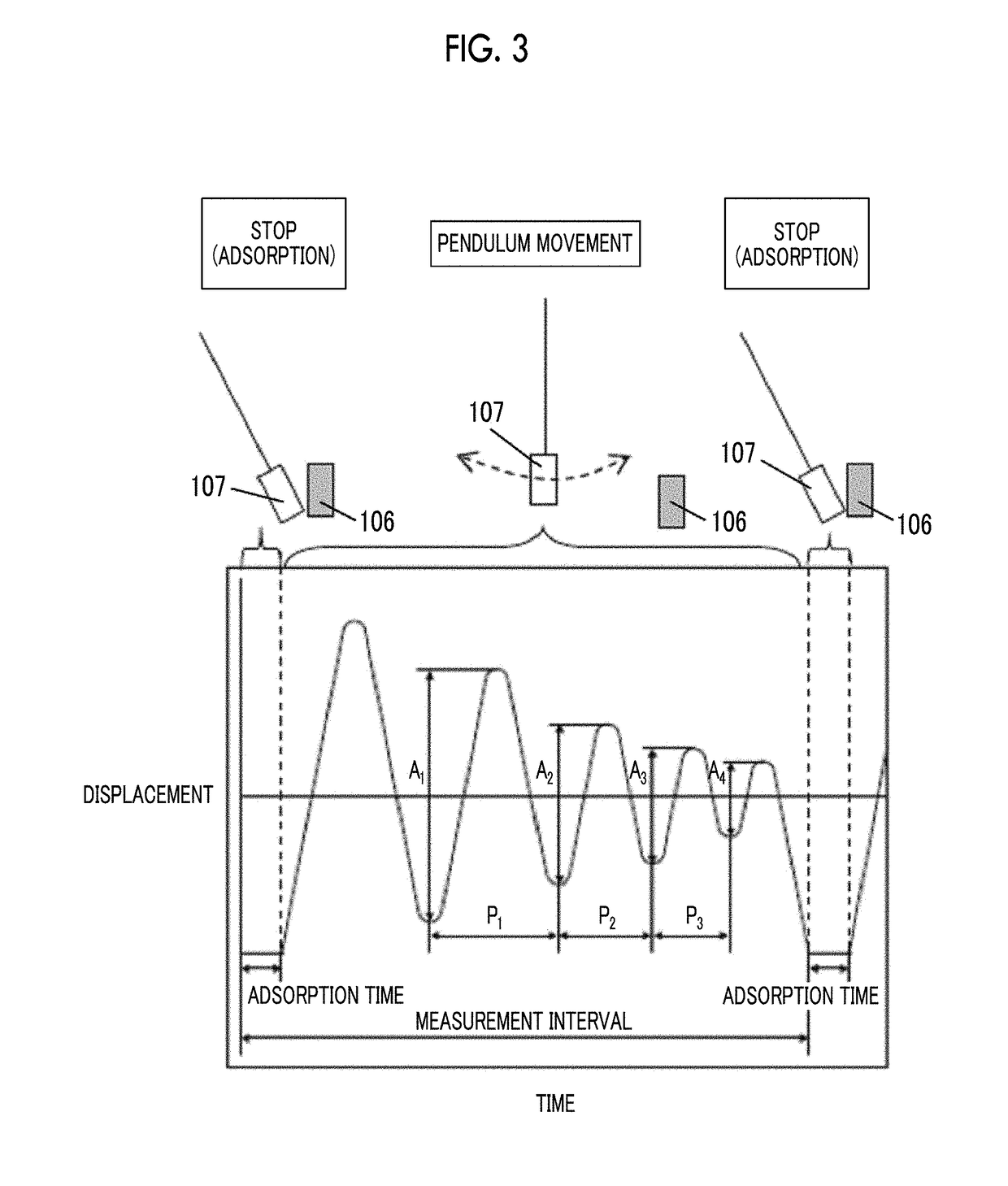
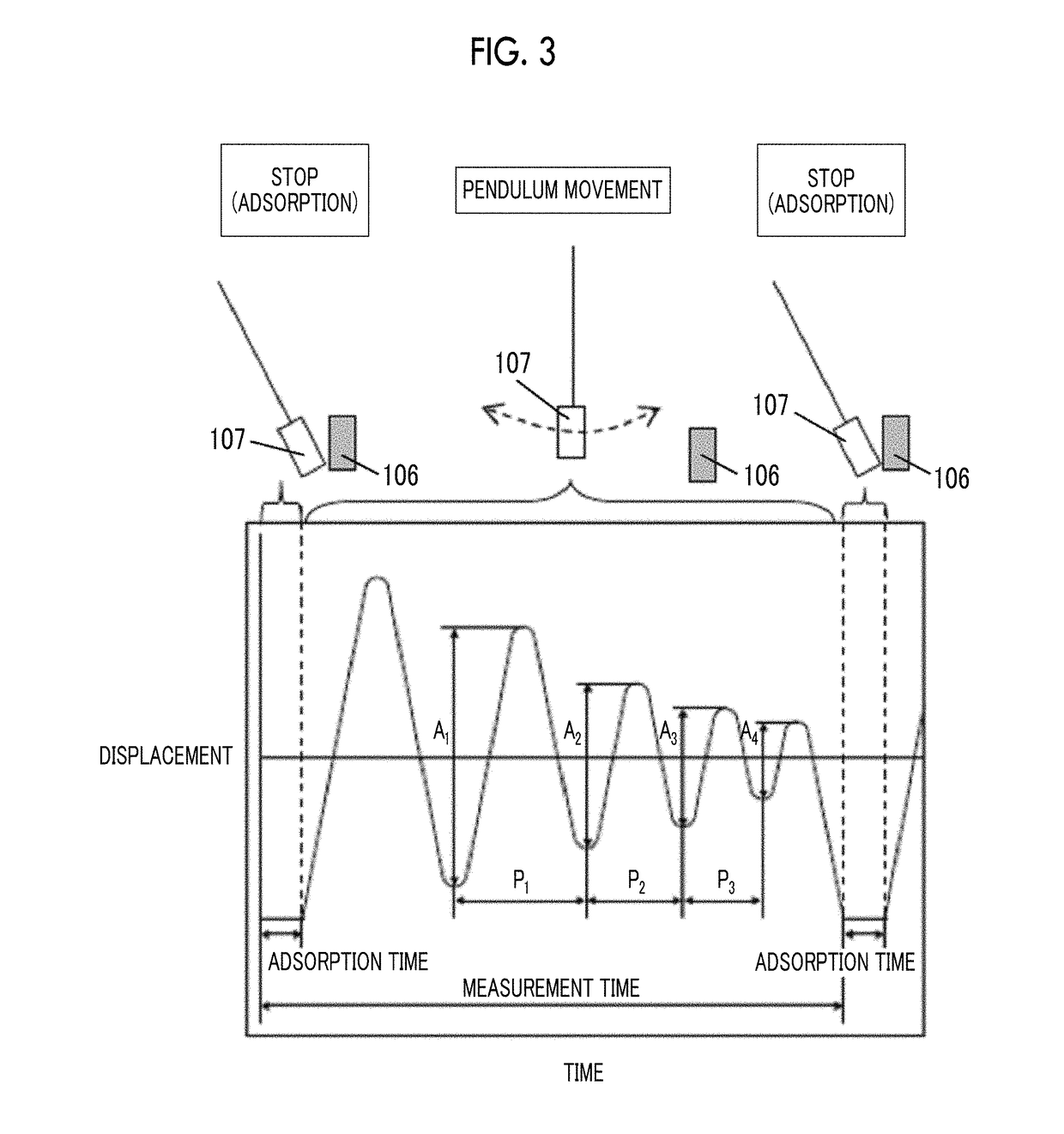
The magnetic tape device includes a TMR head as a servo head; and a magnetic tape which includes a magnetic layer including ferromagnetic hexagonal ferrite powder and a binding agent, and including a servo pattern, an XRD intensity ratio (Int(110) / Int(114)) of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, a center line average surface roughness Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, and a logarithmic decrement acquired by a pendulum viscoelasticity test performed regarding the surface of the magnetic layer is equal to or smaller than 0.050.
Owner:FUJIFILM CORP
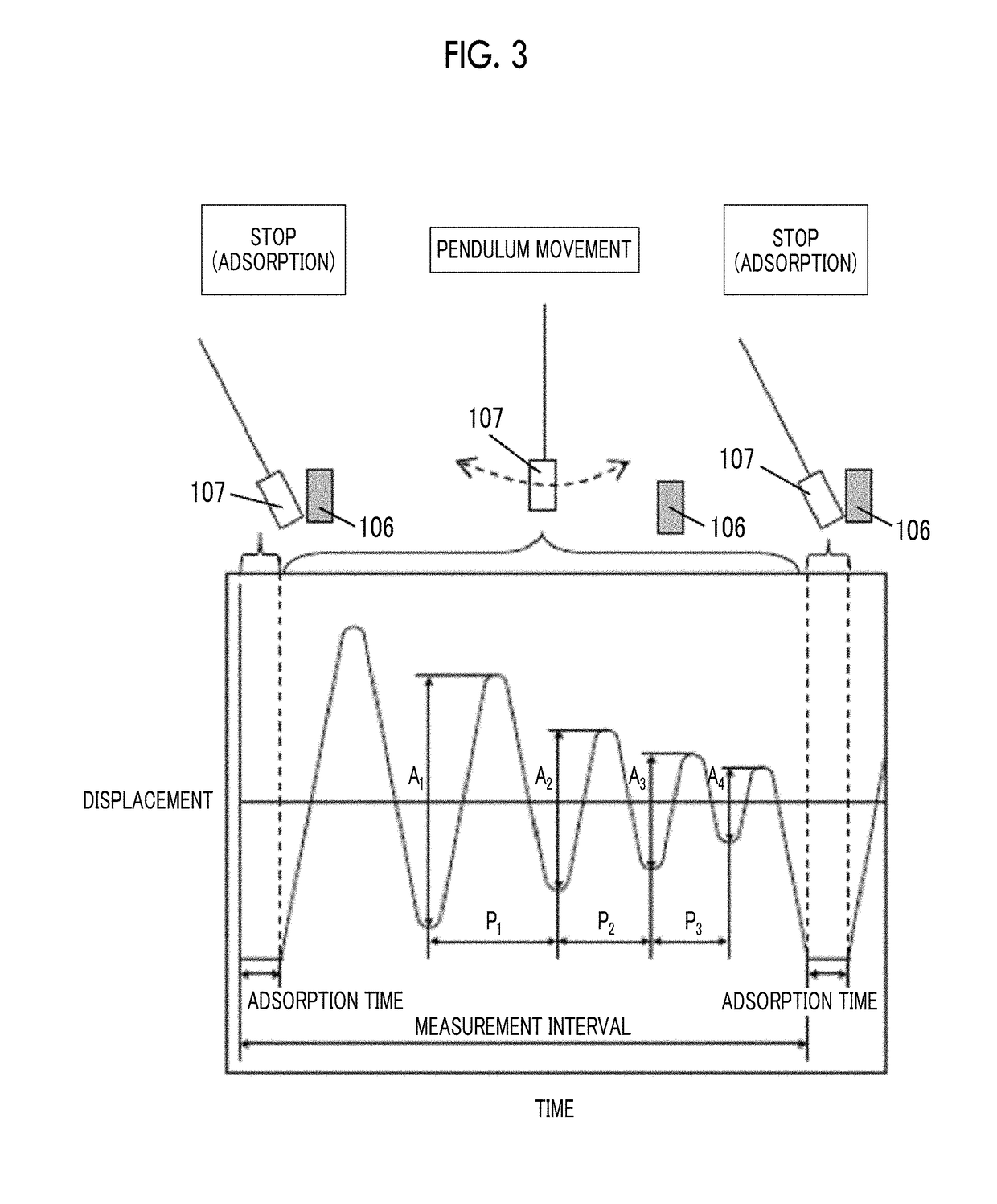
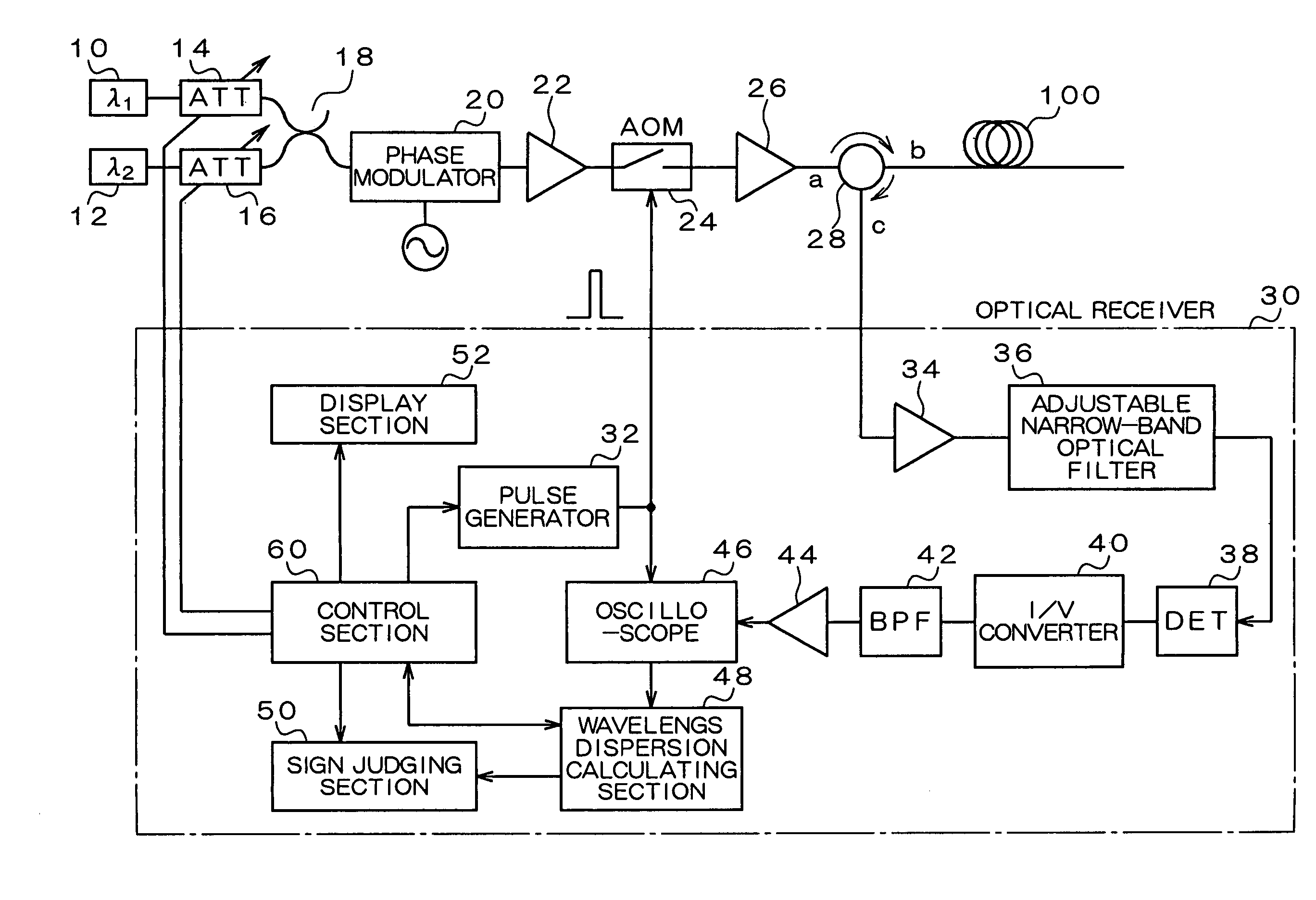
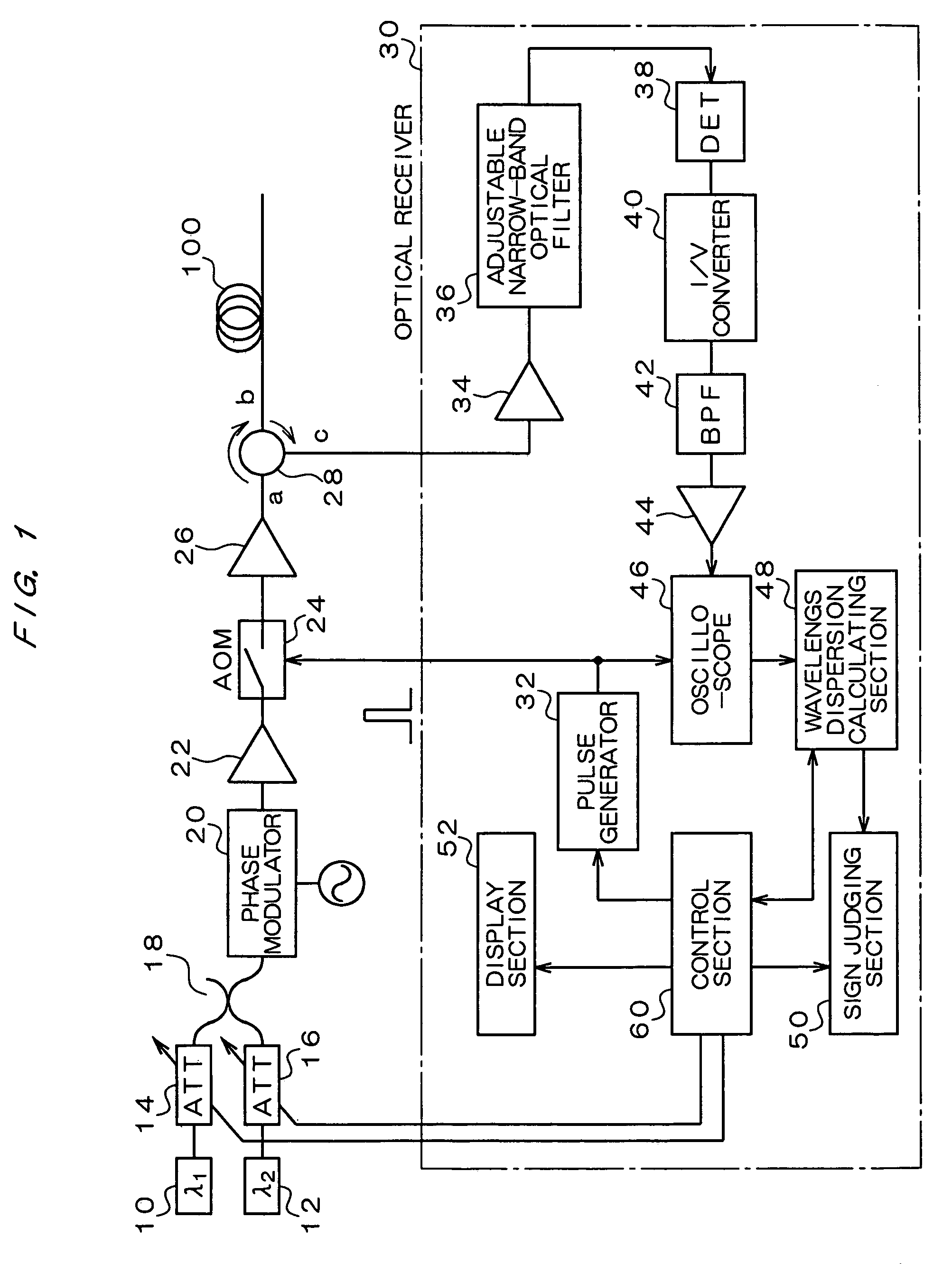
Wavelength dispersion probing system
InactiveUS7020360B2Time and troubleMaterial analysis by optical meansCoupling light guidesMultiplexingMultiplexer
A wavelength dispersion probing system for determining a value of wavelength dispersion and its sign and reducing the trouble and time required for this determination. This wavelength dispersion probing system comprises light sources 10, 12, light attenuators 14, 16, optical multiplexer 18, phase modulator 20, optical amplifiers 22, 26, acoustooptical modulator 24, optical receiver 30. The intensity ratio of two wavelengths is set at 1 to 2 to detect Stokes light or anti-Stokes light included in the return light of an optical fiber 100 by the optical receiver 30, so that wavelength dispersion is probed. The wavelength dispersion is probed by changing the intensity ratio of the two wavelengths to observe the state of the change of the wavelength dispersion, so that the sign of wavelength dispersion is determined by the optical receiver 30.
Owner:ADVANTEST CORP
Magnetic tape device and magnetic reproducing method
ActiveUS20180240478A1Improve smoothnessImprove surface smoothnessMagnetic materials for record carriersBase layers for recording layersIn planeX-ray
The magnetic tape device includes a TMR head as a reproducing head; and a magnetic tape which includes a non-magnetic support, and a magnetic layer including ferromagnetic hexagonal ferrite powder and a binding agent on the non-magnetic support, an XRD intensity ratio (Int(110) / Int(114)) of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, a center line average surface roughness Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, and a logarithmic decrement acquired by a pendulum viscoelasticity test performed regarding the surface of the magnetic layer is equal to or smaller than 0.050.
Owner:FUJIFILM CORP
Magnetic tape and magnetic tape device
ActiveUS20190027177A1Improve surface smoothnessGeneration frequency can be reducedMaterials with ironAlignment for track following on tapesIn planeX-ray
The magnetic tape includes a magnetic layer including ferromagnetic powder and a binding agent, in which a magnetic tape total thickness is equal to or smaller than 5.30 μm, the magnetic layer has a servo pattern, a center line average surface roughness Ra measured regarding a surface of the magnetic layer is equal to or smaller than 1.8 nm, the ferromagnetic powder is ferromagnetic hexagonal ferrite powder, an intensity ratio of a peak intensity of a diffraction peak of a (110) plane with respect to a peak intensity of a diffraction peak of a (114) plane of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, and a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, and a magnetic tape device including this magnetic tape.
Owner:FUJIFILM CORP
Magnetic tape device and head tracking servo method
ActiveUS20180286446A1Lower the resistance valueAvoid it happening againMaterials with ironAlignment for track following on tapesIn planeFull width at half maximum
The magnetic tape device includes a TMR head (servo head); and a magnetic tape including a magnetic layer including ferromagnetic hexagonal ferrite powder, a binding agent, and fatty acid ester, in which an XRD intensity ratio obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio is 0.65 to 1.00, Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, full widths at half maximum of spacing distribution measured by optical interferometry regarding a surface of the magnetic layer before and after performing a vacuum heating with respect to the magnetic tape are greater than 0 nm and equal to or smaller than 7.0 nm, and a difference between spacings before and after the vacuum heating is greater than 0 nm and equal to or smaller than 8.0 nm.
Owner:FUJIFILM CORP
Magnetic tape
ActiveUS20190027180A1Improve surface smoothnessAvoid it happening againMagnetic materials for record carriersRecord information storageIn planeX-ray
The magnetic tape includes a magnetic layer including ferromagnetic powder, non-magnetic powder, and a binding agent and a back coating layer including non-magnetic powder and a binding agent, in which the ferromagnetic powder is ferromagnetic hexagonal ferrite powder, an Ra measured regarding a surface of the magnetic layer is equal to or smaller than 1.8 nm, an intensity ratio of a peak intensity of a diffraction peak of a (110) plane with respect to a peak intensity of a diffraction peak of a (114) plane of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical squareness ratio of the magnetic tape is 0.65 to 1.00, and a logarithmic decrement acquired by a pendulum viscoelasticity test performed regarding a surface of the hack coating layer is equal to or smaller than 0.060.
Owner:FUJIFILM CORP
Magnetic tape and magnetic tape device
ActiveUS20190027171A1Improve accuracyExact reproductionRecord information storageInorganic material magnetismIn planeX-ray
The magnetic tape includes: a non-magnetic support; a non-magnetic layer including non-magnetic powder and a binding agent on the non-magnetic support; and a magnetic layer including ferromagnetic powder and a binding agent on the non-magnetic layer, in which a total thickness of the non-magnetic layer and the magnetic layer is equal to or smaller than 0.60 μm, the magnetic layer has a servo pattern, the ferromagnetic powder is ferromagnetic hexagonal ferrite powder, an intensity ratio of a peak intensity of a diffraction peak of a (110) plane with respect to a peak intensity of a diffraction peak of a (114) plane of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, and a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, and a magnetic tape device including this magnetic tape.
Owner:FUJIFILM CORP
Magnetic tape and magnetic tape device
ActiveUS20190027172A1Improve surface smoothnessImproved head positioning accuracyInorganic material magnetismRecord information storageIn planeX-ray
Provided are a magnetic tape including: a non-magnetic support; and a magnetic layer including ferromagnetic powder and a binding agent on the non-magnetic support, in which the magnetic layer has a timing-based servo pattern, a center line average surface roughness Ra measured regarding a surface of the magnetic layer is equal to or smaller than 1.8 nm, the ferromagnetic powder is ferromagnetic hexagonal ferrite powder, an intensity ratio of a peak intensity of a diffraction peak of a (110) plane with respect to a peak intensity of a diffraction peak of a (114) plane of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, and a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, and a magnetic tape device including this magnetic tape.
Owner:FUJIFILM CORP
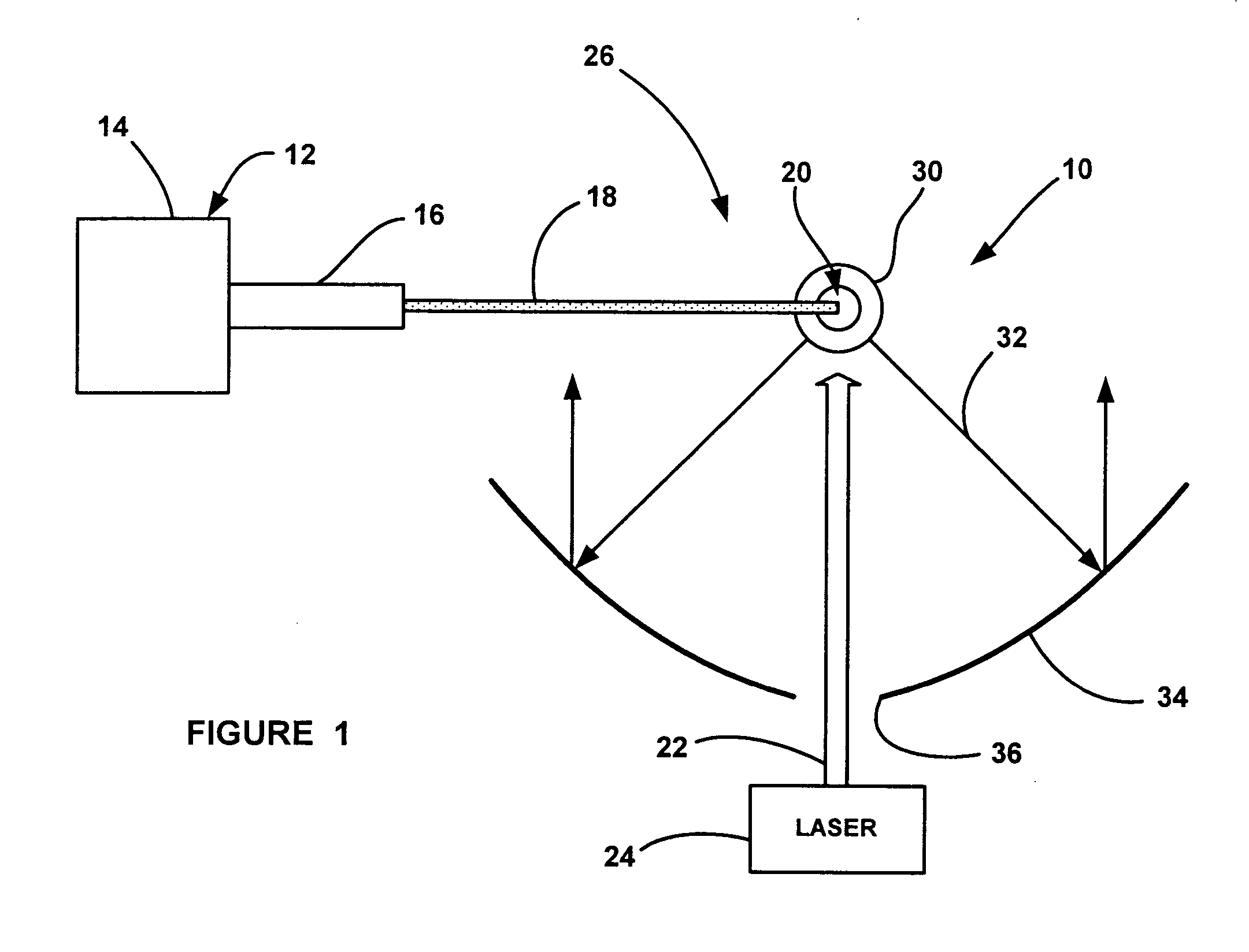
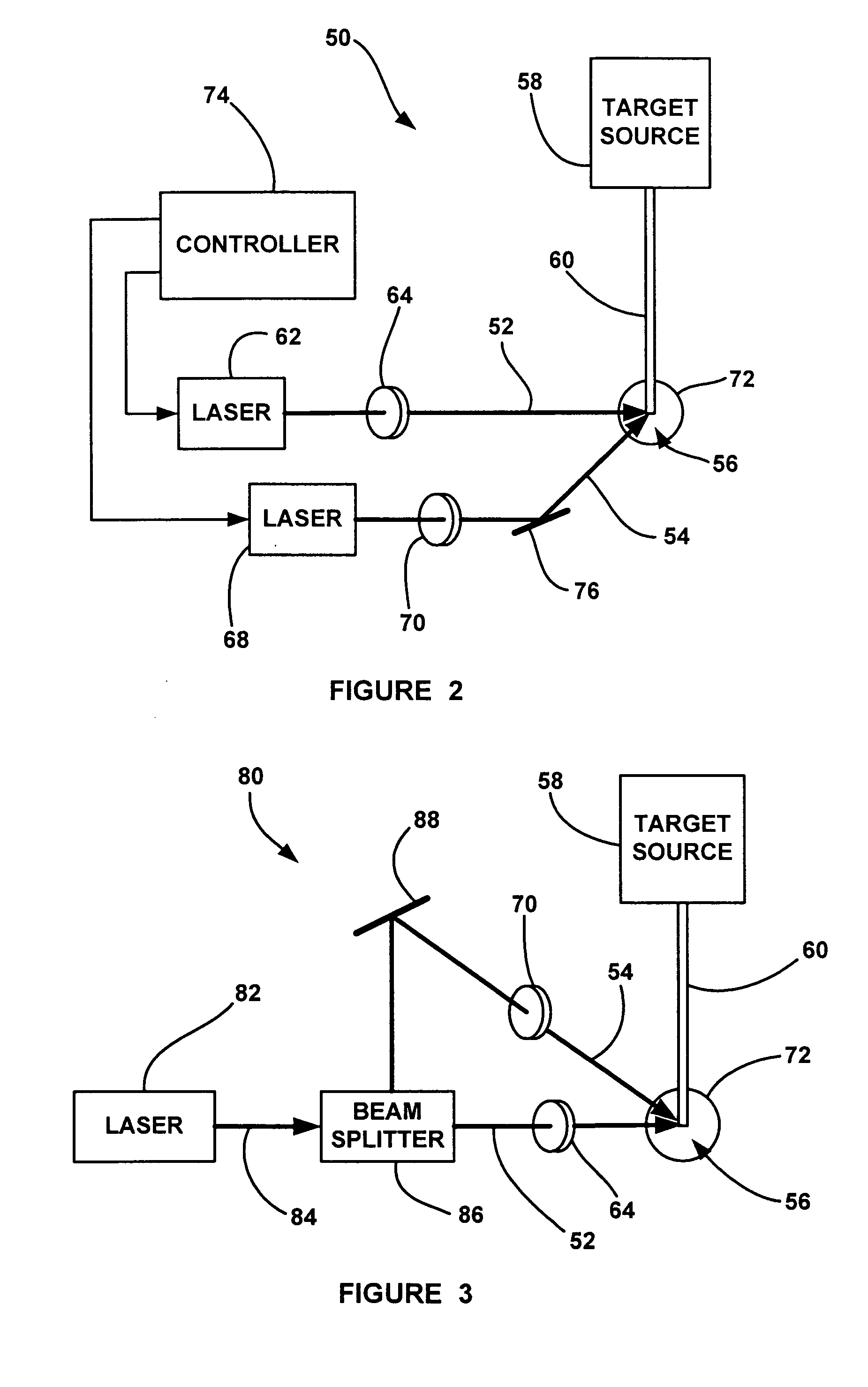
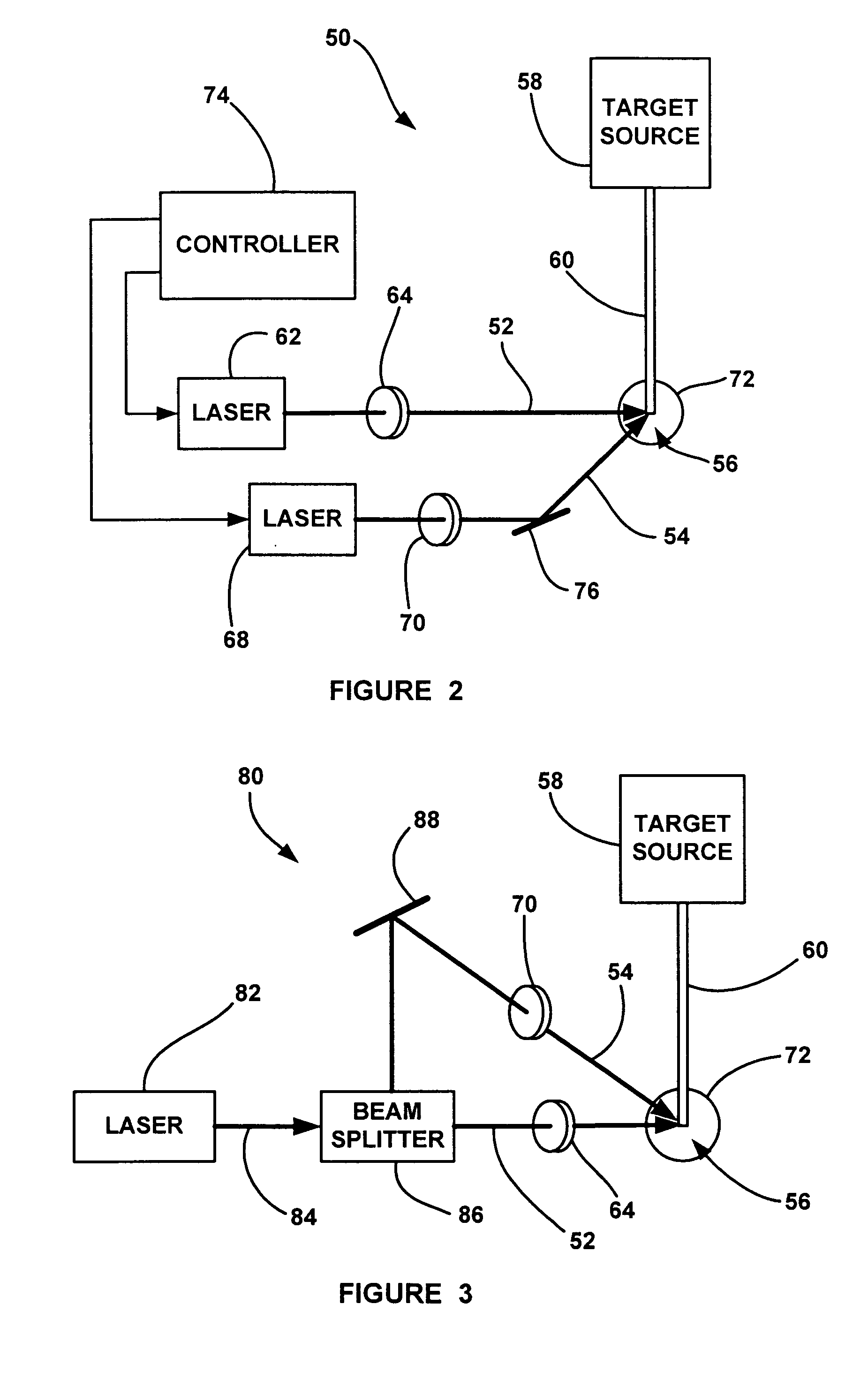
Laser-produced plasma EUV light source with pre-pulse enhancement
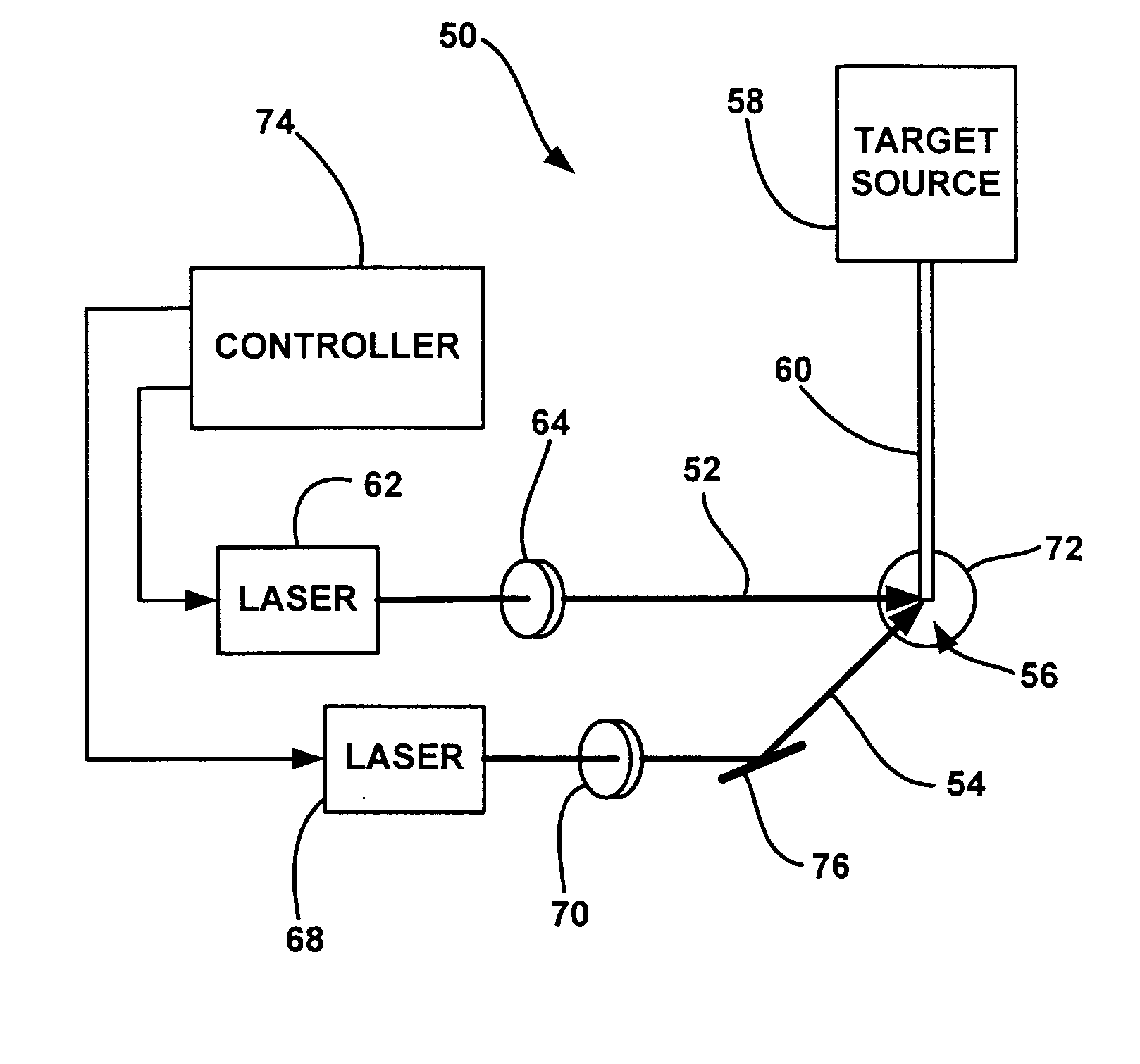
InactiveUS20040264512A1Laser using scattering effectsSemiconductor/solid-state device manufacturingBeam splitterHigh energy
An EUV radiation source that employs a low energy laser pre-pulse and a high energy laser main pulse. The pre-pulse generates a weak plasma in the target area that improves laser absorption of the main laser pulse to improve EUV radiation emissions. High energy ion flux is reduced by collisions in the localized target vapor cloud generated by the pre-pulse. Also, the low energy pre-pulse arrives at the target area 20-200 ns before the main pulse for maximum output intensity. The timing between the pre-pulse and the main pulse can be reduced below 160 ns to provide a lower intensity of the EUV radiation. In one embodiment, the pre-pulse is split from the main pulse by a suitable beam splitter having the proper beam intensity ratio, and the main pulse is delayed to arrive at the target area after the pre-pulse.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Viscosity modifier for lubricating oil and lubricating oil composition
InactiveUS6589920B2Improve the lubrication effectMaintain good propertiesOrganic chemistrySolid fuelsChemical compositionPolystyrene
The lubricating oil composition of the invention comprises a lubricating oil base (A) and a copolymer (B) of ethylene and an alpha-olefin of 3 to 20 carbon atoms. The copolymer (B) of ethylene and an alpha-olefin of 3 to 20 carbon atoms is contained in the composition in an amount of 1 to 20% by weight and has the following properties:(1) the ethylene content (E) is in the range of 40 to 77% by weight,(2) the weight-average molecular weight (Mw) in terms of polystyrene, as measured by GPC, is in the range of 80,000 to 400,000,(3) Mw / Mn is not more than 2.4,(4) the melting point (Tm), as measured by DSC, is not higher than 60° C.,(5) the ethylene content (E, % by weight) and the melting point (Tm, ° C.), as measured by DSC, satisfy the following relation (I):and(6) the intensity ratio, Salphabeta / Salphaalpha, measured by 13C-NMR spectrum is not more than 0.5.
Owner:MITSUI CHEM INC +1
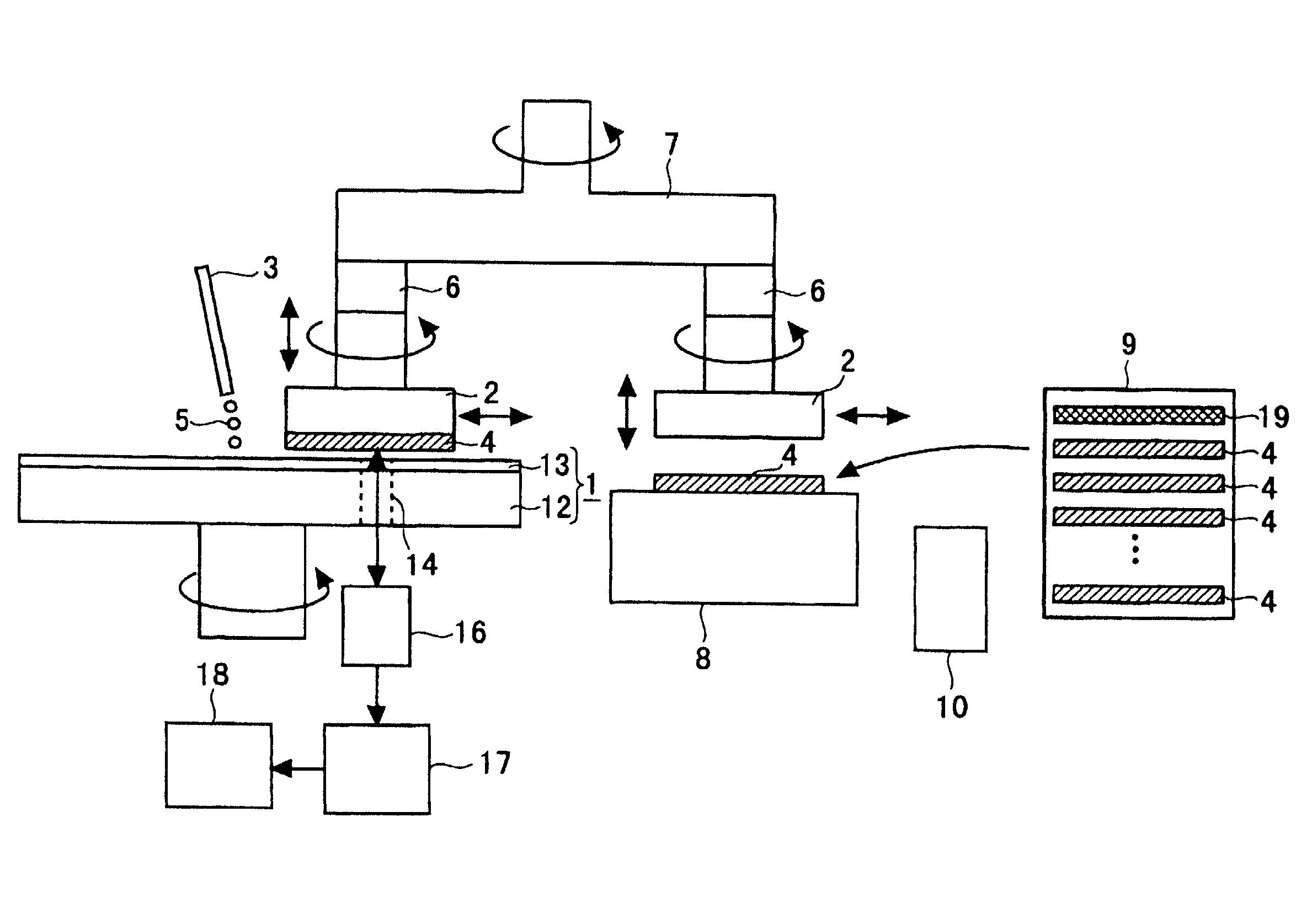
Method of detecting and measuring endpoint of polishing processing and its apparatus and method of manufacturing semiconductor device using the same
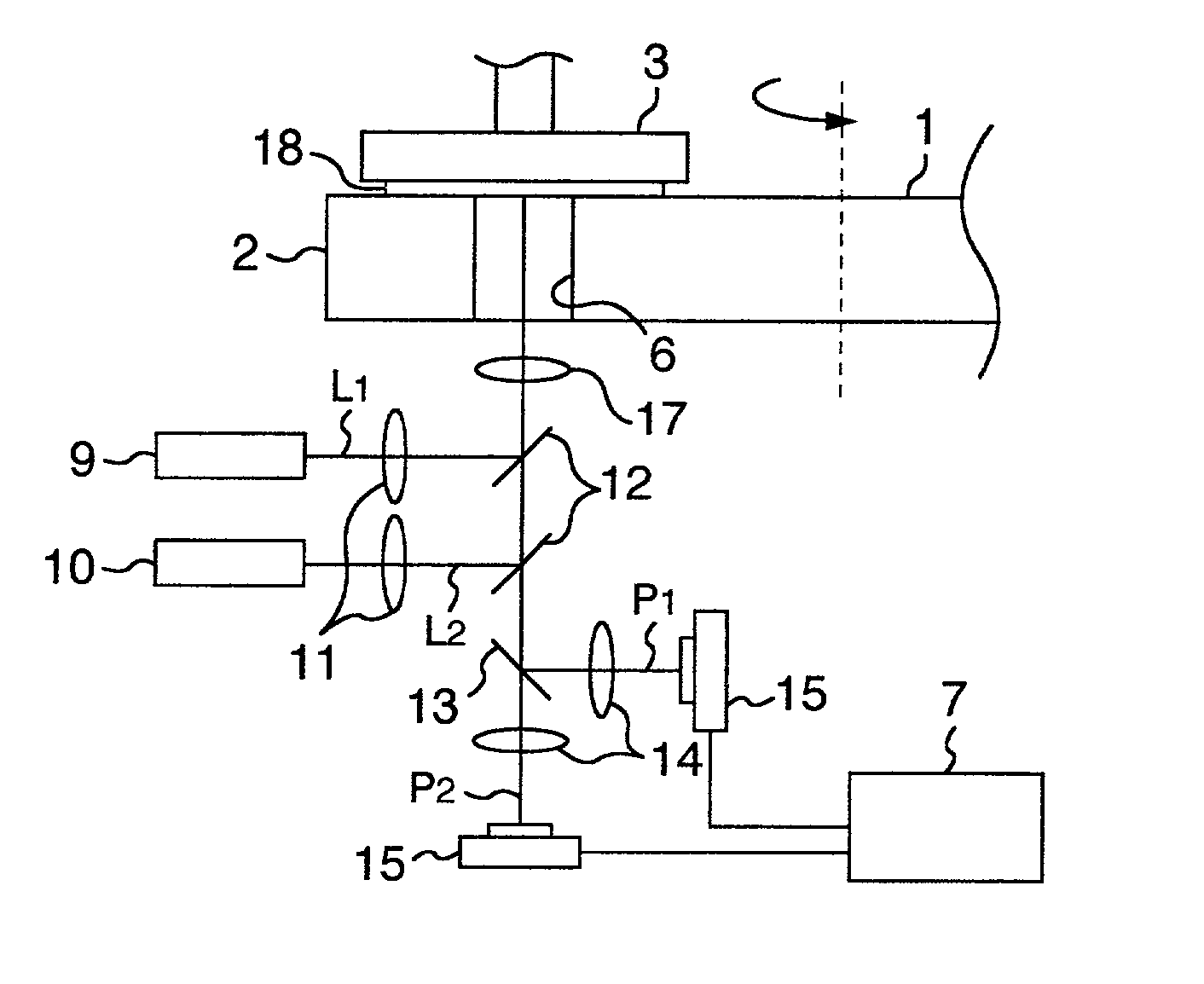
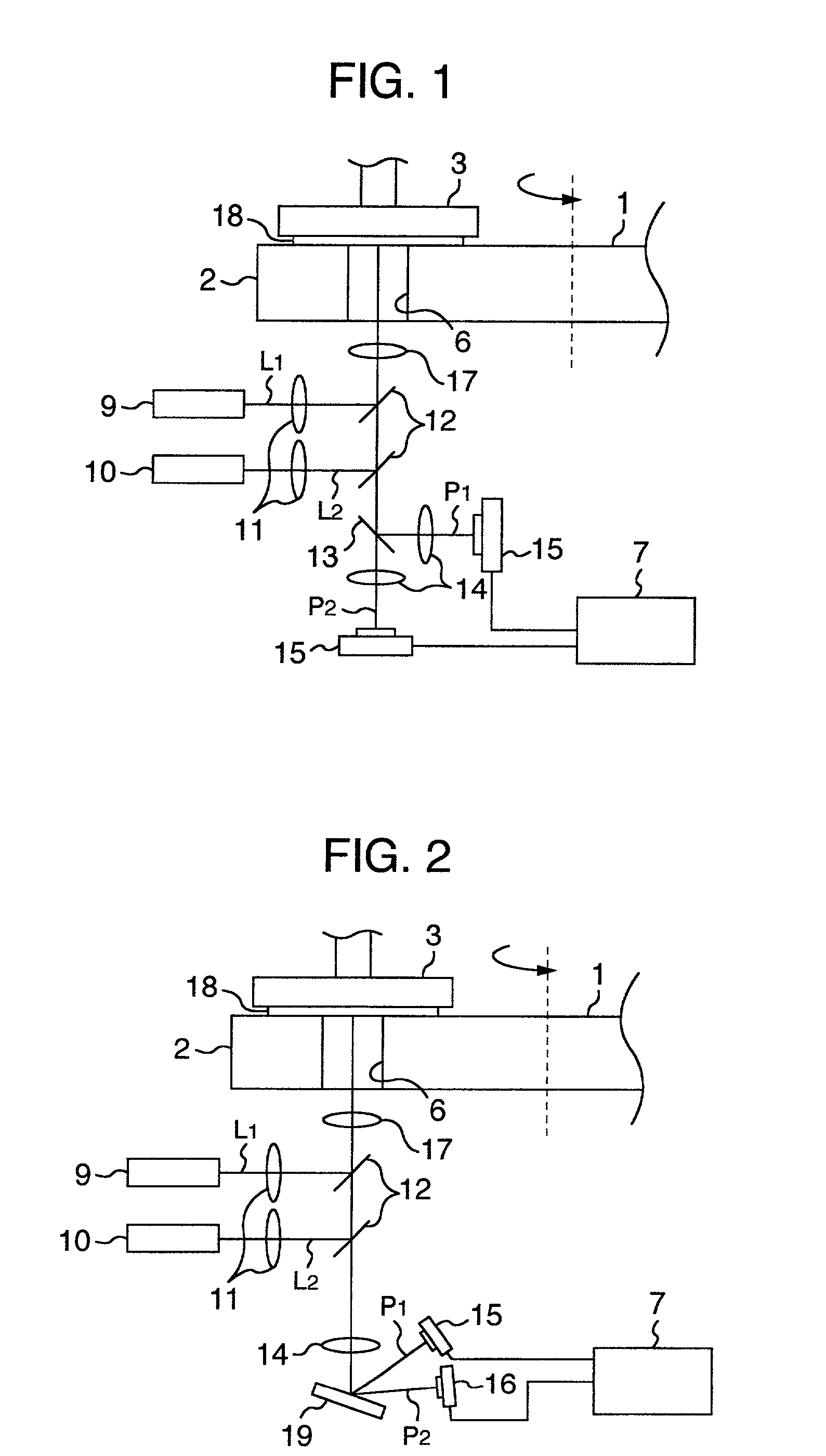
InactiveUS20020127950A1Improve accuracyImprove throughputSemiconductor/solid-state device testing/measurementSemiconductor/solid-state device manufacturingBeam splitterLaser light
Laser sources output laser lights L.sub.1 and L.sub.2 having different wavelengths so as to increase an accuracy of an endpoint detection of polishing processing by enabling an accurate detection of a film thickness of a layer insulating film on a surface of a wafer to be polished by the CMP processing, the lights are emitted from a detection window via a beam splitter to the layer insulating film formed on the surface of the wafer to be polished by a pad, different optical detectors detect interference lights corresponding to the laser lights L.sub.1 and L.sub.2 reflected and generated from a surface of the layer insulating film and a pattern under the surface via the detection window, the beam splitter, and a dichroic mirror, the detection results are supplied to a film thickness evaluation unit 7, a film thickness of the layer insulation film is detected on the basis of a relationship between intensities of the reflected interference lights to the laser lights L.sub.1 and L.sub.2 or the intensity ratio, and an endpoint of polishing processing is determined when the film thickness is equal to a predetermined value.
Owner:HITACHI LTD
Method and apparatus for monitoring polishing state, polishing device, process wafer, semiconductor device, and method of manufacturing semiconductor device
InactiveUS20020127951A1Guaranteed true stateHigh precisionEdge grinding machinesSemiconductor/solid-state device manufacturingEngineeringSemiconductor
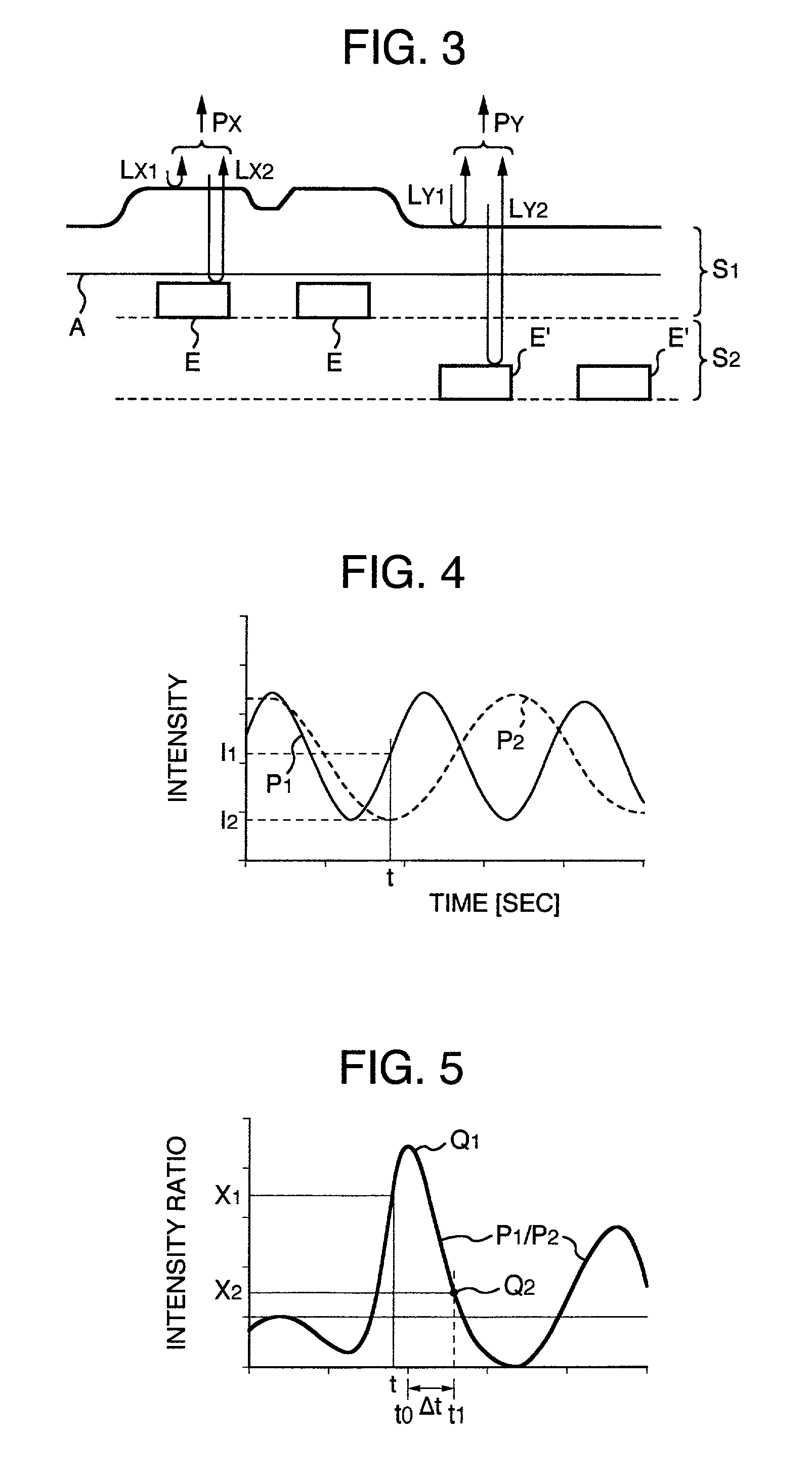
Prior to the polishing of the wafer 4, a reflective body which has the same shape and dimensions as the wafer 4 is held on the polishing head 2 instead of the wafer 4. A polishing agent 5 is interposed between the window 15 of the polishing head 13 and the reflective body, and the reflective body is pressed against the polishing pad 13 with the same pressure as that applied during the polishing of the wafer 4. In this state, the reflective body is irradiated via the window 15 with a probe light emitted from the light source 31, and the spectroscopic intensity of the reflected light is obtained from the sensor 43 as a reference spectrum. During the polishing of the wafer 4, the spectroscopic intensity of the reflected light from the wafer 4 is successively obtained as measured spectra from sensor 43; the intensity ratio of these measured spectra to the above-mentioned reference spectrum is determined, and the polishing state of the wafer 4 is monitored on the basis of this intensity ratio.
Owner:NIKON CORP
Conversion of solid state source output to virtual source
InactiveUS20070138978A1Optical radiation measurementLight source combinationsDownstream processingOptical processing
A light fixture converts source light from one or more solid state light emitting elements to a virtual light source output. An optical element receives and diffuses light from the solid state emitters to form a processed light for the virtual source output. The optical element forms light that is relatively uniform, for example having a substantially Lambertian distribution and / or having a maximum-to-minimum intensity ratio of 2 to 1 or less over the optical area of the virtual source. In the examples, the diffuse optical processing element comprises a cavity having at least one diffusely reflective surface, and the emitting elements supply light into the cavity at locations that result in reflection and diffusion before emission through an aperture of the cavity. The aperture or a downstream processing element appears as the virtual source of the processed light from the cavity.
Owner:ADVANCED OPTICAL TECH
Magnetic tape device employing TMR head and magnetic tape with characterized magnetic layer, and head tracking servo method
ActiveUS10403314B2Improve accuracyAccurate informationMagnetic materials for record carriersAlignment for track following on tapesIn planeX-ray
Owner:FUJIFILM CORP
Magnetic tape device and magnetic reproducing method
ActiveUS10410666B2Improve smoothnessImprove surface smoothnessMaterials with ironRecord information storageIn planeX-ray
The magnetic tape device includes a magnetic tape including a magnetic layer; and a TMR head (reproducing head), in which an intensity ratio of a peak intensity of a diffraction peak of a (110) plane with respect to a peak intensity of a diffraction peak of a (114) plane of a hexagonal ferrite crystal structure obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio of the magnetic tape is 0.65 to 1.00, Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, and a C—H derived C concentration calculated from a C—H peak area ratio of C1s spectra obtained by X-ray photoelectron spectroscopic analysis performed on the surface of the magnetic layer at a photoelectron take-off angle of 10 degrees is 45 to 65 atom %.
Owner:FUJIFILM CORP
Laser-produced plasma EUV light source with pre-pulse enhancement
InactiveUS6973164B2Improves laser absorption of laserLess likely to damageLaser using scattering effectsSemiconductor/solid-state device manufacturingBeam splitterVapor cloud
An EUV radiation source that employs a low energy laser pre-pulse and a high energy laser main pulse. The pre-pulse generates a weak plasma in the target area that improves laser absorption of the main laser pulse to improve EUV radiation emissions. High energy ion flux is reduced by collisions in the localized target vapor cloud generated by the pre-pulse. Also, the low energy pre-pulse arrives at the target area 20–200 ns before the main pulse for maximum output intensity. The timing between the pre-pulse and the main pulse can be reduced below 160 ns to provide a lower intensity of the EUV radiation. In one embodiment, the pre-pulse is split from the main pulse by a suitable beam splitter having the proper beam intensity ratio, and the main pulse is delayed to arrive at the target area after the pre-pulse.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
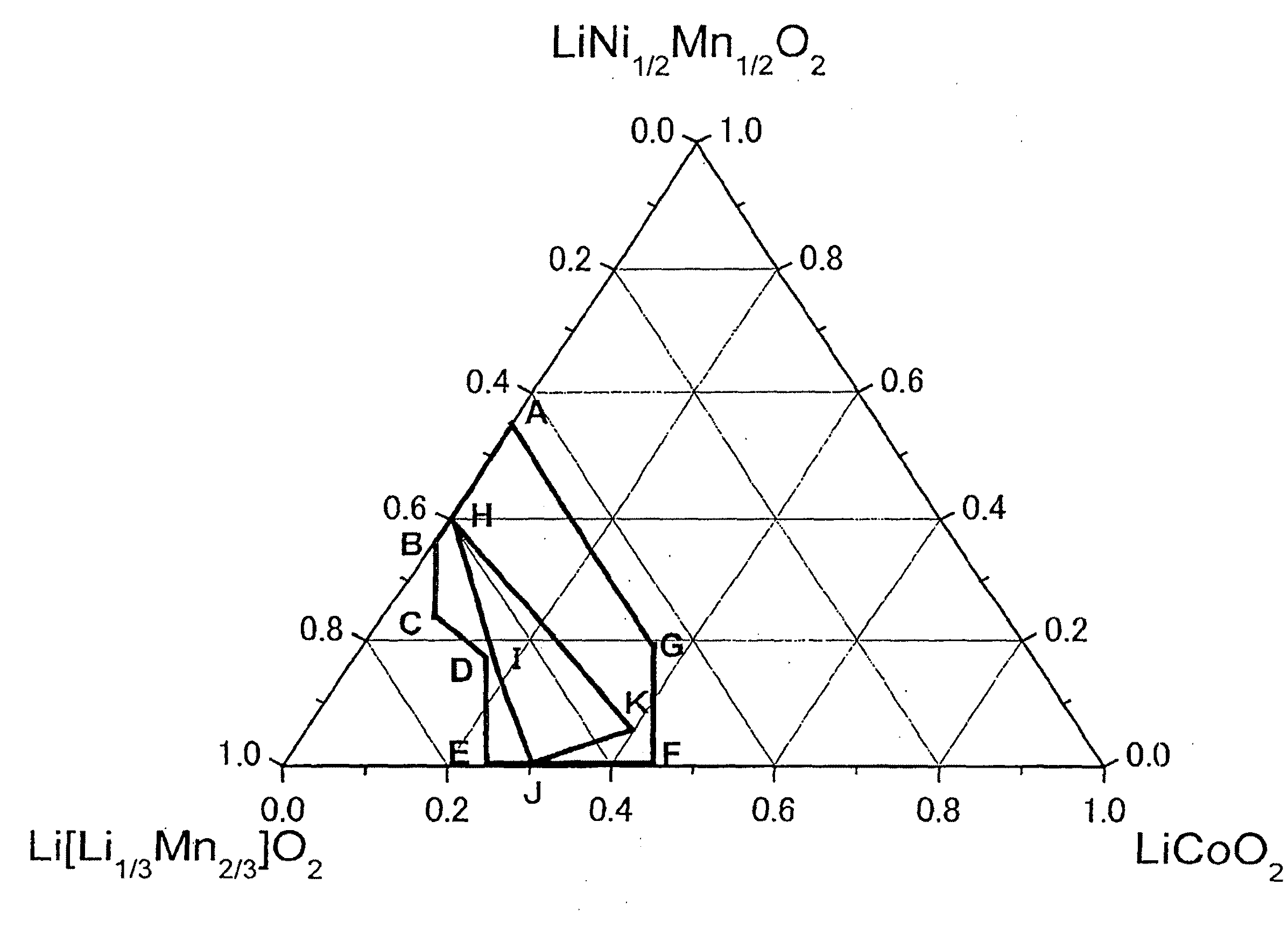
Active material for lithium secondary battery, lithium secondary battery, and method for producing the same
ActiveUS20100233542A1Decrease in battery performanceSufficient discharge capacityBatteries circuit arrangementsElectrode manufacturing processesX-rayCrystal structure
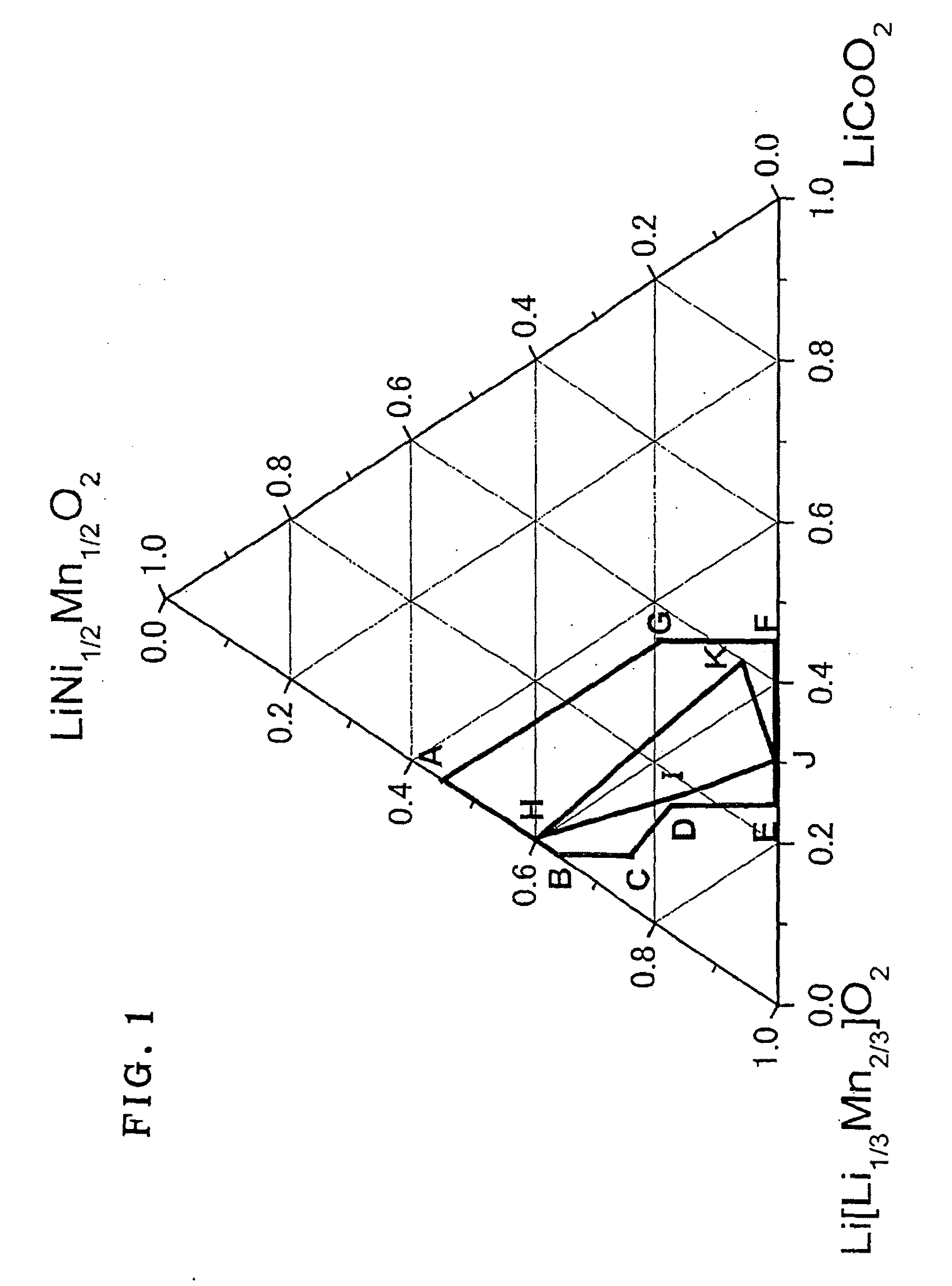
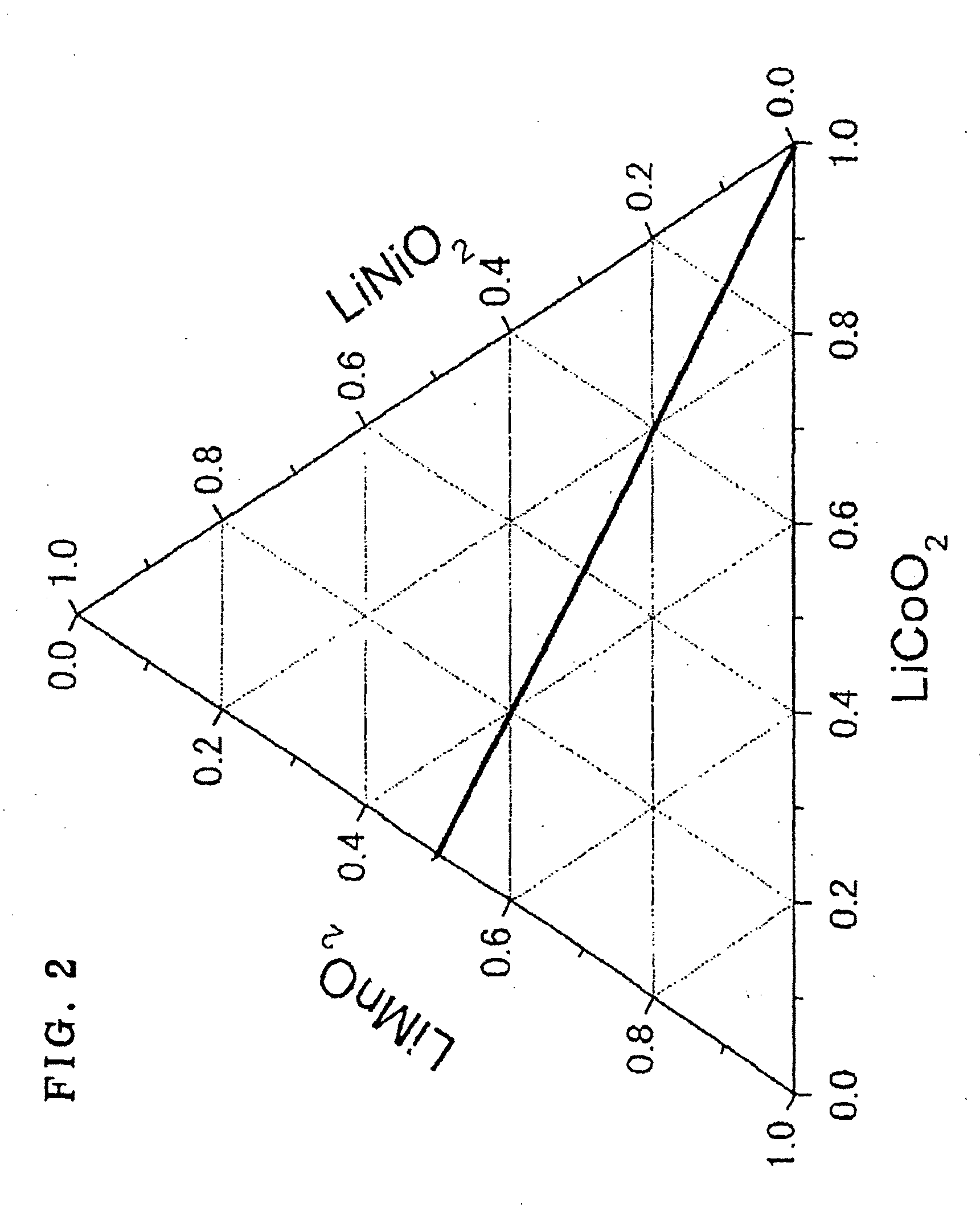
The present invention provides an active material for a lithium secondary battery with a high discharge capacity, particularly for a lithium secondary battery that can increase the discharge capacity in a potential region of 4.3 V or lower, a method for producing the same, a lithium secondary battery having a high discharge capacity, and a method for producing the same. The active material for a lithium secondary battery includes a solid solution of a lithium transition metal composite oxide having an α-NaFeO2 type crystal structure, in which the composition ratio of Li, Co, Ni, and Mn contained in the solid solution satisfies Li1+(1 / 3)xCo1−x−yNi(1 / 2)yMn(2 / 3)x+(1 / 2)y (x+y≦1, 0≦y and 1−x−y=z); in an Li[Li1 / 3Mn2 / 3]O2(x)-LiNi1 / 2Mn1 / 2O2(y)-LiCoO2(z) type ternary phase diagram, (x, y, z) is represented by values in a range present on or within a line of a heptagon (ABCDEFG) defined by the vertexes; point A(0.45, 0.55, 0), point B(0.63, 0.37, 0), point C(0.7, 0.25, 0.05), point D(0.67, 0.18, 0.15), point E(0.75, 0, 0.25), point F(0.55, 0, 0.45), and point G(0.45, 0.2, 0.35); and the intensity ratio between the diffraction peaks on (003) plane and (104) plane measured by X-ray diffractometry before charge-discharge is I(003) / I(104)≧1.56 and at the end of discharge is I(003) / I(104)>1. The invention provides a method for producing the active material for a lithium secondary battery using a coprecipitation method, a lithium secondary battery including a positive electrode containing the active material and a method for producing the lithium secondary battery.
Owner:GS YUASA INT LTD
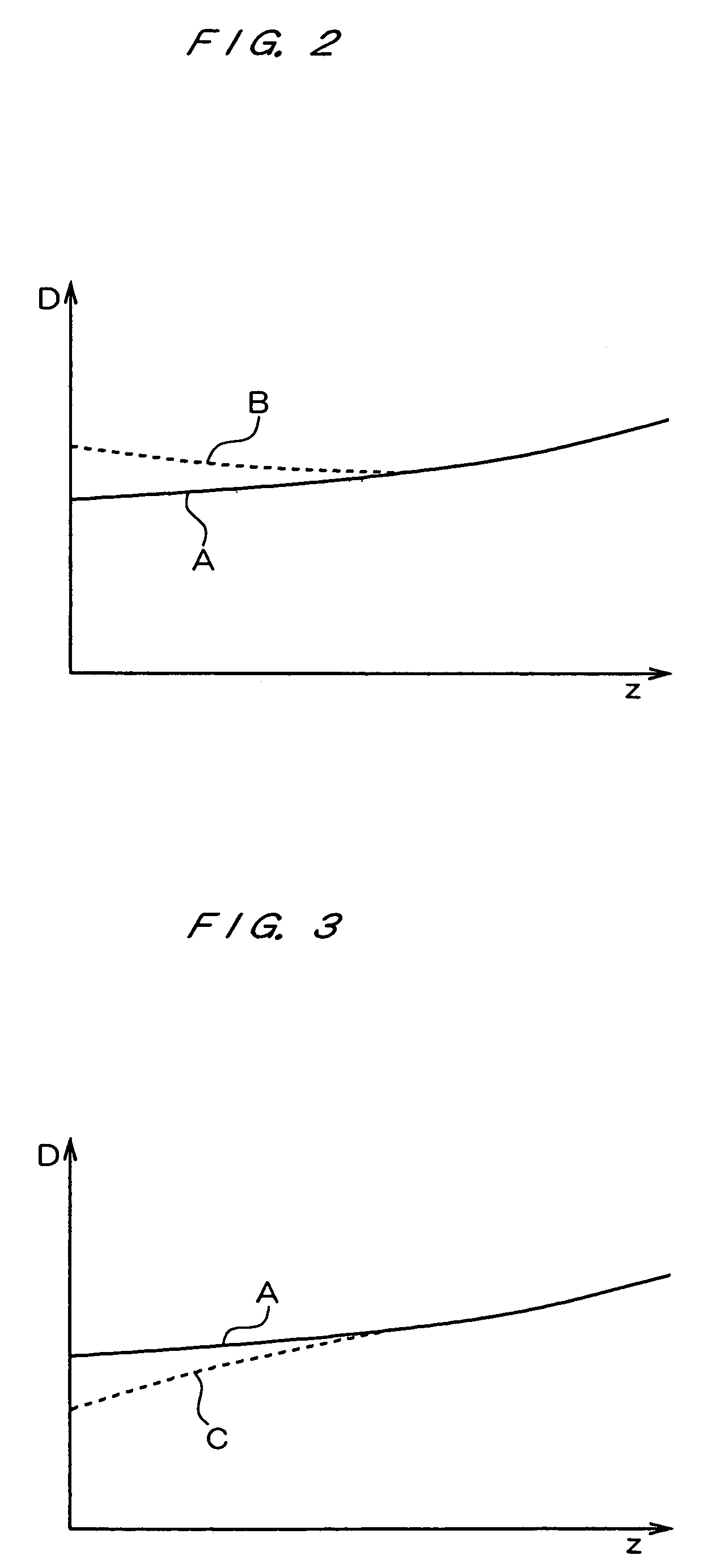
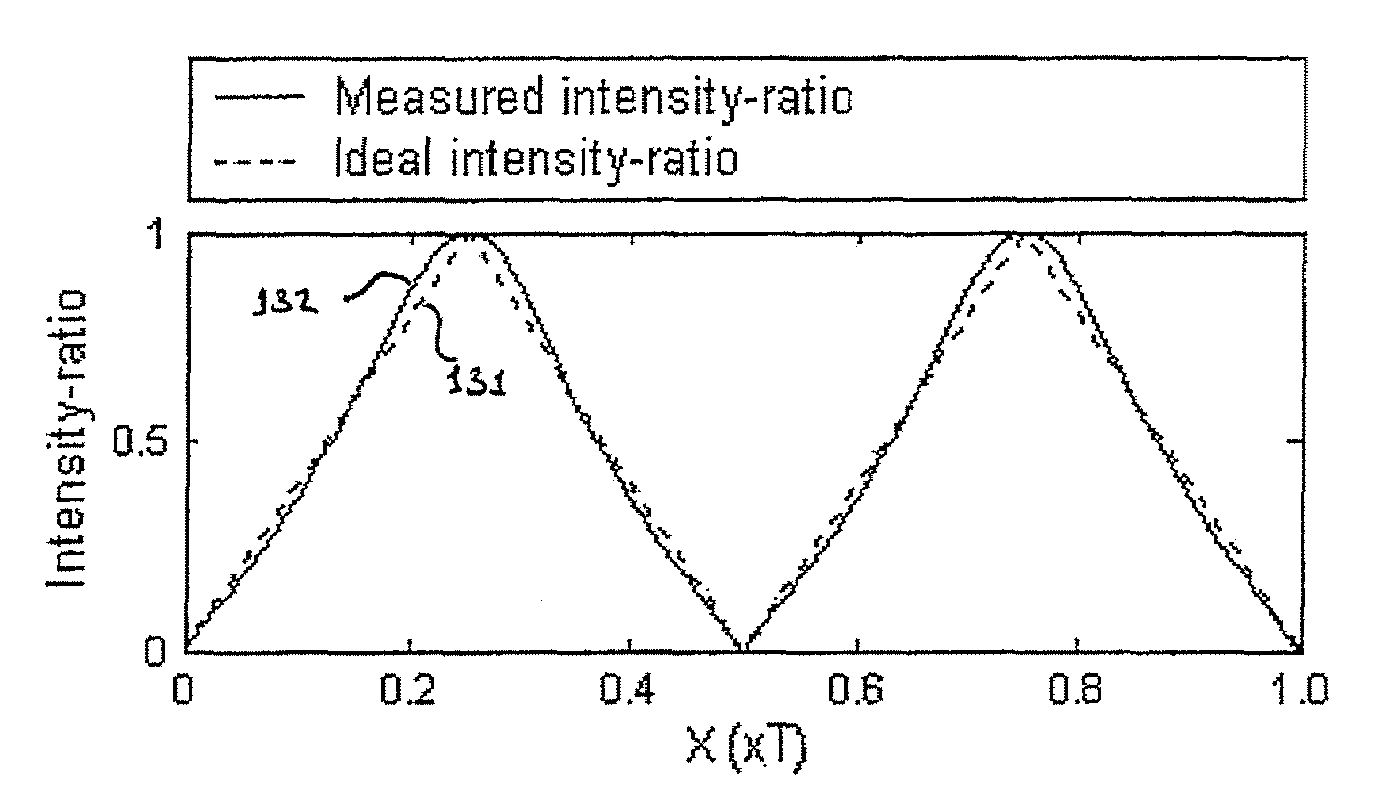
Full-field three-dimensional measurement method
A method and system for full-field fringe-projection for 3-D surface-geometry measurement, referred to as “triangular-pattern phase-shifting” is disclosed. A triangular grey-scale-level-coded fringe pattern is computer generated, projected along a first direction onto an object or scene surface and distorted according to the surface geometry. The 3-D coordinates of points on the surface are calculated by triangulation from distorted triangular fringe-pattern images acquired by a CCD camera along a second direction and a triangular-shape intensity-ratio distribution is obtained from calculation of the captured distorted triangular fringe-pattern images. Removal of the triangular shape of the intensity ratio over each pattern pitch generates a wrapped intensity-ratio distribution obtained by removing the discontinuity of the wrapped image with a modified unwrapping method. Intensity ratio-to-height conversion is used to reconstruct the 3-D surface coordinates of the object. Intensity-ratio error compensation involves estimating intensity-ratio error in a simulation of the measurement process with both real and ideal captured triangular-pattern images obtained from real and ideal gamma non-linearity functions. A look-up table relating the measure intensity-ratio to the corresponding intensity-ratio error is constructed and used for intensity-ratio error compensation. The inventive system is based on two-step phase-shifting but can be extended for multiple-step phase-shifting.
Owner:UNIVERSITY OF WATERLOO
Method and device for simultaneously measuring droplet position, particle sizes and complex refractive index
InactiveCN102003936AReconstruction Spatial PositionEasy to implementPhase-affecting property measurementsParticle size analysisMeasurement deviceRainbow
The invention relates to technology for simultaneously measuring multi-phase flow particles online through multi-parameter, and aims to provide a method and a device for simultaneously measuring droplet positions, particle sizes and a complex refractive index. The method comprises the following steps of: (1) dividing a highly coherent continuous laser beam into two beams after spatial filtering and collimating beam expansion, wherein one beam radiates particles in a detected flow field region, and the other beam is used as a reference beam; (2) mixing scattered light of a lateral 30-degree to 90-degree region of particles in the detected flow field region and the attenuated reference beam to perform interference so as to form a hologram, storing the hologram in a computer after being recorded by a digital camera through an imaging device; (3) acquiring a series of reconstructed images of the detected particles along the depth direction by utilizing digital reconstruction technology; and (4) identifying the reflective spot and the refractive spot of the particles from the reconstructed images by utilizing digital image processing technology so as to acquire space coordinates and scattered light intensity ratios. Compared with rainbow measurement technology, the measurement method has the advantages that: a light path system of the measurement device is relatively simple and is easy to implement.
Owner:ZHEJIANG UNIV
Magnetic tape device and magnetic reproducing method
ActiveUS10410665B2Improve smoothnessImprove surface smoothnessMaterials with ironRecord information storageIn planeX-ray
The magnetic tape device includes a TMR head (reproducing head); and a magnetic tape including a magnetic layer including ferromagnetic hexagonal ferrite powder, a binding agent, and fatty acid ester, in which an XRD intensity ratio obtained by an X-ray diffraction analysis of the magnetic layer by using an In-Plane method is 0.5 to 4.0, a vertical direction squareness ratio is 0.65 to 1.00, Ra measured regarding a surface of the magnetic layer is equal to or smaller than 2.0 nm, full widths at half maximum of spacing distribution measured by optical interferometry regarding the surface of the magnetic layer before and after performing a vacuum heating with respect to the magnetic tape are greater than 0 nm and equal to or smaller than 7.0 nm, and a difference between spacings before and after the vacuum heating is greater than 0 nm and equal to or smaller than 8.0 nm.
Owner:FUJIFILM CORP
Sliding structure and sliding method
InactiveUS20070078067A1Running cost can be reducedCoefficient of frictionVacuum evaporation coatingSputtering coatingSulfurEngineering
According to the present invention, a sliding structure whereby wear resistance of a pair of sliding members that are able to slide relative to each other is improved and the friction coefficient can be reduced is provided. The sliding structure in which a lubricating oil is fed between sliding faces of a pair of sliding members that are able to slide relative to each other such that at least one of the members is allowed to slide is provided. The sliding structure is characterized in that: an amorphous carbon film is formed on the sliding face of at least one sliding member so as to result in a D to G band integrated intensity ratio in the Raman spectrum of between 1.5 and 2.0; and the lubricating oil existing between the sliding faces contains a compound comprising at least molybdenum and sulfur so as to result in a molybdenum content of not less than 400 ppm relative to the lubricating oil.
Owner:TOYOTA JIDOSHA KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com