Solid of tenofovir disoproxil, and preparation method and application thereof
A technology of tenofovir and dipivoxil, applied in the field of pharmaceutical compositions containing these solids, can solve problems such as unstable quality and drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0272] Preparation of Tenofovir Disoproxil
[0273] Add 41.4g of tenofovir monohydrate (commercially available or prepared according to the method disclosed in CN1264387A) at 20~25℃ into 164g of N-methylpyrrolidone, and then add 40g of triethylamine under stirring. After stirring for 0.5 hour at 20-25°C, add 100g of chloromethyl isopropyl carbonate, increase the temperature to 55-65°C and keep the reaction for 5 hours; stop heating, lower the temperature to 20-30°C, add 320g ethyl acetate and purified water 180g, stirred at 0~5℃ and separated, the lower layer was extracted with 110g ethyl acetate at 0~5℃, combined the ethyl acetate layers, washed twice with purified water at 0~5℃, 320g each time, 30~35 Concentrate ethyl acetate at ℃; add 150 mL of cyclohexane to the concentrate, stir at 20-25 ℃ for 10 hours, filter, and rinse with 20 mL of cyclohexane to obtain tenofovir disoproxil as a white solid.
[0274] 1 H NMR(300MHz, DMSO-d 6 )δ: 8.14 (s, 1H), 8.03 (s, 1H), 7.21 (s, 2H), 5....
Embodiment 2
[0277] Preparation of DL-tenofovir disoproxil tartrate and its crystal form A
[0278] At 40~45℃, dissolve 50.0g (96.3mmol) of Tenofovir disoproxil and 14.4g (95.9mmol) of DL-tartaric acid in 4.5L of isopropanol. After the dissolution is complete, stir and cool to 15~20. ℃, continue to stir and crystallize; filter with suction; the filter cake is dried under reduced pressure at 30-35 ℃ to obtain 57.0 g of DL-tenofovir disoproxil tartrate with a yield of 88.5%.
[0279] Tested 1 The H NMR results are: 1 H NMR(300MHz, DMSO-d 6 )δ: 8.13 (s, 1H), 8.03 (s, 1H), 7.30 (s, 2H), 5.57-5.53 (m, 4H), 4.83-4.79 (m, 2H), 4.33-4.32 (d, 2H) , 4.24-4.19 (m, 2H), 4.01-3.96 (m, 3H), 1.24-1.22 (d, 12H), 1.07-1.05 (d, 3H).
[0280] Above 1 In the H NMR results, the signal peaks with chemical shifts at δ8.13 (s, 1H) and 8.03 (s, 1H) were assigned to the two H on the adenine of tenofovir disoproxil, δ4.33- The signal peak at 4.32 (d, 2H) is attributed to the H of the 2 methine groups on DL-tartaric acid....
Embodiment 3
[0290] Preparation of DL-tenofovir disoproxil tartrate and its crystal form A
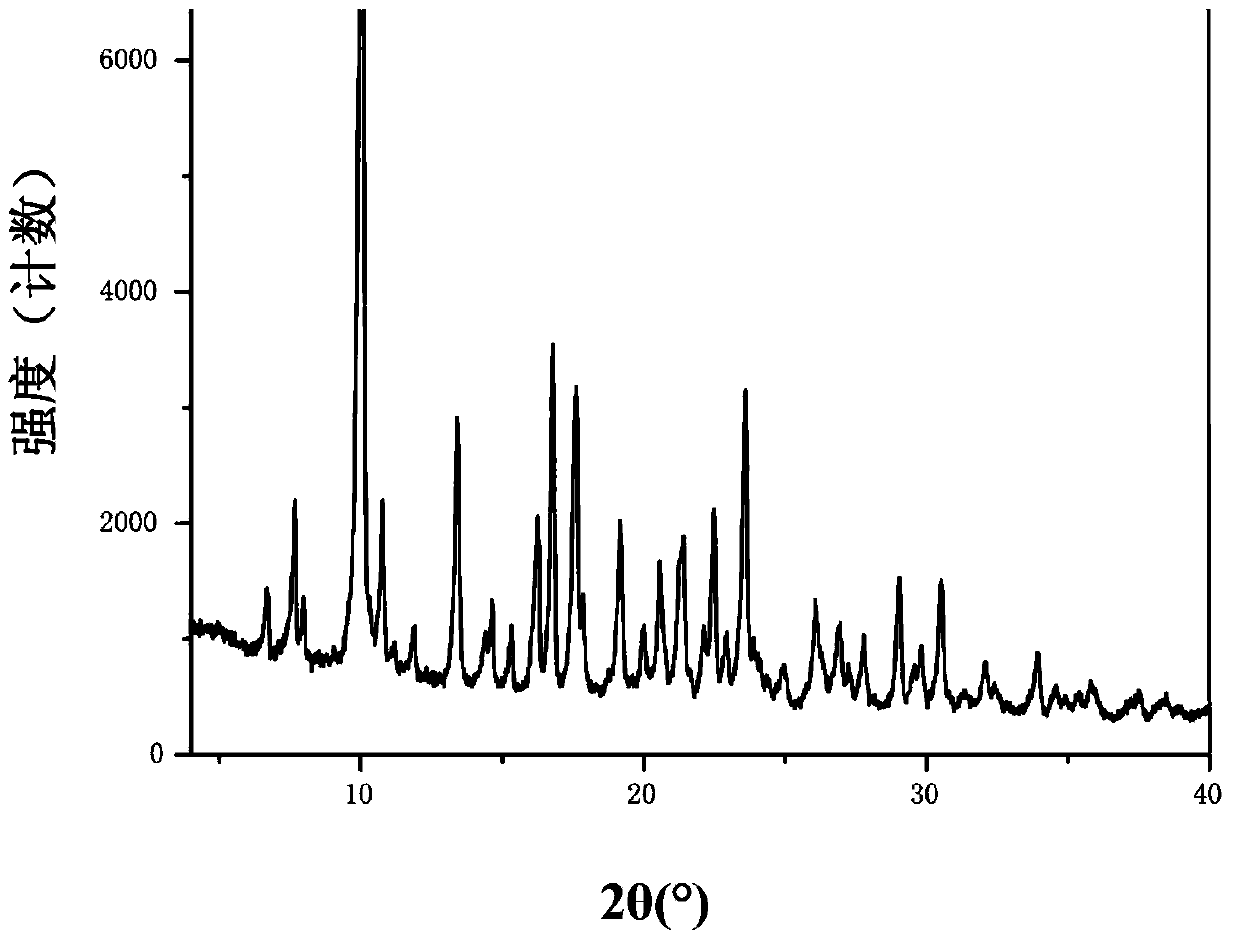
[0291] Dissolve 50.0 g (96.3 mmol) of tenofovir disoproxil and 15.9 g (105.9 mmol) of DL-tartaric acid in a mixed solvent of methanol / ethanol (volume ratio 1 / 1) at 45-50°C, After the dissolution is complete, add 500 mL of isopropyl ether dropwise under the control of the internal temperature of 45~50℃. After the addition is complete, stir and cool to 15~20℃, continue to stir and crystallize; filter with suction; the filter cake is dried under reduced pressure at 35~40℃ DL-Tenofovir disoproxil tartrate crystal form A53.0g was obtained, and the yield was 82.3%. X-ray powder diffraction pattern and figure 1 similar.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com